POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
|
|
|
- Francis Warren
- 9 years ago
- Views:
Transcription
1 Original Issue Date (Created): 7/12/2003 Most Recent Review Date (Revised): 3/29/2016 Effective Date: 8/1/2016 I. POLICY Great or Small Saphenous Veins Treatment of the greater small saphenous veins by surgery (ligation and stripping) or endovenous radiofrequency, laser ablation or microfoam sclerotherapy may be considered medically necessary for symptomatic varicose veins/venous insufficiency when the following criteria have been met: POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY There is demonstrated saphenous reflux and CEAP [Clinical, Etiology, Anatomy, Pathophysiology] class C2 or greater; (see policy guidelines) AND There is documentation of one or more of the following indications: o Ulceration secondary to venous stasis; OR o Recurrent superficial thrombophlebitis; OR o Hemorrhage or recurrent bleeding episodes from a ruptured superficial varicosity; OR o Persistent pain, swelling, itching, burning, or other symptoms are associated with saphenous reflux, AND the symptoms significantly interfere with activities of daily living, AND conservative management including compression therapy for at least 3 months has not improved the symptoms. Treatment of great or small saphenous veins by surgery or endovenous radiofrequency, laser ablation or microfoam sclerotherapy that do not meet the criteria described above is considered not medically necessary. Accessory Saphenous Veins Treatment of accessory saphenous veins by surgery (ligation and stripping) or endovenous radiofrequency, laser ablation or microfoam sclerotherapy may be considered medically necessary for symptomatic varicose veins/venous insufficiency when the following criteria have been met: Page 1
2 Incompetence of the accessory saphenous vein is isolated, OR the great or small saphenous veins had been previously eliminated (at least 3 months); AND There is demonstrated accessory saphenous reflux; AND There is documentation of one or more of the following indications: o Ulceration secondary to venous stasis; OR o o o Recurrent superficial thrombophlebitis; OR Hemorrhage or recurrent bleeding episodes from a ruptured superficial varicosity; OR Persistent pain, swelling, itching, burning, or other symptoms are associated with saphenous reflux, AND the symptoms significantly interfere with activities of daily living, AND conservative management including compression therapy for at least 3 months has not improved the symptoms. Treatment of accessory saphenous veins by surgery, endovenous radiofrequency, laser ablation or microfoam sclerotherapy that do not meet the criteria described above is considered not medically necessary. Symptomatic Varicose Tributaries The following treatments are considered medically necessary as a component of the treatment of symptomatic varicose tributaries. These must be performed either at the same time or following prior treatment (surgical, radiofrequency or laser) of the saphenous veins only when pathology of the saphenous veins exists. (none of these techniques has been shown to be superior to another): Stab avulsion Hook phlebectomy Sclerotherapy Transilluminated powered phlebectomy Treatment of symptomatic varicose tributaries when performed either at the same time or following prior treatment of saphenous veins using any other techniques than noted above is considered investigational. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with this procedure. Treatment of varicose tributaries when there is no demonstrated pathology of the saphenous veins is considered investigational. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with this procedure. Perforator Veins Surgical ligation (including subfascial endoscopic perforator surgery) or endovenous radiofrequency or laser ablation of incompetent perforator veins may be considered medically Page 2
3 necessary as a treatment of leg ulcers associated with chronic venous insufficiency when the following conditions have been met: There is demonstrated perforator reflux; AND The superficial saphenous veins (great, small, or accessory saphenous and symptomatic varicose tributaries) have been previously eliminated; AND Ulcers have not resolved following combined superficial vein treatment and compression therapy for at least 3 months; AND The venous insufficiency is not secondary to deep venous thromboembolism. Ligation or ablation of incompetent perforator veins performed concurrently with superficial venous surgery is not medically necessary. Telangiectasia Treatment of telangiectasia such as spider veins, angiomata, and hemangiomata is considered not medically necessary. Other Techniques for conditions not specifically listed above are investigational, including, but not limited to: Sclerotherapy techniques, other than microfoam sclerotherapy, of great, small or accessory saphenous veins Sclerotherapy of perforator veins Sclerotherapy of isolated tributary veins without prior or concurrent treatment of saphenous veins Stab avulsion, hook phlebectomy, or transilluminated powered phlebectomy of perforator, great or small saphenous or accessory saphenous veins Endovenous radiofrequency or laser ablation of tributary veins Endovenous cryoablation of any vein Mechanochemical ablation of any vein (e.g., ClariVein and similar systems). Cyanoacrylate adhesive of any vein There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with the above procedures. Policy guidelines The standard classification of venous disease is the CEAP (Clinical, Etiologic, Anatomic, Pathophysiologic) classification system. The following is the Clinical portion of the CEAP. Page 3
4 C0 C1 C2 C3 C4a C4b C5 C6 S A Clinical Classification No visible or palpable signs of venous disease Telangiectasies or reticular veins Varicose veins Edema Pigmentation and eczema Lipodermatosclerosis and atrophie blanche Healed venous ulcer Active venous ulcer Symptoms including ache, pain, tightness, skin irritation, heaviness, muscle cramps, as well as other complaints attributable to venous dysfunction Asymptomatic The Etiologic, Anatomic, And Pathophysiologic portions of the classifications are online ( It should be noted that the bulk of the literature discussing the role of ultrasound guidance refers to sclerotherapy of the saphenous vein, as opposed to the varicose tributaries. When ultrasound guidance is used to guide sclerotherapy of the varicose tributaries, it would be considered either not medically necessary or incidental to the injection procedure II. PRODUCT VARIATIONS TOP [N] = No product variation, policy applies as stated [Y] = Standard product coverage varies from application of this policy, see below [N] Capital Cares 4 Kids [N] PPO [N] HMO [Y] Senior Blue HMO* [Y] Senior Blue PPO* [N] Indemnity [N] Special Care [N] POS [Y] FEP PPO** * Refer to Novitas Solutions Inc. Local Coverage Determination (LCD) L34924 Treatment of Varicose Veins and Venous Stasis Disease of Lower Extremities ** Refer to FEP Medical Policy Manual MP Treatment of Varicose Veins/Venous Insufficiency. The FEP Medical Policy manual can be found at: III. DESCRIPTION/BACKGROUND TOP The venous system of the lower extremities consists of the superficial veins (this includes the great and small saphenous and accessory, or duplicate, veins that travel in parallel with the great and small saphenous veins), the deep system (popliteal and femoral veins), and perforator veins Page 4
5 that cross through the fascia and connect the deep and superficial systems. One-way valves are present within all veins to direct the return of blood up the lower limb. Because venous pressure in the deep system is generally greater than that of the superficial system, valve incompetence at any level may lead to backflow (venous reflux) with pooling of blood in superficial veins. Varicose veins with visible varicosities may be the only sign of venous reflux, although itching, heaviness, tension, and pain may also occur. Chronic venous insufficiency secondary to venous reflux can lead to thrombophlebitis, leg ulcerations, and hemorrhage. The CEAP classification considers the clinical, etiologic, anatomic, and pathologic (CEAP) characteristics of venous insufficiency, ranging from class 0 (no visible sign of disease) to class 6 (active ulceration). Treatment of venous reflux/venous insufficiency is aimed at reducing abnormal pressure transmission from the deep to the superficial veins. Conservative medical treatment consists of elevation of the extremities, graded compression, and wound care when indicated. Conventional surgical treatment consists of identifying and correcting the site of reflux by ligation of the incompetent junction followed by stripping of the vein to redirect venous flow through veins with intact valves. While most venous reflux is secondary to incompetent valves at the saphenofemoral or saphenopopliteal junctions, reflux may also occur at incompetent valves in the perforator veins or in the deep venous system. The competence of any single valve is not static and may be pressure-dependent. For example, accessory saphenous veins may have independent saphenofemoral or saphenopopliteal junctions that become incompetent when the great or small saphenous veins are eliminated and blood flow is diverted through the accessory veins. Saphenous Veins and Tributaries Saphenous veins include the great and small saphenous and accessory saphenous veins that travel in parallel with the great or small saphenous veins. Tributaries are veins that empty into a larger vein. Treatment of venous reflux typically includes the following: 1. Identification by preoperative Doppler ultrasonography of the valvular incompetence 2. Control of the most proximal point of reflux, traditionally by suture ligation of the incompetent saphenofemoral or saphenopopliteal junction 3. Removal of the superficial vein from circulation, for example by stripping of the great and/or small saphenous veins 4. Removal of varicose tributaries (at the time of the initial treatment or subsequently) by stab avulsion (phlebectomy) or injection sclerotherapy. Minimally invasive alternatives to ligation and stripping have been investigated. These include sclerotherapy, TIPP, and thermal ablation using cryotherapy, high-frequency radio waves ( khz), or laser energy. Sclerotherapy The objective of sclerotherapy is to destroy the endothelium of the target vessel by injecting an irritant solution (either a detergent, osmotic solution, or chemical irritant), ultimately resulting in the occlusion of the vessel. The success of the treatment depends on accurate injection of the vessel, an adequate injectate volume and concentration of sclerosant, and compression. Page 5
6 Historically, larger veins and very tortuous veins were not considered to be good candidates for sclerotherapy due to technical limitations. Technical improvements in sclerotherapy have included the routine use of Duplex ultrasound to target refluxing vessels, luminal compression of the vein with anesthetics, and a foam/sclerosant injectate in place of liquid sclerosant. Foam sclerosants are commonly produced by forcibly mixing a gas (eg, air or carbon dioxide) with a liquid sclerosant (eg, polidocanol or sodium tetradecyl sulfate). The foam is produced at the time of treatment. Varithena (previously known as Varisolve; BTG Plc, London) is a proprietary microfoam sclerosant that is dispersed from a canister with a controlled density and more consistent bubble size. Endovenous Mechanochemical Ablation Endovenous mechanochemical ablation (MOCA ) uses both sclerotherapy and mechanical damage to the lumen. Following ultrasound imaging, a disposable catheter with a motor drive is inserted into the distal end of the target vein and advanced to the saphenofemoral junction. As the catheter is pulled back, a wire rotates at 3500 rpm within the lumen of the vein, abrading the lumen. At the same time, a liquid sclerosant (sodium tetradecyl sulfate) is infused near the rotating wire. It is proposed that mechanical ablation allows for better efficacy of the sclerosant, and results in less pain and risk of nerve injury without need for the tumescent anesthesia used with thermal endovenous ablation techniques (radiofrequency ablation [RFA] and endovenous laser treatment [EVLT]). Thermal Ablation RFA is performed by means of a specially designed catheter inserted through a small incision in the distal medial thigh to within 1 to 2 cm of the saphenofemoral junction. The catheter is slowly withdrawn, closing the vein. Laser ablation is performed similarly; a laser fiber is introduced into the great saphenous vein under ultrasound guidance; the laser is activated and slowly removed along the course of the saphenous vein. Cryoablation uses extreme cold to cause injury to the vessel. The objective of endovenous techniques is to cause injury to the vessel, causing retraction and subsequent fibrotic occlusion of the vein. Technical developments since thermal ablation procedures were initially introduced include the use of perivenous tumescent anesthesia, which allows successful treatment of veins larger than 12 mm in diameter and helps to protect adjacent tissue from thermal damage during treatment of the small saphenous vein. Cyanoacrylate Adhesive Cyanoacrylate adhesive is a clear, free-flowing liquid that polymerizes in the vessel via an anionic mechanism (ie, polymerizes into a solid material on contact with body fluids or tissue). The adhesive is gradually injected along the length of the vein in conjunction with ultrasound and manual compression. The acute coaptation halts blood flow through the vein until the implanted adhesive becomes fibrotically encapsulated and establishes chronic occlusion of the treated vein. Cyanoacrylate glue has been used as a surgical adhesive and sealant for a variety of indications, including gastrointestinal bleeding, embolization of brain arteriovenous malformations, and to seal surgical incisions or other skin wounds. Page 6
7 Transilluminated Powered Phlebectomy TIPP is an alternative to stab avulsion or hook phlebectomy. This procedure uses 2 instruments: an illuminator, which also provides irrigation, and a resector, which has an oscillating tip and can perform suction. Following removal of the saphenous vein, the illuminator is introduced via a small incision in the skin and tumescence solution (anesthetic and epinephrine) is infiltrated along the course of the varicosity. The resector is then inserted under the skin from the opposite direction, and the oscillating tip is placed directly beneath the illuminated veins to fragment and loosen the veins from the supporting tissue. Irrigation from the illuminator is used to clear the vein fragments and blood through aspiration and additional drainage holes. The illuminator and resector tips may then be repositioned, thereby reducing the number of incisions needed when compared with stab avulsion or hook phlebectomy. It has been proposed that TIPP might result in decreased operative time, decreased complications such as bruising, and faster recovery compared with the established procedures. Treatment of Perforator Veins Perforator veins cross through the fascia and connect the deep and superficial venous systems. Incompetent perforating veins were originally addressed with an open surgical procedure, called the Linton procedure, which involved a long medial calf incision to expose all posterior, medial, and paramedial perforators. While this procedure was associated with healing of ulcers, it was largely abandoned due to a high incidence of wound complications. The Linton procedure was subsequently modified by using a series of perpendicular skin flaps instead of a longitudinal skin flap to provide access to incompetent perforator veins in the lower part of the leg. The modified Linton procedure may occasionally be used for the closure of incompetent perforator veins that cannot be reached by less invasive procedures. Subfascial endoscopic perforator surgery is a less invasive surgical procedure for treatment of incompetent perforators and has been reported since the mid-1980s. Guided by Duplex ultrasound scanning, small incisions are made in the skin, and the perforating veins are clipped or divided by endoscopic scissors. The operation can be performed as an outpatient procedure. Endovenous ablation of incompetent perforator veins with sclerotherapy and RFA has also been reported. Other Deep vein valve replacement is being investigated. Outcomes of interest for venous interventions include healing and recurrence, recannulation of the vein, and neovascularization. Recannulation (recanalization) is the restoration of the lumen of a vein after it has been occluded; this occurs more frequently following treatment with endovenous techniques. Neovascularization is the proliferation of new blood vessels in tissue and occurs more frequently following vein stripping. Direct comparisons of durability for endovenous and surgical procedures are complicated by these different mechanisms of recurrence. Relevant safety outcomes include the incidence of paresthesia, thermal skin injury, thrombus formation, thrombophlebitis, wound infection, and transient neurologic effects. Page 7
8 Regulatory Status In 2015, the VenaSeal Closure System (Sapheon, a part of Medtronic) was approved by the U.S. Food and Drug Administration (FDA) through the premarket approval process for the permanent closure of clinically significant venous reflux through endovascular embolization with coaptation. The VenaSeal Closure System seals the vein using a cyanoacrylate adhesive agent. FDA product code: PJQ. In 2013, Varithena (formerly known as Varisolve ; BTG Plc, London), a sclerosant microfoam made with a proprietary gas mix, was approved by FDA under a new drug application for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein system above and below the knee. The following devices were cleared for marketing by FDA through the 501(k) process for the endovenous treatment of superficial vein reflux: In 1999, the VNUS Closure System (a radiofrequency device) received FDA clearance through the 510(k) process for "endovascular coagulation of blood vessels in patients with superficial vein reflux." The VNUS RFS and RFSFlex devices received FDA clearance in 2005 for use in vessel and tissue coagulation including: treatment of incompetent (ie, refluxing) perforator and tributary veins. The modified VNUS ClosureFast Intravascular Catheter received FDA clearance through the 510(k) process in FDA product code: GEI. In 2002, the Diomed 810 nm surgical laser and EVLT (endovenous laser therapy) procedure kit received FDA clearance through the 510(k) process, " for use in the endovascular coagulation of the great saphenous vein of the thigh in patients with superficial vein reflux." FDA product code: GEX. A modified Erbe Erbokryo cryosurgical unit (Erbe USA) received FDA clearance for marketing in A variety of clinical indications are listed, including cryostripping of varicose veins of the lower limbs. FDA product code: GEH. The Trivex system (InaVein LLC) is a device for transilluminated powered phlebectomy that received FDA clearance through the 510(k) process in October According to the label, the intended use is for ambulatory phlebectomy procedures for the resection and ablation of varicose veins. FDA product code: DNQ. The ClariVein Infusion Catheter (Vascular Insights) received marketing clearance through the 510(k) process in 2008 (K071468). It is used for mechanochemical ablation. Predicate devices were listed as the Trellis Infusion System (K013635) and the Slip-Cath Infusion Catheter (K882796). The system includes an infusion catheter, motor drive, stopcock and syringe and is intended for the infusion of physician-specified agents in the peripheral vasculature. FDA product code: KRA. Page 8
9 IV. RATIONALE TOP Treatment of Saphenous Reflux Compression Therapy A 2009 Cochrane review on compression for venous leg ulcers included a total of 39 randomized controlled trials (RCTs), with 47 different comparisons. 1 The review was updated in 2012, and included 48 RCTs with 59 different comparisons. 2 Most of the RCTs were small. Objective measures of healing were the time to complete healing, the proportion of ulcers healed within the trial period (typically 12 weeks), the change in ulcer size, and the rate of change in ulcer size. Evidence from 8 trials indicated that venous ulcers healed more rapidly with compression than without. Findings suggested that multicomponent systems (bandages or stockings) were more effective than single-component compression. In addition, multicomponent systems containing an elastic bandage appeared more effective than those composed mainly of inelastic constituents. Although these meta-analyses did not include time to healing, studies included in the review reported that the mean time to ulcer healing was approximately 2 months, while the median time to healing in other reports was 3 to 5 months. A Cochrane review on compression stockings for the initial treatment of varicose veins in patients without venous ulceration was published in Included in the review were 7 studies involving 356 participants with varicose veins without healed or active venous ulceration (CEAP [Clinical, Etiology, Anatomy, Pathophysiology] classification C2 to C4). Six of the studies compared different types or pressures of stockings. Subjectively, participants symptoms improved, but results were not compared with a control arm. Due primarily to inadequate reporting, the methodologic quality of the included trials was unclear. Meta-analyses were not performed due to inadequate reporting and suspected heterogeneity. The authors concluded that there is insufficient high-quality evidence to determine whether or not compression stockings are effective as the sole and initial treatment of varicose veins in patients without venous ulceration, or whether any type of stocking is superior to any other type. Ligation and Stripping Systematic literature reviews published in 2008 indicate a similar healing rate of venous ulcers with superficial vein surgery and conservative compression treatments but a reduction in ulcer recurrence rate with surgery. 4,5 In general, recurrence rates after ligation and stripping are estimated at around 20% in short-term follow-up. Jones et al reported on the results of a study that randomized 100 patients with varicose veins to undergo either ligation alone or ligation in conjunction with stripping. 6 At 1 year, reflux was detected in 9% of patients, rising to 26% at 2 years. Rutgers and Kitslaar reported on the results of a trial that randomized 181 limbs to undergo either ligation and stripping or ligation combined with sclerotherapy. 7 At 2 years, Doppler ultrasound demonstrated reflux in approximately 10% of patients after ligation and stripping, increasing to 15% at 3 years. Comparison of Endovenous Ablation With Ligation and Stripping An updated Cochrane review from 2014 compared endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus ligation/stripping for saphenous vein varices. 8 Included in Page 9
10 the review were 13 randomized studies with a combined total of 3081 patients. The overall quality of the evidence was moderate. There was no significant difference between sclerotherapy and surgery in the rate of recurrence as rated by clinicians (odds ratio [OR], 1.74; p=0.06) or for symptomatic recurrence (OR=1.28). For endovenous laser ablation (EVLA) versus surgery, there were no significant differences between the treatment groups for clinician noted or symptomatic recurrence, or for recanalization. Neovascularization and technical failure were reduced in the laser group (OR=0.05, p<0.001; OR=0.29, p<0.001, respectively). For endovenous radiofrequency ablation (RFA) versus surgery, there were no significant differences between the groups in clinician noted recurrence, recanalization, neovascularization, or technical failure. The authors concluded that sclerotherapy, EVLA, and RFA are at least as effective as surgery in the treatment of great saphenous varicose veins. In 2014, Brittenden et al reported a multicenter randomized trial that compared foam sclerotherapy, EVLA, and surgical treatment in 798 patients. 9 The study was funded by U.K. s Health Technology Assessment Programme of the National Institute for Health Research. Veins greater than 15 mm were excluded from the study. At the 6-week follow-up visit, patients who were assigned to treatment with foam or laser had the option of treatment with foam for any residual varicosities; this was performed in 38% of patients in the foam group and 31% of patients in the EVLA group. Six months after treatment, mean disease-specific quality of life (QOL) was slightly worse after sclerotherapy than after surgery (p=0.006), and there were more residual varicose veins, although the differences were small. Disease-specific QOL was similar for the laser and surgery groups. The frequency of procedural complications was similar for the foam sclerotherapy (6%) and surgery (7%) groups, but was lower in the laser group (1%). The rate of complications at 6 months (primarily lumpiness, skin staining), was highest for the sclerotherapy group. Endovenous RFA In 2008, Luebke et al reported a meta-analysis of 8 studies that included a total of 224 patients who underwent RFA and 204 patients who underwent stripping. 10 There was no significant difference between RFA and surgery in immediate or complete great saphenous vein occlusion, incomplete great saphenous vein closure, freedom from reflux, recurrent varicose veins, recanalization, or neovascularization between the 2 treatments. There were significant reductions in tenderness and ecchymosis at 1 week and fewer hematomas at 72 hours, 1 week, and 3 weeks with RFA. QOL results, including return to normal activity and return to work, favored RFA over surgery. The authors noted that rates of recanalization, retreatment, occlusion, and reflux may alter with longer follow-up and that further RCTs with longer follow-up are needed. Long-term outcomes of endovenous RFA were reported from the Closure Study Group clinical registry in Thirty-four centers (1006 patients, 1222 limbs) participated in the registry, with 12 centers contributing 5-year data (406 limbs). The registry included data on the treatment of 52 small saphenous veins and 16 accessory saphenous veins. Follow-up at 1 week showed a 97% anatomic success rate and a decrease in pain in 50% (from 85% to 30%) of patients. An additional 162 failures were identified over the 5 years of follow-up; 129 veins were found to have recanalization, and 33 limbs had reflux in the groin. Logistic regression analysis (risk Page 10
11 factors of sex, age, body mass index [BMI]), vein diameter, and catheter pullback speed) showed that BMI was associated with long-term failure. The rate of pull-back speed of the catheter during treatment was associated with failure to occlude or recanalization. Endovenous Laser Ablation The largest trial on EVLA is the 2012 RELACS study, which was a pragmatic design that randomized 400 patients to EVLA performed by a surgeon at 1 site or to ligation and stripping performed by a different surgeon at a second location. 12 At 2-year follow-up, there was no significant difference between the groups for clinically recurrent varicose veins, medical condition on the Homburg Varicose Vein Severity Score, or disease-related QOL. Saphenofemoral reflux was detected by ultrasonography more frequently after endovenous laser treatment (EVLT) (17.8% vs 1.3%). This study will follow patients for 5 years. Another trial compared EVLA with ligation and stripping in 200 limbs (100 in each group). 13 At 1-year follow-up, 98% of the limbs were reported to be free of symptoms. At 2-year follow-up, the EVLA group had 2 veins completely reopened and 5 partially reopened, which was significantly greater than in the ligation and stripping group. In the 2013 MAGNA trial, 223 consecutive patients (240 legs) with great saphenous vein reflux were randomized to EVLA, ligation and stripping, or foam sclerotherapy. 14 At 1-year follow-up, the anatomic success rates were similar between EVLA and stripping (88.5% and 88.2%, respectively), which were superior to foam sclerotherapy (72.2%). Ten percent of the stripping group showed neovascularization. Healthrelated QOL improved in all groups. The CEAP classification improved in all groups with no significant difference between the groups. Transient adverse events were reported in 11 patients after stripping, 7 after EVLA, and 5 after sclerotherapy. At 5-year follow-up, Kaplan-Meier analysis showed obliteration or absence of the great saphenous vein in 85% of patients who underwent conventional surgery and 77% of patients who underwent EVLA (not significantly different). 15 Grade I neovascularization was higher in the conventional surgery group (27% vs 3%, p<0.001), while grade II neovascularization was similar in the 2 groups (17% vs 13%). Literature on isolated treatment of the anterior accessory saphenous vein is limited. In a 2009 study, outcomes from a cohort of 33 patients who underwent EVLA of the anterior accessory saphenous vein were compared with 33 matched controls undergoing EVLA of the great saphenous vein. 16 In 21 of the patients (64%) in the accessory saphenous vein group, there had been no previous treatment of the great saphenous vein. At 12-month follow-up, there was no evidence of reflux in these patients, and the treated accessory saphenous vein was not visible with ultrasound. The Aberdeen Varicose Vein Questionnaire (AVVQ) had improved in both groups, with no significant difference between the 2 groups. Patient satisfaction scores were also similar. Sclerotherapy In the 2013 MAGNA trial (previously described), 223 consecutive patients (240 legs) with great saphenous vein reflux were randomized to EVLA, ligation and stripping, or physician compounded foam sclerotherapy (1 cc aethoxysclerol 3%: 3 cc air). 14 At 1-year follow-up, the anatomic success rate of foam sclerotherapy (72.2%) was inferior to both EVLA and stripping (88.5% and 88.2%, respectively). Twenty-one patients in the sclerotherapy group had partial Page 11
12 occlusion with reflux, though the clinical complaint was completely relieved. At 5-year followup, obliteration or absence of the great saphenous vein was observed in only 23% of patients treated with sclerotherapy compared with 85% of patients who underwent conventional surgery and 77% of patients who underwent EVLA. 15 Thirty-two percent of legs treated initially with sclerotherapy required 1 or more reinterventions during follow-up compared with 10% in the conventional surgery and EVLA groups. However, clinically relevant grade II neovascularization was higher in the conventional surgery and EVLA groups (17% and 13%, respectively) compared with the sclerotherapy group (4%). EuroQol-5D scores improved equally in all groups. A 2012 study was a noninferiority trial of foam sclerotherapy versus ligation and stripping in 430 patients. 17 Analysis was per protocol. Forty patients (17%) had repeat sclerotherapy. At 2 years, the probability of clinical recurrence was similar in the 2 groups (11.3% sclerotherapy vs 9.0% ligation and stripping), although reflux was significantly more frequent in the sclerotherapy group (35% vs 21%). Thrombophlebitis occurred in 7.4% of patients after sclerotherapy. There were 2 serious adverse events in the sclerotherapy group (deep venous thrombosis and pulmonary emboli) that occurred within 1 week of treatment. In 2013, Varithena microfoam was approved under a new drug application for the treatment of varicose veins. Efficacy data were from 2 randomized, blinded, multicenter studies. 18 One evaluated Varithena at 0.5%, 1.0%, and 2.0% polidocanol and the second evaluated Varithena at 0.5% and 1.0% polidocanol compared with endovenous placebo or a subtherapeutic dose of polidocanol foam. The primary end point was improvement in symptoms at week 8, as measured by the Varicose Vein Symptoms Questionnaire. The improvement in symptoms was greater in the pooled Varithena treatment group (p<0.001) and in each of the individual dose-concentration groups compared with vehicle alone. Secondary and tertiary end points (appearance, duplex ultrasound response, QOL) were also significantly better for the Varithena groups compared with controls. This second study, called VANISH-2, was published in At the 8-week assessment, there was elimination of reflux and/or occlusion of the previously incompetent vein in 85.6% of the combined 0.5% and 1.0% groups, 59.6% of patients in the 0.125% group, and 1.8% of the placebo group. Analysis of data from both studies showed a dose response from 0.5% to 2.0% for improvement in appearance and from 0.5% to 1.0% for Duplex responders. The 1.0% dose of Varithena was selected for the U.S. Food and Drug Administration (FDA) approval. Safety analysis found deep vein thrombosis detected by ultrasound in 2.8% of Varithena -treated patients with 1% of patients having proximal symptomatic thrombi; these were treated with anticoagulants. There was no signal of an increase in neurological adverse events, and there were no adverse cardiac or cardiopulmonary effects following treatment with Varithena injectable foam. Rates of occlusion with Varithena are similar to those reported for EVLA or stripping. A randomized trial comparing EVLA and stripping with this new preparation of foam sclerotherapy is needed to evaluate its comparative effectiveness. Evaluation out to 5 years is continuing. Mechanochemical Ablation Several prospective series and cohort studies have been reported. One prospective multicenter series evaluated the efficacy of mechanochemical ablation (MCA) of the great saphenous vein in Page 12
13 126 patients in a community setting. 20 Veins were selected that were greater than 4 mm and less than 12 mm in diameter, with an average diameter of 7.3 mm. Closure rates were 100% at 1 week, 98% at 3 months, and 94% at 6 months. The Venous Clinical Severity Score decreased from a score of approximately 9 pretreatment to about 3 at 6 months. In 2012, Elias and Raines reported an industry-sponsored safety and efficacy study of MCA with the ClariVein system. 21 Thirty great saphenous veins in 29 patients were treated with this device. Great saphenous veins with diameters greater than 12 mm were excluded. At 6-month follow-up, 1 vein had recanalized, for a primary closure rate of 96.7%. No pain during the procedure or adverse events was reported. Another prospective series evaluated MCA of the small saphenous vein in 50 consecutive patients. 22 Only patients with a vein diameter of 2.5 to 11 mm were included. The dose of sclerosant was increased after the first 15 patients. At the 6-week assessment, all treated veins were occluded and at 1-year follow-up, 94% remained occluded. The median visual analog scale score for pain during the procedure was 2 of 10. There were no major complications. Controlled studies with a greater number of subjects and longer follow-up are needed. A 2013 review of MCA notes that a 5-year 840 patient randomized trial comparing ClariVein with RFA began in 2012 in Europe. 23 This trial will provide needed data on the comparative effectiveness of MCA, measured at a longer duration and in a larger population. Early results from this trial (119 patients) indicate that intraprocedural pain is slightly lower with MCA (13.4 mm on a 100-mm scale) compared with RFA (24.4 mm; p=0.001). 24 Cyanoacrylate Adhesion The VenaSeal pivotal study (VeClose) was a multicenter noninferiority trial with 222 patients that compared VenaSeal versus RFA for the treatment of venous reflux. 25,26 The primary end point, the proportion of patients with complete closure of the target great saphenous vein at 3 months measured by ultrasound, was noninferior to RFA, with a 99% closure rate for VenaSeal compared with 96% for RFA. The secondary end point of intraoperative pain was similar for the 2 groups (2.2 on a 10-point scale for VenaSeal and 2.4 for RFA, p=0.11). Ecchymosis at day 3 was significantly lower in the cyanoacrylate group; 67.6% of patients treated with cyanoacrylate had no ecchymosis compared with 48.2% of patients following RFA (p<0.01). Scores on the Aberdeen Varicose Veins Questionnaire (AVVQ) and Venous Clinical Severity Score improved to a similar extent in the 2 groups. Longer term follow-up is needed to permit conclusions on the durability of this procedure. Twenty-four-month follow-up was reported for 24 of 38 patients enrolled in a study by Almeida et al. 27 Thirty-three-month follow-up was reported in 467 veins out of a series of 795 veins (58.7%) treated at 1 institution in Germany. 28 An inflammatory reddening of the skin was observed at approximately 1 week after treatment in 11.7% of cases. No permanent skin responses were observed. Of the 467 veins reexamined, the sealing rate was 97.7%. This series is limited by the high loss to follow-up. Endovenous Cryoablation Klem et al reported a randomized trial in 2009 that found endovenous cryoablation (n=249) to be inferior to conventional stripping (n=245) for treating patients with symptomatic varicose veins. 29 The percentage of patients with great saphenous vein remaining was 44% in the Page 13
14 endovenous cryoablation group and 15% in the conventional stripping group. The AVVQ also showed better results for conventional stripping (score, 11.7) in comparison with cryoablation (score, 8.0). There were no differences between the groups in 36-Item Short-Form Health Survey summary scores, and neural damage was the same (12%) in both groups. Disselhoff et al reported 2 and 5 year outcomes from a randomized trial that compared cryoablation with EVLA. 30,31 Included were 120 patients with symptomatic uncomplicated varicose veins (CEAP C2) with saphenofemoral incompetence and great saphenous vein reflux. At 10 days after treatment, EVLA had better results than cryoablation with respect to pain score over the first 10 days (2.9 vs 4.4), resumption of normal activity (75% vs 45%) and induration (15% vs 52%). At 2-year follow-up, freedom from recurrent incompetence was observed in 77% of patients after EVLA and 66% of patients after cryoablation (not significantly different). At 5 years, 36.7% of patients were lost to follow-up; freedom from incompetence and neovascularization was found in 62% of patients treated with EVLA and 51% of patients treated with cryoablation (not significantly different). Neovascularization was more common after cryoablation, but incompetent tributaries were more common after EVLA. There was no significant difference between groups in the Venous Clinical Severity Score or AVVQ at either 2 or 5 years. Other Treatments Both steam injection and microwave ablation for endovenous treatment of varicose veins were reported outside of the United States These procedures have not been approved or cleared for marketing by FDA. Section Summary: Treatment of Saphenous Reflux There are a number of large randomized trials on endovenous ablation of the saphenous veins. Comparison with ligation and stripping at 2-year follow-up supports use of both RFA and EVLA. Evidence suggests that ligation and stripping may lead to neovascularization, while thermal ablation may lead to recanalization. Controlled studies with longer follow-up are needed to determine the long-term efficacy of these treatments with greater certainty. Two RCTs suggest that cryotherapy is not as effective as available alternatives. For physician-compounded sclerotherapy, there is high variability in success rates of the procedure and reports of serious adverse events. Rates of occlusion with the FDA-approved microfoam sclerotherapy are similar to those reported for EVLA or stripping. MCA is a combination of liquid sclerotherapy with mechanical abrasion. A potential advantage of this procedure compared with thermal ablation techniques is that it does not require tumescent anesthesia and may result in less postoperative pain. To date, the ClariVein procedure has been assessed in relatively few patients and for short durations. Thus, there is insufficient evidence to permit conclusions regarding the safety and efficacy of MCA. Short-term efficacy of cyanoacrylate adhesion has been shown to be noninferior to RF in a large multicenter RCT at 3 months. Longer term follow-up is needed to determine durability of this treatment. Page 14
15 Treatment of Tributary Varicosities Sclerotherapy and Phlebectomy Early studies established ligation and stripping as the criterion standard for the treatment of saphenofemoral incompetence based on improved long-term recurrence rates, with sclerotherapy used primarily as an adjunct to treat varicose tributaries. A 2006 Cochrane Review, based primarily on RCTs from the 1980s, concluded that: The evidence supports the current place of sclerotherapy in modern clinical practice, which is usually limited to treatment of recurrent varicose veins following surgery and thread veins. 35 Sclerotherapy and phlebectomy are considered appropriate in the absence of reflux of the saphenous system, eg, post- or adjunctive treatment to other procedures such as surgery. 36 In 2014, El-Sheikha et al reported a small randomized trial of concomitant or sequential (if needed) phlebectomy following EVLA for varicose veins. 37 QOL and clinical severity scores were similar between the groups by 1 year, with 16 of 24 patients (67%) in the sequential phlebectomy group receiving a secondary intervention. A small proportion of patients may present with tributary varicosities in the absence of saphenous reflux. For example, of 1009 patients recruited for an RCT, 64 patients were found to have minor varicose veins without reflux, 34 of whom agreed to be randomized to sclerotherapy or conservative treatment. 38 At baseline, 92% had symptoms of heaviness, 69% had cosmetic concerns, 53% reported itching, and 30% reported relief of symptoms through the use of compression hosiery. At 1-year follow-up, there was an improvement in clinicians assessment of the anatomic extent of varicose veins, with 85% of patients in the sclerotherapy group improved compared with 29% of patients in the conservative-therapy group. Symptoms of aching were better or eliminated in 69% of the sclerotherapy group and 28% of the group treated with conservative therapy. Cosmetic concerns were improved in 85% of the sclerotherapy patients and 14% of controls. The bulk of the literature discussing the role of ultrasound guidance refers to sclerotherapy of the saphenous vein, as opposed to the varicose tributaries. In 2012, Yamaki et al reported a prospective RCT that compared visual foam sclerotherapy versus ultrasound-guided foam sclerotherapy of the great saphenous vein together with visual foam sclerotherapy for varicose tributary veins. 39 A total of 51 limbs in 48 patients were treated with ultrasound-guided foam sclerotherapy plus visual foam sclerotherapy of the varicose tributaries, and 52 limbs in 49 patients were treated with foam sclerotherapy alone. At 6-month follow-up, complete occlusion was found in 23 limbs (45.1%) treated with ultrasound-guided and visual-guided foam sclerotherapy and in 22 limbs (42.3%) treated with visual sclerotherapy alone. Reflux was absent in 30 limbs (58.8%) treated with ultrasound and visual guidance and in 37 (71.2%) treated with visual guidance alone (not significantly different). The authors note that for the treatment of tributary veins in clinical practice, most patients receive direct injection of foam without ultrasound guidance. Transilluminated Powered Phlebectomy A 2008 meta-analysis included 5 studies that compared TIPP with conventional surgery. 40 Results showed a significant advantage of TIPP over the conventional treatment for number of Page 15
16 incisions, mean cosmetic score, and duration of the procedure. However, TIPP also increased the incidence of hematoma and resulted in worse mean pain scores. Included in the meta-analysis was an RCT by Chetter et al that compared TIPP (n=29) with a multiple stab incision procedure (n=33). 41 A single surgeon performed all but 2 of the procedures, and there was no difference in operating time. Patients treated with TIPP had an average of 5 incisions, compared with 20 for the multiple stab procedure. However, blinded evaluation revealed that bruising or discoloration was higher for the TIPP group at both 1 and 6 weeks after surgery. At 6 weeks after surgery, patients in the TIPP group showed no improvement in pain (-2 points on the Burford Pain Scale), while patients in the multiple stab incision group had a significant improvement in pain score compared with presurgical baseline (-20 points). Six weeks after surgery, QOL measures had improved in the multiple-stab incision group but not in the TIPP group. Thus, although TIPP had the advantage of fewer surgical incisions, in this single-center study, it was associated with a more prolonged recovery due to more extensive bruising, prolonged pain, and reduced early postoperative QOL. The current literature does not show an advantage of TIPP over conventional treatment. Section Summary: Treatment of Tributary Varicosities The literature indicates that sclerotherapy of tributaries following occlusion of the saphenofemoral or saphenopopliteal junction and saphenous veins may be considered medically necessary. Evidence is insufficient to evaluate the health benefit of sclerotherapy as a sole treatment of varicose tributaries without prior or concurrent treatment of the saphenous veins. No studies have been identified that compare RF or laser ablation of tributary veins with standard procedures (microphlebectomy and/or sclerotherapy). Transilluminated powered phlebectomy (TIPP) is effective at removing varicosities; outcomes are comparable with available alternatives such as stab avulsion and hook phlebectomy. However, there is limited evidence that TIPP is associated with more pain, bruising, discoloration, and a longer recovery. Treatment of Perforator Reflux A systematic literature review published in 2008 indicates insufficient evidence for the role of incompetent perforator vein surgery. 5 These conclusions were based on 4 RCTs published since 2000 that compared superficial vein surgery with conservative therapy in advanced chronic venous insufficiency (CEAP C5 to C6). The 4 trials included 2 level I (large subject population) and 2 level II (small subject population) studies. Two of the trials combined surgical treatment of the incompetent perforator veins with concurrent or prior treatment of the superficial saphenous veins; the other 2 treated the great saphenous vein alone. The 2 randomized studies in which the great saphenous vein alone was treated (including the ESCHAR trial) showed a significant reduction in ulcer recurrence in comparison with conservative therapy. 42,43 A 2011 community hospital-based multicenter, double-blind, randomized trial found no clinical benefit (selfreported symptoms) from adding subfascial endoscopic perforator surgery (SEPS) to saphenous surgery in 75 patients with varicose ulcers (CEAP C5 or C6) and incompetent perforators. 44 Treatment of the great saphenous vein alone has been reported to improve perforator function. For example, 1 study showed that reversal of perforator vein incompetence (41% of 68 previously incompetent perforators) was more common than new perforator vein incompetence Page 16
17 (22% of 183 previously competent perforators) following superficial vein surgery. 45 O Donnell discusses additional (lower quality) evidence to suggest deep venous valvular involvement rather than incompetent perforators in venous insufficiency. 5 Thus, although incompetence of perforator veins is frequently cited as an important etiologic factor in the pathogenesis of venous ulcer, current evidence does not support the routine ligation or ablation of perforator veins. Subfascial Endoscopic Perforator Surgery In 2004, Tenbrook et al published a review of the literature of SEPS, which included 19 case series and 1 randomized trial. 46 In total, the reviewed studies included 1031 patients with 1140 treated limbs. The authors concluded that SEPS was associated with excellent results in terms of ulcer healing and prevention of recurrence. However, the authors also noted that randomized trials are required to define the relative contributions of compression therapy, superficial venous surgery, and SEPS in the management of severe venous disease. In 2015, Van Gent et al reported 10-year follow-up of a randomized trial that compared conservative treatment versus SEPS for venous leg ulcers. 47 Patients (196 legs) returned to the clinic on an annual basis and analysis was conducted with the last-observation-carried-forward. The primary outcome, incidence ulcer-free, was significantly higher in the surgical group than in the conservative treatment group (58.9% vs 39.6%, p=0.007). The number of incompetent perforator veins at follow-up was a risk factor for not being ulcer free (OR=18.5, p<0.001). The relatively high rate of recurrence of the surgically treated group may be due to limited/no stripping of the superficial veins at the time of SEPS. A 2009 meta-analysis of SEPS for chronic venous insufficiency concludes that Its [SEPS] use should not be employed routinely and could only be justified in patients with persistent ulceration thought to be of venous origin, and in whom any superficial reflux has already been ablated and postthrombotic changes excluded. 48 The authors also state that introduction of less invasive techniques for perforator vein ablation, such as ultrasound-guided sclerotherapy or radiofrequency ablation, may diminish the role of SEPS in the future." Other Treatments A 2008 review of procedures for management of varicose veins recommends duplex-guided foam sclerotherapy, microincision phlebectomy, or thermal ablation using a new short RFA catheter for the treatment of symptomatic residual perforator vein incompetence. 49 Ablation of incompetent perforator veins with laser or RFA had been shown to be technically feasible, although no studies had been identified that showed an improvement in clinical outcomes (eg, ulcer healing or recurrence). The 2011 literature update identified 1 study of EVLA for perforating veins in 33 patients with a CEAP classification of 4 (skin changes), 5 (healed ulcer), or 6 (active ulcer). 50 All incompetent saphenous trunks were treated simultaneously (63% of limbs). At 3-month follow-up, occlusion was achieved in 78% of the perforating veins. Five patients (15%) had active ulcers at baseline; 4 of the 5 ulcers had healed by 6 weeks after EVLA. Evidence regarding the treatment of perforator veins with ultrasound-guided sclerotherapy is limited, and there is a risk of deep venous occlusion. 51 Page 17
18 Section Summary: Treatment of Perforator Reflux The literature indicates that the routine ligation/ablation of incompetent perforator veins is not medically necessary for the treatment of varicose veins/venous insufficiency at the time of superficial vein procedures. However, when combined, superficial vein procedures and compression therapy have failed to improve symptoms (ie, ulcers), treatment of perforator vein reflux may be as beneficial as any alternative (eg, deep vein valve replacement). Therefore, treatment of incompetent perforator veins may be considered medically necessary in this specific situation. Comparative studies are needed to determine the most effective method of ligating/ablating incompetent perforator veins. SEPS has been shown to be as effective as the Linton procedure with a reduction in adverse events. Although only 1 case series has been identified showing an improvement in health outcomes, endovenous ablation with specialized laser or RF probes has been shown to effectively ablate incompetent perforator veins with a potential decrease in morbidity in comparison with surgical interventions. For sclerotherapy, concerns have been raised about the risk of deep vein occlusion, and evidence is currently insufficient to evaluate the safety or efficacy of this treatment for incompetent perforator veins. Ongoing and Unpublished Clinical Trials Some currently unpublished trials that might influence this review are listed in Table 1. Table 1. Summary of Key Trials NCT No. Trial Name Planned Enrollment Ongoing NCT a Randomized Control Trial Comparing VenaSeal Sapheon Closure System With Radiofrequency Ablation (Pivotal Study) NCT Mechanochemical Endovenous Ablation of Great Saphenous Vein Incompetence Using the ClariVein Device: a Prospective Study NTR4613 a Mechanochemical endovenous ablation versus radiofrequency ablation in the treatment of primary small saphenous vein insufficiency (MESSI trial) NCT: national clinical trial. NTR: Nederlands Trial Registry. a Denotes industry-sponsored or cosponsored trial. Completion Date 244 Sep Dec Apr 2020 Summary of Evidence The evidence on mechanochemical ablation, cyanoacrylate adhesive, and cryoablation in patients with varicose veins/venous insufficiency includes randomized controlled trials (RCTs) and multicenter series. Relevant outcomes are symptoms, morbid events, functional outcomes, and change in disease status. Several series have been reported on mechanochemical ablation (MCA), and a large RCT comparing MCA with radiofrequency (RF) ablation is ongoing. Efficacy of cyanoacrylate adhesion at 3 months has been shown to be noninferior to RF in a multicenter RCT. Longer term follow-up is needed to determine durability of this treatment. Results from a recent RCT of cryoablation indicate that this therapy is inferior to conventional stripping. The evidence is insufficient to determine the effects of the technology on health outcomes. Page 18
19 Clinical Input Received Through Academic Medical Centers and Specialty Medical Societies While the various Academic Medical Centers and Specialty Medical Societies may collaborate with and make recommendations during this process, through the provision of appropriate reviewers, input received does not represent an endorsement or position statement by the Academic Medical Centers or Specialty Medical Societies, unless otherwise noted Input In response to requests, input was received from 4 physician specialty societies while this policy was under review in There was no agreement on the need to treat varicose tributaries to improve functional outcomes in the absence of saphenous vein disease. Input was also mixed on the use of mechanochemical ablation or use of cyanoacrylate adhesive. Practice Guidelines and Position Statements Society for Vascular Surgery and American Venous Forum The Society for Vascular Surgery and the American Venous Forum (AVF) published clinical practice guidelines in The recommendations are rated as strong = 1 or weak = 2, based on a level of evidence that is either high quality = A, moderate quality = B, or low quality = C, and include the following: Compression therapy for venous ulcerations and varicose veins: Compression therapy is recommended as the primary treatment to aid healing of venous ulceration (grade 1B, strong recommendation, moderate quality evidence). To decrease the recurrence of venous ulcers, they recommend ablation of the incompetent superficial veins in addition to compression therapy (grade 1A, strong recommendation, high-quality evidence). They recommend use of compression therapy for patients with symptomatic varicose veins (grade 2C, weak recommendation, low-quality evidence) but recommend against compression therapy as the primary treatment if the patient is a candidate for saphenous vein ablation (grade 1B, strong recommendation, moderate quality evidence). Treatment of the incompetent great saphenous vein: Endovenous thermal ablation (radiofrequency or laser) is recommended over chemical ablation with foam (grade 1B, strong recommendation, moderate quality evidence) or high ligation and stripping (grade 1B, strong recommendation, moderate quality evidence) due to reduced convalescence and less pain and morbidity. Cryostripping is a technique that is new in the United States, and it has not been fully evaluated. Varicose tributaries: Phlebectomy or sclerotherapy are recommended to treat varicose tributaries (grade 1B, strong recommendation, moderate quality evidence). Transilluminated powered phlebectomy using lower oscillation speeds and extended tumescence is an alternative to traditional phlebectomy (grade 2C, weak recommendation, low-quality evidence). Perforating vein incompetence: Selective treatment of perforating vein incompetence in patients with simple varicose veins is not recommended (CEAP class C2; grade 1B, strong recommendation, moderate quality evidence), but there is a grade 2B Page 19
20 recommendation (weak recommendation, moderate quality evidence) for treatment of pathologic perforating veins (outward flow of 500 ms duration, with a diameter of 3.5 mm) located underneath healed or active ulcers (CEAP class C5-C6) by subfascial endoscopic perforating vein surgery, sclerotherapy, or thermal ablations (grade 1C, weak recommendation, low-quality evidence). American College of Radiology In 2012, the American College of Radiology published its latest version of appropriateness criteria for the treatment of lower-extremity venous insufficiency. 53 The following is a summary of treatment options: Compression Stockings: Graduated compression stockings are routinely used to control venous insufficiency symptoms. They provide external support that can constrict dilated veins and restore competence to incompetent valves. Compression stockings are particularly helpful during pregnancy, and they are frequently used following venous ablation treatment. Surgery: Great saphenous vein (GSV) stripping with branch ligation had historically been the primary treatment option for venous insufficiency. The GSV is ligated near the groin. Ligation alone can preserve the vein for subsequent harvesting in case of arterial bypass; however, ligation alone has proven unsatisfactory for preventing the occurrence of reflux, so it is often supplemented by vein stripping. Ambulatory phlebectomy is primarily used to treat surface varicose veins. It can be performed as an adjunct to endovenous ablation or stripping. This procedure involves making tiny punctures or incisions through which the varicose veins are removed. Other surgical methods to treat venous insufficiency have been described, including SEPS for treating venous ulcers and valvular surgery for treating reflux caused by incompetent valves of the deep veins. Injection Sclerotherapy: Injection sclerotherapy is a common treatment for telangiectasias and can be used to treat smaller varicose veins. The sclerotherapy solution can be in liquid form or can be injected as "foam" (mixed with a gas such as air). Sclerotherapy has not been shown to have long-term effectiveness for large veins, such as the GSV. Endovenous Ablation: Endovenous ablation is a minimally invasive alternative to surgery. It is a percutaneous procedure that can be used to treat the GSV, small saphenous vein, and other superficial veins. Endovenous ablation uses RFA or laser energy (EVLA) applied inside the vein to cause occlusion. Small prospective trials comparing EVLA and RFA with conventional surgery in patients with GSV reflux have shown favorable results. One study demonstrated that EVLA is comparable to surgery in abolishing reflux and improving disease-specific quality of life and that it allows earlier return to normal activity. A recent systematic literature review comparing the safety and efficacy of EVLA and surgery involving saphenous ligation and stripping as treatments for varicose veins showed few differences in clinical effectiveness outcomes, although long-term follow-up was lacking. A meta-analysis suggested that EVLA and RFA are at least as effective as surgery in treating lower-extremity varicose veins. After 3 years, the Page 20
21 estimated pooled success rates for treatment were 78% for surgical stripping, 77% for foam sclerotherapy, 84% for RFA, and 94% for laser therapy. Adjunctive Treatments: Adjunctive treatments may be required to help eliminate venous insufficiency. Patients with venous insufficiency and associated venous occlusion or stenosis of the common iliac vein (eg, May-Thurner syndrome) may require venous recanalization with angioplasty and stenting to achieve a patent conduit for venous return. Patients with pelvic venous insufficiency may require percutaneous embolization of the ovarian veins. Patients with deep venous thrombosis are typically treated with anticoagulation to reduce the risk of thrombus propagation, embolization, and postthrombotic syndrome. One study suggested that endovenous ablation of the saphenous vein can be considered as a viable treatment alternative in patients with venous insufficiency and previous deep venous thrombosis. Complications: All forms of lower-extremity venous insufficiency treatment are subject to recurrence. Additional risks of vein ligation and stripping surgery include: anesthetic risk, scarring, pain, bleeding, deep venous injury or thrombosis, nerve injury, and infection. Complications of the endovenous ablation procedure include bruising, swelling, transient numbness, and rarely deep venous thrombosis. Society of Interventional Radiography In 2003, the Society of Interventional Radiography (SIR) published a position statement 54 that considered endovenous ablation therapy, using either laser or radiofrequency devices under imaging guidance and monitoring, an effective treatment of extremity venous reflux and varicose veins under the following conditions: 1. The endovenous treatment of varicose veins may be medically necessary when one of the following indications (A E) is present: A. Persistent symptoms interfering with activities of daily living in spite of conservative/nonsurgical management. Symptoms include aching, cramping, burning, itching, and/or swelling during activity or after prolonged standing. B. Significant recurrent attacks of superficial phlebitis C. Hemorrhage from a ruptured varix D. Ulceration from venous stasis where incompetent varices are a contributing factor E. Symptomatic incompetence of the great or small saphenous veins (symptoms as in A above) and; 2. A trial of conservative, nonoperative treatment has failed. This would include mild exercise, avoidance of prolonged immobility, periodic elevation of legs, and compressive stockings. and; 3. The patient's anatomy is amenable to endovenous ablation. In a joint statement published in 2007, AVF and SIR recommended reporting standards for endovenous ablation for the treatment of venous insufficiency. 55 The document recommended that reporting in clinical studies should include the symptoms of venous disease, history of Page 21
22 disease and prior treatment, the presence of major comorbidities, and any exclusion criteria. It was noted that potential candidates for endovenous ablation may include patients with reflux in an incompetent great saphenous vein or smaller saphenous vein or in a major tributary branch of the great or smaller saphenous veins such as the anterior thigh circumflex vein, posterior thigh circumflex vein, or anterior accessory great saphenous vein. The presence of reflux in these veins is important to document using duplex ultrasound imaging, and the ultrasound criteria used to define reflux should be indicated. It was also stated that in current practice, most vascular laboratories consider the presence of venous flow reversal for greater than 0.5 to 1.0 second with proximal compression, Valsalva maneuver, or distal compression and release to represent pathologic reflux. National Institute for Health and Care Excellence In 2003 and 2004, the U.K. s National Institute for Health and Care Excellence (NICE) published guidance on radiofrequency ablation of varicose veins and on endovenous laser treatment of the long saphenous vein. 56,57 NICE concluded that the evidence on the safety and efficacy appeared adequate to support the use of these procedures provided that the normal arrangements were in place for consent, audit, and clinical governance. The evidence on efficacy at this time was limited to case series with limited follow-up. Clinicians were encouraged to collect longer-term follow up data. NICE issued updated guidance on ultrasound-guided foam sclerotherapy for varicose veins in The guidance states that: 1.1 Current evidence on the efficacy of ultrasound-guided foam sclerotherapy for varicose veins is adequate. The evidence on safety is adequate, and provided that patients are warned of the small but significant risks of foam embolization (see section 1.2), this procedure may be used with normal arrangements for clinical governance, consent and audit. 1.2 During the consent process, clinicians should inform patients that there are reports of temporary chest tightness, dry cough, headaches and visual disturbance, and rare but significant complications including myocardial infarction, seizures, transient ischaemic attacks and stroke. NICE issued guidance on endovenous mechanochemical ablation in 2013, concluding that current evidence on the safety and efficacy of endovenous mechanochemical ablation for varicose veins is inadequate in quantity and quality. 59 Therefore this procedure should only be used with special arrangements for clinical governance, consent, and audit or research. In 2013, NICE published a practice guideline on the diagnosis and management of varicose veins in the leg. 60 NICE recommends a study of the clinical and cost effectiveness of Concurrent phlebectomies or foam sclerotherapy for varicose tributaries during truncal endothermal ablation for varicose veins Truncal endothermal ablation without concurrent phlebectomies or foam sclerotherapy Page 22
23 Truncal endothermal ablation with phlebectomies or foam sclerotherapy, if needed, 6-12 weeks later. In 2015, NICE published a technology assessment on the clinical effectiveness and costeffectiveness of foam sclerotherapy, endovenous laser ablation, and surgery for varicose veins. 61 Cost-effectiveness was based on a large multicenter randomized trial comparing treatments for varicose veins (described previously). 9 Five-year trial results are currently being evaluated. Not applicable. U.S. Preventive Services Task Force Recommendations V. DEFINITIONS TOP ABLATION is the removal of a part, pathway, or function by surgery, chemical destruction, electrocautery or radiofrequency. BASIC ACTIVITIES OF DAILY LIVING- Include and are limited to walking in the home, eating, bathing, dressing, and homemaking. CHRONIC VENOUS INSUFFICIENCY refers to a collection of venous disorders that includes reflux disease and obstructive physiology. Symptoms include pain, edema and skin irritation. Physical exam reveals ankle edema, subcutaneous fibrosis, hyperpigmentation, lipodermatosclerosia, eczema and dilation of subcutaneous veins and ulcers COSMETIC SURGERY is an elective procedure performed primarily to restore a person s appearance by surgically altering a physical characteristic that does not prohibit normal function, but is considered unpleasant or unsightly. ECHOSCLEROTHERAPY is the use of a duplex ultrasound during sclerotherapy to guide the injections and enhance the precision of saphenous vein sclerotherapy. ENDOLUMINAL means within the lumen of a tubular structure, such as a blood vessel. ENDOSCOPIC refers to a medical procedure that uses a device with a light attached to look at the inside of a body cavity or organ. FASCIA is the fibrous connective tissue of the body that can be separated from other specifically organized structures, such as tendons and ligaments. MECHANOCHEMICAL ABLATION (ENDOVENOUS) IS a procedure where a tube with a rotating hollow wire at its tip is inserted into the affected vein in the leg. As the tube is pulled back out of the vein, the wire is rotated, damaging the lining of the vein. At the same time, a chemical is injected through the hollow wire into the vein. This causes the vein to become inflamed, then shrivel and close. MICROVASCULAR pertains to the portion of the circulatory system that is composed of the capillary network. Page 23
24 NECROTIZING refers to causing the death of tissues or organisms. SUBFASCIAL means beneath a fascia. TELANGIECTASIA is a vascular lesion formed by dilation of a group of small blood vessels VI. BENEFIT VARIATIONS TOP The existence of this medical policy does not mean that this service is a covered benefit under the member's contract. Benefit determinations should be based in all cases on the applicable contract language. Medical policies do not constitute a description of benefits. A member s individual or group customer benefits govern which services are covered, which are excluded, and which are subject to benefit limits and which require preauthorization. Members and providers should consult the member s benefit information or contact Capital for benefit information. VII. DISCLAIMER TOP Capital s medical policies are developed to assist in administering a member s benefits, do not constitute medical advice and are subject to change. Treating providers are solely responsible for medical advice and treatment of members. Members should discuss any medical policy related to their coverage or condition with their provider and consult their benefit information to determine if the service is covered. If there is a discrepancy between this medical policy and a member s benefit information, the benefit information will govern. Capital considers the information contained in this medical policy to be proprietary and it may only be disseminated as permitted by law. VIII. CODING INFORMATION TOP Note: This list of codes may not be all-inclusive, and codes are subject to change at any time. The identification of a code in this section does not denote coverage as coverage is determined by the terms of member benefit information. In addition, not all covered services are eligible for separate reimbursement. Cosmetic and therefore not covered: CPT Codes Current Procedural Terminology (CPT) copyrighted by American Medical Association. All Rights Reserved. Page 24
25 Not covered: HCPCS Code Description S2202 Echosclerotherapy Covered when medically necessary: CPT Codes Current Procedural Terminology (CPT) copyrighted by American Medical Association. All Rights Reserved. ICD-10-CM Diagnosis Description Code* I Varicose veins of right lower extremity with ulcer of thigh I Varicose veins of right lower extremity with ulcer of calf I Varicose veins of right lower extremity with ulcer of ankle I Varicose veins of right lower extremity with ulcer of heel and midfoot I Varicose veins of right lower extremity with ulcer other part of foot I Varicose veins of right lower extremity with ulcer other part of lower leg I Varicose veins of right lower extremity with ulcer of unspecified site I Varicose veins of left lower extremity with ulcer of thigh I Varicose veins of left lower extremity with ulcer of calf I Varicose veins of left lower extremity with ulcer of ankle I Varicose veins of left lower extremity with ulcer of heel and midfoot I Varicose veins of left lower extremity with ulcer other part of foot I Varicose veins of left lower extremity with ulcer other part of lower leg I Varicose veins of left lower extremity with ulcer of unspecified site I83.11 Varicose veins of right lower extremity with inflammation I83.12 Varicose veins of left lower extremity with inflammation I Varicose veins of right lower extremity with both ulcer of thigh and inflammation I Varicose veins of right lower extremity with both ulcer of calf and inflammation I Varicose veins of right lower extremity with both ulcer of ankle and inflammation I Varicose veins of right lower extremity with both ulcer of heel and midfoot and inflammation Page 25
26 ICD-10-CM Diagnosis Description Code* I Varicose veins of right lower extremity with both ulcer other part of foot and inflammation I Varicose veins of right lower extremity with both ulcer of other part of lower extremity and inflammation I Varicose veins of right lower extremity with both ulcer of unspecified site and inflammation I Varicose veins of left lower extremity with both ulcer of thigh and inflammation I Varicose veins of left lower extremity with both ulcer of calf and inflammation I Varicose veins of left lower extremity with both ulcer of ankle and inflammation I Varicose veins of left lower extremity with both ulcer of heel and midfoot and inflammation I Varicose veins of left lower extremity with both ulcer other part of foot and inflammation I Varicose veins of left lower extremity with both ulcer of other part of lower extremity and inflammation I Varicose veins of left lower extremity with both ulcer of unspecified site and inflammation I Varicose veins of right lower extremities with pain I Varicose veins of left lower extremities with pain I Varicose veins of bilateral lower extremities with pain I Varicose veins of right lower extremities with other complications I Varicose veins of left lower extremities with other complications I Varicose veins of bilateral lower extremities with other complications I87.2 Venous insufficiency (chronic) (peripheral) *If applicable, please see Medicare LCD or NCD for additional covered diagnoses. IX. REFERENCES TOP 1. O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009(1):CD PMID O'Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11:CD PMID Shingler S, Robertson L, Boghossian S, et al. Compression stockings for the initial treatment of varicose veins in patients without venous ulceration. Cochrane Database Syst Rev. 2011;11:CD PMID Howard DP, Howard A, Kothari A, et al. The role of superficial venous surgery in the management of venous ulcers: a systematic review. Eur J Vasc Endovasc Surg. Oct 2008;36(4): PMID Page 26
27 5. O'Donnell TF, Jr. The present status of surgery of the superficial venous system in the management of venous ulcer and the evidence for the role of perforator interruption. J Vasc Surg. Oct 2008;48(4): PMID Jones L, Braithwaite BD, Selwyn D, et al. Neovascularisation is the principal cause of varicose vein recurrence: results of a randomised trial of stripping the long saphenous vein. Eur J Vasc Endovasc Surg. Nov 1996;12(4): PMID Rutgers PH, Kitslaar PJ. Randomized trial of stripping versus high ligation combined with sclerotherapy in the treatment of the incompetent greater saphenous vein. Am J Surg. Oct 1994;168(4): PMID Nesbitt C, Bedenis R, Bhattacharya V, et al. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus open surgery for great saphenous vein varices. Cochrane Database Syst Rev. 2014;7:CD PMID Brittenden J, Cotton SC, Elders A, et al. A randomized trial comparing treatments for varicose veins. N Engl J Med. Sep ;371(13): PMID Luebke T, Gawenda M, Heckenkamp J, et al. Meta-analysis of endovenous radiofrequency obliteration of the great saphenous vein in primary varicosis. J Endovasc Ther. Apr 2008;15(2): PMID Merchant RF, Pichot O. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg. Sep 2005;42(3): ; discussion 509. PMID Rass K, Frings N, Glowacki P, et al. Comparable effectiveness of endovenous laser ablation and high ligation with stripping of the great saphenous vein: two-year results of a randomized clinical trial (RELACS study). Arch Dermatol. Jan 2012;148(1): PMID Christenson JT, Gueddi S, Gemayel G, et al. Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. J Vasc Surg. Nov 2010;52(5): PMID Biemans AA, Kockaert M, Akkersdijk GP, et al. Comparing endovenous laser ablation, foam sclerotherapy, and conventional surgery for great saphenous varicose veins. J Vasc Surg. Sep 2013;58(3): e721. PMID van der Velden SK, Biemans AA, De Maeseneer MG, et al. Five-year results of a randomized clinical trial of conventional surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy in patients with great saphenous varicose veins. Br J Surg. Sep 2015;102(10): PMID Theivacumar NS, Darwood RJ, Gough MJ. Endovenous laser ablation (EVLA) of the anterior accessory great saphenous vein (AAGSV): abolition of sapheno-femoral reflux with preservation of the great saphenous vein. Eur J Vasc Endovasc Surg. Apr 2009;37(4): PMID Shadid N, Ceulen R, Nelemans P, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. Br J Surg. Aug 2012;99(8): PMID U.S. Food and Drug Administration Center for Drug Evaluation and Research Varithena Summary Review. 2013; Page 27
28 Accessed December 28, Todd KL, 3rd, Wright D, for the V-IG. The VANISH-2 study: a randomized, blinded, multicenter study to evaluate the efficacy and safety of polidocanol endovenous microfoam 0.5% and 1.0% compared with placebo for the treatment of saphenofemoral junction incompetence. Phlebology. Oct 2014;29(9): PMID Bishawi M, Bernstein R, Boter M, et al. Mechanochemical ablation in patients with chronic venous disease: A prospective multicenter report. Phlebology. Jul PMID Elias S, Raines JK. Mechanochemical tumescentless endovenous ablation: final results of the initial clinical trial. Phlebology. Mar 2012;27(2): PMID Boersma D, van Eekeren RR, Werson DA, et al. Mechanochemical endovenous ablation of small saphenous vein insufficiency using the ClariVein((R)) device: one-year results of a prospective series. Eur J Vasc Endovasc Surg. Mar 2013;45(3): PMID Mueller RL, Raines JK. ClariVein mechanochemical ablation: background and procedural details. Vasc Endovascular Surg. Apr 2013;47(3): PMID Bootun R, Lane T, Dharmarajah B, et al. Intra-procedural pain score in a randomised controlled trial comparing mechanochemical ablation to radiofrequency ablation: The Multicentre Venefit versus ClariVein(R) for varicose veins trial. Phlebology. Sep PMID U.S. Food and Drug Administration. VenaSeal Closure System - P ; Accessed December 28, Morrison N, Gibson K, McEnroe S, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. Apr 2015;61(4): PMID Almeida JI, Javier JJ, Mackay EG, et al. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology. Jul 2015;30(6): PMID Zierau U. Sealing Veins with the VenaSeal Sapheon Closure System: Results for 795 Treated Truncal Veins after 1000 Days. Vasomed. 2015;27: PMID 29. Klem TM, Schnater JM, Schutte PR, et al. A randomized trial of cryo stripping versus conventional stripping of the great saphenous vein. J Vasc Surg. Feb 2009;49(2): PMID Disselhoff BC, der Kinderen DJ, Kelder JC, et al. Randomized clinical trial comparing endovenous laser with cryostripping for great saphenous varicose veins. Br J Surg. Oct 2008;95(10): PMID Disselhoff BC, der Kinderen DJ, Kelder JC, et al. Five-year results of a randomized clinical trial comparing endovenous laser ablation with cryostripping for great saphenous varicose veins. Br J Surg. Aug 2011;98(8): PMID Milleret R, Huot L, Nicolini P, et al. Great saphenous vein ablation with steam injection: results of a multicentre study. Eur J Vasc Endovasc Surg. Apr 2013;45(4): PMID Page 28
29 33. Yang L, Wang XP, Su WJ, et al. Randomized clinical trial of endovenous microwave ablation combined with high ligation versus conventional surgery for varicose veins. Eur J Vasc Endovasc Surg. Oct 2013;46(4): PMID van den Bos RR, Malskat WS, De Maeseneer MG, et al. Randomized clinical trial of endovenous laser ablation versus steam ablation (LAST trial) for great saphenous varicose veins. Br J Surg. Aug 2014;101(9): PMID Tisi PV, Beverley C, Rees A. Injection sclerotherapy for varicose veins. Cochrane Database Syst Rev. 2006(4):CD PMID Leopardi D, Hoggan BL, Fitridge RA, et al. Systematic review of treatments for varicose veins. Ann Vasc Surg. Mar 2009;23(2): PMID El-Sheikha J, Nandhra S, Carradice D, et al. Clinical outcomes and quality of life 5 years after a randomized trial of concomitant or sequential phlebectomy following endovenous laser ablation for varicose veins. Br J Surg. Aug 2014;101(9): PMID Michaels JA, Campbell WB, Brazier JE, et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial). Health Technol Assess. Apr 2006;10(13):1-196, iii-iv. PMID Yamaki T, Hamahata A, Soejima K, et al. Prospective Randomised Comparative Study of Visual Foam Sclerotherapy Alone or in Combination with Ultrasound-guided Foam Sclerotherapy for Treatment of Superficial Venous Insufficiency: Preliminary Report. Eur J Vasc Endovasc Surg. Jan PMID Luebke T, Brunkwall J. Meta-analysis of transilluminated powered phlebectomy for superficial varicosities. J Cardiovasc Surg (Torino). Dec 2008;49(6): PMID Chetter IC, Mylankal KJ, Hughes H, et al. Randomized clinical trial comparing multiple stab incision phlebectomy and transilluminated powered phlebectomy for varicose veins. Br J Surg. Feb 2006;93(2): PMID Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. Jun ;363(9424): PMID Gohel MS, Barwell JR, Taylor M, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. Jul ;335(7610):83. PMID Nelzen O, Fransson I. Early results from a randomized trial of saphenous surgery with or without subfascial endoscopic perforator surgery in patients with a venous ulcer. Br J Surg. Apr 2011;98(4): PMID Blomgren L, Johansson G, Dahlberg-Akerman A, et al. Changes in superficial and perforating vein reflux after varicose vein surgery. J Vasc Surg. Aug 2005;42(2): PMID Tenbrook JA, Jr., Iafrati MD, O'Donnell T F, Jr., et al. Systematic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg. Mar 2004;39(3): PMID Page 29
30 47. van Gent WB, Catarinella FS, Lam YL, et al. Conservative versus surgical treatment of venous leg ulcers: 10-year follow up of a randomized, multicenter trial. Phlebology. Mar 2015;30(1 Suppl): PMID Luebke T, Brunkwall J. Meta-analysis of subfascial endoscopic perforator vein surgery (SEPS) for chronic venous insufficiency. Phlebology. Feb 2009;24(1):8-16. PMID Hirsch SA, Dillavou E. Options in the management of varicose veins, J Cardiovasc Surg (Torino). Feb 2008;49(1): PMID Hissink RJ, Bruins RM, Erkens R, et al. Innovative treatments in chronic venous insufficiency: endovenous laser ablation of perforating veins: a prospective short-term analysis of 58 cases. Eur J Vasc Endovasc Surg. Sep 2010;40(3): PMID Myers KA, Jolley D. Factors affecting the risk of deep venous occlusion after ultrasoundguided sclerotherapy for varicose veins. Eur J Vasc Endovasc Surg. Nov 2008;36(5): PMID Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. May 2011;53(5 Suppl):2S-48S. PMID Silberzweig JE, Funaki BS, Ray CE, Jr., et al. ACR appropriateness criteria treatment of lower-extremity venous insufficiency. 2012; Accessed December 28, Society of Interventional Radiology. Position Statement on Endovenous Ablation. 2003; Accessed December 28, Kundu S, Lurie F, Millward SF, et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: joint statement of the American Venous Forum and the Society of Interventional Radiology. J Vasc Interv Radiol. Sep 2007;18(9): PMID National Institute for Health and Care Excellence (NICE). Radiofrequency Ablation of Varicose Veins; Interventional Procedure Guidance IPG8 2003; Accessed April, National Institute for Health and Care Excellence (NICE). Endovenous Laser Treatment of the Long Saphenous Vein. Interventional Procedure Guidance IPG ; Accessed April, National Institute for Health and Care Excellence (NICE). Ultrasound-guided foam sclerotherapy for varicose veins; IPG ; Accessed April, National Institute for Health and Care Excellence (NICE). IPG435 Endovenous mechanochemical ablation for varicose veins: guidance. 2013; Accessed December 28, National Institute for Health and Care Excellence (NICE). NICE Clinical Guideline 168: Varicose veins in the legs. 2013; Accessed December 28, Page 30
31 61. Brittenden J, Cotton SC, Elders A, et al. Clinical effectiveness and cost-effectiveness of foam sclerotherapy, endovenous laser ablation and surgery for varicose veins: results from the Comparison of LAser, Surgery and foam Sclerotherapy (CLASS) randomised controlled trial. Health Technol Assess. Apr 2015;19(27): PMID Other Sources: Novitas Solutions Inc. Local Coverage Determination (LCD) L34924: Treatment of Varicose Veins and Venous Stasis Disease of the Lower Extremities Effective 10/1/15 [Website]: 924&bc=iAAAAAgAAAAAAA%3d%3d& Accessed December 28, X. POLICY HISTORY TOP MP CAC 11/26/02 CAC 2/25/03 CAC 2/24/04 CAC 12/14/04 CAC 1/31/06 CAC 1/30/07 CAC 1/29/08 CAC 5/27/08 CAC 11/25/08 CAC 7/28/09 CAC 11/24/09 Minor revision- added sclerosing agent must be FDAapproved. CAC 11/30/10 Consensus review CAC 7/26/11 Adopt BCBSA. Documentation requirement for the treatment of the greater or lesser saphenous vein varicosities that the veins are noted to be at least five (5) millimeters in diameter by visual inspection or ultrasound was removed from the policy. Documentation requirement for sclerotherapy for the treatment of superficial varices that the bulging veins are by visual inspection or ultrasound are noted to be 3mm but less than 6mm in diameter was also removed from the policy. Through adopting BCBSA, the policy is now organized into the following sections: Treatment of Greater or Lesser Saphenous Veins, Accessory Saphenous Veins, Symptomatic Varicose Tributaries, Perforator Veins, Telangiectasia, and Other which will facilitate administration. An FEP variation was added. Page 31
32 CAC 8/28/12 Wording changes for clarification to section on Treatment of symptomatic varicose tributaries. No other changes to policy statements. References updated. FEP variation changed to reference FEP policy manual MP Treatment of Varicose Veins/Venous Insufficiency. Codes reviewed 8/13/12 CAC 7/30/13 Minor. Policy statement on accessory saphenous veins was modified to include isolated incompetence of the accessory saphenous vein as medically necessary. Endovenous mechanochemical ablation for varicose veins was added to the policy as investigational. Added rationale section. Admin code review complete. CAC 5/20/14 Consensus review. References and rationale updated. No changes to the policy statements. Coding reviewed. 9/25/14 Admin update- removal of cosmetic language. No change to medical necessity. 12/1/14 Administrative Change. LCD number in Medicare variation changed to reference L Was L /13/15 Admin update- Section -Symptomatic Varicose Tributaries These must be performed either at the same time or following prior treatment (surgical, radiofrequency or laser) of the saphenous veins only when pathology of the saphenous veins exists. (For clarity) CAC 6/2/15 Major review. Added Microfoam sclerotherapy as medically necessary for the treatment of greater or lesser saphenous veins and accessory saphenous veins. Added clarification to indicate treatment of varicose tributaries when there is no demonstrated pathology of the saphenous veins is considered investigational. Updated rationale and references. Coding reviewed. 11/2/15 Administrative change. LCD number changed from L32678 to L34924 due to Novitas update to ICD-10 12/16/15 Administrative change. Added "(e.g., ClariVein and similar systems) to Mechanochemical ablation of any vein. CAC 3/29/16 Minor review. The requirement of failure of compression therapy was removed from the policy statements on ulceration secondary to venous stasis and recurrent superficial thrombophlebitis. The terminology throughout the policy was changed from greater and lesser saphenous veins to great and small saphenous veins. CEAP classification criteria were added to the medically necessary policy statement on great and small saphenous veins and basic information about the CEAP classification system was added to the Policy Guidelines section. Cyanoacrylate adhesive of any vein added as investigational. Updated rationale and references. Coding reviewed and updated. Page 32
33 Top Health care benefit programs issued or administered by Capital BlueCross and/or its subsidiaries, Capital Advantage Insurance Company, Capital Advantage Assurance Company and Keystone Health Plan Central. Independent licensees of the BlueCross BlueShield Association. Communications issued by Capital BlueCross in its capacity as administrator of programs and provider relations for all companies. Page 33
Treatment of Varicose Veins/Venous Insufficiency
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Treatment of Varicose Veins/Venous Insufficiency Number 7.01.519
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Treatment of Varicose Veins/Venous Insufficiency Number 7.01.519
Clinical Review Criteria
 Clinical Review Criteria Treatment of Varicose Veins Radiofrequency Catheter Closure Sclerotherapy Surgical Stripping Trivex System for Outpatient Varicose Vein Surgery VenaSeal Closure System VNUS Closure
Clinical Review Criteria Treatment of Varicose Veins Radiofrequency Catheter Closure Sclerotherapy Surgical Stripping Trivex System for Outpatient Varicose Vein Surgery VenaSeal Closure System VNUS Closure
Medicare C/D Medical Coverage Policy
 Varicose Vein Treatment Origination Date: June 1, 1993 Review Date: July 20, 2016 Next Review: July, 2018 Medicare C/D Medical Coverage Policy DESCRIPTION OF PROCEDURE OR SERVICE Varicose veins of the
Varicose Vein Treatment Origination Date: June 1, 1993 Review Date: July 20, 2016 Next Review: July, 2018 Medicare C/D Medical Coverage Policy DESCRIPTION OF PROCEDURE OR SERVICE Varicose veins of the
Medicare C/D Medical Coverage Policy
 Varicose Vein Treatment Medicare C/D Medical Coverage Policy Origination Date: June 1, 1993 Review Date: September 16, 2015 Next Review: September, 2017 DESCRIPTION OF PROCEDURE OR SERVICE Varicose veins
Varicose Vein Treatment Medicare C/D Medical Coverage Policy Origination Date: June 1, 1993 Review Date: September 16, 2015 Next Review: September, 2017 DESCRIPTION OF PROCEDURE OR SERVICE Varicose veins
Effective Date: March 2, 2016
 Medical Review Criteria Varicose Vein Procedures Effective Date: March 2, 2016 Treatmetn Subject: Varicose Vein Procedures VeinTreatment of Varicose Policy: Veins HPHC covers specific non-experimental
Medical Review Criteria Varicose Vein Procedures Effective Date: March 2, 2016 Treatmetn Subject: Varicose Vein Procedures VeinTreatment of Varicose Policy: Veins HPHC covers specific non-experimental
Yes when meets criteria below
 Vein Disease Treatment MP9241 Covered Service: Prior Authorization Required: Additional Information: Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes None Dean Health Plan covers
Vein Disease Treatment MP9241 Covered Service: Prior Authorization Required: Additional Information: Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes None Dean Health Plan covers
MEDICAL COVERAGE POLICY. SERVICE: Varicose Veins of the Lower Extremities. PRIOR AUTHORIZATION: Required.
 Page 1 of 5 MEDICAL COVERAGE POLICY Important note Even though this policy may indicate that a particular service or supply may be considered covered, this conclusion is not based upon the terms of your
Page 1 of 5 MEDICAL COVERAGE POLICY Important note Even though this policy may indicate that a particular service or supply may be considered covered, this conclusion is not based upon the terms of your
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES SCLEROTHERAPY TREATMENT OF SUPERFICIAL VARICOSE VEINS OF THE LEGS The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance
PROVIDER POLICIES & PROCEDURES SCLEROTHERAPY TREATMENT OF SUPERFICIAL VARICOSE VEINS OF THE LEGS The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance
Medical Coverage Policy Treatment for Varicose Veins-PREAUTH
 Medical Coverage Policy Treatment for Varicose Veins-PREAUTH Device/Equipment Drug Medical Surgery Test Other Effective Date: 9/1/2001 Policy Last Updated: 12/20/2011 Prospective review is recommended/required.
Medical Coverage Policy Treatment for Varicose Veins-PREAUTH Device/Equipment Drug Medical Surgery Test Other Effective Date: 9/1/2001 Policy Last Updated: 12/20/2011 Prospective review is recommended/required.
Surgical Options for Venous Disease. Sandra C Carr MD Vascular Surgery Meriter Wisconsin Heart
 Surgical Options for Venous Disease Sandra C Carr MD Vascular Surgery Meriter Wisconsin Heart Chronic Venous Disease Approximately 23% of adults in the US have varicose veins Estimated 22 million women
Surgical Options for Venous Disease Sandra C Carr MD Vascular Surgery Meriter Wisconsin Heart Chronic Venous Disease Approximately 23% of adults in the US have varicose veins Estimated 22 million women
Clinical Medical Policy Varicose Vein Treatment
 Benefit Coverage Covered Benefit for lines of business including: Health Benefits Exchange (HBE), Rite Care (MED), Children with Special Needs (CSN), Substitute Care (SUB), Rhody Health Partners (RHP),
Benefit Coverage Covered Benefit for lines of business including: Health Benefits Exchange (HBE), Rite Care (MED), Children with Special Needs (CSN), Substitute Care (SUB), Rhody Health Partners (RHP),
Corporate Medical Policy Treatment of Varicose Veins/Venous Insufficiency. Medical Policy
 Corporate Medical Policy Treatment of Varicose Veins/Venous Insufficiency File name: Treatment of Varicose Veins/ Venous Insufficiency File code: UM.SURG.03 Origination: 9/01/2010 Last Review: 07/15/2010,
Corporate Medical Policy Treatment of Varicose Veins/Venous Insufficiency File name: Treatment of Varicose Veins/ Venous Insufficiency File code: UM.SURG.03 Origination: 9/01/2010 Last Review: 07/15/2010,
Medical Coverage Policy Varicose Vein Treatment sad
 Medical Coverage Policy Varicose Vein Treatment sad EFFECTIVE DATE: 10 28 2001 POLICY LAST UPDATED: 05 07 2013 OVERVIEW Varicose veins are large superficial veins that have become swollen. These veins
Medical Coverage Policy Varicose Vein Treatment sad EFFECTIVE DATE: 10 28 2001 POLICY LAST UPDATED: 05 07 2013 OVERVIEW Varicose veins are large superficial veins that have become swollen. These veins
Spotlight Series: Interventional Radiology. Varicose Veins and Venous Insufficiency
 Spotlight Series: Interventional Radiology Varicose Veins and Venous Insufficiency What is venous insufficiency? Spectrum of Disease Spider veins and telangiectasias Small reddish and purple veins near
Spotlight Series: Interventional Radiology Varicose Veins and Venous Insufficiency What is venous insufficiency? Spectrum of Disease Spider veins and telangiectasias Small reddish and purple veins near
MEDICAL COVERAGE POLICY SERVICE: Varicose Veins of the Lower Extremities. SERVICE: Treatment of Varicose Veins of the Lower Extremities
 Important note Even though this policy may indicate that a particular service or supply may be considered covered, this conclusion is not based upon the terms of your particular benefit plan. Each benefit
Important note Even though this policy may indicate that a particular service or supply may be considered covered, this conclusion is not based upon the terms of your particular benefit plan. Each benefit
Venous Reflux Disease and Current Treatments VN20-87-A 01/06
 Venous Reflux Disease and Current Treatments Leg Vein Anatomy Your legs are made up of a network of veins and vessels that carry blood back to the heart The venous system is comprised of: Deep veins Veins
Venous Reflux Disease and Current Treatments Leg Vein Anatomy Your legs are made up of a network of veins and vessels that carry blood back to the heart The venous system is comprised of: Deep veins Veins
TREATMENT OF VARICOSE VEINS: CAN IT BE IMPROVED BY MECHANOCHEMICAL ABLATION USING THE CLARIVEIN DEVICE?
 TREATMENT OF VARICOSE VEINS: CAN IT BE IMPROVED BY MECHANOCHEMICAL ABLATION USING THE CLARIVEIN DEVICE? Michel MJP Reijnen Rijnstate Hospital, Arnhem The Netherlands [email protected] Disclosures
TREATMENT OF VARICOSE VEINS: CAN IT BE IMPROVED BY MECHANOCHEMICAL ABLATION USING THE CLARIVEIN DEVICE? Michel MJP Reijnen Rijnstate Hospital, Arnhem The Netherlands [email protected] Disclosures
Non-surgical treatment of severe varicose veins
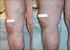 Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Modern Varicose Vein Treatments: What Every Patient Should Know
 The Skin and Vein Center Oneonta Laser Derm & Day Spa Natural Good Looks and Leg Veins Our Specialty Dr Eric Dohner, MD 41-45 Dietz St Oneonta, NY 13820 607/431-2525 www.oneontalaserderm.com Modern Varicose
The Skin and Vein Center Oneonta Laser Derm & Day Spa Natural Good Looks and Leg Veins Our Specialty Dr Eric Dohner, MD 41-45 Dietz St Oneonta, NY 13820 607/431-2525 www.oneontalaserderm.com Modern Varicose
Modern Management of Varicose Veins
 Modern Management of Varicose Veins GPCME 2008 David Ferrar Vascular and Endovascular Surgeon Vascular Ultrasound Specialist Multimodality treatment Compression stockings Sclerotherapy Ultrasound guided
Modern Management of Varicose Veins GPCME 2008 David Ferrar Vascular and Endovascular Surgeon Vascular Ultrasound Specialist Multimodality treatment Compression stockings Sclerotherapy Ultrasound guided
RANDOMIZED CONTROL TRIALS (RCT s) on VARICOSE VEIN ENDOVENOUS TREATMENT
 RANDOMIZED CONTROL TRIALS (RCT s) on VARICOSE VEIN ENDOVENOUS TREATMENT M. PERRIN Lyon - France CONTROVERSIES AND UPDATES IN VASCULAR SURGERY JANUARY 19-21, 2012 Paris B. Eklof Helsingborg, Sweden Thirty
RANDOMIZED CONTROL TRIALS (RCT s) on VARICOSE VEIN ENDOVENOUS TREATMENT M. PERRIN Lyon - France CONTROVERSIES AND UPDATES IN VASCULAR SURGERY JANUARY 19-21, 2012 Paris B. Eklof Helsingborg, Sweden Thirty
VARICOSE VEINS AND VENOUS INSUFFICIENCY
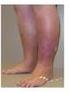 VARICOSE VEINS AND VENOUS INSUFFICIENCY SURGICAL AND ABLATION TREATMENTS Policy Number: 2014M0064A Effective Date: September 1, 2014 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION
VARICOSE VEINS AND VENOUS INSUFFICIENCY SURGICAL AND ABLATION TREATMENTS Policy Number: 2014M0064A Effective Date: September 1, 2014 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION
Peninsula Commissioning Priorities Group. Commissioning Policy Varicose Vein Referral
 NHS Devon NHS Plymouth Torbay Care Trust Peninsula Commissioning Priorities Group Commissioning Policy Varicose Vein Referral Varicose Vein Referral Guidelines 1. Description of service/treatment Most
NHS Devon NHS Plymouth Torbay Care Trust Peninsula Commissioning Priorities Group Commissioning Policy Varicose Vein Referral Varicose Vein Referral Guidelines 1. Description of service/treatment Most
Microfoam Ultrasound-Guided Sclerotherapy of Varicose Veins in 100 Legs
 Microfoam Ultrasound-Guided Sclerotherapy of Varicose Veins in 1 Legs JOHN M. BARRETT, FRNZCGP, n BRUCE ALLEN, FRACR, n ANNE OCKELFORD, FRNZCGP, n AND MITCHEL P. GOLDMAN, MD w n Palm Clinic, Auckland,
Microfoam Ultrasound-Guided Sclerotherapy of Varicose Veins in 1 Legs JOHN M. BARRETT, FRNZCGP, n BRUCE ALLEN, FRACR, n ANNE OCKELFORD, FRNZCGP, n AND MITCHEL P. GOLDMAN, MD w n Palm Clinic, Auckland,
Medical Affairs Policy
 Service: Varicose Vein Treatments PUM 250-0032 Medical Affairs Policy Implemented 04/04/14, 04/01/15, 4/1/16 Reviewed 12/12/14 Revised 12/12/14, 12/11/15 Developed Arise/WPS Policy 12/12/14, 12/11/15 Committee
Service: Varicose Vein Treatments PUM 250-0032 Medical Affairs Policy Implemented 04/04/14, 04/01/15, 4/1/16 Reviewed 12/12/14 Revised 12/12/14, 12/11/15 Developed Arise/WPS Policy 12/12/14, 12/11/15 Committee
Recurrent Varicose Veins. Vineet Mishra, MD Director of Mohs Surgery and Procedural Dermatology University of Texas Health Science Center San Antonio
 Recurrent Varicose Veins Vineet Mishra, MD Director of Mohs Surgery and Procedural Dermatology University of Texas Health Science Center San Antonio Disclosures None Possible Causes of Recurrence DNA:
Recurrent Varicose Veins Vineet Mishra, MD Director of Mohs Surgery and Procedural Dermatology University of Texas Health Science Center San Antonio Disclosures None Possible Causes of Recurrence DNA:
Patient Information Understanding Varicose Veins
 Patient Information Understanding Varicose Veins The Circulatory System Arteries carry oxygenated blood to your legs and the veins carry de-oxygenated blood away from your legs. The blood returns to the
Patient Information Understanding Varicose Veins The Circulatory System Arteries carry oxygenated blood to your legs and the veins carry de-oxygenated blood away from your legs. The blood returns to the
Varicose veins - 1 -
 Varicose veins - 1 - Varicose Veins About 3 in 10 adults develop varicose veins at some time in their life. Most people with varicose veins do not have an underlying disease and they usually occur for
Varicose veins - 1 - Varicose Veins About 3 in 10 adults develop varicose veins at some time in their life. Most people with varicose veins do not have an underlying disease and they usually occur for
Varicose Vein Therapy: An Introduction to Surgical and Endovascular Treatment
 Varicose Vein Therapy: An Introduction to Surgical and Endovascular Treatment Lower Extremity Venous Anatomy Deep, Superficial, Perforating, Reticular veins Located in two separate compartments (deep and
Varicose Vein Therapy: An Introduction to Surgical and Endovascular Treatment Lower Extremity Venous Anatomy Deep, Superficial, Perforating, Reticular veins Located in two separate compartments (deep and
TREATMENT OF VARICOSE AND SPIDER VEINS Patient Info
 TREATMENT OF VARICOSE AND SPIDER VEINS Patient Info www.heartofthevillages.com TIRED & ACHING LEGS? If you suffer from varicose and spider veins, you are not alone. It is estimated that there are more
TREATMENT OF VARICOSE AND SPIDER VEINS Patient Info www.heartofthevillages.com TIRED & ACHING LEGS? If you suffer from varicose and spider veins, you are not alone. It is estimated that there are more
treatment of varicose and spider veins patient information SAMPLE a publication by advancing vein care
 treatment of varicose and spider veins patient information a publication by advancing vein care Since most veins lie deep to the skin s surface, vein disorders are not always visible to the naked eye.
treatment of varicose and spider veins patient information a publication by advancing vein care Since most veins lie deep to the skin s surface, vein disorders are not always visible to the naked eye.
X-Plain Varicose Veins Reference Summary
 X-Plain Varicose Veins Reference Summary Introduction Varicose veins are very common, in both women and men. Varicose veins can be painful and unattractive. Vein doctors use non-invasive ultrasound imaging
X-Plain Varicose Veins Reference Summary Introduction Varicose veins are very common, in both women and men. Varicose veins can be painful and unattractive. Vein doctors use non-invasive ultrasound imaging
The Treatment of Varicose Veins and Spider Veins
 The Treatment of Varicose Veins and Spider Veins St. Joseph Vein Center Surgical Specialists of St. Joseph, P.C. Glen H. Hastings, M.D., F.A.C.S. 820 Lester Ave., Suite 105 St. Joseph, MI 49085 (269) 983-3368
The Treatment of Varicose Veins and Spider Veins St. Joseph Vein Center Surgical Specialists of St. Joseph, P.C. Glen H. Hastings, M.D., F.A.C.S. 820 Lester Ave., Suite 105 St. Joseph, MI 49085 (269) 983-3368
CHAPTER 15 SCLEROTHERAPY FOR VENOUS DISEASE
 Introduction CHAPTER 15 SCLEROTHERAPY FOR VENOUS DISEASE Original authors: Niren Angle, John J. Bergan, Joshua I. Greenberg, and J. Leonel Villavicencio Abstracted by Teresa L. Carman New technology has
Introduction CHAPTER 15 SCLEROTHERAPY FOR VENOUS DISEASE Original authors: Niren Angle, John J. Bergan, Joshua I. Greenberg, and J. Leonel Villavicencio Abstracted by Teresa L. Carman New technology has
VARICOSE VEINS. Information Leaflet. Your Health. Our Priority. VTE Ambulatory Clinic Stepping Hill Hospital
 VARICOSE VEINS Information Leaflet Your Health. Our Priority. Page 2 of 7 Varicose Veins There are no accurate figures for the number of people with varicose veins. Some studies suggest that 3 in 100 people
VARICOSE VEINS Information Leaflet Your Health. Our Priority. Page 2 of 7 Varicose Veins There are no accurate figures for the number of people with varicose veins. Some studies suggest that 3 in 100 people
Local Coverage Determination (LCD): Varicose Veins of the Lower Extremities (L31796)
 Local Coverage Determination (LCD): Varicose Veins of the Lower Extremities (L31796) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L31796 LCD Title Varicose
Local Coverage Determination (LCD): Varicose Veins of the Lower Extremities (L31796) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L31796 LCD Title Varicose
LOWER EXTREMITY VENOUS DUPLEX ULTRASOUND:
 LOWER EXTREMITY VENOUS DUPLEX ULTRASOUND: Chronic Venous Insufficiency Phillip J Bendick, PhD William Beaumont Hospital Royal Oak, Michigan Prevalence: Carotid ASO ~ 3M Peripheral Arterial Dz ~ 5M + CAD
LOWER EXTREMITY VENOUS DUPLEX ULTRASOUND: Chronic Venous Insufficiency Phillip J Bendick, PhD William Beaumont Hospital Royal Oak, Michigan Prevalence: Carotid ASO ~ 3M Peripheral Arterial Dz ~ 5M + CAD
DEBATE ON Management of primary Varicose Veins SURGERY. Adel Husseiny, Kamhawy Vascular Surgery Unit Tanta University
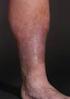 DEBATE ON Management of primary Varicose Veins SURGERY Adel Husseiny, Kamhawy Vascular Surgery Unit Tanta University Efficacy of treatment alternatives Durability of results Cosmetically acceptable results
DEBATE ON Management of primary Varicose Veins SURGERY Adel Husseiny, Kamhawy Vascular Surgery Unit Tanta University Efficacy of treatment alternatives Durability of results Cosmetically acceptable results
Health Technology Assessment of Scheduled Surgical Procedures
 HTA of Scheduled Surgical Procedures April 2013 Health Technology Assessment of Scheduled Surgical Procedures Varicose Vein Surgery April 2013 Safer Better Care About the The (HIQA) is the independent
HTA of Scheduled Surgical Procedures April 2013 Health Technology Assessment of Scheduled Surgical Procedures Varicose Vein Surgery April 2013 Safer Better Care About the The (HIQA) is the independent
Jose Almeida MD, FACS, RVT Jeff Raines PhD, RVT. Oliver Göckeritz MD Christian Wentzel MD. Tim Richards MD Daniel F. Hoheim, MD.
 Final Results 1 Jose Almeida MD, FACS, RVT Jeff Raines PhD, RVT Miami Vein Center Miami, Florida Oliver Göckeritz MD Christian Wentzel MD Venenzentrum Elserpark Leipzig, Germany John Kaufman MD Dotter
Final Results 1 Jose Almeida MD, FACS, RVT Jeff Raines PhD, RVT Miami Vein Center Miami, Florida Oliver Göckeritz MD Christian Wentzel MD Venenzentrum Elserpark Leipzig, Germany John Kaufman MD Dotter
Beaumont Hospital. Varicose Veins. and their TREATMENT. Professor Austin Leahy, MCh, FRCS, FRCSI WWW.VEINCLINICSOFIRELAND.COM
 Beaumont Hospital Varicose Veins and their TREATMENT Professor Austin Leahy, MCh, FRCS, FRCSI WWW.VEINCLINICSOFIRELAND.COM Department of Surgery Beaumont Hospital and Royal College of Surgeons in Ireland
Beaumont Hospital Varicose Veins and their TREATMENT Professor Austin Leahy, MCh, FRCS, FRCSI WWW.VEINCLINICSOFIRELAND.COM Department of Surgery Beaumont Hospital and Royal College of Surgeons in Ireland
Varicose Vein Treatment (Endovenous Ablation of Varicose Veins)
 Scan for mobile link. Varicose Vein Treatment (Endovenous Ablation of Varicose Veins) Varicose vein treatment, also known as endovenous ablation, uses radiofrequency or laser energy to cauterize and close
Scan for mobile link. Varicose Vein Treatment (Endovenous Ablation of Varicose Veins) Varicose vein treatment, also known as endovenous ablation, uses radiofrequency or laser energy to cauterize and close
National Medical Policy
 National Medical Policy Subject: Policy Number: Varicose Veins Surgical Interventions NMP148 Effective Date*: September 2003 Updated: July 2016 Please contact the Medical Policy Department for an archive
National Medical Policy Subject: Policy Number: Varicose Veins Surgical Interventions NMP148 Effective Date*: September 2003 Updated: July 2016 Please contact the Medical Policy Department for an archive
SEDICO Newsletter Issue 8. Varicose veins
 SEDICO Newsletter Issue 8 Definition: Varicose veins Varicose veins are veins that have become enlarged and twisted. The term commonly refers to the veins on the leg, although varicose veins occur elsewhere.
SEDICO Newsletter Issue 8 Definition: Varicose veins Varicose veins are veins that have become enlarged and twisted. The term commonly refers to the veins on the leg, although varicose veins occur elsewhere.
LONG TERM FOLLOW-UP AFTER ENDOVENOUS LASER ABLATION
 LONG TERM FOLLOW-UP AFTER ENDOVENOUS LASER ABLATION Giorgio Spreafico Centro Multidisciplinare Day Surgery Azienda Ospedaliera Università Padova - Italy Faculty Disclosure I have no financial relationships
LONG TERM FOLLOW-UP AFTER ENDOVENOUS LASER ABLATION Giorgio Spreafico Centro Multidisciplinare Day Surgery Azienda Ospedaliera Università Padova - Italy Faculty Disclosure I have no financial relationships
Provided by the American Venous Forum: veinforum.org
 CHAPTER 17 SURGICAL THERAPY FOR DEEP VALVE INCOMPETENCE Original author: Seshadri Raju Abstracted by Gary W. Lemmon Introduction Deep vein valvular incompetence happens when the valves in the veins (tubes
CHAPTER 17 SURGICAL THERAPY FOR DEEP VALVE INCOMPETENCE Original author: Seshadri Raju Abstracted by Gary W. Lemmon Introduction Deep vein valvular incompetence happens when the valves in the veins (tubes
Suffering from varicose veins? Patient Information. ELVeS Radial Minimally invasive laser therapy of venous insufficiency
 Suffering from varicose veins? Patient Information ELVeS Radial Minimally invasive laser therapy of venous insufficiency Do you suffer from heavy legs or visible veins? This makes diseases of the veins
Suffering from varicose veins? Patient Information ELVeS Radial Minimally invasive laser therapy of venous insufficiency Do you suffer from heavy legs or visible veins? This makes diseases of the veins
Varicose Veins: Causes, Symptoms and Management. Andrew C. Stanley MD Section of Vascular Surgery
 Varicose Veins: Causes, Symptoms and Management Andrew C. Stanley MD Section of Vascular Surgery Circulation Heart (Pump)-MI, Sudden Death Arteries (Stroke, Aneurysms, Walking dysfunction, Limb loss) Veins
Varicose Veins: Causes, Symptoms and Management Andrew C. Stanley MD Section of Vascular Surgery Circulation Heart (Pump)-MI, Sudden Death Arteries (Stroke, Aneurysms, Walking dysfunction, Limb loss) Veins
Varicose Veins Operation. Patient information Leaflet
 Varicose Veins Operation Patient information Leaflet 22 nd August 2014 WHAT IS VARICOSE VEIN SURGERY (HIGH LIGATION AND MULTIPLE AVULSIONS) The operation varies from case to case, depending on where the
Varicose Veins Operation Patient information Leaflet 22 nd August 2014 WHAT IS VARICOSE VEIN SURGERY (HIGH LIGATION AND MULTIPLE AVULSIONS) The operation varies from case to case, depending on where the
Treatment by LASER. Understanding Varicose veins and. Dr. Rajesh Mundhada (MD. DNB) Dr. Atul Rewatkar (MD)
 Understanding Varicose veins and Treatment by LASER Dr. Rajesh Mundhada (MD. DNB) Dr. Atul Rewatkar (MD) Orange city Hospital & Research Institute 19, Pandey Lay out, Veer Sawarkar Square, Nagpur 440015
Understanding Varicose veins and Treatment by LASER Dr. Rajesh Mundhada (MD. DNB) Dr. Atul Rewatkar (MD) Orange city Hospital & Research Institute 19, Pandey Lay out, Veer Sawarkar Square, Nagpur 440015
Recurrent Varicose Veins
 Information for patients Recurrent Varicose Veins Sheffield Vascular Institute Northern General Hospital You have been diagnosed as having Varicose Veins that have recurred (come back). This leaflet explains
Information for patients Recurrent Varicose Veins Sheffield Vascular Institute Northern General Hospital You have been diagnosed as having Varicose Veins that have recurred (come back). This leaflet explains
CHINOOK VASCULAR - ENDOVENOUS LASER ABLATION PROCEDURE
 CHINOOK VASCULAR - ENDOVENOUS LASER ABLATION PROCEDURE Endovenous Laser Ablation is a minimally invasive option for treating great saphenous vein incompetence (leaky valves). The first stage of your surgery
CHINOOK VASCULAR - ENDOVENOUS LASER ABLATION PROCEDURE Endovenous Laser Ablation is a minimally invasive option for treating great saphenous vein incompetence (leaky valves). The first stage of your surgery
New approaches for the treatment of varicose veins
 Surg Clin N Am 84 (2004) 1397 1417 New approaches for the treatment of varicose veins Theodore H. Teruya, MD, FACS a, Jeffrey L. Ballard, MD, FACS b,c, * a Division of Vascular Surgery, Loma Linda University,
Surg Clin N Am 84 (2004) 1397 1417 New approaches for the treatment of varicose veins Theodore H. Teruya, MD, FACS a, Jeffrey L. Ballard, MD, FACS b,c, * a Division of Vascular Surgery, Loma Linda University,
Varicose veins and spider veins
 Varicose veins and spider veins Summary Varicose veins are knobbly, twisted and darkish-blue in appearance, and are most commonly found on people s legs. Varicose veins are caused by faulty valves within
Varicose veins and spider veins Summary Varicose veins are knobbly, twisted and darkish-blue in appearance, and are most commonly found on people s legs. Varicose veins are caused by faulty valves within
LASER TREATMENT FOR VARICOSE VEINS
 LASER TREATMENT FOR VARICOSE VEINS How can varicose veins be treated by laser? Laser treatment of varicose veins, Endovascular Laser Ablation (known as EVLA), is a minimally invasive procedure for treating
LASER TREATMENT FOR VARICOSE VEINS How can varicose veins be treated by laser? Laser treatment of varicose veins, Endovascular Laser Ablation (known as EVLA), is a minimally invasive procedure for treating
Endovenous Laser Therapy
 Information for patients Endovenous Laser Therapy Northern General Hospital You have been given this leaflet because you will be having Endovenous Laser Therapy (EVLT). This leaflet explains more about
Information for patients Endovenous Laser Therapy Northern General Hospital You have been given this leaflet because you will be having Endovenous Laser Therapy (EVLT). This leaflet explains more about
THE VEIN CENTER. State-of-the-Art Treatment for Varicose Veins and Spider Veins
 THE VEIN CENTER State-of-the-Art Treatment for Varicose Veins and Spider Veins Vein Disorders Nearly 50 percent of the adult population suffers from undesirable, sometimes painful vein disease. The most
THE VEIN CENTER State-of-the-Art Treatment for Varicose Veins and Spider Veins Vein Disorders Nearly 50 percent of the adult population suffers from undesirable, sometimes painful vein disease. The most
V03 Varicose Veins Surgery
 V03 Varicose Veins Surgery What are varicose veins? Varicose veins are enlarged and twisted veins in the leg. They are common and affect up to 3 in 10 people. More women than men ask for treatment, with
V03 Varicose Veins Surgery What are varicose veins? Varicose veins are enlarged and twisted veins in the leg. They are common and affect up to 3 in 10 people. More women than men ask for treatment, with
AMERICAN VENOUS FORUM
 Revised Venous Clinical Severity Score AMERICAN VENOUS FORUM Pain : 0 Mild: 1 or other discomfort (ie, aching, heaviness, fatigue, soreness, burning) origin Occasional pain or other discomfort (ie, not
Revised Venous Clinical Severity Score AMERICAN VENOUS FORUM Pain : 0 Mild: 1 or other discomfort (ie, aching, heaviness, fatigue, soreness, burning) origin Occasional pain or other discomfort (ie, not
Diagnosis and Treatment of Venous Insufficiency and Varicose Veins
 Diagnosis and Treatment of Venous Insufficiency and Varicose Veins Joseph Conley, MD Scott and White Memorial Hospital Temple, TX September 22, 2012 Objectives Anatomy and Pathophysiology of venous insufficiency
Diagnosis and Treatment of Venous Insufficiency and Varicose Veins Joseph Conley, MD Scott and White Memorial Hospital Temple, TX September 22, 2012 Objectives Anatomy and Pathophysiology of venous insufficiency
SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS
 SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Protocol: SUR037 Effective Date: 5/1/2015 Table of Contents COMMERCIAL INDICATIONS FOR COVERAGE DEFINITIONS MEDICARE AND MEDICAID
SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Protocol: SUR037 Effective Date: 5/1/2015 Table of Contents COMMERCIAL INDICATIONS FOR COVERAGE DEFINITIONS MEDICARE AND MEDICAID
Dr. Linde is an international beauty expert and an acclaimed keynote speaker at conferences all over the world. He is specialized in the treatment of
 VEINS Dr. Linde is an international beauty expert and an acclaimed keynote speaker at conferences all over the world. He is specialized in the treatment of fat pads, wrinkles and transformations of the
VEINS Dr. Linde is an international beauty expert and an acclaimed keynote speaker at conferences all over the world. He is specialized in the treatment of fat pads, wrinkles and transformations of the
EndoVenous Laser Therapy (EVLT) Information Booklet. Dr. Dueck. Varicose Veins
 EndoVenous Laser Therapy (EVLT) Information Booklet Dr. Dueck Varicose Veins Varicose veins are abnormally large veins that protrude from under the skin in the legs. They can be associated with a brown
EndoVenous Laser Therapy (EVLT) Information Booklet Dr. Dueck Varicose Veins Varicose veins are abnormally large veins that protrude from under the skin in the legs. They can be associated with a brown
Information for patients who require Foam Sclerotherapy for Varicose Veins.
 Information for patients who require Foam Sclerotherapy for Varicose Veins. Why do I need this procedure? Everybody has two sets of veins in the legs. These include the superficial and deep veins. Their
Information for patients who require Foam Sclerotherapy for Varicose Veins. Why do I need this procedure? Everybody has two sets of veins in the legs. These include the superficial and deep veins. Their
Treatment of Varicose Veins
 Varicose Vein Treatment of Varicose Veins JMAJ 47(3): 146 151, 2004 Osamu SATO Associate Professor, Department of Surgery, Saitama Medical Center Abstract: Varicose vein is a condition that was first described
Varicose Vein Treatment of Varicose Veins JMAJ 47(3): 146 151, 2004 Osamu SATO Associate Professor, Department of Surgery, Saitama Medical Center Abstract: Varicose vein is a condition that was first described
SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS
 CLINICAL POLICY SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Policy Number: OUTPATIENT 013.24 T2 Effective Date: July 1, 2014 Table of Contents CONDITIONS OF COVERAGE...
CLINICAL POLICY SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Policy Number: OUTPATIENT 013.24 T2 Effective Date: July 1, 2014 Table of Contents CONDITIONS OF COVERAGE...
Venous and Lymphatic Disorders
 Venous and Lymphatic Disorders. ก. Venous and Lymphatic Disorders Varicose Veins Deep Vein Thrombosis (DVT) Lymphedema What Is Varicose Veins? Latin: Varicose = Varix = twisted Abnormal venous dilatation
Venous and Lymphatic Disorders. ก. Venous and Lymphatic Disorders Varicose Veins Deep Vein Thrombosis (DVT) Lymphedema What Is Varicose Veins? Latin: Varicose = Varix = twisted Abnormal venous dilatation
What are spider veins? What is the best treatment for spider veins?
 Laser Spider/Leg Vein Q & A What are spider veins? Millions of women and men are bothered by unsightly spider veins on their faces and legs. Spider veins or telangiectasias are those small red, blue and
Laser Spider/Leg Vein Q & A What are spider veins? Millions of women and men are bothered by unsightly spider veins on their faces and legs. Spider veins or telangiectasias are those small red, blue and
Provided by the American Venous Forum: veinforum.org
 CHAPTER 1 NORMAL VENOUS CIRCULATION Original author: Frank Padberg Abstracted by Teresa L.Carman Introduction The circulatory system is responsible for circulating (moving) blood throughout the body. The
CHAPTER 1 NORMAL VENOUS CIRCULATION Original author: Frank Padberg Abstracted by Teresa L.Carman Introduction The circulatory system is responsible for circulating (moving) blood throughout the body. The
SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS
 COVERAGE POLICY SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Policy Number: 237.E Effective Date: March 1, 2015 Table of Contents BENEFIT CONSIDERATIONS. COVERAGE RATIONALE...
COVERAGE POLICY SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Policy Number: 237.E Effective Date: March 1, 2015 Table of Contents BENEFIT CONSIDERATIONS. COVERAGE RATIONALE...
Varicose Veins and Spider Veins
 page 1 Varicose Veins and Spider Veins Q: What are varicose veins and spider A: Varicose (VAR-i-kos) veins are enlarged veins that can be blue, red, or fleshcolored. They often look like cords and appear
page 1 Varicose Veins and Spider Veins Q: What are varicose veins and spider A: Varicose (VAR-i-kos) veins are enlarged veins that can be blue, red, or fleshcolored. They often look like cords and appear
Evidence review. Endovascular treatment of varicose veins CEP09017
 Evidence review Endovascular treatment of varicose veins CEP09017 August 2009 Contents 2 Summary... 3 Introduction... 4 Methods... 11 Evidence review... 19 Conclusions... 27 Acknowledgements... 28 Glossary...
Evidence review Endovascular treatment of varicose veins CEP09017 August 2009 Contents 2 Summary... 3 Introduction... 4 Methods... 11 Evidence review... 19 Conclusions... 27 Acknowledgements... 28 Glossary...
Australian Safety and Efficacy Register of New Interventional Procedures Surgical. Rapid review ASERNIP-S REPORT NO. 66
 ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures Surgical Rapid review Treatments for varicose veins ASERNIP-S REPORT NO. 66 Australian Safety & Efficacy Register of New
ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures Surgical Rapid review Treatments for varicose veins ASERNIP-S REPORT NO. 66 Australian Safety & Efficacy Register of New
Emory Healthcare s model for a PA based vein clinic
 Emory Healthcare s model for a PA based vein clinic Stephen Konigsberg PA-C No relationships to disclose Society for Vascular Surgery 2011 Vascular Annual Meeting Chicago, IL June 17, 2011 Division of
Emory Healthcare s model for a PA based vein clinic Stephen Konigsberg PA-C No relationships to disclose Society for Vascular Surgery 2011 Vascular Annual Meeting Chicago, IL June 17, 2011 Division of
Successful Endovenous Ablation
 Successful Endovenous Ablation Is it the technology or the technique? PANEL Moderator: Steve Elias, MD, is Director, Center for Vein Disease, Englewood Hospital and Medical Center, New Jersey; and Assistant
Successful Endovenous Ablation Is it the technology or the technique? PANEL Moderator: Steve Elias, MD, is Director, Center for Vein Disease, Englewood Hospital and Medical Center, New Jersey; and Assistant
SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS
 MEDICAL POLICY SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Policy Number: 2015T0447O Effective Date: March 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE
MEDICAL POLICY SURGICAL AND ABLATIVE PROCEDURES FOR VENOUS INSUFFICIENCY AND VARICOSE VEINS Policy Number: 2015T0447O Effective Date: March 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE
Endovenous Treatment of Varicose Veins
 Supplement to Produced under an unrestricted educational grant from Diomed, Inc. November/December 2004 Endovenous A comprehensive guide to the techniques and technology necessary to establish and maintain
Supplement to Produced under an unrestricted educational grant from Diomed, Inc. November/December 2004 Endovenous A comprehensive guide to the techniques and technology necessary to establish and maintain
Confirmed Deep Vein Thrombosis (DVT)
 Confirmed Deep Vein Thrombosis (DVT) Information for patients What is deep vein thrombosis? Blood clotting provides us with essential protection against severe loss of blood from an injury to a vein or
Confirmed Deep Vein Thrombosis (DVT) Information for patients What is deep vein thrombosis? Blood clotting provides us with essential protection against severe loss of blood from an injury to a vein or
Prevalence and surgical outcomes of varicose veins at Regional Institute of Medical Sciences, Imphal
 ORIGINAL ARTICLE JIACM 2013; 14(3-4): 209-13 Prevalence and surgical outcomes of varicose veins at Regional Institute of Medical Sciences, Imphal Ksh Kala Singh*, A Surjyalal Sharma**, L Sunil Singh***,
ORIGINAL ARTICLE JIACM 2013; 14(3-4): 209-13 Prevalence and surgical outcomes of varicose veins at Regional Institute of Medical Sciences, Imphal Ksh Kala Singh*, A Surjyalal Sharma**, L Sunil Singh***,
CMS Limitations Guide - Radiology Services
 CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Tired, Aching Legs? Swollen Ankles? Varicose Veins? An informative guide for patients
 Tired, Aching Legs? Swollen Ankles? Varicose Veins? An informative guide for patients Are You at Risk? Leg problems are widespread throughout the world, but what most people don t know is that approximately
Tired, Aching Legs? Swollen Ankles? Varicose Veins? An informative guide for patients Are You at Risk? Leg problems are widespread throughout the world, but what most people don t know is that approximately
SUMMARY OF GUIDELINES FOR MANAGEMENT OF PATIENTS WITH VARICOSE VEINS AND ASSOCIATED CHRONIC VENOUS DISEASES
 The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum Peter Gloviczki, MD, a Anthony
The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum Peter Gloviczki, MD, a Anthony
A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO)
 A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO) Types of Catheter Related Thrombotic A catheter-related thrombus may be intraluminal (inside the catheter)
A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO) Types of Catheter Related Thrombotic A catheter-related thrombus may be intraluminal (inside the catheter)
The anthropometric characteristics in the two groups were not significantly different.
 Studio prospettico, randomizzato monocentrico per il trattamento sintomatico dell insufficienza venosa cronica degli arti inferiori in pazienti trattati con calze preventive con filato SpikEnergy rispetto
Studio prospettico, randomizzato monocentrico per il trattamento sintomatico dell insufficienza venosa cronica degli arti inferiori in pazienti trattati con calze preventive con filato SpikEnergy rispetto
The veins of the lower limbs can be subdivided into three subtypes: deep veins, superficial veins, and connecting veins (figure 1).
 VEIN DISEASE Vein disease may seem insignificant and harmless at first, but if left untreated, it can develop into a major problem. It occurs when veins have trouble transporting blood back to the heart
VEIN DISEASE Vein disease may seem insignificant and harmless at first, but if left untreated, it can develop into a major problem. It occurs when veins have trouble transporting blood back to the heart
