Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Endpoints:
|
|
|
- Cecil Mathews
- 7 years ago
- Views:
Transcription
1 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product. Before prescribing any product mentioned in this Register, healthcare professionals should consult prescribing information for the product approved in their country. Study No.: TRX Title: An Open Label, Single Dose, Parallel Group Pilot Study to Evaluate Absorption and Transit Characteristics of TREXIMA* and Relpax in Patients Inside and Outside of an Acute Migraine Attack *currently known as TREXIMET (sumatriptan and naproxen sodium) Rationale: A single combination (sumatriptan and naproxen sodium) contains sumatriptan 85mg (as the succinate salt) formulated with RT Technology and naproxen sodium 500mg (referred to as sumatriptan/naproxen sodium or ). The RT Technology formulation of the sumatriptan portion of the absorbs water more quickly and disintegrates in the stomach allowing for faster dissolution than a conventional sumatriptan. There is reason to believe that liquids have a faster transition time through the stomach even in presence of significant gastric stasis. And hence, a triptan formulated to rapidly dissolve into a liquid state might reach the small intestine, and get absorbed, much faster than a triptan in a conventionally formulated. Phase: III Study Period: 29 August November 2006 Study Design: Open Label, Single Dose, Parallel Group Pilot Study Centers: One study center in the United States. Indication: Acute Treatment of Migraine Treatment: Study medication (either sumatriptan/naproxen sodium or eletriptan) was given orally as a. Each of the administration occasions was separated by > 7 days. Subjects received either sumatriptan/naproxen sodium or eletriptan but not both. All subjects received study medication when they were not experiencing an active migraine attack and the first 5 of the ten subjects in each treatment group who experienced a migraine attack and could come to the clinic for dosing received study medication when they were experiencing an acute migraine attack. A radioactive isotope (indium-111 chloride, approximately 25 microcuries in a 1 microliter volume) was incorporated into the naproxen half of the bilayer sumatriptan/naproxen sodium while the sumatriptan side of the was radiolabeled with technetium (Tc)-99m diethylenetriaminepentaacetate (DTPA) (approximately 50 microcuries in a 1 microliter volume). The eletriptan was radiolabeled with a single isotope (technetium [Tc]-99m DTPA). Since the radioactive isotopes were not absorbed, the process of disintegration and transit through the gastrointestinal (GI) tract could be monitored using a gamma camera that captured images at frequent intervals after ingestion of the dose. Objectives: The primary objective of this study was to evaluate the rate of dispersion, gastric transit time and relative onset of absorption of sumatriptan and naproxen when delivered from sumatriptan/naproxen sodium in migraineurs (International Classification of Headache Disorders [ICHD] II 1.1 and ICHDII 1.2.1) with and without the presence of active (moderate to severe) migraine. Endpoints: The following endpoints were measured in the presence and absence of a migraine attack: Pharmacokinetic parameters for sumatriptan and naproxen, specifically, AUC(0-2), AUC(0-24), AUC(0- ), Cmax, and tmax Pharmacokinetic parameters for eletriptan, specifically, AUC(0-2), AUC(0- ), Cmax, and tmax Time to 10%, 50%, and 90% of the radioactive markers representing sumatriptan, naproxen and eletriptan Time to complete of the radioactive markers representing sumatriptan, naproxen and eletriptan 1
2 Time to complete dispersion of the sumatriptan and naproxen portions of the and the eletriptan Time to first appearance of sumatriptan, naproxen and eletriptan at the proximal small intestine. Small intestine transit and residence of the radioactive markers representing sumatriptan, naproxen and eletriptan. Statistical Methods: Pharmacokinetic parameters, AUC(0-2), AUC(0- ), Cmax and tmax, of sumatriptan, naproxen and eletriptan were summarized descriptively by means of n, arithmetic mean, standard deviation, minimum, median, maximum, 95% confidence intervals about the arithmetic mean. Additionally, AUC (0-24) of sumatriptan and naproxen were summarized in the same manner. Geometric means, 95% confidence intervals and between-subject coefficients of variation (CVb) were derived for log e-transformed parameters, AUC(0-2), AUC(0- ), Cmax and AUC(0-24) for sumatriptan and naproxen. The geometric means and coefficients of variation were calculated. Scintigraphic images were analyzed in a time-lapse format and regions of interest (ROI) were drawn to include the stomach and small intestine. If applicable, regions of interest could also be drawn for the early and distal small intestine and colon. Each image was corrected for radioactive decay, compton scatter and background radiation. The following parameters were determined as necessary: time for the tracer to leave the stomach (T10%, T50% and T90% ), small intestine transit time (SITT50%, if applicable), and time for half of the tracer to arrive at the colon (T50% colon arrival, if applicable). Safety data were summarized using descriptive statistics. Study Population: Male and female subjects, years of age inclusive, who had at least a 6- month history of migraine with or without aura according to the International Headache Society (IHS) criteria ICHD-II 1.1 and Number of Subjects: Total Planned, N Enrolled, N Completed, n (%) 10 (100%) 10 (100%) 20 (100%) Total Number Subjects Withdrawn, N (%) Demographics Total N (All Subjects) Females: Males 10 : 0 9 : 1 19 : 1 Mean Age, years (SD) 30.6 (7.50) 33.3 (7.17) 32.0 (7.27) White/Caucasian/European Heritage, n 10 (100%) 10 (100%) 20 (100%) (%) Pharmacokinetic Results: (PK Population) Geometric Mean (CVb%) Pharmacokinetic Parameters Migraine status N Cmax (ng/ml) non migraine (33.0) migraine (30.8) 1. (range) 2. n = 9 3. n = 4 tmax (hr) ( ) 1.50 ( ) AUC(0-2) (nghr/ml) AUC(0-24) (nghr/ml) AUC(0- ) (nghr/ml) 65.2 (36.2) 232(25.6) 232 (24.8) (22.3) 166 (38.0) 158 (41.7) 3 2
3 Geometric Mean (CVb%) Pharmacokinetic Parameters Migraine status N Cmax tmax AUC(0-2) AUC(0-24) AUC(0- ) (μg/ml) (hr) 1 (μghr/ml) (μghr/ml) (μghr/ml) non migraine (25.5) 4.50 ( ) 23.2 (79.3) 571 (15.0) 901 (21.9) 2 migraine (28.1) 4.00 ( ) 24.4 (117) 627 (20.6) 978 (23.4) 1. (range) 2. n = 9 Geometric Mean (CVb%) Pharmacokinetic Parameters Migraine status N Cmax tmax AUC(0-2) AUC(0- ) (ng/ml) (hr) 1 (nghr/ml) (nghr/ml) non migraine (61.2) 2.50 ( ) 70.2 (97.3) 541 (78.7) 2 migraine (74.8) 2.00 ( ) 78.1 (121) 571 (94.2) 1. (range) 2. n = 9 3
4 Pharmacodynamic Results: (Scintigraphy or PD Population): Time to endpoint Treatment Component Non-Migraine Time to 10% Time to 50% Time to 90% Time to start of (0.1706) ( ) (0.4939) ( ) (0.1833) ( ) (0.5143) ( ) (0.6497) ( ) (0.8381) ( ) (0.8632) ( ) (1.0913) ( ) (1.5014) ( ) (0.1679) ( ) (0.5474) ( ) (0.2028) ( ) Migraine N= (0.2618) ( ) (0.7529) ( ) (0.2888) ( ) (0.5855) ( ) (1.1297) ( ) (0.9052) ( ) (0.9016) ( ) (2.3349) ( ) (1.8197) ( ) (0.1304) ( ) (0.8442) ( ) (0.2909) ( ) 4
5 Time to complete Time to Start of Dispersion Time to Complete Dispersion Time to First Appearance at the Proximal Small Intestine (50% to arrive in the cecum) (1.0397) ( ) (1.7192) ( ) (2.6615) ( ) (0.0169) ( ) (0.5353) ( ) (0.1874) ( ) (0.0738) ( ) (1.1399) ( ) (0.2320) ( ) (1.4415) ( ) (1.1711) ( ) (3.4274) ( ) (1.9445) ( ) (2.6148) ( ) (2.0519) ( ) (0.0626) ( ) (0.6174) ( ) (0.1945) ( ) (0.0671) ( ) (0.9354) ( ) (0.3125) ( ) (0.8894) ( ) (0.9973) ( ) (1.3040) ( ) 5
6 Small Intestine Transit and Residence (Time to 50% through small intestine) (1.4956) ( ) (1.1572) ( ) (3.6643) ( ) (1.1777) ( ) (0.6377) ( ) (1.7037) ( ) Safety Results (Safety population): Adverse events were collected during the study period. Due to the small number of subjects with AEs, all AEs reported in the study are tabulated below, rather than just the most frequent AEs. Most Frequent Adverse Events Subjects with any AE(s), n (%) 8 (80) 3 (30) Joint stiffness 3 (30) 0 Muscle tightness 3 (30) 0 Paresthesia 3 (30) 0 Musculoskeletal pain 1 (10) 1 (10) Diarrhea 2 (20) 0 Nausea 2 (20) 0 Dissociation 1 (10) 1 (10) Arthralgia 1 (10) 0 Flank pain 1 (10) 0 Neck pain 1 (10) 0 Abdominal pain 0 1 (10) Toothache 1 (10) 0 Dizziness 0 1 (10) Pharyngitis streptococcal 1 (10) 0 Nephrolithiasis 1 (10) 0 Swelling face 0 1 (10) Flushing 1 (10) 0 Serious Adverse Events - On-Therapy n (%) [n considered by the investigator to be related to study medication] Subjects with non-fatal SAEs, n (%) 0 0 Subjects with fatal SAEs, n (%) 0 0 Conclusion: See publications below. Publications: Kori S, Byrd S, Doll W, Page R, and Sandefer E. Gastroscintigraphic Evaluation of Gastric Emptying and Absorption of Another Conventionally Formulated Triptan. Cephalalgia 2007; Vol 27; 730. Kori S, Byrd S, Doll W, Page R, and Sandefer E. Gastric Emptying and Absorption of a with RT Technology 85mg and Sodium 500mg Tablet. Cephalalgia 2007; Vol 27; 649. Kori S, Doll WJ, Page RC, Byrd SC, Sandefer EP. Gastric Transit and Absorption of, 6
7 another Conventionally Formulated Triptan, in Migraineurs both During and Outside a Migraine Attack: Evaluation by Gastric Scintigraphy. Headache 2007; 47(5):752. Kori S, Byrd SC, Doll WJ, Page RC, and Sandefer EP. Gastric Transit and Absorption of and from a Fixed Single-Tablet RT Technology 85mg and Sodium 500mg in Migraineurs both During and Outside a Migraine Attack: Evaluation by Gastric Scintigraphy. Headache 2007; 47(5):751. 7
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Patient Information ONZETRA TM (On ze' trah) Xsail TM (Eks'-seil) (sumatriptan nasal powder) 11 mg
 Patient Information ONZETRA TM (On ze' trah) Xsail TM (Eks'-seil) (sumatriptan nasal powder) 11 mg Read this Patient Information before you start using ONZETRA Xsail and each time you get a refill. There
Patient Information ONZETRA TM (On ze' trah) Xsail TM (Eks'-seil) (sumatriptan nasal powder) 11 mg Read this Patient Information before you start using ONZETRA Xsail and each time you get a refill. There
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Sponsor / Company: Sanofi Drug substance(s): HOE901 (insulin glargine)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
NIMULID MD. 1. Introduction. 2. Nimulid MD Drug delivery system
 NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
Overview of Dissolution for BA/BE
 Biopharmaceutics Classification System based on Solubility/Permeability Biowaivers for BCS I Drugs Discussion of BCS III Drugs Models establishing in vivo-in vitro Correlations (IVIVC Levels A-C) 1 Biopharmaceutics
Biopharmaceutics Classification System based on Solubility/Permeability Biowaivers for BCS I Drugs Discussion of BCS III Drugs Models establishing in vivo-in vitro Correlations (IVIVC Levels A-C) 1 Biopharmaceutics
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008
 CLINICAL REVIEW Application Type NDA Submission Number 22-157 Submission Code Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008 Reviewer Name Review Completion Date
CLINICAL REVIEW Application Type NDA Submission Number 22-157 Submission Code Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008 Reviewer Name Review Completion Date
U.S. Scientific Update Aricept 23 mg Tablets. Dr. Lynn Kramer President NeuroScience Product Creation Unit Eisai Inc.
 U.S. Scientific Update Aricept 23 mg Tablets Dr. Lynn Kramer President NeuroScience Product Creation Unit Eisai Inc. Unmet Need in Moderate to Severe Alzheimer s Disease (AD) Ongoing clinical deterioration
U.S. Scientific Update Aricept 23 mg Tablets Dr. Lynn Kramer President NeuroScience Product Creation Unit Eisai Inc. Unmet Need in Moderate to Severe Alzheimer s Disease (AD) Ongoing clinical deterioration
Phase: IV. Study Period: 20 Jan. 2006-17 Sep. 2008
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Opadry II / Opadry / Opaglos 2
 Opadry II / Opadry / Opaglos 2 Application Data High Productivity Film Coating / Complete Film Coating System / High Gloss Film Coating Systems Modern Tablet Film Coatings and Influence on Ease of Swallowing
Opadry II / Opadry / Opaglos 2 Application Data High Productivity Film Coating / Complete Film Coating System / High Gloss Film Coating Systems Modern Tablet Film Coatings and Influence on Ease of Swallowing
Guidance for Industry
 Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
5.5 Pharmacokinetics - Sublingual
 5.5 Pharmacokinetics - Sublingual 5.5.1 Single Dose Pharmacokinetics - Dose Linearity Two studies in young healthy males were dedicated to examining sublingual single rising dose (SRD) pharmacokinetics
5.5 Pharmacokinetics - Sublingual 5.5.1 Single Dose Pharmacokinetics - Dose Linearity Two studies in young healthy males were dedicated to examining sublingual single rising dose (SRD) pharmacokinetics
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
QUESTIONS TO ASK MY DOCTOR
 Be a part of the treatment decision by asking questions QUESTIONS TO ASK MY DOCTOR FOR PATIENTS WITH ADVANCED STOMACH OR GASTROESOPHAGEAL JUNCTION (GEJ) CANCER CYRAMZA (ramucirumab) is used alone or in
Be a part of the treatment decision by asking questions QUESTIONS TO ASK MY DOCTOR FOR PATIENTS WITH ADVANCED STOMACH OR GASTROESOPHAGEAL JUNCTION (GEJ) CANCER CYRAMZA (ramucirumab) is used alone or in
Doxylamine succinate belongs to the ethanolamine class of antihistamines with sedative properties.
 Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
MEDICATION GUIDE. TRINTELLIX [trin -tel-ix] (vortioxetine) Tablets
![MEDICATION GUIDE. TRINTELLIX [trin -tel-ix] (vortioxetine) Tablets MEDICATION GUIDE. TRINTELLIX [trin -tel-ix] (vortioxetine) Tablets](/thumbs/40/21035524.jpg) MEDICATION GUIDE TRINTELLIX [trin -tel-ix] (vortioxetine) Tablets Read this Medication Guide before you start taking TRINTELLIX and each time you get a refill. There may be new information. This information
MEDICATION GUIDE TRINTELLIX [trin -tel-ix] (vortioxetine) Tablets Read this Medication Guide before you start taking TRINTELLIX and each time you get a refill. There may be new information. This information
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Disclosure. This presentation contains forward-looking statements.
 Disclosure This presentation contains forward-looking statements. These forward-looking statements are based on management's current expectations and assumptions as of the date of this presentation, and
Disclosure This presentation contains forward-looking statements. These forward-looking statements are based on management's current expectations and assumptions as of the date of this presentation, and
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
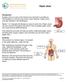 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
SYNOPSIS. Risperidone: Clinical Study Report CR003274
 SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Medication Guide. Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
 Medication Guide MoviPrep (moo-vee-prěp) (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid for oral solution) Read this Medication Guide before you start
Medication Guide MoviPrep (moo-vee-prěp) (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid for oral solution) Read this Medication Guide before you start
Statistics and Pharmacokinetics in Clinical Pharmacology Studies
 Paper ST03 Statistics and Pharmacokinetics in Clinical Pharmacology Studies ABSTRACT Amy Newlands, GlaxoSmithKline, Greenford UK The aim of this presentation is to show how we use statistics and pharmacokinetics
Paper ST03 Statistics and Pharmacokinetics in Clinical Pharmacology Studies ABSTRACT Amy Newlands, GlaxoSmithKline, Greenford UK The aim of this presentation is to show how we use statistics and pharmacokinetics
Sporadic attacks of severe tension-type headaches may respond to analgesics.
 MEDICATIONS While we are big advocates of non-drug treatments, many people do require the use of medications to control headaches. Headache medications are divided into two categories. Abortive drugs are
MEDICATIONS While we are big advocates of non-drug treatments, many people do require the use of medications to control headaches. Headache medications are divided into two categories. Abortive drugs are
Phase of development:
 Title of study: A randomised, placebo-controlled, double-blind, double-dummy, four-way crossover, single-centre study to investigate the effects of 2 mg and 10 mg intravenously administered NRL972 on the
Title of study: A randomised, placebo-controlled, double-blind, double-dummy, four-way crossover, single-centre study to investigate the effects of 2 mg and 10 mg intravenously administered NRL972 on the
Headaches in Children How to Manage Difficult Headaches
 Headaches in Children How to Manage Difficult Headaches Peter Procopis Childhood headaches Differential diagnosis Migraine Psychological Raised Pressure Childhood headaches Other causes: Constitutional
Headaches in Children How to Manage Difficult Headaches Peter Procopis Childhood headaches Differential diagnosis Migraine Psychological Raised Pressure Childhood headaches Other causes: Constitutional
SIGN. Diagnosis and management of headache in adults. Quick Reference Guide. Scottish Intercollegiate Guidelines Network
 SIGN Scottish Intercollegiate Guidelines Network 107 iagnosis and management of headache in adults Quick Reference Guide November 2008 opies of all SIGN guidelines are available online at www.sign.ac.uk
SIGN Scottish Intercollegiate Guidelines Network 107 iagnosis and management of headache in adults Quick Reference Guide November 2008 opies of all SIGN guidelines are available online at www.sign.ac.uk
None related to the presentation Grants to conduct clinical trials from:
 Chronic Daily Headache Bassel F. Shneker, MD, MBA Associate Professor Vice Chair, OSU Neurology The Ohio State University Wexner Medical Center Financial Disclosures None related to the presentation Grants
Chronic Daily Headache Bassel F. Shneker, MD, MBA Associate Professor Vice Chair, OSU Neurology The Ohio State University Wexner Medical Center Financial Disclosures None related to the presentation Grants
What is chronic daily headache? Information for patients Neurology
 What is chronic daily headache? Information for patients Neurology What is chronic daily headache (CDH)? Chronic daily headache (CDH) is the term used when a person has a headache on 15 days a month or
What is chronic daily headache? Information for patients Neurology What is chronic daily headache (CDH)? Chronic daily headache (CDH) is the term used when a person has a headache on 15 days a month or
8/23/2015 A PRACTICAL OPTOMETRIC HEADACHE APPROACH A PRACTICAL OPTOMETRIC HEADACHE APPROACH A PRACTICAL OPTOMETRIC HEADACHE APPROACH
 8/23/2015 A Practical Optometric Approach To Headaches Leonid Skorin, Jr., OD, DO, MS, FAAO, FAOCO Consultant, Department of Surgery Community Division of Ophthalmology Mayo Clinic Health System in Albert
8/23/2015 A Practical Optometric Approach To Headaches Leonid Skorin, Jr., OD, DO, MS, FAAO, FAOCO Consultant, Department of Surgery Community Division of Ophthalmology Mayo Clinic Health System in Albert
Introduction to Enteris BioPharma
 Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Post-Concussive Headaches and Dizziness Louise M. Klebanoff, MD
 Post-Concussive Headaches and Dizziness Louise M. Klebanoff, MD Associate Professor and Vice Chairman for Operations Chief, General Neurology Department of Neurology Disclosures: None Introduction: Headaches
Post-Concussive Headaches and Dizziness Louise M. Klebanoff, MD Associate Professor and Vice Chairman for Operations Chief, General Neurology Department of Neurology Disclosures: None Introduction: Headaches
Updated guidelines on headache management for use by the pharmacist
 M I P C A MIGRAINE IN PRIMARY CARE ADVISORS Updated guidelines on headache management for use by the pharmacist Introduction N U M B E R 2 1, O C T O B E R 2 0 1 2 Headache is a major public health problem,
M I P C A MIGRAINE IN PRIMARY CARE ADVISORS Updated guidelines on headache management for use by the pharmacist Introduction N U M B E R 2 1, O C T O B E R 2 0 1 2 Headache is a major public health problem,
Post Traumatic and other Headache Syndromes. Danielle L. Erb, MD Brain Rehabilitation Medicine, LLC Brain Injury Rehab Center, PRA
 Post Traumatic and other Headache Syndromes Danielle L. Erb, MD Brain Rehabilitation Medicine, LLC Brain Injury Rehab Center, PRA Over 45 million Americans have chronic, recurring headaches 62% of these
Post Traumatic and other Headache Syndromes Danielle L. Erb, MD Brain Rehabilitation Medicine, LLC Brain Injury Rehab Center, PRA Over 45 million Americans have chronic, recurring headaches 62% of these
Public Assessment Report. UK National Procedure. Perindopril 2 mg Tablets. Perindopril 4 mg Tablets. Perindopril 8 mg Tablets PL 20075/0294-0296
 Public Assessment Report UK National Procedure Perindopril 2 mg Tablets Perindopril 4 mg Tablets Perindopril 8 mg Tablets PL 20075/0294-0296 Accord Healthcare Limited 1 LAY SUMMARY This is a summary of
Public Assessment Report UK National Procedure Perindopril 2 mg Tablets Perindopril 4 mg Tablets Perindopril 8 mg Tablets PL 20075/0294-0296 Accord Healthcare Limited 1 LAY SUMMARY This is a summary of
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Improved migraine management in primary care: results of a patient treatment experience study using zolmitriptan orally disintegrating tablet
 ORIGINAL PAPER doi: 1.1111/j.1742-1241.26.128.x Improved migraine management in primary care: results of a patient treatment experience study using zolmitriptan orally disintegrating tablet G. SHAPERO,
ORIGINAL PAPER doi: 1.1111/j.1742-1241.26.128.x Improved migraine management in primary care: results of a patient treatment experience study using zolmitriptan orally disintegrating tablet G. SHAPERO,
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
CLINICAL STUDY REPORT SYNOPSIS
 CLINICAL STUDY REPORT SYNOPSIS Document No.: EDMS-PSDB-6511351:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Protocol No.: CR002353 Johnson & Johnson Pharmaceutical
CLINICAL STUDY REPORT SYNOPSIS Document No.: EDMS-PSDB-6511351:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Protocol No.: CR002353 Johnson & Johnson Pharmaceutical
BIOAVAILABILITY & BIOEQUIVALENCE TRIALS
 BIOAVAILABILITY & BIOEQUIVALENCE TRIALS Shubha Rani,, Ph.D. Technical Director & Head-Biometrics and Data Management Synchron Research Services Pvt. Ltd. Ahmedabad 380 054 drshubha@synchronresearch.com
BIOAVAILABILITY & BIOEQUIVALENCE TRIALS Shubha Rani,, Ph.D. Technical Director & Head-Biometrics and Data Management Synchron Research Services Pvt. Ltd. Ahmedabad 380 054 drshubha@synchronresearch.com
Zomig Nasal Spray. Zolmitriptan 5 mg Nasal Spray Solution. CONSUMER MEDICINE INFORMATION
 Zomig Nasal Spray Zolmitriptan 5 mg Nasal Spray Solution. CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some of the common questions people ask about It does not contain all
Zomig Nasal Spray Zolmitriptan 5 mg Nasal Spray Solution. CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some of the common questions people ask about It does not contain all
Tracy Walker, RN, BSN, CCRP Research Nurse OHSU Knight Cancer Institute. 503-346-1183 walkertr@ohsu.edu
 Tracy Walker, RN, BSN, CCRP Research Nurse OHSU Knight Cancer Institute 503-346-1183 walkertr@ohsu.edu Exercise Questions to Keep in Mind Is there an adverse event? What is the severity? What is the relationship
Tracy Walker, RN, BSN, CCRP Research Nurse OHSU Knight Cancer Institute 503-346-1183 walkertr@ohsu.edu Exercise Questions to Keep in Mind Is there an adverse event? What is the severity? What is the relationship
Migraine The Problem: Common Symptoms:
 Migraine The Problem: A combination of genetic and environmental factors alter pain mechanisms in your brain Transient changes in brain chemicals such as serotonin and neuropeptides affect the membranes
Migraine The Problem: A combination of genetic and environmental factors alter pain mechanisms in your brain Transient changes in brain chemicals such as serotonin and neuropeptides affect the membranes
Upper Gastrointestinal Tract KNH 406
 Upper Gastrointestinal Tract KNH 406 Upper GI A&P GI tract long tube ~ 15 ft. Upper GI mouth, pharynx, esophagus, stomach Accessory organs pancreas, biliary system, liver Four basic functions: motility,
Upper Gastrointestinal Tract KNH 406 Upper GI A&P GI tract long tube ~ 15 ft. Upper GI mouth, pharynx, esophagus, stomach Accessory organs pancreas, biliary system, liver Four basic functions: motility,
Paxil/Paxil-CR (paroxetine)
 Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Oral Bioavailability of Creatine Supplements: Is There Room for Improvement?
 Oral Bioavailability of Creatine Supplements: Is There Room for Improvement? Donald W. Miller Associate Professor Department of Pharmacology and Therapeutics University of Manitoba Acknowledgements ISSN
Oral Bioavailability of Creatine Supplements: Is There Room for Improvement? Donald W. Miller Associate Professor Department of Pharmacology and Therapeutics University of Manitoba Acknowledgements ISSN
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Abstral Prescriber and Pharmacist Guide
 Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
IMIGRAN injection is also used for treatment of cluster headache. Before you use your IMIGRAN injection. When you must not use it
 Imigran Injection Sumatriptan succinate 6mg/0.5mL Consumer Medicine Information What is in this leaflet? Please read this leaflet carefully before you start using. This leaflet answers some common questions
Imigran Injection Sumatriptan succinate 6mg/0.5mL Consumer Medicine Information What is in this leaflet? Please read this leaflet carefully before you start using. This leaflet answers some common questions
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Public assessment Report EU worksharing project paediatric data. Imigran Sumatriptan
 Public assessment Report EU worksharing project paediatric data Imigran Sumatriptan Rapporteur: Medicines Evaluation Board, The Netherlands Co-Rapporteur: Medical Product Agency, Sweden Start 1st round
Public assessment Report EU worksharing project paediatric data Imigran Sumatriptan Rapporteur: Medicines Evaluation Board, The Netherlands Co-Rapporteur: Medical Product Agency, Sweden Start 1st round
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Human ADME and Studies with Radiolabeled Compounds: Phase I-IIa
 WHITE PAPER Human ADME and Studies with Radiolabeled Compounds: Phase I-IIa AD ROFFEL, PhD Director, Global Scientific Affairs PRA Health Sciences Authors: HENK POELMAN, MSc Project Manager, Laboratory
WHITE PAPER Human ADME and Studies with Radiolabeled Compounds: Phase I-IIa AD ROFFEL, PhD Director, Global Scientific Affairs PRA Health Sciences Authors: HENK POELMAN, MSc Project Manager, Laboratory
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium)
 MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
Chemotherapy Induced Nausea & Vomiting
 Chemotherapy Induced Nausea & Vomiting A Nurse s Perspective Michael Flynn MSc, PG Cert, RGN Chemotherapy Nurse Consultant Guy s and St Thomas NHS Foundation Trust Guy s and St Thomas NHS Foundation Trust
Chemotherapy Induced Nausea & Vomiting A Nurse s Perspective Michael Flynn MSc, PG Cert, RGN Chemotherapy Nurse Consultant Guy s and St Thomas NHS Foundation Trust Guy s and St Thomas NHS Foundation Trust
Safety Information Card for Xarelto Patients
 Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
Emergency and inpatient treatment of migraine: An American Headache Society
 Emergency and inpatient treatment of migraine: An American Headache Society survey. The objective of this study was to determine the practice preferences of AHS members for acute migraine treatment in
Emergency and inpatient treatment of migraine: An American Headache Society survey. The objective of this study was to determine the practice preferences of AHS members for acute migraine treatment in
The TIRF REMS Access program is a Food and Drug Administration (FDA) required risk management program
 Subject: Important Drug Warning Announcement of a single shared REMS (Risk Evaluation and Mitigation Strategy) program for all Transmucosal Immediate Release Fentanyl (TIRF) products due to the potential
Subject: Important Drug Warning Announcement of a single shared REMS (Risk Evaluation and Mitigation Strategy) program for all Transmucosal Immediate Release Fentanyl (TIRF) products due to the potential
Bioequivalence Study Design Considerations. Dr. John Gordon
 Bioequivalence Study Design Considerations Dr. John Gordon Key Output of Programme A list of prequalified medicinal products used for treatment of HIV/AIDS, malaria, tuberculosis, influenza, and for reproductive
Bioequivalence Study Design Considerations Dr. John Gordon Key Output of Programme A list of prequalified medicinal products used for treatment of HIV/AIDS, malaria, tuberculosis, influenza, and for reproductive
Nonsteroidal. Drugs (NSAIDs) Anti-Inflammatory. North American Spine Society Public Education Series
 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
New appendix criteria open for a broader concept of chronic migraine
 Blackwell Publishing LtdOxford, UKCHACephalalgia0333-1024Blackwell Science, 20062006266742746Original ArticleA broader concept of chronic migrainej Olesen et al. BRIEF REPORT New appendix criteria open
Blackwell Publishing LtdOxford, UKCHACephalalgia0333-1024Blackwell Science, 20062006266742746Original ArticleA broader concept of chronic migrainej Olesen et al. BRIEF REPORT New appendix criteria open
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Chronic migraine and medication overuse headache: clarifying the current International Headache Society classification criteria
 Chronic migraine and medication overuse headache: clarifying the current International Headache Society classification criteria doi:10.1111/j.1468-2982.2008.01753.x C Sun-Edelstein 1, ME Bigal 2 & AM Rapoport
Chronic migraine and medication overuse headache: clarifying the current International Headache Society classification criteria doi:10.1111/j.1468-2982.2008.01753.x C Sun-Edelstein 1, ME Bigal 2 & AM Rapoport
NHS FORTH VALLEY Guidelines for use of high dose Intravenous Esomeprazole in Adults (Previously called the Hong Kong Protocol)
 NHS FORTH VALLEY Guidelines for use of high dose Intravenous Esomeprazole in Adults (Previously called the Hong Kong Protocol) Date of First Issue 10/05/2010 Approved 16/06/2010 Current Issue Date 18/11/2015
NHS FORTH VALLEY Guidelines for use of high dose Intravenous Esomeprazole in Adults (Previously called the Hong Kong Protocol) Date of First Issue 10/05/2010 Approved 16/06/2010 Current Issue Date 18/11/2015
PATIENT MEDICATION INFORMATION
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr CYRAMZA ramucirumab Read this carefully before you receive CYRAMZA (pronounced "si ram - ze"). This leaflet is a
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr CYRAMZA ramucirumab Read this carefully before you receive CYRAMZA (pronounced "si ram - ze"). This leaflet is a
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors
 Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
American Headache Society Evidence Assessment
 Headache 2015 American Headache Society ISSN 0017-8748 doi: 10.1111/head.12499 Published by Wiley Periodicals, Inc. American Headache Society Evidence Assessment The Acute Treatment of Migraine in Adults:
Headache 2015 American Headache Society ISSN 0017-8748 doi: 10.1111/head.12499 Published by Wiley Periodicals, Inc. American Headache Society Evidence Assessment The Acute Treatment of Migraine in Adults:
MILD TO MODERATE NOTE Medication is listed in increasing order of strength. Ascriptin (Aspirin) (P1-B1,2) - Pain reliever, anti-inflammatory
 Page 1 of 6 pages Contact Surgeon before giving any medication marked with an asterisk. In an emergency or during Loss of Signal, begin appropriate treatment; then call Surgeon as soon as possible. MILD
Page 1 of 6 pages Contact Surgeon before giving any medication marked with an asterisk. In an emergency or during Loss of Signal, begin appropriate treatment; then call Surgeon as soon as possible. MILD
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
It is important that you tell your family and the people closest to you of this increased sensitivity to opioids and the risk of overdose.
 MEDICATION GUIDE VIVITROL (viv-i-trol) (naltrexone for extended-release injectable suspension) Read this Medication Guide before you start receiving VIVITROL injections and each time you receive an injection.
MEDICATION GUIDE VIVITROL (viv-i-trol) (naltrexone for extended-release injectable suspension) Read this Medication Guide before you start receiving VIVITROL injections and each time you receive an injection.
Neuroendocrine Tumors
 Neuroendocrine Tumors Neuroendocrine tumors arise from cells that release a hormone in response to a signal from the nervous system. Neuro refers to the nervous system. Endocrine refers to the hormones.
Neuroendocrine Tumors Neuroendocrine tumors arise from cells that release a hormone in response to a signal from the nervous system. Neuro refers to the nervous system. Endocrine refers to the hormones.
Presenting the SUTENT Patient Call Center.
 Presenting the SUTENT Patient Call Center. Please see patient Medication Guide and full prescribing information attached. We re here to support you. Dealing with cancer is a journey. Along the way, you
Presenting the SUTENT Patient Call Center. Please see patient Medication Guide and full prescribing information attached. We re here to support you. Dealing with cancer is a journey. Along the way, you
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure TELMISARTAN DR REDDY S 20 MG TABLETS TELMISARTAN DR REDDY S 40 MG TABLETS TELMISARTAN DR REDDY S 80 MG TABLETS (telmisartan) Procedure No: UK/H/5034/001-003/DC
Public Assessment Report Decentralised Procedure TELMISARTAN DR REDDY S 20 MG TABLETS TELMISARTAN DR REDDY S 40 MG TABLETS TELMISARTAN DR REDDY S 80 MG TABLETS (telmisartan) Procedure No: UK/H/5034/001-003/DC
One Day at a Time: When Headaches Become Chronic. Robert Shapiro, MD, PhD
 One Day at a Time: When Headaches Become Chronic Robert Shapiro, MD, PhD Disclosures Scientific/Medical Advisory Boards (since 10/12) Transcept Pharmaceuticals Chronic Headaches: Overview What is a chronic
One Day at a Time: When Headaches Become Chronic Robert Shapiro, MD, PhD Disclosures Scientific/Medical Advisory Boards (since 10/12) Transcept Pharmaceuticals Chronic Headaches: Overview What is a chronic
X-Plain Abdominal Aortic Aneurysm Vascular Surgery Reference Summary
 X-Plain Abdominal Aortic Aneurysm Vascular Surgery Reference Summary Ballooning of the aorta, also known as an "abdominal aortic aneurysm," can lead to life threatening bleeding. Doctors may recommend
X-Plain Abdominal Aortic Aneurysm Vascular Surgery Reference Summary Ballooning of the aorta, also known as an "abdominal aortic aneurysm," can lead to life threatening bleeding. Doctors may recommend
Medication Guide. Cymbalta. (duloxetine delayed-release capsules)
 Medication Guide 1 Cymbalta [sim-ball-tah] (duloxetine delayed-release capsules) Read this Medication Guide before you start taking Cymbalta and each time you get a refill. There may be new information.
Medication Guide 1 Cymbalta [sim-ball-tah] (duloxetine delayed-release capsules) Read this Medication Guide before you start taking Cymbalta and each time you get a refill. There may be new information.
Contents Page. 1. What is IV DHE? 3. 2. Medication Licence 3. 3. How can a course of IV DHE help? 4. 4. What are the side effects of IV DHE?
 If you would like this document in another language or format, or if you require the services of an interpreter, please contact us on extension 84299 or 83926. Switchboard: 0845 155 5000 020 3456 7890
If you would like this document in another language or format, or if you require the services of an interpreter, please contact us on extension 84299 or 83926. Switchboard: 0845 155 5000 020 3456 7890
LEVETIRACETAM MONOTHERAPY
 LEVETIRACETAM MONOTHERAPY Beth Korby, RN C Patricia E. Penovich, MD John R. Gates, MD Deanna L. Dickens, MD Gerald L. Moriarty, MD This paper has been prepared specifically for: American Epilepsy Society
LEVETIRACETAM MONOTHERAPY Beth Korby, RN C Patricia E. Penovich, MD John R. Gates, MD Deanna L. Dickens, MD Gerald L. Moriarty, MD This paper has been prepared specifically for: American Epilepsy Society
Efficacy and Safety of Insulin Aspart in Patients with Type 1 Diabetes Mellitus
 Clin Pediatr Endocrinol 2002; 11(2), 87-92 Copyright 2002 by The Japanese Society for Pediatric Endocrinology Original Efficacy and Safety of Insulin Aspart in Patients with Type 1 Diabetes Mellitus Toshikazu
Clin Pediatr Endocrinol 2002; 11(2), 87-92 Copyright 2002 by The Japanese Society for Pediatric Endocrinology Original Efficacy and Safety of Insulin Aspart in Patients with Type 1 Diabetes Mellitus Toshikazu
The Prevalence of Neck Pain in Migrainehead_1608
 1..5 Headache 2010 the Authors Journal compilation 2010 American Headache Society ISSN 0017-8748 doi: 10.1111/j.1526-4610.2009.01608.x Published by Wiley Periodicals, Inc. Research Submission The Prevalence
1..5 Headache 2010 the Authors Journal compilation 2010 American Headache Society ISSN 0017-8748 doi: 10.1111/j.1526-4610.2009.01608.x Published by Wiley Periodicals, Inc. Research Submission The Prevalence
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Digestive System (continued) Digestive System. Stomach. Peptic Ulcer Disease
 Digestive System Digestive System (continued) Responsible for breaking down food, absorbing nutrients, eliminating wastes Alimentary canal Also known as gastrointestinal tract Reaches from mouth to anus
Digestive System Digestive System (continued) Responsible for breaking down food, absorbing nutrients, eliminating wastes Alimentary canal Also known as gastrointestinal tract Reaches from mouth to anus
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Objective: To investigate the hepatic clearance of NRL972 in patients undergoing alcohol withdrawal therapy
 Title of the Study: A multi-centre, open, short term follow-up Phase II study to evaluate the clearance of NRL972 in patients undergoing alcohol withdrawal commencing in a controlled clinical setting Short
Title of the Study: A multi-centre, open, short term follow-up Phase II study to evaluate the clearance of NRL972 in patients undergoing alcohol withdrawal commencing in a controlled clinical setting Short
How to Submit a School Epinephrine Report
 1. INTRODUCTION AND INSTRUCTIONS Dear School Nurse, The revised Regulations Governing the Administration of Prescription Medications in Public and Private Schools (105 CMR 210.000) require schools to submit
1. INTRODUCTION AND INSTRUCTIONS Dear School Nurse, The revised Regulations Governing the Administration of Prescription Medications in Public and Private Schools (105 CMR 210.000) require schools to submit
Proper Diagnosis and Treatment for the Headache Patient Alexander Feoktistov MD, PhD
 Proper Diagnosis and Treatment for the Headache Patient Alexander Feoktistov MD, PhD Director of Clinical Research Diamond Headache Clinic Chicago, IL 2014 Objectives Get familiar with primary headache
Proper Diagnosis and Treatment for the Headache Patient Alexander Feoktistov MD, PhD Director of Clinical Research Diamond Headache Clinic Chicago, IL 2014 Objectives Get familiar with primary headache
CENTER FOR DRUG EVALUATION AND RESEARCH. APPLICATION NUMBER: 022571Orig1s000 LABELING
 CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 022571Orig1s000 LABELING Page 6 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUVPOSA
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 022571Orig1s000 LABELING Page 6 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUVPOSA
Open the Flood Gates Urinary Obstruction and Kidney Stones. Dr. Jeffrey Rosenberg Dr. Emilio Lastarria Dr. Richard Kasulke
 Open the Flood Gates Urinary Obstruction and Kidney Stones Dr. Jeffrey Rosenberg Dr. Emilio Lastarria Dr. Richard Kasulke Nephrology vs. Urology Nephrologist a physician who has been trained in the diagnosis
Open the Flood Gates Urinary Obstruction and Kidney Stones Dr. Jeffrey Rosenberg Dr. Emilio Lastarria Dr. Richard Kasulke Nephrology vs. Urology Nephrologist a physician who has been trained in the diagnosis
Using the Triptans to Treat: Migraine Headaches. Comparing Effectiveness, Safety, and Price
 Using the Triptans to Treat: Migraine Headaches Comparing Effectiveness, Safety, and Price Contents Our Recommendations........................................... 3 Welcome....................................................
Using the Triptans to Treat: Migraine Headaches Comparing Effectiveness, Safety, and Price Contents Our Recommendations........................................... 3 Welcome....................................................
Means, standard deviations and. and standard errors
 CHAPTER 4 Means, standard deviations and standard errors 4.1 Introduction Change of units 4.2 Mean, median and mode Coefficient of variation 4.3 Measures of variation 4.4 Calculating the mean and standard
CHAPTER 4 Means, standard deviations and standard errors 4.1 Introduction Change of units 4.2 Mean, median and mode Coefficient of variation 4.3 Measures of variation 4.4 Calculating the mean and standard
