Intravenous cefazolin plus oral probenecid vs. oral cephalexin for the treatment of cellulitis: a randomized controlled trial STUDY PROTOCOL
|
|
|
- Bryan Page
- 8 years ago
- Views:
Transcription
1 Intravenous cefazolin plus oral probenecid vs. oral cephalexin for the treatment of cellulitis: a randomized controlled trial STUDY PROTOCOL Co-Primary Investigators: Dawn Dalen, BSP, ACPR, PharmD Clinical Pharmacist Specialist Emergency Medicine, Kelowna General Hospital, Kelowna, BC, Canada. Clinical Assistant Professor, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC, Canada. Peter J. Zed, BSc., BSc.(Pharm), ACPR, PharmD, FCSHP Clinical Coordinator, Department of Pharmacy & Pharmacotherapeutic Specialist - Emergency Medicine, Queen Elizabeth II Health Sciences Centre, Capital Health; Associate Professor, College of Pharmacy & Department of Emergency Medicine, Dalhousie University, Halifax, NS, Canada Associate Investigators: Denise Sprague, BSc. (Pharm), ACPR, PharmD Clinical Pharmacist Specialist Infectious Diseases, Kelowna General Hospital, Kelowna, BC, Canada. Clinical Instructor, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC, Canada. Jeffrey Eppler, FRCP(C) Emergency Medicine Physician, Department of Emergency Medicine, Kelowna General Hospital, Kelowna, BC, Canada
2 Samuel G. Campbell, MB BCh, CCFP(EM), Dip PEC (SA), CHE Associate Professor, Department of Emergency Medicine, Dalhousie University, Chief, Department of Emergency Medicine, Queen Elizabeth II Health Sciences Centre, Halifax, NS, Canada (ID number: NCT ) Final Dec 22, 2009 DDJan 19,
3 Research Significance This project will evaluate the efficacy and safety of oral cephalexin compared to intravenous cefazolin plus oral probenecid for the treatment of uncomplicated skin and soft tissue infections (SSTIs), in patients that present to the Emergency Department (ED). We will attempt to identify if this patient population, which would under current Canadian practice patterns, be treated as ambulatory patients receiving cefazolin and probenecid, are able to be managed with oral antibiotics at home. It is our belief that, by establishing the equivalent efficacy and safety of these two antimicrobial regimens that in patients who present to the ED with SSTIs many more patients will be successfully treated at home rather than need to return to the ED daily. This will allow many patients to be treated without disruption of their normal daily activities (e.g. work, school) and will potentially reduce the overwhelming burden of ED overcrowding and overall healthcare costs. Results of our study will be used to inform patients, clinicians, researchers and policy makers on optimizing care for patients with mild-moderate SSTIs and ultimately to improve patient care throughout Canada. Final Dec 22, 2009 DDJan 19,
4 Project Abstract Skin and soft tissue infections (SSTIs) are a common reason for presentation to an Emergency Department (ED) in Canada. Although many patients with mild SSTI are able to be managed at home with oral antibiotics, those with mild-moderate infections are often treated with parenteral antibiotics. Current practice patterns in Canadian EDs indicate this patient population is often treated with intravenous cefazolin once daily along with oral probenecid and return to the ED or other ambulatory setting for daily medication administration and assessment. This parenteral regimen has been found to result in success rates comparable to studies which have evaluated treatment success with oral antibiotics in this patient population (89-97%). Although successful outcome can be achieved with this approach, it is often inconvenient for the patient to return to the ED/ambulatory care unit daily and does contribute to overall ED/ambulatory care visit volumes and overall health care costs. Unfortunately, there has never been a study which has evaluated the relative efficacy and safety or oral antibiotics to the aforementioned parenteral approach in this patient population and thus there remains a significant knowledge gap which must be addressed before a change in current practice can be explored. The objectives of the study is to determine whether oral cephalexin is equivalent to intravenous cefazolin plus oral probenecid for the treatment of uncomplicated SSTIs in patients that present to the ED. This study will be a prospective, multi-centered, randomized controlled non-inferiority trial comparing cephalexin 500 mg orally four times to cefazolin 2 g IV plus probenecid 1 g orally, in patients presenting to the ED with presumed diagnosis of mild to moderate SSTI. The primary outcome will be to compare the proportion of patients failing therapy for their cellulitis after 72 hours of antibiotic treatment with oral cephalexin or IV cefazolin/oral probenecid 1 g daily. Secondary outcomes include the clinical cure rate at 7 days, percentage of patients requiring hospital admission, percentage of patients stepped down to oral antibiotics on or before day 7 of therapy, percentage of patients requiring an additional antibiotic prescription on day 7, and the frequency of adverse events. Final Dec 22, 2009 DDJan 19,
5 Background and Rationale: Skin and soft tissue infections (SSTIs) include infections in any layer of the skin, subcutaneous tissue, fat and muscle. Cellulitis is the most common type of SSTI defined as an infection of the dermis and subcutaneous tissue that is characterized by symptoms such as pain, tenderness, swelling, induration, purulent drainage or discharge, erythema, and/or localized warmth. Grampositive cocci are the causal pathogens in the majority of cellulitis cases, specifically Staphylococcus aureus and streptococci.[1,2,3] The prevalence of cellulitis is difficult to measure and published data is limited. Approximately 7-10% of hospitalizations in North America are a result of skin and soft tissue infections [4] and the estimated cellulitis (ICD-9 codes ) incidence rate is 24.6/1000 person-years with an increased prevalence in males and in patients aged 45 to 64.[5,6] An estimated 20.5% of cellulitis cases are seen in acute care settings such as EDs and outpatient hospitals.[6] Although many patients with mild SSTI are able to be managed at home with oral antibiotics, those with mild-moderate infections are often treated with parenteral antibiotics. Current practice patterns in Canada EDs indicate this patient population is often treated with intravenous cefazolin once daily along with oral probenecid and return to the ED or other ambulatory setting for daily medication administration and assessment.[7] Although cefazolin is traditionally administered every 8 hours, the use of probenecid allows for once daily administration of cefazolin by inhibiting renal secretion, slowing renal elimination and prolonging the period of therapeutic serum concentrations. Brown et al. found that serum concentrations of cefazolin achieved following probenecid administration were sufficient at 24 hours to exceed the minimum inhibitory concentration (MIC) of cefazolin for Staphylococcus aureus and streptococci, and at 12 hours to exceed the MIC for Gram-negative pathogens normally sensitive to cefazolin, such as Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis. [8] A small pharmacokinetic study was then conducted comparing cefazolin 2 g IV every 8 hours or 2 g IV once daily with Final Dec 22, 2009 DDJan 19,
6 probenecid 500 mg orally four times daily, where they found that there was no significant difference between peak cefazolin concentrations on day 1 and 5.[9] A systematic review of the literature has been previously performed by a member of the investigative team.[10] There have been only two studies performed to date which have evaluated clinical outcomes in patients treated with cefazolin and probenecid for the management of SSTI. Brown et al performed a prospective, randomized, double-blind study involving 194 patients compared the efficacy of ceftriaxone to cefazolin with probenecid in the treatment of SSTIs.[11] Results indicated there was no significant difference in failure rates between the two treatment arms between ceftriaxone and cefazolin/probenecid at 7% and 8%, respectively (p=ns). In a second study by Grayson et al. performed a prospective, randomized, double-blinded study in 132 patients to evaluate the efficacy of once-daily cefazolin with probenecid compared to ceftriaxone for the management of moderate-severe cellulitis.[12] Patients were given 1 g probenecid or placebo orally, followed minutes later with 2 g IV cefazolin or ceftriaxone, respectively. A clinical cure was achieved at the end of therapy in 51 of 59 cases (86%) treated with cefazolin plus probenecid and 55 of 57 cases (96%) treated with ceftriaxone plus placebo, p=ns. This parenteral regimen of single daily cefazolin and probenecid has been found to result in success rates which are comparable to studies that have evaluated treatment success with oral antibiotics in this patient population (89-97%).[2,13,14,15] Although successful outcome can be achieved with this approach, it is often inconvenient for the patient to return to the ED/ambulatory care unit daily and does contribute to overall ED/ambulatory care visit volumes and overall health care costs. Unfortunately, there has never been a study which has evaluated Final Dec 22, 2009 DDJan 19,
7 the relative efficacy and safety of oral antibiotics to the aforementioned parenteral approach in this patient population and thus there remains a significant knowledge gap which must be addressed before a change in current practice can be explored. Research Question The question this study will seek to answer is: Is cephalexin 500 mg orally four times daily equivalent to cefazolin 2 g IV daily plus probenecid 1 g orally daily in the management of uncomplicated mild-moderate skin and soft tissue infections in patients presenting to the emergency department? Objectives Primary Objective To compare the proportion of patients failing antibiotic therapy after 72 hours of treatment between the oral cephalexin and intravenous cefazolin plus probenecid arms. Secondary Objectives To compare the cure rate of antibiotic therapy at 7 days between the oral cephalexin and intravenous cefazolin plus probenecid arms. To compare the percentage of patients requiring hospital admission between the two study groups, at any point during the study. To compare the number of patients stepped down to oral therapy over the study period, between the two treatment arms. Final Dec 22, 2009 DDJan 19,
8 To compare between the two studies arms, the number of patients requiring an additional prescription, prescription type and why they are requiring the prescription on day 7 of therapy. To compare the frequency of adverse events during the study period in each arm. Methodology Setting and Design The study will be a prospective, multi-center, double-blind, randomized controlled non-inferiority trial evaluating oral cephalexin compared to intravenous cefazolin and oral probenecid for the treatment of mild-moderate cellulitis in patients presenting to the ED. The study will be conducted in the ED at both the Kelowna General Hospital (KGH) and Queen Elizabeth II Health Sciences Center (QEII HSC) Halifax Infirmary (HI). KGH is a 350 bed tertiary care teaching hospital affiliated with the University of British Columbia. The KGH ED has an annual ED census of 56,000 patients. The QEII HSC is a 1034-bed adult tertiary care and referral center within Capital Health and teaching hospital affiliated with Dalhousie University. The HI has an annual ED census of 62,000 patient visits. Ethics approval for this study is currently being sought through the Interior Health Research Ethics Board, the University of British Columbia Clinical Research Ethics Board and the Capital Health Research Ethics Board. Patient consent will be sought for eligible patients. (See Appendix IV) Patient Selection Patients will be considered for inclusion into this study if they present to KGH or QEII HSC ED Monday to Friday between 0800h-1600h with presumed diagnosis of mild-moderate SSTI and they are deemed well enough to be treated as outpatients. The planned recruitment period is Final Dec 22, 2009 DDJan 19,
9 May to October 2010 which, based on historical data from both centers will allow for sufficient time to enrol the desired sample size. There are no known validated grading tools to determine the severity of a specific patients cellulitis. Due to this factor, this trial was designed to closely mirror real life practice where there is some inter-physician variability in determining the severity of cellulitis and subsequent treatment. To decrease this variability we have used objective criteria to exclude patients with the most mild and most severe cases of cellulitis. For example, patients who require only oral antibiotics (mild cellulitis) and patients who meet the criteria for sepsis (severe cellulitis) will be excluded. Inclusion criteria include, (i) males and females 19 years of age or older; (ii) patients having 2 or more of the following symptoms: pain/tenderness, fever, swelling, erythema, localized warmth, purulent drainage/discharge, induration, regional lymph node swelling or tenderness, and/or extension of redness; and (iii) believed to be secondary to an infection; (iv) can be treated as an outpatient and return daily to the Emergency Department.[13] Exclusion criteria include: (i) patients in which oral antibiotic therapy are indicated but intravenous antibiotics are not requiredknown allergy to study drugs; (ii) known chronic kidney disease with a creatinine clearance <30 ml/min; (iii) known previous methicillin-resistant staphylococcus aureus (MRSA) infection; (iv) use of antibiotics for greater than 24 hours in the past 7 days prior to study enrolment; (v) wound/abscess requiring operative debridement or incision and drainage; (vi) suspected necrotizing fasciitis, osteomyelitis or septic arthritis; (vii) febrile neutropenia; (vii) concomitant documented bacteremia; (viii) two or more signs of systemic sepsis: temperature >38 C or <36 C; heart rate >90 beats/min; respiratory rate >20 breaths/min; WBC <4,000 cells/mm³ or >12,000 cells/mm³ or >10% band forms (if available, but not necessary); (ix) new altered mental status; (x) infections at a site involving prosthetic materials; (xi) animal or human bite wound infections; (xii) post-operative wound infections; (xiii) known peripheral vascular Final Dec 22, 2009 DDJan 19,
10 disease; (xiv) superficial thrombophlebitis; (xv) pregnant/breastfeeding; (xvi) obesity (BMI > 30 kg/m 2 ); (xvii) known allergy to study medication.. Intervention Eligible patients will be randomized to one of two treatment groups using a double-dummy blind approach. One treatment group will receive therapy with cephalexin 500 mg orally four times daily plus saline IV and oral probenecid placebo daily and the second treatment group will receive therapy with intravenous cefazolin 2 g IV plus probenecid 1 g daily plus cephalexin placebo orally four times daily. Probenecid or probenecid placebo will be administered minutes prior to the administration of cefazolin. Cefazolin will be administered by slow IV bolus over 3-5 minutes. Outcome Measures The primary outcome of this study is the proportion of patients failing therapy after 72 hours of antibiotic treatment with oral cephalexin or intravenous cefazolin plus oral probenecid. Failure is defined as hospital admission, change in antibiotics (not due to allergy), or persistent or worsening signs and symptoms of cellulitis following at least 72 hours of antibiotic therapy, to be assessed between 72 and 96 hours after antibiotic therapy is started. Hospital admission or a change in antibiotics (not due to allergy) less than 72 h after the initial ED visit will also constitute treatment failure. Secondary outcomes include the clinical cure rate of cellulitis after 7 days of antibiotic therapy, The percentage of patients requiring hospital admission at any point during the study, the percentage of patients stepped down on or before day 7 of therapy, the percentage of patients requiring an additional antibiotic prescription, what antibiotic and why on day 7, and the frequency of adverse events in the study population during the study period. Final Dec 22, 2009 DDJan 19,
11 Study Procedures Eligible patients presenting to the ED with presumed SSTI who are well enough to be treated as outpatients, will be approached for enrolment. A research assistant will obtain written informed consent on the form found in Appendix IV. Patients who do not give written consent or those who meet any one or more of the exclusion criteria will not be enrolled in the study. During the initial ED visit, each patient that is enrolled will be assigned a unique study number. Study numbers will be randomized in blocks of eight and stratified according to each center to either treatment group via a randomized computer sequence prior to the start of the study by the KGH pharmacy manager who will keep this information in a sealed enveloped confidential locked in a filing cabinet within the pharmacy department. The respective in-patient pharmacy will be informed of each patient s study number upon enrolment and will dispense the corresponding treatment box with the intravenous medication prepared, labelled only with the study number. Patient demographics and clinical data will be collected using a standardized data collection form. Data collected will include: (i) recruitment site; (ii) age; (iii) gender; (iv) weight; (v) past medical history; (vi) current medications; (vii) location of cellulitis; (viii) duration of infection prior to ED visit; (ix) any antibiotic therapy in the last 7 days; (x) social history (recreational drug use, alcohol); (xi) IV drug use; (xii) homelessness; (xiii) previous contact with the health care system over the past year. To preserve confidentiality and anonymity, the data collection form will be labelled only with the study number assigned to the patient upon enrolment and will not contain patient identifiers, including patient initials or medical record number. Collected data will be stored in binders that will be kept in a locked cabinet in a locked office that only the investigators and research assistant have access. Completed consent forms will also be stored in these binders. Any data Final Dec 22, 2009 DDJan 19,
12 that will be electronically recorded, such as information being transferred to an Excel spreadsheet, will be saved in a file folder with select permissions for the investigators only. Both print and electronic data will be preserved for a minimum of years in these locations. The study investigators will destroy the data after years, by shredding the files or doubledeleting the information from the computer. Study drug boxes for both treatment groups will be prepared by the study investigators at a single site (KGH) and then those required will be shipped to QEII HSC. The drug boxes for the first treatment group (A) will contain enough drug for 7 days of therapy with cephalexin 500 mg four times daily as well as for 7 days of placebo IV cefazolin and placebo probenecid daily. The drug boxes for the second treatment group (B) will contain enough drugs for 7 days of therapy with 2g IV cefazolin and 1 g probenecid daily as well as for 7 days of placebo cephalexin four times daily. The cefazolin and placebo syringes as well as the patient s vial of probenecid or placebo capsules will be kept in the minor treatment fridge in the respective emergency departments for the duration of the patient s treatment. At the initial visit, 28 tablets of cephalexin 500 mg (group A) or placebo (group B) will be given to the patient in a vial to self-administer at home four times daily (Figure 1). Patients will be required to return to the Emergency Department daily for assessment and treatment with IV placebo with oral placebo (group A) or IV cefazolin and oral probenecid (group B). Patients will be instructed to bring in all medication with them every day to the emergency department. The number of tablets left in the vial at study completion will be assessed by the research assistant or study investigator. Daily intravenous treatment and assessment of signs and symptoms will continue until 72 hours have passed from the first dose of study drug. If, during any assessment occurring in the first 72 Final Dec 22, 2009 DDJan 19,
13 hours, the managing physician feels that a change in antibiotic therapy or hospital admission is required, the patient will be considered a treatment failure and will be unblinded from the trial. In all other cases, clinical success and failure will be assessed during the ED visit occurring between 72 and 96 hours after the first study dose. If the patient has persistent or worsening signs and symptoms, or requires hospital admission or a change in antibiotics, they will be considered a treatment failure and their therapy will be unblinded at that visit. If the patient satisfies the requirements for step-down from IV to oral antibiotic therapy, they will be considered a treatment success, the physician will write an order for oral antibiotics, and their therapy will be unblinded at that visit. Clinical experience and limited research suggest that a switch from intravenous to oral antimicrobial agents can be safely made after 3 to 4 days.[1] If patients are not returning to the ED on day 7, they will be contacted by telephone to assess 7 day cure rate based on signs and symptoms of their cellulitis, visits a physician or emergency department. If the patient is not a clinical failure but does not meet the requirements for stepdown from IV to oral antibiotic therapy, they will continue on the study drug and return daily for IV therapy and assessments until either success or failure can be determined, up to 7 days after the first dose of study drug. As it has been shown that 5 days of antibiotic therapy is as effective as 10 days of therapy in the treatment of uncomplicated cellulitis [16], we feel that 7 days of therapy will be an adequate length of time during which treatment success or failure can be accurately determined. Evaluation of Study Outcomes Patients will receive study cards and be instructed to present these at each visit to the triage nurse, who will call the number on the card to notify the research assistant of the patient s arrival. A study summary sheet will be placed on the patients chart (Appendix IV). The data collection form found in Appendix I will be used for the initial Emergency Department visit and up Final Dec 22, 2009 DDJan 19,
14 to 6 additional data collection forms (Appendix II) will be completed at each subsequent visit by the research assistant. Presence of the following signs and symptoms will be recorded and include pain/tenderness using a visual analog scale, swelling, erythema, localized warmth, purulent drainage/discharge, induration, regional lymph node swelling or tenderness, extension of redness, temperature, heart rate, blood pressure and respiratory rate. During the initial visit, demographics, clinical history and baseline signs and symptoms of each patient will be recorded by the research assistant. At each subsequent assessment, the presence of signs and symptoms will be recorded and used to determine the efficacy of antibiotic therapy. The resolution of signs and symptoms will be part of the criteria used to determine treatment success and the persistence of signs and symptoms will be part of the criteria used to determine failure. The data collection form will also have an area in which the physician s plan will be recorded. Options will include: (i) continue study drug therapy; (ii) step down from IV to oral therapy; (iii) change antibiotic therapy; and (iv) admit to hospital. The research assistant will assess compliance with oral therapy at end of study using the number of capsules remaining in each patient s vial and an exit survey will be conducted to assess blinding of both patients and physician on that day (Appendix III). After the blinding assessment is completed, the patient may be informed which study treatment they received. If the patients is stepped down to oral therapy and is not returning to the ED on day 7 for assessment, the research assistant will contact the patient for a telephone interview to ask about: (i) any visit to a physician since discharge, and why; (ii) any visit to an ED, and why; and (iii) signs and symptoms of their cellulitis including: pain/tenderness, fever, swelling, erythema, localized warmth, purulent drainage/discharge, induration, regional lymph node swelling or tenderness, and/or extension of redness (Appendix III). Final Dec 22, 2009 DDJan 19,
15 . Statistical Analysis Using a clinically important difference of 10%, a two sided alpha of 0.05, and 80% power, 157 patients would be required in each arm. This calculation assumes an 85% cure rate in each group.[12] The data from the collection forms will be entered into an Excel spreadsheet for analysis. Patients will be analyzed on an intention to treat basis as the primary endpoint. A per protocol analysis will also be conducted as a secondary endpoint if necessary. Descriptive statistics will be used to describe patient demographic information, the primary outcome, and the secondary outcomes. The t-test for independent groups (if data are parametric) or the Mann- Whitney test (if data are non-parametric) will be used to compare continuous data and the Fisher s test for discrete data. Patients that are lost to follow-up will be assumed to have failed therapy. There are no interim analyses planned for this study given the short enrolment period and interventions that are already well established in the treatment of skin and soft tissue infections. Study Timeline Objective Date Ethics Application October 1, 2009 (3 ethics boards) CHSP Grant Application October 16, 2009 Assembly of study drug boxes February-Mar 2010 (Investigators) Random Number Generation Feb-Mar 2010 (Independent Pharmacy Contact) Shipment of study drug boxes to Halifax April 2010 Informing ED Physicians -initial information on potential study Sept 2009 Final Dec 22, 2009 DDJan 19,
16 -once approved, full information and education on study protocol, roll-out, study timeline March 2010 Dissemination Plan We propose to use a multi-layered approach for knowledge exchange and dissemination which will involve: (i) targeted communication with both internal and external stakeholders; (ii) academic publications and presentation; and (iii) project development. (i) Targeted Communication with Key Stakeholder Groups: A one page summary highlighting key findings and messages will be developed with information tailored (increasing detail, complexity and technical specialization) for internal and external stakeholders which will include but not be limited to physicians (partcularly family practitioners and emergency physicians), pharmacists and hospital administrators. Distribution will occur through family physician offices, emergency departments and professional associations. Results will be presented to the Pharmacy and Emergency Medicine communities through Grand Rounds presentations. (ii) Academic Publications: We will present our findings at provincial (e.g. Dalhousie University Annual Refresher Courses for both Medicine and Pharmacy) and national (e.g. Canadian Society of Hospital Pharmacists (CSHP) and Canadian Association of Emergency Physicians (CAEP) venues. We will also prepare one medical (e.g. Canadain Journal of Hospital Pharmacy or Canadian Medical Association Journal) and one methods based (e.g. American Journal of Epidemiology or Canadian Pharmacy Journal) manuscript for high-impact readership. (iii) Project Development: Study results will be integral to future project development. We plan to use the findings from this study to design further studies to further explore opportunities to improve care in this patient population. Relevance This study will represent the first prospective study ever performed to evaluate the relative efficacy and safety of cephalexin compared to cefazolin plus probenecid in the management of SSTI in patients presenting to the ED. In this regard this study is unique and necessary to ensure optimal care is being provided to this patient population. These results will also lay the foundation for future studies in this area. Final Dec 22, 2009 DDJan 19,
17 If our results demonstrate that in this patient population oral antibiotics are equivalent to parenteral antibiotics in the outpatient management of SSTI, these findings will be applicable in all jurisdictions throughout Canada and have significant impact in current practice. If the management of SSTI is further shifted to care at home with oral antibiotics there are several additional intangible benefits from this work. First, it may place further increased attention and focus on optimal prescribing, best pharmacotherapy practice and drug therapy monitoring in this patient population. Second, the ability to shift care to home rather than in the ED or ambulatory care setting, should improve overall patient satisfaction and result in less disruption to patient daily activities (e.g. work, school). Finally, care at home with oral antibiotics for this patient population may help reduce the ever-growing problem of ED overcrowding and overall heath care costs. Final Dec 22, 2009 DDJan 19,
18 Budget Justification A. PERSONNEL ($12,977) ($11,577 Requested) 1. Research Assistants(s) ($10,577) This study will require two research assistants using senior pharmacy students. Each center will require 0.5 FTE student for 16 weeks. Research assistants will be required for this study to carry out the described methodology including patient enrollment and follow-up. The current rate of pay for Interior Health Authority for student research assistant inclusive of all benefits, for 16 weeks at 36 hours/week at 0.5 FTE ($5668). The current rate of pay for QEII HSC for a student research assistant inclusive of all benefits is $14.00/hour. For 16 weeks at 36 hours/week at 0.5 FTE ($4200). In addition, one student research assistant will be required in IHA for 36 hours to prepare study drug kits. At $19.68/hour x 36 hours ($709). Overall research assistant costs would be $ Research Coordinator ($1,400) ($0 Requested) Support from a Research Coordinator from the Department of Emergency Medicine, Dalhousie University. This position will provide administrative support to the principal investigators and research assistants throughout the study. Salary for this position as per Dalhousie University Collective Bargaining Agreement (CBA) at $35 per hour for 40 hours is $1400 but will be provided in-kind as part of existing infrastructure. 3. Statistical Analysis ($1,000) Support for statistical analysis will be contracted to a PhD-trained statistician. Cost estimate comprised of statistical consultation and technical support at standard rate of $100 per hour as per the Biostatistical Consulting Service in the Department of Community Health and Epidemiology, Dalhousie University for 10 hours ($1000) B. Drug Costs ($ ) ($3142 Requested) Drug costs consist of acquisition, preparation and packaging of all study medications and associated materials. Drug costs consist of cephalexin 500 mg tablets ($522), cephalexin placebo ($269), probenecid 500 mg tablets ($445), probenecid placebo tablets ($135), cefazolin 2g vials ($1904) and cefazolin placebo ($1076). Total drug cost $4351. Supplies to prepare and package study drug and placebos include capsules ($745), vials ($128), syringes ($420), boxes for study kits ($640). Total supplies ($1933) and total drug and supplies $6284. IHA will provide Final Dec 22, 2009 DDJan 19,
19 in-kind funding for 50% of drug costs, capsules, vials & syringes ($3142) reducing costs for drug and supplies to $3142. C. MATERIALS AND SUPPLIES ($400) These items would include preparation of study materials, advertisements, miscellaneous office supplies, stationary and photocopying, courier charges, mailing expenses and long distance telephone. ($400) D. RESEARCH EQUIPMENT ($3,000) ($0 Requested) The overall costs include one personal computer, printer and software (MS Office for Mac) ($3000 Provided) which will be provided by existing infrastructure of the Department of Emergency Medicine at QEII HSC. E. OTHER ($2,000) ($0 Requested) 1. Data Management ($2,000) ($0 Requested) Data management costs will be provided in-kind by the Department of Emergency Medicine, IHA ($2000 Provided). Total Required: $24,661 Total In-Kind: $9,542 Total Requested: $15,119 Acknowledgements: Thank you to Leah Neumann, KGH 3 rd year UBC summer student for assisting with drafting the protocol. Final Dec 22, 2009 DDJan 19,
20 References 1. Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Managing skin and soft tissue infections: expert panel recommendations on key decision points. Journal of Antimicrobial Chemotherapy. 2003;52(S1):i Deery HG II. Outpatient parenteral anti-infective therapy for skin and soft-tissue infections. Infectious Disease Clinics of North America. 1998;12(4): Abrahamian FM, Talan DA, Moran GJ. Management of skin and soft-tissue infections in the Emergency Department. Infectious Disease Clinics of North America. 2008;22(1): Vinh DC, Embil JM. Rapidly progressive soft tissue infections. Lancet Infect Dis 2005;5(8): Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. The Canadian Journal of Infectious Diseases & Medical Microbiology. 2008;19(2): Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann TK, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134: Dong SL, Kelly KD, Oland RC, Holroyd BR, Rowe BH. ED management of cellulitis: a review of 5 urban centres Am J Emerg Med 2001;19: Brown G, Zemcov SJ, Clarke AM. Effect of probenecid on cefazolin serum concentrations. Journal of Antimicrobial Chemotherapy. 1993;31(6): Spina SP, Dillon EC Jr. Effect of chronic probenecid therapy on cefazolin serum concentrations. The Annals of Pharmacotherapy. 2003;37: Cox VC, Zed PJ. Once-daily cefazolin and probenecid for skin and soft tissue infections. Ann Pharmacother 2004;38: Final Dec 22, 2009 DDJan 19,
21 11. Brown G, Chamberlain R, Goulding J, Clarke A. Ceftriaxone versus cefazolin with probenecid for severe skin and soft tissue infections. The Journal of Emergency Medicine. 1996;14(5): Grayson ML, McDonald M, Gibson K, Athan E, Munckhof WJ, Paull P, et al. Once-daily intravenous cefazolin plus oral probenecid is equivalent to once-daily intravenous ceftriaxone plus oral placebo for the treatment of moderate-to-severe cellulitis in adults. Clinical Infectious Diseases. 2002;34(11): Giordano PA, Elston D, Akinlade BK, Weber K, Notario GF, Busman TA, et al. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Current Medical Research and Opinion. 2006;22(12): Kiani R. Double-blind, double-dummy comparison of azithromycin and cephalexin in the treatment of skin and skin structure infections. European Journal of Clinical Microbiology & Infectious Diseases. 1991;10(10): Dillon HC Jr. Treatment of staphylococcal skin infections: a comparison of cephalexin and dicloxacillin. Journal of the American Academy of Dermatology. 1983;8(2): Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Archives of Internal Medicine. 2004;164(15): Final Dec 22, 2009 DDJan 19,
22 Figure 1: Patient Flow Diagram Final Dec 22, 2009 DDJan 19,
23 Appendix I: Initial Visit Cefazolin/Probenecid vs Cephalexin Data Collection Form Location of Presentation: KGH QEII HSC Study Number: Date of Initial Presentation: Age: Gender: Weight: Location of Cellulitis: Vitals: Arm Leg temperature: blood pressure: Hand Foot Head/Neck Other Duration of cellulitis prior to ED visit: weeks days hours Antibiotic therapy in the past 7 days: Y N heart rate: resp rate: Symptoms: pain/tenderness (value out of 10 ) fever swelling cephalexin clindamycin Concomitant use of: topical antibiotics septra other: systemic steroids erythema localized warmth purulent drainage/discharge induration medications causing immunosuppression Known Diagnosis of: regional lymph node swelling/tenderness extension of redness diabetes HIV new altered mental status haematological malignancy IV drug user: Homeless: Y N Y N Signs of Systemic Sepsis: temperature >38 C or <36 C heart rate > 90 beats/min Contact with the healthcare system in the past year: Y N respiratory rate > 20 breaths/min WBC <4,000 cells/mm³ or >12,000 cells/mm³ or >10% band forms Final Dec 22, 2009 DDJan 19,
24 Appendix II: Subsequent Visit Cefazolin/Probenecid vs Cephalexin Data Collection Form Study Number: Visit (circle day): Vitals: temp: heart rate: Symptoms: blood pressure: resp rate: Signs of Systemic Sepsis: temperature >38 C or <36 C heart rate > 90 beats/min respiratory rate > 20 breaths/min pain/tenderness (value out of 10 ) WBC <4,000 cells/mm³ or >12,000 cells/mm³ or >10% band forms fever swelling erythema localized warmth purulent drainage/discharge induration regional lymph node swelling/tenderness extension of redness new altered mental status Plan: Continue study drug therapy Step-down from IV to PO therapy Admit to hospital Change antibiotic therapy to: clindamycin septra other: Final Dec 22, 2009 DDJan 19,
25 Appendix III: Assessment at discharge and 7 days Complete AFTER success or failure has been concluded and BEFORE patient is unblinded: Suspected Treatment Group: Patient: oral cephalexin iv cefazolin plus probenecid Number of capsules left in patient vial: Physician: oral cephalexin iv cefazolin plus probenecid If discharged from study PRIOR to day 7, telephone interview conducted on day 7: not applicable yes, contacted date/time: If yes, does the patient have any: visits to a physician since discharge? if so why: visits to an ED since discharge? If so why: signs or symptoms of cellulitis present? pain or tenderness fever redness localized warmth drainage extension of redness swelling/ tenderness around infection Other: Further prescription written for patient on day 7? N Y If Yes: Cephalexin Clindamycin Septra Other: Why antibiotic given? inadequate response MRSA coverage abscess formation Other: Final Dec 22, 2009 DDJan 19,
26 Appendix IV: Patient chart information sheet for return visits Date of Enrolment: TRIAL NAME: Intravenous cefazolin plus oral probenecid vs. oral cephalexin for the treatment of cellulitis: a randomized controlled trial SUMMARY: It has been determined that this patient has cellulitis. As a participant in this trial, this patient is taking: Cephalexin 500 mg 4 times daily OR Cefazolin 2 g IV daily plus Probenecid 1 g PO PURPOSE: The purpose of this study is to determine how well cephalexin works to treat cellulitis compared to cefazolin and probenecid. Currently, the choice of which drug to use is left up to the physician and usually depends on past experience. The results of this study may be able to provide physicians with scientific evidence to guide their decision. PROCEDURES: Initial Visit Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Background Information X Collected Physician Assessment X X X ~ ~ ~ ~ Patient Receives IV X X X ~ ~ ~ ~ Treatment Patient Receives Oral X X X ~ ~ ~ ~ Treatment 4 times a day End of Study Survey (One Question) End of Study X POSSIBLE DRUG THERAPY: Group Drug Therapy Placebo A Cephalexin 500 mg 4 times a day placebo probenecid capsule plus IV saline once daily B Probenecid 1 g PO plus Cefazolin 2 g IV once daily placebo cephalexin capsules 4 times a day If you have any questions or concerns regarding the participation of this patient in this trial. For KGH please call Dr. Dawn Dalen at For QEII HSC please call Dr. Peter Zed at A signed copy of the patient s consent can be found in the patient s medical record. Final Dec 22, 2009 DDJan 19,
27 Appendix IV: Consent Form (Please refer to attached consent forms) Final Dec 22, 2009 DDJan 19,
Publication Year: 2013
 IMPACT OF A CLINICAL DECISION SUPPORT TOOL IN THE EMERGENCY DEPARTMENT ON ANTIMICROBIAL PRESCRIBING PATTERNS FOR THE TREATMENT OF PNEUMONIA UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Publication Year: 2013
IMPACT OF A CLINICAL DECISION SUPPORT TOOL IN THE EMERGENCY DEPARTMENT ON ANTIMICROBIAL PRESCRIBING PATTERNS FOR THE TREATMENT OF PNEUMONIA UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Publication Year: 2013
Hemodialysis catheter infection
 Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
PG Certificate / PG Diploma / MSc in Clinical Pharmacy
 PG Certificate / PG Diploma / MSc in Clinical Pharmacy Programme Information September 2014 Entry School of Pharmacy Queen s University Belfast Queen s University Belfast - Clinical Pharmacy programme
PG Certificate / PG Diploma / MSc in Clinical Pharmacy Programme Information September 2014 Entry School of Pharmacy Queen s University Belfast Queen s University Belfast - Clinical Pharmacy programme
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Antibiotic Prophylaxis for the Prevention of Infective Endocarditis and Prosthetic Joint Infections for Dentists
 PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
ANTIBIOTICS IN SEPSIS
 ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
UNIVERSITY OF WISCONSIN HOSPITAL AND CLINICS DEPARTMENT OF PHARMACY SCOPE OF PATIENT CARE SERVICES FY 2014 October 1 st, 2014
 UNIVERSITY OF WISCONSIN HOSPITAL AND CLINICS DEPARTMENT OF PHARMACY SCOPE OF PATIENT CARE SERVICES FY 2014 October 1 st, 2014 Department Name: Department of Pharmacy Department Director: Steve Rough, MS,
UNIVERSITY OF WISCONSIN HOSPITAL AND CLINICS DEPARTMENT OF PHARMACY SCOPE OF PATIENT CARE SERVICES FY 2014 October 1 st, 2014 Department Name: Department of Pharmacy Department Director: Steve Rough, MS,
How Can We Get the Best Medication History?
 How Can We Get the Best Medication History? Stephen Shalansky, Pharm.D., FCSHP Pharmacy Department, St. Paul s Hospital Faculty of Pharmaceutical Sciences, UBC How Are We Doing Now? Completeness of Medication
How Can We Get the Best Medication History? Stephen Shalansky, Pharm.D., FCSHP Pharmacy Department, St. Paul s Hospital Faculty of Pharmaceutical Sciences, UBC How Are We Doing Now? Completeness of Medication
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Antibiotic Guidelines: Ear Nose and Throat (ENT) Infections. Contents
 Antibiotic Guidelines: Ear Nose and Throat (ENT) Infections. Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): Authors Division: DCSS & Tertiary Medicine
Antibiotic Guidelines: Ear Nose and Throat (ENT) Infections. Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): Authors Division: DCSS & Tertiary Medicine
Health Professions Act BYLAWS SCHEDULE F. PART 2 Hospital Pharmacy Standards of Practice. Table of Contents
 Health Professions Act BYLAWS SCHEDULE F PART 2 Hospital Pharmacy Standards of Practice Table of Contents 1. Application 2. Definitions 3. Drug Distribution 4. Drug Label 5. Returned Drugs 6. Drug Transfer
Health Professions Act BYLAWS SCHEDULE F PART 2 Hospital Pharmacy Standards of Practice Table of Contents 1. Application 2. Definitions 3. Drug Distribution 4. Drug Label 5. Returned Drugs 6. Drug Transfer
POAC CLINICAL GUIDELINE
 POAC CLINICAL GUIDELINE Acute Pylonephritis DIAGNOSIS COMPLICATED PYELONEPHRITIS EXCLUSION CRITERIA: Male Known or suspected renal impairment (egfr < 60) Abnormality of renal tract Known or suspected renal
POAC CLINICAL GUIDELINE Acute Pylonephritis DIAGNOSIS COMPLICATED PYELONEPHRITIS EXCLUSION CRITERIA: Male Known or suspected renal impairment (egfr < 60) Abnormality of renal tract Known or suspected renal
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Post-surgical V.A.C. VeraFlo Therapy with Prontosan Instillation on Inpatient Infected Wounds * COLLECTION OF CASE STUDIES
 COLLECTION OF CASE STUDIES Post-surgical V.A.C. VeraFlo Therapy with Prontosan Instillation on Inpatient Infected Wounds * *All patients were treated with systemic antibiotics Post-surgical V.A.C. VeraFlo
COLLECTION OF CASE STUDIES Post-surgical V.A.C. VeraFlo Therapy with Prontosan Instillation on Inpatient Infected Wounds * *All patients were treated with systemic antibiotics Post-surgical V.A.C. VeraFlo
A case of concurrent deep venous thrombosis, pseudoaneurysm, and extremity abscess in an intravenous methamphetamine abuser
 A case of concurrent deep venous thrombosis, pseudoaneurysm, and extremity abscess in an intravenous methamphetamine abuser BY MATTHEW L HARRISON Abstract Introduction. Intravenous drug abuse is a global
A case of concurrent deep venous thrombosis, pseudoaneurysm, and extremity abscess in an intravenous methamphetamine abuser BY MATTHEW L HARRISON Abstract Introduction. Intravenous drug abuse is a global
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial
 Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
 Skin and Soft tissue Infections: new bugs, old drugs Disclosure Statement Sponsor: Goodman Photographic Presented by: Dr. Kristopher Wiebe, MD, CCFP (EM) Presented to: BC Chapter, Canadian Society of Hospital
Skin and Soft tissue Infections: new bugs, old drugs Disclosure Statement Sponsor: Goodman Photographic Presented by: Dr. Kristopher Wiebe, MD, CCFP (EM) Presented to: BC Chapter, Canadian Society of Hospital
Investigational Drugs: Investigational Drugs and Biologics
 : I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
: I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
Symptom Based Alcohol Withdrawal Treatment
 Symptom Based Alcohol Withdrawal Treatment -Small Rural Hospital- Presenter CDR Dwight Humpherys, DO dwight.humpherys@ihs.gov Idaho State University Baccalaureate Nursing Program Lake Erie College of Osteopathic
Symptom Based Alcohol Withdrawal Treatment -Small Rural Hospital- Presenter CDR Dwight Humpherys, DO dwight.humpherys@ihs.gov Idaho State University Baccalaureate Nursing Program Lake Erie College of Osteopathic
RN PRESCRIBING AND ORDERING DIAGNOSTIC TESTS: REQUIREMENTS AND STANDARDS. (Date TBD)
 RN PRESCRIBING AND ORDERING DIAGNOSTIC TESTS: REQUIREMENTS AND STANDARDS (Date TBD) This document has not been approved by CARNA Provincial Council, it is a draft only for review and not for use. Once
RN PRESCRIBING AND ORDERING DIAGNOSTIC TESTS: REQUIREMENTS AND STANDARDS (Date TBD) This document has not been approved by CARNA Provincial Council, it is a draft only for review and not for use. Once
Drug Utilization and Evaluation of Cephalosporin s At Tertiary Care Teaching Hospital, Bangalore
 Research Article Drug Utilization and Evaluation of Cephalosporin s At Tertiary Care Teaching Hospital, Bangalore HS. Shekar 1*, HR. Chandrashekhar 2, M. Govindaraju 3, P. Venugopalreddy 1, Chikkalingiah
Research Article Drug Utilization and Evaluation of Cephalosporin s At Tertiary Care Teaching Hospital, Bangalore HS. Shekar 1*, HR. Chandrashekhar 2, M. Govindaraju 3, P. Venugopalreddy 1, Chikkalingiah
Non-Inpatient Parenteral Antibiotic Therapy : clinical and cost effective option
 Non-Inpatient Parenteral Antibiotic Therapy : clinical and cost effective option Dilip Nathwani Ninewells Hospital & Medical School Dundee DD1 9SY TAYSIDE OHPAT EXPERIENCE Case Study Seen in A&E or AMU
Non-Inpatient Parenteral Antibiotic Therapy : clinical and cost effective option Dilip Nathwani Ninewells Hospital & Medical School Dundee DD1 9SY TAYSIDE OHPAT EXPERIENCE Case Study Seen in A&E or AMU
2. The prescribing clinician will register with the designated manufacturer.
 Clozapine Management Program Description Magellan of Arizona Pharmacy Program Background: Magellan Health Services of Arizona recognizes the importance of a clozapine program. Clozapine received increased
Clozapine Management Program Description Magellan of Arizona Pharmacy Program Background: Magellan Health Services of Arizona recognizes the importance of a clozapine program. Clozapine received increased
GENERAL INFORMATION. Adverse Event (AE) Definition (ICH GUIDELINES E6 FOR GCP 1.2):
 Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Etiology and treatment of chronic bacterial prostatitis the Croatian experience
 Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Breathe With Ease. Asthma Disease Management Program
 Breathe With Ease Asthma Disease Management Program MOLINA Breathe With Ease Pediatric and Adult Asthma Disease Management Program Background According to the National Asthma Education and Prevention Program
Breathe With Ease Asthma Disease Management Program MOLINA Breathe With Ease Pediatric and Adult Asthma Disease Management Program Background According to the National Asthma Education and Prevention Program
ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D.
 ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D. ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS ACTIVITY DESCRIPTION Antibiotic use in the safest
ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D. ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS ACTIVITY DESCRIPTION Antibiotic use in the safest
Person Centered Care: Walk the Talk
 Person Centered Care: Walk the Talk Integration of Nurse Practitioner (NP) Role into Extendicare Michener Hill Long Term Care (LTC) Presented by: Sandi Engi MN, NP Michener Hill Extendicare November 25
Person Centered Care: Walk the Talk Integration of Nurse Practitioner (NP) Role into Extendicare Michener Hill Long Term Care (LTC) Presented by: Sandi Engi MN, NP Michener Hill Extendicare November 25
healthcare associated infection 1.2
 healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
Electronic Health Record (EHR) Data Analysis Capabilities
 Electronic Health Record (EHR) Data Analysis Capabilities January 2014 Boston Strategic Partners, Inc. 4 Wellington St. Suite 3 Boston, MA 02118 www.bostonsp.com Boston Strategic Partners is uniquely positioned
Electronic Health Record (EHR) Data Analysis Capabilities January 2014 Boston Strategic Partners, Inc. 4 Wellington St. Suite 3 Boston, MA 02118 www.bostonsp.com Boston Strategic Partners is uniquely positioned
HealthCare Partners of Nevada. Heart Failure
 HealthCare Partners of Nevada Heart Failure Disease Management Program 2010 HF DISEASE MANAGEMENT PROGRAM The HealthCare Partners of Nevada (HCPNV) offers a Disease Management program for members with
HealthCare Partners of Nevada Heart Failure Disease Management Program 2010 HF DISEASE MANAGEMENT PROGRAM The HealthCare Partners of Nevada (HCPNV) offers a Disease Management program for members with
A pharmacist s guide to Pharmacy Services compensation
 Alberta Blue Cross Pharmaceutical Services A pharmacist s guide to Pharmacy Services compensation 83443 (2015/12) GENERAL DESCRIPTION... 3 Details... 3 ASSESSMENT CRITERIA... 3 Assessment for a Prescription
Alberta Blue Cross Pharmaceutical Services A pharmacist s guide to Pharmacy Services compensation 83443 (2015/12) GENERAL DESCRIPTION... 3 Details... 3 ASSESSMENT CRITERIA... 3 Assessment for a Prescription
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults
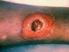 1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
Musculoskeletal Infection Care Process Model
 Musculoskeletal Infection Care Process Model Musculoskeletal infections are serious and potentially life-threatening. Musculoskeletal infections include necrotizing fasciitis, septic arthritis, osteomyelitis,
Musculoskeletal Infection Care Process Model Musculoskeletal infections are serious and potentially life-threatening. Musculoskeletal infections include necrotizing fasciitis, septic arthritis, osteomyelitis,
United 2020: Measuring Impact
 United 2020: Measuring Impact Health The Institute for Urban Policy Research At The University of Texas at Dallas Kristine Lykens, PhD United 2020: Measuring Impact Health Overview In the Dallas area,
United 2020: Measuring Impact Health The Institute for Urban Policy Research At The University of Texas at Dallas Kristine Lykens, PhD United 2020: Measuring Impact Health Overview In the Dallas area,
Agency # 070.00 REGULATION 9 PHARMACEUTICAL CARE/PATIENT COUNSELING
 Agency # 070.00 REGULATION 9 PHARMACEUTICAL CARE/PATIENT COUNSELING 09-00: PATIENT COUNSELING 09-00-0001--PATIENT INFORMATION, DRUG USE EVALUATION, AND PATIENT COUNSELING The intent of this regulation
Agency # 070.00 REGULATION 9 PHARMACEUTICAL CARE/PATIENT COUNSELING 09-00: PATIENT COUNSELING 09-00-0001--PATIENT INFORMATION, DRUG USE EVALUATION, AND PATIENT COUNSELING The intent of this regulation
FACTORS ASSOCIATED WITH HEALTHCARE COSTS AMONG ELDERLY PATIENTS WITH DIABETIC NEUROPATHY
 FACTORS ASSOCIATED WITH HEALTHCARE COSTS AMONG ELDERLY PATIENTS WITH DIABETIC NEUROPATHY Luke Boulanger, MA, MBA 1, Yang Zhao, PhD 2, Yanjun Bao, PhD 1, Cassie Cai, MS, MSPH 1, Wenyu Ye, PhD 2, Mason W
FACTORS ASSOCIATED WITH HEALTHCARE COSTS AMONG ELDERLY PATIENTS WITH DIABETIC NEUROPATHY Luke Boulanger, MA, MBA 1, Yang Zhao, PhD 2, Yanjun Bao, PhD 1, Cassie Cai, MS, MSPH 1, Wenyu Ye, PhD 2, Mason W
Monash University - Master of Clinical Pharmacy
 Monash University - Master of Clinical The Master of Clinical is a 48 credit point program, equivalent to one year of full time study (generally completed in 2 years part time). It comprises 1200 hours
Monash University - Master of Clinical The Master of Clinical is a 48 credit point program, equivalent to one year of full time study (generally completed in 2 years part time). It comprises 1200 hours
Medicare Supplement plan application
 Medicare Supplement plan application SECTION 1 Personal information Last name First name Middle initial Social Security number - - Primary Street address City State ZIP code Mailing Street address (if
Medicare Supplement plan application SECTION 1 Personal information Last name First name Middle initial Social Security number - - Primary Street address City State ZIP code Mailing Street address (if
Why Do Some Antibiotics Fail?
 Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Humulin R (U500) insulin: Prescribing Guidance
 Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Medical College of Georgia SOP NUMBER: 03 INVESTIGATIONAL DRUG HANDLING Version Number: 1.0, 1.1 Effective Date: 09/12/06, 08/02/10, 3/2/11
 Effective Date: 09/12/06, 08/02/10, 3/2/11 Title: 1.0 OBJECTIVE: 1.1 This SOP describes the methods and policies for: Handling investigational drug Dispensing investigational drug 1.2. This procedure applies
Effective Date: 09/12/06, 08/02/10, 3/2/11 Title: 1.0 OBJECTIVE: 1.1 This SOP describes the methods and policies for: Handling investigational drug Dispensing investigational drug 1.2. This procedure applies
PPP 1. Continuation of a medication to ensure continuity of care
 PRESCRIBING POLICIES: 4.7 PHARMACIST AUTHORITY The College of Pharmacists of BC Professional Practice Policy (PPP) 58 Medication Management (Adapting a Prescription) became effective April 1, 2009. The
PRESCRIBING POLICIES: 4.7 PHARMACIST AUTHORITY The College of Pharmacists of BC Professional Practice Policy (PPP) 58 Medication Management (Adapting a Prescription) became effective April 1, 2009. The
Use of Packing for Surgical Wounds. Maggie Benson Clinical Problem Solving II
 Use of Packing for Surgical Wounds Maggie Benson Clinical Problem Solving II Purpose Present patient management s/p Incision and Drainage in an outpatient setting Examine evidence for the use of wound
Use of Packing for Surgical Wounds Maggie Benson Clinical Problem Solving II Purpose Present patient management s/p Incision and Drainage in an outpatient setting Examine evidence for the use of wound
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Outcome of Drug Counseling of Outpatients in Chronic Obstructive Pulmonary Disease Clinic at Thawangpha Hospital
 Mahidol University Journal of Pharmaceutical Sciences 008; 35(14): 81. Original Article Outcome of Drug Counseling of Outpatients in Chronic Obstructive Pulmonary Disease Clinic at Thawangpha Hospital
Mahidol University Journal of Pharmaceutical Sciences 008; 35(14): 81. Original Article Outcome of Drug Counseling of Outpatients in Chronic Obstructive Pulmonary Disease Clinic at Thawangpha Hospital
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
Requirements for Drug Information Centres
 FIP Pharmacy Information Section Requirements for Drug Information Centres Summary All countries should provide drug information services either independently or as part of a regional network. The service
FIP Pharmacy Information Section Requirements for Drug Information Centres Summary All countries should provide drug information services either independently or as part of a regional network. The service
Results of all scientific investigations with the A.Vogel Sore Throat Spray. Issue 2 - June 2008
 Results of all scientific investigations with the A.Vogel Sore Issue 2 - June 2008 Antiviral Antibacterial Anti-inflammatory Analgetic Immunemodulatory Authors: Andy Suter, Medical Department Roland Schoop,
Results of all scientific investigations with the A.Vogel Sore Issue 2 - June 2008 Antiviral Antibacterial Anti-inflammatory Analgetic Immunemodulatory Authors: Andy Suter, Medical Department Roland Schoop,
Guidance for Industry Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment
 Guidance for Industry Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guideline for Developing Randomization Procedures RPG-03
 The Clinical Research Center Research Practice Manual Guideline for Developing Randomization Procedures RPG-03 Purpose Guideline The purpose of this Guideline is to outline the Clinical Research Center
The Clinical Research Center Research Practice Manual Guideline for Developing Randomization Procedures RPG-03 Purpose Guideline The purpose of this Guideline is to outline the Clinical Research Center
NCT00272090. sanofi-aventis HOE901_3507. insulin glargine
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
UTCVM PHARMACY STANDARD OPERATING PROCEDURES
 UTCVM PHARMACY STANDARD OPERATING PROCEDURES Updated: 4/5/2004 I. General Procedures A. Hours: The Pharmacy will be open Monday through Friday, 8:00AM to 6:00PM; Saturday 8:00AM to 1:00PM. The Pharmacy
UTCVM PHARMACY STANDARD OPERATING PROCEDURES Updated: 4/5/2004 I. General Procedures A. Hours: The Pharmacy will be open Monday through Friday, 8:00AM to 6:00PM; Saturday 8:00AM to 1:00PM. The Pharmacy
CHRONIC KIDNEY DISEASE MANAGEMENT GUIDE
 CHRONIC KIDNEY DISEASE MANAGEMENT GUIDE Outline I. Introduction II. Identifying Members with Kidney Disease III. Clinical Guidelines for Kidney Disease A. Chronic Kidney Disease B. End Stage Renal Disease
CHRONIC KIDNEY DISEASE MANAGEMENT GUIDE Outline I. Introduction II. Identifying Members with Kidney Disease III. Clinical Guidelines for Kidney Disease A. Chronic Kidney Disease B. End Stage Renal Disease
CONNECTICUT. Downloaded January 2011 19 13 D8T. CHRONIC AND CONVALESCENT NURSING HOMES AND REST HOMES WITH NURSING SUPERVISION
 CONNECTICUT Downloaded January 2011 19 13 D8T. CHRONIC AND CONVALESCENT NURSING HOMES AND REST HOMES WITH NURSING SUPERVISION (d) General Conditions. (6) All medications shall be administered only by licensed
CONNECTICUT Downloaded January 2011 19 13 D8T. CHRONIC AND CONVALESCENT NURSING HOMES AND REST HOMES WITH NURSING SUPERVISION (d) General Conditions. (6) All medications shall be administered only by licensed
Addressing forecasted oncologist shortage: A Cancer Education Program for Pharmacy Students (CEPPS)
 Addressing forecasted oncologist shortage: A Cancer Education Program for Pharmacy Students (CEPPS) Dr. Department of Pharmacy Practice State University of New York School of Pharmacy and Pharmaceutical
Addressing forecasted oncologist shortage: A Cancer Education Program for Pharmacy Students (CEPPS) Dr. Department of Pharmacy Practice State University of New York School of Pharmacy and Pharmaceutical
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Expedited Partner Therapy (EPT) for Sexually Transmitted Diseases Protocol for Health Care Providers in Oregon
 Expedited Partner Therapy (EPT) for Sexually Transmitted Diseases Protocol for Health Care Providers in Oregon Oregon Health Authority Center for Public Health Practice HIV/STD/TB Section Principles of
Expedited Partner Therapy (EPT) for Sexually Transmitted Diseases Protocol for Health Care Providers in Oregon Oregon Health Authority Center for Public Health Practice HIV/STD/TB Section Principles of
Understanding Diseases and Treatments with Canadian Real-world Evidence
 Understanding Diseases and Treatments with Canadian Real-world Evidence Real-World Evidence for Successful Market Access WHITEPAPER REAL-WORLD EVIDENCE Generating real-world evidence requires the right
Understanding Diseases and Treatments with Canadian Real-world Evidence Real-World Evidence for Successful Market Access WHITEPAPER REAL-WORLD EVIDENCE Generating real-world evidence requires the right
Keeping patients safe when they transfer between care providers getting the medicines right
 PART 1 Keeping patients safe when they transfer between care providers getting the medicines right Good practice guidance for healthcare professions July 2011 Endorsed by: Foreword Taking a medicine is
PART 1 Keeping patients safe when they transfer between care providers getting the medicines right Good practice guidance for healthcare professions July 2011 Endorsed by: Foreword Taking a medicine is
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
Opioids in Chronic Non Cancer Pain: The Basics
 Opioids in Chronic Non Cancer Pain: The Basics List of Suggested Resources This document is a list of useful resources discovered during the development of the Opioids in Chronic Non Cancer Pain topic.
Opioids in Chronic Non Cancer Pain: The Basics List of Suggested Resources This document is a list of useful resources discovered during the development of the Opioids in Chronic Non Cancer Pain topic.
Health Professions Act BYLAWS SCHEDULE F. PART 3 Residential Care Facilities and Homes Standards of Practice. Table of Contents
 Health Professions Act BYLAWS SCHEDULE F PART 3 Residential Care Facilities and Homes Standards of Practice Table of Contents 1. Application 2. Definitions 3. Supervision of Pharmacy Services in a Facility
Health Professions Act BYLAWS SCHEDULE F PART 3 Residential Care Facilities and Homes Standards of Practice Table of Contents 1. Application 2. Definitions 3. Supervision of Pharmacy Services in a Facility
Human Clinical Study for Free Testosterone & Muscle Mass Boosting
 Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
Assessment of Drug Utilization Patterns in Some Health Insurance Outpatient Clinics in Alexandria 1Ibrahem, Samaa Zenhom; 1Amer, N.; 2Ghoneim, M.
 Assessment of Drug Utilization Patterns in Some Health Insurance Outpatient Clinics in Alexandria 1Ibrahem, Samaa Zenhom; 1Amer, N.; 2Ghoneim, M.; 1Abou El Enein N 1High Institute of Public Health, Egypt
Assessment of Drug Utilization Patterns in Some Health Insurance Outpatient Clinics in Alexandria 1Ibrahem, Samaa Zenhom; 1Amer, N.; 2Ghoneim, M.; 1Abou El Enein N 1High Institute of Public Health, Egypt
Pharmacy Interchange. The EHR and Pharmacy Integration: A Preferred Future. Benjamin M. Bluml, R.Ph. Vice President, Research APhA Foundation
 Pharmacy Interchange The EHR and Pharmacy Integration: A Preferred Future Benjamin M. Bluml, R.Ph. Vice President, Research APhA Foundation Quality Health Care Empowers patients Includes the environment
Pharmacy Interchange The EHR and Pharmacy Integration: A Preferred Future Benjamin M. Bluml, R.Ph. Vice President, Research APhA Foundation Quality Health Care Empowers patients Includes the environment
Learning Objectives. Introduction to Reconciling Medication Information. Background. Elements of Performance NPSG.03.06.01
 Pharmacy Evaluation of Medication Reconciliation Initiated in the Emergency Department Manuel A. Calvin, Pharm.D. PGY1 Pharmacy Resident Saint Francis Hospital, Tulsa, OK OSHP Annual Meeting Residency
Pharmacy Evaluation of Medication Reconciliation Initiated in the Emergency Department Manuel A. Calvin, Pharm.D. PGY1 Pharmacy Resident Saint Francis Hospital, Tulsa, OK OSHP Annual Meeting Residency
New York State Office of Alcoholism & Substance Abuse Services Addiction Services for Prevention, Treatment, Recovery
 New York State Office of Alcoholism & Substance Abuse Services Addiction Services for Prevention, Treatment, Recovery USING THE 48 HOUR OBSERVATION BED USING THE 48 HOUR OBSERVATION BED Detoxification
New York State Office of Alcoholism & Substance Abuse Services Addiction Services for Prevention, Treatment, Recovery USING THE 48 HOUR OBSERVATION BED USING THE 48 HOUR OBSERVATION BED Detoxification
BC PALLIATIVE CARE BENEFITS PRESCRIBER GUIDE
 BC PALLIATIVE CARE BENEFITS PRESCRIBER GUIDE VERSION 2.5 OCTOBER 29, 2015 BC PALLIATIVE CARE BENEFITS PRESCRIBER GUIDE CHANGE RECORD DATE VERSION CHANGE DETAILS Dec 1, 2009 1.0 Original version Jun 21,
BC PALLIATIVE CARE BENEFITS PRESCRIBER GUIDE VERSION 2.5 OCTOBER 29, 2015 BC PALLIATIVE CARE BENEFITS PRESCRIBER GUIDE CHANGE RECORD DATE VERSION CHANGE DETAILS Dec 1, 2009 1.0 Original version Jun 21,
Course Curriculum for Master Degree in Clinical Pharmacy
 Course Curriculum for Master Degree in Clinical Pharmacy The Master Degree in Clinical Pharmacy is awarded by the Faculty of Graduate studies at Jordan University of Science and Technology (JUST) upon
Course Curriculum for Master Degree in Clinical Pharmacy The Master Degree in Clinical Pharmacy is awarded by the Faculty of Graduate studies at Jordan University of Science and Technology (JUST) upon
Community health care services Alternatives to acute admission & Facilitated discharge options. Directory
 Community health care services Alternatives to acute admission & Facilitated discharge options Directory Introduction The purpose of this directory is to provide primary and secondary health and social
Community health care services Alternatives to acute admission & Facilitated discharge options Directory Introduction The purpose of this directory is to provide primary and secondary health and social
Research Ethics Review Committee (WHO ERC)
 Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Treatment of Opioid Dependence: A Randomized Controlled Trial. Karen L. Sees, DO, Kevin L. Delucchi, PhD, Carmen Masson, PhD, Amy
 Category: Heroin Title: Methadone Maintenance vs 180-Day psychosocially Enriched Detoxification for Treatment of Opioid Dependence: A Randomized Controlled Trial Authors: Karen L. Sees, DO, Kevin L. Delucchi,
Category: Heroin Title: Methadone Maintenance vs 180-Day psychosocially Enriched Detoxification for Treatment of Opioid Dependence: A Randomized Controlled Trial Authors: Karen L. Sees, DO, Kevin L. Delucchi,
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital
 Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
PharmaCare is BC s public drug insurance program that assists BC residents in paying for eligible prescription drugs and designated medical supplies.
 PHARMANET AND PHARMACARE DATA DICTIONARY Date Range: September 1, 1995 to present date, data is provided by calendar year Data Source: BC Ministry of Health Description The PharmaNet system is an online,
PHARMANET AND PHARMACARE DATA DICTIONARY Date Range: September 1, 1995 to present date, data is provided by calendar year Data Source: BC Ministry of Health Description The PharmaNet system is an online,
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
GP SERVICES COMMITTEE Conferencing and Telephone Management INCENTIVES. Revised 2015. Society of General Practitioners
 GP SERVICES COMMITTEE Conferencing and Telephone Management INCENTIVES Revised 2015 Society of General Practitioners Conference & Telephone Fees (G14077, G14015, G14016, G14017, G14018, G14019, G14021,
GP SERVICES COMMITTEE Conferencing and Telephone Management INCENTIVES Revised 2015 Society of General Practitioners Conference & Telephone Fees (G14077, G14015, G14016, G14017, G14018, G14019, G14021,
A Outpatient Services A1 AMBULATORY CARE CENTRE A1(d) Cancer Centre Clinical Trials Office
 A1(d) CANCER CENTRE CLINICAL TRIALS OFFICE A1(d).1 SERVICE DESCRIPTION A1(d).1.1 Scope of Clinical Services This section A1(d) sets out the requirements for the centralized facilities for the Clinical
A1(d) CANCER CENTRE CLINICAL TRIALS OFFICE A1(d).1 SERVICE DESCRIPTION A1(d).1.1 Scope of Clinical Services This section A1(d) sets out the requirements for the centralized facilities for the Clinical
HEALTH SERVICES UNIT ORIENTATION. 1. Sick call is to be available to all inmates five days per week.
 TI 15.11.01 Appendix D 4/03 Page 1 of 8 HEALTH SERVICES UNIT ORIENTATION A. SICK CALL 1. Sick call is to be available to all inmates five days per week. 2. Sick call provides access for requested medical
TI 15.11.01 Appendix D 4/03 Page 1 of 8 HEALTH SERVICES UNIT ORIENTATION A. SICK CALL 1. Sick call is to be available to all inmates five days per week. 2. Sick call provides access for requested medical
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION
 LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
UW School of Dentistry Comprehensive Medication Policy
 UNIVERSITY OF WASHINGTON SCHOOL OF DENTISTRY Subject: UW School of Dentistry Comprehensive Medication Policy Policy Number: Effective Date: December 2014 Revision Dates: June 2015 PURPOSE This policy provides
UNIVERSITY OF WASHINGTON SCHOOL OF DENTISTRY Subject: UW School of Dentistry Comprehensive Medication Policy Policy Number: Effective Date: December 2014 Revision Dates: June 2015 PURPOSE This policy provides
Tuberculosis Prevention and Control Protocol, 2008
 Tuberculosis Prevention and Control Protocol, 2008 Preamble The Ontario Public Health Standards (OPHS) are published by the Minister of Health and Long- Term Care under the authority of the Health Protection
Tuberculosis Prevention and Control Protocol, 2008 Preamble The Ontario Public Health Standards (OPHS) are published by the Minister of Health and Long- Term Care under the authority of the Health Protection
Headache - What is Your Migraine Size?
 Headache The Pharmacist s Role in Assessment & Peter Loewen, B.Sc.(Pharm), Pharm.D. Vancouver Hospital & Health Sciences Centre University of British Columbia ETC, Headache. Nan Quintin www.vhpharmsci.com
Headache The Pharmacist s Role in Assessment & Peter Loewen, B.Sc.(Pharm), Pharm.D. Vancouver Hospital & Health Sciences Centre University of British Columbia ETC, Headache. Nan Quintin www.vhpharmsci.com
Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer )
 Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
Guideline for Developing Randomization Procedures RPG-03
 The Clinical Research Center Research Practice Manual Guideline for Developing Randomization Procedures RPG-03 Purpose Guideline The purpose of this Guideline is to outline the Clinical Research Center
The Clinical Research Center Research Practice Manual Guideline for Developing Randomization Procedures RPG-03 Purpose Guideline The purpose of this Guideline is to outline the Clinical Research Center
PROTOCOL TITLE: Ambulatory Initiation and Management of Warfarin for Adults
 PROTOCOL NUMBER: 7 PROTOCOL TITLE: Ambulatory Initiation and Management of Warfarin for Adults THIS PROTOCOL APPLIES TO: UW Health Clinics: all adult outpatients with an active order for warfarin TARGET
PROTOCOL NUMBER: 7 PROTOCOL TITLE: Ambulatory Initiation and Management of Warfarin for Adults THIS PROTOCOL APPLIES TO: UW Health Clinics: all adult outpatients with an active order for warfarin TARGET
Pharmacists Allied Health Professionals
 10.0 MOC SECTION 1 MAINPRO-M1 BRINGING BEST EVIDENCE TO CLINICIANS COMBINING EVIDENCE & CLINICAL PHARMACOLOGY TO IMPROVE DRUG THERAPY OCT 23 & 24, 2015 FRI-SAT SURREY MEMORIAL HOSPITAL WHO SHOULD ATTEND
10.0 MOC SECTION 1 MAINPRO-M1 BRINGING BEST EVIDENCE TO CLINICIANS COMBINING EVIDENCE & CLINICAL PHARMACOLOGY TO IMPROVE DRUG THERAPY OCT 23 & 24, 2015 FRI-SAT SURREY MEMORIAL HOSPITAL WHO SHOULD ATTEND
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
A cluster randomized trial evaluating electronic prescribing in an ambulatory care setting
 A cluster randomized trial evaluating electronic prescribing in an ambulatory care setting Merrick Zwarenstein, MBBCh, MSc Senior Scientist Institute for Clinically Evaluative Science 2075 Bayview Avenue
A cluster randomized trial evaluating electronic prescribing in an ambulatory care setting Merrick Zwarenstein, MBBCh, MSc Senior Scientist Institute for Clinically Evaluative Science 2075 Bayview Avenue
Pragmatic Seamless Design for Efficacy Trial of Asthma Management with reduced Cost. Mei Lu, PhD Christine Joseph, Ph.D
 Pragmatic Seamless Design for Efficacy Trial of Asthma Management with reduced Cost Mei Lu, PhD Christine Joseph, Ph.D Henry Ford Health System May 19, 2013 Puff City Pragmatic RCT: Partners HFHS Clinical
Pragmatic Seamless Design for Efficacy Trial of Asthma Management with reduced Cost Mei Lu, PhD Christine Joseph, Ph.D Henry Ford Health System May 19, 2013 Puff City Pragmatic RCT: Partners HFHS Clinical
Section 6. Medical Management Program
 Section 6. Medical Management Program Introduction Molina Healthcare maintains a medical management program to ensure patient safety as well as detect and prevent fraud, waste and abuse in its programs.
Section 6. Medical Management Program Introduction Molina Healthcare maintains a medical management program to ensure patient safety as well as detect and prevent fraud, waste and abuse in its programs.
