PRESCRIBING INFORMATION. Only. Lactulose Solution USP DESCRIPTION
|
|
|
- Coral Lynch
- 9 years ago
- Views:
Transcription
1 PRESCRIBING INFORMATION Only Lactulose Solution USP DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 ml of Lactulose Solution USP contains: 10 g lactulose (and not more than 1.6 g galactose, not more than 1.2 g lactose, not more than 0.8 g epilactose, and not more than 0.1 g fructose). It also contains D&C Yellow No. 10, FD & C Yellow No. 6 and purified water. The ph range is 2.5 to 6.5. Lactulose is a colonic acidifier which promotes laxation. The chemical name for lactulose is 4-0-β-D-galactopyranosyl-D-fructofuranose and the molecular formula is C 12 H 22 O 11. It has the following structural formula: The molecular weight is It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired
2 bowel movement. Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours. INDICATIONS AND USAGE For the treatment of constipation. In patients with a history of chronic constipation, lactulose solution therapy increases the number of bowel movements per day and the number of days on which bowel movements occur. CONTRAINDICATIONS Since lactulose solution contains galactose (not more than 1.6 g/15 ml), it is contraindicated in patients who require a low galactose diet. WARNINGS A theoretical hazard may exist for patients being treated with lactulose solution who may be required to undergo electrocautery procedures during proctoscopy or colonoscopy. Accumulation of H 2 gas in significant concentration in the presence of an electrical spark may result in an explosive reaction. Although this complication has not been reported with lactulose, patients on lactulose therapy undergoing such procedures should have a thorough bowel cleansing with a nonfermentable solution. Insufflation of CO 2 as an additional safeguard may be pursued but is considered to be a redundant measure. PRECAUTIONS General: Since lactulose solution contains galactose (not more than 1.6 g/15 ml) and lactose (not more than 1.2 g/15 ml), it should be used with caution in diabetics. Information for Patients: In the event that an unusual diarrheal condition occurs, contact your physician. Laboratory Tests: Elderly, debilitated patients who receive lactulose for more than 6 months should have serum electrolytes (potassium, chloride, carbon dioxide) measured periodically. Drug Interactions: Results of preliminary studies in humans and rats suggest that nonabsorbable antacids given concurrently with lactulose may inhibit the desired lactulose-induced drop in colonic ph. Therefore, a possible lack of desired effect of treatment should be taken into consideration before such drugs are given concomitantly with lactulose.
3 Carcinogenesis, Mutagenesis, Impairment of Fertility: There are no known human data on long-term potential for carcinogenicity, mutagenicity, or impairment of fertility. There are no known animal data on long-term potential for mutagenicity. Administration of lactulose solution in the diet of mice for 18 months in concentrations of 3% and 10% (v/w) did not produce any evidence of carcinogenicity. In studies in mice, rats, and rabbits, doses of lactulose solution up to 6 or 12 ml/kg/day produced no deleterious effects in breeding, conception, or parturition. Pregnancy: Teratogenic Effects. Pregnancy Category B. Reproduction studies have been performed in mice, rats, and rabbits at doses up to three or six times the usual human oral dose and have revealed no evidence of impaired fertility or harm to the fetus due to lactulose. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lactulose is administered to a nursing woman. Pediatric Use: Safety and effectiveness in pediatric patients have not been established. Precise frequency data are not available. ADVERSE REACTIONS Initial dosing may produce flatulence and intestinal cramps, which are usually transient. Excessive dosage can lead to diarrhea with potential complications such as loss of fluids, hypokalemia, and hypernatremia. Nausea and vomiting have been reported. OVERDOSAGE Signs and Symptoms: There have been no reports of accidental overdosage. In the event of overdosage, it is expected that diarrhea and abdominal cramps would be the major symptoms. Medication should be terminated. Oral LD 50 : The acute oral LD 50 of the drug is 48.8 ml/kg in mice and greater than 30 ml/kg in rats. Dialysis: Dialysis data are not available for lactulose. Its molecular similarity to sucrose, however, would suggest that it should be dialyzable.
4 DOSAGE AND ADMINISTRATION The usual dose is 1 to 2 tablespoonfuls (15 to 30 ml, containing 10 g to 20 g of lactulose) daily. The dose may be increased to 60 ml daily if necessary. Twenty-four to 48 hours may be required to produce a normal bowel movement. Note: Some patients have found that lactulose solution may be more acceptable when mixed with fruit juice, water, or milk. HOW SUPPLIED Lactulose Solution USP, a clear, yellow to golden yellow solution, contains lactulose 667 mg/ml (10 g/15 ml), available as: NDC , 237 ml (8 fl. oz.) NDC , 473 ml (16 fl. oz.) NDC , 946 ml (32 fl. oz.) NDC , 1.89 L (64 fl. oz.) Store at C (68-77 F) [See USP Controlled Room Temperature]. Do not freeze. Keep tightly closed. Under recommended storage conditions, a normal darkening of color may occur. Such darkening is characteristic of sugar solutions and does not affect therapeutic action. Prolonged exposure to temperatures above 86 F (30 C) or to direct light may cause extreme darkening and turbidity which may be pharmaceutically objectionable. If this condition develops, do not use. Prolonged exposure to freezing temperatures may cause change to a semisolid, too viscous to pour. Viscosity will return to normal upon warming to room temperature. Manufactured by: Apotex Inc. Toronto, Ontario Canada M9L 1T9 Manufactured for: Apotex Corp. Weston, FL 33326
5 May 2005
FOR ORAL OR RECTAL ADMINISTRATION FOR THE PREVENTION AND TREATMENT OF PORTAL-SYSTEMIC ENCEPHALOPATHY
 FORM NO. 1360 VC3074 Rev.2/08 LACTULOSE SOLUTION, USP 10 g/15 ml FOR ORAL OR RECTAL ADMINISTRATION FOR THE PREVENTION AND TREATMENT OF PORTAL-SYSTEMIC ENCEPHALOPATHY Rx Only DESCRIPTION Lactulose is a
FORM NO. 1360 VC3074 Rev.2/08 LACTULOSE SOLUTION, USP 10 g/15 ml FOR ORAL OR RECTAL ADMINISTRATION FOR THE PREVENTION AND TREATMENT OF PORTAL-SYSTEMIC ENCEPHALOPATHY Rx Only DESCRIPTION Lactulose is a
Actilax Lactulose PRODUCT INFORMATION
 Actilax Lactulose PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Lactulose Structural formula: Molecular formula: C 12 H 22 O 11 Mol. Wt. 342.3 CAS Registry no.: 4618-18-2 DESCRIPTION Lactulose
Actilax Lactulose PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Lactulose Structural formula: Molecular formula: C 12 H 22 O 11 Mol. Wt. 342.3 CAS Registry no.: 4618-18-2 DESCRIPTION Lactulose
ENZAR FORTE TABLETS. (derived from Pancreatin USP) Sodium tauroglycocholate BPC 65mg (with sugar coating containing essential carminative oils)
 ENZAR FORTE TABLETS COMPOSITION Each enteric-coated tablet contains: Amylase activity -15,000 USP units Lipase activity -4,000 USP units Protease activity -15,000 USP units of (derived from Pancreatin
ENZAR FORTE TABLETS COMPOSITION Each enteric-coated tablet contains: Amylase activity -15,000 USP units Lipase activity -4,000 USP units Protease activity -15,000 USP units of (derived from Pancreatin
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
PHENYLEPHRINE HYDROCHLORIDE INJECTION USP
 PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
2 DOSAGE AND ADMINISTRATION 2.1 Dosing and Dose Adjustment
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
Revised: 9/2014. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use. Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container
 Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Section 1 - Chemical Product and Company Identification 20242-0000, 20242-0010, 20242-0050, 20242-5000, 41999-0000, 41999-1000, 41999-5000
 Material Safety Data Sheet Tannic acid MSDS Name: Catalog Numbers: Synonyms: Section 1 - Chemical Product and Company Identification Tannic acid 20242-0000, 20242-0010, 20242-0050, 20242-5000, 41999-0000,
Material Safety Data Sheet Tannic acid MSDS Name: Catalog Numbers: Synonyms: Section 1 - Chemical Product and Company Identification Tannic acid 20242-0000, 20242-0010, 20242-0050, 20242-5000, 41999-0000,
PA 2286 AMP INFORMATION FOR THE PHYSICIAN H 2
 1 INFORMATION FOR THE PHYSICIAN PA 2286 AMP GLUCAGON FOR INJECTION (rdna ORIGIN) DESCRIPTION Glucagon for Injection (rdna origin) is a polypeptide hormone identical to human glucagon that increases blood
1 INFORMATION FOR THE PHYSICIAN PA 2286 AMP GLUCAGON FOR INJECTION (rdna ORIGIN) DESCRIPTION Glucagon for Injection (rdna origin) is a polypeptide hormone identical to human glucagon that increases blood
Medication Guide. Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
 Medication Guide MoviPrep (moo-vee-prěp) (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid for oral solution) Read this Medication Guide before you start
Medication Guide MoviPrep (moo-vee-prěp) (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid for oral solution) Read this Medication Guide before you start
MATERIAL SAFETY DATA SHEET Weiman Jewelry Cleaner
 Product Name: Product Number: Product Use: Manufacturer/Supplier: MATERIAL SAFETY DATA SHEET Section 1: PRODUCT AND COMPANY IDENTIFICATION 2306C Jewelry cleaner. Weiman Products 755 Tristate Parkway Gurnee,
Product Name: Product Number: Product Use: Manufacturer/Supplier: MATERIAL SAFETY DATA SHEET Section 1: PRODUCT AND COMPANY IDENTIFICATION 2306C Jewelry cleaner. Weiman Products 755 Tristate Parkway Gurnee,
Dacarbazine for Injection, USP Dacarbazine Antineoplastic
 MATERIAL SAFETY DATA SHEET SECTION 1 - PRODUCT & COMPANY IDENTIFICATION 1501 East Woodfield Road Suite 300 East Schaumburg, IL 60173 (847) 969-2700 Product Identifier: Chemical Name: Category: Emergency
MATERIAL SAFETY DATA SHEET SECTION 1 - PRODUCT & COMPANY IDENTIFICATION 1501 East Woodfield Road Suite 300 East Schaumburg, IL 60173 (847) 969-2700 Product Identifier: Chemical Name: Category: Emergency
CVP Chemotherapy Regimen for Lymphoma Information for Patients
 CVP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cyclophosphamide (Cytoxan ) V: Vincristine (Oncovin ) P: Prednisone How Is This Regimen Given? CVP is given every
CVP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cyclophosphamide (Cytoxan ) V: Vincristine (Oncovin ) P: Prednisone How Is This Regimen Given? CVP is given every
X-ray (Radiography), Lower GI Tract
 Scan for mobile link. X-ray (Radiography), Lower GI Tract What is Lower GI Tract X-ray Radiography (Barium Enema)? Lower gastrointestinal (GI) tract radiography, also called a lower GI or barium enema,
Scan for mobile link. X-ray (Radiography), Lower GI Tract What is Lower GI Tract X-ray Radiography (Barium Enema)? Lower gastrointestinal (GI) tract radiography, also called a lower GI or barium enema,
INFORMATION FOR PATIENT. WARNINGS Using more than one enema in 24 hours can be harmful.
 FLEET ENEMA, A SALINE LAXATIVE - ready-to-use squeeze bottle FLEET ENEMA EXTRA, A SALINE LAXATIVE - ready-to-use squeeze bottle FLEET PEDIA-LAX ENEMA, A SALINE LAXATIVE - ready-to-use squeeze bottle FLEET
FLEET ENEMA, A SALINE LAXATIVE - ready-to-use squeeze bottle FLEET ENEMA EXTRA, A SALINE LAXATIVE - ready-to-use squeeze bottle FLEET PEDIA-LAX ENEMA, A SALINE LAXATIVE - ready-to-use squeeze bottle FLEET
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 SECTION I: PRODUCT INFORMATION REVISION DATE: April 27 th, 2015 USAGE: SUPPLIER: SIKA CANADA INC. 601, avenue Delmar Pointe Claire, QC H9R 4A9 EMERGENCY TELEPHONE NUMBER: CANUTEC (collect)
Page 1 of 5 SECTION I: PRODUCT INFORMATION REVISION DATE: April 27 th, 2015 USAGE: SUPPLIER: SIKA CANADA INC. 601, avenue Delmar Pointe Claire, QC H9R 4A9 EMERGENCY TELEPHONE NUMBER: CANUTEC (collect)
PRODUCT ID: LIDOCAINE HYDROCHLORIDE AND EPINEPHRINE INJECTION
 SECTION 15: REGULATORY INFORMATION This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations and the MSDS contains all the information required by the
SECTION 15: REGULATORY INFORMATION This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations and the MSDS contains all the information required by the
Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection
 NEW ZEALAND DATA SHEET Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection Each 1mL contains Glycopyrrolate USP 0.5mg (Glycopyrronium Bromide) and Neostigmine Metilsulfate
NEW ZEALAND DATA SHEET Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection Each 1mL contains Glycopyrrolate USP 0.5mg (Glycopyrronium Bromide) and Neostigmine Metilsulfate
1. NAME OF THE MEDICINAL PRODUCT. Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION. 1 capsule contains:
 1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Material Safety Data Sheet Buffer Solution ph 10.0. Section 1 - Chemical Product and Company Identification
 ISO9001:2000 Certified Material Safety Data Sheet Section 1 - Chemical Product and Company Identification MSDS Name: Catalog Numbers: LC12500, LC12510 Synonyms: None Company Identification: LabChem Inc
ISO9001:2000 Certified Material Safety Data Sheet Section 1 - Chemical Product and Company Identification MSDS Name: Catalog Numbers: LC12500, LC12510 Synonyms: None Company Identification: LabChem Inc
Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules
 Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking Tasigna and each time you get a refill. There may be new information. This information does
Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking Tasigna and each time you get a refill. There may be new information. This information does
1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING
 SIGMA-ALDRICH SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 Version 3.3 Revision Date 11.12.2008 Print Date 16.01.2009 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA 1. IDENTIFICATION
SIGMA-ALDRICH SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 Version 3.3 Revision Date 11.12.2008 Print Date 16.01.2009 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA 1. IDENTIFICATION
OFF! CLIP-ON MOSQUITO REPELLENT
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and Son, Limited 1 Webster Street Brantford ON N3T 5R1
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and Son, Limited 1 Webster Street Brantford ON N3T 5R1
Emergency Telephone (800) 551-7176
 MATERIAL SAFETY DATA SHEET SECTION 1 - PRODUCT & COMPANY IDENTIFICATION 1501 East Woodfield Road Suite 300 East Schaumburg, IL 60173 (847) 969-2700 Product Identifier: Chemical Name: Category: Mesna Detoxifying
MATERIAL SAFETY DATA SHEET SECTION 1 - PRODUCT & COMPANY IDENTIFICATION 1501 East Woodfield Road Suite 300 East Schaumburg, IL 60173 (847) 969-2700 Product Identifier: Chemical Name: Category: Mesna Detoxifying
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients
 VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients The Regimen contains: V = vincristine (Oncovin ) A = Adriamycin (doxorubicin) D = Decadron (dexamethasone) How Is This Regimen Given?
VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients The Regimen contains: V = vincristine (Oncovin ) A = Adriamycin (doxorubicin) D = Decadron (dexamethasone) How Is This Regimen Given?
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 SECTION I: PRODUCT INFORMATION REVISION DATE: February 1 st, 2015 USAGE: ONE COMPONENT URETHANE BASED SEALANT MANUFACTURER/SUPPLIER: SIKA CANADA INC. 601, avenue Delmar Pointe Claire, QC H9R
Page 1 of 5 SECTION I: PRODUCT INFORMATION REVISION DATE: February 1 st, 2015 USAGE: ONE COMPONENT URETHANE BASED SEALANT MANUFACTURER/SUPPLIER: SIKA CANADA INC. 601, avenue Delmar Pointe Claire, QC H9R
: Water and Glycol Leak Detection Solution
 Water and Glycol Leak Detection Solution Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 05/05/2015 Version: 1 SECTION
Water and Glycol Leak Detection Solution Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 05/05/2015 Version: 1 SECTION
OFF! DEEP WOODS SPORTSMEN INSECT REPELLENT I (PUMP SPRAY)
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : OFF! DEEP WOODS SPORTSMEN INSECT REPELLENT I (PUMP Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : OFF! DEEP WOODS SPORTSMEN INSECT REPELLENT I (PUMP Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and
Material Safety data sheet
 EMERGENCY OVERVIEW METFORMIN HYDROCHLORIDE TABLET contain Metformin and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions of normal occupational
EMERGENCY OVERVIEW METFORMIN HYDROCHLORIDE TABLET contain Metformin and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions of normal occupational
Problems of the Digestive System
 The American College of Obstetricians and Gynecologists f AQ FREQUENTLY ASKED QUESTIONS FAQ120 WOMEN S HEALTH Problems of the Digestive System What are some common digestive problems? What is constipation?
The American College of Obstetricians and Gynecologists f AQ FREQUENTLY ASKED QUESTIONS FAQ120 WOMEN S HEALTH Problems of the Digestive System What are some common digestive problems? What is constipation?
Patient Medication Guide Brochure
 Patient Medication Guide Brochure 1 MEDICATION GUIDE TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking TASIGNA and each time you get a refill. There may be new
Patient Medication Guide Brochure 1 MEDICATION GUIDE TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking TASIGNA and each time you get a refill. There may be new
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Patients who fail to bring a driver/someone to stay with them for the night will have their procedure cancelled immediately.
 Preparing for your Colonoscopy You must have someone and/or a driver accompany you and stay with you for 24 hours after your procedure. Patients who fail to bring a driver/someone to stay with them for
Preparing for your Colonoscopy You must have someone and/or a driver accompany you and stay with you for 24 hours after your procedure. Patients who fail to bring a driver/someone to stay with them for
PATIENT MEDICATION INFORMATION
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr CYRAMZA ramucirumab Read this carefully before you receive CYRAMZA (pronounced "si ram - ze"). This leaflet is a
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr CYRAMZA ramucirumab Read this carefully before you receive CYRAMZA (pronounced "si ram - ze"). This leaflet is a
MEDICATION GUIDE JUXTAPID (JUKS-tuh-pid) (lomitapide) capsules
 MEDICATION GUIDE JUXTAPID (JUKS-tuh-pid) (lomitapide) capsules Read this Medication Guide before your treatment. What is the most important information I should know about JUXTAPID? JUXTAPID is available
MEDICATION GUIDE JUXTAPID (JUKS-tuh-pid) (lomitapide) capsules Read this Medication Guide before your treatment. What is the most important information I should know about JUXTAPID? JUXTAPID is available
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
C. difficile Infections
 C. difficile Infections Introduction C. difficile is a type of bacteria that can cause diarrhea and infection of the colon. This bacterium is more likely to infect patients at hospitals and other healthcare
C. difficile Infections Introduction C. difficile is a type of bacteria that can cause diarrhea and infection of the colon. This bacterium is more likely to infect patients at hospitals and other healthcare
CHOC CHILDREN SUROLOGY CENTER. Constipation
 Constipation What is constipation? Constipation is a condition in which a person has uncomfortable or infrequent bowel movements. Generally, a person is considered to be constipated when bowel movements
Constipation What is constipation? Constipation is a condition in which a person has uncomfortable or infrequent bowel movements. Generally, a person is considered to be constipated when bowel movements
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
MACROGOL COMPOUND ORAL POWDER (sodium chloride, sodium hydrogen carbonate, potassium chloride, macrogol 3350) PL 33579/0001 UKPAR TABLE OF CONTENTS
 MACROGOL COMPOUND ORAL POWDER (sodium chloride, sodium hydrogen carbonate, potassium chloride, macrogol 3350) PL 33579/0001 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps
MACROGOL COMPOUND ORAL POWDER (sodium chloride, sodium hydrogen carbonate, potassium chloride, macrogol 3350) PL 33579/0001 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps
Perrigo Pharmaceuticals Co.
 Ibuprofen Page 1 of 7 Perrigo Pharmaceuticals Co. U.S. Pharmaceuticals Division MATERIAL SAFETY DATA SHEET NDC Product(s) Code(s): 0121-4774-05 0121-4774-10 Product Name: Ibuprofen Oral Suspension 100mg/5mL
Ibuprofen Page 1 of 7 Perrigo Pharmaceuticals Co. U.S. Pharmaceuticals Division MATERIAL SAFETY DATA SHEET NDC Product(s) Code(s): 0121-4774-05 0121-4774-10 Product Name: Ibuprofen Oral Suspension 100mg/5mL
CHOP Chemotherapy Regimen for Lymphoma Information for Patients
 CHOP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cytoxan (cyclophosphamide) H: Adriamycin (hydroxy doxorubicin) O: vincristine (Oncovin ) P: Prednisone How Is This
CHOP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cytoxan (cyclophosphamide) H: Adriamycin (hydroxy doxorubicin) O: vincristine (Oncovin ) P: Prednisone How Is This
Independent Forensics of Illinois
 Independent Forensics of Illinois RSID -Semen Lab kit Universal Buffer #0230 Material Safety Data Sheet Date Updated: 01/2014 SECTION 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION MSDS Name: RSID -SEMEN
Independent Forensics of Illinois RSID -Semen Lab kit Universal Buffer #0230 Material Safety Data Sheet Date Updated: 01/2014 SECTION 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION MSDS Name: RSID -SEMEN
Lesson 3 Managing Food Allergies
 Lesson 3 Managing Food Allergies Lesson at a Glance Time Allowed (1 hour) 5 minutes Introduction to Managing Food Allergies 15 minutes Objective 1: Creating a Food Allergy Policy Topic Activity Materials
Lesson 3 Managing Food Allergies Lesson at a Glance Time Allowed (1 hour) 5 minutes Introduction to Managing Food Allergies 15 minutes Objective 1: Creating a Food Allergy Policy Topic Activity Materials
MATERIAL SAFETY DATA SHEET
 The Procter & Gamble Company P&G Household Care Fabric & Home Care Innovation Center 5299 Spring Grove Avenue Cincinnati, OH 45217-1087 MATERIAL SAFETY DATA SHEET MSDS #: RQ1311500 Issue Date: 11/14/2013
The Procter & Gamble Company P&G Household Care Fabric & Home Care Innovation Center 5299 Spring Grove Avenue Cincinnati, OH 45217-1087 MATERIAL SAFETY DATA SHEET MSDS #: RQ1311500 Issue Date: 11/14/2013
MATERIAL SAFETY DATA SHEET Goo Gone Spray Gel
 Product Name: Product Number: Product Use: Manufacturer/Supplier: MATERIAL SAFETY DATA SHEET Section 1: PRODUCT AND COMPANY IDENTIFICATION 2137C Cleaner. GOO GONE 755 Tristate Parkway Gurnee, IL 60031
Product Name: Product Number: Product Use: Manufacturer/Supplier: MATERIAL SAFETY DATA SHEET Section 1: PRODUCT AND COMPANY IDENTIFICATION 2137C Cleaner. GOO GONE 755 Tristate Parkway Gurnee, IL 60031
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
SAFETY DATA SHEET. according to 1907/2006/EC, Article 31. Zenith 6B Toilet Cleaner (072200)
 SAFETY DATA SHEET according to 190/2006/EC, Article 31 Page 1/5 Zenith 6B Toilet Cleaner (02200) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier
SAFETY DATA SHEET according to 190/2006/EC, Article 31 Page 1/5 Zenith 6B Toilet Cleaner (02200) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Chronic Diarrhea in Children
 Chronic Diarrhea in Children National Digestive Diseases Information Clearinghouse What is chronic diarrhea? Diarrhea is loose, watery stools. Chronic, or long lasting, diarrhea typically lasts for more
Chronic Diarrhea in Children National Digestive Diseases Information Clearinghouse What is chronic diarrhea? Diarrhea is loose, watery stools. Chronic, or long lasting, diarrhea typically lasts for more
Material Safety Data Sheet
 Material Safety Data Sheet acc. to OSHA and ANSI 1 Identification of substance: Product details: Product name: Zero-Valent Iron Nanoparticles Stock number: 8001NJ Manufacturer/Supplier: Nanostructured
Material Safety Data Sheet acc. to OSHA and ANSI 1 Identification of substance: Product details: Product name: Zero-Valent Iron Nanoparticles Stock number: 8001NJ Manufacturer/Supplier: Nanostructured
OSMITROL - mannitol injection, solution Baxter Healthcare Corporation
 OSMITROL - mannitol injection, solution Baxter Healthcare Corporation DESCRIPTION OSMITROL Injection (Mannitol Injection, USP) is a sterile, nonpyrogenic solution of Mannitol, USP in a single dose container
OSMITROL - mannitol injection, solution Baxter Healthcare Corporation DESCRIPTION OSMITROL Injection (Mannitol Injection, USP) is a sterile, nonpyrogenic solution of Mannitol, USP in a single dose container
MATERIAL SAFETY DATA SHEET Finished Product.
 MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 - CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Gillette Fresh n Clean Free Fall Body Wash (99897878) Company Identification:
MATERIAL SAFETY DATA SHEET Finished Product. SECTION 1 - CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Gillette Fresh n Clean Free Fall Body Wash (99897878) Company Identification:
Material Safety Data Sheet
 0 1 0 Material Safety Data Sheet Sodium Dodecyl Sulfate, 20% MSDS He a lt h Fire Re a c t iv it y Pe rs o n a l Pro t e c t io n 1 0 0 G Section 1: Chemical Product and Company Identification Product Name:
0 1 0 Material Safety Data Sheet Sodium Dodecyl Sulfate, 20% MSDS He a lt h Fire Re a c t iv it y Pe rs o n a l Pro t e c t io n 1 0 0 G Section 1: Chemical Product and Company Identification Product Name:
23.4% Sodium Chloride Injection, USP (4 meq/ml) Glass Fliptop Vials Rx only Pharmacy Bulk Package Not for Direct Infusion
 CONCENTRATE CAUTION: MUST BE DILUTED FOR I.V. USE. 23.4% Sodium Chloride Injection, USP (4 meq/ml) Glass Fliptop Vials Rx only Pharmacy Bulk Package Not for Direct Infusion DESCRIPTION 23.4% Sodium Chloride
CONCENTRATE CAUTION: MUST BE DILUTED FOR I.V. USE. 23.4% Sodium Chloride Injection, USP (4 meq/ml) Glass Fliptop Vials Rx only Pharmacy Bulk Package Not for Direct Infusion DESCRIPTION 23.4% Sodium Chloride
Material Safety Data Sheet Sulfuric Acid Solutions 0.01N 0.125N (>1% w/w) Section 1 - Chemical Product and Company Identification
 An ISO9001 Certified Company Material Safety Data Sheet Section 1 - Chemical Product and Company Identification MSDS Name: Catalog Numbers: LC25655, LC25660, LC25670, LC25675, LC25680, LC25685 Synonyms:
An ISO9001 Certified Company Material Safety Data Sheet Section 1 - Chemical Product and Company Identification MSDS Name: Catalog Numbers: LC25655, LC25660, LC25670, LC25675, LC25680, LC25685 Synonyms:
Material Safety Data Sheet Aluminum Hydroxide Suspension. Section 1 - Chemical Product and Company Identification
 An ISO9001 Certified Company Material Safety Data Sheet Section 1 - Chemical Product and Company Identification MSDS Name: Catalog Numbers: LC10840 Synonyms: None Company Identification: LabChem Inc 200
An ISO9001 Certified Company Material Safety Data Sheet Section 1 - Chemical Product and Company Identification MSDS Name: Catalog Numbers: LC10840 Synonyms: None Company Identification: LabChem Inc 200
Material Safety Data Sheet BZK Antiseptic Towelettes / Wipes
 Issue date Oct. 02/2013 1. Product and Company Identification Product Name BZK Antiseptic Towelettes CAS # Mixture Product use Antiseptic Manufacturer Lernapharm (Loris) Inc 2323 Halpern, St-Laurent (Montreal)
Issue date Oct. 02/2013 1. Product and Company Identification Product Name BZK Antiseptic Towelettes CAS # Mixture Product use Antiseptic Manufacturer Lernapharm (Loris) Inc 2323 Halpern, St-Laurent (Montreal)
SHOUT TRIPLE-ACTING LAUNDRY STAIN REMOVER
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : Use of the : Spot & Stain Remover Substance/Mixture Company : S.C. Johnson & Son, Inc. 1525 Howe Street Racine WI 53403-2236 Emergency
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : Use of the : Spot & Stain Remover Substance/Mixture Company : S.C. Johnson & Son, Inc. 1525 Howe Street Racine WI 53403-2236 Emergency
MATERIAL SAFETY DATA SHEET RedGard Waterproofing and Crack Prevention Membrane
 Product Name: Product Use: Manufacturer/Supplier: Section 1: PRODUCT AND COMPANY IDENTIFICATION Sealant. Custom Building Products 13001 Seal Beach Blvd Seal Beach, CA 90740 Phone Number: (562) 598-8808
Product Name: Product Use: Manufacturer/Supplier: Section 1: PRODUCT AND COMPANY IDENTIFICATION Sealant. Custom Building Products 13001 Seal Beach Blvd Seal Beach, CA 90740 Phone Number: (562) 598-8808
MATERIAL SAFETY DATA SHEET Complies with ANSI Z400.1 Format SECTION 1: PRODUCT IDENTIFICATION
 Staples the Office Superstore, LLC. MSDS 500 Staples Drive Hazard Rating HMIS NFPA Framingham, MA 01702 USA Health Business Phone: 1.800.322.0912 Flammability 24-HR MEDICAL AND DOT EMERGENCIES: 1.800.322.0912
Staples the Office Superstore, LLC. MSDS 500 Staples Drive Hazard Rating HMIS NFPA Framingham, MA 01702 USA Health Business Phone: 1.800.322.0912 Flammability 24-HR MEDICAL AND DOT EMERGENCIES: 1.800.322.0912
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET SECTION 1 - CHEMICAL PRODUCT & COMPANY IDENTIFICATION Pfizer Consumer Healthcare Pfizer Inc 201 Tabor Road Morris Plains, New Jersey 07950 Emergency telephone Hours of operation
MATERIAL SAFETY DATA SHEET SECTION 1 - CHEMICAL PRODUCT & COMPANY IDENTIFICATION Pfizer Consumer Healthcare Pfizer Inc 201 Tabor Road Morris Plains, New Jersey 07950 Emergency telephone Hours of operation
SAFETY DATA SHEET. 4628 West Skyhawk Drive West Jordan, UT 84084-4501 U.S.A. Phone 877.236.4408
 20% Carbamide Peroxide Whitening Film SAFETY DATA SHEET Common Name: Manufacturer: Distributor: Recommended Use: Restrictions on Use: Chemical Name: Chemical Family: Chemical Formula: CAS#: TSCA 8(b):
20% Carbamide Peroxide Whitening Film SAFETY DATA SHEET Common Name: Manufacturer: Distributor: Recommended Use: Restrictions on Use: Chemical Name: Chemical Family: Chemical Formula: CAS#: TSCA 8(b):
Material Safety Data Sheet Calcium bromide hydrate
 Material Safety Data Sheet Calcium bromide hydrate Section 1 - Chemical Product and Company Identification MSDS Name: Calcium bromide hydrate Synonyms: Calcium dibromide dihydrate. Section 2 - Composition,
Material Safety Data Sheet Calcium bromide hydrate Section 1 - Chemical Product and Company Identification MSDS Name: Calcium bromide hydrate Synonyms: Calcium dibromide dihydrate. Section 2 - Composition,
Calcium Folinate Ebewe Data Sheet
 NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
Care and Problems of the Digestive System. Chapter 18 Lesson 2
 Care and Problems of the Digestive System Chapter 18 Lesson 2 Care of the Digestive System Good eating habits are the best way to avoid or minimize digestive system problems. Eat a variety of foods Avoid
Care and Problems of the Digestive System Chapter 18 Lesson 2 Care of the Digestive System Good eating habits are the best way to avoid or minimize digestive system problems. Eat a variety of foods Avoid
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Patient Information Once Weekly FOSAMAX (FOSS-ah-max) (alendronate sodium) Tablets and Oral Solution
 Patient Information Once Weekly FOSAMAX (FOSS-ah-max) (alendronate sodium) Tablets and Oral Solution Read this information before you start taking FOSAMAX *. Also, read the leaflet each time you refill
Patient Information Once Weekly FOSAMAX (FOSS-ah-max) (alendronate sodium) Tablets and Oral Solution Read this information before you start taking FOSAMAX *. Also, read the leaflet each time you refill
Healthy Weight Loss Program
 Healthy Weight Loss Program NEW! SlimStyles PGX Granules SlimStyles PGX SlimStyx: Available in convenient single-serving Slimstyx packets (2.5 g each) All the products in this brochure are available at
Healthy Weight Loss Program NEW! SlimStyles PGX Granules SlimStyles PGX SlimStyx: Available in convenient single-serving Slimstyx packets (2.5 g each) All the products in this brochure are available at
PRESCRIBING INFORMATION K-10. (Potassium Chloride Oral Solution, 10% USP) POTASSIUM REPLACEMENT THERAPY
 PRESCRIBING INFORMATION K-10 (Potassium Chloride Oral Solution, 10% USP) POTASSIUM REPLACEMENT THERAPY GlaxoSmithKline Inc Date of Revision: 7333 Mississauga Road North April 14, 2015 Mississauga, OntarioL5N
PRESCRIBING INFORMATION K-10 (Potassium Chloride Oral Solution, 10% USP) POTASSIUM REPLACEMENT THERAPY GlaxoSmithKline Inc Date of Revision: 7333 Mississauga Road North April 14, 2015 Mississauga, OntarioL5N
Selected Requirements of Prescribing Information
 The Selected Requirements of Prescribing Information (SRPI) is a checklist of 42 important format prescribing information (PI) items based on labeling regulations [21 CFR 201.56(d) and 201.57] and guidances.
The Selected Requirements of Prescribing Information (SRPI) is a checklist of 42 important format prescribing information (PI) items based on labeling regulations [21 CFR 201.56(d) and 201.57] and guidances.
45813E/Revised: April 2008 CYANOCOBALAMIN INJECTION, USP
 45813E/Revised: April 2008 CYANOCOBALAMIN INJECTION, USP DESCRIPTION: Cyanocobalamin Injection, USP is a sterile solution of cyanocobalamin for intramuscular or subcutaneous use. Each ml contains 1000
45813E/Revised: April 2008 CYANOCOBALAMIN INJECTION, USP DESCRIPTION: Cyanocobalamin Injection, USP is a sterile solution of cyanocobalamin for intramuscular or subcutaneous use. Each ml contains 1000
MATERIAL SAFETY DATA SHEET Buffer solution ph 4 (phthalate), traceable to NIST, colored red
 MATERIAL SAFETY DATA SHEET Buffer solution ph 4 (phthalate), traceable to NIST, colored red MSDS Name: Section 1 Chemical Product and Company Identification Catalog Numbers: 38384 0000, 38384 0010 Synonyms:
MATERIAL SAFETY DATA SHEET Buffer solution ph 4 (phthalate), traceable to NIST, colored red MSDS Name: Section 1 Chemical Product and Company Identification Catalog Numbers: 38384 0000, 38384 0010 Synonyms:
FUNCTIONAL BOWEL DISORDERS
 FUNCTIONAL BOWEL DISORDERS Contributed by the International Foundation for Functional Gastrointestinal Disorders (IFFGD) and edited by the Patient Care Committee of the ACG. INTRODUCTION Doctors use the
FUNCTIONAL BOWEL DISORDERS Contributed by the International Foundation for Functional Gastrointestinal Disorders (IFFGD) and edited by the Patient Care Committee of the ACG. INTRODUCTION Doctors use the
MEDICATION GUIDE ACTOPLUS MET (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets
 MEDICATION GUIDE (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets Read this Medication Guide carefully before you start taking and each time you get a refill. There may
MEDICATION GUIDE (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets Read this Medication Guide carefully before you start taking and each time you get a refill. There may
Frequently Asked Questions: Ai-Detox
 What is Ai-Detox? Frequently Asked Questions: Ai-Detox Ai-Detox is a Chinese herbal medicinal formula, produced using state of the art biotechnology, which ensures the utmost standards in quality and safety.
What is Ai-Detox? Frequently Asked Questions: Ai-Detox Ai-Detox is a Chinese herbal medicinal formula, produced using state of the art biotechnology, which ensures the utmost standards in quality and safety.
Irritable Bowel Syndrome
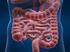 What I need to know about Irritable Bowel Syndrome NATIONAL INSTITUTES OF HEALTH National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services What I need to know
What I need to know about Irritable Bowel Syndrome NATIONAL INSTITUTES OF HEALTH National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services What I need to know
FANTASTIK SCRUBBING BUBBLES ALL PURPOSE CLEANER HEAVY DUTY
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : FANTASTIK SCRUBBING BUBBLES ALL PURPOSE CLEANER Use of the : Hard Surface Cleaner Substance/Mixture Company : S.C. Johnson & Son,
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : FANTASTIK SCRUBBING BUBBLES ALL PURPOSE CLEANER Use of the : Hard Surface Cleaner Substance/Mixture Company : S.C. Johnson & Son,
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Module Three. Risk Assessment
 Module Three Risk Assessment 136 Module Three Introduction to Risk Assessment Time Allotted: 90 Minutes Objectives: Upon completion of this module, the learner will be able to # Define and understand the
Module Three Risk Assessment 136 Module Three Introduction to Risk Assessment Time Allotted: 90 Minutes Objectives: Upon completion of this module, the learner will be able to # Define and understand the
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. Product and Supplier Identification Product Name: Product: Chemical Name: Product Use: Glass Fiber Woven Cloth Orca Composites Woven Cloth (All) E-Glass FRP Manufacturing
MATERIAL SAFETY DATA SHEET 1. Product and Supplier Identification Product Name: Product: Chemical Name: Product Use: Glass Fiber Woven Cloth Orca Composites Woven Cloth (All) E-Glass FRP Manufacturing
WINDEX COMMERCIAL LINE GLASS CLEANER WITH AMMONIA- D
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : WINDEX COMMERCIAL LINE GLASS CLEANER WITH AMMONIA- Use of the : Hard Surface Cleaner Substance/Mixture Company : S.C. Johnson & Son,
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : WINDEX COMMERCIAL LINE GLASS CLEANER WITH AMMONIA- Use of the : Hard Surface Cleaner Substance/Mixture Company : S.C. Johnson & Son,
