BJD. Summary. British Journal of Dermatology THERAPEUTICS
|
|
|
- Kerry Potter
- 8 years ago
- Views:
Transcription
1 THERAPEUTICS BJD British Journal of Dermatology Cortexolone 17a-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0Æ05% cream V. Trifu, G.-S. Tiplica, E. Naumescu, L. Zalupca,à L. Moro and G. Celasco Clinic of Dermatology, Central Emergency Clinical Military Hospital Dr Carol Davila, Bucharest, Romania Clinic II of Dermatology, Clinical Hospital Colentina, Bucharest, Romania Clinic of Dermatology, Clinical Hospital of Dermato-Venerology Prof. Dr Scarlat Longhin, Bucharest, Romania àoutpatient Centre of Diagnosis and Treatment Dr N. Kretzulescu, Bucharest, Romania Cosmo S.p.A., Lainate (MI), Italy Cosmo Research & Development S.p.A., Lainate (MI), Italy Summary Correspondence Giuseppe Celasco. gcelasco@cosmopharma.com Accepted for publication 12 March 2011 Funding sources The work was entirely supported by a grant of Cosmo S.p.A., Lainate (MI), Italy. Conflicts of interest L.M. is an employee at Cosmo S.p.A., Lainate (MI), Italy. G.C. is a consultant at Cosmo Research & Development S.p.A., Lainate (MI), Italy. DOI /j x Background Acne vulgaris is a disorder of the pilosebaceous unit in which the androgens contribute to its onset and persistence. The use of antiandrogens is therefore potentially effective; however, antiandrogens for topical use are not available on the market. Cortexolone 17a-propionate (; Cosmo S.p.A, Lainate, Italy) is a new potent topical antiandrogen potentially useful in acne vulgaris. Objectives To evaluate the safety and the topical efficacy of 1% cream in acne vulgaris as compared with placebo and with tretinoin 0Æ05% cream (Retin-A Ò ; Janssen-Cilag). Methods Seventy-seven men with facial acne scored 2 3 according to Investigator s Global Assessment (IGA) were randomized to receive placebo cream (n = 15), or 1% cream (n = 30), or tretinoin 0Æ05% cream (n = 32) once a day at bedtime for 8 weeks. Clinical efficacy was evaluated every 2 weeks including total lesion count (TLC), inflammatory lesion count (ILC), acne severity index (ASI) and IGA. Safety assessment included local irritancy score, laboratory tests, physical examination, vital signs and recording of adverse events. Results 1% cream was very well tolerated, and was significantly better than placebo regarding TLC (P =0Æ0017), ILC (P =0Æ0134) and ASI (P = 0Æ0090), and also clinically more effective than comparator. The product also induced a faster attainment of 50% improvement in all the above parameters. Conclusions This pilot study supports the rationale for the use of topical antiandrogens in the treatment of acne vulgaris. 1% cream seems to fit with the profile of an ideal antiandrogen for topical use. Acne vulgaris is a chronic disorder of the pilosebaceous unit in which genetic predisposition, endocrine factors, follicular hyperkeratinization and bacterial infection contribute to its onset and persistence. As sebaceous glands and sebum production are undoubtedly stimulated by the androgen hormones, antiandrogens appear to offer a rational approach to the management of acne vulgaris. 1,2 Nevertheless, the use of antiandrogens could be hampered by the potential occurrence of endocrine side-effects also when applied locally. 3 Thus the profile of an ideal antiandrogen for topical use should include strong activity confined to skin, absence of systemic effects and good tolerability. However, antiandrogens for topical use, endowed with all the above characteristics, are not available on the market. Cortexolone 17a-propionate (; Cosmo S.p.A, Lainate, Italy) is a new steroidal antiandrogen endowed with strong topical antiandrogenic activity associated with mild anti-inflammatory properties. 4,5 competes at the human androgen-receptor level 6 without inhibiting the skin 5a-reductase. 7 The steroid easily penetrates human skin 8 and is quickly and extensively metabolized to free inactive cortexolone, thus being devoid of systemic antiandrogenic 1
2 2 Cortexolone 17a-propionate in acne vulgaris, V. Trifu et al. effects. The toxicological profile showed that is well tolerated in rat and rabbit in repeated subcutaneous and dermal toxicities, is not mutagenic, and is not a skin sensitizer. In adult male volunteers a single skin application of various volumes of 1% cream was very well tolerated with negligible absorption in the bloodstream. 9 All these preclinical and clinical results prompted us to evaluate, in a pilot clinical study, the potential efficacy and the safety of 1% cream in acne vulgaris compared with placebo and with topical tretinoin 0Æ05% cream (Retin-A Ò ; Janssen-Cilag). Materials and methods Study aim and objectives The study aim was to evaluate the clinical efficacy and tolerability of 1% cream vs. its vehicle alone (placebo) and vs. local tretinoin 0Æ05% cream in acne vulgaris of mild-to-moderate severity. The primary objective was to demonstrate the clinical and statistical superiority of CB % cream against placebo in improving total lesion count (TLC), inflammatory lesion count (ILC), acne severity index (ASI), and in the proportion of subjects with success outcome at Investigator s Global Assessment (IGA). The secondary objective was to assess the efficacy of 1% cream compared with tretinoin 0Æ05% cream regarding the same parameters, and evaluating the effect regarding time in days to reach improvement of 50% in TLC, ILC and ASI. Ethical considerations The trial was performed in compliance with the ethical principles stated in the Declaration of Helsinki and its subsequent revisions. Study protocol and all other relevant documentation were approved by the Romanian National Authorities and by the relevant local Ethical Committees. The trial was conducted in agreement with Good Clinical Practice and with the applicable European and Romanian regulatory requirements. Prior to participation in the trial, each subject gave his written, informed and witnessed consent. Participants Between January and July 2009, three dermatological hospital centres and one outpatient dermatological centre in Bucharest, Romania, screened 83 white-skinned men (age range years), 77 of whom were randomized to the treatments. The subjects were affected by acne vulgaris of the face of mild-to-moderate severity, with a score of 2 or 3 on IGA, and with TLC between 20 and 100, and ILC between 10 and 50. Exclusion criteria were: women, presence of facial lesions other than acne vulgaris, use of systemic antiacne medications or any kind of light treatment in the month before starting the study, or topical application of acne medications in the last 2 weeks, history of hypersensitivity to any ingredient of the trial drugs, severe liver or renal impairment, presence of diabetes, glaucoma, psychoses, or severe diseases in other organs including viral or bacterial infections. Study design and treatment This pilot clinical study (EudraCT no ) was a randomized, double-blind, parallel-group, comparative trial. After completion of screening, the 77 eligible subjects were randomly assigned to three parallel groups. Each group received 1% cream (n = 30), or tretinoin 0Æ05% cream (n = 32), or placebo (n = 15) constituted by the same excipients of 1% cream (cetyl alcohol, glyceryl monostearate, liquid paraffin, propylene glycol, tocopherol, sodium edetate, polysorbate 80, water). The treatments were thereafter shortly indicated as, tretinoin and placebo, respectively. The products were self-applied in adequate amounts only to the affected areas of the face, once a day at bedtime for 8 weeks. The products were assigned to the subjects in tubes totally indistinguishable according to a blinded randomization list, stratified every six subjects, generated by the sponsor and undisclosed to the investigators and the subjects. Throughout the study duration no additional local or systemic antiacne treatments were allowed, and the subjects were discouraged from starting any new medication without consulting the investigator. Treatment compliance Subjects were requested to return, at each visit, all used nonused medication tubes. The investigator weighed the returned medication tubes, and recorded the returned amounts on drug accountability logs. Efficacy assessment The following four variables were assessed at the screening visit and at the end of weeks 2 (visit 1), 4 (visit 2), 6 (visit 3) and 8 (visit 4) of treatment: TLC and ILC. 10 The count was performed on the right and left sides of the face including the chin, forehead, left and right cheeks. No other regions were considered. TLC included both noninflammatory (comedones) and inflammatory (papules, pustules and nodules) lesions, whereas ILC included only inflammatory lesions. At the screening visit both TLC and ILC values were considered as 100%, and any decrease in the following visits was calculated and regarded as percentage improvement. ASI. 11 The severity of acne was assessed considering, for each type of lesion, the following correction factors: comedones 0Æ5, papules 1, pustules 2, and nodules 3. The total individual severity score was obtained by multiplying the number of each type of lesion with its correction factor, and adding them together. At the screening visit the ASI value obtained was considered as 100%, and any decrease in the
3 Cortexolone 17a-propionate in acne vulgaris, V. Trifu et al. 3 following visits was calculated and regarded as percentage improvement. IGA. 12 The assessment was made on an ordinal scale with five severity grades, as follows: grade 0, clear skin without inflammatory or noninflammatory lesions; grade 1, almost clear, with rare noninflammatory lesions and no more than one small inflammatory lesion; grade 2, mild severity greater than grade 1, with some noninflammatory lesions and no more than a few inflammatory lesions (papules pustules only, no nodular lesions); grade 3, moderate severity greater than grade 2, up to many noninflammatory lesions and may have some inflammatory lesions, but no more than one small nodular lesion; grade 4, severe, greater than grade 3, up to many noninflammatory and inflammatory lesions, but no more than a few nodular lesions. The success outcome at IGA was defined as grade 1 with a grade 0 or 1 for the subjects whose baseline score was 3, and grade 0 for the subjects with baseline score 2. Safety assessment The local tolerability was evaluated at weeks 2, 4, 6 and 8 applying an irritancy score (IS) considering redness, peeling, dryness, swelling, itching and burning, each scored from 0 to 3 according to the severity. Systemic tolerability was evaluated by mean of standard haematology, clinical laboratory, urinalysis, physical examination and vital signs performed at screening and at the end of treatment. Occurrence of adverse events (AEs) was recorded and monitored throughout the study, and during 2 weeks after treatment discontinuation. Statistical analysis Data management and statistical analysis were performed by an independent contract research organization (InnoPharma s.r.l., Desio, Italy). The following analysis populations were used: intent to treat (ITT) population, including all randomized subjects who were dispensed study medication with at least one postbaseline assessment, and safety population including all subjects randomized and treated at least once. Main and secondary variables in the comparison between treatments were analysed at a two-sided significance level of P =0Æ05. Treatment differences in the percentage improvement of TLC, ILC and ASI at various weeks, and compared with the screening visit, were assessed by analysis of variance with repeated measures together with a 95% confidence interval (CI). The proportion of subjects with IGA success outcome at various weeks was assessed by the Cochran Mantel Haenszel method. The Kaplan Meier method was used in the survival analysis to analyse the time in days to reach 50% improvement in TLC, ILC and ASI using the twosided log-rank test. Safety was assessed by the incidence of AEs and by evaluation of vital signs, physical examination, laboratory values and local tolerability. Index of tolerability to the medications was assessed by the same methodology as primary efficacy endpoints or, if the necessary assumptions were not satisfied, in a nonparametric way. Results Subject disposition, characteristics and compliance Subject disposition, characteristics and compliance are reported in Table 1. Eighty-three subjects were screened. Six screen failures occurred, so that 77 subjects were randomized. Of the 77 randomized, 10 subjects did not complete the study as per protocol (four consent withdrawal, six lack of compliance). Seventy-two subjects were analysed both as ITT and safety population (14 in placebo group, 30 in tretinoin group, and 28 in group). The groups were well balanced regarding demographic characteristics, severity of clinical parameters and proportion of subjects with IGA grade 2 and IGA grade 3. Good and comparable compliance was detected among the groups regarding exposure duration, amount of study drug used, and daily amount of medication self-applied. No concomitant medication potentially interfering with acne outcome or its evaluation was reported. Efficacy assessment Total lesion count The results are reported in Figure 1. Starting from week 2 onwards, the mean reduction of TLC was greater in the group than in the placebo group, whereas tretinoin showed a profile of activity intermediate between and placebo groups. The mean ± SD percentage improvement at the final visit was 65Æ70 ± 31Æ42 in the group, 52Æ51 ± 25Æ70 in the tretinoin group, and 37Æ0 ± 33Æ31 in the placebo group. Considering the improvement at the different visits, was significantly more active than placebo at weeks 2 (17Æ9%, 95% CI 0Æ42 35Æ37%, P =0Æ0447), 4 (19Æ9%, 95% CI 2Æ49 37Æ44%, P = 0Æ0254), 6 (22Æ4%, 95% CI 4Æ89 39Æ85%, P =0Æ0124) and 8 (28Æ3%, 95% CI 10Æ75 45Æ82%, P =0Æ0017). In the comparison with tretinoin, was always clinically more effective without reaching a statistically significant level. No significant differences, at any time point, were noticed between placebo and tretinoin groups. The time to reach 50% improvement showed significant differences among the groups (log rank test: P = 0Æ0199) with a median time of 43Æ5 days for, 57Æ0 days for tretinoin, and 58Æ0 days for placebo (data not shown). Inflammatory lesion count The results are reported in Figure 2. Starting from week 2 onwards, the mean ± SD reduction of ILC was greater in the group than in the placebo and tretinoin groups. The percentage improvement at final visit was 67Æ26 ± 32Æ03 in the group, 50Æ71 ± 34Æ46 in the tretinoin group and 38Æ98 ± 33Æ22 in the placebo group. Considering the improvement at the different visits, was significantly more effective than placebo at weeks 4 (22Æ7%, 95% CI 0Æ78 44Æ62%, P =0Æ0424), 6 (22Æ2%, 95% CI 0Æ27 44Æ10%,
4 4 Cortexolone 17a-propionate in acne vulgaris, V. Trifu et al. Table 1 Disposition, characteristics of subjects and compliance Tretinoin Randomized subjects Subjects completing the study per protocol Subjects not completing the study Consent withdrawal 3 1 Lack of compliance Subjects analysed as ITT population Subjects analysed as safety population Age (years), mean ± SD 20Æ4 ± 1Æ7 21Æ2 ± 3Æ4 20Æ6 ± 3Æ5 Weight (kg), mean ± SD 72Æ9 ± 10Æ2 77Æ5 ± 11Æ9 74Æ8 ± 9Æ8 Height (cm), mean ± SD 175Æ9 ± 7Æ7 179Æ5 ± 8Æ1 178Æ1 ± 7Æ5 TLC, mean ± SD 50Æ6 ± 15Æ9 48Æ5 ± 17Æ2 46Æ2 ± 15Æ0 ILC, mean ± SD 33Æ5 ± 11Æ4 29Æ1 ± 10Æ4 28Æ5 ± 11Æ1 ASI, mean ± SD 51Æ4 ± 19Æ0 48Æ2 ± 17Æ1 45Æ7 ± 17Æ4 IGA grade 2, % IGA grade 3, % Exposure duration (days), mean ± SD (range) 50Æ9 ± 3Æ6 (39 55) 46Æ8 ± 13Æ5 (13 55) 51Æ9 ± 1Æ8 (46 56) Total amount of cream used (g), mean ± SD (range) 97Æ3 ± 48Æ8 (23Æ2 203Æ2) 100Æ9 ± 65Æ6 (2Æ3 221Æ9) 116Æ4 ± 65Æ1 (13Æ2 221Æ0) Daily dose (g), mean ± SD (range) 1Æ9 ± 0Æ9 (0Æ5 3Æ9) 2Æ1 ± 1Æ2 (0Æ2 4Æ4) 2Æ2 ± 1Æ2 (0Æ3 4Æ2) Concomitant medications ITT, intent to treat; TLC, total lesion count; ILC, inflammatory lesion count; ASI, acne severity index; IGA, Investigator s Global Assessment. P =0Æ0472) and 8 (27Æ9%, 95% CI 5Æ85 49Æ82%, P = 0Æ0134). When compared with tretinoin, was superior at each observation time, and also statistically better at week 6 (19Æ2%, 95% CI 1Æ13 37Æ32%, P =0Æ0374). No significant differences, at any time point, were noticed between the placebo and tretinoin groups. The time to reach 50% improvement showed significant differences among the groups (log rank test: P =0Æ0490) with a median time of 36Æ5 days for, 44Æ0 days for tretinoin and 58Æ0 days for placebo (data not shown). Acne severity index The results are reported in Figure 3. Starting from week 2 onwards, mean ASI improvement was more evident in the group than in the placebo group. The improvement in the tretinoin group was intermediate between that observed in the and placebo groups. The mean ± SD percentage improvement at the end of treatment was 68Æ35 ± 30Æ58 in the group, 53Æ07 ± 33Æ49 in the tretinoin group and 39Æ52 ± 31Æ63 in the placebo group. Considering the Mean reduction from baseline (%) Retin -A P < 0 05 vs. P < 0 01 vs. Mean reduction from baseline (%) Retin-A P < 0 05 vs. < 0 05 vs. Retin-A Weeks 2 Weeks 4 Weeks 6 Weeks 8 0 Weeks 2 Weeks 4 Weeks 6 Weeks 8 Fig 1. Improvement in total lesion count. Fig 2. Improvement in inflammatory lesion count.
5 Cortexolone 17a-propionate in acne vulgaris, V. Trifu et al. 5 improvement at the different visits, was always better than placebo, and this was statistically significant at weeks 2 (22Æ2%, 95% CI 5Æ08 39Æ37%, P =0Æ0113), 6 (22Æ3%, 95% CI 1Æ19 43Æ50%, P =0Æ0385) and 8 (23Æ4%, 95% CI 7Æ17 49Æ62%, P =0Æ0090). When compared with tretinoin, was clinically but not statistically more effective at each observation time. No significant differences at any time Mean improvement from baseline (%) Retin-A P < 0 05 vs. P < 0 01 vs. Weeks 2 Weeks 4 Weeks 6 Weeks 8 Fig 3. Improvement in acne severity index. Table 2 Effect on Investigator s Global Assessment Treatment Subjects achieving success, n (%) (n = 14) 1 (7) 2 (14) Tretinoin (n = 26) 3 (12) 7 (27) (n = 27) 6 (22) 11 (41) Subjects shifted from score 2 3 to score 0 1, n (%) point were noticed between the placebo and tretinoin groups. The time to reach 50% improvement showed significant differences among the groups (log rank test: P =0Æ0438) with a median time of 42Æ5 days for, 44Æ0 days for tretinoin and 57Æ0 days for placebo (data not shown). Investigator s Global Assessment The results are reported in Table 2. The statistical analysis applied to IGA success did not show significant differences among the treatments. Nevertheless, in the group a higher proportion of success was detected (22%) as compared with the tretinoin (12%) and placebo (7%) groups. In agreement with these findings, the proportion of subjects reducing to IGA grade between 0 and 1 was notably higher in the group (41%) than in the tretinoin (27%) and placebo (14%) groups. Safety assessment The local tolerability of was good. In contrast, in the groups treated with tretinoin and placebo an evident worsening of IS was observed within the first 2 weeks. This effect gradually abated to near normal value ( 1) at the end of the treatment period (Table 3). An exploratory analysis showed a statistically significant difference between the and placebo groups at visit 1 (P =0Æ0412). Regarding systemic tolerability, no clinically important abnormalities were detected in any treated group in haematology, clinical laboratory, urinalysis, vital signs and other observations related to safety. A total of eight subjects (11%) experienced 14 AEs which were distributed as follows: five in the placebo group, six in the tretinoin group, and three in the group (Table 4). The most frequently reported AEs were laryngitis, herpes simplex, pruritus and acne worsening. All AEs were mild or moderate, and none was judged as either serious or requiring withdrawal from the study or treatment discontinuation. Table 3 Irritancy score improvement Treatment Visit 1 (2 weeks) Visit 2 (4 weeks) Visit 3 (6 weeks) Visit 4 (8 weeks) n Mean ± SD 2Æ14 ± 2Æ18 2Æ14 ± 3Æ92 1Æ79 ± 4Æ46 0Æ86 ± 2Æ14 Range Tretinoin n Mean ± SD 2Æ20 ± 3Æ17 1Æ04 ± 2Æ13 0Æ73 ± 1Æ43 0Æ62 ± 1Æ20 Range P-value vs. placebo 0Æ3809 0Æ2834 0Æ5257 0Æ8261 n Mean ± SD 1Æ29 ± 2Æ42 0Æ75 ± 1Æ48 0Æ57 ± 1Æ03 0Æ30 ± 0Æ61 Range P-value vs. placebo 0Æ0412 0Æ1015 0Æ2772 0Æ6046
6 6 Cortexolone 17a-propionate in acne vulgaris, V. Trifu et al. Table 4 Adverse events (AEs) by system organ class AEs (n = 14) Tretinoin (n = 30) (n = 28) Events, n Patients with AEs, n (%) Events, n Patients with AEs, n (%) Events, n Patients with AEs, n (%) AEs and patients with any AEs 5 3 (21) 6 2 (7) 3 3 (11) AEs by system organ class Infection and infestations 2 2 (14) 1 1 (3) 1 1 (4) Metabolism and nutrition disorders (3) 0 0 Musculoskeletal and connective tissue disorders (3) 0 0 Skin and subcutaneous tissue disorders 3 3 (21) 3 2 (7) 2 2 (7) Discussion is a new potent steroidal antiandrogen, with additional anti-inflammatory properties, which was demonstrated in animal models to act selectively at the skin level without systemic effects. 4,5 The present study was a pilot, randomized, double-blind, comparative trial evaluating the efficacy and the tolerability of 1% cream in acne vulgaris of mild-to-moderate severity, compared with placebo and topical tretinoin. As this trial was the first experience of repeated administration of to humans, it was deemed prudent to enrol only a limited number of adult males, and not to expose them for too long a period of treatment exceeding 8 weeks. Considering the concerns raised from some Ethical Committees about the need to treat patients only with placebo, the size of the placebo group was reduced. was significantly more effective than placebo in improving TLC, ILC and ASI. Worthy of interest is the effect particularly evident on the inflammatory lesions, probably due to the ancillary anti-inflammatory activity of the molecule. All the above effects had already become evident after 2 4 weeks of treatment and increased proportionally thereafter. The faster effect of, as compared with that of placebo and tretinoin, has been also confirmed by the survival analyses showing an evident reduction of number of days required to reach 50% improvement in all parameters. Also in the comparison with tretinoin, was globally more effective in all above parameters. This finding is of particular interest as topical retinoids have a well-established clinical efficacy in the treatment of acne vulgaris. 13 In our study the clinical activity of tretinoin was very similar to that described in other clinical trials but, surprisingly, it was found not significantly better than placebo. This inconsistency could be attributed to the small and unbalanced groups of patients, thus preventing detection of statistically significant differences between tretinoin and placebo despite evident differences of activity. The study did not show statistically significant differences among treatments regarding the success achievement in the IGA at the end of the treatment. Nevertheless, induced a higher proportion of IGA success than placebo and tretinoin, and also induced a notably higher proportion of subjects in whom the IGA grade 2 3 at screening was reduced to grade 0 1 at the end of the treatment period. During the study, no concerns were raised regarding the local and systemic safety of. Worthy of interest is the better tolerability of as compared with tretinoin, already evident at visit 1. This is of practical importance considering that one of the main concerns in using topical retinoids is the appearance of skin irritation in the first weeks of treatment, frequently leading to discontinuation or reduction of the number of applications. No serious AEs were detected through the study, no drop-outs occurred for safety reasons, and no differences among the groups were noticed concerning the nature and the incidence of AEs. In conclusion, the data provided by this pilot study support the rationale for the use of topical antiandrogens in the treatment of acne vulgaris of mild-to-moderate severity. was found to be significantly more effective than placebo, clinically better than tretinoin, very quick in onset of clinical effect, and mostly well tolerated. Considering the preliminary nature of this trial, additional studies including more patients and more international study centres are needed to evaluate the potential of 1% cream in the treatment of acne vulgaris. What s already known about this topic? Antiandrogens are considered potentially effective in treatment of acne vulgaris; nevertheless, antiandrogens for topical use are not yet on the market. What does this study add? This study demonstrated, for the first time, that the topical antiandrogen cortexolone 17a-propionate 1% cream is safe and effective in the treatment of acne vulgaris, as compared with vehicle and topical tretinoin. These data contribute to support the use of topical antiandrogens in the treatment of acne vulgaris.
7 Cortexolone 17a-propionate in acne vulgaris, V. Trifu et al. 7 References 1 Simpson NB, Cunliffe WJ. Disorders of the sebaceous glands. In: Rook s Textbook of Dermatology (Burns DA, Breathnach SM, Cox NH, Griffiths CEM, eds), 7th edn, Vol. 3. Oxford: Blackwell Science, 2004; Thiboutot D, Chen WC. Update and future of hormonal therapy in acne. Dermatology 2003; 206: Chen C, Puy LA, Simarg J et al. Local and systemic reduction by topical finasteride or flutamide of hamster flank organ size and enzyme activity. J Invest Dermatol 1995; 105: Celasco G, Moro L, Bozzella R et al. Biological profile of cortexolone 17a-propionate (), a new topical and peripherally selective androgen antagonist. Arzneimittelforschung 2004; 54: Celasco G. Compound profile, September Data on Cosmo file. 6 Neliat G. In vitro pharmacology: human androgen receptor study of and CB Cerep Study Report 10631, January Data on Cosmo file. 7 Juchaux F. Effect of the compound on testosterone metabolism in reconstructed human epidermis. Bioalternatives Study Report GT050709, December Data on Cosmo file. 8 Ford G. : in vitro dermal penetration studies. BioDynamics Research Ltd Study Report CPS 01, October Data on Cosmo file. 9Müller M. First dose in man of ; a new topical antiandrogen drug. Cross S.A. Study Report CRO-07-89, September Data on Cosmo file. 10 Burke BM, Cunliffe WJ. The assessment of acne vulgaris the Leeds technique. Br J Dermatol 1984; 111: Tucker SB, Tausend R, Cochran R et al. Comparison of topical clindamycin phosphate, benzoyl peroxide, and a combination of the two for the treatment of acne vulgaris. Br J Dermatol 1984; 110: Center for Drug Evaluation and Research. Guidance for Industry. Acne Vulgaris: Developing Drugs for Treatment. Rockville, MD: CDER, Thieliz A, Abdel-Naser MB, Fluhr JW et al. Topical retinoids in acne an evidence-based overview. J Dtsch Dermatol Ges 2010; 8 (Suppl. 1):S Berger R, Barba A, Fleischer A et al. A double-blinded, randomized, vehicle-controlled, multicenter, parallel-group study to assess the safety and efficacy of tretinoin gel microsphere 0Æ04% in the treatment of acne vulgaris in adults. Cutis 2007; 80: Nighland M, Grossman R. Tretinoin microsphere gel in facial acne vulgaris: a meta-analysis. J Drugs Dermatol 2008; 8 (Suppl.):S Webster G, Cargill DI, Quiring J et al. A combined analysis of 2 randomized clinical studies of tretinoin gel 0Æ05% for the treatment of acne. Cutis 2009; 83:
Comparison of the Effect of Azelaic Acid 20% And Clindamycin 1% In the Treatment of Mild And Moderate Acne. Abstract
 Original Article Comparison of the Effect of Azelaic Acid 20% And Clindamycin 1% In the Treatment of Mild And Moderate Acne Soudabeh Tirgar Tabari, MD Ali Akbar Moghadam Nia, PharmD Karimollah Hajian Amirmajid
Original Article Comparison of the Effect of Azelaic Acid 20% And Clindamycin 1% In the Treatment of Mild And Moderate Acne Soudabeh Tirgar Tabari, MD Ali Akbar Moghadam Nia, PharmD Karimollah Hajian Amirmajid
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Available online www.jocpr.com. Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(2):736-741 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 A comparative study of efficacy and safety of combination
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(2):736-741 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 A comparative study of efficacy and safety of combination
New Developments in the Topical Management of Acne
 Clinical Review New Developments in the Topical Management of Acne Abstract Adapalene 0.1%/BPO 2.5% (adapalene/bpo) gel is a novel agent for acne therapy that has recently become available in Canada. This
Clinical Review New Developments in the Topical Management of Acne Abstract Adapalene 0.1%/BPO 2.5% (adapalene/bpo) gel is a novel agent for acne therapy that has recently become available in Canada. This
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of. Superoxidized solution (SOS) is an electrochemically processed aqueous
 Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
1. ACNE 1. Lisa Schmidt, MPH, Eve A. Kerr, MD, and Kenneth Clark, MD
 1. ACNE 1 Lisa Schmidt, MPH, Eve A. Kerr, MD, and Kenneth Clark, MD The general approach to summarizing the key literature on acne was to review relevant sections of two medical text books (Vernon and
1. ACNE 1 Lisa Schmidt, MPH, Eve A. Kerr, MD, and Kenneth Clark, MD The general approach to summarizing the key literature on acne was to review relevant sections of two medical text books (Vernon and
A comparison of efficacy and safety of topical 0.1% adapalene and 4% benzoyl peroxide in the treatment of mild to moderate acne vulgaris
 Original Article A comparison of efficacy and safety of topical 0.1% adapalene and 4% benzoyl peroxide in the treatment of mild to moderate acne vulgaris Usma Iftikhar, Shahbaz Aman, Muhammad Nadeem, Atif
Original Article A comparison of efficacy and safety of topical 0.1% adapalene and 4% benzoyl peroxide in the treatment of mild to moderate acne vulgaris Usma Iftikhar, Shahbaz Aman, Muhammad Nadeem, Atif
Comparative study between topical Zanco J. Med. Sci., Vol. 18, No. (3), 2014 http://dx.doi.org/10.15218/zjms.2014.0036
 Comparative study between topical clindamycin solution (1%) versus combination of clindamycin (1%)/adapalene (0.1%) gel in the treatment of mild to moderate acne vulgaris Received: 27/4/2013 Accepted:
Comparative study between topical clindamycin solution (1%) versus combination of clindamycin (1%)/adapalene (0.1%) gel in the treatment of mild to moderate acne vulgaris Received: 27/4/2013 Accepted:
Smoothbeam Laser Treatment of Acne Vulgaris. Emerging Applications
 Smoothbeam Laser Treatment of Acne Vulgaris Emerging Applications About Acne Vulgaris Very common - Affects 80% of population Almost every person experiences acne Most common reason to visit dermatologist
Smoothbeam Laser Treatment of Acne Vulgaris Emerging Applications About Acne Vulgaris Very common - Affects 80% of population Almost every person experiences acne Most common reason to visit dermatologist
A 3-PRODUCT SALICYLIC ACID REGIMEN EFFECTIVELY TREATS ACNE VULGARIS IN POST- ADOLESCENT WOMEN
 A 3-PRODUCT SALICYLIC ACID REGIMEN EFFECTIVELY TREATS ACNE VULGARIS IN POST- ADOLESCENT WOMEN J. R. Kaczvinsky 1, C. E. Mack 1, L. L. Griffin 1, J. Li 1, R. A. Rose-Mansfield 1, S. C. Weitz 1, M. J. Marmor
A 3-PRODUCT SALICYLIC ACID REGIMEN EFFECTIVELY TREATS ACNE VULGARIS IN POST- ADOLESCENT WOMEN J. R. Kaczvinsky 1, C. E. Mack 1, L. L. Griffin 1, J. Li 1, R. A. Rose-Mansfield 1, S. C. Weitz 1, M. J. Marmor
Therapeutics for the Clinician
 A Multicenter, Double-blind Study to Evaluate the Efficacy and Safety of 2 Treatments in articipants With Mild to Moderate Acne Vulgaris Zoe Diana Draelos, MD; Alan R. Shalita, MD; Diane Thiboutot, MD;
A Multicenter, Double-blind Study to Evaluate the Efficacy and Safety of 2 Treatments in articipants With Mild to Moderate Acne Vulgaris Zoe Diana Draelos, MD; Alan R. Shalita, MD; Diane Thiboutot, MD;
+++#,# & %!"#$%& '"#()*
 +++#,#! "#$#%&"##$!'#&("&!"# $ %& %& & %!"#$%& '"#()* +++#,# Research Article Pharmacology International Journal of Pharma and Bio Sciences ISSN 0975-6299 COMPARISION OF CLINICAL EFFICACY OF TOPICAL CLINDAMYCIN
+++#,#! "#$#%&"##$!'#&("&!"# $ %& %& & %!"#$%& '"#()* +++#,# Research Article Pharmacology International Journal of Pharma and Bio Sciences ISSN 0975-6299 COMPARISION OF CLINICAL EFFICACY OF TOPICAL CLINDAMYCIN
Acne (Acne Vulgaris) A common type of bacteria that lives on the skin, known as Propionibacterium acnes, sometimes
 Acne (Acne Vulgaris) Acne, clinically known as acne vulgaris, is the most common skin disease. It affects 85% of teenagers, some as young as 12, and often continues into adulthood. It is also called pimples,
Acne (Acne Vulgaris) Acne, clinically known as acne vulgaris, is the most common skin disease. It affects 85% of teenagers, some as young as 12, and often continues into adulthood. It is also called pimples,
74 Full Text Available On www.ijupls.com. Medical Sciences. Original Article!!!
 International Journal of Universal Pharmacy and Life Sciences 2(2): March-April 2012 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND LIFE SCIENCES Medical Sciences Original Article!!! Received: 18-04-2012;
International Journal of Universal Pharmacy and Life Sciences 2(2): March-April 2012 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND LIFE SCIENCES Medical Sciences Original Article!!! Received: 18-04-2012;
Indication under review: cutaneous treatment of acne vulgaris when comedones, papules and pustules are present.
 Resubmission adapalene 0.1%/benzoyl peroxide 2.5% gel (Epiduo ) SMC No. (682/11) Galderma UK Ltd 07 March 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Resubmission adapalene 0.1%/benzoyl peroxide 2.5% gel (Epiduo ) SMC No. (682/11) Galderma UK Ltd 07 March 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Acne. Sofia Chaudhry M.D. Histology of an inflamed comedo. Bolognia, 2008.
 Acne Sofia Chaudhry M.D. Histology of an inflamed comedo. Bolognia, 2008. Acne Vulgaris (Common Acne) Multifactorial disorder of pilosebaceous unit Affects 40-50 million individuals annually in U.S. alone
Acne Sofia Chaudhry M.D. Histology of an inflamed comedo. Bolognia, 2008. Acne Vulgaris (Common Acne) Multifactorial disorder of pilosebaceous unit Affects 40-50 million individuals annually in U.S. alone
Acne breakouts vulgaris is one of the commonest skin conditions, which dermatologists treat and it
 Introduction Acne breakouts vulgaris is one of the commonest skin conditions, which dermatologists treat and it mostly impacts teens, though it might appear at any sort of age. Acne breakouts cause necessarily
Introduction Acne breakouts vulgaris is one of the commonest skin conditions, which dermatologists treat and it mostly impacts teens, though it might appear at any sort of age. Acne breakouts cause necessarily
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
A novel, effective, skin-friendly fixed-dose combination topical formulation for adolescents with acne
 A novel, effective, skin-friendly fixed-dose combination topical formulation for adolescents with acne From a satellite symposium held on June 13 th, 2014 at the 12 th Congress of the European Society
A novel, effective, skin-friendly fixed-dose combination topical formulation for adolescents with acne From a satellite symposium held on June 13 th, 2014 at the 12 th Congress of the European Society
Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment
 Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Acne vulgaris is a chronic skin disease of the
 Adapalene gel 0.3% for the treatment of acne vulgaris: A multicenter, randomized, double-blind, controlled, phase III trial Diane Thiboutot, MD, a DavidM.Pariser,MD,FACP, b Nancy Egan, MD, c Javier Flores,
Adapalene gel 0.3% for the treatment of acne vulgaris: A multicenter, randomized, double-blind, controlled, phase III trial Diane Thiboutot, MD, a DavidM.Pariser,MD,FACP, b Nancy Egan, MD, c Javier Flores,
D.RIGOPOULOS, D.IOANNIDES,* D.KALOGEROMITROS, S.GREGORIOU AND A.KATSAMBAS
 British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
Comparative Study of Effectiveness of Clindamycin Monotherapy and Clindamycin - Benzoyl Peroxide Combination Therapy in Grade II Acne Patients
 Indian Journal of Pharmacy Practice Association of Pharmaceutical Teachers of India Comparative Study of Effectiveness of Clindamycin Monoerapy and Clindamycin - Benzoyl Peroxide Combination Therapy in
Indian Journal of Pharmacy Practice Association of Pharmaceutical Teachers of India Comparative Study of Effectiveness of Clindamycin Monoerapy and Clindamycin - Benzoyl Peroxide Combination Therapy in
Annex II. Scientific conclusions and grounds for refusal presented by the European Medicines Agency
 Annex II Scientific conclusions and grounds for refusal presented by the European Medicines Agency 5 Scientific conclusions Overall summary of the scientific evaluation of Ethinylestradiol-Drospirenone
Annex II Scientific conclusions and grounds for refusal presented by the European Medicines Agency 5 Scientific conclusions Overall summary of the scientific evaluation of Ethinylestradiol-Drospirenone
Summary. Introduction. Pablo Gonzalez, MD, 1 Ricardo Vila, PharmD, 2 & Marcela Cirigliano, MD 3
 Original Contribution Journal of Cosmetic Dermatology,, 25--26 The tolerability profile of clindamycin %/benzoyl peroxide 5% gel vs. adapalene.%/benzoyl peroxide 2.5% gel for facial acne: results of a
Original Contribution Journal of Cosmetic Dermatology,, 25--26 The tolerability profile of clindamycin %/benzoyl peroxide 5% gel vs. adapalene.%/benzoyl peroxide 2.5% gel for facial acne: results of a
Name of Policy: Laser Treatment of Active Acne
 Name of Policy: Laser Treatment of Active Acne Policy #: 394 Latest Review Date: January 2015 Category: Surgery Policy Grade: Effective January 1, 2015: Active Policy but no longer scheduled for regular
Name of Policy: Laser Treatment of Active Acne Policy #: 394 Latest Review Date: January 2015 Category: Surgery Policy Grade: Effective January 1, 2015: Active Policy but no longer scheduled for regular
Acne vulgaris is a complex skin disorder
 SKINmed: Dermatology for the Clinician TM (ISSN 154-974) is published bimonthly (Jan., March, May, July, Sept., Nov.) by Le Jacq Ltd., Three Parklands Drive, Darien, CT 682-3652. Copyright 25 reflect those
SKINmed: Dermatology for the Clinician TM (ISSN 154-974) is published bimonthly (Jan., March, May, July, Sept., Nov.) by Le Jacq Ltd., Three Parklands Drive, Darien, CT 682-3652. Copyright 25 reflect those
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
SYNOPSIS. Risperidone: Clinical Study Report CR003274
 SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
ACNE AND ROSACEA. Disclosure. Distribution of Acne 6/12/2014. NJAFP 2014 Summer Celebration & Scientific Assembly 1
 ACNE AND ROSACEA Everett Schlam, MD Assistant Director, Mountainside Hospital Family Medicine Residency Program, Verona, NJ Disclosure Dr. Schlam has indicated that he has nothing to disclose relevant
ACNE AND ROSACEA Everett Schlam, MD Assistant Director, Mountainside Hospital Family Medicine Residency Program, Verona, NJ Disclosure Dr. Schlam has indicated that he has nothing to disclose relevant
***Department of Dermatology and Venereology, Baghdad Teaching Hospital. Baghdad, Iraq.
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 14, Issue 9 Ver. III (Sep. 2015), PP 70-76 www.iosrjournals.org A Comparative Study of a Topical Active
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 14, Issue 9 Ver. III (Sep. 2015), PP 70-76 www.iosrjournals.org A Comparative Study of a Topical Active
Acne Vulgaris: Basic Facts
 Acne 1 Module Instructions The following module contains hyperlinked information which serves to offer more information on topics you may or may not be familiar with. We encourage that you read all the
Acne 1 Module Instructions The following module contains hyperlinked information which serves to offer more information on topics you may or may not be familiar with. We encourage that you read all the
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
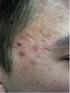
Pathogenesis 10/04/2015. Acne
 Deepani Rathnayake MBBS, MD, FACD Acne Affects >80% of adolescents >40% of adults Associated with Disfigurement Loss of confidence Depression Affects quality of life Pathogenesis i) increased sebum production,
Deepani Rathnayake MBBS, MD, FACD Acne Affects >80% of adolescents >40% of adults Associated with Disfigurement Loss of confidence Depression Affects quality of life Pathogenesis i) increased sebum production,
How To Treat Acne
 THE IMPORTANCE OF EARLY DIAGNOSIS AND TREATMENT OF ACNE* Adelaide Ann Hebert, MD, FAAD ABSTRACT Several topical and systemic agents are available for acne treatment. Commonly used topical therapies include
THE IMPORTANCE OF EARLY DIAGNOSIS AND TREATMENT OF ACNE* Adelaide Ann Hebert, MD, FAAD ABSTRACT Several topical and systemic agents are available for acne treatment. Commonly used topical therapies include
Advances in Acne Management. ABP Objectives for ACNE. Case 1 5/13/11
 Advances in Acne Management Advances and Controversies in Pediatrics 2011 Erin Mathes, MD Assistant Clinical Professor of Dermatology and Pediatrics UCSF ABP Objectives for ACNE Plan for the treatment
Advances in Acne Management Advances and Controversies in Pediatrics 2011 Erin Mathes, MD Assistant Clinical Professor of Dermatology and Pediatrics UCSF ABP Objectives for ACNE Plan for the treatment
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Product: Tazarotene, cream, 500 micrograms per g (0.05%) and 1.0 mg per g (0.1%), 30 g, Zorac
 PUBLIC SUMMARY DOCUMENT Product: Tazarotene, cream, 500 micrograms per g (0.05%) and 1.0 mg per g (0.1%), 30 g, Zorac Sponsor: Genepharm Australasia Ltd Date of PBAC Consideration: July 2007 1. Purpose
PUBLIC SUMMARY DOCUMENT Product: Tazarotene, cream, 500 micrograms per g (0.05%) and 1.0 mg per g (0.1%), 30 g, Zorac Sponsor: Genepharm Australasia Ltd Date of PBAC Consideration: July 2007 1. Purpose
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
COMPOUNDING PHARMACY SOLUTIONS PRESCRIPTION COMPOUNDING FOR DERMATOLOGY
 JUNE 2012 COMPOUNDING PHARMACY SOLUTIONS PRESCRIPTION COMPOUNDING WWW.CPSRXS. COM We customize individual prescriptions for the specific needs of our patients. INSIDE THIS ISSUE: Acne 2 Cutaneous Candidiasis
JUNE 2012 COMPOUNDING PHARMACY SOLUTIONS PRESCRIPTION COMPOUNDING WWW.CPSRXS. COM We customize individual prescriptions for the specific needs of our patients. INSIDE THIS ISSUE: Acne 2 Cutaneous Candidiasis
Summary Acne vulgaris is a chronic, treatable disease but response to treatment may be delayed.
 TREATMENT OF ACNE VULGARIS Summary Acne vulgaris is a chronic, treatable disease but response to treatment may be delayed. Topical agents are useful as monotherapy for mild acne and in combination with
TREATMENT OF ACNE VULGARIS Summary Acne vulgaris is a chronic, treatable disease but response to treatment may be delayed. Topical agents are useful as monotherapy for mild acne and in combination with
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C
 FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
Acne. Objectives. Conflicts of Interest. Acne 05/18/2015
 Objectives Acne Jonathan A. Dyer, MD Associate Professor of Dermatology and Child Health University of Missouri Discuss acne pathogenesis Recognize common acne subtypes Implement a treatment plan based
Objectives Acne Jonathan A. Dyer, MD Associate Professor of Dermatology and Child Health University of Missouri Discuss acne pathogenesis Recognize common acne subtypes Implement a treatment plan based
acne Dr. M. Goeteyn Dermatologie AZ Sint-Jan Brugge-Oostende AV marleen.goeteyn@azsintjan.be
 acne Dr. M. Goeteyn Dermatologie AZ Sint-Jan Brugge-Oostende AV marleen.goeteyn@azsintjan.be Why should you treat acne? -Impact on the quality of life -scarring What is acne? Acne is an inflammatory disease
acne Dr. M. Goeteyn Dermatologie AZ Sint-Jan Brugge-Oostende AV marleen.goeteyn@azsintjan.be Why should you treat acne? -Impact on the quality of life -scarring What is acne? Acne is an inflammatory disease
THE SCIENCE WHITE PAPER SERIES OF IMAGE SKINCARE: Benzoyl Peroxide for treatment of acne vulgaris
 THE SCIENCE WHITE PAPER SERIES OF IMAGE SKINCARE: Benzoyl Peroxide for treatment of acne vulgaris by Marc A. Ronert MD PhD, Clinical Director Image Skincare ABSTRACT Image Skincare offers products with
THE SCIENCE WHITE PAPER SERIES OF IMAGE SKINCARE: Benzoyl Peroxide for treatment of acne vulgaris by Marc A. Ronert MD PhD, Clinical Director Image Skincare ABSTRACT Image Skincare offers products with
ACUPUNCTURE AND ACNE. About Acne. References Garner SE. Acne vulgaris. In: Williams H (Ed). Evidence Based Dermatology. London: BMJ, 2003.
 ACUPUNCTURE AND ACNE About Acne Acne vulgaris, the most common type of acne, is a chronic inflammatory skin disease affecting hair follicles, and sebaceous glands and ducts. It occurs on the face in 99%
ACUPUNCTURE AND ACNE About Acne Acne vulgaris, the most common type of acne, is a chronic inflammatory skin disease affecting hair follicles, and sebaceous glands and ducts. It occurs on the face in 99%
B. Disorders of sebaceous glands
 Go Back to the Top To Order, Visit the Purchasing Page for Details B. Disorders of sebaceous glands 1. Acne vulgaris It most frequently occurs on the face of adolescent men and women. Comedones, folliculitis,
Go Back to the Top To Order, Visit the Purchasing Page for Details B. Disorders of sebaceous glands 1. Acne vulgaris It most frequently occurs on the face of adolescent men and women. Comedones, folliculitis,
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
The effective treatment of acne vulgaris by a high-intensity, narrow band 405 420 nm light source
 J Cosmetic & Laser Ther 2003; 5: 111 116 # J Cosmetic & Laser Ther. All rights reserved ISSN 1476-4172 DOI: 10.1080/14764170310001276 111 The effective treatment of acne vulgaris by a high-intensity, narrow
J Cosmetic & Laser Ther 2003; 5: 111 116 # J Cosmetic & Laser Ther. All rights reserved ISSN 1476-4172 DOI: 10.1080/14764170310001276 111 The effective treatment of acne vulgaris by a high-intensity, narrow
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
Salicylic Acid Peel Incorporating Triethyl Citrate and Ethyl Linoleate in the Treatment of Moderate Acne: A New Therapeutic Approach
 ORIGINAL ARTICLES Salicylic Acid Peel Incorporating Triethyl Citrate and Ethyl Linoleate in the Treatment of Moderate Acne: A New Therapeutic Approach BEATRICE RAONE, MD,* STEFANO VERALDI, MD, ROBERTA
ORIGINAL ARTICLES Salicylic Acid Peel Incorporating Triethyl Citrate and Ethyl Linoleate in the Treatment of Moderate Acne: A New Therapeutic Approach BEATRICE RAONE, MD,* STEFANO VERALDI, MD, ROBERTA
J of Evolution of Med and Dent Sci/ eissn- 2278-4802, pissn- 2278-4748/ Vol. 3/ Issue 52/Oct 13, 2014 Page 12179
 PRESCRIPTION AUDIT OF ACNE VULGARIS IN SKIN OUTPATIENT DEPARTMENT OF A TERTIARY CARE TEACHING HOSPITAL Vishal Prakash Giri 1, Shubhra Kanodia 2, Om Prakash Giri 3, Ataul Haque 4 HOW TO CITE THIS ARTICLE:
PRESCRIPTION AUDIT OF ACNE VULGARIS IN SKIN OUTPATIENT DEPARTMENT OF A TERTIARY CARE TEACHING HOSPITAL Vishal Prakash Giri 1, Shubhra Kanodia 2, Om Prakash Giri 3, Ataul Haque 4 HOW TO CITE THIS ARTICLE:
First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases
 First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases Andrew E. Mulberg, MD, FAAP Division Deputy Director OND/ODE3/DGIEP FDA Partnership is the Key Coming together is a beginning;
First In Human Pediatric Trials and Safety Assessment for Rare and Orphan Diseases Andrew E. Mulberg, MD, FAAP Division Deputy Director OND/ODE3/DGIEP FDA Partnership is the Key Coming together is a beginning;
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
4/18/2014 ACNE: THEN & NOW 25 YEARS OF INNOVATION ACNE VULGARIS ETIOLOGY OF ACNE. Jenifer Lloyd, DO, FAAD Professor of Internal Medicine
 ACNE: THEN & NOW 25 YEARS OF INNOVATION Jenifer Lloyd, DO, FAAD Professor of Internal Medicine ACNE VULGARIS The most common disease of the pilosebaceous unit. Commonly affects the face, chest & back.
ACNE: THEN & NOW 25 YEARS OF INNOVATION Jenifer Lloyd, DO, FAAD Professor of Internal Medicine ACNE VULGARIS The most common disease of the pilosebaceous unit. Commonly affects the face, chest & back.
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Topical therapies are the mainstay of treatment
 Study of the Efficacy, Tolerability, and Safety of 2 Fixed-Dose Combination Gels in the Management of Acne Vulgaris Christos C. Zouboulis, MD, PhD; Tanja C. Fischer, MD; Johannes Wohlrab, MD, PhD; Jo Barnard,
Study of the Efficacy, Tolerability, and Safety of 2 Fixed-Dose Combination Gels in the Management of Acne Vulgaris Christos C. Zouboulis, MD, PhD; Tanja C. Fischer, MD; Johannes Wohlrab, MD, PhD; Jo Barnard,
Guidelines of Care for Acne Vulgaris Management Technical Report
 Guidelines of Care for Acne Vulgaris Management Technical Report 2 Acne Vulgaris Table of Contents Page No. ntroduction... 3 Clinical questions... 3 evaluation of evidence... 4 Grading and classification
Guidelines of Care for Acne Vulgaris Management Technical Report 2 Acne Vulgaris Table of Contents Page No. ntroduction... 3 Clinical questions... 3 evaluation of evidence... 4 Grading and classification
The Effectiveness oftopical Antibacterials in Acne: A Double-Blind Clinical Study
 J Int Med Res (1978) 6, 72 The Effectiveness oftopical Antibacterials in Acne: A Double-Blind Clinical Study E Franz, MD, 8 Rohde, MD and S Weidner-Strahl, MD, University Department of Dermatology, 34
J Int Med Res (1978) 6, 72 The Effectiveness oftopical Antibacterials in Acne: A Double-Blind Clinical Study E Franz, MD, 8 Rohde, MD and S Weidner-Strahl, MD, University Department of Dermatology, 34
Name of Policy: Laser Treatment of Active Acne
 Name of Policy: Laser Treatment of Active Acne Policy #: 394 Latest Review Date: December 2009 Category: Surgery Policy Grade: B Background: As a general rule, benefits are payable under Blue Cross and
Name of Policy: Laser Treatment of Active Acne Policy #: 394 Latest Review Date: December 2009 Category: Surgery Policy Grade: B Background: As a general rule, benefits are payable under Blue Cross and
ACNE TOP TIPS FOR GPs! Louise Moss GP Moss Valley Medical Practice, Eckington, GPwSI for NDCCG Sept 2013
 ACNE TOP TIPS FOR GPs! Louise Moss GP Moss Valley Medical Practice, Eckington, GPwSI for NDCCG Sept 2013 Acne-an important condition Aim for today To have a better understanding of how to treat Acne well,
ACNE TOP TIPS FOR GPs! Louise Moss GP Moss Valley Medical Practice, Eckington, GPwSI for NDCCG Sept 2013 Acne-an important condition Aim for today To have a better understanding of how to treat Acne well,
PowerLight LED Light Therapy. The FUTURE of corrective skin
 PowerLight LED Light Therapy The FUTURE of corrective skin care TODAY LED facial treatments Effective when used with correct protocols Non thermal stimulation of collagen Increases circulation and lymphatic
PowerLight LED Light Therapy The FUTURE of corrective skin care TODAY LED facial treatments Effective when used with correct protocols Non thermal stimulation of collagen Increases circulation and lymphatic
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition
 Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Latasha Weeks, B.A., B.S. Doctor of Pharmacy Candidate, 2007 University of Maryland School of Pharmacy Learning
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Latasha Weeks, B.A., B.S. Doctor of Pharmacy Candidate, 2007 University of Maryland School of Pharmacy Learning
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition
 Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Dr. Mohamed Emam Lecturer in Clinical Pharmacy Department Faculty of Pharmacy Beni Suef University Hair Pilosebaceous
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Dr. Mohamed Emam Lecturer in Clinical Pharmacy Department Faculty of Pharmacy Beni Suef University Hair Pilosebaceous
ACNE. Nisakorn Saewan, Ph.D.
 ACNE, Ph.D. Contents Definition Cause of acne Type of acne Acne grades Acne treatments Objectives To explain the definition of acne To elucidate cause of acne To identify type and grade of acne To explain
ACNE, Ph.D. Contents Definition Cause of acne Type of acne Acne grades Acne treatments Objectives To explain the definition of acne To elucidate cause of acne To identify type and grade of acne To explain
X-Plain Acne Reference Summary
 X-Plain Acne Reference Summary Introduction Nearly 17 million people in the United States have acne, making it one of the most common skin diseases in the USA. Although acne is not a serious health threat,
X-Plain Acne Reference Summary Introduction Nearly 17 million people in the United States have acne, making it one of the most common skin diseases in the USA. Although acne is not a serious health threat,
Therapeutic Overview Topical Acne Agents
 Therapeutic Overview Topical Acne Agents Overview/Summary Acne vulgaris, a disease of the pilosebaceous follicles, is the most common cutaneous dermatological disorder and primarily affects adolescents
Therapeutic Overview Topical Acne Agents Overview/Summary Acne vulgaris, a disease of the pilosebaceous follicles, is the most common cutaneous dermatological disorder and primarily affects adolescents
Immunex Corporation Novantrone (Mitoxantrone HCL) P&CNS Advisory Committee Briefing Document. Page 020
 Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Is Monotherapy Treatment of Etanercept Effective Against Plaque Psoriasis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Psoriasis, Incidence, Quality of Life, Psoriatic Arthritis, Prevalence
 1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Preetha selva et al. / International Journal of Phytopharmacology. 6(1), 2015, 42-46. International Journal of Phytopharmacology
 International Journal of Phytopharmacology Journal homepage: www.onlineijp.com 42 e- ISSN 0975 9328 Print ISSN 2229 7472 IJP A CLINICAL STUDY TO EVALUATE THE EFFECT OF TOPICAL TAZAROTENE IN THE TREATMENT
International Journal of Phytopharmacology Journal homepage: www.onlineijp.com 42 e- ISSN 0975 9328 Print ISSN 2229 7472 IJP A CLINICAL STUDY TO EVALUATE THE EFFECT OF TOPICAL TAZAROTENE IN THE TREATMENT
Comparison efficacy of azithromycin vs. doxycycline in patients with acne vulgaris
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 1002-1009 http://www.ijcmas.com Original Research Article Comparison efficacy of azithromycin
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 1002-1009 http://www.ijcmas.com Original Research Article Comparison efficacy of azithromycin
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Human Clinical Study for Free Testosterone & Muscle Mass Boosting
 Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
The treatment of acne is often hampered by
 168 Clinical Pharmacist April 2009 Vol 1 Treatment can help clear acne, minimising scarring and relieving psychosocial stress Acne treatment For personal use only. Not to be reproduced without permission
168 Clinical Pharmacist April 2009 Vol 1 Treatment can help clear acne, minimising scarring and relieving psychosocial stress Acne treatment For personal use only. Not to be reproduced without permission
Acne is a cutaneous pleomorphic disorder of the pilosebaceous unit involving abnormalities in sebum
 Available Online through ISSN: 0975-766X Review Article www.ijptonline.com NOVEL DRUG DELIVERY SYSTEM AND ITS USE IN THE TREATMENT OF ACNE M.Jotish*, Chodavarapu Naga Pranitha, Ponnam Kundana, G.Sarath
Available Online through ISSN: 0975-766X Review Article www.ijptonline.com NOVEL DRUG DELIVERY SYSTEM AND ITS USE IN THE TREATMENT OF ACNE M.Jotish*, Chodavarapu Naga Pranitha, Ponnam Kundana, G.Sarath
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Efficacy of octenidine dihydrochloride and 2-phenoxyethanol in the topical treatment of inflammatory acne
 P i l o t s t u d y Efficacy of octenidine dihydrochloride and 2-phenoxyethanol in the topical treatment of inflammatory acne S. Mayr-Kanhäuser, B. Kränke, and W. Aberer K E Y WORDS acne, antibiotic resistance,
P i l o t s t u d y Efficacy of octenidine dihydrochloride and 2-phenoxyethanol in the topical treatment of inflammatory acne S. Mayr-Kanhäuser, B. Kränke, and W. Aberer K E Y WORDS acne, antibiotic resistance,
The Cause of Acne. Caring for Acne
 Sebaceous (oil) glands generate oil. The nose area is typically affected by acne because the sebaceous glands are larger and more active in this vicinity than in any other area of the face. In order to
Sebaceous (oil) glands generate oil. The nose area is typically affected by acne because the sebaceous glands are larger and more active in this vicinity than in any other area of the face. In order to
..,oi..' Advance to the. head of the êlass ~ Optimized tretinoin plus. proven tolerabilty-for a smart start they can stay with!
 FOR THE TREATMENT OF ACNE VULGARIS!J ii II " 'T/ ~~ I., -- o '~(1 001.. _!! í '"..,oi..', Q,~~ ~. Advance to the '" head of the êlass ~ Optimized tretinoin plus proven tolerabilty-for a smart start they
FOR THE TREATMENT OF ACNE VULGARIS!J ii II " 'T/ ~~ I., -- o '~(1 001.. _!! í '"..,oi..', Q,~~ ~. Advance to the '" head of the êlass ~ Optimized tretinoin plus proven tolerabilty-for a smart start they
Disease overview. Acne is a skin disorder, due to many intrinsic factors. Acne improves mainly upon reaching adulthood
 Innovative treatment for acneic skin Disease overview Acne is a skin disorder, due to many intrinsic factors Acne improves mainly upon reaching adulthood Acne affects 80% of adolescents & 20% of adults
Innovative treatment for acneic skin Disease overview Acne is a skin disorder, due to many intrinsic factors Acne improves mainly upon reaching adulthood Acne affects 80% of adolescents & 20% of adults
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
D E R M A T O L O G Y
 We customize individual prescriptions for the specific needs of our patients. J A N U A R Y 2 0 1 3 I N S I D E T H I S I S S U E : Warts 2 Melasma 3 P R E S C R I P T I O N C O M P O U N D I N G F O R
We customize individual prescriptions for the specific needs of our patients. J A N U A R Y 2 0 1 3 I N S I D E T H I S I S S U E : Warts 2 Melasma 3 P R E S C R I P T I O N C O M P O U N D I N G F O R
Antipsychotic drugs are the cornerstone of treatment
 Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Glossary of Clinical Trial Terms
 Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Acne Skin: Lifestyle or Genetic?
 Acne Skin: Lifestyle or Genetic? Yuechan Chen ID: 6786032339 Professor Weiss May 05 2014 ACNE STUDIES CHEN 1 Acne Skin: Lifestyle or Genetic? How can I cure my acne, clear its roots?! Acne is a stubborn
Acne Skin: Lifestyle or Genetic? Yuechan Chen ID: 6786032339 Professor Weiss May 05 2014 ACNE STUDIES CHEN 1 Acne Skin: Lifestyle or Genetic? How can I cure my acne, clear its roots?! Acne is a stubborn
ACNE GUIDELINES: Management of Acne Vulgaris: Clinical Recommendations & Isotretinoin Position Statement. American Academy of Dermatology
 O ACNE GUIDELINES: Management of Acne Vulgaris: Clinical s & Isotretinoin Position Statement American Academy of Dermatology s s for systems for the grading and classification of acne Clinicians may find
O ACNE GUIDELINES: Management of Acne Vulgaris: Clinical s & Isotretinoin Position Statement American Academy of Dermatology s s for systems for the grading and classification of acne Clinicians may find
Preparation of new formulations of anti-acne creams and their efficacy
 African Journal of Pharmacy and Pharmacology Vol. 4(6), pp. 298-303, June 2010 Available online http://www.academicjournals.org/ajpp ISSN 1996-0816 2010 Academic Journals Full Length Research Paper Preparation
African Journal of Pharmacy and Pharmacology Vol. 4(6), pp. 298-303, June 2010 Available online http://www.academicjournals.org/ajpp ISSN 1996-0816 2010 Academic Journals Full Length Research Paper Preparation
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Acne. Normal skin. What is acne?
 Acne Acne is extremely common in varying degrees of severity. About 9 out of 10 teenagers develop some degree of acne. However, this does not mean it should be ignored. Acne is a distressing condition
Acne Acne is extremely common in varying degrees of severity. About 9 out of 10 teenagers develop some degree of acne. However, this does not mean it should be ignored. Acne is a distressing condition
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Riociguat Clinical Trial Program
 Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
