Antifungals and their use in veterinary ophthalmology
|
|
|
- Phillip Price
- 9 years ago
- Views:
Transcription
1 Vet Clin Small Anim 34 (2004) Antifungals and their use in veterinary ophthalmology Marnie M. Ford, PhD, DVM Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, University of Missouri, 379 East Campus Drive, Columbia, MO 65211, USA Mycotic infections can be divided into those that result in superficial disease and those that result in systemic disease. Factors determining the ocular tissue predilection and type of disease caused by a particular fungal organism include fungal species characteristics and host predisposition (large ocular surface area [1], prominent eyes [1], local or systemic immunoprotection [2], and geographic location [3]). Additionally, superficial corneal disease may be exacerbated by exposure to vegetative material (hay, grasses, shavings, and straw) and dust [2], whereas concurrent illness or immunocompromise may predispose to systemic disease. Although horses are most commonly affected with ocular surface fungal infections (keratomycosis), dogs and cats are predisposed to internal ocular infections (anterior uveitis, chorioretinitis, retinal detachment, and secondary glaucoma) and systemic disease. The aim of this article is to provide a general outline of the current knowledge of antifungal agents in veterinary ophthalmology. Few agents are approved by the US Food and Drug Administration (FDA) for the treatment of companion animal fungal infections. Therefore, extralabel use of products approved for human beings and compounding of specific agents may be necessary, particularly in the treatment of keratomycoses. The biology of fungi The fungal kingdom comprises yeasts, molds, fungal rusts, and mushrooms [2,4]. Fungi are heterotrophic, nonmotile, multicellular, eukaryotic organisms with a definitive cell wall and no chlorophyll [2,4]. Within the fungal kingdom, pathogens can be divided into three groups: multinucleate address: [email protected] /04/$ - see front matter Ó 2004 Elsevier Inc. All rights reserved. doi: /j.cvsm
2 670 M.M. Ford / Vet Clin Small Anim 34 (2004) septate (distinct divisions between cellular elements) filamentous fungi, nonseptate filamentous fungi, and yeasts [5]. Dimorphic species are typified by manifesting a single morphism under specific environmental conditions (eg, the yeast form in the vertebrate host tissue and a hyphal/mycelial form in vitro) [4]. Examples of dimorphic organisms include Blastomyces dermatitidis, Coccioides immitis, and Histoplasma capsulatum. The mold forms give rise to spores that germinate and produce slender, filamentous, branched hyphae that may be septate or nonseptate [2,4]. The mycelial form, present in soil or decaying organic material, is composed of a collection of hyphae. Mycelia produce infective spores that are responsible for inoculating vertebrate tissues [4]. Soil is considered the true reservoir for many fungal organisms because it is the site for essential phases of development [4]. Each hypha has a surrounding cell wall made up of chitins, glucans, and mannans. Chitin is a structural polysaccharide (N-acetylglucosamine) that is absent in the vertebrate systems [2]. The cell wall contains complex polysaccharides and glycoproteins, and the nuclear envelope is a porous double membrane containing abundant RNA [4]. Within the cell wall, the plasma membrane contains ergosterol, a cell membrane sterol that is frequently targeted by antifungal agents [5]. Ergosterol regulates permeability of the cell membrane and the activity of membrane-bound enzymes [6,7]. Chitin synthesis is stimulated by low ergosterol content and inhibited by a high concentration. Patchy chitin formation occurs as a result of ergosterol biosynthesis inhibition [6]. Ocular manifestations of fungal infection Keratomycosis Keratomycosis is a serious sight-threatening disease in all species; however, species differences exist. It is most commonly reported in the horse and is rare in dogs and cats [8 10]; therefore, information and experience regarding veterinary treatment are based predominantly on literature reports of equine keratomycoses. Fungi are a normal component of the equine conjunctival microflora [1,11,12] but become pathogenic after corneal injury [2,13]. Topical corticosteroids exacerbate keratomycosis [13 18]. After invasion of compromised corneal epithelium, fungi migrate to the deep stroma by hyphal tip elongation. Proliferation occurs toward the glycosaminoglycan (GAG)-rich environment of the deep stroma adjacent to Descemet s membrane. This allows the organism to escape natural host ocular surface immune responses. Fungal pathogens or budding yeasts that infect the equine cornea are usually opportunistic [14]. A study conducted over a 10-year period found isolates from 13 different genera and 20 different species [19]. Aspergillus [14] and Fusarium [14] organisms are the most frequently isolated filamentous fungi [19,20]. Others, including Penicillium, Cladosporium, Mucor, Rhizopus,
3 M.M. Ford / Vet Clin Small Anim 34 (2004) Candida, Cylindrocarpon, Pseudallescheria, and dematiaceous Alternaria and Culveria organisms, are occasionally isolated [14]. Diagnosis of keratomycosis should be based on history, results of ophthalmic examination, and cytologic findings. Specific antifungal therapy can be initiated after isolation of pathogenic fungi or may be empiric based on known prevalence of unique ocular fungi in specific geographic areas [3]. Frequent topical applications of antibiotics and antifungals in combination with aggressive daily corneal epithelial scrapings are reported to have successful outcomes [21]. Surgical intervention, including lamellar keratectomy with conjunctival grafting or penetrating keratoplasty, is indicated in cases of middle to deep stromal involvement. Surgical management of keratomycoses is discussed elsewhere [15,22]. Ocular manifestations of systemic mycoses Unlike horses, small companion animals are most commonly affected with systemic infections with ocular manifestations, such as uveitis, chorioretinitis, retinal detachment, and secondary glaucoma [4]. The reported frequency of ocular manifestations in dogs is 20% to 50% [23]. In addition to systemic administration of antifungal medication(s), specific therapy for anterior uveitis or secondary glaucoma is often required [23 26]. The four most common fungal organisms that cause systemic mycoses with ocular manifestations include B dermatitidis, C immitis, Cryptococcus neoformans, and H capsulatum. Limited therapeutic options exist for systemic mycoses. Most drugs are not approved by the FDA for use in dogs and cats. Drawbacks of systemic antifungal therapy include expense and toxicity of certain drug regimens. Classes of antifungal drugs Antifungals may be categorized into chemical groups based on structure and mode of action. Classes of antifungal agents used in veterinary ophthalmology include polyenes, azoles, allylamines, lipopeptides, and pyrimidines. Other substances used for local control of fungal infections include silver sulfadiazine (SSD) and povidone iodine. The mode of action may be divided into those that target fungal cell membrane synthesis (azoles, allylamines, lipopeptides, and chitin synthase inhibitors) or function (polyenes and lipopeptides) and those that target nucleic acid synthesis (pyrimidines and SSD). The classes of antifungal agents most commonly used in the treatment of equine keratomycoses are the azoles (miconazole, itraconazole, and fluconazole) and the polyene agent natamycin. The most commonly used classes for treatment of canine and feline systemic mycoses are the polyenes (amphotericin B [AMB] and lipid-complexed AMB) and the azoles (ketoconazole, itraconazole, and fluconazole).
4 672 M.M. Ford / Vet Clin Small Anim 34 (2004) Pathogenic mechanisms, growth requirements, and antifungal drug susceptibility vary considerably among fungal pathogens [27,28]. Initial treatment of patients with suspected fungal keratitis may be empiric, and choice of therapy is defined by previous clinical experience, available drugs, and financial constraints [14]. Because of risk of toxicity associated with systemic administration of antifungal agents, development of safer broadspectrum antifungal antibiotics with greater potency is ongoing. Progress in development of new agents has been slow [29,30], because fungi are eukaryotic organisms; therefore, agents that inhibit protein, RNA, or DNA biosynthesis in fungi have a greater potential for host toxicity [31,32]. In addition, the incidence of life-threatening fungal infections has been reported as being too low to warrant aggressive product development [33]. The range of antifungal drugs available for systemic use has been limited to a few agents, the most effective of which (AMB) is highly nephrotoxic [34]. Polyenes Polyene macrolide antibiotics, the first major group of antifungal agents to be discovered [35], are a group of structurally similar products of Streptomyces spp. Since the discovery of nystatin (previously known as fungicidin) in 1950 [36], more than 60 members of the class have been described [35]; however, only AMB, natamycin, and nystatin are of practical interest for the treatment of ocular fungal infections [35,37]. Polyenes are unstable insoluble chemicals that are poorly absorbed, with limited penetration into the eye, cerebrospinal fluid (CSF), and joint capsule [35,37 39]. Natamycin, the only topical antifungal agent approved for use on the eye and the only commercially available drug for treatment of ocular fungal keratitis, penetrates the intact cornea poorly [40]. Gastrointestinal absorption of AMB is minimal; therefore, parenteral administration is recommended [41]. After intravenous administration, AMB is highly protein bound and redistributes from the blood to the tissues; however, central nervous system (CNS) penetration is poor. Less toxic formulations of AMB and nystatin have been developed with the use of lipid incorporation, which has been reported to enhance efficacy in treating ocular disease [37,42]. The exact metabolic pathways of AMB are not known; however, a biphasic elimination occurs with an initial half-life of 2 to 4 days, followed by a terminal half-life of 15 days [43], with minimal elimination occurring via the hepatic and renal pathways [41]. Mechanism of action Polyene antibiotics are fungicidal [5,44] with several proposed mechanisms that have not been fully elucidated. The most widely accepted mechanism of action is membrane barrier disruption. Susceptible cells (fungi, protozoa, and mammalian cells) selectively and irreversibly bind polyenes to ergosterol, the principal sterol in the plasma membrane of fungi [35,45,46]. Binding of
5 M.M. Ford / Vet Clin Small Anim 34 (2004) polyenes to ergosterol results in altered membrane permeability [46] and inhibition of cytochrome P-450 and the electron transport chain [39]. As a consequence of increased membrane permeability, leakage of potassium and essential cytoplasmic metabolites [47 49] is followed by impairment of concentrating mechanisms [46] and loss of ammonium, inorganic phosphate, low-molecular-weight carboxylic acids, phosphate esters, nucleotides, and proteins [46,50 52]. Cation leakage secondarily inhibits aerobic and anaerobic glycolysis and respiration [46]. Replacement of lost intracellular cations by hydrogen ions results in a decrease in internal ph that, when less than 5.5, causes lysosome disruption, autolysis, and cell death [46]. The susceptibility or resistance of fungi to these drugs is determined by the relative amounts of sterol in the cell membrane; the most susceptible cells have high sterol/phospholipid ratios, and the least susceptible have low ratios [46]. Spectrum Polyene antibiotics have the broadest spectrum of antifungal activity of any of the clinically available agents [44]. Specific filamentous fungi include most species of Aspergillus [5,18,35,53 58], yeast (especially Candida sp [5,44,46,55,59,60]) except for Candida lusitaniae [44,46]), Zygomycetes (Mucor and Rhizopus [5]), Cryptococcus [46,55], Blastomyces, Coccidioides, Histoplasma [5], Paracoccidioides brasiliensis, Sporotrichum sp, Torulopsis, (Candida) glabrata [5,44], and Sporothrix schenckii [5,46]. Trichosporon beigelii and Pseudoallescheria boydii are often resistant [33,61 63]. Activity against Prototheca [5], Curvularia, Alternaria, Wangiella, and Cladosporium varies [64]. In addition, natamycin is also active against Trichophyton sp, Acremonium sp, and P boydii [37], whereas Aspergillus species are frequently resistant [37]. Nystatin is active against Cryptococcus [5,46], Prototheca [5], some filamentous fungi [5], some dimorphic fungi [5], and Trichophyton [46]. The liposomal formulation of nystatin seems to be as active as free nystatin [65]. Systemic doses and ophthalmic preparations of the commonly used polyene antibiotics are listed in Table 1. Adverse effects The plasma membranes of mammalian cells contain sterols in the form of cholesterol; therefore, all polyenes are toxic to mammalian cells to some degree [35]. This toxicity may be decreased by the higher affinity of the polyenes for ergosterol in fungal cells than for cholesterol in mammalian cells [33,44,52,66,67]. Amphotericin B. Nephrotoxicity after parenteral administration of AMB remains the greatest adverse effect associated with the polyene antibiotics [44,55,68 70]. The proposed mechanism of action is renal vasoconstriction with a subsequent reduction in glomerular filtration rate or direct renal epithelial cell toxicity [40]. The occurrence of nephrotoxicity may be reduced by pretreatment with 0.9% saline intravenously [71] or concurrent
6 Table 1 Systemic doses and ophthalmic preparations of antifungal drugs Systemic route (IV, SQ, PO) AMB (Fungizone; IV: Canine: dose, IV a, 3 times per week Apothecon) (cumulative dose) Blastomycosis: 0.5 mg/kg (4 6 mg/kg) Histoplasmosis: mg/kg (5 10 mg/kg) Cryptococcus: mg/kg (4 10 mg/kg) b Coccidiomycosis: mg/kg (8 11 mg/kg) Feline: dose, IV a, 3 times per week (cumulative dose) Blastomycosis: 0.25 mg/kg (4 mg/kg) Cryptococcosis: mg/kg (4 10 mg/kg) b Histoplasmosis: mg/kg (4 8 mg/kg) SQ: Canine: mg/kg/d Feline: mg/kg/d in 400 ml of 5% dextrose or 0.45% saline c (cumulative dose: 4 10 mg/kg) AMB liposomal preparation (AmBisome; Fujisawa) (Albecet; The Liposome Co.) Natamycin (Natacyn; Alcon) IV: Canine: Blastomycosis/cryptococcosis: Albecet 1 mg/kg, q48 hours (cumulative dose: 8 12 mg/kg) No reported experience with other lipid-based formulations in animals (eg, cholesteryl, Amphotec; colloidal, Amphocil; liposomal, AmBiosome), but recommended human IV dosage might be appropriate in dogs Ophthalmic route Topical: 5 mg/ml = 0.5% colloidal suspension 1. Inject 10 ml of sterile water or 5% dextrose solution into the bottle with dry AMB, 50 mg; AMB is incompatible with saline or other electrolyte solutions 2. Shake until transparent, refrigerate, do not filter Subconjunctival: mg Intravitreal: 5 lg Intracameral: 25 lg in 0.05 ml of distilled H 2 O 7.4 lg in 0.1 ml of distilled H 2 O Topical: 1 drop of 5% suspension, q1 2 hours initially, then decreasing to 6 8 times daily after a few days for equine fungal keratomycosis 674 M.M. Ford / Vet Clin Small Anim 34 (2004)
7 Nystatin (Mycostatin; Apothecon) Miconazole (Monistat; Janssen) Ketoconazole (Nizoral; Janssen) Too toxic for parenteral administration Not absorbed after oral administration IV: 20 mg/kg of BW PO: Canine: dose f, q12 hours months or 30 day after resolution Blastomycosis: 5 15 mg/kg d q12 hours, 3 months Histoplasmosis: 10 mg/kg q12 24 hours, 3 months Cryptococcosis: 10 mg/kg de q12 24 hours, 3 months Coccidiomycosis: 5 10 mg/kg q12 hours, 8 12 months Feline: Blastomycosis: 10 mg/kg q12 hours d, 3 months Histoplasmosis: 10 mg/kg q12 24 hours, 3 months Cryptococcosis: 10 mg/kg de q12 24 hours, 3 months Coccidiomycosis: 50 mg/cat q12 24 hours, 12 months Topical: Eyedrop suspension of pure nystain, 100,000 IU, in 5 ml of sterile isotonic, isohydric phosphate buffer solution (20 ml of NaH 2 PO 4 (8.0 g in 1000 ml of H 2 O) and 80 ml NaH 2 PO 4 (9.47 g in 1000 ml of H 2 O)) are mixed; NaCl (0.44 g) is added, and the solution is sterilized Topical: Equine: 1% IV miconazole (Monistat, 10 mg/ml) q4 6 hours Miconazole 2% dermatologic creams (Conofite) q6 12 hours for up to 3 weeks without adverse effects Subconjunctival: 1% IV solution, not recommended for use in horses Subtenons: 5 10 mg has been used in horses Intracameral: miconazole, 0.1 mg, with 0.1 ml of sterile 0.9% NaCl Topical: 1% 2% solution from the oral tablets (continued on next page) M.M. Ford / Vet Clin Small Anim 34 (2004)
8 Table 1 (continued ) Itraconazole (Sporonox; Janssen) Fluconazole (UK 49,858) (Diflucan; Pfizer) Econazole (Spectazole; Ortho Dermatological) Systemic route (IV, SQ, PO) PO: Canine f Blastomycosis: 5 mg/kg/d, 2 months 5 mg/kg q12 hours for 5 days, then q24 hours h Histoplasmosis: 5 mg/kg q12 hours, 4 6 months h Coccidiomycosis: 5 mg/kg q12 hours, 12 months Feline f Blastomycosis: 5 10 mg/kg q12 hours, 2 months Histoplasmosis: 5 mg/kg q12 hours, 4 6 months h Cryptococcosis: 5 10 mg/kg q12 hours, 6 10 months g 20 mg/kg q24 hours for 6 10 months g mg/cat q12 24 hours up to 12 months PO: Canine: dose, frequency, duration Blastomycosis: 5 mg/kg q12 hours, 2 months g Histoplasmosis: mg/kg q12 24 hours, 4 6 months g Cryptococcosis: 5 15 mg/kg q12 24 hours, 6 12 months g Coccidiomycosis: 5 mg/kg q12 hours, up to 12 months Feline: dose, frequency, duration Histoplasmosis: mg/kg q12 24 hours, 4 6 months g Cryptococcosis: 5 15 mg/kg q12 24 hours, 6 12 months g Coccidiomycosis: mg/cat q12 24 hours, up to 12 months Ophthalmic route Topical: Equine: 1% itraconazole with 30% dimethyl sulfoxide ointment q4 6 hours = 7.9 lg/g corneal concentration of itraconazole, mean duration of treatment = 34.6 days Topical: 0.2% saline-based injectable solution Subconjunctival: 0.2% saline-based injectable solution Intravitreal: 100 lg/0.1 ml Topical: 1% solution 1% dermatologic cream 676 M.M. Ford / Vet Clin Small Anim 34 (2004)
9 Clotrimazole (Mycelex; Bayer) (Canesten; Bayer) Thiabendazole (Mintezol; Merck) Silver sulfadiazine (Silvadene; Marion) Povidone iodine (Betadine 10%; Purdue Frederick) Flucytosine (Ancobon) PO: 25 mg/kg/d SQ: Canine: Cryptococcosis: 50 mg/kg q6 8 hours, 1 2 months Feline: Cryptococcosis: mg/cat q6 12 hours, 1 9 months PO: Canine: Cryptococcosis: 50 mg/kg q6 hours dg Feline: Cryptococcosis: 200 mg/kg/d divided q6 hours dg Topical: concentrations greater than 10 lg/ml are fungicidal, 1% 2% dermatologic cream or the topical preparation in 1% arachis oil has been shown to be useful Topical: 1% 4% solution of oral worming mixture and artificial tears or water Topical: 1% cream, q4 hours Topical: 0.2% (1:50 solution) Topical: 1% solution Abbreviations: AMB, amphotericin B; BW, body weight; IV, intravenous; PO, orally; SQ, subcutaneous. a Measure serum blood urea nitrogen (BUN) and urine sediment for evidence of kidney damage before administration of each dose. On day 1, the total dose is diluted to 5% dextrose 20 ml and 5 ml is given; if no acute anaphylactic response develops in 1 minute, the remainder is given over 45 seconds. Thereafter the total dose is given over 1 minute in 5% dextrose 20 ml for 6 12 weeks, 3 times a week. If BUN exceeds 50 mg/dl, the dose is discontinued or reduced 25% 50% until BUN falls below 40 mg/dl. Treatment of dogs and cats should be continued for a minimum of 60 days or at least 1 month beyond clinical or radiographic resolution of clinical signs. b May be used in combination with flucytosine. c 2 3 times weekly. d With AMB initially. e After AMB/flucytosine for 4 6 months. f Take with food. g For 30 days beyond resolution. h For 60 days beyond resolution. Data from Refs. [5,24,35,41,44,63,73,76,78,85, ,119,132, ]. M.M. Ford / Vet Clin Small Anim 34 (2004)
10 678 M.M. Ford / Vet Clin Small Anim 34 (2004) administration of AMB with 5% dextrose over 1 to 5 hours [41,70]. The risk of nephrotoxicity is reduced approximately 8 to 10 times by the use of lipidcomplexed AMB drugs [40,44,72,73]; however, these drugs are significantly more expensive [41]. Other adverse effects include anorexia, vomiting, hypokalemia, distal renal tubular acidosis, hypomagnesemia, thrombophlebitis, cardiac arrhythmias, nonregenerative anemia, and fever [40]. In an attempt to delay absorption and reduce toxicity, subcutaneous administration of higher doses of AMB has been used [74,75]. Topical, subconjunctival, or intraocular administration of AMB has been associated with local mild to severe irritation of the tissues [76,77], transitory and reversible iritis, and slight clouding of the lens without permanent sequelae [78]. If injected subconjunctivally, AMB should only be administered after appropriate dilution (see Table 1). Natamycin. Topical administration of the 5% commercial natamycin suspension is nontoxic; however, low-grade inflammation and local irritation [5] may develop with prolonged use [35]. Toxicity prohibits subconjunctival or intraocular administration. Nystatin. Occasional gastrointestinal upset has been reported when nystatin is administered systemically at high doses [40]. Nystatin may be compounded for topical ophthalmic use (see Table 1 for formulation). Resistance AMB resistance is rare and slow to develop [38]. Known cases of resistance to polyene antibiotics are not commonly reported (see references [79 83]). Azoles Azoles are the most widely used of the antifungal agents [37] and are divided into two subclasses: imidazoles and triazoles. Imidazoles include ketoconazole, miconazole, bifonazole, butoconazole, clotrimazole, econazole, enilconazole, fenticonazole, isoconazole, and parconazole. Triazoles include itraconazole, fluconazole, terconazole, and voriconazole. Azoles have activity against dermatophytes, Cryptococcus, Blastomyces, Histoplasma, Aspergillus, and Candida sp [40,84]. Azoles are water soluble with variable absorption after oral administration. Fluconazole, in contrast to itraconazole, is poorly protein bound with good CSF penetration. Renal elimination occurs after fluconazole administration, whereas itraconazole is eliminated in the bile. Fluconazole has better penetration into the eye and CNS than itraconazole [85] and should be considered with CNS involvement or in individuals refractory to treatment with AMB and itraconazole [41]. The spectrum of activity for fluconazole includes many species of Candida and Cryptococcus as well as dimorphic fungi, such as Histoplasma, Blastomyces,
11 M.M. Ford / Vet Clin Small Anim 34 (2004) and Coccioides; however, it has no efficacy against other fungi, such as Aspergillus. Mechanism of action Azoles are fungistatic agents used to treat ophthalmic mycoses [46]. Antifungal activity arises from a complex multimechanistic process initiated by the inhibition of ergosterol biosynthesis and the disturbance of lipid organization in cell membranes [86 88]. Specifically, azoles inhibit the fungal cytochrome P450 3A (CYP3A) enzyme lanosterol 14-a-demethylase. This prevents the conversion of lanosterol to ergosterol and disrupts the integrity of membrane-bound enzymes [5] and fungal cell membranes [46,89], which results in increased membrane permeability [35] and leakage of small ions, amino acids, and protein from the fungi [47]. Mammalian cells can be affected as well but can compensate for the temporary effects of the imidazoles by using dietary cholesterol [90]. Variations in affinity for the mammalian CYP3A receptor are the basis for drug drug interactions with other CYP3Adependent drugs, such as cyclosporine [91]. Azole inhibition of cytochrome function may also be the basis of interference with steroid biosynthesis [38]. These changes are reversible unless a high dose is administered [92]. Spectrum Azoles have a broad spectrum of activity against yeasts and filamentous fungi [5,14,35,76,90,93,94], including Coccidioides, Candida [95], Cryptococcus [96], Histoplasma sp [48,97], Paracoccidia, Paecilomyces lilacinus [98,99], Scopulariopsis brevicaulis [100], Aspergillus, Mucor, Fusarium sp [12,46,101, 102], Sporothrix, Alternaria, Blastomyces, Sporotrichum sp, and Prototheca [5]. Ketoconazole, considered to be the traditional treatment of choice for coccidiomycosis [41], is less effective than itraconazole, with lower response rates, higher relapse rates, and longer treatment periods in dogs [24,94]. Itraconazole has a similar spectrum of activity as fluconazole but includes Aspergillus and is not active against the Zygomycetes or Fusarium spp. Itraconazole is the treatment of choice for blastomycosis [41], with similar response and recurrence rates between dogs treated with AMB and itraconazole [103]. Itraconazole is also the treatment of choice for histoplasmosis [85,104]. In cats, itraconazole is more effective for histoplasmosis than ketoconazole, with fewer adverse effects [104]. The spectrum of thiabendazole includes Cladosporium, Fusarium, Penicillium, and Phialophora. Topical and systemic doses of the azole drugs are listed in Table 1. A limited spectrum of activity and resistance makes use of this drug uncommon. Adverse effects Topical formulations of the azoles (miconazole and econazole) are well tolerated [35,63]. Itraconazole (1%)/dimethyl sulfoxide (DMSO; 30%) ointment can be compounded with good results when applied to the cornea [40]. Toxicity is most commonly associated with intravenous administration
12 680 M.M. Ford / Vet Clin Small Anim 34 (2004) of miconazole because of the presence of the solubilizing agent required [5,47,49]. These effects are not seen with subcutaneous injections [105]. Oral administration of ketoconazole, itraconazole, and clotrimazole has been associated with inappetence and vomiting [5,35,90]. Skin changes have also been associated with azole administration and include pruritus, alopecia, reversible leukotrichia (ketoconazole) [90], and drug eruption (itraconazole and fluconazole) [75,106]. Cataracts [107] and hepatitis [90] have been associated with ketoconazole administration in dogs. Cortisol and testosterone suppression and increased progesterone concentrations in dogs have been associated with ketoconazole and itraconazole administration [5,108]; therefore, their use is contraindicated in pregnancy. Cats are more sensitive to ketoconazole and may develop anorexia, depression, weight loss, diarrhea, and fever [5]. Resistance Known cases of azole resistance are uncommonly reported (see references [79 83]). Pyrimidines (flucytosine, 5-fluorocytosine, 5-FC) Flucytosine, a fluorine analogue of a normal cell constituent cytosine, is water soluble and weakly protein bound, with oral absorption that is unaffected by acid [5]. Flucytosine has excellent tissue penetration, including into the CNS. Mechanism of action After oral administration, flucytosine is taken up into the cell by a cytosine permease and is rapidly deaminated into 5-fluorouracil (5-FU) by cytosine deaminase, a fungus-specific enzyme [40,109]. 5-FU has fungicidal and fungistatic properties. 5-FU can be converted to 5-fluoro-dUMP, which indirectly inhibits DNA synthesis (-cidal), or 5-fluoroUTP, which disrupts protein synthesis after incorporation into RNA (-static) [109]. Spectrum Flucytosine is principally active against strains of Cryptococcus and Candida [40] and is fungistatic against Aspergillus flavus and A fumigatus in laboratory animals (see Table 1 for doses) [76]. Adverse effects Reported adverse effects include gastrointestinal disturbances (nausea, vomiting, and diarrhea), bone marrow suppression (anemia, leukopenia, and thrombocytopenia), cutaneous eruption and rash primarily seen on the scrotum and nasal planum (dogs), oral ulceration, and increased levels of hepatic enzymes. Flucytosine should be used with extreme caution in patients with renal impairment, preexisting bone marrow disease, hepatic disease,
13 M.M. Ford / Vet Clin Small Anim 34 (2004) hematologic diseases, or treatment with other bone marrow suppressing drugs. A report of aberrant behavior and seizures in a cat without concurrent CNS infection has also been noted after flucytosine administration [40]. Resistance Resistance to flucytosine can develop quite rapidly, especially when it is used alone against Cryptococcus. Recommendations for the use of AMB, a synergistic drug, in combination with flucytosine have been made for the treatment of cryptococcosis [40]. Lipopeptides (candins) Candins, cyclic hexamers of amino acids with a lipophilic side chain, are semisynthetic derivatives of pneumocandin and were first isolated from A nidulans in 1974 [91]. Both the ring and the side chain are critical for antifungal activity, but variations in the location and composition of small side groups attached to ring members can either enhance or reduce antifungal activity [ ]. Currently, these antifungal agents are investigational and undergoing clinical trials. Mechanism of action Candins inhibit the synthesis of 1,3-D-glucan, a glucose polysaccharide essential for the structural integrity of many fungal cell walls [5, ]. This inhibition causes structural damage to the cell wall and, ultimately, cell lysis [5,89]. Spectrum Although the spectrum of activity of candins is still being defined, in vitro, they are known to include Candida spp, Aspergillus spp, H capsulatum, B dermatitidis, Pneumocystis carinii, some lesser known filamentous fungi, and possibly C immitis and S schenckii [89]. Fungi with only small amounts of (1,3)-B-glucan synthase are resistant (eg, C neoformans) [89]. Of the candins, echinocandin B and pneumocandin B are likely to be introduced into clinical use [5]. Pneumocandin is named for its activity against Pneumocystis and Candida [5]. These drugs, which can be used in combination with AMB, are active against fluconazole-resistant Candida as well as Aspergillus sp and other important filamentous fungi. They are not active against B dermatitidis, C neoformans, or Fusarium sp, because these species lack 1,3-D-glucan synthase [5]. Adverse effects Candins have a relatively low toxicity compared with the polyenes [116,117]; however, reports in human literature describe thrombophlebitis, vein irritation, hypersensitivity reactions, and anaphylaxis.
14 682 M.M. Ford / Vet Clin Small Anim 34 (2004) Other Silver sulfadiazine (sulfadiazine silver, Silvadene) SSD was developed in 1968 by Fox [118], who combined the heavy metal antibacterial action of silver with the antibacterial and antifungal action of sulfadiazine [2,76, ]. SSD derives synergistic benefits from sulfonamides and heavy metals and functions as an organic base heavy metal release system by liberating silver [121]. SSD reacts rapidly by binding silver to the DNA of microorganisms and prevents the unzipping of the DNA helix, inhibiting replication [122]. Because no reduced silver is released within the tissues, the risk of argyrosis caused by silver deposition is minimal [123]. Spectrum. SSD has been found to be effective in Fusarium keratitis (human) [123], an organism to which miconazole is resistant [35,124]. In addition, SSD can be used alone or in combination with polyenes or imidazoles for topical treatment of fungal keratitis [120]. SSD has been found to be effective against most fungi, namely, Aspergillus, yeast-like fungi, and the brown dematiaceous filamentous fungi [123]. In a prospective, randomized, crossover trial conducted in human beings, 1% SSD was compared with 1% miconazole drops in 40 patients with keratomycosis [123]. Both drops were well tolerated, but SSD was superior to miconazole (80% versus 50% success rate) [123]. Adverse effects. SSD is an inexpensive medication with wide availability and without adverse effects [76,123]. It is most commonly used in horses with keratomycosis and seems to be well tolerated and effective, despite the fact that there are warnings on the package against use on the eye [40,76]. Conspicuous epithelial regeneration occurs in the presence of SSD [123],most likely related to the greater amount of DNA in mammalian cells, resulting in a ratio of SSD to microorganismal DNA that is high enough to prevent their division [122]. The corresponding ratio of SSD to epithelial cell DNA is too low to block epithelial cell regeneration (healing), which, in turn, is facilitated by the suppression of microorganismal proliferation [122]. Iodides Systemic administration. The antimicrobial mechanism of iodides is unknown but may result from enhancement of the immune response of the host by spurring the halide peroxide killing system of phagocytic cells [5]. AMB and imidazoles also affect the immune system in a similar manner [5]. Sodium iodide has traditionally been the treatment of choice in sporotrichosis before FDA approval of itraconazole [5]. Ketoconazole and sodium iodide administered together seem to have additive effects against sporotrichosis [5]. Topical iodine. Povidone iodine is an antiseptic agent effective against bacteria, fungi, viruses, and protozoa [125] and can be used therapeutically on corneal ulcers [125]. Iodines are used to treat infectious keratitis in horses [76].
15 M.M. Ford / Vet Clin Small Anim 34 (2004) A 1:10 solution to contain a final concentration of 0.1% available povidoneiodine has been shown to be effective against A niger in rabbits [126]. Povidone iodine has recently received attention as a topical antifungal agent, especially against Fusarium isolates [127]. Seven percent tincture of iodine is more irritating than organic iodine but penetrates the cornea more effectively and may serve as a stimulus for fibrovascular infiltrates [76]. Because of the presence of alcohol in tincture of iodine, corneal application should be performed with caution [76]. Inadvertent contact with the conjunctiva or palpebral margin may result in transient ocular irritation with conjunctival hyperemia and chemosis [76]. After initial application, iodine tincture may be reapplied in 24 to 48 hours and at 2- to 3-day intervals until neovascularization of the lesion is evident [76]. Chitin synthesis inhibitors Because chitin is present in fungal cell walls but not mammalian cell walls, chitin synthesis inhibitors are being investigated as new antifungals [128,129]. Chitin synthesis inhibitors are fungicidal because of interference with fungal cell wall formation [39,41]. Chitin and glucan have been targeted directly or indirectly via the enzymes responsible for their synthesis [39,130,131]. Lufenuron (Program) is a chitin synthesis inhibitor approved for control of ectoparasites [41]. An extralabel use has been shown clinically to improve dogs affected with coccidiomycosis and to minimize treatment times [132,133]. Currently, research is being conducted to determine the pharmacokinetic activity of lufenuron in horses [134]. Preliminary studies have demonstrated that lufenuron is absorbed into the equine circulation after oral administration in a dose-dependent manner; however, lufenuron showed no effect on the rate of growth of Aspergillus spp in vitro [134]. These studies demonstrate a potential of use in the treatment of equine keratomycosis. Determination of fungal susceptibility Minimum inhibitory concentration (MIC) testing has become a useful aid to select the most appropriate therapy [89], because there is a growing incidence of fungal disease, an expanding availability of antifungal drugs, and an increasing development of fungal resistance [89]. Unfortunately, susceptibility of fungi to various drugs is not always predictable [34] because of laboratory variation and the slow development of interpretive criteria of MIC data based on in vivo/in vitro correlation [34]. Fungal MIC determination can vary more than 50,000-fold [34,89,135,136]. Because the underlying immunocompromised state of many patients with fungal infections is an important determinant of the outcome of treatment, correlation of sensitivity to response to therapy may be difficult to determine [34]. In 1983, the National Committee for Clinical Laboratory Standards
16 684 M.M. Ford / Vet Clin Small Anim 34 (2004) (NCCLS) established a subcommittee to standardize fungal MIC determination [34,89,135,136]. This committee arose from the need to standardize inoculum size and preparation, incubation time and temperature, media, and end point determination [34,135, ]. Acceptable methods for susceptibility testing of filamentous fungi and criteria for MIC interpretation are under development. At this time, the relevance or pharmacodynamic correlate of fungal MICs is not firmly established [89]; therefore, it has been suggested to use fungal MIC as a predictor of failure rather than of success [89]. An excellent review of reported MIC values may be found in the report by Vanden Bossche et al [37]. In the absence of veterinary-specific criteria, standards developed for human medicine may be useful [34]. Antifungal drug resistance Antifungal drug resistance is well recognized and can be intrinsic or acquired after infection (ie, intrinsically resistant fungi), selection amplification of a resistant strain from a population of many strains, or mutation of initially susceptible fungi [34]. The mechanism by which resistance develops depends on the mode of action of the class of antifungal drug and includes reduced drug uptake, drug export through efflux pumps, or reduced affinity of target enzymes [34]. Fortunately, unlike bacterial resistance, transferable drug resistance has not been recognized with fungi, and the spread of resistance has been considerably slower [34]. Prevention of emergence and spread of resistant fungi depends on maximizing the pharmacodynamic properties of the particular drug class, use of local rather than systemic treatment to reduce general exposure of the patient s normal fungal flora to antifungal agents, and practicing good hygiene [34]. This point may be punctuated by the use of topical antifungal agents rather than systemic agents for the treatment of mycotic keratitis. In the case of the flucytosine, combination antifungal therapy is a well-recognized strategy to prevent emergence of flucytosine resistance [34]. For an excellent review of the proposed mechanisms of the development of azole resistance, the reader is referred to the article by Vanden Bossche et al [37]. Summary Many variables affect the outcome of keratomycosis and systemic fungal infections in animals. These include pathogenicity of the fungal organism (toxins, trophisms, and evasion of host response); previous treatment with topical or systemic corticosteroids, which can have a dramatic negative impact on host defense mechanisms; concurrent systemic illness or immunocompromise; severity/extent of infection; and degree of pain (ie, increased reflex tearing dilutes topical medication) [14]. Experimental work
17 M.M. Ford / Vet Clin Small Anim 34 (2004) suggests that antibiotics may occasionally exacerbate fungal infections [142], and some researchers advocate that concurrent antibiotic therapy is contraindicated in horses with yeast infections and septate fungal infections unless bacterial infection is also suspected [14]. Nevertheless, given that normal conjunctival flora often include bacteria and fungi and because care of keratomycoses often includes mixed bacterial and fungal infections, the possible dynamics (natural influences and local competition) between ocular surface microorganisms merit further investigation. There are many unanswered questions regarding the accuracy of in vitro susceptibilities and corneal concentration capabilities for antifungal topical medications [14]. Inherent host resistance or other immune interactions between the patient and fungus are perhaps the most important determinants of the outcome but are currently difficult to measure or assess except by subjective clinical observation [14]. Acknowledgments The author extends thanks to the following individuals for careful review of this article: Dr Cecil Moore, Dr Marie Kerl, Dr William Fales, Dr Gia Klauss, Dr Heather Streppa, and Dr Peter Bondy. References [1] Samuelson D, Andresen B, Gwin R. Conjunctival fungal flora in horses, cattle, dogs, and cats. J Am Vet Med Assoc 1984;184: [2] Andrew SE, Brooks DE, Smith PJ, Gelatt KN, Chmielewski NT, Whittaker CJ. Equine ulcerative keratomycosis: visual outcome and ocular survival in 39 cases ( ). Equine Vet J 1998;30(2): [3] Brooks D, Andrew S, Dillavou C, Ellis G, Kubilis P. Antimicrobial susceptibility patterns of fungi isolated from horses with ulcerative keratomycosis. Am J Vet Res 1998;59(2): [4] Ramsey D. Bacterial, fungal, and algal microbiology and the eye. In: Basic science course notes. Raleigh: North Carolina State University; [5] Johns K, O Day D. Pharmacologic management of keratomycoses. Surv Ophthalmol 1988;33(3): [6] Pesti M, Campbell J, Peberdy J. Alteration of ergosterol content and chitin synthase activity in Candida albicans. Curr Microbiol 1981;5: [7] Marichal P. Mechanisms of resistance to azole antifungal compounds. Curr Opin Antiinfect Invest Drugs 1999;1: [8] Grahn B, Wolfer J, Keller C, Wilcock B. Equine keratomycosis: clinical and laboratory findings in 23 cases. Prog Vet Comp Ophthalmol 1993;3:2 7. [9] Friedman D, Schoster J, Pickett J, Dubielzig R, Czuprynski C, Knoll J, et al. Pseudallescheria boydii keratomycosis in a horse. J Am Vet Med Assoc 1989;195: [10] Mitchell J, Attleberger M. Fusarium keratomycosis in the horse. Vet Med Small Anim Clin 1973;68(11): [11] Whitley R, Burgess E, Moore C. Microbial isolates of the normal equine eye. Equine Vet J 1983;2(Suppl 2): [12] Moore C, Heller N, Majors N, Whitley R, Burgess E, Weber J. Prevalence of ocular microorganisms in hospitalized and stabled horses. Am J Vet Res 1988;49(6):773 7.
18 686 M.M. Ford / Vet Clin Small Anim 34 (2004) [13] Moore CP. Antibacterial susceptibility patterns for microbial isolates associated with infectious keratitis in horses: 63 cases ( ). J Am Vet Med Assoc 1995;207(7): [14] Ball M. Equine fungal keratitis. Compend Contin Educ Pract Vet 2000;22: [15] Hendrix D, Brooks D, Smith P, Gelatt K, Miller T, Whittaker C, et al. Corneal stromal abscesses in the horse: a review of 24 cases. Equine Vet J 1995;27(6): [16] Barton M. Equine keratomycosis. Compend Contin Educ Pract Vet 1992;14(7): [17] Moore C, Fales W, Whittington P, Bauer L. Bacterial and fungal isolates from Equidae with ulcerative keratitis. J Am Vet Med Assoc 1983;182(6): [18] Beech J, Sweeney C, Irby N. Keratomycoses in 11 horses. Equine Vet J 1983;(Suppl 2): [19] Coad CT, Robinson NM, Wilhelmus KR. Antifungal sensitivity testing for equine keratomycosis. Am J Vet Res 1985;46(3): [20] Gaarder J, Rebhun W, Ball M, Patten V, Shin S, Erb H. Clinical appearances, healing patterns, risk factors, and outcomes of horses with fungal keratitis: 53 cases ( ). J Am Vet Med Assoc 1998;213(1): [21] Sweeney R, Sweeny R, Roby K, Irby N. Corneal stromal abscess in two horses. Compend Contin Educ Pract Vet 1984;6(Suppl):S [22] Whittaker CJG, Smith PJ, Brooks DE, Hendrix DV, Chmielewski NT, Andrew SE, et al. Therapeutic penetrating keratoplasty for deep corneal stromal abscesses in eight horses. Vet Comp Ophthalmol 1997;7: [23] Krohne S. Canine systemic fungal infections. Vet Clin North Am Small Anim Pract 2000; 30(5): [24] Taboda J. Systemic mycoses. In: Ettinger S, Feldman E, editors. Textbook of veterinary internal medicine. Philadelphia: WB Saunders; p [25] Bloom J, Hamor R, Gerding PJ. Ocular blastomycosis in dogs: 73 cases, 108 eyes ( ). J Am Vet Med Assoc 1996;209(7): [26] Gionfriddo J, Powell C. Disseminated blastomycosis with ocular involvement in a dog. Vet Med 2002;97(6): [27] Vartivarian S. Virulence properties and non-immune pathogenetic mechanisms of fungi. Clin Infect Dis 1992;14(Suppl 1):S30 6. [28] Rinaldi M. Laboratory evaluation of antifungal agents: a brief overview. Clin Infect Dis 1992;14(Suppl 1):S [29] Kobayashi G, Medoff G. Antifungal agents: recent developments. Annu Rev Microbiol 1977;31: [30] Martin J. Biosynthesis of polyene macrolide antibiotics. Annu Rev Microbiol 1977;31: [31] Georgopapadakou N, Walsh T. Human mycoses: drugs and targets for emerging pathogens. Science 1994;264: [32] Brown M, Gronwall R, Houston A. Pharmacokinetics and body fluid and endometrial concentrations of cephapirin in mares. Am J Vet Res 1986;47: [33] Georgopapadakou N, Walsh T. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother 1996;40: [34] Shuter J. Antifungal and antiviral agents: a review. Cancer Invest 1999;17(2): [35] O Day DM. Antifungal agents. In: Leibowitz H, editor. Corneal disorders: clinical diagnosis and management. Philadelphia: WB Saunders; p [36] Hazen E, Brown R. Two antifungal agents produced by soil actinomycete. Science 1950; 112:423. [37] Vanden Bossche H, Engelen M, Rochette F. Antifungal agents of use in animal health chemical, biochemical and pharmacological aspects. J Vet Pharmacol Ther 2003; 26(1):5 29. [38] DeVoe A. Keratomycosis. Am J Ophthalmol 1971;71(1): [39] Gupte M, Kulkarni P, Ganguli B. Antifungal antibiotics. App Microbiol Biotechnol 2002;58:46 57.
19 M.M. Ford / Vet Clin Small Anim 34 (2004) [40] Plumb D. Veterinary drug handbook. 4th edition. Ames: Iowa State University Press; [41] Kerl M. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract 2003;33: [42] Dupont B. Overview of the lipid formulations of amphotericin B. J Antimicrob Chemother 2002;49(Suppl S1):31 6. [43] Boothe D. Treatment of fungal infections. In: Boothe D, editor. Small animal clinical pharmacology and therapeutics. Philadelphia: WB Saunders; p [44] Andriole V. Current and future antifungal therapy: new targets for antifungal agents. J Antimicrob Chemother 1999;44: [45] Kerridge D, Whelan W. The polyene macrolide antibiotics and 5-fluorocytosine: molecular actions and interactions. In: Ryley J, editor. Mode of action of antifungal agents. Cambridge: Cambridge University Press; p [46] Brumbaugh G. Rational selection of antimicrobial drugs for treatment of infections of horses. Vet Clin North Am Equine Pract 1987;3(1): [47] Edwards D. Antimicrobial drug action. Baltimore: University Park Press; [48] Hermans P, Keys T. Antifungal agents used for deep-seated mycotic infections. Mayo Clin Proc 1983;58: [49] Sande M, Mandell G. Antimicrobial agents: miscellaneous antibacterial agents; antifungal and antiviral agents. In: Gilman G, Goodman L, Gilman A, editors. The pharmacologic basis of therapeutics. New York: MacMillan Publishing Company; p [50] Vanden Bosscle H, Willemsens G, Marichal P. Anti-candida drugs the biochemical basis for their activity. CRC Crit Rev Microbiol 1987;15: [51] Brajtburg J, Powderly W, Kobayashi G, Mendoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother 1990;34: [52] Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochem Biophys Acta 1986;864: [53] Chin G, Hyndiuk R, Kwasny G, Schultz R. Keratomycosis in Wisconsin. Am J Ophthalmol 1975;79: [54] Prasad S, Nema H. Mycotic infections of cornea (drug sensitivity study). Indian J Ophthalmol 1982;30:81 5. [55] Gallis H, Drew R, Pickard W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 1990;12: [56] Jones D, Sexton R, Rebell G. Mycotic keratitis in South-Florida: a review of thirty nine cases. Trans Ophthalmol Soc UK 1969;89: [57] Malik S, Mitter S. Medical treatment in keratomycosis. Indian J Ophthalmol 1979;27: [58] Forster R. Infectious diseases: fungal diseases. In: Smolin G, Thoft R, editors. The cornea. Boston: Little, Brown and Company; p [59] Boke W, Thiel H. Zur konservativen therapie der hypopyonkeratitis und des hornhautabszesses. Klin Monatsbl Augenheilkunde 1973;163: [60] Rosen R, Friedman A. Successfully treated postoperative Candida parakrusei endophthalmitis. Am J Ophthalmol 1973;76: [61] Andriole V. Current and future therapy of invasive fungal infections. In: Remington J, Swartz M, editors. Current clinical topics in infectious diseases. Malden, MA: Blackwell Sciences; p [62] Andriole V, Bodey G. Pocket guide to systemic antifungal therapy. Springfield, NJ: Scientific Therapeutics; [63] Behrens-Baumann W. Topical antimycotics in ophthalmology. Ophthalmologica 1997; 211(Suppl 1):33 8. [64] Walsh T, Pizzo A. Treatment of systemic fungal infections: recent progress and current problems. Eur Clin Microbiol Infect Dis 1988;7:
20 688 M.M. Ford / Vet Clin Small Anim 34 (2004) [65] Johnson E, Ojwang J, Szekely A, Wallace T, Warnock D. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother 1998;42: [66] Vazquez J, Sanchez V, Dmuchowski C, Dembry L, Sobel J, Zervos M. Nosocomial acquisition of Candida albicans: an epidemiologic study. J Infect Dis 1993;168: [67] Warnock D. Amphotericin B: an introduction. J Antimicrob Chemother 1991;28(Suppl B): [68] Bodey G. Antifungal agents. In: Candidiasis: pathogenesis, diagnosis and treatment. 2nd edition. New York: Raven Press; p [69] Andriole V, Kravetz H. The use of amphotericin B in man. JAMA 1962;180: [70] Legendre A, Selcer B, Edwards D, Stevens R. Treatment of canine blastomycosis with amphotericin B and ketoconazole. J Am Vet Med Assoc 1984;184(10): [71] Branch R. Prevention of amphotericin B-induced renal impairment. A review on the use of sodium supplementation. Arch Intern Med 1988;148(11): [72] Wingard J. Conventional versus lipid formulations of amphotericin B. Presented at Focus on Fungal Infections VII, Imedex Inc. San Antonio, March 12 14, [73] Krawiec D, McKiernan B, Twardock A, Swenson C, Itkin R, Johnson L, et al. Use of an amphotericin B lipid complex for treatment of blastomycosis in dogs. J Am Vet Med Assoc 1996;209(12): [74] Greene C, Watson A. Antifungal chemotherapy. In: Greene C, editor. Infectious diseases of the dog and cat. Philadelphia: WB Saunders; p [75] Malik R, Craig AJ, Wigney DI, Martin P, Love DN. Combination chemotherapy of canine and feline cryptococcosis using subcutaneously administered amphotericin B. Aust Vet J 1996;73(4): [76] Moore C, Collins B, Fales W, Halenda R. Antimicrobial agents for treatment of infectious keratitis in horses. J Am Vet Med Assoc 1995;207(7): [77] Leopold I. Antibiotics and antifungal agents. Invest Ophthalmol 1964;3: [78] Havener W. Ocular pharmacology. 5th edition. St. Louis: CV Mosby Company; p [79] Vanden Bossche H, Marichal P, Odds F. Molecular mechanisms of drug resistance in fungi. Trends Microbiol 1994;2: [80] Vanden Bossche H, Koymans L. Cytochromes P450 in fungi. Mycoses 1998;41(Suppl 1): [81] White T, Marr K, Bowden R. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998;11: [82] Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother 2002; 49(Suppl S1):7 10. [83] Espinel-Ingroff A, Warnock D, Vasquez J, Arthington-Skaggs B. In vitro antifungal susceptibility methods and clinical implications of antifungal resistance. Med Mycol 2000; 38(1): [84] Fromtling R. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1988;1: [85] Wolf A. Histoplasmosis. In: Green C, editor. Infectious diseases of the dog and cat. Philadelphia: WB Saunders; p [86] Vanden Bossche H. Biochemical targets for antifungal azole derivatives: hypothesis on the mode of action. Curr Top Med Mycol 1985;1: [87] Vanden Bossche H. Mode of action of pyridine, pyrimidine and azole antifungals. In: Plempel M, editor. Sterol biosynthesis inhibitors. Chichester: Horwood Ltd; p [88] Brasseur R, Vandenbosch C, Vanden Bossche H, Ruysschaert J. Mode of insertion of miconazole, ketoconazole and deacylated ketoconazole in lipid layers. Biochem Pharmacol 1983;32:
21 M.M. Ford / Vet Clin Small Anim 34 (2004) [89] Neely M, Ghannoum M. The exciting future of antifungal therapy. Eur J Clin Microbiol Infect Dis 2000;19: [90] Moriello K. Ketoconazole: clinical pharmacology and therapeutic recommendations. J Am Vet Med Assoc 1986;188: [91] Groll A, Piscitelli S, Walsh T. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds and putative targets for antifungal drug development. Adv Pharmacol 1988;44: [92] Iwata K, Yamaguchi H, Hiratoni T. Mode of action of clotrimazole. Sabouraudia 1973; 11: [93] Van Cutsem J, Van Gerven F, Janssen P. Activity of orally, topically, and parenterally administered itraconazole in the treatment of superficial and deep mycoses: animal models. Rev Infect Dis 1987;9(Suppl):S [94] Dunbar MJ, Pyle R, Boring J, McCoy C. Treatment of canine blastomycosis with ketoconazole. J Am Vet Med Assoc 1983;182(2): [95] Fitzsimons R, Peters AL. Miconazole and ketoconazole a satisfactory first-line treatment for keratomycosis. Am J Ophthalmol 1986;101: [96] Perry H, Dounenfeld E. Cryptococcus keratitis after keratoplasty. Am J Ophthalmol 1990;110: [97] Harvey S. Antiseptics and disinfectants; fungicides; ectoparasiticides. In: Gilman A, Goodman L, editors. The pharmacological basis of therapeutics. New York: MacMillan Publishing Company; p [98] Kozarsky A, Stulting R, Waring G, Cornell F, Wilson L, Cavanach H. Penetrating keratoplasty for exogenous Paecilomyces keratitis followed by postoperative endophthalmitis. Am J Ophthalmol 1984;98: [99] Minogue MJ, Francis IC, Quatermass P, Kappagoda MB, Bradbury R, Walls RS, et al. Successful treatment of fungal keratitis caused by Paecilomyces lilacinus. Am J Ophthalmol 1984;98: [100] Del Prete A, Sepe G, Ferrante M, Loffredo C, Masciello M, Sebastiani A. Fungal keratitis due to Scopulariopsis brevicaulis in an eye previously suffering from herpetic keratitis. Ophthalmologica 1994;208: [101] Jones B. Principles in the management of oculomycosis. Trans Am Acad Ophthalmol Otolaryngol 1975;79: [102] Matsumoto I, Soejima N. Keratomycosis. Mykosen 1976;19: [103] Legendre AM, Rohrbach B, Toal R, Rinaldi M, Grace L, Jones J. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10(6): [104] Hodges R, Legendre A, Adams L, Willard M, Pitts R, Monce K, et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med 1994;8(6): [105] Foster C, Stefanyszyn M. Intraocular penetration of miconazole in rabbits. Arch Ophthalmol 1979;97: [106] Plotnick A, Boshoven E, Rosychuk R. Primary cutaneous coccidioidomycosis and subsequent drug eruption to itraconazole in a dog. J Am Anim Hosp Assoc 1997;33(2): [107] Da Costa P, Merideth R, Sigler R. Cataracts in dogs after long-term ketoconazole treatment. Vet Comp Ophthalmol 1996;6: [108] Willard M, Nachreiner R, McDonald R, Roudebush P. Ketoconazole-induced changes in selected canine hormone concentrations. Am J Vet Res 1986;47(12): [109] Polak A. Mode of action studies. In: Ryley J, editor. Chemotherapy of fungal diseases. Berlin: Springer-Verlag; p [110] Rex J, Walsh T, Sobel J, Filler S, Pappas P, Dismukes W, et al. Practice guidelines for the treatment of candidiasis. Clin Infect Dis 2000;30: [111] Sobel J. Practice guidelines for the treatment of fungal infections. For the Mycosis Study Group. Infectious Diseases Society of America. Clin Infect Dis 2000;30:652. [112] Galgiani J, Ampel N, Catanzaro A, Johnson R, Stevens D, Williams P. Practice guidelines for the treatment of coccidioidomycosis. Clin Infect Dis 2000;30:
22 690 M.M. Ford / Vet Clin Small Anim 34 (2004) [113] De Lucca A, Walsh T. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 1999;43:1 11. [114] Douglas C, D Ippolito J, Shei G, Meinz M, Onishi J, Marrinan J, et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1, 3-beta-D-glucan synthase inhibitors. Antimicrob Agents Chemother 1997;41: [115] Tang J, Parr JJ, Turner W, Debono M, Lagrandeur L, Burkhardt F, et al. LY303366: a noncompetitive inhibitor of (1, 3)-b-D-glucan synthases from Candida albicans and Aspergillus fumigatus [abstract 367]. Progr Abstr 33rd Intersci Conf Antimicrob Agents Chemother 1993:186. [116] Decker H, Heitsch H, Konig W, Fiedler H. Structure activity relationships of the nikkomycins. J Gen Microbiol 1991;137: [117] Bartizal K, Gill C, Abruzzo G, Flattery A, Kong L, Scott P, et al. In vitro preclinical evaluation of studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob Agents Chemother 1997;41: [118] Fox C. Silver sulphadiazine: a new topical therapy for Pseudomonas in burns. Arch Surg 1968;96: [119] Ball M, Rebhun W, Gaarder J, Patten V. Evaluation of itraconazole-dimethyl sulfoxide ointment for the treatment of keratomycosis in nine horses. J Am Vet Med Assoc 1997; 211(2): [120] Wlodkowski T, Rosenkranz H. Antifungal activity of silver sulfadiazine. Lancet 1973;2: [121] Harrison H. Pharmacology of sulfadiazine silver: its attachment to burned human and rat skin and studies of gastrointestinal absorption and extension. Arch Surg 1979;114: [122] Fox C. Topical therapy and the development of silver sulfadiazine. Surg Gynecol Obstet 1983;157:82 8. [123] Mohan M, Gupta S, Kalra V, Vajpayee R, Sachdev M. Topical silver sulphadiazine a new drug for ocular keratomycosis. Br J Ophthalmol 1988;72: [124] Foster C. Miconazole therapy for keratomycosis. Am J Ophthalmol 1981;91: [125] Hale L. The treatment of corneal ulcer with povidone-iodine (Betadine). NC Med J 1969; 30:54 6. [126] White J, Stephens G, Cinotti A. The use of povidone-iodine for treatment of fungi in rabbit eyes. Ann Ophthalmol 1972;4(10): [127] Bates E, McCartney D. A study of the potential effect of povidone-iodine solution in treating ocular fungal pathogens. Invest Ophthalmol Vis Sci 1996;37(Suppl):872. [128] Cabib E, Bowers B, Sburlati A, Silverman S. Fungal cell-wall synthesis: the construction of a biological structure. Microbiol Sci 1988;5: [129] Calderone R, Brown P. Adherence and receptor relationships of Candida albicans. Microbiol Rev 1991;55:1 20. [130] Shaw J, Bowers B, Silverman S, Valdivieso M, Duran A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol 1991;114: [131] Sudoh M, Nagahashi S, Doi M, Ohta A, Takagi M, Arisawa M. Cloning of the chitin synthase 3 gene from Candida albicans and its expression during yeast-hyphal transition. Mol Gen Genet 1993;241: [132] Greene R. Coccidiomycosis. In: Greene C, editor. Infectious diseases of the dog and cat. Philadelphia: WB Saunders; p [133] Bartsch R, Greene R. New treatment of coccidiomycosis. Vet Forum 1997;14:50 2. [134] Scotty N, Johnson P, Giuliano E, Evans T, Rottinghaus G, Fothergill A, et al. Lufenuron: determination of antifungal activity in vitro and measurement of blood concentrations after oral administration in horses. Proc Amer Coll Vet Ophthalmol 2003; 6:351. [135] National Committee for Clinical Laboratory Standards (NCCLS). Antifungal susceptibility testing committee report. Reference method for broth dilution antifungal
23 M.M. Ford / Vet Clin Small Anim 34 (2004) susceptibility testing of yeast approved standard. 2nd edition. NCCLS Document M27 A ;22(No. 15):1 51. [136] Rex J, Pfaller M, Rinaldi M, Polak A, Galgiani J. Antifungal susceptibility testing. Clin Microbiol Rev 1993;6: [137] Denning D. Echinocandins and pneumocandins a new antifungal class with a novel mode of action. J Antimicrob Chemother 1997;40(5): [138] Espinel-Ingroff A. Clinical relevance of antifungal resistance. Infect Dis Clin North Am 1997;11(4): [139] Rex J, Pfaller M, Galgiani J, Bartlett M, Espinel-Ingroff A, Ghannoum M, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin Infect Dis 1997;24: [140] National Committee for Clinical Laboratory Standards (NCCLS). Antifungal susceptibility testing committee report. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi approved standard. 2nd edition. NCCLS Document M38-A. 2002;22(No. 15):1 7. [141] Odds F, Van Gerven F, Espinel-Ingroff A, Bartlett M, Ghannoum M, Lancaster M, et al. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother 1988;42: [142] Rippon J. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. Philadelphia: WB Saunders; [143] Legendre A. Blastomycosis. In: Green C, editor. Infectious diseases of the dog and cat. Philadelphia: WB Saunders; p [144] Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases ( ). J Am Vet Med Assoc 1998;213(5): [145] Bistner S, Riis R. Clinical aspects of mycotic keratitis in the horse. Cornell Vet 1979;(69): [146] Roberts SM, Severin GA, Lavach JD. Antibacterial activity of dilute povidone-iodine solutions used for ocular surface disinfection in dogs. Am J Vet Res 1986;47:
Polyene structure. Antifungal agents. Available classes. Polyene action. 1. Polyenes MID 27. Polyene mechanism of action
 Antifungal agents A history of pharmaceutical neglect: Rare Difficult to devise Difficult to test in vitro Not renumerative Escalating pace of research but Old gold standard Polyene structure Lipophilic,
Antifungal agents A history of pharmaceutical neglect: Rare Difficult to devise Difficult to test in vitro Not renumerative Escalating pace of research but Old gold standard Polyene structure Lipophilic,
ANTIFUNGAL AGENTS. Most common eg. Athlete s foot, Ringworm, and Tinea cruris. Cutaneous = skin, hair and nails
 ANTIFUNGAL AGENTS Objectives: 1. To learn the general classes of fungal infections 2. To learn the subclassification of antifungal drugs 3. To know the mechanism of action and basic uses for antifungal
ANTIFUNGAL AGENTS Objectives: 1. To learn the general classes of fungal infections 2. To learn the subclassification of antifungal drugs 3. To know the mechanism of action and basic uses for antifungal
Chapter 20: Antimicrobial Drugs
 Chapter 20: Antimicrobial Drugs 1. Overview of Antimicrobial Drugs 2. Antibacterial Drugs 3. Antiviral Drugs 4. Drugs for Eukaryotic Pathogens 1. Overview of Antimicrobial Drugs Antibiotics An antibiotic
Chapter 20: Antimicrobial Drugs 1. Overview of Antimicrobial Drugs 2. Antibacterial Drugs 3. Antiviral Drugs 4. Drugs for Eukaryotic Pathogens 1. Overview of Antimicrobial Drugs Antibiotics An antibiotic
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
How antifungal drugs kill fungi and cure disease
 How antifungal drugs kill fungi and cure disease Snake-oil Salesmen New Product Devours Candida-Yeast Without Major Dietary Change Do you suffer from depression, anxiety, irritability, heartburn, indigestion
How antifungal drugs kill fungi and cure disease Snake-oil Salesmen New Product Devours Candida-Yeast Without Major Dietary Change Do you suffer from depression, anxiety, irritability, heartburn, indigestion
REFRACTIVE SURGERY NIGHTMARES Dr.ATHIYA AGARWAL
 REFRACTIVE SURGERY NIGHTMARES Dr.ATHIYA AGARWAL POST LASIK INFECTION Infection occurring after photorefractive keratectomy (PRK) may be 1. Secondary to the defect in the epithelium as well as the use of
REFRACTIVE SURGERY NIGHTMARES Dr.ATHIYA AGARWAL POST LASIK INFECTION Infection occurring after photorefractive keratectomy (PRK) may be 1. Secondary to the defect in the epithelium as well as the use of
CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT
 Page 1 of 6 TITLE: PATIENTS CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT GUIDELINES: These are the 2011 Guidelines for the Treatment of Candida species infections in adult patients. These recommendations
Page 1 of 6 TITLE: PATIENTS CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT GUIDELINES: These are the 2011 Guidelines for the Treatment of Candida species infections in adult patients. These recommendations
Approaches to Infection Control
 Approaches to Infection Control Considerations for PTAs in the Clinic Objectives Describe the basic characteristics of bacteria, viruses, fungi, and parasites. Discuss the locations, advantages, and disadvantages
Approaches to Infection Control Considerations for PTAs in the Clinic Objectives Describe the basic characteristics of bacteria, viruses, fungi, and parasites. Discuss the locations, advantages, and disadvantages
Fungal Infection in Total Joint Arthroplasty. Dr.Wismer Dr.Al-Sahan
 Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
COMPOUNDING PHARMACY SOLUTIONS PRESCRIPTION COMPOUNDING FOR DERMATOLOGY
 JUNE 2012 COMPOUNDING PHARMACY SOLUTIONS PRESCRIPTION COMPOUNDING WWW.CPSRXS. COM We customize individual prescriptions for the specific needs of our patients. INSIDE THIS ISSUE: Acne 2 Cutaneous Candidiasis
JUNE 2012 COMPOUNDING PHARMACY SOLUTIONS PRESCRIPTION COMPOUNDING WWW.CPSRXS. COM We customize individual prescriptions for the specific needs of our patients. INSIDE THIS ISSUE: Acne 2 Cutaneous Candidiasis
 EQUINE CORNEAL DISEASES & SURGERY: Stromal Corneal Diseases Gwendolyn Lynch, DVM, DACVO Eye Care for Animals at TLC The Life Centre & Marion DuPont Scott Equine Medical Center, Leesburg, Virginia Ulcerative
EQUINE CORNEAL DISEASES & SURGERY: Stromal Corneal Diseases Gwendolyn Lynch, DVM, DACVO Eye Care for Animals at TLC The Life Centre & Marion DuPont Scott Equine Medical Center, Leesburg, Virginia Ulcerative
Cells are tiny building blocks that make up all living things. Cells are so small that you need a microscope to see them.
 FC01 CELLS s are tiny building blocks that make up all living things. s are so small that you need a microscope to see them. ANIMAL CELL PLANT CELL This is the control centre of the cell. It contains chromosomes
FC01 CELLS s are tiny building blocks that make up all living things. s are so small that you need a microscope to see them. ANIMAL CELL PLANT CELL This is the control centre of the cell. It contains chromosomes
Tracy Jane Wetter. A dissertation submitted in partial fulfillment of the requirements for the degree. Doctor of Philosophy.
 ADVANCES IN YEAST AND MOLD MONODRUG AND COMBINATION DRUG ANTIFUNGAL SUSCEPTIBILITY TESTING by Tracy Jane Wetter A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor
ADVANCES IN YEAST AND MOLD MONODRUG AND COMBINATION DRUG ANTIFUNGAL SUSCEPTIBILITY TESTING by Tracy Jane Wetter A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor
WHY IS THIS IMPORTANT?
 CHAPTER 1 WHAT IS MICROBIOLOGY AND WHY IS IT IMPORTANT? WHO / TDR / Crump WHY IS THIS IMPORTANT? Microbiology is more relevant than ever in today s world. Infectious diseases are a leading health-related
CHAPTER 1 WHAT IS MICROBIOLOGY AND WHY IS IT IMPORTANT? WHO / TDR / Crump WHY IS THIS IMPORTANT? Microbiology is more relevant than ever in today s world. Infectious diseases are a leading health-related
1) Siderophores are bacterial proteins that compete with animal A) Antibodies. B) Red blood cells. C) Transferrin. D) White blood cells. E) Receptors.
 Prof. Lester s BIOL 210 Practice Exam 4 (There is no answer key. Please do not email or ask me for answers.) Chapters 15, 16, 17, 19, HIV/AIDS, TB, Quorum Sensing 1) Siderophores are bacterial proteins
Prof. Lester s BIOL 210 Practice Exam 4 (There is no answer key. Please do not email or ask me for answers.) Chapters 15, 16, 17, 19, HIV/AIDS, TB, Quorum Sensing 1) Siderophores are bacterial proteins
Surveillance cultures PRO. Kurt Espersen ICU 4131 Rigshospitalet Copenhagen
 Kurt Espersen ICU 4131 Rigshospitalet Copenhagen Difficult to Diagnose Systemic Candidal Infection Immunsuppression in critically ill patients Frequent manifestation of fungus in ICU Fungi were isolated
Kurt Espersen ICU 4131 Rigshospitalet Copenhagen Difficult to Diagnose Systemic Candidal Infection Immunsuppression in critically ill patients Frequent manifestation of fungus in ICU Fungi were isolated
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
Nursing college, Second stage Microbiology Dr.Nada Khazal K. Hendi L14: Hospital acquired infection, nosocomial infection
 L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Oncology Pharmacotherapy
 Oncology Pharmacotherapy THE USE OF LIPID FORMULATIONS OF AMPHOTERICIN B IN CANCER PATIENTS Rod Quilitz, PharmD Department of Pharmacy, H. Lee Moffitt Cancer Center & Research Institute Questions relating
Oncology Pharmacotherapy THE USE OF LIPID FORMULATIONS OF AMPHOTERICIN B IN CANCER PATIENTS Rod Quilitz, PharmD Department of Pharmacy, H. Lee Moffitt Cancer Center & Research Institute Questions relating
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
 GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Methods of Grading S/N Style of grading Percentage Score 1 Attendance, class work and assignment 10 2 Test 20 3 Examination 70 Total 100
 COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Controversies in Hospital Medicine: Antifungal Therapy. Empiric Therapy, Combination Therapy, and the Question of Central Venous Catheters
 Controversies in Hospital Medicine: Antifungal Therapy Empiric Therapy, Combination Therapy, and the Question of Central Venous Catheters Douglas Fish, Pharm.D. Professor of Pharmacy, University of Colorado
Controversies in Hospital Medicine: Antifungal Therapy Empiric Therapy, Combination Therapy, and the Question of Central Venous Catheters Douglas Fish, Pharm.D. Professor of Pharmacy, University of Colorado
Filamentous fungal infections and the role of amphotericin B
 Filamentous fungal infections and the role of amphotericin B David W. Denning Director, National Aspergillosis Centre University Hospital of South Manchester The University of Manchester AGENDA Increasing
Filamentous fungal infections and the role of amphotericin B David W. Denning Director, National Aspergillosis Centre University Hospital of South Manchester The University of Manchester AGENDA Increasing
ANTIFUNGAL AND ANTIPARASITIC AGENTS. Dr Deliwe Nkosi Microbiology
 ANTIFUNGAL AND ANTIPARASITIC AGENTS Dr Deliwe Nkosi Microbiology FUNGAL INFECTIONS Type Body site Disease Aetiological agent SUPERFICIAL hair, skin, nail Tinea (ringworm) Microsporum, Trichophyton, Epidermophyton
ANTIFUNGAL AND ANTIPARASITIC AGENTS Dr Deliwe Nkosi Microbiology FUNGAL INFECTIONS Type Body site Disease Aetiological agent SUPERFICIAL hair, skin, nail Tinea (ringworm) Microsporum, Trichophyton, Epidermophyton
DRUGS FOR GLUCOSE MANAGEMENT AND DIABETES
 Page 1 DRUGS FOR GLUCOSE MANAGEMENT AND DIABETES Drugs to know are: Actrapid HM Humulin R, L, U Penmix SUNALI MEHTA The three principal hormones produced by the pancreas are: Insulin: nutrient metabolism:
Page 1 DRUGS FOR GLUCOSE MANAGEMENT AND DIABETES Drugs to know are: Actrapid HM Humulin R, L, U Penmix SUNALI MEHTA The three principal hormones produced by the pancreas are: Insulin: nutrient metabolism:
CIBMTR Infection Data and the New Infection Inserts.
 CIBMTR Infection Data and the New Infection Inserts. Marcie Tomblyn, MD, MS Scientific Director, CIBMTR Infection and Immune Reconstitution Working Committee Overview Indication for expanded data collection
CIBMTR Infection Data and the New Infection Inserts. Marcie Tomblyn, MD, MS Scientific Director, CIBMTR Infection and Immune Reconstitution Working Committee Overview Indication for expanded data collection
New strategies in anticancer therapy
 癌 症 診 療 指 引 簡 介 及 臨 床 應 用 New strategies in anticancer therapy 中 山 醫 學 大 學 附 設 醫 院 腫 瘤 內 科 蔡 明 宏 醫 師 2014/3/29 Anti-Cancer Therapy Surgical Treatment Radiotherapy Chemotherapy Target Therapy Supportive
癌 症 診 療 指 引 簡 介 及 臨 床 應 用 New strategies in anticancer therapy 中 山 醫 學 大 學 附 設 醫 院 腫 瘤 內 科 蔡 明 宏 醫 師 2014/3/29 Anti-Cancer Therapy Surgical Treatment Radiotherapy Chemotherapy Target Therapy Supportive
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
Influence of ph Most local anesthetics are weak bases.
 Local anesthetics The agent must depress nerve conduction. The agent must have both lipophilic and hydrophilic properties to be effective by parenteral injection. Structure-activity relationships The typical
Local anesthetics The agent must depress nerve conduction. The agent must have both lipophilic and hydrophilic properties to be effective by parenteral injection. Structure-activity relationships The typical
ELISA BIO 110 Lab 1. Immunity and Disease
 ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
Understanding ph and Osmolarity. Marc Stranz, PharmD
 Understanding ph and Osmolarity Marc Stranz, PharmD Outline ph and osmolarity tolerance guidelines, Definitions of ph and osmolarity, ph and osmolarity in vitro data, ph and osmolarity of common infusions,
Understanding ph and Osmolarity Marc Stranz, PharmD Outline ph and osmolarity tolerance guidelines, Definitions of ph and osmolarity, ph and osmolarity in vitro data, ph and osmolarity of common infusions,
INSULIN PRODUCTS. Jack DeRuiter
 INSULIN PRODUCTS Jack DeRuiter The number and types of insulin preparations available in the United States is constantly changing, thus students should refer to recent drug resources for a current list
INSULIN PRODUCTS Jack DeRuiter The number and types of insulin preparations available in the United States is constantly changing, thus students should refer to recent drug resources for a current list
CEFA-DROPS AND CEFA-TABS
 Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
C. difficile Infections
 C. difficile Infections Introduction C. difficile is a type of bacteria that can cause diarrhea and infection of the colon. This bacterium is more likely to infect patients at hospitals and other healthcare
C. difficile Infections Introduction C. difficile is a type of bacteria that can cause diarrhea and infection of the colon. This bacterium is more likely to infect patients at hospitals and other healthcare
Probiotics for the Treatment of Adult Gastrointestinal Disorders
 Probiotics for the Treatment of Adult Gastrointestinal Disorders Darren M. Brenner, M.D. Division of Gastroenterology Northwestern University, Feinberg School of Medicine Chicago, Illinois What are Probiotics?
Probiotics for the Treatment of Adult Gastrointestinal Disorders Darren M. Brenner, M.D. Division of Gastroenterology Northwestern University, Feinberg School of Medicine Chicago, Illinois What are Probiotics?
WARNING LETTER. According to its approved product labeling (PI) (in pertinent part, emphasis original):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
Course Outline and Syllabus for Students
 Course Outline and Syllabus for Students Name: Ian Crandall Course Number: PHM242H1 Course Title: Microbiology of Infectious Diseases Course Description: The course provides a brief introduction to the
Course Outline and Syllabus for Students Name: Ian Crandall Course Number: PHM242H1 Course Title: Microbiology of Infectious Diseases Course Description: The course provides a brief introduction to the
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Immunity and how vaccines work
 1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT*
 THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT* BY JAMES M. ORTEN AND HENRY B. DEVLINt (From the Deparkment of Physiological Chemistry, Wayne University College of Medicine,
THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT* BY JAMES M. ORTEN AND HENRY B. DEVLINt (From the Deparkment of Physiological Chemistry, Wayne University College of Medicine,
 ASSESSMENT ON THE EFFICACY OF SKUDO IN ELIMINATING ECTOPARASITES AND ON ITS EFFECTS ON DOGS HEALTH. Investigator: Prof. Dr. Gisele Zoccal Mingoti Veterinary Medicine State University Paolista (Unesp) Araçatuba
ASSESSMENT ON THE EFFICACY OF SKUDO IN ELIMINATING ECTOPARASITES AND ON ITS EFFECTS ON DOGS HEALTH. Investigator: Prof. Dr. Gisele Zoccal Mingoti Veterinary Medicine State University Paolista (Unesp) Araçatuba
Feline Lymphoma Chemotherapy and Chemotherapy Protocols
 Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Analytical Specifications RIVAROXABAN
 Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
The present study reports on the application of silver anode in root canals for disinfection of
 Murat AYDIN *, The antibacterial effect of silver anode in root canals Oral Microbiol Immunol Abstract The present study reports on the application of silver anode in root canals for disinfection of infected
Murat AYDIN *, The antibacterial effect of silver anode in root canals Oral Microbiol Immunol Abstract The present study reports on the application of silver anode in root canals for disinfection of infected
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
Canine Lymphoma Frequently Asked Questions by Pet Owners
 Canine Lymphoma Frequently Asked Questions by Pet Owners What is lymphoma? The term lymphoma describes a diverse group of cancers in dogs that are derived from white blood cells called lymphocytes. Lymphocytes
Canine Lymphoma Frequently Asked Questions by Pet Owners What is lymphoma? The term lymphoma describes a diverse group of cancers in dogs that are derived from white blood cells called lymphocytes. Lymphocytes
LYMPHOMA IN DOGS. Diagnosis/Initial evaluation. Treatment and Prognosis
 LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
PRINIPLES OF RADIATION THERAPY Adarsh Kumar. The basis of radiation therapy revolve around the principle that ionizing radiations kill cells
 PRINIPLES OF RADIATION THERAPY Adarsh Kumar The basis of radiation therapy revolve around the principle that ionizing radiations kill cells Radiotherapy terminology: a. Radiosensitivity: refers to susceptibility
PRINIPLES OF RADIATION THERAPY Adarsh Kumar The basis of radiation therapy revolve around the principle that ionizing radiations kill cells Radiotherapy terminology: a. Radiosensitivity: refers to susceptibility
Rheumatoid Arthritis. Outline. Treatment Goal 4/10/2013. Clinical evaluation New treatment options Future research Discussion
 Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
Osteosarcoma: treatment beyond surgery
 Osteosarcoma: treatment beyond surgery Eric Chow, DVM DACVS Sue Downing, DVM DACVIM-Oncology Providing the best quality care and service for the patient, the client, and the referring veterinarian. Osteosarcoma
Osteosarcoma: treatment beyond surgery Eric Chow, DVM DACVS Sue Downing, DVM DACVIM-Oncology Providing the best quality care and service for the patient, the client, and the referring veterinarian. Osteosarcoma
CONSENT FORM. Procedure: Descemet s Stripping Automated Endothelial Keratoplasty (DSAEK)
 CONSENT FORM Procedure: Descemet s Stripping Automated Endothelial Keratoplasty (DSAEK) Surgeon: Jeffrey W. Liu, M.D. Peninsula Laser Eye Medical Group 1174 Castro Street, Ste. 100 Mountain View, CA 94040
CONSENT FORM Procedure: Descemet s Stripping Automated Endothelial Keratoplasty (DSAEK) Surgeon: Jeffrey W. Liu, M.D. Peninsula Laser Eye Medical Group 1174 Castro Street, Ste. 100 Mountain View, CA 94040
PATIENT INFORMATION ABOUT ADJUVANT THERAPY AFTER THE WHIPPLE OPERATION FOR ADENOCARCINOMA ( CANCER ) OF THE PANCREAS AND RELATED SITES.
 PATIENT INFORMATION ABOUT ADJUVANT THERAPY AFTER THE WHIPPLE OPERATION FOR ADENOCARCINOMA ( CANCER ) OF THE PANCREAS AND RELATED SITES. Radiation Oncology Sidney Kimmel Cancer Center at Johns Hopkins Last
PATIENT INFORMATION ABOUT ADJUVANT THERAPY AFTER THE WHIPPLE OPERATION FOR ADENOCARCINOMA ( CANCER ) OF THE PANCREAS AND RELATED SITES. Radiation Oncology Sidney Kimmel Cancer Center at Johns Hopkins Last
Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com
 Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com Natasha L. Herz, MD INFORMED CONSENT FOR DESCEMET S STRIPPING and AUTOMATED ENDOTHELIAL KERATOPLASTY (DSAEK)
Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com Natasha L. Herz, MD INFORMED CONSENT FOR DESCEMET S STRIPPING and AUTOMATED ENDOTHELIAL KERATOPLASTY (DSAEK)
1. Give the name and functions of the structure labeled A on the diagram. 2. Give the name and functions of the structure labeled B on the diagram.
 2013 ANATOMY & PHYSIOLOGY Sample Tournament Station A: Use the diagram in answering Questions 1-5. 1. Give the name and functions of the structure labeled A on the diagram. 2. Give the name and functions
2013 ANATOMY & PHYSIOLOGY Sample Tournament Station A: Use the diagram in answering Questions 1-5. 1. Give the name and functions of the structure labeled A on the diagram. 2. Give the name and functions
Protocol for the treatment of oral candida at E.M.H.
 Protocol for the treatment of oral candida at E.M.H. Introduction Candidosis and Xerostomia [a dry mouth] are the two most common oral problems found in palliative care. Cancer patients are very susceptible
Protocol for the treatment of oral candida at E.M.H. Introduction Candidosis and Xerostomia [a dry mouth] are the two most common oral problems found in palliative care. Cancer patients are very susceptible
A t f e t r e r th t is s lec e t c u t re r e t h t e e st s u t den e t t sh s ould b e e a b a le e t o t :
 Dermatopharmacology Prof Werner Sinclair Department of Dermatology University of the Free State Outcomes for this Lecture After this lecture the student should be able to: Name the most important characteristics
Dermatopharmacology Prof Werner Sinclair Department of Dermatology University of the Free State Outcomes for this Lecture After this lecture the student should be able to: Name the most important characteristics
Course Curriculum for Master Degree in Clinical Pharmacy
 Course Curriculum for Master Degree in Clinical Pharmacy The Master Degree in Clinical Pharmacy is awarded by the Faculty of Graduate studies at Jordan University of Science and Technology (JUST) upon
Course Curriculum for Master Degree in Clinical Pharmacy The Master Degree in Clinical Pharmacy is awarded by the Faculty of Graduate studies at Jordan University of Science and Technology (JUST) upon
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
7- Master s Degree in Public Health and Public Health Sciences (Majoring Microbiology)
 7- Master s Degree in Public Health and Public Health Sciences (Majoring Microbiology) Students should fulfill a total of 38 credit hours: 1- Basic requirements: 10 credit hours. 150701, 150702, 150703,
7- Master s Degree in Public Health and Public Health Sciences (Majoring Microbiology) Students should fulfill a total of 38 credit hours: 1- Basic requirements: 10 credit hours. 150701, 150702, 150703,
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Tuberculosis And Diabetes. Dr. hanan abuelrus Prof.of internal medicine Assiut University
 Tuberculosis And Diabetes Dr. hanan abuelrus Prof.of internal medicine Assiut University TUBERCULOSIS FACTS More than 9 million people fall sick with tuberculosis (TB) every year. Over 1.5 million die
Tuberculosis And Diabetes Dr. hanan abuelrus Prof.of internal medicine Assiut University TUBERCULOSIS FACTS More than 9 million people fall sick with tuberculosis (TB) every year. Over 1.5 million die
PACUC Guidelines. Rodent Survival Surgery
 PACUC Guidelines Rodent Survival Surgery Guidelines for rodent survival surgery are based on the National Research Council Guide for the Care and Use of Laboratory Animals 8 th edition and the Animal Welfare
PACUC Guidelines Rodent Survival Surgery Guidelines for rodent survival surgery are based on the National Research Council Guide for the Care and Use of Laboratory Animals 8 th edition and the Animal Welfare
Course Descriptions. I. Professional Courses: MSEG 7216: Introduction to Infectious Diseases (Medical Students)
 Course Descriptions I. Professional Courses: MSEG 7216: Introduction to Infectious Diseases (Medical Students) This course is offered during the first semester of the second year of the MD Program. It
Course Descriptions I. Professional Courses: MSEG 7216: Introduction to Infectious Diseases (Medical Students) This course is offered during the first semester of the second year of the MD Program. It
Tuula Putus, M.D. Professor in Occupational Health Care and Occupational Medicine, University of Turku, Finland
 Tuula Putus, M.D. Professor in Occupational Health Care and Occupational Medicine, University of Turku, Finland The concepts sick buildings and SBS (sick building syndrome) were introduced in the 70 ies
Tuula Putus, M.D. Professor in Occupational Health Care and Occupational Medicine, University of Turku, Finland The concepts sick buildings and SBS (sick building syndrome) were introduced in the 70 ies
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
9/16/2014. Anti-Immunoglobulin E (IgE) Omalizumab (Xolair ) Dosing Guidance
 Disclosure Statement of Financial Interest New Therapies for Asthma Including Omalizumab and Anti-Cytokine Therapies Marsha Dangler, PharmD, BCACP Clinical Pharmacy Specialist James H. Quillen VA Medical
Disclosure Statement of Financial Interest New Therapies for Asthma Including Omalizumab and Anti-Cytokine Therapies Marsha Dangler, PharmD, BCACP Clinical Pharmacy Specialist James H. Quillen VA Medical
Descemet s Stripping Endothelial Keratoplasty (DSEK)
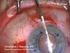 Descemet s Stripping Endothelial Keratoplasty (DSEK) Your doctor has decided that you will benefit from a corneal transplant operation. This handout will explain your options to you. It explains the differences
Descemet s Stripping Endothelial Keratoplasty (DSEK) Your doctor has decided that you will benefit from a corneal transplant operation. This handout will explain your options to you. It explains the differences
1400 Telegraph Bloomfield Hills, MI 48302 248-334-6877-Phone number/248-334-6877-fax Number CANCER TREATMENT
 1400 Telegraph Bloomfield Hills, MI 48302 248-334-6877-Phone number/248-334-6877-fax Number CANCER TREATMENT Learning that your pet has a diagnosis of cancer can be overwhelming. We realize that your pet
1400 Telegraph Bloomfield Hills, MI 48302 248-334-6877-Phone number/248-334-6877-fax Number CANCER TREATMENT Learning that your pet has a diagnosis of cancer can be overwhelming. We realize that your pet
Required Text: Tortora, Funke, and Case. Microbiology, An Introduction, 9 th ed. Benjamin Cummings, 2007.
 Department of Biology Introduction to Microbiology Biol 132 (3 credit hours) Dr. Kathryn Sutton, Assistant Professor of Biology Spring, 2007 M 9-9:50; CBH Room 203 General Information Office Location CBH
Department of Biology Introduction to Microbiology Biol 132 (3 credit hours) Dr. Kathryn Sutton, Assistant Professor of Biology Spring, 2007 M 9-9:50; CBH Room 203 General Information Office Location CBH
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Immune-Mediated Low Platelet or Thrombocyte Count
 rally is Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Immune-Mediated Low Platelet or Thrombocyte Count (Thrombocytopenia)
rally is Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Immune-Mediated Low Platelet or Thrombocyte Count (Thrombocytopenia)
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Overview of Refractive Surgery
 Overview of Refractive Surgery Michael N. Wiggins, MD Assistant Professor, College of Health Related Professions and College of Medicine, Department of Ophthalmology Jones Eye Institute University of Arkansas
Overview of Refractive Surgery Michael N. Wiggins, MD Assistant Professor, College of Health Related Professions and College of Medicine, Department of Ophthalmology Jones Eye Institute University of Arkansas
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Avastin (Bevacizumab) Intravitreal Injection
 Avastin (Bevacizumab) Intravitreal Injection This handout describes how Avastin may be used to treat wet age related macular degeneration (AMD) or macular edema due to retinal vascular disease such as
Avastin (Bevacizumab) Intravitreal Injection This handout describes how Avastin may be used to treat wet age related macular degeneration (AMD) or macular edema due to retinal vascular disease such as
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Fungal infections of the eye - laboratory diagnosis and treatment
 Review Article Nepal Med Coll J 2008; 10(1): 48-63 Fungal infections of the eye - laboratory diagnosis and treatment N Nayak Corresponding author: Additional Professor of Ocular Microbiology, Dr. Rajendra
Review Article Nepal Med Coll J 2008; 10(1): 48-63 Fungal infections of the eye - laboratory diagnosis and treatment N Nayak Corresponding author: Additional Professor of Ocular Microbiology, Dr. Rajendra
Introduction to Enteris BioPharma
 Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Certificate of Mold Analysis
 , Tel: (954) 384-4446 Fax: (954) 384-4838 Toll Free: 800-427-0550 AIHA Lab ID # 163230 Prepared for: Phone Number: (800) 427-0550 Fax Number: (555) 555-5555 Email Address: Test Location: [email protected]
, Tel: (954) 384-4446 Fax: (954) 384-4838 Toll Free: 800-427-0550 AIHA Lab ID # 163230 Prepared for: Phone Number: (800) 427-0550 Fax Number: (555) 555-5555 Email Address: Test Location: [email protected]
10. Treatment of peritoneal dialysis associated fungal peritonitis
 10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
