Characteristics Desired in Clinical Data Warehouse for Biomedical Research
|
|
|
- Godwin McCoy
- 8 years ago
- Views:
Transcription
1 Original Article Healthc Inform Res April;20(2): pissn eissn X Characteristics Desired in Clinical Data Warehouse for Biomedical Research Soo-Yong Shin, PhD 1, Woo Sung Kim, MD, PhD 1,2, Jae-Ho Lee, MD, PhD 1,3,4 1 Department of Biomedical Informatics, Asan Medical Center, Seoul, Korea; 2 Departments of Pulmonary and Critical Care Medicine, 3 Department of Emergency Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea; 4 Division of General Internal Medicine, Brigham and Women s Hospital, Boston, MA, USA Objectives: Due to the unique characteristics of clinical data, clinical data warehouses (CDWs) have not been successful so far. Specifically, the use of CDWs for biomedical research has been relatively unsuccessful thus far. The characteristics necessary for the successful implementation and operation of a CDW for biomedical research have not clearly defined yet. Methods: Three examples of CDWs were reviewed: a multipurpose CDW in a hospital, a CDW for independent multi-institutional research, and a CDW for research use in an institution. After reviewing the three CDW examples, we propose some key characteristics needed in a CDW for biomedical research. Results: A CDW for research should include an honest broker system and an Institutional Review Board approval interface to comply with governmental regulations. It should also include a simple query interface, an anonymized data review tool, and a data extraction tool. Also, it should be a biomedical research platform for data repository use as well as data analysis. Conclusions: The proposed characteristics desired in a CDW may have limited transfer value to organizations in other countries. However, these analysis results are still valid in Korea, and we have developed clinical research data warehouse based on these desiderata. Keywords: Information Storage and Retrieval, Research Design, Biomedical Research, Privacy, Research Ethics I. Introduction There are many ways to define a data warehouse (DW) due to its widespread adoption [1-4]; a good working definition Submitted: January 13, 2014 Revised: March 22, 2014 Accepted: April 6, 2014 Corresponding Author Jae-Ho Lee, MD, PhD Department of Emergency Medicine, Asan Medical Center, 88 Olympic-ro 43-gil, Songpa-gu, Seoul , Korea. Tel: , Fax: , rufiji@gmail.com This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. c 2014 The Korean Society of Medical Informatics of a DW is a dedicated computer system or database that consolidates subject-oriented, time-variant, and non-volatile data from multiple sources to support decision-making processes. Recently, DWs have become invaluable resources in various domains, and they are used to analyze trends over time or to extract valuable information. Based on the success of DWs in other fields, hospitals have started to adopt a DW system. A survey indicated that the adoption rate of DWs in Clinical and Translational Science Award (CTSA) institutions has increased from 64% (18 of 28 institutions) in 2008 to 86% (30 of 35) in 2010 [5]. DWs in hospitals, which are usually called clinical data warehouses (CDWs) [4,6,7], are used for various purposes, including administration, management, clinical practice, and research. These can be categorized as either conventional usage or hospital-specific usage. Conventional usage includes administration, operation, and management. Therefore, such a DW in a hospital is usually called an enterprise DW. It is
2 Soo-Yong Shin et al an earlier type of DW in hospitals [8]. The hospital-specific usage consists of clinical practice, quality improvement, and biomedical research [9]. However, research usage cannot be efficiently supported by conventional DW technology due to the complexity and heterogeneity of clinical and research data [10]. In addition, as electronic health records (EHRs) have been adopted in many hospitals, research using EHR data has been highlighted recently [11,12]. EHR is the legacy and live system that generates the raw data used to record clinical data of patients. Governmental regulations, such as the requirement of de-identification, limits the direct use of EHR data [13]. Also, EHR data must be extracted, transformed, and loaded to other databases for analysis. CDWs integrate and reconstruct raw data from EHRs and other legacy systems for analysis, and they can adopt several interfaces needed for research compliance. Therefore, the importance of CDWs in accessing and analyzing EHR data for research has been increasing. Until now, CDWs have not been successful for hospital management compared to their promise because conventional DWs do not satisfy the needs of some unique hospital environments [9,10]. For example, an intensive care unit has many sets of continuous patient monitoring data and pointof-care device data, so their integration requires special concerns [14]. Radiology and other image data warehouses also require special features [4]. Recently, the term big data has also been introduced into DWs in the biomedicine field [15]. Therefore, we need to develop a special type of DW or DW for research to satisfy a hospital s individual needs, not just incorporate a conventional DW technology. However, the characteristics of CDWs for research have not been discussed widely and have not been well differentiated from conventional CDWs, although the characteristics of CDW were well described by Huser and Cimino [16]. In this paper, we focused on how to build a CDW for research; we use the term, clinical research data warehouse (CRDW) because one of the most important reasons to build a CDW is to support research. II. Methods To clarify the key elements needed in a CRDW, we reviewed the various types of CRDW-related terms such as the CDW, the research data warehouse and the integrated data repository, defined CRDW-related terms, and compared them. First, we searched PubMed with the keywords clinical data warehouse, and we found 89 articles in total. We classified these into three types, namely, research usage of CDWs, multi-institutional research data warehouses, and single institution research CDWs. From the three kinds of CDWs, two researchers selected the following three well-known CDWs based on their own knowledge to determine both the issues and benefits of current CRDWs: the Ohio State University Medical Center (OSUMC) Information Warehouse (IW), a research usage of CDWs in a hospital [17,18]; the Informatics for Integrating Biology and the Bedside (i2b2), a DW for an independent multi-institutional research [19,20]; and the Stanford Translational Research Integrated Database Environment (STRIDE), a CDW of single-institution research in a hospital [21,22]. From the literature review, CDW definitions, and a comparison of CDW cases, we propose some essential characteristics desired in a CRDW. III. Results 1. Clinical Research Data Warehouse and Its Related Terminologies Usually, the term CDW refers to an enterprise data warehouse in a hospital, which is used for administration, management, clinical practice, and research [23]. Here, we use the term CRDW to refer to a data warehouse in a hospital or other organization that is used only for research [24]. Therefore, a CDW is a place where healthcare providers can gain access to clinical data gathered during the patient care process [25] that may provide information for users in diverse areas [17-22]. The data in a CDW include any information related to patient care, such as specific demographics, vital signs, input and output data recorded for the patient, treatments and procedures performed, supplies used, and costs associated with the patient s care. The differences between a DW in a hospital and a DW in other domains were well described by Inmon [9]. He claimed that the information needs of medicine and healthcare are fundamentally different than those of other areas, and these fundamental differences in information gathering and storage make it difficult to implement successful data warehousing in hospitals [9]. The different perspectives for data warehousing between the healthcare domain and other domains are summarized in Table 1. First, each transaction or encounter in healthcare is relatively unique, as opposed to the business world in which each transaction is very repetitive. The data will even have different characteristics for each department in the hospital, including the emergency room, operation room, or the clinics. The second difference is in the types of data. Most healthcare data include textual descriptions of the various medical encounters of a patient. Additionally, data warehousing requires metadata or a common vocabulary, which
3 Characteristics Desired in CDW Table 1. Differences between a data warehouse of the healthcare domain and those of other domains Category Health care Other domain (bank, finance) Transaction Unique Repetitive Data type Mixed (text, code, number) Number Common vocabulary Normalization required Existing Time value of information Significant Not significant External category Essential Not significant Summarized and modified from Inmon [9]. is already well defined and used in other domains, such as banking or finance. Although there are many common vocabularies and related standards in medicine, the usage rate of common jargon in a hospital is low. Another major difference is that DWs in hospitals are primarily used as research data repositories. A recent CTSA survey reported that CDWs have shifted from a primarily administrative focus to a role incorporating more of the data contained in electronic medical records and the support systems for biomedical research [5]. Therefore, there is some precedent for CRDWs in ideas like research data warehouses and integrated data repositories [5]. The descriptions of both terms highlight the integration of multiple data sources, including hospital-generated data and genetic data, as essential for research. 2. Representative Examples of Clinical Research Data Warehouses When we reviewed the current CDWs or CRDWs, we found that the current data warehouses that support research can be classified into three different categories, namely, research usage of CDW, multi-institutional research data warehouse, and single-institution research data warehouse. The first type of CDW can support research as well as clinical practice and management. Representative examples are the OSUMC IW and Emory Healthcare [17,18,26]. Usually, this type of CDW has little institutional conflict and is able to gather information from clinical data sources since it supports hospital administration and business. Data marts are implemented to allow for research data search and extraction. Multi-institutional research data warehouses and singlehospital research data warehouses are designed for research purposes only, not for management. Lots of hospitals in the United States have adopted independent multi-institutional CRDW projects using the i2b2 platform [19,27]. There are over 60 hospitals that operate CDWs based on the i2b2 platform, including Cincinnati Children s Hospital [20,27]. This approach has several benefits by using an open-source platform, such as reducing implementation costs and guaranteeing the success of the project since there are many reference sites. Using the i2b2 architecture, their system integrates data from multiple sources, combines research data with clinical data, focuses on cohorts and patient populations, and has the potential for de-identified queries. The representative example of single hospital research data warehouse is Stanford University s STRIDE [21,22]. Since STRIDE is implemented in a university, not a hospital, the STRIDE project itself was prioritized independently, regardless of the complexities of hospital IT, and it can easily implement the necessary regulations. 1) Ohio State University Medical Center Information Warehouse The OSUMC IW seems to be a CDW drawing from diverse and disparate information systems throughout OSUMC [17,18]. Though it has been used for diverse areas including business, clinical, and research, we reviewed the OSUMC IW to determine the characteristics of a CDW that make it useful specifically for research. The OSUMC IW has little institutional conflict and is thus able to gather information from diverse clinical data sources. Data marts have been implemented to allow for research data extraction and getting the information out to users. In addition, data from several external sources are regularly incorporated into the OSUMC IW to assist in translational research. Therefore, this IW is a core asset that facilitates translational research and advances personalized healthcare. In 2006, the Ohio State University Institutional Review Board (IRB) approved a protocol recognizing the IW as an honest broker of clinical data, meaning that the IW can provide de-identified, limited, and coded data for use in research. 2) Cincinnati Children s clinical research data warehouse Cincinnati Children s CRDW is based on the i2b2 architecture, which is a research project designed to build an institutional-independent research data repository [19,20]. The Cincinnati Children s Hospital adopted the open-source platform i2b2 to reduce their costs and guarantee the success of Vol. 20 No. 2 April
4 Soo-Yong Shin et al the project. Using i2b2 architecture, their system integrates data from multiple sources, combines research data with clinical data, focuses on cohorts and patient populations, and has the potential for de-identified queries. The Cincinnati Children s CRDW includes patient demographics, diagnoses, procedures and medication orders for all inpatient and ambulatory encounters, including lab results, discharge summaries, and reports from pathology, cardiology, and radiology. This CRDW can integrate researchers own data by serving as a platform for research registries. This approach has several benefits. First, many of the queries asked of a registry are essentially forms of cohort identification. Second, integrating the registration information removes the need to load the data into multiple database systems or have users manually re-enter the relevant EHR data [19]. 3) Stanford Translational Research Integrated Database Environment STRIDE is a research and development project at Stanford University meant to create a standards-based informatics Table 2. A comparison of CRDW and CDW Category CRDW CDW (data warehouse in a hospital) Aim Clinical & translational research General, hospital management Need System Interface Essential function Subject area Data extraction & review De-identification Data extraction system Data (chart) review system Database IRB approval process Biologic specimen search Research design Cohort discovery De-identified data review Data extraction Research data (e.g., disease, laboratory test, medication) Integrated report Data extraction system Database None Reporting & review Ad-hoc query User Researchers Administrative staffs Internal data External data EHR data CPOE data LIMS data Bio-specimen data e-crf data Public research database Researcher-owned database Hospital management (e.g., nursing practice) All in-hospital data None Metadata Mandatory Selective Privacy Location De-identifiable data Identifiable data (after IRB approval) Hospital Research center Medical school Identifiable data Hospital Project priority High Low Implementation easiness Hard Easy Regulation Easy to follow Hard to follow CRDW: clinical research data warehouse, CDW: clinical data warehouse, EHR: Electronic Health Record, CPOE: computerized physician order entry, LIMS: laboratory information management system, e-crf: electronic case report form, IRB: Institutional Review Board
5 Characteristics Desired in CDW platform that supports clinical and translational research [21,22]. Because STRIDE is implemented in a university rather than a hospital, the STRIDE project itself was prioritized independently, regardless of the complexities of hospital IT, and it can easily implement the necessary regulations. STRIDE consists of three main databases, including a clinical data warehouse, a bio-specimen database, and a research database. Working upon those database systems are an anonymous cohort identification tool, a patient cohort data review tool, clinical data extraction, research data management, and bio-specimen data management. STRIDE is an IRB approved project, and some processes, such as data extraction require IRB approval. 3. Comparisons between a Clinical Research Data Warehouse and an Enterprise Data Warehouse in a Hospital Setting The results of a comparison between a CRDW and a CDW are summarized in Table 2. Essentially, CDWs are similar to conventional DWs, though there are some differences (as described in Table 1). However, a CRDW has significantly different characteristics. The purpose of a CRDW is to aid clinical and translational studies, not hospital management [5]. All data in a CRDW should be anonymized to protect the patients privacy. Additionally, IRB approval is required to process and search interfaces for ad-hoc queries. The most essential functions of a CRDW are research design, chart review, and data extraction, so it focuses on tasks like cohort identification and hypothesis generation and analysis [11,28]. Therefore, research data are the main subject area, though all related data and processes for clinical practice and research can also be incorporated. The primary sources of data for a CRDW are hospital information systems, such as EHR, laboratory information management system (LIMS), and computerized physician order entry (CPOE). Clinical trial registries and other researcher-owned databases (i.e., cohort or genomic data) should be integrated into the CRDW as well [18-21]. Other public research databases should also be interfaced to promote research. Its users are researchers and clinicians, not hospital administrative staff members. However, metadata and structured formats are still required for the easy and accurate retrieval of data. A CRDW can be located within a hospital, research center, or medical school, although a hospital should also contain a CDW for administration purposes. However, the location of a CRDW is problematic. A CRDW in a hospital is likely to rank lower in priority schemes than the more urgent hospital IT projects, making it likely to be neglected. A hospital also has to acquire additional funding for a CRDW project. However, a CRDW that is not located in a hospital requires a long developmental period and intra-institutional agreements must be made for clinical data to be obtained. Still, a CRDW outside of a hospital has some merits. It is much easier to incorporate non-hospital public data sources. Most importantly, the necessary regulations, such as the Health Insurance Portability and Accountability Act (HIPAA) compliance, IRB approval, and the honest broker system could be implemented more easily. IV. Discussion Based on the results of our comparisons, the ideal characteristics of a CRDW are defined in Table 3. The ultimate goal of a CRDW might be to serve as a biomedical research platform that is useful for data analysis in addition to functioning as a data repository. Therefore, interfaces for queries, honest bro- Table 3. Desired characteristics of a clinical research data warehouse Key element Explanation Remark Honest broker Query interface Chart review Protecting patient privacy based on hospital policy and HIPAA compliance Direct ad-hoc queries Data analysis tools Reviewing the de-identified EHR charts De-identification Cohort discovery Hypothesis design and analysis Data extraction Extracting the necessary (de-identifiable) data DRM module for access control IRB interface Research approvals Waivers Virtual desktop environment HIPAA: Health Insurance Portability and Accountability Act, EHR: Electronic Health Record, DRM: digital rights management, IRB: Institutional Review Board. Vol. 20 No. 2 April
6 Soo-Yong Shin et al ker services, data extraction, chart review, and IRB approval may be the key elements. The honest broker is an individual/organization/system which acts on the tissue bank and database [29]. The honest broker protects patients privacy based on institutional policy and government regulations, such as HIPAA, through de-identification [13,17,29,30]. It de-identifies all of the necessary patient-related data and serves as an interface to extract the requested bio-specimen samples or clinical data. The identifiable data can be extracted with IRB approval. Therefore, an interface with the IRB system should also be prepared. If an electronic IRB system may be used, the research approval or waiver should be automatically transferred into the CRDW. In the case of a paper-based IRB, the necessary information should be entered into the database by the researchers. For research hypothesis design and analysis, an easy interface for queries and data review tools should be implemented. A query interface allows a user to find the candidate number of a study group and to search the necessary bio-specimen samples. By reviewing query results, researchers could design study hypotheses. A chart review tool is also needed to confirm the cohort size and analyze the hypothesis manually, displaying the de-identified patient data; however, if the IRB approves, the necessary identifiable data could be delivered. A data extraction tool is necessary to obtain desired data for further use. For the extraction of identifiable data, digital rights management tools, which control data access, should be considered to protect privacy. Alternatively, a virtual desktop environment could be used. If the virtual desktop environment used is based on cloud computing technology, several security concerns can be easily solved. The cloud can also offer the powerful computing resources required to handle genomic data processing, such as next-generation sequencing. Finally, a CRDW needs to integrate data analysis programs by supporting the seamless transfer of extracted data. The analysis programs could be statistical packages and machine learning toolkits. Figure 1 shows a schematic diagram of a CRDW containing the key elements described above. Clinical and research data and processes can all be incorporated into this CRDW. The clinical data come from hospital information systems, including EHR, LIMS, and CPOE, and the research data are from electronic case reports or the users research database. Requirements of patient safety, privacy and security should be implemented within the system. The CRDW is accessed by research tools for data extraction, a chart review system, data mining, and other analyses that allow the data to be better understood and used. The mandatory features of a CRDW are easy interfaces for queries and data extraction. An easy query interface helps a user design a hypothesis by finding the number of a study group and possible clinical data. Data extraction is important to test a hypothesis by analyzing extracted data. The remaining three characteristics, namely, an honest broker system, chart review, and IRB interface help a user to perform research more conveniently while obeying the necessary regulations. Many older DWs in hospitals focus on hospital management, not on clinical research. However, the number of requests from researchers seeking access to CDWs has been increasing. Here, we describe a set of desired characteristics for a CRDW used for research purposes. A CRDW should include an honest broker system and an IRB approval interface Figure 1. A diagram of an ideal clinical research data warehouse. EMR: Electronic Medical Record, EHR: Electronic Health Record, IRB: Institutional Review Board, DB: database, OLAP: online analytic processing, e-crf: electronic case report form
7 Characteristics Desired in CDW to comply with governmental regulations, as well a simple query interface, an anonymized data review tool, and a data extraction tool. A CRDW should serve as a biomedical research platform for data analysis as well as a data repository. However, CRDWs have diverse development obstacles, including funding and sponsorship, data ownership and access issues, and staffing issues [5]. To overcome these obstacles, open-source systems are gaining popularity over in-house systems [5]. The use of in-house developed front-facing business intelligence tools has decreased, while the adoption of open-source data warehouse tools, such as i2b2, has increased because it reduces costs and guarantees the success of a CRDW project. However, many CRDW projects are still based on in-house systems because open-source systems also need customization to satisfy the unique requirements of each hospital. Conflict of Interest No potential conflict of interest relevant to this article was reported. References 1. Inmon WH. Tech topic: what is a data warehouse. [place unknown]: Prism Solutions; Inmon WH, Derek S, Neushloss G. DW 2.0: the architecture for the next generation of data warehousing. Amsterdam: Morgan Kaufman; Chaudhuri S, Dayal U. An overview of data warehousing and OLAP technology. ACM SIGMOD 1997;26(1): Rubin DL, Desser TS. A data warehouse for integrating radiologic and pathologic data. J Am Coll Radiol 2008;5(3): MacKenzie SL, Wyatt MC, Schuff R, Tenenbaum JD, Anderson N. Practices and perspectives on building integrated data repositories: results from a 2010 CTSA survey. J Am Med Inform Assoc 2012;19(e1):e Prather JC, Lobach DF, Goodwin LK, Hales JW, Hage ML, Hammond WE. Medical data mining: knowledge discovery in a clinical data warehouse. Proc AMIA Annu Symp 1997;1997: Ledbetter CS, Morgan MW. Toward best practice: leveraging the electronic patient record as a clinical data warehouse. J Healthc Inf Manag 2001;15(2): Dewitt JG, Hampton PM. Development of a data warehouse at an academic health system: knowing a place for the first time. Acad Med 2005;80(11): Inmon B. Data warehousing in a healthcare environment. Pittsburgh (PA): The Data Administration Newsletter; 2007 [cited at 2014 Mar 20]. Available from: Evans RS, Lloyd JF, Pierce LA. Clinical use of an enterprise data warehouse. Proc AMIA Annu Symp 2012;2012: Embi PJ, Kaufman SE, Payne P. Biomedical informatics and outcomes research: enabling knowledge-driven health care. Circulation 2009;120(23): Jensen PB, Jensen LJ, Brunak S. Mining electronic health records: towards better research applications and clinical care. Nat Rev Genet 2012;13(6): US Department of Health & Human Services. Guidance regarding methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) privacy rule. Washington (DC): US Department of Health & Human Service; c2013 [cited at 2013 Apr 12]. Available from: understanding/coveredentities/de-identification/guidance.html. 14. de Mul M, Alons P, van der Velde P, Konings I, Bakker J, Hazelzet J. Development of a clinical data warehouse from an intensive care clinical information system. Comput Methods Programs Biomed 2012;105(1): Bernstam EV, Hersh WR, Johnson SB, Chute CG, Nguyen H, Sim I, et al. Synergies and distinctions between computational disciplines in biomedical research: perspective from the Clinical and Translational Science Award programs. Acad Med 2009;84(7): Huser V, Cimino JJ. Desiderata for healthcare integrated data repositories based on architectural comparison of three public repositories. Proc AMIA Annu Symp 2013;2013: Liu J, Erdal S, Silvey SA, Ding J, Riedel JD, Marsh CB, et al. Toward a fully de-identified biomedical information warehouse. Proc AMIA Annu Symp 2009;2009: Kamal J, Liu J, Ostrander M, Santangelo J, Dyta R, Rogers P, et al. Information warehouse: a comprehensive informatics platform for business, clinical, and research applications. Proc AMIA Annu Symp 2010;2010: Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). J Am Med Inform Assoc 2010;17(2): Cincinnati Children s Hospital Medical Center. i2b2 Research data warehouse. Cincinnati (OH): Cincinnati Children s Hospital Medical Center; c2011 [cited at 2014 Vol. 20 No. 2 April
8 Soo-Yong Shin et al Jan 8]. Available from: Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE: an integrated standards-based translational research informatics platform. Proc AMIA Annu Symp 2009;2009: Hernandez P, Podchiyska T, Weber S, Ferris T, Lowe H. Automated mapping of pharmacy orders from two electronic health record systems to RxNorm within the STRIDE clinical data warehouse. Proc AMIA Annu Symp 2009;2009: Grant A, Moshyk A, Diab H, Caron P, de Lorenzi F, Bisson G, et al. Integrating feedback from a clinical data warehouse into practice organisation. Int J Med Inform 2006;75(3-4): The University of Chicago, Center for Research Informatics. Clinical Research Data Warehouse (CRDW). Chicago (IL): The University of Chicago; c2014 [cited at 2014 Mar 21]. Available from: edu/?page_id=772/. 25. Gray GW. Challenges of building clinical data analysis solutions. J Crit Care 2004;19(4): Emory University. Clinical data warehouse - healthcare. Atlanta (GA): Emory University; c2014 [cited at 2014 Mar 21]. Available from: ehc_clinical_data_warehouse/. 27. Kohane IS, Churchill SE, Murphy SN. A translational engine at the national scale: informatics for integrating biology and the bedside. J Am Med Inform Assoc 2012;19(2): Kahn MG, Weng C. Clinical research informatics: a conceptual perspective. J Am Med Inform Assoc 2012;19(e1):e36-e Dhir R, Patel AA, Winters S, Bisceglia M, Swanson D, Aamodt R, et al. A multidisciplinary approach to honest broker services for tissue banks and clinical data: a pragmatic and practical model. Cancer 2008;113(7): El Emam K. Methods for the de-identification of electronic health records for genomic research. Genome Med 2011;3(4):
STRIDE An Integrated Standards-Based Translational Research Informatics Platform
 STRIDE An Integrated Standards-Based Translational Research Informatics Platform Henry J. Lowe MD, Todd A. Ferris MD, MS Penni M. Hernandez ND, RN, Susan C. Weber PhD Stanford Center for Clinical Informatics,
STRIDE An Integrated Standards-Based Translational Research Informatics Platform Henry J. Lowe MD, Todd A. Ferris MD, MS Penni M. Hernandez ND, RN, Susan C. Weber PhD Stanford Center for Clinical Informatics,
Use of the Research Patient Data Registry at Partners Healthcare, Boston
 Use of the Research Patient Data Registry at Partners Healthcare, Boston Advancing Clinical Research with Hospital Clinical Records Shawn Murphy MD, Ph.D. Massachusetts General Hospital Outline of presentation
Use of the Research Patient Data Registry at Partners Healthcare, Boston Advancing Clinical Research with Hospital Clinical Records Shawn Murphy MD, Ph.D. Massachusetts General Hospital Outline of presentation
i2b2 Clinical Research Chart
 i2b2 Clinical Research Chart Shawn Murphy MD, Ph.D. Griffin Weber MD, Ph.D. Michael Mendis Vivian Gainer MS Lori Phillips MS Rajesh Kuttan Wensong Pan MS Henry Chueh MD Susanne Churchill Ph.D. John Glaser
i2b2 Clinical Research Chart Shawn Murphy MD, Ph.D. Griffin Weber MD, Ph.D. Michael Mendis Vivian Gainer MS Lori Phillips MS Rajesh Kuttan Wensong Pan MS Henry Chueh MD Susanne Churchill Ph.D. John Glaser
Strategic Management System for Effective Health Care Planning (SMS-EHCP)
 674 Strategic Management System for Effective Health Care Planning (SMS-EHCP) 1 O. I. Omotoso, 2 I. A. Adeyanju, 3 S. A. Ibraheem 4 K. S. Ibrahim 1,2,3,4 Department of Computer Science and Engineering,
674 Strategic Management System for Effective Health Care Planning (SMS-EHCP) 1 O. I. Omotoso, 2 I. A. Adeyanju, 3 S. A. Ibraheem 4 K. S. Ibrahim 1,2,3,4 Department of Computer Science and Engineering,
Healthcare Professional. Driving to the Future 11 March 7, 2011
 Clinical Analytics for the Practicing Healthcare Professional Driving to the Future 11 March 7, 2011 Michael O. Bice Agenda Clinical informatics as context for clinical analytics Uniqueness of medical
Clinical Analytics for the Practicing Healthcare Professional Driving to the Future 11 March 7, 2011 Michael O. Bice Agenda Clinical informatics as context for clinical analytics Uniqueness of medical
i2b2 Clinical Research Chart
 i2b2 Clinical Research Chart Shawn Murphy MD, Ph.D. Griffin Weber MD, Ph.D. Michael Mendis Andrew McMurry Vivian Gainer MS Lori Phillips MS Rajesh Kuttan Wensong Pan MS Henry Chueh MD Susanne Churchill
i2b2 Clinical Research Chart Shawn Murphy MD, Ph.D. Griffin Weber MD, Ph.D. Michael Mendis Andrew McMurry Vivian Gainer MS Lori Phillips MS Rajesh Kuttan Wensong Pan MS Henry Chueh MD Susanne Churchill
Practical Implementation of a Bridge between Legacy EHR System and a Clinical Research Environment
 Cross-Border Challenges in Informatics with a Focus on Disease Surveillance and Utilising Big-Data L. Stoicu-Tivadar et al. (Eds.) 2014 The authors. This article is published online with Open Access by
Cross-Border Challenges in Informatics with a Focus on Disease Surveillance and Utilising Big-Data L. Stoicu-Tivadar et al. (Eds.) 2014 The authors. This article is published online with Open Access by
The Use of Patient Records (EHR) for Research
 The Use of Patient Records (EHR) for Research Mary Devereaux, Ph.D. Director, Biomedical Ethics Seminars Assistant Director, Research Ethics Program & San Diego Research Ethics Consortium Abstract The
The Use of Patient Records (EHR) for Research Mary Devereaux, Ph.D. Director, Biomedical Ethics Seminars Assistant Director, Research Ethics Program & San Diego Research Ethics Consortium Abstract The
The clinical data pipeline Fueling analytics for researchers and physicians from a single source
 The clinical data pipeline Fueling analytics for researchers and physicians from a single source Contents Introduction 4 Enabling access to data 6 The data trust for clinical operations 8 The data trust
The clinical data pipeline Fueling analytics for researchers and physicians from a single source Contents Introduction 4 Enabling access to data 6 The data trust for clinical operations 8 The data trust
DATA WAREHOUSING IN THE HEALTHCARE ENVIRONMENT. By W H Inmon
 DATA WAREHOUSING IN THE HEALTHCARE ENVIRONMENT By W H Inmon For years organizations had unintegrated data. With unintegrated data there was a lot of pain. No one could look across the information of the
DATA WAREHOUSING IN THE HEALTHCARE ENVIRONMENT By W H Inmon For years organizations had unintegrated data. With unintegrated data there was a lot of pain. No one could look across the information of the
From Fishing to Attracting Chicks
 The Greater Plains Collaborative: a PCORNet Clinical Data Research Network s Strategies for Creating an Interoperable Architecture From Fishing to Attracting Chicks Russ Waitman, PhD Associate Professor,
The Greater Plains Collaborative: a PCORNet Clinical Data Research Network s Strategies for Creating an Interoperable Architecture From Fishing to Attracting Chicks Russ Waitman, PhD Associate Professor,
Implementing Honest Broker System(s) in Academic Medical Centers: The Pittsburgh Experience
 Implementing Honest Broker System(s) in Academic Medical Centers: The Pittsburgh Experience Christopher Ryan, Ph.D., CIP IRB Director Professor of Psychiatry University of Pittsburgh ryancm@upmc.edu The
Implementing Honest Broker System(s) in Academic Medical Centers: The Pittsburgh Experience Christopher Ryan, Ph.D., CIP IRB Director Professor of Psychiatry University of Pittsburgh ryancm@upmc.edu The
Achieving Value from Diverse Healthcare Data
 Achieving Value from Diverse Healthcare Data Paul Bleicher, MD, PhD Chief Medical Officer Humedica Boston MA The Information Environment in Healthcare Pharma, Biotech, Devices Hospitals Physicians Pharmacies
Achieving Value from Diverse Healthcare Data Paul Bleicher, MD, PhD Chief Medical Officer Humedica Boston MA The Information Environment in Healthcare Pharma, Biotech, Devices Hospitals Physicians Pharmacies
BUILDING PRIMARY CARE RESEARCH INFRASTRUCTURE AT YOUR COMMUNITY HEALTH CENTER
 BUILDING PRIMARY CARE RESEARCH INFRASTRUCTURE AT YOUR COMMUNITY HEALTH CENTER Harvard Catalyst Community Health Innovation and Research Program Grant # 1 UL1 RR025758-04 First Edition 1 Editors Shalini
BUILDING PRIMARY CARE RESEARCH INFRASTRUCTURE AT YOUR COMMUNITY HEALTH CENTER Harvard Catalyst Community Health Innovation and Research Program Grant # 1 UL1 RR025758-04 First Edition 1 Editors Shalini
EMR Systems and the Conduct of Clinical Research. Daniel E Ford, MD, MPH Vice Dean for Clinical Investigation Johns Hopkins School of Medicine
 EMR Systems and the Conduct of Clinical Research Daniel E Ford, MD, MPH Vice Dean for Clinical Investigation Johns Hopkins School of Medicine Clinical Research Environment Research protocols are becoming
EMR Systems and the Conduct of Clinical Research Daniel E Ford, MD, MPH Vice Dean for Clinical Investigation Johns Hopkins School of Medicine Clinical Research Environment Research protocols are becoming
i2b2 Clinical Research Chart
 i2b2 Clinical Research Chart Shawn Murphy MD, Ph.D. Griffin Weber MD, Ph.D. Michael Mendis Vivian Gainer MS Lori Phillips MS Rajesh Kuttan Wensong Pan MS Henry Chueh MD Susanne Churchill Ph.D. John Glaser
i2b2 Clinical Research Chart Shawn Murphy MD, Ph.D. Griffin Weber MD, Ph.D. Michael Mendis Vivian Gainer MS Lori Phillips MS Rajesh Kuttan Wensong Pan MS Henry Chueh MD Susanne Churchill Ph.D. John Glaser
Personalized Medicine: Humanity s Ultimate Big Data Challenge. Rob Fassett, MD Chief Medical Informatics Officer Oracle Health Sciences
 Personalized Medicine: Humanity s Ultimate Big Data Challenge Rob Fassett, MD Chief Medical Informatics Officer Oracle Health Sciences 2012 Oracle Corporation Proprietary and Confidential 2 3 Humanity
Personalized Medicine: Humanity s Ultimate Big Data Challenge Rob Fassett, MD Chief Medical Informatics Officer Oracle Health Sciences 2012 Oracle Corporation Proprietary and Confidential 2 3 Humanity
A National Clinical Decision Support Infrastructure to Enable Genetically-Guided Population Health Management at Scale
 A National Clinical Decision Support Infrastructure to Enable Genetically-Guided Population Health Management at Scale September 23, 2010 Kensaku Kawamoto, MD, PhD Assistant Professor Department of Community
A National Clinical Decision Support Infrastructure to Enable Genetically-Guided Population Health Management at Scale September 23, 2010 Kensaku Kawamoto, MD, PhD Assistant Professor Department of Community
Using Big Data to Advance Healthcare Gregory J. Moore MD, PhD February 4, 2014
 Using Big Data to Advance Healthcare Gregory J. Moore MD, PhD February 4, 2014 Sequencing Technology - Hype Cycle (Gartner) Gartner - Hype Cycle for Healthcare Provider Applications, Analytics and Systems,
Using Big Data to Advance Healthcare Gregory J. Moore MD, PhD February 4, 2014 Sequencing Technology - Hype Cycle (Gartner) Gartner - Hype Cycle for Healthcare Provider Applications, Analytics and Systems,
Innovation in the LIS: Implications for Design, Procurement and Management
 Innovation in the LIS: Implications for Design, Procurement and Management Ulysses J. Balis, M.D. Director, Division of Pathology Informatics & Director, Pathology Informatics Fellowship Program Department
Innovation in the LIS: Implications for Design, Procurement and Management Ulysses J. Balis, M.D. Director, Division of Pathology Informatics & Director, Pathology Informatics Fellowship Program Department
Accelerating Clinical Trials Through Shared Access to Patient Records
 INTERSYSTEMS WHITE PAPER Accelerating Clinical Trials Through Shared Access to Patient Records Improved Access to Clinical Data Across Hospitals and Systems Helps Pharmaceutical Companies Reduce Delays
INTERSYSTEMS WHITE PAPER Accelerating Clinical Trials Through Shared Access to Patient Records Improved Access to Clinical Data Across Hospitals and Systems Helps Pharmaceutical Companies Reduce Delays
Data Warehousing and Data Mining in Business Applications
 133 Data Warehousing and Data Mining in Business Applications Eesha Goel CSE Deptt. GZS-PTU Campus, Bathinda. Abstract Information technology is now required in all aspect of our lives that helps in business
133 Data Warehousing and Data Mining in Business Applications Eesha Goel CSE Deptt. GZS-PTU Campus, Bathinda. Abstract Information technology is now required in all aspect of our lives that helps in business
EHR Recruitment Process and Methods Workgroup. John Buse, UNC-CH On behalf of Marie Rape, Chunhua Weng and Peter Embi
 EHR Recruitment Process and Methods Workgroup John Buse, UNC-CH On behalf of Marie Rape, Chunhua Weng and Peter Embi REDCap Survey INTRODUCTION The Methods and Process Domain Task Force has established
EHR Recruitment Process and Methods Workgroup John Buse, UNC-CH On behalf of Marie Rape, Chunhua Weng and Peter Embi REDCap Survey INTRODUCTION The Methods and Process Domain Task Force has established
Medical Informatics An Overview Saudi Board For Community Medicine
 Medical Informatics An Overview Saudi Board For Community Medicine Ahmed AlBarrak PhD Medical Informatics Associate Professor of Health Informatics, Family & Community Med, Chairman, Medical Informatics,
Medical Informatics An Overview Saudi Board For Community Medicine Ahmed AlBarrak PhD Medical Informatics Associate Professor of Health Informatics, Family & Community Med, Chairman, Medical Informatics,
Electronic Medical Record Customization and the Impact Upon Chart Completion Rates
 338 May 2010 Family Medicine Improving Workflow Electronic Medical Record Customization and the Impact Upon Chart Completion Rates Kevin J. Bennett, PhD; Christian Steen, MD Objective: The study s objctive
338 May 2010 Family Medicine Improving Workflow Electronic Medical Record Customization and the Impact Upon Chart Completion Rates Kevin J. Bennett, PhD; Christian Steen, MD Objective: The study s objctive
Developing a Self-Service Query Interface for Re-Using De-Identified Electronic Health Record Data
 632 MEDINFO 2013 C.U. Lehmann et al. (Eds.) 2013 IMIA and IOS Press. This article is published online with Open Access by IOS Press and distributed under the terms of the Creative Commons Attribution Non-Commercial
632 MEDINFO 2013 C.U. Lehmann et al. (Eds.) 2013 IMIA and IOS Press. This article is published online with Open Access by IOS Press and distributed under the terms of the Creative Commons Attribution Non-Commercial
Secondary use of Clinical Data for Medical Research
 Secondary use of Clinical Data for Medical Research Pr Marc CUGGIA INSERM UMR 936 Faculté de médecine Université de Rennes 1 - CHU de Rennes CrREDIBLE Workshop Agenda Context and needs in medical research
Secondary use of Clinical Data for Medical Research Pr Marc CUGGIA INSERM UMR 936 Faculté de médecine Université de Rennes 1 - CHU de Rennes CrREDIBLE Workshop Agenda Context and needs in medical research
Clinical Data Services
 Clinical Data Services Data Storage, Data Collection Data Management Human Research Academy October 2014 CTS Research Development Services 706.721.6247 www.ctsrds@gru.edu Objectives Participants will:
Clinical Data Services Data Storage, Data Collection Data Management Human Research Academy October 2014 CTS Research Development Services 706.721.6247 www.ctsrds@gru.edu Objectives Participants will:
Big Data Analytics in Health Care
 Big Data Analytics in Health Care S. G. Nandhini 1, V. Lavanya 2, K.Vasantha Kokilam 3 1 13mss032, 2 13mss025, III. M.Sc (software systems), SRI KRISHNA ARTS AND SCIENCE COLLEGE, 3 Assistant Professor,
Big Data Analytics in Health Care S. G. Nandhini 1, V. Lavanya 2, K.Vasantha Kokilam 3 1 13mss032, 2 13mss025, III. M.Sc (software systems), SRI KRISHNA ARTS AND SCIENCE COLLEGE, 3 Assistant Professor,
www.ijreat.org Published by: PIONEER RESEARCH & DEVELOPMENT GROUP (www.prdg.org) 28
 Data Warehousing - Essential Element To Support Decision- Making Process In Industries Ashima Bhasin 1, Mr Manoj Kumar 2 1 Computer Science Engineering Department, 2 Associate Professor, CSE Abstract SGT
Data Warehousing - Essential Element To Support Decision- Making Process In Industries Ashima Bhasin 1, Mr Manoj Kumar 2 1 Computer Science Engineering Department, 2 Associate Professor, CSE Abstract SGT
Addressing the State of the Electronic Health Record (EHR)
 Addressing the State of the Electronic Health Record (EHR) Agenda Definitions Attributes Differences Adoption Model Current State Challenges Implementation considerations What is it? EMR CMR EHR EPR PHR
Addressing the State of the Electronic Health Record (EHR) Agenda Definitions Attributes Differences Adoption Model Current State Challenges Implementation considerations What is it? EMR CMR EHR EPR PHR
Find the signal in the noise
 Find the signal in the noise Electronic Health Records: The challenge The adoption of Electronic Health Records (EHRs) in the USA is rapidly increasing, due to the Health Information Technology and Clinical
Find the signal in the noise Electronic Health Records: The challenge The adoption of Electronic Health Records (EHRs) in the USA is rapidly increasing, due to the Health Information Technology and Clinical
Lab Orders Made Easy.
 EMR-Link Lab Orders Made Easy. Liaison Healthcare Informatics: P r ov i d i n g c o n n ec ti v it y & i n t e r o p e r a b i l it y th r o u g h s ec u r e c lo u d - b a s e d s e rv i c e s Liaison
EMR-Link Lab Orders Made Easy. Liaison Healthcare Informatics: P r ov i d i n g c o n n ec ti v it y & i n t e r o p e r a b i l it y th r o u g h s ec u r e c lo u d - b a s e d s e rv i c e s Liaison
Research Resources at Partners Hospitals
 Research Resources at Partners Hospitals Barbara E. Bierer, M.D. SVP Research, BWH bbierer@partners.org (617) 732-8990 Rick Bringhurst, M.D. SVP Research, MGH rbringhurst@partners.org (617) 724-8549 Agenda
Research Resources at Partners Hospitals Barbara E. Bierer, M.D. SVP Research, BWH bbierer@partners.org (617) 732-8990 Rick Bringhurst, M.D. SVP Research, MGH rbringhurst@partners.org (617) 724-8549 Agenda
Investigation of Decision Making Issues in the use of Current Clinical Information Systems
 Investigation of Decision Making Issues in the use of Current Clinical Information Systems Pubudika Mawilmada Computer Science Discipline Faculty of Science and Technology QUT Susan Smith Cardiac Surgery
Investigation of Decision Making Issues in the use of Current Clinical Information Systems Pubudika Mawilmada Computer Science Discipline Faculty of Science and Technology QUT Susan Smith Cardiac Surgery
Research Data Extraction Service. Research and Woodruff Health Sciences IT
 Research Data Extraction Service Research and Woodruff Health Sciences IT 1 Topics Emory s Clinical Data Warehouse Types of data available Accessing the data Key Points Clinical Data Warehouse initiated
Research Data Extraction Service Research and Woodruff Health Sciences IT 1 Topics Emory s Clinical Data Warehouse Types of data available Accessing the data Key Points Clinical Data Warehouse initiated
ehealth, HIS, etc ehealth All information about health HMIS mhealth HIS Statistical IS Credited: Karl Brown, Rockefeller Foundation
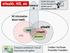 ehealth, HIS, etc Statistical IS Health Informatics: Formal discipline that studies use of information in health All information about health HIS HMIS ehealth mhealth Tele-medicine Enterprise architecture:
ehealth, HIS, etc Statistical IS Health Informatics: Formal discipline that studies use of information in health All information about health HIS HMIS ehealth mhealth Tele-medicine Enterprise architecture:
Application of Data Mining Methods in Health Care Databases
 6 th International Conference on Applied Informatics Eger, Hungary, January 27 31, 2004. Application of Data Mining Methods in Health Care Databases Ágnes Vathy-Fogarassy Department of Mathematics and
6 th International Conference on Applied Informatics Eger, Hungary, January 27 31, 2004. Application of Data Mining Methods in Health Care Databases Ágnes Vathy-Fogarassy Department of Mathematics and
Master of Science in Computer Science. Option Health Information Systems
 Master of Science in Computer Science Option Health Information Systems 1. The program Currently, in the Lebanese and most of Middle East s hospitals, the management of health information systems is handled
Master of Science in Computer Science Option Health Information Systems 1. The program Currently, in the Lebanese and most of Middle East s hospitals, the management of health information systems is handled
Data Mining for Successful Healthcare Organizations
 Data Mining for Successful Healthcare Organizations For successful healthcare organizations, it is important to empower the management and staff with data warehousing-based critical thinking and knowledge
Data Mining for Successful Healthcare Organizations For successful healthcare organizations, it is important to empower the management and staff with data warehousing-based critical thinking and knowledge
Introduction to Information and Computer Science: Information Systems
 Introduction to Information and Computer Science: Information Systems Lecture 1 Audio Transcript Slide 1 Welcome to Introduction to Information and Computer Science: Information Systems. The component,
Introduction to Information and Computer Science: Information Systems Lecture 1 Audio Transcript Slide 1 Welcome to Introduction to Information and Computer Science: Information Systems. The component,
Transformational Data-Driven Solutions for Healthcare
 Transformational Data-Driven Solutions for Healthcare Transformational Data-Driven Solutions for Healthcare Today s healthcare providers face increasing pressure to improve operational performance while
Transformational Data-Driven Solutions for Healthcare Transformational Data-Driven Solutions for Healthcare Today s healthcare providers face increasing pressure to improve operational performance while
Kaiser Permanente Comments on Health Information Technology, by James A. Ferguson
 Kaiser Permanente Comments on Health Information Technology, by James A. Ferguson FTC Public Workshop: Innovations in Health Care Delivery 24 April, 2008 Kaiser Permanente Overview Established in 1945,
Kaiser Permanente Comments on Health Information Technology, by James A. Ferguson FTC Public Workshop: Innovations in Health Care Delivery 24 April, 2008 Kaiser Permanente Overview Established in 1945,
The HSCC Clinical Data Warehouse: The South Carolina Biomedical Research Tool of the Future
 The HSCC Clinical Data Warehouse: The South Carolina Biomedical Research Tool of the Future November 2013 About Health Sciences South Carolina The HSSC Clinical Data Warehouse: Health Sciences South Carolina
The HSCC Clinical Data Warehouse: The South Carolina Biomedical Research Tool of the Future November 2013 About Health Sciences South Carolina The HSSC Clinical Data Warehouse: Health Sciences South Carolina
Center for. Clinical Informatics. Clinical Informatics. Mission Statement
 Clinical Informatics Center for Clinical Informatics Clinical Informatics transforms health care by analyzing, designing, implementing, and evaluating information and communication systems that enhance
Clinical Informatics Center for Clinical Informatics Clinical Informatics transforms health care by analyzing, designing, implementing, and evaluating information and communication systems that enhance
Records and Clinical Trials
 Integrating Electronic Health Records and Clinical Trials The Children s Hospital An Examination of Pragmatic Issues Affiliated with University of Colorado Health Sciences Center Denver, Colorado Michael
Integrating Electronic Health Records and Clinical Trials The Children s Hospital An Examination of Pragmatic Issues Affiliated with University of Colorado Health Sciences Center Denver, Colorado Michael
Use of Electronic Health Record Data in Clinical Investigations
 Use of Electronic Health Record Data in Clinical Investigations Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Use of Electronic Health Record Data in Clinical Investigations Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Of EHRs and Meaningful Use. Pat Wise, RN, MA, MS FHIMSS COL (USA ret d) VP, Healthcare Information Systems, HIMSS
 Of EHRs and Meaningful Use Pat Wise, RN, MA, MS FHIMSS COL (USA ret d) VP, Healthcare Information Systems, HIMSS 1 MU: Where We are Today From www.cms.gov As of the end of January 31, 2013: >210,000 EPs
Of EHRs and Meaningful Use Pat Wise, RN, MA, MS FHIMSS COL (USA ret d) VP, Healthcare Information Systems, HIMSS 1 MU: Where We are Today From www.cms.gov As of the end of January 31, 2013: >210,000 EPs
Environmental Health Science. Brian S. Schwartz, MD, MS
 Environmental Health Science Data Streams Health Data Brian S. Schwartz, MD, MS January 10, 2013 When is a data stream not a data stream? When it is health data. EHR data = PHI of health system Data stream
Environmental Health Science Data Streams Health Data Brian S. Schwartz, MD, MS January 10, 2013 When is a data stream not a data stream? When it is health data. EHR data = PHI of health system Data stream
EMR Systems and the Conduct of Clinical Research. Daniel E Ford, MD, MPH Vice Dean for Clinical Investigation Johns Hopkins School of Medicine
 EMR Systems and the Conduct of Clinical Research Daniel E Ford, MD, MPH Vice Dean for Clinical Investigation Johns Hopkins School of Medicine Clinical Research Environment Research protocols are becoming
EMR Systems and the Conduct of Clinical Research Daniel E Ford, MD, MPH Vice Dean for Clinical Investigation Johns Hopkins School of Medicine Clinical Research Environment Research protocols are becoming
Design and Implementation of an Automated Geocoding Infrastructure for the Duke Medicine Enterprise Data Warehouse
 Design and Implementation of an Automated Geocoding Infrastructure for the Duke Medicine Enterprise Data Warehouse Shelley A. Rusincovitch, Sohayla Pruitt, MS, Rebecca Gray, DPhil, Kevin Li, PhD, Monique
Design and Implementation of an Automated Geocoding Infrastructure for the Duke Medicine Enterprise Data Warehouse Shelley A. Rusincovitch, Sohayla Pruitt, MS, Rebecca Gray, DPhil, Kevin Li, PhD, Monique
Integration Information Model
 Release 1.0.1 The openehr Reference Model a. Ocean Informatics Editors: T Beale a Revision: 0.6 Pages: 15 Date of issue: 22 Jul 2006 Keywords: EHR, reference model, integration, EN13606, openehr EHR Extract
Release 1.0.1 The openehr Reference Model a. Ocean Informatics Editors: T Beale a Revision: 0.6 Pages: 15 Date of issue: 22 Jul 2006 Keywords: EHR, reference model, integration, EN13606, openehr EHR Extract
Secondary Use of EMR Data View from SHARPn AMIA Health Policy, 12 Dec 2012
 Secondary Use of EMR Data View from SHARPn AMIA Health Policy, 12 Dec 2012 Christopher G. Chute, MD DrPH, Professor, Biomedical Informatics, Mayo Clinic Chair, ISO TC215 on Health Informatics Chair, International
Secondary Use of EMR Data View from SHARPn AMIA Health Policy, 12 Dec 2012 Christopher G. Chute, MD DrPH, Professor, Biomedical Informatics, Mayo Clinic Chair, ISO TC215 on Health Informatics Chair, International
HIT AND HSR FOR ACTIONABLE KNOWLEDGE: HEALTH SYSTEM SUMMARY. PARTNER: Veterans Health Administration (VHA) Organization and IT Infrastructure
 HIT AND HSR FOR ACTIONABLE KNOWLEDGE: HEALTH SYSTEM SUMMARY PARTNER: Veterans Health Administration (VHA) Organizational Description And History Organization and IT Infrastructure The U.S. Department of
HIT AND HSR FOR ACTIONABLE KNOWLEDGE: HEALTH SYSTEM SUMMARY PARTNER: Veterans Health Administration (VHA) Organizational Description And History Organization and IT Infrastructure The U.S. Department of
Lection 3-4 WAREHOUSING
 Lection 3-4 DATA WAREHOUSING Learning Objectives Understand d the basic definitions iti and concepts of data warehouses Understand data warehousing architectures Describe the processes used in developing
Lection 3-4 DATA WAREHOUSING Learning Objectives Understand d the basic definitions iti and concepts of data warehouses Understand data warehousing architectures Describe the processes used in developing
SPATIAL DATA CLASSIFICATION AND DATA MINING
 , pp.-40-44. Available online at http://www. bioinfo. in/contents. php?id=42 SPATIAL DATA CLASSIFICATION AND DATA MINING RATHI J.B. * AND PATIL A.D. Department of Computer Science & Engineering, Jawaharlal
, pp.-40-44. Available online at http://www. bioinfo. in/contents. php?id=42 SPATIAL DATA CLASSIFICATION AND DATA MINING RATHI J.B. * AND PATIL A.D. Department of Computer Science & Engineering, Jawaharlal
Design of a Multi Dimensional Database for the Archimed DataWarehouse
 169 Design of a Multi Dimensional Database for the Archimed DataWarehouse Claudine Bréant, Gérald Thurler, François Borst, Antoine Geissbuhler Service of Medical Informatics University Hospital of Geneva,
169 Design of a Multi Dimensional Database for the Archimed DataWarehouse Claudine Bréant, Gérald Thurler, François Borst, Antoine Geissbuhler Service of Medical Informatics University Hospital of Geneva,
A Survey on Data Warehouse Architecture
 A Survey on Data Warehouse Architecture Rajiv Senapati 1, D.Anil Kumar 2 1 Assistant Professor, Department of IT, G.I.E.T, Gunupur, India 2 Associate Professor, Department of CSE, G.I.E.T, Gunupur, India
A Survey on Data Warehouse Architecture Rajiv Senapati 1, D.Anil Kumar 2 1 Assistant Professor, Department of IT, G.I.E.T, Gunupur, India 2 Associate Professor, Department of CSE, G.I.E.T, Gunupur, India
Syllabus. HMI 7437: Data Warehousing and Data/Text Mining for Healthcare
 Syllabus HMI 7437: Data Warehousing and Data/Text Mining for Healthcare 1. Instructor Illhoi Yoo, Ph.D Office: 404 Clark Hall Email: muteaching@gmail.com Office hours: TBA Classroom: TBA Class hours: TBA
Syllabus HMI 7437: Data Warehousing and Data/Text Mining for Healthcare 1. Instructor Illhoi Yoo, Ph.D Office: 404 Clark Hall Email: muteaching@gmail.com Office hours: TBA Classroom: TBA Class hours: TBA
Adam Wilcox, PhD PhD Columbia University
 Adam Wilcox, PhD Adam Wilcox, PhD Columbia University Intermountain Healthcare Columbia University Medical Center/ NewYork Presbyterian Hospital Evolving EHR Data Needs for Research, Business Intelligence
Adam Wilcox, PhD Adam Wilcox, PhD Columbia University Intermountain Healthcare Columbia University Medical Center/ NewYork Presbyterian Hospital Evolving EHR Data Needs for Research, Business Intelligence
Data warehousing and data mining an overview
 Data warehousing and data mining an overview Abstract Dr. Suman Bhusan Bhattacharyya MBBS, ADHA, MBA With continuous advances in technology, increasing number of clinicians are using electronic medical
Data warehousing and data mining an overview Abstract Dr. Suman Bhusan Bhattacharyya MBBS, ADHA, MBA With continuous advances in technology, increasing number of clinicians are using electronic medical
A Design and implementation of a data warehouse for research administration universities
 A Design and implementation of a data warehouse for research administration universities André Flory 1, Pierre Soupirot 2, and Anne Tchounikine 3 1 CRI : Centre de Ressources Informatiques INSA de Lyon
A Design and implementation of a data warehouse for research administration universities André Flory 1, Pierre Soupirot 2, and Anne Tchounikine 3 1 CRI : Centre de Ressources Informatiques INSA de Lyon
INLS 725 INLS 725 Electronic Health Records (Online)
 Fall 2015 Online Class INLS 725 INLS 725 Electronic Health Records (Online) Instructor: GRACE SHIN E-Mail: gshin6@live.unc.edu Phone: 404.216.2934 School of Information and Library Science UNC-Chapel Hill
Fall 2015 Online Class INLS 725 INLS 725 Electronic Health Records (Online) Instructor: GRACE SHIN E-Mail: gshin6@live.unc.edu Phone: 404.216.2934 School of Information and Library Science UNC-Chapel Hill
E-Governance in Higher Education: Concept and Role of Data Warehousing Techniques
 E-Governance in Higher Education: Concept and Role of Data Warehousing Techniques Prateek Bhanti Asst. Professor, FASC, MITS Deemed University, Lakshmangarh-332311, Sikar, Rajasthan, INDIA Urmani Kaushal
E-Governance in Higher Education: Concept and Role of Data Warehousing Techniques Prateek Bhanti Asst. Professor, FASC, MITS Deemed University, Lakshmangarh-332311, Sikar, Rajasthan, INDIA Urmani Kaushal
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW. Dingcheng Li Mayo Clinic, USA 20-Dec-2015
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
Tertiary Use of Electronic Health Record Data. Maggie Lohnes, RN, CPHIMS, FHIMSS VP Provider Relations Anolinx, LLC October 26, 2015
 Tertiary Use of Electronic Health Record Data Maggie Lohnes, RN, CPHIMS, FHIMSS VP Provider Relations Anolinx, LLC October 26, 2015 Disclosure of Conflicts of Interest No relevant conflicts of interest
Tertiary Use of Electronic Health Record Data Maggie Lohnes, RN, CPHIMS, FHIMSS VP Provider Relations Anolinx, LLC October 26, 2015 Disclosure of Conflicts of Interest No relevant conflicts of interest
MED 2400 MEDICAL INFORMATICS FUNDAMENTALS
 MED 2400 MEDICAL INFORMATICS FUNDAMENTALS NEW YORK CITY COLLEGE OF TECHNOLOGY The City University Of New York School of Arts and Sciences Biological Sciences Department Course title: Course code: MED 2400
MED 2400 MEDICAL INFORMATICS FUNDAMENTALS NEW YORK CITY COLLEGE OF TECHNOLOGY The City University Of New York School of Arts and Sciences Biological Sciences Department Course title: Course code: MED 2400
Supporting Clinical and Translational Research with Informatics
 Supporting Clinical and Translational Research with Informatics Thomas R. Campion, Jr., Ph.D. Assistant Professor of Healthcare Policy and Research Assistant Professor of Healthcare Policy and Research
Supporting Clinical and Translational Research with Informatics Thomas R. Campion, Jr., Ph.D. Assistant Professor of Healthcare Policy and Research Assistant Professor of Healthcare Policy and Research
Leveraging Devices, Data and Discovery for Smarter Healthcare in Japan
 Application Healthc Inform Res. 2011 September;17(3):184-189. http://dx.doi.org/10.4258/hir.2011.17.3.184 pissn 2093-3681 eissn 2093-369X Leveraging Devices, Data and Discovery for Smarter Healthcare in
Application Healthc Inform Res. 2011 September;17(3):184-189. http://dx.doi.org/10.4258/hir.2011.17.3.184 pissn 2093-3681 eissn 2093-369X Leveraging Devices, Data and Discovery for Smarter Healthcare in
Clinical Decision Support using a Terminology Server to improve Patient Safety
 150 Digital Healthcare Empowering Europeans R. Cornet et al. (Eds.) 2015 European Federation for Medical Informatics (EFMI). This article is published online with Open Access by IOS Press and distributed
150 Digital Healthcare Empowering Europeans R. Cornet et al. (Eds.) 2015 European Federation for Medical Informatics (EFMI). This article is published online with Open Access by IOS Press and distributed
Design and Implementation of Hospital Management System Using Java
 IOSR Journal of Mobile Computing & Application (IOSR-JMCA) e-issn: 2394-0050, P-ISSN: 2394-0042.Volume 2, Issue 1. (Mar. - Apr. 2015), PP 32-36 www.iosrjournals.org Design and Implementation of Hospital
IOSR Journal of Mobile Computing & Application (IOSR-JMCA) e-issn: 2394-0050, P-ISSN: 2394-0042.Volume 2, Issue 1. (Mar. - Apr. 2015), PP 32-36 www.iosrjournals.org Design and Implementation of Hospital
BUILDING OLAP TOOLS OVER LARGE DATABASES
 BUILDING OLAP TOOLS OVER LARGE DATABASES Rui Oliveira, Jorge Bernardino ISEC Instituto Superior de Engenharia de Coimbra, Polytechnic Institute of Coimbra Quinta da Nora, Rua Pedro Nunes, P-3030-199 Coimbra,
BUILDING OLAP TOOLS OVER LARGE DATABASES Rui Oliveira, Jorge Bernardino ISEC Instituto Superior de Engenharia de Coimbra, Polytechnic Institute of Coimbra Quinta da Nora, Rua Pedro Nunes, P-3030-199 Coimbra,
Hospital IT Expenses and Budgets Related to Clinical Sophistication. Market Findings from HIMSS Analytics
 Hospital IT Expenses and Budgets Related to Clinical Sophistication Market Findings from HIMSS Analytics Table of Contents 2 3 4 8 13 14 Executive Summary Expense Metrics Used for this Research Operating
Hospital IT Expenses and Budgets Related to Clinical Sophistication Market Findings from HIMSS Analytics Table of Contents 2 3 4 8 13 14 Executive Summary Expense Metrics Used for this Research Operating
EHR Adoption and Vision for HIM
 EHR Adoption and Vision for HIM Christina M. Janus, MBA, RHIA EOHIMA Spring Seminar April 14, 2007 1 Content Covered Key EHR Functions Adoption Model Group Share of Current Technologies & Vision for the
EHR Adoption and Vision for HIM Christina M. Janus, MBA, RHIA EOHIMA Spring Seminar April 14, 2007 1 Content Covered Key EHR Functions Adoption Model Group Share of Current Technologies & Vision for the
An EVIDENCE-ENHANCED HEALTHCARE ECOSYSTEM for Cancer: I/T perspectives
 An EVIDENCE-ENHANCED HEALTHCARE ECOSYSTEM for Cancer: I/T perspectives Chalapathy Neti, Ph.D. Associate Director, Healthcare Transformation, Shahram Ebadollahi, Ph.D. Research Staff Memeber IBM Research,
An EVIDENCE-ENHANCED HEALTHCARE ECOSYSTEM for Cancer: I/T perspectives Chalapathy Neti, Ph.D. Associate Director, Healthcare Transformation, Shahram Ebadollahi, Ph.D. Research Staff Memeber IBM Research,
KNOWLEDGENT WHITE PAPER. Big Data Enabling Better Pharmacovigilance
 Big Data Enabling Better Pharmacovigilance INTRODUCTION Biopharmaceutical companies are seeing a surge in the amount of data generated and made available to identify better targets, better design clinical
Big Data Enabling Better Pharmacovigilance INTRODUCTION Biopharmaceutical companies are seeing a surge in the amount of data generated and made available to identify better targets, better design clinical
BUILDING A HEALTH CARE DATA WAREHOUSE FOR CANCER DISEASES
 BUILDING A HEALTH CARE DATA WAREHOUSE FOR CANCER DISEASES Dr.Osama E.Sheta 1 and Ahmed Nour Eldeen 2 1,2 Department of Mathematics Faculty of Science, Zagazig University, Zagazig, Elsharkia, Egypt. 1 oesheta75@gmail.com,
BUILDING A HEALTH CARE DATA WAREHOUSE FOR CANCER DISEASES Dr.Osama E.Sheta 1 and Ahmed Nour Eldeen 2 1,2 Department of Mathematics Faculty of Science, Zagazig University, Zagazig, Elsharkia, Egypt. 1 oesheta75@gmail.com,
Improved Outcomes from Data and Knowledge Driven Insights Anil Jain, MD, FACP
 Improved Outcomes from Data and Knowledge Driven Insights Anil Jain, MD, FACP April 20, 2016 Housekeeping 1. Using the control panel - Use the control panel on the right side of your screen to minimize
Improved Outcomes from Data and Knowledge Driven Insights Anil Jain, MD, FACP April 20, 2016 Housekeeping 1. Using the control panel - Use the control panel on the right side of your screen to minimize
A Data Warehouse Architecture for Clinical Data Warehousing
 A Data Warehouse Architecture for Clinical Data Warehousing Tony R. Sahama and Peter R. Croll Faculty of Information Technology Queensland University of Technology PO Box 2434, Brisbane 4001, Queensland
A Data Warehouse Architecture for Clinical Data Warehousing Tony R. Sahama and Peter R. Croll Faculty of Information Technology Queensland University of Technology PO Box 2434, Brisbane 4001, Queensland
IBM Software. IBM Initiate: Delivering Accurate Patient and Provider Identification for Canadian Electronic Health Records
 IBM Software IBM Initiate: Delivering Accurate Patient and Provider Identification for Canadian Electronic Health Records IBM Initiate: Delivering Accurate Patient and Provider Identification for Canadian
IBM Software IBM Initiate: Delivering Accurate Patient and Provider Identification for Canadian Electronic Health Records IBM Initiate: Delivering Accurate Patient and Provider Identification for Canadian
How To Design An Electronic Medical Record
 65 Users Needs and Expectations of Electronic Medical Record Systems in Family Medicine Residence Settings George Demiris a, Karen L. Courtney a, Steven E. Waldren b a University of Missouri-Columbia,
65 Users Needs and Expectations of Electronic Medical Record Systems in Family Medicine Residence Settings George Demiris a, Karen L. Courtney a, Steven E. Waldren b a University of Missouri-Columbia,
Using Public Health Evaluation Models to Assess Health IT Implementations
 Using Public Health Evaluation Models to Assess Health IT Implementations September 2011 White Paper Prepared for Healthcare Information and Management Systems Society 33 West Monroe Street Suite 1700
Using Public Health Evaluation Models to Assess Health IT Implementations September 2011 White Paper Prepared for Healthcare Information and Management Systems Society 33 West Monroe Street Suite 1700
A Service Oriented Approach for Guidelines-based Clinical Decision Support using BPMN
 e-health For Continuity of Care C. Lovis et al. (Eds.) 2014 European Federation for Medical Informatics and IOS Press. This article is published online with Open Access by IOS Press and distributed under
e-health For Continuity of Care C. Lovis et al. (Eds.) 2014 European Federation for Medical Informatics and IOS Press. This article is published online with Open Access by IOS Press and distributed under
Cerner i2b2 User s s Guide and Frequently Asked Questions. v1.3
 User s s Guide and v1.3 Contents General Information... 3 Q: What is i2b2?... 3 Q: How is i2b2 populated?... 3 Q: How often is i2b2 updated?... 3 Q: What data is not in our i2b2?... 3 Q: Can individual
User s s Guide and v1.3 Contents General Information... 3 Q: What is i2b2?... 3 Q: How is i2b2 populated?... 3 Q: How often is i2b2 updated?... 3 Q: What data is not in our i2b2?... 3 Q: Can individual
An Introduction to Data Warehousing. An organization manages information in two dominant forms: operational systems of
 An Introduction to Data Warehousing An organization manages information in two dominant forms: operational systems of record and data warehouses. Operational systems are designed to support online transaction
An Introduction to Data Warehousing An organization manages information in two dominant forms: operational systems of record and data warehouses. Operational systems are designed to support online transaction
Meaningful Use Stage 2 Update: Deploying SNOMED CT to provide decision support in the EHR
 Meaningful Use Stage 2 Update: Deploying SNOMED CT to provide decision support in the EHR James R. Campbell MD University of Nebraska Medical Center Implementation Showcase October 31, 2014 Disclosure
Meaningful Use Stage 2 Update: Deploying SNOMED CT to provide decision support in the EHR James R. Campbell MD University of Nebraska Medical Center Implementation Showcase October 31, 2014 Disclosure
IT Infrastructure Components to support clinical care and translational research projects in a comprehensive cancer center
 IT Infrastructure Components to support clinical care and translational research projects in a comprehensive cancer center Prof. Dr. H.U. Prokosch CCC Erlangen-Nuremberg Chair of Medical Informatics, University
IT Infrastructure Components to support clinical care and translational research projects in a comprehensive cancer center Prof. Dr. H.U. Prokosch CCC Erlangen-Nuremberg Chair of Medical Informatics, University
The American Academy of Ophthalmology Adopts SNOMED CT as its Official Clinical Terminology
 The American Academy of Ophthalmology Adopts SNOMED CT as its Official Clinical Terminology H. Dunbar Hoskins, Jr., M.D., P. Lloyd Hildebrand, M.D., Flora Lum, M.D. The road towards broad adoption of electronic
The American Academy of Ophthalmology Adopts SNOMED CT as its Official Clinical Terminology H. Dunbar Hoskins, Jr., M.D., P. Lloyd Hildebrand, M.D., Flora Lum, M.D. The road towards broad adoption of electronic
Medical Informatics: A Fishing Story
 Medical Informatics: A Fishing Story Russ Waitman, PhD Director of Medical Informatics, Associate Professor, Department of Biostatistics Director, Frontiers Biomedical Informatics Assistant Vice Chancellor,
Medical Informatics: A Fishing Story Russ Waitman, PhD Director of Medical Informatics, Associate Professor, Department of Biostatistics Director, Frontiers Biomedical Informatics Assistant Vice Chancellor,
Meaningful Use Stage 2 Certification: A Guide for EHR Product Managers
 Meaningful Use Stage 2 Certification: A Guide for EHR Product Managers Terminology Management is a foundational element to satisfying the Meaningful Use Stage 2 criteria and due to its complexity, and
Meaningful Use Stage 2 Certification: A Guide for EHR Product Managers Terminology Management is a foundational element to satisfying the Meaningful Use Stage 2 criteria and due to its complexity, and
HIMSS Electronic Health Record Definitional Model Version 1.0
 HIMSS Electronic Health Record Definitional Model Version 1.0 Prepared by HIMSS Electronic Health Record Committee Thomas Handler, MD. Research Director, Gartner Rick Holtmeier, President, Berdy Systems
HIMSS Electronic Health Record Definitional Model Version 1.0 Prepared by HIMSS Electronic Health Record Committee Thomas Handler, MD. Research Director, Gartner Rick Holtmeier, President, Berdy Systems
Data Warehousing and OLAP Technology for Knowledge Discovery
 542 Data Warehousing and OLAP Technology for Knowledge Discovery Aparajita Suman Abstract Since time immemorial, libraries have been generating services using the knowledge stored in various repositories
542 Data Warehousing and OLAP Technology for Knowledge Discovery Aparajita Suman Abstract Since time immemorial, libraries have been generating services using the knowledge stored in various repositories
1. Introduction to ehealth:
 1. Introduction to ehealth: E-Health is one of the fastest growing areas within the health sector. The scope of e- Health involves application of the knowledge, skills and tools, which enable information
1. Introduction to ehealth: E-Health is one of the fastest growing areas within the health sector. The scope of e- Health involves application of the knowledge, skills and tools, which enable information
Strategies for De-Identification and Anonymization of Electronic Health Record Data for Use in Multicenter Research Studies
 Strategies for De-Identification and Anonymization of Electronic Health Record Data for Use in Multicenter Research Studies Clete A. Kushida, M.D., Ph.D. Professor, Stanford University Medical Center Overview
Strategies for De-Identification and Anonymization of Electronic Health Record Data for Use in Multicenter Research Studies Clete A. Kushida, M.D., Ph.D. Professor, Stanford University Medical Center Overview
Summary of the Proposed Rule for the Medicare and Medicaid Electronic Health Records (EHR) Incentive Program (Eligible Professionals only)
 Summary of the Proposed Rule for the Medicare and Medicaid Electronic Health Records (EHR) Incentive Program (Eligible Professionals only) Background Enacted on February 17, 2009, the American Recovery
Summary of the Proposed Rule for the Medicare and Medicaid Electronic Health Records (EHR) Incentive Program (Eligible Professionals only) Background Enacted on February 17, 2009, the American Recovery
The i2b2 Hive and the Clinical Research Chart
 The i2b2 Hive and the Clinical Research Chart Henry Chueh Shawn Murphy The i2b2 Hive is centered around two concepts. The first concept is the existence of services provided by applications that are wrapped
The i2b2 Hive and the Clinical Research Chart Henry Chueh Shawn Murphy The i2b2 Hive is centered around two concepts. The first concept is the existence of services provided by applications that are wrapped
To Protect and Validate: Use of Clinical Data for Simulation
 To Protect and Validate: Use of Clinical Data for Simulation Stephanie H. Hoelscher MSN, RN, CHISP/Texas Tech University Health Sciences Center Justin Fair MBA, CPHIMS/University Medical Center March 1,
To Protect and Validate: Use of Clinical Data for Simulation Stephanie H. Hoelscher MSN, RN, CHISP/Texas Tech University Health Sciences Center Justin Fair MBA, CPHIMS/University Medical Center March 1,
National Nursing Informatics Deep Dive Program
 National Nursing Informatics Deep Dive Program What is Nursing Informatics and Why is it Important? Connie White Delaney, PhD, RN, FAAN, FACMI Dean and Professor, School of Nursing Director, Clinical and
National Nursing Informatics Deep Dive Program What is Nursing Informatics and Why is it Important? Connie White Delaney, PhD, RN, FAAN, FACMI Dean and Professor, School of Nursing Director, Clinical and
E-government Supported by Data warehouse Techniques for Higher education: Case study Malaysian universities
 E-government Supported by Data warehouse Techniques for Higher education: Case study Malaysian universities Mohammed Abdulameer Mohammed Universiti Utara Malaysia Kedah, Malaysia Ahmed Rasol Hasson Babylon
E-government Supported by Data warehouse Techniques for Higher education: Case study Malaysian universities Mohammed Abdulameer Mohammed Universiti Utara Malaysia Kedah, Malaysia Ahmed Rasol Hasson Babylon
