AIR FORCE SPECIALTY CODE: 4P0X1 PHARMACY TECHNICIAN
|
|
|
- Erik Kelly
- 8 years ago
- Views:
Transcription
1 AIR FORCE SPECIALTY CODE: 4P0X1 PHARMACY TECHNICIAN 4P0X1 Qualification Training Package (QTP) 8.4. Controlled Substances Management QTP 4P0X1-8.4 Oct 2007 OPR: CMSgt Maurial/TSgt Rueckert
2 AIR FORCE SPECIALTY CODE: 4P0X1 PHARMACY TECHNICIAN CONTROLLED SUBSTANCES MANAGEMENT TABLE OF CONTENTS MODULE OBJECTIVE PAGES 1. Controlled Substance Management 3-24
3 INTRODUCTION 1. This Qualification Training Package (QTP) was developed to enhance and standardize on-the-job training for 4P0X1 personnel. As a trainer, the QTPs provide teachable elements of task breakdowns. The teachable elements will assist in guiding the trainee towards independent task performance, proficiency, and serve as an evaluation tool for task certifiers/certification officials. 2. Review the volume(s) of the Career Development Course (CDC) and identify which module(s) of the QTP are needed for the trainee s job position or upgrade skill-level training. The QTP training for each module should be accomplished in the order which most closely mirrors the area the trainee is working in. Items in column 2 of the Pharmacy Career Field Education and Training Plan (CFETP) marked with a 5 or 7 are the core tasks for the 4P career field. Additional proficiency training may be required for these tasks at the supervisor s discretion. 3. Ensure the trainee reviews the training references in each module prior to attempting any task or QTP evaluation. Review the performance checklist and training objective with the trainee. If the trainee has questions about the objective, clarify the desired outcome/results of performance, demonstration or completion for the task. Remember the objective of each QTP is to standardize training and allow sufficient time for the trainee to learn each task thoroughly in order to perform the task independently. 4. When the trainee has received sufficient training and is ready to be evaluated on the objective, follow the evaluation instructions. The performance checklist must be used as you evaluate each task objective. When the trainee successfully demonstrates and accomplishes the objective, document task completion appropriately in the member s 6- part training/competency folder. 5. The QTP task completion is to be annotated on an AF Form 1098 Special Task Certification and Recurring Training, filed in part 3, section A of the 6-part training/competency folder. NOTE: The individual checklists and final evaluations are not filed in each member s 6-part training/competency folder. A master checklist is filed in part 3, section A of the Master Training Plan (MTP). 6. If the trainee does not accomplish the objective, review the areas needing further instruction. Conduct feedback for each module with the trainee, and document appropriately in the member s 6-part training/competency folder. As the trainer, once you are satisfied the trainee is ready to perform the task, he/she will be re-evaluated until the objective is met. 7. If a task being trained requires third party certification by a task certifier/certifying official, the trainer ensures the trainee is qualified to perform the task independently. The trainee will then be evaluated by certifier/certifying official. Tasks requiring third party certification are identified in column 2 of CFETP with a number sign (#). The third
4 party certifier will ensure documentation in column 3E of the CFETP and appropriately documented in the members 6-part training/competency folder. 8. The QTPs are a necessary tool for standardizing task qualifications for upgrade training or job position training. Such standardization benefits the CFETP training concept throughout a member s career. These documents also may be used in assessing/certifying pharmacy technicians upon arrival at a new duty station. 9. Feedback is a vital and important part of improving our educational process for pharmacy technicians. Your first-hand expertise is valued and feedback is highly encouraged ensuring we have the most up-to-date information and training possible. Please direct all inquires to your immediate supervisor.
5 SUBJECT AREA: Controlled Substances Management TASK NAME(S): Procedures Adding drug to CHCS; Issues; Turn-ins (decrement); Increment; Adjustment; Complete RX Transaction; Return RX Transaction; Remove RX Transaction; Cancel Function; Discontinue Function CFETP/STS REFERENCE(S): EQUIPMENT REQUIRED: 1. Composite Healthcare System Program (CHCS) TRAINING REFERENCE(S): CDC 4P051A, Volume 3 and CHCS User s Manual REMARKS/NOTES: Controlled Substances Management is vital to compliance with all guidelines and the success of accreditation inspections. Reports for CII and reports for CIII-CV must be filed separately. Trainee must have Narcotic System Reports key enabled by systems to perform task(s) OBJECTIVE: 1. Effectively and accurately manage controlled substances according to Air Force, Federal, State, and local laws and instructions. EVALUATION INSTRUCTIONS: 1. After the trainee has received instructions, allow sufficient practice on each part of the task. 2. Use the performance checklist to ensure all steps of the task are accomplished without assistance and without error. 3. Document task competency upon completion of the evaluation in the trainee s 6-part training/competency folder. Initial evaluation should be documented in the CFETP. All recurring evaluations should be documented on AF Form STEPS IN TASK PERFORMANCE: 1. Identify appropriate need to Add, Issue, Turn-in (decrement), Increment, Adjust, Complete RX Transaction, Return RX Transaction, Remove RX Transaction, Cancel RX Transaction or Discontinue Transaction in CHCS. 2. Identify information required to Add, Issue, Turn-in (decrement), Increment, Adjust, Complete RX Transaction, Return RX Transaction, Cancel RX Transaction or Discontinue Transaction in CHCS. 3. Insert pertinent information in required fields in CHCS. ATTACHMENT(S): None
6 PERFORMANCE CHECKLIST: 1. Add Medications to CHCS PROCEDURES PERFORMANCE ITEMS SAT UNSAT a. Identify appropriate need to add medication to inventory (new medication added to formulary) b. Identify information required to add medication to CHCS c. Follow menu path SFM FOM ADN d. Drug name e. Route f. Dosage strength g. Content unit h. Dosage form i. Insert pertinent information in required fields of CHCS j. Drug name k. Route l. Dosage strength m. Content unit n. Dosage form o. Drug check p. Legal status q. Label print name r. Synonym s. Drug Authorization key (under ordering restrictions enter doc if POE) 2. Issue Medications a. Identify appropriate need to issue controlled medication b. Receive order/issue request c. Identify information required to issue medication d. Follow menu path NAR ISM - ISI e. Medication f. Requestor s Name g. Issue to Location h. Units Dispensed i. Received by j. Expiration Date of Product k. Manufacturer l. Lot Number m. Insert pertinent information in required fields in CHCS
7 n. Medication o. Requestor s Name p. Issue to Location q. Units Dispensed r. Received by s. Expiration Date of Product t. Manufacturer u. Lot Number 3. Turn-Ins (decrement) a. Identify appropriate need to decrement from controlled inventory (return medication to medical logistics) b. Identify information required to decrement inventory c. Follow menu path NAR INV - DCI d. Medication e. Delivery list from logistics f. Voucher number for medication g. Quantity h. Insert pertinent information in required fields in CHCS i. Voucher number for medication j. Medication being decremented k. Quantity 4. Increment a. Identify appropriate need to increment controlled inventory (receive medication from medical logistics) b. Identify information required to increment inventory c. Follow menu path NAR INV - ADD d. Medication e. Delivery list from logistics f. Voucher number for medication g. Quantity h. Insert pertinent information in required fields in CHCS i. Voucher number for medication j. Medication being incremented k. Quantity 5. Adjustment a. Identify appropriate need to adjust controlled inventory (manufacturer overage or shortage) b. Identify information required to adjust controlled inventory c. Follow menu path SFM NAR ADJ d. Controlled item
8 e. Vault f. Increment or Decrement g. Quantity h. Insert pertinent information in required fields of CHCS i. Controlled item j. Vault for adjustment k. Increment or Decrement inventory l. Quantity m. Reason for adjustment (be exact) 6. Complete RX Transaction a. Identify appropriate need to complete RX transaction b. Identify information required to complete RX transaction c. Follow menu path NSM CPM CRT d. RX number e. Insert pertinent information in required fields of CHCS f. Select appropriate RX number g. Complete process as required 7. Return RX Transaction a. Identify appropriate need to return RX transaction b. Identify information required to return RX transaction c. Follow menu path NSM CPM RRT d. RX number e. Insert pertinent information in required fields of CHCS f. Select appropriate RX number g. Complete process as required 8. Remove RX Transaction a. Identify appropriate need to remove RX transaction b. Identify information required to remove RX transaction c. Follow menu path NSM CPM REM d. RX number e. Insert pertinent information in required fields of CHCS f. Select appropriate RX number g. Complete process as required 9. Cancel Function a. Identify appropriate need to cancel a prescription b. Identify information required to cancel a prescription c. Follow menu path OPM PM SPM - CAP d. RX number e. Insert pertinent information in required fields of CHCS f. Select appropriate RX number g. Complete process as required 10. Discontinue Function a. Identify appropriate need to discontinue a prescription b. Identify information required to discontinue a prescription c. Follow menu path OPM PM DAP d. RX number e. Insert pertinent information in required fields of CHCS
9 f. Select appropriate RX number g. Complete process as required FINAL RESULTS: Trainee: Trainer: Certifier: Date: FEEDBACK: Using the performance checklist as a review reference, discuss the trainee s performance, indicating strengths, weaknesses, suggested improvement, etc. If trainee performed all task steps satisfactorily, document appropriately in trainee s 6-part training/competency folder.
10 SUBJECT AREA: Controlled Substances Management TASK NAME(S): Reports...Supply Voucher Reports; Drug Utilization Review; Specific/General Transaction Reports; Outstanding Issue Report; Narcotic Movement Report; Inventory Report CFETP/STS REFERENCE(S): EQUIPMENT REQUIRED: 1. Composite Healthcare System Program (CHCS) 2. Printer TRAINING REFERENCE(S): CDC 4P051A, Volume 3 and CHCS User s Manual REMARKS/NOTES: Controlled Substances Management is vital to compliance with all guidelines and the success of accreditation inspections. Reports for CII and reports for CIII-CV must be filed separately. Trainee must have Narcotic System Reports key enabled by Systems to perform task(s) OBJECTIVE: 1. Generate Controlled Substance Reports EVALUATION INSTRUCTIONS: 1. After the trainee has received instructions, allow sufficient practice on each part of the task. 2. Use the performance checklist to ensure all steps of the task are accomplished without assistance and without error. 3. Document task competency upon completion of the evaluation in the trainee s 6-part training/competency folder. Initial evaluation should be documented in the CFETP. All recurring evaluations should be documented on AF Form STEPS IN TASK PERFORMANCE: 1. Identify appropriate need to generate Supply Voucher Reports, Drug Utilization Review, Specific/General Transaction Reports, Outstanding Issue Report, Narcotic Movement Report or Inventory Report in CHCS. 2. Identify information required to generate Supply Voucher Reports, Drug Utilization Review, Specific/General Transaction Reports, Outstanding Issue Report, Narcotic Movement Report or Inventory Report in CHCS. 3. Insert pertinent information in required fields in CHCS. ATTACHMENT(S): None
11 PERFORMANCE CHECKLIST: 1. Supply Voucher Report REPORTS PERFORMANCE ITEMS SAT UNSAT a. Identify need to generate supply voucher report (ex. Disinterested inventory, trouble shooting perpetual inventory etc.) NOTE: This option will print the Supply Voucher information. By (R)eceipts: by (T)urn-ins: (to logistics or returns company) b. Identify information required to generate Supply Voucher Report c. Follow menu path PRM NRR - SVR d. Insert pertinent information in required fields in CHCS e. Select either receipts or turn-ins (when doing a disinterested inventory both would be provided to the inspector) f. Select vault for review g. Select first date logged (depending on how far back you need to review) h. Select last date logged (normally that would be today--if it's for a disinterested inventory it would be the actual date string (1 July 2006 to 31 July 2006 etc.) i. Select device to print on. j. Print report 2. Drug Utilization Review a. Identify the need to generate an outpatient pharmacy drug utilization report (may use when needing to call patients a particular drug was dispensed to provides the same information as an STR with the addition of the patient s name, social security number, doctors name and the SIG of each prescription) b. Identify information required to generate Drug Utilization Review c. Follow menu path PRM DUR ODU d. Insert pertinent information in required fields of CHCS e. Select sort option (1-6): 1 f. Select report option combination: 1 g. Select medical center division: (Name of your medical center/clinic) then hit enter h. Select outpatient site: all// or the pharmacy you need the report to compile from then hit enter i. Select medical center division--hit enter j. Select patient: typically ALL//ENTER k. Select Drug: <drug name> then enter (if other drugs are needed add them here. l. Select physician: typically all...but may need to do by name m. Select earliest fill date:
12 n. Select latest fill date: o. Queue on device: select appropriate printer you wish to print from p. Requested start time: Now or a later time 3. Specific/General Transaction Report a. Identify need to generate specific or general transaction report(s) b. Identify information required to generate Specific/General Transaction Report(s) c. Follow menu path PRM NRR STR or GTR d. Insert pertinent information in required fields in CHCS e. Select (D)rug, (I)ssue, (P)rescription, or (S)upply Vouchers f. Select Controlled Item, Issue Number, Prescription RX #, or Supply Transaction Voucher Number g. Select Vault Dispensed From h. Enter Earliest Date Transaction Completed i. Enter Latest Date Transaction Completed j. Input Printer Device 4. Outstanding Issue Report NOTE: This option will print outstanding issues in the following formats. (this report is useful when performing biennial inventory and when determining where controlled drugs are in your facility) (A)ll (D)rug - Specific (L)ocation - Specific (R)ange of Dates Asked a. Identify need to generate outstanding issue report (new calendar year) b. Identify information required to generate Outstanding Issue Report c. Follow menu path PRM NRR - OIR d. Select from All/Drug Specific/Location Specific/Range of Dates Asked) e. Queue on device: select appropriate printer you wish to print from f. Requested start time: Now or a later time g. Print report 5. Narcotic Movement Report a. Identify need to generate narcotic movement report b. Identify information required to generate narcotic movement report c. Follow menu path PRM NRR- NMR d. Select Vault for movement report e. Enter Earliest Date f. Enter Latest Date g. Select Queue on Device h. Request start time i. Print document 6. Inventory Report a. Identify need to generate inventory report (biennial, disinterested inventory, perpetual inventory) b. Identify information required to generate inventory report. c. Follow menu path PRM NRR - INR d. Sort by (A)lphabetic Name, (D)EA Schedule, or (N)ational Stock Number
13 e. Select Vault Name f. Select Printer Device FINAL RESULTS: Trainee: Trainer: Certifier: Date: FEEDBACK: Using the performance checklist as a review reference, discuss the trainee s performance, indicating strengths, weaknesses, suggested improvement, etc. If trainee performed all task steps satisfactorily, document appropriately in trainee s 6-part training/competency folder.
14 SUBJECT AREA: Controlled Substances Management TASK NAME(S): Forms and Files 85; 579; 781; 582; 701; 702; 115a CFETP/STS REFERENCE(S): EQUIPMENT REQUIRED: 1. Computer Terminal 2. Access to Air Force Forms and Publications Website 3. Printer 4. Blue or Black Pen TRAINING REFERENCE(S): AFI AFI CDC 4PO51A, Volume Two AFMAN Pharmacy Practice Manual, Chapter Three AFRIMS REMARKS/NOTES: Controlled Substances Management is vital to compliance with all guidelines and the success of accreditation inspections. Reports for CII and reports for CIII-CV must be filed separately. OBJECTIVE: 1. Accurately complete Air Forms as directed by Air Force Instructions (AFI) and local policies 2. Disposition forms IAW AFI s and local policies EVALUATION INSTRUCTIONS: 1. After the trainee has received instructions, allow sufficient practice on each part of the task. 2. Use the performance checklist to ensure all steps of the task are accomplished without assistance and without error. 3. Document task competency upon completion of the evaluation in the trainee s 6-part training/competency folder. Initial evaluation should be documented in the CFETP. All recurring evaluations should be documented on AF Form STEPS IN TASK PERFORMANCE: 1. Identify appropriate need for AF Forms 85, 579, 781, 582, and Standard Forms 701, 702 and 115a. 2. Identify information required for AF Forms 85, 579, 582, and Standard Forms 701, 702 and 115a.
15 3. Retrieve the correct form 4. Complete information as required 5. Disposition as required ATTACHMENT(S): None
16 PERFORMANCE CHECKLIST: 1. AF Form 85 FORMS AND FILES PERFORMANCE ITEMS SAT UNSAT a. Identify appropriate need for AF Form 85 (adjust inventory) b. Identify information required for AF Form 85 Inventory Adjustment Voucher c. Adjustment justification d. Quantity over/short e. Voucher number (may create local policy/form for voucher number tracking--see attachment below) f. Medication name g. Actual Inventory Count h. Actual recorded balance in CHCS or automation unit (PYXIS Etc.) i. Actual cost per unit short/over j. Retrieve form k. Complete form l. Route for approval (flight commander and MTF commander) m. File AF Form 85 Adjustment voucher as indicated on file plan. Technician should be able to describe time period required to keep voucher as indicated by AF RIMS and AF MAN AF Form 579 a. Identify appropriate need for AF Form 579 (issue controlled substance to ward, clinic, remote site) b. Identify information required to complete AF Form 579 Controlled Substances Register c. Requesting unit/clinic d. Date initiated e. Individual initiated by f. Medical Treatment Facility g. Register number h. Article nomenclature i. Register number of 579 that balance was transferred from (if new state initial issue) j. Balance forwarded (if zero state zero) k. Initials of individual that transferred/transcribed balance l. Date of issue m. Time of issue n. Identify if item was issued from pharmacy o. Identify if item was returned to pharmacy p. Identify the amount issued or returned q. Identify balance of item r. Identify Initials of pharmacy personnel and unit/clinic representative
17 s. Identify if balance will be forwarded to new 579 register document register number forwarded to t. Retrieve form u. Complete form (issue from CHCS) or return form (in CHCS) v. File AF Form 579 as indicated on file plan. Technician should be able to describe time period required to keep controlled substances register as indicated by AF RIMS and AFMAN AF Form 781 a. Identify appropriate need for AF Form 781 (controlled substance prescription) b. Identify information required to complete AF Form 781 Multiple Item Prescription form c. Medication nomenclature d. Strength e. Amount (up to 90 days depending on local policy) must be identified by both alpha and numeric documentation f. Directions g. Refills (up to 6 months or 5 refills on Sch III-V only, Sch II-no refills) h. Full name of patient (age and weight if under 12) i. SSN of sponsor j. Work/home telephone number k. Address l. Date m. Signature of prescriber n. Prescriber Identification (Name, SSN or BNDD, Grade, Degree, Service, Facility, and DEA # if civilian provider) o. Enter pertinent information into CHCS p. Attach appropriate pharmacy generated label to AF Form 781 q. File AF Form 579 as indicated on file plan. Technician should be able to describe time period required to keep multiple item prescription as indicated by AF RIMS and AFMAN AF Form 582 a. Identify appropriate need for AF Form 582 (controlled substances activity) b. Identify information required to complete AF Form 582 Pharmacy Stock Record c. Article d. Unit of issue e. Date f. Pharmacy tracking number g. Name of provider h. Patient or inpatient unit i. Voucher number j. Amount received k. Amount dispensed l. Recorded balance m. Retrieve form n. Complete form (Transfer information to new form once all fields have
18 been completed on current form or in other words full) o. File AF Form 582 as indicated on file plan. Technician should be able to describe time period required to keep pharmacy stock record as indicated by AF RIMS and AFMAN Standard Form 701 a. Identify appropriate need for SF 701 (pharmacy security) b. Identify information required to complete SF 701 Security Checklist c. Division/branch/office d. Room number e. Month/year f. Time security inspection was accomplished g. Retrieve form h. Complete form (checkmark appropriate blocks to indicate item was inspected, initials of individual that performed inspection, annotate time security inspection was accomplished) i. Physically check identified items to ensure security or resource protection j. File SF 701 as indicated on file plan. Technician should be able to describe time period required to keep security checklist as indicated by AF RIMS and AFMAN Standard Form 702 a. Identify appropriate need for SF 702 (open, close, check security container) b. Identify information required to complete SF 702 Security Container Check Sheet c. Room number d. Building e. Container number f. Month/year g. Date h. Opened by initials/time i. Closed by initials/time j. Checked by initials/time k. Physically check to ensure security container is open/closed according with pertinent regulations and operating instructions l. File SF 702 as indicated on file plan. Technician should be able to describe time period required to keep security container check sheet as indicated by AF RIMS and AFMAN Standard Form 115a a. Identify appropriate need for SF 115a (track 579 or issued controlled substances) b. Identify information required to complete SF 155a register of controlled numbers c. Organization or unit d. Control number on 579 e. Location issued to or received from f. Nomenclature of issued medication g. Date issued or returned h. Initials/signature of individuals involved in transaction
19 i. File SF 115a as indicated on file plan. Technician should be able to describe time period required to keep register of controlled numbers as indicated by AF RIMS and AFMAN FINAL RESULTS: Trainee: Trainer: Certifier: Date: FEEDBACK: Using the performance checklist as a review reference, discuss the trainee s performance, indicating strengths, weaknesses, suggested improvement, etc. If trainee performed all task steps satisfactorily, document appropriately in trainee s 6-part training/competency folder.
20 SUBJECT AREA: Controlled Substances Management TASK NAME(S): Inventories Biennial; Disinterested; Perpetual CFETP/STS REFERENCE(S): EQUIPMENT REQUIRED: 1. Computer terminal with Composite Healthcare Computer System (CHCS) access 2. Printer TRAINING REFERENCE(S): CDC 4P051A, Volume 3 and CHCS User s Manual REMARKS/NOTES: Controlled Substances Management is vital to compliance with all guidelines and the success of accreditation inspections. Reports for CII and reports for CIII-CV must be filed separately. Trainee must have Narcotic System Reports key enabled by Systems to perform task(s) OBJECTIVE: 1. Correctly perform inventory procedures 2. Disposition inventories IAW AFI s and local policies EVALUATION INSTRUCTIONS: 1. After the trainee has received instructions, allow sufficient practice on each part of the task. 2. Use the performance checklist to ensure all steps of the task are accomplished without assistance and without error. 3. Document task competency upon completion of the evaluation in the trainee s 6-part training/competency folder. Initial evaluation should be documented in the CFETP. All recurring evaluations should be documented on AF Form STEPS IN TASK PERFORMANCE: 1. Identify appropriate need for completing biennial, disinterested or perpetual inventories. 2. Identify information required for completing biennial, disinterested or perpetual inventories. 3. Insert pertinent information into required fields in CHCS. 4. Perform biennial, disinterested or perpetual inventory functions. 5. Disposition as required.
21 ATTACHMENT(S): None
22 PERFORMANCE CHECKLIST: 1. Biennial Inventory INVENTORIES PERFORMANCE ITEMS SAT UNSAT a. Identify appropriate need for completing biennial inventory (required 1 May every odd year). b. Identify information required for completing biennial inventory. c. Outstanding Issue Report d. PYXIS inventory reports e. CHCS inventory reports f. Insert pertinent information into required fields in CHCS (see reports checklists) g. Perform biennial inventory (physically count on-hand quantity of every controlled substances) h. File Biennial Inventory as indicated on file plan. Technician should be able to describe time period required to keep biennial inventory as indicated by AF RIMS and AF MAN Disinterested Inventory a. Identify appropriate need for completing disinterested inventory (Monthly requirement, report of survey or command directed) b. Identify information required for completing disinterested inventory c. Insert pertinent information required for completing disinterested inventory d. Perform disinterested inventory e. File Disinterested Inventory as indicated on file plan. Technician should be able to describe time period required to keep disinterested inventory as indicated by AF RIMS and AF MAN Perpetual Inventory a. Identify appropriate need for completing perpetual inventory (daily or shift change) b. Identify information required for completing perpetual inventory c. Insert pertinent information into required fields in CHCS (see reports checklists) d. Perform perpetual inventory e. File perpetual inventory as indicated on file plan. Technician should be able to describe time period required to keep perpetual inventory as indicated by AF RIMS and AF MAN *** NOTE*** If any numbers do not match up, an explanation of why must be at the bottom of inventory report. Here are 3 examples of what may be written at the bottom:
23 1. Medication +1 pending AF Form 85 for signature 2. RXJ not CRT d before inventory 3. RXJ filled and CRT d after inventory FINAL RESULTS: Trainee: Trainer: Certifier: Date: FEEDBACK: Using the performance checklist as a review reference, discuss the trainee s performance, indicating strengths, weaknesses, suggested improvement, etc. If trainee performed all task steps satisfactorily, document appropriately in trainee s 6-part training/competency folder.
24 BIBLIOGRAPHY AND OTHER REFERENCES 1. McAllister, Everett B. Lt Col., et al. Pharmacy Practice Manual. Andrews Air Force Base, Washington, DC: Associate Chief, Biomedical Science Corps for Pharmacy. 2. Pharmacy Law Digest. St. Louis, Missouri: Facts and Comparisons. 3. AFMAN , USAF Supply Manual, 1 July AFI , The Air Force Installation Security Program, 1 March AFI , Medical Logistics Support, 10 March AFI , Medical Care Management, 1 May AFRIMS 8. Pharmacy Technician Career Development Course (CDC) Set A and Set B 9. CHCS Users Manual
AIR FORCE SPECIALTY CODE: 4P0X1 PHARMACY TECHNICIAN
 AIR FORCE SPECIALTY CODE: 4P0X1 PHARMACY TECHNICIAN 4P0X1 Qualification Training Package (QTP) 9.7 Prepare Workload Reports, Report of Patients, and Cost Reports QTP 4P0X1-9.7 Oct 2007 OPR: TSgt Jennifer
AIR FORCE SPECIALTY CODE: 4P0X1 PHARMACY TECHNICIAN 4P0X1 Qualification Training Package (QTP) 9.7 Prepare Workload Reports, Report of Patients, and Cost Reports QTP 4P0X1-9.7 Oct 2007 OPR: TSgt Jennifer
QTP 4P0X1-3 15 September 2014 PHARMACY TECHNICIAN. Inspect drug storage in patient care areas. OPR: CMSgt Shawn M. Sill. 1 Page
 QTP 4P0X1-3 15 September 2014 PHARMACY TECHNICIAN Inspect drug storage in patient care areas OPR: CMSgt Shawn M. Sill 1 Page TABLE OF CONTENTS MODULE OBJECTIVE PAGES 1. Inspect drug storage areas 3 6 2.
QTP 4P0X1-3 15 September 2014 PHARMACY TECHNICIAN Inspect drug storage in patient care areas OPR: CMSgt Shawn M. Sill 1 Page TABLE OF CONTENTS MODULE OBJECTIVE PAGES 1. Inspect drug storage areas 3 6 2.
QTP 4P0X1-9 15 September 2014 PHARMACY TECHNICIAN. Non-Controlled Medication Audits. OPR: SMSgt Libby, MSgt Frisby and Maj Olech
 QTP 4P0X1-9 15 September 2014 PHARMACY TECHNICIAN Non-Controlled Medication Audits OPR: SMSgt Libby, MSgt Frisby and Maj Olech TABLE OF CONTENTS MODULE OBJECTIVE PAGES 1. Five Medication Audit Process
QTP 4P0X1-9 15 September 2014 PHARMACY TECHNICIAN Non-Controlled Medication Audits OPR: SMSgt Libby, MSgt Frisby and Maj Olech TABLE OF CONTENTS MODULE OBJECTIVE PAGES 1. Five Medication Audit Process
DENTAL ASSISTANT SPECIALTY SUPERVISION AND TRAINING
 QTP 4Y0X1-8 24 March 2015 Supersedes all previous versions DENTAL ASSISTANT SPECIALTY SUPERVISION AND TRAINING Volume 8 381st Training Squadron 2931 Harney Road Fort Sam Houston, TX 78234 QTP 4Y0X1-8 DENTAL
QTP 4Y0X1-8 24 March 2015 Supersedes all previous versions DENTAL ASSISTANT SPECIALTY SUPERVISION AND TRAINING Volume 8 381st Training Squadron 2931 Harney Road Fort Sam Houston, TX 78234 QTP 4Y0X1-8 DENTAL
BUDGET AND FINANCIAL PLANNING
 QTP 4Y0X1 20 March 2015 Supersedes all previous versions DENTAL ASSISTANT SPECIALTY BUDGET AND FINANCIAL PLANNING Volume 7 381st Training Squadron 2931 Harney Road Fort Sam Houston, TX 78234 QTP 4Y0X1-7
QTP 4Y0X1 20 March 2015 Supersedes all previous versions DENTAL ASSISTANT SPECIALTY BUDGET AND FINANCIAL PLANNING Volume 7 381st Training Squadron 2931 Harney Road Fort Sam Houston, TX 78234 QTP 4Y0X1-7
NOTE: In the event that the seal is accidentally broken, the narcotic may be wasted via narcotic wastage procedures.
 HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER NARCOTICS AND CONTROLLED DRUGS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER NARCOTICS AND CONTROLLED DRUGS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
AF Substance Abuse Policy and Programs
 Air Force Medical Operations Agency AF Substance Abuse Policy and Programs 30 March 2011 IOM Committee on Prevention, Diagnosis, Treatment, and Management of Substance Use Disorders 1 Overview SUD Policies
Air Force Medical Operations Agency AF Substance Abuse Policy and Programs 30 March 2011 IOM Committee on Prevention, Diagnosis, Treatment, and Management of Substance Use Disorders 1 Overview SUD Policies
EMR Technology Checklist
 Patient Accessibility/Scheduling/Account Maintenance: Able to interact with schedule through an online portal pre register VIP status to move patient to the front of the line Access and pre registration
Patient Accessibility/Scheduling/Account Maintenance: Able to interact with schedule through an online portal pre register VIP status to move patient to the front of the line Access and pre registration
Abacus Rx, Inc. 8000 SW 117 Ave PH-G Visit us at Miami, FL 33183 (305) 220-0400 Fax (305) 220-4900
 Abacus Rx, Inc. 8000 SW 117 Ave PH-G Visit us at Miami, FL 33183 (305) 220-0400 Fax (305) 220-4900 www.abacusrx.com Thank you for your inquire! We are committed to provide Pharmacies with the finest technology
Abacus Rx, Inc. 8000 SW 117 Ave PH-G Visit us at Miami, FL 33183 (305) 220-0400 Fax (305) 220-4900 www.abacusrx.com Thank you for your inquire! We are committed to provide Pharmacies with the finest technology
Vendor Configuration, Ordering, and Receiving. Kroll 9.1. Kroll 9.1
 Vendor Configuration, Ordering, and Receiving Kroll 9.1 Kroll 9.1 Contents Vendor Configuration... 1 Catalog Tab... 3 Ordering Tab... 6 Receiving Tab... 7 General Tab... 9 Preventing OTC Inventory Adjustments...
Vendor Configuration, Ordering, and Receiving Kroll 9.1 Kroll 9.1 Contents Vendor Configuration... 1 Catalog Tab... 3 Ordering Tab... 6 Receiving Tab... 7 General Tab... 9 Preventing OTC Inventory Adjustments...
AEROSPACE MEDICAL SERVICE SPECIALTY INDEPENDENT DUTY MEDICAL TECHNICIAN EMERGENCY MEDICINE PROCEDURES
 QTP4N0X1C-9 02 July 2015 AEROSPACE MEDICAL SERVICE SPECIALTY INDEPENDENT DUTY MEDICAL TECHNICIAN EMERGENCY MEDICINE PROCEDURES Volume 9 TOTAL FORCE, TOTAL CARE EVERYTIME, ANYWHERE 383d TRAINING SQUADRON/XUFB
QTP4N0X1C-9 02 July 2015 AEROSPACE MEDICAL SERVICE SPECIALTY INDEPENDENT DUTY MEDICAL TECHNICIAN EMERGENCY MEDICINE PROCEDURES Volume 9 TOTAL FORCE, TOTAL CARE EVERYTIME, ANYWHERE 383d TRAINING SQUADRON/XUFB
GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL
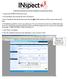 GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL 1. Log in to your INSPECT WebCenter Account. 2. Go to the Requests tab on the left, and select New Request. 3. Select Practitioner from the drop-down
GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL 1. Log in to your INSPECT WebCenter Account. 2. Go to the Requests tab on the left, and select New Request. 3. Select Practitioner from the drop-down
POS Helpdesk Operational Procedure
 POS Helpdesk Operational Procedure Purpose: To describe the tools and scenarios associated with IME Pharmacy Point of Sale (POS) Help Desk operations. Identification of Roles: Pharmacy Point of Sale (POS)
POS Helpdesk Operational Procedure Purpose: To describe the tools and scenarios associated with IME Pharmacy Point of Sale (POS) Help Desk operations. Identification of Roles: Pharmacy Point of Sale (POS)
University of Miami Clinical Enterprise Technologies
 Nursing Manual Our Mission: To design and deliver ongoing support for a network of Business and Clinical Information Management Systems which enhance the academic and research vision while implementing
Nursing Manual Our Mission: To design and deliver ongoing support for a network of Business and Clinical Information Management Systems which enhance the academic and research vision while implementing
Enhance Controlled Substance Inventory Control While Improving Workflow Efficiency
 WHITE PAPER Enhance Controlled Substance Inventory Control While Improving Workflow Efficiency Most ambulatory pharmacies still use a manual process to record the receipt and disposition of controlled
WHITE PAPER Enhance Controlled Substance Inventory Control While Improving Workflow Efficiency Most ambulatory pharmacies still use a manual process to record the receipt and disposition of controlled
Donald H. Williams, Pharmacy Board. D. E. A. Rules 21 C.F.R. 1300. DEA Rules - Objectives. DEA Rules
 D. E. A. Rules 21 C.F.R. 1300 Everything that you need to know but were afraid to ask DEA Rules - Objectives The student will be able to discuss the manner in which the DEA regulates the distribution of
D. E. A. Rules 21 C.F.R. 1300 Everything that you need to know but were afraid to ask DEA Rules - Objectives The student will be able to discuss the manner in which the DEA regulates the distribution of
GEORGIA ~ STATUTE. Georgia Code Annotated 16-13-21 (DEA registration); 43-34-100 et seq; 43-34-21 et seq (medical board provisions; PA committee)
 GEORGIA ~ STATUTE STATUTE DATE Enacted 1972 REGULATORY BODY PA DEFINED SCOPE OF PRACTICE PRESCRIBING/DISPENSING SUPERVISION DEFINED Georgia Code Annotated 16-13-21 (DEA registration); 43-34-100 et seq;
GEORGIA ~ STATUTE STATUTE DATE Enacted 1972 REGULATORY BODY PA DEFINED SCOPE OF PRACTICE PRESCRIBING/DISPENSING SUPERVISION DEFINED Georgia Code Annotated 16-13-21 (DEA registration); 43-34-100 et seq;
UTCVM PHARMACY STANDARD OPERATING PROCEDURES
 UTCVM PHARMACY STANDARD OPERATING PROCEDURES Updated: 4/5/2004 I. General Procedures A. Hours: The Pharmacy will be open Monday through Friday, 8:00AM to 6:00PM; Saturday 8:00AM to 1:00PM. The Pharmacy
UTCVM PHARMACY STANDARD OPERATING PROCEDURES Updated: 4/5/2004 I. General Procedures A. Hours: The Pharmacy will be open Monday through Friday, 8:00AM to 6:00PM; Saturday 8:00AM to 1:00PM. The Pharmacy
Exceptions to the Rule: A Pharmacy Law Presentation. Objectives DISCLAIMER 10/16/2015
 Exceptions to the Rule: A Pharmacy Law Presentation Eric Roath, Pharm.D. Director of Professional Practice Michigan Pharmacists Association Objectives 1. Identify basic legal frameworks that govern the
Exceptions to the Rule: A Pharmacy Law Presentation Eric Roath, Pharm.D. Director of Professional Practice Michigan Pharmacists Association Objectives 1. Identify basic legal frameworks that govern the
BOARD OF PHARMACY DIVISION 41 OPERATION OF PHARMACIES (RETAIL AND INSTITUTIONAL DRUG OUTLETS) CONSULTING PHARMACISTS AND OPERATION OF DRUG ROOMS
 BOARD OF PHARMACY DIVISION 41 OPERATION OF PHARMACIES (RETAIL AND INSTITUTIONAL DRUG OUTLETS) CONSULTING PHARMACISTS AND OPERATION OF DRUG ROOMS 855-041-6050 Definitions Hospitals with Pharmacies (1) In
BOARD OF PHARMACY DIVISION 41 OPERATION OF PHARMACIES (RETAIL AND INSTITUTIONAL DRUG OUTLETS) CONSULTING PHARMACISTS AND OPERATION OF DRUG ROOMS 855-041-6050 Definitions Hospitals with Pharmacies (1) In
MENTAL HEALTH SERVICE SPECIALTY THE TWELVE CORE FUNCTIONS OF SUBSTANCE ABUSE COUNSELING
 DEPARTMENT OF THE AIR FORCE QTP 4C0X1-1 Headquarters US Air Force September 1998 Washington, DC 20330-1030 MENTAL HEALTH SERVICE SPECIALTY THE TWELVE CORE FUNCTIONS OF SUBSTANCE ABUSE COUNSELING TRAINING
DEPARTMENT OF THE AIR FORCE QTP 4C0X1-1 Headquarters US Air Force September 1998 Washington, DC 20330-1030 MENTAL HEALTH SERVICE SPECIALTY THE TWELVE CORE FUNCTIONS OF SUBSTANCE ABUSE COUNSELING TRAINING
Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population
 Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population Prescriber FAQs Update January 22, 2015 1. What information is needed
Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population Prescriber FAQs Update January 22, 2015 1. What information is needed
Heath Shield Heath Care Management System
 Heath Shield Heath Care Management System Introduction Heath Shield will be an integrated, modular client server based system which can be extended to a web based solution also. The programs will have
Heath Shield Heath Care Management System Introduction Heath Shield will be an integrated, modular client server based system which can be extended to a web based solution also. The programs will have
Communications Officer Specialty Track Study Guide
 Communications Officer Specialty Track Study Guide NATIONAL HEADQUARTERS CIVIL AIR PATROL Maxwell Air Force Base, Alabama CAPP 214 20 AUGUST 2012 Table of Contents Preface... 3 Training Objectives... 4
Communications Officer Specialty Track Study Guide NATIONAL HEADQUARTERS CIVIL AIR PATROL Maxwell Air Force Base, Alabama CAPP 214 20 AUGUST 2012 Table of Contents Preface... 3 Training Objectives... 4
NH Laws / Rules Regarding Limited Retail Drug Distributors
 NH Laws / Rules Regarding Limited Retail Drug Distributors 318:1, VII-a. "Limited retail drug distributor'' means a distributor of legend devices or medical gases delivered directly to the consumer pursuant
NH Laws / Rules Regarding Limited Retail Drug Distributors 318:1, VII-a. "Limited retail drug distributor'' means a distributor of legend devices or medical gases delivered directly to the consumer pursuant
Technician Training and Roles in Institutional Pharmacy Practice
 Technician Training and Roles in Institutional Pharmacy Practice Cindy Wilson, Pharm.D. Harborview Medical Center Learning Objectives Understand laws regarding technician training, certification, licensure,
Technician Training and Roles in Institutional Pharmacy Practice Cindy Wilson, Pharm.D. Harborview Medical Center Learning Objectives Understand laws regarding technician training, certification, licensure,
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 BY ORDER OF THE COMMANDER OKLAHOMA CITY AIR LOGISTICS COMPLEX AIR FORCE INSTRUCTION 36-2650 OKLAHOMA CITY AIR LOGISTICS COMPLEX Supplement 21 OCTOBER 2014 Personnel TECHNOLOGY CENTER PROGRAM PROCEDURES
BY ORDER OF THE COMMANDER OKLAHOMA CITY AIR LOGISTICS COMPLEX AIR FORCE INSTRUCTION 36-2650 OKLAHOMA CITY AIR LOGISTICS COMPLEX Supplement 21 OCTOBER 2014 Personnel TECHNOLOGY CENTER PROGRAM PROCEDURES
Drug Diversion. Controlled Substance Prescribing and Diversion
 Drug Diversion Provider Presentation Controlled Substance Prescribing and Diversion Angela Wallace, R.Ph. Coastal District Inspector, PMP Law Enforcement Officer DHEC Bureau of Drug Control Presentation
Drug Diversion Provider Presentation Controlled Substance Prescribing and Diversion Angela Wallace, R.Ph. Coastal District Inspector, PMP Law Enforcement Officer DHEC Bureau of Drug Control Presentation
DISTRICT OF COLUMBIA MUNICIPAL REGULATIONS FOR PHARMACY TECHNICIANS
 Title 17 District of Columbia Municipal Regulations DISTRICT OF COLUMBIA MUNICIPAL REGULATIONS FOR PHARMACY TECHNICIANS Effective CHAPTER 99 PHARMACY TECHNICIANS Secs. 9900 General Provisions 9901 Term
Title 17 District of Columbia Municipal Regulations DISTRICT OF COLUMBIA MUNICIPAL REGULATIONS FOR PHARMACY TECHNICIANS Effective CHAPTER 99 PHARMACY TECHNICIANS Secs. 9900 General Provisions 9901 Term
Pharmacy Technician Structured Practical Training Program MANUAL AND SUBMISSION FORMS. December 2014 (Updated July 2015)
 Pharmacy Technician Structured Practical Training Program MANUAL AND SUBMISSION FORMS December 2014 (Updated July 2015) *To be reviewed by Supervisor and Pharmacy Technician-in-Training and used in conjunction
Pharmacy Technician Structured Practical Training Program MANUAL AND SUBMISSION FORMS December 2014 (Updated July 2015) *To be reviewed by Supervisor and Pharmacy Technician-in-Training and used in conjunction
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 BY ORDER OF THE COMMANDER AIR FORCE RESERVE COMMAND AIR FORCE RESERVE COMMAND INSTRUCTION 36-2003 1 JULY 2014 Personnel AIR FORCE RESERVE ADVERTISING PROGRAM COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
BY ORDER OF THE COMMANDER AIR FORCE RESERVE COMMAND AIR FORCE RESERVE COMMAND INSTRUCTION 36-2003 1 JULY 2014 Personnel AIR FORCE RESERVE ADVERTISING PROGRAM COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
Medication Reconciliation Training Packet. Legacy Health System
 Medication Reconciliation Training Packet Legacy Health System 1 Objectives To identify the key elements of the medication reconciliation process To describe the role of the nurse in the medication reconciliation
Medication Reconciliation Training Packet Legacy Health System 1 Objectives To identify the key elements of the medication reconciliation process To describe the role of the nurse in the medication reconciliation
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS TABLE OF CONTENTS
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS TABLE OF CONTENTS Section 1. Authority.... 1 Section 2. Definitions.... 1 Section 3. Qualifications and Requirements for Pharmacy Technicians and Pharmacy Technicians-in-Training....
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS TABLE OF CONTENTS Section 1. Authority.... 1 Section 2. Definitions.... 1 Section 3. Qualifications and Requirements for Pharmacy Technicians and Pharmacy Technicians-in-Training....
Eight Important Principles For Managing Prescription Medications In the Athletic Training Room
 Eight Important Principles For Managing Prescription Medications In the Athletic Training Room Robert Nickell, RPh, FAPO, FCPHA, FACA Sports Pharmacist USA Team Pharmacist President and CEO, Sportpharm
Eight Important Principles For Managing Prescription Medications In the Athletic Training Room Robert Nickell, RPh, FAPO, FCPHA, FACA Sports Pharmacist USA Team Pharmacist President and CEO, Sportpharm
Medical College of Georgia SOP NUMBER: 03 INVESTIGATIONAL DRUG HANDLING Version Number: 1.0, 1.1 Effective Date: 09/12/06, 08/02/10, 3/2/11
 Effective Date: 09/12/06, 08/02/10, 3/2/11 Title: 1.0 OBJECTIVE: 1.1 This SOP describes the methods and policies for: Handling investigational drug Dispensing investigational drug 1.2. This procedure applies
Effective Date: 09/12/06, 08/02/10, 3/2/11 Title: 1.0 OBJECTIVE: 1.1 This SOP describes the methods and policies for: Handling investigational drug Dispensing investigational drug 1.2. This procedure applies
Pharmacy Operations. Assisting the Pharmacist. Pharmacy Technician Training Systems Passassured, LLC
 Pharmacy Operations Assisting the Pharmacist Pharmacy Technician Training Systems Passassured, LLC Pharmacy Operations, Assisting the Pharmacist PassAssured's Pharmacy Technician Training Program Pharmacy
Pharmacy Operations Assisting the Pharmacist Pharmacy Technician Training Systems Passassured, LLC Pharmacy Operations, Assisting the Pharmacist PassAssured's Pharmacy Technician Training Program Pharmacy
Pharmacy Auto Refill System
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Auto Refill System (BEX) Version 1.0 Information Technology Support Center Division of Information Resources Albuquerque, New Mexico Preface This document
RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Auto Refill System (BEX) Version 1.0 Information Technology Support Center Division of Information Resources Albuquerque, New Mexico Preface This document
- 1 - First Time Pharmacy Managers (Revised 02/02/2011)
 State of Connecticut Department of Consumer Protection Commission of Pharmacy 165 Capitol Avenue, Room 147 Hartford, CT 06106 - Telephone: 860-713-6070 ALL FIRST-TIME PHARMACY MANAGERS ARE REQUIRED TO
State of Connecticut Department of Consumer Protection Commission of Pharmacy 165 Capitol Avenue, Room 147 Hartford, CT 06106 - Telephone: 860-713-6070 ALL FIRST-TIME PHARMACY MANAGERS ARE REQUIRED TO
Medicare Part D Plan Finder instructions
 Medicare Part D Plan Finder instructions These instructions may help you find the lowest cost Part D coverage (in both stand alone and Advantage plans). You may need these detailed instructions to get
Medicare Part D Plan Finder instructions These instructions may help you find the lowest cost Part D coverage (in both stand alone and Advantage plans). You may need these detailed instructions to get
Medicare Part D Plan Finder instructions
 Medicare Part D Plan Finder instructions You can use these instructions to help you find the lowest cost Part D plans (both stand alone and Advantage plans) for the prescription drugs that you take. You
Medicare Part D Plan Finder instructions You can use these instructions to help you find the lowest cost Part D plans (both stand alone and Advantage plans) for the prescription drugs that you take. You
Purpose: To provide guidelines for the safe administration, proper documentation and auditing of controlled substances by nursing personnel.
 University of Kentucky / UK HealthCare Policy and Procedure Policy # ND14-03 Title/Description: Administration, Documentation, and Auditing of Controlled Substances Purpose: To provide guidelines for the
University of Kentucky / UK HealthCare Policy and Procedure Policy # ND14-03 Title/Description: Administration, Documentation, and Auditing of Controlled Substances Purpose: To provide guidelines for the
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 Template modified: 27 May 1997 14:30 BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE INSTRUCTION 46-101 25 JULY 1994 Nursing NURSING OPERATIONS COMPLIANCE WITH THIS PUBLICATION IS MANDATORY NOTICE:
Template modified: 27 May 1997 14:30 BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE INSTRUCTION 46-101 25 JULY 1994 Nursing NURSING OPERATIONS COMPLIANCE WITH THIS PUBLICATION IS MANDATORY NOTICE:
Patient Portal Users Guide
 e-mds Solution Series Patient Portal Users Guide Version 7.0 How to Use the Patient Portal CHARTING THE FUTURE OF HEALTHCARE e-mds 9900 Spectrum Drive. Austin, TX 78717 Phone 512.257.5200 Fax 512.335.4375
e-mds Solution Series Patient Portal Users Guide Version 7.0 How to Use the Patient Portal CHARTING THE FUTURE OF HEALTHCARE e-mds 9900 Spectrum Drive. Austin, TX 78717 Phone 512.257.5200 Fax 512.335.4375
Advancing Technology: Enhancing the Current EHR
 COL Michael Greenly, PM EHR Core Solution Delivery Division 2015 Defense Health Information Technology Symposium Advancing Technology: Enhancing the Current EHR 1 DHA Vision A joint, integrated, premier
COL Michael Greenly, PM EHR Core Solution Delivery Division 2015 Defense Health Information Technology Symposium Advancing Technology: Enhancing the Current EHR 1 DHA Vision A joint, integrated, premier
Prescribing a new medication
 Pulse Rx Solutions Prescribing a new medication The core of Pulse s Rx solution, the Rx Pad is designed to make the prescribing of patient medications straightforward, precise, and convenient. pulseinc.com
Pulse Rx Solutions Prescribing a new medication The core of Pulse s Rx solution, the Rx Pad is designed to make the prescribing of patient medications straightforward, precise, and convenient. pulseinc.com
Specialty drug program. Save time. Save money. Feel good.
 Specialty drug program Save time. Save money. Feel good. What are specialty drugs? Specialty drugs are used to treat serious or ongoing medical conditions such as multiple sclerosis, hemophilia, hepatitis
Specialty drug program Save time. Save money. Feel good. What are specialty drugs? Specialty drugs are used to treat serious or ongoing medical conditions such as multiple sclerosis, hemophilia, hepatitis
Pharmacy Operating Guidelines & Information
 Pharmacy Operating Guidelines & Information RxAMERICA PHARMACY BENEFIT MANAGEMENT Pharmacy Operating Guidelines & Information Table of Contents I. Quick Reference List...3 C. D. E. Important Phone Numbers...
Pharmacy Operating Guidelines & Information RxAMERICA PHARMACY BENEFIT MANAGEMENT Pharmacy Operating Guidelines & Information Table of Contents I. Quick Reference List...3 C. D. E. Important Phone Numbers...
Technician Training and Roles in Institutional Pharmacy Practice
 Technician Training and Roles in Institutional Pharmacy Practice Joshua DeSilvey, Pharm.D., M.S. University of Washington Medical Center January 23, 2007 Learning Objectives Understand laws regarding technician
Technician Training and Roles in Institutional Pharmacy Practice Joshua DeSilvey, Pharm.D., M.S. University of Washington Medical Center January 23, 2007 Learning Objectives Understand laws regarding technician
DEPARTMENT OF HEALTH NOTICE OF PROPOSED RULEMAKING
 DEPARTMENT OF HEALTH NOTICE OF PROPOSED RULEMAKING The Director of the Department of Health ( Department ), pursuant to the District of Columbia Health Occupations Revision Act of 1985, effective March
DEPARTMENT OF HEALTH NOTICE OF PROPOSED RULEMAKING The Director of the Department of Health ( Department ), pursuant to the District of Columbia Health Occupations Revision Act of 1985, effective March
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS. These regulations are promulgated as authorized by the Act.
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
eprescribe FAQs General Application FAQs
 Allscripts eprescribe eprescribe FAQs General Application FAQs What does eprescribe allow me to do? Based on Allscripts' award winning EMR technology, eprescribe enables physicians to improve patient care
Allscripts eprescribe eprescribe FAQs General Application FAQs What does eprescribe allow me to do? Based on Allscripts' award winning EMR technology, eprescribe enables physicians to improve patient care
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS. These regulations are promulgated as authorized by the Act.
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. Direct supervision means that a licensed pharmacist
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. Direct supervision means that a licensed pharmacist
CHAPTER 6 PROCUREMENT AND SUPPLY OF PHARMACEUTICAL PRODUCTS IN THE PUBLIC AND PRIVATE MEDICAL SECTORS
 CHAPTER 6 PROCUREMENT AND SUPPLY OF PHARMACEUTICAL PRODUCTS IN THE PUBLIC AND PRIVATE MEDICAL SECTORS Overview 6.1 This chapter sets out the Review Committee s findings and recommendations on procurement
CHAPTER 6 PROCUREMENT AND SUPPLY OF PHARMACEUTICAL PRODUCTS IN THE PUBLIC AND PRIVATE MEDICAL SECTORS Overview 6.1 This chapter sets out the Review Committee s findings and recommendations on procurement
8.0.0.0.0 Accounts Receivable. I. Overview Definitions and Abbreviations. Equipment and Supplies Required
 8.0.0.0.0 Accounts Receivable I. Overview Definitions and Abbreviations Equipment and Supplies Required Page 1 of 36 Revised October 2015 Version 4 II. Job Position(s) Accounts Receivable Clerk Page 2
8.0.0.0.0 Accounts Receivable I. Overview Definitions and Abbreviations Equipment and Supplies Required Page 1 of 36 Revised October 2015 Version 4 II. Job Position(s) Accounts Receivable Clerk Page 2
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 BY ORDER OF THE COMMANDER 45TH SPACE WING 45TH SPACE WING INSTRUCTION 33-114 18 DECEMBER 2012 Communications and Information SOFTWARE MANAGEMENT COMPLIANCE WITH THIS PUBLICATION IS MANDATORY ACCESSIBILITY:
BY ORDER OF THE COMMANDER 45TH SPACE WING 45TH SPACE WING INSTRUCTION 33-114 18 DECEMBER 2012 Communications and Information SOFTWARE MANAGEMENT COMPLIANCE WITH THIS PUBLICATION IS MANDATORY ACCESSIBILITY:
247 CMR: BOARD OF REGISTRATION IN PHARMACY
 247 CMR 9.00: CODE OF PROFESSIONAL CONDUCT; PROFESSIONAL STANDARDS FOR REGISTERED PHARMACISTS, PHARMACIES AND PHARMACY DEPART- MENTS Section 9.01: Code of Professional Conduct for Registered Pharmacists,
247 CMR 9.00: CODE OF PROFESSIONAL CONDUCT; PROFESSIONAL STANDARDS FOR REGISTERED PHARMACISTS, PHARMACIES AND PHARMACY DEPART- MENTS Section 9.01: Code of Professional Conduct for Registered Pharmacists,
Welcome to OptumRx Your Prescription Benefit Program
 Welcome to OptumRx Your Prescription Benefit Program OptumRx offers you more ways to improve your health, while keeping medications more affordable and accessible. Welcome to OptumRx OptumRx manages your
Welcome to OptumRx Your Prescription Benefit Program OptumRx offers you more ways to improve your health, while keeping medications more affordable and accessible. Welcome to OptumRx OptumRx manages your
HEALTHCARE SOLUTIONS INTEGRATED MEDICATION MANAGEMENT SOLUTIONS FOR INNOVATIVE HOSPITALS
 HEALTHCARE SOLUTIONS INTEGRATED MEDICATION MANAGEMENT SOLUTIONS FOR INNOVATIVE HOSPITALS A LONG HISTORY OF INNOVATION IN SAFE AND EFFICIENT TAILORED AUTOMATED DRUG MANAGEMENT SYSTEMS «SWISSLOG, AN ESTABLISHED
HEALTHCARE SOLUTIONS INTEGRATED MEDICATION MANAGEMENT SOLUTIONS FOR INNOVATIVE HOSPITALS A LONG HISTORY OF INNOVATION IN SAFE AND EFFICIENT TAILORED AUTOMATED DRUG MANAGEMENT SYSTEMS «SWISSLOG, AN ESTABLISHED
REVIEW OF FEDERAL LAW FOR PHARMACY TECHNICIANS DR. SULLIVAN S MONOGRAPH
 REVIEW OF FEDERAL LAW FOR PHARMACY TECHNICIANS DR. SULLIVAN S MONOGRAPH REVIEW OF FEDERAL LAW FOR PHARMACY TECHNICIANS ACTIVITY DESCRIPTION This program will assist pharmacy technicians to understand the
REVIEW OF FEDERAL LAW FOR PHARMACY TECHNICIANS DR. SULLIVAN S MONOGRAPH REVIEW OF FEDERAL LAW FOR PHARMACY TECHNICIANS ACTIVITY DESCRIPTION This program will assist pharmacy technicians to understand the
EPCS FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES. Revised: January 2016
 FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: January 2016 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: January 2016 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
Investigational Drugs: Investigational Drugs and Biologics
 : I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
: I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
Optum Physician EMR v 8.0 Release Notes
 Optum Physician EMR v 8.0 Release Notes OptumInsight 70 Royal Little Drive Providence, RI 02904 Copyright 2002-2014 OptumInsight. All rights reserved. Document Information Author(s) Release Date G.Caldera
Optum Physician EMR v 8.0 Release Notes OptumInsight 70 Royal Little Drive Providence, RI 02904 Copyright 2002-2014 OptumInsight. All rights reserved. Document Information Author(s) Release Date G.Caldera
PHARMACY TECHNICIAN SERIES
 PHARMACY TECHNICIAN SERIES Occ. Work Prob. Effective Last Code No. Class Title Area Area Period Date Action 4058 Pharmacy Technician I 12 446 6 mo. 02/15/04 Revised 4059 Pharmacy Technician II 12 446 6
PHARMACY TECHNICIAN SERIES Occ. Work Prob. Effective Last Code No. Class Title Area Area Period Date Action 4058 Pharmacy Technician I 12 446 6 mo. 02/15/04 Revised 4059 Pharmacy Technician II 12 446 6
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 BY ORDER OF THE COMMANDER AIR FORCE SPECIAL OPERATIONS COMMAND (AFSOC) AIR FORCE INSTRUCTION 33-114 AIR FORCE SPECIAL OPERATIONS COMMAND Supplement 16 OCTOBER 2008 Incorporating Change 1, 23 November 2011
BY ORDER OF THE COMMANDER AIR FORCE SPECIAL OPERATIONS COMMAND (AFSOC) AIR FORCE INSTRUCTION 33-114 AIR FORCE SPECIAL OPERATIONS COMMAND Supplement 16 OCTOBER 2008 Incorporating Change 1, 23 November 2011
DAISY 4.2. New features. Electronic prescribing with erx Integrated credit card processing with DAISY InCharge
 DAISY 4.2 New features Electronic prescribing with erx Integrated credit card processing with DAISY InCharge DAISY 4.2 Update Release Notes 1 What s New in DAISY 4.2... 1 New Features... 1 Other Enhancements
DAISY 4.2 New features Electronic prescribing with erx Integrated credit card processing with DAISY InCharge DAISY 4.2 Update Release Notes 1 What s New in DAISY 4.2... 1 New Features... 1 Other Enhancements
MEDITECH TRAINING MANUAL PHARMACY APPLICATION. General Information
 MEDITECH PHARMACY TRAINING MANUAL ELLSWORTH COUNTY MEDICAL CENTER September 2004 MEDITECH TRAINING MANUAL PHARMACY APPLICATION General Information Meditech is a fully integrated system consisting of applications
MEDITECH PHARMACY TRAINING MANUAL ELLSWORTH COUNTY MEDICAL CENTER September 2004 MEDITECH TRAINING MANUAL PHARMACY APPLICATION General Information Meditech is a fully integrated system consisting of applications
INSTRUCTIONS FOR USE: OA-RX
 P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
Using Electronic Prescriptions (erx)
 Using Electronic Prescriptions (erx) In order to send Electronic Prescriptions, upgrade to Version 14.2 or higher. Electronic Prescriptions only work in the United States and its territories, including
Using Electronic Prescriptions (erx) In order to send Electronic Prescriptions, upgrade to Version 14.2 or higher. Electronic Prescriptions only work in the United States and its territories, including
This revision replaces AF Form 40A with AF Form 1289 in Attachment 3. A bar ( ) indicates a revision from the previous version.
 BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE INSTRUCTION 36-8002 1 JULY 1998 Personnel TELECOMMUTING GUIDELINES FOR AIR FORCE RESERVISTS AND THEIR SUPERVISORS COMPLIANCE WITH THIS PUBLICATION IS
BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE INSTRUCTION 36-8002 1 JULY 1998 Personnel TELECOMMUTING GUIDELINES FOR AIR FORCE RESERVISTS AND THEIR SUPERVISORS COMPLIANCE WITH THIS PUBLICATION IS
Introduction 2. 1. The Role of Pharmacy Within a NHS Trust 3. 2. Pharmacy Staff 4. 3. Pharmacy Facilities 5. 4. Pharmacy and Resources 6
 Index Index Section Page Introduction 2 1. The Role of Pharmacy Within a NHS Trust 3 2. Pharmacy Staff 4 3. Pharmacy Facilities 5 4. Pharmacy and Resources 6 5. Prescription Charges 7 6. Communication
Index Index Section Page Introduction 2 1. The Role of Pharmacy Within a NHS Trust 3 2. Pharmacy Staff 4 3. Pharmacy Facilities 5 4. Pharmacy and Resources 6 5. Prescription Charges 7 6. Communication
Meaningful Use Qualification Plan
 Meaningful Use Qualification Plan Overview Certified EHR technology used in a meaningful way is one piece of a broader Health Information Technology infrastructure intended to reform the health care system
Meaningful Use Qualification Plan Overview Certified EHR technology used in a meaningful way is one piece of a broader Health Information Technology infrastructure intended to reform the health care system
California State Board of Pharmacy and Medical Board of California
 California State Board of Pharmacy and Medical Board of California Transmission and Receipt of Electronic Controlled Substance Prescriptions Pursuant to DEA Interim Final Rule (IFR): Electronic Prescriptions
California State Board of Pharmacy and Medical Board of California Transmission and Receipt of Electronic Controlled Substance Prescriptions Pursuant to DEA Interim Final Rule (IFR): Electronic Prescriptions
Controlled II Substance Rules & Regulations. Controlled II Substance Rules & Regulations
 North Carolina Board of Pharmacy Controlled Substance Pocketcard A brief summary of pertinent rules and regulations impacting the practice of pharmacy ~ Controlled II Substance Controlled II Substance
North Carolina Board of Pharmacy Controlled Substance Pocketcard A brief summary of pertinent rules and regulations impacting the practice of pharmacy ~ Controlled II Substance Controlled II Substance
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS. These regulations are promulgated as authorized by the Act.
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 BY ORDER OF THE COMMANDER AIR COMBAT COMMAND AIR FORCE INSTRUCTION 21-103 AIR COMBAT COMMAND Supplement ADDENDUM_R 17 JULY 2013 Current 28 August 2015 Maintenance MISSION STATUS REPORTING TOOL (MSRT),
BY ORDER OF THE COMMANDER AIR COMBAT COMMAND AIR FORCE INSTRUCTION 21-103 AIR COMBAT COMMAND Supplement ADDENDUM_R 17 JULY 2013 Current 28 August 2015 Maintenance MISSION STATUS REPORTING TOOL (MSRT),
CHAPTER 6 OUTPATIENT SURGICAL CENTERS 6. 1
 CHAPTER 6 OUTPATIENT SURGICAL CENTERS 6. 1 OUTPATIENT SURGICAL CENTERS I. LICENSURE 1. Modified II B Pharmacy license 2. DEA license II. REQUIREMENTS 1. Formulary may be any drugs needed to meet the medical
CHAPTER 6 OUTPATIENT SURGICAL CENTERS 6. 1 OUTPATIENT SURGICAL CENTERS I. LICENSURE 1. Modified II B Pharmacy license 2. DEA license II. REQUIREMENTS 1. Formulary may be any drugs needed to meet the medical
DC DEPARTMENT OF HEALTH Pharmaceutical Procurement and Distribution Pharmaceutical Warehouse. DC Health Care Safety Net ALLIANCE PROGRAM
 DC DEPARTMENT OF HEALTH Pharmaceutical Warehouse DC Health Care Safety Net ALLIANCE PROGRAM OPERATIONAL PROTOCOLS Operational protocols for the DC Health Care Alliance program through the DOH Pharmaceutical
DC DEPARTMENT OF HEALTH Pharmaceutical Warehouse DC Health Care Safety Net ALLIANCE PROGRAM OPERATIONAL PROTOCOLS Operational protocols for the DC Health Care Alliance program through the DOH Pharmaceutical
Philosophy: Although sample medication may be helpful in patient care, drug samples:
 Title: Policy on Sample Medication Policy #: 450.80 Adopted: 1/90, 7/00 Revised: 3/95, 5/98, 7/00, 10/01 Reviewed: 7/00, 10/01, 02/03 This policy is applicable to the following Henry Ford Health System
Title: Policy on Sample Medication Policy #: 450.80 Adopted: 1/90, 7/00 Revised: 3/95, 5/98, 7/00, 10/01 Reviewed: 7/00, 10/01, 02/03 This policy is applicable to the following Henry Ford Health System
Michael D. Bullek BSP Rph. Commissioner, New Hampshire Board of Pharmacy
 Michael D. Bullek BSP Rph. Commissioner, New Hampshire Board of Pharmacy Legislative changes PDMP program changes 503B outsourcing facilities Rules changes Ph800 review Ph300 Ph400 Ph700 Current issues
Michael D. Bullek BSP Rph. Commissioner, New Hampshire Board of Pharmacy Legislative changes PDMP program changes 503B outsourcing facilities Rules changes Ph800 review Ph300 Ph400 Ph700 Current issues
Facilities Planning & Management Maintenance Repair Parts Policies & Procedures
 Facilities Planning & Management Policies & Procedures Revised 10/2013 Facilities Planning & Management Policies & Procedures PURCHASE REQUESTS cspolicy1.wpd 1-4 ISSUES FROM STOCK cspolicy2.wpd 5-7 MONTHLY
Facilities Planning & Management Policies & Procedures Revised 10/2013 Facilities Planning & Management Policies & Procedures PURCHASE REQUESTS cspolicy1.wpd 1-4 ISSUES FROM STOCK cspolicy2.wpd 5-7 MONTHLY
May 2010 Rhonda Dash, MPH Director, Research Compliance
 May 2010 Rhonda Dash, MPH Director, Research Compliance Introduce the new Director of Research Compliance Review Applicable Federal Regulations Review Applicable State Regulations Review UNTHSC new Policies
May 2010 Rhonda Dash, MPH Director, Research Compliance Introduce the new Director of Research Compliance Review Applicable Federal Regulations Review Applicable State Regulations Review UNTHSC new Policies
WellDyneRx Mail Service General Questions and Answers
 WellDyneRx Mail Service General Questions and Answers I. Location/ Hours of Operation 1. Where is WellDyneRx Mail Pharmacy located? WellDyneRx mail pharmacy has two locations: 1) Centennial, CO, a suburb
WellDyneRx Mail Service General Questions and Answers I. Location/ Hours of Operation 1. Where is WellDyneRx Mail Pharmacy located? WellDyneRx mail pharmacy has two locations: 1) Centennial, CO, a suburb
USE OF CONTROLLED SUBSTANCES AN OVERVIEW FOR RESEARCHERS
 USE OF CONTROLLED SUBSTANCES AN OVERVIEW FOR RESEARCHERS APPLICABILITY (WITH REGARD TO CONTROLLED SUBSTANCES) This presentation is applicable to: Animal and non-animal research This presentation is mostly
USE OF CONTROLLED SUBSTANCES AN OVERVIEW FOR RESEARCHERS APPLICABILITY (WITH REGARD TO CONTROLLED SUBSTANCES) This presentation is applicable to: Animal and non-animal research This presentation is mostly
16.19.10.11 PUBLIC HEALTH CLINICS: A. CLINIC LICENSURE: (1) All clinics where dangerous drugs are administered, distributed or dispensed shall obtain
 16.19.10.11 PUBLIC HEALTH CLINICS: A. CLINIC LICENSURE: (1) All clinics where dangerous drugs are administered, distributed or dispensed shall obtain a limited drug permit as described in Section 61-11-14
16.19.10.11 PUBLIC HEALTH CLINICS: A. CLINIC LICENSURE: (1) All clinics where dangerous drugs are administered, distributed or dispensed shall obtain a limited drug permit as described in Section 61-11-14
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES
 FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: April 2015 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: April 2015 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
Policies and Procedures. Number: 1127
 Policies and Procedures Title: NARCOTIC CONTROL: DOCUMENTATION AND COUNT Authorization: [X] Pharmacy/Nursing Committee [X] SHR Nursing Practice Committee Number: 1127 Source: Pharmacy/Nursing Cross Index:
Policies and Procedures Title: NARCOTIC CONTROL: DOCUMENTATION AND COUNT Authorization: [X] Pharmacy/Nursing Committee [X] SHR Nursing Practice Committee Number: 1127 Source: Pharmacy/Nursing Cross Index:
SURGICAL SERVICE SPECIALTY UROLOGY SURGICAL SPECIALTY
 DEPARTMENT OF THE AIR FORCE Headquarters US Air Force Washington, DC 20330-5000 QTP 4N1X1B-1 24 March 2015 SURGICAL SERVICE SPECIALTY UROLOGY SURGICAL SPECIALTY ACCESSIBILITY: Publications and forms are
DEPARTMENT OF THE AIR FORCE Headquarters US Air Force Washington, DC 20330-5000 QTP 4N1X1B-1 24 March 2015 SURGICAL SERVICE SPECIALTY UROLOGY SURGICAL SPECIALTY ACCESSIBILITY: Publications and forms are
Query and Reports Tutorial. Minnesota Board of Pharmacy Prescription Monitoring Program
 Minnesota Board of Pharmacy Prescription Monitoring Program August 2015 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 Accessing RxSentry... 3 Request an Account...
Minnesota Board of Pharmacy Prescription Monitoring Program August 2015 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 Accessing RxSentry... 3 Request an Account...
Harris CareTracker Training Tasks Workbook Clinical Today eprescribing Clinical Tool Bar Health History Panes Progress Notes
 Harris CareTracker Training Tasks Workbook Clinical Today eprescribing Clinical Tool Bar Health History Panes Progress Notes Practice Name: Name: / Date Started: Date : Clinical Implementation Specialist:
Harris CareTracker Training Tasks Workbook Clinical Today eprescribing Clinical Tool Bar Health History Panes Progress Notes Practice Name: Name: / Date Started: Date : Clinical Implementation Specialist:
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY. LCB File No. R047-15. September 15, 2015
 PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R047-15 September 15, 2015 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R047-15 September 15, 2015 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
COMPLIANCE WITH THIS PUBLICATION IS MANDATORY
 BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE INSTRUCTION 44-159 1 AUGUST 2000 Medical DEMAND REDUCTION PROGRAM COMPLIANCE WITH THIS PUBLICATION IS MANDATORY NOTICE: This publication is available
BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE INSTRUCTION 44-159 1 AUGUST 2000 Medical DEMAND REDUCTION PROGRAM COMPLIANCE WITH THIS PUBLICATION IS MANDATORY NOTICE: This publication is available
SAULT COLLEGE OF APPLIED ARTS AND TECHNOLOGY SAULT STE. MARIE, ONTARIO COURSE OUTLINE
 SAULT COLLEGE OF APPLIED ARTS AND TECHNOLOGY SAULT STE. MARIE, ONTARIO COURSE OUTLINE COURSE TITLE: Institutional Pharmacy Dispensing Lab CODE NO. : PTN302 SEMESTER: 3 PROGRAM: AUTHOR: Pharmacy Technician
SAULT COLLEGE OF APPLIED ARTS AND TECHNOLOGY SAULT STE. MARIE, ONTARIO COURSE OUTLINE COURSE TITLE: Institutional Pharmacy Dispensing Lab CODE NO. : PTN302 SEMESTER: 3 PROGRAM: AUTHOR: Pharmacy Technician
Electronic Medical Records (EMR) Centricity EMR 2005. ---------------- Updating the Patient Record
 Electronic Medical Records (EMR) Centricity EMR 2005 ---------------- Updating the Patient Record Table of Contents Introduction...1 The Chart Update...1 The Process...1 Accessing a Chart Update via your
Electronic Medical Records (EMR) Centricity EMR 2005 ---------------- Updating the Patient Record Table of Contents Introduction...1 The Chart Update...1 The Process...1 Accessing a Chart Update via your
Understanding Specialty Training Tracks
 Understanding Specialty Training Tracks The purpose of this lesson is for students to comprehend the use of CAP's specialty training tracks. Desired Learning Outcomes 1. Describe the organizational structure
Understanding Specialty Training Tracks The purpose of this lesson is for students to comprehend the use of CAP's specialty training tracks. Desired Learning Outcomes 1. Describe the organizational structure
SUMMARY OF CHANGES This revision aligns the instruction with AFPD 36-1, General Civilian Personnel Provisions and Authorities.
 Template modified: 27 May 1997 14:30 BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE PAMPHLET 36-106 20 DECEMBER 1993 Personnel SUPERVISOR S RECORDS NOTICE: This publication is available digitally
Template modified: 27 May 1997 14:30 BY ORDER OF THE SECRETARY OF THE AIR FORCE AIR FORCE PAMPHLET 36-106 20 DECEMBER 1993 Personnel SUPERVISOR S RECORDS NOTICE: This publication is available digitally
INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
 HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER MEDICATIONS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES APPROVED BY APPLIES
HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER MEDICATIONS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES APPROVED BY APPLIES
Inventory Management
 Inventory Management Chapter Outline Inventory Management Inventory Systems Computer & Inventory Ordering Forms Stocking & Storing Inventory Management Inventory A listing of medication of the goods or
Inventory Management Chapter Outline Inventory Management Inventory Systems Computer & Inventory Ordering Forms Stocking & Storing Inventory Management Inventory A listing of medication of the goods or
Consolidated Storage Program (CSP) Defense Property Accountability System (DPAS) Warehouse Module Reference Guide
 Consolidated Storage Program (CSP) Defense Property Accountability System (DPAS) Warehouse Module Reference Guide Table of Contents Purpose & Background... 3 Login to DPAS. 4 Individual Issue Process Member
Consolidated Storage Program (CSP) Defense Property Accountability System (DPAS) Warehouse Module Reference Guide Table of Contents Purpose & Background... 3 Login to DPAS. 4 Individual Issue Process Member
Managing Submissions via ExpressO: A Guide for Law Review Editors
 : A Guide for Law Review Editors Table of Contents List of Figures... 3 Welcome to ExpressO... 4 Contacting bepress Consulting Services... 4 Accessing ExpressO... 5 Editorial Privileges... 5 Editor Tools:
: A Guide for Law Review Editors Table of Contents List of Figures... 3 Welcome to ExpressO... 4 Contacting bepress Consulting Services... 4 Accessing ExpressO... 5 Editorial Privileges... 5 Editor Tools:
Texas Pharmacy Association's Annual Meeting 8/1/2014. Texas State Board of Pharmacy 1. Goals. Board of Pharmacy Update. Board of Pharmacy Members
 Board of Pharmacy Update Gay Dodson, R.Ph. Executive Director/Secretary Texas Pharmacy Association s Annual San Marcos August 1, 2014 2 Goals Review recent changes to pharmacy rules; Talk about some issues
Board of Pharmacy Update Gay Dodson, R.Ph. Executive Director/Secretary Texas Pharmacy Association s Annual San Marcos August 1, 2014 2 Goals Review recent changes to pharmacy rules; Talk about some issues
