Date: 1 December 2006 Trial ID: MS Version: 1.0 Clinical Trial Report Report Synopsis Page: 1 of 5
|
|
|
- Logan Henderson
- 7 years ago
- Views:
Transcription
1 Report Synopsis Page: 1 of 5 Synopsis Trial Registration ID-number NCT IND Number No IND number. NovoFine Autocover needle received 510(k) clearance on 30 March 2005 Title of Trial An Open-label, Multicenter, Prospective Cross-Over Study of Preference and Safety of NovoFine Autocover Needles compared to BD SafetyGlide Syringes in the Hospital Setting Investigators Six Investigators in the U.S. Trial Sites Six sites in the U.S. Publications None Trial Period FPFV: 21 July 2005; LPLV: 02 June 2006 Development Phase IV Objectives Primary Objective: To assess the preference of the NovoFine Autocover Needle compared to the BD SafetyGlide Syringe by nurses in the hospital setting. Secondary Objectives: To assess the safety of the NovoFine Autocover Needle compared to the BD SafetyGlide Syringe as to the incidence of accidental needlestick injury. To assess device preference of NovoFine Autocover Needle compared to the BD SafetyGlide Syringe as measured by the Sharps-Safety and Needlestick Prevention Questionnaires. Methodology This was a Phase IV, multicenter, prospective, open-label, randomized, two-period crossover, comparative study of 18 weeks duration (comprising a 2-week screening period and a 16-week treatment period). The trial population included nurses and hospitalized patients with diabetes. Nurses were the primary population of interest. Patients were included because the study design required that the trial devices be used to administer insulin subcutaneously to patients. At the start of the study, each hospital was randomized to a device sequence by Inc. The nurses administered insulin injections subcutaneously to the eligible patients whose prescribed insulin was compatible with the device assigned to the nurse. Patients prescribed insulin was not altered at any time during the study. Each participating nurse was asked to administer a minimum of 100 subcutaneous insulin injections over the 16 weeks of the study (50 injections per period per device). Nurses were instructed in proper handling of trial devices. Only nurses demonstrating device proficiency were allowed to participate in the study.
2 Report Synopsis Page: 2 of 5 Number of Subjects Planned and Analysed Planned: 100 Nurses, 10,000 injections. Each participating nurse was asked to administer a minimum of 100 insulin injections subcutaneously over the 16 weeks of the study. Actual Subject Population: NovoFine b BD Syringe BD Syringe c NovoFine Total Subjects a Enrolled/randomized Subjects who performed no injection 9 (20.9%) 3 (5.7%) 12 (12.5%) Subjects who performed 1 injection 34 (79.1%) 50 (94.3%) 84 (87.5%) Discontinued Other d 6 (14.0%) 11 (20.8%) 17 (17.7%) Completed Trial 28 (65.1%) 39 (73.6%) 67 (69.8%) a. Subject refers to nurse. b. NovoFine BD Syringe refers to the sequence where NovoFine Autocover Needle is in the first period and BD SafetyGlide Syringe is in the second period. c. BD Syringe NovoFine refers to the sequence where BD SafetyGlide Syringe is in the first period and NovoFine Autocover Needle is in the second period. d. Other includes: low number of patients consenting; nurse changed her job, went to another facility, or was not willing to continue the study; no qualified patients for nurse to inject; or the nurse was pregnant and discontinued the study when her baby was born. Diagnosis and Main Criteria for Inclusion Nurses: Registered nurse with competence in subcutaneous (SC) injections for at least 4 weeks prior to the start of the trial must demonstrate proficiency with the insulin delivery device (NovoFine Autocover Needle or BD SafetyGlide Syringe) prior to using the device in patients. Patients: Hospitalized patients, age 18 years, with type 1 or type 2 diabetes mellitus requiring treatment with insulin by subcutaneous injection. Test Product, Dose and Mode of Administration, Batch Number NovoFine Autocover Needles 30 G x 8 mm (Lot Number 05B11E). The patients received their prescribed insulin by subcutaneous injection with the NovoFine Autocover Needle. Duration of Treatment 18 weeks (comprising a 2-week screening period and a 16-week treatment period) Reference Therapy, Dose and Mode of Administration, Batch Number BD SafetyGlide Syringes 29 G x 1/2 inch (Lot Numbers: and ). The patients received their prescribed insulin by subcutaneous injection with the BD SafetyGlide Syringe. Criteria for Evaluation Efficacy Primary Efficacy Endpoint To assess the preference of the NovoFine Autocover Needle compared to BD SafetyGlide Syringe by nurses in the hospital setting using the Device Preference Questionnaire for NovoFine Autocover Needle and BD Safety Glide Syringe. Secondary Efficacy Endpoints There were no secondary efficacy endpoints for this study.
3 Report Synopsis Page: 3 of 5 Criteria for Evaluation Safety The frequency of adverse events (needlestick injuries) related to the NovoFine Autocover Needle compared to the BD SafetyGlide Syringe when handled by nurses. Nurses responses on the Clinical Technical Complaint that included any comments regarding the NovoFine Autocover Needle or BD SafetyGlide Syringe, or any malfunctions or suspected malfunctions of either device when used during the study. Device preference of the NovoFine Autocover Needle compared to the BD SafetyGlide Syringe as measured by the Sharps-Safety and Needlestick-Prevention Questionnaires. Statistical Methods Efficacy analysis was performed on Per-Protocol population. The answer to the primary question about nurse s preference was binary: 1 (the NovoFine Autocover needle) or 0 (the BD Safety Glide syringe). The P-value for testing whether NovoFine Autocover is preferred over BD Safety Glide Syringe was presented using the Fisher s exact testing. Unless otherwise specified, all analyses were performed at 5% significance level and the confidence interval was 95% confidence interval. The incidence of needlestick injuries was compared between NovoFine Autocover Needle and BD Safety Glide Syringe using a Poisson log-linear model. In the log-linear model, number of needlestick injuries served as response variable, device, period and sequence served as the explanatory variable. Sharps-Safety and Needlestick-Prevention Questionnaire was completed for each device by the nurse. For the analysis of questions in the Sharps-Safety and Needlestick-Prevention Device Assessment Questionnaire, the response for each question was dichotomized (scores 1, 2 vs. 4 and 5; score 3 was treated as neutral or was not included in the analysis), and logistic regression was performed. Demography of Trial Population In this study, no baseline characteristics and demographics were collected for either nurses or patients. Efficacy Results Among the participating nurses, 40% of the nurses preferred the NovoFine Autocover Needle versus 60% who preferred the BD SafetyGlide Syringe. The difference in device preference between the two devices was not statistically significant irrespective of the device sequence. The preference rate of the NovoFine Autocover was > 60% at 3 sites, Atlanta Medical Center [67%], Genesis Good Samaritan Hospital [60%], and the Southern Ohio Medical Center (64%). However, the other 3 sites showed a lower preference rate for NovoFine Autocover needle (Baylor Medical Center at Irving [27%], Mt. Carmel St. Ann's Hospital [25%], and Cleveland Clinic Foundation [17%]). The low preference by the nurses may be due to the bias for the BD SafetyGlide Syringe over the FlexPen NovoFine Autocover needle, since the vial/syringe method is the predominant form for administration of insulin in the United Sates and is used by majority of the hospital staff. The other possibility may be due to the fact that the nurses performed fewer injections using the Autocover Needle (mean: about 21 injections; median: 15 injections) than using the BD SafetyGlide Syringe (mean: about 26 injections; median: 22 injections). Safety Results No needlestick injuries were reported in this study; no nurses discontinued due to adverse events. Three Clinical Technical Complaints were reported with the use of a device for the nurses and the patients. One nurse reported that after injecting insulin she noticed the insulin was leaking. The event was not related to the use of NovoFine Autocover Needle. For one patient, the nurse reported 3 events of excessive bleeding at the injection site. The event was evaluated as possibly related to the study device NovoFine Autocover Needle. One patient died of cardiac arrest. The design of the study required that only those clinical technical complaints reported by the patients that were related to the device be considered an adverse event. The death of this patient was not related to the device, and was therefore not an adverse event as defined by criteria in the protocol. No treatment-emergent adverse events (TEAEs) were reported by nurses. Four (1.8%) patients reported a total of 7 TEAE: infusion site pain (3 patients, 4 events) and injection site haemorrhage (1 patient, 3 events) while using the NovoFine Autocover Needle. All the adverse events reported were mild in severity. No TEAEs were reported by patients when using the BD SafetyGlide Syringe.
4 Report Synopsis Page: 4 of 5 No serious adverse events (SAE) resulting with the use of a device were reported by nurses or patients when using the NovoFine Autocover Needle or the BD SafetyGlide Syringe. Preference of safety features of NovoFine Autocover Needle in preventing needlestick injuries was confirmed based on the nurses response: Question Number a Question Autocover Needle b n (%) SafetyGlide Syringe b n (%) Q1 The safety feature can be activated using a one-handed technique. 58 (86.6) 63 (88.7) Q2 Use of this product requires you to use the safety feature. 62 (92.5) 43 (62.3) Q3 This product requires less time to use than a standard syringe. 47 (69.1) 60 (87.0) Q4 The safety feature works well with my hand size. 55 (80.9) 65 (90.3) Q5 The device is easy to handle while wearing gloves. 63 (92.6) 63 (88.7) Q6 The device provides a better alternative to traditional recapping. 61 (89.7) 64 (92.8) Q7 There is a clear and unmistakable change (either audible or visible) that 62 (91.2) 67 (94.4) occurs when the safety feature is activated. Q8 The safety feature operates reliably. 64 (94.1) 68 (94.4) Q9 The exposed sharp is permanently blunted or covered after use and prior 67 (98.5) 61 (89.7) to disposal. Q10 The user does not need extensive training for correct operation. 63 (92.6) 67 (95.7) Q11 The design of the device suggests proper use. 62 (88.6) 59 (85.5) Q12 It is difficult to skip a crucial step when using the device. 55 (79.7) 48 (69.6) Q13 During use of the device the user s hands remain behind the needle until 68 ( 100) 59 (83.1) the injection is completed and the safety mechanism is locked. Q14 The device minimizes the risk of user exposure to blood. 67 (97.1) 56 (78.9) Q15 The device minimizes the risk of infection (e.g. through crosscontamination). 66 (95.7) 59 (84.3) Q16 The device is easy to dispose of after use. 66 (95.7) 64 (91.4) Q17 The safety feature of the device helps to reduce my anxiety about 62 (91.2) 62 (86.1) accidental needlesticks. Q18 Overall, I would recommend using the device (possible to recommend both). 47 (69.1) 62 (86.1) a. Answer scores of Q1-Q18: On a 5-point scale, 1and 2 = agree, 3=neither agree or disagree, 4 and 5 = disagree b. The responses to the questions indicate agree (rating 1 and 2) Characteristics favorable to the use of the NovoFine Autocover needle included: Question 2: the requirement to use the safety device, Question 9 the permanent blunting or covering of the needle after use and prior to disposal, Question 14: the minimized risk of exposure to blood, and Question 15: the minimized risk of infection through cross-contamination from a needlestick. Characteristics favorable to the use of the SafetyGlide needle included: Question 3 the requirement of less time to use than a standard syringe and Question 18: greater recommendation of the SafetyGlide needle (86%) versus the Autocover (69%). All the nurses agreed that, during the use of the NovoFine Autocover Needle, the user s hands remained behind the needle until the injection was completed and the safety mechanism was locked. More nurses responded that they experienced some difficulty associated with the use of the NovoFine Autocover Needle versus the BD SafetyGlide Syringe. Difficulties experienced in the use of the NovoFine Autocover Needle included: it was difficult to determine whether the patient actually received the insulin dose indicated since you cannot visualize if the needle penetrated the patient s skin because of the safety device; it appeared that one had to apply more
5 Report Synopsis Page: 5 of 5 pressure for the needle to enter the patient's skin; with some skin types it was difficult to fully engage Autocover after the injection; and medicine was noted on skin after injection. Difficulties experienced in the use of the BD-SafetyGlide Syringe included: safety feature of the SafetyGlide syringe was not automatic; difficulty reading smaller units of syringe due to safety device in the way; and syringe can be awkward at times. Conclusions No needlestick injuries were reported in this study indicating that the NovoFine Autocover Needle was as safe as the BD-SafetyGlide Syringe. No significant difference in preference to either safety device was reported by the participating nurses, irrespective of the device sequence. In general, the nurses responded favorably for the handling and acceptance of both the devices. Sixty-nine percent of the nurses said they would recommend the use of the NovoFine Autocover Safety needle and gave many advantageous benefits to the Autocover needle in preventing needlestick injuries. The trial was conducted in accordance with the Declaration of Helsinki and ICH Good Clinical Practice.
SYNOPSIS. Risperidone: Clinical Study Report CR003274
 SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics
 Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Guideline for the Administration of Insulin by Nursing Staff
 Guideline for the Administration of Insulin by Nursing Staff Aims and objectives In Lanarkshire the number of people with Diabetes on insulin treatment is growing, as both the population ages and people
Guideline for the Administration of Insulin by Nursing Staff Aims and objectives In Lanarkshire the number of people with Diabetes on insulin treatment is growing, as both the population ages and people
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Phase 4: Evaluate Safer Medical Device(s)
 NIOSH recommends that health care facilities use safer medical devices to protect workers from needlestick and other sharps injuries. Since the passage of the Needlestick Safety and Prevention Act in 2000
NIOSH recommends that health care facilities use safer medical devices to protect workers from needlestick and other sharps injuries. Since the passage of the Needlestick Safety and Prevention Act in 2000
NCT00272090. sanofi-aventis HOE901_3507. insulin glargine
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
An introduction to the principles and practice of safe and effective administration of injections
 An introduction to the principles and practice of safe and effective administration of injections Introduction Giving an injection safely is considered to be a routine nursing activity. However it requires
An introduction to the principles and practice of safe and effective administration of injections Introduction Giving an injection safely is considered to be a routine nursing activity. However it requires
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Summary ID# 13614. Clinical Study Summary: Study F3Z-JE-PV06
 CT Registry ID# Page 1 Summary ID# 13614 Clinical Study Summary: Study F3Z-JE-PV06 INSIGHTS; INSulin-changing study Intending to Gain patients insights into insulin treatment with patient-reported Health
CT Registry ID# Page 1 Summary ID# 13614 Clinical Study Summary: Study F3Z-JE-PV06 INSIGHTS; INSulin-changing study Intending to Gain patients insights into insulin treatment with patient-reported Health
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Healthcare Waste Management Training
 WHO Regional Office for Europe Healthcare Waste Management Training Module 5 Sharps: Handling & Mitigation Measures Content Introduction Needle Stick accidents: Tanzania Mitigation measures Safer design
WHO Regional Office for Europe Healthcare Waste Management Training Module 5 Sharps: Handling & Mitigation Measures Content Introduction Needle Stick accidents: Tanzania Mitigation measures Safer design
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
My Doctor Says I Should Learn To Use Insulin... What Do I Do Next? BD Getting Started. Drawing and Injecting Insulin
 My Doctor Says I Should Learn To Use Insulin... What Do I Do Next? BD Getting Started Drawing and Injecting Insulin It is important to know how to draw and inject insulin so that you can give your injection
My Doctor Says I Should Learn To Use Insulin... What Do I Do Next? BD Getting Started Drawing and Injecting Insulin It is important to know how to draw and inject insulin so that you can give your injection
My Doctor Says I Need to Mix Insulins... How Do I Begin? BD Getting Started. Mixing Insulins
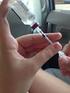 My Doctor Says I Need to Mix Insulins... How Do I Begin? BD Getting Started Mixing Insulins When your doctor tells you to use two types of insulin for an injection, they can be mixed in the same insulin
My Doctor Says I Need to Mix Insulins... How Do I Begin? BD Getting Started Mixing Insulins When your doctor tells you to use two types of insulin for an injection, they can be mixed in the same insulin
Safe use of insulin e- learning module
 Safe use of insulin e- learning module Page 1 Introduction Insulin is a hormone produced by the beta cells in the pancreas, it is released when blood glucose levels are raised for example after a meal.
Safe use of insulin e- learning module Page 1 Introduction Insulin is a hormone produced by the beta cells in the pancreas, it is released when blood glucose levels are raised for example after a meal.
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS
 105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS Section 210.001: Purpose 210.002: Definitions 210.003: Policies Governing the Administration of Prescription
105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS Section 210.001: Purpose 210.002: Definitions 210.003: Policies Governing the Administration of Prescription
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
New 7/1/2015 MCFRS 1
 New 7/1/2015 MCFRS 1 The providers will summarize the need for this change from an epinephrine auto injector The provider will define the proper dosage of epinephrine for the adult and pediatric patient
New 7/1/2015 MCFRS 1 The providers will summarize the need for this change from an epinephrine auto injector The provider will define the proper dosage of epinephrine for the adult and pediatric patient
Using a Graseby MS26 Syringe Driver for Continuous Subcutaneous Infusions (CSCI) Protocol
 Using a Graseby MS26 Syringe Driver for Continuous Subcutaneous Infusions (CSCI) Protocol Who Division 1 Registered Nursing staff for the purposes of administering and monitoring of infusion Division 2
Using a Graseby MS26 Syringe Driver for Continuous Subcutaneous Infusions (CSCI) Protocol Who Division 1 Registered Nursing staff for the purposes of administering and monitoring of infusion Division 2
Guide to the European Union
 Guide to the European Union (Prevention of Sharps Injuries in the Healthcare Sector) Regulations 2014 Our vision: A country where worker safety, health and welfare and the safe management of chemicals
Guide to the European Union (Prevention of Sharps Injuries in the Healthcare Sector) Regulations 2014 Our vision: A country where worker safety, health and welfare and the safe management of chemicals
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Appendix G STATISTICAL METHODS INFECTIOUS METHODS STATISTICAL ROADMAP. Prepared in Support of: CDC/NCEH Cross Sectional Assessment Study.
 Appendix G STATISTICAL METHODS INFECTIOUS METHODS STATISTICAL ROADMAP Prepared in Support of: CDC/NCEH Cross Sectional Assessment Study Prepared by: Centers for Disease Control and Prevention National
Appendix G STATISTICAL METHODS INFECTIOUS METHODS STATISTICAL ROADMAP Prepared in Support of: CDC/NCEH Cross Sectional Assessment Study Prepared by: Centers for Disease Control and Prevention National
New Evidence reports on presentations given at EULAR 2012. Rituximab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
Parenteral Dosage of Drugs
 Chapter 11 Parenteral Dosage of Drugs Parenteral Route of administration other than gastrointestinal Intramuscular (IM) Subcutaneous (SC) Intradermal (ID) IV Parenteral Most medications prepared in liquid
Chapter 11 Parenteral Dosage of Drugs Parenteral Route of administration other than gastrointestinal Intramuscular (IM) Subcutaneous (SC) Intradermal (ID) IV Parenteral Most medications prepared in liquid
Humulin R (U500) insulin: Prescribing Guidance
 Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
A patient guide to the use of insulin for diabetes
 A patient guide to the use of insulin for diabetes Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
A patient guide to the use of insulin for diabetes Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Administration of Medications & Fluids via a Peripheral Intravenous Cannula
 Administration of Medications & Fluids via a Peripheral Intravenous Cannula Clinical S.O.P. No.: 22.0 Compiled by: Approved by: Review date: November 2016 Administration of Medications & Fluids via S.O.P.
Administration of Medications & Fluids via a Peripheral Intravenous Cannula Clinical S.O.P. No.: 22.0 Compiled by: Approved by: Review date: November 2016 Administration of Medications & Fluids via S.O.P.
ROCHESTER AREA SCHOOL DISTRICT
 No. 210 SECTION: PUPILS ROCHESTER AREA SCHOOL DISTRICT TITLE: USE OF MEDICATIONS ADOPTED: August 11, 2008 REVISED: August 25, 2014 210. USE OF MEDICATIONS 1. Purpose The Board shall not be responsible
No. 210 SECTION: PUPILS ROCHESTER AREA SCHOOL DISTRICT TITLE: USE OF MEDICATIONS ADOPTED: August 11, 2008 REVISED: August 25, 2014 210. USE OF MEDICATIONS 1. Purpose The Board shall not be responsible
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Phase: IV. Study Period: 20 Jan. 2006-17 Sep. 2008
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Bayer HealthCare, LLC Sharps Disposal Submission to CalRecycle July 1, 2014
 Bayer HealthCare, LLC Sharps Disposal Submission to CalRecycle July 1, 2014 1. A pharmaceutical manufacturer that sells or distributes a medication in California that is usually intended to be self-injected
Bayer HealthCare, LLC Sharps Disposal Submission to CalRecycle July 1, 2014 1. A pharmaceutical manufacturer that sells or distributes a medication in California that is usually intended to be self-injected
Is what you know about INSULIN PUMP THERAPY. This educational resource is provided by Medtronic MiniMed, Inc.
 Is what you know about INSULIN PUMP THERAPY MYTH or? This educational resource is provided by Medtronic MiniMed, Inc. You have to know a lot about technology to learn to use a pump Interacting with your
Is what you know about INSULIN PUMP THERAPY MYTH or? This educational resource is provided by Medtronic MiniMed, Inc. You have to know a lot about technology to learn to use a pump Interacting with your
Health Protection Agency INFECTION PREVENTION AND CONTROL GUIDELINES FOR BLOOD GLUCOSE MONITORING IN CARE HOMES
 Health Protection Agency INFECTION PREVENTION AND CONTROL GUIDELINES FOR BLOOD GLUCOSE MONITORING IN CARE HOMES October 2009 1 Introduction Routine diabetes care involves monitoring blood glucose levels
Health Protection Agency INFECTION PREVENTION AND CONTROL GUIDELINES FOR BLOOD GLUCOSE MONITORING IN CARE HOMES October 2009 1 Introduction Routine diabetes care involves monitoring blood glucose levels
OCCUPATIONAL SAFETY AND HEALTH ADMINISTRATION (OSHA)
 OCCUPATIONAL SAFETY AND HEALTH ADMINISTRATION (OSHA) The OSHA/VOSH 1910.1030 Blood borne Pathogens Standard was issued to reduce the occupational transmission of infections caused by microorganisms sometimes
OCCUPATIONAL SAFETY AND HEALTH ADMINISTRATION (OSHA) The OSHA/VOSH 1910.1030 Blood borne Pathogens Standard was issued to reduce the occupational transmission of infections caused by microorganisms sometimes
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Betaferon (interferon beta 1b)
 Betaferon (interferon beta 1b) What is Betaferon? Betaferon (interferon beta-1b) is a type of medicine known as an interferon, which is used to treat MS. Interferons are proteins found naturally in the
Betaferon (interferon beta 1b) What is Betaferon? Betaferon (interferon beta-1b) is a type of medicine known as an interferon, which is used to treat MS. Interferons are proteins found naturally in the
The Importance of Following the PROTOCOL in Clinical Trials
 The Importance of Following the PROTOCOL in Clinical Trials Presentation Objectives: Upon completion of this presentation, participants will be able to: Describe the following terms: Protocol, Protocol
The Importance of Following the PROTOCOL in Clinical Trials Presentation Objectives: Upon completion of this presentation, participants will be able to: Describe the following terms: Protocol, Protocol
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Afrezza Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Afrezza (human insulin) Prime Therapeutics will review Prior Authorization requests Prior Authorization
Afrezza Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Afrezza (human insulin) Prime Therapeutics will review Prior Authorization requests Prior Authorization
AQUATIC ANIMAL MEDICINE RECORD BOOK
 AQUATIC ANIMAL MEDICINE RECORD BOOK FISH HEALTH INSPECTORATE www.efishbusiness.co.uk It is a legal requirement for you to retain these records for at least five years following the administration or other
AQUATIC ANIMAL MEDICINE RECORD BOOK FISH HEALTH INSPECTORATE www.efishbusiness.co.uk It is a legal requirement for you to retain these records for at least five years following the administration or other
Adverse Events in Clinical Trials: Definitions and Documentation
 Clinical and Translational Science Institute / CTSI at the University of California, San Francisco Welcome to Online Training for Clinical Research Coordinators Adverse Events in Clinical Trials: Definitions
Clinical and Translational Science Institute / CTSI at the University of California, San Francisco Welcome to Online Training for Clinical Research Coordinators Adverse Events in Clinical Trials: Definitions
Trial Description. Organizational Data. Secondary IDs
 Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
of the Dossier Volume: Page:
 . -. Baxter Healthcare Corporation ~ 2. SYNOPSIS Name of Company individual Study Table (ForNd&mzfAufhoriiy = 3axter Healthcare Corporation Referring to Part Use Of@) %me of Finished Product: 3iaspirin
. -. Baxter Healthcare Corporation ~ 2. SYNOPSIS Name of Company individual Study Table (ForNd&mzfAufhoriiy = 3axter Healthcare Corporation Referring to Part Use Of@) %me of Finished Product: 3iaspirin
Introcan Safety. 1 billion times protection. Vascular Access
 Introcan Safety 1 billion times protection Vascular Access The B. Braun Introcan Safety IV Catheter Reduces Needlestick Injuries Passive Safety Technology Established worldwide: B. Braun has minimized
Introcan Safety 1 billion times protection Vascular Access The B. Braun Introcan Safety IV Catheter Reduces Needlestick Injuries Passive Safety Technology Established worldwide: B. Braun has minimized
NIH Clinical Center Patient Education Materials Giving a subcutaneous injection
 NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
OSHA s Bloodborne Pathogens Standard 1910.1030
 OSHA s Bloodborne Pathogens Standard 1910.1030 Jens Nissen & Kennan Arp Iowa OSHA Enforcement 515-281-3122 nissen.jens@dol.gov or arp.kennan@dol.gov Bloodborne Pathogens Standard Federal Law 29 CFR 1910.1030
OSHA s Bloodborne Pathogens Standard 1910.1030 Jens Nissen & Kennan Arp Iowa OSHA Enforcement 515-281-3122 nissen.jens@dol.gov or arp.kennan@dol.gov Bloodborne Pathogens Standard Federal Law 29 CFR 1910.1030
In the inpatient setting, a common practice for
 Hosp Pharm 2014;49(11):1033 1038 2014 Thomas Land Publishers, Inc. www.hospital-pharmacy.com doi: 10.1310/hpj4911-1033 Original Article Economic Impact of Converting from Pen and 10- Vial to 3- Vial for
Hosp Pharm 2014;49(11):1033 1038 2014 Thomas Land Publishers, Inc. www.hospital-pharmacy.com doi: 10.1310/hpj4911-1033 Original Article Economic Impact of Converting from Pen and 10- Vial to 3- Vial for
A P P E N D I X SAMPLE FORMS
 A P P E N D I X A SAMPLE FORMS Authorization for Disclosure Consent for HBV/HCV Antigens, HIV Antibody Documentation of Staff Education Employees Eligible for Hepatitis-B Vaccination Hepatitis-A Consent
A P P E N D I X A SAMPLE FORMS Authorization for Disclosure Consent for HBV/HCV Antigens, HIV Antibody Documentation of Staff Education Employees Eligible for Hepatitis-B Vaccination Hepatitis-A Consent
CLINICAL STUDY REPORT SYNOPSIS
 CLINICAL STUDY REPORT SYNOPSIS Document No.: EDMS-PSDB-6511351:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Protocol No.: CR002353 Johnson & Johnson Pharmaceutical
CLINICAL STUDY REPORT SYNOPSIS Document No.: EDMS-PSDB-6511351:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Protocol No.: CR002353 Johnson & Johnson Pharmaceutical
INSULIN INJECTION KNOW-HOW
 0-0- INSULIN INJECTION KNOW-HOW Learning how to Congratulations for making the move to insulin therapy. It won t be long before you start enjoying better blood sugar control, more energy, and a host of
0-0- INSULIN INJECTION KNOW-HOW Learning how to Congratulations for making the move to insulin therapy. It won t be long before you start enjoying better blood sugar control, more energy, and a host of
Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic Chemotherapy for Adults in the Community Setting
 Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic Chemotherapy for Adults in the Community Setting Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic
Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic Chemotherapy for Adults in the Community Setting Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
Sponsor / Company: Sanofi Drug substance(s): HOE901 (insulin glargine)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
to Part of Dossier: Name of Active Ingredient: Title of Study: Quality of life study with adalimumab in rheumatoid arthritis. ESCALAR.
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Section 10. Guidelines for the Safe Handling and Disposal of Needles and Sharps
 Section 10 Guidelines for the Safe Handling and Disposal of Needles and Sharps On behalf of Infection Control Policy Review Group NHS Ayrshire and Arran Warning - this document is uncontrolled when printed
Section 10 Guidelines for the Safe Handling and Disposal of Needles and Sharps On behalf of Infection Control Policy Review Group NHS Ayrshire and Arran Warning - this document is uncontrolled when printed
Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text)
 Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text) Key features of RCT (randomized controlled trial) One group (treatment) receives its treatment at the same time another
Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text) Key features of RCT (randomized controlled trial) One group (treatment) receives its treatment at the same time another
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Insulin Administration by Syringe 10/24/2012 1
 Insulin Administration by Syringe 10/24/2012 1 This PowerPoint and test will satisfy the knowledge portion of medication training for High Alert/High Risk Medication - Insulin. This PowerPoint covers basic
Insulin Administration by Syringe 10/24/2012 1 This PowerPoint and test will satisfy the knowledge portion of medication training for High Alert/High Risk Medication - Insulin. This PowerPoint covers basic
HUMULIN (HU-mu-lin) N
 Instructions for Use HUMULIN (HU-mu-lin) N (human insulin [rdna origin] isophane suspension) vial (100 Units/mL, U-100) Read the Instructions for Use before you start taking HUMULIN N and each time you
Instructions for Use HUMULIN (HU-mu-lin) N (human insulin [rdna origin] isophane suspension) vial (100 Units/mL, U-100) Read the Instructions for Use before you start taking HUMULIN N and each time you
REPUBLIC OF KENYA MINISTRY OF HEALTH NATIONAL POLICY ON INJECTION SAFETY AND MEDICAL WASTE MANAGEMENT
 REPUBLIC OF KENYA MINISTRY OF HEALTH NATIONAL POLICY ON INJECTION SAFETY AND MEDICAL WASTE MANAGEMENT MINISTRY OF HEALTH NATIONAL POLICY INJECTION SAFETY AND MEDICAL WASTE MANAGEMENT FEBRUARY 2007 National
REPUBLIC OF KENYA MINISTRY OF HEALTH NATIONAL POLICY ON INJECTION SAFETY AND MEDICAL WASTE MANAGEMENT MINISTRY OF HEALTH NATIONAL POLICY INJECTION SAFETY AND MEDICAL WASTE MANAGEMENT FEBRUARY 2007 National
Frequently Asked Questions (FAQs)
 Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
Scottish Medicines Consortium
 Scottish Medicines Consortium insulin glulisine for subcutaneous injection 100 units/ml (Apidra ) No. (298/06) Sanofi Aventis 4 August 2006 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium insulin glulisine for subcutaneous injection 100 units/ml (Apidra ) No. (298/06) Sanofi Aventis 4 August 2006 The Scottish Medicines Consortium (SMC) has completed its assessment
PATIENT INFORMATION. Medicine To Treat: D iabetes. What You Need to Know About. Insulin
 PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
FERNDALE AREA SCHOOL DISTRICT
 No. 210 SECTION: PUPILS FERNDALE AREA SCHOOL DISTRICT TITLE: MEDICATIONS ADOPTED: AUGUST 1985 REVISED: DECEMBER 6, 2000 MAY 9, 2007 JUNE 17, 2009 JUNE 18, 2014 210. MEDICATIONS REVISED: 1. Purpose The
No. 210 SECTION: PUPILS FERNDALE AREA SCHOOL DISTRICT TITLE: MEDICATIONS ADOPTED: AUGUST 1985 REVISED: DECEMBER 6, 2000 MAY 9, 2007 JUNE 17, 2009 JUNE 18, 2014 210. MEDICATIONS REVISED: 1. Purpose The
Frequently Asked Questions
 Frequently Asked Questions 1. What is the purpose of this research study?... 2 2. Who can participate?... 2 3. How is the medication given?... 2 4. Are there any other medications administered as part
Frequently Asked Questions 1. What is the purpose of this research study?... 2 2. Who can participate?... 2 3. How is the medication given?... 2 4. Are there any other medications administered as part
Manor Park Primary School
 Manor Park Primary School Managing Medical Needs and Medicines in School Policy September 2015. Managing Medical Needs and Medicines in School Policy (Refer to DSEN, Inclusion, Safeguarding policies) At
Manor Park Primary School Managing Medical Needs and Medicines in School Policy September 2015. Managing Medical Needs and Medicines in School Policy (Refer to DSEN, Inclusion, Safeguarding policies) At
Study Design and Statistical Analysis
 Study Design and Statistical Analysis Anny H Xiang, PhD Department of Preventive Medicine University of Southern California Outline Designing Clinical Research Studies Statistical Data Analysis Designing
Study Design and Statistical Analysis Anny H Xiang, PhD Department of Preventive Medicine University of Southern California Outline Designing Clinical Research Studies Statistical Data Analysis Designing
Medicare Drug Coverage Under Part A, Part B, and Part D
 Medicare Drug Coverage Under Part A, Part B, and Part D Medicare Part A and Part B generally do not cover outpatient prescription drugs, most of which are now covered under Part D. This document and the
Medicare Drug Coverage Under Part A, Part B, and Part D Medicare Part A and Part B generally do not cover outpatient prescription drugs, most of which are now covered under Part D. This document and the
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
 SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
PHARMACOTHERAPY HOW TO INJECT INSULIN. Living your life as normal as possible. www.lilly-pharma.de www.lilly-diabetes.de
 PHARMACOTHERAPY HOW TO INJECT INSULIN Living your life as normal as possible www.lilly-pharma.de www.lilly-diabetes.de In Germany about 1.9 million people with diabetes are being treated with insulin.
PHARMACOTHERAPY HOW TO INJECT INSULIN Living your life as normal as possible www.lilly-pharma.de www.lilly-diabetes.de In Germany about 1.9 million people with diabetes are being treated with insulin.
Media Kit Injections Without Needles
 Media Kit Injections Without Needles Company Overview PharmaJet is a medical device company dedicated to the development of needle-free injection technology, with the goal of reducing the use of needles
Media Kit Injections Without Needles Company Overview PharmaJet is a medical device company dedicated to the development of needle-free injection technology, with the goal of reducing the use of needles
Medications or therapeutic solutions may be injected directly into the bloodstream
 Intravenous Therapy Medications or therapeutic solutions may be injected directly into the bloodstream for immediate circulation and use by the body. State practice acts designate which health care professionals
Intravenous Therapy Medications or therapeutic solutions may be injected directly into the bloodstream for immediate circulation and use by the body. State practice acts designate which health care professionals
POSSIBLE NURSING DIAGNOSIS: Pain Potential for Infection / Infection Fluid volume deficit
 1 Procedure for Subcutaneous Over-the-needle Cannula Insertion, Removal, Medication Administration, and Fluid Administration for the Individual in the Home PURPOSE: To provide medication via the subcutaneous
1 Procedure for Subcutaneous Over-the-needle Cannula Insertion, Removal, Medication Administration, and Fluid Administration for the Individual in the Home PURPOSE: To provide medication via the subcutaneous
Blood/Bodily Fluid Exposure and Needlestick Injury Policy Statement & HIV PEP Kit Dispensing Guideline
 Blood/Bodily Fluid Exposure and Needlestick Injury Policy Statement & HIV PEP Kit Dispensing Guideline Providing certain services in a pharmacy, especially injections, carries a risk for the pharmacist
Blood/Bodily Fluid Exposure and Needlestick Injury Policy Statement & HIV PEP Kit Dispensing Guideline Providing certain services in a pharmacy, especially injections, carries a risk for the pharmacist
Bloodborne Pathogens. San Diego Unified School District Nursing & Wellness Program August 2013
 Bloodborne Pathogens San Diego Unified School District Nursing & Wellness Program August 2013 Why Another In-service?? Cal/OSHA mandates that employees with occupational exposure are informed at the time
Bloodborne Pathogens San Diego Unified School District Nursing & Wellness Program August 2013 Why Another In-service?? Cal/OSHA mandates that employees with occupational exposure are informed at the time
Medication Guide. SYMLIN (SĬM-lĭn) (pramlintide acetate) injection
 Page 1 Medication Guide SYMLIN (SĬM-lĭn) (pramlintide acetate) injection Read the Medication Guide and the Patient Instructions for Use that come with your SYMLIN product before you start using it and
Page 1 Medication Guide SYMLIN (SĬM-lĭn) (pramlintide acetate) injection Read the Medication Guide and the Patient Instructions for Use that come with your SYMLIN product before you start using it and
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE
 ICH E6 GCP: Consolidated Guideline: Investigator 1/7 Institutional Review Board Services ICH HARMONIZED TRIPARTITE GUIDELINE E6: GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE 4. INVESTIGATOR 4.1 Investigator's
ICH E6 GCP: Consolidated Guideline: Investigator 1/7 Institutional Review Board Services ICH HARMONIZED TRIPARTITE GUIDELINE E6: GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE 4. INVESTIGATOR 4.1 Investigator's
Study Protocol Template
 Study Protocol Template (Chart Reviews) Instructions: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. It
Study Protocol Template (Chart Reviews) Instructions: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. It
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Medication Administration for Non-Licensed School Staff
 Medication Administration for Non-Licensed School Staff School Health Issues A federal mandate created in the 1970s obligated schools to provide children with medical services, including medication administration.
Medication Administration for Non-Licensed School Staff School Health Issues A federal mandate created in the 1970s obligated schools to provide children with medical services, including medication administration.
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Harmony Clinical Trial Medical Media Factsheet
 Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
8/5/2015. Double Checking Practices. Background. Does a Double Checking Insulin Procedure Improve Patient Safety?
 Disclosure to Participants Notice of Requirements For Successful Completion Please refer to learning goals and objectives Learners must attend the full activity and complete the evaluation in order to
Disclosure to Participants Notice of Requirements For Successful Completion Please refer to learning goals and objectives Learners must attend the full activity and complete the evaluation in order to
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Hand Hygiene and Infection Control
 C Hand Hygiene and Infection Control Sirius Business Services Ltd www.siriusbusinessservices.co.uk Tel 01305 769969 info@siriusbusinessservices.co.uk Whatever your First Aid, Fire Safety or Health & Safety
C Hand Hygiene and Infection Control Sirius Business Services Ltd www.siriusbusinessservices.co.uk Tel 01305 769969 info@siriusbusinessservices.co.uk Whatever your First Aid, Fire Safety or Health & Safety
INTRODUCTION TO INSULIN
 INTRODUCTION TO INSULIN This section will give you some practical advice about using insulin. Your diabetes team will answer any particular questions that you may have e.g. dose of insulin. TYPES OF INSULIN
INTRODUCTION TO INSULIN This section will give you some practical advice about using insulin. Your diabetes team will answer any particular questions that you may have e.g. dose of insulin. TYPES OF INSULIN
National Patient Safety Goals Effective January 1, 2015
 National Patient Safety Goals Goal 1 Nursing are enter ccreditation Program Improve the accuracy of patient and resident identification. NPSG.01.01.01 Use at least two patient or resident identifiers when
National Patient Safety Goals Goal 1 Nursing are enter ccreditation Program Improve the accuracy of patient and resident identification. NPSG.01.01.01 Use at least two patient or resident identifiers when
Health and Safety (Sharp Instruments in Healthcare) Regulations 2013
 Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 Guidance for employers and employees HSE information sheet Health Services Information Sheet 7 This information sheet is for healthcare
Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 Guidance for employers and employees HSE information sheet Health Services Information Sheet 7 This information sheet is for healthcare
