Spondyloarthritis is a general term for a group of rheumatic
|
|
|
- Regina Thomas
- 10 years ago
- Views:
Transcription
1 Rheumatic Disease Clinics of North America Supplement 1 11 Current Treatment for Psoriatic Arthritis and Other Spondyloarthritides Spondyloarthritis is a general term for a group of rheumatic diseases, including: psoriatic arthritis (PsA), ankylosing spondylitis (AS), reactive arthritis, undifferentiated spondyloarthritis, juvenile spondyloarthritis, and inflammatory bowel disease associated spondyloarthritis. Three clinical features that characterize the spondyloarthritides, in addition to inflammatory peripheral arthritis, are enthesitis, inflammatory back pain, and dactylitis. Synovitis is the primary lesion in rheumatoid arthritis (RA), whereas both synovitis and enthesitis are important in spondyloarthritis. 1, 2 Spondyloarthritides share certain genetic linkages, such as common expression of human leukocyte antigen (HLA)-B27, as well as common histopathologic elements that may be somewhat distinct from those typically seen in RA. Some of these features include relatively decreased synovial lining layer cellularity and increased synovial vascularity, more prominent involvement of polymorphonuclear cells and macrophages, and an increase in the expression of Toll-like receptors 2 and 4, suggesting a role for innate immunity in the spondyloarthritides. 3, 4 Currently, there is no specific diagnostic test for these conditions. Thus, diagnosis is based on an appropriate constellation of clinical, laboratory, and imaging features. Recent studies are beginning to provide further insight into the histopathology of spondyloarthritis as well as demonstrate the effectiveness and safety of new treatments. This article provides a brief overview of each condition and a comprehensive summary of current available treatments. PHILIP MEASE, MD large parts of the body. Other psoriasis variants, such as guttate, pustular, and erythrodermic, are less common. 13, 14 Psoriasis usually develops before joint involvement, typically by many years. Whereas the severity of the psoriasis is not predictive of the severity of PsA, recent studies suggest that there may be a correlation between psoriasis severity and the occurrence of PsA. 8, 15 Joint involvement often is asymmetric, with frequent inflammation of the distal interphalangeal (DIP), as well as other joints. Other characteristic features include enthesitis, dactylitis, and spine inflammation, particularly in the sacroiliac joints. Enthesitis involves inflammation at sites where tendons, ligaments, and joint capsule fibers insert into bone for example, at the insertions of the Achilles tendon and plantar fascia as well as at ligaments around the rib cage and pelvis. Dactylitis, swelling of a whole digit, includes both synovitis of the joints and enthesitis of tendon and ligament attachments in the digit. Other features may include episodic iritis and frequent nail psoriasis, evidenced by pitting or gross onycholysis. Distinct from RA, serum test results for rheumatoid factor (RF) usually are negative. Elevations in levels of the acute phase reactants, such as the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP), are variable. Radiographic features that help distinguish PsA from RA include both lytic changes such as the pencil-in-cup change, which reflects gross bone and cartilage lysis as well as evidence of new bone formation, such as complete ankylosis of joints and juxta-articular osteitis. A significant 2, 11, 16 proportion of patients experience functional impairment. PSORIATIC ARTHRITIS Psoriatic arthritis (PsA) is a chronic and progressive form of inflammatory arthritis. It occurs in individuals with psoriasis, which affects approximately 2% to 3% of the general population. The reported range of prevalence of PsA in psoriasis patients is 6% to 39%, 5 and recent large telephone surveys in Europe and in the United States suggest ranges of 30% 6 and 11%, respectively. 7-9 This wide range is related to differing methods of ascertainment and heterogeneity of the populations studied. There currently is no predictive marker to indicate in which psoriasis patients arthritis will develop. 10 Nearly one half of patients have a family history of PsA, 11 although the concordance of PsA in identical twins is only 30% to 40%. 12 About 25% of PsA patients have spinal involvement. The condition often is classified as one of the subtypes of spondyloarthropathy, given shared HLA associations among those with spinal involvement, and characteristic inflammatory clinical and immunopathologic features. 4 PsA generally is considered an autoimmune disease, albeit with unknown antigenic determinants. Disease Features The key clinical feature of the most common form of psoriasis, plaque psoriasis, is thickened, erythematous, hyperkeratotic skin lesions. These scaly patches usually occur over extensor surfaces, such as the elbows or knees, and may coalesce to cover Immunopathology Because nearly all of the newest treatments for PsA involve biologic agents that target underlying immunologic pathways of this condition, it is useful to briefly review its immunopathology. T cells are known to play a strong role in psoriasis and in PsA. A number of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), also serve important roles in the disease. TNF-α has gained much recent attention, as agents that block its activity have become available. TNF-α is produced by macrophages, keratinocytes, mast cells, monocytes, dendritic cells, and activated T cells. It up-regulates nuclear transcription factors, including nuclear factor κb (NFKB), resulting in enhanced expression of many molecules central to the inflammatory response, including other cytokines (eg, IL-1, IL-6) and chemokines (eg, IL-8). TNF-α also induces the expression of endothelial, keratinocyte, and dendritic cell surface adhesion receptors that are involved in the trafficking of leukocytes to inflammatory lesions, such as intercellular adhesion molecule (ICAM)-1 and E-selectin (CD62E). TNF-α also mediates numerous other biologic processes that can result in joint and bone damage, such as expression of metalloproteinases by fibroblasts, maturation of activated osteoclasts from monocytic stem cells, and angiogenesis. A comprehensive discussion of the immunohistochemical features of 11, PsA is given elsewhere. Philip Mease, MD, is Chief, Division of Rheumatology Research, Swedish Medical Center, and Clinical Professor, University of Washington School of Medicine, Seattle. Address correspondence to Philip J. Mease, MD, Seattle Rheumatology Associates, 1101 Madison St., Suite 1000, Seattle, WA 98104; [email protected]. This issue of Rheumatic Disease Clinics Updates is sponsored by an unrestricted educational grant from Abbott Laboratories. The opinions and/or clinical experience outlined herein are those of the author and do not necessarily represent the views of the publisher or sponsor. Philip Mease, MD, is a consultant for and on the speaker s bureau for Abbott Laboratories, Amgen, Biogen Idec, Centocor, Genentech, and Wyeth. Dr. Mease is a stock shareholder for Amgen. Copyright 2006 Elsevier Inc. All rights reserved. Printed in Canada.
2 12 Rheumatic Disease Clinics of North America Supplement 1 Classification of PsA New classification criteria for PsA have been developed by an international working group (CASPAR) to improve the sensitivity and specificity of the classification of this disorder. 21 Although a number of classification criteria for PsA have been proposed since the initial Moll & Wright criteria, 22, 23 it is the latter criteria that primarily have been used. By this criteria set, PsA is classified as inflammatory arthritis in a patient with psoriasis and (usually) negative RF presenting with one of the following five clinical subsets: (1) oligoarticular (< 5 tender and swollen joints) asymmetric arthritis, (2) polyarticular arthritis, (3) predominant DIP joints, (4) spondylitis predominant, and (5) arthritis mutilans. Outcome Measures in PsA For the most part, outcome measures for PsA have been adapted from similar measures used in assessment of RA and psoriasis. These are used both in clinical treatment trials, as well as in longitudinal studies of the natural history of PsA. Although these measures can effectively assess peripheral joint and skin inflammation, function, and quality of life, they have yet to be fully refined and validated in PsA and do not adequately assess domains such as enthesitis, spine inflammation, dactylitis, and fatigue. A brief synopsis of these scales is given in Table 1. Most have not been specifically validated in PsA, although their performance characteristics in clinical trials have been good. These measures have been reviewed in detail Several studies have documented the effectiveness of ultrasound and magnetic resonance imaging (MRI) in detecting inflammation in the joints and entheses of patients with spondyloarthritis, as well as the extent of structural damage. 30, 31 As these tools become more refined, they will enhance our ability to assess the effectiveness of new therapies on the progression of joint damage in PsA. TABLE 1. A Synopsis of Scales Measuring Psoriatic Arthritis Outcome Measures of PsA Outcome ACR Response Criteria: 20%, 50%, 70% (validated in RA, not PsA) Tender and swollen joint counts (modified for PsA to include CMC joints) 3/5: patient global, physician global, patient pain, HAQ, acute phase reactant (sed rate, CRP) Psoriatic Arthritis Response Criteria (PsARC) (not validated) Improvement in at least 2 of 4 criteria, including: Physician global assessment (0 5) Patient global assessment (0 5) Tender joint score (>30%) Swollen joint score (>30%) Improvement in at least 1 of 2 joint scores No worsening in any criteria DAS (Exploratory, adapted from RA) Skin assessment (PASI, Target Lesion, Static Global) QOL/function/disability indices (HAQ, SF-36, DLQI) Radiographic (Modified Sharp, Modified van der Heijde/Sharp, Modified Steinbrocker, Wassenberg/Rau) ACR = American College of Rheumatology; CMC = carpal metacarpal; CRP = C-reactive protein; DAS = Disease Activity Score; DLQI = Dermatology Life Quality Index; HAQ = Health Assessment Questionnaire; PASI = Psoriasis Area and Severity Index; PsA = psoriatic arthritis; QOL = quality of life; RA = rheumatoid arthritis; SF-36 = Short Form Health Survey general questionnaire. Data from Gladman et al 24 and Mease et al. 25 Treatment Treatment of PsA depends on the severity of the condition and the features involved. The approach to treatment uses similar principles as RA treatment, on the one hand, and psoriasis treatment on the other. One challenge in determining which medications are the most useful for an individual patient is that some medications may preferentially impact psoriasis symptoms, but not joint problems, and vice versa (Fig. 1). Another challenge is matching the potency of the medication to the severity of the disease as well as the likelihood of future progressive damage. If the skin lesions of psoriasis are mild, topical creams such as vitamin D or steroid cream or various forms of light therapy may be used. Traditional nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, corticosteroids, or traditional disease-modifying anti-rheumatic drugs (DMARDs) can help mild to moderate, and occasionally severe, arthritis manifestations. For moderate to severely affected patients who do not respond adequately to traditional DMARDs, biologic agents, such as the anti-tnf-α medications, have contributed greatly to our ability to improve joint and skin disease, to inhibit progression of structural damage to the joints, and to improve patients functional ability and quality of life. Physical therapy also is an important component of treatment that helps to maintain or improve joint mobility, muscle strength, and range of motion. Occupational therapists focus on procedures and devices to aid hand and wrist function. Orthopedic procedures, including joint modification or replacement, can help with more severely damaged joints. Figure 1. Typical treatment modalities used for different features of psoriatic arthritis (PsA). 1, 2 Treatment for PsA is dictated by the type and severity of features involved. CsA = cyclosporine; DMARD = disease-modifying anti-rheumatic drug; IA = intraarticular; Lef = leflunomide; MTX = methotrexate; NSAID = nonsteroidal antiinflammatory drug; PT = physical therapy; PUVA = psoralen ultraviolet A; SSZ = sulfasalazine; TNF = tumor necrosis factor; UVB = ultraviolet B. Traditional Medications: NSAIDs, COX-2 Inhibitors, Corticosteroids and DMARDs NSAIDs are used to treat mild cases of PsA that involve fewer joints and little or no disability and that demonstrate no evidence of progressive structural damage. These medications help reduce inflammation and relieve pain, and thus restore joint mobility. However, gastrointestinal side effects render these medications intolerable for some patients. 32 Selective COX-2 inhibitors, such as celecoxib, and relatively selective COX-2 inhibitors, such as meloxicam, may be associated with less gastrointestinal toxicity than other NSAIDs. 32 Corticosteroids must be used cautiously in patients with PsA because withdrawal may trigger a flare of psoriasis. 33, 34 The DMARDs traditionally used in PsA are methotrexate (MTX) and sulfasalazine, although cyclosporine, azathioprine, and antimalarial drugs also are used. 32 Methotrexate Most of the published data for MTX pertain to its safety and efficacy in treating RA. In the few controlled trials involving the
3 Rheumatic Disease Clinics of North America Supplement 1 13 use of MTX in PsA, there has been only marginal benefit shown in the joints or skin. 35, 36 However, more satisfactory results have been obtained in clinical practice when higher doses of MTX are used compared with those used in older clinical trials. In a 26-year retrospective study of long-term, low-dose MTX therapy in 157 patients with severe psoriasis, 76% of patients had a good response to MTX. However, 61% experienced side effects, including liver function abnormalities, bone marrow suppression, and gastric complaints, and 20% discontinued treatment. 37 In a 12-week, prospective, double-blind trial, Willkens and colleagues 35 found that PsA patients receiving either 7.5 mg or 15 mg of MTX per week experienced a greater decrease in psoriasis skin surface area than patients receiving placebo. Patients receiving MTX also rated better on the physician s global assessment in joint activity than patients receiving placebo. There was, however, no difference between MTX and placebo in terms of joint pain, tenderness, and swelling or duration of morning stiffness. Although patients with PsA receiving MTX showed a great decrease in skin surface area involvement, there was no difference in terms of lesion induration or scaling. In a retrospective open-label study designed to examine the disease-modifying effects of MTX, there were no differences in radiographic progression between patients treated with MTX (n = 38) and matched controls. 38 MTX Versus Cyclosporine In a prospective, randomized study of 35 patients treated for 1 year, Spadaro and colleagues concluded that MTX was as effective as cyclosporine in treating PsA. 39 Cyclosporine dosages of 3 to 5 mg/kg per day and MTX at 7.5 to 15 mg per week were associated with several clinical improvements, such as fewer painful or swollen joints, decreased Ritchie index score, shorter duration of morning stiffness, improvement in Psoriasis Area and Severity Index (PASI), improved grip strength, and improved patient and physician global assessments. The ESR improved to a greater extent with MTX than with cyclosporine, but MTX was associated with elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes. Fewer patients given MTX withdrew from the study, despite issues with hepatotoxicity. 39 The most significant adverse effect of prolonged, intensive use of either MTX or leflunomide, another DMARD, is the potential for liver toxicity. This seems to occur more frequently in patients with psoriasis than among those with RA. 40 A number of reasons have been speculated, including a proclivity toward obesity in psoriasis patients, increasing their risk for hepatic steatosis (fatty liver) as a concomitant problem. Leflunomide Leflunomide, a recently approved synthetic DMARD for RA, also has been shown to be effective in treating PsA. In a randomized clinical trial (n = 190), leflunomide was found to be statistically superior to placebo in reducing the symptoms of PsA, with Psoriatic Arthritis Response Criteria (PsARC) of 59% for the treatment group and 29.7% for the placebo group. 41 The drug achieved clinically significant but modest results in modified ACR 20 and PASI measurements. Adverse effects for leflunomide include diarrhea and elevations in ALT. Cyclosporine In a double-blind trial comparing cyclosporine with placebo in patients with PsA, 80% of patients in the highest-dosage group of cyclosporine (7.5 mg/kg) exhibited clearing or near-clearing of psoriasis. 42 A recent meta-analysis of three major randomized studies of patients with severe psoriasis concluded that cyclosporine is highly effective and well tolerated in the short term for management of skin disease. 43 Cyclosporine Versus Sulfasalazine In the absence of placebo-controlled trials, cyclosporine appears to be at least as effective as sulfasalazine in treating PsA, and it may be more effective than sulfasalazine in providing pain relief. For example, a 24-week, open-label study of 99 patients who had PsA compared cyclosporine with sulfasalazine and with symptomatic therapy (eg, analgesics, NSAIDs). Patients receiving cyclosporine experienced significantly greater improvement in pain after 6 months than did patients in the other two groups (p <.05). Treatment with cyclosporine also was associated with a reduction in CRP. 44 Sulfasalazine Sulfasalazine has been used in more published PsA trials than most conventional therapies. In 221 patients with PsA whose disease was resistant to NSAIDs, Clegg and colleagues 45 administered either 2 g/day of sulfasalazine or placebo for 36 weeks. Patients 24, 25, 45 response to therapy was calculated according to PsARC. Although individual elements of the response criteria did not show a significant benefit for sulfasalazine, the distinction of the treatment and placebo groups did achieve statistical significance when using the composite criteria (58% vs. 45%, p =.05). When these patients were included in a larger retrospective re-analysis of data from various studies on treatment of spondyloarthritides (n = 619), sulfasalazine was found to be more effective than placebo in overall response to therapy, but its effectiveness was limited to peripheral articular symptoms, not axial manifestations. 16 In a 6- month trial with 351 patients who had spondyloarthritis, highdose (3 g/day) sulfasalazine was superior to placebo only in patients overall assessment. However, when the subgroup of PsA patients was analyzed, Dougados and colleagues found significant benefits from sulfasalazine in the patients and physician s global assessments, overall pain, joint pain and swelling, and duration of morning stiffness. 46 Other studies have shown marginal benefits of sulfasalazine compared with placebo in PsA Other DMARDs Etretinate, a retinoid medication traditionally used for psoriasis, has been shown to have some marginal benefit for patients with PsA. 51 In a study of 82 patients with PsA, intramuscular gold provided more relief of overall pain and improvement in the Ritchie Index than did oral gold, but it was not statistically superior to placebo. 52 The use of antimalarial medications is controversial because psoriasis flares have been associated anecdotally with use of these drugs. 53 A 1999 review of 18 English-language publications revealed that up to 18% of patients who had psoriasis developed an exacerbation of their disease following antimalarial therapy. 54 However, with this caution in mind, antimalarial medications can be used for mild PsA. 32 New DMARDs: Biologic Agents Biologic agents are designed to target specific immunologic mechanisms operative in a variety of inflammatory diseases. Rheumatologists are using these drugs increasingly to treat RA (see Volume 1/Issue 1 in this series). Dermatologists are using these agents to treat moderate to severe psoriasis. Both specialists are using these agents to treat PsA that has not responded adequately to NSAID or DMARD therapy alone (Fig. 2). Biologic agents differ in their mechanisms of action but share an ability to interrupt specific parts of the cascade of immunologic cell activation involved in rheumatic diseases. In PsA, these agents, particularly those targeting the proinflammatory cytokine TNF-α, have produced dramatic 20, improvements in arthritis, enthesitis, and psoriatic skin lesions.
4 14 Rheumatic Disease Clinics of North America Supplement 1 Figure 2. Biologic agents in trials or in use for treatment of psoriatic arthritis and psoriasis. EU = Europe; US = United States. Current anti TNF-α agents include etanercept (Enbrel, Immunex Corporation, Thousand Oaks, CA), infliximab (Remicade, Centocor, Inc., Malvern, PA), and adalimumab (Humira, Abbott Laboratories, Chicago, IL). The precise mechanism of action responsible for the efficacy of these agents has not been fully delineated. Some of the observed histologic and immunohistochemical effects of TNF inhibition include reductions of: synovial lining layer thickness, vascularity, endothelial expression of avb3 and vascular cell adhesion molecule (VCAM)-1, sublayer expression of ICAM-1 and E-selectin, T-cell and macrophage infiltration, vascular endothelial growth factor (VEGF) expression, synovial Flk-1 expression and reduction in the numbers of osteoclast precursors, as well as in osteoclast differentiation. Several studies have shown correlative findings in skin biopsies. 20, These studies reaffirm the central role of TNF-α in the inflammation of PsA and psoriasis, and enhance our understanding of the pathophysiology of these conditions. Clinical trials of these medications have shown consistently positive results in both joints and skin and consequent improvement in function and in quality of life. Notably, for the first time in PsA, radiographic assessment has shown that these medications also inhibit progression of joint damage , 64 Studies of patients with RA show that a substantial number of those who have inadequate efficacy with one anti TNF-α medication may switch to another and attain at least an ACR 20 response Although interesting, this has not been formally studied in patients with PsA. Etanercept Etanercept is a soluble receptor TNF-α antagonist currently approved for the treatment of RA, JA, PsA, AS, and psoriasis. Dosage is typically 25 mg given subcutaneously twice a week or 50 mg given once a week, although when a higher dose is used in skin psoriasis. The approval of etanercept in PsA was based on two placebo-controlled trials. In a single-center trial, 60 patients with PsA, about half of whom (47%) were receiving concomitant stable dose of MTX, were randomized to etanercept or to placebo. 56 On average, patients had nearly a 20-year history of psoriasis and a 15-year history of inflammatory arthritis. In the 3-month placebocontrolled phase of the study, 87% of patients receiving etanercept and 23% of patients receiving placebo improved according to the composite outcome measure PsARC. In addition, 73% of patients receiving etanercept and 13% of patients receiving placebo improved according to the composite ACR 20 response criteria, a standard for assessing treatment response in rheumatoid arthritis (p =.015). Skin lesions also improved. The concomitant use of MTX did not affect the results, suggesting that etanercept can be effective as monotherapy or in combination with a traditional DMARD. In a larger, multicenter study of similar design, 205 patients with PsA received etanercept or placebo. Concomitant MTX was used by 42% of patients. 57 Fifty-nine percent of etanercept patients and 15% of placebo patients achieved an ACR 20 response. PsARC response was observed in 72% and 31%, respectively. The treatment group showed significant changes in all individual measures of the composite criteria. Skin responses were good at 6 months; the etanercept group achieved 47% PASI 50 and 23% PASI 75, respectively, and the placebo group achieved 18% PASI 50 and 3% PASI 75 (p.001). Also, there were significant improvements in measures of quality of life (Short Form Health Survey general questionnaire [SF-36], Dermatology Life Quality Index [DLQI]) and function (Health Assessment Questionnaire [HAQ]). Inhibition of disease progression, as documented radiographically, also was shown, the first demonstration of such an effect in PsA. Thus, after 48 weeks, there was no worsening of Total Sharp Score (TSS) in the active treatment group, a significant benefit compared with placebo, where worsening was observed. 57, 68 As in the previous trial, background treatment with MTX did not affect outcome. Infliximab Infliximab, a chimeric monoclonal antibody, is currently approved for RA, Crohn s disease, PsA, AS, and ulcerative colitis. It typically is administered as an intravenous infusion every 8 weeks. There have been two major placebo-controlled trials using this drug at a dose of 5 mg/kg intravenously in PsA: the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT, n = 104) 58 and a phase III study, IMPACT II (n = 200). 59 In both studies, background DMARD use was allowed but not required. In IMPACT II, MTX was the only DMARD allowed. In both studies, about half of enrolled patients received treatment with concomitant MTX, and this did not have an impact on efficacy. IMPACT showed an ACR 20 response in 69% of patients receiving infliximab and 8% of patients receiving placebo at 16 weeks and a PsARC response in 78% and 18%, respectively. Patients with a good or moderate European League Against Rheumatism (EULAR) response were 85% and 23%, respectively. Enthesitis and dactylitis improved significantly. Thirty-nine patients had a baseline PASI of 2.5. Of these, 67% had at least a PASI 75 response compared with 0.05% in the placebo group (all p values =.001). In an open-label, 1-year follow up of this group, patients originally taking placebo quickly achieved similar results with equivalent efficacy. 69 Radiographic data of hands and feet (including the DIP joints of the hand) were analyzed according to the modified van der Heijde-Sharp method. These showed no disease progression in both groups over 50 weeks. Because of the short duration of placebo treatment (14 weeks), no difference in the treatment groups could be shown over 1 year. However, the calculated annual progression rate was reduced, indicating that even delayed treatment with infliximab after 14 weeks inhibited radiographic progression of PsA. 70 Results from IMPACT II showed ACR 20 response in 58% of patients receiving infliximab and 11% of patients receiving placebo, PsARC in 77% and 27%, and PASI 75 in 65% and 2%, respectively (all p values =.001). 59 Median PASI improvement was 87% in ACR 20 responders and 74% in ACR 20 non-responders, suggesting that skin response could be achieved even when joint response was not demonstrated. 71 Radiographic data showed significantly less disease progression in patients receiving infliximab at week 24 than in patients receiving placebo. 64 When PsA-specific radiographic features including pencil-in-cup deformities and gross osteolysis were examined, there was no difference between the treatment groups, a result found in radiographic results of the other anti TNFα agents as well. Adalimumab Adalimumab is a human anti TNF-α monoclonal antibody. It is administered subcutaneously, 40 mg every other week. It currently is approved for use in RA and PsA. It was first shown
5 Rheumatic Disease Clinics of North America Supplement 1 15 effective for PsA in an open trial of 15 patients. 72 In a larger placebo-controlled trial (n = 313) in PsA, in which 50% of patients were on background MTX, 58% of the treatment group and 14% of the placebo group showed ACR 20 response at 3 months, the primary endpoint (p <.001). Initial response in skin was rapid (as early as 2 weeks) and significant. As in the studies of the other TNF inhibitors, concomitant use of MTX did not have an effect on efficacy. At 6 months, PASI 50/75/90 responses were 75%, 59%, and 42% in the adalimumab group and 12%, 1%, and 0% in the placebo group, respectively (all p values <.001). 60 Radiographic progression of disease was significantly inhibited by adalimumab. 60 A number of other anti TNF-α agents are in various phases of development for PsA, including a human monoclonal antibody for subcutaneous administration (golimumab), a PEGylated Fab fragment of a monoclonal antibody (certolizumab pegol [CDP870]), and even oral agents that have TNF-α inhibiting capability. Safety of Anti TNF-α Agents An in-depth review of safety issues regarding anti TNF-α agents was provided in Volume 1/Number 1 of this Updates series. No significant new safety issues have developed in PsA trials, other than those reported in RA trials (such as administration reactions and infection). However, the experience to date with TNF-α inhibitors in PsA is relatively small. Of note, there has been no observed increase in frequency of Koebner phenomenon or cellulitis in patients with PsA. Other Biologic Agents Several agents being developed for use in RA or psoriasis eventually will be assessed for use in PsA and other spondyloarthritis (see Fig. 2). The common feature of these agents is their ability to target different key cells and cytokines of the immune response and inflammatory processes. Agents currently being tested for efficacy in PsA include drugs that inhibit T cells by blocking the second signal of T-cell activation. The first signal is provided by the cognate interaction of antigen appropriately presented in the context of major histocompatibility complex molecules on the surface of antigen-presenting cells, with specific T-cell receptors on the surface of T cells. There are several pairs of receptor/counter-receptors capable of providing stimulatory second signals. Several of these are the targets of immunomodulatory therapies for autoimmune diseases and are characterized below. Alefacept Alefacept is a human fusion protein that blocks the interaction between leukocyte function associated antigen-3 (LFA-3) on antigen-presenting cells and CD2 on T cells. It is given as a weekly (15 mg) intramuscular injection and currently is approved for use in psoriasis. 73, 74 Treatment leads to a depletion of T cells, preferentially memory T cells, via interactions through the molecule s Fc piece. The drug is given as a regimen of 12 weeks on and 12 weeks off, partly to allow recovery of CD4 counts, which must be monitored during therapy. Despite the transient depletion of CD4 cells, there has been no increased risk of infection in psoriasis trials. 73, 74 In a small (n = 11) open-label trial of this compound in PsA, more than half of patients showed ACR 20 responses. Synovial biopsies showed a decrease in CD4, CD8, and CD68 (macrophage) cells in the synovial lining. 75 A recent randomized clinical trial (n = 185) to evaluate the efficacy and safety of alefacept in combination with MTX for patients with PsA 76 found that this combination provided significant clinical improvement in PsA. Patients given a 12-week course of alefacept and MTX had significantly greater response rates in both PsA (ACR 20) and psoriasis (PASI 50) than patients given placebo plus MTX. There was incremental improvement in ACR 20 response during the treatment-free phase, indicating that response to alefacept continues even when the drug is not being administered. 77 Efalizumab Efalizumab is a humanized antibody to the CD11 subunit of LFA-1 that inhibits the interaction of LFA-1 with its counter-receptors, including ICAM-1. It can interfere with the activation of T lymphocytes, as well as migration of cells from the circulation to sites of inflammation. Demonstrated efficacy in psoriasis has led to its approval for this condition. 78 In a 12-week trial of patients with PsA, in which efalizumab was administered subcutaneously once a week, 28% of patients achieved an ACR 20 response versus 19% in the placebo group (p =.2717). 79 Abatacept Abatacept, previously known as CTLA4-Ig, is a recombinant human fusion protein comprising the extracellular domain of human CTLA4 along with the Fc piece of a human immunoglobulin G (IgG)1 molecule. CTLA4 is a naturally occurring inhibitor molecule that binds to CD80 and CD86 on antigen-presenting cells, thereby inhibiting the ability of CD28 to bind to these molecules and provide an activating signal. Recently approved for use in RA, abatacept is administered intravenously once per month. 80 A Phase II trial for psoriasis has been conducted. 81 It is anticipated that further assessment of this drug will be conducted for PsA and for psoriasis. Other Potential Treatments A number of other agents are being tested or potentially could be tested in PsA, including a number of cytokine inhibitors. A pilot trial of an IL-15 inhibitor in PsA has shown efficacy. 82 A trial currently is underway to assess the efficacy and safety of an IL-1 antagonist, anakinra, in PsA (Fitzgerald O, personal communication, 2005). A humanized antibody to the α- subunit (CD25) of the IL-2 receptor that blocks IL-2 binding to the T-cell receptor has been tried in psoriasis, albeit with some loss of efficacy noted over time. 83, 84 A monoclonal antibody to the IL-6 receptor (MRA) is in phase III development for the treatment of RA; benefit has been shown in phase II, and this antibody will likely be tested in PsA. 85 Several inhibitors of IL-12 are undergoing active evaluation in psoriasis, with good early success (Leonardi C, personal communication, 2005); these are likely to be assessed in PsA as well. Similarly, it is anticipated that inhibitors of IL-18 will be studied. Conversely, some cytokines may have anti-inflammatory effects, and thus, their administration may be therapeutic. A recombinant IL-10 agent has been studied in psoriasis, with demonstration of preliminary benefit. 86 However, a controlled study with human IL-10 in patients with PsA showed benefit in the skin but not in joints. 87 Similarly, a recombinant human IL-11 has been used to treat psoriasis, with preliminary benefit noted clinically and histologically. 88 huokt 3γ1, a monoclonal antibody to CD3 and a component of the T-cell receptor complex, has demonstrated some benefit in PsA, although issues such as transient T-cell depletion and mild cytokine release symptoms have been noted. 89 Pioglitazone is a ligand for peroxisome proliferator-activated receptor (PPAR)γ that is administered orally. It originally was developed to treat diabetes but has been considered as a potential therapy for inflammatory autoimmune disease, such as PsA, due to observations that it led to a marked inhibition of angiogenesis and down-regulation of proinflammatory cytokines. 90 In an uncontrolled trial of pioglitazone treatment, 60% of patients met the
6 16 Rheumatic Disease Clinics of North America Supplement 1 PsARC criteria and 50% achieved an ACR 20 response after 12 weeks. 91 This agent may be beneficial for treating PsA, but its efficacy must be evaluated in a well-controlled study. ANKYLOSING SPONDYLITIS Ankylosing spondylitis (AS) is the most prevalent of the spondyloarthritides. Among the spondyloarthritides, AS and PsA have the most severe disease course. The pathophysiologic features of AS are similar to those of PsA and, indeed, at a cellular and immunohistochemical level, appear to be identical. 92, 93 Progression of inflammation in AS produces radiographic damage in the spine and in peripheral joints, leading to pain, loss of function, and loss of mobility for patients. Many patients with AS also show gut lesions similar to those seen in Crohn s disease. 94 Disability and consequent absence from work is increased threefold for patients with AS Thus, the condition results in substantial costs for the patient and society. 98 Treatment Treatment for AS traditionally has included medications, such as NSAIDs, that largely relieve symptoms, although they may also modify the disease. Recent trials demonstrated the efficacy of 99, 100 celecoxib and etoricoxib (not approved or marketed) in AS. Traditional synthetic DMARDs have not proved effective for spinal aspects of AS, the predominant clinical issue for these patients. 101 Physical therapy traditionally has played an important role in treatment and has been shown effective for patients with AS As with PsA and RA, perhaps the most significant advance in pharmacologic treatment for AS has been the development of biologic agents that may modify progression of the disease. As in RA and PsA, the three currently available anti-tnf-α agents used in clinical trials in AS include etanercept, infliximab, and adalimumab. Of these, etanercept and infliximab have gained approval thus far. In contrast to RA, clinical studies in AS have not included MTX in combination with biologic agents because the efficacy of MTX in AS is doubtful. 101 A comprehensive discussion of biologic agents in AS is given by Braun et al. 101 TNF Inhibitors in AS Etanercept has demonstrated efficacy in AS. An initial randomized, double-blind, placebo-controlled study of etanercept (25 mg administered biweekly subcutaneously for 4 months) demonstrated significant improvement in disease activity over baseline values, as measured by assessment of spinal pain, morning stiffness, enthesitis, ESR, CRP, chest expansion, functioning, and quality of life. 105 Improvement was rapid and sustained. There was no significant difference in rates of adverse events between the etanercept and placebo groups. A larger, multicenter, double-blind, placebo-controlled study confirmed these results in patients with moderate to severe AS. 106 Patients treated with etanercept achieved significant improvement in clinical features, with a greater percentage achieving ASAS (Assessment in Ankylosing Spondylitis) 20 by week 12 compared with patients receiving placebo. Improvement was significant within 2 weeks and was sustained through 2 years of treatment Several randomized clinical trials have documented the efficacy of infliximab in AS A key outcome has been the regression of spinal inflammation, as documented by MRI studies. 116 In a recent international, multicenter randomized clinical trial, 64, % of patients receiving infliximab achieved ASAS 20 compared with 19.2% of patients receiving placebo. In the same study, 22.4% of patients taking infliximab achieved ASAS criteria for partial remission, compared with 1.3% of patients taking placebo. Long-term results with infliximab 120 indicate continuous improvement over 3 years, including reduction of NSAID dosage in 70% of patients. All patients were withdrawn from treatment after 3 years to assess the nature of the subsequent disease course. 121 Two thirds of these patients relapsed within the first 6 months, with a mean relapse time of 3 months. When infliximab was reinstituted, efficacy as originally noted was re-achieved. A recent study 122 comparing the effectiveness of etanercept and infliximab in RA compared with AS found that patients with AS experienced health-related quality-of-life improvement that was comparable to, and sometimes greater than, that observed in patients with RA. Preliminary data from the only current study of adalimumab in AS show significant improvement in pain and function. 123 ASAS/EULAR Recommendations The Assessment in Ankylosing Spondylitis (ASAS) international working groups and the European League Against Rheumatism (EULAR) recently released recommendations for the management of AS. 124, 125 These were developed using evidencebased recommendations from a multidisciplinary development committee, as well as a systematic literature search. The group developed 10 key recommendations (Table 2) to assist clinicians in assessing both clinical and observational data, thus enabling them to select the most effective treatment for specific patients. The recommendations specify that optimal treatment of AS includes both pharmacologic and nonpharmacologic therapies. The group also emphasizes these are recommendations, not guidelines, and that the recommendations will be modified as new clinical and patient evidence is elucidated. TABLE 2. ASAS/EULAR Recommendations for the Treatment of Ankylosing Spondylitis 1. Treatment of AS should be tailored according to: current manifestations of the disease; level of current symptoms, clinical findings and prognostic indicators; general clinical status; and wishes and expectations of the patient. 2. Disease monitoring of patients with AS should include: patient history (eg, questionnaires), clinical parameters, laboratory presentation, as well as the ASAS core set. The frequency of monitoring should be decided on an individual basis, depending on symptoms, severity, and drug treatment. 3. Optimal management of AS requires a combination of nonpharmacological and pharmacological treatments. 4. Nonpharmacological treatment of AS should include patient education and regular exercise. Individual and group physical therapy should be considered. Patient associations and self-help groups may be useful. 5. NSAIDs are recommended as first-line drug treatment for patients with AS with pain and stiffness. In those with increased gastrointestinal (G) risk, nonselective NSAIDs plus a gastroprotective agent, or a selective COX-2 inhibitor could be used. 6. Analgesics, such as acetaminophen and opioids, might be considered for pain control in patients in whom NSAIDs are insufficient, contraindicated, and/or poorly tolerated. 7. Corticosteroid injections directed to the local site of musculoskeletal inflammation may be considered. The use of systemic corticosteroids for axial disease is not supported by evidence. 8. There is no evidence for the efficacy of DMARDs, including sulfasalazine and methotrexate, for the treatment of axial disease. Sulfasalazine may be considered in patients with peripheral arthritis. 9. Anti-TNF treatment should be given to patients with persistently high disease activity, despite conventional treatments according to the ASAS recommendations. There is no evidence to support the obligatory use of DMARDs before, or concomitant with, anti-tnf treatment in patients with axial disease. 10. Total hip arthroplasty should be considered in patients with refractory pain or disability and radiographic evidence of structural damage, independent of age. Spinal surgery for example, corrective osteotomy and stabilization procedures may be of value in selected patients. AS = ankylosing spondylitis; ASAS = Assessment in Ankylosing Spondylitis; COX-2 = cyclooxygenase-2; DMARD = disease-modifying anti-rheumatic drug; EULAR = European League Against Rheumatism; NSAID = nonsteroidal anti-inflammatory drug; TNF = tumor necrosis factor. From Zochling et al. 124
7 Rheumatic Disease Clinics of North America Supplement 1 17 Recommendations for Use of Anti-TNF-α Agents in AS The ASAS international working group also has developed specific guidelines for use of biologic agents in treating AS. These have been modified for use in the United States by the Spondyloarthritis Research and Treatment Network (SPARTAN). Table 3 lists the ASAS guidelines and US modifications. TREATMENT OF OTHER SPONDYLOARTHRITIDES Other forms of spondyloarthritis, including reactive arthritis, undifferentiated spondyloarthritis, and inflammatory bowel disease associated spondyloarthritis, affect relatively smaller populations and are clinically heterogeneous. Thus, there are fewer studies documenting effective treatments. Reactive arthritis (previously known as Reiter syndrome) is a term used to describe arthritis that can include spondyloarthritis and that, in some cases, is triggered by infectious agents. This includes those infecting the urogenital or gastroenteral tract, such as Campylobacter, Salmonella, Versinia, and Chlamydia. 126 Poststreptococcal reactive arthritis appears to be a heterogeneous condition, with a group that resembles rheumatic fever and a group with spondyloarthropathy traits. 127 There are numerous theories regarding a link between infectious triggers and resultant prolonged activation of the immunologic and inflammatory cascades, even when the infectious agent is no longer present. 128 Mechanisms suggested to be potentially relevant to the etiology and pathogenesis of reactive arthritis include molecular mimicry, arthritogenic peptides, and genetic factors. 129 Undifferentiated spondyloarthritis includes conditions that have spondyloarthritis features but do not fit into any other defined spondyloarthritis subgroup. Spondyloarthritis associated with inflammatory bowel disease may be associated with peripheral joints, including a pauciarticular, asymmetrical, transitory form, along with enthesopathy. It is observed in 10% to 20% of patients with inflammatory bowel disease associated arthritis 130 and frequently coincides with a flare-up of intestinal disease. Demonstration of the benefit of treatments for these other subtypes of spondyloarthritis has been limited. As in PsA, physical therapy may play an important role. NSAIDs relieve pain and inflammation and have been proved effective in a recent trial. 131 Sulfasalazine may be used to treat patients with gut disease or peripheral arthritis in early and active stages of these other subtypes of spondyloarthritis. 132 Use of Biologic Agents in Other Spondyloarthritides Preliminary studies suggest that infliximab and etanercept are effective in treating arthritis associated with inflammatory bowel disease TNF-α inhibition with infliximab and adalimumab, but not etanercept (at the doses studied), have proved effective in Crohn s disease 136 and in ulcerative colitis, which can be associated with AS. Rapid, substantial, and sustained improvement in symptoms has been reported following treatment with infliximab, suggesting an essential role for TNF-α in spondyloarthropathy, similar to that observed in Crohn s disease. 130 Biologic Agents for Juvenile-Onset Spondyloarthritis Although the experience with anti-tnf-α agents is limited in children and adolescents with juvenile-onset spondyloarthritis, current results suggest that infliximab and etanercept are at least as effective in children as in adults. 137 Most patients are able to stop taking NSAIDs and other medications. In a recent study, administration of 0.2 to 0.8 mg/kg of etanercept subcutaneously twice weekly in eight patients with juvenile-onset AS produced a prolonged reduction in the number of active joints, morning stiffness, TABLE 3. ASAS/EULAR Guidelines for Use of Biologic Agents to Treat Ankylosing Spondylitis and US Modifications of These Guidelines ASAS Diagnosis Modified New York Criteria Disease Activity BASDAI Score > 4 (scale 0 10 cm) and Physician Global Assessment by expert opinion (yes/no) Previous Treatment Failure by lack of response or intolerability to > 2 NSAIDs for 3 months for all clinical 3 presentations: axial, peripheral arthritis, and enthesitis Patients with peripheral arthritis also having a lack of response to a local steroid injection (for those with oligoarticular involvement) must have had a lack of response to both NSAIDs and sulfasalazine Patients with enthesitis must have had an adequate trial of at least 2 local steroid injections, unless contraindicated Dosing Etanercept 25 mg SQ twice a week Infliximab 5 mg/kg IV q 6 8 weeks Responder Criteria 50% improvement of BASDAI or absolute change of 2 on 0- to 10- cm scale and expert opinion Time of Evaluation Between 6 12 weeks TB Precaution Use country-specific guidelines US Modifications of the ASAS Guidelines Modified New York Criteria BASDAI Score of > 4 cm (scale 0 10 cm) and Physician Global Assessment of 2 or greater on Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) Failure by lack of response or intolerability to > 2 NSAIDs for 3 months for all clinical 3 presentations: axial, peripheral arthritis, and enthesitis Patients with peripheral arthritis must have had a lack of response or intolerability to > 1 DMARD (sulfasalazine preferred) for peripheral arthritis; not required for axial disease or enthesitis (steriod injection not required) Etanercept 25 mg SQ twice a week Infliximab 5 mg/kg IV q 6 8 weeks Improvement in BASDAI by at least 2 units and Physician Global of > 1 Between 6 8 weeks TB screening and treatment per the American Thoracic Association recommendations From the Spondylitis Association of America. Available at: ASAS = Assessment in Ankylosing Spondylitis; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; DMARD = disease-modifying anti-rheumatic drug; EULAR = European League Against Rheumatism; IV = intravenous; NSAID = nonsteroidal anti-inflammatory drug; SQ = subcutaneously; TB = tuberculosis. and ESR. 138 The mean age of the group was 15.9 years (range, years), and the mean follow-up of these patients was 15.4 months. All patients tolerated etanercept without side effects. In unpublished observations, Burgos-Vargas 137 has treated six patients with juvenile-onset spondyloarthritis with 5 mg/kg of infliximab at weeks 0, 2, 6, and then every 2 months for nearly 1 year. There were significant reductions in peripheral and axial signs of disease after the first and second infusion of the drug, including a decrease in the number of peripheral joints with active arthritis and tender entheses. A decrease in CRP values, in pain as evaluated by visual analog scale (VAS), and in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI) scores also was observed. Long-term follow up is required to determine safety and efficacy
8 18 Rheumatic Disease Clinics of North America Supplement 1 of these medications in children, and whether anti-tnf-α agents can halt the progressive destruction characteristic of juvenile-onset spondyloarthritis. Cost-Effectiveness Analysis There is an emerging focus on the economic implications of these biologic therapies, whose acquisition costs exceed those of older anti-rheumatic therapies. Appropriate pharmacoeconomic assessment must take into consideration the costs of the therapies themselves, but also the costs of the disease, in terms of work disability and the interference with the ability to perform activities of daily living. Highly effective therapies may be shown to be cost-effective if they are able to avoid the costs of rheumatic diseases. Several recent studies have demonstrated the potential cost-effectiveness of anti-tnf-α agents in PsA. 139, 140 Similar studies have been accomplished for AS CONCLUSION As emerging treatments for PsA, AS, and other spondyloarthritides show significant benefit for clinical signs and symptoms, quality of life, functional status, and the inhibition of joint damage as assessed by radiographic progression, there has been increasing interest in accurate diagnosis and classification of these diseases. This would facilitate the institution of appropriate therapy in a timely fashion. Additionally, numerous studies have increased our understanding of the basic pathophysiology of the diseases, providing support for the clinical effects of targeted therapy, for example, inhibition of TNF-α. There are fewer studies documenting these benefits with traditional DMARDs, whose effectiveness does not seem as great as TNF-α inhibitors. In AS, recent trials suggest that DMARDs are not effective in the spine. Targeted therapies that inhibit proinflammatory cytokines, such as the TNFα inhibitors, have proved highly effective in managing joint and enthesial disease. Agents that block the cell cell interactions required to activate T cells are effective in the skin and may benefit the joints, as well. Observation of the effectiveness of these agents has helped elucidate the pathogenesis of PsA and psoriasis, which in turn may lead to more novel and effective interventions. Significant efforts are underway to develop and validate outcome measures that accurately assess the effect of therapies and determine the natural history of these diseases. This effort, along with the development of evidence-based treatment guidelines and general educational initiatives, is being led by international research consortia such as the ASAS international working group and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). The benefits of biologic agents must be weighed against their cost patient improvement and inhibition of disease progression versus allocation of limited resources. Comprehensive health economic analyses are being developed to determine the full impact of these more effective treatments on patient function, productivity, and quality of life in the context of society as a whole. REFERENCES 1. Nash P, Mease P, Braun J, et al. Seronegative spondyloarthropathies to lump or split? Ann Rheum Dis. 2005;64(suppl 2):ii9-ii Gladman D, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis, 2005;64(suppl 2):ii14-ii De Rycke L VB, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005;52: Kruithof E, Baeten D, De Rycke L, et al. Synovial histopathology of psoriatic arthritis, either oligo-or polyarticular, resembles more spondyloarthropathy than rheumatoid arthritis. Arthritis Res Ther. 2005;7:R Helliwell PS, Wright V. Psoriatic arthritis: clinical features. In Klippel J, Dieppe P, eds. Rheumatology. St Louis: Mosby; 2000: Salonen S. The EUROPSO Psoriasis Patient Study: Treatment History and Satisfaction Reported by 17,900 Members of European Psoriasis Patients Associations. Presented at the Spring Symposium of the European Academy of Dermatology and Venereology, 2003, Malta. 7. Leonard DG, O Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalized for psoriasis. Mayo Clin Proc. 1978;53: Gelfand J, Gladman D, Mease P, et al. Epidemiology of psoriatic arthritis in the United States population. J Am Acad Dermatol. 2005;53: Shbeeb M, Uramoto KM, Gibson LE, et al. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, J Rheumatol. 2000;27: Mease P. Targeting therapy in psoriatic arthritis. Drug Discovery Today. 2004;1: Mease PJ. Psoriatic arthritis/psoriasis. In Smolen J, Lipsky PE, eds. Targeted Therapies in Rheumatology. London: Martin Dunitz; 2003: Sege-Peterson K WR. Psoriatic arthritis. In Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick s Dermatology in General Medicine. New York: McGraw Hill; 1999: Langley R, Krueger G, Griffiths C. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii Menter A. The pathogenesis and treatment of psoriasis. In Schiff M, ed. Rheumatology Educational Initiative (REDI): Making a Difference in Rheumatology. Early and Aggressive Treatment = Better Outcomes for Today s Patients. Hasbrouck Heights, NJ: Veritas Institute for Medical Education; 2004: Gladman DD. Psoriatic arthritis. Rheum Dis Clin North Am. 1998;24: Mease PJ, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol. 2005;52: Krueger J, Bowcock A. Psoriasis pathophysiology:current concepts of pathogenesis. Ann Rheum Dis. 2005;64(suppl 2):ii30-ii Ritchlin CT. Pathogenesis of psoriatic arthritis. Curr Opin Rheumatol. 2005;17: Veale D, Ritchlin C, FitzGerald O. Immunopathology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;65(suppl 2):ii26-ii Mease P. TNF alpha therapy in psoriatic arthritis and psoriasis. Ann Rheum Dis. 2004;63: Taylor WS Helliwell P, Gladman DD, Mease P, Mielants M, Marchesoni A. A validation of current classification criteria for the diagnosis of psoriatic arthritis preliminary results of the CASPAR study. Ann Rheum Dis. 2005;64(suppl III): Taylor W, Marchesoni A, Arreghini M, et al. A comparison of the performance characteristics of classification criteria for the diagnosis of psoriatic arthritis. Semin Arthritis Rheum. 2004;34: Moll J, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3: Gladman DD, Helliwell P, Mease PJ, et al. Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum. 2004;50: Mease P, Antoni C, Gladman DD, et al. Psoriatic arthritis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(suppl 2):ii49-ii van der Heijde D, Sharp J, Wassenberg S, et al. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis. 2005;64(suppl 2):ii61-ii Klauser A, Springer P, Frauscher F, et al. Comparison between magnetic resonance imaging, unenhanced and contrast-enhanced ultrasound in the diagnosis of active sacroilitis [abstract 115]. Arthritis Rheum. 2002;46(suppl 9):S Kamel M, Mansour R, Eid H, et al. Ultrasound detection of patellar enthesitis: a comparison with MRI [abstract 113]. Arthritis Rheum. 2002;46(suppl 9):S Alcade M, Cruz M, Bordoy C, et al. Assessment of entheseal injury in ankylosing spondylitits by ultrasound [abstract 1117]. Arthritis Rheum. 2002;46(suppl 9):S Bukulmez H, Dunn R, Pratt R, et al. Magnetic resonance imagining (MRI) in HLA-B27 transgenic rat model of spondyloarthropathy:image findings prior to clinically detectable arthritis [abstract 982]. Arthritis Rheum. 2002;46(suppl 9):S Braun J, Baraliakos X, Golder W, et al. Ankylosing spondylitis (AS)-development and evaluation of a spinal scoring system (ASspiMRI) using magnetic resonance imaging (MRI) in patients with active disease [abstract 114]. Arthritis Rheum. 2002;46(suppl 9):S Nash P, Clegg DO. Psoriatic arthritis therapy:nsaids and traditional DMARDs. Ann Rheum Dis. 2005;64(suppl 2):ii74-ii Christophers E, Mrowietz U. Psoriasis. In Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick s Dermatology in General Medicine. New York: McGraw Hill; 1999: Witman P. Topical therapies for localized psoriasis. Mayo Clin Proc. 2001;76: Willkens RF, Williams HJ, Ward JR, et al. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum. 1984;27: Black RL, O Brien WM, Vanscott EJ, et al. Methotrexate therapy in psoriatic arthritis: double-blind study on 21 patients. JAMA. 1964;189: Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years experience with lowdose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14: Abu-Shakra M, Gladman DD, Thorne JC, et al. Longterm methotrexate therapy in psoriatic arthritis: clinical and radiological outcome. J Rheumatol. 1995;22:
9 Rheumatic Disease Clinics of North America Supplement Spadaro A, Riccieri V, Sili-Scavalli A, et al. Comparison of cyclosporin A and methotrexate in the treatment of psoriatic arthritis: a one-year prospective study. Clin Exp Rheumatol. 1995;13: Whiting-O Keefe QE, Fye KH, Sack KD, Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am J Med. 1991;90: Kaltwasser JP, Nash P, Gladman D, et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis. Arthritis Rheum. 2004;50: Ellis CN FM, Messana JM, et al. Cyclosporine for plaque-type psoriasis: results of a multidose, double-blind trial. N Engl J Med. 1991;324: Faerber L, Braeutigam M, Weidinger G, et al. Cyclosporine in severe psoriasis: results of a meta-analysis in 579 patients. Am J Clin Dermatol, 2001;2: Salavarani C, Macchioni P, Olivieri I, et al. A comparison of cyclosporine, sulfasalazine and symptomatic therapy in the treatment of psoriatic arthritis. J Rheumatol. 2001;28: Clegg DO, Reda DJ, Mejias E, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis: a Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39: Dougados M, vam der Linden S, Leirisalo-Repo M, et al. Sulfasalazine in the treatment of spondylarthropathy: a randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38: Combe B, Goupille P, Kuntz JL, et al. Sulphasalazine in psoriatic arthritis: a randomized, multicentre, placebo-controlled study. Br J Rheumatol. 1996;35: Gupta AK, Grober JS, Hamilton TA, et al. Sulfasalazine therapy for psoriatic arthritis: a double blind, placebo controlled trial. J Rheumatol, 1995;22: Fraser SM, Hopkins R, Hunter JA, et al. Sulphasalazine in the management of psoriatic arthritis. Br J Rheumatol. 1993;32: Farr M, Kitas GD, Waterhouse L, et al. Sulphasalazine in psoriatic arthritis: a double-blind placebo-controlled study. Br J Rheumatol. 1990;29: Hopkins R, Bird HA, Jones H, et al. A double-blind controlled trial of etretinate (Tigason) and ibuprofen in psoriatic arthritis. Ann Rheum Dis. 1985;44: Palit J, Hill J, Capell HA, et al. A multicentre double-blind comparison of auranofin, intramuscular gold thiomalate and placebo in patients with psoriatic arthritis. Br J Rheumatol. 1990;29: Vine JE, Hymes SR, Warner NB, et al. Pustular psoriasis induced by hydroxychloroquine: a case report and review of the literature. J Dermatol. 1996;23: Wolf R, RuoccoV. Triggered psoriasis. Adv Exp Med Biol. 1999;455: Mease P, Antoni C. Psoriatic arthritis treatment: biologicial response modifiers. Ann Rheum Dis. 2005;64(suppl 2):ii78-ii Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356: Mease P, Kivitz A, Burch F, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50: Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IM- PACT). Arthritis Rheum. 2005;52: Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64: Mease P, Gladman D, Ritchlin C. Adalimumab in the treatment of patients with moderately to severely active psoriatic arthritis: results of ADEPT. Arthritis Rheum. 2005;58: Ritchlin CT, Haas-Smith SA, Li P, et al. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111: Goedkoop AY, Kraan MC, Teunissen MB, et al. Early effects of tumour necrosis factor alpha blockade on skin and synovial tissue in patients with active psoriasis and psoriatic arthritis. Ann Rheum Dis. 2004;63: Veale D, Markham T, Fearon U, et al. Anti-TNF alpha therapy in psoriasis and psoriatic arthritis: clinical and angiogenic responses [abstract]. Arthritis Rheum. 2003;48(suppl 9):S van der Heijde D, Kavanaugh A, Beutler A, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic damage in patients with active psoriatic arthritis: results from IMPACT 2 trial. Ann Rheum Dis. 2005;64(suppl 3): Hansen K, Cush J, Patel S, et al. The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis [abstract]. Arthritis Rheum. 2002;46(suppl 9):S Haraoui B, Keystone EC, Thorne J, et al. The Canadian biologic observational switchover survey (BOSS): switching from infliximab to etanercept leads to successful treatment of rheumatoid arthritis [abstract]. Ann Rheum Dis. 2003;62(suppl 1): Bombardieri S, Tzioufas AG, McKenna F, et al. Efficacy evaluation of adalimumab (Humira) in patients with single and multiple prior biologics in the ReAct trial [abstract]. Arthritis Rheum. 2004;50(suppl 9):S Mease P, Ruderman EM, Ritchlin C, et al. Etanercept in psoriatic arthritis: sustained improvement in joint and skin disease and inhibition of radiographic progression at 2 years [abstract]. Ann Rheum Dis. 2004;63(suppl 1)(OP0136): Antoni C, Kavanaugh A, Kirkham B, et al. The one year results of the infliximab multinational psoriatic arthritis controlled trial (IMPACT): substantial efficacy on synovitis and psoriatic lesions with or without concomitant DMARD therapy [abstract]. Arthritis Rheum. 2003;48(suppl 9):S Kavanaugh A, Antoni C, Gladman D, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis. (Epub ahead of print). 71. Mease P, Kavanaugh A, Krueger G, et al. Infliximab improves psoriasis regardless of arthritis response in patients with active psoriatic arthritis: results from IMPACT 2 trial [abstract]. Arthritis Rheum. 2004;50(suppl 9):S Ritchlin C, Anandarajaha A, Totterman S, et al. Preliminary data from a study of adalimumab in the treatment of psoriatic arthritis [abstract]. Ann Rheum Dis. 2004;63(suppl 1): Krueger GG, Papp KA, Stough DB, et al. A randomized, double-blind, placebocontrolled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2002;47: Lebwohl M, Christophers E, Langley R, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139: Kraan MC, van Kuijk AW, Dinant HJ, et al. Alefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritis. Arthritis Rheum. 2002;46: Mease P, Gladman D, Keystone E. Efficacy of alefacept in combination with methotrexate in the treatment of psoriatic arthritis [abstract]. Ann Rheum Dis. 2005;64(suppl 3): Mease P, Gladman D, Keystone E. Alefacept in combination with methotrexate for the treatment of psoriatic arthritis: results of a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2005;52(suppl 9):S Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349: Papp K, Mease P, Garovoy M, et al. Efalizumab in Patients with Psoriatic Arthritis: Results of a Phase II Randomized Double-Blind Placebo Controlled Study. Presented at the International Psoriasis Symposium; 2004; Toronto. 80. Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349: Abrams JR, Lebwohl M Guzzo C. CTLA4Ig-mediated blockade of T cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103: McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4: Krueger JG, Walters IB, Miyazawa M, et al. Successful in vivo blockade of CD25 (high-affinity interleukin 2 receptor) on T cells by administration of humanized anti-tac antibody to patients with psoriasis. J Am Acad Dermatol. 2000;43: Jung JH ZT, Tutuncu Z, Other biologic therapy. In Gordon KB, Ruderman EM, eds. Psoriasis and Psoriatic Arthritis: An Integrated Approach. Heidelberg: Springer; 2005: Nishimoto N Yoshizaki K, Miyasaka N, et al. Long-term safety and efficacy of anti-interleukin 6 receptor antibody (MRA) in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(suppl 9):S Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/cxcr2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116: McInnes IB, Illei GG, Danning CL, et al. IL-10 improves skin disease and modulates endothelial activation and leukocyte effector function in patients with psoriatic arthritis. J Immunol. 2001;167: Trepicchio WL, Ozawa M, Walters IB, et al. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest. 1999;104: Utset TO, Auger JA, Peace D, et al. Modified anti-cd3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol. 2002;29: Mease PJ. Recent advances in the management of psoriatic arthritis. Curr Opin Rheumatol. 2004;16: Bongartz T, Coras B, Vogt T, Scholmerich J, Muller-Ladner U. Treatment of active psoriatic arthritis with the PPARgamma ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford). 2005;44: Keller C WA, Davis J. Cytokines in the seronegative spondyloarthropathies and their modification by TNF blockade: a brief report and literature review. Ann Rheum Dis. 2003;62: Kruithof E, Baeten D, Van den Bosch F, et al. Down-regulation of synovial inflammation by TNF alpha blockade with infliximab in spondyloarthropathy: a histopathological study in 20 patients. Ann Rheum Dis. 2003;62(suppl 1): Mielants H,Veys EM. HLA-B27 related arthritis and bowel inflammation. Part 1. Sulfasalazine (salazopyrin) in HLA-B27 related reactive arthritis. J Rheumatol. 1985;12: Zink A, Listing J, Klindworth C, et al. The national database of the German Collaborative Arthritis Centres: I. Structure, aims, and patients. Ann Rheum Dis. 2001;60:
10 20 Rheumatic Disease Clinics of North America Supplement Boonen A, Chorus A, Miedema H, et al. Employment, work disability, and work days lost in patients with ankylosing spondylitis: a cross sectional study of Dutch patients. Ann Rheum Dis. 2001;60: Chorus AM, Boonen A, Miedema HS, et al. Employment perspectives of patients with ankylosing spondylitis. Ann Rheum Dis. 2002;61: Boonen A, van der Heijde D, Landewe R, et al. Costs of ankylosing spondylitis in three European countries: the patient s perspective. Ann Rheum Dis. 2003;62: Dougados M, Behier JM, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44: Melian A, van der Heijde D, James M, et al. Etoricoxib in the treatment of ankylosing spondylitis (AS) [abstract 1131]. Arthritis Rheum. 2002;46(suppl 9):S Braun J, Baraliakos X, Brandt J, Sieper J. Therapy of ankylosing spondylitis. Part II: biological therapies in the spondyloarthritides. Scand J Rheumatol. 2005;34: Ward MM. Predictors of the progression of functional disability in patients with ankylosing spondylitis. J Rheumatol. 2002;29: van Tubergen A, Landewe R, van der Heijde D, et al. Combined spa-exercise therapy is effective in patients with ankylosing spondylitis: a randomized controlled trial. Arthritis Rheum. 2001;45: Braun J, Sieper J. Therapy of ankylosing spondylitis and other spondyloarthritides: established medical treatment, anti-tnf-alpha therapy and other novel approaches. Arthritis Res. 2002;4: Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346: Davis JC Jr, van der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48: Davis JC, van der Heijde D, Braun J, et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis. 2005;64: Baraliakos X, Davis J, Tsuji W, Braun J. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor alpha receptor fusion protein etanercept. Arthritis Rheum. 2005;52: Kruithof E, Van den Bosch F, Baeten D, et al. Repeated infusions of infliximab, a chimeric anti-tnfalpha monoclonal antibody, in patients with active spondyloarthropathy: one year follow up. Ann Rheum Dis. 2002;61: Stone M, Salonen D, Lax M, et al. Clinical imaging correlates of response to treatment with infliximab in patients with ankylosing spondylitis. J Rheumatol. 2001;28: Maksymowych WP, Jhangri GS, Lambert RG, et al. Infliximab in ankylosing spondylitis: a prospective observational inception cohort analysis of efficacy and safety. J Rheumatol. 2002;29: Breban M, Vignon E, Claudepierre P, et al. Efficacy of infliximab in refractory ankylosing spondylitis: results of a six-month open-label study. Rheumatology (Oxford). 2002;41: Braun J, Brandt J, Listing J, et al. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum. 2003;48: Temekonidis TI, Alamanos Y, Nikas SN, et al. Infliximab therapy in patients with ankylosing spondylitis: an open-label, 12-month study. Ann Rheum Dis. 2003;62: Collantes-Estevez E, Munoz-Villanueva M, Canete-Crespillo JD, et al. Infliximab in refractory spondyloarthropathies: a multicentre 38-week open study. Ann Rheum Dis. 2003;62: Braun J, van der Heijde D. Imaging and scoring in ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16: van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52: van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a 24-week randomized, placebo-controlled trial (ASSERT). Ann Rheum Dis. 2004;50(suppl 9) Braun J, Kellner H, Deodhar A, et al. Infliximab improves quality of life in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Ann Rheum Dis. 2004;63(suppl 1): Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359: Baraliakos X, Listing J, Brandt J, et al. Clinical response to withdrawal of anti- TNF therapy in patients with ankylosing spondylitis (AS) after 3 years of continuous treatment with infliximab. Arthritis Res Ther. 2005;7:R439-R Heiberg MS, Nordvag BY, Mikkelsen K, et al. The comparative effectiveness of tumor necrosis factor-blocking agents in patients with rheumatoid arthritis and patients with ankylosing spondylitis: a six-month, longitudinal, observational, multicenter study. Arthritis Rheum. 2005;52: Haibel H, Brandt H, Baraliakos X, Adalimumab in the treatment of active ankylosing spondylitis: results of an open-label, 52-week trial. Ann Rheum Dis, 2005;64(suppl 3): Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis Sep 15. Epub ahead of print: available from: Zochling J, van der Heijde D, Dougados M, et al. Current evidence for the management of ankylosing spondylitis a systematic literature review for the ASAS/EULAR management recommendations in ankylosing spondylitis. Ann Rheum Dis 2005 Sep 15. Epub ahead of print: available from: t=citation&list_uids= Sieper J. Disease mechanisms in reactive arthritis. Curr Rheumatol Rep. 2004;6: Healy PJ, Helliwell PS. Classification of the spondyloarthropathies. Curr Opin Rheumatol. 2005;17: Leirisalo-Repo M. Reactive arthritis. Scand J Rheumatol. 2005;34: Kim TH, Uhm WS, Inman RD. Pathogenesis of ankylosing spondylitis and reactive arthritis. Curr Opin Rheumatol. 2005;17: De Vos M. Review article: joint involvement in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(suppl 4): Wanders A, van der Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis. Arthritis Rheum. 2005;52: Braun J, Sieper J. Biological therapies in the spondyloarthritides the current state. Rheumatology (Oxford). 2004;43: Baraliakos X, Braun J. Current concepts in the therapy of the spondyloarthritides. BioDrugs. 2004;18: Brandt J, Sieper J, Braun J. [Current therapy with TNFalpha blocking agents for ankylosing spondylitis and undifferentiated spondyloarthritis]. [German]. Z Rheumatol. 2004;63: Brandt J, Haibel H, Reddig J, Sieper J, Braun J. Successful short-term treatment of severe undifferentiated spondyloarthropathy with the anti-tumor necrosis factor-alpha monoclonal antibody infliximab. J Rheumatol. 2002;29: Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn s disease. N Engl J Med. 1999;340: Burgos-Vargas R. Juvenile onset spondyloarthropathies: therapeutic aspects. Ann Rheum Dis. 2002;61: Reiff A, Henrickson M. Prolonged efficacy of etanercept in refractory juvenile ankylosing spondylitis. Arthritis Rheum 2001;44(suppl):292S Marra CA, et al. The cost-effectiveness of adding infliximab to usual therapy in the treatment of psoriatic arthritis. Ann Rheum Dis. 2005;64(suppl 3): Barnsback NB, et al. The economic implications of TNF-a inhibitors in the treatment of psoriatic arthritis. Ann Rheum Dis. 2004;50(suppl 9):S Boonen A. Measuring socioeconomic outcome in AS clinical trials. Ann Rheum Dis. 2005;64: Kobelt G A-SP, Brophy S, Jonsson L, Calin A, Braun J, The burden of AS and the cost-effectiveness of treatment with infliximab. Rheumatology. 2004;43: Listing J, Brandt J, Rudwaleit M, et al. Impact of anti-tumor necrosis factor treatment on admissions to hospital and days of sick leave in patients with ankylosing spondylitis. Ann Rheum Dis. 2004;63:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
subcutaneous initially every 4 weeks then every 12 weeks Coverage Criteria: Express Scripts, Inc. monograph dated 02/24/2010
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Humira (adalimumab subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Humira (adalimumab subcutaneous injection) Commercial HMO/PPO/CDHP
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd
 ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Rheumatoid Arthritis. Outline. Treatment Goal 4/10/2013. Clinical evaluation New treatment options Future research Discussion
 Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
Patient Input Information Clinical Trials Outcomes Common Drug Review
 CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists
 MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
Immune modulation in rheumatology. Geoff McColl University of Melbourne/Australian Rheumatology Association
 Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists
 Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Arthritis and Rheumatology Clinics of Kansas Patient Education. Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis
 Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Evidence-based Management of Rheumatoid Arthritis (2009)
 CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
Rheumatoid Arthritis
 Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
Is Monotherapy Treatment of Etanercept Effective Against Plaque Psoriasis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
The Most Common Autoimmune Disease: Rheumatoid Arthritis. Bonita S. Libman, M.D.
 The Most Common Autoimmune Disease: Rheumatoid Arthritis Bonita S. Libman, M.D. Disclosures Two googled comics The Normal Immune System Network of cells and proteins that work together Goal: protect against
The Most Common Autoimmune Disease: Rheumatoid Arthritis Bonita S. Libman, M.D. Disclosures Two googled comics The Normal Immune System Network of cells and proteins that work together Goal: protect against
Psoriasis. Psoriasis. Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine
 Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Understanding Rheumatoid Arthritis
 Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis
 Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
ABOUT RHEUMATOID ARTHRITIS
 MEDIA BACKGROUNDER ABOUT RHEUMATOID ARTHRITIS Rheumatoid arthritis (RA) is a type of arthritis (chronic inflammatory polyarthritis) that typically affects hands and feet, although any joint in the body
MEDIA BACKGROUNDER ABOUT RHEUMATOID ARTHRITIS Rheumatoid arthritis (RA) is a type of arthritis (chronic inflammatory polyarthritis) that typically affects hands and feet, although any joint in the body
SECTION 3. Criteria for Special Authorization of Select Drug Products. Section 3 Criteria for Special Authorization of Select Drug Products
 SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
Psoriatic Arthritis. Title. Understanding and Managing. in All the Wrong Places. Clinical Features. Etiology of Psoriatic Arthritis
 Focus on CME at Memorial University Understanding and Managing Title Psoriatic Arthritis in All the Wrong Places Proton Rahman MD, MSc, FRCPC Although Baron Jean-Luis Aubert offered the first case description
Focus on CME at Memorial University Understanding and Managing Title Psoriatic Arthritis in All the Wrong Places Proton Rahman MD, MSc, FRCPC Although Baron Jean-Luis Aubert offered the first case description
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis
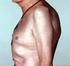 Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Rheumatoid Arthritis Information
 Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Psoriatic Arthritis Current Guidelines. Linda Sekhon, DHSc, PA-C
 Psoriatic Arthritis Current Guidelines Linda Sekhon, DHSc, PA-C Learning Objectives At the conclusion of this lecture, participants should be able to: Define Psoriatic Arthritis and briefly describe the
Psoriatic Arthritis Current Guidelines Linda Sekhon, DHSc, PA-C Learning Objectives At the conclusion of this lecture, participants should be able to: Define Psoriatic Arthritis and briefly describe the
Drug Therapy Guidelines: Humira (adalimumab)
 Drug Therapy Guidelines: Humira (adalimumab) Effective Date: 5/1/08 Committee Review Date: 1/6/01, 9/18/01, 1/15/02, 1/7/03, 1/20/04, 1/18/05, 12/7/05, 10/15/06, 7/2/07, 11/5/07, 3/25/08 Policy Statements:
Drug Therapy Guidelines: Humira (adalimumab) Effective Date: 5/1/08 Committee Review Date: 1/6/01, 9/18/01, 1/15/02, 1/7/03, 1/20/04, 1/18/05, 12/7/05, 10/15/06, 7/2/07, 11/5/07, 3/25/08 Policy Statements:
Rheumatoid Arthritis:
 Rheumatoid Arthritis Update 2014 Mark Hulsey, MD FACR Rheumatoid Arthritis Key Features Symptoms >6 weeks duration Often lasts the remainder of the patient s life Inflammatory synovitis Palpable synovial
Rheumatoid Arthritis Update 2014 Mark Hulsey, MD FACR Rheumatoid Arthritis Key Features Symptoms >6 weeks duration Often lasts the remainder of the patient s life Inflammatory synovitis Palpable synovial
PRACTICAL HELP FROM THE ARTHRITIS FOUNDATION. www.arthritis.org 800-283-7800. Psoriatic Arthritis
 Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Rheumatoid Arthritis. Nicole Klett,, M.D.
 Rheumatoid Arthritis Nicole Klett,, M.D. Rheumatoid Arthritis Systemic Chronic Inflammatory Primarily targets the synovium of diarthrodial joints Etiology likely combination genetic and environmental Diarthrodial
Rheumatoid Arthritis Nicole Klett,, M.D. Rheumatoid Arthritis Systemic Chronic Inflammatory Primarily targets the synovium of diarthrodial joints Etiology likely combination genetic and environmental Diarthrodial
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
How To Choose A Biologic Drug
 North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
Rheumatoid Arthritis. Disease RA Final.indd 2 15. 6. 10. 11:23
 Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 17 December 2003 CPMP/EWP/556/95 rev 1/Final COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 17 December 2003 CPMP/EWP/556/95 rev 1/Final COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
Psoriatic Arthritis www.arthritis.org.nz
 Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Rheumatoid Arthritis
 What is Rheumatoid Arthritis? Rheumatoid Arthritis (RA) is a chronic and systemic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints
What is Rheumatoid Arthritis? Rheumatoid Arthritis (RA) is a chronic and systemic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints
Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy
 Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon
 Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon Rheumatology Specialist Registrar & PhD Research Fellow 2 Overview Back ground on psoriatic arthritis (PsA) Epidemiology
Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon Rheumatology Specialist Registrar & PhD Research Fellow 2 Overview Back ground on psoriatic arthritis (PsA) Epidemiology
New Evidence reports on presentations given at EULAR 2012. Rituximab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011
 Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011 Working party: Eva Baecklund, Helena Forsblad d Elia, Carl Turesson Background Our purpose
Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011 Working party: Eva Baecklund, Helena Forsblad d Elia, Carl Turesson Background Our purpose
RHEUMATOID ARTHRITIS. Dr Bruce Kirkham Rheumatology Clinical Lead
 RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital
 Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
EVIDENCE BASED TREATMENT OF CROHN S DISEASE. Dr E Ndabaneze
 EVIDENCE BASED TREATMENT OF CROHN S DISEASE Dr E Ndabaneze PLAN 1. Case presentation 2. Topic on Evidence based Treatment of Crohn s disease - Introduction pathology aetiology - Treatment - concept of
EVIDENCE BASED TREATMENT OF CROHN S DISEASE Dr E Ndabaneze PLAN 1. Case presentation 2. Topic on Evidence based Treatment of Crohn s disease - Introduction pathology aetiology - Treatment - concept of
Rheumatoid Arthritis: Constantly Evolving Treatment Approaches
 Rheumatoid Arthritis: Constantly Evolving Treatment Approaches Jody Garry, Pharm.D. Primary Care Pharmacy Resident VA Medical Center - Iowa City Presentation Overview Pathophysiology & epidemiology Diagnostic
Rheumatoid Arthritis: Constantly Evolving Treatment Approaches Jody Garry, Pharm.D. Primary Care Pharmacy Resident VA Medical Center - Iowa City Presentation Overview Pathophysiology & epidemiology Diagnostic
Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology
 Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Dr. Leonard Calabrese: I want to welcome the
Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Exploring Care for Psoriatic Arthritis: Bridging Dermatology and Rheumatology Dr. Leonard Calabrese: I want to welcome the
What s new in clinical assesment of ankylosing spondylitis?
 What s new in clinical assesment of ankylosing spondylitis? Désirée van der Heijde Professor of Rheumatology Leiden University Medical Center, the Netherlands Diakonhjemmet Hospital, Oslo, Norway Content
What s new in clinical assesment of ankylosing spondylitis? Désirée van der Heijde Professor of Rheumatology Leiden University Medical Center, the Netherlands Diakonhjemmet Hospital, Oslo, Norway Content
Media Release. Basel, 11 June 2009. RA patients with enhanced response identified
 Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Adalimumab for the treatment of psoriasis
 DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
Psoriatic Arthritis. Ewa Olech, MD Division of Rheumatology University of Nevada School of Medicine Las Vegas
 Psoriatic Arthritis Ewa Olech, MD Division of Rheumatology University of Nevada School of Medicine Las Vegas The Spectrum of Spondyloarthritis Characteristics of the Spondyloarthritis Sacroiliac & spinal
Psoriatic Arthritis Ewa Olech, MD Division of Rheumatology University of Nevada School of Medicine Las Vegas The Spectrum of Spondyloarthritis Characteristics of the Spondyloarthritis Sacroiliac & spinal
Information on Rheumatoid Arthritis
 Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Can Rheumatoid Arthritis treatment ever be stopped?
 Can Rheumatoid Arthritis treatment ever be stopped? Robert L. DiGiovanni, DO, FACOI Program Director Largo Medical Center Rheumatology Fellowship [email protected] Do not pour strange medicines
Can Rheumatoid Arthritis treatment ever be stopped? Robert L. DiGiovanni, DO, FACOI Program Director Largo Medical Center Rheumatology Fellowship [email protected] Do not pour strange medicines
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Once the immune system is triggered, cells migrate from the blood into the joints and produce substances that cause inflammation.
 HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Multiple Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
Psoriasis Treatment Transition Pathway
 Psoriasis Treatment Transition Pathway A Treatment Support Tool Adapted from Circle Nottingham NHS Treatment Centre Psoriasis Pathway (under consultation) with support from Abbvie Ltd Treatment Pathways
Psoriasis Treatment Transition Pathway A Treatment Support Tool Adapted from Circle Nottingham NHS Treatment Centre Psoriasis Pathway (under consultation) with support from Abbvie Ltd Treatment Pathways
Treatment of Severe Rheumatoid Arthritis
 Treatment of Severe Rheumatoid Arthritis Zhanguo Li Department of Rheumatology and Immunology, People s Hospital Beijing University Medical School, China Contents Background Challenges Treatment strategies
Treatment of Severe Rheumatoid Arthritis Zhanguo Li Department of Rheumatology and Immunology, People s Hospital Beijing University Medical School, China Contents Background Challenges Treatment strategies
Methotrexate Is Not Disease Modifying In Psoriatic Arthritis
 Methotrexate Is Not Disease Modifying In Psoriatic Arthritis A New Treatment Paradigm Is Required Gabrielle H Kingsley*, Jonathan Packham, Neil McHugh, Diarmuid Mulherin George Kitas, Kuntal Chakravarty,
Methotrexate Is Not Disease Modifying In Psoriatic Arthritis A New Treatment Paradigm Is Required Gabrielle H Kingsley*, Jonathan Packham, Neil McHugh, Diarmuid Mulherin George Kitas, Kuntal Chakravarty,
Arthritis in Children: Juvenile Rheumatoid Arthritis By Kerry V. Cooke
 Reading Comprehension Read the following essay on juvenile rheumatoid arthritis. Then use the information in the text to answer the questions that follow. Arthritis in Children: Juvenile Rheumatoid Arthritis
Reading Comprehension Read the following essay on juvenile rheumatoid arthritis. Then use the information in the text to answer the questions that follow. Arthritis in Children: Juvenile Rheumatoid Arthritis
Psoriatic arthritis FACTSHEET
 1 What is psoriatic arthritis? Psoriatic arthritis (PsA) is a disease where joints around the body become inflamed and sore. It can make moving about difficult and painful. People who have PsA also have
1 What is psoriatic arthritis? Psoriatic arthritis (PsA) is a disease where joints around the body become inflamed and sore. It can make moving about difficult and painful. People who have PsA also have
Psoriatic Arthritis. What is psoriatic arthritis? Understanding joints. Who gets psoriatic arthritis? Page 1 of 5
 Page 1 of 5 Psoriatic Arthritis Psoriatic arthritis causes inflammation, pain, and swelling of joints in some people who have psoriasis. Other parts of the body may also be affected. For example, in many
Page 1 of 5 Psoriatic Arthritis Psoriatic arthritis causes inflammation, pain, and swelling of joints in some people who have psoriasis. Other parts of the body may also be affected. For example, in many
Phenotypes and Classification of Psoriasis
 Rheumatology 2010 Birmingham 21 April 2010 Phenotypes and Classification of Psoriasis Christopher E.M. Griffiths Abbott Centocor Incyte Galderma Janssen-Cilag Leo Pharma Lynxx Novartis Pfizer Schering-Plough
Rheumatology 2010 Birmingham 21 April 2010 Phenotypes and Classification of Psoriasis Christopher E.M. Griffiths Abbott Centocor Incyte Galderma Janssen-Cilag Leo Pharma Lynxx Novartis Pfizer Schering-Plough
Medicines for Psoriatic Arthritis. A Review of the Research for Adults
 Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy
 Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
In the last decade, there have been major changes in the
 233 Promising New Treatments for Rheumatoid Arthritis The Kinase Inhibitors Yusuf Yazici, M.D., and Alexandra L. Regens, B.A. Abstract Three major advances over the last decade have impacted the way we
233 Promising New Treatments for Rheumatoid Arthritis The Kinase Inhibitors Yusuf Yazici, M.D., and Alexandra L. Regens, B.A. Abstract Three major advances over the last decade have impacted the way we
PSORIATIC ARTHRITIS. » Diagnosis» Symptoms» Treatments» + more
 PSORIATIC ARTHRITIS» Diagnosis» Symptoms» Treatments» + more WHAT IS PSORIASIS? PSORIASIS is pronounced sore-eye-ah-sis. It is an autoimmune disease, meaning that certain triggers cause the immune system
PSORIATIC ARTHRITIS» Diagnosis» Symptoms» Treatments» + more WHAT IS PSORIASIS? PSORIASIS is pronounced sore-eye-ah-sis. It is an autoimmune disease, meaning that certain triggers cause the immune system
NURS 821 Alterations in the Musculoskeletal System. Rheumatoid Arthritis. Type III Hypersensitivity Response
 NURS 821 Alterations in the Musculoskeletal System Margaret H. Birney PhD, RN Lecture 12 Part 2 Joint Disorders (cont d) Rheumatoid Arthritis Definition: Autoimmune disorder occurring in genetically sensitive
NURS 821 Alterations in the Musculoskeletal System Margaret H. Birney PhD, RN Lecture 12 Part 2 Joint Disorders (cont d) Rheumatoid Arthritis Definition: Autoimmune disorder occurring in genetically sensitive
Rheumatoid Arthritis www.arthritis.org.nz
 Rheumatoid Arthritis www.arthritis.org.nz Did you know? RA is the second most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most often appears between
Rheumatoid Arthritis www.arthritis.org.nz Did you know? RA is the second most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most often appears between
ENBREL (Etanercept) 25 mg and 50 mg powder for injection and water for injections
 DATA SHEET ENBREL Etanercept (rch) NAME OF THE MEDICINE ENBREL (Etanercept) 25 mg and 50 mg powder for injection and water for injections ENBREL (Etanercept) 25 mg and 50 mg solution for injection in pre-filled
DATA SHEET ENBREL Etanercept (rch) NAME OF THE MEDICINE ENBREL (Etanercept) 25 mg and 50 mg powder for injection and water for injections ENBREL (Etanercept) 25 mg and 50 mg solution for injection in pre-filled
PSORIATIC ARTHRITIS. Chryssanthie Kafkala, M.D. INTRODUCTION:
 PSORIATIC ARTHRITIS Chryssanthie Kafkala, M.D. INTRODUCTION: Psoriatic arthritis is a disease with generally good prognosis. Both ocular and systemic involvement is usually benign, however, the following
PSORIATIC ARTHRITIS Chryssanthie Kafkala, M.D. INTRODUCTION: Psoriatic arthritis is a disease with generally good prognosis. Both ocular and systemic involvement is usually benign, however, the following
Adalimumab for the treatment of psoriatic arthritis
 Adalimumab for the treatment of psoriatic arthritis Erratum for Premeeting briefing and ERG report After issuing the premeeting briefing and ERG report to the Appraisal Committee the following error was
Adalimumab for the treatment of psoriatic arthritis Erratum for Premeeting briefing and ERG report After issuing the premeeting briefing and ERG report to the Appraisal Committee the following error was
Rheumatic Diseases, Psoriasis, and Crohn s Disease
 Rheumatic Diseases, Psoriasis, and Crohn s Disease What does this handout cover? This handout has information about rheumatic disease, psoriasis, and Crohn s disease. It also has information on how these
Rheumatic Diseases, Psoriasis, and Crohn s Disease What does this handout cover? This handout has information about rheumatic disease, psoriasis, and Crohn s disease. It also has information on how these
Rheumatoid Arthritis
 Rheumatoid Arthritis Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Autoimmune diseases are illnesses that occur when the body's tissues are mistakenly
Rheumatoid Arthritis Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Autoimmune diseases are illnesses that occur when the body's tissues are mistakenly
Psoriatic arthritis (PsA) is an inflammatory arthritis
 25 Psoriatic Arthritis Update Philip Mease, M.D. Abstract Psoriatic arthritis is an inflammatory arthritis occurring in up to 30% of patients with psoriasis. Its clear distinction from rheumatoid arthritis
25 Psoriatic Arthritis Update Philip Mease, M.D. Abstract Psoriatic arthritis is an inflammatory arthritis occurring in up to 30% of patients with psoriasis. Its clear distinction from rheumatoid arthritis
Treatment of Rheumatoid Arthritis in the New Millennium. Neal I. Shparago, D.O., FACP, FACR
 Treatment of Rheumatoid Arthritis in the New Millennium Neal I. Shparago, D.O., FACP, FACR What is RA? Symmetric inflammatory polyarthritis Rheumatoid factor positive in 80% Anti-CCP positive in approximately
Treatment of Rheumatoid Arthritis in the New Millennium Neal I. Shparago, D.O., FACP, FACR What is RA? Symmetric inflammatory polyarthritis Rheumatoid factor positive in 80% Anti-CCP positive in approximately
Speaking Plainly. Biologic treatment options for rheumatoid arthritis
 in association with Plain English Campaign Speaking Plainly Biologic treatment options for rheumatoid arthritis A guide to help healthcare professionals talking to patients with rheumatoid arthritis Foreword
in association with Plain English Campaign Speaking Plainly Biologic treatment options for rheumatoid arthritis A guide to help healthcare professionals talking to patients with rheumatoid arthritis Foreword
How To Treat Ankylosing Spondylitis
 Inflammatory Arthritis and Biologic therapy Ministry of Health(MOH) Recommendations Biologic Therapy for Inflammatory Arthritis Ministry of Health Recommendations Introduction Chronic inflammatory arthropathies
Inflammatory Arthritis and Biologic therapy Ministry of Health(MOH) Recommendations Biologic Therapy for Inflammatory Arthritis Ministry of Health Recommendations Introduction Chronic inflammatory arthropathies
Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
PSORIATIC ARTHRITIS. Elvia Moreta, MD St. Paul Rheumatology 2012
 PSORIATIC ARTHRITIS Elvia Moreta, MD St. Paul Rheumatology 2012 RESEARCH DISCLOSURE ABBOTT BMS CENTOCOR GENENTEC LILLY NOVARTIS ROCHE SAVIENT UCB CONSULTANT ABBOTT UCB MEMBER ABIM fellow CORRONA Consortium
PSORIATIC ARTHRITIS Elvia Moreta, MD St. Paul Rheumatology 2012 RESEARCH DISCLOSURE ABBOTT BMS CENTOCOR GENENTEC LILLY NOVARTIS ROCHE SAVIENT UCB CONSULTANT ABBOTT UCB MEMBER ABIM fellow CORRONA Consortium
CLINICAL POLICY Department: Medical Management Document Name: Rheumatoid & Juvenile Arthritis and Ankylosing Spondylitis Treatments
 Page: 1 of 18 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Page: 1 of 18 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Rheumatoid Arthritis www.arthritis.org.nz
 Rheumatoid Arthritis www.arthritis.org.nz Did you know? Rheumatoid arthritis (RA) is the third most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most
Rheumatoid Arthritis www.arthritis.org.nz Did you know? Rheumatoid arthritis (RA) is the third most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most
Do I need a physician referral? Yes, we see patients on referral from a health care provider.
 FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
to Part of Dossier: Name of Active Ingredient: Title of Study: Quality of life study with adalimumab in rheumatoid arthritis. ESCALAR.
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
