Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 4-Year Follow Up
|
|
|
- Morgan Richard
- 9 years ago
- Views:
Transcription
1 Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 4-Year Follow Up October March 2015
2 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year Follow-up Report OVERVIEW A Prospective, 10-year follow-up clinical evaluation is being conducted to confirm Motiva Implant Matrix Silicone Breast Implants safety and effectiveness in 220 augmentation and revision patients. Patient follow-up was established at 24 hours, 4 days, 2 weeks, 1 month, 3, 6 and 12 months, and will continue to evaluate the long- term (10 years) safety and effectiveness of Motiva products. The Clinical Evaluation Plan for the Evaluation of Motiva Implant Matrix Silicone Breast Implants in Aesthetic Breast Augmentation was initially designed to include 40 patients, however addenda to the initial protocol were approved to include up to 220 patients in the study. Safety was assessed by the presence of complications, such as implant rupture, capsular contracture, double capsules or late seromas that lead to reoperation. The efficacy of Motiva Implant Matrix Silicone Breast Implants was measured by the surgeon and patient through an assessment of their level of satisfaction with the aesthetic results, as well as by the Quality of Life (QoL) Tool. Surgeon s level of satisfaction with the augmentation procedure was measured on a 5-point Likert scale, while the patient s satisfaction at the time of the follow-up visits assessment was based on 6-point Likert scale. The QoL measures were a combination of the Rosenberg Self Esteem Scale, the Body Esteem Scale, the Tennessee Self Concept Scale, and the SF-36, as explained in the Protocol. Data was collected before implantation and at all scheduled follow-up visits. This evaluation was monocentric, conducted at a Plastic Surgery Center accredited by the American Association For Accreditation of Surgery Facilities, by a group of board certified Plastic and Reconstructive Surgeons. Between September and October 2010, 36 augmentation patients (called group A) were operated on, using general anesthesia, for the following indications (as defined by the treating surgeons): Mammary hypotrophy and hypotrophy plus ptosis grade II or I. Regarding the reason for the surgery, 10 of the patients of group A were primary augmentation cases, with no cases of reconstructive surgery (according to the Protocol for this evaluation). Four revision surgeries were performed three, six, ten and 24 months after the initial augmentation procedure. Two of the four revision surgeries were performed on the same patient. The median age at surgery was 31 years. The average weight at surgery was 55.8 kg, and 4 out of 36 implanted patients were smokers. Most of the patients requested a moderate augmentation, with an average cup size change of one cup. The estimated average implant size calculated by the surgeon prior the surgery was 318 cc, which was close to the actual average volume used across the 36 patients: cc. The most frequently used projection family was the Full family ( cm), in 75% of the cases. The average implant base (diameter) was 11.1 cm, which corresponds to a value very similar to the median in the Motiva Implant Matrix catalog. The most common incision site was inframammary (97%) with only one case of transaxillary approach. The most frequent site of placement was submuscular (69%), followed by the subfascial placement (14%), the subglandular placement (14%) and the dual plane placement (3%). Drains were not applied in any of the cases and no intraoperatory complications were reported. The perioperatory was characterized by the absence of infection, hematoma, and suture dehiscence; implant exposure, intra-operatory or immediate post-operatory complications. According to the Protocol and subsequent addenda, the Motiva Implant Matrix Silicone Breast Implants used are either SilkSurface (also commercially known as SmoothSilk ), or VelvetSurface. These surfaces are manufactured by negative imprinting using 3D technology, in direct contrast with surfaces made with the projection of salt or sugar crystals. These are not conventional surfaces: SilkSurface has contact points per cm 2 of an average size of 16 microns each and a profile roughness parameter (RA) of The VelvetSurface used in part for Group B, has contact points of microns depth per cm 2 and a profile roughness parameter (RA) of The characterization of both implant surfaces was performed in collaboration with the Plastic and Reconstructive Surgery Research Center, at the University of Manchester, United Kingdom. Figures 1 and 2 show Optical and Scanning Electron Microscopies characterizations of SilkSurface and VelvetSurface. Figure 1. Surface Optical Characterization (a) and Scanning Electron Microscopy (b) of Motiva Implant Matrix Round SilkSurface. Figure 2. Surface Optical Characterization (a) and Scanning Electron Microscopy (b) of Motiva Implant Matrix Round VelvetSurface. Surface 360 Surface Consistency 50x Surface 360 Surface Consistency 50x PAGE 2 OF 12 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED
3 Prospective Clinical Evaluation: 4-Year Follow-up Report Motiva Implant Matrix Silicone Breast Implants CLINICAL RESULTS SAFETY OUTCOMES - GROUP A Table 1 shows some of the main characteristics of the patients treated/procedures performed. The patient s clinical evolution 4 years after the breast augmentation procedure was highly satisfactory, as shown in Table 2. This 4-year summary includes the results for 32 of the 36 patients originally implanted (Group A). Due to the fact that it is not possible to analyze the safety and efficacy of the original implants in the three reimplanted (3) patients, their results are detailed and reported separately. Table 1: Breast Augmentation with Motiva Implant Matrix Silicone Breast Implants (n=32), Group A. Average age 31 (21 to 51 range) Average weight 55.8 kg Surface SmoothSilk / SilkSurface (10) Most frequent incision Inframammary (97%) Most frequent placement Submuscular (69% traditional, 14% subfascial, 3% dual plane) Average volume 326.7cc (235 cc to 400 cc range used) Most frequent Projection Full projection (medium height) in 75% of the implantations Most frequent Base 11.1 cm (similar to Matrix median) It is important to note that, for the entire Group A, no cases of pain, edema, erythema, inflammatory reaction, hematoma, seroma, infection, scaring, calcification, granuloma, stretch marks, pruritus, tightness, symmastia, extrusion, asymmetry, rippling, malposition of the implant or irregular appearance were observed during the 48- month follow-up visit. Table 2: Relevant Clinical Data Recorded During the 4-Year Follow-Up Visit (n=32*), Group A. Safety Changes in nipple sensitivity 15.6% of the patients (5 patients out of 32) 1 Twinges 6.25% of the patients (2 out of 32) 2 Ptosis 15.6% of the patients (5 out of 32 in both surgical sites) Reported loss of volume % of the patients (2 out of 32 in both surgical sites) 1 One patient reported hypersensitivity in one surgical site, for a 1.6% of the surgical sites. Four patients reported loss of nipple sensitivity, one in both surgical sites, two in the left nipple and one in the right nipple, for a total of 7.8% of the surgical sites. 2 Two patients reported twinges in a single breast, for a 3.1% of the surgical sites. 3 Subjective perception not confirmed by imaging or 3D scans. * n=32: The three revision surgeries are reported separately. Revision Surgery Cases Of the 36 patients originally implanted, one patient requested a reoperation three months after the primary surgery to correct an aesthetic dissatisfaction due to a larger than desired breast size. Safety data for this patient up to month 48, indicate that no other events were observed and her reported level of satisfaction at this visit is high (extremely satisfied). A second patient was diagnosed with Grade II ptosis and her satisfaction level with her augmentation procedure had decreased significantly in comparison with the 3-month follow-up data (from extremely satisfied on month 3 to satisfied on month 6). The patient was reoperated on and a mastopexia with implants replacement was performed 6 months after the primary procedure. The patient reported to be satisfied with the outcome during the 1-year and 2-year follow-up visits. Safety data for this patient up to month 24 indicate that no adverse events were observed, however, the patient requested a new surgery to replace her implants for a larger size, a criteria not shared by the surgeons. The patient did not attend the 3-year and 4-year follow-up visits. ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED PAGE 3 OF 12
4 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year Follow-up Report The third reoperation case, in which a periareolar round block mammoplasty was performed, was a patient with tubular breasts who, in spite of being satisfied with her aesthetic result after primary implantation, was considered by the treating surgeon to have a good improvement opportunity, as shown in Figure 3. The Motiva breast implants originally implanted were not removed or replaced during the round block procedure, performed 10 months after the primary augmentation surgery. In her 2-year follow-up visit, this patient reports a high level of satisfaction with her aesthetic result (extremely satisfied). However, and in spite of being extremely satisfied with the aesthetic result, the surgeon recommended a breast implant replacement, which was performed 26 months after the primary surgery, to improve the aesthetic result even further Up to date, 24 months after her last surgery, no adverse events were reported for this patient. Both patients and surgeons reported a high level of satisfaction. In the three cases, histological analysis of the removed implants pseudo-capsule did not show signs of contracture or any other anomaly (Figures 3, 4 and 5 depict the above-mentioned reoperation cases). Figure 3: and Postoperatory with Motiva Implant Matrix 375 cc, Full Projection, and After Reoperation and Replacement with Motiva Implant Matrix 275 cc, Full Projection (3 months, 24 months, 36 months and 48 months). Primary Augmentation, 375cc Full 275cc Full, 3 months 275cc Full, 24 months 275cc Full, 36 months 275cc Full, 48 months Figure 4: and Postoperatory with Motiva Implant Matrix 375 cc Full Projection, and After Reoperation and New Implantation with Motiva Implant Matrix 375 cc Full (3 months and 24 months).* *This patient abandoned the casuistic two years after the primary implantation. Primary Augmentation Before Mastopexia Revision Surgery After Mastopexia Revision 24 Months After Mastopexia Figure 5: Breast Augmentation with Motiva Implant Matrix 335 cc Full Projection, Subsequent Periareolar Round Block Mammoplasty and New Breast Augmentation with Motiva Implant Matrix 350 cc Corsé Projection, Performed on a Patient with Tubular Breasts. After Primary Augmentation After 24 months round block mammoplasty New breast augmentation after 3 months New breast augmentation after 12 months New breast augmentation after 24 months PAGE 4 OF 12 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED
5 Prospective Clinical Evaluation: 4-Year Follow-up Report Motiva Implant Matrix Silicone Breast Implants SAFETY OUTCOMES - GROUP B Between November 2011 and March 2014, 73 additional patients (group B) have been implanted with Motiva Implant Matrix Silicone Breast Implants in the same clinical center and following the same guidelines and procedures stated in the original Clinical Evaluation Plan for the Evaluation of Motiva Implant Matrix Silicone Breast Implants in Aesthetic Breast Augmentation. 39 out of 73 patients have received SilkSurface and SilkSurface PLUS Motiva Implant Matrix implants and 34 patients have been implanted with VelvetSurface and VelvetSurface PLUS Motiva Implant Matrix Implants. To the date of submission of this evaluation, of the total 73 additional patients implanted with Motiva Implant Matrix Silicone Breast Implants 71, 67, 61, 53 and 18 patients have attended the first month, third month, sixth month, 1-year and 2-year follow-up visits, respectively. Table 3 shows some of the main characteristics of group B patients (n=73). Table 3: Breast Augmentation with SilkSurface (n= 39) and VelvetSurface (n=34) Motiva Implant Matrix Silicone Breast Implants, Group B. Average age 34 (21 to 58 range) Most frequent incision Inframammary (78%) Most frequent placement Submuscular (6) Average volume Most frequent Projection Most frequent Base cc (135 cc to 500 cc range used) Full projection (medium height) in 51% of the implantations 10.5 cm For this group of patients, the perioperatory was also characterized by the absence of infection, hematoma, suture dehiscence, implant exposure or intra-operatory or immediate post-operatory complications. The results of the 1-year follow-up visit, available for 73% of the additional patients (n=53), are described below. No cases of pain, edema, erythema, inflammatory reaction, hematoma, seroma, infection, calcification, granuloma, stretch marks, ptosis, extrusion, insufficient aesthetic result, asymmetry, rippling, malposition or irregular appearance were observed during the 1-year follow up visit. It is important to note that no cases of rupture, capsular contracture, double capsules, late seromas or ALCL were reported during the 1-year period evaluated. Table 4: Relevant Clinical Data Recorded During the 1-Year Follow-Up Visit (n=53) of the Additional Cases Implanted with SilkSurface (n= 28) and VelvetSurface (n=25) Motiva Implant Matrix. Safety Changes in nipple sensitivity 18.9% (10 out of 53) 1 Pruritus Tightness Twinges 17% (9 out of 53) 2 Bilateral loss of volume 1.88% (1 out of 53) Bilateral symmastia 1.88% (1 out of 53) Bilateral scarring 1.88% (1 out of 53) 1.88% (1 out of 53 in both surgical sites) 5.7% (3 out of 53 in both surgical sites) 1 One patient reported hypersensitivity in one breast, for a total of 1% of the surgical sites. One patient reported loss of nipple sensitivity in a single surgical site and eight patients in both breasts, for a total of 16% of the surgical sites. 2 One patient reported twinges in a single breast and eight patients in both breasts, for a 17% of the surgical sites. ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED PAGE 5 OF 12
6 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year Follow-up Report SAFETY OUTCOMES GROUPS A AND B As shown in Table 5, there are no cases of rupture, capsular contracture, double capsules, late seromas reported during the 4-year period evaluated for patients in group A at Year 1, 2, 3 and 4 or group B at Year 1. Given the special nature of these surfaces, the clinical results observed and the survival rate of the complications are very promising. These surfaces were engineered to have very low roughness parameters, theoretically with the aim of reducing inflammation in the post-operative period and preventing chronic inflammation after the recovery period. A first level of evidence is shown in Figure 6, in two cases of patients requesting breast implant replacement for aesthetic reasons and who originally had SilkSurface implants for (a) 6 months and (b) 26 months. In both cases a very thin pseudocapsule is the result of a breast implant surface characterized by a multitude of contact points with a low roughness parameter. It is significant that the group of surgeons participating in the clinical evaluation follows a best-practice protocol 1 for breast augmentation that in combination with these surfaces, has so far resulted in no capsular contracture or rupture cases. 1 Tebbets, J.B. Achieving a zero percent reoperation rate at 3 years in 50 consecutive case augmentation mammaplasty PMA study. Plast. Reconstr. Surg. 108(6): , Table 5: Percentage and Total Number of Adverse Events Reported in Groups A and B. Year 1 After Surgery Year 2 After Surgery Year 3 After Surgery Year 4 After Surgery Capsular Contracture (0/172) (0/104) (0/66) (0/64) Rupture (0/172) (0/104) (0/66) (0/64) Double Capsules (0/172) (0/104) (0/66) (0/64) Late Seromas (0/172) (0/104) (0/66) (0/64) Figures 6A and 6B Show the Resulting Pseudocapsules After Primary Breast Augmentation with Silksurface in Two Different Patients Requesting a Reoperation for Aesthetic Reasons, At 6 Months and 26 Months Respectively. A Very Thin Capsule Demonstrates the Positive Clinical Outcome of these Surfaces. 6a 6b PAGE 6 OF 12 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED
7 Prospective Clinical Evaluation: 4-Year Follow-up Report Motiva Implant Matrix Silicone Breast Implants EFFECTIVENESS OUTCOMES - GROUP A Patient and Surgeon s Level of Satisfaction In general, the level of satisfaction for both patients and surgeons was high at all endpoints during all the follow-up visits, up to month 48. The results show that a 10 of the patients were satisfied to extremely satisfied with the aesthetic results, as depicted in Charts #1 and #2. Additionally, the surgeons were very satisfied to extremely satisfied with the aesthetic results in 94% of the cases, as shown in Charts #3 and #4. Chart #1: Patient s Level of Satisfaction with the Breast Augmentation Procedure Aesthetic Results, 48 Months After The Implantation (n=32), Group A. Nivel de Satisfacción del Paciente Grupo A 16% 53% Extremadamente satisfecha - 53% Muy satisfecha - 31% Satisfecha - 16% 31% Insatisfecha - Muy Insatisfecha - Extremadamente insatisfecha - Chart #2: Patient s Level of Satisfaction 48 Months After the Implantation, Using Gaussian Distribution of the Data (n=32), Group A. Chart #2 shows that the median for patient s level of satisfaction is 1.625, which means that the vast majority of the implanted patients are extremely to very satisfied with the aesthetic results provided by their breast augmentation surgical procedure. This is statistically significant, with a 95% level of confidence (SD: ). Frecuency Patients' Satisfaction Media Desv. Est. N Group A Range Chart #3: Surgeon s Level of Satisfaction with the Breast Augmentation Aesthetic Results, 48 Months After the Implantation (n=32), Group A. Surgeon's Level of Satisfaction Group A 3% 3% 3% 69% Extremely Satisfied - 69% Very Satisfied - 22% 22% Satisfied - 3% Dissatisfied - 3% Very Dissatisfied - 3% Extremely Dissatisfied - Chart #4: Surgeon s Level of Satisfaction 48 Months After the Implantation Using Gaussian Distribution of the Data (n=32), Group A. Chart #4 shows that the median for surgeon s level of satisfaction is 1.5, which means that the treating surgeons are extremely to very satisfied with the use of Motiva Implant Matrix in the implanted population. This is statistically significant, with a 95% level of confidence (SD: ). It should be noted that the surgeons report dissatisfaction with the aesthetic results on the fourth year visit in 6% of the cases (2 out of 32 patients) due to the patient s significant weight gain. Frecuency Surgeon's Satisfaction Media Desv. Est. N Range Group A ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED PAGE 7 OF 12
8 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year Follow-up Report Reoperation Cases Satisfaction Level Up to date, satisfaction data is only available for two of the three reoperation cases. For these two cases, both patient and surgeon report to be at least very satisfied with the aesthetic result. Quality of Life and Self-Esteem As stated in previous reports, an improvement in the self-esteem and quality of life scores has been observed in comparison to the preoperative values. Preoperatively, the patient s average score on the 9 self-esteem and quality of life variables evaluated was 8.86 (1 being the lowest value and 10 the highest). The average scores on all 9 variables recorded preoperatively and during the 2-week, 1-month, 2-month, 3-month, 6-month, 12-month, 24-month, 36-month and 48-month follow-up visits were 8.86, 8.88, 9.07, 9.19, 9.22, 9.25, 9.34, 9.27, 9.17 and 8.98, respectively. A 1.2% increase in overall self-esteem / quality of life scores was observed 48 months after the implantation, compared to the baseline level. Although a reduction in overall self-esteem / quality of life scores was observed 48 months after the implantation, compared to the previous post-operatory follow-ups, this is believed to be due to individual variables such as weight gain, pregnancies and other personal situations at the moment of evaluation. In fact, 5 out of the 32 evaluated patients reported an important weight gain and significant reductions in their self-esteem / quality of life scores, causing the average scores to be lower. The following chart shows the change of quality of life / self-esteem average scores from the preoperative visit up to the 48-month follow-up visit. 9,4 9,3 9,2 y= 0,0278x + 8,97 R 2 = 0,2529 9,19 9,22 9,25 9,34 9,27 9,17 9,1 9 9,07 8,98 8,9 8,86 8,88 8,8 8,7 8,6 Pre-Surgery 2 Weeks 1 Month 2 Months 3 Months 6 Months 12 Months 24 Months 36 Months 48 Months Chart 5: Average Scores on the Self-esteem and Quality of Life Scales (from a 1 to 10 scale) Before the Augmentation Surgery and 2 Weeks, 1 Month, 2 Months, 3 Months, 6 Months, 12 Months, 24 Months, 36 Months and 48 Months After the Breast Augmentation Procedure (n=32). Figures 7 and 8 depict some of the patients that underwent breast augmentation with Motiva Implant Matrix Silicone Breast Implants within this prospective clinical evaluation. Figure 7: Breast Augmentation Results with Motiva Implant Matrix Silicone Breast Implants 355 Full Projection. 6 Months 1 Year 2 Years 3 Years 4 Years Figure 8: Breast Augmentation Results with Motiva Implant Matrix Silicone Breast Implants 325 Corsé Projection. 6 Months 1 Year 2 Years 3 Years 4 Years PAGE 8 OF 12 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED
9 Prospective Clinical Evaluation: 4-Year Follow-up Report Motiva Implant Matrix Silicone Breast Implants ADDITIONAL EFFICACY EVIDENCE EFFECTIVENESS OUTCOMES - GROUP B Patient and Surgeon s Level of Satisfaction In general, the level of satisfaction of both patients and surgeons was high at all endpoints during all the follow-up visits, up to year 1 (Group B). The results show that a 98% of the patients were satisfied to extremely satisfied with the aesthetic results, as depicted in Charts #6 and #7. Additionally, the surgeons were very satisfied to extremely satisfied with the aesthetic results in 10 of the cases, as shown in Charts #8 and #9. Chart #6: Patient s Level of Satisfaction with the Breast Augmentation Procedure Aesthetic Results, 1 Year After the Implantation (n=53), Group B. *The only case of dissatisfaction corresponds to a patient who suffered a case of symmastia, which is unrelated to efficacy or safety of the implants. 13% Patients' Level of Satisfaction Group B 2% Extremely Satisfied - 53% Very Satisfied - 32% 53% Satisfied - 13% 32% Dissatisfied - 2%* Very Dissatisfied - Extremely Dissatisfied - Chart #7: Patient s Level of Satisfaction 1 Year After the Implantation, using Gaussian Distribution of the Data (n=53), Group B. Chart #7 shows that the median for patient s level of satisfaction is 1.660, which means that the vast majority of the implanted patients are very to extremely satisfied with the aesthetic results provided by their breast augmentation surgical procedure. This is statistically significant, with a 95% level of confidence (SD: ). Frecuency Patients' Satisfaction Media Desv. Est. N Group B Range Chart #8: Surgeon s Level of Satisfaction with the Breast Augmentation Aesthetic Results, 1 Year After the Implantation (n=53), Group B. Surgeon's Level of Satisfaction Group B 26% 74% Extremely Satisfied - 74% Very Satisfied - 26% Satisfied - Dissatisfied - Very Dissatisfied - Extremely Dissatisfied - Chart #9: Surgeon s Level of Satisfaction 1 Year After the Implantation, using Gaussian Distribution of the Data (n=53), Group B. Chart #9 shows that the median for surgeon s level of satisfaction is 1.264, which means that the treating surgeons are very to extremely satisfied with the use of Motiva Implant Matrix in the implanted population. This is statistically significant, with a 95% level of confidence (SD: ). Frecuency Surgeon's Satisfaction Media Desv. Est. N Group B Range ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED PAGE 9 OF 12
10 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year Follow-up Report Figures 9, 10 and 11 depict some of the patients (group B) that underwent breast augmentation with Motiva Implant Matrix Silicone Breast Implants within this prospective clinical evaluation. Figure 9. Breast Augmentation Results with Motiva Implant Matrix Silicone Breast Implants VelvetSurface, 325 cc, Corsé. 3 Months 6 Months 12 Months Figure 10. Breast Augmentation Results with Motiva Implant Matrix Silicone Breast Implants SilkSurface, 355 cc, Full. 3 Months 6 Months 12 Months Figure 11. Breast Augmentation Results with Motiva Implant Matrix Silicone Breast Implants VelvetSurface, 230 cc, Demi. 3 Months 6 Months 12 Months 24 Months PAGE 10 OF 12 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED
11 Prospective Clinical Evaluation: 4-Year Follow-up Report Motiva Implant Matrix Silicone Breast Implants CONCLUSION After 4 years of being implanted, the 64 Motiva implants in group A evaluated to date, have shown to be safe and effective in the aesthetic breast augmentation procedures performed in this prospective evaluation. The rate of implant replacement in this group at four years is 8.33% (3 out of the originally 36 recruited patients) and the main reason for requesting a new surgical procedure was the patient s or surgeon s criteria of a possible improvement over the aesthetic results obtained with the primary surgery. None of the above cases were associated to the implants themselves. The rate of implant replacement for safety reasons in this group remains unchanged after four years. For the 64 implants evaluated at 4 years, patients and surgeons reported a high rate of satisfaction with the aesthetic results (10 of the patients were satisfied, very satisfied or extremely satisfied with the results and the surgeons were very satisfied or extremely satisfied with the augmentation procedure aesthetic result in 94% of the cases). The adverse events frequency has behaved as expected, with no serious illness or poor aesthetical results attributable to the implants. No incidents or deaths have occurred since the beginning of the clinical evaluation. The evaluation at 1 year of 106 out of 146 additional implants in group B, included after the recruitment and treatment dates stated in the Clinical Evaluation Plan for the Evaluation of Motiva Implant Matrix Silicone Breast Implants in Aesthetic Breast Augmentation, provide further evidence that Motiva Implant Matrix Silicone Breast Implants are safe and effective in breast augmentation surgical procedures. For the 106 implants in Group B evaluated at 1 year, patients and surgeons reported a high rate of satisfaction with the aesthetic results (98% of the patients were satisfied, very satisfied or extremely satisfied with the results and the surgeons were very satisfied or extremely satisfied with the augmentation procedure aesthetic result in 10 of the cases). The adverse events frequency has behaved as expected, with no serious illness or poor aesthetical results attributable to the implants. No incidents or deaths have occurred in any group. It is relevant to mention that in both groups, A and B, no cases of rupture, capsular contracture, double capsules, late seromas or ALCL were reported during the periods evaluated. Both SilkSurface and VelvetSurface have shown an optimal safety record. This is an ongoing study and further evaluations will follow, but the clinical results so far are very promising for these innovative implant surfaces. Prepared by: Rita Albertazzi - Clinical Affairs Consultant Bernardo Garro - Regulatory Affairs Executive Establishment Labs March 2015 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED PAGE 11 OF 12
12 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 4-Year Follow-up Report Motiva 4 Year Study EN Establishment Labs: Coyol Free Zone, Alajuela, COSTA RICA [email protected] Exclusive Distribution Motiva European Distribution Center: Sint Jansveld 11A, 2160 Wommelgem, BELGIUM [email protected] Motiva USA: 712 Fifth Avenue, 14 th Floor, New York, NY , USA [email protected] PAGE 12 OF 12 ESTABLISHMENT LABS S.A. PROPERTY - ALL RIGHTS RESERVED
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants
 Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction
 Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction About This Brochure This brochure is intended to provide you with a high level overview of the facts about
Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction About This Brochure This brochure is intended to provide you with a high level overview of the facts about
Breast Augmentation Primer
 Breast Augmentation Primer Breast augmentation is typically a very rewarding procedure. In order to achieve the highest level of patient satisfaction, many decisions must be carefully made. We have put
Breast Augmentation Primer Breast augmentation is typically a very rewarding procedure. In order to achieve the highest level of patient satisfaction, many decisions must be carefully made. We have put
Breast Augmentation. If you are dissatisfied with your breast size, augmentation surgery is a choice to consider. Breast augmentation can:
 Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
A Girlfriend s Guide. Breast Augmentation WWW.RPSMD.COM
 A Girlfriend s Guide to Breast Augmentation 1 Your Breast Augmentation Surgery: This is an important decision that you are considering. Have you always thought about increasing the size of your breasts,
A Girlfriend s Guide to Breast Augmentation 1 Your Breast Augmentation Surgery: This is an important decision that you are considering. Have you always thought about increasing the size of your breasts,
IS BREAST AUGMENTATION RIGHT FOR YOU?
 IS BREAST AUGMENTATION RIGHT FOR YOU? (816) 286-4126 www.kansascitysurgicalarts.com For many women, having shapely, full breasts is an important part of feeling feminine. The breasts give the body proportion,
IS BREAST AUGMENTATION RIGHT FOR YOU? (816) 286-4126 www.kansascitysurgicalarts.com For many women, having shapely, full breasts is an important part of feeling feminine. The breasts give the body proportion,
BREAST IMPLANTS (enlargement, augmentation) Dr. Benjamin Van Raalte TYPES OF IMPLANTS saline round implants high profiles low profile shaped
 BREAST IMPLANTS (enlargement, augmentation) Dr. Benjamin Van Raalte has 20 years of experience with breast enlargement including axillary incisions and gel implants. Dr. Van Raalte is the first Quad City
BREAST IMPLANTS (enlargement, augmentation) Dr. Benjamin Van Raalte has 20 years of experience with breast enlargement including axillary incisions and gel implants. Dr. Van Raalte is the first Quad City
Outcome Assessment of Breast Distortion Following Submuscular Breast Augmentation
 Aesth Plast Surg (2009) 33:44 48 DOI 10.1007/s00266-008-9275-y ORIGINAL ARTICLE Outcome Assessment of Breast Distortion Following Submuscular Breast Augmentation Scott L. Spear Æ Jaime Schwartz Æ Joseph
Aesth Plast Surg (2009) 33:44 48 DOI 10.1007/s00266-008-9275-y ORIGINAL ARTICLE Outcome Assessment of Breast Distortion Following Submuscular Breast Augmentation Scott L. Spear Æ Jaime Schwartz Æ Joseph
Breast Implants: Local Complications and Adverse Outcomes
 Breast Implants: Local Complications and Adverse Outcomes This booklet highlights the most common problems associated with silicone gel-filled and saline-filled breast implants: those that occur in the
Breast Implants: Local Complications and Adverse Outcomes This booklet highlights the most common problems associated with silicone gel-filled and saline-filled breast implants: those that occur in the
COPYRIGHT ASPS. Breast Augmentation. The Symbol of Excellence in Plastic Surgery
 Breast Augmentation The Symbol of Excellence in Plastic Surgery A public education service of the American Society of Plastic Surgeons and the American Society for Aesthetic Plastic Surgery. This brochure
Breast Augmentation The Symbol of Excellence in Plastic Surgery A public education service of the American Society of Plastic Surgeons and the American Society for Aesthetic Plastic Surgery. This brochure
FDA Update on the Safety of Silicone Gel-Filled Breast Implants
 FDA Update on the Safety of Silicone Gel-Filled Breast Implants June 2011 Center for Devices and Radiological Health U.S. Food and Drug Administration FDA Update on the Safety of Silicone Gel-Filled Breast
FDA Update on the Safety of Silicone Gel-Filled Breast Implants June 2011 Center for Devices and Radiological Health U.S. Food and Drug Administration FDA Update on the Safety of Silicone Gel-Filled Breast
Breast Augmentation and Lifts Explained. Breast Augmentation and Lifts explained
 Breast Augmentation and Lifts explained 1 Your breasts, your choices Whether you are a mother whose breasts have lost their fullness and perkiness due to breastfeeding, or a woman who simply wants to feel
Breast Augmentation and Lifts explained 1 Your breasts, your choices Whether you are a mother whose breasts have lost their fullness and perkiness due to breastfeeding, or a woman who simply wants to feel
Effect of Incision Choice on Outcomes in Primary Breast Augmentation
 Breast Surgery Effect of Incision Choice on Outcomes in Primary Breast Augmentation Jeffrey M. Jacobson, MD; Margaret E. Gatti, MD, MPH; Adam D. Schaffner, MD; Lauren M. Hill, MD; and Scott L. Spear, MD
Breast Surgery Effect of Incision Choice on Outcomes in Primary Breast Augmentation Jeffrey M. Jacobson, MD; Margaret E. Gatti, MD, MPH; Adam D. Schaffner, MD; Lauren M. Hill, MD; and Scott L. Spear, MD
Tissue Reinforcement with Strattice Reconstructive Tissue Matrix following Correction of Severe Breast Deformity
 Tissue Reinforcement with Strattice Reconstructive Tissue Matrix following Correction of Severe Breast Deformity Robert Cohen, MD, FACS* Paradise Valley, AZ Case summary A 41-year old woman with a history
Tissue Reinforcement with Strattice Reconstructive Tissue Matrix following Correction of Severe Breast Deformity Robert Cohen, MD, FACS* Paradise Valley, AZ Case summary A 41-year old woman with a history
1 of 6 2/10/12 10:15 AM
 Home Dr. Bitar About Us Events Media Specialties Non Surgical Gallery Fees Contact Us Plastic Surgery Products Magazine, October 2007 Issue Breast Augmentation: The Axillary Approach by George John Bitar,
Home Dr. Bitar About Us Events Media Specialties Non Surgical Gallery Fees Contact Us Plastic Surgery Products Magazine, October 2007 Issue Breast Augmentation: The Axillary Approach by George John Bitar,
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with Natrelle Silicone-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with Natrelle Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R A c c e p t a n c e O
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with Natrelle Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R A c c e p t a n c e O
Aestheticare Cosmetic Surgery Institute Dr. Ronald E. Moser 30260 Rancho Viejo Rd. San Juan Capistrano, CA 92675 (800) 662-1055
 Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy, weight changes, sun exposure,
Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy, weight changes, sun exposure,
Breast Augmentation Information Sheet
 Breast Augmentation Information Sheet Are you thinking about Breast Augmentation? If you are considering surgery, Dr. Peterson wants you to be thoroughly informed about this procedure. Reading this brochure
Breast Augmentation Information Sheet Are you thinking about Breast Augmentation? If you are considering surgery, Dr. Peterson wants you to be thoroughly informed about this procedure. Reading this brochure
XXXXX File No. 108655-001 Petitioner v. Issued and entered this 28 th day of June 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX File No.
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX File No.
SUBJECT: MANAGEMENT OF BREAST EFFECTIVE DATE: 12/16/99 IMPLANTS REVISED DATE:
 MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants
 Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
NATRELLE 410 Highly Cohesive
 Breast Augmentation NATRELLE 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants Important Factors Breast Augmentation Patients Should Consider Highly Cohesive Anatomically Shaped Silicone-Filled
Breast Augmentation NATRELLE 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants Important Factors Breast Augmentation Patients Should Consider Highly Cohesive Anatomically Shaped Silicone-Filled
Product Reference Guide
 Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Breast Augmentation Options CANADA
 Breast Augmentation Options CANADA Important Safety Information MemoryGel Breast Implants: MemoryGel Breast Implants are indicated for breast augmentation in women at least 22 years old or for breast reconstruction.
Breast Augmentation Options CANADA Important Safety Information MemoryGel Breast Implants: MemoryGel Breast Implants are indicated for breast augmentation in women at least 22 years old or for breast reconstruction.
Implant Selection: Getting it right the first time. Bruce Cunningham MD, MSc Professor of Surgery University of Minnesota
 Implant Selection: Getting it right the first time Bruce Cunningham MD, MSc Professor of Surgery University of Minnesota USA Surgeon Survey Mentor Memory-Gel implants became available in November of 2006
Implant Selection: Getting it right the first time Bruce Cunningham MD, MSc Professor of Surgery University of Minnesota USA Surgeon Survey Mentor Memory-Gel implants became available in November of 2006
Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants
 Augmentation Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants Table of Contents Section Page Glossary...1 1. Considering Silicone Gel-filled Breast
Augmentation Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants Table of Contents Section Page Glossary...1 1. Considering Silicone Gel-filled Breast
Patient Educational Brochure AUGMENTATION. Breast Augmentation with MENTOR MemoryGel Silicone Gel Breast Implants
 Patient Educational Brochure AUGMENTATION Breast Augmentation with MENTOR MemoryGel Silicone Gel Breast Implants 1 PATIENT EDUCATIONAL BROCHURE AUGMENTATION BREAST AUGMENTATION WITH MENTOR MEMORYGEL SILICONE
Patient Educational Brochure AUGMENTATION Breast Augmentation with MENTOR MemoryGel Silicone Gel Breast Implants 1 PATIENT EDUCATIONAL BROCHURE AUGMENTATION BREAST AUGMENTATION WITH MENTOR MEMORYGEL SILICONE
Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant
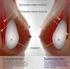 Breast Surgery Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant Larry S. Nichter, MD; and Robert S. Hamas, MD Aesthetic Surgery Journal 32(7) 861 867 2012 The American Society
Breast Surgery Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant Larry S. Nichter, MD; and Robert S. Hamas, MD Aesthetic Surgery Journal 32(7) 861 867 2012 The American Society
NATRELLE Silicone-Filled Breast Implants
 Directions for Use NATRELLE Silicone-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician DFU Allergan Rev.
Directions for Use NATRELLE Silicone-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician DFU Allergan Rev.
It is important that you read this information carefully and completely.
 Placement of Permanent Breast Implant Following Tissue Expansion 1. I hereby authorize Dr. John P. Stratis and such assistants as may be selected to perform the following procedure or treatment INFORMED-
Placement of Permanent Breast Implant Following Tissue Expansion 1. I hereby authorize Dr. John P. Stratis and such assistants as may be selected to perform the following procedure or treatment INFORMED-
INTERNATIONAL. Breast Augmentation. Options
 INTERNATIONAL Breast Augmentation Options Important Safety Information: Mentor MemoryGel and Mentor saline-filled breast implants are indicated for breast augmentation - in women who are at least 18 years
INTERNATIONAL Breast Augmentation Options Important Safety Information: Mentor MemoryGel and Mentor saline-filled breast implants are indicated for breast augmentation - in women who are at least 18 years
CONSENT FOR BREAST IMPLANT REMOVAL
 CONSENT FOR BREAST IMPLANT REMOVAL GENERAL INFORMATION The removal of breast implants that have been placed either for cosmetic or reconstructive purposes is a surgical operation. Breast implant removal
CONSENT FOR BREAST IMPLANT REMOVAL GENERAL INFORMATION The removal of breast implants that have been placed either for cosmetic or reconstructive purposes is a surgical operation. Breast implant removal
Intracapsular Allogenic Dermal Grafts for Breast Implant Related Problems
 Cosmetic Intracapsular Allogenic Dermal Grafts for Breast Implant Related Problems Richard A. Baxter, M.D. Mountlake Terrace, Wash. Despite advances in surgical techniques and breast implant design, certain
Cosmetic Intracapsular Allogenic Dermal Grafts for Breast Implant Related Problems Richard A. Baxter, M.D. Mountlake Terrace, Wash. Despite advances in surgical techniques and breast implant design, certain
Breast Reconstruction Options. Department of Plastic Surgery #290 Santa Clara Homestead Campus
 Breast Reconstruction Options Department of Plastic Surgery #290 Santa Clara Homestead Campus Importance of Breast Reconstruction As successes in treating breast cancer have grown, more women have been
Breast Reconstruction Options Department of Plastic Surgery #290 Santa Clara Homestead Campus Importance of Breast Reconstruction As successes in treating breast cancer have grown, more women have been
Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants
 Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled
Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Reconstruction Patients about Mentor MemoryGel Silicone Gel-Filled
Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants
 Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants W. Grant Stevens, MD; Salvatore J. Pacella, MD; Andrew J. L. Gear, MD;
Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants W. Grant Stevens, MD; Salvatore J. Pacella, MD; Andrew J. L. Gear, MD;
INFORMED-CONSENT BREAST AUGMENTATION
 RICHARD A. BARTLETT, M.D. Board Certified-American Board of Plastic Surgery Member-American Society of Plastic Surgeons Member-American Society for Aesthetic Plastic Surgery INFORMED-CONSENT BREAST AUGMENTATION
RICHARD A. BARTLETT, M.D. Board Certified-American Board of Plastic Surgery Member-American Society of Plastic Surgeons Member-American Society for Aesthetic Plastic Surgery INFORMED-CONSENT BREAST AUGMENTATION
Augmentation. Style 68HP BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED NATRELLE INSPIRA BREAST IMPLANTS CRYSTAL AMBER CASEY MINELA MINELA CASEY
 Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST AMBER IMPLANTS CRYSTAL AND Nurse NATRELLE Style 68HP Singer NATRELLE Style 20 NATRELLE INSPIRA BREAST IMPLANTS MINELA Student NATRELLE
Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST AMBER IMPLANTS CRYSTAL AND Nurse NATRELLE Style 68HP Singer NATRELLE Style 20 NATRELLE INSPIRA BREAST IMPLANTS MINELA Student NATRELLE
Directions for Use. NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants
 Directions for Use Directions for Use NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by
Directions for Use Directions for Use NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by
Jennifer L. Walden, MD Aesthetic Plastic Surgery 5656 Bee Caves Rd, Suite E201, Austin, TX 78746 Phone: 512-328-4100 Fax: 512-328-2132
 Jennifer L. Walden, MD Aesthetic Plastic Surgery 5656 Bee Caves Rd, Suite E201, Austin, TX 78746 Phone: 512-328-4100 Fax: 512-328-2132 Breast Augmentation Frequently Asked Questions Please read over these
Jennifer L. Walden, MD Aesthetic Plastic Surgery 5656 Bee Caves Rd, Suite E201, Austin, TX 78746 Phone: 512-328-4100 Fax: 512-328-2132 Breast Augmentation Frequently Asked Questions Please read over these
An Approach that Integrates Patient Education and Informed Consent in Breast Augmentation
 Techniques in Cosmetic Surgery An Approach that Integrates Patient Education and Informed Consent in Breast Augmentation John B. Tebbetts, M.D., and Terrye B. Tebbetts Dallas, Texas Informed consent requires
Techniques in Cosmetic Surgery An Approach that Integrates Patient Education and Informed Consent in Breast Augmentation John B. Tebbetts, M.D., and Terrye B. Tebbetts Dallas, Texas Informed consent requires
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery
 Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
The enhancement of buttock volume with silicone COSMETIC. Buttock Augmentation with Silicone Implants: A Multicenter Survey Review of 2226 Patients
 COSMETIC Buttock Augmentation with Silicone Implants: A Multicenter Survey Review of 2226 Patients M. Mark Mofid, M.D. Raul Gonzalez, M.D. José Abel de la Peña, M.D. Constantino G. Mendieta, M.D. Douglas
COSMETIC Buttock Augmentation with Silicone Implants: A Multicenter Survey Review of 2226 Patients M. Mark Mofid, M.D. Raul Gonzalez, M.D. José Abel de la Peña, M.D. Constantino G. Mendieta, M.D. Douglas
Patients pursuing breast augmentation have
 COSMETIC Preoperative Sizing in Breast Augmentation David A. Hidalgo, M.D. Jason A. Spector, M.D. New York, N.Y. Background: Implant size selection in breast augmentation patients is one of many variables
COSMETIC Preoperative Sizing in Breast Augmentation David A. Hidalgo, M.D. Jason A. Spector, M.D. New York, N.Y. Background: Implant size selection in breast augmentation patients is one of many variables
Eurosilicone. Options for Breast Augmentation
 Eurosilicone Options for Breast Augmentation Introduction Along with the trend towards the increasing importance of the way you look and because of changes in lifestyle, more and more women are concerned
Eurosilicone Options for Breast Augmentation Introduction Along with the trend towards the increasing importance of the way you look and because of changes in lifestyle, more and more women are concerned
3D Analysis of Breast Augmentation Defines Operative Changes and Their Relationship to Implant Dimensions
 NORTHEASTERN SOCIETY OF PLASTIC SURGEONS 3D Analysis of Breast Augmentation Defines Operative Changes and Their Relationship to Implant Dimensions Oren M. Tepper, MD, Kevin H. Small, MD, Jacob G. Unger,
NORTHEASTERN SOCIETY OF PLASTIC SURGEONS 3D Analysis of Breast Augmentation Defines Operative Changes and Their Relationship to Implant Dimensions Oren M. Tepper, MD, Kevin H. Small, MD, Jacob G. Unger,
TOM J. POUSTI, MD, F.A.C.S. PLASTIC AND RECONSTRUCTION SURGERY
 TOM J. POUSTI, MD, F.A.C.S. PLASTIC AND RECONSTRUCTION SURGERY INFORMED CONSENT FOR BREAST AUGMENTATION SURGERY INSTRUCTIONS This is an informed consent document that has been prepared to help inform you
TOM J. POUSTI, MD, F.A.C.S. PLASTIC AND RECONSTRUCTION SURGERY INFORMED CONSENT FOR BREAST AUGMENTATION SURGERY INSTRUCTIONS This is an informed consent document that has been prepared to help inform you
Tumescent Anesthetic Breast Surgery (TABS)
 Tumescent Anesthetic Breast Surgery (TABS) A Consecutive Case Series Review of Primary and Secondary Breast Augmentation Surgery in an Office Based Surgical Center Julio C. Novoa, M.D. Introduction: Review
Tumescent Anesthetic Breast Surgery (TABS) A Consecutive Case Series Review of Primary and Secondary Breast Augmentation Surgery in an Office Based Surgical Center Julio C. Novoa, M.D. Introduction: Review
Breast Augmentation Amsterdam Plastic Surgery Breast Augmentation Overview
 Breast Augmentation Amsterdam Plastic Surgery Breast Augmentation Overview The long-lasting results of breast augmentation are not limited to just physical changes as data documents that many patients
Breast Augmentation Amsterdam Plastic Surgery Breast Augmentation Overview The long-lasting results of breast augmentation are not limited to just physical changes as data documents that many patients
Breast Anatomy. Patient Information on Breast Augmentation Surgery
 Patient Information on Breast Augmentation Surgery Breast Anatomy In order to understand breast augmentation surgery, a general knowledge of breast anatomy is often helpful. This section will explain the
Patient Information on Breast Augmentation Surgery Breast Anatomy In order to understand breast augmentation surgery, a general knowledge of breast anatomy is often helpful. This section will explain the
Sientra Patient Planning Guide. Breast Augmentation
 Sientra Patient Planning Guide Breast Augmentation Table Of Contents Introduction Smart Choices, Beautiful Results The Safety of Silicone About Breasts The Right Surgeon to Help Achieve Your Vision Your
Sientra Patient Planning Guide Breast Augmentation Table Of Contents Introduction Smart Choices, Beautiful Results The Safety of Silicone About Breasts The Right Surgeon to Help Achieve Your Vision Your
TrueMonobloc BluSeal Controlled Surfaces ProgressiveGel Ultima 3D Simulation TwinPack Qinside Safety Technology TrueTissue Technology Ergonomix
 TrueMonobloc BluSeal Controlled Surfaces ProgressiveGel Ultima 3D Simulation TwinPack Qinside Safety Technology TrueTissue Technology Ergonomix The perfect harmony between quality, technology and safety.
TrueMonobloc BluSeal Controlled Surfaces ProgressiveGel Ultima 3D Simulation TwinPack Qinside Safety Technology TrueTissue Technology Ergonomix The perfect harmony between quality, technology and safety.
Plastic Surgery @ it s Best
 Plastic Surgery @ it s Best The single most important factor in the success of aesthetic (cosmetic) surgery is the surgeon you select and his team. Dr. Tariq Saeed is the renowned Bahraini, Plastic, Aesthetic
Plastic Surgery @ it s Best The single most important factor in the success of aesthetic (cosmetic) surgery is the surgeon you select and his team. Dr. Tariq Saeed is the renowned Bahraini, Plastic, Aesthetic
Transumbilical breast augmentation (TUBA) was described
 ORIGINAL ARTICLE A Practical Review of a Growing Technique William A. Brennan, MD, and Jacob Haiavy, MD Background: The transumbilical breast augmentation procedure has been described in the literature
ORIGINAL ARTICLE A Practical Review of a Growing Technique William A. Brennan, MD, and Jacob Haiavy, MD Background: The transumbilical breast augmentation procedure has been described in the literature
Page 1 of 7 Patient s Initials 10-01-00 version
 INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
Breast Cancer No studies have ever shown this to be a concern for humans. There is no evidence that implants cause cancer.
 What is the risk of complications? o Silicone Material Silicone is derived from silicon, a semi-metallic or metal-like element that in nature combines with oxygen to form silicon dioxide, or silica. Beach
What is the risk of complications? o Silicone Material Silicone is derived from silicon, a semi-metallic or metal-like element that in nature combines with oxygen to form silicon dioxide, or silica. Beach
SALINE-FILLED BREAST IMPLANT SURGERY: MAKING AN INFORMED DECISION
 SALINE-FILLED BREAST IMPLANT SURGERY: MAKING AN INFORMED DECISION So You re Considering Saline-Filled Breast Implant Surgery The purpose of this brochure is to assist you in making an informed decision
SALINE-FILLED BREAST IMPLANT SURGERY: MAKING AN INFORMED DECISION So You re Considering Saline-Filled Breast Implant Surgery The purpose of this brochure is to assist you in making an informed decision
Plastic Surgery & Diving
 Plastic Surgery & Diving Breast Augmentation (Breast Implants) SCOTT JOHNSON PHOTO In the previous issue we discussed plastic surgeries such as nose jobs, liposuction and face lifts, noting that the topic
Plastic Surgery & Diving Breast Augmentation (Breast Implants) SCOTT JOHNSON PHOTO In the previous issue we discussed plastic surgeries such as nose jobs, liposuction and face lifts, noting that the topic
Surgeons are documenting and discussing the SPECIAL TOPIC. Breast Implant Complication Review: Double Capsules and Late Seromas
 SPECIAL TOPIC Breast Implant Complication Review: Double Capsules and Late Seromas Elizabeth J. Hall-Findlay, M.D. Banff, Alberta, Canada Background: The problem of double s and late seromas is a relatively
SPECIAL TOPIC Breast Implant Complication Review: Double Capsules and Late Seromas Elizabeth J. Hall-Findlay, M.D. Banff, Alberta, Canada Background: The problem of double s and late seromas is a relatively
Discover your. Breast
 Discover your Breast Augmentation Options We are proud to Be the ONLY BREAST IMPLANT Company that manufactures in the usa. WHy Does THAT MATTer To you? For over 25 years, Mentor has been recognized worldwide
Discover your Breast Augmentation Options We are proud to Be the ONLY BREAST IMPLANT Company that manufactures in the usa. WHy Does THAT MATTer To you? For over 25 years, Mentor has been recognized worldwide
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
 Product Insert Data Sheet MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS 102872-001 Rev. C Effective November 2006 102872-001 Rev A effective June 2006 CAUTION: Federal (USA) law restricts this device
Product Insert Data Sheet MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS 102872-001 Rev. C Effective November 2006 102872-001 Rev A effective June 2006 CAUTION: Federal (USA) law restricts this device
Informed Consent for Breast Augmentation with Silicone Implants
 INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty surgery with silicone gel-filled implants, its risks, as well as alternative treatment(s).
INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty surgery with silicone gel-filled implants, its risks, as well as alternative treatment(s).
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SILICONE GEL-FILLED IMPLANTS
 2006 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use
2006 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use
Directions for Use (DFU) Natrelle. Silicone-Filled Breast Implants. Physician Labeling THE SCIENCE OF REJUVENATION
 Directions for Use (DFU) Natrelle Silicone-Filled Breast Implants Physician Labeling THE SCIENCE OF REJUVENATION TABLE OF CONTENTS Page Introduction... 1 Directions to the Surgeon... 1 Information to
Directions for Use (DFU) Natrelle Silicone-Filled Breast Implants Physician Labeling THE SCIENCE OF REJUVENATION TABLE OF CONTENTS Page Introduction... 1 Directions to the Surgeon... 1 Information to
In terms of cup size, how much of an enlargement would you like to achieve?
 Information for patients considering breast augmentation surgery under the care of Mr C. Stone FRCS(Plast) Consultant Reconstructive & Aesthetic Plastic Surgeon About Mr Stone Mr Stone is a Consultant
Information for patients considering breast augmentation surgery under the care of Mr C. Stone FRCS(Plast) Consultant Reconstructive & Aesthetic Plastic Surgeon About Mr Stone Mr Stone is a Consultant
INFORMED CONSENT - AUGMENTATION MAMMAPLASTY WITH SILICONE GEL-FILLED IMPLANTS
 Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
GLOBAL. Frequently Asked Questions
 GLOBAL Frequently Asked Questions about Breast Implants 2 Contents 1 Silicone Material 1 Safety of Silicone 2 Capsular Contracture 2 Breast Cancer 3 Mammography 3 Silicone Allergy 3 Implant Life Expectancy
GLOBAL Frequently Asked Questions about Breast Implants 2 Contents 1 Silicone Material 1 Safety of Silicone 2 Capsular Contracture 2 Breast Cancer 3 Mammography 3 Silicone Allergy 3 Implant Life Expectancy
We re here to help you make a confident, informed decision.
 We re here to help you make a confident, informed decision. For over 20 years, Mentor Corporation has been recognized worldwide as a leading manufacturer of the highest quality breast implants. This brochure
We re here to help you make a confident, informed decision. For over 20 years, Mentor Corporation has been recognized worldwide as a leading manufacturer of the highest quality breast implants. This brochure
irections for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture Directions for Use THE SCIENCE OF REJUVENATION
 irections for Use Directions for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture THE SCIENCE OF REJUVENATION Caution: Federal (USA) law restricts this device to sale by or on the order
irections for Use Directions for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture THE SCIENCE OF REJUVENATION Caution: Federal (USA) law restricts this device to sale by or on the order
Breast Implant Guide
 Breast Implant Guide Shapes, Sizes, Textures & More Brought to you by: 801.479.5722 Copyright 2013 P2P. All Rights Reserved Contents Overview 5 History & Background 6 Implant Types 8 Implant Shapes 13
Breast Implant Guide Shapes, Sizes, Textures & More Brought to you by: 801.479.5722 Copyright 2013 P2P. All Rights Reserved Contents Overview 5 History & Background 6 Implant Types 8 Implant Shapes 13
Your Guide to breast augmentation
 Your Guide to breast augmentation 1 Contents Foreword 01 Introduction 02 Implant techniques 06 About the implant 08 What complications can occur? 10 Further information 16 Notes 17 Published by BAPRAS
Your Guide to breast augmentation 1 Contents Foreword 01 Introduction 02 Implant techniques 06 About the implant 08 What complications can occur? 10 Further information 16 Notes 17 Published by BAPRAS
[email protected]
 [email protected] 1 What is it? Remedial cover from the 29th day up to 24 months post surgery. Who is the insured person? You the surgeon are the insured person. How do I apply? You may apply
[email protected] 1 What is it? Remedial cover from the 29th day up to 24 months post surgery. Who is the insured person? You the surgeon are the insured person. How do I apply? You may apply
How To Use A Saline Filled Breast Implant
 Directions for Use (DFU) Natrelle Saline-Filled Breast Implants Physician Labeling Table of Contents Page Introduction... 1 Directions to the Physician... 1 Device Description... 2 Indications... 3 Contraindications...
Directions for Use (DFU) Natrelle Saline-Filled Breast Implants Physician Labeling Table of Contents Page Introduction... 1 Directions to the Physician... 1 Device Description... 2 Indications... 3 Contraindications...
INFORMED-CONSENT-OPEN CAPSULECTOMY AND BREAST IMPLANT EXCHANGE
 INFORMED-CONSENT-OPEN CAPSULECTOMY AND BREAST IMPLANT EXCHANGE Instructions This is an informed-consent document that has been prepared to help inform you about open capsulectomy and breast implant exchange,
INFORMED-CONSENT-OPEN CAPSULECTOMY AND BREAST IMPLANT EXCHANGE Instructions This is an informed-consent document that has been prepared to help inform you about open capsulectomy and breast implant exchange,
DOI: 10.1097/01.PRS.0000135945.02642.8B
 CME Breast Augmentation Scott L. Spear, M.D., Erwin J. Bulan, M.D., and Mark L. Venturi, M.D. Washington, D.C. Learning Objectives: After studying this article, the participant should be able to: 1. Understand
CME Breast Augmentation Scott L. Spear, M.D., Erwin J. Bulan, M.D., and Mark L. Venturi, M.D. Washington, D.C. Learning Objectives: After studying this article, the participant should be able to: 1. Understand
BREAST AUGMENTATION PATIENT INFORMATION
 BREAST AUGMENTATION PATIENT INFORMATION Elizabeth J. Hall-Findlay, MD, FRCSC 340-317 Banff Avenue, Box 2009, Banff, Alberta, Canada T1L 1B7 403-762-2055 tel 403-762-8297 fax www.banffplasticsurgery.com
BREAST AUGMENTATION PATIENT INFORMATION Elizabeth J. Hall-Findlay, MD, FRCSC 340-317 Banff Avenue, Box 2009, Banff, Alberta, Canada T1L 1B7 403-762-2055 tel 403-762-8297 fax www.banffplasticsurgery.com
The Silicone Breast Implant Controversy
 The Silicone Breast Implant Controversy by Susan E. Kolb, M.D., F.A.C.S. There has been a great deal of controversy regarding the safety of silicone breast implants. For the women who have implants, conflicting
The Silicone Breast Implant Controversy by Susan E. Kolb, M.D., F.A.C.S. There has been a great deal of controversy regarding the safety of silicone breast implants. For the women who have implants, conflicting
Breast Implants and Reconstruction
 Last Review Date: October 9, 2015 Number: MG.MM.SU.fv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: October 9, 2015 Number: MG.MM.SU.fv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
BREAST AUGMENTATION INFORMATION BOOKLET GENE SLOAN, MD, FACS
 BREAST AUGMENTATION INFORMATION BOOKLET GENE SLOAN, MD, FACS AESTHETIC PLASTIC SURGERY, LLC 8315 CANTRELL ROAD, #120 LITTLE ROCK, ARKANSAS 72227 501-224-1300 www.gsloanmd.com October 2012 INTRODUCTION
BREAST AUGMENTATION INFORMATION BOOKLET GENE SLOAN, MD, FACS AESTHETIC PLASTIC SURGERY, LLC 8315 CANTRELL ROAD, #120 LITTLE ROCK, ARKANSAS 72227 501-224-1300 www.gsloanmd.com October 2012 INTRODUCTION
Breast reconstruction using an implant after risk-reducing surgery
 Breast reconstruction using an implant after risk-reducing surgery This information is from the booklet Understanding riskreducing breast surgery. You may find the full booklet helpful. We can send you
Breast reconstruction using an implant after risk-reducing surgery This information is from the booklet Understanding riskreducing breast surgery. You may find the full booklet helpful. We can send you
Product Catalogue. 2013 Edition
 roduct Catalogue 2013 Edition Eurosilicone is a leading manufacturer within the global plastic surgery community. e are an innovative developer and manufacturer of a complete range of quality surgical
roduct Catalogue 2013 Edition Eurosilicone is a leading manufacturer within the global plastic surgery community. e are an innovative developer and manufacturer of a complete range of quality surgical
Chapter 24. Evolution of Procedures
 Chapter 24 BREAST SURGERY KEY FIGURES: Saline implant reconstruction Latissimus dorsi reconstruction Free TRAM reconstruction In the developed world, breast reconstruction after mastectomy and breast reduction
Chapter 24 BREAST SURGERY KEY FIGURES: Saline implant reconstruction Latissimus dorsi reconstruction Free TRAM reconstruction In the developed world, breast reconstruction after mastectomy and breast reduction
INFORMED CONSENT-AUGMENTATION MAMMAPLASTY
 INFORMED CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
INFORMED CONSENT-AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks, and alternative treatments.
Complications in breast augmentation: maximizing patient outcomes with some surgical solutions to common problems
 18 Complications in breast augmentation: maximizing patient outcomes with some surgical solutions to common problems Bradley P. Bengtson Key points We learn most from complications and our difficult revision
18 Complications in breast augmentation: maximizing patient outcomes with some surgical solutions to common problems Bradley P. Bengtson Key points We learn most from complications and our difficult revision
Dr. Justin B. Maxhimer, M.D. Boulder Plastic Surgery: 303-443-2277. IV Seasons Skin Care: 303-938-1666 www.boulderplasticsurgery.
 Dr. Hans R. Kuisle, M.D., F.A.C.S Dr. Winfield Hartley, M.D., F.A.C.S Dr. Justin B. Maxhimer, M.D. 2525 4 th Street, Suite 200, Boulder, CO 80304 Boulder Plastic Surgery: 303-443-2277 IV Seasons Skin Care:
Dr. Hans R. Kuisle, M.D., F.A.C.S Dr. Winfield Hartley, M.D., F.A.C.S Dr. Justin B. Maxhimer, M.D. 2525 4 th Street, Suite 200, Boulder, CO 80304 Boulder Plastic Surgery: 303-443-2277 IV Seasons Skin Care:
Breast Augmentation Information Sheet
 Breast Augmentation Information Sheet This information sheet is a general guide for patients undergoing breast augmentation (enlargement) under the care of Paul Harris. It should help clarify some questions
Breast Augmentation Information Sheet This information sheet is a general guide for patients undergoing breast augmentation (enlargement) under the care of Paul Harris. It should help clarify some questions
