CHEMOTHERAPY PROTOCOLS V10.1
|
|
|
- Evelyn Melvyn Beasley
- 9 years ago
- Views:
Transcription
1 CHEMOTHERAPY PROTOCOLS V10.1 Issue Date: 10 th July 2014 Page 1 of 42 Filename: MCHACPROTO Issue No: 10.1
2 CCC Chemotherapy Protocols Haematological Malignancies... 3 Hodgkins disease...3 Non-Hodgkins Lymphoma...5 Head and Neck Cancer Nasopharyngeal Carcinoma Thyroid Cancer Sarcomas Soft Tissue Sarcoma Adjuvant Neo-adjuvant Osteosarcoma / MFH of bone / Leiomyosarcoma of Bone Advanced Osteosarcoma Ewings Sarcoma Neoadjuvant Aggressive fibromatosis Rhabdomyosarcoma IVADo Regime for High Risk Rhabdomyosarcoma Gastro-intestinal Stromal Tumours (GIST) Germ Cell Tumours Adjuvant Primary CNS Lymphoma Adenocarcinoma of Unknown Primary Origin CCC Emergency Chemotherapy Drugs Bone Metastases Surface Area Nomogram Issue Date: 10 th July 2014 Page 2 of 42 Filename: MCHACPROTO Issue No: 10.1
3 Haematological Malignancies Hodgkins disease Early HD PET/CT as part of staging Group I Involved field XRT Group II ABVD x 3 + IF XRT Group III Three cycles full dose chemotherapy + IF XRT or 6 cycles full dose chemotherapy + IF XRT if residual abnormality Advanced HD Stages III / IV or I / II with mediastinal bulk + / - B symptoms ABVD Doxorubicin 25mg/m 2 IV day 1 and 15 Bleomycin 10000iu/m 2 IV day 1 and 15 Hydrocortisone 100mg IV day 1 and 15 Vinblastine 6mg/m 2 IV day1 and 15 (max 10mg) Dacarbazine 350mg/m 2 IV day 1 and 15 Repeat at 28 day intervals to CR + 2, min 6 max 8 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated ChlVPP Vinblastine 6mg/m 2 IV days 1 and 8 (max 10mg) Chlorambucil 6mg/m 2 po days 1-14 Prednisolone 40mg po days 1-14 Procarbazine 50mg oral tds days 1-14 Repeat 28 days from day 1 x 6 cycles) Issue Date: 10 th July 2014 Page 3 of 42 Filename: MCHACPROTO Issue No: 10.1
4 Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Issue Date: 10 th July 2014 Page 4 of 42 Filename: MCHACPROTO Issue No: 10.1
5 Non-Hodgkins Lymphoma Low grade First line CVP-R Cyclophosphamide 600mg/m2 IV day 1 Vincristine 1.4mg/m2 IV day 1 (maximum 2mg) Prednisolone 50mg orally days 1-5 Rituximab 375mg/m2 IV day 1 Repeat at 21 day intervals to a maximum of 6-8 cycles. Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated *Bendamustine 120mg/m 2 IV days 1 and 2 Repeat at 21 day intervals to a maximum of 8 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated *NB available via the Cancer Drug Fund CHOP-R Cyclophosphamide 750mg/m 2 IV day 1 Doxorubicin 50mg/m 2 IV day 1 Vincristine 1.4mg/m 2 IV day 1 Prednisolone 50mg po days 1-5 Rituximab 375mg/m 2 IV day 1 Repeat at 21 day intervals for 6 cycles Criteria: fit patients with bulky disease Issue Date: 10 th July 2014 Page 5 of 42 Filename: MCHACPROTO Issue No: 10.1
6 Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated *Maintenance Rituximab Rituximab 375mg/m 2 IV every 3 months for 2 years Criteria CR or PR following first line therapy Must commence within 2 months of completion of first line therapy *NB available via the Cancer Drug Fund Mantle Cell Lymphoma *Bendamustine 120mg/m 2 IV days 1 and 2 Repeat at 21 day intervals to a maximum of 8 cycles Criteria Option for first line therapy Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Second Line *NB available via the Cancer Drug Fund *Bendamustine 120mg/m 2 IV days 1 and 2 Issue Date: 10 th July 2014 Page 6 of 42 Filename: MCHACPROTO Issue No: 10.1
7 Repeat at 21 day intervals to a maximum of 8 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Criteria Unable to receive R-CHOP Unable to receive high dose therapy *NB available via the Cancer Drug Fund Second / third line Chlorambucil +/- Prednisolone Chlorambucil + Prednisolone 10mg daily orally for 14 days 20mg oral daily for 14 days Repeat at 28 day intervals for up to 6 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Fludarabine Fludarabine 40mg/m 2 orally days 1-5 (only 3 days if heavily pretreated) Issue Date: 10 th July 2014 Page 7 of 42 Filename: MCHACPROTO Issue No: 10.1
8 Repeat at 28 day intervals max 6 cycles Criteria Progressed after alkylating agents and anthracyclines Age < 75yrs PS 0-1 Normal marrow function NB: patients receiving fludarabine should have all blood products Irradiated Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Rituximab Rituximab 375mg/m 2 in 500ml Sodium chloride 0.9% IV weekly x 4 Criteria Stage III / IV follicular / Mantle cell NHL Chemoresistant (prior alkylating agent and anthracycline chemotherapy) Normal renal / hepatic function Anticipated survival > 3 months Age < 65yrs PS 0-2 Not eligible for a clinical trial Intermediate / High grade Stage I CHOP x 3 + involved field XRT Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Issue Date: 10 th July 2014 Page 8 of 42 Filename: MCHACPROTO Issue No: 10.1
9 Stage II-IV CHOP-R Cyclophosphamide 750mg/m 2 IV day 1 Doxorubicin 50mg/m 2 IV day 1 Vincristine 1.4mg/m 2 IV day 1 (max. 1mg if aged >70) Prednisolone 50mg po days 1-5 Rituximab 375mg/m 2 IV day 1 Repeat at 21 day intervals for 6 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Relapsed Intermediate / High grade A proportion of patients with relapsed intermediate / high grade NHL may be salvaged with high dose chemotherapy + marrow / PBSC rescue. Patients in this situation should be discussed with the NHL MDT at the Royal Liverpool Hospital. Issue Date: 10 th July 2014 Page 9 of 42 Filename: MCHACPROTO Issue No: 10.1
10 Head and Neck Cancer Locally advanced disease XRT +Cisplatin/5FU Cisplatin 80mg/m 2 IV day 1 Fluorouracil 1g/m 2 over 24hrs IV days 1-4 Repeat at 21 day intervals for 2-4 cycles prior to XRT Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines or XRT + Cisplatin Cisplatin 100mg/m 2 IV days 1, 22, and 43 with concomitant XRT Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines or XRT + Cisplatin / 5FU / Docetaxel Cisplatin 80mg/m 2 IV day 1 Fluorouracil 1000mg/m 2 over 24hrs IV days 1-4 Docetaxel 75mg/m 2 IV day 1 Repeat at 21 day intervals for 3 cycles prior to CTX/XRT Issue Date: 10 th July 2014 Page 10 of 42 Filename: MCHACPROTO Issue No: 10.1
11 Criteria Locally advanced SCC suitable for CTX/XRT PS 0/1 Creatinine clearance > 50mls/min Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines XRT + Cetuximab Cetuximab 400mg/m 2 IV loading dose 1 week prior to XRT 250mg/m 2 IV weekly during XRT Criteria Locally advanced SCC suitable for CTX/XRT Unsuitable for cisplatin eg creatinine clearance < 50mls/min PS 0/1 Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Recurrent or Metastatic Disease Cisplatin/5FU Cisplatin 80mg/m 2 IV day 1 Fluorouracil 1g/m 2 IV over 24hrs days 1-4 or Cisplatin Cisplatin 100mg/m 2 IV Repeat at 21 day intervals max 6 cycles Laboratory investigations Issue Date: 10 th July 2014 Page 11 of 42 Filename: MCHACPROTO Issue No: 10.1
12 Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines *Cisplatin or Carboplatin + /5FU + Cetuximab Cisplatin/5FU Cisplatin 80mg/m 2 IV day 1 5Fluorouracil 1g/m 2 IV over 24hrs days 1-4 Repeat at 21 day intervals max 6 cycles Or Carboplatin/5FU Carboplatin AUC 5 Day 1 Fluorouracil 1g/m 2 IV over 24hrs Days 1-4 Repeat at 21 day intervals max 6 cycles until Cetuximab 400mg/m 2 IV loading dose week 1 250mg/m 2 IV weekly during chemotherapy and may be continued progression Criteria Ist line chemotherapy for advanced disease PS 0/1 No prior treatment with cetuximab Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Where renal / hepatic function are abnormal treatment is at physician *Available via the cancer drugs fund 2 nd Line Chemotherapy for advanced disease Issue Date: 10 th July 2014 Page 12 of 42 Filename: MCHACPROTO Issue No: 10.1
13 Paclitaxel mg/m 2 IV over 3hrs Dose depends on PS and extent of prior therapy Premedication Chlorphenamine 10mg IV Dexamethasone 20mg IV Ranitidine 50mg IV Repeat at 21 day intervals, max 6 cycles Criteria PS 0-1 Nasopharyngeal Carcinoma Chemoradiation + Adjuvant Chemotherapy Criteria Nasopharyngeal carcinoma Stage III/IV Creatinine clearance > 50ml/min Chemoradiation Cisplatin 100mg/m2 days 1, 22, 43 to start prior to XRT Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines followed by: Adjuvant chemotherapy Commencing 21 days after 3rd cycle of cisplatin Cisplatin 80mg/m 2 IV day 1 Fluorouracil 1g.m 2 IV over 24 hrs days 1-4 Repeat at 21 day intervals for 3 cycles Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Issue Date: 10 th July 2014 Page 13 of 42 Filename: MCHACPROTO Issue No: 10.1
14 Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Issue Date: 10 th July 2014 Page 14 of 42 Filename: MCHACPROTO Issue No: 10.1
15 Thyroid Cancer Medullary Thyroid Cancer *Vandetanib Continue until disease progression Criteria PS 0-2 Locally advanced unresectable / metastatic First line Laboratory investigations FBC, U/Es, LFTs prior to each cycle Discontinue if deteriorating renal or liver function Normal FBC limits for administration apply *NB available via the Cancer Drug Papillary or Follicular Thyroid Cancer Sorafenib 400mg bd oral continuously Continue until disease progression Criteria PS 0-2 Refractory to radioiodine Laboratory investigations FBC, U/Es, LFTs prior to each cycle Discontinue if deteriorating renal or liver function *NB available via the Cancer Drugs fund Issue Date: 10 th July 2014 Page 15 of 42 Filename: MCHACPROTO Issue No: 10.1
16 Sarcomas Soft Tissue Sarcoma Adjuvant There is no proven role for adjuvant chemotherapy for soft tissue sarcomas. However treatment may be considered when chemo-sensitive tumours such as rhabdomyosarcoma, synovial sarcoma are resected with close margins. Neo-adjuvant Suggested protocol for down sizing prior to surgery Doxorubicin 20mg/m2 day 1,2,3 Mesna prior to ifosfamide 3g/m2 day 1,2,3 Ifosfamide + Mesna 3g/m2 / 3g/m2 day 1,2,3 Mesna post ifos/mesna infusion 3g/m2 day 1,2,3 Advanced There is no evidence that combinations are superior to single agents as palliative chemotherapy First line Doxorubicin Doxorubicin 75mg/m 2 IV q 21 days x 6 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Second line Ifosfamide Mesna prior to ifosfamide 3g/m 2 days 1,2,3 Ifosfamide/Mesna 3g/m 2 days 1,2,3 Mesna post ifos/mesna infusion 3g/m 2 days 1,2,3 Repeat at 21 day intervals for up to 6 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Issue Date: 10 th July 2014 Page 16 of 42 Filename: MCHACPROTO Issue No: 10.1
17 Dacarbazine 800mg/m 2 IV day 1 Repeat at 21 day intervals for up to 6 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated *Trabectedin Consider in select cases of advanced STS- Failure after treatment with anthracyclines and ifosfamide or intolerant/contraindications to anthracyclines and ifosfamide. Consider in Myxoid liposarcomas and leiomyosarcomas. PS mg/m 2 as IV infusion over 24 hours every 21 days. *NB Available via the off-protocol mechanism Issue Date: 10 th July 2014 Page 17 of 42 Filename: MCHACPROTO Issue No: 10.1
18 Osteosarcoma / MFH of bone / Leiomyosarcoma of Bone Neoadjuvant / Post operative schedule PAM x 2 -> Surgery (week 10) -> PAM x 2 -> Doxorubicin - Methotrexate x 2 PAM Cisplatin 60mg/m 2 IV day 1, 2 Doxorubicin 25mg/m 2 IV days 1, 2, 3 Methotrexate 12g/m 2 IV days 22 and 29 Folinic acid rescue Start 24 hrs post start of Methotrexate infusion with 30mg IV 6 hourly Switch to oral after 6 doses if not vomiting Methotrexate levels at 24, 48, 72 hrs etc. Continue until Methotrexate undetectable ie <0.1M usually 4-5 days Methotrexate level Folinic acid dose 6hrly < 0.1M Stop rescue <0.5-5M 15-30mg 6hrly 5-50M 200mg/m 2 6hrly >50M 1000mg/m 2 6hrly In addition patients urinary ph should be >7 prior to starting Methotrexate infusion NB: do not give methotrexate if renal function is abnormal or in the presence of a third space. Also avoid all non-steroidal anti-inflammatory agents prior to treatment and until Methotrexate undetectable. Doxorubicin /Methotrexate Doxorubicin 37.5mg/m 2 IV days 1, 2 Methotrexate 12g/m 2 IV days 15 and 22 Folinic acid rescue see above Criteria Age < 40yrs PS 0-2 Issue Date: 10 th July 2014 Page 18 of 42 Filename: MCHACPROTO Issue No: 10.1
19 Cisplatin/Doxorubicin Cisplatin 100mg/m 2 IV day 1 60yrs) Doxorubicin 25mg/m 2 IV days 1, 2, 3 (20mg/m 2 days 1-3 age > Repeat at 21 days x 3 cycles then surgery then 3 further cycles. Criteria Not suitable for PAM schedule. PS 0-2 Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Issue Date: 10 th July 2014 Page 19 of 42 Filename: MCHACPROTO Issue No: 10.1
20 Advanced Osteosarcoma Cisplatin/Doxorubicin Cisplatin 100mg/m 2 IV day 1 Doxorubicin 25mg/m 2 IV days 1, 2, 3 Repeat at 21 days x 6 cycles Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines LV ejection fraction prior to cycle 1 if history of cardiac problems Issue Date: 10 th July 2014 Page 20 of 42 Filename: MCHACPROTO Issue No: 10.1
21 Ewings Sarcoma Non-metastatic Ewings Sarcoma/PNET/Askin tumour / Rhabdomyosarcoma Laboratory Investigations FBC/Biochemistry/Ca/Mg/Cl/HCO3 each cycle Early morning urine PO4, Creatinine, osmolarity at baseline and repeat every other cycle of VIDE Echo/MUGA baseline/cycle 4 if indicated/cycle 6/ end Rx / pregnant Bone scan/ct chest/mri primary/marrow biopsy at baseline MRI primary after cycles 2, 4, 6(omit after 2 if good response) Neoadjuvant VIDE (cycles 1-6) Vincristine 1.5mg/m 2 (max 2mg) IV day 1 Doxorubicin 20mg/m 2 IV days 1-3 Mesna 1g/m 2 day 1 Etoposide 150mg/m 2 IV days 1-3 Ifosfamide/Mesna 1.5g/m 2 / 1.5g/m 2 IV days 1-3 Mesna 1.5g/m 2 IV days 3 Pegfilgrastim 6mg subcutaneous injection day 4 Evaluation after cycle 4: If surgical resection likely proceed to cycles 5 and 6 If radiotherapy to be definitive local therapy proceed to cycles 5 and 6 If disease progression discontinue and consider surgery or radiotherapy If pre-surgery XRT planned proceed to cycles 5 and 6 omitting doxorubicin Dose modifications Haematological toxicity Delayed recovery of wbc / platelets > 6 days reduce etoposide 20% Neutropenic sepsis grade 3 or 4 reduce etoposide 20% Further episodes repeat etoposide 20% reductions GI / Mucositis Graded 3 or 4 reduce etoposide by 20% Cardiac function Issue Date: 10 th July 2014 Page 21 of 42 Filename: MCHACPROTO Issue No: 10.1
22 LVEF < 40% omit doxorubicin and substitute Dactinomycin1.5mg/m 2 Repeat echo after next cycle and consider reintroducing doxorubicin if LVEF has recovered. Definitive local treatment Surgery should occur 21 days after cycle 6 or as soon as recovery allows. Radiotherapy should commence concurrent with cycle 7 omitting Dactinomycin from concurrent cycles. If radiation is required following surgery it should commence after cycle 8 omitting Dactinomycin from concurrent cycles. VIA (cycles 7-14) Vincristine 1.5mg/m 2 (max 2mg) IV day 1 Dactinomycin 0.75mg/m 2 (max 1.5mg)IV days 1-2 Mesna prior to ifosfamide 1g/m 2 days 1-2 Ifosfamide/Mesna 1.5g/m 2 / 1.5g/m 2 IV days 1-2 Mesna post ifos/mesna infusion 1.5g/m 2 days 1-2 NB: omit Dactinomycin during radiotherapy Haematological toxicity Delayed recovery of wbc/platelets > 6 days reduce Dactinomycin & Ifosfamide by 20% Neutropenic sepsis grade 3 /4 reduce Dactinomycin & Ifosfamide by 20% + add GCSF Further episodes should be managed with serial 20% reductions GI / Mucositis Grade 3 or 4 reduce Ifosfamide + Dactinomycin by 20% Renal Toxicity GFR > 60 no change GFR reduce Ifosfamide by 30%, reduce etoposide by 30% GFR < 40 switch Ifosfamide to cyclophosphamide 1500mg/m 2 on day 1 only reduce etoposide by 30% Cardiac function LVEF < 40% or 10% decrease from previous level, delay chemotherapy and repeat in 7 days. If recovered proceed with chemotherapy. If still impaired consider omission or dose reduction of Ifosfamide. Issue Date: 10 th July 2014 Page 22 of 42 Filename: MCHACPROTO Issue No: 10.1
23 VAC Vincristine 1.5mg/m 2 IV (max 2mg) day 1 Dactinomycin 0.75mg/m 2 IV (max 1.5mg) day 1-2 Mesna prior to cyclophosphamide 500mg /m 2 IV pre cyclophosphamide day 1 Cyclophosphamide/Mesna 1500mg/m 2 / 1500mg/m 2 IV day 1 Mesna post cyclo/mesna infusion 1500mg/m 2 IV post cyclophosphamide/mesna day 1 Repeat at 21 days for 4 6 cycles Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated VACA (In patients unsuitable for VIDE previously NOT exposed to anthracyclines. Dactinomycin and doxorubicin on alternate cycles) Vincristine 1.5mg/m 2 IV (max 2mg) day 1 Mesna prior to cyclophosphamide 500mg /m 2 IV day 1 Cyclophosphamide/Mesna 1200mg/m 2 / 1200mg/m 2 IV day 1 Mesna post cyclos/mesna infusion 1200mg/m 2 IV day 1 Dactinomycin 0.5mg/m 2 IV (max 1mg) days 1-3 Alternating with Doxorubicin 20mg/m 2 IV days 1-3 Etopside / Ifosfamide Etoposide 120mg/m2 IV days 1-3 Mesna prior to ifosfamide 500mg/m2 days 1-3 Ifosfamide/Mesna 3g/m2 / 3g/m2 IV days 1-3 Mesna post ifos/mesna infusion 1.5g/m2 IV days 1-3 Issue Date: 10 th July 2014 Page 23 of 42 Filename: MCHACPROTO Issue No: 10.1
24 Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Palliative Ewings Etoposide / cisplatin Etoposide 120mg/m 2 IV days 1-3 Cisplatin 50mg/m 2 IV days 1-2 or Carboplatin AUC 5 IV day 1 Etoposide 120mg/m 2 IV day1 Etoposide 240mg/m 2 PO days 2,3 Repeat at 21 day intervals max 6 cycles Criteria PS 0-1 Cr cl > 50ml/min for cisplatin Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Cyclophosphamide/ Topotecan Relapsed Ewing s sarcoma Relapsed/ 2 nd line rhabdomyosarcoma Topotecan 0.75 mg/m 2 Days 1-5 Cyclophosphamide 250 mg/m 2 Days 1-5 Cycle repeated every 21 days Issue Date: 10 th July 2014 Page 24 of 42 Filename: MCHACPROTO Issue No: 10.1
25 Gemcitabine/Docetaxel Relapsed metastatic osteosarcoma Selected metastatic soft tissue sarcomas (3 rd line)/ uterine leiomyosarcoma Relapsed Ewings (if other 2 nd line is not suitable) Day 1 Gemcitabine 675 mg/m 2 IV over 90 minutes Day 8 Gemcitabine 675 mg/m2 IV over 90 minutes followed by Docetaxel mg/m2 IV over 60 minutes Dexamethasone 8 mg bd for 3 days to start 24 hours pre docetaxel (ie Days 7-9) Cycle repeated every 21 days Irinotecan/Temozolomide Relapsed Ewing s sarcoma Relapsed/ 2nd line rhabdomyosarcoma Irinotecan 20 mg/m2 IV Days 1-5, Days 8-12 Temozolomide 100mg/m2 po Days 1-5 Cycle repeated every days Paclitaxel Angiosarcomas (2nd line or 1st line if not suitable for doxorubicin) 80 mg/m 2 weekly up to 12 weeks 175 mg/m 2 every 21 days (4-6 cycles, review after cycle 3) Oral Etoposide Palliative metastatic Ewings or rhabdomyosarcoma Etoposide mg bd 7-14 days (at clinician s ) Issue Date: 10 th July 2014 Page 25 of 42 Filename: MCHACPROTO Issue No: 10.1
26 Aggressive fibromatosis 1 st Line Tamoxifen +/- NSAID s 2 nd Line Methotrexate 30 mg/m 2 (usually 50mg total dose) Vinblastine 6 mg/m 2 (usually 10mg total dose) Every 1-2 weeks Duration of course at clinician s Vinorelbine can replace vinblastine if neuropathy is a problem. Rhabdomyosarcoma Baseline investigations: - FBC, U+E s, LFT s Bone chemistry - CT thorax / Abdo staging - Bone marrow aspirate and trephine - Bone Scan - If paramningeal site- CSF - Consider early morning urine for phosphate, creatinine, osmolarity for Ifosfamide containing regimes For patients aged < 40 years: IVADo regime for high risk rhabdomyosarcoma (see separate regime) Maintenance therapy: Vinorelbine 25 mg/m 2 IV D 1, 8, 15 Cyclophosphamide 25 mg/m 2 PO OD D 1-28 Every 28 days Maintenance therapy following IVADo to be used in: 1. For Alveolar Rhabdomyosarcoma maintenance therapy following IVADo for 6 cycles ( i.e. 6 months) 2. For metastatic disease on intensive treatment, if no residual disease or limited residual disease, IVADo to be followed by maintenance treatment for 12 cycles Issue Date: 10 th July 2014 Page 26 of 42 Filename: MCHACPROTO Issue No: 10.1
27 For patients > 40 years: IVAD Vincristine 1.4 mg/m2 (max dose 2mg) D1 Doxorubicin 30 mg/m2 D1, D2 Mesna 1.2g/m 2 pre ifosfamide D1,D2 Ifosfamide 3g/m 2 D1,D2 Mesna 2.4g/m 2 post ifosfamide in 2 divided doses D1,D2 Repeat at 21 day intervals for 6 cycles VAC Vincristine 1.5mg/m 2 IV (max 2mg) Day 1 Dactinomycin 0.75mg/m 2 IV (max 1.5mg) Day 1 and 2 Mesna prior to cyclophosphamide 500mg /m 2 IV pre cyclophosphamide Day 1 Cyclophosphamide/Mesna 1500mg/m 2 / 1500mg/m 2 IV Day 1 Mesna post cyclo/mesna infusion 1500mg/m 2 IV post cyclophosphamide/mesna Day 1 Repeat at 21 day intervals for 4-6 cycles IVA Vincristine 1.5mg/m2 (max dose 2mg) Day 1 Dactinomycin 1.5 mg/m2 (max single dose 2mg) Day 1 Mesna prior to ifosfamide 1.2g/m 2 day Days 1-2 Ifosfamide/Mesna 3 g/m 2 / 3 g/m2 Days 1-2 Mesna post ifos/mesna infusion 2.4g/m 2 split into 2 doses Day 2 Repeat at 21 day intervals for 6 cycles Issue Date: 10 th July 2014 Page 27 of 42 Filename: MCHACPROTO Issue No: 10.1
28 IVADo Regime for High Risk Rhabdomyosarcoma Cycle 1 Cycle 2 Cycle 3 Cycle 4 IVADo V V IVADo V V IVADo IVADo Surgery/ Radiotherapy Week Cycle Cycle Cycle Cycle Cycle IVA IVA IVA IVA IVA Week I= Mesna prior to ifosfamide 1.2g/m 2 over 1 hour Ifosfamide/Mesna 3g/ m 2 / 3g/m 2 over 3 hours Days 1-2 Mesna post ifos/mesna infusion 2.4g/m 2 over 8 hours (split into 2 doses) V= Vincristine 1.5mg/m 2 (max single dose 2mg) Day 1 A= Dactinomycin 1.5 mg/m 2 (max single dose 2mg) Day 1 Do= Doxorubicin 30 mg/m 2 Days 1-2 for cycles 1-4 Each cycle: WCC>2 Neutrophils> 1.0 ( or physician s ) Platelets > 80 Weekly vincristine to be given irrespective of pancytopenia unless unwell Reassess after cycle 3. If not CR or PR > 1/3 rd consider 2 nd line treatment + RT Issue Date: 10 th July 2014 Page 28 of 42 Filename: MCHACPROTO Issue No: 10.1
29 PAM Chemotherapy for Resectable Osteosarcoma Cycle 1 Cycle 2 SURGERY Cycle 3 AP M M AP M M AP M M Week Cycle 4 Cycle 5 Cycle 6 AP M M A M M A M M Week AP - Doxorubicin 25 mg/m2 Days 1-3 Cisplatin 60mg/m2 Days 1-2 M- Methotrexate 12 g/m2 (With folinic acid rescue) Day 1 A- Doxorubicin 37.5 mg/m 2 Days 1-2 Issue Date: 10 th July 2014 Page 29 of 42 Filename: MCHACPROTO Issue No: 10.1
30 Gastro-intestinal Stromal Tumours (GIST) *Adjuvant Imatinib po 400mg daily for 36 months Criteria Tumour > 5cm Mitoses > 5 mitoses/50 HPF SI / colonic primary *Available via the Cancer Drugs Fund Imatinib (Glivec ) Imatinib 400mg daily orally until progression Criteria PS 0-2 c-kit positive locally advanced / metastatic disease Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated FBC / biochemistry prior to each visit Dose reduce if significant toxicity / rising hepatic transaminases Sunitinib (Sutent )* Sunitinib 50mg orally daily for 4 weeks followed by a two week break Criteria PS 0-2 c-kit positive locally advanced / metastatic disease previous response to imatinib Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated FBC / biochemistry prior to each visit Issue Date: 10 th July 2014 Page 30 of 42 Filename: MCHACPROTO Issue No: 10.1
31 *Available via the off protocol mechanism Issue Date: 10 th July 2014 Page 31 of 42 Filename: MCHACPROTO Issue No: 10.1
32 Germ Cell Tumours Adjuvant Stage I Pure Seminoma Carboplatin Carboplatin AUC x 7 IV - one dose only Laboratory investigations EDTA clearance required to calculate AUC Ensure normal hepatic function prior to treatment Stage I Non-seminomatous testicular GCT Criteria: vascular or lymphatic invasion BEP 3 Bleomycin 30000iu IV day 1, 8, 15 Etoposide 165mg/m 2 IV days 1-3 Cisplatin 50mg/m 2 IV days 1-2 Repeat at 21 day intervals for 2 cycles Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Tumour markers: HCG,AFP,LDH where appropriate prior to each cycle Low risk all GCT BEP 3 Bleomycin 30000iu IV day 1, 8, 15 Etoposide 165mg/m 2 IV days 1-3 Cisplatin 50mg/m 2 IV days 1-2 Repeat at 21 day intervals for 3 cycles. Issue Date: 10 th July 2014 Page 32 of 42 Filename: MCHACPROTO Issue No: 10.1
33 Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Tumour markers: HCG,AFP,LDH where appropriate prior to each cycle Intermediate / High risk all GCT BEP 5 Bleomycin 30000iu IV days 1, 5, 15 cycles 1-3 Etoposide 100mg/m 2 IV days 1-5 Cisplatin 20mg/m 2 IV days 1-5 Repeat at 21 day intervals x 3 cycles then EP 5 for a further 3 cycles (i.e. omit bleomycin) Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Tumour markers: HCG,AFP,LDH where appropriate prior to each cycle CNS disease POMB / ACE + Intrathecal (IT) Methotrexate POMB Day 1 Vincristine 2mg IV Methotrexate 1g/m 2 IV over 24hrs (Standard dose is 300mg/m 2 ) Issue Date: 10 th July 2014 Page 33 of 42 Filename: MCHACPROTO Issue No: 10.1
34 Folinic acid 15mg 6hrly x 12 doses start 12 hrs after completion of Methotrexate Day 2 Bleomycin 15mg IV over 24hr Day 3 Bleomycin 15mgIV over 24 hr Day 4 Cisplatin 120mg/m 2 IV over 12hr Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Tumour markers: HCG,AFP,LDH where appropriate prior to each cycle ACE Dactinomycin 0.5mg IV days 1-3 Etoposide 100mg/m 2 IV days 1-3 Cyclophosphamide 500mg/m 2 IV day 3 Intrathecal (IT) Methotrexate 12.5mg flat dose (folinic acid rescue 15mg 6hrly x 4 start at 24hrs) Laboratory Investigations Ensure normal renal and hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Repeat cycles at 14 days from day 1, POMB, POMB, ACE, POMB, ACE etc 4-5 cycles of POMB. Initial organ failure Low dose cisplatin / etoposide Cisplatin 20mg/m 2 IV Etoposide 100mg/m 2 IV Repeat daily x 2-3days depending on clinical situation Issue Date: 10 th July 2014 Page 34 of 42 Filename: MCHACPROTO Issue No: 10.1
35 Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Tumour markers: HCG,AFP,LDH where appropriate prior to each cycle Relapsed NSGCT TIP Paclitaxel 175mg/m 2 IV 3hr infusion day 1 Ifosfamide 1000mg/m 2 (+mesna) IV days 1-5 Cisplatin 20mg/m 2 IV days 1-5 Premedication Chlorphenamine 10mg IV Dexamethasone 20mg IV Ranitidine 50mg IV Repeat at 21 day intervals x 4 cycles Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Tumour markers: HCG,AFP,LDH where appropriate prior to each cycle High dose chemotherapy May be curative in selected patients with drug sensitive relapsed disease. Issue Date: 10 th July 2014 Page 35 of 42 Filename: MCHACPROTO Issue No: 10.1
36 Primary CNS Lymphoma De Angelis Weeks 1, 5, 9 Day -1-7 allopurinol 300mg/day (Modified) Vincristine 1.4mg/m 2 (max 2mg) IV bolus weeks 2, Methotrexate 3500mg/m 2 IV over 6 hours With adequate folinic acid rescue (see below) and urinary alkalinisation Procarbazine 100mg/m 2 oral daily for 7 days Weeks 3, 7 Vincristine 1.4mg/m 2 (max 2mg) IV bolus weeks 2, Methotrexate 3500mg/m 2 IV over 6 hours With adequate folinic acid rescue (see below) and urinary alkalinisation Dexamethasone 16mg/day week 1 12mg / day week 2 8mg/day week 3 6mg/day week 4 4mg/day week 5 2mg/day week 6 Folinic acid rescue Start 24 hrs post start of Methotrexate infusion with 30mg IV 6 hourly Switch to oral after 6 doses if not vomiting Methotrexate levels at 24, 48, 72 hrs etc. Continue until Methotrexate undetectable ie <0.1M usually 4-5 days Methotrexate level Folinic acid dose 6hrly < 0.1M Stop rescue <0.5-5M 15-30mg 6hrly 5-50M 200mg/m 2 6hrly >50M 1000mg/m 2 6hrly Issue Date: 10 th July 2014 Page 36 of 42 Filename: MCHACPROTO Issue No: 10.1
37 In addition patients urinary ph should be >7 prior to starting Methotrexate infusion NB: do not give methotrexate if renal function is abnormal or in the presence of a third space. Also avoid all non-steroidal anti-inflammatory agents prior to Rx and until Methotrexate undetectable. Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer methotrexate only if clearance is > 50mls/min Issue Date: 10 th July 2014 Page 37 of 42 Filename: MCHACPROTO Issue No: 10.1
38 Adenocarcinoma of Unknown Primary Origin Possible GI primary MdG / capecitabine / gemcitabine Possible breast primary Manage as for similar stage breast cancer Possible ovarian primary Carboplatin 5 x (GFR+25) Possible lung primary Carboplatin / Gemcitabine Midline nodal disease +/- lung metastases BEP 3 x max 4 cycles depending on response Undifferentiated carcinoma Etoposide / platinum Etoposide 120mg/m 2 days 1-3 (or oral 240mg/m 2 ) Cisplatin 70mg/m 2 days 1 Repeat at 21 day intervals max 6 cycles Laboratory investigations Ensure normal hepatic function prior to cycle 1 and repeat during subsequent cycles if clinically indicated Calculate creatinine clearance prior to each cycle and administer cisplatin according to guidelines Issue Date: 10 th July 2014 Page 38 of 42 Filename: MCHACPROTO Issue No: 10.1
39 CCC Emergency Chemotherapy Drugs Likely cancers requiring treatment: Lymphoma Germ Cell Tumours Small Cell Lung Cancer Drugs Available Cisplatin Etoposide Doxorubicin Cyclophosphamide Vincristine Pegfilgrastim 30mg in 250ml Sodium chloride 0.9% x 2 doses 100mg x 2 doses 50mg 800mg 2mg 6mg These drugs will be stored in oncology pharmacy in the fridge in the dispensary area labelled Fridge 3. The fridge will be labelled as containing Emergency Chemotherapy Drugs. Cisplatin will be stored at room temperature on top of fridge 3. Emergency chemotherapy should be prescribed by a consultant and entry to the pharmacy will be via the CCC bleep holder only. Issue Date: 10 th July 2014 Page 39 of 42 Filename: MCHACPROTO Issue No: 10.1
40 Bone Metastases There is increasing evidence from studies in a number of malignancies that intravenous bisphosphonate therapy can ameliorate bone pain and reduce the risk of skeletal complications in patients with bone metastases. At present for suitable patients the recommended treatment is zelodronate + Adcal D3 until progression. Bisphosphonates Zoledronic acid 4mg IV in 100mls Sodium chloride 0.9% over minutes repeated at 28 day intervals. Criteria: Performance status 0-2 Symptomatic / extensive bone metastases Calcium supplements: Patients should have their serum calcium measured every four weeks and Adcal D3 prescribed as necessary. Renal impairment: Cr clearance Dose of zoledronic acid (Cockcroft-Gault) >60 4.0mg mg mg mg < 30 no treatment Serum creatinine should be repeated every 4 weeks and if it rises significantly during treatment zoledronic acid should be witheld until the creatinine has returned to within 10% of the baseline prior to starting. Ibandronate (Bondranat ) Bondranat has yet to be shown to be as effective as zoledronic acid in reducing the incidence of skeletal events and thus we cannot recommend it as routine treatment. However for patients who have difficulties with venous access or renal impairment it may be requested via the off protocol mechanism. Issue Date: 10 th July 2014 Page 40 of 42 Filename: MCHACPROTO Issue No: 10.1
41 *Denosumab Denosumab 120mg sc monthly Criteria Patients ineligible for IV bisphosphonate due to poor venous access or renal impairment *NB Available via the Cancer Drugs Fund Issue Date: 10 th July 2014 Page 41 of 42 Filename: MCHACPROTO Issue No: 10.1
42 Surface Area Nomogram Issue Date: 10 th July 2014 Page 42 of 42 Filename: MCHACPROTO Issue No: 10.1
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Cycle frequency: Every three weeks Total number of cycles: 3 or 4
 BEP 3-day (Bleomycin/Etoposide/Cisplatin) Germ cell tumours Bleomycin 30,000iu 200mls N. Saline/30mins Days 2, 8 & 15 Etoposide 165mg/m 2 1L N. Saline/1hr Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/4hrs
BEP 3-day (Bleomycin/Etoposide/Cisplatin) Germ cell tumours Bleomycin 30,000iu 200mls N. Saline/30mins Days 2, 8 & 15 Etoposide 165mg/m 2 1L N. Saline/1hr Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/4hrs
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Schedule: Drug Dose iv/infusion/oral q Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2
 Doxorubicin/Cisplatin Osteosarcoma - neoadjuvant Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2 (3 before surgery) Ensure adequate renal function Pre & post-hydration,
Doxorubicin/Cisplatin Osteosarcoma - neoadjuvant Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2 (3 before surgery) Ensure adequate renal function Pre & post-hydration,
Schedule: Drug Dose iv/infusion/oral q Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8
 Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Lung Pathway Group Pemetrexed and Cisplatin in Non-Small Cell Lung Cancer (NSCLC)
 Indication: NICE TA181 First line treatment option in advanced or metastatic non-squamous NSCLC (histology confirmed as adenocarcinoma or large cell carcinoma) Performance status 0-1 Regimen details: Pemetrexed
Indication: NICE TA181 First line treatment option in advanced or metastatic non-squamous NSCLC (histology confirmed as adenocarcinoma or large cell carcinoma) Performance status 0-1 Regimen details: Pemetrexed
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP)
 BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
Thames Valley Cancer Network. Network Chemotherapy Protocols Breast Cancer
 Network Chemotherapy Protocols Breast Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.nhs.uk. Any correspondence about the protocols
Network Chemotherapy Protocols Breast Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.nhs.uk. Any correspondence about the protocols
Network Chemotherapy Regimens
 Network Chemotherapy Regimens Lung Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.org.uk. Any correspondence about the protocols
Network Chemotherapy Regimens Lung Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.org.uk. Any correspondence about the protocols
Pediatric Oncology for Otolaryngologists
 Pediatric Oncology for Otolaryngologists Frederick S. Huang, M.D. Division of Hematology/Oncology Department of Pediatrics The University of Texas Medical Branch Grand Rounds Presentation to Department
Pediatric Oncology for Otolaryngologists Frederick S. Huang, M.D. Division of Hematology/Oncology Department of Pediatrics The University of Texas Medical Branch Grand Rounds Presentation to Department
NORDIC. Indication Mantle cell lymphoma (for patients suitable for subsequent chemotherapy with haematopoietic stem cell rescue)
 NORDIC C) CODOX (R) CODOX M/ (R) IVAC Indication Mantle cell lymphoma (for patients suitable for subsequent chemotherapy with haematopoietic stem cell rescue) ICD-10 code C83.13 Regimen details (R) maxi-chop
NORDIC C) CODOX (R) CODOX M/ (R) IVAC Indication Mantle cell lymphoma (for patients suitable for subsequent chemotherapy with haematopoietic stem cell rescue) ICD-10 code C83.13 Regimen details (R) maxi-chop
CHEMOTHERAPY FOR ADVANCED UROTHELIAL CANCER OF THE BLADDER. Walter Stadler, MD University of Chicago
 CHEMOTHERAPY FOR ADVANCED UROTHELIAL CANCER OF THE BLADDER Walter Stadler, MD University of Chicago Chemotherapy Doctor Terms Drugs used to treat cancer Will attack cancer no matter where it is located
CHEMOTHERAPY FOR ADVANCED UROTHELIAL CANCER OF THE BLADDER Walter Stadler, MD University of Chicago Chemotherapy Doctor Terms Drugs used to treat cancer Will attack cancer no matter where it is located
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab Emtansine (KADCYLA)
 BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab Emtansine (KADCYLA) Protocol Code Tumour Group Contact Physician UBRAVKAD Breast Dr Stephen Chia ELIGIBILITY:
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab Emtansine (KADCYLA) Protocol Code Tumour Group Contact Physician UBRAVKAD Breast Dr Stephen Chia ELIGIBILITY:
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Thames Valley Chemotherapy Regimens
 Chemotherapy Regimens Lung Cancer Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts,
Chemotherapy Regimens Lung Cancer Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts,
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA
 2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
Breast Pathway Group FEC 60 (Fluorouracil / Epirubicin / Cyclophosphamide) in Early Breast Cancer in Elderly / Frail
 Breast Pathway Group FEC 60 (Fluorouracil / Epirubicin / Cyclophosphamide) in Early Breast Cancer in Elderly / Frail Indication: Neoadjuvant or adjuvant therapy for elderly and frail patients with breast
Breast Pathway Group FEC 60 (Fluorouracil / Epirubicin / Cyclophosphamide) in Early Breast Cancer in Elderly / Frail Indication: Neoadjuvant or adjuvant therapy for elderly and frail patients with breast
ADJUVANT TREATMENT CLINICAL EVALUATION NEOADJUVANT TREATMENT
 te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
Carcinoma of the vagina is a relatively uncommon disease, affecting only about 2,000 women in
 EVERYONE S GUIDE FOR CANCER THERAPY Malin Dollinger, MD, Ernest H. Rosenbaum, MD, Margaret Tempero, MD, and Sean Mulvihill, MD 4 th Edition, 2001 Vagina Jeffrey L. Stern, MD Carcinoma of the vagina is
EVERYONE S GUIDE FOR CANCER THERAPY Malin Dollinger, MD, Ernest H. Rosenbaum, MD, Margaret Tempero, MD, and Sean Mulvihill, MD 4 th Edition, 2001 Vagina Jeffrey L. Stern, MD Carcinoma of the vagina is
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
West of Scotland Cancer Network Chemotherapy Protocol. Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021)
 West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT )
 BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT ) Protocol Code Tumour Group Contact Physician UGIPNSUNI Gastrointestinal Dr. Hagen
BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT ) Protocol Code Tumour Group Contact Physician UGIPNSUNI Gastrointestinal Dr. Hagen
BREAST CANCER - METASTATIC & LOCALLY ADVANCED CHEMOTHERAPY REGIMENS Capecitabine. Capecitabine + Docetaxel. Capecitabine + Vinorelbine
 Capecitabine Capecitabine 1000-1250mg/m 2 oral TWICE daily for 14 days Until disease progression Capecitabine + Docetaxel Capecitabine 750-1000mg/m 2 oral TWICE daily for 14 days Up to 6 cycles Capecitabine
Capecitabine Capecitabine 1000-1250mg/m 2 oral TWICE daily for 14 days Until disease progression Capecitabine + Docetaxel Capecitabine 750-1000mg/m 2 oral TWICE daily for 14 days Up to 6 cycles Capecitabine
PREMEDICATIONS: Agent(s) Dose Route Schedule
 BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
Chemotherapy Order Assessment and Review
 Chemotherapy Order Assessment and Review Contents Introduction... 2 Step 1: Verify Patient Information... 2 Step 2: Confirm Protocol Matches Clinical Indication and Eligibility for Treatment... 2 Step
Chemotherapy Order Assessment and Review Contents Introduction... 2 Step 1: Verify Patient Information... 2 Step 2: Confirm Protocol Matches Clinical Indication and Eligibility for Treatment... 2 Step
Docetaxel, Carboplatin and Trastuzumab (TCH i.e. Taxotere Carboplatin, Herceptin ) for Early Breast Cancer
 Regimen: Docetaxel, Carboplatin and Trastuzumab (TCH i.e. Taxotere Carboplatin, Herceptin ) for Early Breast Cancer Indication Approved for the treatment of early and locally advanced breast cancer in
Regimen: Docetaxel, Carboplatin and Trastuzumab (TCH i.e. Taxotere Carboplatin, Herceptin ) for Early Breast Cancer Indication Approved for the treatment of early and locally advanced breast cancer in
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
LYMPHOMA IN DOGS. Diagnosis/Initial evaluation. Treatment and Prognosis
 LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
5-Fluorouracil & Radiotherapy for Adjuvant Oesophageal or Gastric Cancer (Modified Macdonald Protocol)
 5-Fluorouracil & Radiotherapy for Adjuvant Oesophageal or Gastric Cancer (Modified Macdonald Protocol) Indication: Adjuvant Chemo-radiotherapy for patients with resectable adenocarcinoma of the stomach
5-Fluorouracil & Radiotherapy for Adjuvant Oesophageal or Gastric Cancer (Modified Macdonald Protocol) Indication: Adjuvant Chemo-radiotherapy for patients with resectable adenocarcinoma of the stomach
Regimen : Fludarabine Cyclophosphamide Rituximab (FCR-oral)
 Regimen : Fludarabine Cyclophosphamide Rituximab (FCR-oral) Indication CLL 1st line as per NICE TAG 174 CLL (relapsed) as per NICE TAG 193 Regimen details Day Drug Dose Route Cycle 1 1 Rituximab 375mg/m
Regimen : Fludarabine Cyclophosphamide Rituximab (FCR-oral) Indication CLL 1st line as per NICE TAG 174 CLL (relapsed) as per NICE TAG 193 Regimen details Day Drug Dose Route Cycle 1 1 Rituximab 375mg/m
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Cyclophosphamide/Rabbit Anti-Thymocyte Globulin for Allograft
 INDICATIONS Cyclophosphamide/Rabbit Anti-Thymocyte Globulin for Allograft Aplastic Anaemia (patients under 40 years) PRE-ASSESSMENT Ensure pre-transplant work-up form is complete Ensure patient has triple
INDICATIONS Cyclophosphamide/Rabbit Anti-Thymocyte Globulin for Allograft Aplastic Anaemia (patients under 40 years) PRE-ASSESSMENT Ensure pre-transplant work-up form is complete Ensure patient has triple
Adjuvant Chemotherapy 1. Vinorelbine-25/Cisplatin-80 (chemo alone) CTIS 1722 4 2. Gemcitabine-1250/Carboplatin-5AUC CTIS 1601 5
 LUNG CANCER Section by: Dr Conrad Lewanski, Dr Danielle Power, Dr Tom Newsom-Davis Version; Lung Cancer Regimens v4.3 NWLCN 20Feb12 Section last reviewed: 1 st July 2011 Section last corrected: 20 th February
LUNG CANCER Section by: Dr Conrad Lewanski, Dr Danielle Power, Dr Tom Newsom-Davis Version; Lung Cancer Regimens v4.3 NWLCN 20Feb12 Section last reviewed: 1 st July 2011 Section last corrected: 20 th February
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
West of Scotland Cancer Network Systemic Anti-Cancer Therapy Protocol
 West of Scotland Cancer Network Systemic Anti-Cancer Therapy Protocol FEC-DH in the adjuvant treatment of Breast Cancer (BRWOS- 031/1) Indication Adjuvant chemotherapy for HER2+ve Early Breast Cancer Eligibility
West of Scotland Cancer Network Systemic Anti-Cancer Therapy Protocol FEC-DH in the adjuvant treatment of Breast Cancer (BRWOS- 031/1) Indication Adjuvant chemotherapy for HER2+ve Early Breast Cancer Eligibility
Metastatic Breast Cancer 201. Carolyn B. Hendricks, MD October 29, 2011
 Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
Treating myeloma. Dr Rachel Hall Royal Bournemouth Hospital
 Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
National Cancer Drugs Fund List (Updated 13 February 2014)
 National Cancer Drugs Fund List (Updated 13 February 2014) DRUG Abiraterone Aflibercept Axitinib Bendamustine NCDF APPROVED CRITERIA The treatment of metastatic castration resistant prostate cancer where
National Cancer Drugs Fund List (Updated 13 February 2014) DRUG Abiraterone Aflibercept Axitinib Bendamustine NCDF APPROVED CRITERIA The treatment of metastatic castration resistant prostate cancer where
Avastin: Glossary of key terms
 Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Managing Lymphoma. Professor Clare Knottenbelt BVSc MSc DSAM MRCVS
 Managing Lymphoma Professor Clare Knottenbelt BVSc MSc DSAM MRCVS Lymphoma Common cancer (18% of dog cancers) DOGS: Multicentric CATS: Alimentary Presentation varies with site of LSA and paraneoplastic
Managing Lymphoma Professor Clare Knottenbelt BVSc MSc DSAM MRCVS Lymphoma Common cancer (18% of dog cancers) DOGS: Multicentric CATS: Alimentary Presentation varies with site of LSA and paraneoplastic
Clinical Management Protocol Chemotherapy Breast Cancer. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: BREAST CANCER Patient information given at each stage following agreed information pathway
Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: BREAST CANCER Patient information given at each stage following agreed information pathway
Feline Lymphoma Chemotherapy and Chemotherapy Protocols
 Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Guidelines for the Management of. Nausea and Vomiting in Cancer Patients
 Guidelines for the Management of Nausea and Vomiting in Cancer Patients Guidelines for the Management of Nausea and Vomiting in Cancer Patients Management of chemotherapy-induced nausea and vomiting includes
Guidelines for the Management of Nausea and Vomiting in Cancer Patients Guidelines for the Management of Nausea and Vomiting in Cancer Patients Management of chemotherapy-induced nausea and vomiting includes
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including anaplastic, gliosarcoma and glioblastoma multiforme)
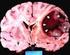 Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
2014-06-10 Approved Bendamustine - Treatment of relapsed low grade NHL in patients unable to receive standard therapy 2014-05-
 Sub. Sub. Status -06-16 Approved Lenalidomide - 2nd line treatment of multiple myeloma in patients who have contraindications to the use of bortezomib -06-16 Approved Lenalidomide - 2nd line treatment
Sub. Sub. Status -06-16 Approved Lenalidomide - 2nd line treatment of multiple myeloma in patients who have contraindications to the use of bortezomib -06-16 Approved Lenalidomide - 2nd line treatment
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Cytotoxic and Biotherapies Credentialing Programme Module 2
 Cytotoxic and Biotherapies Credentialing Programme Module 2 1. The Cell Cycle 2. Cancer Therapies 3. Adjunctive Therapies On completion of this module the RN will State the difference between a normal
Cytotoxic and Biotherapies Credentialing Programme Module 2 1. The Cell Cycle 2. Cancer Therapies 3. Adjunctive Therapies On completion of this module the RN will State the difference between a normal
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Leukaemia and lymphoma what s the difference?
 Freephone helpline 0808 808 5555 [email protected] www.lymphomas.org.uk Leukaemia and lymphoma what s the difference? This is a difficult question to answer simply but it is one that is often
Freephone helpline 0808 808 5555 [email protected] www.lymphomas.org.uk Leukaemia and lymphoma what s the difference? This is a difficult question to answer simply but it is one that is often
Breast Pathway Group FEC(100)-Docetaxel: Fluorouracil / Epirubicin / Cyclophosphamide followed by Docetaxel in Early Breast Cancer
 Breast Pathway Group FEC(100)-Docetaxel: Fluorouracil / Epirubicin / Cyclophosphamide followed by Docetaxel in Indication: Neoadjuvant or adjuvant therapy in node positive patients. Also considered in
Breast Pathway Group FEC(100)-Docetaxel: Fluorouracil / Epirubicin / Cyclophosphamide followed by Docetaxel in Indication: Neoadjuvant or adjuvant therapy in node positive patients. Also considered in
Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD]
![Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD] Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD]](/thumbs/29/13852629.jpg) Approved Haematology Chemotherapy Protocol Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD] MCCN Ref OPCS Proc OPCS Del CLFLUD X70.5 X72.3 IV CLFC X70.4 X72.2 IV X73.1 O CLFCR X71.5 X72.1 LYFMD
Approved Haematology Chemotherapy Protocol Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD] MCCN Ref OPCS Proc OPCS Del CLFLUD X70.5 X72.3 IV CLFC X70.4 X72.2 IV X73.1 O CLFCR X71.5 X72.1 LYFMD
This regimen has low emetogenic potential refer to local protocol None required routinely. Baseline results valid for 7 days. Results valid for 72 hrs
 Regimen : Ipilimumab for Advanced Melanoma ICD10 code Codes pre-fixed with C43. Indication Regimen detail Ipilimumab is recommended as an option for treating advanced (unresectable or metastatic) melanoma
Regimen : Ipilimumab for Advanced Melanoma ICD10 code Codes pre-fixed with C43. Indication Regimen detail Ipilimumab is recommended as an option for treating advanced (unresectable or metastatic) melanoma
Osteosarcoma: treatment beyond surgery
 Osteosarcoma: treatment beyond surgery Eric Chow, DVM DACVS Sue Downing, DVM DACVIM-Oncology Providing the best quality care and service for the patient, the client, and the referring veterinarian. Osteosarcoma
Osteosarcoma: treatment beyond surgery Eric Chow, DVM DACVS Sue Downing, DVM DACVIM-Oncology Providing the best quality care and service for the patient, the client, and the referring veterinarian. Osteosarcoma
Breast Cancer. Breast Cancer Page 1
 Breast Cancer Summary Breast cancers which are detected early are curable by local treatments. The initial surgery will give the most information about the cancer; such as size or whether the glands (or
Breast Cancer Summary Breast cancers which are detected early are curable by local treatments. The initial surgery will give the most information about the cancer; such as size or whether the glands (or
AC-Docetaxel: Doxorubicin / Cyclophosphamide followed by Docetaxel in Breast Cancer
 AC-Docetaxel: Doxubicin / Cyclophosphamide followed by Docetaxel in Breast Cancer Indication: Neoadjuvant therapy f high risk and fit Breast Cancer patients, suitable f a taxane containing regimen AC Regimen
AC-Docetaxel: Doxubicin / Cyclophosphamide followed by Docetaxel in Breast Cancer Indication: Neoadjuvant therapy f high risk and fit Breast Cancer patients, suitable f a taxane containing regimen AC Regimen
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Cytotoxic Therapy in Metastatic Breast Cancer
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Version 2002: von Minckwitz Versions
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Version 2002: von Minckwitz Versions
New Treatment Options for Breast Cancer
 New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
Network Chemotherapy Protocols
 Network Chemotherapy Protocols Sarcoma Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.nhs.uk. Any correspondence about the protocols should
Network Chemotherapy Protocols Sarcoma Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.nhs.uk. Any correspondence about the protocols should
.org. Metastatic Bone Disease. Description
 Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Hydration Protocol for Cisplatin Chemotherapy
 Betsi Cadwaladr University Health Version: 1.3 CSPM2 Hydration Protocol for Cisplatin Chemotherapy Date to be reviewed: July 2018 No of pages: 9 Author(s): Tracy Parry-Jones Author(s) title: Lead Cancer
Betsi Cadwaladr University Health Version: 1.3 CSPM2 Hydration Protocol for Cisplatin Chemotherapy Date to be reviewed: July 2018 No of pages: 9 Author(s): Tracy Parry-Jones Author(s) title: Lead Cancer
London Cancer Systemic Treatment for Breast Cancer
 London Cancer Systemic Treatment for Breast Cancer July 2013 Revise every 6 months Full review April 2015 Version 1.1 (January 2014 update) Contents 1. Neo-adjuvant or Primary Medical Treatment... 3 1.1.
London Cancer Systemic Treatment for Breast Cancer July 2013 Revise every 6 months Full review April 2015 Version 1.1 (January 2014 update) Contents 1. Neo-adjuvant or Primary Medical Treatment... 3 1.1.
Treatment options for recurrent ovarian cancer
 Treatment options for recurrent ovarian cancer There are a number of treatment options for women with recurrent ovarian cancer. Chemotherapy is the treatment most commonly offered and on occasion, surgery
Treatment options for recurrent ovarian cancer There are a number of treatment options for women with recurrent ovarian cancer. Chemotherapy is the treatment most commonly offered and on occasion, surgery
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre
 Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre An audit of neutropenic complications in breast cancer patients receiving adjuvant or neo-adjuvant chemotherapy with FEC-D in
Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre An audit of neutropenic complications in breast cancer patients receiving adjuvant or neo-adjuvant chemotherapy with FEC-D in
Oncology. Objectives. Cancer Nomenclature. Cancer is a disease of the cell Cancer develops when certain cells begin to grow out of control
 Oncology Objectives Describe the etiology and pathophysiological mechanisms of cancer Discuss medical and family history findings relevant to cancer Identify general signs and symptoms associated with
Oncology Objectives Describe the etiology and pathophysiological mechanisms of cancer Discuss medical and family history findings relevant to cancer Identify general signs and symptoms associated with
Locoregional & advanced esophagus or esophagogastric junction cancer
 Eloxatin (oxaliplatin) Prior Authorization Request (For Maryland Only) Send completed form to: Case Review Unit CVS/caremark Specialty Programs Fax: 866-249-6155 CVS/caremark administers the prescription
Eloxatin (oxaliplatin) Prior Authorization Request (For Maryland Only) Send completed form to: Case Review Unit CVS/caremark Specialty Programs Fax: 866-249-6155 CVS/caremark administers the prescription
Regimen: Fluorouracil, Epirubicin and Cyclophosphamide + Docetaxel (FEC/T) for Early Breast Cancer
 Regimen: Fluorouracil, Epirubicin and Cyclophosphamide + Docetaxel (FEC/T) for Early Breast Cancer Indication Adjuvant treatment for node positive early breast cancer This protocol is subject to local
Regimen: Fluorouracil, Epirubicin and Cyclophosphamide + Docetaxel (FEC/T) for Early Breast Cancer Indication Adjuvant treatment for node positive early breast cancer This protocol is subject to local
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
Releasing Nuclear Medicine Patients to the Public: Dose Calculations and Discharge Instructions
 Educational Objectives Releasing Nuclear Medicine Patients to the Public: Dose Calculations and Discharge Instructions Robert E. Reiman, MD Radiation Safety Division Duke University Medical Center Durham,
Educational Objectives Releasing Nuclear Medicine Patients to the Public: Dose Calculations and Discharge Instructions Robert E. Reiman, MD Radiation Safety Division Duke University Medical Center Durham,
The Role of Bisphosphonates in Multiple Myeloma: 2007 Update Clinical Practice Guideline
 The Role of Bisphosphonates in Multiple Myeloma: 2007 Update Clinical Practice Guideline Introduction ASCO convened an Update Committee to review and update the 2002 recommendations for the role of bisphosphonates
The Role of Bisphosphonates in Multiple Myeloma: 2007 Update Clinical Practice Guideline Introduction ASCO convened an Update Committee to review and update the 2002 recommendations for the role of bisphosphonates
This 2-part article will discuss 9 anticancer. Commonly Used Chemotherapy Drugs Part 1. M e d i c a t i o n s. Clinician s Brief.
 M e d i c a t i o n s O N C O L O G Y Peer Reviewed Antony Moore, BVSc, MVSc, Diplomate ACVIM (Oncology) Veterinary Oncology Consultants, Sydney, Australia Commonly Used Chemotherapy Drugs Part 1 This
M e d i c a t i o n s O N C O L O G Y Peer Reviewed Antony Moore, BVSc, MVSc, Diplomate ACVIM (Oncology) Veterinary Oncology Consultants, Sydney, Australia Commonly Used Chemotherapy Drugs Part 1 This
