MOSAIC HIV Prophylactic Vaccine
|
|
|
- Elizabeth Fields
- 9 years ago
- Views:
Transcription
1 MOSAIC HIV Prophylactic Vaccine Overview Development Program Hanneke Schuitemaker Head Viral Vaccines Discovery & Translational Medicine March 16, 2015 Melinda, Goddess of Healing Melinda s artwork reflects her journey living with HIV.
2 HIV Vaccine Efficacy Trials Four Concepts Tested in >30 years VaxGen Merck Sanofi VaxGen VRC Gp120 Ad5 ALVAC AIDSVAX DNA-Ad5 Env protein gag pol nef gag and env gag pol env AIDSVAX trial STEP & HVTN 503 Thai trial (RV144) HVTN 505 Humoral immunity Cellular immunity Humoral and cellular immunity Humoral and cellular immunity No efficacy No efficacy Adverse trend 31% reduction of HIV-1 acquisition No efficacy
3 Consumer Healthcare Pharmaceuticals Medical Devices and Diagnostics Therapeutic Areas Cardiovascular and Metabolic Diseases Neuroscience Infectious Diseases & Vaccines Immunology Oncology Global Public Health Vaccines Therapeutics
4 The Mosaic HIV vaccine research program Funded by NIH/NIAID through the Integrated Pre clinical/clinical AIDS Vaccine Development (IPCAVD) to Dr Dan Barouch at Beth Israel Deaconess Medical Center/Harvard University. Current partners BIDMC MHRP IAVI Additional future (potential) partners Ragon NIAID/HVTN
5 Lessons learned from the Thai trial Thai trial/rv144 study suggests that an HIV-1 vaccine is possible But.improvement is needed Key features desired include: Delivery vehicles and antigens that induce both humoral and cellular immunity Antigens optimized for immunologic coverage of global virus diversity
6 Best-in-class HIV Vaccine Offering Optimal Protection Against all Clades of HIV-1 Different HIV 1 clades dominate in different geographic regions Adolescents (11 17 years) /Adults (18 65 years) in endemic countries and populations at risk in Western world Vectors that elicit optimal immune responses Low seroprevalent Ad26 Ad26.HIV-Gag-Pol Ad26.HIV-Env (MVA.HIV-Gag-Pol-Env) Mosaic inserts for global coverage Trimeric env protein for improved humoral immunity Dan H Barouch et al, 2013 Dan H Barouch et al., 2010
7 Heterologous Prime-Boost with Adeno and Pox vectors harbouring gag-pol-env Mosaic Inserts Elicit Protective Immunity Against heterologous SHIV-SF162P3 Challenges Ad.mos Note: SHIV challenge model ~100 fold more infectious than HIV in humans P Value vs Sham* Per Exposure Risk Reduction Ad/MVA % Ad/Ad % Sham N/A N/A *Cox proportional hazard model Barouch et al. Cell 2013 Correlates of Protection Assay P Value ELISA ADCP NAb
8 Current status and plans: Preclinical NHP studies have demonstrated Proof-of-Concept Heterologous prime-boost regimens delivering mosaic antigens afford partial protection against SHIV-SF162P3 repetitive intrarectal challenges Substantial increase of humoral immunity by gp140 boost affording partial protection in stringent SHIV-SF162P3 and SIVmac251 challenge models Challenge study evaluating regimens as in Phase 1/2a ongoing, data expected in Q3, 2015 Two GLP-Toxicity studies completed Clade C gp140 trimer alone Heterologous prime-boost regimens Conclusions: no toxicologically relevant findings observed; all vaccine regimens well tolerated and immunogenic
9 Aim: Ongoing NHP study #13-19: study design (similar to HIV-V-A004) Gr (#) To determine the best vaccine boost components to achieve broad humoral and cellular immunogenicity and to protect against SHIV SF162P3 challenge in rhesus macaques 0 Mo (2Dec13) 3 Mo (24Feb 2014) 6 Mo (19May 2014) 12 Mo (1Dec 2014) I (n=12) Ad26 mos Ad26 mos Ad26 mos + protein Ad26 mos + protein II (n=12) Ad26 mos Ad26 mos protein protein III (n=12) Ad26 mos Ad26 mos MVA mos + protein MVA mos + protein IV (n=12) Ad26 mos Ad26 mos MVA mos MVA mos V (n=12) Placebo Placebo Placebo Placebo VI (n=12) Ad26 mos Ad26 mos Ad26 mos Ad26 mos Ad26 mos = Ad26.mos1Gag Pol + Ad26.mos1Env + Ad26.mos2Gag Pol (5x10 10 vp in total) Protein (clade C gp140) dosed with adjuvant (250 μg protein μg AdjuPhos) Placebo = saline Challenge with SHIV SF162P3 to start in May 2015 Collaboration with Prof. Dan Barouch, BIDMC, Harvard
10 A prime-boost vaccine regimen aiming at global coverage Prime Boost Ad26 Mosaic vectors gag pol env Ad26 Mosaic vectors gag pol env +/ Soluble trimer gp140 env protein AdAd26.Mos1.gag.pol AdAd26.Mos2.gag.pol AdAd26.Mos1.env MVA Mosaic vectors gag pol env or +/ Soluble trimer gp140 env protein months Regimen to be selected after Phase 1/2a Confidential information proprietary to
11 Clinical Development Program Overview Phase 1/2a Phase 2b/3 Phase 3/ USA, Africa, Asia Safety Regimen selection Dose confirmation USA Evaluation of Mosaic trimer (in parallel) Africa and Asia Efficacy in high risk population USA, LatAm, Europe Efficacy in high risk population Additional trials Lot to lot, bridging Long term efficacy Persistence of Immunity Additional trials popula ons countries BLA MAA submissions
12 Current status and plans on CTM manufacturing Ad26.Mos trivalent drug product for Phase 1/2a manufactured and released Ad26.Mos1.env, Ad26.Mos1.gagpol, Ad26.Mos2.gagpol in 2:1:1 ratio Late stage development activities initiated Clade C GP140 and adjuvant (Aluminum Phosphate) for Phase 1/2a manufactured and released Mosaic GP140 program started Late stage development activities initiated MVA.Mos1 and MVA.Mos2 drug products for Phase 1/2a provided by the MHRP Final release completed by MHRP and Janssen Late stage supply under Janssen responsibility
13 Overall Early Clinical Development Plan Target vaccine regimen will have 2 or 3 components Adeno MVA Protein Establish safety of each component separate FIH studies HIV-V-A002/MENSCH HIV-V-A003 HIV-V-A004/APPROACH HPX2003: evaluation of Mosaic GP140
14 Overall Early Clinical Development Plan FIH safety of MVA-Mosaic in HIV-V-A002/MENSCH To assess the safety of MVA Mosaic when given as a late boost to subjects previously vaccinated with Ad26.ENVA (in IPCAVD001) and naïve subjects Clinical site: Brigham and Women s Hospital, Boston Population: healthy subjects, yo; N= up to 32 IND sponsor: Crucell-Janssen Co-funder: Ragon Institute Vaccinations ongoing Immuno interim analysis 3Q15
15 Overall Early Clinical Development Plan FIH safety of Clade C gp140 protein in HIV-V-A003 To assess the safety of 2 dose levels of GP140 Clade C with Aluminum phosphate Clinical site: single site in USA Population: healthy subjects, yo; N= 50 Low dose groups fully enrolled High dose groups have begun dosing mid March 2015
16 Overall Early Clinical Development Plan FIH safety of Ad26.Mos.HIV in HIV-V-A004/APPROACH To assess the safety and immunogenicity of the 3 components in prime boost regimens; regimen selection study Clinical sites: USA, Uganda, Rwanda, South Africa, Thailand Population: healthy subjects, age yrs; N= 400 Vaccinations ongoing in US since January African sites on track to start March April Thailand sites to start June
17 HIV-V-A004 = APPROACH The path or route to the start of a technical climb. Although this is generally a walk or, at most, a scramble it is occasionally as challenging as the climb itself
18 Study Design Wk 0 Wk 12 Wk24 Wk48 boost Healthy volunteers 18 to 50 yo 400 subjects, equal randomization to one of 7 regimens and placebo follow-up 4 wk screening 48 wks Note: for a subset of subjects who consent, mucosal samples will be collected (cervicovaginal, ano-rectal, ejaculate) and/or microbiome analysis will be performed Confidential information Confidential proprietary For internal to Janssen/Crucell use only
19 APPROACH Trial Design: a multicenter, randomized, parallel group, placebo-controlled, double-blind clinical trial in healthy HIVuninfected adults Primary endpoint Group N Month 0 (baseline) Week 12 Week 24 Week 48 Booster Group 1 50 Ad26.Mos.HIV Ad26.Mos.HIV Ad26.Mos.HIV + gp140 (250 µg/adj*) Ad26.Mos.HIV + gp140 (250 µg/adj) Group 2 50 Ad26.Mos.HIV Ad26.Mos.HIV Ad26.Mos.HIV + gp140 (50 µg/adj) Ad26.Mos.HIV + gp140 (50 µg/adj) Group 3 50 Ad26.Mos.HIV Ad26.Mos.HIV Ad26.Mos.HIV Ad26.Mos.HIV Group 4 50 Ad26.Mos.HIV Ad26.Mos.HIV MVA Mos + gp140 (250 µg/adj) MVA Mos + gp140 (250 µg/adj) 12 month follow-up Group 5 50 Ad26.Mos.HIV Ad26.Mos.HIV MVA Mos + gp140 (50 µg/adj) MVA Mos + gp140 (50 µg/adj) Group 6 50 Ad26.Mos.HIV Ad26.Mos.HIV MVA Mos MVA Mos Group 7 50 Ad26.Mos.HIV Ad26.Mos.HIV gp140 (250 µg/adj gp140 (250 µg/adj) Group 8 50 Placebo Placebo Placebo Placebo *Adj=AdjuPhos
20 (Anticipated) Clinical sites for HIV-V-A004 APPROACH US 1. University of Colorado, Anshultz Medical Campus 2. Miami Research Associates (MRA) 3. Brigham and Women s Hospital (BWH) THAILAND 4. Armed Forces Research Institute of Medical Sciences (AFRIMS) 5. Vaccine Trial Centre (Mahidol) UGANDA 6. Makerere University Walter Reed Project (MUWRP) 7. Uganda Virus Research Institute (UVRI) SOUTH AFRICA 8. Desmund Tutu HIV Centre (DTHC) 9. AURUM - Klerksdorp site 10. Perinatal HIV Research Centre (PHRU) 11. Centre for the AIDS Programme of Research in South Africa (CAPRISA) RWANDA 12. Projet San Francisco (PSF)
21 From APPROACH to Efficacy trials Developing criteria for regimen selection and Go/no Go The optimal regimen is hypothesized to elicit a well balanced immune response with both antibody and T cell immunity broad coverage of HIV clades A, B and C For the choice of regimen, emphasis will be on immunological correlates that have been identified to correlate with a reduced risk of SIV/SHIV infection in NHP and on immunological correlates that have been identified to correlate with a reduced risk of HIV infection in RV144 For the Go/No Go criteria, emphasis will be on Antibodies and cellular responses as a measure of vaccine take Providing an indication that the elicited antibodies are functional
RSA & HIV vaccine efficacy studies. Glenda Gray 15 March 2016
 RSA & HIV vaccine efficacy studies Glenda Gray 15 March 2016 3 strategies that are advancing Efficacy Studies P5 Clade C approach using ALVAC & gp120/mf59 (HVTN 702) Multi-clade approach using rad26/mva/gp140
RSA & HIV vaccine efficacy studies Glenda Gray 15 March 2016 3 strategies that are advancing Efficacy Studies P5 Clade C approach using ALVAC & gp120/mf59 (HVTN 702) Multi-clade approach using rad26/mva/gp140
TOWARDS AN HIV VACCINE
 why is it so hard to make an HIV vaccine and where are we now? Neal Nathanson, MD Emeritus Professor Department of Microbiology University of Pennsylvania School of Medicine 1 Estimated number of persons
why is it so hard to make an HIV vaccine and where are we now? Neal Nathanson, MD Emeritus Professor Department of Microbiology University of Pennsylvania School of Medicine 1 Estimated number of persons
Regulatory Pathways for Licensure and Use of Ebola Virus Vaccines During the Current Outbreak FDA Perspective
 Regulatory Pathways for Licensure and Use of Ebola Virus Vaccines During the Current Outbreak FDA Perspective Office of Vaccines Research and Review Center for Biologics Evaluation and Research U.S. Food
Regulatory Pathways for Licensure and Use of Ebola Virus Vaccines During the Current Outbreak FDA Perspective Office of Vaccines Research and Review Center for Biologics Evaluation and Research U.S. Food
Third Meeting of Developing Countries' Vaccine Regulators Network Regulatory Forum on HIV Vaccines November 17-18, 2005
 Third Meeting of Developing Countries' Vaccine Regulators Network Regulatory Forum on HIV Vaccines November 17-18, 2005 HIV Vaccines in Asia:- Welcome Address given by WR Thailand. Introduction of attendants,
Third Meeting of Developing Countries' Vaccine Regulators Network Regulatory Forum on HIV Vaccines November 17-18, 2005 HIV Vaccines in Asia:- Welcome Address given by WR Thailand. Introduction of attendants,
Overview of Phase 1 Oncology Trials of Biologic Therapeutics
 Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
FAST TRACK DEVELOPMENT OF EBOLA VACCINES: FDA REGULATORY PERSPECTIVE
 FAST TRACK DEVELOPMENT OF EBOLA VACCINES: FDA REGULATORY PERSPECTIVE Marion Gruber, Ph.D. Director Office of Vaccines Research & Review Center for Biologics Evaluation and Research US Food and Drug Administration
FAST TRACK DEVELOPMENT OF EBOLA VACCINES: FDA REGULATORY PERSPECTIVE Marion Gruber, Ph.D. Director Office of Vaccines Research & Review Center for Biologics Evaluation and Research US Food and Drug Administration
Prospects for Vaccines against Hepatitis C Viruses. T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS
 Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
The Cell Therapy Catapult
 The Cell Therapy Catapult Keith Thompson CEO January 29 2013 [email protected] Catapult is a Technology Strategy Board programme The Launch of Catapults Hauser 2 Hauser Report Creating new manufacturing
The Cell Therapy Catapult Keith Thompson CEO January 29 2013 [email protected] Catapult is a Technology Strategy Board programme The Launch of Catapults Hauser 2 Hauser Report Creating new manufacturing
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
HSA Consumer Guide. Understanding Vaccines, Vaccine Development and Production. www.hsa.gov.sg November 2009. How a Vaccine Works.
 November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO
 Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO Statistically significant difference in progression-free survival continues to be seen in non-squamous
Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO Statistically significant difference in progression-free survival continues to be seen in non-squamous
William Atkinson, MD, MPH Hepatitis B Vaccine Issues June 16, 2016
 William Atkinson, MD, MPH Hepatitis B Vaccine Issues June 16, 2016 Advisory Committee on Immunization Practices (ACIP) The recommendations to be discussed are primarily those of the ACIP composed of 15
William Atkinson, MD, MPH Hepatitis B Vaccine Issues June 16, 2016 Advisory Committee on Immunization Practices (ACIP) The recommendations to be discussed are primarily those of the ACIP composed of 15
Sheffield Kidney Institute. Planning a Clinical Trial
 Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
R HIV Vaccine Safety and Adleviation of Virus Vector
 a Publication of the HIV Vaccine Trials Network Volume 5, issue 1 OCTOBER, 2013 CLOSING IN ON Probing scientific questions to advance HIV vaccine development Tracey Day and Cecilia Morgan Several HVTN
a Publication of the HIV Vaccine Trials Network Volume 5, issue 1 OCTOBER, 2013 CLOSING IN ON Probing scientific questions to advance HIV vaccine development Tracey Day and Cecilia Morgan Several HVTN
Trial Description. Organizational Data. Secondary IDs
 Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Mid-Clinical Stage Antiviral Drug Development Company
 BIOTRON LIMITED (ASX:BIT) Mid-Clinical Stage Antiviral Drug Development Company Investor Update 20 August 2015 Dr Michelle Miller Managing Director +61 412 313329 [email protected] www.biotron.com.au
BIOTRON LIMITED (ASX:BIT) Mid-Clinical Stage Antiviral Drug Development Company Investor Update 20 August 2015 Dr Michelle Miller Managing Director +61 412 313329 [email protected] www.biotron.com.au
Frequently Asked Questions (FAQs)
 Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
HIV (Human Immunodeficiency Virus) Screening and Pre-Exposure Prophylaxis Guideline
 HIV (Human Immunodeficiency Virus) Screening and Pre-Exposure Prophylaxis Guideline Background... 2 Screening... 2 Recommendations... 2 Ordering and consent... 2 Indications for Periodic HIV Screening...
HIV (Human Immunodeficiency Virus) Screening and Pre-Exposure Prophylaxis Guideline Background... 2 Screening... 2 Recommendations... 2 Ordering and consent... 2 Indications for Periodic HIV Screening...
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
New Advances in Cancer Treatments. March 2015
 New Advances in Cancer Treatments March 2015 Safe Harbour Statement This presentation document contains certain forward-looking statements and information (collectively, forward-looking statements ) within
New Advances in Cancer Treatments March 2015 Safe Harbour Statement This presentation document contains certain forward-looking statements and information (collectively, forward-looking statements ) within
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
VACCINATIONS FOR ADULT HORSES
 VACCINATIONS FOR ADULT HORSES **ALL VACCINATION PROGRAMS SHOULD BE DEVELOPED IN CONSULTATION WITH A LICENSED VETERINARIAN** CORE VACCINATIONS protect against diseases that are endemic to a region, are
VACCINATIONS FOR ADULT HORSES **ALL VACCINATION PROGRAMS SHOULD BE DEVELOPED IN CONSULTATION WITH A LICENSED VETERINARIAN** CORE VACCINATIONS protect against diseases that are endemic to a region, are
How To Kill Jesuva
 Summary of Key Points WHO Position Paper on Vaccines against Japanese Encephalitis (JE) February 2015 1 Background l Japanese Encephalitis Virus (JEV) is the leading cause of viral encephalitis in Asia
Summary of Key Points WHO Position Paper on Vaccines against Japanese Encephalitis (JE) February 2015 1 Background l Japanese Encephalitis Virus (JEV) is the leading cause of viral encephalitis in Asia
MIAMI DADE COLLEGE MEDICAL CAMPUS SCHOOL OF HEALTH SCIENCES EMERGENCY MEDICAL SERVICES Emergency Medical Technician (EMT) Application Packet
 MEDICAL CAMPUS SCHOOL OF HEALTH SCIENCES EMERGENCY MEDICAL SERVICES Emergency Medical Technician (EMT) Application Packet Student Name (Print) Student Number The information in this 8 - page packet must
MEDICAL CAMPUS SCHOOL OF HEALTH SCIENCES EMERGENCY MEDICAL SERVICES Emergency Medical Technician (EMT) Application Packet Student Name (Print) Student Number The information in this 8 - page packet must
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Are Booster Doses of Hepatitis B Vaccine Necessary?
 Are Booster Doses of Hepatitis B Vaccine Necessary? Current CDC Recommendations And Gaps in Knowledge Division of Viral Hepatitis Centers for Disease Control and Prevention, USA Current United States Recommendations
Are Booster Doses of Hepatitis B Vaccine Necessary? Current CDC Recommendations And Gaps in Knowledge Division of Viral Hepatitis Centers for Disease Control and Prevention, USA Current United States Recommendations
Clinical trials in haemophilia
 Clinical trials in haemophilia Dr. Paul Giangrande Oxford Haemophilia and Thrombosis Centre & Nuffield Department of Clinical Medicine University of Oxford [email protected] Why do clinical
Clinical trials in haemophilia Dr. Paul Giangrande Oxford Haemophilia and Thrombosis Centre & Nuffield Department of Clinical Medicine University of Oxford [email protected] Why do clinical
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
A clinical research organization
 A clinical research organization About Us State of art facility. All clinical trials carried out in accordance with ICH GCP guidelines. Quality services within stipulated time period. Team of experienced
A clinical research organization About Us State of art facility. All clinical trials carried out in accordance with ICH GCP guidelines. Quality services within stipulated time period. Team of experienced
2015-10-26 10:58 FOR UK AND EMEA MEDICAL MEDIA ONLY. (Logo: http://photos.prnewswire.com/prnh/20140324/ny88746logo)
 2015-10-26 10:58 Janssen Receives Positive CHMP Opinion Recommending EDURANT(R)Black Triangle Drug (rilpivirine) for the Treatment of Adolescents Aged 12 to
2015-10-26 10:58 Janssen Receives Positive CHMP Opinion Recommending EDURANT(R)Black Triangle Drug (rilpivirine) for the Treatment of Adolescents Aged 12 to
Immunity and how vaccines work
 1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
ICH Topic E 8 General Considerations for Clinical Trials. Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95)
 European Medicines Agency March 1998 CPMP/ICH/291/95 ICH Topic E 8 General Considerations for Clinical Trials Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95) TRANSMISSION
European Medicines Agency March 1998 CPMP/ICH/291/95 ICH Topic E 8 General Considerations for Clinical Trials Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95) TRANSMISSION
Glossary of Clinical Trial Terms
 Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Summary of Key Points WHO Position Paper on Vaccines against Human Papillomavirus (HPV) October 2014
 Summary of Key Points WHO Position Paper on Vaccines against Human Papillomavirus (HPV) October 2014 1 Background l Selected types of HPV cause cervical cancer, anogenital warts, and other anogenital and
Summary of Key Points WHO Position Paper on Vaccines against Human Papillomavirus (HPV) October 2014 1 Background l Selected types of HPV cause cervical cancer, anogenital warts, and other anogenital and
Study Design. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D. Ellis Unger, M.D. Ghanshyam Gupta, Ph.D. Chief, Therapeutics Evaluation Branch
 BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
INTERPRETATION INFORMATION SHEET
 Creative Testing Solutions 2424 West Erie Dr. 2205 Highway 121 10100 Martin Luther King Jr. St. No. Tempe, AZ 85282 Bedford, TX 76021 St. Petersburg, FL 33716 INTERPRETATION INFORMATION SHEET Human Immunodeficiency
Creative Testing Solutions 2424 West Erie Dr. 2205 Highway 121 10100 Martin Luther King Jr. St. No. Tempe, AZ 85282 Bedford, TX 76021 St. Petersburg, FL 33716 INTERPRETATION INFORMATION SHEET Human Immunodeficiency
Session 6 Clinical Trial Assessment Phase I Clinical Trial
 L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
Diploma Course on Research & Development of Products to Meet Public Health Needs
 Diploma Course on Research & Development of Products to Meet Public Health Needs Organized by Nagasaki University, Japan and Faculty of Allied Health Sciences, Thammasat University, in cooperation with
Diploma Course on Research & Development of Products to Meet Public Health Needs Organized by Nagasaki University, Japan and Faculty of Allied Health Sciences, Thammasat University, in cooperation with
Alzheimer s and Herpes Zoster
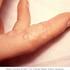 The relationship between Alzheimer's and herpes zoster should be studied. A solid link between herpes simplex virus-1 (HSV1) and Alzheimer's has been found. See: "Cold Sore Virus Linked To Alzheimer's
The relationship between Alzheimer's and herpes zoster should be studied. A solid link between herpes simplex virus-1 (HSV1) and Alzheimer's has been found. See: "Cold Sore Virus Linked To Alzheimer's
When an occupational exposure occurs, the source patient should be evaluated for both hepatitis B and hepatitis C. (AII)
 XI. OCCUPATIONAL EXPOSURES TO HEPATITIS B AND C RECOMMENDATION: When an occupational exposure occurs, the source patient should be evaluated for both hepatitis B and hepatitis C. (AII) The risk of transmission
XI. OCCUPATIONAL EXPOSURES TO HEPATITIS B AND C RECOMMENDATION: When an occupational exposure occurs, the source patient should be evaluated for both hepatitis B and hepatitis C. (AII) The risk of transmission
Oxford BioMedica. Annual Results 2008
 Oxford BioMedica Annual Results 2008 12 March 2009 Disclaimer This presentation does not constitute an offer to sell or a solicitation of offers to buy Ordinary Shares (the Securities ). Although reasonable
Oxford BioMedica Annual Results 2008 12 March 2009 Disclaimer This presentation does not constitute an offer to sell or a solicitation of offers to buy Ordinary Shares (the Securities ). Although reasonable
Understanding the Clinical Research Process and Principles of Clinical Research
 Understanding the Clinical Research Process and Principles of Clinical Research Participant Guide Name INTRODUCTION... 1 What Is AIDS?...1 What Is the History of AIDS?...2 What Are the Division of AIDS
Understanding the Clinical Research Process and Principles of Clinical Research Participant Guide Name INTRODUCTION... 1 What Is AIDS?...1 What Is the History of AIDS?...2 What Are the Division of AIDS
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549. Report of Foreign Private Issuer
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
exactly. The need for efficiency in developing effective new therapeutics has never been greater.
 exactly. The need for efficiency in developing effective new therapeutics has never been greater. As demands on the global healthcare system increase and treating disease becomes more complex, the research,
exactly. The need for efficiency in developing effective new therapeutics has never been greater. As demands on the global healthcare system increase and treating disease becomes more complex, the research,
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Human Clinical Study for Free Testosterone & Muscle Mass Boosting
 Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
ADMEDUS INVESTOR PRESENTATION ASX:AHZ. March, 2015. www.admedus.com
 ADMEDUS INVESTOR PRESENTATION March, 2015 ASX:AHZ DISCLAIMER This presentation is the property of Admedus Ltd ( Admedus ). This presentation is not and does not constitute an offer, invitation or recommendation
ADMEDUS INVESTOR PRESENTATION March, 2015 ASX:AHZ DISCLAIMER This presentation is the property of Admedus Ltd ( Admedus ). This presentation is not and does not constitute an offer, invitation or recommendation
Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products (NIMPs)
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products
Overview of Drug Development: the Regulatory Process
 Overview of Drug Development: the Regulatory Process Roger D. Nolan, PhD Director, Project Operations Calvert Research Institute November, 2006 Adapted from course taught by Cato Research Background: Roger
Overview of Drug Development: the Regulatory Process Roger D. Nolan, PhD Director, Project Operations Calvert Research Institute November, 2006 Adapted from course taught by Cato Research Background: Roger
Treatment for. Malaria: DHA/PQP. Science Day MMV Stakeholders Meeting. Defeating Malaria Together 1
 ANewOnce-a-day Treatment for Uncomplicated Malaria: DHA/PQP Science Day MMV Stakeholders Meeting Dr. Ambrose Talisuna Former Director Global Access, MMV & Field Coordinator, African Eurartesim Registration
ANewOnce-a-day Treatment for Uncomplicated Malaria: DHA/PQP Science Day MMV Stakeholders Meeting Dr. Ambrose Talisuna Former Director Global Access, MMV & Field Coordinator, African Eurartesim Registration
A Letter from MabVax Therapeutics President and Chief Executive Officer
 A Letter from MabVax Therapeutics President and Chief Executive Officer Dear Fellow Stockholder: You have invested in MabVax Therapeutics because you share our passion for finding new therapies for the
A Letter from MabVax Therapeutics President and Chief Executive Officer Dear Fellow Stockholder: You have invested in MabVax Therapeutics because you share our passion for finding new therapies for the
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY. Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds
 NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy
 New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy Policy Statement The accelerated four-dose schedule for combined hepatitis A and B vaccine
New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy Policy Statement The accelerated four-dose schedule for combined hepatitis A and B vaccine
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
Graduate Certificate Pre-Med Program Course Descriptions For Year 2015-2016 FALL
 Graduate Certificate Pre-Med Program Course Descriptions For Year 2015-2016 FALL COURSE TITLE: BIOCHEMISTRY COURSE NUMBER: 5104 This course emphasizes biochemical compounds, processes and systems, designed
Graduate Certificate Pre-Med Program Course Descriptions For Year 2015-2016 FALL COURSE TITLE: BIOCHEMISTRY COURSE NUMBER: 5104 This course emphasizes biochemical compounds, processes and systems, designed
Guidance for Industry
 Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
Ethics and Scientific Oversight for Phase 1 Clinical Trials in Hong Kong. Sydney TANG Chairman, HKU/HA HKW IRB November 21, 2015
 Ethics and Scientific Oversight for Phase 1 Clinical Trials in Hong Kong Sydney TANG Chairman, HKU/HA HKW IRB November 21, 2015 Clinical Trials at HKU Phase 1 Phase II Phase III Phase IV Conducted on patient
Ethics and Scientific Oversight for Phase 1 Clinical Trials in Hong Kong Sydney TANG Chairman, HKU/HA HKW IRB November 21, 2015 Clinical Trials at HKU Phase 1 Phase II Phase III Phase IV Conducted on patient
Montanide TM adjuvants for stimulation of cellular immune response in FMD vaccines
 Montanide TM adjuvants for stimulation of cellular immune response in FMD vaccines Sébastien Deville, Juliette Ben Arous, François Bertrand, Laurent Dupuis FAO-ICAR Conference Feb 13-15, New Delhi An affiliate
Montanide TM adjuvants for stimulation of cellular immune response in FMD vaccines Sébastien Deville, Juliette Ben Arous, François Bertrand, Laurent Dupuis FAO-ICAR Conference Feb 13-15, New Delhi An affiliate
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND
 EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
BVD qpcr Bulk Milk Test
 BVD qpcr Bulk Milk Test NML launches a new method for BVD screening... NML launched their new bulk milk BVD qpcr service at the BCVA in mid November 2012. The service offers a simple and easy method to
BVD qpcr Bulk Milk Test NML launches a new method for BVD screening... NML launched their new bulk milk BVD qpcr service at the BCVA in mid November 2012. The service offers a simple and easy method to
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines
 FINAL ENGLISH ONLY Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines World Health Organization 2013 All rights reserved. Publications of the World Health Organization
FINAL ENGLISH ONLY Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines World Health Organization 2013 All rights reserved. Publications of the World Health Organization
How To Follow Up After Treatment With Gene Therapy
 European Medicines Agency London, 30 May 2008 Doc. Ref. EMEA/CHMP/GTWP/60436/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON FOLLOW-UP OF PATIENTS ADMINISTERED WITH GENE THERAPY
European Medicines Agency London, 30 May 2008 Doc. Ref. EMEA/CHMP/GTWP/60436/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON FOLLOW-UP OF PATIENTS ADMINISTERED WITH GENE THERAPY
Proof-of-Concept Studies and the End of Phase IIa Meeting with the FDA
 Medpace Discovery Series presents Proof-of-Concept Studies and the End of Phase IIa Meeting with the FDA DR. JIM WEI: Today my topic is going to be Proof-of-Concept Studies and FDA End of Phase 2a Meetings
Medpace Discovery Series presents Proof-of-Concept Studies and the End of Phase IIa Meeting with the FDA DR. JIM WEI: Today my topic is going to be Proof-of-Concept Studies and FDA End of Phase 2a Meetings
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE E15
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE DEFINITIONS FOR GENOMIC BIOMARKERS, PHARMACOGENOMICS,
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE DEFINITIONS FOR GENOMIC BIOMARKERS, PHARMACOGENOMICS,
MASTERS PROGRAMME IN VACCINOLOGY AND PHARMACEUTICAL CLINICAL DEVELOPMENT
 Siena, Italy June 2011 December 2012 MASTERS PROGRAMME IN VACCINOLOGY Programme Organized by: University of Siena, Medical School Novartis Vaccines Novartis Vaccines Institute for Global Health Siena -
Siena, Italy June 2011 December 2012 MASTERS PROGRAMME IN VACCINOLOGY Programme Organized by: University of Siena, Medical School Novartis Vaccines Novartis Vaccines Institute for Global Health Siena -
Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA
 Media Release Basel, 28 June 2016 Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA OCREVUS is the first investigational medicine
Media Release Basel, 28 June 2016 Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA OCREVUS is the first investigational medicine
Not All Clinical Trials Are Created Equal Understanding the Different Phases
 Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Up to $402,000. Insight HIV. Drug Class. 1.2 million people in the United States were living with HIV at the end of 2011 (most recent data).
 HIV Background, new developments, key strategies Drug Class Insight INTRODUCTION Human Immunodeficiency Virus (HIV) is the virus that can lead to Acquired Immunodeficiency Syndrome, or AIDS. No safe and
HIV Background, new developments, key strategies Drug Class Insight INTRODUCTION Human Immunodeficiency Virus (HIV) is the virus that can lead to Acquired Immunodeficiency Syndrome, or AIDS. No safe and
DAIDS Bethesda, MD USA POLICY
 Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
MIAMI DADE COLLEGE MEDICAL CAMPUS SCHOOL OF HEALTH SCIENCES EMERGENCY MEDICAL SERVICES Emergency Medical Technician (EMT) Application Packet
 SCHOOL O HEALTH SCIENCES EMERGENCY MEDICAL SERVICES Emergency Medical Technician (EMT) Application Packet Student Name (Print) Student Number The information in this 8 - page packet must be completed to
SCHOOL O HEALTH SCIENCES EMERGENCY MEDICAL SERVICES Emergency Medical Technician (EMT) Application Packet Student Name (Print) Student Number The information in this 8 - page packet must be completed to
Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
 September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases
 First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases Andrew E. Mulberg, MD, FAAP Division Deputy Director OND/ODE3/DGIEP FDA Partnership is the Key Coming together is a beginning;
First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases Andrew E. Mulberg, MD, FAAP Division Deputy Director OND/ODE3/DGIEP FDA Partnership is the Key Coming together is a beginning;
Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO
 Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO 1 PCI BIOTECH Important notice and disclaimer This presentation may contain certain forward-looking statements and forecasts based
Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO 1 PCI BIOTECH Important notice and disclaimer This presentation may contain certain forward-looking statements and forecasts based
TBE vaccines: immunogenicity, effectiveness and safety
 TBE vaccines: immunogenicity, effectiveness and safety Prof. H.Kollaritsch, MD., DTM., Associate professor for Specific Prophylaxis and Tropical Medicine, Center for Pathophysiology, Infectiology and Immunology,
TBE vaccines: immunogenicity, effectiveness and safety Prof. H.Kollaritsch, MD., DTM., Associate professor for Specific Prophylaxis and Tropical Medicine, Center for Pathophysiology, Infectiology and Immunology,
HBV screening and management in HIV-infected children and adolescents
 HBV screening and management in HIV-infected children and adolescents Linda Aurpibul M.D. Research Institute for Health Sciences, Chiang Mai University 8% HIV and Hepatitis B Co-infection Among Perinatally
HBV screening and management in HIV-infected children and adolescents Linda Aurpibul M.D. Research Institute for Health Sciences, Chiang Mai University 8% HIV and Hepatitis B Co-infection Among Perinatally
Institute for OneWorld Health
 Institute for OneWorld Health a nonprofit pharmaceutical company Oxymoron no more: nonprofits can deliver medicines for the poor of the world January 10, 2005 Victoria Hale, PhD CEO, OneWorld Health The
Institute for OneWorld Health a nonprofit pharmaceutical company Oxymoron no more: nonprofits can deliver medicines for the poor of the world January 10, 2005 Victoria Hale, PhD CEO, OneWorld Health The
18.5 Percent Overall Response Rate Observed in Pembrolizumab-Treated Patients with this Aggressive Form of Breast Cancer
 News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
Guidance for Industry
 Guidance for Industry IND Exemptions for Studies of Lawfully Marketed Drug or Biological Products for the Treatment of Cancer U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry IND Exemptions for Studies of Lawfully Marketed Drug or Biological Products for the Treatment of Cancer U.S. Department of Health and Human Services Food and Drug Administration Center
Acknowledgements. PAH in Children: Natural History. The Sildenafil Saga
 The Sildenafil Saga David L. Wessel, MD Executive Vice President Chief Medical Officer Ikaria Distinguished Professor of Critical Care Children s National Medical Center Washington, DC, USA Acknowledgements
The Sildenafil Saga David L. Wessel, MD Executive Vice President Chief Medical Officer Ikaria Distinguished Professor of Critical Care Children s National Medical Center Washington, DC, USA Acknowledgements
