Comments on: Draft Guidance for Industry Developing Products for Weight Management
|
|
|
- Stephanie Phillips
- 8 years ago
- Views:
Transcription
1 Comments on: Draft Guidance for Industry Developing Products for Weight Management Pertaining to Docket No: 2007D Submitted by: David B. Allison, Ph.D. Professor & Head, Section on Statistical Genetics, Dept of Biostatistics & Director, Clinical Nutrition Research Center, Department of Nutrition Sciences Ryals Public Health Building, Suite 414 University of Alabama at Birmingham 1665 University Boulevard Birmingham, Alabama Phone: Fax: Kishore M. Gadde, M.D. Director, Obesity Clinical Trials Programme Box 3292 Duke University Medical Centre Durham, NC Phone: Fax: Date: February, 2007 Submitted to: Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD We are please to see the FDA attend to this important issue and produce the Draft Guidance (hereafter DG). The high prevalence of obesity, its substantial adverse impact on health and quality and quantity of life, and the substantial consumer demand for weight loss therapies all speak to the need to evaluate novel therapeutics in the most rigorous and effective manner possible. In fact, inspired in part by the September 2004 FDA advisory committee meeting that discussed this topic, we and other colleagues recently applied for and obtained an NIH grant to host a 2-day conference on Design, Analysis, and Interpretation of Randomized Clinical Trials for Obesity. The meeting was held in December of 2006 and the complete videotaped proceedings are available for free viewing at We encourage the FDA staff working on this guidance document to view these videos as they contain much pertinent information from leading experts. We offer specific comments below. Page 1 of 7
2 1. Body Fat as a Primary Endpoint. The DG states This guidance applies to products intended to be used for medical weight loss, which can be defined as a long-term reduction in fat mass [emphasis added] with a goal of reduced morbidity and mortality through quantifiable improvements in biomarkers such as blood pressure, lipids, and HbA1c. We applaud this statement. Obesity can be defined as an excess of body fat where excess denotes an amount sufficient to impair heal or longevity. Unfortunately, largely for practical reasons, in much obesity research body fat is not measured and weight or BMI are used as surrogates. While this is justified and necessary in some cases (e.g., some large epidemiologic studies), in the 21 st century virtually every major university and medical center has access to equipment, such as dual energy x-ray absorptiometers (DXAs) that allow precise determination of body fat. Given this and the fact that there is reason to believe that it is loss of fat mass and not loss of weight per se that confers health benefits among obese persons 1, we believe it should be the expectation, rather than the exception that studies use body fat measurements rather than body weight measurements as the primary endpoint. We were therefore disappointed by the DG s later statement that The primary efficacy endpoints should be a comparison of the mean absolute or percent change in body weight between the active-product and placebo-treated groups and the proportion of patients in each treatment group who lose greater than or equal to 5 percent of baseline weight. This seems to contradict the earlier statement we quoted. We urge FDA to replace weight with fat mass in statements about desired primary endpoints. This is an opportunity for FDA to lead the field. 2. Is Specifying a Mechanism of Health Benefit Necessary? Returning to the DG s statement This guidance applies to products intended to be used for medical weight loss, which can be defined as a long-term reduction in fat mass with a goal of reduced morbidity and mortality through quantifiable improvements in biomarkers such as blood pressure, lipids, and HbA1c [emphasis added], we question whether the phrase we have highlighted in necessary and useful. A drug that caused weight or fat loss and reduced morbidity and mortality but did so through mechanisms other than those listed above and even through unknown and therefore unquantified mechanisms would not necessarily be less valuable. Yet the statement might imply that it were and hamper development or approval of such drugs. We therefore recommend eliminating this phrase. 3. Criterion for Weight Maintenance. We find the text however, weight loss and weight maintenance should be demonstrated over the course of at least 1 year before a product can be considered effective for weight management. Thus, the weight management indication incorporates and signifies weight loss and weight maintenance to be vague and potentially confusing. For example, it might be taken to imply that a treatment group must lose a given amount of weight prior to the end of the first year and then either achieve weight stability in the group mean 1 Allison, D. B., Zannolli, R., Faith, M. S., Heo, M., Pietrobelli, A., VanItallie, T. B., Pi-Sunyer, F. X., & Heymsfield, S. B. (1999). Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. International Journal of Obesity, 23, Page 2 of 7
3 or experience no more than a certain amount of weight regain by the end of 1 year to be considered efficacious. This would seem to eliminate from consideration treatments that produce continued weight loss throughout the 1 year period because no maintenance has been demonstrated. Some rewriting of this text for clarity might be useful. 4. Statistical Analysis. The DG states The analysis should be applied to the last observation carried forward on treatment in the modified ITT population defined as subjects who received at least one dose of study drug and have at least one post-baseline assessment of body weight. Sensitivity analyses employing other imputation strategies should assess the effect of dropouts on the results. While we strongly agree with the use of ITT approaches, we believe that the use of last observation carried forward (LOCF) is markedly out of step with modern statistical thinking. This perhaps reflects the fact that the 2004 FDA advisory meeting addressing this topic did not include a statistician with clinical trial expertise. A review of the video tapes referred to above will show that several leading statisticians all eschewed LOCF and suggested alternatives. These alternatives are now well established 2 and available in major statistical packages. We have a paper nearing completion that compares the performance of these various approaches in multiple real obesity trials and will be glad to share a copy with FDA upon request. LOCF does not have a sound statistical foundation and can be biased in either direction (i.e., it is not necessarily conservative). Our own work suggests that multiple imputation may be the best method for conducting ITT analyses in obesity trials and that standard mixed models also work quite well in reasonably sized studies. 5. The Logic of Requiring Prior Attempts at Lifestyle Therapy. The DG states Because all drug and biological therapies impose some risk for adverse events, the use of a weight-management product should be contemplated only after a sufficient trial of lifestyle modification has failed and the risks of excess adiposity and the anticipated benefits of weight loss are expected to outweigh the known and unknown risks of treatment with a particular weight-management product. We do not see how the premise (even if accepted) that all drug and biological therapies impose some risk for adverse events leads to the conclusion that the use of a weight-management product should be contemplated only after a sufficient trial of lifestyle modification has failed and the risks of excess adiposity and the anticipated benefits of weight loss are expected to outweigh the known and unknown risks of treatment with a particular weight-management product. This conclusion seems to presuppose several other premises that may not be valid. Such premises include (but may not be limited to): (a) All patients in need of and desirous of weight loss are willing to try a lifestyle intervention first; (b) All lifestyle interventions have lesser risks for known and unknown adverse events than do pharmaceuticals; and (c) For a person in need of and desirous of treatment, there is a sufficiently large probability that they could lose a sufficient 2 Gadbury, G. L., Coffey, C. S., & Allison, D. B. (2003). Modern Statistical Methods For Obesity Trial Data: Beyond LOCF. Obesity Reviews, 4, Page 3 of 7
4 amount of weight with a lifestyle intervention that a pharmaceutical would no longer be valuable. With respect to premise (a), we think it is not likely to be true. There will be some individuals that simply refuse to first try a lifestyle intervention without pharmaceutical support. Though one might advise them otherwise, if they persist in their opinion, it seems inappropriate to deny them treatment. With respect to premise (b), while this may be true, it seems more an article of faith than a matter of demonstrated fact. With respect to premise (c), lifestyle treatments for obesity on average produce no more than a 12% loss of baseline weight 3. Therefore, assuming height remains constant, anyone with a baseline BMI greater than, at most, 30/(1-.12) or 34.1 would still be predicted to have a BMI greater than 30 even after lifestyle treatment. We emphasize that this is in fact conservative as a recent systematic review concluded that lifestyle modification therapies lead to less than 5 kg of weight loss after 2-4 years 4 which corresponds to a loss of less than about 5% for the average size person in an obesity trial. Thus, for these people, it would seem unwise to put off the very likely need for additional treatment. Finally, the DG is the guidance for industry for developing products for weight management, and it is not clear that there is a benefit to also trying to make it a policy statement on a hierarchy of appropriate interventions suggested to an average obese individual living in the community wishing to lose weight. For these reasons, we question whether use of a weight-management product should be contemplated only after a sufficient trial of lifestyle modification has failed. 6. Inclusion of Very Obese Subjects. The DG states Because excess adiposity may influence a product s metabolism and disposition, the pharmacokinetics profile of a weight-management product should be examined in patients with a broad range of BMIs (e.g., 27 kg/m 2 to 35 kg/m 2 ). Because so substantial a proportion of the US adult population now have BMIs greater than 35 and even 40, we suggest replacing (e.g., 27 kg/m 2 to 35 kg/m 2 ) with (e.g., > 27 kg/m 2 and representative of the distribution of BMIs among persons in the United States with BMIs > 27 kg/m 2 ). 7. Sample Size for Safety Evaluation. Section 2 of the DG addresses the sample size needed for safety evaluation, stating A reasonable estimation of the safety of a weight-management product upon which to base approval generally can be made when a total of approximately 3,000 subjects are randomized to active doses of the product and no fewer than 1,500 subjects are randomized to placebo for 1 year of treatment. For example, the above sample size will provide 80 percent power to rule out with 95 percent confidence an approximately 50 percent increase in the incidence of an adverse event that occurs at a rate of 3 percent in the placebo 3 Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord Oct;21(10): Duoketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes. 2005;29: Page 4 of 7
5 group (i.e., 4.5 percent versus 3 percent). While we are not necessarily advocating a different sample size, we wish to mention some recent results from a survey we conducted of patients entering obesity treatment trials 5. These patients were asked what risk of death or another severe adverse event they would be willing to accept for various magnitudes of weight loss. The median self-defined tolerable risks were only slightly above zero in most cases and well below 1.5% absolute increase in risk listed above. Thus, more than 50% pf patents entering into obesity treatment trials would seem to want studies that had greater power to detect smaller risks than will be offered by the sample sizes suggested in the DG. 8. Testing for Weight-Loss Independent Effects. The DG states Improvements in blood pressure, lipids, glycemia, or other areas commensurate with the degree of weight lost are expected in patients treated with an effective weight-management product. Therefore, changes in common weight-related comorbidities should be factored into the efficacy assessment of investigational weight-management products. We were not certain we understood the import of these sentences. It seems to imply that investigators should evaluate whether drugs have beneficial effects on risk factors above and beyond the effects of weight loss, as stated later in the DG Thus, for a weight-management product to obtain a stand-alone indication for the prevention or treatment of type 2 diabetes, dyslipidemia, hypertension, or any other weight-related comorbidity, it should be shown that the product effectively prevents or treats the comorbidity through a mechanism that is independent of weight loss. We agree that this is a valuable and interesting question worthy of investigation. A key question is how should one analyze data or design studies to address such questions. To our knowledge, this has not yet been addressed in the published literature. This was the subject of Dr. Allison s talk at the aforementioned December meeting for which the videos are available. 9. Seeking positive synergy in combination therapy. The DG states a fixed-dose combination that is associated with at least twice the weight loss observed with that of each of the individual components will be viewed more favorably than combinations that do not achieve this degree of relative weight loss. If drug A produces a weight loss of δ A and drug B produces a weight loss of δ B, this suggestion would imply that the combination of drugs A and B, should produce a weight loss of 2*max(δ A,δ B ). In contrast, even if the drugs were purely additive, the combination would only produce an effects of (δ A +δ B ). Thus, this suggestion asks for positive synergy which seems like an excessively high demand. A combination product that fails to demonstrate a positive synergy might still offer greater efficacy than either drug alone and might also be superior to individual components in other aspects such as improved tolerability; we suggest that this could be a consideration for approval. 5 Allison, D, Faith, M., Sargent, S., Berkowitz, R., Cutter, G., McVie, T., Gadde, KM., Foster, G. Sample Size and Risk Assessment in Obesity Trials: Patient Perspective vs. Current Practice. (2006). Abstract 667- P Obesity Vol. (14), Supp. A212 Page 5 of 7
6 10. Criterion for treating drug-induced weight gain. The DG states Patients eligible for participation in trials examining the efficacy and safety of products for the treatment of medication-induced weight gain should have a documented increase in body weight of at least 5 percent within 6 months of starting a drug known to cause weight gain. We agree that drug-induced weight gain is a major problem. 6 In fact, for individuals taking certain atypical antipsychotic agents, it is such a large problem that we wonder if it is wise to wait for subjects to show weight gain. We advocate studies of prophylactic use of antiobesity agents from the outset of treatment with agents that cause major weight gain. 11. Use of Run-in Periods. The DG does not mention use of run-in periods. We believe that the decision to neither advocate for or against them is wise because, at present, there are no published data suggesting that the use of run-in periods does or does not have any particular desirable or undesirable effect. We believe it is worth mentioning this explicitly in the DG. Minor Points. 12. The DG states Obesity is a chronic, relapsing health risk defined by excess body fat. A risk is generally defined as a probability. Obesity is a physical condition and therefore cannot be called a risk. Rather it is a condition that can increase the risk of undesirable events. 13. We suggest changing Total body fat can be accurately measured using hydrodensitometry and dual-energy x-ray absorptiometry (DEXA) to Total body fat can be accurately measured using hydrodensitometry, dual-energy x-ray absorptiometry (DEXA) and other methods. 14. We suggest changing Excess body fat increases the risk of death and major comorbidities such as type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, osteoarthritis of the knee, sleep apnea, and some cancers to Excess body fat increases the rate of death and risk of major comorbidities such as type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, osteoarthritis of the knee, sleep apnea, and some cancers... Alas, the risk of death is 1.0 for all of us. 15. Similarly, we suggest changing the word risk to rate whenever referring to mortality as the outcome (unless you are specifying a precise finite period of time, e.g. the risk of dying in 10 years or the risk of death before age 50 ). 16. We suggest changing in general, are lowest in individuals with BMIs of 18.5 kg/m 2 to 24.9 kg/m 2 and increase in a curvilinear or linear manner with BMIs of 25 kg/m 2 to approximately 40 kg/m 2. to in general, are lowest in individuals 6 Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychoticinduced weight gain: a comprehensive research synthesis. Am J Psychiatry Nov;156(11): Page 6 of 7
7 with BMIs of 18.5 kg/m 2 to 24.9 kg/m 2 and increase in a monotonic manner with BMIs > 25 kg/m In Table 1, how would a person with a BMI of, for example, be classified? Should the categories not be amended to not leave intervals (however small) out by use of the < symbol at the appropriate points? 18. The DG offers a standard definition of childhood overweight or obesity as being above the 95 percentile of an age and sex-matched BMI distribution. It is important to note that the particular distribution referred to is one that is fixed to that of a past US population, rather than the current population distribution. Use of the latter would tautologically always result in 5% of the childhood population always being obese. 19. We suggest changing Secondary efficacy endpoints should include, but are not limited to, changes in the following metabolic parameters: to Secondary efficacy endpoints should include, but are not limited to, changes in the following metabolic variables:. Similarly, in other places where the word parameter is used to mean variable, it should be replaced by the word variable. 20. The DG states Because the evaluation of investigational weight-management products routinely includes assessment of changes in patients metabolic profiles, and in some cases may involve measurement of visceral fat content by CT or MRI, waist circumference should not serve as a surrogate for visceral fat content when measured in a clinical trial investigating the efficacy of a product for weight loss. Rather, it can be a means to confirm that reductions in waist circumference following treatment with a weight-management product are associated with the expected improvements in metabolic parameters. We are unclear why it is important to confirm that reductions in waist circumference following treatment with a weight-management product are associated with the expected improvements in metabolic parameters nor exactly how to determine the expected improvements in metabolic variables. 21. We suggest changing The difference in mean weight loss between the activeproduct and placebo-treated groups is at least 5 percent and the difference is statistically significant to The difference in mean weight loss between the active-product and placebo-treated groups is at least 5 percent of baseline body weight [assuming that this is what is meant] and the difference is statistically significant. Page 7 of 7
Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI)
 Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) The American Society for Gastrointestinal Endoscopy PIVI on Endoscopic Bariatric Procedures (short form) Please see related White
Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) The American Society for Gastrointestinal Endoscopy PIVI on Endoscopic Bariatric Procedures (short form) Please see related White
Guidance for Industry Developing Products for Weight Management
 Guidance for Industry Developing Products for Weight Management DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry Developing Products for Weight Management DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Treating Patients with PRE-DIABETES David Doriguzzi, PA-C First Valley Medical Group. Learning Objectives. Background. CAPA 2015 Annual Conference
 Treating Patients with PRE-DIABETES David Doriguzzi, PA-C First Valley Medical Group Learning Objectives To accurately make the diagnosis of pre-diabetes/metabolic syndrome To understand the prevalence
Treating Patients with PRE-DIABETES David Doriguzzi, PA-C First Valley Medical Group Learning Objectives To accurately make the diagnosis of pre-diabetes/metabolic syndrome To understand the prevalence
Bariatric Surgery. OHTAC Recommendation. Bariatric Surgery
 OHTAC Recommendation Bariatric Surgery January 21, 2005 1 The Ontario Health Technology Advisory Committee (OHTAC) met on January 21, 2005 and reviewed bariatric surgery for morbid obesity. Obesity is
OHTAC Recommendation Bariatric Surgery January 21, 2005 1 The Ontario Health Technology Advisory Committee (OHTAC) met on January 21, 2005 and reviewed bariatric surgery for morbid obesity. Obesity is
The Link Between Obesity and Diabetes The Rapid Evolution and Positive Results of Bariatric Surgery
 The Link Between Obesity and Diabetes The Rapid Evolution and Positive Results of Bariatric Surgery Michael E. Farkouh, MD, MSc Peter Munk Chair in Multinational Clinical Trials Director, Heart and Stroke
The Link Between Obesity and Diabetes The Rapid Evolution and Positive Results of Bariatric Surgery Michael E. Farkouh, MD, MSc Peter Munk Chair in Multinational Clinical Trials Director, Heart and Stroke
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
WHAT IS THE CORE RECOMMENDATION OF THE ACSM/AHA PHYSICAL ACTIVITY GUIDELINES?
 PHYSICAL ACTIVITY AND PUBLIC HEALTH GUIDELINES FREQUENTLY ASKED QUESTIONS AND FACT SHEET PHYSICAL ACTIVITY FOR THE HEALTHY ADULT WHAT IS THE CORE RECOMMENDATION OF THE ACSM/AHA PHYSICAL ACTIVITY GUIDELINES?
PHYSICAL ACTIVITY AND PUBLIC HEALTH GUIDELINES FREQUENTLY ASKED QUESTIONS AND FACT SHEET PHYSICAL ACTIVITY FOR THE HEALTHY ADULT WHAT IS THE CORE RECOMMENDATION OF THE ACSM/AHA PHYSICAL ACTIVITY GUIDELINES?
Clinical Research on Lifestyle Interventions to Treat Obesity and Asthma in Primary Care Jun Ma, M.D., Ph.D.
 Clinical Research on Lifestyle Interventions to Treat Obesity and Asthma in Primary Care Jun Ma, M.D., Ph.D. Associate Investigator Palo Alto Medical Foundation Research Institute Consulting Assistant
Clinical Research on Lifestyle Interventions to Treat Obesity and Asthma in Primary Care Jun Ma, M.D., Ph.D. Associate Investigator Palo Alto Medical Foundation Research Institute Consulting Assistant
TYPE 2 DIABETES MELLITUS: NEW HOPE FOR PREVENTION. Robert Dobbins, M.D. Ph.D.
 TYPE 2 DIABETES MELLITUS: NEW HOPE FOR PREVENTION Robert Dobbins, M.D. Ph.D. Learning Objectives Recognize current trends in the prevalence of type 2 diabetes. Learn differences between type 1 and type
TYPE 2 DIABETES MELLITUS: NEW HOPE FOR PREVENTION Robert Dobbins, M.D. Ph.D. Learning Objectives Recognize current trends in the prevalence of type 2 diabetes. Learn differences between type 1 and type
Weight Loss Surgery DA participants- 18 months later. By: Caitlyn Patrick and Evan Morgan
 Weight Loss Surgery DA participants- 18 months later By: Caitlyn Patrick and Evan Morgan Outline Background Obesity Comorbidities Treatments Barriers to care Kylee Miller s work PDSA Plan: Systematic follow
Weight Loss Surgery DA participants- 18 months later By: Caitlyn Patrick and Evan Morgan Outline Background Obesity Comorbidities Treatments Barriers to care Kylee Miller s work PDSA Plan: Systematic follow
DISCLOSURES RISK ASSESSMENT. Stroke and Heart Disease -Is there a Link Beyond Risk Factors? Daniel Lackland, MD
 STROKE AND HEART DISEASE IS THERE A LINK BEYOND RISK FACTORS? D AN IE L T. L AC K L AN D DISCLOSURES Member of NHLBI Risk Assessment Workgroup RISK ASSESSMENT Count major risk factors For patients with
STROKE AND HEART DISEASE IS THERE A LINK BEYOND RISK FACTORS? D AN IE L T. L AC K L AN D DISCLOSURES Member of NHLBI Risk Assessment Workgroup RISK ASSESSMENT Count major risk factors For patients with
Antipsychotic drugs are the cornerstone of treatment
 Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Statins and Risk for Diabetes Mellitus. Background
 Statins and Risk for Diabetes Mellitus Kevin C. Maki, PhD, FNLA Midwest Center for Metabolic & Cardiovascular Research and DePaul University, Chicago, IL 1 Background In 2012 the US Food and Drug Administration
Statins and Risk for Diabetes Mellitus Kevin C. Maki, PhD, FNLA Midwest Center for Metabolic & Cardiovascular Research and DePaul University, Chicago, IL 1 Background In 2012 the US Food and Drug Administration
Mortality Assessment Technology: A New Tool for Life Insurance Underwriting
 Mortality Assessment Technology: A New Tool for Life Insurance Underwriting Guizhou Hu, MD, PhD BioSignia, Inc, Durham, North Carolina Abstract The ability to more accurately predict chronic disease morbidity
Mortality Assessment Technology: A New Tool for Life Insurance Underwriting Guizhou Hu, MD, PhD BioSignia, Inc, Durham, North Carolina Abstract The ability to more accurately predict chronic disease morbidity
Antipsychotic Medications and the Risk of Diabetes and Cardiovascular Disease
 Antipsychotic Medications and the Risk of Diabetes and Cardiovascular Disease Professional Tool #1: Screening and Monitoring in a High-Risk Population: Questions and Answers Overview of Cardiometabolic
Antipsychotic Medications and the Risk of Diabetes and Cardiovascular Disease Professional Tool #1: Screening and Monitoring in a High-Risk Population: Questions and Answers Overview of Cardiometabolic
PEER REVIEW HISTORY ARTICLE DETAILS
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
OBESITY DRUG OUTCOME MEASURES
 OBESITY DRUG OUTCOME MEASURES A Consensus Report of Considerations Regarding Pharmacologic Intervention A Product Of: The George Washington University School of Public Health and Health Services Department
OBESITY DRUG OUTCOME MEASURES A Consensus Report of Considerations Regarding Pharmacologic Intervention A Product Of: The George Washington University School of Public Health and Health Services Department
Adult Weight Management Training Summary
 Adult Weight Management Training Summary The Commission on Dietetic Registration, the credentialing agency for the Academy of Nutrition and Dietetics Marilyn Holmes, MS, RDN, LDN About This Presentation
Adult Weight Management Training Summary The Commission on Dietetic Registration, the credentialing agency for the Academy of Nutrition and Dietetics Marilyn Holmes, MS, RDN, LDN About This Presentation
Comment of Consumer Reports Regarding Draft Guidance for Industry: Arsenic In Apple Juice Action Level Docket No. FDA-2012-D-0322
 November 12, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville, MD 20852. Comment of Consumer Reports Regarding Draft Guidance for Industry:
November 12, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville, MD 20852. Comment of Consumer Reports Regarding Draft Guidance for Industry:
Bariatric Surgery in Class I Obesity
 OBES SURG (2014) 24:487 519 DOI 10.1007/s11695-014-1214-1 OTHER Bariatric Surgery in Class I Obesity A Position Statement from the International Federation for the Surgery of Obesity and Metabolic Disorders
OBES SURG (2014) 24:487 519 DOI 10.1007/s11695-014-1214-1 OTHER Bariatric Surgery in Class I Obesity A Position Statement from the International Federation for the Surgery of Obesity and Metabolic Disorders
The weight of the world.
 The weight of the world. SONY ANTHONY Obesity Derived from the Latin word obesus to devour Definition: having a very high amount of body fat in relation to lean body mass Classifications using Body Mass
The weight of the world. SONY ANTHONY Obesity Derived from the Latin word obesus to devour Definition: having a very high amount of body fat in relation to lean body mass Classifications using Body Mass
Main Effect of Screening for Coronary Artery Disease Using CT
 Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Role of Body Weight Reduction in Obesity-Associated Co-Morbidities
 Obesity Role of Body Weight Reduction in JMAJ 48(1): 47 1, 2 Hideaki BUJO Professor, Department of Genome Research and Clinical Application (M6) Graduate School of Medicine, Chiba University Abstract:
Obesity Role of Body Weight Reduction in JMAJ 48(1): 47 1, 2 Hideaki BUJO Professor, Department of Genome Research and Clinical Application (M6) Graduate School of Medicine, Chiba University Abstract:
PROTOCOL ACTION FOR HEALTH IN DIABETES. Look AHEAD. Clinical Trial
 PROTOCOL ACTION FOR HEALTH IN DIABETES Look AHEAD Clinical Trial Approved by the Look AHEAD Protocol Review Committee First Issue February 06, 2001 First Revision, May 10, 2001 Second Revision, October
PROTOCOL ACTION FOR HEALTH IN DIABETES Look AHEAD Clinical Trial Approved by the Look AHEAD Protocol Review Committee First Issue February 06, 2001 First Revision, May 10, 2001 Second Revision, October
Karen Kovach M.S, R.D Chief Scientific Officer Weight Watchers International Inc.
 Waking up to real solutions to Chronic Disease: Tackling obesity can reduce the burden of chronic disease and deliver substantial cost savings to struggling European healthcare systems Karen Kovach M.S,
Waking up to real solutions to Chronic Disease: Tackling obesity can reduce the burden of chronic disease and deliver substantial cost savings to struggling European healthcare systems Karen Kovach M.S,
Summary ID# 13614. Clinical Study Summary: Study F3Z-JE-PV06
 CT Registry ID# Page 1 Summary ID# 13614 Clinical Study Summary: Study F3Z-JE-PV06 INSIGHTS; INSulin-changing study Intending to Gain patients insights into insulin treatment with patient-reported Health
CT Registry ID# Page 1 Summary ID# 13614 Clinical Study Summary: Study F3Z-JE-PV06 INSIGHTS; INSulin-changing study Intending to Gain patients insights into insulin treatment with patient-reported Health
Not All Clinical Trials Are Created Equal Understanding the Different Phases
 Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products
 Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Diabetes mellitus is a chronic condition that occurs as a result of problems with the production and/or action of insulin in the body.
 International Diabetes Federation Diabetes Background Information Diabetes mellitus is a chronic condition that occurs as a result of problems with the production and/or action of insulin in the body.
International Diabetes Federation Diabetes Background Information Diabetes mellitus is a chronic condition that occurs as a result of problems with the production and/or action of insulin in the body.
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Guidance for Industry Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations
 Guidance for Industry Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations DRAFT GUIDANCE This guidance document is being distributed
Communicating uncertainty about benefits and
 Communicating uncertainty about benefits and harms of pharmaceutical products Lisa M. Schwartz, MD, MS Steven Woloshin, MD, MS The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth
Communicating uncertainty about benefits and harms of pharmaceutical products Lisa M. Schwartz, MD, MS Steven Woloshin, MD, MS The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth
Diabetes and Obesity. The diabesity epidemic
 Diabetes and Obesity Frank B. Diamond, Jr. M.D. Professor of Pediatrics University of South Florida College of Medicine The diabesity epidemic Prevalence of diabetes worldwide was over 135 million people
Diabetes and Obesity Frank B. Diamond, Jr. M.D. Professor of Pediatrics University of South Florida College of Medicine The diabesity epidemic Prevalence of diabetes worldwide was over 135 million people
Obesity and risk of breast cancer in the WHI clinical trial MS 1895; accepted by JAMA-Oncology
 Obesity and risk of breast cancer in the WHI clinical trial MS 1895; accepted by JAMA-Oncology Marian L Neuhouser, PhD, RD WHI Clinical Coordinating Center Fred Hutchinson Cancer Research Center WHI 2015
Obesity and risk of breast cancer in the WHI clinical trial MS 1895; accepted by JAMA-Oncology Marian L Neuhouser, PhD, RD WHI Clinical Coordinating Center Fred Hutchinson Cancer Research Center WHI 2015
A starting point for today 30/04/2015. Obesity does matter. Obesity is modifiable. Reducing weight has demonstrable health benefit
 Obesity Intervention for Front-Line Healthcare Providers Welcome to our MBTelehealth sites! A special evaluation plan for the day Linking Learning to Practice for FPs: Earn 2 Mainpro-C and 2 bonus Mainpro
Obesity Intervention for Front-Line Healthcare Providers Welcome to our MBTelehealth sites! A special evaluation plan for the day Linking Learning to Practice for FPs: Earn 2 Mainpro-C and 2 bonus Mainpro
AHS s Headache Coding Corner A user-friendly guide to CPT and ICD coding
 AHS s Headache Coding Corner A user-friendly guide to CPT and ICD coding Stuart Black, MD Part 3 - Medical Decision Making (MDM) coding in Headache As stated in the CPT codebook, the classification of
AHS s Headache Coding Corner A user-friendly guide to CPT and ICD coding Stuart Black, MD Part 3 - Medical Decision Making (MDM) coding in Headache As stated in the CPT codebook, the classification of
Catholic Medical Center & Androscoggin Valley Hospital. Surgical Weight Loss Options For a Healthier Tomorrow
 Catholic Medical Center & Androscoggin Valley Hospital Surgical Weight Loss Options For a Healthier Tomorrow Presentation Overview Obesity Health Related Risks Who Qualifies for Weight Loss Surgery? Gastric-bypass
Catholic Medical Center & Androscoggin Valley Hospital Surgical Weight Loss Options For a Healthier Tomorrow Presentation Overview Obesity Health Related Risks Who Qualifies for Weight Loss Surgery? Gastric-bypass
Body Composition & Longevity. Ohan Karatoprak, MD, AAFP Clinical Assistant Professor, UMDNJ
 Body Composition & Longevity Ohan Karatoprak, MD, AAFP Clinical Assistant Professor, UMDNJ LONGEVITY Genetic 25% Environmental Lifestyle Stress 75% BMI >30 OBESE 25-30 OVERWEIGHT 18-25 NORMAL WEIGHT 18
Body Composition & Longevity Ohan Karatoprak, MD, AAFP Clinical Assistant Professor, UMDNJ LONGEVITY Genetic 25% Environmental Lifestyle Stress 75% BMI >30 OBESE 25-30 OVERWEIGHT 18-25 NORMAL WEIGHT 18
Title:An association between liraglutide treatment and reduction in excessive daytime sleepiness in obese subjects with type 2 diabetes
 Author's response to reviews Title:An association between liraglutide treatment and reduction in excessive daytime sleepiness in obese subjects with type 2 diabetes Authors: Fernando Gomez-Peralta (fgomezperalta@gmail.com)
Author's response to reviews Title:An association between liraglutide treatment and reduction in excessive daytime sleepiness in obese subjects with type 2 diabetes Authors: Fernando Gomez-Peralta (fgomezperalta@gmail.com)
Guidance for Industry
 Guidance for Industry End-of-Phase 2A Meetings U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) September 2009 Procedural Guidance
Guidance for Industry End-of-Phase 2A Meetings U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) September 2009 Procedural Guidance
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Choices Around Bariatric Surgery
 Choices Around Bariatric Surgery What should you know? Richard Stubbs MD FRCS FRACS Wakefield Obesity Clinic, Wellington 152 kg / BMI 59 74 kg / BMI 29 Indications (NIH Consensus Statement 1991) BMI >
Choices Around Bariatric Surgery What should you know? Richard Stubbs MD FRCS FRACS Wakefield Obesity Clinic, Wellington 152 kg / BMI 59 74 kg / BMI 29 Indications (NIH Consensus Statement 1991) BMI >
ESCMID Online Lecture Library. by author
 Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Master of Science. Obesity and Weight Management
 Department of Clinical Sciences and Nutrition Master of Science in Obesity and Weight Management Dublin Part-Time Taught Modular Masters Programme Module Descriptor Outlines XN7201 The Obesity Epidemic
Department of Clinical Sciences and Nutrition Master of Science in Obesity and Weight Management Dublin Part-Time Taught Modular Masters Programme Module Descriptor Outlines XN7201 The Obesity Epidemic
Section 2. Overview of Obesity, Weight Loss, and Bariatric Surgery
 Section 2 Overview of Obesity, Weight Loss, and Bariatric Surgery What is Weight Loss? How does surgery help with weight loss? Short term versus long term weight loss? Conditions Improved with Weight Loss
Section 2 Overview of Obesity, Weight Loss, and Bariatric Surgery What is Weight Loss? How does surgery help with weight loss? Short term versus long term weight loss? Conditions Improved with Weight Loss
General Wellness: Policy for Low Risk Devices. Draft Guidance for Industry and Food and Drug Administration Staff
 General Wellness: Policy for Low Risk Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
General Wellness: Policy for Low Risk Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
PREOPERATIVE MANAGEMENT FOR BARIATRIC PATIENTS. Adrienne R. Gomez, MD Bariatric Physician St. Vincent Bariatric Center of Excellence
 PREOPERATIVE MANAGEMENT FOR BARIATRIC PATIENTS Adrienne R. Gomez, MD Bariatric Physician St. Vincent Bariatric Center of Excellence BARIATRIC SURGERY Over 200,000 bariatric surgical procedures are performed
PREOPERATIVE MANAGEMENT FOR BARIATRIC PATIENTS Adrienne R. Gomez, MD Bariatric Physician St. Vincent Bariatric Center of Excellence BARIATRIC SURGERY Over 200,000 bariatric surgical procedures are performed
CLINICAL GUIDELINES IDENTIFICATION, EVALUATION, AND TREATMENT OF OVERWEIGHT AND OBESITY IN ADULTS. Executive Summary. Obesity Education Initiative
 Obesity Education Initiative PREPRINT JUNE 1998 CLINICAL GUIDELINES ON THE IDENTIFICATION, EVALUATION, AND TREATMENT OF OVERWEIGHT AND OBESITY IN ADULTS Executive Summary N A T I O N A L I N S T I T U
Obesity Education Initiative PREPRINT JUNE 1998 CLINICAL GUIDELINES ON THE IDENTIFICATION, EVALUATION, AND TREATMENT OF OVERWEIGHT AND OBESITY IN ADULTS Executive Summary N A T I O N A L I N S T I T U
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Traditional View of Diabetes. Are children with type 1 diabetes obese: What can we do? 8/9/2012. Change in Traditional View of Diabetes
 Are children with type 1 diabetes obese: What can we do? Traditional View of Diabetes Type 1 Diabetes ( T1DM) Onset Juvenile Lean Type 2 Diabetes ( T2DM) Onset Adult Obese QI Project Indrajit Majumdar
Are children with type 1 diabetes obese: What can we do? Traditional View of Diabetes Type 1 Diabetes ( T1DM) Onset Juvenile Lean Type 2 Diabetes ( T2DM) Onset Adult Obese QI Project Indrajit Majumdar
ECONOMIC COSTS OF PHYSICAL INACTIVITY
 ECONOMIC COSTS OF PHYSICAL INACTIVITY This fact sheet highlights the prevalence and health-consequences of physical inactivity and summarises some of the key facts and figures on the economic costs of
ECONOMIC COSTS OF PHYSICAL INACTIVITY This fact sheet highlights the prevalence and health-consequences of physical inactivity and summarises some of the key facts and figures on the economic costs of
Dieting and Gallstones
 Dieting and Gallstones WIN Weight-control Information Network What are Gallstones are clusters of solid material that form in the gallbladder. The most common type is made mostly of cholesterol. Gallstones
Dieting and Gallstones WIN Weight-control Information Network What are Gallstones are clusters of solid material that form in the gallbladder. The most common type is made mostly of cholesterol. Gallstones
Barriers to Healthcare Services for People with Mental Disorders. Cardiovascular disorders and diabetes in people with severe mental illness
 Barriers to Healthcare Services for People with Mental Disorders Cardiovascular disorders and diabetes in people with severe mental illness Dr. med. J. Cordes LVR- Klinikum Düsseldorf Kliniken der Heinrich-Heine-Universität
Barriers to Healthcare Services for People with Mental Disorders Cardiovascular disorders and diabetes in people with severe mental illness Dr. med. J. Cordes LVR- Klinikum Düsseldorf Kliniken der Heinrich-Heine-Universität
Fixed-Effect Versus Random-Effects Models
 CHAPTER 13 Fixed-Effect Versus Random-Effects Models Introduction Definition of a summary effect Estimating the summary effect Extreme effect size in a large study or a small study Confidence interval
CHAPTER 13 Fixed-Effect Versus Random-Effects Models Introduction Definition of a summary effect Estimating the summary effect Extreme effect size in a large study or a small study Confidence interval
Diabetes and Weight-Loss Surgery
 WHITE PAPER Diabetes and Weight-Loss Surgery Treat the cause. Cure the symptom. Center of Excellence BARIATRIC SURGERY Written July 2011 Bariatric Surgery: The Cure for Type II Diabetes? For most individuals
WHITE PAPER Diabetes and Weight-Loss Surgery Treat the cause. Cure the symptom. Center of Excellence BARIATRIC SURGERY Written July 2011 Bariatric Surgery: The Cure for Type II Diabetes? For most individuals
Summary 1. Comparative-effectiveness
 Cost-effectiveness of Delta-9-tetrahydrocannabinol/cannabidiol (Sativex ) as add-on treatment, for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately
Cost-effectiveness of Delta-9-tetrahydrocannabinol/cannabidiol (Sativex ) as add-on treatment, for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately
Metabolic Syndrome Overview: Easy Living, Bitter Harvest. Sabrina Gill MD MPH FRCPC Caroline Stigant MD FRCPC BC Nephrology Days, October 2007
 Metabolic Syndrome Overview: Easy Living, Bitter Harvest Sabrina Gill MD MPH FRCPC Caroline Stigant MD FRCPC BC Nephrology Days, October 2007 Evolution of Metabolic Syndrome 1923: Kylin describes clustering
Metabolic Syndrome Overview: Easy Living, Bitter Harvest Sabrina Gill MD MPH FRCPC Caroline Stigant MD FRCPC BC Nephrology Days, October 2007 Evolution of Metabolic Syndrome 1923: Kylin describes clustering
Roux-en-Y Gastric Bypass
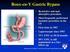 Roux-en-Y Gastric Bypass Restrictive and malabsorptive procedure Most frequently performed bariatric procedure in the US First done in 1967 Laparoscopic since 1993 75% EWL in 18-24 months 50% EWL is still
Roux-en-Y Gastric Bypass Restrictive and malabsorptive procedure Most frequently performed bariatric procedure in the US First done in 1967 Laparoscopic since 1993 75% EWL in 18-24 months 50% EWL is still
General and Abdominal Adiposity and Risk of Death in Europe
 Deutsches Institut für Ernährungsforschung Potsdam-Rehbrücke General and Abdominal Adiposity and Risk of Death in Europe Tobias Pischon Department of Epidemiology German Institute of Human Nutrition Potsdam-Rehbruecke
Deutsches Institut für Ernährungsforschung Potsdam-Rehbrücke General and Abdominal Adiposity and Risk of Death in Europe Tobias Pischon Department of Epidemiology German Institute of Human Nutrition Potsdam-Rehbruecke
Technology Assessment
 Technology Assessment Lifestyle Interventions for Four Conditions: Type 2 Diabetes, Metabolic Syndrome, Breast Cancer, and Prostate Cancer Technology Assessment Program Prepared for: Agency for Healthcare
Technology Assessment Lifestyle Interventions for Four Conditions: Type 2 Diabetes, Metabolic Syndrome, Breast Cancer, and Prostate Cancer Technology Assessment Program Prepared for: Agency for Healthcare
Improving cardiometabolic health in Major Mental Illness
 Improving cardiometabolic health in Major Mental Illness Dr. Adrian Heald Consultant in Endocrinology and Diabetes Leighton Hospital, Crewe and Macclesfield Research Fellow, Manchester University Metabolic
Improving cardiometabolic health in Major Mental Illness Dr. Adrian Heald Consultant in Endocrinology and Diabetes Leighton Hospital, Crewe and Macclesfield Research Fellow, Manchester University Metabolic
Study Design and Statistical Analysis
 Study Design and Statistical Analysis Anny H Xiang, PhD Department of Preventive Medicine University of Southern California Outline Designing Clinical Research Studies Statistical Data Analysis Designing
Study Design and Statistical Analysis Anny H Xiang, PhD Department of Preventive Medicine University of Southern California Outline Designing Clinical Research Studies Statistical Data Analysis Designing
TYPE 2 DIABETES IN CHILDREN DIAGNOSIS AND THERAPY. Ines Guttmann- Bauman MD Clinical Associate Professor, Division of Pediatric Endocrinology, OHSU
 TYPE 2 DIABETES IN CHILDREN DIAGNOSIS AND THERAPY Ines Guttmann- Bauman MD Clinical Associate Professor, Division of Pediatric Endocrinology, OHSU Objectives: 1. To discuss epidemiology and presentation
TYPE 2 DIABETES IN CHILDREN DIAGNOSIS AND THERAPY Ines Guttmann- Bauman MD Clinical Associate Professor, Division of Pediatric Endocrinology, OHSU Objectives: 1. To discuss epidemiology and presentation
Is Insulin Effecting Your Weight Loss and Your Health?
 Is Insulin Effecting Your Weight Loss and Your Health? Teressa Alexander, M.D., FACOG Women s Healthcare Associates www.rushcopley.com/whca 630-978-6886 Obesity is Epidemic in the US 2/3rds of U.S. adults
Is Insulin Effecting Your Weight Loss and Your Health? Teressa Alexander, M.D., FACOG Women s Healthcare Associates www.rushcopley.com/whca 630-978-6886 Obesity is Epidemic in the US 2/3rds of U.S. adults
CLINICAL TRIALS SHOULD YOU PARTICIPATE? by Gwen L. Nichols, MD
 CLINICAL TRIALS SHOULD YOU PARTICIPATE? by Gwen L. Nichols, MD Gwen L. Nichols, M.D., is currently the Oncology Site Head of the Roche Translational Clinical Research Center at Hoffman- LaRoche. In this
CLINICAL TRIALS SHOULD YOU PARTICIPATE? by Gwen L. Nichols, MD Gwen L. Nichols, M.D., is currently the Oncology Site Head of the Roche Translational Clinical Research Center at Hoffman- LaRoche. In this
Drug Treatment for Weight Loss: New Advances
 Drug Treatment for Weight Loss: New Advances William G. Haynes FRCP MD Professor of Internal Medicine Divisions of Endocrinology and Cardiovascular Medicine University of Iowa Outline Why should we use
Drug Treatment for Weight Loss: New Advances William G. Haynes FRCP MD Professor of Internal Medicine Divisions of Endocrinology and Cardiovascular Medicine University of Iowa Outline Why should we use
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes
 Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
With Big Data Comes Big Responsibility
 With Big Data Comes Big Responsibility Using health care data to emulate randomized trials when randomized trials are not available Miguel A. Hernán Departments of Epidemiology and Biostatistics Harvard
With Big Data Comes Big Responsibility Using health care data to emulate randomized trials when randomized trials are not available Miguel A. Hernán Departments of Epidemiology and Biostatistics Harvard
Issues Regarding Use of Placebo in MS Drug Trials. Peter Scott Chin, MD Novartis Pharmaceuticals Corporation
 Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Understanding Diseases and Treatments with Canadian Real-world Evidence
 Understanding Diseases and Treatments with Canadian Real-world Evidence Real-World Evidence for Successful Market Access WHITEPAPER REAL-WORLD EVIDENCE Generating real-world evidence requires the right
Understanding Diseases and Treatments with Canadian Real-world Evidence Real-World Evidence for Successful Market Access WHITEPAPER REAL-WORLD EVIDENCE Generating real-world evidence requires the right
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
The Prevention and Treatment of Missing Data in Clinical Trials: An FDA Perspective on the Importance of Dealing With It
 nature publishing group The Prevention and Treatment of Missing Data in Clinical Trials: An FDA Perspective on the Importance of Dealing With It RT O Neill 1 and R Temple 2 At the request of the Food and
nature publishing group The Prevention and Treatment of Missing Data in Clinical Trials: An FDA Perspective on the Importance of Dealing With It RT O Neill 1 and R Temple 2 At the request of the Food and
Why have new standards been developed?
 Why have new standards been developed? Fitnessgram is unique (and widely accepted) because the fitness assessments are evaluated using criterion-referenced standards. An advantage of criterion referenced
Why have new standards been developed? Fitnessgram is unique (and widely accepted) because the fitness assessments are evaluated using criterion-referenced standards. An advantage of criterion referenced
Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence
 Guidelines Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence Stroke/TIA Nearly 700,000 ischemic strokes and 240,000 TIAs every year in the United States Currently, the risk for
Guidelines Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence Stroke/TIA Nearly 700,000 ischemic strokes and 240,000 TIAs every year in the United States Currently, the risk for
Listen to your heart: Good Cardiovascular Health for Life
 Listen to your heart: Good Cardiovascular Health for Life Luis R. Castellanos MD, MPH Assistant Clinical Professor of Medicine University of California San Diego School of Medicine Sulpizio Family Cardiovascular
Listen to your heart: Good Cardiovascular Health for Life Luis R. Castellanos MD, MPH Assistant Clinical Professor of Medicine University of California San Diego School of Medicine Sulpizio Family Cardiovascular
Am I at Risk for type 2 Diabetes? Taking Steps to Lower the Risk of Getting Diabetes NATIONAL DIABETES INFORMATION CLEARINGHOUSE
 NATIONAL DIABETES INFORMATION CLEARINGHOUSE Am I at Risk for type 2 Diabetes? Taking Steps to Lower the Risk of Getting Diabetes U.S. Department of Health and Human Services National Institutes of Health
NATIONAL DIABETES INFORMATION CLEARINGHOUSE Am I at Risk for type 2 Diabetes? Taking Steps to Lower the Risk of Getting Diabetes U.S. Department of Health and Human Services National Institutes of Health
How To Treat Dyslipidemia
 An International Atherosclerosis Society Position Paper: Global Recommendations for the Management of Dyslipidemia Introduction Executive Summary The International Atherosclerosis Society (IAS) here updates
An International Atherosclerosis Society Position Paper: Global Recommendations for the Management of Dyslipidemia Introduction Executive Summary The International Atherosclerosis Society (IAS) here updates
Weight Loss Surgery. Our Surgeons. A Patient s Guide
 Our Surgeons Our bariatric surgeons and support staff provide the information and support necessary to achieve substantial and sustainable weight loss. Our surgeons: Weight Loss Surgery A Patient s Guide
Our Surgeons Our bariatric surgeons and support staff provide the information and support necessary to achieve substantial and sustainable weight loss. Our surgeons: Weight Loss Surgery A Patient s Guide
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
EMEA RM DRAFT GUIDANCE ISPE RESPONSE 1
 EMEA RM DRAFT GUIDANCE ISPE RESPONSE 1 October 4, 2005 Guideline on Risk Management Systems for Medicinal Products for Human Use DRAFT London, 6 September 2005. This guideline will be included as chapter
EMEA RM DRAFT GUIDANCE ISPE RESPONSE 1 October 4, 2005 Guideline on Risk Management Systems for Medicinal Products for Human Use DRAFT London, 6 September 2005. This guideline will be included as chapter
Likelihood Approaches for Trial Designs in Early Phase Oncology
 Likelihood Approaches for Trial Designs in Early Phase Oncology Clinical Trials Elizabeth Garrett-Mayer, PhD Cody Chiuzan, PhD Hollings Cancer Center Department of Public Health Sciences Medical University
Likelihood Approaches for Trial Designs in Early Phase Oncology Clinical Trials Elizabeth Garrett-Mayer, PhD Cody Chiuzan, PhD Hollings Cancer Center Department of Public Health Sciences Medical University
Guideline for Industry
 Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Appendix: Description of the DIETRON model
 Appendix: Description of the DIETRON model Much of the description of the DIETRON model that appears in this appendix is taken from an earlier publication outlining the development of the model (Scarborough
Appendix: Description of the DIETRON model Much of the description of the DIETRON model that appears in this appendix is taken from an earlier publication outlining the development of the model (Scarborough
HEDIS CY2012 New Measures
 HEDIS CY2012 New Measures TECHNICAL CONSIDERATIONS FOR NEW MEASURES The NCQA Committee on Performance Measurement (CPM) approved five new measures for HEDIS 2013 (CY2012). These measures provide feasible
HEDIS CY2012 New Measures TECHNICAL CONSIDERATIONS FOR NEW MEASURES The NCQA Committee on Performance Measurement (CPM) approved five new measures for HEDIS 2013 (CY2012). These measures provide feasible
CSAPH Rep. 3-A-13 -- page 2 of 14
 CSAPH Rep. 3-A-13 -- page 2 of 14 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 psychological disorders
CSAPH Rep. 3-A-13 -- page 2 of 14 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 psychological disorders
Adoption by CHMP for release for consultation November 2010. End of consultation (deadline for comments) 31 March 2011
 1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
The National Diabetes Prevention Program: An Update on Efforts in Michigan
 The National Diabetes Prevention Program: An Update on Efforts in Michigan Gretchen A. Piatt, PhD, MPH Assistant Professor Department of Medical Education University of Michigan 10/11/12 WHY PREVENT DIABETES?
The National Diabetes Prevention Program: An Update on Efforts in Michigan Gretchen A. Piatt, PhD, MPH Assistant Professor Department of Medical Education University of Michigan 10/11/12 WHY PREVENT DIABETES?
NICE made the decision not to commission a cost effective review, or de novo economic analysis for this guideline for the following reasons:
 Maintaining a healthy weight and preventing excess weight gain in children and adults. Cost effectiveness considerations from a population modelling viewpoint. Introduction The Centre for Public Health
Maintaining a healthy weight and preventing excess weight gain in children and adults. Cost effectiveness considerations from a population modelling viewpoint. Introduction The Centre for Public Health
CASE B1. Newly Diagnosed T2DM in Patient with Prior MI
 Newly Diagnosed T2DM in Patient with Prior MI 1 Our case involves a gentleman with acute myocardial infarction who is newly discovered to have type 2 diabetes. 2 One question is whether anti-hyperglycemic
Newly Diagnosed T2DM in Patient with Prior MI 1 Our case involves a gentleman with acute myocardial infarction who is newly discovered to have type 2 diabetes. 2 One question is whether anti-hyperglycemic
First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases
 First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases Andrew E. Mulberg, MD, FAAP Division Deputy Director OND/ODE3/DGIEP FDA Partnership is the Key Coming together is a beginning;
First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases Andrew E. Mulberg, MD, FAAP Division Deputy Director OND/ODE3/DGIEP FDA Partnership is the Key Coming together is a beginning;
The opinions expressed herein are our own and do not necessarily reflect the views of The Johns Hopkins University.
 Page 1 of 5 Johns Hopkins Center for a Livable Future 615 North Wolfe Street, W7010 Baltimore, MD 21205 July 12, 2012 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
Page 1 of 5 Johns Hopkins Center for a Livable Future 615 North Wolfe Street, W7010 Baltimore, MD 21205 July 12, 2012 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
ACCELERATING BIOTECHNOLOGY INNOVATION
 ACCELERATING BIOTECHNOLOGY INNOVATION FOR RARE DISEASES: Challenges and solutions Emil D. Kakkis, M.D., Ph.D. President and Founder, EFRD Also CEO, Ultragenyx Pharmaceutical Inc. April 17, 2013 1 EveryLife
ACCELERATING BIOTECHNOLOGY INNOVATION FOR RARE DISEASES: Challenges and solutions Emil D. Kakkis, M.D., Ph.D. President and Founder, EFRD Also CEO, Ultragenyx Pharmaceutical Inc. April 17, 2013 1 EveryLife
Recommendations for Prescribing Exercise to Overweight and Obese Patients
 10 Recommendations for Prescribing Exercise to Overweight and Obese Patients 10 10 Recommendations for Prescribing Exercise to Overweight and Obese Patients Effects of Exercise The increasing prevalence
10 Recommendations for Prescribing Exercise to Overweight and Obese Patients 10 10 Recommendations for Prescribing Exercise to Overweight and Obese Patients Effects of Exercise The increasing prevalence
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Corporate Presentation May 13, 2015
 Corporate Presentation May 13, 2015 Creating the Next Generation of CNS Drugs Forward-Looking Statement This presentation contains forward-looking statements. These statements relate to future events and
Corporate Presentation May 13, 2015 Creating the Next Generation of CNS Drugs Forward-Looking Statement This presentation contains forward-looking statements. These statements relate to future events and
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
The first endoscopically-delivered device therapy for obese patients with type 2 diabetes
 DIABETES WEIGHT ENDOBARRIER THERAPY The first endoscopically-delivered device therapy for obese patients with type 2 diabetes Restore the metabolic health of your patients with EndoBarrier Therapy. Dual
DIABETES WEIGHT ENDOBARRIER THERAPY The first endoscopically-delivered device therapy for obese patients with type 2 diabetes Restore the metabolic health of your patients with EndoBarrier Therapy. Dual
