Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections
|
|
|
- Conrad Walsh
- 8 years ago
- Views:
Transcription
1 Journal of Antimicrobial Chemotherapy (2005) 55, doi: /jac/dkh546 Advance Access publication 10 February 2005 Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections Judith N. Steenbergen*, Jeff Alder, Grace M. Thorne and Francis P. Tally Cubist Pharmaceuticals, Inc., 65 Hayden Avenue, Lexington, MA 02421, USA JAC Infections caused by drug-resistant pathogens are on the rise. Daptomycin, a cyclic lipopeptide with activity against most Gram-positive pathogens, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, is a newly US-FDA approved antimicrobial for complicated skin and skin structure infections (csssi). Daptomycin has a unique mechanism of action that results in destruction of the membrane potential. The rapid bactericidal activity of daptomycin makes it an attractive antibiotic for serious Gram-positive infections. Keywords: MRSA, csssi, vancomycin Introduction The increase in infections caused by Gram-positive pathogens and the rise in antibiotic-resistant bacterial strains have prompted the need for novel antibiotics. 1,2 Recent reports indicate that more than 25% of Staphylococcus aureus infections in Europe are caused by methicillin-resistant S. aureus (MRSA), and the majority of these isolates are resistant to additional antibiotics. 3 The incidence of MRSA varies greatly by country. Over 50% of S. aureus isolates in Portugal and Italy are methicillin-resistant, isolates in England, Greece, and France have MRSA rates around 25%, whereas the Netherlands and Switzerland have the lowest incidence of MRSA. 3 Vancomycin has been an effective antibiotic against MRSA; however, the increased use of vancomycin has led to the development of isolates with reduced susceptibility. The mechanism of reduced susceptibility to vancomycin in S. aureus has not been fully elucidated and appears to be heterogeneous. Reduced susceptibility to vancomycin is correlated with alterations in the bacterial cell wall leading to significantly thicker and more disorganized cell walls. 4 These thicker cell walls may sequester the vancomycin from reaching the target nascent cell wall precursors. 4 Additional in vitro studies have linked development of vancomycin reduced susceptibility with phenotypic changes such as loss of haemolysis and the meca gene, and genotypic changes such as the presence of either the group I or group II polymorphism in the agr gene locus. 5 7 To date, three vancomycin-resistant S. aureus strains (VRSA) have been isolated in the United States Both the Pennsylvania and the New York strains were isolated from patients not on vancomycin therapy. 9,12 Therefore, the need for new potent antimicrobial agents with MRSA activity is essential. Mechanism of action Daptomycin, a fermentation product produced by Streptomyces roseosporus, is a cyclic lipopeptide antibiotic with potent bactericidal activity against most Gram-positive organisms including multiple antibiotic-resistant and -susceptible strains Daptomycin was recently approved in the United States for the treatment of complicated skin and skin structure infections (csssi) associated with S. aureus (methicillin-susceptible, MSSA, and methicillin-resistant, MRSA), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis and Enterococcus faecalis (vancomycin-susceptible only). Below, we discuss the in vitro potency of daptomycin against a range of other organisms including vancomycin-resistant E. faecalis and Enterococcus faecium. The unique structure of daptomycin consists of a 13-member amino acid cyclic lipopeptide with a decanoyl side-chain (Figure 1). This distinctive structure confers a novel mechanism of action. 22 The proposed mechanism involves insertion of the lipophilic daptomycin tail into the bacterial cell membrane, causing rapid membrane depolarization and a potassium ion efflux. This is followed by arrest of DNA, RNA and protein synthesis resulting in bacterial cell death (Figure 2) The bactericidal effect of daptomycin is rapid with greater than 99.9% of both MRSA and MSSA bacteria dead in less than 1 h. 25,26 This rapid cell death does not result in rapid bacterial cell lysis. 24 Daptomycin also remains bactericidal (99.9% kill within 24 h) against stationary phase cultures of both MSSA and MRSA present at high density (10 9 cfu) in a simulated endocardial vegetation model *Corresponding author. Tel: ; Fax: ; Judith.steenbergen@cubist.com JAC vol.55 no.3 q The British Society for Antimicrobial Chemotherapy 2005; all rights reserved.
2 Figure 1. Daptomycin chemical structure. Figure 2. Daptomycin mechanism of action. Hypothetical steps: step 1, daptomycin binds to the cytoplasmic membrane in a calcium-dependent manner; step 2, daptomycin oligomerizes, disrupting the membrane; step 3, the release of intracellular ions and rapid cell death. Microbiology In vitro potency has been demonstrated for daptomycin against a range of aerobic and anaerobic Gram-positive bacteria including multidrug-resistant strains ,28 MIC 90 values along with MIC ranges for select pathogens can be found in Table 1. The table shows data from two recent studies illustrating the conserved MIC ranges and values for both European strains and isolates collected worldwide. Daptomycin s spectrum of activity encompasses the difficult to treat antibiotic-resistant organisms including methicillin-resistant and -susceptible Staphylococcus aureus (MRSA, MSSA), glycopeptide-intermediate S. aureus (GISA), methicillin-resistant coagulase-negative Staphylococcus spp. (CoNS), and vancomycin-resistant enterococci (VRE) Daptomycin demonstrated potency against the recently isolated vancomycin-resistant S. aureus as well as linezolid and quinupristin/dalfopristin-resistant S. aureus and E. faecium. 14,17 20,24 Furthermore, daptomycin is also effective against a variety of streptococcal groups such as the b-haemolytic streptococci including S. pyogenes (Group A) and S. agalactiae (Group B) as 13 15,20,21 well as other Streptococcus spp. Along with the commonly isolated Gram-positive organisms, daptomycin is also potent against Corynebacterium jeikeium, and a variety of anaerobic species including Peptostreptococcus spp., Clostridium perfringens, Clostridium difficile, and Propionibacterium acnes (Table 1). 28 Drug synergy with daptomycin has been described in vitro with aminoglycosides and rifampicin antibiotics. 29 Resistance Daptomycin s efficacy is enhanced by the near absence of antibiotic resistance as verified by both in vitro and clinical studies. 30 Resistance to daptomycin has been difficult to generate in the laboratory both in single passage and serial passage experi ments. 30 The emergence of resistance was <0.2% across the entire set of Phase II and III clinical trials with over 1000 daptomycin-treated patients. The reason for this decrease in susceptibility is unknown and no transferable elements conferring daptomycin resistance have been isolated. Pharmacology Analysis of daptomycin pharmacodynamics determined that a once-daily dosing regimen increases the efficacy and safety of daptomycin. 31 In vitro and in vivo analysis established that daptomycin is effective in a concentration-dependent manner, has a long half-life (8 h), and demonstrates a prolonged post-antibiotic effect up to 6.8 h (Table 2). 32 These findings resulted in a once a day dosing regimen recommendation of 4 mg/kg for complicated skin and skin structure infections (csssi) in the United States. Once-daily dosing of daptomycin results in linear pharmacokinetics with minimal drug accumulation. 31 Daptomycin distributes primarily in the plasma, with penetration to vascular tissues (Table 3). Daptomycin does not cross the blood brain barrier and does not penetrate the cerebrospinal fluid of normal individuals. However, there was a 5% penetration (relative to 284
3 Table 1. In vitro activity of daptomycin against select Gram-positive bacteria Organism No. of strains MIC range (mg/l) MIC 90 (mg/l) Reference Select aerobic pathogens Staphylococcus aureus oxacillin-resistant European isolates worldwide isolates 1247 <_ oxacillin-susceptible European isolates 888 <_ worldwide isolates 1955 <_ Coagulase-negative staphylococci a European isolates worldwide isolates 838 <_ b-haemolytic streptococci European isolates b worldwide isolates 247 <_ Enterococcus faecalis vancomycin-susceptible European isolates 1789 <_ worldwide isolates 626 <_ vancomycin-resistant European isolates 40 <_ worldwide isolates Enterococcus faecium vancomycin-susceptible European isolates worldwide isolates 97 <_ vancomycin-resistant European isolates worldwide isolates Enterococcus spp. c European isolates 160 <_ worldwide isolates Corynebacterium jeikeium Select anaerobic pathogens Actinomyces group Bifidobacterium spp. 13 < Clostridium difficile Clostridium perfringens Lactobacillus spp. d 37 < Peptostreptococcus spp Propionibacterium spp a Includes methicillin-resistant isolates vancomycin-resistant isolates. b European isolates were S. agalactiae only. c Includes vancomycin-resistant isolates. d All Lactobacillus spp. were grown anaerobically. Table 2. Mean (S.D.) daptomycin pharmacokinetic parameters in healthy volunteers on day 7 Dose (mg/kg) C max (mg/l) T max a (h) AUC 0 24 (mg h/l) t 1/2 (h) V (L/kg) CL T (ml/h/kg) CL R (ml/h/kg) Ae 24 (%) 4(n=6) 57.8 (3.0) 0.8 (0.5, 1.0) 494 (75) 8.1 (1.0) (0.009) 8.3 (1.3) 4.8 (1.3) 53.0 (10.8) 6(n=6) 98.6 (12) 0.5 (0.5,1.0) 747 (91) 8.9 (1.3) (0.013) 8.1 (1.0) 4.4 (0.3) 47.4 (11.5) 8(n=6) 133 (13.5) 0.5 (0.5,1.0) 1130 (117) 9.0 (1.2) (0.012) 7.2 (0.8) 3.7 (0.5) 52.1 (5.19) C max, maximum plasma concentration; T max, time to C max ; AUC 0 24, area under the concentration time curve from 0 to 24 h; t 1/2, terminal elimination half-life; V, apparent volume of distribution; CL T, systemic clearance; CL R, renal clearance; Ae 24, percentage of dose recovered in urine over 24 h as unchanged daptomycin following the first dose. a Median (minimum, maximum). 285
4 Table 3. Daptomycin tissue penetration Tissue Species Maximum concentration Percent relative to serum Reference Blister fluid human 27.6 mg/l Blood clot tissue rat, rabbit 3.5 mg/g Peritoneal tissue chamber rat 11.8 mg/l Lung mouse, rat 5 mg/l BAL-ELF mouse, rat, sheep 1 mg/l 2 45 CSF rabbit 5.2 mg/l BAL-ELF, bronchoalveolar lavage epithelial lining fluid. serum) of daptomycin into the cerebrospinal fluid of rabbits with Streptococcus pneumoniae meningitis, resulting in clearance of the infection in this model. 33 Daptomycin is primarily renally excreted, with the majority of the drug remaining intact in the Table 4. Incidence of adverse events that occurred in >_ 2% of patients in either daptomycin or comparator treatment groups in Phase III csssi studies Adverse event Daptomycin % (n=534) Comparator a % (n=558) Gastrointestinal disorders constipation nausea diarrhoea vomiting dyspepsia General disorders injection site reactions fever Nervous system disorders headache insomnia dizziness Skin/subcutaneous disorders rash pruritus Diagnostic investigations abnormal liver function tests elevated CPK Infections fungal infections urinary tract infections Vascular disorders hypotension hypertension Renal/urinary disorders renal failure Blood/lymphatic disorders anaemia Respiratory disorders dyspnoea Musculoskeletal disorders limb pain arthralgia a Comparators included vancomycin (1 g iv every 12 h) and antistaphylococcal penicillins (i.e. nafcillin, oxacillin, cloxacillin, flucloxacillin; 4 12 g/day in divided doses). urine. 31 Since daptomycin is excreted through the kidneys, the dosing interval is increased to every 48 h in patients with severe renal impairment defined as a creatinine clearance of <30 ml/min. Because of daptomycin s unique mechanism of action and because it is not metabolized by cytochrome p450 or other hepatic enzymes, no antagonistic drug interactions have been observed. Pre-clinical studies In pre-clinical studies, daptomycin treatment has been linked to fully reversible skeletal muscle toxicity with no effect on smooth or cardiac muscle. Animal studies determined that both degenerative and regenerative changes are observed in skeletal muscle with no rhabdomyolysis. 31 These effects, which can be associated with elevated creatine phosphokinase (CPK) levels, are fully reversible after cessation of daptomycin use and were not statistically significant when compared with comparator. 31 The numbers of side effects for patients receiving daptomycin were comparable to standard therapy and less than 2% of patients receiving daptomycin discontinued therapy. 34 The most common adverse events from the Phase III csssi clinical trials for daptomycin and comparator drugs are listed in Table 4. The efficacy of daptomycin against a range of infections has been demonstrated in animal studies. Using a variety of antibiotic-resistant and -sensitive Gram-positive bacteria, daptomycin eradicated infections in the blood, muscle, kidney, heart and bone tissues of animals. These results show promise for daptomycin therapy for further clinical indications. An ongoing Phase III clinical trial is in progress to determine the efficacy of 6 mg/kg daptomycin once a day for endocarditis and bacteraemia caused by S. aureus. Clinical studies Daptomycin was evaluated in two large investigator-blinded, randomized, multicentre csssi studies in Europe, South Africa and the United States. 34 Adults with csssi of known or suspected Gram-positive aetiology were enrolled. The predominant csssi infections studied included wound infections, major abscesses and ulcer infections. Daptomycin was compared with conventional therapy of a semi-synthetic penicillin (e.g. nafcillin, oxacillin, cloxacillin, or flucloxacillin) or vancomycin (for suspected MRSA). The clinical success rates for each treatment group (intent to treat, modified intent to treat, clinically evaluable, and microbiologically evaluable) are shown in Table
5 Table 5. Clinical success rates by treatment group, in Phase III csssi studies Daptomycin Comparator a Population n % success n % success 95% CI b Intent-to-treat ( 5.8, 5.0) Modified intent-to-treat ( 5.5, 5.9) Clinically evaluable ( 4.0, 5.6) Microbiologically evaluable ( 3.8, 6.3) a Cloxacillin, flucloxacillin, nafcillin, oxacillin or vancomycin. b 95% confidence interval around the difference in success rate (comparator daptomycin). Table 6. Clinical success rates by infecting pathogen, in Phase III csssi studies The study was designed to determine whether daptomycin was comparable to standard therapy and was not powered to show superiority. Therefore, statistical analysis determined that in the clinical trails, daptomycin was non-inferior to comparator therapy leading to daptomycin approval by the FDA in the United States. 34 Results of the microbiologically evaluable population are detailed by pathogen in Table Over 1000 patients were evaluated, and the following pathogens were the predominant organisms isolated; MSSA, MRSA, S. pyogenes, S. agalactiae, S. dysgalactiae subsp. equisimilis, and E. faecalis. The results from these Phase III trials confirmed the efficacy and safety of daptomycin. Conclusions Success rate, n/n (%) Pathogen Daptomycin comparator a 170/198 (85.9) 180/207 (87.0) Methicillin-susceptible Staphylococcus aureus (MSSA) b Methicillin-resistant 21/28 (75.0) 25/36 (69.4) Staphylococcus aureus (MRSA) b Streptococcus pyogenes 79/84 (94.0) 80/88 (90.9) Streptococcus agalactiae 23/27 (85.2) 22/29 (75.9) Streptococcus dysgalactiae 8/8 (100) 9/11 (81.8) subsp. equisimilis Enterococcus faecalis (vancomycin-susceptible only) b 27/37 (73.0) 40/53 (75.5) a Vancomycin or semi-synthetic penicillins (e.g. nafcillin, oxacillin, cloxacillin or flucloxacillin). b As determined by the central laboratory. In summary, daptomycin is a rapidly bactericidal antibiotic that is active against clinically relevant Gram-positive bacteria including antibiotic-resistant strains. Clinical data demonstrate that daptomycin is highly effective against csssi and ongoing clinical trials including infectious endocarditis caused by S. aureus, should expand treatment indications. The low occurrence of side effects, low resistance rates, and high potency demonstrate that daptomycin has significant clinical utility in the treatment of Gram-positive infections, including those caused by MRSA. References 1. Bell, J. M. & Turnidge, J. D. (2002). High prevalence of oxacillinresistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from SENTRY antimicrobial surveillance program, Antimicrobial Agents and Chemotherapy 46, Stefani, S. & Varaldo, P. E. (2003). Epidemiology of methicillinresistant staphylococci in Europe. Clinical Microbiology and Infection 9, Fluit, A. C., Wielders, C. L., Verhoef, J. et al. (2001). Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. Journal of Clinical Microbiology 39, Cui, L., Ma, X., Sato, K. et al. (2003). Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. Journal of Clinical Microbiology 41, Verdier, I., Reverdy, M. E., Etienne, J. et al. (2004). Staphylococcus aureus isolates with reduced susceptibility to glycopeptides belong to accessory gene regulator group I or II. Antimicrobial Agents and Chemotherapy 48, Sakoulas, G., Eliopoulos, G. M., Moellering, R. C. et al. (2003). Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? Journal of Infectious Diseases 187, Adhikari, R. P., Scales, G. C., Kobayashi, K. et al. (2004). Vancomycin-induced deletion of the methicillin resistance gene meca in Staphylococcus aureus. Journal of Antimicrobial Chemotherapy 54, Centers for Disease Control and Prevention. (2002). Staphylococcus aureus resistant to vancomycin United States, In Morbidity and Mortality Weekly Report, pp Centers for Disease Control and Prevention. (2002). Public Health Dispatch: vancomycin-resistant Staphylococcus aureus Pennsylvania, In Morbidity and Mortality Weekly Report Centers for Disease Control and Prevention. (2004). Brief report: vancomycin-resistant Staphylococcus aureus New York, In Morbidity and Mortality Weekly Report Mutnick, A. H., Biedenbach, D. J. & Jones, R. N. (2003). Geographic variations and trends in antimicrobial resistance among Enterococcus faecalis and Enterococcus faecium in the SENTRY Antimicrobial Surveillance Program ( ). Diagnostic Microbiology and Infectious Disease 46, Kacica, M. A., Scott, C., Johnson, G. et al. (2004). Vancomycinresistant Staphylococcus aureus (VRSA) in a resident of a long-term care facility (LTCF). In Program and Abstracts of the 42 nd Annual Meeting of the Infectious Diseases Society of America, Boston, MA, USA. Abstract 530, p Infectious Diseases Society of America, Alexandria, VA, USA. 13. Barry, A. L., Fuchs, P. C. & Brown, S. D. (2001). In vitro activities of daptomycin against 2,789 clinical isolates from 11 North 287
6 American medical centers. Antimicrobial Agents and Chemotherapy 45, Critchley, I. A., Draghi, D. C., Sahm, D. F. et al. (2003). Activity of daptomycin against susceptible and multidrug-resistant Grampositive pathogens collected in the SECURE study (Europe) during Journal of Antimicrobial Chemotherapy 51, Fluit, A. C., Schmitz, F. J., Verhoef, J. et al. (2004). Daptomycin in vitro susceptibility in European Gram-positive clinical isolates. International Journal of Antimicrobial Agents 24, Fluit, A. C., Schmitz, F. J., Verhoef, J. et al. (2004). In vitro activity of daptomycin against Gram-positive European clinical isolates with defined resistance determinants. Antimicrobial Agents and Chemotherapy 48, Petersen, P. J., Bradford, P. A., Weiss, W. J. et al. (2002). In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrobial Agents and Chemotherapy 46, Richter, S. S., Kealey, D. E., Murray, C. T. et al. (2003). The in vitro activity of daptomycin against Staphylococcus aureus and Enterococcus species. Journal of Antimicrobial Chemotherapy 52, Rybak, M. J., Hershberger, E., Moldovan, T. et al. (2000). In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrobial Agents and Chemotherapy 44, Snydman, D. R., Jacobus, N. V., McDermott, L. A. et al. (2000). Comparative in vitro activities of daptomycin and vancomycin against resistant Gram-positive pathogens. Antimicrobial Agents and Chemotherapy 44, Streit, J. M., Jones, R. N. & Sader, H. S. (2004). Daptomycin activity and spectrum: a worldwide sample of 6737 clinical Grampositive organisms. Journal of Antimicrobial Chemotherapy 53, Silverman, J. A., Perlmutter, N. G. & Shapiro, H. M. (2003). Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 47, Canepari, P., Boaretti, M., del Mar Lleo, M. et al. (1990). Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032). Antimicrobial Agents and Chemotherapy 34, Silverman, J., Harris, B., Cotroneo, N., et al. (2003). Daptomycin (DAP) treatment induces membrane and cell wall alterations in Staphylococcus aureus. In Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL. Abstract C1 2135, p American Society for Microbiology, Washington, DC, USA. 25. Cha, R. & Rybak, M. J. (2004). Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. Journal of Antimicrobial Chemotherapy 54, Fuchs, P. C., Barry, A. L. & Brown, S. D. (2002). In vitro bactericidal activity of daptomycin against staphylococci. Journal of Antimicrobial Chemotherapy 49, Tedesco, K. L. & Rybak, M. J. (2003). Impact of high inoculum Staphylococcus aureus on the activities of nafcillin (NAF), vancomycin (VAN), linezolid (LZD), gentamicin (GEN), and daptomycin (DAP) in an in vitro pharmacodynamic model (IVD). In Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL. Abstract A-1151, p. 14. American Society for Microbiology, Washington, DC, USA. 28. Goldstein, E. J., Citron, D. M., Merriam, C. V. et al. (2003). In vitro activities of daptomycin, vancomycin, quinupristin dalfopristin, linezolid, and five other antimicrobials against 307 gram-positive anaerobic and 31 Corynebacterium clinical isolates. Antimicrobial Agents and Chemotherapy 47, Rand, K. H. & Houck, H. (2004). Daptomycin synergy with rifampicin and ampicillin against vancomycin-resistant enterococci. Journal of Antimicrobial Chemotherapy 53, Silverman, J. A., Oliver, N., Andrew, T. et al. (2001). Resistance studies with daptomycin. Antimicrobial Agents and Chemotherapy 45, Oleson, F. B., Jr, Berman, C. L., Kirkpatrick, J. B. et al. (2000). Once-daily dosing in dogs optimizes daptomycin safety. Antimicrobial Agents and Chemotherapy 44, Safdar, N., Andes, D. & Craig, W. A. (2004). In vivo pharmacodynamic activity of daptomycin. Antimicrobial Agents and Chemotherapy 48, Cottagnoud, P., Pfister, M., Acosta, F. et al. (2004). Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrobial Agents and Chemotherapy 48, Arbeit, R. D., Maki, D., Tally, F. P. et al. (2004). The safety and efficacy of daptomycin for the treatment of complicated skin and skinstructure infections. Clinical Infectious Diseases 38, Alder, J., Li, T., Yu, D. et al. (2003). Analysis of daptomycin efficacy and breakpoint standards in a murine model of Enterococcus faecalis and Enterococcus faecium renal infection. Antimicrobial Agents and Chemotherapy 47, Cha, R., Grucz, R. G., Jr & Rybak, M. J. (2003). Daptomycin dose effect relationship against resistant gram-positive organisms. Antimicrobial Agents and Chemotherapy 47, Dandekar, P. K., Tessier, P. R., Williams, P. et al. (2003). Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. Journal of Antimicrobial Chemotherapy 52, Kaatz, G. W., Seo, S. M., Reddy, V. N. et al. (1990). Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrobial Agents and Chemotherapy 34, Louie, A., Kaw, P., Liu, W. et al. (2001). Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrobial Agents and Chemotherapy 45, Mader, J. T. & Adams, K. (1989). Comparative evaluation of daptomycin (LY146032) and vancomycin in the treatment of experimental methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrobial Agents and Chemotherapy 33, Smith, K., Cobbs, G., Dill, R. et al. (1990). Daptomycin versus vancomycin treatment for Staphylococcus aureus bacteremia in a murine model. Chemotherapy 36, Wise, R., Gee, T., Andrews, J. M. et al. (2002). Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrobial Agents and Chemotherapy 46, Michiels, M. J. & Bergeron, M. G. (1996). Differential increased survival of staphylococci and limited ultrastructural changes in the core of infected fibrin clots after daptomycin administration. Antimicrobial Agents and Chemotherapy 40, Vaudaux, P., Francois, P., Bisognano, C. et al. (2003). Comparative efficacy of daptomycin and vancomycin in the therapy of experimental foreign body infection due to Staphylococcus aureus. Journal of Antimicrobial Chemotherapy 52, Alder, J., Arbeit, R., Eisenstein, B., et al. (2004). Pulmonary epithelial lining fluid (ELF) as a privileged site: daptomycin in pulmonary infections. In International Congress of Infectious Diseases, Cancun, Mexico. 11 th ICID Abstracts, Abstract , p. S195. International Society for Infectious Diseases, Brighton, UK. 288
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
UWHC Guidelines for the Use of Daptomycin (Cubicin )
 UWHC Guidelines for the Use of Daptomycin (Cubicin ) Guidelines developed by: Antimicrobial Use Subcommittee; UWHC Drug Policy Program (DPP) Authors: Barry Fox, MD, Sarah E. Bland, RPh Updated by: Lucas
UWHC Guidelines for the Use of Daptomycin (Cubicin ) Guidelines developed by: Antimicrobial Use Subcommittee; UWHC Drug Policy Program (DPP) Authors: Barry Fox, MD, Sarah E. Bland, RPh Updated by: Lucas
COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent to 150 mg clindamycin base.
 APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
ASHP Therapeutic Position Statements 569
 ASHP Therapeutic Position Statements 569 Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society
ASHP Therapeutic Position Statements 569 Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society
Lecture Outline. Quinolones
 Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
CEFA-DROPS AND CEFA-TABS
 Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
General Trends in Infectious Disease
 General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
The Five Intravenous Antibiotics You Need to Know
 The Five Intravenous Antibiotics You Need to Know Ray Geyer, DO Infectious Disease Specialist Medical Director, Benefis Infection Prevention Empirical Antimicrobial Prescribing: Save the Patient and Kill
The Five Intravenous Antibiotics You Need to Know Ray Geyer, DO Infectious Disease Specialist Medical Director, Benefis Infection Prevention Empirical Antimicrobial Prescribing: Save the Patient and Kill
Cubist Pharmaceuticals, Inc. 65 Hayden Avenue Lexington, MA 02421 (781) 860-8660 www.cubist.com. Confronting Challenges in Acute Care
 Cubist Pharmaceuticals, Inc. 65 Hayden Avenue Lexington, MA 02421 (781) 860-8660 www.cubist.com Confronting Challenges in Acute Care >640,000 Patients Treated with CUBICIN (estimated) as of 12/31/08 $140
Cubist Pharmaceuticals, Inc. 65 Hayden Avenue Lexington, MA 02421 (781) 860-8660 www.cubist.com Confronting Challenges in Acute Care >640,000 Patients Treated with CUBICIN (estimated) as of 12/31/08 $140
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Vancomycin. Beta-lactams. Beta-lactams. Vancomycin (Glycopeptide) Rifamycins (rifampin) MID 4
 Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
ASHP report. that has been in clinical use for nearly 50 years as a penicillin
 ASHP report Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society
ASHP report Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society
Hemodialysis catheter infection
 Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Antimicrobe.org: An Online Reference for the Practicing Infectious Diseases Specialist
 INVITED ARTICLE SURFING THE WEB Victor L. Yu, Section Editor Antimicrobe.org: An Online Reference for the Practicing Infectious Diseases Specialist Steven D. Burdette and Thomas E. Herchline Department
INVITED ARTICLE SURFING THE WEB Victor L. Yu, Section Editor Antimicrobe.org: An Online Reference for the Practicing Infectious Diseases Specialist Steven D. Burdette and Thomas E. Herchline Department
CEFADROXIL CAPSULES, USP 500 mg Rx only
 CEFADROXIL CAPSULES, USP 500 mg Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil capsules and other antibacterial drugs, cefadroxil capsules should
CEFADROXIL CAPSULES, USP 500 mg Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil capsules and other antibacterial drugs, cefadroxil capsules should
Antimicrobial Activity and Spectrum of PPI-0903M (T-91825), a Novel Cephalosporin, Tested against a Worldwide Collection of Clinical Strains
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
Principles of Disease and Epidemiology. Copyright 2010 Pearson Education, Inc.
 Principles of Disease and Epidemiology Pathology, Infection, and Disease Disease: An abnormal state in which the body is not functioning normally Pathology: The study of disease Etiology: The study of
Principles of Disease and Epidemiology Pathology, Infection, and Disease Disease: An abnormal state in which the body is not functioning normally Pathology: The study of disease Etiology: The study of
Vancomycin Therapeutic Drug Monitoring Vancouver Coastal Health & Providence Health Care Regional Guideline. September 27, 2011. (Version 3.
 Vancomycin Therapeutic Drug Monitoring Vancouver Coastal Health & Providence Health Care Regional Guideline Sept. 27, 2011 (Version 3.5) Developed by: Jane de Lemos, Tim Lau, Mike Legal. Endorsed by: Terri
Vancomycin Therapeutic Drug Monitoring Vancouver Coastal Health & Providence Health Care Regional Guideline Sept. 27, 2011 (Version 3.5) Developed by: Jane de Lemos, Tim Lau, Mike Legal. Endorsed by: Terri
CUBICIN RF (daptomycin for injection), for intravenous use Initial U.S. Approval: 2003
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN RF safely and effectively. See full prescribing information for CUBICIN RF. CUBICIN RF (daptomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN RF safely and effectively. See full prescribing information for CUBICIN RF. CUBICIN RF (daptomycin
Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults
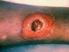 1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Emerging Therapies for Serious Gram-Positive Bacterial Infections: A Focus on Linezolid
 S144 Emerging Therapies for Serious Gram-Positive Bacterial Infections: A Focus on Linezolid Joseph F. Plouffe Division of Infectious Diseases, Ohio State University College of Medicine, Columbus, Ohio
S144 Emerging Therapies for Serious Gram-Positive Bacterial Infections: A Focus on Linezolid Joseph F. Plouffe Division of Infectious Diseases, Ohio State University College of Medicine, Columbus, Ohio
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013. Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M.
 The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
GT-020 Phase 1 Clinical Trial: Results of Second Cohort
 GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
SECTION 6 THERAPEUTIC DRUG MONITORING
 SECTION 6 THERAPEUTIC DRUG MONITORING Kieran Hand Consultant Pharmacist Anti-infectives The objectives of this section are: To test your ability to monitor serum levels for drugs with a narrow therapeutic
SECTION 6 THERAPEUTIC DRUG MONITORING Kieran Hand Consultant Pharmacist Anti-infectives The objectives of this section are: To test your ability to monitor serum levels for drugs with a narrow therapeutic
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Right-sided infective endocarditis:tunisian experience
 Right-sided infective endocarditis:tunisian experience L. Ammari, A. Ghoubontini, A. Berriche, R. Abdelmalek, S.Aissa, F.Kanoun, B.Kilani, H.Tiouiri Benaissa, T.Ben chaabane Department of Infectious diseases,
Right-sided infective endocarditis:tunisian experience L. Ammari, A. Ghoubontini, A. Berriche, R. Abdelmalek, S.Aissa, F.Kanoun, B.Kilani, H.Tiouiri Benaissa, T.Ben chaabane Department of Infectious diseases,
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections
 A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections By Gary R. Skankey, MD, FACP, Infectious Disease, Las Vegas, NV Sponsored
A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections By Gary R. Skankey, MD, FACP, Infectious Disease, Las Vegas, NV Sponsored
Optimizing Antimicrobial Susceptibility Test Reporting
 JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
Analytical Specifications RIVAROXABAN
 Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Lectures and Examinations Schedule
 Howard University College of Pharmacy Pharmacy Biomedical Preview Program: Summer 2012 Coordinator: Emmanuel O. Akala, R.Ph., Ph.D. Lead Tutor: Lectures and s Schedule Week 1: Microbiology 7/9/12 9:00am
Howard University College of Pharmacy Pharmacy Biomedical Preview Program: Summer 2012 Coordinator: Emmanuel O. Akala, R.Ph., Ph.D. Lead Tutor: Lectures and s Schedule Week 1: Microbiology 7/9/12 9:00am
Chapter 20: Antimicrobial Drugs
 Chapter 20: Antimicrobial Drugs 1. Overview of Antimicrobial Drugs 2. Antibacterial Drugs 3. Antiviral Drugs 4. Drugs for Eukaryotic Pathogens 1. Overview of Antimicrobial Drugs Antibiotics An antibiotic
Chapter 20: Antimicrobial Drugs 1. Overview of Antimicrobial Drugs 2. Antibacterial Drugs 3. Antiviral Drugs 4. Drugs for Eukaryotic Pathogens 1. Overview of Antimicrobial Drugs Antibiotics An antibiotic
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Beta-lactam antibiotics - Cephalosporins
 Beta-lactam antibiotics - Cephalosporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability ypropertiesp Stability to bacterial
Beta-lactam antibiotics - Cephalosporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability ypropertiesp Stability to bacterial
Omega-3 fatty acids improve the diagnosis-related clinical outcome. Critical Care Medicine April 2006;34(4):972-9
 Omega-3 fatty acids improve the diagnosis-related clinical outcome 1 Critical Care Medicine April 2006;34(4):972-9 Volume 34(4), April 2006, pp 972-979 Heller, Axel R. MD, PhD; Rössler, Susann; Litz, Rainer
Omega-3 fatty acids improve the diagnosis-related clinical outcome 1 Critical Care Medicine April 2006;34(4):972-9 Volume 34(4), April 2006, pp 972-979 Heller, Axel R. MD, PhD; Rössler, Susann; Litz, Rainer
Outpatient Parenteral Antimicrobial Therapy
 Outpatient Parenteral Antimicrobial Therapy Jason E. Bowling, MD a,b, *, James S. Lewis II, PharmD c,d, Aaron D. Owens, MD a,e KEYWORDS Outpatient parenteral antimicrobial therapy Antibiotics Adverse events
Outpatient Parenteral Antimicrobial Therapy Jason E. Bowling, MD a,b, *, James S. Lewis II, PharmD c,d, Aaron D. Owens, MD a,e KEYWORDS Outpatient parenteral antimicrobial therapy Antibiotics Adverse events
SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS S7A
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS
Course Outline and Syllabus for Students
 Course Outline and Syllabus for Students Name: Ian Crandall Course Number: PHM242H1 Course Title: Microbiology of Infectious Diseases Course Description: The course provides a brief introduction to the
Course Outline and Syllabus for Students Name: Ian Crandall Course Number: PHM242H1 Course Title: Microbiology of Infectious Diseases Course Description: The course provides a brief introduction to the
ANTIBIOTIC RESISTANCE THREATS. in the United States, 2013
 ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
 Skin and Soft tissue Infections: new bugs, old drugs Disclosure Statement Sponsor: Goodman Photographic Presented by: Dr. Kristopher Wiebe, MD, CCFP (EM) Presented to: BC Chapter, Canadian Society of Hospital
Skin and Soft tissue Infections: new bugs, old drugs Disclosure Statement Sponsor: Goodman Photographic Presented by: Dr. Kristopher Wiebe, MD, CCFP (EM) Presented to: BC Chapter, Canadian Society of Hospital
Etiology and treatment of chronic bacterial prostatitis the Croatian experience
 Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Introduction to Antimicrobial Therapy
 Introduction to Antimicrobial Therapy Christine Kubin, Pharm.D., BCPS Clinical Pharmacist, Infectious Diseases Case #1 L.G. is a 78 yo woman admitted for cardiac cath. 3-vessel disease was identified and
Introduction to Antimicrobial Therapy Christine Kubin, Pharm.D., BCPS Clinical Pharmacist, Infectious Diseases Case #1 L.G. is a 78 yo woman admitted for cardiac cath. 3-vessel disease was identified and
NewYork-Presbyterian Hospital Sites: Columbia University Medical Center Guideline: Medication Use Manual Page 1 of 12
 Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey
 2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey Antibiotic resistance is a global issue that has significant impact in the field of infectious diseases.
2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey Antibiotic resistance is a global issue that has significant impact in the field of infectious diseases.
Phase: IV. Study Period: 20 Jan. 2006-17 Sep. 2008
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
{ Rifaximin versus Nonabsorbable Disaccharides for the Treatment of Hepatic Encephalopathy: A Meta Analysis}
 { Rifaximin versus Nonabsorbable Disaccharides for the Treatment of Hepatic Encephalopathy: A Meta Analysis} {Dong Wu, Shu-Mei Wu, Jie Lu, Ying-Qun Zhou, Ling Xu, and Chuan-Yong Guo} Noor Al-Hakami, Pharm
{ Rifaximin versus Nonabsorbable Disaccharides for the Treatment of Hepatic Encephalopathy: A Meta Analysis} {Dong Wu, Shu-Mei Wu, Jie Lu, Ying-Qun Zhou, Ling Xu, and Chuan-Yong Guo} Noor Al-Hakami, Pharm
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
WARNING LETTER. According to its approved product labeling (PI) (in pertinent part, emphasis original):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) COMMUNITY ACQUIRED vs. HEALTHCARE ASSOCIATED
 METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) COMMUNITY ACQUIRED vs. HEALTHCARE ASSOCIATED Recently, there have been a number of reports about methicillin-resistant Staph aureus (MRSA) infections
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) COMMUNITY ACQUIRED vs. HEALTHCARE ASSOCIATED Recently, there have been a number of reports about methicillin-resistant Staph aureus (MRSA) infections
BSc in Medical Sciences with PHARMACOLOGY
 BSc in Medical Sciences with PHARMACOLOGY Course Director Dr Christopher John Module Leaders Dr Robert Dickinson (Module 1) Dr Anabel Varela Carver (Module 2) Dr Sohag Saleh (Module 3) Course Administrator
BSc in Medical Sciences with PHARMACOLOGY Course Director Dr Christopher John Module Leaders Dr Robert Dickinson (Module 1) Dr Anabel Varela Carver (Module 2) Dr Sohag Saleh (Module 3) Course Administrator
Summary of the risk management plan (RMP) for Sirturo (bedaquiline)
 EMA/16634/2014 Summary of the risk management plan (RMP) for Sirturo (bedaquiline) This is a summary of the risk management plan (RMP) for Sirturo, which details the measures to be taken in order to ensure
EMA/16634/2014 Summary of the risk management plan (RMP) for Sirturo (bedaquiline) This is a summary of the risk management plan (RMP) for Sirturo, which details the measures to be taken in order to ensure
Nursing college, Second stage Microbiology Dr.Nada Khazal K. Hendi L14: Hospital acquired infection, nosocomial infection
 L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
Sepsis Awareness Month
 Aon Kenya Insurance Brokers Ltd Aon Hewitt Healthcare Division Sepsis Awareness Month Issue 11 September 2015 In this Issue 2 Getting to understand Sepsis 3 Stages in Sepsis Advancement 4 Diagnosis & Treatment
Aon Kenya Insurance Brokers Ltd Aon Hewitt Healthcare Division Sepsis Awareness Month Issue 11 September 2015 In this Issue 2 Getting to understand Sepsis 3 Stages in Sepsis Advancement 4 Diagnosis & Treatment
SURGICAL ANTIBIOTIC PROPHYLAXIS. Steve Johnson, PharmD, BCPS Prime Therapeutics, Inc
 SURGICAL ANTIBIOTIC PROPHYLAXIS Steve Johnson, PharmD, BCPS Prime Therapeutics, Inc OBJECTIVES Discuss antibiotic use as prophylaxis vs presumptive therapy vs treatment of infections. Discuss risk factors
SURGICAL ANTIBIOTIC PROPHYLAXIS Steve Johnson, PharmD, BCPS Prime Therapeutics, Inc OBJECTIVES Discuss antibiotic use as prophylaxis vs presumptive therapy vs treatment of infections. Discuss risk factors
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis
 Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
C-Difficile Infection Control and Prevention Strategies
 C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer Adrienne.Mims@AlliantQuality.org 1/18/2016 1 Disclosure This educational activity does not have commercial
C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer Adrienne.Mims@AlliantQuality.org 1/18/2016 1 Disclosure This educational activity does not have commercial
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Tuberculousmeningitis: what is the best treatment regimen?
 Tuberculousmeningitis: what is the best treatment regimen? H S Schaaf Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University
Tuberculousmeningitis: what is the best treatment regimen? H S Schaaf Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University
Summary of the risk management plan (RMP) for Ofev (nintedanib)
 EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Targocid 200mg Teicoplanin 200mg Powder for Injection Targocid 400mg Teicoplanin 400mg Powder for Injection 2 QUALITATIVE AND QUANTITATIVE
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Targocid 200mg Teicoplanin 200mg Powder for Injection Targocid 400mg Teicoplanin 400mg Powder for Injection 2 QUALITATIVE AND QUANTITATIVE
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs
 Journal of Antimicrobial Chemotherapy (2006) 57, 326 330 doi:10.1093/jac/dki463 Advance Access publication 29 December 2005 Adjustment of antibiotic treatment according to the results of blood cultures
Journal of Antimicrobial Chemotherapy (2006) 57, 326 330 doi:10.1093/jac/dki463 Advance Access publication 29 December 2005 Adjustment of antibiotic treatment according to the results of blood cultures
Guidelines for Antimicrobial Stewardship in Hospitals in Ireland. A Strategy for the Control of Antimicrobial Resistance in Ireland
 A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
Hialeah Nursing and Rehabilitation Center Combines Technology and Best Practices to Improve Infection Control Specific to C.diff
 RESEARCH ARTICLE Page 1 of 5 Hialeah Nursing and Rehabilitation Center Combines Technology and Best Practices to Improve Infection Control Specific to C.diff ABSTRACT RB Health Partners, Inc., June 24,
RESEARCH ARTICLE Page 1 of 5 Hialeah Nursing and Rehabilitation Center Combines Technology and Best Practices to Improve Infection Control Specific to C.diff ABSTRACT RB Health Partners, Inc., June 24,
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients.
 Dec QMJ VOL.5 No.8 Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients. Radhi F.Alshaibani* الخلاصھ لا زالت حمى التایفوید سببا مھما من اسباب الامراض وفقدان ساعات العمل
Dec QMJ VOL.5 No.8 Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients. Radhi F.Alshaibani* الخلاصھ لا زالت حمى التایفوید سببا مھما من اسباب الامراض وفقدان ساعات العمل
Cardiovascular diseases. pathology
 Cardiovascular diseases pathology Atherosclerosis Vascular diseases A disease that results in arterial wall thickens as a result of build- up of fatty materials such cholesterol, resulting in acute and
Cardiovascular diseases pathology Atherosclerosis Vascular diseases A disease that results in arterial wall thickens as a result of build- up of fatty materials such cholesterol, resulting in acute and
Mini-Medical School on Infectious Diseases. Session #1 - Basic Science
 Mini-Medical School on Infectious Diseases Session #1 - Basic Science The Microbial World Michael V. Norgard, Ph.D., Chairman Department of Microbiology U.T. Southwestern Medical Center The Microbial World
Mini-Medical School on Infectious Diseases Session #1 - Basic Science The Microbial World Michael V. Norgard, Ph.D., Chairman Department of Microbiology U.T. Southwestern Medical Center The Microbial World
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS
 Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
Staphylococcus aureus Bloodstream Infection Treatment Guideline
 Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
Protein Synthesis Inhibitors
 Frank Lowy Protein Synthesis Inhibitors This lecture discusses a diverse group of antibiotics that are grouped together because they all have a common mechanism of action they are protein synthesis inhibitors.
Frank Lowy Protein Synthesis Inhibitors This lecture discusses a diverse group of antibiotics that are grouped together because they all have a common mechanism of action they are protein synthesis inhibitors.
WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses
 WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses 20 August 2009 Table of contents EXECUTIVE SUMMARY... i Other recommendations...iii 1. INTRODUCTION...
WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses 20 August 2009 Table of contents EXECUTIVE SUMMARY... i Other recommendations...iii 1. INTRODUCTION...
Consequences of Discontinuing Rivaroxaban in Patients with Atrial Fibrillation
 A summary of current literature of interest to pharmacists. March 2013 Alan Hopefl, Pharm.D., Amerinet Clinical Manager Consequences of Discontinuing Rivaroxaban in Patients with Atrial Fibrillation Patients
A summary of current literature of interest to pharmacists. March 2013 Alan Hopefl, Pharm.D., Amerinet Clinical Manager Consequences of Discontinuing Rivaroxaban in Patients with Atrial Fibrillation Patients
Open and Honest Care in your Local Hospital
 Open and Honest Care in your Local Hospital The Open and Honest Care: Driving Improvement Programme aims to support organisations to become more transparent and consistent in publishing safety, experience
Open and Honest Care in your Local Hospital The Open and Honest Care: Driving Improvement Programme aims to support organisations to become more transparent and consistent in publishing safety, experience
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION
 PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
Preoperative Laboratory and Diagnostic Studies
 Preoperative Laboratory and Diagnostic Studies Preoperative Labratorey and Diagnostic Studies The concept of standardized testing in all presurgical patients regardless of age or medical condition is no
Preoperative Laboratory and Diagnostic Studies Preoperative Labratorey and Diagnostic Studies The concept of standardized testing in all presurgical patients regardless of age or medical condition is no
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
TEN MYTHS OF MASTITIS THERAPY. Ron Erskine College of Veterinary Medicine Michigan State University E. Lansing, Michigan
 TEN MYTHS OF MASTITIS THERAPY Ron Erskine College of Veterinary Medicine Michigan State University E. Lansing, Michigan 1. Once a Staph aureus cow, always a Staph aureus cow. Although a difficult therapeutic
TEN MYTHS OF MASTITIS THERAPY Ron Erskine College of Veterinary Medicine Michigan State University E. Lansing, Michigan 1. Once a Staph aureus cow, always a Staph aureus cow. Although a difficult therapeutic
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The Legal Cost of Getting Infection Prevention and Control Wrong
 The Legal Cost of Getting Infection Prevention and Control Wrong Phil Barnes, Associate Anthony Collins LLP What Are Healthcare Associated Infections? Infections acquired as a consequence of receiving
The Legal Cost of Getting Infection Prevention and Control Wrong Phil Barnes, Associate Anthony Collins LLP What Are Healthcare Associated Infections? Infections acquired as a consequence of receiving
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Why Do Some Antibiotics Fail?
 Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2008 (part I)
 ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2008 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus bacteraemia annual report, part I The format
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2008 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus bacteraemia annual report, part I The format
PATIENT INFORMATION LEAFLET. CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN
 PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
MERREM I.V. (meropenem for injection) FOR INTRAVENOUS USE ONLY
 Page 3 MERREM I.V. (meropenem for injection) FOR INTRAVENOUS USE ONLY To reduce the development of drug-resistant bacteria and maintain the effectiveness of MERREM I.V. (meropenem for injection) and other
Page 3 MERREM I.V. (meropenem for injection) FOR INTRAVENOUS USE ONLY To reduce the development of drug-resistant bacteria and maintain the effectiveness of MERREM I.V. (meropenem for injection) and other
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Fungal Infection in Total Joint Arthroplasty. Dr.Wismer Dr.Al-Sahan
 Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
Diagnostics: Page 2 of 5
 Proteinuria Proteinuria is a condition in which there are increased amounts of protein in the urine. There are a number of different diseases which can result in proteinuria. In the early stages of the
Proteinuria Proteinuria is a condition in which there are increased amounts of protein in the urine. There are a number of different diseases which can result in proteinuria. In the early stages of the
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES
 APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009 (part I)
 ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus Bacteraemia Annual Report, Part I The annual
ANNUAL REPORT ON STAPHYLOCOCCUS AUREUS BACTERAEMIA CASES IN DENMARK 2009 (part I) STAPHYLOCOCCUS LABORATORY, STATENS SERUM INSTITUT 1 Staphylococcus aureus Bacteraemia Annual Report, Part I The annual
2012 Anatomy & Physiology C Division. National Competition-Science
 2012 Anatomy & Physiology C Division National Competition-Science Olympiad 1. The alimentary canal includes 8 different parts and 4 accessory organs. Name 5 parts from the alimentary canal and 3 accessory
2012 Anatomy & Physiology C Division National Competition-Science Olympiad 1. The alimentary canal includes 8 different parts and 4 accessory organs. Name 5 parts from the alimentary canal and 3 accessory
