UWHC Guidelines for the Use of Daptomycin (Cubicin )
|
|
|
- Meryl Webb
- 9 years ago
- Views:
Transcription
1 UWHC Guidelines for the Use of Daptomycin (Cubicin ) Guidelines developed by: Antimicrobial Use Subcommittee; UWHC Drug Policy Program (DPP) Authors: Barry Fox, MD, Sarah E. Bland, RPh Updated by: Lucas Schulz, PharmD Coordination: Sara Shull, PharmD, MBA, Manager, DPP Reviewed by: David Andes, MD; Barry Fox, MD; Dennis Maki, MD, Antimicrobial Use Subcommittee; Carol A. Spiegel, PhD, Marie Pietruska, PharmD Originally Approved by P&T Committee: July 2010 Updated: February 2011 Scheduled For Review: February 2013 A. Background Antimicrobial resistance among Gram-positive organisms, particularly the sharply rising incidence of life-threatening infections caused by methicillin-resistant staphylococci (MRSA) and vancomycin-resistant enterococci (VRE), has become a major concern. New antimicrobial drugs that are effective against these organisms are available; however, to preserve their utility, minimize their unique toxicities and provide cost-effective therapy, it is essential to ensure that these newer agents are used selectively and appropriately. Daptomycin (Cubicin, Cubist Pharmaceuticals) is the first antibiotic in the new cyclic lipopeptide class. It is bactericidal in a concentration-dependent manner against a wide variety of Gram-positive organisms, including those resistant to vancomycin. B. Appropriate Indications Appropriate use of daptomycin for extended therapy will usually require a culture/susceptibility test with identification of an organism that exhibits resistance to other possible antimicrobial agents. Based on the potential for bacterial resistance associated with the use of daptomycin, the UW Pharmacy and Therapeutics Committee has approved its use in the following conditions: 1.0 For patients with bacteremia with suspected MRSA, (i.e., coagulase-positive grampositive cocci in a blood culture with identification and sensitivities not yet completed) with daptomycin dosed at 6 mg/kg. Step-down therapy to vancomycin should usually be made if the vancomycin MIC of the organism is 1.5 mcg/ml. Under certain circumstances approved by Infectious Diseases, daptomycin may be continued unless the vancomycin MIC is 1.0 mcg/ml, usually in more serious staphylococcal infections. 2.0 Life-threatening infection, usually septic shock, endocarditis, complex deep skin and soft tissue infections (SSTIs) or intraabdominal sepsis, where there is strong suspicion that the patient is infected by MRSA or VRE (e.g., the patient is known to have been or is currently colonized by these organisms). If the cultures do not confirm MRSA or VRE infection within 72 hours, daptomycin should be switched to vancomycin or other antibiotics. 3.0 Invasive infections caused by vancomycin-resistant Enterococcus faecium such as bacteremia, intraabdominal infection, or deep SSTIs. 4.0 SSTIs or osteomyelitis caused by MRSA with a vancomycin MIC >1.5 mcg/ml. Under certain circumstances approved by Infectious Diseases, daptomycin may be continued unless the vancomycin MIC is 1.0 mcg/ml. 5.0 Other MRSA/VRE indications : 5.1 Unable to tolerate vancomycin therapy. Combination therapy may be warranted if gram-negative organisms are present. A history of vancomycin red man syndrome (the histamine-release reaction) should generally not be considered a reason to use daptomycin for primary treatment; in that circumstance,
2 pretreatment with an H1-histamine blocker, such as diphenhydramine, and slowing the infusion rate can allow vancomycin to be used safely. 5.2 When the infecting MRSA strain is known to have a vancomycin MIC >1.5 mcg/ml. 5.3 Single-dose procedural prophylaxis in patients known to be colonized with VRE or with an MRSA isolate with vancomycin MIC 2.0 mcg/ml, in a normally sterile body site undergoing an invasive interventional radiology or surgical procedure of that site (dose =4 mg/kg).. C. Inappropriate Uses 1.0 Daptomycin should not be used to treat pneumonia. 2.0 Daptomycin should not be used for empiric therapy of non-life-threatening infections where there is little evidence that MRSA or VRE are infecting or colonizing microorganisms. 3.0 Daptomycin should generally not be used for treating coagulase-negative staphylococcal infections where vancomycin is the drug of choice. Daptomycin may be considered if the vancomycin MIC is 4 mcg/ml 4.0 Daptomycin should not be used for inpatients for the simple convenience of oncedaily dosing. (except for transition of patients on vancomycin to daptomycin at or near the time of discharge to facilitate once-daily outpatient therapy). 5.0 Daptomycin should not be used to treat asymptomatic catheter or non catheterassociated bacteriuria. 6.0 MSSA infections, unless there are serious reactions to all appropriate betalactams, and the vancomycin MIC is >1.5 mcg/ml, or concomitant serious adverse reaction to vancomycin. 7.0 Under most circumstances, SSTIS or osteomyelitis with MRSA where the vancomycin MIC is 1.5 mcg/ml 8.0 Antimicrobial prophylaxis for surgical procedures in patients colonized with MRSA, in the absence of a severe vancomycin allergy. D. Contraindications 1.0 Daptomycin is contraindicated in any person with a known hypersensitivity to daptomycin or any of the product components. E. Precautions 1.0 Concomitant use of daptomycin and HMG-CoA reductase inhibitors may increase the risk of the development of myopathy. HMG-CoA inhibitors may be considered for temporary suspension. 2.0 The use of antimicrobial agents may promote the overgrowth of nonsusceptible organisms. Appropriate measures should be taken if superinfection occurs during the course of treatment. 3.0 Development of diarrhea following administration of daptomycin may indicate the complicating pseudomembranous colitis.
3 4.0 Dose adjustment is required in renal insufficiency. Patients with creatinine clearances of less than 30 ml/min should receive the normal dose, but the dosing interval should be increased to once every 48 hours. 5.0 Avoid using except in cases of strongly suspected or documented infection to reduce the development of resistant organisms F. Adverse Effects / Drug Interactions 1.0 Daptomycin has been associated with myalgia, increased creatine kinase and rhabdomyolysis. 2.0 Gastrointestinal side effects associated with daptomycin in clinical trials may include diarrhea, nausea, constipation and vomiting. 3.0 Other side effects observed in clinical trials (> 2% of patients) include headache, insomnia, rash, dizziness and fever. 4.0 Less common side effects in clinical trials (at least 1% of patients) include hypotension, pruritus, rash, hyperkalemia, hypokalemia, anemia, increased liver function tests, dizziness, headache, insomnia, renal failure, dyspnea and fungal infection. 5.0 Therapeutic levels of daptomycin may falsely prolong prothrombin time and elevate INR. If abnormal results are obtained, a specimen should be drawn just prior to the next daptomycin dose and tested for PT/INR. If the results are still abnormal, further investigation as to the cause is warranted. G. Monitoring Parameters/Documentation 1.0 Therapeutic 1.1 Culture and susceptibility of pathogen from site of infection 1.2 White blood cell count with differential 1.3 Physical exam to monitor for resolution of signs/symptoms of infection 2.0 Toxic 2.1 CBC and blood chemistry periodically 2.2 Baseline serum CPK and recheck at least weekly while on therapy H. Infectious Disease Authorization 1.0 Use of daptomycin generally requires approval by Infectious Diseases. 2.0 The physician wishing to prescribe daptomycin for an adult patient will contact pager #3333 between the hours of 0700 and 2200 to reach the ID physician on call. If ID approval is given, the ordering physician will then inform the unit pharmacist that approval has been given. A formal consult is not required, but the prescribing physician or the pharmacist should document the name of the authorizing Infectious Diseases physician in the appropriate questions field when entering the order into HealthLink. 3.0 The physician wishing to prescribe daptomycin for a pediatric patient will contact the Pediatric Infectious Disease physician on call between the hours of 0700 and 2200 for approval of the order for daptomycin. If ID approval is given, the ordering physician will then inform the unit pharmacist that approval has been given. A
4 formal consult is not required, but the prescribing physician or the pharmacist should document the name of the authorizing Infectious Diseases physician in the appropriate questions field when entering the order into HealthLink. 4.0 In the event of an emergency or if there is an expected delay in the approval process, such as an order written between 2200 and 0700, a single dose of drug may be dispensed, if the appropriate criteria for treatment are met, by the Pharmacy without ID approval. Subsequent doses will not be dispensed until ID approval has been obtained. I. Dose and Administration 1.0 Doses of daptomycin are administered every 24 hours and should be based on ideal body weight (IBW) to reduce the risk of myopathy. 2.0 If the dose entered is not based on IBW, the pharmacist is authorized to change the dose to one based on IBW unless the prescriber indicates Dose as written or the Infectious Diseases physician s note indicates that the dose is to be based on a weight other than IBW. 3.0 If patient s actual body weight is less than IBW (ABW < IBW), dose based on ABW. 4.0 For MRSA and MSSA bacteremia, endocarditis, 6 mg/kg, based on IBW, for 2-6 weeks. 5.0 Vancomycin-resistant Enterococcus faecium infections involving bacteremia: 6 mg/kg IV, based on IBW, every 24 hours for up to 14 consecutive days. 6.0 Vancomycin-resistant Enterococcus faecium infections NOT with bacteremia, such as urinary tract infections and SSTIs: 4 mg/kg IV, based on IBW, every 24 hours for up to 14 days. 7.0 Shorter courses of therapy (i.e., 3-7 days) may be suitable for other types of infections. Complicated skin and skin structure infections due to MRSA may be treated with 4 mg/kg, based on IBW, IV every 24 hours for a maximum of 8 to10 consecutive days. 8.0 Doses greater than 6 mg/kg, based on IBW, are not recommended at this time. In unusual circumstances, larger doses may rarely be indicated. Practitioners requesting the use of larger doses must get authorization through the #3333 pager. 9.0 Dosing during inpatient hemodialysis is 4mg/kg after HD for SSTIs or 6 mg/kg after HD for bloodstream infections. For inpatients, doses are given after the patient returns from HD For outpatients receiving HD, a 20% increase (~1mg/kg) may be given during the last 30 minutes of each HD session (5 mg/kg or 7 mg/kg). A dose increase on the HD session prior to 68 hour intra-dialytic period (Friday on a Monday, Wednesday, Friday schedule or Saturday on a Tuesday, Thursday, Saturday schedule) is NOT necessary but may be prescribed at the physicians discretion. Alternative dosing, see # Patients receiving HD with residual renal function ( 100ml in 24 hours) should strongly be considered for an increased dose (50%) before an inter-dialytic period greater than 48 hours (ie. 4/4/6 or 6/6/9).
5 12.0 Dosing during continuous veno-venous hemodialysis (CVVHD) is 8mg/kg every 48 hours or 4mg/kg every 24 hours. Both regimens yield similar AUC/MIC ratios (PK/PD characteristic associated with efficacy) at steady-state; however, the 8mg/kg dose lower minimum concentration (PK parameter associated with safety). Dosing in alternative continous renal replacement therapies has not been studied Daptomycin injection should be administered over a period of 30 minutes. Do not use the intravenous infusion bag in series connections. Concomitant drugs should be administered separately. Diluents containing dextrose should not be used In the outpatient setting, a two-minute infusion may be used and is preferred The IV line should be flushed before administration of any other medications Important Note: Daptomycin may take minutes to prepare due to the amount of time it takes to go into solution. Therefore, a STAT dose may not be available for 90 minutes or longer after the order is entered. J. Cost 1.0 Cost* comparison of linezolid, quinupristin/dalfopristin, daptomycin and vancomycin Drug Route, Dose Cost/Day ($) Cost/14 Days ($) Daptomycin 4 mg/kg IBW IV Q24h per 65 kg per 65 kg 6 mg/kg IBW IV Q24h per 65 kg per 65 kg Vancomycin 1 g IV Q12h Linezolid 600 mg IV Q12h Linezolid 600 mg PO Q12h Linezolid Quinupristin / Dalfopristin (nonformulary at UWHC) *Costs current as of 1/11/ mg IV Q12h x 7 days plus 600 mg PO Q12h x 7 days mg IV Q8h
6 K. References 1.0 Bliziotis IA, Plessa E, Pappas G, Falagas ME. Daptomycin versus other antimicrobial agents for the treatment of skin and soft tissue infections: A meta-analysis. Annals Pharmacother 2010;44: Fowler VG, Boucher HW, Corey RG, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fätkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE, S. aureus Endocarditis and Bacteremia Study Group. Daptomycin vs. standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; Mohr JF, Friedrich LV, Yankelev S, Lamp KC. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int J Antimicrob Agents 2009;33: Bhavnani SM, Prakhya A, Hammel JP, Ambrose PG. Cost-effectiveness of daptomycin versus vancomycin and gentamicin for patients with methicillin-resistant Staphylococcus aureus bacteremia or endocarditis. Clin Infect Dis 2009;49: Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: Data from a randomized trial of patients with bacteremia and endocarditis. Clin Infect Dis 2010;50: Salama NN, Segal JH, Churchwell MD, et al. Single-dose daptomycin pharmacokinetics in chronic haemodialysis patients. Nephrol Dial Transplant 2010;25: Salama NN, Segal JH, Churchwell MD, et al. Intradialytic administration of daptomycin in end stage renal disease patients on hemodialysis. Clin J Am Soc Nephrol. 2009;4: Vilay AM, Brio M, DePestel DD, et al. Daptomycin pharmacokinetics in critically ill patients receiving continous venovenous hemodialysis. Crit Care Med. 2011;39:19-25.
Hemodialysis catheter infection
 Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Staphylococcus aureus Bloodstream Infection Treatment Guideline
 Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
ANTIBIOTICS IN SEPSIS
 ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
Questions and Answers for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV
 Questions and Answers for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV The purpose of this document is to provide additional clarification to the existing information
Questions and Answers for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV The purpose of this document is to provide additional clarification to the existing information
Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults
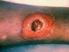 1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
Outpatient Parenteral Antimicrobial Therapy
 Outpatient Parenteral Antimicrobial Therapy Jason E. Bowling, MD a,b, *, James S. Lewis II, PharmD c,d, Aaron D. Owens, MD a,e KEYWORDS Outpatient parenteral antimicrobial therapy Antibiotics Adverse events
Outpatient Parenteral Antimicrobial Therapy Jason E. Bowling, MD a,b, *, James S. Lewis II, PharmD c,d, Aaron D. Owens, MD a,e KEYWORDS Outpatient parenteral antimicrobial therapy Antibiotics Adverse events
Vancomycin Therapeutic Drug Monitoring Vancouver Coastal Health & Providence Health Care Regional Guideline. September 27, 2011. (Version 3.
 Vancomycin Therapeutic Drug Monitoring Vancouver Coastal Health & Providence Health Care Regional Guideline Sept. 27, 2011 (Version 3.5) Developed by: Jane de Lemos, Tim Lau, Mike Legal. Endorsed by: Terri
Vancomycin Therapeutic Drug Monitoring Vancouver Coastal Health & Providence Health Care Regional Guideline Sept. 27, 2011 (Version 3.5) Developed by: Jane de Lemos, Tim Lau, Mike Legal. Endorsed by: Terri
Infectious Diseases @ EUHM Learning Activities:
 Infectious Diseases @ EUHM Learning Activities: Preceptor: Steve Mok, PharmD, BCPS (AQ-ID) Office: EUHM Clinical Pharmacy office, 2 nd fl Peachtree Building Hours: 8:00 17:00 Desk: 404-686-8904 Pager:
Infectious Diseases @ EUHM Learning Activities: Preceptor: Steve Mok, PharmD, BCPS (AQ-ID) Office: EUHM Clinical Pharmacy office, 2 nd fl Peachtree Building Hours: 8:00 17:00 Desk: 404-686-8904 Pager:
C-Difficile Infection Control and Prevention Strategies
 C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer [email protected] 1/18/2016 1 Disclosure This educational activity does not have commercial
C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer [email protected] 1/18/2016 1 Disclosure This educational activity does not have commercial
Staphyloccus aureus sepsis: follow- up practice guidelines
 Staphyloccus aureus sepsis: follow- up practice guidelines March 17, 2012 National Study Day Hospital Antibiotic Stewardship prof. dr. Dirk Vogelaers, Ghent University Hospital apr. Franky Buyle, Ghent
Staphyloccus aureus sepsis: follow- up practice guidelines March 17, 2012 National Study Day Hospital Antibiotic Stewardship prof. dr. Dirk Vogelaers, Ghent University Hospital apr. Franky Buyle, Ghent
WARNING LETTER. According to its approved product labeling (PI) (in pertinent part, emphasis original):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS
 Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
POAC CLINICAL GUIDELINE
 POAC CLINICAL GUIDELINE Acute Pylonephritis DIAGNOSIS COMPLICATED PYELONEPHRITIS EXCLUSION CRITERIA: Male Known or suspected renal impairment (egfr < 60) Abnormality of renal tract Known or suspected renal
POAC CLINICAL GUIDELINE Acute Pylonephritis DIAGNOSIS COMPLICATED PYELONEPHRITIS EXCLUSION CRITERIA: Male Known or suspected renal impairment (egfr < 60) Abnormality of renal tract Known or suspected renal
Bachir K. Younes, M.D., M.P.H.
 Work: 36923 Cook St. # 103 Palm Desert, CA 92211 Phone (760) 636-1336 Fax (760) 636-1335 Bachir K. Younes, M.D., M.P.H. Personal Born: Jan. 1 st, 1971 in Lebanon Marital Status: Married to Roula Sleilati
Work: 36923 Cook St. # 103 Palm Desert, CA 92211 Phone (760) 636-1336 Fax (760) 636-1335 Bachir K. Younes, M.D., M.P.H. Personal Born: Jan. 1 st, 1971 in Lebanon Marital Status: Married to Roula Sleilati
SECTION 6 THERAPEUTIC DRUG MONITORING
 SECTION 6 THERAPEUTIC DRUG MONITORING Kieran Hand Consultant Pharmacist Anti-infectives The objectives of this section are: To test your ability to monitor serum levels for drugs with a narrow therapeutic
SECTION 6 THERAPEUTIC DRUG MONITORING Kieran Hand Consultant Pharmacist Anti-infectives The objectives of this section are: To test your ability to monitor serum levels for drugs with a narrow therapeutic
CUBICIN RF (daptomycin for injection), for intravenous use Initial U.S. Approval: 2003
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN RF safely and effectively. See full prescribing information for CUBICIN RF. CUBICIN RF (daptomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN RF safely and effectively. See full prescribing information for CUBICIN RF. CUBICIN RF (daptomycin
Inpatient Anticoagulation Safety. To provide safe and effective anticoagulation therapy through a collaborative approach.
 Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Beaumont Hospital Department of Nephrology and Renal Nursing. Guideline for administering Ferinject
 Beaumont Hospital Department of Nephrology and Renal Nursing Guideline Name: Guideline for administering Ferinject Guideline Number: 18 Guideline Version: a Developed By: Louise Kelly CNM 1 Renal Day Care
Beaumont Hospital Department of Nephrology and Renal Nursing Guideline Name: Guideline for administering Ferinject Guideline Number: 18 Guideline Version: a Developed By: Louise Kelly CNM 1 Renal Day Care
Develop an understanding of the differential diagnosis of pseudomembranous colitis
 Update on Clostridium difficile Colitis Clostridium difficile infection has recently emerged in populations without any known risk factors. This presentation will focus on the historical background, diagnosis,
Update on Clostridium difficile Colitis Clostridium difficile infection has recently emerged in populations without any known risk factors. This presentation will focus on the historical background, diagnosis,
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT
 Page 1 of 6 TITLE: PATIENTS CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT GUIDELINES: These are the 2011 Guidelines for the Treatment of Candida species infections in adult patients. These recommendations
Page 1 of 6 TITLE: PATIENTS CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT GUIDELINES: These are the 2011 Guidelines for the Treatment of Candida species infections in adult patients. These recommendations
CEFADROXIL CAPSULES, USP 500 mg Rx only
 CEFADROXIL CAPSULES, USP 500 mg Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil capsules and other antibacterial drugs, cefadroxil capsules should
CEFADROXIL CAPSULES, USP 500 mg Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil capsules and other antibacterial drugs, cefadroxil capsules should
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
CEFA-DROPS AND CEFA-TABS
 Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Fungal Infection in Total Joint Arthroplasty. Dr.Wismer Dr.Al-Sahan
 Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
ORTHOPAEDIC INFECTION PREVENTION AND CONTROL: AN EMERGING NEW PARADIGM
 ORTHOPAEDIC INFECTION PREVENTION AND CONTROL: AN EMERGING NEW PARADIGM AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77th Annual Meeting March 9-12, 2010 New Orleans, Louisiana COMMITTEE ON PATIENT SAFETY PREPARED
ORTHOPAEDIC INFECTION PREVENTION AND CONTROL: AN EMERGING NEW PARADIGM AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77th Annual Meeting March 9-12, 2010 New Orleans, Louisiana COMMITTEE ON PATIENT SAFETY PREPARED
DVT/PE Management with Rivaroxaban (Xarelto)
 DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer )
 Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
10. Treatment of peritoneal dialysis associated fungal peritonitis
 10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
healthcare associated infection 1.2
 healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
The Five Intravenous Antibiotics You Need to Know
 The Five Intravenous Antibiotics You Need to Know Ray Geyer, DO Infectious Disease Specialist Medical Director, Benefis Infection Prevention Empirical Antimicrobial Prescribing: Save the Patient and Kill
The Five Intravenous Antibiotics You Need to Know Ray Geyer, DO Infectious Disease Specialist Medical Director, Benefis Infection Prevention Empirical Antimicrobial Prescribing: Save the Patient and Kill
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
Etiology and treatment of chronic bacterial prostatitis the Croatian experience
 Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Antibiotic Prophylaxis for the Prevention of Infective Endocarditis and Prosthetic Joint Infections for Dentists
 PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
PROTOCOL TITLE: Ambulatory Initiation and Management of Warfarin for Adults
 PROTOCOL NUMBER: 7 PROTOCOL TITLE: Ambulatory Initiation and Management of Warfarin for Adults THIS PROTOCOL APPLIES TO: UW Health Clinics: all adult outpatients with an active order for warfarin TARGET
PROTOCOL NUMBER: 7 PROTOCOL TITLE: Ambulatory Initiation and Management of Warfarin for Adults THIS PROTOCOL APPLIES TO: UW Health Clinics: all adult outpatients with an active order for warfarin TARGET
National Patient Safety Goals Effective January 1, 2015
 National Patient Safety Goals Goal 1 Nursing are enter ccreditation Program Improve the accuracy of patient and resident identification. NPSG.01.01.01 Use at least two patient or resident identifiers when
National Patient Safety Goals Goal 1 Nursing are enter ccreditation Program Improve the accuracy of patient and resident identification. NPSG.01.01.01 Use at least two patient or resident identifiers when
Drug Shortage Alert 11/15/2012
 Drug Shortage Alert 11/15/2012 Recommendations and information provided in Drug Shortage Alerts are compiled by experts in the field. Practitioners always are advised to consult with staff to ensure response
Drug Shortage Alert 11/15/2012 Recommendations and information provided in Drug Shortage Alerts are compiled by experts in the field. Practitioners always are advised to consult with staff to ensure response
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections
 A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections By Gary R. Skankey, MD, FACP, Infectious Disease, Las Vegas, NV Sponsored
A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections By Gary R. Skankey, MD, FACP, Infectious Disease, Las Vegas, NV Sponsored
National Patient Safety Goals Effective January 1, 2015
 National Patient Safety Goals Effective January 1, 2015 Goal 1 Improve the accuracy of resident identification. NPSG.01.01.01 Long Term are ccreditation Program Medicare/Medicaid ertification-based Option
National Patient Safety Goals Effective January 1, 2015 Goal 1 Improve the accuracy of resident identification. NPSG.01.01.01 Long Term are ccreditation Program Medicare/Medicaid ertification-based Option
ALBERTA IMMUNIZATION POLICY GUIDELINES
 ALBERTA IMMUNIZATION POLICY GUIDELINES Hepatitis Vaccines. Hepatitis A Vaccines Refer to the vaccine product monograph and the Canadian Immunization Guide for further Product monographs are available on
ALBERTA IMMUNIZATION POLICY GUIDELINES Hepatitis Vaccines. Hepatitis A Vaccines Refer to the vaccine product monograph and the Canadian Immunization Guide for further Product monographs are available on
Venous Thromboembolism: Long Term Anticoagulation. Dan Johnson, Pharm.D.
 Venous Thromboembolism: Long Term Anticoagulation Dan Johnson, Pharm.D. Disclosures No financial relationships with products discussed Off-label use of drug therapy always discussed Objectives Review clinical
Venous Thromboembolism: Long Term Anticoagulation Dan Johnson, Pharm.D. Disclosures No financial relationships with products discussed Off-label use of drug therapy always discussed Objectives Review clinical
Guidelines for Antimicrobial Stewardship in Hospitals in Ireland. A Strategy for the Control of Antimicrobial Resistance in Ireland
 A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
MSHP Annual Meeting 11/7/2014
 Using Clinical Decision Support (CDS): Meeting Quality Measures and Beyond Michael D. Kraft, PharmD, BCNSP Clinical Associate Professor University of Michigan College of Pharmacy Assistant Director-Education
Using Clinical Decision Support (CDS): Meeting Quality Measures and Beyond Michael D. Kraft, PharmD, BCNSP Clinical Associate Professor University of Michigan College of Pharmacy Assistant Director-Education
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
James T. Dwyer DO, FACOI
 Antibiotics in the Surgical Patient James T. Dwyer DO, FACOI Objectives Define current prophylactic recommendations for the use of antibiotics in the surgical patient List current antibiotics available
Antibiotics in the Surgical Patient James T. Dwyer DO, FACOI Objectives Define current prophylactic recommendations for the use of antibiotics in the surgical patient List current antibiotics available
PPP 1. Continuation of a medication to ensure continuity of care
 PRESCRIBING POLICIES: 4.7 PHARMACIST AUTHORITY The College of Pharmacists of BC Professional Practice Policy (PPP) 58 Medication Management (Adapting a Prescription) became effective April 1, 2009. The
PRESCRIBING POLICIES: 4.7 PHARMACIST AUTHORITY The College of Pharmacists of BC Professional Practice Policy (PPP) 58 Medication Management (Adapting a Prescription) became effective April 1, 2009. The
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP)
 BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
NewYork-Presbyterian Hospital Sites: Columbia University Medical Center Guideline: Medication Use Manual Page 1 of 12
 Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
Evolution of a Closed Loop Medication Use Process
 Evolution of a Closed Loop Medication Use Process Paul J. Vitale, Pharm.D. [email protected] Vice President and Chief Pharmacy Officer The Mercy Medical Center Baltimore, Maryland Agenda Hospital Background
Evolution of a Closed Loop Medication Use Process Paul J. Vitale, Pharm.D. [email protected] Vice President and Chief Pharmacy Officer The Mercy Medical Center Baltimore, Maryland Agenda Hospital Background
Cubist Pharmaceuticals, Inc. 65 Hayden Avenue Lexington, MA 02421 (781) 860-8660 www.cubist.com. Confronting Challenges in Acute Care
 Cubist Pharmaceuticals, Inc. 65 Hayden Avenue Lexington, MA 02421 (781) 860-8660 www.cubist.com Confronting Challenges in Acute Care >640,000 Patients Treated with CUBICIN (estimated) as of 12/31/08 $140
Cubist Pharmaceuticals, Inc. 65 Hayden Avenue Lexington, MA 02421 (781) 860-8660 www.cubist.com Confronting Challenges in Acute Care >640,000 Patients Treated with CUBICIN (estimated) as of 12/31/08 $140
COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent to 150 mg clindamycin base.
 APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis
 Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
Practice Guidelines for Outpatient Parenteral Antimicrobial Therapy
 IDSA GUIDELINES Practice Guidelines for Outpatient Parenteral Antimicrobial Therapy Alan D. Tice, 1 Susan J. Rehm, 2 Joseph R. Dalovisio, 3 John S. Bradley, 4 Lawrence P. Martinelli, 5 Donald R. Graham,
IDSA GUIDELINES Practice Guidelines for Outpatient Parenteral Antimicrobial Therapy Alan D. Tice, 1 Susan J. Rehm, 2 Joseph R. Dalovisio, 3 John S. Bradley, 4 Lawrence P. Martinelli, 5 Donald R. Graham,
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) COMMUNITY ACQUIRED vs. HEALTHCARE ASSOCIATED
 METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) COMMUNITY ACQUIRED vs. HEALTHCARE ASSOCIATED Recently, there have been a number of reports about methicillin-resistant Staph aureus (MRSA) infections
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) COMMUNITY ACQUIRED vs. HEALTHCARE ASSOCIATED Recently, there have been a number of reports about methicillin-resistant Staph aureus (MRSA) infections
8. Strategies in Patients at Risk for Allergic Reactions to IV Contrast for CT
 Updated 7/14/09 UAB Department of Radiology Guidelines for Administering Gadolinium-based and Iodine-based Intravenous Contrast Agents in Patients with Renal Dysfunction or at Risk for Adverse Reactions
Updated 7/14/09 UAB Department of Radiology Guidelines for Administering Gadolinium-based and Iodine-based Intravenous Contrast Agents in Patients with Renal Dysfunction or at Risk for Adverse Reactions
IV solutions may be given either as a bolus dose or infused slowly through a vein into the plasma at a constant or zero-order rate.
 د.شيماء Biopharmaceutics INTRAVENOUS INFUSION: IV solutions may be given either as a bolus dose or infused slowly through a vein into the plasma at a constant or zero-order rate. The main advantage for
د.شيماء Biopharmaceutics INTRAVENOUS INFUSION: IV solutions may be given either as a bolus dose or infused slowly through a vein into the plasma at a constant or zero-order rate. The main advantage for
Upstate University Health System Medication Exam - Version A
 Upstate University Health System Medication Exam - Version A Name: ID Number: Date: Unit: Directions: Please read each question below. Choose the best response for each of the Multiple Choice and Medication
Upstate University Health System Medication Exam - Version A Name: ID Number: Date: Unit: Directions: Please read each question below. Choose the best response for each of the Multiple Choice and Medication
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Antibiotic Lock Therapy Guideline
 Antibiotic Lock Therapy Guideline I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition, chemotherapy, or hemodialysis,
Antibiotic Lock Therapy Guideline I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition, chemotherapy, or hemodialysis,
Guideline. Treatment of tuberculosis in renal disease. Version 3.0
 Guideline Treatment of tuberculosis in renal disease Version 3.0 Key critical points Renal failure is recognised as a risk factor for developing tuberculosis. Renal failure is recognised as a risk factor
Guideline Treatment of tuberculosis in renal disease Version 3.0 Key critical points Renal failure is recognised as a risk factor for developing tuberculosis. Renal failure is recognised as a risk factor
2. The prescribing clinician will register with the designated manufacturer.
 Clozapine Management Program Description Magellan of Arizona Pharmacy Program Background: Magellan Health Services of Arizona recognizes the importance of a clozapine program. Clozapine received increased
Clozapine Management Program Description Magellan of Arizona Pharmacy Program Background: Magellan Health Services of Arizona recognizes the importance of a clozapine program. Clozapine received increased
Safety Information Card for Xarelto Patients
 Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION
 PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
Urinary Tract Infections
 Urinary Tract Infections Leading cause of morbidity and health care expenditures in persons of all ages. An estimated 50 % of women report having had a UTI at some point in their lives. 8.3 million office
Urinary Tract Infections Leading cause of morbidity and health care expenditures in persons of all ages. An estimated 50 % of women report having had a UTI at some point in their lives. 8.3 million office
Gloucestershire Hospitals
 Gloucestershire Hospitals NHS Foundation Trust TRUST GUIDELINE INFLIXIMAB PRESCRIBING AND ADMINISTRATION 1. INTRODUCTION Infliximab is an anti-tumour necrosis factor-α (Anti-TNF) antibody. It is from a
Gloucestershire Hospitals NHS Foundation Trust TRUST GUIDELINE INFLIXIMAB PRESCRIBING AND ADMINISTRATION 1. INTRODUCTION Infliximab is an anti-tumour necrosis factor-α (Anti-TNF) antibody. It is from a
Addressing the challenge of healthcare associated infections (HCAIs) in Europe
 POSITION PAPER 05 January 2011 Addressing the challenge of healthcare associated infections (HCAIs) in Europe A Call for Action Page 1 of 8 A holistic approach to combating HCAIs in Europe We must rise
POSITION PAPER 05 January 2011 Addressing the challenge of healthcare associated infections (HCAIs) in Europe A Call for Action Page 1 of 8 A holistic approach to combating HCAIs in Europe We must rise
Antimicrobial Stewardship for Hospital Acquired Infection Prevention: Focus on C. difficile infection
 Antimicrobial Stewardship for Hospital Acquired Infection Prevention: Focus on C. difficile infection Emi Minejima, PharmD Assistant Professor of Clinical Pharmacy USC School of Pharmacy [email protected]
Antimicrobial Stewardship for Hospital Acquired Infection Prevention: Focus on C. difficile infection Emi Minejima, PharmD Assistant Professor of Clinical Pharmacy USC School of Pharmacy [email protected]
Electronic Medical Record (EMR) Safety Results of CAH Testing. Tom Johns, PharmD Shands at the University of Florida
 Electronic Medical Record (EMR) Safety Results of CAH Testing Tom Johns, PharmD Shands at the University of Florida Vendors Evaluated CPSI Healthland Cerner Test Patients Test Patient A Age: 76 y/o Sex:
Electronic Medical Record (EMR) Safety Results of CAH Testing Tom Johns, PharmD Shands at the University of Florida Vendors Evaluated CPSI Healthland Cerner Test Patients Test Patient A Age: 76 y/o Sex:
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
William B. Smith, MD President. Patrick R. Ayd, RN, MBA Chief Operating Officer. SNBL-CPC Baltimore, Maryland
 Patrick R. Ayd, RN, MBA Chief Operating Officer SNBL-CPC Baltimore, Maryland William B. Smith, MD President New Orleans Center for Clinical Research / Volunteer Research Group University of Tennessee Medical
Patrick R. Ayd, RN, MBA Chief Operating Officer SNBL-CPC Baltimore, Maryland William B. Smith, MD President New Orleans Center for Clinical Research / Volunteer Research Group University of Tennessee Medical
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES
 APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
Carla Duff, CPNP MSN CCRP Clinical Advanced Registered Nurse Practitioner University of South Florida Division of Allergy, Immunology, and
 Carla Duff, CPNP MSN CCRP Clinical Advanced Registered Nurse Practitioner University of South Florida Division of Allergy, Immunology, and Rheumatology Intravenous Subcutaneous IVIg SCIg What should you
Carla Duff, CPNP MSN CCRP Clinical Advanced Registered Nurse Practitioner University of South Florida Division of Allergy, Immunology, and Rheumatology Intravenous Subcutaneous IVIg SCIg What should you
Prostate Assessment Pathway Prostate Biopsy Alerts
 Prostate Assessment Pathway Prostate Biopsy Alerts Guidelines for the Management of Patient Preparation, Medications and Complications July 2015 Table of Contents Roles and Responsibilities. 1 SECTION
Prostate Assessment Pathway Prostate Biopsy Alerts Guidelines for the Management of Patient Preparation, Medications and Complications July 2015 Table of Contents Roles and Responsibilities. 1 SECTION
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: infusion_therapy_in_the_home 3/1998 2/2016 2/2017 2/2016 Description of Procedure or Service Home infusion
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: infusion_therapy_in_the_home 3/1998 2/2016 2/2017 2/2016 Description of Procedure or Service Home infusion
POST-EXPOSURE PROPHYLAXIS IN THE HEALTH CARE SETTING
 MARCH 2014 A Quick Guide to POST-EXPOSURE PROPHYLAXIS IN THE HEALTH CARE SETTING HIV PROVIDER REFERENCE SERIES A PUBLICATION OF THE MOUNTAIN PLAINS AIDS EDUCATION AND TRAINING CENTER MountainPlains AIDS
MARCH 2014 A Quick Guide to POST-EXPOSURE PROPHYLAXIS IN THE HEALTH CARE SETTING HIV PROVIDER REFERENCE SERIES A PUBLICATION OF THE MOUNTAIN PLAINS AIDS EDUCATION AND TRAINING CENTER MountainPlains AIDS
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Drug Utilization and Evaluation of Cephalosporin s At Tertiary Care Teaching Hospital, Bangalore
 Research Article Drug Utilization and Evaluation of Cephalosporin s At Tertiary Care Teaching Hospital, Bangalore HS. Shekar 1*, HR. Chandrashekhar 2, M. Govindaraju 3, P. Venugopalreddy 1, Chikkalingiah
Research Article Drug Utilization and Evaluation of Cephalosporin s At Tertiary Care Teaching Hospital, Bangalore HS. Shekar 1*, HR. Chandrashekhar 2, M. Govindaraju 3, P. Venugopalreddy 1, Chikkalingiah
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
MANAGEMENT OF COMMON SIDE EFFECTS of INH (Isoniazid), RIF (Rifampin), PZA (Pyrazinamide), and EMB (Ethambutol)
 MANAGEMENT OF COMMON SIDE EFFECTS of INH (Isoniazid), RIF (Rifampin), PZA (Pyrazinamide), and EMB (Ethambutol) 1. Hepatotoxicity: In Active TB Disease a. Background: 1. Among the 4 standard anti-tb drugs,
MANAGEMENT OF COMMON SIDE EFFECTS of INH (Isoniazid), RIF (Rifampin), PZA (Pyrazinamide), and EMB (Ethambutol) 1. Hepatotoxicity: In Active TB Disease a. Background: 1. Among the 4 standard anti-tb drugs,
WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses
 WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses 20 August 2009 Table of contents EXECUTIVE SUMMARY... i Other recommendations...iii 1. INTRODUCTION...
WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses 20 August 2009 Table of contents EXECUTIVE SUMMARY... i Other recommendations...iii 1. INTRODUCTION...
PGY-2 ONCOLOGY PHARMACY RESIDENCY
 PGY-2 ONCOLOGY PHARMACY RESIDENCY Program Description and Requirements Providence Health System (PAMC) in Anchorage consists of 394 acute care beds and is part of an integrated health system including
PGY-2 ONCOLOGY PHARMACY RESIDENCY Program Description and Requirements Providence Health System (PAMC) in Anchorage consists of 394 acute care beds and is part of an integrated health system including
Prevention of CAUTI is discussed in the CDC/HICPAC document, Guideline for Prevention of Catheter-associated Urinary Tract Infection 4.
 Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [] and Non-Catheter-Associated Urinary Tract Infection [UTI]) and Other Urinary System Infection [USI]) Events Introduction: Urinary
Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [] and Non-Catheter-Associated Urinary Tract Infection [UTI]) and Other Urinary System Infection [USI]) Events Introduction: Urinary
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections
 Journal of Antimicrobial Chemotherapy (2005) 55, 283 288 doi:10.1093/jac/dkh546 Advance Access publication 10 February 2005 Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive
Journal of Antimicrobial Chemotherapy (2005) 55, 283 288 doi:10.1093/jac/dkh546 Advance Access publication 10 February 2005 Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive
Massachusetts Department of Developmental Services MRSA, VRE, and C. Diff Management Protocol
 Massachusetts Department of Developmental Services MRSA, VRE, and C. Diff Management Protocol PURPOSE: To provide guidance for personnel in order to prevent the spread of Antibiotic Resistant Microorganisms
Massachusetts Department of Developmental Services MRSA, VRE, and C. Diff Management Protocol PURPOSE: To provide guidance for personnel in order to prevent the spread of Antibiotic Resistant Microorganisms
Lecture Outline. Quinolones
 Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Why Do Some Antibiotics Fail?
 Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Hematologic Malignancies/Stem Cell Transplantation Program Clinical Section UCLA Health System Los Angeles, CA 90095
 Hematologic Malignancies/Stem Cell Transplantation Program Clinical Section UCLA Health System Los Angeles, CA 90095 CS 6.4 DIAGNOSIS AND MANAGEMENT OF CYTOMEGALOVIRUS (CMV) INFECTION AND DISEASE Location:
Hematologic Malignancies/Stem Cell Transplantation Program Clinical Section UCLA Health System Los Angeles, CA 90095 CS 6.4 DIAGNOSIS AND MANAGEMENT OF CYTOMEGALOVIRUS (CMV) INFECTION AND DISEASE Location:
