Breast Implants A Review
|
|
|
- Earl Short
- 10 years ago
- Views:
Transcription
1 Acta chir belg, 2004, 104, Breast Implants A Review D. Van Zele, O. Heymans Department of Plastic, Reconstructive & Aesthetic surgery, Surgery of the hand and Burncenter, University Hospital Sart Tilman, Liège, Belgium. Key words. History ; review ; breast implant ; breast prosthesis ; silicone ; saline ; complications. Abstract. Breast implants have been used for about four decades for both reconstructive and aesthetic purposes. In 1963, the quality of the artificial implants was revolutionized by the introduction of the silicone gel-filled implant. Since, this modern prosthesis has gone through an evolution of change and improvement with several types of devices with many variations and styles within each class. Actually, for the last three decades, approximately one million women have received silicone breast implants in the USA. But, in 1992, the American FDA banned silicone from the market, leaving saline implants as the only product generally available as an alternative until now. Other filler materials were introduced, but have never progressed beyond the experimental stage in the USA (in contrast with Europe). The evolution of the different implants through time, with their advantages and disadvantages will be discussed, but also the controversy on silicone implants in the USA and their suspected association with systemic diseases. Review In 1854, VELPEAU was the first surgeon who described in his essay Traité des maladies de sein et de la region mammaire techniques which modified and corrected altered shapes and sizes of the breast. GERSUNY proposed, in 1889, local paraffin injections to increase the volume of the breast. Although previously, a cosmetic amelioration could be noted, on he long run numerous complications appeared as solid massesgranulomas, cutaneous erosion and cutaneous fistulas, migration, blindness or pulmonary embolism. In 1895, CZERNY described a successful transplantation of a lipoma, which had the size of a fist, from the back into a breast after excision of a fibro adenoma, and this for pure aesthetic purposes. Other autologous materials like dermis grafts and fatdermis grafts (taken from the gluteal folds) were examined for their potential by E. LEXER in 1925 and by BERSON in 1944, but the results appeared to be too unpredictable, with atrophy noted up to 50%. Giant calcifications and cysts of fat necrosis were frequently observed (1). In the fifties, the injection of liquid silicone appeared and was performed for augmentation of the breast and facial contours. At that time, surgeons thought that silicone was a completely inert substance with the advantage that its consistence approached that of normal breast tissue. In many cases though, non medical-grade silicone was used, under dubious conditions. Eventually, as was noticed before with the paraffin injections, important complications occurred such as hard, painful nodules and/or inflammation. These phenomena could be that serious that the only solution to the problem was a SC mastectomy to relieve the patient! (2-4). It is still performed in some Asian countries. In the past, implants made of ivory or glass have been unsuccessfully utilized. Since 1951, an increasing number of plastic surgeons became interested in the use of implants for augmentation mammaplasty, when PANGMANN introduced a sponge-like implant made of polyvinyl alcohol (PVA) (5-6). The goal of this spongy structure was to promote integration of the implant into the surrounding breast tissue by growth into the pores of the sponge. But what was thought to be an advantage, appeared to be a disadvantage because incrustation and scar retraction reduced the volume and softness of the implant. Calcification and asymmetry were frequently seen and sometimes the implant contracted into a rock-hard formation. So, the implant was modified and the sponge encapsulated in a polyethylene sac. This sac was supposed to block the infiltration and thus prevent the complications mentioned above. But nevertheless, the same problems appeared because of the non- water tightness of the envelope.
2 Breast Implants 159 In the sixties, the quality of the artificial implants was improved to a degree that rendered these acceptable as a method of treatment. The design of the implant was revolutionized by T. CRONIN and F. GEROW (7). They presented their experience with a new round implant. They wrapped a smooth and thin envelope of rubbery silicone elastomer (polysiloxisanes) around a soft but firm silicone-gel compound (Fig. 1). Silicone is one of the least bioreactive materials used in medical devices. It is used in tracheotomy tubes, the coating of surgical needles, suture thread or pacemakers, heart valves, facial implants for birth defects, artificial lenses for the eyes, CSF shunts, artificial joints or catheters. Compared to the already existing implants, these new implants conserved better their form, softness and also provoked less local tissue reaction. Quickly this natural feel Silastic gel prosthesis conquered the whole world and became the implant of choice and the golden standard. In 1968, DEMPSEY and LATHAM proposed a submuscular- retropectoral-implantation of the implant in order to diminish capsular formation and/or breast shape changes (8). This technique reduced the capsular contracture rate from 40% to 5%. A year later, Cronin and GEROW modified the design of the implant by making the envelope thinner and stronger and redesigning the shape to a tear drop, resembling a normal looking breast (9). In addition, Dacron patches were integrated on the posterior side of the implants in the hope it would stabilize its position by the integration of these patches to the pectoralis fascia. A few years later, it was found that this tear dropshaped Dacron-backed prosthesis reacted unfavourably to any tendency to capsular contracture. For this reason thin-walled discoid non-backed prostheses progressively became the favoured shape and design. Fig. 2 Development of capsular contracture bilaterally following breast augmentation resulting in breast firmness and implant malposition. In the seventies, animal research done on the implantation of synthetic materials in living tissue showed that a textured implant surface lowered the capsular contracture incidence around the implant (Fig. 2). ASHLEY described a new textured gel-filled implant with a polyurethane foam coating the silicone shell (10). Although the coating was originally planned as a fixation layer, this foam cover resulted in decreased capsular contracture (2-15% when placed sub-glandularly compared to 20-59% when smooth implants are used) and remained soft more consistently than the smooth surface implant (fibroblast proliferation into the polyurethane in many directions) (11). But other studies showed that the polyurethane would degrade and release 2-toluene diamine (TDA), a breakdown product that was known to cause hepatomas in highly cancer-prone rats when fed with high-dose TDA (12, 13). Fig. 1 Smooth silicone-filled prosthesis Fig. 3 Close up view of the textured surface of a breast implant
3 160 D. Van Zele and O. Heymans The manufacturer voluntarily withdrew the polyurethane-coated implants from the market in 1991, although TDA never has been documented to be a human carcinogen nor was there evidence of TDA being formed in vivo from polyurethane. Actually, these charges have proved to be unfounded! In the early 80 s, new formulations of the shell became available that were stronger and minimized the bleed or diffusion of what is usually tiny amounts of the silicone oil fraction of the gel contents. These newer barrier coat devices, also called lowbleed implants, bleed as little as 1 :10 the rate of the older models. H. BECKER introduced a permanent inflatable implant to augment the breast for aesthetic reasons in the early eighties (14-15). The technique was an improvement compared to that previously described by RADOVAN, in 1978, in which a temporary tissue expander needed to be replaced with a permanent implant (16). The advantages of this kind of implant were a shorter scar and the possibility to adjust the volume peroperatively and postoperatively to obtain a perfect symmetry. A variation of the inflatable implant is brought by the double lumen implant, first introduced by J. HARTLEY in 1973, which is generally constructed with an inner silicone-filled elastomer bag and an outer saline-filled bag. The reverse configuration also exists and is mostly used as a tissue expander after mastectomy. Because the implant surface morphology appeared to be the determining factor of the importance of periprosthetic contracture (which was noticed using the polyurethane-coated implants), research was oriented towards the fabrication of textured envelopes that avoided the long-term doubted surfaced to textured-surfaced envelopes, which seem to minimize the incidence of unwanted firmness from capsular contracture (16-21) (Fig. 3). In 1990, the implant with an anatomical shape, based on the teardrop design introduced by CRONIN and GEROW was reintroduced. This design was inspired by the natural form of the breast and was supposed to bring a better aesthetic result to the upper pole of the breast, which is often too full when round implants are used. January, the 6th 1992, the FDA Administration Commissioner D. KESSLER mandated a temporary moratorium on the use of silicone gel implants.. Manufacturers of implants should provide additional evidence to the already existing one to prove their safety (many studies in the early literature had already concluded that some local complications could occur, and safety studies already suggested no systemic toxicity in animals). The General and Plastic Surgery Devices Panel recommended that the further use of implants should be limited for reconstruction only, that women receiving the implants participate in scientific protocols and that epidemiological studies be conducted to assess the risk of auto-immune diseases. Their decision did not apply to saline-filled implants, although many of the local complications of gel-filled implants were also associated with saline implants. The latter were permitted to remain on the market unrestrictely for both reconstruction and augmentation. The FDA considered saline-filled implants less risky, because although they have the same silicone rubber envelope as gel-filled implants, leakage or rupture would release only salt water, not silicone gel, into the body. How did it get so far? In 1977, a Houston attorney, R. MITHOFF, won the first lawsuit for a Cleveland woman who claimed that her ruptured implants and subsequent operations had caused pain and suffering. She received a $ settlement from the Dow Corning Company. In the beginning of the 80 s The Public Citizen Research Group sent out warning signals that silicone implants could cause cancer in women. At that time, only a dozen isolated case reports in medical literature were published about the possible association of silicone implants and the induction of illnesses. Consequently, these reports were followed by the publication of some case series which received a rush of publicity. This made the FDA decide, in 1988, to reclassify the silicone breast implants into a Class III category which required manufacturers to prove their safety in order to keep their products on the market. Valid scientific data would be evaluated. The questions on implant safety exploded nationally in 1990 when a talk show Face-to-face with Connie Chung detailed the horrors of breast implants. Her report sparked a wave of concern among women with implants and increased pressure on government officials to act. The FDA called a halt to the use of silicone gelfilled implants in January, the 6th of This led the way for a variety of litigation and class action lawsuits against implant manufacturers, before sufficient scientific data were gathered to prove or disprove the symptoms attributed to implants. Almost every disease and symptom complex from scleroderma to chronic fatigue syndrome to multiple sclerosis and amyotrophic lateral sclerosis have been blamed on the breast implant (22). In 1994 already individual lawsuits have been filed against the Dow Corning Company, compared to 3558 two years before. The company is facing bankrupt, with 20000
4 Breast Implants 161 lawsuits and about potential claims having been filed in the global settlement. In that same year, a Mayo Clinic epidemiological study is published in the New England Journal of Medicine, which found no increased risk of connective tissue disease and other disorders studied in women with silicone implants (23). The American College of Rheumatology came also to the conclusion, in 1995, that implants did not cause systemic diseases (24). The American Academy of Neurology reviewed, in 1997, existing silicone implant studies and reported that the existing research showed no link between silicone implants and neurological disorders (25). The Journal of National Cancer Institute published a review of scores of medical studies concluding that breast implants did not cause cancer (26). The Institute of Medicine of the National Academy of Sciences released its final report after a 2-year investigation on the possible involvement of silicone implants in systemic or connective tissue diseases as lupus, cancer and scleroderma (27). In that report, it is clearly stated that there is no causal relationship between silicone implants and systemic diseases. Furthermore, an increase in recurrent breast cancer could NOT be proven, some of the studies reviewed even suggest lower rates of breast cancer in implanted women (28). In 2000, JANOWSKY et al. published the results of their metaanalysis of the relationship between silicone implants and the risk of connective tissue diseases in the New England Journal of Medicine (29). They analysed the results of 20 major studies on this subject but could not find evidence of an association between breast implants. Today, the silicone gel-filled implants remain off the market in the USA pending manufacturer safety studies and this at least until What are the forces driving the implant controversy despite of the weight of scientific evidence (12) : A. The media (shows, magazines, hysterical behaviour) This type of press has created an undue amount of anxiety in many women, which in turn has provoked litigious behaviour and inappropriate demands for explant surgery! B. Class Action Plaintiffs attorneys The suing of implant manufacturers became a boom industry in the US $10 million or more may be owed to you!!, with some lawyers out to convince women that even though they may feel just fine, they are really sick and must be properly compensated. C. Radical Feminist Activist Groups It was their opinion that the preference for big breasts represented female oppression! And if preferring large breasts was oppression, then the implants used to enlarge one s breasts are tools of oppression. After having finished this special topic on silicone implants, we will continue and pick up the thread in Since the ban in that same year on silicone gel implants, research has been conducted to find a suitable substitute. Implants with a silicone elastomer shell containing normal saline have been used as an alternative. Salinefilled implants have been introduced for the first time by ARION in 1965, but since then they comprised only 10% of the implants placed (30). The controversy on the safety of silicone implants has led to a spectacular increase of the use of saline-filled implants in the USA (10% à 95%) because saline is wholly harmless and if the silicone elastomer ruptures, the saline solution is rapidly absorbed by the surrounding tissues. They also had the advantage of being harmless and also resulted in very low capsular contracture rates, especially when placed behind the pectoralis muscle (1-6%) (20, 31-35). But, on the other hand, they have a less natural appearance (folds, consistence) and an incidence rate of deflation of 1-16% (20, 31, 36) They are actually available in round or contoured shapes and textured or smooth shells, single or double lumen. The double lumen implants are generally constructed with an inner silicone-filled elastomer bag and an outer saline-filled bag. The reverse configuration also exists and is mostly used as a tissue expander after mastectomy. There are several valve types : anterior diaphragm, posterior leaf, or postoperative adjustable valves. As already mentioned, substances as oils (peanut / triglycerids / soybean - Trilucent ) and hydrogels (Biooncotic gel) (36) were also presented as alternatives but these potential substitutes have not progressed yet beyond the experimental stage in the USA. Oils have excellent radiolucency and their consistency approach that one of the breast. On the contrary, their biocompatibility is questionable (if the implant were to break, oil would be dispersed in a multitude of immersible droplets because oil is hydrophobic and not readily soluble. It may clog lymphatics and macrophages for weeks or months until it might be dispersed), bacteria may grow in some vegetable oils and oxygen transmission across the shell may in time cause degradation of oils or fats. In the United Kingdom, more than 5000 women had been implanted with the Trilucent implant between 1995 and 1999 (37-39). In June 2000, the MDA (Medical Device Agency) issued a hazard notice recommending that all women who had been implanted with Trilucent implants, which were sold in the UK between 1995 and 1999, consider having their implants removed as a precautionary measure and that until those implants can be removed, women should not conceive or breastfeed.
5 162 D. Van Zele and O. Heymans Toxicological preliminary data suggested that genotoxic products as aldehyde products (n-alkanals) may be created when the soybean breaks down. (diffusion/rupture) : based on current scientific knowledge, there is no reason to think that such risk exists (12). A recent series on explanted Trilucent breast implants confirmed the suspicion that a high percentage of these implants bleed, 34% in this series! Patients may be asymptomatic, or they may present with rippling if they have moderate bleed, or with implant deflation when the oil leak is significant enough to manifest as volume loss (40). The rate of significant capsular contracture (Baker 3-4) was approximately as high as 45% (41). The hydrogel-filled implant has a textured surface and is pre-filled with polyvinylpyrrolidone (PVP) hydrogel or bio-oncotic gel. Hydrogel has better lubricating properties than saline, some increased viscosity and does not harbour bacteria (42). On the contrary, a recent series concluded that the MISTI Gold implants did not fully fill the criteria of safe implants. This was based on the findings that 59% of the implants placed, were removed after 4 years. The main reason for the removal was an increase in volume of 38%, followed by capsular contracture in 17% (43). Other potential fillers as air or even breast tissue were tried but abandoned. Cohesive silicone gel is the latest filler innovation on the market (not in the USA) (44). Cohesive gel was already used in anatomical implants, but are recently also available in round implants. The advantages of cohesive gel implants are primarily that they provide a proportionate sized breast augmentation with a very soft and natural-feeling breast implant. The implant maintains its shape in an upright position (has a memory ) which decreases the incidence of folding of the implant shell and the development of rippling. If a cohesive gel implant ruptures, it maintains both its shape and integrity. As well, there are nine models of anatomical cohesive gel implants which vary with respect to height, projection and base width. This provides flexibility for the patient and surgeon in determining the appropriate implant to balance body proportions and breast aesthetics. One disadvantage of cohesive gel implants is that they require a slightly larger incision for insertion. Because of this, insertion is usually performed through an incision in the fold under the breast or occasionally through an incision around the areola. Cohesive gel implants are very difficult to insert through an incision in the armpit. This large diversity of implants also means that none of these implants is perfect. Today, the possible complications that might occur after a breast augmentation are still the same as before ; with that difference that their frequency diminished. In contrast to the use of breast implants for purely aesthetic purposes (Fig. 4, 5), they are also routinely used for breast reconstruction after a mastectomy for breast cancer. A variety of reconstructive techniques exist and the choice of the technique depends both on the present local tissue conditions of the breast and the surgeon s preference. In general, the reconstruction of the breast is performed as a delayed procedure and is not done immediately after the mastectomy in a single operation. In selected cases though (very small tumours, prophylactic breast ablation, excellent local tissue conditions), an immediate reconstruction is possible, using a definitive breast prosthesis or a inflatable expander, which will be filled postoperatively with a saline solution. This expander can be temporary or definitive, depending on the surgeon s preference. In case of a temporary expansion, a second intervention is planned a few months after the first one : the expander is replaced by a definitive implant and more preferably an anatomical shaped textured gel-filled prosthesis. This gel-filled prosthesis is exactly the same as the one used for aesthetical breast augmentation. The advantages and disadvantages of each type of filler material (saline vs gel) have already been discussed previously. When the breast reconstruction is performed as a delayed procedure, several reconstructive possibilities exist. Here, the local tissue quality is a very important factor to consider preoperatively. If the skin has been previously irradiated or if several local unfavourable conditions are present (adherent scar onto the thoracic wall, absent subcutaneous tissues and/or pectoralis muscle), the choice of introducing an expander alone is contraindicated, because of the very high extrusion rate. In this situation, to avoid potential implant exposition, healthy tissue should be added to cover the expander. This coverage is provided by a pedicled latissimus dorsi musculocutaneous flap (Fig. 6, 7). More recently, other techniques have been developed using only autologous tissue to avoid the afore mentioned disadvantages of breast implants. Autologous tissue taken from the abdomen or gluteal region is transferred as a free flap into the mastectomy site. In loco, a microsurgical anastomosis is then realized between the artery and vein of the flap and local recipient vessels (internal mammary or thoracodorsal vessels). These free perforator flaps include mainly the DIEP (deep Inferior epigastric perforator) and SGAP (superior gluteal artery perforator) flap and free TRAM flap (45-47) (Fig. 8, 9).
6 Breast Implants 163 Fig. 4 Preoperative view Fig. 5 Postoperative view of an aesthetic breast augmentation with subpectoral insertion of an anatomically shaped textured silicone-filled breast prosthesis. Fig. 6 Preoperative view of the mastectomy site on the left side Fig. 7 Postoperative view of a breast reconstruction with a pedicled latissimus dorsiflap and prosthesis with subsequent nipple-areola reconstruction and tattooing. Contralateral breast reduction and symmetrization. Fig. 8 Preoperative view of the mastectomy site at the right side Fig. 9 Postoperative view of a breast reconstruction by autologous tissue only by means of a DIEP flap and after subsequent nipple-areola reconstruction.
7 164 D. Van Zele and O. Heymans Today, this operation is also performed as an immediate procedure after mastectomy. Conclusion Breast implants have been used for about four decades for both reconstructive and aesthetic purposes. The use of silicone-gel implants in breast surgery dates back to 1963, when two doctors wrapped a thin envelope of rubbery silicone elastomer around a soft but firm siliconegel compound. Over the years, a variety of improvements have been made in both the gel compound and containing envelope, but today the basic implant design remains the same. Since the ban on silicone gel implants in the USA (in Belgium, on the contrary, silicone implants remained on the market and were never banned because lack of proved scientific evidence), research has been conducted to find a suitable substitute and implants with a silicone elastomer shell containing normal saline ( saltwater ) have been used as an alternative. However, saline breast implants are generally thought not to have as natural a feel or appearance as do silicone gel breast implants. Additionally, they have a high incidence rate of deflation. Furthermore, none of the other potential substitutes, such as soy or peanut oil-filled prostheses, have progressed beyond the experimental stage in the USA. Nothing man made has eternal life and implants are not an exception to this rule. Inherent in the process of implant aging is a range of complications based on scientific evidence, that should be discussed with the patient. Silicone gel breast implants may rupture and cause local symptoms, but they have not been demonstrated to be a systemic health hazard for patients who have undergone augmentation mammaplasty or postmastectomy reconstruction. In search of reducing furthermore the possibility of rupture and bleeding, the cohesive silicone gel implant was recently introduced on the market. The great advantage of this implant is that it maintains both its shape and integrity If it ruptures. References 1. BERSON M. Dermal-fat transplants used in building up the breast. Surgery, 1945, 15 : CHEN Th. Silicone injection granulomas of the breast : treatment by subcutaneous mastectomy and immediate subpectoral breast implant. Br J Plast Surg, 1995, 48 : PARSONS R. W., THERING H. R. Management of the siliconeinjected breast. Plast Reconstr Surg, 1997, 60 : MORGENSTERN L., GLEISHMAN S., MICHEL S., ROSENBERG J., KNIGHT I., GOODMAN D. Relation of free Silicone to the Human Breast Carcinoma. Arch Surg, 1985, 120 : GLATT B. S., AFIFI GHADA, NOONE R. B. Long-Term Follow-Up of a Sponge Breast Implant and Review of the Literature. Ann Plast Surg, 1999, 42 : PANGMAN W. J., WALLACE R. M. The Use of Plastic Prosthesis in Breast Plasic and Other Soft Tissue Surgery. West J Surg, 1955, 63 : CRONIN T. D., GEROW F. J. Augmentation Mammoplasty : a New natural feel prosthesis. Transactions of the IIIrd Congress of Plastic Surgery, 1963 : DEMPSEY W. C., LATHAM W. D. Subpectoral implants in augmentation mammoplasty. Plast Reconst Surg, 1968, 42 : CRONIN T. D., GREENBERG R. L. Our Experience with the Silastic Gel Braest Prosthesis. Plast Reconst Surg, 1970, 46 : ASHLEY F. L. Further Studies on the Natural-Y Breast Prosthesis. Plast Reconst Surg, 1972, 49 : PENNISI V. R. Long-Term Use of Polyuerthane Breast Prosthese : A 14-year Experience. Plast Reconst Surg, 1990, 86 : ROHRICH R. J., MUZAFFAR A. R. Silicone-Gel Breast Implants : Health and Regulatory Update Prepared for the American Council on Science and Health (ACSH). 13. FDA, FDA Talkpaper TDA and polyurethane breast implants, BECKER H. Breast Augmentation Using the Expander Mammary Prosthesis. Plast Reconst Surg, 1987, 79 : CAMILLERI I. G., MALATA C. M., STAVRIANOS, MCLEAN N. R. A Review of 120 Becker Permanent Tissue Expanders in Reconstruction of the Breast. Br J Plast Surg, 1996, 49 : RADOVAN C. Breast Reconstruction after mastectomy using the temporary expander. Plast Reconst Surg, 1982, 69 :, MAHLER D., HAUBEN D. J. Retromammary vs Retropectoral Breast Augmentation- A Comparative Study. Ann Plast Surg, 1982, 8 : SCHUSTER D. I., LAVINE D. M. Nine-Year Experience with Subpectoral Breast Reconstruction after Subcutaneous Mastectomy in 98 patients Utilizing Saline-inflatable Prostheses. Ann Plast Surg, 1988, 21 : SHAPIRO M. A. Smooth vs Rough : An 8-Year Survey of Mammary Protheses. Plast Reconst Surg, 1989, 84 : GYLBERT L., ASPLUND O., JURELL G. Capsular Contracture after Breast Reconstruction with Silicone-Gel and Saline-Filled Implants : A 6-Year Follow-Up. Plast Reconst Surg, 1990, 85 : ASPLUND O., GYLBERT L., JURELL G., WARD C. Textured or Smooth Implants for submuscular Breast Augmentation : A Controlled Study. Plast Reconst Surg, 1996, 97 : VASEY F., FELDSTEIN J. The Silicone Breast Implant Controversy. Freddom, CA : Crossing Press, 1993 : pp GABRIEL S. E., O FALLON W. M. et al. Risk of Connective-Tissue Diseases and other Disorders after Breast Implantation. NEJM, 1994, 330 : BRODY G. S., DEAPEN D. M. et al. Concensus Statement on the Relationship of Breast Implants to Connective Tissue Disorders. Plast Reconst Surg, 1996, 90 : FERGUSON J. H. Silicone Breast Implants and Neurologic Disorders. Report of the Practice Committee of the American Academy of Neurology. Neurology, 1997, 48 : GRIGG M., BONDURANT S. et al. Information for Women About the Safety of Silicone Implants. Institute of Medecine, National Academy of Sciences. National Academy Press Institute of Medecine, National Academy of Sciences. National Academy Press, DEAPEN D. M., BERNSTEIN L., BRODY G. Are Breast Implants Anticarcinogenic? A 14-year Follow-up of the Los Angeles Study. Plast Reconst Surg, 1997, 99 : JANOWSKY E. C., KUPPER L. L., HULKA B. S. Meta-Analysis of the Relation Between Silicone Breast implants and the risk of Connective Tissue Diseases. NEJM 2000, 342 : ARION H. G. Présentation d une prothese retromammaire. J Soc Fr Gynecol, 1965, 35 : GUTOWSKI K. A., MESNA G. T., CUNNINGHAM B. L. Saline-Filled Breast Implants : A Plastic Surgery Educational Foundation Multicenter Outcomes Study. Plast Reconst Surg, 1997, 100 :
8 Breast Implants HART D. Women and Saline Breast Implant Surgery. Plast Surg Nursing, 1995, 15 (3) : SPEAR S. L., ELMARAGHY M., HESS C. Textured-Surface Saline- Filled Silicone Breast Implants for Augmentation Mammoplasty. Plast Reconst Surg 2000, 105 : MLADICK R. A. No-Touch Submuscular Saline Breast Augmentation Technique. Aesthetic Plast Surg, 1993, 17 : MLADICK R. A. Inflatable Breast Implants. Plast Reconst Surg, 1995, 95 : ERSEK R. A., SALISBURY A. V. Textured Surface, Nonsilicone Gel Breast Implants : Four Years Clinical Outcome. Plast Reconst Surg, 1997, 100 : YOUNG L., LUND H., UEDA K., PIDGEON L., WATSON SCHORR M., KREEGER J. Bleed of and Biologic Response to Triglyceride Filler Used in Radiolucent Breast Implants. Plast Reconst Surg, 1996, 97 : RIZKALIA M., WEBB J., CHUO C. B., MATTHEWS R. N. Experience of explantation of Trilucent breast implants. Br J Plast Surg, 2002, 55, CICCHETTI S., SPINA B., NICOLO G., SANTI P. L. Trilucent Breast implants Five year s Experience from an Italian perspective. Ann Plast Surg, 2003, 51 : BENEDIKTSSON K., PERBECK L. G. Fluid Retention in Bioplasty Misti Gold II Breast Prostheses with Development of Capular Contracture. Scand J Plast Reconst Hand Surg, 2000, 34 : PIZA-KATZER H., PUHL P., BALOGH B., WESCHELBERGER G. Longterm results of MISTI Gold breast implants : a retrospective study. PRS 2002, 110 : HEDEN P., JEMBECK J., HOBER M. Breast augmentation with anatomical cohesive gel implants. The world s largest current experience. Clin Plast Surg, 2001, 28 : BLONDEEL P. N. The sensate free superior gluteal artery perforator (S-GAP) flap : a valuable alternative in autologous breast reconstruction. Br J Plast Surg, 1999, 52 : BLONDEEL P. N. One hundred free DIEP flap breast reconstructions : a personal experience. Br J Plast Surg, 1999, 52 : SCHUSTERMAN M. A., KROLL S. S., MILLER M. J. et al. Ann Plast Surg., 1994, 32 : , discussion : D. Van Zele Department of Plastic, Reconstructive & Aesthetic surgery Surgery of the hand and Burncenter University Hospital Sart Tilman B-4000 Liège, Belgium
Breast Implant Guide
 Breast Implant Guide Shapes, Sizes, Textures & More Brought to you by: 801.479.5722 Copyright 2013 P2P. All Rights Reserved Contents Overview 5 History & Background 6 Implant Types 8 Implant Shapes 13
Breast Implant Guide Shapes, Sizes, Textures & More Brought to you by: 801.479.5722 Copyright 2013 P2P. All Rights Reserved Contents Overview 5 History & Background 6 Implant Types 8 Implant Shapes 13
Breast Implants and Reconstruction
 Last Review Date: October 9, 2015 Number: MG.MM.SU.fv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: October 9, 2015 Number: MG.MM.SU.fv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
FACTS about. MENTOR MemoryGel. Silicone Gel Filled Breast Implants
 FACTS about MENTOR MemoryGel Silicone Gel Filled Breast Implants Are you considering breast implant surgery but not certain which type of implant to choose? You re not alone. Science-based information
FACTS about MENTOR MemoryGel Silicone Gel Filled Breast Implants Are you considering breast implant surgery but not certain which type of implant to choose? You re not alone. Science-based information
THE MENTOR COMMITMENT
 THE MENTOR COMMITMENT As a leading global manufacturer of the highest quality breast implants, we Mentor and our 1,200 worldwide employee associates strive to provide high-quality products and services
THE MENTOR COMMITMENT As a leading global manufacturer of the highest quality breast implants, we Mentor and our 1,200 worldwide employee associates strive to provide high-quality products and services
Breast Reconstruction Frequently Asked Questions
 Breast Reconstruction Frequently Asked Questions GENERAL Do I need to have breast reconstruction? It is never medically necessary to have breast reconstruction. This is considered an elective procedure,
Breast Reconstruction Frequently Asked Questions GENERAL Do I need to have breast reconstruction? It is never medically necessary to have breast reconstruction. This is considered an elective procedure,
Breast Implants: Local Complications and Adverse Outcomes
 Breast Implants: Local Complications and Adverse Outcomes This booklet highlights the most common problems associated with silicone gel-filled and saline-filled breast implants: those that occur in the
Breast Implants: Local Complications and Adverse Outcomes This booklet highlights the most common problems associated with silicone gel-filled and saline-filled breast implants: those that occur in the
BREAST IMPLANTS (enlargement, augmentation) Dr. Benjamin Van Raalte TYPES OF IMPLANTS saline round implants high profiles low profile shaped
 BREAST IMPLANTS (enlargement, augmentation) Dr. Benjamin Van Raalte has 20 years of experience with breast enlargement including axillary incisions and gel implants. Dr. Van Raalte is the first Quad City
BREAST IMPLANTS (enlargement, augmentation) Dr. Benjamin Van Raalte has 20 years of experience with breast enlargement including axillary incisions and gel implants. Dr. Van Raalte is the first Quad City
Breast reconstruction using an implant after risk-reducing surgery
 Breast reconstruction using an implant after risk-reducing surgery This information is from the booklet Understanding riskreducing breast surgery. You may find the full booklet helpful. We can send you
Breast reconstruction using an implant after risk-reducing surgery This information is from the booklet Understanding riskreducing breast surgery. You may find the full booklet helpful. We can send you
Textured-Surface Saline-Filled Silicone Breast Implants for Augmentation Mammaplasty
 Cosmetic Special Topic Textured-Surface Saline-Filled Silicone Breast Implants for Augmentation Mammaplasty Scott L. Spear, M.D., Mohamed Elmaraghy, M.D., and Christopher Hess, M.D. Washington, D.C. The
Cosmetic Special Topic Textured-Surface Saline-Filled Silicone Breast Implants for Augmentation Mammaplasty Scott L. Spear, M.D., Mohamed Elmaraghy, M.D., and Christopher Hess, M.D. Washington, D.C. The
SUBJECT: MANAGEMENT OF BREAST EFFECTIVE DATE: 12/16/99 IMPLANTS REVISED DATE:
 MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Breast Augmentation. If you are dissatisfied with your breast size, augmentation surgery is a choice to consider. Breast augmentation can:
 Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
Breast Augmentation Primer
 Breast Augmentation Primer Breast augmentation is typically a very rewarding procedure. In order to achieve the highest level of patient satisfaction, many decisions must be carefully made. We have put
Breast Augmentation Primer Breast augmentation is typically a very rewarding procedure. In order to achieve the highest level of patient satisfaction, many decisions must be carefully made. We have put
The Silicone Breast Implant Controversy
 The Silicone Breast Implant Controversy by Susan E. Kolb, M.D., F.A.C.S. There has been a great deal of controversy regarding the safety of silicone breast implants. For the women who have implants, conflicting
The Silicone Breast Implant Controversy by Susan E. Kolb, M.D., F.A.C.S. There has been a great deal of controversy regarding the safety of silicone breast implants. For the women who have implants, conflicting
Medical Policy Original Effective Date: 11-19-08 Revised Date: 1-27-16 Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
INFORMED-CONSENT BREAST AUGMENTATION
 RICHARD A. BARTLETT, M.D. Board Certified-American Board of Plastic Surgery Member-American Society of Plastic Surgeons Member-American Society for Aesthetic Plastic Surgery INFORMED-CONSENT BREAST AUGMENTATION
RICHARD A. BARTLETT, M.D. Board Certified-American Board of Plastic Surgery Member-American Society of Plastic Surgeons Member-American Society for Aesthetic Plastic Surgery INFORMED-CONSENT BREAST AUGMENTATION
COPYRIGHT ASPS. Breast Augmentation. The Symbol of Excellence in Plastic Surgery
 Breast Augmentation The Symbol of Excellence in Plastic Surgery A public education service of the American Society of Plastic Surgeons and the American Society for Aesthetic Plastic Surgery. This brochure
Breast Augmentation The Symbol of Excellence in Plastic Surgery A public education service of the American Society of Plastic Surgeons and the American Society for Aesthetic Plastic Surgery. This brochure
Intracapsular Allogenic Dermal Grafts for Breast Implant Related Problems
 Cosmetic Intracapsular Allogenic Dermal Grafts for Breast Implant Related Problems Richard A. Baxter, M.D. Mountlake Terrace, Wash. Despite advances in surgical techniques and breast implant design, certain
Cosmetic Intracapsular Allogenic Dermal Grafts for Breast Implant Related Problems Richard A. Baxter, M.D. Mountlake Terrace, Wash. Despite advances in surgical techniques and breast implant design, certain
Note: For information related to the medical necessity criteria for mammaplasty procedures, see SURG.00086 Reduction Mammaplasty.
 Subject: Document#: Current Effective Date: 10/01/2008 Status: Revised Last Review Date: 08/28/2008 Description/Scope Reconstructive breast surgery refers to surgical procedures to rebuild the contour
Subject: Document#: Current Effective Date: 10/01/2008 Status: Revised Last Review Date: 08/28/2008 Description/Scope Reconstructive breast surgery refers to surgical procedures to rebuild the contour
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction
 Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction About This Brochure This brochure is intended to provide you with a high level overview of the facts about
Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction About This Brochure This brochure is intended to provide you with a high level overview of the facts about
Page 1 of 7 Patient s Initials 10-01-00 version
 INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
TOM J. POUSTI, MD, F.A.C.S. PLASTIC AND RECONSTRUCTION SURGERY
 TOM J. POUSTI, MD, F.A.C.S. PLASTIC AND RECONSTRUCTION SURGERY INFORMED CONSENT FOR BREAST AUGMENTATION SURGERY INSTRUCTIONS This is an informed consent document that has been prepared to help inform you
TOM J. POUSTI, MD, F.A.C.S. PLASTIC AND RECONSTRUCTION SURGERY INFORMED CONSENT FOR BREAST AUGMENTATION SURGERY INSTRUCTIONS This is an informed consent document that has been prepared to help inform you
Breast Reconstruction Following Mastectomy or Lumpectomy
 Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.pr Go to Comunicados a Proveedores, and click Cartas
Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.pr Go to Comunicados a Proveedores, and click Cartas
Aestheticare Cosmetic Surgery Institute Dr. Ronald E. Moser 30260 Rancho Viejo Rd. San Juan Capistrano, CA 92675 (800) 662-1055
 Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy, weight changes, sun exposure,
Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy, weight changes, sun exposure,
It is important that you read this information carefully and completely.
 Placement of Permanent Breast Implant Following Tissue Expansion 1. I hereby authorize Dr. John P. Stratis and such assistants as may be selected to perform the following procedure or treatment INFORMED-
Placement of Permanent Breast Implant Following Tissue Expansion 1. I hereby authorize Dr. John P. Stratis and such assistants as may be selected to perform the following procedure or treatment INFORMED-
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery
 Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
Breast Augmentation with Silicone Gel Prosthesis: Smooth or Textured Envelopes?
 Breast Augmentation with Silicone Gel Prosthesis: Smooth or Textured Envelopes? São Paulo - Brasil Areola and Breast dimensions Breast Type Access Type of Prosthesis Expectations and Reality No breast
Breast Augmentation with Silicone Gel Prosthesis: Smooth or Textured Envelopes? São Paulo - Brasil Areola and Breast dimensions Breast Type Access Type of Prosthesis Expectations and Reality No breast
Breast Reconstruction Options. Department of Plastic Surgery #290 Santa Clara Homestead Campus
 Breast Reconstruction Options Department of Plastic Surgery #290 Santa Clara Homestead Campus Importance of Breast Reconstruction As successes in treating breast cancer have grown, more women have been
Breast Reconstruction Options Department of Plastic Surgery #290 Santa Clara Homestead Campus Importance of Breast Reconstruction As successes in treating breast cancer have grown, more women have been
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants
 Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Looking for answers about breast reconstruction?
 Looking for answers about breast reconstruction? Start here. Start today. { 1 } { 2 } Taking the mystery out of breast reconstruction If you or someone you care about is considering breast reconstruction
Looking for answers about breast reconstruction? Start here. Start today. { 1 } { 2 } Taking the mystery out of breast reconstruction If you or someone you care about is considering breast reconstruction
IS BREAST AUGMENTATION RIGHT FOR YOU?
 IS BREAST AUGMENTATION RIGHT FOR YOU? (816) 286-4126 www.kansascitysurgicalarts.com For many women, having shapely, full breasts is an important part of feeling feminine. The breasts give the body proportion,
IS BREAST AUGMENTATION RIGHT FOR YOU? (816) 286-4126 www.kansascitysurgicalarts.com For many women, having shapely, full breasts is an important part of feeling feminine. The breasts give the body proportion,
INTERNATIONAL. Breast Augmentation. Options
 INTERNATIONAL Breast Augmentation Options Important Safety Information: Mentor MemoryGel and Mentor saline-filled breast implants are indicated for breast augmentation - in women who are at least 18 years
INTERNATIONAL Breast Augmentation Options Important Safety Information: Mentor MemoryGel and Mentor saline-filled breast implants are indicated for breast augmentation - in women who are at least 18 years
Dr. Justin B. Maxhimer, M.D. Boulder Plastic Surgery: 303-443-2277. IV Seasons Skin Care: 303-938-1666 www.boulderplasticsurgery.
 Dr. Hans R. Kuisle, M.D., F.A.C.S Dr. Winfield Hartley, M.D., F.A.C.S Dr. Justin B. Maxhimer, M.D. 2525 4 th Street, Suite 200, Boulder, CO 80304 Boulder Plastic Surgery: 303-443-2277 IV Seasons Skin Care:
Dr. Hans R. Kuisle, M.D., F.A.C.S Dr. Winfield Hartley, M.D., F.A.C.S Dr. Justin B. Maxhimer, M.D. 2525 4 th Street, Suite 200, Boulder, CO 80304 Boulder Plastic Surgery: 303-443-2277 IV Seasons Skin Care:
Chapter 24. Evolution of Procedures
 Chapter 24 BREAST SURGERY KEY FIGURES: Saline implant reconstruction Latissimus dorsi reconstruction Free TRAM reconstruction In the developed world, breast reconstruction after mastectomy and breast reduction
Chapter 24 BREAST SURGERY KEY FIGURES: Saline implant reconstruction Latissimus dorsi reconstruction Free TRAM reconstruction In the developed world, breast reconstruction after mastectomy and breast reduction
Breast Augmentation Amsterdam Plastic Surgery Breast Augmentation Overview
 Breast Augmentation Amsterdam Plastic Surgery Breast Augmentation Overview The long-lasting results of breast augmentation are not limited to just physical changes as data documents that many patients
Breast Augmentation Amsterdam Plastic Surgery Breast Augmentation Overview The long-lasting results of breast augmentation are not limited to just physical changes as data documents that many patients
Implant Selection: Getting it right the first time. Bruce Cunningham MD, MSc Professor of Surgery University of Minnesota
 Implant Selection: Getting it right the first time Bruce Cunningham MD, MSc Professor of Surgery University of Minnesota USA Surgeon Survey Mentor Memory-Gel implants became available in November of 2006
Implant Selection: Getting it right the first time Bruce Cunningham MD, MSc Professor of Surgery University of Minnesota USA Surgeon Survey Mentor Memory-Gel implants became available in November of 2006
Medical Policy Reconstructive Breast Surgery/Management of Breast Implants
 Medical Policy Reconstructive Breast Surgery/Management of Breast Implants Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References
Medical Policy Reconstructive Breast Surgery/Management of Breast Implants Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References
INFORMED-CONSENT-OPEN CAPSULECTOMY AND BREAST IMPLANT EXCHANGE
 INFORMED-CONSENT-OPEN CAPSULECTOMY AND BREAST IMPLANT EXCHANGE Instructions This is an informed-consent document that has been prepared to help inform you about open capsulectomy and breast implant exchange,
INFORMED-CONSENT-OPEN CAPSULECTOMY AND BREAST IMPLANT EXCHANGE Instructions This is an informed-consent document that has been prepared to help inform you about open capsulectomy and breast implant exchange,
Fat Injection to Correct Contour Deformities in the Reconstructed Breast
 Fat Injection to Correct Contour Deformities in the Reconstructed Breast Scott L. Spear, M.D., Henry B. Wilson, M.D., and Michelle D. Lockwood, M.D. Washington, D.C. Background: A ten-year, single-surgeon
Fat Injection to Correct Contour Deformities in the Reconstructed Breast Scott L. Spear, M.D., Henry B. Wilson, M.D., and Michelle D. Lockwood, M.D. Washington, D.C. Background: A ten-year, single-surgeon
Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 4-Year Follow Up
 Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 4-Year Follow Up October 2010 - March 2015 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year
Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 4-Year Follow Up October 2010 - March 2015 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year
A Girlfriend s Guide. Breast Augmentation WWW.RPSMD.COM
 A Girlfriend s Guide to Breast Augmentation 1 Your Breast Augmentation Surgery: This is an important decision that you are considering. Have you always thought about increasing the size of your breasts,
A Girlfriend s Guide to Breast Augmentation 1 Your Breast Augmentation Surgery: This is an important decision that you are considering. Have you always thought about increasing the size of your breasts,
BREAST RECONSTRUCTION POST MASTECTOMY
 BREAST RECONSTRUCTION POST MASTECTOMY CLINICAL POLICY Policy Number: SURGERY 095.11 T2 Effective Date: January 1, 2016 Table of Contents CONDITIONS OF COVERAGE... BENEFIT CONSIDERATIONS... COVERAGE RATIONALE...
BREAST RECONSTRUCTION POST MASTECTOMY CLINICAL POLICY Policy Number: SURGERY 095.11 T2 Effective Date: January 1, 2016 Table of Contents CONDITIONS OF COVERAGE... BENEFIT CONSIDERATIONS... COVERAGE RATIONALE...
DOI: 10.1097/01.PRS.0000135945.02642.8B
 CME Breast Augmentation Scott L. Spear, M.D., Erwin J. Bulan, M.D., and Mark L. Venturi, M.D. Washington, D.C. Learning Objectives: After studying this article, the participant should be able to: 1. Understand
CME Breast Augmentation Scott L. Spear, M.D., Erwin J. Bulan, M.D., and Mark L. Venturi, M.D. Washington, D.C. Learning Objectives: After studying this article, the participant should be able to: 1. Understand
Breast Reconstruction
 Breast Reconstruction by Editorial Staff and Contributors En Español (Spanish Version) Click here to view an animated version of this procedure. Definition Breast reconstruction is plastic surgery to rebuild
Breast Reconstruction by Editorial Staff and Contributors En Español (Spanish Version) Click here to view an animated version of this procedure. Definition Breast reconstruction is plastic surgery to rebuild
Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants
 Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled
Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled
Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant
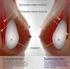 Breast Surgery Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant Larry S. Nichter, MD; and Robert S. Hamas, MD Aesthetic Surgery Journal 32(7) 861 867 2012 The American Society
Breast Surgery Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant Larry S. Nichter, MD; and Robert S. Hamas, MD Aesthetic Surgery Journal 32(7) 861 867 2012 The American Society
Product Catalogue. 2013 Edition
 roduct Catalogue 2013 Edition Eurosilicone is a leading manufacturer within the global plastic surgery community. e are an innovative developer and manufacturer of a complete range of quality surgical
roduct Catalogue 2013 Edition Eurosilicone is a leading manufacturer within the global plastic surgery community. e are an innovative developer and manufacturer of a complete range of quality surgical
Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants
 Augmentation Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants Table of Contents Section Page Glossary...1 1. Considering Silicone Gel-filled Breast
Augmentation Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants Table of Contents Section Page Glossary...1 1. Considering Silicone Gel-filled Breast
NATRELLE 410 Highly Cohesive
 Breast Augmentation NATRELLE 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants Important Factors Breast Augmentation Patients Should Consider Highly Cohesive Anatomically Shaped Silicone-Filled
Breast Augmentation NATRELLE 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants Important Factors Breast Augmentation Patients Should Consider Highly Cohesive Anatomically Shaped Silicone-Filled
Product Reference Guide
 Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants
 Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants
 Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants W. Grant Stevens, MD; Salvatore J. Pacella, MD; Andrew J. L. Gear, MD;
Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants W. Grant Stevens, MD; Salvatore J. Pacella, MD; Andrew J. L. Gear, MD;
BREAST AUGMENTATION PATIENT INFORMATION
 BREAST AUGMENTATION PATIENT INFORMATION Elizabeth J. Hall-Findlay, MD, FRCSC 340-317 Banff Avenue, Box 2009, Banff, Alberta, Canada T1L 1B7 403-762-2055 tel 403-762-8297 fax www.banffplasticsurgery.com
BREAST AUGMENTATION PATIENT INFORMATION Elizabeth J. Hall-Findlay, MD, FRCSC 340-317 Banff Avenue, Box 2009, Banff, Alberta, Canada T1L 1B7 403-762-2055 tel 403-762-8297 fax www.banffplasticsurgery.com
Breast Augmentation and Lifts Explained. Breast Augmentation and Lifts explained
 Breast Augmentation and Lifts explained 1 Your breasts, your choices Whether you are a mother whose breasts have lost their fullness and perkiness due to breastfeeding, or a woman who simply wants to feel
Breast Augmentation and Lifts explained 1 Your breasts, your choices Whether you are a mother whose breasts have lost their fullness and perkiness due to breastfeeding, or a woman who simply wants to feel
Breast Implant Information Booklet. 4th edition
 Breast Implant Information Booklet 4th edition This booklet has been prepared to provide guidance for persons considering the use of silicone gel-filled breast implants. These implants are associated with
Breast Implant Information Booklet 4th edition This booklet has been prepared to provide guidance for persons considering the use of silicone gel-filled breast implants. These implants are associated with
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with Natrelle Silicone-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with Natrelle Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R A c c e p t a n c e O
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with Natrelle Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R A c c e p t a n c e O
Tissue Reinforcement with Strattice Reconstructive Tissue Matrix following Correction of Severe Breast Deformity
 Tissue Reinforcement with Strattice Reconstructive Tissue Matrix following Correction of Severe Breast Deformity Robert Cohen, MD, FACS* Paradise Valley, AZ Case summary A 41-year old woman with a history
Tissue Reinforcement with Strattice Reconstructive Tissue Matrix following Correction of Severe Breast Deformity Robert Cohen, MD, FACS* Paradise Valley, AZ Case summary A 41-year old woman with a history
Eurosilicone. Options for Breast Augmentation
 Eurosilicone Options for Breast Augmentation Introduction Along with the trend towards the increasing importance of the way you look and because of changes in lifestyle, more and more women are concerned
Eurosilicone Options for Breast Augmentation Introduction Along with the trend towards the increasing importance of the way you look and because of changes in lifestyle, more and more women are concerned
Breast Augmentation Information Sheet
 Breast Augmentation Information Sheet Are you thinking about Breast Augmentation? If you are considering surgery, Dr. Peterson wants you to be thoroughly informed about this procedure. Reading this brochure
Breast Augmentation Information Sheet Are you thinking about Breast Augmentation? If you are considering surgery, Dr. Peterson wants you to be thoroughly informed about this procedure. Reading this brochure
XXXXX File No. 108655-001 Petitioner v. Issued and entered this 28 th day of June 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX File No.
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX File No.
Comparison of Breast Implant Deflation for Mentor Anterior and Posterior Valve Designs in Aesthetic and Reconstructive Patients
 BREAST Comparison of Breast Implant Deflation for Mentor Anterior and Posterior Valve Designs in Aesthetic and Reconstructive Patients Benjamin Levi, M.D. Alfred W. Rademaker, Ph.D. Neil A. Fine, M.D.
BREAST Comparison of Breast Implant Deflation for Mentor Anterior and Posterior Valve Designs in Aesthetic and Reconstructive Patients Benjamin Levi, M.D. Alfred W. Rademaker, Ph.D. Neil A. Fine, M.D.
1 of 6 2/10/12 10:15 AM
 Home Dr. Bitar About Us Events Media Specialties Non Surgical Gallery Fees Contact Us Plastic Surgery Products Magazine, October 2007 Issue Breast Augmentation: The Axillary Approach by George John Bitar,
Home Dr. Bitar About Us Events Media Specialties Non Surgical Gallery Fees Contact Us Plastic Surgery Products Magazine, October 2007 Issue Breast Augmentation: The Axillary Approach by George John Bitar,
Reshaping You. Breast Reconstruction for Breast Cancer Patients
 Reshaping You Breast Reconstruction for Breast Cancer Patients Foreword Women diagnosed with breast cancer begin a journey that requires making health care decisions that can have profound effects on their
Reshaping You Breast Reconstruction for Breast Cancer Patients Foreword Women diagnosed with breast cancer begin a journey that requires making health care decisions that can have profound effects on their
Breast Reconstruction Surgery
 Breast Reconstruction Surgery Breast Cancer Centre of Hope Breast Cancer Navigator 204-788-8080 Toll-free in Manitoba1-888-660-4866 Types of mastectomies Steps of breast reconstruction Breast reconstruction
Breast Reconstruction Surgery Breast Cancer Centre of Hope Breast Cancer Navigator 204-788-8080 Toll-free in Manitoba1-888-660-4866 Types of mastectomies Steps of breast reconstruction Breast reconstruction
Since the introduction of breast implants, COSMETIC
 COSMETIC Textured Surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: A Meta-Analysis of Randomized Controlled Trials G. Philip Barnsley, M.D. Leif J.
COSMETIC Textured Surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: A Meta-Analysis of Randomized Controlled Trials G. Philip Barnsley, M.D. Leif J.
BREAST RECONSTRUCTION POST MASTECTOMY
 COVERAGE DETERMINATION GUIDELINE BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: CDG.003.05 Effective Date: January 1, 2016 Table of Contents COVERAGE RATIONALE... DEFINITIONS... APPLICABLE CODES...
COVERAGE DETERMINATION GUIDELINE BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: CDG.003.05 Effective Date: January 1, 2016 Table of Contents COVERAGE RATIONALE... DEFINITIONS... APPLICABLE CODES...
IMPLANT MENTOR EFFECTIVE JANUARY 2012 BREAST IMPLANTS NOT FOR DISTRIBUTION IN USA APPROVED FOR EMEA REGION. Mentor Worldwide LLC 2012 0907007 Rev C
 IMPLANT CATALOGUE EFFECTIVE JANUARY 2012 MENTOR BREAST IMPLANTS NOT FOR DISTRIBUTION IN USA APPROVED FOR EMEA REGION Mentor Worldwide LLC 2012 0907007 Rev C In 2002, we completed a new plant in Leiden,
IMPLANT CATALOGUE EFFECTIVE JANUARY 2012 MENTOR BREAST IMPLANTS NOT FOR DISTRIBUTION IN USA APPROVED FOR EMEA REGION Mentor Worldwide LLC 2012 0907007 Rev C In 2002, we completed a new plant in Leiden,
INFORMED CONSENT-AUGMENTATION MAMMAPLASTY
 INFORMED CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
INFORMED CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
THE DECISION GUIDE TO BREAST RECONSTRUCTION
 THE DECISION GUIDE TO BREAST RECONSTRUCTION Breast reconstruction is the process of making a new breast after mastectomy (removal of the breast) for breast cancer treatment or prevention ( therapeutic
THE DECISION GUIDE TO BREAST RECONSTRUCTION Breast reconstruction is the process of making a new breast after mastectomy (removal of the breast) for breast cancer treatment or prevention ( therapeutic
3D Analysis of Breast Augmentation Defines Operative Changes and Their Relationship to Implant Dimensions
 NORTHEASTERN SOCIETY OF PLASTIC SURGEONS 3D Analysis of Breast Augmentation Defines Operative Changes and Their Relationship to Implant Dimensions Oren M. Tepper, MD, Kevin H. Small, MD, Jacob G. Unger,
NORTHEASTERN SOCIETY OF PLASTIC SURGEONS 3D Analysis of Breast Augmentation Defines Operative Changes and Their Relationship to Implant Dimensions Oren M. Tepper, MD, Kevin H. Small, MD, Jacob G. Unger,
Hydrogel Competence in Eye Surgery
 Hydrogel Competence in Eye Surgery Self-inflating tissue expander osmed self-inflating tissue expanders are made of a specially developed hydrogel that uses the osmotic principle to gain volume. These
Hydrogel Competence in Eye Surgery Self-inflating tissue expander osmed self-inflating tissue expanders are made of a specially developed hydrogel that uses the osmotic principle to gain volume. These
Discover your. Breast
 Discover your Breast Augmentation Options We are proud to Be the ONLY BREAST IMPLANT Company that manufactures in the usa. WHy Does THAT MATTer To you? For over 25 years, Mentor has been recognized worldwide
Discover your Breast Augmentation Options We are proud to Be the ONLY BREAST IMPLANT Company that manufactures in the usa. WHy Does THAT MATTer To you? For over 25 years, Mentor has been recognized worldwide
body breast augmentation
 face body skin breast augmentation Rowena After years of self confidence issues and feeling out of proportion to the rest of my body, I felt I needed to make a change for me! breast augmentation considering
face body skin breast augmentation Rowena After years of self confidence issues and feeling out of proportion to the rest of my body, I felt I needed to make a change for me! breast augmentation considering
Breast Reconstruction. What You Should Know
 Breast Reconstruction What You Should Know M astectomy for treating breast cancer is the most common reason that women have breast reconstruction. In fact, the number of women undergoing this reconstructive
Breast Reconstruction What You Should Know M astectomy for treating breast cancer is the most common reason that women have breast reconstruction. In fact, the number of women undergoing this reconstructive
Breast Augmentation Options CANADA
 Breast Augmentation Options CANADA Important Safety Information MemoryGel Breast Implants: MemoryGel Breast Implants are indicated for breast augmentation in women at least 22 years old or for breast reconstruction.
Breast Augmentation Options CANADA Important Safety Information MemoryGel Breast Implants: MemoryGel Breast Implants are indicated for breast augmentation in women at least 22 years old or for breast reconstruction.
breast augmentation Cosmetic Surgery
 breast augmentation Cosmetic Surgery breast augmentation Welcome to Birkdale - Our appearance shouldn t bind us, it should set us free The desire to improve our appearance is completely natural. How we
breast augmentation Cosmetic Surgery breast augmentation Welcome to Birkdale - Our appearance shouldn t bind us, it should set us free The desire to improve our appearance is completely natural. How we
What You Need to Know About Breast Reconstruction Surgery
 Millard Fillmore Suburban Hospital A Kaleida Health Facility What You Need to Know About Breast Reconstruction Surgery This packet of information contains choices that are available to you regarding breast
Millard Fillmore Suburban Hospital A Kaleida Health Facility What You Need to Know About Breast Reconstruction Surgery This packet of information contains choices that are available to you regarding breast
FDA Update on the Safety of Silicone Gel-Filled Breast Implants
 FDA Update on the Safety of Silicone Gel-Filled Breast Implants June 2011 Center for Devices and Radiological Health U.S. Food and Drug Administration FDA Update on the Safety of Silicone Gel-Filled Breast
FDA Update on the Safety of Silicone Gel-Filled Breast Implants June 2011 Center for Devices and Radiological Health U.S. Food and Drug Administration FDA Update on the Safety of Silicone Gel-Filled Breast
Breast prosthesis implantation for reconstructive and cosmetic surgery: a rapid review
 ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures-Surgical Rapid Review Breast prosthesis implantation for reconstructive and cosmetic surgery: a rapid review ASERNIP-S
ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures-Surgical Rapid Review Breast prosthesis implantation for reconstructive and cosmetic surgery: a rapid review ASERNIP-S
CONSENT FOR BREAST IMPLANT REMOVAL
 CONSENT FOR BREAST IMPLANT REMOVAL GENERAL INFORMATION The removal of breast implants that have been placed either for cosmetic or reconstructive purposes is a surgical operation. Breast implant removal
CONSENT FOR BREAST IMPLANT REMOVAL GENERAL INFORMATION The removal of breast implants that have been placed either for cosmetic or reconstructive purposes is a surgical operation. Breast implant removal
Patient Educational Brochure AUGMENTATION. Breast Augmentation with MENTOR MemoryGel Silicone Gel Breast Implants
 Patient Educational Brochure AUGMENTATION Breast Augmentation with MENTOR MemoryGel Silicone Gel Breast Implants 1 PATIENT EDUCATIONAL BROCHURE AUGMENTATION BREAST AUGMENTATION WITH MENTOR MEMORYGEL SILICONE
Patient Educational Brochure AUGMENTATION Breast Augmentation with MENTOR MemoryGel Silicone Gel Breast Implants 1 PATIENT EDUCATIONAL BROCHURE AUGMENTATION BREAST AUGMENTATION WITH MENTOR MEMORYGEL SILICONE
NATRELLE Silicone-Filled Breast Implants
 Directions for Use NATRELLE Silicone-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician DFU Allergan Rev.
Directions for Use NATRELLE Silicone-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician DFU Allergan Rev.
We re here to help you make a confident, informed decision.
 We re here to help you make a confident, informed decision. For over 20 years, Mentor Corporation has been recognized worldwide as a leading manufacturer of the highest quality breast implants. This brochure
We re here to help you make a confident, informed decision. For over 20 years, Mentor Corporation has been recognized worldwide as a leading manufacturer of the highest quality breast implants. This brochure
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
 Product Insert Data Sheet MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS 102872-001 Rev. C Effective November 2006 102872-001 Rev A effective June 2006 CAUTION: Federal (USA) law restricts this device
Product Insert Data Sheet MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS 102872-001 Rev. C Effective November 2006 102872-001 Rev A effective June 2006 CAUTION: Federal (USA) law restricts this device
Augmentation. Style 68HP BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED NATRELLE INSPIRA BREAST IMPLANTS CRYSTAL AMBER CASEY MINELA MINELA CASEY
 Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST AMBER IMPLANTS CRYSTAL AND Nurse NATRELLE Style 68HP Singer NATRELLE Style 20 NATRELLE INSPIRA BREAST IMPLANTS MINELA Student NATRELLE
Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST AMBER IMPLANTS CRYSTAL AND Nurse NATRELLE Style 68HP Singer NATRELLE Style 20 NATRELLE INSPIRA BREAST IMPLANTS MINELA Student NATRELLE
Product Catalogue 2013
 Product Catalogue 2013 Founded in 1979, Nagor is the only British company dedicated to the specialist design, manufacture and supply of high quality breast implants and related medical devices. Nagor's
Product Catalogue 2013 Founded in 1979, Nagor is the only British company dedicated to the specialist design, manufacture and supply of high quality breast implants and related medical devices. Nagor's
Outcome Assessment of Breast Distortion Following Submuscular Breast Augmentation
 Aesth Plast Surg (2009) 33:44 48 DOI 10.1007/s00266-008-9275-y ORIGINAL ARTICLE Outcome Assessment of Breast Distortion Following Submuscular Breast Augmentation Scott L. Spear Æ Jaime Schwartz Æ Joseph
Aesth Plast Surg (2009) 33:44 48 DOI 10.1007/s00266-008-9275-y ORIGINAL ARTICLE Outcome Assessment of Breast Distortion Following Submuscular Breast Augmentation Scott L. Spear Æ Jaime Schwartz Æ Joseph
