Articles. Funding Deutsche Krebshilfe.
|
|
|
- Annabelle Griffin
- 10 years ago
- Views:
Transcription
1 Conventional chemotherapy () with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL -1) Norbert Schmitz, Maike Nickelsen, Marita Ziepert, Mathias Haenel, Peter Borchmann, Christian Schmidt, Andreas Viardot, Martin Bentz, Norma Peter, Gerhard Ehninger, Gottfried Doelken, Christian Ruebe, Lorenz Truemper, Andreas Rosenwald, Michael Pfreundschuh, Markus Loeffler*, Bertram Glass*, for the German High-Grade Lymphoma Study Group (DSHNHL) Summary Background High-dose therapy (HDT) followed by transplantation of autologous haemopoietic stem cells is frequently done as part of first-line therapy in young patients with high-risk aggressive B-cell lymphoma. We investigated whether HDT with cytotoxic agents identical to those used for conventional therapy followed by autologous stem-cell transplantation (ASCT) improved survival outcome compared with conventional chemotherapy when rituximab was added to both modalities. Methods We did an open-label, randomised trial comparing conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone) and rituximab (R-) with dose-escalated sequential HDT and rituximab () followed by repetitive ASCT in high-risk (age-adjusted International Prognostic Index [IPI] or 3) patients aged 18 6 years with aggressive B-cell lymphoma. Eligible patients received radiotherapy for bulky, extranodal disease, or both. Randomisation (1:1) used the Pocock minimisation algorithm; patients were stratified by age-adjusted IPI factors, bulky disease, and centre. The primary endpoint was event-free survival. All analyses were done on the intention-to-treat population. This trial is registered with ClinicalTrials.gov, number NCT199. Findings 136 patients were randomly assigned to R- and 139 to. 13 patients in the R- group and 13 in the group were included in the intention-to-treat population. After a median of 4 months (IQR 9 59), 3-year event-free survival was 69 5% (95% CI ) in the R- group and 61 4% (5 8 7 ) in the group (p= 14; hazard ratio 1 3, 95% CI 9 ). All 18 evaluable patients treated with had grade 4 leucopenia, as did 48 (58 5%) of 8 patients with documented blood counts in the R- group. All 18 evaluable patients in the group had grade 3 4 thrombocytopenia, as did 6 (33 8%) of 77 patients in the R- group with documented blood counts. The most important non-haematological grade 3 or 4 adverse event was infection, which occurred in 96 (75 %) of 18 patients treated with and in 4 (31 3%) of 18 patients treated with R-. Interpretation In young patients with high-risk aggressive B-cell lymphoma, was not superior to conventional R-CHOEP therapy and was associated with significantly more toxic effects. R- with or without radiotherapy remains a treatment option for these patients, with encouraging efficacy. Funding Deutsche Krebshilfe. Introduction Diffuse large B-cell lymphoma is the most common subtype of clinically aggressive lymphomas and comprises about a third of all B-cell lymphomas. With modern treatment strategies survival varies between about 5% and more than 9%, 1 largely dependent on clinical risk factors first described by the International Prognostic Index (IPI). For young patients aged 18 to 6 years with high-risk disease many investigators worldwide use high-dose therapy (HDT) followed by transplantation of autolo gous blood-derived haemopoietic stem cells (ASCT) as part of first-line therapy Rituximab, a monoclonal anti-cd antibody, has substantially improved treatment outcomes for both old and young low-risk patients with diffuse large B-cell lymphoma. 1,13 No published study specifically looked into the efficacy of adding rituximab to standard CHOP (cyclophospha mide, doxorubicin, vincristine, prednisone), CHOP-like chemotherapy, or HDT/ASCT in young, high-risk patients with aggressive B-cell lymphoma. To assess the efficacy of high-dose chemotherapy necessitating transplantation of autologous haemopoi etic stem cells, the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) initiated the -1 trial, which com pared aggressive conventional chemotherapy (cyclophosphamide, doxorubicin, vin cristine, etoposide, prednisone []) with HDT comprising multiple cycles of identical, but dose-escalated cytotoxic agents Published Online November 16, 1 S147-45(1) See Online/Comment S147-45(1)757-7 *ML and BG contributed equally to this Article Trial investigators listed in the appendix Asklepios Hospital St Georg, Hamburg, Germany (Prof N Schmitz MD, M Nickelsen MD, B Glass MD); Institute for Medical Informatics, Statistics and Epidemiology, University of Leipzig, Germany (M Ziepert PhD, Prof M Loeffler MD); Department Internal Medicine III, Municipal Hospital Chemnitz, Germany (M Haenel MD); Department Internal Medicine I, University of Cologne, Germany (P Borchmann MD); Department Internal Medicine III, Ludwig-Maximilians University of Munich, Germany (C Schmidt MD); Department Internal Medicine III, University of Ulm, Germany (A Viardot MD); Municipal Hospital, Karlsruhe, Germany (M Bentz MD); Carl-Thiem- Hospital, Cottbus, Germany (N Peter MD); Department Internal Medicine I, University Hospital Carl Gustav Carus, Dresden, Germany (Prof G Ehninger MD); Department Internal Medicine C, University Hospital Greifswald, Germany (Prof G Doelken MD); Department Radiotherapy, University Hospital Saarland, Homburg,Germany (Prof C Ruebe MD); Department Haematology and Oncology, University of Göttingen, Published online November 16, 1 1
2 PBSC PBSC PBSC Randomisation CYC 15 mg/m² DOX 7 mg/m² VCR mg ETO 6 mg/m² PRD 5 mg CYC 45 mg/m² DOX 7 mg/m² VCR mg ETO 96 mg/m² PRD 5 mg CYC 45 mg/m² DOX 7 mg/m² VCR mg ETO 96 mg/m² PRD 5 mg CYC 6 mg/m² DOX 7 mg/m² VCR mg ETO 148 mg/m² PRD 5 mg Days Radiotherapy for bulky or extranodal disease Figure 1: Study design Doses of the drugs administered with the MegaCHOEP programme (CHOEP with escalated the doses of cyclophosphamide, etoposide, and doxorubicin) varied with each treatment cycle as indicated. Vincristine and prednisone are absolute doses. Doses for cyclophosphamide, doxorubicin, and etoposide are reported as mg/m². Stars represent one infusion of rituximab. CYC=Cyclophosphamide. DOX=doxorubicin. ETO=etoposide. PRD=prednisone. VCR=vincristine. =cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone. PBSC=peripheral blood stem cells. 36 patients randomly assigned to a treatment group 15 patients assigned to without rituximab; treatment arm stopped April, 4 75 randomly assigned to a treatment group 136 assigned to R- 139 assigned to R-Mega 4 withdrew consent missing data 4 withdrew consent 3 missing data 13 in the intent-to-treat population 13 in the intent-to-treat population 15 did not complete chemotherapy 7 treatment failures change of diagnosis 1 patient decision for unknown reasons 3 had toxic effects 16 patients assigned to MegaCHOEP without rituximab; treatment arm stopped April, completed chemotherapy 94 completed chemotherapy 63 received radiotherapy 54 received radiotherapy 1 did not receive any study treatment* 37 did not complete chemotherapy 4 treatment failures change of diagnosis 1 patient decision for unknown reasons 6 had toxic effects 15 changed to R- 5 changed to R- at end of study 1 bone marrow involvement >75% 1 intercurrent disease Figure : Trial profile The doses of the drugs administered with the MegaCHOEP regimen (CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin) varied with each treatment cycle as shown in figure 1. R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R- CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. *Due to CNS disease detected after randomisation. Due to incoming reference pathology. The protocol stipulated for a change of treatment arm in case of mobilisation failure or excessive toxic effects. As recommended by the data safety and monitoring committee. (MegaCHOEP) and combined both regimens with rituximab (R- and ). The trial was designed as a proof-of-principle study to address the role of dose-intensity in the rituximab era. Methods Patients Between March 3, 3, and April 7, 9, we did a prospective, randomised, open-label, phase 3 study at 61 centres experienced in lymphoma treatment including ASCT. Eligible patients were between 18 years and 6 years of age who presented with biopsy-proven, untreated, CD-positive, aggressive B-cell lymphoma. 14 The diagnosis was re viewed by a panel of six reference pathologists. Only patients with two or three risk factors (Ann Arbor stage III or IV, elevated lactate dehydrogenase, Eastern Co operative Oncology Group [ECOG] performance status or 3) of the age-adjusted IPI were eligible. Patients with diagnosis of any malignancy other than aggressive B-cell lymphoma, substantial impairment of cardiac, pulmon ary, hepatic, or renal function, bonemarrow infiltration more than 5%, active hepatitis, known HIV-positivity, or hyper sensitivity to any study drug, or simultaneous participation in other clinical studies were excluded. No lymphoma-directed therapy except for prednisone (1 mg orally for 3 days) and vincristine ( mg) was allowed before study entry. Our study complied with the declaration of Helsinki and respected the guidelines of good clinical practice. The institutional review board or ethics committee of each participating centre approved the study protocol and its amendment. All patients gave written informed consent. Randomisation and masking The trial was not masked. After obtaining informed consent investigators faxed the registration form to the Published online November 16, 1
3 study office in Hamburg, Germany, where inclusion and exclusion criteria were checked. Randomisation was done in a 1:1 ratio with the Pocock minimisation algorithm 15 at the Institute for Medical Informatics, Statistics, and Epidemiology in Leipzig, Germany. Treatment allocation was stratified by age-adjusted IPI factors, presence of bulky disease, and centre. The study started as a four-arm study with two arms identical to the ones described below but without rituximab. The study group met in April 17, 4, and decided to stop enrolment into arms without rituximab. Since June 3, 4, the study continued as outlined below. Procedures Patients had baseline assessments including history, clinical status, laboratory tests, CT scans of neck, chest, and abdomen, and a bone-marrow biopsy. Further investigations were done when indicated. Tumour measurements were done by the treating physician or the local radiologist. Performance status was defined according to the ECOG scale, 16 lactate dehydrogenase levels were expressed as the ratio of observed over the upper normal value. The stage of disease was defined according to the Ann Arbor classification. Figure 1 shows the study design. Conventional chemotherapy consisted of eight cycles of CHOEP at -week intervals () supported by granulocyte colony-stimulating factor. Each cycle comprised 75 mg/m² of cyclophosphamide, 5 mg/m² of doxorubicin, mg of vincristine admin istered on day 1, 1 mg/m² of etoposide on days 1 3, and 1 mg of prednisone on days 1 5. The MegaCHOEP regimen used identical cytotoxic agents but escalated the doses of cyclophosphamide, etoposide, and doxorubicin. 17 The dose of doxorubicin was increased to 7 mg/m² in all four cycles. The doses of cyclophosphamide and etoposide were escalated to 15 mg/m² and 6 mg/m² in cycle 1, 45 mg/m² and 96 mg/m² in cycles and 3, and 6 mg/m² and 148 mg/m² in cycle 4. To allow for continuation of treatment every 3 weeks blood-derived haemopoietic stem cells were harvested after treatment cycles 1,, and (optionally) 3. Granulocyte colony-stimulating factor ( 5 μg/kg per day) was started on day 6 after cycle 1 and continued until a minimum of 1⁶ CD34- positive progenitor cells per kg bodyweight had been collected for reinfusion after cycle. The mobilisation procedure was repeated after cycle and the collection product was split into two each of which needed to contain more than 1⁶ CD34-positive cells per kg bodyweight to be transplanted after cycle 3 and cycle 4. If the yield of the second collection was insufficient, another harvest was done after cycle 3. Patients with failure to mobilise stem cells or who had excessive toxic effects on were requested to continue therapy with R- until eight cycles had been administered. Rituximab (375 mg/m²) was administered on day of cycles 1 4, 6, and 8 of the R- and on days, 14, 36, 56, 77, and 98 of the regimen. R- (n=13) (n=13) Sex Male 8 (63 1%) 8 (6 1%) Female 48 (36 9%) 5 (37 9%) Age, years 5 (18 6) 47 (19 6) Lactate dehydrogenase level Elevated more than normal 17 (97 7%) 18 (97 %) Ann Arbor stage III or IV 16 (96 9%) 17 (96 %) ECOG performance status 1 88 (67 7%) 88 (66 7%) >1 4 (3 3%) 44 (33 3%) Number of extranodal sites 1 74 (56 9%) 75 (56 8%) >1 56 (43 1%) 57 (43 %) B-symptoms* Yes 71 (55 %) 81 (61 8%) No 58 (45 %) 5 (38 %) Bulky disease Yes 77 (59 %) 81 (61 4%) No 53 (4 8%) 51 (38 6%) Bone marrow involvement Yes 16 (1 3%) 1 (7 6%) No 114 (87 7%) 1 (9 4%) Age-adjusted International Prognostic Index 95 (73 1%) 97 (73 5%) 3 35 (6 9%) 35 (6 5%) Histology Not reviewed 8 (6 %) 3 ( 3%) Reviewed 1 (93 8%) 19 (97 7%) DLBCL 11 (8 8%) 11 (78 3%) Follicular lymphoma (grade III) 4 (3 3%) 6 (4 7%) Follicular lymphoma and DLBCL 3 ( 5%) 3 ( 3%) Burkitt s lymphoma 1 ( 8%) Burkitt-like lymphoma 1 ( 8%) 1 ( 8%) Blastic mantle-cell lymphoma 1 ( 8%) 1 ( 8%) Aggressive marginal-zone lymphoma (1 6%) 1 ( 8%) Unclassified B-cell lymphoma 7 (5 7%) 1 (7 8%) No aggressive B-cell lymphoma 1 ( 8%) 4 (3 1%) Technically insufficient material 1 ( 8%) (1 6%) Data are n (%) or media (range). R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R-CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. ECOG=Eastern Cooperative Oncology Group. DLBCL=Diffuse large B-cell lymphoma. *One missing value per arm. Table 1: Baseline characteristics Thus, doses and dose intensities of rituximab were identical and timing was very similar in both treatment arms (figure 1). Radiotherapy (36 Gy, administered at daily doses of 1 8 to Gy over 4 weeks) was mandatory for all patients with bulky disease defined as any mass of 7 5 cm or larger at the largest diameter or extranodal involve ment. Patients with meningeosis cerebri received Germany (Prof L Truemper MD); Institute of Pathology, University of Würzburg, Germany (Prof A Rosenwald MD); and Department Internal Medicine I, University Hospital Saarland, Homburg, Germany (Prof M Pfreundschuh MD) Correspondence to: Prof Norbert Schmitz, Department of Haematology, Oncology and Stem Cell Transplantation, Asklepios Hospital St Georg, Lohmühlenstrasse 5, D-99 Hamburg [email protected] See Online for appendix Published online November 16, 1 3
4 Cyclophosphamide (mg/m²) Doxorubicin (mg/m²) Vincristine (mg) Etoposide (mg/m²) Prednisone (mg) MegaCHOEP CHOEP MegaCHOEP CHOEP MegaCHOEP CHOEP MegaCHOEP CHOEP MegaCHOEP CHOEP Planned dose Dose received* 98% 98% 99% 99% 1% 1% 96% 98% 1% 1% Planned dose per week Dose received* 85% 9% 87% 9% 88% 85% 8% 91% 91% 93% *Median % of planned dose or dose intensity. Table : Dose and dose intensities R- (n=17) (n=16) Complete and unconfirmed response 1 (78 7%) 9 (71 4%) Complete and unconfirmed response 1 ( 8%) (1 6%) and additional treatment Partial response (1 6%) 5 (4 %) No change (1 6%) (1 6%) Progressive disease 13 (1 %) 15 (11 9%) Therapy-associated death 4 (3 1%) 7 (5 6%) Unknown 5 (3 9%) 5 (4 %) Data are n (%). Response to treatment was assessed in accordance with the International Workshop 1999 Critera. 18 R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R- CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. Table 3: Response to treatment by treatment group 15 mg of methotrexate, 4 mg of cytosine arabinoside, and 4 mg of dexamethasone intrathecally on days 1 and days 5 of each treatment cycle with leucovorin rescue until no lymphoma cells could be detected in cerebrospinal fluid. CNS prophylaxis was mandatory for any patient with involvement of bone marrow, testes, or the skull region and consisted of 15 mg of methotrexate intrathecally on days 1 and 5 of cycles 1 and. Tumour response was assessed months after end of therapy in both treatment arms. Responses were classified as complete remission, unconfirmed complete remission, partial remission, stable disease, and progressive disease using international workshop criteria. 18 Follow-up visits were scheduled every 3 months for the first years after end of therapy, every 6 months for years 3 to 5, and annually thereafter. All adverse events defined as any adverse change from baseline characteristics were retrieved in predefined categories from case report forms. Each event was graded according to the National Cancer Institute Common Toxicity Criteria (version 3.). 19 Statistical analysis The trial was initially planned in a factorial design to compare patients randomly assigned to receive eight cycles of with those assigned to the MegaCHOEP regimen. We originally wanted to also compare patients randomly assigned to rituximab or no rituximab. However, on June 3, 4, the study arms without rituximab were closed. A 15% difference between R- and was regarded as clinically relevant to justify the additional toxic effects and effort of HDT. Therefore, we aimed to identify a difference of 15% in 3-year event-free survival (hazard ratio 563) with a two-sided significance level of 5% and a power of 8%, requiring 38 patients for the intentionto-treat analysis. To achieve full power for the planned per-protocol analysis, 368 patients needed to be included (software for sample size calculations: nquery Advisor.). The first planned interim analysis was done after 185 patients had been enrolled and showed that the stopping rule according to O Brien and Fleming used to show the superiority of over R-CHOEP was not fulfilled. We also did conditional power calculations to test whether the study aim could still be achieved. The probability to show superiority of over R- at the end of trial was only 6 %. Following a decision of the data monitoring and safety committee, the trial was stopped in April 7, 9, with 36 patients randomly assigned. Because the steering committee recommended switching patients on treatment with to R-, response assess ment was restricted to 17 patients treated with R- and 16 patients treated with. For Kaplan-Meier analyses patients on treatment were censored on the date of change. Primary analyses were done by intention-to-treat. Event-free survival, progression-free survival, and overall survival were measured from the date of randomisation, estimated according to Kaplan-Meier, and differences between groups were compared by the log-rank test. The primary endpoint was event-free survival (defined as time from randomisation to disease progression, start of salvage treatment, additional, unplanned treatment, relapse, or death from any cause). Secondary endpoints were response, progression during treatment, frequency of toxic effects, progression-free survival (defined as time from randomisation to progression, relapse, or death from any cause; patients with complete response or unconfirmed complete response and additional treat ment were censored) and overall survival (defined as time from randomisation to death from any cause). Kaplan- 4 Published online November 16, 1
5 Event-free survival (%) R- Overall survival (%) A 1 R p= C R Months since randomisation p= Progression-free survival (%) Overall survival (%) B p= D p= Months since randomisation Figure 3: Kaplan-Meier estimates of outcomes by treatment group in the intention-to-treat population Event-free survival (A), progression-free survival (B), and overall survival (C) for the intention-to-treat population. Overall survival for the 19 patients with age-adjusted IPI (D). R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R-CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin Meier estimates at 3 years, with 95% CIs, were calculated for event-free survival, progres sion-free survival, and overall survival. Multivariable analyses were done with Cox proportional-hazard models adjusted for stratification variables. Sensitivity analyses (ie, per-protocol analyses, complete treatment analyses) were done to assess the robustness of the results. Subgroup analyses according to the age-adjusted IPI were done as planned in the study protocol to investigate whether the treatment effects were homo geneous. Baseline charac teristics were reported as per centages except for age, which was reported as the median. Qualitative data (eg, non-haematological toxic effects) were analysed by use of χ² test and, if necessary, by Fisher s exact test. Relative dose and relative doseintensity were assessed according to the Kaplan-Meier method as described elsewhere. 1 Differences between groups were classed as significant for p values less than or equal to 5. Statistical analyses of efficacy were done with SPSS PASW 18. This study is registered with ClinicalTrials.gov, number NCT199. Role of the funding source Staff members of the DSHNHL were responsible for distribution and collection of case report forms, data entry, and validation, coordination of monitoring procedures, elaboration of queries, adverse event reporting, statistical analyses, and production of the study report. Annual study group meetings served as platforms for progress reports and decisions on trial conduct. Deutsche Krebshilfe, who provided funding for study, had no role in study design, data collection and analysis, interpretation or writing the report. All authors had full access to the raw data in this study and the corresponding author had final responsibility for the decision to submit for publication. Results We enrolled 36 patients, 31 of whom were treated with out rituximab (figure ). 6 patients with CD-positive, aggressive B-cell lymphoma received chemotherapy and rituximab and formed the intention-to-treat-population of this analysis. More than 9% of those patients received prephase therapy with vincristine and prednisone. Published online November 16, 1 5
6 Event-free survival (%) Progression-free survival (%) A R B 1 1 p= R C 1 Overall survival (%) p= R p= Months since randomisation R- Patient characteristics did not differ significantly between arms (table 1). Close to 8% of patients in both treatment arms had diffuse large B-cell lymphoma; the other subtypes of aggressive B-cell lymphoma are specified in table 1. Most patients scored an age-adjusted IPI of ; age-adjusted IPI was 3 in about 7% of patients, mainly reflecting their poor performance status. 15 patients (11 5%) did not complete R- and 38 (8 8%) did not complete for reasons detailed in the trial profile (figure ). Besides lymphoma progression, changes of diagnosis, and individual patient decisions, three patients in the conventional arm and 1 patients in the experimental arm did not complete therapy as randomised because of toxic effects. Six of these 1 patients stopped study treatment while 15 patients continued therapy with R- as stipulated in the protocol. At the end of study, five patients on treatment with were switched to R- because of ethical concerns expressed by the data monitoring and safety committee and study group members. The planned administered doses and dose intensities for all drugs are compared in table. According to protocol, 63 (48 5%) of 13 patients treated with R- and 54 (4 9%) of 13 patients treated with were irradiated for bulky or extranodal disease. There was no significant difference in the proportion of patients who achieved a complete or unconfirmed response (p= 18) or an overall response (p= 35; table 3). After a median of 4 months (IQR 9 59), 3-year event-free survival was estimated at 69 5% (95% CI ) for patients treated with R- and 61 4% (5 8 7 ) for patients treated with (p= 14; hazard ratio 1 3, 95% CI 9 ). 3-year progression-free survival was 73 7% (95% CI ) after treatment with R- and 69 8% ( ) after treatment with (p= 48). 3-year overall survival was 84 6% (95% CI ) for patients treated with R- compared with 77 % ( ) for patients treated with (p= 8; figure 3). Patients with age-adjusted IPI had significantly better event-free survival if treated with R- (75 5% [95% CI ] in the R- group vs 63 5% [ ] in the group; p= 59) and overall survival (91 % [95% CI ] vs 77 1% [ ]; p= 1; figure 3) while no significant differences were seen if patients with ageadjusted IPI 3 only were assessed (event-free survival: 53 9% [95% CI ] vs 55 5% [ ]; Figure 4: Kaplan-Meier estimates of outcomes by treatment group in patients who received all treatment as per protocol Event-free survival (A), progression-free survival (B), and overall survival (C). R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R-CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. 6 Published online November 16, 1
7 p= 9; overall survival: 68 1% [95% CI ] vs 76 5% [6 9 8]; p= 75). Because a sizeable fraction of patients randomly assigned to were unable to complete treatment we did a further analysis restricting the comparison of R- and to 15 patients who had received all treatment per protocol. No significant differences were seen in event-free survival (p= 51), progression-free survival (p= 41), or overall survival (p= 51) between treatment arms (figure 4). In multivariate analyses of event-free survival and overall survival adjusted for strata no other factors significantly influencing treatment outcome were found (data not shown). Figure 5 shows the event-free survival of 93 patients treated on study including the 31 patients who did not receive rituximab before these treatment arms were closed. A median of 6 5 1⁶ CD34-positive blood cells were transplanted after cycle of, 4 6 1⁶ after cycle 3, and 4 1⁶ after cycle 4. Neutrophil (absolute neutrophil count > 5 1⁹ per L) and platelet (platelets >5 1⁹ per L) recovery occurred after a median of 13 days and 14 days after cycle 1, 15 days and 16 days after cycle, 15 days and 18 days after cycle 3, and 16 days and days after cycle 4; no graft failures were reported. All evaluable patients (n=18) in the group had WHO grade 4 leucopenia; granulocyte colony-stimulating factor was used in 95 3% of all treatment cycles and in 18 of 19 patients (99 %) on R-, but 48 (58 5%) of 8 patients with documented blood counts still developed grade 4 leucopenia. All evaluable patients in the group had grade 3 4 thrombocytopenia; 6 (33 8 %) of 77 patients on R- with documented blood counts had grade 3 or 4 thrombocytopenia. Platelet transfusions were needed in 115 (91 3%) of 18 patients in the group and 13 (1 5%) of 14 patients in the R- group with documented platelet counts. Anaemia was frequent and necessitated the transfusion of red blood cells in 117 (9 9%) of 16 in the arm and 8 (63 5%) of 16 patients in the R- arm. Patients on developed more mucositis, diarrhoea, nausea, and vomiting, which together with the substantial neutropenia contributed to the 75 % incidence of severe infections in these patients (table 4). Sensory neurological side-effects were more common after R-, which might be due to the higher cumulative dose of vincristine administered (p= ). Overall, 3 patients on and 1 patients on R- died; causes of death are listed in table 5. not only caused more deaths related to toxic effects than did R- (5 6% vs 3 1%) but also more patients died from lymphoma (16 patients as opposed to nine patients after R-). Nine secondary malignancies have occurred to date (3 6%) with no significant differences Event-free survival (%) MegaCHOEP R Months since randomisation between treatment arms: four patients were diagnosed with myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML), or solid tumours (n=) after R-, five patients were diagnosed with MDS, AML, and epidermoid cancer or solid tumours (n=3) after. Discussion In young patients aged 18 to 6 years with high-risk (age-adjusted IPI or 3) aggressive B-cell lymphoma R- administered every weeks was associated with high remission rates. Our results for event-free, progression-free, and overall survival after R- in such patients are the most encouraging to date (panel). However, was no better than MegaCHOEP R- Figure 5: Kaplan-Meier estimates of event-free survival for patients who received R- or with or without rituximab =cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone. MegaCHOEP= with escalated doses of cyclophosphamide, etoposide, and doxorubicin. R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R-CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. R- (n=13) (n=13) Infection 4/18 (31 3%) 96/18 (75 %) Mucositis 1/11 (8 3%) 81/15 (64 8%) Nausea 1/1 ( 8%) 1/13 (17 1%) Diarrhoea 4/1 (3 3%) 14/1 (11 5%) Vomiting /119 (1 7%) 13/13 (1 6%) Psychiatric disorders /119 (1 7%) 7/1 (5 7%) Arrhythmia 5/1 (4 1%) Sensory 1/14 (8 1%) /11 (1 7%) Data are number (%) of patients with a documented event during 1 treatment cycle. R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R-CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. Table 4: Grade 3 4 non-haematological adverse events by treatment arm Published online November 16, 1 7
8 R- Number of deaths (%)* 1/17 (16 5%) 3/16 (5 4%) Tumour related 9 16 Therapy-related (only study 4 7 treatment) Therapy-related (including salvage) 1 1 Secondary neoplasia 3 Concomitant disease 1 3 Other or unknown R-=cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, and rituximab. =R-CHOEP with escalated doses of cyclophosphamide, etoposide, and doxorubicin. *In some cases more than one cause of death was documented. Table 5: Cause of death Panel: Research in context Systematic review We searched Medline from January,, to June, 1, with search terms aggressive B-cell lymphoma or diffuse large B-cell lymphoma and rituximab and young patients or patients younger than 6 for reports published in English and German. We identified one trial comparing chemoimmunotherapy (R-CHOEP) with chemotherapy alone 13 and one trial comparing two chemoimmunotherapy regimens. Neither trial assessed young, high-risk patients with age-adjusted International Prognostic Index (IPI) or 3. We did not identify any trial comparing conventional therapy with high-dose therapy both combined with rituximab. 3 5 These latter studies compared conventional chemotherapy (R-CHOP or R-CHOP-like 3 5 ) to classic high-dose therapy (BEAM [BCNU, etoposide, cytosinearabinoside, melphalan] or total-body-irradiation-based) in combination with rituximab. Interpretation Our study shows that aggressive conventional chemoimmunotherapy (R-) gives excellent results in young, high-risk patients with aggressive B-cell lymphoma, which cannot be improved with high-dose therapy necessitating autologous stem cell transplantation. This finding is strengthened by the fact that we used identical cytotoxic drugs for both conventional and high-dose therapy. R- in terms of efficacy, and was associated with significantly more toxicity, suggesting that in the rituximab era HDT followed by ASCT does not improve outcome for this group of patients. In the pre-rituximab era, survival of young, high-risk patients with diffuse large B-cell lymphoma varied from 55% to 67% 3 11 after conventional chemotherapy whereas full reports in randomised trials with rituximab-containing regimens have not been published yet. Recent abstracts with short follow-up showed -year progressionfree survival of 63% and overall survival of 75% after (R)- CHOP-1, 3 event-free survival of 56% and overall survival of 83% after R-CHOP-14 for patients with age adjusted IPI 3, 4 and progression-free survival of 59% and overall survival of 83% after R-CHOP-14 or R-Mega- CHOP. 5 While survival rates after CHOP-1 or R-CHOP-1 were not separately reported by Stiff and colleagues, 3 the event-free survival after R-CHOP-14 for patients with age adjusted IPI or 3 in Le Gouill and colleagues study 4 was 13 5 % lower, progression-free survival after R-MegaCHOP 5 was 14 5% lower than reported here for R-. We speculate that the higher event-free survival and progression-free survival reported for R- reflects the integration of etoposide into the CHOP regimen. The addition of etoposide to CHOP was pioneered by Koeppler and colleagues 6 more than 5 years ago and since that time CHOEP has continuously been used to treat high-risk patients with aggressive B-cell and T-cell lymphoma in Germany. 7 A prospective, randomised study from the pre-rituximab era adopted this strategy and confirmed the superiority of CHOEP over CHOP in young patients with normal lactate dehydrogenase levels. 8 Although a randomised comparison of R-CHOP to R-CHOEP has not been done, the study group members of the DSHNHL had voted in favour of R- and against R-CHOP-14 when the design of the current study was previously discussed in 1. In addition to the results of the ran domised study by Pfreundschuh and colleagues 8 the major reasons for this decision were two-fold: first, we wanted to make sure that a potentially superior survival of patients treated with HDT/ASCT could not be explained by the poor results obtained with conventional chemotherapy. This strategy had been successfully implemented when we compared HDT/ASCT to an aggressive conventional salvage chemotherapy in patients with relapsed Hodgkin s disease. 9 Second, and most importantly, the study group members expressed strong ethical concerns that CHOP chemotherapy would show unsatisfactory activity in young, high-risk patients. For this reason, the precursor of the current DSHNHL study from the prerituximab era had already used CHOEP instead of CHOP in the conventional treatment arm. 8 R-CHOEP has also been used in other countries, for instance, an early report from Sweden 3 and a population-based analysis from Denmark 31 support that etoposide significantly adds to the efficacy of R-CHOP-14. Finally, the Groupe d Etude des Lymphomes de l Adulte (GELA) recently reported superior results in young patients with age-adjusted IPI 1 comparing their R-ACVBP (rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone) regimen to R-CHOP-14. Like the CHOEP regimen, ACVBP uses higher doses of cyclo phosphamide and doxorubicin and adds additional cytotoxic agents (vindesine and bleomycin). Radiotherapy for bulky and extranodal disease might also have contributed to the favourable outcome of our patients Published online November 16, 1
9 In the studies mentioned above, patients were randomly assigned to either conventional or high-dose therapy. While the study done in the USA lacked the statistical power to compare the results of R-CHOP-1 with R-CHOP-1 followed by HDT/ASCT, 3 the study done in France came to the same conclusion as our study. 4 In patients who were PET-negative after four courses of R-CHOP-14, HDT/ASCT did not improve progressionfree survival or overall survival as compared with four more courses of R-CHOP-14. Another study in Italy reported a significantly better progression-free survival for chemo sensitive patients who proceeded to transplantation; overall survival was not significantly different. 5 However, neither -year progression-free survival (63%) nor overall survival (8%) after HDT/ASCT were better than the -year progressionfree survival (75%) and overall (85%) after R-. We therefore suggest adding a standard dose of etoposide to CHOP and giving local radiotherapy rather than exposing patients to the higher risks and discomfort of HDT/ASCT. We did not address the point that classic HDT with BEAM (BCNU, etoposide, cytosin-arabinoside, melphalan) followed by ASCT might improve progressionfree survival in patients who respond to a limited number of conventional chemotherapy courses with the R-CHOP regimen, and therefore cannot rule it out. While all other studies used established high-dose regimens (BEAM or total-body-irradiation-based), we chose to use identical drugs in both the conventional and the high-dose regimen to address the proof-of-principle question of whether dose escalation of cytotoxic agents improves outcome also in the rituximab era. HDT was administered to all patients who could possibly receive it and was not restricted to patients achieving complete remission or partial remission with conventional chemotherapy. Extensive phase studies with 33 and without 34 rituximab have shown that the time interval between cycles of MegaCHOEP could not be further shortened and six cycles were not superior to four cycles. 17 Current analyses show that even patients able to receive the full programme did not have better outcomes than after R-. We conclude that further escalation of dose or doseintensity within a high-dose regimen is not possible and would not be effective. Because of the high efficacy of R-CHOEP we stopped using HDT/ASCT as part of firstline therapy in high-risk (age-adjusted IPI, 3) patients with aggressive B-cell lymphoma. In these young pa tients, R- was no more toxic than R-CHOP, was feasible in an outpatient basis, and was highly effective. R-, therefore, represents a valid alternative to other regimens. Further improvement is certainly necessary, especially in patients with age-adjusted IPI 3. Ongoing investigations will show if those patients not responding to rituximab and combination chemotherapy belong to biological high-risk groups characterised by gene-expression profiling 35 or the pres ence of MYC and BCL translocations. 36 We recently showed that variations in rituximab dosing can improve event-free survival and overall survival in elderly high-risk patients with diffuse large B-cell lymphoma. 37 Therefore, we are investigating if doubling the number of rituximab infusions from six to 1 will also improve outcome of younger patients. Contributors NS, MZ, ML, and BG designed the study. NS, MN, MZ, MH, PB, CS, AV, MB, NP, GE, GD, LT, MP, and BG recruited patients and obtained study data. AR was responsible for histological review. CR designed radiotherapy and was responsible for radiological review. MZ and ML did the biometric analyses. NS, MN, MZ, ML, and BG analysed and interpreted the data and wrote the report. All authors reviewed and approved the final report. Conflicts of interest NS and MP were members of Roche advisory boards. BG, NS, MP, and AR have received research support from Roche. All other authors declare that they have no conflicts of interest. Acknowledgments This work was supported by the Deutsche Krebshilfe (7-73-pf4). We thank Ulrike Schoenwiese, Beate Mann (Institute for Medical Informatics, Statistics, and EpidemiologyLeipzig), and Corinna Endler (Asklepios Hospital St Georg Hamburg) for data management, Martina Kunert and Barbara Wicklein for data base development (IMISE Leipzig), and Huei-Shan Wu (Asklepios Hospital St Georg Hamburg) for secretarial assistance. References 1 Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD+ B-cell lymphoma in the rituximab era. J Clin Oncol 1; 8: The International Non-Hodgkin s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-hodgkin s lymphoma. N Engl J Med 1993; 39: Haioun C, Lepage E, Gisselbrecht C, et al. Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non-hodgkin s lymphoma: updated results of the prospective study LNH87-. Groupe d Etude des Lymphomes de l Adulte. J Clin Oncol 1997; 15: Gianni AM, Bregni M, Siena S, et al. High-dose chemotherapy and autologous bone marrow transplantation compared with MACOP-B in aggressive B-cell lymphoma. N Engl J Med 1997; 336: Santini G, Salvagno L, Leoni P, et al. VACOP-B versus VACOP-B plus autologous bone marrow transplantation for advanced diffuse non-hodgkin s lymphoma: results of a prospective randomized trial by the non-hodgkin s Lymphoma Cooperative Study Group. J Clin Oncol 1998; 16: Kluin-Nelemans HC, Zagonel V, Anastasopoulou A, et al. Standard chemotherapy with or without high-dose chemotherapy for aggressive non-hodgkin s lymphoma: randomized phase III EORTC study. J Natl Cancer Inst 1; 93: 3. 7 Gisselbrecht C, Lepage E, Molina T, et al, and the Groupe d Etude des Lymphomes de l Adulte. Shortened first-line high-dose chemotherapy for patients with poor-prognosis aggressive lymphoma. J Clin Oncol ; : Kaiser U, Uebelacker I, Abel U, et al. Randomized study to evaluate the use of high-dose therapy as part of primary treatment for aggressive lymphoma. J Clin Oncol ; : Martelli M, Gherlinzoni F, De Renzo A, et al. Early autologous stemcell transplantation versus conventional chemotherapy as front-line therapy in high-risk, aggressive non-hodgkin s lymphoma: an Italian multicenter randomized trial. J Clin Oncol 3; 1: Milpied N, Deconinck E, Gaillard F, et al, and the Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med 4; 35: Betticher DC, Martinelli G, Radford JA, et al. Sequential high dose chemotherapy as initial treatment for aggressive sub-types of non-hodgkin lymphoma: results of the international randomized phase III trial (MISTRAL). Ann Oncol 6; 17: Published online November 16, 1 9
10 1 Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-b-cell lymphoma. N Engl J Med ; 346: Pfreundschuh M, Trümper L, Osterborg A, et al, and the MabThera International Trial Group. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-b-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 6; 7: Jaffe ES, Harris NL, Stein H, et al. Pathology and genetics: Neoplasm of hematopoietic and lymphoid tissue. In Kleihues P, Sobin LH, eds. World Health Organization classification of tumours. Lyon: IARC Press Pocock SJ. Clinical trials. John Wiley & Sons, Chichester (1983). 16 Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 198; 5: Schmitz N, Kloess M, Reiser M, et al, and the German High-Grade Non Hodgkin s Lymphoma Study Group (DSHNHL). Four versus six courses of a dose-escalated cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen plus etoposide (megachoep) and autologous stem cell transplantation: early dose intensity is crucial in treating younger patients with poor prognosis aggressive lymphoma. Cancer 6; 16: Cheson BD, Horning SJ, Coiffier B, et al, and the NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-hodgkin s lymphomas. J Clin Oncol 1999; 17: National Cancer Institute. Common terminology criteria for adverse events v3.. electronic_applications/docs/ctcaev3.pdf (accessed Oct 11, 11). O Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35: Wunderlich A, Kloess M, Reiser M, et al, and the German High-Grade Non-Hodgkin s Lymphoma Study Group (DSHNHL). Practicability and acute haematological toxicity of - and 3-weekly CHOP and CHOEP chemotherapy for aggressive non-hodgkin s lymphoma: results from the NHL-B trial of the German High-Grade Non-Hodgkin s Lymphoma Study Group (DSHNHL). Ann Oncol 3; 14: Récher C, Coiffier B, Haioun C, et al, and the Groupe d Etude des Lymphomes de l Adulte. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH3-B): an open-label randomised phase 3 trial. Lancet 11; 378: Stiff PJ, Unger JM, Cook J, et al. Randomized phase III U.S./ Canadian intergroup trial (SWOG S974) comparing CHOP ± R for eight cycles to CHOP ± R for six cycles followed by autotransplant for patients with high-intermediate (H-Int) or high IPI grade diffuse aggressive non-hodgkin lymphoma (NHL). Proc Am Soc Clin Oncol 11; 9: abstr Le Gouill S, Milpied NJ, Lamy T, et al. First-line rituximab (R) high-dose therapy (R-HDT) versus R-CHOP14 for young adults with diffuse large B-cell lymphoma: preliminary results of the GOELAMS 75 prospective multicenter randomized trial. Proc Am Soc Clin Oncol 11; 9: abstr Vitolo U, Chiappella A, Brusamolino E, et al. A randomized multicentre phase III study for first line treatment of young patients with high risk (aaipi -3) diffuse large B-cell lymphoma (DLBCL): rituximab (R) plus dose-dense chemotherapy CHOP-14/MEGACHOP 14 with or without intensified high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT). Results of DLCL4 trial of Italian lymphoma foundation (FIL). Ann Oncol 11; (suppl 4): abstr 7. 6 Köppler H, Pflüger KH, Eschenbach I, et al. CHOP-VP16 chemotherapy and involved field irradiation for high grade non-hodgkin s lymphomas: a phase II multicentre study. Br J Cancer 1989; 6: Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 1; 116: Pfreundschuh M, Trümper L, Kloess M, et al, and the German High-Grade Non-Hodgkin s Lymphoma Study Group. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood 4; 14: Schmitz N, Pfistner B, Sextro M, et al, and the German Hodgkin s Lymphoma Study Group, and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin s disease: a randomised trial. Lancet ; 359: Adde M, Enblad G, Hagberg H, Sundström C, Laurell A. Outcome for young high-risk aggressive B-cell lymphoma patients treated with and rituximab (R-). Med Oncol 6; 3: Gang AO, Strøm C, Pedersen M, et al. R- improves overall survival in young high-risk patients with diffuse large B-cell lymphoma compared with R-CHOP-14. A population-based investigation from the Danish Lymphoma Group. Ann Oncol 1; 3: Held G, Murawski N, Ziepert M, et al. Role of radiotherapy for elderly DLBCL patients in the rituximab (R) era: final results of the RICOVER-6-NO-RX study of the DSHNHL. Haematologica 1; 97: 458 (abstract 1114a). 33 Glass B, Ziepert M, Reiser M, et al, and the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). High-dose therapy followed by autologous stem-cell transplantation with and without rituximab for primary treatment of high-risk diffuse large B-cell lymphoma. Ann Oncol 1; 1: Glass B, Kloess M, Bentz M, et al, and the German High-Grade Non-Hodgkin Lymphoma Study Group. Dose-escalated CHOP plus etoposide (MegaCHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk non-hodgkin lymphoma. Blood 6; 17: Lenz G, Wright G, Dave SS, et al, and the Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-b-cell lymphomas. N Engl J Med 8; 359: Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 1; 3: Pfreundschuh M, Held G, Zeynalova S, et al. Improved outcome of elderly poor-prognosis DLBCL patients with 6 x CHOP-14 and 8 applications of rituximab (R) given over an extended period: results of the SMARTE-R-CHOP-14 Trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 11; 118: 59a. 1 Published online November 16, 1
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
Diffuse large B-cell lymphoma: the curable disease?
 Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
DIFFUSE LARGE B-CELL LYMPHOMA
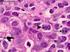 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
Lymphoma. Measurability of Quality Performance Indicators Version 2.0
 Lymphoma Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Lymphoma Clinical Quality Performance Indicators (September 2013) Lymphoma Data Definitions V2.0 (September
Lymphoma Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Lymphoma Clinical Quality Performance Indicators (September 2013) Lymphoma Data Definitions V2.0 (September
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
Rituximab in Non - Hodgkins Lymphoma. Fatima Bassa, Dept. of Haematology October 2008
 Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
J Clin Oncol 23:8447-8452. 2005 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics
 Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up
 Annals of Oncology 23 (Supplement 7): vii78 vii82, 2012 doi:10.1093/annonc/mds273 Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines f diagnosis, and follow-up H. Tilly 1, U. Vitolo
Annals of Oncology 23 (Supplement 7): vii78 vii82, 2012 doi:10.1093/annonc/mds273 Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines f diagnosis, and follow-up H. Tilly 1, U. Vitolo
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES
 NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Audience Response Question?
 Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Audience Response Question? Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management. Presenter Disclosure Information
 Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
J Clin Oncol 22:4302-4311. 2004 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 22 NUMBER 21 NOVEMBER 1 2004 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Incidence and Predictors of Low Chemotherapy Dose-Intensity in Aggressive Non-Hodgkin s Lymphoma: A Nationwide
VOLUME 22 NUMBER 21 NOVEMBER 1 2004 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Incidence and Predictors of Low Chemotherapy Dose-Intensity in Aggressive Non-Hodgkin s Lymphoma: A Nationwide
Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma
 IMAJ VOL 11 January 9 Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma Ariela Dortort Lazar MD 1,, Ofer Shpilberg
IMAJ VOL 11 January 9 Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma Ariela Dortort Lazar MD 1,, Ofer Shpilberg
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Supplementary Online Content
 Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH)
 Session 3 : Epidemiology and public health Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH) Le Guyader-Peyrou Sandra Bergonie Institut Context:
Session 3 : Epidemiology and public health Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH) Le Guyader-Peyrou Sandra Bergonie Institut Context:
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Lymphoma Diagnosis and Classification
 Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA
 2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
Histopathologic results
 Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Guidelines for the Management of Follicular Lymphoma
 Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Collaboration to collect Autologous transplant outcomes in Lymphoma and Myeloma (CALM) Additional Questionnaire (MED C) INCLUSION CRITERIA CALM STUDY
 Additional Questionnaire (MED C) CALM study Inclusion period: 01/01/2008 to 31/12/2011 PATIENT REGISTRATION FORM Disease Diagnosis Lymphoma S Non Hodgkin Lymphoma (NHL) Mature B-cell neoplasm Follicular
Additional Questionnaire (MED C) CALM study Inclusion period: 01/01/2008 to 31/12/2011 PATIENT REGISTRATION FORM Disease Diagnosis Lymphoma S Non Hodgkin Lymphoma (NHL) Mature B-cell neoplasm Follicular
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy
 Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
How I treat patients with diffuse large B-cell lymphoma
 How I treat How I treat patients with diffuse large B-cell lymphoma James O. Armitage 1 1 The Joe Shapiro Professor of Medicine, University of Nebraska Medical Center, Omaha Introduction The disease we
How I treat How I treat patients with diffuse large B-cell lymphoma James O. Armitage 1 1 The Joe Shapiro Professor of Medicine, University of Nebraska Medical Center, Omaha Introduction The disease we
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Outline of thesis and future perspectives.
 Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
STUDY PROTOCOL. Fabio Ciceri M.D. Istituto Scientifico H. San Raffaele Dept. of of Oncology, Haematology/Transplant Unit I-20132 Milan
 STUDY PROTOCOL Clinical phase II trial to evaluate the safety and efficacy of treosulfan combined with cytarabine and fludarabine prior to autologous haematopoietic stem cell transplantation in elderly
STUDY PROTOCOL Clinical phase II trial to evaluate the safety and efficacy of treosulfan combined with cytarabine and fludarabine prior to autologous haematopoietic stem cell transplantation in elderly
Lymphoma Overview Joseph Leach, MD
 Lymphoma Overview Joseph Leach, MD 71 year old male presents with complaints of mild fa5gue and a visible mass in the le: supraclavicular region PE demonstrates a firm easily palpable mass in the le: supraclavicular
Lymphoma Overview Joseph Leach, MD 71 year old male presents with complaints of mild fa5gue and a visible mass in the le: supraclavicular region PE demonstrates a firm easily palpable mass in the le: supraclavicular
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited
 rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Stem Cell Transplantation
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Update on Follicular Lymphoma. Brad Kahl, M.D.
 Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
MEDICAL COVERAGE POLICY
 Important note Even though this policy may indicate that a particular service or supply is considered covered, this conclusion is not necessarily based upon the terms of your particular benefit plan. Each
Important note Even though this policy may indicate that a particular service or supply is considered covered, this conclusion is not necessarily based upon the terms of your particular benefit plan. Each
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Summary & Conclusion
 The prognostic value of angiogenesis markers in patients with non-hodgkin lymphoma. Summary & Conclusion The current study aims to asses the prognostic value of some angiogenesis markers in patients with
The prognostic value of angiogenesis markers in patients with non-hodgkin lymphoma. Summary & Conclusion The current study aims to asses the prognostic value of some angiogenesis markers in patients with
Treatment results with Bortezomib in multiple myeloma
 Treatment results with Bortezomib in multiple myeloma Prof. Dr. Orhan Sezer Hamburg University Medical Center Circulating proteasome levels are an independent prognostic factor in MM 1.0 Probability of
Treatment results with Bortezomib in multiple myeloma Prof. Dr. Orhan Sezer Hamburg University Medical Center Circulating proteasome levels are an independent prognostic factor in MM 1.0 Probability of
Chemoimmunotherapy resistant follicular lymphoma A single institutional study
 Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
cancer cancer Hessamfar-Bonarek M et al. Int. J. Epidemiol. 2010;39:135-146
 Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
New Targets and Treatments for Follicular Lymphoma. Disclosures
 Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Relapsed Diffuse Large B-Cell Lymphoma 10 Years Later
 H & 0 C l i n i c a l C a s e S t u d i e s Relapsed Diffuse Large B-Cell Lymphoma 10 Years Later Shikha Jain, MD Neel Shah, MD Stephanie Gregory, MD Rush University Medical Center, Chicago, Illinois Introduction
H & 0 C l i n i c a l C a s e S t u d i e s Relapsed Diffuse Large B-Cell Lymphoma 10 Years Later Shikha Jain, MD Neel Shah, MD Stephanie Gregory, MD Rush University Medical Center, Chicago, Illinois Introduction
Hematopoietic Stem Cell Transplantation. Imad A. Tabbara, M.D. Professor of Medicine
 Hematopoietic Stem Cell Transplantation Imad A. Tabbara, M.D. Professor of Medicine Hematopoietic Stem Cells Harvested from blood, bone marrow, umbilical cord blood Positive selection of CD34 (+) cells
Hematopoietic Stem Cell Transplantation Imad A. Tabbara, M.D. Professor of Medicine Hematopoietic Stem Cells Harvested from blood, bone marrow, umbilical cord blood Positive selection of CD34 (+) cells
Long Term Low Dose Maintenance Chemotherapy in the Treatment of Acute Myeloid Leukemia
 Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Treating myeloma. Dr Rachel Hall Royal Bournemouth Hospital
 Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
GLSG/OSHO Study Group. Supported by Deutsche Krebshilfe
 GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
STEM CELL TRANSPLANTATION IN MULTIPLE MYELOMA
 STEM CELL TRANSPLANTATION IN MULTIPLE MYELOMA Sundar Jagannath MD Professor of Medicine St. Vincent s Comprehensive Cancer Center New York, NY Where is transplant today in the management of Myeloma? Autologous
STEM CELL TRANSPLANTATION IN MULTIPLE MYELOMA Sundar Jagannath MD Professor of Medicine St. Vincent s Comprehensive Cancer Center New York, NY Where is transplant today in the management of Myeloma? Autologous
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy
 Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Endpoint Selection in Phase II Oncology trials
 Endpoint Selection in Phase II Oncology trials Anastasia Ivanova Department of Biostatistics UNC at Chapel Hill [email protected] Primary endpoints in Phase II trials Recently looked at journal articles
Endpoint Selection in Phase II Oncology trials Anastasia Ivanova Department of Biostatistics UNC at Chapel Hill [email protected] Primary endpoints in Phase II trials Recently looked at journal articles
Lauren Berger: Why is it so important for patients to get an accurate diagnosis of their blood cancer subtype?
 Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; [email protected] (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; [email protected] (2) Steven
DE Tsai 1, HCF Moore 1, CL Hardy 2, DL Porter 1, EY Loh 1, DJ Vaughn 1, S Luger 1, SJ Schuster 1 and EA Stadtmauer 1
 Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm
 Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Cure versus control: Which is the best strategy?
 Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need?
 What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Management of low grade glioma s: update on recent trials
 Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Health Disparities in Multiple Myeloma. Kenneth R. Bridges, M.D. Senior Medical Director Onyx Pharmaceuticals, Inc.
 Health Disparities in Multiple Myeloma Kenneth R. Bridges, M.D. Senior Medical Director Onyx Pharmaceuticals, Inc. Multiple Myeloma Overview Multiple myeloma (MM) is a type of blood cancer that develops
Health Disparities in Multiple Myeloma Kenneth R. Bridges, M.D. Senior Medical Director Onyx Pharmaceuticals, Inc. Multiple Myeloma Overview Multiple myeloma (MM) is a type of blood cancer that develops
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
1. Introduction. 2. Clinical aspects
 1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
Many people with non-hodgkin lymphoma have found an educational support group helpful. Support
 Track 2: Treatment Options [Narrator] Many people with non-hodgkin lymphoma have found an educational support group helpful. Support groups take many forms: some meet the needs of people with all kinds
Track 2: Treatment Options [Narrator] Many people with non-hodgkin lymphoma have found an educational support group helpful. Support groups take many forms: some meet the needs of people with all kinds
The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma
 The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma 11 th Annual National Leadership Summit on Health Disparities Innovation Towards Reducing Disparities Congressional Black
The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma 11 th Annual National Leadership Summit on Health Disparities Innovation Towards Reducing Disparities Congressional Black
Interesting Case Series. Periorbital Richter Syndrome
 Interesting Case Series Periorbital Richter Syndrome MarkGorman,MRCS,MSc, a Julia Ruston, MRCS, b and Sarath Vennam, BMBS a a Division of Plastic Surgery, Royal Devon and Exeter Hospital, Exeter, Devon,
Interesting Case Series Periorbital Richter Syndrome MarkGorman,MRCS,MSc, a Julia Ruston, MRCS, b and Sarath Vennam, BMBS a a Division of Plastic Surgery, Royal Devon and Exeter Hospital, Exeter, Devon,
Bone Marrow/Stem Cell Transplant
 Blue Distinction Centers for Transplants Program Selection Criteria for 2010 Mid-Point Designations To qualify as a Blue Distinction Center for Transplants (), each facility must satisfy s quality based
Blue Distinction Centers for Transplants Program Selection Criteria for 2010 Mid-Point Designations To qualify as a Blue Distinction Center for Transplants (), each facility must satisfy s quality based
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
