Psoriatic Arthritis in Iceland
|
|
|
- Shanon Rich
- 10 years ago
- Views:
Transcription
1 Psoriatic Arthritis in Iceland a study of the population of Reykjavik Þorvarður Jón Löve Thesis for the degree of Philosophiae Doctor University of Iceland School of Health Sciences Faculty of Medicine March 2013
2
3 Psoriatic Arthritis in Iceland a study of the population of Reykjavik Þorvarður Jón Löve Thesis for the degree of Philosophiae Doctor Supervisor: Björn Guðbjörnsson Doctoral committee: Bárður Sigurgeirsson Helgi Jónsson Helgi Valdimarsson Kristján Erlendsson University of Iceland School of Health Sciences Faculty of Medicine March 2013
4
5 Sóragigt á Íslandi rannsókn á íbúum Reykjavíkur Þorvarður Jón Löve Ritgerð til doktorsgráðu Umsjónarkennari: Björn Guðbjörnsson Doktorsnefnd: Bárður Sigurgeirsson Helgi Jónsson Helgi Valdimarsson Kristján Erlendsson Háskóli Íslands Heilbrigðisvísindasvið Læknadeild Mars 2013
6 Thesis for a doctoral degree at the University of Iceland. All right reserved. No part of this publication may be reproduced in any form without the prior permission of the copyright holder. Þorvarður Jón Löve 2013 ISBN Printing by Leturprent Reykjavík, Iceland 2013
7 Ágrip Sóragigt er sjúkdómur sem veldur bólgu og verkjum í liðum og festum. Hún er nátengd húðsjúkdómnum sóra eins og nafnið gefur til kynna. Undanfarinn áratug hefur orðið hraður vöxtur í fjölda birtra rannsókna um sóragigt, og hluti þeirra rannsókna hefur farið fram á Íslandi. Markmið þessarar rannsóknar var að finna flesta og helst alla sjúklinga með þegar greinda sóragigt á Íslandi til þess að meta algengi sjúkdómsins, birtingarform hans, erfðir og möguleg tengsl naglbreytinga við önnur sjúkdómseinkenni. Leitað var að sjúklingum á landinu öllu, en aðeins sá hluti Íslendinga sem býr á höfuðborgarsvæðinu var lagður til grundvallar algengistölum. Notast var við lista frá fyrri rannsóknum á húðsjúkdómnum sóra til þess að finna sjúklinga með sóragigt, en einnig var leitað í skrám Landspítala. Öllum sjúklingum var boðið til skoðunar og 71% þeirra sem bjuggu á höfðuborgarsvæðinu tóku þátt. Til þess að svara rannsóknarspurningum varðandi naglbreytingar voru gögn um sóragigtarsjúklinga sem safnað var í þessari rannsókn borin saman við gögn um sömu sjúklinga frá þeim tíma sem þeir tóku þátt í rannsókn á húðsjúkdómnum sóra. Alls fundust upplýsingar um 346 einstaklinga með greinda sóragigt, en af þeim voru 301 lifandi með búsetu á Íslandi, og 220 þeirra bjuggu á höfuðborgarsvæðinu árið Til samanburðar voru upplýsingar um 1116 einstaklinga með sóra. Algengi sóragigtar byggt á skráðum greiningum var 0.16%, en 0.14% ef leiðrétt var fyrir þeim hluta sem ekki var hægt að staðfesta sjúkdóminn hjá við skoðun. Nær tvöfalt fleiri konur en karlar voru með sóragigt. Ættingjar sóragigtarsjúklinga voru með hækkaða hlutfallsáhættu (RR) á að fá sjúkdóminn sem var 39.2, 12.2, 3.6, og 2.6 fyrir fyrstu til fjórðu gráðu ættingja (p<0.0001). Naglbreytingar voru algengari í sóragigt en í sóra, og greining á þremur helstu undirflokkum naglbreytinga leiddi í ljós að eingöngu nagllos hefur klíníska þýðingu í sóragigt, með gagnlíkindahlutfall (OR) 2.1 fyrir sóragigt á meðal sórasjúklinga, OR 3.8 fyrir smáliðasjúkdóm í sóragigt og jákvæða fylgni milli naglloss og fjölda aumra liða (p=0.02). Sóragigt er álíka algeng á Íslandi og í nágrannalöndum okkar og er mjög ættlæg yfir marga ættliði. Nagllos er mun algengara meðal sóragigtarsjúklinga en sórasjúklinga, tengist smáliðasjúkdómi sérstaklega og sóragigtarsjúklingar með nagllos hafa fleiri auma liði. Lykilorð: Sóragigt, sóri, algengi, ættgengi, nagllos. 3
8 4
9 Abstract Psoriatic arthritis causes inflammation and pain in joints and entheses. It is closely linked to the skin disease psoriasis as its name suggests. Over the past decade the number of published research papers on psoriatic arthritis has grown rapidly, and some of those studies have been performed in Iceland. The goal of this study was to find most or all patients in Iceland with previously diagnosed psoriatic arthritis to study the prevalence, clinical presentation, inheritance, and the possible relationship between nail changes and other clinical symptoms. Although cases were sought from all of Iceland, only those living in the Reykjavik area were included in prevalence estimates. All patients were invited for a physical examination. Information on psoriasis patients collected for previous studies was used to find patients with psoriatic arthritis, and a search of the records of Landspitali hospital was performed as well. Data on psoriatic arthritis patients collected for this study were compared to data previously collected on psoriasis patients to analyze the connection between nail changes and clinical symptoms in psoriatic arthritis. Information on 346 individuals with diagnosed psoriatic arthritis was found, of whom 301 were alive in 2003, with 220 living in the Reykjavik area; 71% of them participated in the physical examination portion of the study. Information on 1116 psoriasis patients was available for comparison. The prevalence of diagnosed psoriatic arthritis was 0.16%, but 0.14% when the proportion of individuals without confirmable disease at examination was taken into account. Nearly twice as many women as men had the disease. Family members of psoriatic arthritis patients had a relative risk (RR) of 39.2, 12.2, 3.6, and 2.6 for having the disease for first- to fourth degree relatives (p<0.0001). Nail changes were more common in psoriatic arthritis than psoriasis and only onycholysis was clinically important in psoriatic arthritis, with an odds ratio (OR) of 2.1 for developing arthritis among psoriasis patients, OR of 3.8 for having small joint involvement among psoriatic arthritis patients, and a positive correlation between the presence of onycholysis and the number of tender joints (p=0.02) Psoriatic arthritis has a similar prevalence in Iceland as in neighbouring countries and is very familial over multiple meioses. Onycholysis is more prevalent in psoriatic arthritis than in psoriasis, is linked to small joint disease, and correlates with having more tender joints. Keywords: Psoriatic arthritis, psoriasis, prevalence, heritability, onycholysis. 5
10 6
11 Acknowledgements To Guðrún Inga, the love of my life, without whose support this thesis would not have been possible, and to our perfect twins Jakob Rafn and Tómas Karl This thesis is the result of a decade of collaboration, at times across the Atlantic involving people in Ann Arbor, Boston, and Reykjavik. I owe a debt of gratitude to many who supported me, guided me, and challenged me to continue, particularly when time and other resources were in short supply. First, I would like to thank professor emeritus Helgi Valdimarsson for generously replying, when I came to him for career advice in 2002: Come work for me. He proceeded to introduce me to the world of psoriasis research, and then suggested that I focus on psoriatic arthritis research. I am forever grateful for the opportunity he provided, and for his continuing mentorship ever since. Professor Björn Guðbjörnsson has been instrumental in my work, providing guidance, encouragement, and much needed resources. His continuing faith in the importance of this project has been crucial to its completion and I look forward to future collaboration. Assistant professor Jóhann Elí Guðjónsson was the fellow I replaced at the Helgi s lab in 2002, when he went off to Ann Arbor. He showed me the ropes, lent me his books, and was always inviting and helpful when I got stuck in the early stages of the project. I am also grateful for the use of all of the rich data he collected in the years before I joined the group. To my teachers of statistics, epidemiology and genetics at the Harvard School of Public Health and Harvard Medical School, I owe thanks for broadening my horizon and for providing feedback on proposed projects, large and small. Special thanks go to my mentor, Elizabeth Karlsson and my training program director Simon Helfgott for providing me with time and resources to pursue research and a master s degree during my time at Harvard. Sincere thanks go to my thesis committee for their contribution, and in particular to Kristján Erlendsson who protected me from distractions that might have delayed this thesis. This work was supported by the Department of Immunology and the Centre for Rheumatology Research, both at Landspitali University Hospital. I am also grateful for the expertise and data access provided by decode genetics for the analysis of familiality, and the work of Ari Kárason. Finally, I want to thank my mother Margrét Jónsdóttir for all her support, love, and generosity, my brother Karl, my sister Laufey, and my late father Jakob Löve for igniting within me a curiosity for the wonders of the natural world. 7
12 Contents Ágrip... 3 Abstract... 5 Acknowledgements... 7 Contents... 8 List of abbreviations List of figures List of tables List of papers Declaration of contribution Introduction Psoriatic arthritis Pathology Familiality Genetics Clinical features Clinical subgroups Peripheral disease Axial disease Enthesitis Arthritis mutilans Nail disease Other features Disease activity Diagnosis Treatment Summary Aims
13 3 Materials and methods Study population Statistical analysis Data analysis section from paper I Data analysis section from paper II Assessment of inheritance Kinship coefficients RR Data analysis section from paper III Data analysis section from paper IV Results Study group Prevalence Demographics and disease activity Disease course and treatment Familiality Nail disease and small joint involvement Onycholysis and arthritis in psoriasis Discussion Study group Prevalence Demographics and disease activity Disease course and treatment Familiality Nail disease and small joint involvement Onycholysis and arthritis in psoriasis Conclusions References Original publications
14 10
15 List of abbreviations accp... anti-cyclic Citrullinated Peptide CASPAR... ClASsification criteria for Psoriatic ARthritis CI... Confidence Interval CLA... Cutaneous Lymphocyte Antigen DIP... Distal InterPhalangeal DMARD... Disease-Modifying AntiRheumatic Drug DMD... Disease Modifying Drug EULAR... European League Against Rheumatism FACIT... Functional Assessment of Chronic Illness Therapy FDR... First Degree Relative GWAS... Genome Wide Association Study HAQ... Health Assessment Questionnaire HLA... Heuman Leukocyte Antigen IF... InterFeron IL... InterLeukin IQR... Inter-Quartile Range KC... Kinship Coefficient KFSS... Krupp s Fatigue Severity Scale LDI... Leeds Dactylitis Index LEI... Leeds Enthesitis Index MCP... MetaCarpoPhalangeal MHC... Major Histocompatibility Complex mnapsi... modified Nail Psoriasis Severity Index MRI... Magnetic Resonance Imaging ND... Not Determined during OMERACT 8 discussions NSAIDS... Non-Steroidal Anti-Inflammatory Drugs OMERACT... Outcome Measures in Rheumatoid Arthritis Clinical Trials OR... Odds Ratio PASI... Psoriasis Area and Severity Index PIP... Proximal InterPhalangeal PsAQoL... Psoriatic Arthritis Quality of Life Index RR... Relative Risk SCID... Severe Combined Immune Deficiency SF36... Medical Outcomes Study 36-Item Short-Form Health Survey SNP... Single Nucleotide Polymorphism TNF... Tumor Necrosis Factor VAS... Visual Analog Scale VIF... Variance Inflation Factor 11
16 12
17 List of figures Figure 1 - The number of articles on psoriatic arthritis in PubMed from Following a stable period from there has been a rapid increase in the number of publications. (Corlan, 2011) Figure 2 - The left panel shows the tortuous pattern associated with psoriatic synovium and the right panel shows the straight, regular hypervascularity of rheumatoid arthritis. Arthroscopic images reproduced with the kind permission of Dr. Douglas Veale (Reece et al., 1999) Figure 3 - Fluorescent optical images of the hands of patients with arthritis. (Image reproduced with the kind permission of Dr. Werner (Werner, et al., 2010)) Figure 4 - From left: Onycholysis, pitting, and subungual hyperkeratosis. Images kindly provided by Jóhann Elí Guðjónsson Figure 5 - Fluorescent optical image showing dactylitis of the fourth digit of the left hand involving the MCP, PIP, and DIP joints simultaneously as well as the soft tissues between them. (Image reproduced with the kind permission of Dr. Werner (Werner et al., 2010)) Figure 6 - The CASPAR criteria. (Taylor et al., 2006). Current psoriasis is assigned a score of 2; all other features are assigned a score of Figure 7 - The study group was formed by combining two data sources with dissimilar backgrounds to ensure maximum coverage. Only patients who had died before the study began were excluded from this list Figure 8 - Selection process for inclusion in study group (Love et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology Figure 9 - Prevalence of psoriatic arthritis by age and gender (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology Figure 10 - Pedigree of a family where psoriasis and psoriatic arthritis are common. White indicates healthy individual, black psoriasis and blue psoriatic arthritis. Disease status for earlier generations is not known Figure 11 - Kinship coefficients (KCs) for the risk of developing psoriatic arthritis. For excluded meioses 0-7 the p-value is <
18 14
19 List of tables Table 1 - Domains for assessment of psoriatic arthritis and preferred methods for assessing each domain. Core domains as defined by OMERACT 8 are in boldface Table 2 Demographic information on 131 patients included in the study. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology Table 3 - Frequency and severity of skin and joint involvement. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology Table 4 - Subcategories of PsA at disease onset and at the time of evaluation. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology Table 5 - Relative risk (RR) estimates for having psoriatic arthritis over five generations. (Karason et al., 2009) Reprinted with the kind permission of Rheumatology (Oxford) Table 6 - Clinical characteristics of 154 patients with psoriatic arthritis. (Love et al., 2010) Reprinted with the kind permission of the Scandinavian Journal of Rheumatology Table 7 - Predictors of small joint involvement presented as odds ratios with 95% confidence intervals. (Love, et al., 2010) Reprinted with the kind permission of the Scandinavian Journal of Rheumatology Table 8 - Baseline characteristics of the study group. (Love, et al., 2012) Reprinted with the kind permission of the Journal of Rheumatology Table 9 - Results of the multivariate regression model. (Love, Gudjonsson, et al., 2012) Reprinted with the kind permission of the Journal of Rheumatology Table 10 - Univariate analysis of disease and treatment characteristics of 139 psoriatic arthritis patients at followup. Results of the chi-square test for DMARD use and t test for all other variables, presented as mean (95% CI). (Love, et al., 2012). Reprinted with the kind permission of the Journal of Rheumatology Table 11 - Results of 3 multivariate linear regression analyses showing the association of age, sex, and subtypes of nail changes with each of 3 disease activity measures. Regression results are presented as -coefficient (95% CI). (Love, et al., 2012). Reprinted with the kind permission of the Journal of Rheumatology
20 16
21 List of papers This thesis is based on the following original publications, which are referred to in the text by their Roman numerals (I-IV) as needed: I. Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2007). Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. The Journal of Rheumatology, 34(10), II. Karason, A., Love, T., & Gudbjornsson, B. (2009). A strong heritability of psoriatic arthritis over four generations - the Reykjavik Psoriatic Arthritis Study. Rheumatology (Oxford, England), 48(11), III. Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2010). Small joint involvement in psoriatic arthritis is associated with onycholysis: the Reykjavik Psoriatic Arthritis Study. Scandinavian Journal of Rheumatology, 34(10), IV. Love, T. J., Gudjonsson, J. E., Valdimarsson, H., & Gudbjornsson, B. (2012). Psoriatic Arthritis and Onycholysis - Results from the Cross-sectional Reykjavik Psoriatic Arthritis Study. The Journal of Rheumatology, 39(7), In addition, some unpublished data may be presented. All papers are reprinted by kind permission of the publishers. 17
22 18
23 Declaration of contribution I, Þorvarður Jón Löve [Thorvardur Jon Love], declare that I have participated in the planning and execution of all of the research described in this thesis, unless indicated by references to the work of others. Specifically, I contributed to the published papers in the following manner: Paper I: Paper II: Paper III: Paper IV: Planning, data collection, statistical analysis, writing, editing Planning, data collection, writing, editing Planning, data collection, statistical analysis, writing, editing Planning, data collection, statistical analysis, writing, editing 19
24 20
25 Number of indexed articles 1 Introduction Autoimmune diseases are a group of over 80 diseases that the National Institutes of Health in the United States estimates affect 5% - 8% of the population of that country (Autoimmune Diseases Coordinating Committee, 2005). Simple extrapolation suggests that as many as 520 million people around the world may suffer from autoimmune diseases at any given time. Most are chronic conditions, with no known cure. However, over the past two decades new medications have emerged that hold The growing literature on psoriatic arthritis Year Figure 1 - The number of articles on psoriatic arthritis in PubMed from Following a stable period from there has been a rapid increase in the number of publications. (Corlan, 2011). the promise of a better life for patients suffering from many of these conditions. This progress in the field of treatment has invigorated clinical research into autoimmune diseases, with increased funds becoming available and previously neglected diseases receiving appropriate attention. Psoriatic arthritis is an example of a disease which had received little attention for many years, but has seen exponential growth in interest and activity in the first decade of the twenty first century, as illustrated in figure 1 This thesis summarizes the current literature on the clinical features, diagnosis, treatment, and epidemiology of psoriatic arthritis, and then describes research on psoriatic arthritis performed from in the setting of the general population of Reykjavik, Iceland and subsequently published in peer reviewed journals, resulting in new insights regarding the prevalence, familiality, and clinical features of psoriatic arthritis. 1.1 Psoriatic arthritis Psoriasis is one of the most common autoimmune diseases. Its prevalence varies between societies from around 0% in Samoa to 11.8% in Arctic-Kasach'ye (Christophers, 2001). In a recent review it was reported that the prevalence among adults ranged from 0.91% to 8.5% in 18 studies (Parisi et al., 2012). Interestingly, half of the 12 studies based on self-report 21
26 reported a prevalence of 3% or higher, while four of six studies based on records and/or physical examination found the prevalence was <2% (Parisi, et al., 2012). Based on these data it seems reasonable to conclude that the prevalence of psoriasis is 1%-3% in the general population, and that less than 2% of the adult population has been diagnosed with psoriasis in Northern European countries such as Norway and the United Kingdom. Psoriasis is a T-cell-mediated, chronic, autoimmune, inflammatory skin disease that results in skin thickening, erythema, and scaling, often resulting in pruritus (Nestle et al., 2009). Even when limited to the skin it causes a significant reduction in quality of life, with physical and mental functioning reduced similarly to that seen in cancer, hypertension, heart disease, diabetes, and depression (Rapp et al., 1999). It is furthermore associated with the metabolic syndrome, hypertension and diabetes, and severe psoriasis is associated with an increased risk of myocardial infarction (Gelfand et al., 2006; Love et al., 2011; Qureshi et al., 2009), a risk that may be mitigated with systemic treatment, although conflicting results have been reported (Abuabara et al., 2011; Ahlehoff et al., 2012). Psoriasis is considered a skin disease and is not known to directly involve organs other than the skin and nails, with one exception: arthritis. Psoriatic arthritis is an inflammatory arthritis present in 6.25% - 48% of psoriasis patients (Gladman, 2009; Reich et al., 2009). In studies conducted prior to 2000 it was found to affect 0.02% % of the general population (Hellgren, 1969; Lomholt, 1963; van Romunde et al., 1984). However, more recent studies have placed the prevalence between 0.06% and 0.25%, and in all studies published in 2005 or later the prevalence has been found to be higher than 0.15% (De Angelis et al., 2007; Gelfand et al., 2005; Madland et al., 2005; Pedersen et al., 2008; Reich, et al., 2009; Trontzas et al., 2005; Wilson et al., 2009b). Reasons for this increased prevalence have not been pursued directly, but it has been suggested that they may include increased detection of psoriatic arthritis, differences in case definition, and study design (Setty & Choi, 2007). It should be noted that all of the population based studies are based on diagnosed psoriatic arthritis only, and the actual prevalence is no doubt higher, as some cases are never diagnosed by a physician. However, it is unlikely that more accurate figures will become available as it is not feasible to perform population based studies with screening using interviews, physical examination and imaging studies for a disease as rare as psoriatic arthritis. In addition to prevalence studies there have been attempts to define the incidence of psoriatic arthritis, both among psoriasis patients and in the general population. These studies suggest an incidence ranging from 0.1 to 23.1 per 100,000 in the general population 22
27 (Alamanos et al., 2003; Hukuda et al., 2001; Kaipiainen-Seppänen, 1996; Savolainen et al., 2003; Shbeeb et al., 2000; Söderlin et al., 2002), with four of the six studies reporting a range from new cases per 100,000 individuals annually. Most studies have shown an equal gender distribution among both psoriasis and psoriatic arthritis, with some notable exceptions as in Sweden where the male/female ratio was found to be 0.4 (Söderlin, et al., 2002). However, the general consensus is that there is no clear gender predilection in this disease (Gladman, 2009; Setty & Choi, 2007). Psoriatic arthritis causes substantial additional morbidity compared to that described among psoriasis patients (Rapp, et al., 1999; Zachariae et al., 2002). It has long been held that psoriatic arthritis is associated with an increased standardized mortality rate (Wong et al., 1997) although a recent study did not replicate this finding (Buckley et al., 2010). Data have not conclusively supported an increased risk of cardiovascular disease so far, although cross sectional data and the results of a sub-analysis in a study of psoriasis suggest that this is the case (Gladman et al., 2009; Han et al., 2006; Li et al., 2012). Thus, psoriatic arthritis is a severe, fairly common autoimmune disease with significant comorbidities. 1.2 Pathology Psoriasis is a T-cell driven disease that causes epidermal hyperplasia, with an influx of autoimmune effector cells including monocytes, neutrophils, and dendritic cells (Nestle, et al., 2009). Lymphocytes migrate to the skin via the cutaneous lymphocyte antigen (CLA), a process that may be enhanced by expression of HLA Cw0602 (Johnston et al., 2004). Erythema is also an important feature of psoriasis and is caused by increased growth and dilation of superficial blood vessels in the skin (Nestle, et al., 2009). Joint and synovial findings in psoriatic arthritis are T-cell driven as in the skin, and the prominent hyperplasia of blood vessels in skin is echoed in the synovium (Fearon & Veale, 2001). As in rheumatoid arthritis, psoriatic arthritis causes hyper proliferation of the lining cells of the synovium, resulting in the formation of an invasive, inflammatory tissue mass called pannus (Anandarajah & Ritchlin, 2004). However, some pathological features differentiate psoriatic arthritis clearly from rheumatoid arthritis, the most prominent being the distinct patterns of hyper vascularity seen in these two diseases. While the vascular growth in rheumatoid arthritis is very regular, in psoriatic arthritis the vessels are quite tortuous and chaotic, a difference clearly visible arthroscopically as demonstrated in figure 2 (Reece, et al., 1999). 23
28 Figure 2 - The left panel shows the tortuous pattern associated with psoriatic synovium and the right panel shows the straight, regular hypervascularity of rheumatoid arthritis. Arthroscopic images reproduced with the kind permission of Dr. Douglas Veale (Reece et al., 1999). The end result of pannus formation in rheumatoid arthritis is bony erosions, and these are present in psoriatic arthritis as well. However, the similarities end there. Psoriatic arthritis is associated with the presence of simultaneous bony proliferation and destructive change at the affected joint. The pencil-in-cup deformity is considered pathognomic, and involves a whittling down to a narrow tip of the distal end of a phalangeal bone, while the base of the adjacent phalanx flares out to form a cup-like structure, usually at the distal interphalangeal joint (DIP), a joint rarely if ever involved in rheumatoid arthritis (Wassenberg et al., 2001). While rheumatoid arthritis and psoriatic arthritis are both T-cell driven diseases, the T-cell subsets observed are not the same. In both psoriatic skin and synovium CD8+ cells predominate, while CD4+ cells are more common in rheumatoid arthritis (Reece, et al., 1999). Recent studies suggest that CD8+ T cells play a pivotal role in the pathogenesis of psoriasis, as the great majority of lesional T-cells are CD8+ and psoriasis lesions do not develop when CD8+ cell migration from the dermis into the epidermis is inhibited. Most recently, the important role played by the Th-17 subset of CD4+ T-cells in psoriatic disease has become apparent (Lowes et al., 2008), suggesting that the a role for the Th-17/IL23 axis is a potential target for treatment of psoriatic disease (Maeda et al., 2012). Indeed, it appears that a recently developed monoclonal antibody that blocks interleukin 12 and 23 through their common p40 subunit (ustekinumab) is very effective for psoriasis (Griffiths et al., 2010), effective in psoriatic arthritis (Gottlieb et al., 2009), but is not being tested further as a treatment for rheumatoid arthritis. Direct blocking of IL17 (ixekizumab) and its receptor (brodalumab) have been tested and appear promising in the treatment of psoriasis (Leonardi et al., 2012; Papp et al., 2012), but no studies for psoriatic arthritis are available. 24
29 Psoriatic arthritis is clearly a T-cell driven autoimmune disease, and a host of cytokines is produced, including tumor necrosis factor (TNF) alpha, interferon (IF) gamma, interleukin (IL) 1, IL 8, IL 10, and vascular growth factors, but the only synovial fluid marker shown to have an association with diseases activity on physical examination is the expression of IL23A mrna (Celis et al., 2012). In summary, the pathology of psoriatic arthritis is that of a T-cell driven autoimmune response leading to a complex cascade of cytokine production which most closely resembles that seen in psoriatic skin but is clearly different from rheumatoid arthritis. The importance of these distinctive features in the pathogenesis of psoriatic arthritis is supported by the discovery of treatments that specifically target psoriatic disease. 1.3 Familiality Psoriatic arthritis is a familial disease. Two proband-based studies have reported a lambda-s (also known as K-factor) of , suggesting that first degree relatives of patients with psoriatic arthritis have a nearly 50-fold risk of developing the disease compared to the general population (Moll & Wright, 1973a; Myers et al., 2005). However, these studies are limited by their use of lambda-s, lambda-o, lambda-p or lambda-1 (the Sibling, Offspring, Parent, or 1 st degree relative recurrence risk ratio, respectively) as a surrogate for lambda-r (the true relative recurrence risk ratio). This approach is problematic, as ascertainment bias and over reporting can lead to substantial inflation of these recurrence risk ratios, an effect that is more pronounced in rare conditions (Guo, 1998). Furthermore, proband based studies of familiality are generally restricted to 1 st degree relatives, as proband reports regarding the disease status of more distant relatives are inherently less reliable. In addition to these shortcomings of the proband method, it relies on the prevalence of the disease in the underlying population being known, and is sensitive to this information being correct (Guo, 1998). None of the previously available studies of the familiality of psoriatic arthritis have been performed in populations where the prevalence of psoriatic arthritis was known. Therefore, there exists a need for more accurate estimates of the familiality of psoriatic arthritis, and no information on the risk in other than 1 st degree relatives has previously been made available. 1.4 Genetics There have been many attempts at linking psoriatic arthritis to specific HLA genes, and studies have shown that there is a strong association with HLA B27 and HLA-Cw06, but also with HLA-B13, HLA-B38/39, HLA-B57, and HLA-DRB1. However, it is not clear how 25
30 many of these associations are independent, and how many are a result of linkage disequilibrium in the MHC region (Rahman & Elder, 2012). Recent data suggest that the association between HLA-Cw0602 and PsA may be misleading, as it confers a high risk for psoriasis, but is protective when looking at the risk for developing arthritis after psoriasis has developed (L. Eder et al., 2012). In those PsA patients who do have HLA-Cw06 it is associated with an increased lag from the onset of skin disease to the onset of arthritis as well as a mild form of psoriatic arthritis (Ho et al., 2007; Queiro et al., 2006). Finally, while nail changes in patients with psoriasis are associated with an increased risk of developing psoriatic arthritis (Wilson et al., 2009a), patients carrying HLA- Cw06 are less likely to have nail changes (Gudjonsson et al., 2006). This unexpected pattern of associations clearly illustrates the need to study psoriatic arthritis separately from psoriasis, both among psoriasis patients and outside of psoriasis populations, rather than considering the arthritis as an extension, or severe form, of the skin disease. In addition to HLA genes, psoriatic arthritis has been linked to several other changes in the genome. An area on the arm of chromosome 16q was among the first to show an association with the disease (Karason et al., 2003), and genome wide single nucleotide polymorphism studies have since identified variants in IL23R, IL12B, TRAF3IP2, TNIP1, and TNFAIP3 that are associated with psoriatic arthritis (Bowes & Barton, 2010; Ellinghaus et al., 2010; Hüffmeier, Uebe, et al., 2010; Stuart et al., 2010; Tejasvi et al., 2012). Most recently a metaanalysis of PsA cases extracted from published genome wide association studies (GWAS) found an association near the rel-locus on chromosome 2p16 (Hüffmeier, et al., 2010). The associations found with IL23R and TNFAIP3 are particularly interesting given the association of psoriatic disease with TH17 lymphocytes, and the success of ustekinumab, an IL23 inhibitor, in suppressing psoriasis (Gottlieb, et al., 2009). At least three separate consortia, one based in the United States and two in Europe, are performing large genome wide association studies of psoriatic arthritis, and results should become available soon. To date the only genetic difference between psoriasis patients and psoriatic arthritis patients detected outside of the HLA region is a deletion in the epidermal differentiation complex associated with skin diseases but not arthritis (de Cid et al., 2009; Hüffmeier, Estivill, et al., 2010). In a candidate gene study of 41 known rheumatoid arthritis susceptibility genes only one single nucleotide polymorphism (SNP) was associated with PsA, but while it conferred an increased risk for rheumatoid arthritis it was protective for the development of psoriatic arthritis (Bowes et al., 2012). 26
31 1.5 Clinical features The hallmark clinical feature of psoriatic arthritis is the inflamed synovial joint, a symptom shared by all inflammatory arthritidies. However, the disease has other features, all linked by the theme of inflammation. Some of these help differentiate psoriatic arthritis from rheumatoid arthritis, and many are shared by a group of diseases called the spondyloarthritides. While most patients with psoriatic arthritis develop the disease after the skin disease, close to 20% develop arthritis before any skin disease is evident (Gladman et al., 1987; Olivieri et al., 2009; Scarpa et al., 1984). Indeed, it has been suggested that there exists a subset of psoriatic arthritis patients with no skin disease, psoriatic arthritis sine psoriasis, which can be identified using family history, clinical, and genetic features. It is possible that some of these patients will never develop the skin disease (Olivieri, et al., 2009). Spondylitis, dactylitis, enthesitis and uveitis are all well recognized features of psoriatic arthritis (Gladman, 1998), and in some patients back pain or enthesitis may predominate or even be the only symptom apart from skin disease (Salvarani et al., 1997). The non-articular features are shared with other arthritides such as reactive arthritis, inflammatory bowel disease associated arthritis and ankylosing spondylitis (Gladman, 1998). These diseases form a group named the spondyloarthritides, lumped together because of their similarities, but each has distinguishing features. In the case of psoriatic arthritis, the presence of psoriasis in the patient or a close relative distinguishes it from the other spondyloarthritides Clinical subgroups Although the concept of psoriatic arthritis was known from the early 19 th century, classification of psoriatic arthritis into clinical subsets was first described by Moll and Wright (Moll & Wright, 1973b). They described five subgroups: DIP arthritis, asymmetric oligoarthritis, symmetric polyarthritis, arthritis mutilans, and axial disease. However, later work suggests that the most useful clinical subclasses of psoriatic arthritis are peripheral, axial, entheseal, and mutilating disease as described in detail below. It should be noted that these clinical subgroups are not mutually exclusive, so an individual patient may have symptoms consistent with one or more of them at any given time. 27
32 Peripheral disease Inflammation of large or small synovial joints outside of the axial skeleton in psoriatic arthritis is known as peripheral arthritis and affected 86% of patients in a recent study, making it by far the most common form of psoriatic arthritis (Wilson, et al., 2009b). Peripheral arthritis is the inflammation of the synovium of a joint resulting in swelling, which may be visible or palpable. There is no difference in the clinical appearance of an inflamed peripheral joint in psoriatic arthritis compared to other inflammatory arthritides. However, the joint distribution can provide a clue to the underlying disease, as distal interphalangeal joints (DIP) are rarely swollen in patients with rheumatoid arthritis, while they may be involved in psoriatic arthritis. In their groundbreaking work on psoriatic arthritis, Moll and Wright suggested separate subcategories within peripheral arthritis for symmetric and asymmetric arthritis (Moll & Wright, 1973b). It has since been shown mathematically that the number of involved joints directly affects perceived symmetry (Helliwell et al., 2000). Once this has been taken into account and the data re-examined it has been demonstrated that even in the data presented by Moll and Wright the predominant subgroup was symmetric arthritis or 56% and 84%, rather than the 15% reported in their classic paper, and these revised figures are in line with subsequent series (Helliwell, 2009). Although oligoarthritis and polyarthritis are sometimes still considered separately in psoriatic arthritis, usually with the fifth involved joint marking polyarthritis, recent data indicate that this is not a helpful way of dividing peripheral psoriatic arthritis into further subgroups (Helliwell et al., 2007) Axial disease Inflammation involving the axial skeleton is known as spondylitis or spondyloarthritis. The prototypical disease in this group is ankylosing spondylitis, and the spondyloarthritides draw their name from this presentation of the disease. However, the prevalence of this disease manifestation varies between the individual spondyloarthritides, and in the case of psoriatic arthritis 2-5% have exclusively axial disease while up to 40% have some evidence of spinal involvement (Gladman, 1998). The spondyloarthritis seen in psoriatic arthritis is less symmetrical than in ankylosing spondylitis, marginal and non- marginal syndesmophytes are present rather than strictly marginal as in ankylosing spondylitis, and the disease may skip over vertebrae in psoriatic arthritis. It is considered less severe than the form seen in ankylosing spondylitis (Gladman, 1998). 28
33 Enthesitis Some authors suggest that entheseal involvement is the root pathological mechanism in psoriatic arthritis, and that the observed synovial inflammation is a spillover of inflammation from primary inflammatory involvement of entheseal structures (Benjamin & McGonagle, 2009; McGonagle et al., 2007). Whether that is the case remains to be elucidated, but regardless it is clear that entheseal involvement is often a prominent feature of psoriatic arthritis, and may be the only form of musculoskeletal inflammatory involvement in some patients (Salvarani, et al., 1997). Because enthesitis is difficult to diagnose clinically, it is not as clearly defined as other forms of psoriatic arthritis. However, entheseal and tenosynovial patterns of inflammation seen on magnetic resonance imaging (MRI) may help differentiate between psoriatic arthritis and rheumatoid arthritis (Narváez et al., 2012). It appears that entheseal involvement detected by ultrasound is common among patients with psoriasis as they have an OR of 2.6 for having enthesitis when compared to individuals with other skin diseases. (Naredo et al., 2011). MRI and ultrasound are costly and/or operator dependent Figure 3 - Fluorescent optical images of the hands of patients with arthritis. Upper left: Patient with psoriatic arthritis showing tracking of inflammation along tendon lines with inflammation of DIP and PIP joints. Upper Right: Patient with rheumatoid arthritis showing inflammation mostly confined to individual joints. Bottom: Inflammation in a triangular pattern directly proximal to the fingernails characteristic of psoriatic arthritis, known as the Werner sign. (Image reproduced with the kind permission of Dr. Werner (Werner, et al., 2010)) 29
34 imaging techniques, and therefore the recent introduction of imaging of inflammation using fluorescent dyes shown in figure 3 may help diagnose and evaluate patients with psoriatic arthritis more efficiently and accurately in the future (Werner, et al., 2010) Arthritis mutilans Mutilating arthritis is the least common form of psoriatic arthritis, but clearly the most severe. Recent data from the Nordic countries suggests it may be as rare as 4.5 per 1,000,000 inhabitants (Gudbjornsson et al., 2012). Its features are so striking that although it involves only peripheral joints it is considered a separate clinical subgroup from regular peripheral arthritis. It is a form of arthritis where there is, often rapid, destruction of all bony tissue in a joint or even an entire digit. As a result the affected digit retracts in the same way a telescope would, leading to the descriptive term digital telescoping (Moll & Wright, 1973b). The prevalence of this condition is not clear but it may affect up to 5% of patients with psoriatic arthritis, while this number must be interpreted with caution given different definitions of this condition (Helliwell, 2009). Interestingly, there may be a higher prevalence of positive anticyclic citrullinated peptide (accp) antibodies in these patients (Korendowych et al., 2005). Perhaps in part due to the lack of a clear definition, little is known about this feature in the era of modern disease modifying anti-rheumatic agents outside of case reports and clinical anecdotes reported by clinicians Nail disease Nail changes have traditionally been considered a part of the skin disease psoriasis and are not reported as a feature of other spondyloarthropathies (Jiaravuthisan et al., 2007). The major subtypes of nail changes described in psoriasis and psoriatic arthritis are onycholysis, pitting, and subungual hyperkeratosis, all shown in figure 3. These three subtypes are applied consistently across all texts that address nail change subtypes in psoriatic disease. In addition, oil spots, salmon spots, ridging, and dystrophy are described in various texts but are less clearly defined, are not used across the literature, and the term nail dystrophy is even used interchangeably to describe severe nail damage and as an umbrella term for any structural nail abnormalities (Eastmond & Wright, 1979; Jiaravuthisan, et al., 2007; Jones et al., 1994). To facilitate comparison with the existing literature we have focused on onycholysis, pitting, and subungual hyperkeratosis in our analysis of nail changes in psoriatic patients. 30
35 Nail changes are far more common in patients with psoriatic arthritis than in patients with skin disease as 15% - 50% of patients with psoriasis have nail changes but the same is true of up to 85% of patients with PsA (Gladman et al., 2004; Jiaravuthisan, et al., 2007; Lavaroni et al., 1994). This has led some investigators to focus on this aspect of the disease. Magnetic Resonance Imaging (MRI) studies suggest that nail changes represent a continuation of inflammation originating in entheseal structures and may be a manifestation of arthritis (Scarpa et al., 2006; Tan et al., 2007). Recently it was reported that nail changes are an independent risk factor for later developing arthritis (Wilson, et al., 2009a). Figure 4 - From left: Onycholysis, pitting, and subungual hyperkeratosis. Images kindly provided by Jóhann Elí Guðjónsson. None of these recent studies have explored subtypes of nail changes, even though an association between DIP joint involvement in PsA and onycholysis was reported over 20 years ago. (Torre Alonso et al., 1991) Furthermore, the early literature on psoriatic arthritis suggests that onycholysis is more important than other nail changes in psoriatic arthritis, while nail pitting seems to be fairly common in non-psoriatics, suggesting it may be a poor discriminator between psoriatic and non-psoriatic causes of nail disease (Eastmond & Wright, 1979; Robertson & Braune, 1974; Torre Alonso, et al., 1991). These insights into nail changes, old and new, are exciting as they have the potential to open a window into the otherwise invisible world of entheseal changes in psoriatic arthritis. The need for a clear understanding of the association of specific subtypes of nail changes with psoriasis and psoriatic arthritis is therefore pressing. 31
36 1.5.3 Other features Dactylitis is a term that refers to inflammation of an entire digit. It is associated with all of the spondyloarthritides and is seen in 35% of patients with psoriatic arthritis in cross sectional studies (Gladman, 1998). It is not a feature of rheumatoid arthritis and can therefore be helpful in making a diagnosis of a spondyloarthritis. Figure 5 shows a fluorescent optical image of dactylitis of the fourth digit in an individual with psoriatic arthritis. Uveitis is another clinical feature that is shared across all of the diseases that make up the spondyloarthritides. It is less common in psoriatic arthritis than in ankylosing spondylitis, and seems to have different clinical characteristics. It has been reported that ocular inflammation may be associated with psoriatic skin disease without arthritis (Rehal et al., 2011) Disease activity Figure 5 - Fluorescent optical image showing dactylitis of the fourth digit of the left hand involving the MCP, PIP, and DIP joints simultaneously as well as the soft tissues between them. (Image reproduced with the kind permission of Dr. Werner (Werner et al., 2010)) Because of the heterogeneous presentation of psoriatic arthritis described above, measuring disease activity can be challenging. The difference between the set of clinical features present in two individuals with psoriatic arthritis can present a problem similar to that faced by lupus researchers in terms of how to measure disease activity. It is not clear how disease activity indices can be constructed to reliably capture an improvement in all subtypes of disease, while still having the ability to detect improvement in patients who have disease that is mostly or entirely limited to a subset of features. This presents the clinician with a poser, as it is not clear if the results of a clinical trial for a new compound that shows an improvement in one aspect of disease, such as peripheral arthritis, can be generalized to other aspects such as dactylitis or uveitis. Thus, the study and classification of the clinical features of psoriatic arthritis remains an active field of investigation 40 years after the seminal publication by Moll and Wright, and is likely to remain so for years to come. 32
37 Table 1 - Domains for assessment of psoriatic arthritis and preferred methods for assessing each domain. Domain OMERACT 8 vote results Preferred assessment tool Peripheral joint activity 96% 66/68 joint count a, b Skin activity 86% PASI a Physical function Not voted on separately HAQ, SF36 function a Health related quality of life 92% SF36, PsAQoL a Pain Not voted on separately ND a Global assessment 95% Patient global VAS a Fatigue Not voted on separately ND, candidates include KFSS and FACIT-fatigue a Nails Not voted on separately mnapsi a Spinal disease 59% ND a, but Modified Schöber test, Lumbar side flexion, and Cervival rotation have been shown to be suitable a, c Dactylitis 66% LDI, LDI basic a, d Enthesitis 63% LEI a, e Imaging 72% ND a Acute phase reactants 9% ND a Based on published results from OMERACT 8 regarding core domains and voting results (Gladman, Mease, Strand, et al., 2007), a report on outcome measure discussions at OMERACT 8 (Gladman, Mease, Healy, et al., 2007), and a review of the literature regarding the preferred assessment tools for each domains. a =(Gladman, et al., 2007) b =(Gladman, et al., 2004) c =(Fernandez-Sueiro et al., 2009) d =(Healy & Helliwell, 2007) e =(Healy & Helliwell, 2008). PASI=Psoriasis Area and Severity Index; HAQ=Health Assessment Questionnaire; SF36= Medical Outcomes Study 36-Item Short-Form Health Survey; PsAQoL= Psoriatic Arthritis Quality of Life Index; ND = Not Determined during OMERACT 8 discussions; VAS=Visual Analog Scale; KFSS=Krupp s Fatigue Severity Scale; FACIT=Functional Assessment of Chronic Illness Therapy; mnapsi=modified Nail Psoriasis Severity Index; LDI=Leeds Dactylitis Index; LEI= Leeds Enthestitis Index. Core domains as defined by OMERACT 8 are in boldface. Recently, efforts have been made to arrive at a consensus regarding the most important domains of disease to monitor in clinical trials, and at OMERACT 8 72% of participants agreed on a set of 6 core features, shown in bold typeface in table 1. While the most appropriate tool for each domain has not been formally defined, review of the literature reveals that for most domains there is either a de-facto consensus or strong evidence supporting the use of one tool over other available tools as detailed in table 1. Attempts to create combined skin and joint disease indices include the Modified Composite Psoriatic Disease Activity Index and the Disease Activity index for PSoriatic Arthritis but there is not a consensus regarding their usefulness (FitzGerald et al., 2012; Helliwell et al., 2012; Mease, 2011). Therefore, the 66/68 swollen/tender joint count and the PASI score remain the standard for evaluating joint and skin activity in psoriatic arthritis. 33
38 1.6 Diagnosis Psoriatic arthritis is in most cases a subspecialty diagnosis, as the disease needs to be distinguished from both rheumatoid arthritis and osteoarthritis as well as less common arthritic conditions. However, it is important to recognize that the level of expertise actually applied in the diagnosis of arthritic conditions varies by country, and that within some health care systems primary care physicians may have been trained to diagnose inflammatory arthritides. Furthermore, while it is important to use a specialist diagnosis in trials, it may be appropriate in population based studies to accept the diagnosis of a non-specialist, depending on the type of analysis planned. The gold standard for clinical diagnosis of psoriatic arthritis remains the rheumatologist opinion, most often using as a template the original definition of Moll and Wright of an inflammatory arthritis associated with psoriasis that is usually rheumatoid factor negative. While at least nine different classification and/or diagnostic criteria have been proposed since (Svensson et al., 2002; Taylor, et al., 2006), the CASPAR criteria appear to be gaining acceptance as the standard for clinical studies (Coates et al., 2012; Tillett et al., 2012; van den Berg et al., 2012). To meet the CASPAR (ClASsification criteria for Psoriatic ARthritis) criteria, a patient must have inflammatory articular disease (joint, spine, or entheseal) with 3 points from the following 5 categories: 1. Evidence of current psoriasis, a personal history of psoriasis, or a family history of psoriasis. - Current psoriasis is defined as psoriatic skin or scalp disease present today as judged by a rheumatologist or dermatologist. A personal history of psoriasis is defined as a history of psoriasis that may be obtained from a patient, family physician, dermatologist, rheumatologist, or other qualified health care provider. - A family history of psoriasis is defined as a history of psoriasis in a first- or seconddegree relative according to patient report. 2. Typical psoriatic nail dystrophy including onycholysis, pitting, and hyperkeratosis observed on current physical examination. 3. A negative test result for the presence of rheumatoid factor by any method except latex but preferably by enzyme-linked immunosorbent assay or nephelometry, according to the local laboratory reference range. 4. Either current dactylitis, defined as swelling of an entire digit, or a history of dactylitis recorded by a rheumatologist. 5. Radiographic evidence of juxtaarticular new bone formation, appearing as illdefined ossification near joint margins (but excluding osteophyte formation) on plain radiographs of the hand or foot. Figure 6 - The CASPAR criteria. (Taylor et al., 2006). Current psoriasis is assigned a score of 2; all other features are assigned a score of 1. Applying the CASPAR criteria is fairly straightforward as summarized in figure 6. However, using the CASPAR criteria requires the full set of variables to be available for each individual, as missing data will likely reduce sensitivity. It can still be applied to older datasets with missing data as a sensitivity analysis to get a general idea of how close the 34
39 dataset is to meeting the CASPAR criteria, but if data points are missing the results must be interpreted with appropriate caution. An important benefit of using the CASPAR criteria is that they allow for the use of family history of psoriasis to establish the diagnosis in an individual without skin disease. This allows future studies to include the entity described in the literature as psoriatic arthritis sine psoriasis in a systematic manner (Olivieri, et al., 2009). 1.7 Treatment Psoriatic arthritis used to be considered a mild disease, and the primary treatment was with non-steroidal anti-inflammatory drugs (NSAIDs) (Cuéllar et al., 1994). However, in the 1990s it was conclusively demonstrated that psoriatic arthritis is an erosive disease that causes significant morbidity, and may increase mortality (Gladman et al., 1998; Gladman, et al., 1987). As a consequence the recommended treatment currently involves early use of disease modifying anti-rheumatic drugs (DMARDs), including methotrexate (Gossec et al., 2012). Advances in the treatment of psoriatic arthritis have mostly mirrored those of rheumatoid arthritis. Unfortunately drug trials in psoriatic arthritis have been few and small compared to trials in rheumatoid arthritis, with the exception of the recent introduction of TNF inhibitors discussed below. Due to the lack of large trials of non-biological disease modifying drugs, psoriatic arthritis patients have been treated as if they had rheumatoid arthritis, on the assumption that the effects will be similar. Recent data suggest that this may not always be the case. The first randomized trial of methotrexate did not find that it prevents erosions in psoriatic arthritis as it does in rheumatoid arthritis, at least not in doses of 15mg per week or less (Kingsley et al., 2012). Furthermore, a recent review of the use of DMARDs in psoriatic arthritis concluded that The evidence of efficacy with traditional DMARD is scarce, the effect is small, and the evidence of effect on radiographic progression is even lower. (Soriano, 2012). However, until more data become available the current practice of using similar treatment schemes for psoriatic arthritis and rheumatoid arthritis will likely continue. With the introduction of TNF inhibitors as an effective treatment for arthritis, double blind randomized trials of these medications have been performed in psoriatic arthritis and have clearly demonstrated their efficacy (Ash et al., 2012). More recently, medications targeting other parts of the immune system such as abatacept which targets co-stimulation of T-cells, and ustekinumab which targets IL12/IL23 have shown promising results in trials (Gottlieb, et al., 2009; Griffiths, et al., 2010; Mease et al., 2011). Direct blocking of IL17 (ixekizumab) 35
40 and its receptor (brodalumab) has not been tested in psoriatic arthritis (Leonardi, et al., 2012; Papp, et al., 2012), although studies in psoriasis are promising. In addition to targeting the autoimmune disease directly, recent data on comorbidities of psoriasis suggest that there might be a role for targeting risk factors for cardiovascular disease more aggressively in this patient population (Semb et al., 2012). No specific guidelines on how to approach this have been published, but the European League Against Rheumatism (EULAR) guidelines for approaching this issue for rheumatoid arthritis patients include the suggestion that the same should be applied to patients with ankylosing spondylitis and psoriatic arthritis, despite the lack of data supporting this approach (Peters et al., 2010). 1.8 Summary Psoriatic arthritis is an inflammatory arthritis associated with psoriasis that causes significant morbidity and probably increases mortality. It has received less attention than some other autoimmune diseases, but recently there has been increased research activity and a body of knowledge is being rapidly accumulated. This thesis presents the results of a study of 220 individuals with psoriatic arthritis, of whom the author has personally examined 156 using pre-defined criteria. In addition to this group of patients I have used data on 1116 psoriasis patients from the Icelandic population collected by my colleague Johann Eli Gudjonsson to compare features in psoriasis patients to those seen in psoriatic arthritis patients. 36
41 2 Aims The objective of the study presented in this thesis was to increase our understanding of the epidemiology and disease presentation of psoriatic arthritis. To achieve this we defined the following set of aims: First, we aimed to describe the prevalence, demographics and disease course among psoriatic arthritis patients in Reykjavik, Iceland. Second, we aimed to define the familiality of the disease using novel methods, extending the current knowledge regarding risk in first degree relatives to more distant relatives, and to understand when the risk approaches that of the general population. Third, we aimed to define if there is a link between psoriatic nail disease and small joint disease in general, rather than only distal interphalangeal joint disease as other researchers have previously demonstrated. Fourth, we aimed to explore whether the association between psoriatic arthritis and nail disease varied by the subtype of nail disease. 37
42 3 Materials and methods 3.1 Study population The study population involved all individuals living in the Reykjavik capital area in Individuals living outside of this area were excluded due to limited access to rheumatology services outside of the Reykjavik area. The study group was arrived at through the process shown in figure 7 based on two methods, as described in detail below. Patients with psoriasis in Reykjavik who were asked if they had been diagnosed with psoriatic arthritis Psoriatic arthritis study group Patients who had died before data collection began in 2002 were excluded Patients with a diagnosis of psoriatic arthritis recorded at Landspitali University Hospital from 1981 to 2001 First, we used the method used in previous epidemiological studies of psoriatic arthritis (Shbeeb, et al., 2000) and screened all psoriasis patients who had been located in the Reykjavik area for studies of psoriasis for psoriatic arthritis. However, we were concerned that many psoriatic arthritis patients might not have skin disease that was significant enough to result in a diagnosis of psoriasis, and that consequently many cases would be missed by recruiting from the psoriasis population alone. We therefore used the electronic diagnosis records of Landspitali University Hospital reaching back to 1981 to locate patients who had received a diagnosis consistent with psoriatic arthritis from a rheumatologist. Figure 7 The study group was formed by combining two data sources with dissimilar backgrounds to ensure maximum coverage. Only patients who had died before the study began were excluded from this list. Combining these two methods resulted in the largest list of psoriatic arthritis patients living in the Reykjavik area yet assembled. We considered different methodologies to test the completeness of the list. Our first choice was to apply catch-and-release techniques to estimate the total population. However, further scrutiny revealed that this methodology would be inappropriate, as our methods were consciously designed to capture a different set of patients with the second method (i.e. hospital based records). Thus, our methods failed the basic premise of this useful and often applied methodology, namely that an individual has to have a similar chance of being captured in both the capture and recapture rounds for the method to work. The chosen approach to ascertain the completeness of the list involved contacting all rheumatologists in Iceland, asking them for the number of patients with 38
43 psoriatic arthritis they were following, and comparing that number to the number of patients reporting that rheumatologist as their treating physician. This method showed that our list contained over 90% of the individuals known to be treated by rheumatologists for psoriatic arthritis in the Reykjavik area, as well as some who were no longer following with their rheumatologist. Once the list had been confirmed to be satisfactorily complete as described above we proceeded to invite all of the psoriatic arthritis patients for a study visit. During this visit the patients completed a questionnaire on their background and health, participated in a detailed physical examination performed by a physician, including body measurements, and finally donated a blood sample for genetic and other studies. Those patients who had previously participated in studies of psoriasis already had detailed phenotypic data and biosamples available for study from a previous point in time, but still completed a full evaluation for this study as well. These data were used to perform all of the analyses described in this thesis Additional subjects - aim four For the fourth objective described in this thesis we added data from a previous study of psoriasis to allow for more detailed analysis of nail changes. Cases were located from community sources, primarily a local psoriasis patient group, and were invited for a physical examination over a period of 5 years. Psoriasis patients without active skin disease were excluded, as were children under the age of 18. The details of the recruitment and data collection methods have been published (Gudjonsson, et al., 2006). 3.2 Data collection All data used in pursuing aims 1-3 were collected in by Thorvardur Jon Love and Bjorn Gudbjornsson, except for the geneaology database which was constructed by decode Genetics. To complete aim 4 we compared our new dataset to data collected in by Johann Eli Gudjonsson for a study of psoriasis Psoriatic arthritis Psoriatic arthritis patients who accepted the invitation to participate were invited to schedule a study appointment at a clinical study center in Reykjavik. At the beginning of this appointment subjects completed a detailed questionnaire including information on their childhood, exposure to infections and vaccinations, and medical history. They were then interviewed by a physician using a structured interview form to extract such details as the time of disease onset, time of diagnosis, treating rheumatologist, and more. Next, a structured 39
44 physical examination was performed, including a full 66/68 swollen/tender joint count, PASI skin score, nail change classification, and measurements of height and weight. Only fingernails were evaluated due to the high frequency (17%) of fungal toenail infections in Iceland (Sigurgeirsson et al., 2002). Finally, a blood sample was drawn for genetic analysis Confirmation of diagnosis Several sets of classification criteria for studying psoriatic arthritis were available at the time of our study, but none of them were widely recognized or accepted as a standard. For this study we chose the SweSpa criteria (Svensson, et al., 2002) as a way to arrive at an independently confirmed diagnosis of psoriatic arthritis. As a result, our data are based on two definitions of psoriatic arthritis. First, patients with a diagnosis of psoriatic arthritis by a rheumatologist were considered to have psoriatic arthritis, and this group was used to estimate the prevalence in the Icelandic population in a way that could be compared to the results of other similarly designed studies, and in the analysis of familiality. Second, only those patients meeting the SweSpa criteria were used to calculate a more conservative estimation of prevalence, and this smaller group was then used to study the demographics and disease characteristics further in aims one, three, and four Psoriasis comparison group The details of the data collection performed for the psoriasis group have been published elsewhere (Gudjonsson, et al., 2006). Only a subset of data concerning the skin and nail involvement was used to complete aim four of this thesis. All patients were asked if they had been diagnosed with PsA by a rheumatologist to allow for a comparison between those with skin disease alone and those who had arthritis as well. The analysis of our data from aim one has shown that this self-report of psoriatic arthritis among psoriasis patients is highly predictive of independently confirmable psoriatic arthritis, with 83% meeting the SweSpa criteria (Svensson, et al., 2002) and a minimum of 80% meeting the CASPAR criteria. (Love, et al., 2007; Love, et al., 2010). 3.3 Statistical analysis Statistical methods varied based on the objective and available data, and are described in detail in each paper. Briefly, confidence intervals for prevalence estimates were calculated using poisson distributions and comparisons between groups were performed using the t-test and chi-squared test (Love, et al., 2007). Familiality was ascertained using the kinship 40
45 coefficient (KC) and relative risk (RR), employing the computerized genealogy database at decode genetics (Karason, et al., 2009). Multivariate logistic regression models were constructed to estimate the association between individual types of nail changes and various features of psoriatic arthritis (Love, et al., 2010; Love, et al., 2012). For readability and accuracy, the data analysis sections from the methods of the four papers presented in this thesis are included here verbatim in chapters through Data analysis section from paper I The study data were stripped of information allowing identification of individuals before the analysis of data began and the code for this information was kept in a separate, encrypted database. The study was approved by the National Bioethics Committee of Iceland (approval ) and the Data Protection Committee of Iceland. Informed consent was obtained from all the participants in the study. Data were analyzed using Statview 5.0 statistical software. Chi-squared and t-tests were used for comparisons of dichotomous values and means, respectively. When predicting the number of confirmable cases based on inclusion of examined patients, direct standardization was used to correct for age and sex discrepancies between the group of patients who were examined and those who were not. We calculated 95% confidence intervals for prevalence rates using binomial distribution. All reported p values are based on 2-tailed analysis Data analysis section from paper II Assessment of inheritance The RR for disease in relatives is a measure of the risk of disease in a relative of an affected person as compared with the risk in the population as a whole. Obtaining valid estimates of the RR is, however, not straightforward, because many sampling schemes may lead to biased or inaccurate estimates (Guo, 1998). The use of a population-based group of patients eliminates some of this potential sampling bias. In addition, a near-complete genealogy database facilitates identification of patients who are related to other patients. It is important to note that only probands were used in our analysis, and no attempt was made to recruit relatives of cases of PsA. This design avoids the potential overestimation of familiality when secondary cases are recruited through probands, as described by Sun-Wei Guo (Guo, 1998) Kinship coefficients We incorporate a kinship coefficient (KC), a measure of identical by-descent sharing, to assess whether the affected individuals are more related than a set of matched controls. The 41
46 KC for a pair of individuals is the probability that, for a particular autosomal locus, two randomly selected alleles, one from each individual, are inherited from a common ancestor (Cannon-Albright et al., 1994). We determined the mean pair-wise KC for the set of PsA patients and compared this with the distribution of the mean pair-wise KC for sets of matched controls. Each patient was matched to a single control individual in each control group. The controls were drawn at random from the genealogical database and were matched on the year of birth, gender and the number of ancestors recorded in the genealogy database. Because of the large size of the pedigrees, there was a computational problem in accurately determining the KC. Monte Carlo simulations were used to approximate the average pair-wise KC for each group. A total of simulations were performed to ensure that the Monte Carlo errors had a negligible effect on the results. Empirical P-values were calculated by counting how many sets of controls had average pair-wise KCs larger than the set of affecteds being tested. The contribution of close relatives dominates the KC values. To show that increased relatedness extends beyond FDRs [First Degree Relatives], the results of KC calculations were refined by excluding the genetic contributions from relatives at one or two meioses, up to and including seven meioses. The KC is expressed as a genealogical index of familiality, calculated as the mean KC (Risch, 1990). All P-values reported are nominal Relative Risk To assess the significance of the RR obtained for a given group of patients, we compared their observed values with the RR computed for up to 1000 independently drawn and matched groups of control individuals [25]. The control individuals were selected in the same manner as described above for the KC calculations. Empirical P-values can be calculated using the control groups; thus, a P-value of 0.05 for the RR would indicate that 5% of the matched control groups had values as large as or larger than that for the patient s relatives or spouses. The number of control groups required to obtain a fixed accuracy of the empirical P-values is inversely proportional to the P-value. We therefore selected the number of control groups generated adaptively up to a maximum of When none of the values computed for the maximum number of control groups was larger than the observed value for the patient s relatives and mates, we report the P-value as being < Using a variance stabilizing square-root transform, an approximate CI may be constructed based on the distribution of RR for control groups. 42
47 3.3.3 Data analysis section from paper III The association between small joint involvement and nail disease was evaluated using the 2 test. Statistically significant associations defined by a p-value of 0.05 or less were evaluated further in a multivariate logistic regression model. Age and gender were included as covariates in all multivariate analyses. Other potential covariates were identified based on clinical experience and information in the literature, and then tested for a univariate association with small joint disease, using the 2 test for categorical variables and univariate logistic regression for continuous variables. Odds ratios (ORs) were calculated with 95% confidence intervals (CIs). Those variables that were significantly associated with the main outcome of small joint disease on univariate analysis at a p-value of 0.05 or less were included in the multivariate analysis as covariates. Statistical analysis was performed using JMP version 7 from SAS Data analysis section from paper IV Statistical analysis. The chi-square test was used to test for associations between dichotomous characteristics and the t-test for comparison of the mean of continuous variables. All variables found to be associated with PsA at a p value of 0.05 or less were included in a multivariate logistic regression model. Age and gender were included in all multivariate models. Due to concerns for potential collinearity between subtypes of nail changes, a correlation matrix was constructed to identify any strong correlations between nail change subtypes, and the stability of the model was tested further by calculating the mean variance inflation factor (VIF) as well as the VIF for each independent variable. This was in addition to the collinearity testing built into the statistical analysis package used. Goodness of fit was calculated using the Hosmer Lemeshow method. We furthermore performed post-hoc sensitivity analysis where we divided nail changes into three scenarios: onycholysis without pitting, pitting without onycholysis, and pitting occurring with onycholysis, and tested each for association using the chi-square test. Finally, we tested for univariate associations between subclasses of nail changes and skin activity (measured by the PASI score) and joint activity (measured using tender and swollen joint counts). The results of this analysis were further analysed in three post-hoc linear regression models to estimate the strength of associations of the three subgroups of nail changes with the aforementioned skin and joint measures, adjusting for age and gender. Statistical analysis was performed using STATA version
48 4 Results The results corresponding to the four aims presented in this thesis have been published in peer-reviewed journals, and the resulting papers are included as reprints at the end of this thesis. What follows is an overview of these results, but the full results are in the individual publications. When referring to the results presented here, the individual published papers should be cited rather than this thesis. 4.1 Study group We found 346 patients who had either a diagnosis of psoriatic arthritis in their medical record or a selfreported diagnosis of psoriatic arthritis by a rheumatologist, or both as described in the methods section. Of these, 301 were alive and 220 of them were living in the Reykjavik area at the time of the study (2003). Thirty of these 220 patients were present in both data sources. Figure 8 details how the eligible study group for the Reykjavik study of psoriatic arthritis was defined. Of the 301 patients with a diagnosis of psoriatic arthritis, 187 (62%) participated in the full study including physical examination, and 156 (71%) of the patients living in the Reykjavik area did the same. Figure 8 - Selection process for inclusion in study group (Love et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology 44
49 Different subsets of the Reykjavik study group were used for each aim of the study. For the prevalence estimates we used the full group of 220 patients found in the Reykjavik area to arrive at an estimate of the prevalence of diagnosed psoriatic arthritis. Data on 131 patients from the Reykjavik area with verifiable disease on physical examination in 2003 were used to further evaluate the demographics and disease course as reported in interviews and on questionnaires, and recorded on physical examination. For the familiality analysis we combined data on the 220 patients identified in the Reykjavik area with genealogical data from decode Genetics to compare the relative relatedness of individuals with and without psoriatic arthritis. The analysis of the relationship between nail changes and small joint arthritis made use of patient material from outside the Reykjavik area, increasing the number of eligible patients with confirmed psoriatic arthritis to 154 in this analysis. Finally, we used data on 1116 patients with psoriasis whose nails had been examined in a previous study on psoriasis to determine the association between nail changes and arthritis among patients with psoriasis, and reported data on subsequent nail findings among those with arthritis several years later. 4.2 Prevalence Census data were used to establish that 134,253 individuals were living in the study area of Reykjavik in To arrive at an estimate of prevalence directly comparable to other studies Figure 9 - Prevalence of psoriatic arthritis by age and gender. Numbers are per 100,000 individuals age 18 years and older living in Reykjavik at the time of the study. Data are categorized by age groups and gender. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology 45
50 using self-reported diagnoses and/or medical records to establish the diagnosis of psoriatic arthritis we included all 220 individuals and calculated a prevalence of 164 per 100,000 (95% CI ). This represents our estimate of the prevalence of diagnosed psoriatic arthritis in the Reykjavik area in However, our data set allowed us to refine this estimate further based on the confirmation rate of psoriatic arthritis among our study group. We found that in 84% of patients examined we could confirm the diagnosis of psoriatic arthritis using previously published criteria (Love et al., 2006). Using this confirmation rate, we calculated a revised prevalence rate of 139 per 100,000 (95% CI ). This estimate represents the point prevalence of active or confirmable psoriatic arthritis in the Reykjavik area in The prevalence of diagnosed psoriatic arthritis was higher in women (208 per 100,000, 95% CI ) than in men (118 per 100,000, 95% CI ), and this was also true for the refined estimates of independently confirmed disease (174 per 100,000, 95% CI vs. 101 per 100,000, 95% CI ). When plotted against age the prevalence increased as expected with age, but then dropped after age 67 as illustrated in figure Demographics and disease activity The mean age of patients participating in the study was 54 and 56 years for men and women, respectively, as presented in table 2 along with additional demographic data. Men had onset of skin disease later in life than women, and there was a trend towards the same pattern for arthritis. Eleven patients reported developing arthritis before any skin symptoms appeared, another eleven reported simultaneous presentation of skin and joint symptoms, but the most common presentation was that of skin disease preceding joint disease, reported by 83 patients. The remaining 26 patients could not recall the order of onset of their symptoms. Among those patients who developed skin disease first Table 2 Demographic information on 131 patients included in the study. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology 46
51 the mean time that passed until arthritis developed was 16 years. Those patients who reported arthritis as their first symptom developed skin disease at a mean of 6 years later. The demographic characteristics are summarized in table 2. At the time of evaluation, 98.5% of the included patients had active skin and/or joint disease, but two patients were included because of their clear history of inflammatory arthritis associated Table 3 - Frequency and severity of skin and joint involvement. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology with psoriasis; both were receiving immunosuppressive therapy at the time of their evaluation. Skin disease was active in 115 (88%) with an average PASI score of 4.1, peripheral joint disease was active in 104 (79%) with mean swollen and tender joint counts of 5.1 and 5.5, respectively. One patient had active dactylitis, and 7 patients (5%) had inflammatory back pain without peripheral involvement. Further information on skin and joint involvement is presented in table 3. At the time of examination 42 patients (32%) were taking disease modifying drugs (DMDs), and an additional 29 (22%) had previously used DMDs. Table 4 - Subcategories of PsA at disease onset and at the time of evaluation. (Love, et al., 2007) Reprinted with the kind permission of the Journal of Rheumatology 47
52 4.4 Disease course and treatment Oligoarthritis was the most common presentation of psoriatic arthritis in the study group, and was the predominant pattern in 44% at the time of examination. We found that 78 patients (60%) had a different pattern of psoriatic arthritis at the time of their examination than they recalled having at the onset of their disease. Table 4 presents the number of patients in each disease category at the onset of disease and in 2003, a mean of 20 years after onset of psoriatic arthritis. We analyzed the use of DMDs by disease pattern and found that patients with oligoarthritis, polyarthritis, and inflammatory back pain all had similar odds of having been administered a DMD (47%, 50%, and 44%, respectively, 48% combined). One of 11 patients (9%) with enthesitis reported DMD use (p = 0.01). Patients with any type of peripheral arthritis were twice as likely to have received a DMD as those who had no peripheral arthritis (48% vs 24%, respectively; p = 0.03). 4.5 Familiality Linking the decode genealogy database with our list of 220 patients with psoriatic arthritis we were able to construct pedigrees that illustrate the high penetrance and familial nature of psoriatic arthritis in the Icelandic population. Figure 10 is an example of such a pedigree, demonstrating significant clustering of PsA patients. We calculated the kinship coefficient (KC) for patients and controls, and the results are presented in figure 11. The KC was statistically significantly higher among patients than in the control group, and this was true up to seven meioses from the proband at p< The KC values were 5.0, 3.4, 1.7, 1.3, 1.0, 0.8 and 0.7 for the first seven excluded meioses (p< for all values). Figure 10- Pedigree of a family where psoriasis and psoriatic arthritis are common. White indicates healthy individual, black psoriasis and blue psoriatic arthritis. Disease status for earlier generations is not known. The recurrence risk (RR) in relatives (lambda-r) was calculated for first to fifth degree relatives, and the results are presented in table 5. A statistically significantly increased RR 48
53 KC (x10.000) was observed in first- to fourth degree relatives, with RR values of 39.2, 12.2, 3.6, and 2.6, respectively, all p-values < KC Patients KC Controls In addition to evaluating the KC and RR for the entire group we compared the effect by gender and found no significant effect. We attempted to calculate the RR in spouses, but as no spouse of a psoriatic Excluded meioses Figure 11 - Kinship coefficients (KCs) for the risk of developing psoriatic arthritis. For excluded meioses 0-7 the p-value is < arthritis patient had the disease we were unable to calculate this value. This was to be expected based on our prevalence estimates for psoriatic arthritis, as the expected number of psoriatic arthritis patients among 220 spouses would be Three spouses had psoriasis, or 1.2% (95% CI 0.3% - 3.8%), similar to the background population prevalence of psoriasis. 4.6 Nail disease and small joint involvement Of 301 patients with diagnosed psoriatic arthritis living in Iceland in 2003, 187 participated in this part of the study. For the analysis presented here we used only data on those 154 patients in whom we could verify the diagnosis of psoriatic arthritis using previously published criteria as discussed in the methods section. Table 6 presents data on their disease characteristics. Table 5 - Relative risk (RR) estimates for having psoriatic arthritis over five generations. (Karason et al., 2009) Reprinted with the kind permission of Rheumatology (Oxford) 49
54 Nail changes in general were not associated with the presence of small joint involvement. However, on univariate analysis of individual subtypes of nail changes, onycholysis had an association with small joint disease, and the OR was 2.80 (95% CI ). Other factors that were associated with small joint disease in the univariate analysis were higher numbers of swollen joints and decreased disease duration. The independent contribution of these factors to the association with small joint disease was evaluated using multivariate logistic regression, Table 6 - Clinical characteristics of 154 patients with psoriatic arthritis. (Love et al., 2010) Reprinted with the kind permission of the Scandinavian Journal of Rheumatology adjusting for age, gender, disease duration, and the number of swollen joints. Patients with onycholysis had an OR of 3.42 (95% CI ) for having small joint involvement in this multivariate model. The details of the analysis are presented in table 7. Table 7 - Predictors of small joint involvement presented as odds ratios with 95% confidence intervals. (Love, et al., 2010) Reprinted with the kind permission of the Scandinavian Journal of Rheumatology 50
55 4.7 Onycholysis and arthritis in psoriasis Among 2547 individuals recruited from the community based on self-reported psoriasis or family history of psoriasis, 1116 patients age 18 years and older had their disease verified on physical examination, and data on nail disease recorded. These patients formed the study group in this part of the study. The mean age at psoriasis onset of was 21 (95% CI 20-21) years and 56% were women. Chronic plaque psoriasis was seen in 94%, and arms, scalp, and legs, were most often involved (84%, 70%, and 70%, respectively). Nails were involved in 37% (95% CI 34%-40%) and the most common type of nail change was onycholysis, found in 29% (95% CI 26%-32%). Table 8 summarizes the characteristics of these patients, grouped by the presence or absence of arthritis. Of the 1116 psoriasis patients, 187 or 17% (95% CI 15%-19%) reported a diagnosis of psoriatic arthritis by a rheumatologist. Univariate analysis revealed that age, gender, onycholysis and pitting were associated with arthritis in this group (p<0.05). These five variables were included in a multiple logistic regression model to evaluate the independent contribution of each variable. We tested the model for collinearity as described in the methods section, and found no variance inflation. Onycholysis was the only type of nail change that remained associated with PsA in the multivariate analysis with an OR of 2.06, p < Table 9 summarizes the results of the multivariate model. Table 8 - Baseline characteristics of the study group. (Love, Gudjonsson, et al., 2012) Reprinted with the kind permission of the Journal of Rheumatology 51
56 In addition to building the multivariate model we performed a post-hoc analysis described in the methods section to better illustrate the different contributions of onycholysis and pitting, as they often co-occur in the same individual. We found that the OR for PsA in this analysis was 1.95 in patients with onycholysis without pitting (p<0.001), 1.76 when both onycholysis and pitting were present (p=0.009), and 1.52 when pitting was present without concurrent onycholysis (p=0.16). Of the 187 PsA patients who participated in the original study of psoriasis, 139 (74%) returned for the second study focusing on PsA at a mean of 3.8 years later (median 4, IQR 3-4). These patients all had complete skin, joint, and nail evaluations. Most of them (97%) were seen three or more years after their initial evaluation for the first study. The prevalence of each of the three types of nail changes had increased from the first examination to the second examination, with the overall proportion of patients with some nail involvement rising from approximately 53% to about 80%. Fewer than 4% of those with nail changes at the first visit were without nail changes at the second visit. Questions from one of the reviewers of this paper prompted the design of a post-hoc analysis, intended to give insights into the relationship between nail findings and the severity of skin and joint symptoms. This analysis indicated that there was a univariate relationship between each of the subtypes of nail changes and skin disease activity as captured by the PASI score, but the correlation with joint counts was weaker, with only onycholysis being associated with a higher tender joint count at a p-value of 0.05 or lower. Details of the results of the univariate analysis are presented in table 10. Using a multivariate linear regression including each nail change subtype we found that only onycholysis was independently associated with any of the three disease activity measures at a statistically significant level, namely the tender joint count, but there was an interesting trend towards a positive association between pitting and PASI score, and a negative one between pitting and tender joint count (both p=0.09). The details of this analysis are presented in table 11. Table 9 - Results of the multivariate regression model. (Love, et al., 2012) Reprinted with the kind permission of the Journal of Rheumatology 52
57 53 Table 10 Univariate analysis of disease and treatment characteristics of 139 psoriatic arthritis patients at followup. Results of the chi-square test for DMARD use and t test for all other variables, presented as mean (95% CI). (Love, et al., 2012). Reprinted with the kind permission of the Journal of Rheumatology Table 11 Results of 3 multivariate linear regression analyses showing the association of age, sex, and subtypes of nail changes with each of 3 disease activity measures. Regression results are presented as -coefficient (95% CI). (Love, et al., 2012). Reprinted with the kind permission of the Journal of Rheumatology
58 5 Discussion We have performed a population based study describing the prevalence and characteristics of psoriatic arthritis in Reykjavik, Iceland. Using the Icelandic genealogy database we have expanded on previous findings on the familiality of psoriatic arthritis to show that an increased risk is not limited to first degree relatives but extends to at least four degrees of genetic separation. Finally, we have used our data to study nail disease in psoriatic disorders, both in the context of arthritic symptoms and skin disease, and found associations with onycholysis that have not been previously described. 5.1 Study group The study group was selected to include as many patients with psoriatic arthritis as possible. To achieve this we aimed to find all patients in Iceland who carried this diagnosis at the time of the study. By combining two approaches we found 60% more cases in the Reykjavik area than would have been predicted by extrapolating from the only study available at the time (Shbeeb, et al., 2000). However, it is never possible to exclude the possibility that cases were missed, a potential weakness of this and other studies trying to establish the population prevalence of a disease. It should be noted though that missing cases would primarily affect the first paper presented in this thesis. This study can only speak to the prevalence and characteristics of diagnosed psoriatic arthritis in the general population, and it is conceivable that undiagnosed cases are in some way different from diagnosed cases of psoriatic arthritis, for instance in terms of disease severity. However, the findings presented here should at a minimum be generalizable to cases of diagnosed psoriatic arthritis in populations of Northern European descent. 5.2 Prevalence Our finding of a prevalence of 0.16% for psoriatic arthritis in Iceland fits well with the published literature from similar populations in the 21 st century (Alamanos, et al., 2003; De Angelis, et al., 2007; Gelfand, et al., 2005; Madland, et al., 2005; Pedersen, et al., 2008; Reich, et al., 2009; Shbeeb, et al., 2000; Trontzas, et al., 2005; Wilson, et al., 2009b), lending credibility to the claim that we were successful in locating most patients with diagnosed psoriatic arthritis at the time we collected our data. While one study from Greece suggested a dramatically lower prevalence, this may be an effect of a genetically different population as well as differences in environmental exposures and methodology (Alamanos, et al., 2003). 54
59 As described in the methods section, we first screened patients with psoriasis for psoriatic arthritis, and interestingly using this approach alone would have led to an estimate of approximately 100 per 100,000, which is what had been previously reported at the time based on the population of Rochester county (Shbeeb, et al., 2000). But expanding our methods to find patients with the diagnosis of psoriatic arthritis regardless of their psoriasis status led to a higher prevalence estimate. This is particularly interesting as results based on the same Rochester county data published in 2000, but now adopting the method of looking for a diagnosis of psoriatic arthritis regardless of the presence of a diagnosis of psoriasis as we have done in this thesis, were recently published, and the updated point prevalence for the year 2000 was found to be 158 per 100,000 very close indeed to our point prevalence estimate of 163 per 100,000 in Iceland in 2002 (Wilson, et al., 2009b). Estimating the prevalence of a disease based on diagnosed cases only always excludes from consideration cases that are present in the population but have not yet been diagnosed. We saw evidence of this in our efforts as the prevalence of psoriatic arthritis appeared to decrease linearly with increasing distance from the Reykjavik area, as would be expected given the near complete absence of rheumatology practices outside of Reykjavik. Therefore, our prevalence estimate must be seen as a minimum number, and the true prevalence is likely higher. With increasing awareness and focus on psoriatic arthritis, it is possible that more cases are already being diagnosed than before. It could therefore be interesting to repeat this study and compare the current prevalence of diagnosed disease to what we observed 10 years ago. However, given the time and effort required to repeat a prevalence study it seems prudent to recommend that resources should rather be focused on better understanding the pathology of this complex disorder. 5.3 Demographics and disease activity Our findings regarding the demographic composition of our group of psoriatic arthritis patients were in line with what previous groups have reported with regards to age. However, we found that psoriatic arthritis patients in Iceland are more likely to be women, while most other large series have reported a male:female ratio of close to 1:1 (Kane et al., 2003; Lopez- Montilla et al., 2002; Shbeeb, et al., 2000; Stern, 1985; Svensson, et al., 2002). On the other hand, some smaller patient collections have shown a similar predilection for women (Alenius et al., 2002; Green et al., 1981; McHugh et al., 2003). The most obvious potential explanation for our finding is that due to an unmeasured bias, for instance among patients and/or physicians in Iceland, male patients with psoriatic arthritis are less likely to present to a 55
60 physician and/or are less likely to receive a diagnosis of psoriatic arthritis than women. However, we have no data to support that this is, in fact, the explanation for our observation, nor do we think it would be possible to measure this type of bias reliably. The most prudent interpretation of our finding is therefore that although interesting it should not be generalized to other populations as evidence that this gender bias is an inherent feature of psoriatic arthritis unless similar data surface in other large studies. Age at disease onset and disease activity were similar in our group to that reported from other groups (Lihi Eder et al., 2011; Gladman, et al., 1987; Wilson, et al., 2009a), including an earlier onset of disease in women, both in skin and joints. Our finding that most patients develop joint disease after developing skin disease is also in line with previous reports. Skin and joint activity scores were similar to those reported in other publications on unselected patients with psoriatic arthritis (Lindqvist et al., 2008; Reich, et al., 2009). Perhaps the most striking finding in our prevalence study was that of a dramatic reduction in prevalence after age 67. Among the potential explanations would be that older patients are more likely to have any arthritis dismissed as age-related changes or osteoarthritis, and would therefore be less likely to receive a diagnosis of psoriatic arthritis. Similarly, older patients may have been diagnosed with rheumatoid arthritis due to lack of awareness of the psoriatic phenotype and its distinct features. However, as these hypotheses are difficult to test, one cannot exclude the possibility that psoriatic arthritis patients die at an earlier age than their peers, causing a drop in measured prevalence of the disease. This hypothesis is supported by data from Toronto, showing an increased standardized mortality rate among psoriatic arthritis patients, compared to the general population, and that the excess deaths may be from coronary artery disease (Wong, et al., 1997). Combined with a large and growing literature on the association between autoimmune diseases such as rheumatoid arthritis and psoriasis on the one hand and cardiovascular disease on the other, the hypothesis of psoriatic arthritis causing increased mortality seems increasingly plausible. At this time there is no population based study available on the relative risk for cardiovascular disease in psoriatic arthritis patients compared to the general population, but some cross sectional studies seem to hint at such an association (Gladman, et al., 2009; Han, et al., 2006; Tam et al., 2008). We are currently planning studies of mortality and cardiovascular disease in the Icelandic population, as well as collaborating with others on similar studies using British healthcare databases. Initial findings from the latter cohort did not reveal an increased mortality among psoriatic arthritis patients (unpublished data). 56
61 5.4 Disease course and treatment Oligoarthritis was the most common pattern of initial joint involvement, but was the pattern in less than half the patients at the time they were examined for this study. Patients commonly expressed a different disease pattern at the time of our examination than what they had experienced at the onset of their disease, supporting the idea that psoriatic arthritis is a fluctuating disease and that the number of joints involved and the presence or absence of symmetry may not reflect any inherent properties of the disease (Helliwell, 2009; Helliwell, et al., 2000; Helliwell, et al., 2007). Therefore, these types of disease patterns should not be relied on to guide treatment or to differentiate psoriatic arthritis from other types of arthritis. These observations were limited by our design as we relied on patient recall of the symptoms at the onset of disease, but their agreement with the observations of others suggest the data we present here are indeed accurate (Lindqvist, et al., 2008; McHugh, et al., 2003). More than half of the patients had received some form of oral disease modifying therapy. The high proportion of patients being treated with NSAIDs only may reflect the treatment paradigm at the time, as psoriatic arthritis was regarded a mild form of inflammatory arthritis that often responded well to conservative treatment (Gladman, 2009). However, given the unfavorable long term safety profile of NSAID medications and new insights into the true nature of psoriatic arthritis as an inflammatory, destructive arthritis, it is likely that today we would observe a different treatment pattern (Ash, et al., 2012; Gossec, et al., 2012). 5.5 Familiality Psoriatic arthritis was found to be an order of magnitude more familial than rheumatoid arthritis in our study. Previous proband based studies had suggested that the sibling recurrence ratio was high, but suffered from crude estimates due to uncertainty regarding the true underlying prevalence of psoriatic arthritis in the population. As an example, one study used the prevalence of psoriatic arthritis among siblings of patients in Canada as the nominator and the prevalence of psoriatic arthritis in the United States found in a telephone survey as the denominator to calculate a sibling recurrence rate of 30 (Chandran et al., 2009). However, using the prevalence estimate from Rochester county in 2000 would have resulted in an estimate of 76, while the revised prevalence figure from 2011 from the same data source would have given a figure of 47.5 (Shbeeb, et al., 2000; Wilson, et al., 2009b). This flaw in the traditional method of estimating the sibling recurrence risk has been elegantly demonstrated by others, and should be taken into account when comparing our results to those of other studies (Guo, 1998). 57
62 The population based estimate presented here represents the only study to date of the familiality of psoriatic arthritis performed in a population where the underlying population prevalence was known with reasonable certainty. Even more importantly, the methods we used relied neither on this number nor the traditional method of sibling recurrence risk estimation, and our results are therefore not subject to the shortcomings described above. By taking advantage of the genealogy database of decode genetics we have arrived at a more accurate figure for first degree relative risk than was previously possible, and have furthermore for the first time published data on the recurrence risk in more distantly related individuals than would be possible in a traditional familial recurrence study. Our description of how the genetic effect is attenuated with the number of meioses separating individuals is new and unique in the literature. It is particularly informative as it suggests a very strong genetic effect, yet the pattern is not compatible with a simple genetic disease but rather a complex multigenic disease. This finding of a strong familiality emphasizes the importance of genetic studies as a method of understanding the pathology of this complex disease. But the incomplete penetrance suggests that there is also an important role for a trigger, such as an infectious agent, physical or mental trauma. This author has demonstrated elsewhere that adiposity increases the risk for psoriatic arthritis (Love, Zhu, et al., 2012). Other studies have suggested trauma as a trigger (Doury, 1993; Olivieri et al., 1991; Pages et al., 1992; Punzi et al., 1998). These findings have led us to plan studies to explore trauma, infections, vaccinations, pregnancy, smoking and alcohol as potential risk factor for psoriatic arthritis. 5.6 Nail disease and small joint involvement Our finding of an association between nail disease in psoriatic arthritis and involvement of small joints in general was expected, based on a previous report of an association between distal interphalangeal (DIP) joint involvement and nail changes (Scarpa, et al., 2006). The exclusive association with onycholysis was not expected, although we have since found literature that provided some hints of such a relationship (Torre Alonso, et al., 1991), including descriptions from one of the pioneering descriptive studies of psoriatic arthritis nearly 40 years ago (Eastmond & Wright, 1979). This knowledge appears to have been lost in the interim as most recent studies refer to nail changes as a group without differentiating between the clearly different morphologies involved. The findings from this study caused a change in our planned aim for the final paper presented in this thesis, in that we changed our data analysis plan so that we might more rigorously address the question of differential associations between various subtypes of nail changes and psoriatic arthritis. 58
63 5.7 Onycholysis and arthritis in psoriasis The results from the last paper presented in this thesis clearly demonstrate that onycholysis is more strongly associated with arthritis than either pitting or subungual hyperkeratosis, and that once those patients who have both onycholysis and pitting have been removed from the pitting group it becomes clear that the latter is probably not associated with arthritis at all, as was suggested in early studies of psoriatic arthritis (Eastmond & Wright, 1979). Even more striking is the trend towards a negative association between pitting and the number of affected joints, while the skin score is positively associated with pitting. Finally, the observation that the presence of onycholysis is associated with a higher number of tender joints seems to further underline the importance of this specific nail change in psoriatic arthritis. The results are strengthened by the fact that we present two distinct analyses performed at two points in time by two independent assessors. The first assessment showed an independent association between onycholysis and arthritis, and the second assessment, performed at a different time by a different physician, showed a relationship with disease severity as well. Because we had already shown in our previous paper that onycholysis might be particularly important in psoriatic arthritis, we designed this part of the study rigorously to avoid potential problems with collinearity between different types of nail changes. Therefore, we believe that the statistical methods are sound, and that our findings are not a product of a flaw in our selected methods, but rather a reflection of a true association. A potential weakness of this study was the small size of the follow up group, and we present the post-hoc analysis data with the caveat that we have performed multiple tests in a small group. However, we are comfortable presenting these data in the manner we did due to the fact that they agree with our hypothesis to analyze data on more than 1,000 psoriasis patients, and furthermore they agree with published data from 20 and 40 years ago (Eastmond & Wright, 1979; Torre Alonso, et al., 1991). We would furthermore like to stress that we have not come across any analysis of subtypes of nail changes in psoriatic arthritis that contradicts our finding. Based on these findings we suggest that researchers exploring the relationship between nail changes and psoriatic disease always record and analyze their data based on at least the three subclasses of nail changes reported on here. 59
64 6 Conclusions Psoriatic arthritis is a complex disease which results in significant morbidity and increases mortality. It has received less attention than some other autoimmune diseases in the past, but a rapidly growing part of the medical literature now focuses on this important disease. The results presented in this thesis allow us to conclude that the prevalence of diagnosed psoriatic arthritis in Iceland in 2002 was at least 0.14% in the adult population, and that it was likely 0.16%. However, it does not answer the question of the true prevalence of psoriatic arthritis. Some cases were certainly missed, and undiagnosed psoriatic arthritis was not included. With increased awareness it is plausible that the number of diagnosed patients will grow, and a similar study performed today would perhaps result in a higher estimate. Taken together, these limitations mean that the figure presented should be interpreted as the minimum prevalence at the time of our ascertainment. However, our findings presented here give us and other researchers a starting point for future studies of psoriatic arthritis in Iceland and elsewhere, as well as solid baseline knowledge to build on when discussing the disease from an epidemiological and public health perspective. Although refinement of the prevalence estimate may be feasible, this should not be a high priority. There are many questions regarding the risk factors for and consequences of psoriatic arthritis that are more pressing. We also found that psoriatic arthritis is more common in women. This is at odds with most of the published literature on psoriatic arthritis, although some cohorts have a similar gender ratio. It is possible that this result is a consequence of a diagnostic bias, as men may not seek care as soon for arthritis, or may not bring up their psoriasis when examined by a rheumatologist. However, as these are speculations we cannot rule out based on our data that there is a real gender difference with respect to psoriatic arthritis prevalence and we encourage other researchers to look at their data closely with respect to potential gender differences in the disease phenotype. This should in many cases be possible within existing datasets, making this an effort that may deliver new insights without much financial cost. The disease pattern we describe of a changing phenotype is not a new finding and it is our opinion that it should not be explored further separately in other studies. However, prospective studies should publish these data as a secondary finding when possible, as this will over time clarify and solidify our understanding of the fluctuating nature of this disease Psoriatic arthritis is clearly a highly familial disease, and our results support and add to previously published data. These results emphasize the importance of genetic research in 60
65 psoriatic arthritis, but the need for understanding potential triggers for psoriatic arthritis is also pressing. The findings on familiality presented here should not be understood as evidence that the key to future treatment and prevention of psoriatic arthritis necessarily will be found through the study of genetics. On the contrary, we may find that the only way to understand and prevent this debilitating disease is to find, understand, and then inactivate or intercept the triggers that move an individual from a state of genetic susceptibility to a state of disease. One such insight into potential clues to who is at high risk for psoriatic arthritis and might benefit from preventive treatment comes from the observation that nail involvement can predict later development of arthritis in psoriasis patients (Wilson, et al., 2009a). This adds more weight to the finding we present of the specific importance of onycholysis in the context of arthritis. The next logical step would be to confirm that onycholysis is the most important factor in predicting the onset of arthritis later. Our group of over 800 psoriasis patients without arthritis examined carefully for subtypes of nail changes a decade ago gives us an opportunity to answer this question by conducting a study where we contact all of them to determine who has now developed arthritis. We hope to perform this study soon. Also of interest would be an exploration of the types of cells and cytokines one might find in affected nails compared to unaffected nails, but we have not designed such studies yet. Clearly there may be opportunities to utilize the nail as a window into the pathology that drives psoriatic disease and we have just begun to scratch the surface of understanding the processes involved. The future of research in the field of psoriatic arthritis is bright. With more young researchers adopting this disease for their career in research, partly through the efforts of such organizations as the Group for Research on Psoriasis and Psoriatic Arthritis (GRAPPA), there is a clear momentum towards improved diagnosis and treatment of this debilitating disease. Our research and that of others over the past decade has clarified many aspects of the phenotype of psoriatic arthritis but much work lies ahead in describing risk factors that lead to arthritis and comorbidities that accompany the disease. We intend to continue taking part in this effort and have planned studies that utilize the population presented here, as well as data on populations in the United Kingdom and the United States in collaboration with researchers at the University of Boston, University of Pennsylvania, and Harvard. Hopefully we will be able to continue contributing to a pool of knowledge that will lead us to a day when the diagnosis of psoriatic arthritis no longer carries with it the morbidity we now know it brings with it. 61
66 References Abuabara, K., Lee, H., & Kimball, A. B. (2011). The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br. J. Dermatol., 165(5), Ahlehoff, O., Skov, L., Gislason, G., Lindhardsen, J., Kristensen, S. L., Iversen, L., Lasthein, S., Gniadecki, R., Dam, T. N., Torp-Pedersen, C., & Hansen, P. R. (2012). Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J. Intern. Med. Alamanos, Y., Papadopoulos, N., Voulgari, P., Siozos, C., Psychos, D., Tympanidou, M., & Drosos, A. (2003). Epidemiology of psoriatic arthritis in northwest Greece, J. Rheumatol., 30(12), Alenius, G. M., Jidell, E., Nordmark, L., & Rantapaa Dahlqvist, S. (2002). Disease manifestations and HLA antigens in psoriatic arthritis in northern Sweden. Clin. Rheumatol., 21(5), Anandarajah, A., & Ritchlin, C. (2004). Pathogenesis of psoriatic arthritis. Curr. Opin. Rheumatol., 16(4), Ash, Z., Gaujoux-Viala, C., Gossec, L., Hensor, E. M., FitzGerald, O., Winthrop, K., van der Heijde, D., Emery, P., Smolen, J. S., & Marzo-Ortega, H. (2012). A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis., 71(3), Autoimmune Diseases Coordinating Committee, T. (2005). Progress in Autoimmune Disease Research: National Institutes of Health. Benjamin, M., & McGonagle, D. (2009). The enthesis organ concept and its relevance to the spondyloarthropathies. Adv. Exp. Med. Biol., 649, Bowes, J., & Barton, A. (2010). The genetics of psoriatic arthritis: lessons from genome-wide association studies. Discovery Medicine, 10(52),
67 Bowes, J., Ho, P., Flynn, E., Ali, F., Marzo-Ortega, H., Coates, L., Warren, R., McManus, R., Ryan, A., Kane, D., Korendowych, E., McHugh, N., Fitzgerald, O., Packham, J., Morgan, A., Bruce, I., & Barton, A. (2012). Comprehensive assessment of rheumatoid arthritis susceptibility loci in a large psoriatic arthritis cohort. Ann. Rheum. Dis., 71(8), Buckley, C., Cavill, C., Taylor, G., Kay, H., Waldron, N., Korendowych, E., & McHugh, N. (2010). Mortality in psoriatic arthritis - a single-center study from the UK. J. Rheumatol., 37(10), Cannon-Albright, L., Thomas, A., Goldgar, D., Gholami, K., Rowe, K., Jacobsen, M., McWhorter, W., & Skolnick, M. (1994). Familiality of cancer in Utah. Cancer Res., 54(9), Celis, R., Planell, N., Fernandez-Sueiro, J., Sanmarti, R., Ramirez, J., Gonzalez-Alvaro, I., Pablos, J., & Canete, J. (2012). Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Research & Therapy, 14(2), R93. Chandran, V., Schentag, C. T., Brockbank, J. E., Pellett, F. J., Shanmugarajah, S., Toloza, S. M., Rahman, P., & Gladman, D. D. (2009). Familial aggregation of psoriatic arthritis. Ann. Rheum. Dis., 68(5), Christophers, E. (2001). Psoriasis--epidemiology and clinical spectrum. Clin. Exp. Dermatol., 26(4), Coates, L., Conaghan, P., Emery, P., Green, M., Ibrahim, G., Maciver, H., & Helliwell, P. (2012). Investigating the use of the CASPAR criteria in early psoriatic arthritis. Arthritis Rheum. Corlan, A. D. (2011). Medline trend: automated yearly statistics of PubMed results for any query. Retrieved , 2012, from Cuéllar, M., Citera, G., & Espinoza, L. (1994). Treatment of psoriatic arthritis. Baillière's clinical rheumatology, 8(2),
68 De Angelis, R., Salaffi, F., & Grassi, W. (2007). Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand. J. Rheumatol., 36(1), de Cid, R., Riveira-Munoz, E., Zeeuwen, P., Robarge, J., Liao, W., Dannhauser, E., Giardina, E., Stuart, P., Nair, R., Helms, C., Escaramís, G., Ballana, E., Martín-Ezquerra, G., den Heijer, M., Kamsteeg, M., Joosten, I., Eichler, E., Lázaro, C., Pujol, R., Armengol, L., Abecasis, G., Elder, J., Novelli, G., Armour, J., Kwok, P.-Y., Bowcock, A., Schalkwijk, J., & Estivill, X. (2009). Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat. Genet., 41(2), Doury, P. (1993). Psoriatic arthritis with physical trauma. J. Rheumatol., 20(9), Eastmond, C. J., & Wright, V. (1979). The nail dystrophy of psoriatic arthritis. Ann. Rheum. Dis., 38(3), Eder, L., Chandran, V., Pellet, F., Shanmugarajah, S., Rosen, C. F., Bull, S. B., & Gladman, D. D. (2012). Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis., 71(1), Eder, L., Law, T., Chandran, V., Shanmugarajah, S., Shen, H., Rosen, C., Cook, R., & Gladman, D. (2011). Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis Care Res., 63(8), Ellinghaus, E., Ellinghaus, D., Stuart, P., Nair, R., Debrus, S., Raelson, J., Belouchi, M., Fournier, H., Reinhard, C., Ding, J., Li, Y., Tejasvi, T., Gudjonsson, J., Stoll, S., Voorhees, J., Lambert, S., Weidinger, S., Eberlein, B., Kunz, M., Rahman, P., Gladman, D., Gieger, C., Wichmann, H., Karlsen, T., Mayr, G., Albrecht, M., Kabelitz, D., Mrowietz, U., Abecasis, G., Elder, J., Schreiber, S., Weichenthal, M., & Franke, A. (2010). Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat. Genet., 42(11), Fearon, U., & Veale, D. (2001). Pathogenesis of psoriatic arthritis. Clin. Exp. Dermatol., 26(4),
69 Fernandez-Sueiro, J. L., Willisch, A., Pertega-Diaz, S., Tasende, J. A., Fernandez-Lopez, C., Galdo, F., & Blanco, F. J. (2009). Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum., 61(3), FitzGerald, O., Helliwell, P., Mease, P., Mumtaz, A., Coates, L., Pedersen, R., Nab, H., & Molta, C. (2012). Application of composite disease activity scores in psoriatic arthritis to the PRESTA data set. Ann. Rheum. Dis., 71(3), Gelfand, J. M., Gladman, D. D., Mease, P. J., Smith, N., Margolis, D. J., Nijsten, T., Stern, R. S., Feldman, S. R., & Rolstad, T. (2005). Epidemiology of psoriatic arthritis in the population of the United States. J. Am. Acad. Dermatol., 53(4), Gelfand, J. M., Neimann, A. L., Shin, D. B., Wang, X., Margolis, D. J., & Troxel, A. B. (2006). Risk of myocardial infarction in patients with psoriasis. JAMA, 296(14), Gladman, D. D. (1998). Clinical aspects of the spondyloarthropathies. Am. J. Med. Sci., 316(4), Gladman, D. D. (2009). Psoriatic arthritis from Wright's era until today. J. Rheumatol. Suppl., 83, 4-8. Gladman, D. D., Ang, M., Su, L., Tom, B., Schentag, C., & Farewell, V. (2009). Cardiovascular morbidity in psoriatic arthritis. Ann. Rheum. Dis., 68(7), Gladman, D. D., Farewell, V., Wong, K., & Husted, J. (1998). Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum., 41(6), Gladman, D. D., Helliwell, P., Mease, P. J., Nash, P., Ritchlin, C., & Taylor, W. (2004). Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum., 50(1), Gladman, D. D., Mease, P. J., Healy, P., Helliwell, P. S., Fitzgerald, O., Cauli, A., Lubrano, E., Krueger, G. G., van der Heijde, D., Veale, D. J., Kavanaugh, A., Nash, P., Ritchlin, 65
70 C., Taylor, W., & Strand, V. (2007). Outcome measures in psoriatic arthritis. J. Rheumatol., 34(5), Gladman, D. D., Mease, P. J., Strand, V., Healy, P., Helliwell, P. S., Fitzgerald, O., Gottlieb, A. B., Krueger, G. G., Nash, P., Ritchlin, C. T., Taylor, W., Adebajo, A., Braun, J., Cauli, A., Carneiro, S., Choy, E., Dijkmans, B., Espinoza, L., van der Heijde, D., Husni, E., Lubrano, E., McGonagle, D., Qureshi, A., Soriano, E. R., & Zochling, J. (2007). Consensus on a core set of domains for psoriatic arthritis. J. Rheumatol., 34(5), Gladman, D. D., Shuckett, R., Russell, M., Thorne, J., & Schachter, R. (1987). Psoriatic arthritis (PSA)--an analysis of 220 patients. The Quarterly journal of medicine, 62(238), Gossec, L., Smolen, J., Gaujoux-Viala, C., Ash, Z., Marzo-Ortega, H., van der Heijde, D., FitzGerald, O., Aletaha, D., Balint, P., Boumpas, D., Braun, J., Breedveld, F., Burmester, G., Cañete, J., de Wit, M., Dagfinrud, H., de Vlam, K., Dougados, M., Helliwell, P., Kavanaugh, A., Kvien, T., Landewé, R., Luger, T., Maccarone, M., McGonagle, D., McHugh, N., McInnes, I., Ritchlin, C., Sieper, J., Tak, P., Valesini, G., Vencovsky, J., Winthrop, K., Zink, A., Emery, P., & European League Against, R. (2012). European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann. Rheum. Dis., 71(1), Gottlieb, A., Menter, A., Mendelsohn, A., Shen, Y.-K., Li, S., Guzzo, C., Fretzin, S., Kunynetz, R., & Kavanaugh, A. (2009). Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebocontrolled, crossover trial. Lancet, 373(9664), Green, L., Meyers, O. L., Gordon, W., & Briggs, B. (1981). Arthritis in psoriasis. Ann. Rheum. Dis., 40(4), Griffiths, C., Strober, B., van de Kerkhof, P., Ho, V., Fidelus-Gort, R., Yeilding, N., Guzzo, C., Xia, Y., Zhou, B., Li, S., Dooley, L., Goldstein, N., Menter, A., & Group, A. S. (2010). Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med., 362(2),
71 Gudbjornsson, B., Ejstrup, L., Gran, J. T., L., I., Lindqvist, U., Paimela, L., Ternowitz, T., & Stahle, M. (2012). Psoriatic Arthritis Mutilans in the Nordic Countries; Demographics and Disease Status. The Nordic PAM Study. Scand. J. Rheumatol., 41(s126), 106. Gudjonsson, J. E., Karason, A., Runarsdottir, E., Antonsdottir, A., Hauksson, V., Jónsson, H., Gulcher, J., Stefansson, K., & Valdimarsson, H. (2006). Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients--an analysis of 1019 HLA-C- and HLA-B-typed patients. J. Invest. Dermatol., 126(4), Guo, S. (1998). Inflation of sibling recurrence-risk ratio, due to ascertainment bias and/or overreporting. Am. J. Hum. Genet., 63(1), Han, C., Robinson, D., Hackett, M., Paramore, L., Fraeman, K., & Bala, M. (2006). Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J. Rheumatol., 33(11), Healy, P. J., & Helliwell, P. S. (2007). Measuring dactylitis in clinical trials: which is the best instrument to use? J. Rheumatol., 34(6), Healy, P. J., & Helliwell, P. S. (2008). Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum., 59(5), Hellgren, L. (1969). Association between rheumatoid arthritis and psoriasis in total populations. Acta Rheumatol. Scand., 15(4), Helliwell, P. (2009). Established psoriatic arthritis: clinical aspects. J. Rheumatol. Suppl., 83, Helliwell, P., Fitzgerald, O., & Mease, P. (2012). Development of composite measures for psoriatic arthritis: a report from the GRAPPA 2010 annual meeting. J. Rheumatol., 39(2), Helliwell, P., Hetthen, J., Sokoll, K., Green, M., Marchesoni, A., Lubrano, E., Veale, D., & Emery, P. (2000). Joint symmetry in early and late rheumatoid and psoriatic arthritis: comparison with a mathematical model. Arthritis Rheum., 43(4),
72 Helliwell, P., Porter, G., Taylor, W., & Group, C. S. (2007). Polyarticular psoriatic arthritis is more like oligoarticular psoriatic arthritis, than rheumatoid arthritis. Ann. Rheum. Dis., 66(1), Ho, P., Barton, A., Worthington, J., Thomson, W., Silman, A., & Bruce, I. (2007). HLA-Cw6 and HLA-DRB1*07 together are associated with less severe joint disease in psoriatic arthritis. Ann. Rheum. Dis., 66(6), Hüffmeier, U., Estivill, X., Riveira-Munoz, E., Traupe, H., Wendler, J., Lohmann, J., Böhm, B., Burkhardt, H., & Reis, A. (2010). Deletion of LCE3C and LCE3B genes at PSORS4 does not contribute to susceptibility to psoriatic arthritis in German patients. Ann. Rheum. Dis., 69(5), Hüffmeier, U., Uebe, S., Ekici, A., Bowes, J., Giardina, E., Korendowych, E., Juneblad, K., Apel, M., McManus, R., Ho, P., Bruce, I., Ryan, A., Behrens, F., Lascorz, J., Böhm, B., Traupe, H., Lohmann, J., Gieger, C., Wichmann, H.-E., Herold, C., Steffens, M., Klareskog, L., Wienker, T., Fitzgerald, O., Alenius, G.-M., McHugh, N., Novelli, G., Burkhardt, H., Barton, A., & Reis, A. (2010). Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet., 42(11), Hukuda, S., Minami, M., Saito, T., Mitsui, H., Matsui, N., Komatsubara, Y., Makino, H., Shibata, T., Shingu, M., Sakou, T., & Shichikawa, K. (2001). Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J. Rheumatol., 28(3), Jiaravuthisan, M. M., Sasseville, D., Vender, R. B., Murphy, F., & Muhn, C. Y. (2007). Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J. Am. Acad. Dermatol., 57(1), Johnston, A., Gudjonsson, J. E., Sigmundsdottir, H., Love, T. J., & Valdimarsson, H. (2004). Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin. Exp. Immunol., 138(1),
73 Jones, S. M., J.B., A., M.G., C., C.R., L., G., E., & N.J., M. (1994). Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Br. J. Rheumatol., 33(9), Kaipiainen-Seppänen, O. (1996). Incidence of psoriatic arthritis in Finland. Br. J. Rheumatol., 35(12), Kane, D., Stafford, L., Bresnihan, B., & FitzGerald, O. (2003). A classification study of clinical subsets in an inception cohort of early psoriatic peripheral arthritis--'dip or not DIP revisited'. Rheumatology (Oxford). 42(12), Karason, A., Gudjonsson, J., Upmanyu, R., Antonsdottir, A., Hauksson, V., Runasdottir, E., Jonsson, H., Gudbjartsson, D., Frigge, M., Kong, A., Stefansson, K., Valdimarsson, H., & Gulcher, J. (2003). A susceptibility gene for psoriatic arthritis maps to chromosome 16q: evidence for imprinting. Am. J. Hum. Genet., 72(1), Karason, A., Love, T. J., & Gudbjornsson, B. (2009). A strong heritability of psoriatic arthritis over four generations--the Reykjavik Psoriatic Arthritis Study. Rheumatology (Oxford). 48(11), Kingsley, G., Kowalczyk, A., Taylor, H., Ibrahim, F., Packham, J., McHugh, N., Mulherin, D., Kitas, G., Chakravarty, K., Tom, B., O'Keeffe, A., Maddison, P., & Scott, D. (2012). A randomized placebo-controlled trial of MTX in PsA. Rheumatology (Oxford). 51(8), Korendowych, E., Owen, P., Ravindran, J., Carmichael, C., & McHugh, N. (2005). The clinical and genetic associations of anti-cyclic citrullinated peptide antibodies in psoriatic arthritis. Rheumatology (Oxford). 44(8), Lavaroni, G., Kokelj, F., Pauluzzi, P., & Trevisan, G. (1994). The nails in psoriatic arthritis. Acta Derm. Venereol. Suppl. (Stockh). 186, 113. Leonardi, C., Matheson, R., Zachariae, C., Cameron, G., Li, L., Edson-Heredia, E., Braun, D., & Banerjee, S. (2012). Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med., 366(13),
74 Li, W. Q., Han, J. L., Manson, J., Rimm, E., Rexrode, K., Curhan, G., & Qureshi, A. (2012). Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br. J. Dermatol., 166(4), Lindqvist, U. R., Alenius, G. M., Husmark, T., Theander, E., Holmstrom, G., & Larsson, P. T. (2008). The Swedish early psoriatic arthritis register-- 2-year followup: a comparison with early rheumatoid arthritis. J. Rheumatol., 35(4), Lomholt, G. (1963). Psoriasis: Prevalence, Spontaneous Course and Genetics: a Census Study on the Prevalence of Skin Diseases on the Faroe Islands. Copenhagen: GEC GAD. Lopez-Montilla, M. D., Gonzalez, J., Martinez, F. G., Fernandez-Moreno, J. R., & Collantes, E. (2002). Clinical features of late onset psoriatic arthritis. Exp. Gerontol., 37(2-3), Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2006). Hospital Records and Self Report from Psoriasis Patients Have Similar Specificity for Locating Independently Verifiable Cases of Psoriatic Arthritis. Arthritis Rheum., 54(9S), S753. Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2007). Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J. Rheumatol., 34(10), Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2010). Small joint involvement in psoriatic arthritis is associated with onycholysis: the Reykjavik Psoriatic Arthritis Study. Scand. J. Rheumatol., 39(4), Love, T. J., Gudjonsson, J. E., Valdimarsson, H., & Gudbjornsson, B. (2012). Psoriatic Arthritis and Onycholysis -- Results from the Cross-sectional Reykjavik Psoriatic Arthritis Study. J. Rheumatol., 39(7), Love, T. J., Qureshi, A. A., Karlson, E. W., Gelfand, J. M., & Choi, H. K. (2011). Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, Arch. Dermatol., 147(4),
75 Love, T. J., Zhu, Y., Zhang, Y., Wall-Burns, L., Ogdie, A., Gelfand, J. M., & Choi, H. K. (2012). Obesity and the risk of psoriatic arthritis: a population-based study. Ann. Rheum. Dis., 71(8), Lowes, M., Kikuchi, T., Fuentes-Duculan, J., Cardinale, I., Zaba, L., Haider, A., Bowman, E., & Krueger, J. (2008). Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. The Journal of Investigative Dermatology, 128(5), Madland, T. M., Apalset, E. M., Johannessen, A. E., Rossebo, B., & Brun, J. G. (2005). Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J. Rheumatol., 32(10), Maeda, S., Hayami, Y., Naniwa, T., & Ueda, R. (2012). The Th17/IL-23 Axis and Natural Immunity in Psoriatic Arthritis. International Journal of Rheumatology, 2012(2012). Retrieved from doi: /2012/ McGonagle, D., Lories, R., Tan, A., & Benjamin, M. (2007). The concept of a "synovioentheseal complex" and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum., 56(8), McHugh, N. J., Balachrishnan, C., & Jones, S. M. (2003). Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford). 42(6), Mease, P. (2011). Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mnapsi), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res., 63(S11), S64-S85. 71
76 Mease, P., Genovese, M., Gladstein, G., Kivitz, A., Ritchlin, C., Tak, P., Wollenhaupt, J., Bahary, O., Becker, J.-C., Kelly, S., Sigal, L., Teng, J., & Gladman, D. (2011). Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum., 63(4), Moll, J., & Wright, V. (1973a). Familial occurrence of psoriatic arthritis. Ann. Rheum. Dis., 32(3), Moll, J., & Wright, V. (1973b). Psoriatic arthritis. Semin. Arthritis Rheum., 3(1), Myers, A., Kay, L., Lynch, S., & Walker, D. (2005). Recurrence risk for psoriasis and psoriatic arthritis within sibships. Rheumatology (Oxford). 44(6), Naredo, E., Möller, I., de Miguel, E., Batlle-Gualda, E., Acebes, C., Brito, E., Mayordomo, L., Moragues, C., Uson, J., de Agustín, J., Martínez, A., Rejón, E., Rodriguez, A., Daudén, E., Ultrasound School of the Spanish Society of, R., & Spanish, E. C. O. A. G. (2011). High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford). 50(10), Narváez, J., Narváez, J., de Albert, M., Gómez-Vaquero, C., & Nolla, J. (2012). Can Magnetic Resonance Imaging of the Hand and Wrist Differentiate Between Rheumatoid Arthritis and Psoriatic Arthritis in the Early Stages of the Disease? Semin. Arthritis Rheum. Nestle, F. O., Kaplan, D. H., & Barker, J. (2009). Psoriasis. N. Engl. J. Med., 361(5), Olivieri, I., Gemignani, G., Christou, C., Semeria, R., Giustarini, S., & Pasero, G. (1991). The triggering role of physical injury in the onset of peripheral arthritis in seronegative spondyloarthropathy. Rheumatol. Int., 10(6), Olivieri, I., Padula, A., D'Angelo, S., & Cutro, M. (2009). Psoriatic arthritis sine psoriasis. J. Rheumatol. Suppl., 83,
77 Pages, M., Lassoued, S., Fournie, B., & Fournie, A. (1992). Psoriatic arthritis precipitated by physical trauma: destructive arthritis or associated with reflex sympathetic dystrophy? J. Rheumatol., 19(1), Papp, K. A., Leonardi, C., Menter, A., Ortonne, J. P., Krueger, J. G., Kricorian, G., Aras, G., Li, J., Russell, C. B., Thompson, E. H., & Baumgartner, S. (2012). Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med., 366(13), Parisi, R., Symmons, D. P., Griffiths, C. E., & Ashcroft, D. M. (2012). Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Invest. Dermatol. Pedersen, O. B., Svendsen, A. J., Ejstrup, L., Skytthe, A., & Junker, P. (2008). The occurrence of psoriatic arthritis in Denmark. Ann. Rheum. Dis., 67(10), Peters, M., Symmons, D., McCarey, D., Dijkmans, B., Nicola, P., Kvien, T., McInnes, I., Haentzschel, H., Gonzalez-Gay, M., Provan, S., Semb, A., Sidiropoulos, P., Kitas, G., Smulders, Y., Soubrier, M., Szekanecz, Z., Sattar, N., & Nurmohamed, M. (2010). EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis., 69(2), Punzi, L., Pianon, M., Bertazzolo, N., Fagiolo, U., Rizzi, E., Rossini, P., & Todesco, S. (1998). Clinical, laboratory and immunogenetic aspects of post-traumatic psoriatic arthritis: a study of 25 patients. Clin. Exp. Rheumatol., 16(3), Queiro, R., Gonzalez, S., López-Larrea, C., Alperi, M., Sarasqueta, C., Riestra, J., & Ballina, J. (2006). HLA-C locus alleles may modulate the clinical expression of psoriatic arthritis. Arthritis Research & Therapy, 8(6), R185. Qureshi, A. A., Choi, H. K., Setty, A. R., & Curhan, G. C. (2009). Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch. Dermatol., 145(4), Rahman, P., & Elder, J. (2012). Genetics of psoriasis and psoriatic arthritis: a report from the GRAPPA 2010 annual meeting. J. Rheumatol., 39(2),
78 Rapp, S. R., Feldman, S. R., Exum, M. L., Fleischer, A. B., Jr., & Reboussin, D. M. (1999). Psoriasis causes as much disability as other major medical diseases. J. Am. Acad. Dermatol., 41(3 Pt 1), Reece, R., Canete, J., Parsons, W., Emery, P., & Veale, D. (1999). Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum., 42(7), Rehal, B., Modjtahedi, B. S., Morse, L. S., Schwab, I. R., & Maibach, H. I. (2011). Ocular psoriasis. J. Am. Acad. Dermatol., 65(6), Reich, K., Kruger, K., Mossner, R., & Augustin, M. (2009). Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br. J. Dermatol., 160(5), Risch, N. (1990). Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet, 46(2), Robertson, J. C., & Braune, M. L. (1974). Splinter haemorrhages, pitting, and other findings in fingernails of healthy adults. Br. Med. J., 4(5939), Salvarani, C., Cantini, F., Olivieri, I., Macchioni, P., Niccoli, L., Padula, A., Ferri, S., & Portioli, I. (1997). Isolated peripheral enthesitis and/or dactylitis: a subset of psoriatic arthritis. J. Rheumatol., 24(6), Savolainen, E., Kaipiainen-Seppänen, O., Kröger, L., & Luosujärvi, R. (2003). Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J. Rheumatol., 30(11), Scarpa, R., Oriente, P., Pucino, A., Torella, M., Vignone, L., Riccio, A., & Biondi Oriente, C. (1984). Psoriatic arthritis in psoriatic patients. Br. J. Rheumatol., 23(4), Scarpa, R., Soscia, E., Peluso, R., Atteno, M., Manguso, F., Del Puente, A., Spano, A., Sirignano, C., Oriente, A., Di Minno, M. N., Iervolino, S., & Salvatore, M. (2006). Nail and distal interphalangeal joint in psoriatic arthritis. J. Rheumatol., 33(7),
79 Semb, A., Kvien, T., Demicco, D., Fayyad, R., Wun, C.-C., Larosa, J., Betteridge, J., Pedersen, T., & Holme, I. (2012). Effect of intensive lipid lowering on cardiovascular outcome in patients with and without inflammatory joint disease. Arthritis Rheum. Setty, A. R., & Choi, H. K. (2007). Psoriatic arthritis epidemiology. Current Rheumatology Reports, 9(6), Shbeeb, M., Uramoto, K. M., Gibson, L. E., O'Fallon, W. M., & Gabriel, S. E. (2000). The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, J. Rheumatol., 27(5), Sigurgeirsson, B., Steingrimsson, O., & Sveinsdottir, S. (2002). Prevalence of onychomycosis in Iceland: a population-based study. Acta Derm. Venereol., 82(6), Soriano, E. R. (2012). The actual role of therapy with traditional disease-modifying antirheumatic drugs in psoriatic arthritis. J. Rheumatol. Suppl., 89, Stern, R. S. (1985). The epidemiology of joint complaints in patients with psoriasis. J. Rheumatol., 12(2), Stuart, P., Nair, R., Ellinghaus, E., Ding, J., Tejasvi, T., Gudjonsson, J., Li, Y., Weidinger, S., Eberlein, B., Gieger, C., Wichmann, H., Kunz, M., Ike, R., Krueger, G., Bowcock, A., Mrowietz, U., Lim, H., Voorhees, J., Abecasis, G., Weichenthal, M., Franke, A., Rahman, P., Gladman, D., & Elder, J. (2010). Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet., 42(11), Svensson, B., Holmström, G., Lindqvist, U., & Psoriatric Arthritis Register Group of the Swedish Society for, R. (2002). Development and early experiences of a Swedish psoriatic arthritis register. Scand. J. Rheumatol., 31(4), Söderlin, M., Börjesson, O., Kautiainen, H., Skogh, T., & Leirisalo-Repo, M. (2002). Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann. Rheum. Dis., 61(10), Tam, L. S., Tomlinson, B., Chu, T., Li, M., Leung, Y. Y., Kwok, L. W., Li, T., Yu, T., Zhu, Y. E., Wong, K. C., Kun, E., & Li, E. (2008). Cardiovascular risk profile of patients 75
80 with psoriatic arthritis compared to controls--the role of inflammation. Rheumatology (Oxford). 47(5), Tan, A. L., Benjamin, M., Toumi, H., Grainger, A. J., Tanner, S. F., Emery, P., & McGonagle, D. (2007). The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis--a high-resolution MRI and histological study. Rheumatology (Oxford). 46(2), Taylor, W., Gladman, D., Helliwell, P., Marchesoni, A., Mease, P., Mielants, H., & Group, C. S. (2006). Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum., 54(8), Tejasvi, T., Stuart, P., Chandran, V., Voorhees, J., Gladman, D., Rahman, P., Elder, J., & Nair, R. (2012). TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis. The Journal of Investigative Dermatology, 132(3 Pt 1), Tillett, W., Costa, L., Jadon, D., Wallis, D., Cavill, C., McHugh, J., Korendowych, E., & McHugh, N. (2012). The ClASsification for Psoriatic ARthritis (CASPAR) criteria--a retrospective feasibility, sensitivity, and specificity study. J. Rheumatol., 39(1), Torre Alonso, J. C., Rodriguez Perez, A., Arribas Castrillo, J. M., Ballina Garcia, J., Riestra Noriega, J. L., & Lopez Larrea, C. (1991). Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br. J. Rheumatol., 30(4), Trontzas, P., Andrianakos, A., Miyakis, S., Pantelidou, K., Vafiadou, E., Garantziotou, V., & Voudouris, C. (2005). Seronegative spondyloarthropathies in Greece: a populationbased study of prevalence, clinical pattern, and management. The ESORDIG study. Clin. Rheumatol., 24(6), van den Berg, R., van Gaalen, F., van der Helm-van Mil, A., Huizinga, T., & van der Heijde, D. (2012). Performance of classification criteria for peripheral spondyloarthritis and psoriatic arthritis in the Leiden early arthritis cohort. Ann. Rheum. Dis., 71(8),
81 van Romunde, L. K., Valkenburg, H. A., Swart-Bruinsma, W., Cats, A., & Hermans, J. (1984). Psoriasis and arthritis. I. A population study. Rheumatol. Int., 4(2), Wassenberg, S., Fischer-Kahle, V., Herborn, G., & Rau, R. (2001). A method to score radiographic change in psoriatic arthritis. Z. Rheumatol., 60(3), Werner, S., Langer, H.-E., Kurtz, B., Lind-Albrecht, G., & Bahner, M. (2010). Xiralite, a New Method for Visualization of Inflammation in Finger- and Wrist- joints. Ann. Rheum. Dis., 69(S3), 120. Wilson, F., Icen, M., Crowson, C., McEvoy, M., Gabriel, S., & Kremers, H. (2009a). Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum., 61(2), Wilson, F., Icen, M., Crowson, C., McEvoy, M., Gabriel, S., & Kremers, H. (2009b). Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J. Rheumatol., 36(2), Wong, K., Gladman, D. D., Husted, J., Long, J. A., & Farewell, V. T. (1997). Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum., 40(10), Zachariae, H., Zachariae, R., Blomqvist, K., Davidsson, S., Molin, L., Mork, C., & Sigurgeirsson, B. (2002). Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm. Venereol., 82(2),
82 78
83 Original publications The following pages contain reprints of the original publications that form the basis of this thesis, and are referred to in the text. While the Scandinavian Journal of Rheumatology (III) and Rheumatology (Oxford) (II) granted permission to reprint the published versions of the papers, the Journal of Rheumatology (I & IV) requested that the submitted, rather than the copy-edited, versions be included here. Hence the format changes between portable document format (.pdf) and Microsoft Word. The papers reprinted are: I. pp Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2007). Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. The Journal of Rheumatology, 34(10), II. pp Karason, A., Love, T., & Gudbjornsson, B. (2009). A strong heritability of psoriatic arthritis over four generations--the Reykjavik Psoriatic Arthritis Study. Rheumatology (Oxford, England), 48(11), III. pp Love, T. J., Gudbjornsson, B., Gudjonsson, J. E., & Valdimarsson, H. (2010). Small joint involvement in psoriatic arthritis is associated with onycholysis: the Reykjavik Psoriatic Arthritis Study. Scandinavian Journal of Rheumatology, 34(10), IV. pp Love, T. J., Gudjonsson, J. E., Valdimarsson, H., & Gudbjornsson, B. (2012). Psoriatic Arthritis and Onycholysis -- Results from the Cross-sectional Reykjavik Psoriatic Arthritis Study. The Journal of Rheumatology, 39(7),
84 80
85 81 PAPER I
86 TITLE PAGE Title: Psoriatic Arthritis in Reykjavik, Iceland: Prevalence, Demographics and Disease Course. Authors: 1. Thorvardur Jon Loeve Department of Immunology, Landspitali University Hospital Reykjavik, Iceland 2. Bjorn Gudbjornsson Centre for Rheumatology Research Landspitali University Hospital Reykjavik, Iceland 3. Johann Eli Gudjonsson Department of Dermatology University of Michigan Ann Arbor, MI, USA 4. Helgi Valdimarsson Department of Immunology Landspitali University Hospital Reykjavik, Iceland Abstract: Please refer to the next page for the abstract. Key Indexing Terms: Psoriatic Arthritis; Prevalence; Demographics, Disease Course; Sex. Department(s) and institution(s) to which the work should be attributed: 1. Department of Immunology, Landspitali University Hospital, Iceland. 2. Centre for Rheumatology Research, Landspitali University Hospital, Iceland. Source(s) of support in the form of grants or industrial support: Grant from the Science Fund of Landspitali University Hospital Grant from the Research Fund of the Icelandic College of Rheumatology Initials, surnames, appointments and highest academic degrees of all authors: 1. TJ, LOEVE 3. JE, GUDJONSSON Title: Resident Doctor Title: Resident Doctor Highest Degree: MD Highest Degree: PhD 2. B, GUDBJORNSSON Title: Assoc. Professor Highest Degree: PhD 4. H, VALDIMARSSON Title: Professor Highest Degree: PhD Corresponding Author / Requests for Reprints: Bjorn Gudbjornsson Centre for Rheumatology Research Landspitali University Hospital 101 Reykjavik Iceland Tel: Fax: [email protected] Running footer: Prevalence of Psoriatic Arthritis 82
87 ABSTRACT Objectives: Determine the prevalence, demographics, and course of psoriatic arthritis (PsA) in the Reykjavik area of Iceland. Methods: Two hundred and twenty patients, 18 years of age or older, living in the Reykjavik area of Iceland, were located in a community registry of psoriatic patients and in hospital records. Of these, 156 (71%) were interviewed and examined for verification of skin and joint disease according to published criteria. Results: Prevalence of PsA in the adult population was estimated to be 164 per 100,000 (95% CI ), adjusted to 139 per 100,000 (95% CI ) after exclusion of 25 individuals. The female to male ratio was close to 2:1. The mean age of skin disease onset was 23 years, with significantly earlier onset in women (20 vs. 26 years; p = 0.01) but no significant difference for the onset of joint disease. Mean duration of PsA was 20 years. Oligoarthritis was most common (44%), followed by polyarthritis (31%), enthesitis (8%), and inflammatory back pain (7%). According to patients recall of clinical features at onset, 78 patients (60%) had changed categories of PsA at the time of the study, most frequently from polyarthritis to oligoarthritis (48%), followed by oligoarthritis to polyarthritis (36%). These changes seemed independent of disease modifying drugs (DMD), which 54% had received. Conclusions: Psoriatic arthritis in the Reykjavik area of Iceland has a prevalence of at least 0.14% and is strikingly more common in women. The majority of patients reported a change in the pattern of affected joints during the course of their disease. 83
88 INTRODUCTION Psoriatic arthritis (PsA) is an inflammatory joint disease affecting patients with psoriasis and is an entity separate from rheumatoid arthritis (RA) [1]. Diagnostic criteria are being developed [2], but the definition proposed by Moll and Wright in 1973 of a usually seronegative inflammatory arthritis associated with psoriasis is often used [3]. Population based studies have shown a prevalence of PsA ranging from 57 per 100,000 in Greece to 195 per 100,000 in Norway, while a telephone survey in the US showed a prevalence of 250 per 100,000 [4-7]. On the basis of recent reports on the prevalence of PsA in patients with psoriatic skin lesions and estimates of the population prevalence of psoriasis, a prevalence range of 300 to 1000 cases per 100,000 has been suggested [8]. It should be noted that reports dating back to the 1930s and 1940s in the US indicated a prevalence of arthritis among psoriatic patients as high as 32-40% [9], and studies of psoriasis in various populations have shown a range of prevalence from 0% to 11.8%, with most in the 0.5%-2.5% range [10, 11]. Because of these varying reports and estimates of the population prevalence of both psoriasis and PsA, further studies are needed to directly address the prevalence of PsA. Following the early work on PsA by Wright [12], several attempts have been made to define disease subcategories [13-16]. The initial five subcategories have been modified through time, and it has been suggested that only two or three subsets of disease are relevant, namely: oligoarthritis, polyarthritis and spondylarthritis [14, 16]. A mathematical model recently applied to the clinical classification of RA and PsA confirmed that DIP joint involvement, enthesitis, spinal involvement and dactylitis are useful to differentiate between these two diseases. However, the model also indicates that the symmetrical joint involvement of RA is a consequence of a higher number of 84
89 involved joints, and that symmetrical involvement is just as likely in PsA when the same number of joints are affected [17]. Textbooks and most review articles describe psoriatic arthritis as a disease that affects men and women equally [8]. However, relatively few studies have addressed possible gender differences. Reviewing data from 15 studies published over 20 years reveals male to female ratios ranging from 1.3 to 1.6 [13, 17-20] to female to male ratios of 1.3 to 1.6 [21-23]. However, a ratio close to 1:1 is most commonly reported [7, 15, 24-27]. The goal of this study was to determine the prevalence of psoriatic arthritis in the Reykjavik area of Iceland and to describe the demographics, clinical features, and the disease course in this population. 85
90 METHODS Study group The study involved patients with psoriatic arthritis in the Reykjavik area of Iceland, where 63% of the adult population resides. Landspitali University Hospital (LUH) serves as a primary hospital for Reykjavik and its suburbs, and it is the only secondary and tertiary care hospital in Iceland. Patients were recruited from two sources, as shown in figure 1. First, from a database of 1386 patients with verified psoriasis created during ongoing studies of psoriasis [28, 29]. This database contains information on about 1% of the Reykjavik population and its recruitment sources included all affected members of the Icelandic Psoriasis Foundation (SPOEX), all available relatives and other family members of these patients regardless of whether or not they were reported to have psoriasis, and patients recruited through a publicity campaign. Patients who only had pustular psoriasis were not included. From this database 152 individuals who lived in the Reykjavik area in 2003, and reported that they had been diagnosed with PsA by a rheumatologist, were included in the present study. The second source was an electronic registry of patients admitted to the LUH between 1981 and It yielded 98 patients who had been diagnosed with PsA and lived in the Reykjavik area in As 30 patients were present in both these databases they jointly provided 220 potential PsA patients for evaluation. Clinical examination As indicated in figure 1, these 220 patients were initially contacted by letter, followed by a phone call; 21 individuals (10%) could not be reached or did not respond. Of the remaining 199, 162 (81%) agreed to participate in the study, but 6 patients did not turn up. The remaining 156 patients, or 71% of the original study group, were eventually interviewed and examined by one of two physicians; an experienced 86
91 rheumatologist (BG) or a resident physician (TJL) trained by the rheumatologist for this task. Skin and joints were evaluated for evidence of psoriasis and PsA and a focused history was acquired. The patients also answered a questionnaire regarding their skin disease and their arthritic symptoms. Inclusion criteria The inclusion criteria used for this study are derived from those of the Swedish Psoriatic Arthritis Registry (SwePsA) [27] requiring that the patients fulfill the following two criteria. First, at least six consecutive weeks of one or more of the following: Inflamed joint(s), tenosynovitis, inflammatory back pain, enthesitis or dactylitis. Second, the patients had to have been diagnosed with psoriasis by a dermatologist or have psoriatic skin lesions at the time of the examination. Patients who did not have an active arthritis were included if they had been diagnosed with PsA by a rheumatologist and were on remitting drugs at the time of the study. However, patients who reported a diagnosis of a rheumatic disease other than PsA when interviewed, or who were observed to have another rheumatic condition when examined, were excluded. Of the 156 patients examined, 25 (16%) were excluded because they did not fulfill the above conditions. The remaining 131 patients (84%) had verifiable PsA and were included in the study. Disease Assessment Extent of joint involvement was assessed according to the American College of Rheumatology (ACR) joint count for tenderness and swelling, with 66 and 68 joints evaluated, respectively. Both counts include the DIP joints, and have previously been shown to be useful in PsA [30]. Skin affliction was rated by the PASI score [31]. Radiographs were not evaluated, as radiographic changes were not part of the 87
92 diagnostic criteria used. However, over two thirds of the patients had previously been examined by radiography as a part of diagnostic procedures. The patients were initially divided into the following predefined subgroups based on their joint involvement: Inflammatory back pain, symmetric polyarthritis, asymmetric polyarthritis, oligoarthritis, enthesitis and arthralgia. However, during the course of the analysis the subgroups were reduced to four: oligoarthritis, polyarthritis, inflammatory back pain or enthesitis. This was done by merging the two groups of polyarthritis and defining joint tenderness as a form of joint affliction, distinguishing between oligo- and polyarthritis based on the number of tender joints. This reflects the trend towards simplified classifications referred to in the introduction [17]. Figure 2 shows how subgroups were defined. Thus, the patients were classified as having peripheral arthritis even though they also had inflammatory back pain and/or enthesitis, and inflammatory back pain if they had enhtesitis but no peripheral arthritis. Only those patients with exclusive enthesitis at the time of the study were classified as having enthesitis. Data analysis and ethical considerations The study material was stripped of information allowing identification of individuals before the analysis of the data began and the code for this information was kept in a separate, encrypted database. The study was approved by the National Bioethics Committee of Iceland (approval #03-006) and the Data Protection Committee of Iceland. Informed consent was obtained from all the participants in the study. The data were analyzed using the Statview 5.0 statistical software package on a personal computer. The chi-squared and t-tests were used for comparisons of dichotomous values and means, respectively. When predicting the number of confirmable cases based on inclusion of examined patients, direct standardization was used to correct for 88
93 age and gender discrepancies between the group of patients who were examined and those who were not. We calculated 95% confidence intervals for prevalence rates using binomial distribution. All reported p values are based on two-tailed analysis. 89
94 RESULTS Prevalence According to census data, 134,253 individuals, aged 18 years or older, lived in the Reykjavik area of Iceland in 2003 when the study was done [32]. Based on this, a prevalence of 164 per 100,000 (95% CI ) was calculated when all 220 individuals with self reported or hospital diagnosed PsA were included. Of the 156 patients who could be examined, PsA was confirmed according to the inclusion criteria in 131 or 84% (figure 1) and the confirmation rate was similar for patients recruited from the community and hospital databases. There was no obvious selection bias regarding the individuals who could not be reached for clinical evaluation, so we extrapolated the inclusion ratio for the patients who were examined clinically to all the 220 patients in the original study group, correcting for age and gender, resulting in an adjusted prevalence ratio of 139 per 100,000 (95% CI ). Notably, a higher prevalence was observed among women (208 per 100,000, 95% CI ) than among men (118 per , 95% CI ), and this held true for our adjusted prevalence calculations as well (174 per 100,000, 95% CI vs 101 per 100,000, 95% CI ). Figure 3 shows the adjusted prevalence grouped by gender and age. Demographics Demographic data for the 131 patients who passed the inclusion criteria are presented in table 1. The mean age at onset of psoriatic arthritis was 35 years, with 40% of patients having onset of symptoms in their fourth decade of life. Age at disease onset varied by gender, with the men having an average onset of skin symptoms 5.9 years later than the women (p = 0.01) and joint symptoms 3.4 years later (p = 0.12). For the 83 patients who reported developing skin symptoms before joint symptoms, the arthritis presented on average 16 years later. Eleven patients reported simultaneous 90
95 onset of skin and joint symptoms, another 11 insisted that the joint symptoms preceded the skin lesions while 26 patients did not recall which came first. The mean time from the onset of joint symptoms to the onset of skin symptoms was 6 years among the 11 patients who reported that arthritis preceded the skin disease. It should be noted that 42 patients (32%) were taking a disease modifying drug (DMD) at the time of the study, mostly methotrexate (67%), and a total of 71 patients (54%) reported having used a DMD at some stage. Skin and joint involvement At the time of the evaluation, 115 patients (88%) were found to have active skin involvement with an average PASI score of 4.1. Peripheral joint involvement was observed in 104 (79%) of the patients, 89 (68%) with swelling and 97 (74%) with joint pain on palpation. Both skin and joint involvement was present in 90 patients (69%) while two individuals (1.5%) had neither joint nor skin symptoms at the time of the study. Only one patient was included based solely on the presence of enthesitis but none had tenosynovitis only. Nail involvement was detected in 104 individuals (79%) and small joints were affected in 42 (40%) of patients with nail involvement compared with 5 of the 27 (19%) with no nail involvement (p = 0.059). These findings are summarized in table 2. Dactylitis was observed in one patient (<1%), and another reported a clear past history of dactylitis. Patterns of joint affliction and treatment Oligoarthritis was the most common presentation both at disease onset (66%) and at the time of the examination (44%). However, 78 patients (60%) changed categories during the course of the disease and 13 (10%) had no clinical evidence of active arthritis when they were examined, although they all had a clear history of joint disease verified by their rheumatologist. Enthesitis was observed in 64 (49%), arthritis 91
96 in 104 (79%), and signs of inflammatory back pain, i.e. likely spondylarthritis, were observed in 36 (27%) of the patients. Changes in the disease course are shown in table 3, where joint symptoms at onset as recalled by the patients and findings by objective evaluation at entry to the present study are presented. Peripheral arthritis coexisted with enthesitis in 52 patients (40%), and with inflammatory back pain in 29 patients (22%). While 71 patients reported that they had received a DMD at some stage, 55 (42%) said that their DMD treatment had been targeted at their arthritis. However, patients with oligoarthritis, polyarthritis and inflammatory back pain all had similar odds of having been administered a DMD (47%, 50%, and 44%, respectively, 48% combined). In contrast, only one of eleven patients (9%) presenting with enthesitis reported DMD use (p = 0.01). Patients with peripheral arthritis were twice as likely to have received a DMD as those who did not have this type of PsA (48% vs. 24%, respectively, p = 0.03). 92
97 DISCUSSION This cross-sectional study is based on a cohort of 220 patients who were recruited from both hospital and community based registers and had previously been diagnosed with PsA by rheumatologists. It addresses the prevalence of psoriatic arthritis in the Reykjavik area of Iceland, as well as the clinical and demographic features of these patients. Our finding of an adjusted prevalence of 139 per 100,000 is relatively high when compared with the per 100,000 range reported in previous population based studies [4, 6, 7]. However a recent telephone survey in the US suggested a prevalence as high as 250/100,000 [5], which is considerably higher than even our unadjusted prevalence of 164 per 100,000, a figure based in large part on self report of PsA diagnosis. Explanations for these discordant prevalence findings may include the wide range of prevalence of psoriatic skin disease reported in different populations [10, 11], differences in study design and the lack of universally accepted diagnostic criteria for PsA. Furthermore, psoriatic arthritis remains a diagnostic challenge, and is likely to be under diagnosed. Thus, it has been reported that even rheumatologists may fail to diagnose patients with typical features of PsA [33]. Information obtained from clinical databases without a careful confirmatory evaluation may, on the other hand, overestimate the prevalence as shown by the present study. We are not aware of any previous demographic study in which the majority of patients with self-reported PsA have been carefully examined and the diagnosis verified in accordance with published criteria. It should be noted in this context that the diagnosis could not be verified for 16% of the participants in the present study although they had all previously been diagnosed with PsA. Studies of PsA among patients with psoriatic skin disease have suggested a prevalence ranging from 6% to 48%, with designs varying from prospective referral 93
98 or record review studies [7, 21, 22, 25] to a self reported questionnaire study [34]. Some of these studies were based on psoriasis patients attending hospital clinics, and may therefore be skewed towards those with severe disease who are more likely to develop PsA [21]. The prevalence of PsA amongst the patients in our community based psoriasis register was 16% [29]. Previous studies have shown a wide range of gender ratios in PsA. Our finding of a female to male ratio approaching 2:1 among PsA patients is strikingly different from the female to male ratio for the skin disease in our psoriasis registry (1:1.3, p<0.001). This calls for further scrutiny as it is different from the 1:1 gender ratio most frequently reported for PsA. It is not likely that the relatively high prevalence of PsA amongst women in our study is due to underestimation of axial arthritis in men because the frequency of this form of PsA was similar to previous studies reporting equal gender prevalence [4, 6]. Thus, objective evidence for inflammatory back pain as the exclusive presentation of PsA was found in only 7% of our patients and peripheral disease in 79%, which is in line with some previous studies [4, 6, 7, 21]. Three of the 9 patients classified as PsA on the basis of axial disease alone had been diagnosed with the help of radiographic imaging, and the remaining 6 had all previously been diagnosed by a rheumatologist, very likely with the help of an imaging technique. Reported mean age at onset of psoriatic arthritis ranges from 35 to 41 years [7, 19, 21] and we found it to be 35 years. Skin symptoms of psoriasis been shown to have an earlier age of onset in women [28, 35] and our data support this. No subgroup classification of psoriatic arthritis is universally accepted and our approach takes into account a trend towards reduced numbers of subgroups. In a previous study in which 87 patients were followed prospectively for 5 years it was 94
99 found that 21% of PsA patients changed their disease pattern, most often progressing from oligo- to polyarthritis (43%) [23]. We found that 60% of our patients had changed categories during an average disease course of twenty years, but most commonly from poly- to oligoarthritis (48%) while 36% of the patients had progressed from oligo- to polyarthritis. These changes may partly reflect inherent features of the disease, but could also to some extent result from therapy as more than half of our patients had been treated with a DMD at some stage. It should be noted, however, that this aspect of our study is based on patients recollection of the onset of their joint symptoms which obviously introduces a memory bias, and the data should be interpreted accordingly. The main strength of our study is that all available patients from both community and hospital based data sources, who had all previously been diagnosed with PsA, were recruited. Each source has different selection biases, that complement each other. Thus, hospital based patients tend to have severe disease and, therefore, a relatively high prevalence of PsA [21]. Conversely, PsA may be under diagnosed in population based cohorts, especially in patients with minimal skin lesions. The psoriasis register includes all affected members of the Icelandic Psoriasis Association as well as their relatives and family members, and it should be emphasized in this context that members of extended families are still very interactive in Iceland. When these individuals were re-evaluated according to predefined and published criteria 16% did not have verifiable PsA (figure 2). This is important as prior studies of the prevalence and demographics of PsA have either relied on patient records [4, 6, 7], or self report over the phone [5]. The main shortcoming of the present study is that the participants were not randomly recruited from the population living in the Reykjavik area of Iceland, but such a 95
100 strategy is hardly realistic for complex diseases with prevalence well below 1%. As it is likely that relatively mild PsA is under diagnosed in psoriasis patients, especially those who only attend dermatological clinics or health centers, our adjusted PsA prevalence of 0.14% is probably an underestimate. Further studies are clearly needed to refine epidemiological information on psoriatic arthritis, including gender ratios, as well as prospective studies with multiple follow up visits to monitor the disease course over long periods. Acknowledgments The authors would like to thank Helgi Sigvaldason for his assistance with statistical analysis. This study was supported by grants from the Science Fund of the Landspitali University Hospital, and the Research Fund of the Icelandic College of Rheumatology. 96
101 References 1 Baker H. Epidemiological aspects of psoriasis and arthritis. Br J Dermatol 1966; 78: Helliwell PS and Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis 2005; 64 Suppl 2:ii Moll JM and Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973; 3: Madland TM, Apalset EM, Johannessen AE, Rossebo B and Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol 2005; 32: Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53: Alamanos Y, Papadopoulos NG, Voulgari PV, et al. Epidemiology of psoriatic arthritis in northwest Greece, J Rheumatol 2003; 30: Shbeeb M, Uramoto KM, Gibson LE, O'Fallon WM and Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, J Rheumatol 2000; 27: Gladman DD. Psoriatic arthritis. Dermatol Ther 2004; 17: Leczinsky C. The incidence of of arthropathy in a 10 year series of psoriatic cases. Acta Derm Venereol 1948; 28: Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26: Schafer T. Epidemiology of psoriasis. Review and the German perspective. Dermatology (Basel, Switzerland) 2006; 212: Wright V. Psoriasis and arthritis. Ann Rheum Dis 1956; 15: Koo T, Nagy Z, Sesztak M, et al. Subsets in psoriatic arthritis formed by cluster analysis. Clin Rheumatol 2001; 20: Veale D, Rogers S and Fitzgerald O. Classification of clinical subsets in psoriatic arthritis. Br J Rheumatol 1994; 33: Kane D, Stafford L, Bresnihan B and FitzGerald O. A classification study of clinical subsets in an inception cohort of early psoriatic peripheral arthritis-- 'DIP or not DIP revisited'. Rheumatology (Oxford) 2003; 42:
102 16 Marsal S, Armadans-Gil L, Martinez M, Gallardo D, Ribera A and Lience E. Clinical, radiographic and HLA associations as markers for different patterns of psoriatic arthritis. Rheumatology (Oxford) 1999; 38: Helliwell PS, Hetthen J, Sokoll K, et al. Joint symmetry in early and late rheumatoid and psoriatic arthritis: comparison with a mathematical model. Arthritis Rheum 2000; 43: Kaipiainen-Seppanen O. Incidence of psoriatic arthritis in Finland. Br J Rheumatol 1996; 35: Queiro-Silva R, Torre-Alonso JC, Tinture-Eguren T and Lopez-Lagunas I. A polyarticular onset predicts erosive and deforming disease in psoriatic arthritis. Ann Rheum Dis 2003; 62: Scarpa R, Manguso F, Oriente A, Peluso R, Atteno M and Oriente P. Is the involvement of the distal interphalangeal joint in psoriatic patients related to nail psoriasis? Clin Rheumatol 2004; 23: Alenius GM, Jidell E, Nordmark L and Rantapaa Dahlqvist S. Disease manifestations and HLA antigens in psoriatic arthritis in northern Sweden. Clin Rheumatol 2002; 21: Green L, Meyers OL, Gordon W and Briggs B. Arthritis in psoriasis. Ann Rheum Dis 1981; 40: McHugh NJ, Balachrishnan C and Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford) 2003; 42: van Romunde LK, Valkenburg HA, Swart-Bruinsma W, Cats A and Hermans J. Psoriasis and arthritis. I. A population study. Rheumatol Int 1984; 4: Stern RS. The epidemiology of joint complaints in patients with psoriasis. J Rheumatol 1985; 12: Lopez-Montilla MD, Gonzalez J, Martinez FG, Fernandez-Moreno JR and Collantes E. Clinical features of late onset psoriatic arthritis. Exp Gerontol 2002; 37: Svensson B, Holmstrom G and Lindqvist U. Development and early experiences of a Swedish psoriatic arthritis register. Scand J Rheumatol 2002; 31: Gudjonsson JE, Karason A, Antonsdottir AA, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with Psoriasis vulgaris have distinct clinical features. J Invest Dermatol 2002; 118: Gudjonsson JE, Karason A, Runarsdottir EH, et al. Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients-- 98
103 an analysis of 1019 HLA-C- and HLA-B-typed patients. J Invest Dermatol 2006; 126: Gladman DD, Helliwell P, Mease PJ, Nash P, Ritchlin C and Taylor W. Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum 2004; 50: Fredriksson T and Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978; 157: Online Database, [homepage on the internet]. Population by sex and age Gorter S, van der Heijde DM, van der Linden S, et al. Psoriatic arthritis: performance of rheumatologists in daily practice. Ann Rheum Dis 2002; 61: Zachariae H, Zachariae R, Blomqvist K, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol 2002; 82: Henseler T and Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985; 13:
104 TABLES Table 1 Some relevant demographic features of the PsA patients Males (n=54) Females (n=77) P BMI on day of examination, mean kg/m2 ± SD (n) 28 ± 5 (51) 29 ± 6 (69) 0.42 Age at examination, mean years ± SD (n) 56 ± 13 (54) 54 ± 14 (77) 0.38 Age at onset of skin disease, mean years ± SD (n) 26 ± 12 (48) 20 ± 12 (61) 0.01 Duration of Psoriasis, mean years ± SD (n) 30 ± 12 (48) 34 ± 14 (61) 0.15 Age at onset of Psoriatic Arthritis, mean years ± SD (n) 37 ± 13 (52) 34 ± 12 (75) 0.12 Duration of Psoriatic Arthritis, mean years ± SD (n) 19 ± 10 (52) 21 ± 13 (75) 0.35 Skin to joint onset, mean years, ± SD (n) 11 ± 12 (46) 13 ± 12 (59) 0.24 Demographic information on the 131 patients who satisfied the inclusion criteria. 100
105 Table 2 - Frequency and severity of skin and joint involvement at the time of the study (N=131) Active psoriasis (skin or nails) n (%) 125 (95) Skin involvement n (%) 115 (88) PASI score mean (SD) 4.1 (4.2) median (25-75%) 2.8 ( ) Nail involvement n (%) 104 (79) Active peripheral arthritis n (%) 104 (78) Joint swelling n (%) 89 (68) ACR swollen joint count mean (SD) 5.1 (4.0) median (25-75%) 2.0 ( ) Joint tenderness n (%) 97 (74) ACR tender joint count mean (SD) 5.5 (4.7) median (25-75%) 2.0 ( ) ACR = American College of Rheumatology PASI = Psoriasis Area and Severity Index 101
106 Category at onset as recalled by the patient Table 3 - Subcategories of psoriatic arthritis at onset and at the time of evaluation (n=131) Category at examination as evaluated by rheumatologist Oligoarthritiarthritis back pain Poly- Inflammatory Enthesitis None 2) (n=11) (n=13) (n=58) (n=40) (n=9) Oligoarthritis (n=86) Polyarthritis (n=31) Inflammatory back pain (n=7) Enthesitis (n=1) Unknown 3 (n=6) ) Bold numbers indicate patients who stayed in the same category. 2) This group of patients had no disease activity on examination. 3) This group of patients could not remember their symptoms at the onset of PsA. 102
107 FIGURES Figure 1 This flowchart details how cases were located, contacted, examined and finally included or excluded based on previously published criteria. 103
108 Figure 2 This flowchart illustrates the method used for subgroup classification. As each patient may have multiple symptoms simultaneously, each symptom was assigned a significance, with peripheral joint involvement being the most significant and enthesitis the least significant. Patients who had none of these symptoms were excluded from the study. 104
109 Figure Male Female >67 (n=5) (n=18) (n=33) (n=42) (n=52) (n=36) Adjusted prevalence of psoriatic arthritis, calculated using the number of patients in the entire study group predicted to have verifiable psoriatic arthritis based on the ratio of verifiable disease in the 71% of patients that were examined, with the results adjusted for age and gender. Numbers are per 100,000 individuals age 18 years and older, living in Reykjavik at the time of the present study. Data are presented categorized by age groups and divided by gender. 105
110 Figure Legends Figure 1 This flowchart details how cases were located, contacted, examined and finally included or excluded based on previously published criteria. Figure 2 This flowchart illustrates the method used for subgroup classification. As each patient may have multiple symptoms simultaneously, each symptom was assigned a significance, with peripheral joint involvement being the most significant and enthesitis the least significant. Patients who had none of these symptoms were excluded from the study. Figure 3 Adjusted prevalence of psoriatic arthritis, calculated using the number of patients in the entire study group predicted to have verifiable psoriatic arthritis based on the ratio of verifiable disease in the 71% of patients that were examined, with the results adjusted for age and gender. Numbers are per 100,000 individuals age 18 years and older, living in Reykjavik at the time of the present study. Data are presented categorized by age groups and divided by gender. 106
111 107 PAPER II
112 108
113 109
114 110
115 111
116 112
117 113 PAPER III
118 114
119 115
120 116
121 117
122 118
123 119 PAPER IV
124 Title: Corresponding Author: Psoriatic Arthritis and Onycholysis Results from the Cross-Sectional Reykjavik Psoriatic Arthritis study Thorvardur Jon Love, MD, MMSc VMN 14C Landspitali Haskolasjukrahus, Fossvogi 108 Reykjavik Tel: Fax: Authors: Thorvardur Jon Love, MD, MMSc Centre for Rheumatology Research Landspitali University Hospital Reykjavik Iceland Johann Eli Gudjonsson, MD PhD Department of Dermatology University of Michigan Ann Arbor, MI USA Helgi Valdimarsson, MD PhD Department of Immunology Landspitali University Hospital Reykjavik Iceland Bjorn Gudbjornsson, MD PhD Centre for Rheumatology Research Landspitali University Hospital Reykjavik Iceland Keywords: Word count: Running footline: Psoriatic arthritis, psoriasis, nails, onycholysis, epidemiology 1888 (excluding abstract) Onycholysis and Psoriatic Arthritis 120
125 ABSTRACT Objective To measure the associations between subtypes of nail changes and psoriatic arthritis among psoriasis patients. Materials and Methods Patients age 18 and older with active psoriasis were examined for skin and nail changes and asked if they had been diagnosed with psoriatic arthritis. Patients with arthritis were invited for a separate study 1-6 years after their initial visit. Univariate and multivariate analyses were used to test the strength of associations between subtypes of nail changes and arthritis. Results Of 1116 patients with psoriasis, 37% (95% CI 34%-40%) had nail changes. Age, any nail change, onycholysis, and pitting were each associated with psoriatic arthritis on univariate analysis. Multivariate analysis showed that onycholysis was the only type of nail change independently associated with psoriatic arthritis (OR 2.05, p<0.001). Nail changes persisted and had increased in prevalence at the follow-up examination which occurred a mean of 3.8 (median 4, IQR 3-4) years later. Previously reported associations between psoriasis location and arthritis were not seen in this dataset. Conclusion Psoriatic arthritis is associated with onycholysis. Associations with pitting and subungual hyperkeratosis were not statistically significant. Subtypes of nail changes should be analysed separately in future studies of psoriatic arthritis. 121
126 INTRODUCTION Psoriatic arthritis (PsA) is an inflammatory joint disease associated with psoriasis.(1) While PsA affects % of the general population, it is 100 times more common among patients with psoriasis, affecting 8%-16% in population based studies.(2-4) This high prevalence results in a large absolute risk of PsA even if a risk factor is associated with a modest effect size. Confirming the risk of PsA associated with a clinical or laboratory finding among psoriasis patients requires carefully designed prospective studies, but cross sectional association studies can help identify candidate risk factors. Such associations also have the potential to shed light on the pathophysiology of PsA. Previous studies show that PsA is associated with nail changes.(5) While 15% - 50% of patients with psoriasis have nail changes the same is true of up to 85% of patients with PsA.(2, 5-7) Recent MRI studies suggest that nail changes represent a continuation of inflammation originating in entheseal structures and may be a manifestation of arthritis.(8, 9) These studies have not explored subtypes of nail changes, while an association between DIP joint involvement in PsA and onycholysis has been reported.(10) We have previously reported an association between onycholysis and small joint arthritis not limited to DIP joints.(11) These associations were not seen for either pitting or subungual hyperkeratosis. Thus, there exists a need for a better understanding of the relationship between subtypes of nail changes among psoriasis patients and the presence of PsA. We have performed a cross sectional study of the association between subtypes of nail changes in psoriasis and the presence of arthritis in a large population of psoriasis 122
127 patients. Furthermore, we report the findings of a follow-up evaluation of nail changes among the PsA patients up to 6 years after the initial visit. MATERIALS AND METHODS We performed a cross sectional study of patients with psoriasis and PsA. Psoriasis cases were located from community sources, primarily a local psoriasis patient group. These patients were invited for a physical examination over a period of 5 years. Patients without active skin disease and children under 18 years of age were excluded. Pattern of skin involvement was recorded, as was nail involvement and family history of psoriasis. The details of the recruitment and data collection methods have been described previously.(12) Diagnosis of arthritis. All patients were asked if they had been diagnosed with PsA by a rheumatologist. As commonly used criteria for the diagnosis of PsA were not available until after the initial part of this study had concluded, the gold standard of rheumatologist diagnosis reported by the patient was accepted. However, the majority of patients who reported arthritis (76%) have been examined in a separate study, where 83% met the SweSpa criteria (13) and a minimum of 80% met the CASPAR criteria, confirming that this group of patients is homogenous and has PsA by current definitions. (2, 11) Follow-up. One year after the psoriasis recruitment ended we invited those patients who had reported PsA to participate in a separate study where they were evaluated for skin, nail and joint disease as previously described.(2) The Psoriasis Area and Severity Index (PASI) score was used to evaluate severity of skin disease and tender and swollen joint counts were used to measure the severity of peripheral arthritis. The patients who participated in this second evaluation represent the follow-up group in this report. 123
128 Ascertainment of skin and nail disease. The initial examination was performed by a board certified dermatologist (JEG), in training at the time. The follow-up examination was performed by a rheumatologist in training at the time (TJL), with specific training for this task provided by an experienced rheumatologist (BG). Only fingernails were evaluated due to the high frequency (17%) of fungal toenail infections in Iceland.(14) In addition to nail changes, the location and type of skin lesions was recorded. Statistical analysis. The chi-square test was used to test for associations between dichotomous characteristics and the t-test for comparison of the mean of continuous variables. All variables found to be associated with PsA at a p value of 0.05 or less were included in a multivariate logistic regression model. Age and gender were included in all multivariate models. Due to concerns for potential collinearity between subtypes of nail changes, a correlation matrix was constructed to identify any strong correlations between nail change subtypes, and the stability of the model was tested further by calculating the mean variance inflation factor (VIF) as well as the VIF for each independent variable. This was in addition to the collinearity testing built into the statistical analysis package used. Goodness of fit was calculated using the Hosmer Lemeshow method. We furthermore performed post-hoc sensitivity analysis where we divided nail changes into three scenarios: onycholysis without pitting, pitting without onycholysis, and pitting occurring with onycholysis, and tested each for association using the chi-square test. Finally, we tested for univariate associations between subclasses of nail changes and skin activity (measured by the PASI score) and joint activity (measured using tender and swollen joint counts). The results of this analysis were further analysed in three post-hoc linear regression models to estimate the strength of associations of the three subgroups of 124
129 nail changes with the aforementioned skin and joint measures, adjusting for age and gender. Statistical analysis was performed using STATA version 11. RESULTS We included 1116 patients age 18 or older with psoriatic lesions after examining 2547 patients with self-reported psoriasis or family history of psoriasis from the community. The mean age at onset of psoriasis was 21 (95% CI 20-21) and 56% were women. The most common type of skin disease was chronic plaque psoriasis in 94%, and the most commonly involved sites were the arms, scalp, and legs, with 84%, 70%, and 70% of patients having involvement of these sites, respectively. Nails were involved in 37% (95% CI 34%-40%) of patients, and the most common nail change was onycholysis, found in 29% (95% CI 26%-32%). Table 1 presents these and other characteristics of the study group. Nail changes and arthritis When asked about arthritis, 187 patients or 17% (95% CI 15%-19%) reported having been diagnosed with PsA by a rheumatologist. Age, gender, onycholysis and pitting were each associated with PsA on univariate analysis at a p value of less than These five variables were included in a multiple logistic regression model to evaluate the independent contribution of each variable. We tested the model for collinearity and found a mean VIF of 1.10 with a range of 1.02 to 1.19, confirming variance inflation was not an issue in the model. Goodness of fit testing revealed a Hosmer-Lemeshow chi-square value of 5.25 with p=0.73, indicating there was not a lack of fit in the model. Onycholysis was the only type of nail change that remained associated with PsA in the 125
130 multivariate analysis with an OR of 2.06, p < Table 2 summarizes the results of the multivariate model. Based on the findings from the multivariate model we performed a post-hoc sensitivity analysis as described in the methods section. The OR for PsA in this analysis was 1.95 in patients with onycholysis alone (p<0.001), 1.76 when both onycholysis and pitting were present (p=0.009), and 1.52 when only pitting was present (p=0.16). Follow-up of nail changes At a mean of 3.8 years after the initial physical examination (median 4, IQR 3-4), 139 of the 183 PsA patients (76%) returned to participate in a study of PsA and were evaluated for skin, joint and nail involvement. Most (97%) were seen 3 or more years after their first visit. All types of nail changes had increased in prevalence from the first to the second examination, with the overall prevalence of nail changes rising from about 53% to around 80%. Less than 4% of patients who had nail changes at the initial visit were free from nail changes at their follow-up visit. Further post-hoc analysis of this group suggested by reviewers showed univariate relationships between all subtypes of nail changes and skin disease activity measured with the PASI score, and a weaker relationship between onycholysis and tender joint count as presented in Table 3. Linear regression models incorporating all types of nail changes showed a trend towards an association between pitting and the PASI score and a negative association with the tender joint count (p=0.09 for both), as well as an association between onycholysis and the tender joint count (p=0.02), as shown in table
131 DISCUSSION We performed an analysis of associations between subtypes of nail changes and PsA in a large population based sample of psoriasis patients. Our findings show that onycholysis has a stronger association with arthritis than other types of nail changes, including pitting. Nail changes among PsA patients tended to persist, and increased in frequency over time. We did not observe previously reported associations between psoriasis site and arthritis. It has been suggested that pitting and onycholysis are a part of the joint disease in PsA rather than the skin disease based on anatomical observations.(15) We have previously reported that onycholysis, but not other nail changes, is associated with small joint arthritis in PsA patients.(11) The data presented here lend further support to the idea that onycholysis is the subtype of nail changes that is most closely associated with arthritis. We present findings from two points in time. Using multivariate analysis we have shown that at the first time point the presence of arthritis was associated with onycholysis, and that at the second time point the tender joint count, but not the PASI score, was associated with onycholysis. It is important to note that the evaluation at the first timepoint was done by a different physician than at the second timepoint, and that data from the first visit were not available to the physician determining fingernail status at the second visit. The presence of arthritis, arthritis activity and skin activity were not associated with the other two types of nail changes evaluated. This finding is timely given the recent report from a prospective study that nail changes in patients with psoriasis predict the onset of arthritis.(4) Although limited by its cross-sectional design, this is a population based study of a large number of psoriasis patients. While nearly one in five PsA patients did not meet the 127
132 CASPAR criteria, this classification effort was incomplete due to lack of data. Thus, the data presented here are based on rheumatologist diagnosis and should be interpreted accordingly. Prospective studies of the risk of PsA will be needed to confirm our results and calculate the risk for PsA associated with onycholysis, but considering the prior literature on this subject,(10, 11, 16) future studies of the risk of arthritis associated with nail changes should collect and analyse data on the subtypes of nail changes. Both the nail matrix and the nail bed are in direct contact with entheseal structures,(9, 15) yet we found only an association between onycholysis and arthritis. Although pitting may also be entheseal-related, it is a very common type of nail change in the general population, affecting as much as 58% of healthy individuals in one study whereas less than 2% had onycholysis.(17) Furthermore, the presence of nail pitting alone is a poor discriminator between psoriatic and other causes, while onycholysis favours a psoriatic origin.(16) Thus, pitting is less specific to psoriatic disease than onycholysis, perhaps explaining the complete lack of association seen in our sensitivity analysis of patients with pitting but no onycholysis. In summary, we found an association between onycholysis and psoriatic arthritis in patients with psoriasis independent of other subtypes of nail changes. Future studies should report on the subtypes of nail changes. 128
133 REFERENCES 1. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1): Love TJ, Gudbjornsson B, Gudjonsson JE, Valdimarsson H. Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J Rheumatol Oct;34(10): Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum Oct 15;61(10): Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum Feb 15;61(2): Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol Jul;57(1): Lavaroni G, Kokelj F, Pauluzzi P, Trevisan G. The nails in psoriatic arthritis. Acta Derm Venereol Suppl (Stockh). 1994;186: Gladman DD, Helliwell P, Mease PJ, Nash P, Ritchlin C, Taylor W. Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum Jan;50(1): Scarpa R, Soscia E, Peluso R, Atteno M, Manguso F, Del Puente A, et al. Nail and distal interphalangeal joint in psoriatic arthritis. J Rheumatol Jul;33(7): Tan AL, Benjamin M, Toumi H, Grainger AJ, Tanner SF, Emery P, et al. The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis--a high-resolution MRI and histological study. Rheumatology (Oxford) Feb;46(2): Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, Ballina Garcia J, Riestra Noriega JL, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, 129
134 immunological and radiological study of 180 patients. British journal of rheumatology Aug;30(4): Love TJ, Gudjonsson JE, Valdimarsson H, Gudbjornsson B. Small joint involvement in psoriatic arthritis is associated with onycholysis: the Reykjavik Psoriatic Arthritis Study. Scand J Rheumatol Aug;39(4): Gudjonsson JE, Karason A, Runarsdottir EH, Antonsdottir AA, Hauksson VB, Jonsson HH, et al. Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients--an analysis of 1019 HLA-C- and HLA-B-typed patients. J Invest Dermatol Apr;126(4): Svensson B, Holmstrom G, Lindqvist U. Development and early experiences of a Swedish psoriatic arthritis register. Scand J Rheumatol. 2002;31(4): Sigurgeirsson B, Steingrimsson O, Sveinsdottir S. Prevalence of onychomycosis in Iceland: a population-based study. Acta Derm Venereol. 2002;82(6): McGonagle D, Tan AL, Benjamin M. The nail as a musculoskeletal appendage-- implications for an improved understanding of the link between psoriasis and arthritis. Dermatology (Basel, Switzerland). 2009;218(2): Eastmond CJ, Wright V. The nail dystrophy of psoriatic arthritis. Ann Rheum Dis Jun;38(3): Robertson JC, Braune ML. Splinter haemorrhages, pitting, and other findings in fingernails of healthy adults. British medical journal. 1974;4(5939):
135 TABLE 1 All Skin only Skin and arthritis p Age at examination, mean (95% CI) 47 (46-48) 46 (45-47) 51 (49-53) <0.001* Age at psoriasis onset, mean (95% CI) 21 (20-21) 21 (20-21) 21 (19-23) 0.51 Male (n, %) 492 (44%) 427 (46%) 65 (35%) 0.005* Skin involvement (n, %) 1116 (100%) 933 (84%) 183 (16%) NA Scalp 774 (70%) 648 (70%) 126 (68%) Ear 261 (23%) 212 (23%) 49 (26%) Trunk 473 (43%) 389 (42%) 84 (45%) Axillae 61 (5%) 52 (6%) 9 (5%) Arms 935 (84%) 780 (84%) 155 (83%) Groins 81 (7%) 70 (8%) 11 (6%) Legs 780 (70%) 646 (70%) 134 (72%) Perianal 74 (7%) 59 (6%) 15 (8%) Nail involvement (n, %) 415 (37%) 316 (34%) 99 (54%) <0.001* Pitting 204 (18%) 155 (17%) 49 (26%) 0.002* Onycholysis 322 (29%) 241 (26%) 81 (43%) <0.001* Subungual hyperkeratosis 137 (12%) 106 (11%) 31 (17%) Table 1 - Baseline characteristics of the study group and the results of a univariate comparison between patients with psoriasis only and psoriasis with arthritis. P values represent results of the chi-square test for dichotomous variables and the t-test for age, and * indicates significance at p=0.05 level. TABLE 2 OR 95% CI P Age at examination <0.001 Male <0.001 Onycholysis <0.001 Pitting Table 2 Results of the multivariate logistic regression model. OR=Odds Ratio, CI=Confidence Interval 131
136 Table 4. Results of three multivariate linear regression analyses showing the association of age, gender, and subtypes of nail changes with each of three disease activity measures. Regression results are presented as the -coefficient with 95% confidence intervals in parentheses, followed by the p-value, * Indicates significance at p=0.05 level. TABLE 3 No nail changes Onycholysis p Pitting p Subungual hyperkeratosis p n NA 87 NA 73 NA PASI score 2.55 ( ) 4.66 ( ) 0.02* 4.84 ( ) 0.01* 5.07 ( ) 0.01* Tender joint count 2.22 ( ) 4.30 ( ) 0.05* 3.46 ( ) ( ) 0.09 Swollen joint count 1.89 ( ) 3.45 ( ) ( ) ( ) 0.07 Current DMARD use 5 (19%) 30 (31%) (26%) (33%) Table 3 Univariate analysis of disease and treatment characteristics of 139 psoriatic arthritis patients at follow-up. Results of the chisquare test for DMARD use and the t-test for all other variables, presented as the mean followed by the 95% confidence interval in parentheses. P-values represent a comparison between a nail group subtype and the group with no nail changes, and * indicates significance at p=0.05 level. PASI= Psoriasis Area and Severity Index, DMARD= Disease Modifying Antirheumatic Drug. TABLE Association with PASI score 2 - Association with tender joint count 3 - Association with swollen joint count Age at time of examination 0.00 ( ) ( ) ( ) 0.82 Gender 0.22 ( ) ( ) ( ) 0.71 Pitting 1.40 ( ) ( ) ( ) 0.87 Onycholysis 0.72 ( ) ( ) 0.02* 0.94 ( ) 0.34 Subungual hyperkeratosis 1.26 ( ) ( ) ( ) 0.96
137
Psoriatic Arthritis. Ewa Olech, MD Division of Rheumatology University of Nevada School of Medicine Las Vegas
 Psoriatic Arthritis Ewa Olech, MD Division of Rheumatology University of Nevada School of Medicine Las Vegas The Spectrum of Spondyloarthritis Characteristics of the Spondyloarthritis Sacroiliac & spinal
Psoriatic Arthritis Ewa Olech, MD Division of Rheumatology University of Nevada School of Medicine Las Vegas The Spectrum of Spondyloarthritis Characteristics of the Spondyloarthritis Sacroiliac & spinal
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
Psoriatic Arthritis Current Guidelines. Linda Sekhon, DHSc, PA-C
 Psoriatic Arthritis Current Guidelines Linda Sekhon, DHSc, PA-C Learning Objectives At the conclusion of this lecture, participants should be able to: Define Psoriatic Arthritis and briefly describe the
Psoriatic Arthritis Current Guidelines Linda Sekhon, DHSc, PA-C Learning Objectives At the conclusion of this lecture, participants should be able to: Define Psoriatic Arthritis and briefly describe the
Psoriatic Arthritis. Title. Understanding and Managing. in All the Wrong Places. Clinical Features. Etiology of Psoriatic Arthritis
 Focus on CME at Memorial University Understanding and Managing Title Psoriatic Arthritis in All the Wrong Places Proton Rahman MD, MSc, FRCPC Although Baron Jean-Luis Aubert offered the first case description
Focus on CME at Memorial University Understanding and Managing Title Psoriatic Arthritis in All the Wrong Places Proton Rahman MD, MSc, FRCPC Although Baron Jean-Luis Aubert offered the first case description
Rheumatoid Arthritis. Nicole Klett,, M.D.
 Rheumatoid Arthritis Nicole Klett,, M.D. Rheumatoid Arthritis Systemic Chronic Inflammatory Primarily targets the synovium of diarthrodial joints Etiology likely combination genetic and environmental Diarthrodial
Rheumatoid Arthritis Nicole Klett,, M.D. Rheumatoid Arthritis Systemic Chronic Inflammatory Primarily targets the synovium of diarthrodial joints Etiology likely combination genetic and environmental Diarthrodial
It is worth noting that people with psoriasis can also develop other forms of arthritis such as rheumatoid arthritis and osteoarthritis.
 Psoriatic Arthritis Main Colour - pantone 2597u Research - pantone 206u Children - pantone 123 4 What is psoriatic arthritis? Psoriatic arthritis is an inflammatory joint disease associated with psoriasis.
Psoriatic Arthritis Main Colour - pantone 2597u Research - pantone 206u Children - pantone 123 4 What is psoriatic arthritis? Psoriatic arthritis is an inflammatory joint disease associated with psoriasis.
Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon
 Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon Rheumatology Specialist Registrar & PhD Research Fellow 2 Overview Back ground on psoriatic arthritis (PsA) Epidemiology
Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon Rheumatology Specialist Registrar & PhD Research Fellow 2 Overview Back ground on psoriatic arthritis (PsA) Epidemiology
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis
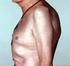 Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
NURS 821 Alterations in the Musculoskeletal System. Rheumatoid Arthritis. Type III Hypersensitivity Response
 NURS 821 Alterations in the Musculoskeletal System Margaret H. Birney PhD, RN Lecture 12 Part 2 Joint Disorders (cont d) Rheumatoid Arthritis Definition: Autoimmune disorder occurring in genetically sensitive
NURS 821 Alterations in the Musculoskeletal System Margaret H. Birney PhD, RN Lecture 12 Part 2 Joint Disorders (cont d) Rheumatoid Arthritis Definition: Autoimmune disorder occurring in genetically sensitive
Psoriatic arthritis in practice : How to detect? How to diagnose? Pascal RICHETTE Hôpital Lariboisière, Paris. Copyright
 Psoriatic arthritis in practice : How to detect? How to diagnose? Pascal RICHETTE Hôpital Lariboisière, Paris The patient: a 57 year-old man, with a history of psoriatic nail dystrophy for 10 years Past
Psoriatic arthritis in practice : How to detect? How to diagnose? Pascal RICHETTE Hôpital Lariboisière, Paris The patient: a 57 year-old man, with a history of psoriatic nail dystrophy for 10 years Past
Understanding Rheumatoid Arthritis
 Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Do I need a physician referral? Yes, we see patients on referral from a health care provider.
 FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
Rheumatoid Arthritis
 Rheumatoid Arthritis Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Autoimmune diseases are illnesses that occur when the body's tissues are mistakenly
Rheumatoid Arthritis Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Autoimmune diseases are illnesses that occur when the body's tissues are mistakenly
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
(Intro to Arthritis with a. Arthritis) Manager of Education & Services for the Vancouver Island Region of The Arthritis Society
 Arthritis 101 (Intro to Arthritis with a Focus on Rheumatoid Arthritis) by Cari Taylor by Cari Taylor Manager of Education & Services for the Vancouver Island Region of The Arthritis Society What You Will
Arthritis 101 (Intro to Arthritis with a Focus on Rheumatoid Arthritis) by Cari Taylor by Cari Taylor Manager of Education & Services for the Vancouver Island Region of The Arthritis Society What You Will
Rheumatoid Arthritis
 Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
How To Choose A Biologic Drug
 North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
Psoriasis. Psoriasis. Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine
 Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Rheumatology Labs for Primary Care Providers. Robert Monger, M.D., F.A.C.P. 2015 Frontiers in Medicine
 Rheumatology Labs for Primary Care Providers Robert Monger, M.D., F.A.C.P. 2015 Frontiers in Medicine Objectives Review the Indications for and Interpretation of lab testing for the following diseases:
Rheumatology Labs for Primary Care Providers Robert Monger, M.D., F.A.C.P. 2015 Frontiers in Medicine Objectives Review the Indications for and Interpretation of lab testing for the following diseases:
Rheumatoid arthritis: an overview. Christine Pham MD
 Rheumatoid arthritis: an overview Christine Pham MD RA prevalence Chronic inflammatory disease affecting approximately 0.5 1% of the general population Prevalence is higher in North America (approaching
Rheumatoid arthritis: an overview Christine Pham MD RA prevalence Chronic inflammatory disease affecting approximately 0.5 1% of the general population Prevalence is higher in North America (approaching
Psoriasis, Incidence, Quality of Life, Psoriatic Arthritis, Prevalence
 1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
PSORIATIC ARTHRITIS. Elvia Moreta, MD St. Paul Rheumatology 2012
 PSORIATIC ARTHRITIS Elvia Moreta, MD St. Paul Rheumatology 2012 RESEARCH DISCLOSURE ABBOTT BMS CENTOCOR GENENTEC LILLY NOVARTIS ROCHE SAVIENT UCB CONSULTANT ABBOTT UCB MEMBER ABIM fellow CORRONA Consortium
PSORIATIC ARTHRITIS Elvia Moreta, MD St. Paul Rheumatology 2012 RESEARCH DISCLOSURE ABBOTT BMS CENTOCOR GENENTEC LILLY NOVARTIS ROCHE SAVIENT UCB CONSULTANT ABBOTT UCB MEMBER ABIM fellow CORRONA Consortium
The Most Common Autoimmune Disease: Rheumatoid Arthritis. Bonita S. Libman, M.D.
 The Most Common Autoimmune Disease: Rheumatoid Arthritis Bonita S. Libman, M.D. Disclosures Two googled comics The Normal Immune System Network of cells and proteins that work together Goal: protect against
The Most Common Autoimmune Disease: Rheumatoid Arthritis Bonita S. Libman, M.D. Disclosures Two googled comics The Normal Immune System Network of cells and proteins that work together Goal: protect against
Genetic and Environmental Risk Factors for Psoriatic Arthritis among patients with Psoriasis
 Genetic and Environmental Risk Factors for Psoriatic Arthritis among patients with Psoriasis by Lihi Eder A thesis submitted in conformity with the requirements for the degree of PhD Institute for Medical
Genetic and Environmental Risk Factors for Psoriatic Arthritis among patients with Psoriasis by Lihi Eder A thesis submitted in conformity with the requirements for the degree of PhD Institute for Medical
Case 13 A 30 - year - old man with painful swollen fingers
 Case 13 A 30 - year - old man with painful swollen fingers David Smiles, a 30 - year - old man presents to his GP with painful swollen fingers. They have been getting progressively worse over the previous
Case 13 A 30 - year - old man with painful swollen fingers David Smiles, a 30 - year - old man presents to his GP with painful swollen fingers. They have been getting progressively worse over the previous
What s new in clinical assesment of ankylosing spondylitis?
 What s new in clinical assesment of ankylosing spondylitis? Désirée van der Heijde Professor of Rheumatology Leiden University Medical Center, the Netherlands Diakonhjemmet Hospital, Oslo, Norway Content
What s new in clinical assesment of ankylosing spondylitis? Désirée van der Heijde Professor of Rheumatology Leiden University Medical Center, the Netherlands Diakonhjemmet Hospital, Oslo, Norway Content
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
RHEUMATOID ARTHRITIS. Dr Bruce Kirkham Rheumatology Clinical Lead
 RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
Information on Rheumatoid Arthritis
 Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
PSORIATIC ARTHRITIS. Chryssanthie Kafkala, M.D. INTRODUCTION:
 PSORIATIC ARTHRITIS Chryssanthie Kafkala, M.D. INTRODUCTION: Psoriatic arthritis is a disease with generally good prognosis. Both ocular and systemic involvement is usually benign, however, the following
PSORIATIC ARTHRITIS Chryssanthie Kafkala, M.D. INTRODUCTION: Psoriatic arthritis is a disease with generally good prognosis. Both ocular and systemic involvement is usually benign, however, the following
Rheumatoid Arthritis www.arthritis.org.nz
 Rheumatoid Arthritis www.arthritis.org.nz Did you know? RA is the second most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most often appears between
Rheumatoid Arthritis www.arthritis.org.nz Did you know? RA is the second most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most often appears between
Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology
 Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Dr. Leonard Calabrese: I want to welcome the
Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Dr. Leonard Calabrese: I want to welcome the
Phenotypes and Classification of Psoriasis
 Rheumatology 2010 Birmingham 21 April 2010 Phenotypes and Classification of Psoriasis Christopher E.M. Griffiths Abbott Centocor Incyte Galderma Janssen-Cilag Leo Pharma Lynxx Novartis Pfizer Schering-Plough
Rheumatology 2010 Birmingham 21 April 2010 Phenotypes and Classification of Psoriasis Christopher E.M. Griffiths Abbott Centocor Incyte Galderma Janssen-Cilag Leo Pharma Lynxx Novartis Pfizer Schering-Plough
Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease
 DOI: 10.1111/j.1468-3083.2009.03363.x JEADV Blackwell Publishing Ltd REVIEW ARTICLE Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease D McGonagle* NIHR, Leeds
DOI: 10.1111/j.1468-3083.2009.03363.x JEADV Blackwell Publishing Ltd REVIEW ARTICLE Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease D McGonagle* NIHR, Leeds
Rheumatoid Arthritis. Disease RA Final.indd 2 15. 6. 10. 11:23
 Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES
 DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES GOAL #1 develop the ability to order and understand interpretation
DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES GOAL #1 develop the ability to order and understand interpretation
HLA-Cw*0602 associates with a twofold higher prevalence. of positive streptococcal throat swab at the onset of
 1 HLA-Cw*0602 associates with a twofold higher prevalence of positive streptococcal throat swab at the onset of psoriasis: a case control study Lotus Mallbris, MD, PhD, Katarina Wolk, MD, Fabio Sánchez
1 HLA-Cw*0602 associates with a twofold higher prevalence of positive streptococcal throat swab at the onset of psoriasis: a case control study Lotus Mallbris, MD, PhD, Katarina Wolk, MD, Fabio Sánchez
GENETIC ANALYSIS OF PSORIASIS AND PSORIATIC ARTHRITIS Department of Dermatology, University of Michigan
 GENETIC ANALYSIS OF PSORIASIS AND PSORIATIC ARTHRITIS Department of Dermatology, University of Michigan SELF ASSESSMENT FORM FOR STUDY SUBJECTS AND CONTROLS Accession Number (will be filled in by lab)
GENETIC ANALYSIS OF PSORIASIS AND PSORIATIC ARTHRITIS Department of Dermatology, University of Michigan SELF ASSESSMENT FORM FOR STUDY SUBJECTS AND CONTROLS Accession Number (will be filled in by lab)
Psoriasis Co-morbidities: Changing Clinical Practice. Theresa Schroeder Devere, MD Assistant Professor, OHSU Dermatology. Psoriatic Arthritis
 Psoriasis Co-morbidities: Changing Clinical Practice Theresa Schroeder Devere, MD Assistant Professor, OHSU Dermatology Psoriatic Arthritis Psoriatic Arthritis! 11-31% of patients with psoriasis have psoriatic
Psoriasis Co-morbidities: Changing Clinical Practice Theresa Schroeder Devere, MD Assistant Professor, OHSU Dermatology Psoriatic Arthritis Psoriatic Arthritis! 11-31% of patients with psoriasis have psoriatic
2010 ACR/EULAR Classification Criteria for Rheumatoid Arthritis
 2010 ACR/EULAR Classification Criteria for Rheumatoid Arthritis Published in the September 2010 Issues of A&Rand ARD Phases of the Project Phase 1 Data analysis Phase 2 Consensus process Predictors of
2010 ACR/EULAR Classification Criteria for Rheumatoid Arthritis Published in the September 2010 Issues of A&Rand ARD Phases of the Project Phase 1 Data analysis Phase 2 Consensus process Predictors of
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
Development and Validation of a Screening Questionnaire for Psoriatic Arthritis
 Development and Validation of a Screening Questionnaire for Psoriatic Arthritis Dafna D. Gladman 1, Catherine T. Schentag 1, Brian D. Tom 2, Vinod Chandran 1, Cheryl F. Rosen 1 Vernon T. Farewell 2 1 University
Development and Validation of a Screening Questionnaire for Psoriatic Arthritis Dafna D. Gladman 1, Catherine T. Schentag 1, Brian D. Tom 2, Vinod Chandran 1, Cheryl F. Rosen 1 Vernon T. Farewell 2 1 University
The Functional but not Nonfunctional LILRA3 Contributes to Sex Bias in Susceptibility and Severity of ACPA-Positive Rheumatoid Arthritis
 The Functional but not Nonfunctional LILRA3 Contributes to Sex Bias in Susceptibility and Severity of ACPA-Positive Rheumatoid Arthritis Yan Du Peking University People s Hospital 100044 Beijing CHINA
The Functional but not Nonfunctional LILRA3 Contributes to Sex Bias in Susceptibility and Severity of ACPA-Positive Rheumatoid Arthritis Yan Du Peking University People s Hospital 100044 Beijing CHINA
Media Release. Basel, 11 June 2009. RA patients with enhanced response identified
 Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Psoriatic Arthritis. What is psoriatic arthritis? Understanding joints. Who gets psoriatic arthritis? Page 1 of 5
 Page 1 of 5 Psoriatic Arthritis Psoriatic arthritis causes inflammation, pain, and swelling of joints in some people who have psoriasis. Other parts of the body may also be affected. For example, in many
Page 1 of 5 Psoriatic Arthritis Psoriatic arthritis causes inflammation, pain, and swelling of joints in some people who have psoriasis. Other parts of the body may also be affected. For example, in many
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital
 Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
teaching hospital and research centre, Himayath sagar road, Hyderabad, Telangana state, India 2
 Scholars Journal of Applied Medical Sciences (SJAMS) Sch. J. App. Med. Sci., 15; 3(A):215-219 Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources)
Scholars Journal of Applied Medical Sciences (SJAMS) Sch. J. App. Med. Sci., 15; 3(A):215-219 Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources)
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd
 ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Autoimmunity and immunemediated. FOCiS. Lecture outline
 1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
Psoriatic Arthritis: a Critical Review
 Clinic Rev Allerg Immunol (2013) 44:141 148 DOI 10.1007/s12016-012-8302-6 Psoriatic Arthritis: a Critical Review Varun Dhir & Amita Aggarwal Published online: 1 February 2012 # Springer Science+Business
Clinic Rev Allerg Immunol (2013) 44:141 148 DOI 10.1007/s12016-012-8302-6 Psoriatic Arthritis: a Critical Review Varun Dhir & Amita Aggarwal Published online: 1 February 2012 # Springer Science+Business
Rheumatoid Arthritis: Symptoms, Causes, and Treatments of Rheumatoid Foot and Ankle
 Rheumatoid arthritis is the most common form of inflammatory arthritis, affecting about two to three million Americans. Rheumatoid arthritis is a symmetric disease, meaning that it will usually involve
Rheumatoid arthritis is the most common form of inflammatory arthritis, affecting about two to three million Americans. Rheumatoid arthritis is a symmetric disease, meaning that it will usually involve
X-Plain Rheumatoid Arthritis Reference Summary
 X-Plain Rheumatoid Arthritis Reference Summary Introduction Rheumatoid arthritis is a fairly common joint disease that affects up to 2 million Americans. Rheumatoid arthritis is one of the most debilitating
X-Plain Rheumatoid Arthritis Reference Summary Introduction Rheumatoid arthritis is a fairly common joint disease that affects up to 2 million Americans. Rheumatoid arthritis is one of the most debilitating
Psoriatic Arthritis www.arthritis.org.nz
 Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Arthritis and Rheumatology Clinics of Kansas Patient Education. Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis
 Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Am I likely to develop. rheumatoid Arthritis? A guide for people with joint symptoms
 Am I likely to develop Rheumatoid rheumatoid Arthritis? arthritis? A guide for people with joint symptoms At a glance Rheumatoid arthritis (RA) is a common disease that causes painful and swollen joints,
Am I likely to develop Rheumatoid rheumatoid Arthritis? arthritis? A guide for people with joint symptoms At a glance Rheumatoid arthritis (RA) is a common disease that causes painful and swollen joints,
Arthritis of the Hands
 Arthritis of the Hands On the Agenda Normal Osteoarthitis Rheumatoid arthritis CPPD crystal deposition Gout Psoriatic arthritis Normal Hand X-ray Osteoarthritis (DJD) Gradual degeneration of articular
Arthritis of the Hands On the Agenda Normal Osteoarthitis Rheumatoid arthritis CPPD crystal deposition Gout Psoriatic arthritis Normal Hand X-ray Osteoarthritis (DJD) Gradual degeneration of articular
Once the immune system is triggered, cells migrate from the blood into the joints and produce substances that cause inflammation.
 HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
History and Physical Examination for Rheumatic Disease for MUSC Students
 History and Physical Examination for Rheumatic Disease for MUSC Students Inflammatory vs. non-inflammatory arthritis Inflammatory Prolonged stiffness after rest Stiffness improved with use Warmth Prolonged
History and Physical Examination for Rheumatic Disease for MUSC Students Inflammatory vs. non-inflammatory arthritis Inflammatory Prolonged stiffness after rest Stiffness improved with use Warmth Prolonged
Latvian Early Intervention Concept development
 Latvian Early Intervention Concept development Professor Daina Andersone, Medical Faculty of Latvian University Fit for Work Medical team leader in Latvia Latvian Presidency of the EU Council Healthcare
Latvian Early Intervention Concept development Professor Daina Andersone, Medical Faculty of Latvian University Fit for Work Medical team leader in Latvia Latvian Presidency of the EU Council Healthcare
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 17 December 2003 CPMP/EWP/556/95 rev 1/Final COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 17 December 2003 CPMP/EWP/556/95 rev 1/Final COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
Immune modulation in rheumatology. Geoff McColl University of Melbourne/Australian Rheumatology Association
 Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Developing a National Audit for Rheumatoid and Early Inflammatory Arthritis Ian Rowe
 Developing a National Audit for Rheumatoid and Early Inflammatory Arthritis Ian Rowe Chair Clinical Affairs Committee, BSR Outline The British Society for Rheumatology Challenges in improving patient outcomes
Developing a National Audit for Rheumatoid and Early Inflammatory Arthritis Ian Rowe Chair Clinical Affairs Committee, BSR Outline The British Society for Rheumatology Challenges in improving patient outcomes
Critical Issues in School Health Arthritis in the School Setting. Lawrence Zemel MD Tegan Willard RN Connecticut Children s
 Critical Issues in School Health Arthritis in the School Setting Lawrence Zemel MD Tegan Willard RN Connecticut Children s Juvenile Rheumatoid Idiopathic Arthritis Definition of JRA (JIA) Persistent arthritis
Critical Issues in School Health Arthritis in the School Setting Lawrence Zemel MD Tegan Willard RN Connecticut Children s Juvenile Rheumatoid Idiopathic Arthritis Definition of JRA (JIA) Persistent arthritis
RECOGNISING INFLAMMATORY BACK PAIN. This programme is supported and funded by Pfizer Date of preparation: December 2011 Project code: ENB 248
 RECOGNISING INFLAMMATORY BACK PAIN This programme is supported and funded by Pfizer Date of preparation: December 2011 Project code: ENB 248 Contents Inflammatory back pain: overview Spondyloarthropathies
RECOGNISING INFLAMMATORY BACK PAIN This programme is supported and funded by Pfizer Date of preparation: December 2011 Project code: ENB 248 Contents Inflammatory back pain: overview Spondyloarthropathies
ABOUT RHEUMATOID ARTHRITIS
 MEDIA BACKGROUNDER ABOUT RHEUMATOID ARTHRITIS Rheumatoid arthritis (RA) is a type of arthritis (chronic inflammatory polyarthritis) that typically affects hands and feet, although any joint in the body
MEDIA BACKGROUNDER ABOUT RHEUMATOID ARTHRITIS Rheumatoid arthritis (RA) is a type of arthritis (chronic inflammatory polyarthritis) that typically affects hands and feet, although any joint in the body
Rheumatoid arthritis
 Rheumatoid arthritis Rheumatoid arthritis Chronic multisystem disease Unknown cause Characteristic feature persistent inflammation of synovia in symmetric peripheral joints Synovial inflammation cartilage
Rheumatoid arthritis Rheumatoid arthritis Chronic multisystem disease Unknown cause Characteristic feature persistent inflammation of synovia in symmetric peripheral joints Synovial inflammation cartilage
Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis
 Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Cutaneous Lymphoma FAST FACTS
 Cutaneous Lymphoma FAST FACTS What is Cutaneous Lymphoma? Cutaneous lymphomas are types of non-hodgkin s lymphomas (NHL) that originate in the lymphocytes (white blood cells). Unlike most other types of
Cutaneous Lymphoma FAST FACTS What is Cutaneous Lymphoma? Cutaneous lymphomas are types of non-hodgkin s lymphomas (NHL) that originate in the lymphocytes (white blood cells). Unlike most other types of
Arthritis Mutilans in a Patient with Psoriasis
 Case Report Arthritis Mutilans in a Patient with Psoriasis Mubina Gaffar, MD Arthritis is reported to be a feature of psoriasis in approximately 7% of cases. 1 The most dramatic and severe form of arthritis
Case Report Arthritis Mutilans in a Patient with Psoriasis Mubina Gaffar, MD Arthritis is reported to be a feature of psoriasis in approximately 7% of cases. 1 The most dramatic and severe form of arthritis
2.1 Who first described NMO?
 History & Discovery 54 2 History & Discovery 2.1 Who first described NMO? 2.2 What is the difference between NMO and Multiple Sclerosis? 2.3 How common is NMO? 2.4 Who is affected by NMO? 2.1 Who first
History & Discovery 54 2 History & Discovery 2.1 Who first described NMO? 2.2 What is the difference between NMO and Multiple Sclerosis? 2.3 How common is NMO? 2.4 Who is affected by NMO? 2.1 Who first
Stickler Syndrome and Arthritis
 Stickler Syndrome and Arthritis Arthritis Foundation Pacific Region, Nevada Office Presented by: Crystal Schulz, MPH Community Development Manager Arthritis Foundation Improving lives through leadership
Stickler Syndrome and Arthritis Arthritis Foundation Pacific Region, Nevada Office Presented by: Crystal Schulz, MPH Community Development Manager Arthritis Foundation Improving lives through leadership
Predictors of Physical Therapy Use in Patients with Rheumatoid Arthritis
 Predictors of Physical Therapy Use in Patients with Rheumatoid Arthritis Maura Iversen,, PT, DPT, SD, MPH 1,2,3 Ritu Chhabriya,, MSPT 4 Nancy Shadick, MD 2,3 1 Department of Physical Therapy, Northeastern
Predictors of Physical Therapy Use in Patients with Rheumatoid Arthritis Maura Iversen,, PT, DPT, SD, MPH 1,2,3 Ritu Chhabriya,, MSPT 4 Nancy Shadick, MD 2,3 1 Department of Physical Therapy, Northeastern
Arthritis and Rheumatology. Antoni Chan MBChB, FRCP, PhD Consultant Rheumatologist Royal Berkshire NHS Foundation Trust
 Antoni Chan MBChB, FRCP, PhD Consultant Rheumatologist Royal Berkshire NHS Foundation Trust Rheumatology Investigation, Diagnosis, Treatment The challenge 8 billion a year in cost 700,000 people suffering
Antoni Chan MBChB, FRCP, PhD Consultant Rheumatologist Royal Berkshire NHS Foundation Trust Rheumatology Investigation, Diagnosis, Treatment The challenge 8 billion a year in cost 700,000 people suffering
Autoimmune Diseases More common than you think Randall Stevens, MD
 Autoimmune Diseases More common than you think Randall Stevens, MD picture placeholder Autoimmune Diseases More than 60 different disorders Autoimmune disorders (AID) diseases caused by the immune system
Autoimmune Diseases More common than you think Randall Stevens, MD picture placeholder Autoimmune Diseases More than 60 different disorders Autoimmune disorders (AID) diseases caused by the immune system
ARTHRITIS INTRODUCTION
 ARTHRITIS INTRODUCTION Arthritis is the most common disease affecting the joints. There are various forms of arthritis but the two that are the most common are osteoarthritis (OA), and rheumatoid arthritis
ARTHRITIS INTRODUCTION Arthritis is the most common disease affecting the joints. There are various forms of arthritis but the two that are the most common are osteoarthritis (OA), and rheumatoid arthritis
PRACTICAL HELP FROM THE ARTHRITIS FOUNDATION. www.arthritis.org 800-283-7800. Psoriatic Arthritis
 Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Arthritis in Children: Juvenile Rheumatoid Arthritis By Kerry V. Cooke
 Reading Comprehension Read the following essay on juvenile rheumatoid arthritis. Then use the information in the text to answer the questions that follow. Arthritis in Children: Juvenile Rheumatoid Arthritis
Reading Comprehension Read the following essay on juvenile rheumatoid arthritis. Then use the information in the text to answer the questions that follow. Arthritis in Children: Juvenile Rheumatoid Arthritis
Spondyloarthritis is a general term for a group of rheumatic
 Rheumatic Disease Clinics of North America Supplement 1 11 Current Treatment for Psoriatic Arthritis and Other Spondyloarthritides Spondyloarthritis is a general term for a group of rheumatic diseases,
Rheumatic Disease Clinics of North America Supplement 1 11 Current Treatment for Psoriatic Arthritis and Other Spondyloarthritides Spondyloarthritis is a general term for a group of rheumatic diseases,
Nail disorders. Prof. MUDr. Petr Arenberger, DrSc, MBA
 Nail disorders Prof. MUDr. Petr Arenberger, DrSc, MBA Baseline Nail Disease in Patients with Moderate-to-Severe Psoriasis and Response to Treatment with Infliximab over One Year Phoebe Rich, Christopher
Nail disorders Prof. MUDr. Petr Arenberger, DrSc, MBA Baseline Nail Disease in Patients with Moderate-to-Severe Psoriasis and Response to Treatment with Infliximab over One Year Phoebe Rich, Christopher
16. ARTHRITIS, OSTEOPOROSIS, AND CHRONIC BACK CONDITIONS
 16. ARTHRITIS, OSTEOPOROSIS, AND CHRONIC BACK CONDITIONS Goal Reduce the impact of several major musculoskeletal conditions by reducing the occurrence, impairment, functional limitations, and limitation
16. ARTHRITIS, OSTEOPOROSIS, AND CHRONIC BACK CONDITIONS Goal Reduce the impact of several major musculoskeletal conditions by reducing the occurrence, impairment, functional limitations, and limitation
GP Symposium Dermatology Dr Seow Hoong Foo Dr Shireen Velangi March 6th 2014
 Psoriasis : It s not just skin de eep NICE Guidelines and Quality Standards: a collaboration to deliver quality care GP Symposium Dermatology y p gy Dr Seow Hoong Foo Dr Shireen Velangi March 6th 2014
Psoriasis : It s not just skin de eep NICE Guidelines and Quality Standards: a collaboration to deliver quality care GP Symposium Dermatology y p gy Dr Seow Hoong Foo Dr Shireen Velangi March 6th 2014
Rheumatoid Arthritis: Diagnosis, Management and Monitoring
 Rheumatoid Arthritis: Diagnosis, Management and Monitoring Effective Date: September 30, 2012 Scope This guideline is intended to aid in early recognition, intervention and management of patients with
Rheumatoid Arthritis: Diagnosis, Management and Monitoring Effective Date: September 30, 2012 Scope This guideline is intended to aid in early recognition, intervention and management of patients with
Rheumatoid Arthritis Information
 Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy
 Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
