The Janssen Biotech Support System. Getting patients started on STELARA
|
|
|
- Charleen Goodwin
- 9 years ago
- Views:
Transcription
1 The Janssen Biotech Support System Getting patients started on STELARA
2 3 STEPS after identifying patients appropriate for STELARA (ustekinumab) STEP 1: Determine the correct dose STELARA is administered by subcutaneous injection. 1 Psoriatic Arthritis The recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks For patients with co-existent moderate-to-severe plaque psoriasis weighing >100 kg (220 lbs), the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks Plaque Psoriasis For patients weighing 100 kg (220 lbs), the recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks For patients weighing >100 kg (220 lbs), the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks. In subjects weighing >100 kg (220 lbs), 45 mg was also shown to be efficacious. However, 90 mg resulted in greater efficacy in these subjects STEP 2: Complete the Prescription Form Complete the Prescription Form for STELARA including previous therapy, patient weight, insurance information, and dosage information Request Pharmacy and Medical insurance cards Submit copies of Pharmacy and Medical insurance cards with the Prescription Form to specialty pharmacy or StelaraSupport for benefit verification Request investigation of benefits most appropriate for patient Provide eligible commercially insured patients with the StelaraSupport Instant Savings Program card for activation STEP 3: Receive STELARA Once benefits are verified, the specialty pharmacy will coordinate shipment with you and your patient: To patient s home or other convenient location, for patient self-injection To your office or to a location convenient for your patient, for injection by a Healthcare Provider (HCP) Coordinate shipment with patient appointment/ injection date or patient s next scheduled self-injection treatment, as appropriate Please note: Instruct patients in proper storage and handling of STELARA Keep STELARA refrigerated at 2 C to 8 C (36 F to 46 F). Keep the product in the original carton to protect from light until the time of use. Do not freeze. Do not shake. STELARA does not contain a preservative; discard any unused portion 1 STELARA, available as 45 mg and 90 mg, is a subcutaneous injection intended for use under the guidance and supervision of a physician with patients who will be closely monitored and have regular follow-up. Patients may self-inject with STELARA after physician approval and proper training. Patients should be instructed to follow the directions provided in the Medication Guide Please see coding and billing information on page STELARA Prescribing Information. Janssen Biotech, Inc. Indications or older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. or older) with active psoriatic arthritis alone or in combination with methotrexate (MTX). The Janssen Biotech Support System Selected Important Safety Information STELARA is an immunosuppressant and may increase the risk of infections, reactivation of latent infections, and malignancies. Serious adverse reactions have been reported in STELARA -treated patients, including bacterial, fungal, and viral infections, malignancies, hypersensitivity reactions and one case of Reversible Posterior Leukoencephalopathy Syndrome (RPLS). STELARA should not be given to patients who have had clinically significant hypersensitivity to ustekinumab (or excipients) or patients with any clinically important active infection. Patients should be evaluated for tuberculosis prior to initiating treatment with STELARA. Live vaccines should not be given to patients receiving STELARA. If RPLS is suspected, discontinue STELARA. Please see related and other Important Safety Information on pages 7 and
3 Patient Affordability Benefits Verification Alternative sources of financial support for STELARA (ustekinumab) vary depending on the patient s insurance coverage. Patients with commercial insurance Provide eligible patients with StelaraSupport Instant Savings Program information and encourage immediate card activation. See StelaraSupport Instant Savings Program materials for additional information and program restrictions, or visit Patients with Medicare, Medicaid, TRICARE, or commercial insurance Foundation Support may be available: The Assistance Fund theassistancefund.org The HealthWell Foundation healthwellfoundation.org Patient Access Network Foundation panfoundation.org Patient Advocate Foundation patientadvocate.org Other resources Johnson & Johnson Patient Assistance Foundation, Inc. (JJPAF) is committed to providing access to uninsured patients that lack the financial resources to pay for their medicines. If your patient needs STELARA, and is uninsured and unable to pay for their medicine, please have them contact a JJPAF program specialist at or visit the foundation website at to see if they might qualify for assistance. For a complete list of alternative funding options, please direct your patients to JanssenPrescriptionAssistance.com/STELARA. Knowing the differences between Pharmacy and Medical coverage can be beneficial. Helps facilitate smoother, more efficient administrative processing Helps ensure that patients have appropriate access to the medications they need Remember, check both Pharmacy and Medical insurance coverage cards. Pharmacy benefit coverage for STELARA Coverage for prescription biologic medications may vary from the medical benefit: Coverage requirements may vary Patient out-of-pocket requirement may vary Distribution may be through the specialty pharmacy, retail pharmacy, or mail order Medical benefit coverage for STELARA Coverage for medical procedures, hospital care, and Healthcare Provider administered medications may vary from the pharmacy benefit: Coverage requirements may vary Patient out-of-pocket requirement may vary Distribution may be through the specialty pharmacy or Buy and Bill Preparing & submitting claims for STELARA * Code Type Code Number Description ICD ICD-10 L40 L40.50 L40.51 L40.52 L40.53 L40.54 L40.59 L40.0 L40.1 L40.2 L40.3 L40.4 L40.8 L40.9 Psoriatic arthritis Plaque psoriasis Psoriasis Arthropathic psoriasis, unspecified Distal interphalangeal psoriatic arthropathy Psoriatic arthritis mutilans Psoriatic spondylitis Psoriatic juvenile arthropathy Other psoriatic arthropathy Psoriasis vulgaris Generalized pustular psoriasis Acrodermatitis continua Pustulosis palmaris et plantaris Guttate psoriasis Other psoriasis Psoriasis, unspecified HCPCS J3357 Injection, ustekinumab, 1 mg CPT codes and descriptions are copyright 2015 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association (AMA). Coding unit for STELARA Number of units provided 1 unit one 45 mg/0.5 ml single-use prefilled syringe 1 unit one 90 mg/1 ml single-use prefilled syringe *This document is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations, and policies concerning reimbursement are complex and are updated frequently. While we have made an effort to be current as of the issue date of this document, the information may not be as current or comprehensive when you view it. Similarly, all codes are supplied for informational purposes only and this information does not represent any statement, promise, or guarantee by Janssen Biotech, Inc., about coverage, levels of reimbursement, payment, or charge. Please consult with your payer organization(s) for local or actual coverage, reimbursement policies, and determination processes. Please consult with your counsel or reimbursement specialist for any reimbursement or billing questions specific to your institution. CPT Therapeutic, prophylactic, or diagnostic injection, subcutaneous/intramuscular NDC mg/0.5 ml in a single-use, prefilled syringe 90 mg/1 ml in a single-use, prefilled syringe NOTE: To convert a 10-digit NDC to an 11-digit NDC, a leading 0 should be added to the middle set of numbers. 4 5
4 Full Prescribing Information and Medication Guide We help make access easy Support for you Single point of contact for answers to your questions about coverage Assistance with benefit investigation, and the prior authorization and appeal process Easy-to-use, time-saving enhanced provider portal Support for your patients One-to-one counseling on insurance benefits and cost-sharing requirements StelaraSupport Instant Savings Program for eligible patients Nurse support available 7 days a week Safe Returns for proper disposal of prefilled syringes at no cost Medication reminders Support TM The Janssen Biotech Support System STELARA ( ) Monday Friday, 8:00 am 8:00 pm ET One-to-one support for access to treatment Patient insurance benefit investigation is provided as a service by The Lash Group, Inc., under contract for Janssen Biotech, Inc. In this regard, The Lash Group, Inc., assists healthcare professionals in the determination of whether treatment could be covered by the applicable third-party payer based on coverage guidelines provided by the payer and patient information provided by the healthcare provider under appropriate authorization following the provider s exclusive determination of medical necessity. Importantly, insurance verification is the ultimate responsibility of the provider. Third-party reimbursement is affected by many factors. Therefore, The Lash Group, Inc., and Janssen Biotech make no representation or guarantee that full or partial insurance reimbursement or any other payment will be available. This information is provided as an information service only. While The Lash Group, Inc., tries to provide correct information, it and Janssen Biotech make no representations or warranties, expressed or implied, as to the accuracy of the information. In no event shall The Lash Group, Inc., or Janssen Biotech or its employees or agents be liable for any damages resulting from or relating to the services. All providers and other users of this information agree that they accept responsibility for the use of this service. Janssen Biotech assumes no responsibility for, and does not guarantee the quality, scope, or availability of the services including but not limited to reimbursement support services, patient education, and other support services. Each provider, not Janssen Biotech, is responsible for the services they provide. These support services have no independent value to providers apart from the product and are included within the cost of the product. Janssen Biotech, Inc. 6 Janssen Biotech, Inc /
5 Indications or older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. or older) with active psoriatic arthritis alone or in combination with methotrexate (MTX). Important Safety Information Infections STELARA may increase the risk of infections and reactivation of latent infections. Serious bacterial, fungal, and viral infections, some requiring hospitalization, were reported. Serious infections included diverticulitis, cellulitis, pneumonia, appendicitis, cholecystitis, sepsis, osteomyelitis, viral infections, gastroenteritis and urinary tract infections. STELARA should not be given to patients with a clinically important active infection and should not be administered until the infection resolves or is adequately treated. Instruct patients to seek medical advice if signs or symptoms suggestive of an infection occur. Exercise caution when considering use of STELARA in patients with a chronic infection or a history of recurrent infection. Theoretical Risk for Vulnerability to Particular Infections Individuals genetically deficient in IL-12/IL-23 are particularly vulnerable to disseminated infections from mycobacteria, Salmonella, and Bacillus Calmette-Guerin (BCG) vaccinations. Serious infections and fatal outcomes have been reported in such patients. It is not known whether patients with pharmacologic blockade of IL-12/IL-23 from treatment with STELARA will be susceptible to these types of infections. Consider appropriate diagnostic testing as dictated by clinical circumstances. Pre-Treatment Evaluation of Tuberculosis (TB) Evaluate patients for TB prior to initiating treatment with STELARA. STELARA should not be given to patients with active TB. Initiate treatment of latent TB before administering STELARA. Patients should be monitored closely for signs and symptoms of active TB during and after treatment with STELARA. Malignancies STELARA is an immunosuppressant and may increase the risk of malignancy. Malignancies were reported among patients who received STELARA in clinical studies. The safety of STELARA has not been evaluated in patients who have a history of malignancy or who have a known malignancy. There have been reports of the rapid appearance of multiple cutaneous squamous cell carcinomas in patients receiving STELARA who had risk factors for developing non-melanoma skin cancer (NMSC). All patients receiving STELARA, especially those >60 years or those with a history of PUVA or prolonged immunosuppressant treatment, should be monitored for the appearance of NMSC. Hypersensitivity Reactions STELARA is contraindicated in patients with clinically significant hypersensitivity to ustekinumab or excipients. Hypersensitivity reactions, including anaphylaxis and angioedema, have been reported. If an anaphylactic or other clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue STELARA. Reversible Posterior Leukoencephalopathy Syndrome (RPLS) One case of RPLS has been reported in a STELARA -treated patient. If RPLS is suspected, administer appropriate treatment and discontinue STELARA. RPLS is a neurological disorder, which is not caused by an infection or demyelination. RPLS can present with headache, seizures, confusion, and visual disturbances. RPLS has been associated with fatal outcomes. Immunizations Prior to initiating therapy with STELARA, patients should receive all immunizations recommended by current guidelines. Patients being treated with STELARA should not receive live vaccines. BCG vaccines should not be given during treatment or within one year of initiating or discontinuing STELARA. Exercise caution when administering live vaccines to household contacts of STELARA patients, as shedding and subsequent transmission to STELARA patients may occur. Non-live vaccinations received during a course of STELARA may not elicit an immune response sufficient to prevent disease. Concomitant Therapies The safety of STELARA in combination with other immunosuppressive agents or phototherapy has not been evaluated. Ultraviolet-induced skin cancers developed earlier and more frequently in mice. In psoriasis studies, the relevance of findings in mouse models for malignancy risk in humans is unknown. In psoriatic arthritis studies, concomitant MTX use did not appear to influence the safety or efficacy of STELARA. Allergen Immunotherapy STELARA may decrease the protective effect of allergen immunotherapy (decrease tolerance) which may increase the risk of an allergic reaction to a dose of allergen immunotherapy. Therefore, caution should be exercised in patients receiving or who have received allergen immunotherapy, particularly for anaphylaxis. Most Common Adverse Reactions The most common adverse reactions ( 3% and higher than that with placebo) in psoriasis clinical trials for STELARA 45 mg, STELARA 90 mg, or placebo were: nasopharyngitis (8%, 7%, 8%), upper respiratory tract infection (5%, 4%, 5%), headache (5%, 5%, 3%), and fatigue (3%, 3%, 2%), respectively. In psoriatic arthritis (PsA) studies, a higher incidence of arthralgia and nausea was observed in patients treated with STELARA when compared with placebo (3% vs 1% for both). Please see accompanying full Prescribing Information and Medication Guide for STELARA, located in the pocket. Provide the Medication Guide to your patients and encourage discussion
MEDICATION GUIDE STELARA
 MEDICATION GUIDE STELARA (stel ar a) (ustekinumab) Injection What is the most important information I should know about STELARA? STELARA is a medicine that affects your immune system. STELARA can increase
MEDICATION GUIDE STELARA (stel ar a) (ustekinumab) Injection What is the most important information I should know about STELARA? STELARA is a medicine that affects your immune system. STELARA can increase
STELARA (ustekinumab)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STELARA safely and effectively. See full prescribing information for STELARA. injection, for subcutaneous
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STELARA safely and effectively. See full prescribing information for STELARA. injection, for subcutaneous
Reference ID: 3376979
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STELARA safely and effectively. See full prescribing information for STELARA. STELARA (ustekinumab)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STELARA safely and effectively. See full prescribing information for STELARA. STELARA (ustekinumab)
Getting Started With Self-Injection
 Getting Started With Self-Injection When getting started with self-injection, you may have a lot of questions, even after getting trained by a doctor or nurse. Use these instructions to guide you through
Getting Started With Self-Injection When getting started with self-injection, you may have a lot of questions, even after getting trained by a doctor or nurse. Use these instructions to guide you through
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec)
 PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
STELARA INJECTION. What is in this leaflet. Before you use STELARA. What STELARA is used for. Consumer Medicine Information
 STELARA INJECTION Ustekinumab (rmc) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about STELARA (pronounced stel-ahr-uh). It does not contain all the
STELARA INJECTION Ustekinumab (rmc) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about STELARA (pronounced stel-ahr-uh). It does not contain all the
Reimbursement Billing and Coding Guide
 Reimbursement Billing and Coding Guide Please see Indication and Important Safety Information on page 2 and 3 This billing guide is intended to provide healthcare providers with an overview of coding,
Reimbursement Billing and Coding Guide Please see Indication and Important Safety Information on page 2 and 3 This billing guide is intended to provide healthcare providers with an overview of coding,
ACTEMRA (tocilizumab) injection, for intravenous use injection, for subcutaneous use Initial U.S. Approval: 2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ACTEMRA safely and effectively. See full prescribing information for ACTEMRA. ACTEMRA (tocilizumab)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ACTEMRA safely and effectively. See full prescribing information for ACTEMRA. ACTEMRA (tocilizumab)
ACTEMRA Lab Monitoring Guide 1 For DMARD-IR patients with moderate to severe RA
 ath,, interrupt er p infusion es including ld ACTEMRA Lab Monitoring Guide 1 For DMARD-IR patients with moderate to severe RA eving an d laboratory n training and death, permanently s, platelets, d Indication
ath,, interrupt er p infusion es including ld ACTEMRA Lab Monitoring Guide 1 For DMARD-IR patients with moderate to severe RA eving an d laboratory n training and death, permanently s, platelets, d Indication
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Prescription Drug Plan
 Prescription Drug Plan The prescription drug plan helps you pay for prescribed medications using either a retail pharmacy or the mail order program. For More Information Administrative details and procedures
Prescription Drug Plan The prescription drug plan helps you pay for prescribed medications using either a retail pharmacy or the mail order program. For More Information Administrative details and procedures
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Hospital Outpatient Coding and Billing Information Sheet for Neulasta and NEUPOGEN
 Hospital Outpatient Coding and Billing Information Sheet for Neulasta and Neulasta Delivery Kit Neulasta Prefilled Syringe For assistance contact 1-844-MYNEULASTA (1-844-696-3852) or visit www.amgenassistonline.com
Hospital Outpatient Coding and Billing Information Sheet for Neulasta and Neulasta Delivery Kit Neulasta Prefilled Syringe For assistance contact 1-844-MYNEULASTA (1-844-696-3852) or visit www.amgenassistonline.com
Guideline for the use of Biological Therapies in the Treatment of Psoriasis
 1 Date of Production: March1 st 2011 Date of 1 st review: July 10 th 2015 Date for next review: March1 st 2018 Local Contact Dermatology Consultant Shanti Ayob Patient group to which this applies: Patients
1 Date of Production: March1 st 2011 Date of 1 st review: July 10 th 2015 Date for next review: March1 st 2018 Local Contact Dermatology Consultant Shanti Ayob Patient group to which this applies: Patients
MEDICATION GUIDE REMICADE (Rem-eh-kaid) (infliximab) Read the Medication Guide that comes with REMICADE before you receive the first treatment, and
 MEDICATION GUIDE REMICADE (Rem-eh-kaid) (infliximab) Read the Medication Guide that comes with REMICADE before you receive the first treatment, and before each time you get a treatment of REMICADE. This
MEDICATION GUIDE REMICADE (Rem-eh-kaid) (infliximab) Read the Medication Guide that comes with REMICADE before you receive the first treatment, and before each time you get a treatment of REMICADE. This
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
2015 Billing Guide for. REMICADE (infliximab)
 2015 Billing Guide for REMICADE (infliximab) Table of Contents Introduction... 1 Factors That Influence Coverage.... 2 REMICADE (infliximab) Quick Reference Guide... 3 Coding for REMICADE and Drug Administration
2015 Billing Guide for REMICADE (infliximab) Table of Contents Introduction... 1 Factors That Influence Coverage.... 2 REMICADE (infliximab) Quick Reference Guide... 3 Coding for REMICADE and Drug Administration
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd
 ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
How To Take Methotrexate By Injection
 How To Take Methotrexate By Injection 1 how long does it take for methotrexate to work for abortion 2 methotrexate 15 mg hair loss 3 methotrexate injection dosage for rheumatoid arthritis 4 order methotrexate
How To Take Methotrexate By Injection 1 how long does it take for methotrexate to work for abortion 2 methotrexate 15 mg hair loss 3 methotrexate injection dosage for rheumatoid arthritis 4 order methotrexate
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Reimbursement Guide 2011
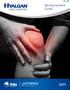 Reimbursement Guide 2011 IMPORTANT SAFETY INFORMATION HYALGAN is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative
Reimbursement Guide 2011 IMPORTANT SAFETY INFORMATION HYALGAN is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative
PART III: CONSUMER INFORMATION
 PART III: CONSUMER INFORMATION Pr REMICADE (Infliximab) This leaflet is part III of a three-part "Product Monograph" published when REMICADE was approved for sale in Canada and is designed specifically
PART III: CONSUMER INFORMATION Pr REMICADE (Infliximab) This leaflet is part III of a three-part "Product Monograph" published when REMICADE was approved for sale in Canada and is designed specifically
M05.9 Rheumatoid arthritis with rheumatoid factor, unspecified M06.00 Rheumatoid arthritis without rheumatoid factor, unspecified site M06.
 Rheumatology ICD-9 ICD-10 V58.69 Z79.3 Long term (current) use of hormonal contraceptives Z79.891 Long term (current) use of opiate analgesic Z79.899 Other long term (current) drug therapy 714.0 M05.40
Rheumatology ICD-9 ICD-10 V58.69 Z79.3 Long term (current) use of hormonal contraceptives Z79.891 Long term (current) use of opiate analgesic Z79.899 Other long term (current) drug therapy 714.0 M05.40
Patient Assistance Application for HUMIRA (adalimumab)
 The AbbVie Patient Assistance Foundation provides AbbVie medicines at no cost to patients experiencing financial difficulties. Eligible patients typically have no healthcare coverage for the requested
The AbbVie Patient Assistance Foundation provides AbbVie medicines at no cost to patients experiencing financial difficulties. Eligible patients typically have no healthcare coverage for the requested
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT STELARA 45 mg solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 45 mg ustekinumab in 0.5
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT STELARA 45 mg solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 45 mg ustekinumab in 0.5
Influenza Vaccine Protocol Agreement (O.C.G.A. Section 43-34-26.1)
 Influenza Vaccine Protocol Agreement (O.C.G.A. Section 43-34-26.1) This Influenza Vaccine Protocol Agreement (the "Protocol") authorizes the Georgia licensed pharmacists (the "Pharmacists") or nurses (
Influenza Vaccine Protocol Agreement (O.C.G.A. Section 43-34-26.1) This Influenza Vaccine Protocol Agreement (the "Protocol") authorizes the Georgia licensed pharmacists (the "Pharmacists") or nurses (
Reimbursement for Physician- Administered Drugs:
 Reimbursement for Physician- Purchased and Physician- Administered Drugs: Understanding the Buy and Bill Process 60889-R5-V1 This information is provided d for your background education and is not intended
Reimbursement for Physician- Purchased and Physician- Administered Drugs: Understanding the Buy and Bill Process 60889-R5-V1 This information is provided d for your background education and is not intended
California SB 486 Needle Disposal Plan for SIMPONI (golimumab)
 California SB 486 Needle Disposal Plan for SIMPONI (golimumab) This describes the process in place for the end-of-life management of SIMPONI self-injection devices. SIMPONI (golimumab) is a drug for treatment
California SB 486 Needle Disposal Plan for SIMPONI (golimumab) This describes the process in place for the end-of-life management of SIMPONI self-injection devices. SIMPONI (golimumab) is a drug for treatment
2006 Provider Coding/Billing Information. www.novoseven-us.com
 2006 Provider Coding/Billing Information 2 3 Contents About NovoSeven...2 Coverage...4 Coding...4 Reimbursement...8 Establishing Medical Necessity and Appealing Denied Claims...10 Claims Materials...12
2006 Provider Coding/Billing Information 2 3 Contents About NovoSeven...2 Coverage...4 Coding...4 Reimbursement...8 Establishing Medical Necessity and Appealing Denied Claims...10 Claims Materials...12
Psoriasis. Psoriasis. Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine
 Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
You can find the PsA treatment support you want. You don t have to do this alone.
 For adults with active Psoriatic arthritis (Ps a) There is a way forward. You can find the PsA treatment support you want. You don t have to do this alone. Indication: CIMZIA is approved for the treatment
For adults with active Psoriatic arthritis (Ps a) There is a way forward. You can find the PsA treatment support you want. You don t have to do this alone. Indication: CIMZIA is approved for the treatment
Medication Guide Enbrel (en-brel) (etanercept)
 Medication Guide Enbrel (en-brel) (etanercept) Read the Medication Guide that comes with Enbrel before you start using it and each time you get a refill. There may be new information. This Medication Guide
Medication Guide Enbrel (en-brel) (etanercept) Read the Medication Guide that comes with Enbrel before you start using it and each time you get a refill. There may be new information. This Medication Guide
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HUMIRA - adalimumab injection, solution Abbott Laboratories ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use HUMIRA safely and effectively.
HUMIRA - adalimumab injection, solution Abbott Laboratories ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use HUMIRA safely and effectively.
NOVARTIS SERVICE REQUEST FORM FOR PATIENT SUPPORT
 NOVARTIS SERVICE REQUEST FORM FOR PATIENT SUPPORT Please complete the Fax Cover Sheet and Service Request Form, and fax all pages to the number specified below. Dear Health Care Professional: The Novartis
NOVARTIS SERVICE REQUEST FORM FOR PATIENT SUPPORT Please complete the Fax Cover Sheet and Service Request Form, and fax all pages to the number specified below. Dear Health Care Professional: The Novartis
These are just some of the eligibility requirements meeting these criteria does not guarantee acceptance.
 BARACLUDE PATIENT ASSISTANCE PROGRAM The Baraclude Patient Assistance Program is designed to provide free medication to qualifying patients who do not have prescription drug coverage and are having a hard
BARACLUDE PATIENT ASSISTANCE PROGRAM The Baraclude Patient Assistance Program is designed to provide free medication to qualifying patients who do not have prescription drug coverage and are having a hard
-------------------------------CONTRAINDICATIONS------------------------------ Sepsis (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Enbrel safely and effectively. See full prescribing information for Enbrel. Enbrel (etanercept) Solution
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Enbrel safely and effectively. See full prescribing information for Enbrel. Enbrel (etanercept) Solution
You can find the PsA treatment support you want. You don t have to do this alone.
 For adults with active Psoriatic arthritis (Ps a) There is a way forward. You can find the PsA treatment support you want. You don t have to do this alone. Indication: CIMZIA is approved for the treatment
For adults with active Psoriatic arthritis (Ps a) There is a way forward. You can find the PsA treatment support you want. You don t have to do this alone. Indication: CIMZIA is approved for the treatment
Is Monotherapy Treatment of Etanercept Effective Against Plaque Psoriasis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Patient Input Information Clinical Trials Outcomes Common Drug Review
 CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
Vaccine Protocol Agreement. Name of Pharmacy: Address: City, State, Zip:
 Vaccine Protocol Agreement Name of Pharmacy: Address: City, State, Zip: This Vaccine Protocol Agreement (the "Protocol") authorizes the Georgia licensed pharmacists (the "Pharmacists") or nurses ( Nurses
Vaccine Protocol Agreement Name of Pharmacy: Address: City, State, Zip: This Vaccine Protocol Agreement (the "Protocol") authorizes the Georgia licensed pharmacists (the "Pharmacists") or nurses ( Nurses
Cytokine and CAM Antagonists
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
REMICADE (infliximab)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use REMICADE safely and effectively. See full prescribing information for REMICADE. REMICADE (infliximab)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use REMICADE safely and effectively. See full prescribing information for REMICADE. REMICADE (infliximab)
Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Medicare Drug Coverage Under Part A, Part B, and Part D
 Medicare Drug Coverage Under Part A, Part B, and Part D Medicare Part A and Part B generally do not cover outpatient prescription drugs, most of which are now covered under Part D. This document and the
Medicare Drug Coverage Under Part A, Part B, and Part D Medicare Part A and Part B generally do not cover outpatient prescription drugs, most of which are now covered under Part D. This document and the
Below, this letter outlines [patient name] s medical history, prognosis, and treatment rationale.
![Below, this letter outlines [patient name] s medical history, prognosis, and treatment rationale. Below, this letter outlines [patient name] s medical history, prognosis, and treatment rationale.](/thumbs/25/5563775.jpg) [Date] [Name of Contact] [Title] [Name of Health Insurance Company] [Address] [City, State, Zip Code] Insured: [Patient Name] Policy Number: [Number] Group Number: [Number] Diagnosis: [Diagnosis and ICD-9-CM
[Date] [Name of Contact] [Title] [Name of Health Insurance Company] [Address] [City, State, Zip Code] Insured: [Patient Name] Policy Number: [Number] Group Number: [Number] Diagnosis: [Diagnosis and ICD-9-CM
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Bristol-Myers Squibb Access Support Program. What Medications does the BMS Access Support Program help with? Program Registration Steps
 Oncology Reimbursement Support Phone: 1-800-861-0048 Fax: 1-888-776-2370 Bristol-Myers Squibb Access Support Program The Bristol-Myers Squibb Access Support Program is designed to help patients with reimbursement
Oncology Reimbursement Support Phone: 1-800-861-0048 Fax: 1-888-776-2370 Bristol-Myers Squibb Access Support Program The Bristol-Myers Squibb Access Support Program is designed to help patients with reimbursement
Medicare Resource Guide
 Medicare Resource Guide Patient Name Dear Patient, Please take the time to read the following sections of this brochure as noted by your healthcare provider. These different components of Medicare deal
Medicare Resource Guide Patient Name Dear Patient, Please take the time to read the following sections of this brochure as noted by your healthcare provider. These different components of Medicare deal
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
SUPPORT PATH PROGRAM INTAKE FORM PHONE: 1-855-769-7284 FAX: 1-855-298-8700
 SUPPORT PATH PROGRAM INTAKE FORM PHONE: 1-855-769-7284 FAX: 1-855-298-8700 1 REQUESTED SERVICE(S) (REQUIRED) CHECK ALL BOXES THAT APPLY Benefits Investigation Prior Authorization and Appeals Support Patient
SUPPORT PATH PROGRAM INTAKE FORM PHONE: 1-855-769-7284 FAX: 1-855-298-8700 1 REQUESTED SERVICE(S) (REQUIRED) CHECK ALL BOXES THAT APPLY Benefits Investigation Prior Authorization and Appeals Support Patient
Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy
 Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists
 MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Nurse Aide Training Program Application Checklist
 Nurse Aide Training Program Application Checklist The following checklist must be completed before enrolling in the Nurse Aide Training course: Complete, sign, and date the Application Form Have the physical
Nurse Aide Training Program Application Checklist The following checklist must be completed before enrolling in the Nurse Aide Training course: Complete, sign, and date the Application Form Have the physical
ALLERGENIC EXTRACT. Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE. U.S. Government License No.
 ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
MEDICATION GUIDE. ACTEMRA (AC-TEM-RA) (tocilizumab) Solution for Intravenous Infusion
 MEDICATION GUIDE ACTEMRA (AC-TEM-RA) (tocilizumab) Solution for Intravenous Infusion ACTEMRA (AC-TEM-RA) (tocilizumab) Injection, Solution for Subcutaneous Administration Read this Medication Guide before
MEDICATION GUIDE ACTEMRA (AC-TEM-RA) (tocilizumab) Solution for Intravenous Infusion ACTEMRA (AC-TEM-RA) (tocilizumab) Injection, Solution for Subcutaneous Administration Read this Medication Guide before
The clinical studies have been performed in children, adolescents and adults, from 4 years up to 55 years of age.
 NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
How Is OnabotulinumtoxinA Reimbursed For Chronic Migraine? Impact Of FDA Approval And The New CPT Code
 How Is OnabotulinumtoxinA Reimbursed For Chronic Migraine? Impact Of FDA Approval And The New CPT Code Effective January 1, 2013, physicians will be able to report the new CPT code 64615 when performing
How Is OnabotulinumtoxinA Reimbursed For Chronic Migraine? Impact Of FDA Approval And The New CPT Code Effective January 1, 2013, physicians will be able to report the new CPT code 64615 when performing
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Lindenwold Board File Code # 5141.21 Of Education Page 1 of 7
 Of Education Page 1 of 7 The Board of Education disclaims any and all responsibility for the diagnosis and treatment of the illness be contingent upon the timely administration of medication duly prescribed
Of Education Page 1 of 7 The Board of Education disclaims any and all responsibility for the diagnosis and treatment of the illness be contingent upon the timely administration of medication duly prescribed
Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
swine flu vaccination:
 swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
A Guide to Patient Services. Cedars-Sinai Health Associates
 A Guide to Patient Services Cedars-Sinai Health Associates Welcome Welcome to Cedars-Sinai Health Associates. We appreciate the trust you have placed in us by joining our dedicated network of independent-practice
A Guide to Patient Services Cedars-Sinai Health Associates Welcome Welcome to Cedars-Sinai Health Associates. We appreciate the trust you have placed in us by joining our dedicated network of independent-practice
FDA Update on the H1N1 Flu Vaccine and Antiviral Medications
 FDA Update on the H1N1 Flu Vaccine and Antiviral Medications Beth Fabian Fritsch, R.Ph., M.B.A. Commander, U.S. Public Health Service Health Programs Coordinator Office of Special Health Issues Food and
FDA Update on the H1N1 Flu Vaccine and Antiviral Medications Beth Fabian Fritsch, R.Ph., M.B.A. Commander, U.S. Public Health Service Health Programs Coordinator Office of Special Health Issues Food and
Bard: Intermittent Catheters. A guide to. Bard: Pelvic Organ Prolapse. An REIMBURSEMENT. overview of OF INTERMITTENT. Prolapse CATHETERS
 Bard: Intermittent Catheters A guide to Bard: Pelvic Organ Prolapse An REIMBURSEMENT overview of Pelvic OF INTERMITTENT Organ Prolapse CATHETERS 1 Intermittent catheterization is a covered Medicare benefit
Bard: Intermittent Catheters A guide to Bard: Pelvic Organ Prolapse An REIMBURSEMENT overview of Pelvic OF INTERMITTENT Organ Prolapse CATHETERS 1 Intermittent catheterization is a covered Medicare benefit
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
ICD-10 FROM A NURSE PERSPECTIVE. Learning Objectives 4/22/2015. Adoption of ICD-10 Classification of Diseases CD-10-CM Diagnostic Codes
 ICD-10 FROM A NURSE PERSPECTIVE Learning Objectives 1. New ICD-10-CM diagnostic system for Dermatology. 2. Impact of new codes on nursing and clinical support staff. 3. Education and resources available.
ICD-10 FROM A NURSE PERSPECTIVE Learning Objectives 1. New ICD-10-CM diagnostic system for Dermatology. 2. Impact of new codes on nursing and clinical support staff. 3. Education and resources available.
Teriflunomide (Aubagio) 14mg once daily tablet
 Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
AMPYRA (dalfampridine) Important Safety Information
 NEWS RELEASE Acorda to Present New rhigm22 and AMPYRA (dalfampridine) Data at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 10/7/2015 ARDSLEY, N.Y.--(BUSINESS
NEWS RELEASE Acorda to Present New rhigm22 and AMPYRA (dalfampridine) Data at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 10/7/2015 ARDSLEY, N.Y.--(BUSINESS
Cytokine and CAM Antagonists
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
SECTION 3. Criteria for Special Authorization of Select Drug Products. Section 3 Criteria for Special Authorization of Select Drug Products
 SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
QUESTIONS TO ASK MY DOCTOR
 Be a part of the treatment decision by asking questions QUESTIONS TO ASK MY DOCTOR FOR PATIENTS WITH ADVANCED STOMACH OR GASTROESOPHAGEAL JUNCTION (GEJ) CANCER CYRAMZA (ramucirumab) is used alone or in
Be a part of the treatment decision by asking questions QUESTIONS TO ASK MY DOCTOR FOR PATIENTS WITH ADVANCED STOMACH OR GASTROESOPHAGEAL JUNCTION (GEJ) CANCER CYRAMZA (ramucirumab) is used alone or in
New York City Office of Labor Relations Employee Benefits Program/Municipal Labor Committee
 New York City Office of Labor Relations Employee Benefits Program/Municipal Labor Committee PICA PRESCRIPTION DRUG PROGRAM Self-Injectable Medications Chemotherapy Medications Questions & Answers Last
New York City Office of Labor Relations Employee Benefits Program/Municipal Labor Committee PICA PRESCRIPTION DRUG PROGRAM Self-Injectable Medications Chemotherapy Medications Questions & Answers Last
EPEC. Education for Physicians on End-of-life Care. Trainer s Guide
 EPEC Education for Physicians on End-of-life Care Trainer s Guide Procedure/Diagnosis Coding and Reimbursement Mechanisms for Physician Services in Palliative Care EPEC Project, The Robert Wood Johnson
EPEC Education for Physicians on End-of-life Care Trainer s Guide Procedure/Diagnosis Coding and Reimbursement Mechanisms for Physician Services in Palliative Care EPEC Project, The Robert Wood Johnson
How to Request an Exception or Appeal a Decision From Your Prescription Drug Plan
 How to Request an Exception or Appeal a Decision From Your Prescription Drug Plan Exceptions What is an Exception? Sometimes you may not be able to obtain a prescription medication that your healthcare
How to Request an Exception or Appeal a Decision From Your Prescription Drug Plan Exceptions What is an Exception? Sometimes you may not be able to obtain a prescription medication that your healthcare
NUVIGIL (armodafinil) oral tablet
 NUVIGIL (armodafinil) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
NUVIGIL (armodafinil) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
this 7^ day of September 2014 by Randall S. Gregg Special Deputy Director
 r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
Severe rheumatoid arthritis (a disease that causes inflammation of the joints),where MabThera is given intravenously together with methotrexate.
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
Overview of the BCBSRI Prescription Management Program
 Definitions Overview of the BCBSRI Prescription Management Program DISPENSING GUIDELINES mean: the prescription order or refill must be limited to the quantities authorized by your doctor not to exceed
Definitions Overview of the BCBSRI Prescription Management Program DISPENSING GUIDELINES mean: the prescription order or refill must be limited to the quantities authorized by your doctor not to exceed
VACCINES FOR CHILDREN (VFC) QUESTIONS AND ANSWERS
 VACCINES FOR CHILDREN (VFC) QUESTIONS AND ANSWERS VACCINES FOR CHILDREN PROGRAM ELIGIBILITY 1. What is the VFC Program? The VFC program is a federally funded program that provides vaccines at no cost to
VACCINES FOR CHILDREN (VFC) QUESTIONS AND ANSWERS VACCINES FOR CHILDREN PROGRAM ELIGIBILITY 1. What is the VFC Program? The VFC program is a federally funded program that provides vaccines at no cost to
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
February 2016. page 1 / 9
 February 2016 page 1 / 9 page 2 / 9 Alternative Medicines Corner Advising on this article: Nicole M. Maisch February 1, 2016 Melatonin supplementation may improve outcomes in children with atopic dermatitis
February 2016 page 1 / 9 page 2 / 9 Alternative Medicines Corner Advising on this article: Nicole M. Maisch February 1, 2016 Melatonin supplementation may improve outcomes in children with atopic dermatitis
HCSP GUIDES A GUIDE TO: PREPARING FOR TREATMENT. A publication of the Hepatitis C Support Project
 HCSP GUIDES T R E AT M E N T I S S U E S A publication of the Hepatitis C Support Project The information in this guide is designed to help you understand and manage HCV and is not intended as medical
HCSP GUIDES T R E AT M E N T I S S U E S A publication of the Hepatitis C Support Project The information in this guide is designed to help you understand and manage HCV and is not intended as medical
NOVARTIS SERVICE REQUEST FORM FOR PATIENT SUPPORT
 NOVARTIS SERVICE REQUEST FORM FOR PATIENT SUPPORT Please complete the Fax Cover Sheet and Service Request Form, and fax all pages to the number specified below. Dear Health Care Professional: The Novartis
NOVARTIS SERVICE REQUEST FORM FOR PATIENT SUPPORT Please complete the Fax Cover Sheet and Service Request Form, and fax all pages to the number specified below. Dear Health Care Professional: The Novartis
Gloucestershire Hospitals
 Gloucestershire Hospitals NHS Foundation Trust TRUST GUIDELINE INFLIXIMAB PRESCRIBING AND ADMINISTRATION 1. INTRODUCTION Infliximab is an anti-tumour necrosis factor-α (Anti-TNF) antibody. It is from a
Gloucestershire Hospitals NHS Foundation Trust TRUST GUIDELINE INFLIXIMAB PRESCRIBING AND ADMINISTRATION 1. INTRODUCTION Infliximab is an anti-tumour necrosis factor-α (Anti-TNF) antibody. It is from a
Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta350
 Secukinumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta350 NICE 2015. All rights reserved. Contents 1 Guidance...
Secukinumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta350 NICE 2015. All rights reserved. Contents 1 Guidance...
CSAC/EIA Health Small Group Access+ HMO 15-0 Inpatient Benefit Summary
 CSAC/EIA Health Small Group Access+ HMO 15-0 Inpatient Benefit Summary (Uniform Health Plan Benefits and Coverage Matrix) Blue Shield of California THIS MATRIX IS INTENDED TO BE USED TO HELP YOU COMPARE
CSAC/EIA Health Small Group Access+ HMO 15-0 Inpatient Benefit Summary (Uniform Health Plan Benefits and Coverage Matrix) Blue Shield of California THIS MATRIX IS INTENDED TO BE USED TO HELP YOU COMPARE
Exceptions and Appeals for Drug Therapies: A Guide for Healthcare Providers
 Exceptions and Appeals for Drug Therapies: A Guide for Healthcare Providers Table of Contents Introduction... 5 Prior Authorization... 7 Overview... 7 Step Therapy... 7 Quantity Limits... 7 The Prior Authorization
Exceptions and Appeals for Drug Therapies: A Guide for Healthcare Providers Table of Contents Introduction... 5 Prior Authorization... 7 Overview... 7 Step Therapy... 7 Quantity Limits... 7 The Prior Authorization
How To Treat A Tumor Necrosis Factor (Tnf) Blocker
 HIGHLIGHTS OF PRESCRIBING INFORMA TION These highlights do not include all the information needed to use SIMPONI safely and effectively. See full prescribing information for SIMPONI. SIMPONI (golimumab)
HIGHLIGHTS OF PRESCRIBING INFORMA TION These highlights do not include all the information needed to use SIMPONI safely and effectively. See full prescribing information for SIMPONI. SIMPONI (golimumab)
INSTRUCTIONS FOR REPORTING IMMUNIZATION SERVICES TO THIRD PARTY PAYERS (Billing Guide)
 INSTRUCTIONS FOR REPORTING IMMUNIZATION SERVICES TO THIRD PARTY PAYERS (Billing Guide) NOTE: All italicized words or phrases are defined at the end of this document. Adults (19 years of age and older)
INSTRUCTIONS FOR REPORTING IMMUNIZATION SERVICES TO THIRD PARTY PAYERS (Billing Guide) NOTE: All italicized words or phrases are defined at the end of this document. Adults (19 years of age and older)
Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer )
 Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
