West Virginia Medicaid PDL Recommended Changes Summary Pharmaceutical and Therapeutics Committee Meeting January 26, 2011
|
|
|
- Madeline Wiggins
- 8 years ago
- Views:
Transcription
1 West Virginia Medicaid PDL Recommended Changes Summary Pharmaceutical and Therapeutics Committee Meeting January 26, 2011 TOPIC Current PDL Status ACNE AGENTS, TOPICAL 4/1/11 Planned PDL Status Recommend Grandfather existing users Comments AVAR (sulfur/sulfacetamide) New Dosage Form Non-Preferred N/A ZENCIA WASH (sulfacetamide sodium/sulfur) New Dosage Form Non-Preferred N/A Thirty (30) day trials each of one preferred retinoid and two unique chemical entities in two other subclasses, including the generic version of a requested non-preferred product, are required before a non-preferred agent will be present. (In cases of pregnancy, a trial of retinoids will not be required.) In addition, thirty day trials of combinations of the corresponding preferred single agents available are required before non-preferred combination agents will be authorized. Thirty (30) day trials each of one preferred retinoid and two unique chemical entities in two other subclasses, including the generic version of a requested non-preferred product, are required before a non-preferred agent will be present. (In cases of pregnancy, a trial of retinoids will not be required.) In addition, thirty day trials of combinations of the corresponding preferred single agents available are
2 required before non-preferred combination agents will be authorized. ANGIOTENSIN MODULATORS TEKAMLO (aliskiren/amlodipine) (Auto PA) AMTURNIDE (aliskiren/amlodipine/hct)( Auto PA) New Drug Combination New Drug combination ANTICOAGULANTS Preferred N/A Tekturna HCT, Valturna, Tekamlo or Amturnide will be approved if the criteria for Tekturna is met and the patient needs the other drugs in the combination. PRADAXA (dabigatran) (Auto PA) New Drug Preferred N/A ANTIEMETICS Pradaxa will be approved for the diagnosis of nonvalvular atrial fibrillation. GRANISOL (granisetron) New Dosage Form Non-Preferred N/A A 3-day trial of a preferred agent is required before a non-preferred agent will be present. PA is required for all agents when limits are exceeded. ANTIPARKINSON S AGENTS (ORAL) LODOSYN (carbidopa) New Dosage Form Non-Preferred N/A Patients starting therapy on drugs in this class must show a documented allergy to all of the preferred agents, in the corresponding class, before a non-preferred agent
3 will be authorized. BONE RESORPTION SUPPRESSION AND RELATED AGENTS ATELVIA (risedronate) New Dosage Form Non-Preferred N/A COUGH & COLD/1 ST GENERATION ANTIHISTAMINES A 30-day trial of the preferred agent is required before a non-preferred agent will be approved. codeine/promethazine Preferred Remove from PDL No DELSYM (dextromethorphan) Not Managed Preferred N/A guaifenesin/codeine Preferred Remove from PDL No phenylephrine/codeine/promethazine Preferred Remove from PDL No phenylephrine/phenyltoloxamine/chlorpheniramine Preferred Remove from PDL No phenylephrine/pyrilamine/chlorpheniramine Preferred Remove from PDL No benzonatate capsules Preferred Remove from PDL No HYPERURICEMIA AND GOUT AGENTS Allopurinol Treatment and prevention (Although allopurinol can be used for treatment or prevention of gouty arthritis attacks, it may be necessary to add colchicines for the first few days of treatment. Acute attacks may increase or intensify during the first few days.) Not Managed Preferred N/A A 30-day trial of one of the preferred agents for the prevention of gouty arthritis attacks is required before a non-preferred agent will be approved unless one of the exceptions on the PA form is present. *In the case of acute gouty attacks, a 10-day ( 20 tablets) supply of Colcrys will be approved. colchicine/probenecid (Used for prevention) Not Managed Preferred N/A COLCRYS (colchicine) (Treatment of acute attacks) Not Managed Non-Preferred N/A
4 Probenecid (Used for prevention only) Not Managed Preferred N/A ULORIC (febuxostat) (Treatment and prevention- May also require colchicines in the first few weeks) Not Managed Non-Preferred N/A ZYLOPRIM (allopurinol) (Treatment and prevention) Not Managed Non-Preferred N/A HYPOGLYCEMICS, INCRETIN MIMETICS/ENHANCERS Januvia/Janumet,Onglyza and Kombiglyze XR will be subject to the following clinical edits: KOMBIGLYZE XR (saxagliptin/metformin) New Drug Combination MULTIPLE SCLEROSIS AGENTS Preferred N/A 1. Previous history of a 30day trial of an oral agent (sulfonylurea, thiazolindinedione (TZD) or metformin) 2. Onglyza and Kombiglyze XR will not be approved for concurrent therapy with insulin. 3 Januvia/Janumet will be approved for concurrent therapy with insulin for three month intervals, For authorization, HgBA1C levels must Be 7. Current lab values must submitted.. GILENYA (fingolimod) Preferred Agents are: Interferons Avonex (interferon beta-1a) Betaseron (interferon beta-1b) Rebif (interferon beta-1a) Non-Interferons Copaxone (glatiramer) New Non-Preferred N/A Currrent Criteria: A 30-day trial of a preferred agent will be required before a non-preferred agent will be approved A 30-day trial of the preferred agent will be required before a nonpreferred agent will be approved. *Amypra will be prior authorized if the following
5 A 30-day trial of a preferred agent will be required before a nonpreferred agent will be approved Non-Preferred Agents Interferons Extavia (interferon beta-1b) Non-Interferons AMPYRA (dalfampridine) GILENYA (fingolimod) TYSABRI (natalizumab conditions are met: 1. Diagnosis of multiple sclerosis 2. No history of seizures 3. No evidence of moderate or severe renal impairment 4. Initial prescription will be approved for 30 days only. Tysabri will only be approved for members who are enrolled in the TOUCH Prescribing Program. AP does not apply. PA Criteria for Gilenya: 1) A diagnosis of relapse remitting multiple sclerosis or progressive secondary multiple sclerosis AND 2) Medication is prescribed by a neurologist AND 3) History of thirty (30) trial of one of the preferred agents for multiple sclerosis unless one of the exceptions on the PA form is present AND 4) Dosage is limited to one tablet per day. OPTHALMIC ANTI-INFLAMMATORIES BROMDAY (bromfenac) New Dosage Form Non-Preferred N/A Five (5) day trials of each of the preferred ophthalmic anti-inflammatory agents are required before nonpreferred agents will be
6 present. MISC. BRAND/GENERIC BEYAZ (ethinyl estradiol/drospirenone/levomefolate) Other agents in this sub-category are: LO SEASONIQUE (ethinyl estradiol/levonorgestrel) SEASONIQUE (ethinyl estradiol/levonorgestrel) YASMIN (ethinyl estradiol/drospirenone) New Drug Non-Preferred N/A A thirty (30) day trial of each preferred unique chemical entity in the corresponding therapeutic category is required before a nonpreferred agent will be authorized.
Drug Utilization Review Board Meeting Minutes March 2, 2011
 Drug Utilization Review Board Meeting Minutes March 2, 2011 The West Virginia Medicaid Drug Utilization Review (DUR) Board meeting was called to order with the following in attendance: Members Present:
Drug Utilization Review Board Meeting Minutes March 2, 2011 The West Virginia Medicaid Drug Utilization Review (DUR) Board meeting was called to order with the following in attendance: Members Present:
Alla chme nl A EFFECTIVE 07/01/2014 BUREAU FOR MEDICAL SERVICES WEST VIRGINIA MEDICAID PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA
 A. Re-Review 1. Bethkis ANTIBIOTICS, INHALED BETHKIS (tobramycin) TOBI (tobramycin) 2. Effient CAYSTON (aztreonam) TOBI POOHALER tobramycin PLATELET AGGREGATION INHIBITORS AGGRENOX (dipyridamole/asa) BRIUNTA
A. Re-Review 1. Bethkis ANTIBIOTICS, INHALED BETHKIS (tobramycin) TOBI (tobramycin) 2. Effient CAYSTON (aztreonam) TOBI POOHALER tobramycin PLATELET AGGREGATION INHIBITORS AGGRENOX (dipyridamole/asa) BRIUNTA
Study Support Materials Cover Sheet
 Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Can an administrative drug claims database be used to understand claimant drug utilization?
 Can an administrative drug claims database be used to understand claimant drug utilization? By Elaine McKenzie, BSP, MBA, Consultant, TELUS Health Analytics Elaine McKenzie is a consultant who works with
Can an administrative drug claims database be used to understand claimant drug utilization? By Elaine McKenzie, BSP, MBA, Consultant, TELUS Health Analytics Elaine McKenzie is a consultant who works with
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Multiple Sclerosis Agents
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Multiple Sclerosis Agents A. Prescriptions That Require Prior Authorization Prescriptions
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Multiple Sclerosis Agents A. Prescriptions That Require Prior Authorization Prescriptions
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants
 Brochure More information from http://www.researchandmarkets.com/reports/1841621/ Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants Description: Introduction Beginning with
Brochure More information from http://www.researchandmarkets.com/reports/1841621/ Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants Description: Introduction Beginning with
Three years ago, the mantra for
 MANAGING MS: Trends, Issues, and Perspectives The entry of oral medications for multiple sclerosis into the marketplace and the high cost of biologic drugs are presenting new challenges for stakeholders.
MANAGING MS: Trends, Issues, and Perspectives The entry of oral medications for multiple sclerosis into the marketplace and the high cost of biologic drugs are presenting new challenges for stakeholders.
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Conflict of Interest Declaration. Overview of New Medications for Multiple Sclerosis. Assessment Question. Objectives 4/1/2011
 Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Tysabri
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Newer Anticoagulants and Newer Diabetic Drug Classes. Nicole N. Nguyen, PharmD Senior Clinical Pharmacist Health Care Services August 21, 2013
 Newer Anticoagulants and Newer Diabetic Drug Classes Nicole N. Nguyen, PharmD Senior Clinical Pharmacist Health Care Services August 21, 2013 Apixaban Newer Anticoagulants Dabigatran etexilate Rivaroxaban
Newer Anticoagulants and Newer Diabetic Drug Classes Nicole N. Nguyen, PharmD Senior Clinical Pharmacist Health Care Services August 21, 2013 Apixaban Newer Anticoagulants Dabigatran etexilate Rivaroxaban
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Multiple Sclerosis. Current and Future Players. GDHC1009FPR/ Published March 2013
 Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE June 22, 2015 SUBJECT EFFECTIVE DATE MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical
ISSUE DATE June 22, 2015 SUBJECT EFFECTIVE DATE MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
The rising cost of prescription drugs to treat multiple sclerosis in upstate New York
 T H E F A C T S A B O U T The rising cost of prescription drugs to treat multiple sclerosis in upstate New York Per-person mean annual prescription drug cost to treat multiple sclerosis Annual drug cost
T H E F A C T S A B O U T The rising cost of prescription drugs to treat multiple sclerosis in upstate New York Per-person mean annual prescription drug cost to treat multiple sclerosis Annual drug cost
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants which meet any of the following conditions
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants which meet any of the following conditions
PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022. Multiple
 Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
Literature Scan: Oral Multiple Sclerosis Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
this 7^ day of September 2014 by Randall S. Gregg Special Deputy Director
 r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
PL CE LIVE December 2011 Forum
 December 2011 PL CE LIVE Kristin W. Weitzel, Pharm.D., CDE, FAPhA Associate Editor Pharmacist s Letter/Prescriber s Letter CE Information Pharmacist's Letter / Therapeutic Research Center is accredited
December 2011 PL CE LIVE Kristin W. Weitzel, Pharm.D., CDE, FAPhA Associate Editor Pharmacist s Letter/Prescriber s Letter CE Information Pharmacist's Letter / Therapeutic Research Center is accredited
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Resources for the Primary Care Provider. Please print these out for reference
 Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
全 球 多 发 性 硬 化 症 治 疗 市 场 研 究 报 告 Multiple Sclerosis Therapeutics - A Global Strategic Business Report
 /wepdwukltuyot 全 球 多 发 性 硬 化 症 治 疗 市 场 研 究 报 告 Multiple Sclerosis Therapeutics - A Global Strategic Business Report 联 系 购 买 电 话 :010-82863480 公 司 名 称 : 佐 思 信 息 公 司 地 址 : 北 京 市 海 淀 区 苏 州 街 18 号 院 长 远 天
/wepdwukltuyot 全 球 多 发 性 硬 化 症 治 疗 市 场 研 究 报 告 Multiple Sclerosis Therapeutics - A Global Strategic Business Report 联 系 购 买 电 话 :010-82863480 公 司 名 称 : 佐 思 信 息 公 司 地 址 : 北 京 市 海 淀 区 苏 州 街 18 号 院 长 远 天
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
MSTAC Initial Application
 MSTAC Initial Application Please send applications to: Facsimile 04 916 7571 Further Contact Details: Address The Co-ordinator MSTAC PHARMAC P O Box 10-254 WELLINGTON Phone 04 460 4990 Email mstaccoordinator@pharmac.govt.nz
MSTAC Initial Application Please send applications to: Facsimile 04 916 7571 Further Contact Details: Address The Co-ordinator MSTAC PHARMAC P O Box 10-254 WELLINGTON Phone 04 460 4990 Email mstaccoordinator@pharmac.govt.nz
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
AUBAGIO. Step Therapy Criteria Health Choice Generations Formulary ID: 15179 Version 19 Effective Date: 11/1/2015. PRODUCT(s) AFFECTED AUBAGIO
 AUBAGIO AUBAGIO Claim will pay automatically for AUBAGIO if enrollee has a paid claim for at least a 1 days supply of COPAXONE, REBIF, TYSABRI, BETASERON OR EXTAVIA in the past 365 days. Otherwise, AUBAGIO
AUBAGIO AUBAGIO Claim will pay automatically for AUBAGIO if enrollee has a paid claim for at least a 1 days supply of COPAXONE, REBIF, TYSABRI, BETASERON OR EXTAVIA in the past 365 days. Otherwise, AUBAGIO
How to S.E.A.R.C.H. for the Right MS Therapy for You!
 How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
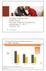 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis Treatment Experience Questionnaire (MSTEQ)
 Multiple Sclerosis Treatment Experience Questionnaire (MSTEQ) Name: Today s date (mm/dd/yy): It is important that you take your as prescribed by your physician. However, from to you may find it difficult
Multiple Sclerosis Treatment Experience Questionnaire (MSTEQ) Name: Today s date (mm/dd/yy): It is important that you take your as prescribed by your physician. However, from to you may find it difficult
Patient Group Input to CADTH
 Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
VA Premier CompleteCare Drugs that Require Step Therapy Last Updated: 09/23/2014
 Atelvia Atelvia Claim will pay automatically for Atelvia if enrollee has a paid claim for at least a 1 days supply of alendronate in the past 365 days. Otherwise, Atelvia requires a step therapy exception
Atelvia Atelvia Claim will pay automatically for Atelvia if enrollee has a paid claim for at least a 1 days supply of alendronate in the past 365 days. Otherwise, Atelvia requires a step therapy exception
ACTEMRA. Step Therapy Criteria HEALTH CHOICE EXCHANGE 2016 Effective Date: 01/01/2016. PRODUCT(s) AFFECTED ACTEMRA
 ACTEMRA ACTEMRA Claim will pay automatically for Actemra if enrollee has a paid claim for at least a 1 days supply of Enbrel and Humira in the past 365 days. Otherwise, Actemra requires a step therapy
ACTEMRA ACTEMRA Claim will pay automatically for Actemra if enrollee has a paid claim for at least a 1 days supply of Enbrel and Humira in the past 365 days. Otherwise, Actemra requires a step therapy
The MS Disease- Modifying Drugs. Gener al information
 The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market
 A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
Aubagio. AgeWell Drugs that Require Step Therapy Last Updated: 08/08/2014. Products Affected. Details AUBAGIO TAB 14MG AUBAGIO TAB 7MG
 Aubagio AUBAGIO TAB 14MG AUBAGIO TAB 7MG Claim will pay automatically for AUBAGIO if enrollee has a paid claim for at least a 1 days supply of COPAXONE, REBIF, TYSABRI, BETASERON OR EXTAVIA in the past
Aubagio AUBAGIO TAB 14MG AUBAGIO TAB 7MG Claim will pay automatically for AUBAGIO if enrollee has a paid claim for at least a 1 days supply of COPAXONE, REBIF, TYSABRI, BETASERON OR EXTAVIA in the past
SOUTH TAMPA MULTIPLE SCLEROSIS CENTER PATIENT/ CARE GIVER QUESTIONNAIRE
 SOUTH TAMPA MULTIPLE SCLEROSIS CENTER PATIENT/ CARE GIVER QUESTIONNAIRE DEMOGRAPHIC INFORMATION Patient Name: Date: Address: City: State: Zip Code Best Phone Number: Marital Status Phone (H): (W) (Cell):
SOUTH TAMPA MULTIPLE SCLEROSIS CENTER PATIENT/ CARE GIVER QUESTIONNAIRE DEMOGRAPHIC INFORMATION Patient Name: Date: Address: City: State: Zip Code Best Phone Number: Marital Status Phone (H): (W) (Cell):
PATIENT / VISIT INFORMATION PATIENT INFORMATION
 PATIENT / VISIT INFORMATION PATIENT INFORMATION Name of Patient: Date of Birth: Date of Visit: VISIT INFORMATION Please complete this form in its entirety, and present it to the registration desk when
PATIENT / VISIT INFORMATION PATIENT INFORMATION Name of Patient: Date of Birth: Date of Visit: VISIT INFORMATION Please complete this form in its entirety, and present it to the registration desk when
Medications for MULTIPLE SCLEROSIS Student Version
 Medications for MULTIPLE SCLEROSIS Student Version DEH 2300 Valencia Community College Summer: 2006-14 Sandra C. Pendergraft DEFINITION / PATHOPHYSIOLOGY Chronic, frequently progressive disease of CNS
Medications for MULTIPLE SCLEROSIS Student Version DEH 2300 Valencia Community College Summer: 2006-14 Sandra C. Pendergraft DEFINITION / PATHOPHYSIOLOGY Chronic, frequently progressive disease of CNS
Resources for the Patient. Please print these out and give them to your patients with MS
 Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
06/06/2012. The Impact of Multiple Sclerosis in the Pacific Northwest. James Bowen, MD. Swedish Neuroscience Institute
 The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
MEDICATION(S) SUBJECT TO STEP THERAPY
 ACE/ARB COMBO AZOR 5-20 MG TABLET, AZOR 5-40 MG TABLET, BENICAR HCT, MICARDIS HCT, TARKA, TEKTURNA HCT, TELMISARTAN-HYDROCHLOROTHIAZID, TRIBENZOR Claims for formulary step 2 ACE Inhibitor combination products
ACE/ARB COMBO AZOR 5-20 MG TABLET, AZOR 5-40 MG TABLET, BENICAR HCT, MICARDIS HCT, TARKA, TEKTURNA HCT, TELMISARTAN-HYDROCHLOROTHIAZID, TRIBENZOR Claims for formulary step 2 ACE Inhibitor combination products
Multiple Sclerosis Step Therapy and Quantity Limit Criteria
 Multiple Sclerosis Step Therapy and Quantity Limit Criteria Tysabri (natalizumab) will NOT be included in this step therapy program for Blue Cross and Blue Shield of Illinois because this plan does not
Multiple Sclerosis Step Therapy and Quantity Limit Criteria Tysabri (natalizumab) will NOT be included in this step therapy program for Blue Cross and Blue Shield of Illinois because this plan does not
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Incretin Mimetic/Enhancer Hypoglycemics (formerly referred to as Other Hypoglycemics) A. Thresholds for Prior Authorization All prescriptions
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Incretin Mimetic/Enhancer Hypoglycemics (formerly referred to as Other Hypoglycemics) A. Thresholds for Prior Authorization All prescriptions
PDL Excerpts Illustrating Proposed Changes
 PDL Excerpts Illustrating Proposed Changes Antidepressants, Other (SNRIs) o Duloxetine added as a preferred agent PREFERRED venlafaxine ER capsules duloxetine capsules SNRIS AP desvenlafaxine ER desvenlafaxine
PDL Excerpts Illustrating Proposed Changes Antidepressants, Other (SNRIs) o Duloxetine added as a preferred agent PREFERRED venlafaxine ER capsules duloxetine capsules SNRIS AP desvenlafaxine ER desvenlafaxine
Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary
 Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary OBJECTIVE The intent of the Multiple Sclerosis (MS) Agents Step Therapy (ST) program is to encourage the use of preferred multiple
Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary OBJECTIVE The intent of the Multiple Sclerosis (MS) Agents Step Therapy (ST) program is to encourage the use of preferred multiple
SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary
 July 1, 2013 Bulletin #141 Drug Plan and Extended Benefits Branch ISSN 1923-0761 SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary Product DIN Pre-Markup ($) New
July 1, 2013 Bulletin #141 Drug Plan and Extended Benefits Branch ISSN 1923-0761 SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary Product DIN Pre-Markup ($) New
Prior Authorization of buprenophine/naloxone (Suboxone ) or buprenorphine (Subutex )
 June 2010 April 2009 Prior Authorization of buprenophine/naloxone (Suboxone ) or buprenorphine (Subutex ) Effective August 1, West Virginia Medicaid will require prior authorization for all Suboxone and
June 2010 April 2009 Prior Authorization of buprenophine/naloxone (Suboxone ) or buprenorphine (Subutex ) Effective August 1, West Virginia Medicaid will require prior authorization for all Suboxone and
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Decisions relating to Multiple Sclerosis treatments
 10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Integrating New Treatments: A Case Based Approach
 Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
Personalised Medicine in MS
 Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
The Basics of Pharmacy Benefits Management (PBM) 2009
 The Basics of Pharmacy Benefits Management (PBM) 2009 Andrew Kingery Pharmacy Account Management Virginia CE Forum 2009 Course# 201719 Objectives & Introduction Provide basic components of a PBM Define
The Basics of Pharmacy Benefits Management (PBM) 2009 Andrew Kingery Pharmacy Account Management Virginia CE Forum 2009 Course# 201719 Objectives & Introduction Provide basic components of a PBM Define
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Review Date: March 2012. Issue Status: Approved Issue No: 2 Issue Date: March 2010
 Title: Multiple Sclerosis guidelines for the use of beta-interferon, glatiramer acetate, natalizumab, mitoxantrone and other disease Authors Name: Dr P Talbot Contact Name: Dr Paul Talbot Contact Phone
Title: Multiple Sclerosis guidelines for the use of beta-interferon, glatiramer acetate, natalizumab, mitoxantrone and other disease Authors Name: Dr P Talbot Contact Name: Dr Paul Talbot Contact Phone
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd.
 teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Multiple Sclerosis Agents Page 1 of 13 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Multiple Sclerosis Agents Tysabri (natalizumab) medical policy
Multiple Sclerosis Agents Page 1 of 13 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Multiple Sclerosis Agents Tysabri (natalizumab) medical policy
Global Multiple Sclerosis Market: Trends and Opportunities (2013-18)
 Global Multiple Sclerosis Market: Trends and Opportunities (2013-18) Scope of the Report The report titled Global Multiple Sclerosis Market: Trends & Opportunities (2013-18) provides an insight into the
Global Multiple Sclerosis Market: Trends and Opportunities (2013-18) Scope of the Report The report titled Global Multiple Sclerosis Market: Trends & Opportunities (2013-18) provides an insight into the
Updates to the Alberta Human Services Drug Benefit Supplement
 Updates to the Alberta Human Services Drug Benefit Supplement Effective December 1, 2014 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone
Updates to the Alberta Human Services Drug Benefit Supplement Effective December 1, 2014 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone
Multiple Sclerosis. Global Drug Forecast and Market Analysis to 2022 Event-Driven Update. GDHC34PIDR / Published April 2013
 Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents
 Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER Objectives List
Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER Objectives List
Uncertainty in Benefit and Risk: Tysabri (natalizumab)
 Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
SPECIAL AUTHORIZATION GUIDELINES
 91B ALBERTA DRUG BENEFIT LIST SPECIAL AUTHORIZATION GUIDELINES 37BSpecial Authorization Policy 90BDrug Products Eligible for Consideration by Special Authorization Drug Products may be considered for coverage
91B ALBERTA DRUG BENEFIT LIST SPECIAL AUTHORIZATION GUIDELINES 37BSpecial Authorization Policy 90BDrug Products Eligible for Consideration by Special Authorization Drug Products may be considered for coverage
The High Prices of Prescription Drugs Increase Costs for Everyone
 The High Prices of Prescription Drugs Increase Costs for Everyone Individuals, Families, States, Taxpayers All Face Higher Costs Due to Rising Drug Prices Prescription drugs are one of the major drivers
The High Prices of Prescription Drugs Increase Costs for Everyone Individuals, Families, States, Taxpayers All Face Higher Costs Due to Rising Drug Prices Prescription drugs are one of the major drivers
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
