Clinical Trials Update 2013
|
|
|
- Jessie Walsh
- 9 years ago
- Views:
Transcription
1 Clinical Trials Update 2013
2 Clinical Trials Update 2013 Dear NET colleagues The mission of the NET subgroup is, through collaboration, to develop and participate in practice-changing clinical studies to improve outcomes for patients with neuroendocrine tumours; linking clinical research with basic science, industry partners, and patient advocacy groups and charities. The past year has seen a change of Chair and review of the subgroup membership. In the pages that follow, you will find a summary of ongoing studies (either open to recruitment; or closed to recruitment, but still in follow-up) as well as studies in advanced stages of planning and due to open contact details of investigators are provided in order to enhance clinician and patient access. The UK is increasingly seen as an important contributor to national and international NET studies; including an enhanced profile with industry through excellent recruitment (e.g. COOPERATE-2, RADIANT-4 and BEZ studies). Well done and thank you to all investigators. In addition, translational research in NETs is being developed alongside the clinical portfolio (TRANSNET group; Chair: Tim Meyer), with the aim of opening translational studies addressing the role of imaging, pathology and biochemistry as potential biomarkers in NETs; the first of these (CALMNET) is due to open imminently. A number of pilot studies will be funded in 2014 following a recent call for proposals for NET Patient Foundation-funded grants. The future strategy of the group is to expand the current portfolio of studies from predominantly oncology-based studies in advanced disease, to include surgical studies (including adjuvant studies), and studies from non-oncology specialties (e.g. gastroenterology, interventional radiology, endocrinology) relevant to patients with NETs; we welcome any enthusiastic investigators to propose ideas or join the group. Best wishes for your respective research activities in Prof Juan W Valle Chair NET subgroup NCRI Upper GI Clinical Studies Group [email protected]
3 NCRI Portfolio Map: Neuroendocrine Tumours
4 NET Studies (in alphabetical order) BEZ235 (2401) 1 ST Line Randomized phase II study of BEZ235 or everolimus in advanced pancreatic neuroendocrine tumours NCRI ID: NCRN379; Registration: NCT Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Advanced (unresectable or metastatic), histologically confirmed well differentiated (low to intermediate grade) pancreatic neuroendocrine tumor (pnet) Radiological documentation of progressive disease within the last 12 months prior to randomization. Measurable disease per RECIST Version 1.0 WHO performance status 2 Exclusion criteria Previous treatment with PI3K and/or mtor pathway inhibitors Uncontrolled cardiac disease, hypertension or diabetes mellitus Gastrointestinal disease that may significantly alter the absorption of BEZ235 Immunocompromised patients (chronic treatment with systemic high dose steroids or other immunosuppressive agent) including known seropositivity for HIV Diarrhea Grade 2 Primary endpoint: PFS Secondary endpoints: safety and tolerability, RR, OS, TTF Exploratory objectives: biochemical response (changes in CgA and NSE levels) and its association with PFS, tumor tissue biomarker analysis, additional exploratory biomarkers Recruiting centres and contact details Date study open: Nov 2012 Planned accrual: Global: 140 patients; UK: 15 patients
5 Actual accrual: Global: 51 patients; UK: 4 patients Planned closure: Closed Oct-2012; on follow up Prof. Juan W Valle, The Christie NHS Foundation Trust, Manchester Dr. Pippa Corrie, Cambridge University Hospitals NHS Foundation Trust, Cambridge Prof. Nick Reed, Beatson Oncology Centre, Glasgow Prof. Tim Meyer, Royal Free London NHS Foundation Trust, London [email protected] [email protected] [email protected] [email protected]
6 BEZ235 (2201) 2 nd line A multicenter, two stage, phase II study, evaluating the efficacy of oral BEZ235 plus best supportive care (BSC) versus placebo plus BSC in the treatment of patients with advanced pancreatic neuroendocrine tumours (pnet) after failure of mtor inhibitor therapy. NCRI ID: NCRN411; Registration: NCT Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Advanced welldifferentiated (grade 1-2) pancreatic pnet with evidence of disease progression after previous treatment with mtor inhibitor. Measurable disease per RECIST Version 1.1 If patient is on treatment with SSA, stable dose at least 2 months prior to study start is needed. Exclusion criteria Previous treatment with any PI3K inhibitor or AKT inhibitor Discontinued prior mtor inhibitor therapy due to toxicity More than 3 prior systemic treatment regimens (including cytotoxic chemotherapy, targeted therapy, immunotherapy). Uncontrolled cardiac disease Gastrointestinal disease that may significantly alter the absorption of BEZ235 Immunocompromised patients (chronic treatment with systemic high dose steroids or other immunosuppressive agent) including known seropositivity for HIV Primary endpoint: Stage 1: Primary: PFS at 16 weeks Secondary: AEs, RR, DCR, DoR
7 Exploratory: correlation with biochem markers, molecular profiling, proteomic / genomic analysis Stage 2: Primary: PFS (local assessment) Secondary: AEs, RR, DCR, DoR, OS Exploratory: population PKs, correlation with biochem markers, molecular profiling, proteomic / genomic analysis Recruiting centres and contact details Date study open: Nov-2012 Planned accrual: UK: 6 patients Actual accrual: UK: 3 patients Planned closure: Stage 1 completed Sept Closed pending the interim analysis Prof. Nick Reed, Beatson Oncology Centre, Glasgow Prof. Juan W Valle, The Christie NHS Foundation Trust, Manchester [email protected] [email protected]
8 CALM-NET A Phase IV, Multicentre, Open label, Single Group Exploratory Study to Assess the Clinical Value of Enumeration of Circulating Tumour Cells (CTCs) to Predict Clinical Symptomatic Response and Progression Free Survival in Patients receiving Deep Subcutaneous Administrations of Somatuline (lanreotide) Autogel to treat the Symptoms of Functioning Midgut NeuroEndocrine Tumours (NET). EudraCT Number: Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Well or moderately differentiated functioning midgut NET. The clinically appropriate treatment for the patient must primarily be monotherapy with a somatostatin analogue. Patients must have had either a positive somatostatin receptor scintigraphy result or a positive 68Gallium-DOTATATE PET imaging result. Patients must have a documented urinary or plasma 5-HIAA result within the year prior to study entry which is above the laboratory reference range. Exclusion criteria The patient has been treated with a somatostatin analogue prior to study entry, unless a washout period of at least 2 weeks for subcutaneous octreotide, or at least 6 weeks for a single dose of long acting somatostatin analogue has occurred. The patient has received interferon, chemotherapy, chemoembolisation or radionuclide therapy within 3 months prior to study entry. Primary endpoint: To assess the clinical value of enumeration of circulating tumour cells (CTCs) to predict clinical symptomatic response in patients receiving Somatuline Autogel. Secondary endpoints: Serum biomarkers and its correlation with CTC numbers. Genomic profile of CTCs and density of somatostatin receptor (SSTR) subtypes 2 and 5 on CTCs. Recruiting centres and contact details Date study open: Planned accrual: Global: 50 patients Prof. Tim Meyer, Royal Free London NHS Foundation Trust, London Dr Christos Toumpanakis, Royal Free London NHS Foundation Trust, London [email protected] [email protected]
9 Prof. Nick Reed, Beatson Oncology Centre, Glasgow Prof. Ashley Grossman, University of Oxford Churchill Hospital Dr Aled Rees, University Hospital of Wales, Cardiff Prof. Juan W Valle, The Christie NHS Foundation Trust, Manchester Prof Andrea Frilling, Hammersmith Hospital, London Dr Gaurav Kapur, Norfolk and Norwich University Hospital NHS Foundation Trust, Norwich Dr Tahir Shah, University Hospital Birmingham NHS Foundation Trust, Birmingham Dr John Newell-Price, Sheffield Teaching Hospital NHS Foundation Trust, Sheffield Dr Alan Anthoney, Leeds Teaching Hospitals NHS Trust, Leeds Dr Daniel Cuthbertson, Aintree University Hospitals NHS Foundation Trust Dr Rajaventhan Srirajaskanthan, Kings College Hospital NHS Foundation Trust, London [email protected] [email protected] [email protected] [email protected] [email protected] [email protected] [email protected] [email protected] [email protected] [email protected] [email protected]
10 COOPERATE-II A randomized, open-label phase II multicenter study evaluating the efficacy of oral everolimus alone or in combination with pasireotide LAR i.m. in advanced progressive pancreatic neuroendocrine tumours (PNET) NCRI ID: NCRN239; Registration: NCT Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Advanced low to intermediate 10 mg Everolimus grade PNET Progression within 12 months PS 0-2 R Measurable disease No need for SSA 60 mg Pasireotide LAR + 10 mg Everolimus Exclusion criteria Patients currently requiring SSA treatment or who received prior therapy with mtor inhibitors. Patients with more than 2 prior systemic treatment regimens Patients receiving chronic treatment with corticosteroids or another immunosuppressive agent. History or active liver disease such as cirrhosis decompensated liver disease, chronic active hepatitis or chronic persistent hepatitis. Stratification factors Prior SSA (yes/no) Elevated biomarkers (yes/no): CgA >2xULN and/or NSE >1xULN Primary endpoint: PFS Secondary endpoints: ORR, DCR, DoR; OS; Safety; PK Recruiting centres and contact details Date study open: Sep-2011 Planned accrual: Global: 150 patients; UK: 7 patients Actual accrual: Global: 160 patients; UK: 14 patients Planned closure: Closed Oct-2012; on follow up Prof. Juan W Valle, The Christie NHS Foundation Trust, Manchester Dr. Pippa Corrie, Cambridge University Hospitals NHS Foundation Trust, Cambridge Prof. Nick Reed, Beatson Oncology Centre, Glasgow [email protected] [email protected] [email protected]
11 LUNA Multicenter 3-arm trial to evaluate the efficacy and safety of Pasireotide LAR or Everolimus alone or in combination in patients with well differentiated neuroendocrine carcinoma of the lung and thymus -LUNA Trial EUDRACT number: Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Histological confirmed advanced (unresectable or metastatic) well differentiated neuroendocrine carcinoma of the lung and thymus (typical and atypical) Previously treated or treatment naïve patients can be included. At least one measurable lesion of disease by RECIST 1.1 criteria with radiological disease progression within 12 months prior to randomization Adequate bone marrow, liver and renal function Exclusion criteria Patients with severe functional disease who require symptomatic treatment with somatostatin analogs can not be included in the protocol.
12 Prior therapy with mtor inhibitors (e.g. sirolimus, temsirolimus, everolimus). Prior therapy with radioligand therapy within 6 months prior to starting study treatment or not recovered from the side effects of such therapy. Mixed tumours are excluded. Severe comorbidity, including HIV infection or hepatitis Patients with symptomatic cholelithiasis. Stratification factors Typical carcinoid tumor (TC) vs. atypical carcinoid tumor (AC) according to WHO classification Line of treatment (1st line vs. others). Primary endpoint: Proportion of patients progression-free at 12 months according to RECIST V 1.1. Secondary endpoints: PFs, Disease control rate, Time to response, Duration of response, Time to progression, Biochemical response rate, safety and tolerability of the combination with primary lung and thymus NET patients Recruiting centres and contact details Planned accrual: Global: 108 patients Prof. Tim Meyer, Royal Free London NHS Foundation Trust, London Dr Was Mansoor, The Christie NHS Foundation Trust, Manchester Prof. Nick Reed, Beatson Oncology Centre, Glasgow [email protected] [email protected] [email protected]
13 NETTER-1 A multi-centre, stratified, open, randomized, comparator-controlled, parallel-group phase III study comparing treatment with 177Lu-DOTA0-Tyr3-Octreotate to Octreotide LAR in patients with inoperable, progressive, somatostatin receptor positive, midgut carcinoid tumours. NCRI ID: NCRN328; Registration: REC12/NW/0251 Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Randomization (1:1) A) 177Lu-DOTA0-Tyr3-Octreotate plus 30 mg Octreotide LAR B) High dose (60 mg) Octreotide LAR Inclusion criteria Presence of inoperable (curative intent), histologically proven, midgut carcinoid tumour. Ki67 index 20% Patients on Octreotide LAR at a fixed dose of 20 mg or 30 mg at 3-4 weeks intervals for at least 12 weeks prior to enrolment in the study. Patients must have progressive disease based on RECIST Criteria, Version 1.1 evidenced with CT scans/mri obtained within 3 years from enrolment compared with a recent scan not older than 4 weeks from the projected randomization date, and while the patient was on a fixed dose of Sandostatin LAR. Confirmed presence of somatostatin receptors on all technically evaluable tumour lesions documented by CT/MRI scans, based on positive OctreoScan imaging within 24 weeks prior to enrolment in the study
14 The tumour uptake observed using OctreoScan normal liver uptake on planar imaging Exclusion criteria Inadequeate renal, liver and bone marrow function Treatment with >30 mg Octreotide LAR at 3-4 weeks intervals within 12 weeks prior to enrolment in the study. Peptide receptor radionuclide therapy (PRRT) at any time prior to enrolment in the study. Uncontrolled congestive heart failure or diabetes mellitus. Stratification factors Centre OctreoScan tumour uptake score (Grade 2, 3 and 4) Length of time on the most recent constant dose of Octreotide prior to enrolment ( 6 and >6 months) Primary endpoint: PFS = time from treatment start to documented (RECIST) progression, or death (any cause).pfs evaluated over fixed period of 76 weeks since treatment start. Secondary endpoints: TTP, ORR, OS. Recruiting centres and contact details Date study open: May 2012 Planned accrual: Global: 230 patients; UK: 35 patients Actual accrual: Global: 87 patients; UK: 13 patients Planned closure: 3 years after last enrolled subject completed wk 76 = Nov 2015 Prof. Ashley Grossman, University of Oxford Churchill Hospital Dr Prakash Manoharan, The Christie NHS Foundation Trust, Manchester Prof. Nick Reed, Beatson Oncology Centre, Glasgow Martyn Caplin, Royal Free Hospital, London [email protected] [email protected] [email protected] [email protected]
15 OBLIQUE A Phase IV, Observational study to assess Quality of Life in patients with Pancreatic Neuroendocrine Tumors receiving treatment with oral 10 mg Everolimus (Afinitor ) o.d.: The OBLIQUE Study CRAD001PGB12 NCRI ID: NCRN572; Registration: Portfolio number Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema This is a non-interventional multi-centre post-marketing study, to document changes in HrQoL, assessed on the EORTC QLQ-C30 patient reported outcomes questionnaire, in adult patients with advanced pancreatic NETs being treated orally with 10 mg everolimus (Afinitor ) o.d. in routine clinical practice. This study does not impose diagnostic or therapeutic interventions on the patients. There is also no mandatory visit schedule. Inclusion criteria Diagnosis of advanced (unresectable or metastatic) well or moderately differentiated neuroendocrine tumors of pancreatic origin with radiologically documented progressive disease Patients for whom a decision has been made to start treatment with 10 mg everolimus (Afinitor ) o.d. following review of their medical history against the known safety profile of everolimus (Afinitor ). Patients taking somatostatin analogues for symptom control should be on a stable dose for at least two months prior to enrollment. Exclusion criteria Patients that have any factor in their medical history that contradicts recommendations for prescription made in the latest version of the summary of product characteristics (SmPC) for Everolimus. Primary endpoint: HrQoL as assessed by the EORTC QLQ-C30 patient reported outcome questionnaire (Global Health Status QoL score) after 6 months of oral treatment with 10 mg everolimus o.d. Secondary endpoints: OS, PFS, biochemical tumour markers, duration of everolimus, safety and tolerability Recruiting centres and contact details Date study open: June 2013 Planned accrual: UK: 33 patients Actual accrual: UK: 2 patients Planned closure: June 2015 Prof. John Ramage, Kent and Hampshire Hospitals NET Centre Prof. Tim Meyer, Royal Free London NHS [email protected] [email protected]
16 Foundation Trust, London Prof. Juan W Valle, The Christie NHS Foundation Trust, Manchester Dr. Judith Cave (Southampton) Dr. Lucy Wall (Edinburgh) Prof Daniel Palmer (Clatterbridge, Liverpool) Prof Andrea Frilling, Hammersmith Hospital, London Dr. Jonathan Wadsley (Sheffield) Dr. Gaurav Kapur (Norfolk & Norwich University Hospital) Dr. Pankaj Punia (Queen Elizabeth Hospital, Birmingham) Dr. Sebastian Cummins (Royal Surrey County Hospital, Guildford) Dr. Ian Chau (Royal Marsden Hospital, Sutton) Dr. Karin Bradley (Bristol Royal Infirmary) [email protected] not available [email protected] [email protected] [email protected] [email protected] [email protected] not available [email protected] not available [email protected]
17 RADIANT-4 Phase III, randomized, double-blind study of everolimus (RAD001) plus best supportive care versus placebo plus best supportive care in the treatment of patients with advanced neuroendocrine tumour (NET) of gastrointestinal (GI) or lung origin without a history of carcinoid syndrome NCRI ID: NCRN333; Registration: NCT Inclusion/exclusion criteria and study schema Inclusion criteria Open (recruiting) Open (in follow-up) Due to be opened Advanced low and intermediate grade (welldifferentiated) nonfunctional NET of GI or lung origin (nonpancreatic) R 2:1 Everolimus 10 mg/day + BSC n=190 Placebo + BSC n=95 Exclusion criteria Patients with history of or active symptoms of carcinoid syndrome More than one prior line of chemotherapy or prior targeted therapy Gastrointestinal disease that may significantly alter the absorption of oral everolimus Liver disease such as cirrhosis, decompensated liver disease, and chronic hepatitis or known history of HIV seropositivity Chronic treatment with corticosteroids or other immunosuppressive agents Stratification factors tumour origin, WHO PS and prior SSA Primary endpoint: PFS by central radiology Cross over to open-label everolimus only after interim analysis and DMC recommendation Recruiting centres and contact details Date study open: Apr-2012 Planned accrual: Global: 280 patients; UK: 12 patients Actual accrual: UK: 20 patients (167%) Planned closure: Closed to accrual Aug-2013; on follow up
18 Recruitment status (global, final): COUNTRY # pts enrolled Italy 66 USA 56 Germany 23 United Kingdom 20 Canada 18 Grand Total 296 Prof. Juan W Valle, The Christie NHS Foundation Trust, Manchester Dr. Pippa Corrie, Cambridge University Hospitals NHS Foundation Trust, Cambridge Prof. Nick Reed, Beatson Oncology Centre, Glasgow Prof. Tim Meyer, Royal Free London NHS Foundation Trust, London [email protected] [email protected] [email protected] [email protected]
19 TELESTAR A Phase 3, Randomized, Placebo-controlled, Parallelgroup, Multicenter, Double-blind Study to Evaluate the Efficacy and Safety of Telotristat Etiprate (LX1606) in Patients with Carcinoid Syndrome Refractory to Somatostatin Analog (SSA) Therapy EudraCT Number: Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Histopathologically-confirmed, advanced, well-differentiated metastatic NET Documented history of carcinoid syndrome, and currently experiencing an average of 4 bowel movements per day. Currently receiving a stable-dose SSA therapy at least 3 months prior to entering the Run-in Period or patients who cannot tolerate SSA therapy. Exclusion criteria Other causes of diarrhea apart from the carcinoid syndrome Presence of more than 12 watery bowel movements per day associated with volume contraction, dehydration, or hypotension compatible with a pancreatic cholera -type clinical syndrome, as judged by the Investigator Karnofsky Performance Status 60% Treatment with any tumor directed therapy A history of substance or alcohol abuse within 2 years prior to Screening Randomization (1:1:1) Stratification factors: Baseline urinary 5-HIAA levels. Primary endpoint: Reduction from baseline in the number of daily bowel movements averaged over the 12-week double-blind portion of the trial Secondary endpoints: other efficacy parameters, safety and quality of life
20 Recruiting centres and contact details Date study open: Sep-2011 Planned accrual: Global: 150 patients Prof Martyn Caplin, Royal Free Hospital, London Prof. Nick Reed, Beatson Oncology Centre, Glasgow Dr Martin Weickert, University Hospital Coventry and Warwickshire
21 VIBRANT Phase I Trial of Vandetanib Combined With 131I-mIBG to Treat Patients With Advanced Phaeochromocytoma and Paraganglioma (VIBRaNT) Registration: NCT Open (recruiting) Open (in follow-up) Due to be opened Inclusion/exclusion criteria and study schema Inclusion criteria Histopathological/cytological diagnosis of advanced phaechromocytoma or paraganglioma or R1 resection post original surgical debulking Positive 123I-mIBG diagnostic scan Stable blood pressure (<140/90mmHg), if appropriate, on anti-hypertensive therapy No previous systemic therapy for advanced or metastatic disease Measurable disease (RECIST v1.1) WHO performance status 0 or 1 Adequate bone marrow and renal function Exclusion criteria Patients undergoing current treatment with curative intent Refractory nausea and vomiting, chronic gastrointestinal disease or significant bowel resection that would preclude adequate absorption Significant cardiac comorbidity Primary endpoint: Dose limiting toxicity for the combination of Vandetanib and 131ImIBG Secondary endpoints: response rate, PFS and toxicity profile
22 Recruiting centres and contact details Date study open: Jan-2014 Planned accrual: Global: 18 patients Dr Debashis Sarker, King s College, London Dr Christina Thirlwell, Royal Free, London Dr Was Mansoor, The Christie NHS Foundation Trust, Manchester [email protected] [email protected] [email protected]
Ching-Yao Yang, Yu-Wen Tien
 Ching-Yao Yang, Yu-Wen Tien Division of General Surgery, Department of Surgery, National Taiwan University Hospital Oct-30-2010 Pancreatic NET have poorer prognosis when presence of liver metastases at
Ching-Yao Yang, Yu-Wen Tien Division of General Surgery, Department of Surgery, National Taiwan University Hospital Oct-30-2010 Pancreatic NET have poorer prognosis when presence of liver metastases at
NOUVEAUTES THERAPEUTIQUES DANS LES TUMEURS NEUROENDOCRINES DIGESTIVES (Radiothérapie vectorisée et loco-régionale exclue) Philippe RUSZNIEWSKI
 NOUVEAUTES THERAPEUTIQUES DANS LES TUMEURS NEUROENDOCRINES DIGESTIVES (Radiothérapie vectorisée et loco-régionale exclue) Réunion APRAMEN, Paris, 2 février 2013 Philippe RUSZNIEWSKI Pôle des Maladies de
NOUVEAUTES THERAPEUTIQUES DANS LES TUMEURS NEUROENDOCRINES DIGESTIVES (Radiothérapie vectorisée et loco-régionale exclue) Réunion APRAMEN, Paris, 2 février 2013 Philippe RUSZNIEWSKI Pôle des Maladies de
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Novel Radionuclide Therapies in Oncology : Promising Area in the Field of Nuclear Medicine
 Novel Radionuclide Therapies in Oncology : Promising Area in the Field of Nuclear Medicine Erik Mittra, MD, PhD Clinical Assistant Professor Radiology / Nuclear Medicine Objectives / Outline 1. Understand
Novel Radionuclide Therapies in Oncology : Promising Area in the Field of Nuclear Medicine Erik Mittra, MD, PhD Clinical Assistant Professor Radiology / Nuclear Medicine Objectives / Outline 1. Understand
Harmony Clinical Trial Medical Media Factsheet
 Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Riociguat Clinical Trial Program
 Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT )
 BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT ) Protocol Code Tumour Group Contact Physician UGIPNSUNI Gastrointestinal Dr. Hagen
BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT ) Protocol Code Tumour Group Contact Physician UGIPNSUNI Gastrointestinal Dr. Hagen
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Gastrointestinal and pancreatic neuroendocrine tumours. Dr. med. Henrik Csaba Horváth
 Gastrointestinal and pancreatic neuroendocrine tumours Dr. med. Henrik Csaba Horváth What is the definition of neuroendocrine tumours? Neuroendocrine tumours are neoplastic lesions, composed either by
Gastrointestinal and pancreatic neuroendocrine tumours Dr. med. Henrik Csaba Horváth What is the definition of neuroendocrine tumours? Neuroendocrine tumours are neoplastic lesions, composed either by
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics
 Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
REF/2011/06/002450 CTRI Website URL - http://ctri.nic.in
 Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 07:52:01 GMT) CTRI Number Last Modified On 08/07/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 07:52:01 GMT) CTRI Number Last Modified On 08/07/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Mechanism Of Action of Palbociclib & PFS Benefit
 A Phase II Randomized Controlled Trial of Palbociclib & Tamoxifen/Fulvestrant in Postmenopausal Women and Men With Hormone-Receptor Positive, HER2- Negative Metastatic Breast Cancer (MBC) Protocol Chair:
A Phase II Randomized Controlled Trial of Palbociclib & Tamoxifen/Fulvestrant in Postmenopausal Women and Men With Hormone-Receptor Positive, HER2- Negative Metastatic Breast Cancer (MBC) Protocol Chair:
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial
 Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Peptide receptor radionuclide treatment of neuroendocrine tumours
 Peptide receptor radionuclide treatment of neuroendocrine tumours Jann Mortensen Dept. of Clinical Physiology, Nuclear Medicine & PET Neuroendocrine Tumor Center of Excellence Rigshospitalet University
Peptide receptor radionuclide treatment of neuroendocrine tumours Jann Mortensen Dept. of Clinical Physiology, Nuclear Medicine & PET Neuroendocrine Tumor Center of Excellence Rigshospitalet University
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ
 OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ Study Overview Inhibition of poly(adenosine diphosphate [ADP]-ribose) polymerase
OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ Study Overview Inhibition of poly(adenosine diphosphate [ADP]-ribose) polymerase
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Kanıt: Klinik çalışmalarda ZYTIGA
 mkdpk de Sonunda Gerçek İlerleme! Kanıt: Klinik çalışmalarda ZYTIGA Dr. Sevil Bavbek 5. Türk Tıbbi Onkoloji Kongresi Mart 214, Antalya Endocrine therapies Adrenals Testis Abiraterone Orteronel Androgen
mkdpk de Sonunda Gerçek İlerleme! Kanıt: Klinik çalışmalarda ZYTIGA Dr. Sevil Bavbek 5. Türk Tıbbi Onkoloji Kongresi Mart 214, Antalya Endocrine therapies Adrenals Testis Abiraterone Orteronel Androgen
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development
 Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes
 Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Aktualne reguły leczenia nowotworów neuroendokrynnych przewodu pokarmowego
 Aktualne reguły leczenia nowotworów neuroendokrynnych przewodu pokarmowego Current treatment options for neuroendocrine GI tumors Archil Aladashvili, GE Current treatment options for neuroendocrine GI
Aktualne reguły leczenia nowotworów neuroendokrynnych przewodu pokarmowego Current treatment options for neuroendocrine GI tumors Archil Aladashvili, GE Current treatment options for neuroendocrine GI
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Cirrhosis and HCV. Jonathan Israel M.D.
 Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
Drug/Drug Combination: Bevacizumab in combination with chemotherapy
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
Management of low grade glioma s: update on recent trials
 Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY. Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds
 NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
Cancer treatment and diabetes
 Cancer treatment and diabetes Dr Daniel Morganstein Consultant Endocrinologist, 1 2 Diabetes and cancer Cancer and its treatment also poses challenges to managing diabetes Surgery Altered appetite Cachexia
Cancer treatment and diabetes Dr Daniel Morganstein Consultant Endocrinologist, 1 2 Diabetes and cancer Cancer and its treatment also poses challenges to managing diabetes Surgery Altered appetite Cachexia
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Trials in Elderly Melanoma Patients (with a focus on immunotherapy)
 Trials in Elderly Melanoma Patients (with a focus on immunotherapy) Where we were Immunotherapy Trials: past and present Relevance for real world practice Where we are SIOG October 2012 James Larkin FRCP
Trials in Elderly Melanoma Patients (with a focus on immunotherapy) Where we were Immunotherapy Trials: past and present Relevance for real world practice Where we are SIOG October 2012 James Larkin FRCP
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Ga-68 PET. John Buscombe
 Ga-68 PET John Buscombe Introduction n Ga-68 a new radionuclide n PET based n Chemistry can be difficult n Used primarily with DOTATATE n Now also used with other agents In-111 octreotide v F-18 FDG in
Ga-68 PET John Buscombe Introduction n Ga-68 a new radionuclide n PET based n Chemistry can be difficult n Used primarily with DOTATATE n Now also used with other agents In-111 octreotide v F-18 FDG in
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Thursday, November 3, 2005
 Thursday, November 3, 2005 8:30-10:30 a. m. Gastric Tumors, Session 1 Chairman: P. Ruszniewski, Clichy, France 9:00-9:30 a. m. Working Group Sessions Pathology and Genetics Group leaders: G. Rindi, Parma,
Thursday, November 3, 2005 8:30-10:30 a. m. Gastric Tumors, Session 1 Chairman: P. Ruszniewski, Clichy, France 9:00-9:30 a. m. Working Group Sessions Pathology and Genetics Group leaders: G. Rindi, Parma,
Neuroendocrine Tumors
 Neuroendocrine Tumors Neuroendocrine tumors arise from cells that release a hormone in response to a signal from the nervous system. Neuro refers to the nervous system. Endocrine refers to the hormones.
Neuroendocrine Tumors Neuroendocrine tumors arise from cells that release a hormone in response to a signal from the nervous system. Neuro refers to the nervous system. Endocrine refers to the hormones.
Foundational Issues Related to Immunotherapy and Melanoma
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Newsletter. WntResearch AB, Medeon Science Park, Per Albin Hanssons väg 41, 205 12 Malmö, Sweden. Primary Objective:
 Newsletter This resume of the results from the phase 1 study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
Newsletter This resume of the results from the phase 1 study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
Re: LUMIGAN 0.03% (bimatoprost ophthalmic solution 0.03%) and LUMIGAN 0.01% (bimatoprost ophthalmic solution 0.01%)
 Allergan Ltd, 1st Floor, Marlow International, Parkway, Marlow, Bucks SL7 1YL Tel: (01628) 494444 Facsimile: (01628) 494449 Ref No: UK15-000677 Mandeep Allingham NW Surrey CCG February 18, 2015 Dear Ms
Allergan Ltd, 1st Floor, Marlow International, Parkway, Marlow, Bucks SL7 1YL Tel: (01628) 494444 Facsimile: (01628) 494449 Ref No: UK15-000677 Mandeep Allingham NW Surrey CCG February 18, 2015 Dear Ms
Clinical Spotlight in Breast Cancer
 2015 European Oncology Congress in Vienna Clinical Spotlight in Breast Cancer Reference Slide Deck Abstract #1815 Impact of Palbociclib Plus Fulvestrant on Global QOL, Functioning, and Symptoms Compared
2015 European Oncology Congress in Vienna Clinical Spotlight in Breast Cancer Reference Slide Deck Abstract #1815 Impact of Palbociclib Plus Fulvestrant on Global QOL, Functioning, and Symptoms Compared
Anticancer Drug Clinical Trial Guideline. (version 2.0)
 Anticancer Drug Clinical Trial Guideline (version 2.0) May, 2007 1 Table of Contents 1. Introduction... 4 2. The Overall Consideration of Clinical Trial... 4 2.1 The Selection of Patients... 4 2.2 Dose
Anticancer Drug Clinical Trial Guideline (version 2.0) May, 2007 1 Table of Contents 1. Introduction... 4 2. The Overall Consideration of Clinical Trial... 4 2.1 The Selection of Patients... 4 2.2 Dose
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
18.5 Percent Overall Response Rate Observed in Pembrolizumab-Treated Patients with this Aggressive Form of Breast Cancer
 News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics
 Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research
Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research
Brigham and Women s Hospital, Boston, MA, USA; 2 Verastem, Inc., Boston, MA, USA
 Determination of Biomarker Response in a Phase II Window of Opportunity Study of Defactinib (VS 6063), a Focal Adhesion Kinase (FAK) Inhibitor, in Patients with Resectable Malignant Pleural Mesothelioma
Determination of Biomarker Response in a Phase II Window of Opportunity Study of Defactinib (VS 6063), a Focal Adhesion Kinase (FAK) Inhibitor, in Patients with Resectable Malignant Pleural Mesothelioma
ESCMID Online Lecture Library. by author
 Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Metastatic Breast Cancer 201. Carolyn B. Hendricks, MD October 29, 2011
 Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Amylase and Lipase Tests
 Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C
 FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
In ELOQUENT-2, Empliciti was evaluated in patients who had received one to three prior
 - First and only immunostimulatory antibody approved in the European Union for multiple myeloma - Accelerated assessment and approval based on long-term data from ELOQUENT-2, which evaluated Empliciti
- First and only immunostimulatory antibody approved in the European Union for multiple myeloma - Accelerated assessment and approval based on long-term data from ELOQUENT-2, which evaluated Empliciti
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center
 What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
NP/PA Clinical Hepatology Fellowship Summary of Year-Long Curriculum
 OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
Series 1 Case Studies Adverse Events that Represent Unanticipated Problems: Reporting Required
 Welcome! This document contains three (3) series of Case Study examples that will demonstrate all four OHSU reporting categories (#1 4) as well as examples of events that are considered not reportable.
Welcome! This document contains three (3) series of Case Study examples that will demonstrate all four OHSU reporting categories (#1 4) as well as examples of events that are considered not reportable.
PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including anaplastic, gliosarcoma and glioblastoma multiforme)
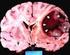 Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products
 Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
2. Background This drug had not previously been considered by the PBAC.
 PUBLIC SUMMARY DOCUMENT Product: Ambrisentan, tablets, 5 mg and 10 mg, Volibris Sponsor: GlaxoSmithKline Australia Pty Ltd Date of PBAC Consideration: July 2009 1. Purpose of Application The submission
PUBLIC SUMMARY DOCUMENT Product: Ambrisentan, tablets, 5 mg and 10 mg, Volibris Sponsor: GlaxoSmithKline Australia Pty Ltd Date of PBAC Consideration: July 2009 1. Purpose of Application The submission
Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases
 I Congresso de Oncologia D Or July 5-6, 2013 Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University
I Congresso de Oncologia D Or July 5-6, 2013 Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
CANCER RESEARCH UK STRATIFIED MEDICINE PROGRAMME IAN WALKER PHD, MBA HEAD OF STRATIFIED MEDICINE
 CANCER RESEARCH UK STRATIFIED MEDICINE PROGRAMME IAN WALKER PHD, MBA HEAD OF STRATIFIED MEDICINE Agenda Introduction to Cancer Research UK CRUK Stratified Medicine Programme Key Challenges and Lessons
CANCER RESEARCH UK STRATIFIED MEDICINE PROGRAMME IAN WALKER PHD, MBA HEAD OF STRATIFIED MEDICINE Agenda Introduction to Cancer Research UK CRUK Stratified Medicine Programme Key Challenges and Lessons
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED
 ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Non-Functioning Pancreatic Neuroendocrine Tumours
 Non-Functioning Pancreatic Neuroendocrine Tumours NET Patient Foundation Non-Functioning Pancreatic Neuroendocrine Tumours This booklet is intended to provide patients and their carers with information
Non-Functioning Pancreatic Neuroendocrine Tumours NET Patient Foundation Non-Functioning Pancreatic Neuroendocrine Tumours This booklet is intended to provide patients and their carers with information
Hepatocellular Carcinoma (HCC)
 Abhishek Vadalia Introduction Chemoembolization is being used with increasing frequency in the treatment of solid hepatic tumors such as Hepatocellular Carinoma (HCC) & rare Cholangiocellular Carcinoma
Abhishek Vadalia Introduction Chemoembolization is being used with increasing frequency in the treatment of solid hepatic tumors such as Hepatocellular Carinoma (HCC) & rare Cholangiocellular Carcinoma
Digital Health: Catapulting Personalised Medicine Forward STRATIFIED MEDICINE
 Digital Health: Catapulting Personalised Medicine Forward STRATIFIED MEDICINE CRUK Stratified Medicine Initiative Somatic mutation testing for prediction of treatment response in patients with solid tumours:
Digital Health: Catapulting Personalised Medicine Forward STRATIFIED MEDICINE CRUK Stratified Medicine Initiative Somatic mutation testing for prediction of treatment response in patients with solid tumours:

 Understanding Clinical Trials HR =.6 (CI :.51.7) p
Understanding Clinical Trials HR =.6 (CI :.51.7) p