Approval of proposals relating to adalimumab, leuprorelin and levothyroxine and other autoimmune biologic funding applications
|
|
|
- Andrew Walters
- 7 years ago
- Views:
Transcription
1 30 June 2009 Approval of proposals relating to adalimumab, leuprorelin levothyroxine other autoimmune biologic funding applications Abbott adalimumab, leuprorelin levothyroxine proposal PHARMAC is pleased to announce its decision to widen funded access to adalimumab for the last-line treatment of ankylosing spondylitis, Crohn s disease, severe chronic plaque psoriasis psoriatic arthritis from 1 August PHARMAC has also approved widening of access to, reference pricing of, leuprorelin funding of a new br of levothyroxine. Key aspects of the decisions in relation to these pharmaceuticals are detailed on the following pages, followed by a summary of some of the common themes raised during consultation. Decline of autoimmune biologic funding applications PHARMAC has declined all outsting community funding proposals for etanercept (for rheumatoid arthritis, ankylosing spondylitis, severe chronic plaque psoriasis psoriatic arthritis), abatacept (for rheumatoid arthritis), infliximab (for rheumatoid arthritis) rituximab (for rheumatoid arthritis). It is important to note that future proposals or applications for these medicines could still be made would be considered in accordance with PHARMAC s Operating Policies Procedures. This decision will not affect the current community funding of etanercept for juvenile idiopathic arthritis, which will continue to be available subject to Special Authority criteria. The decision will also not prevent DHB hospitals from funding infliximab, abatacept or rituximab, should they choose to, for use in the treatment of autoimmune disorders within DHB hospitals. As previously indicated, PHARMAC intends to undertake further economic assessment with the aim of issuing advice to DHB hospitals on the cost-effectiveness of the use of these pharmaceuticals. DHB hospitals currently funding patients on infliximab, adalimumab or etanercept for any of the newly funded adalimumab indications may apply directly to PHARMAC (care of the Medical Director) for consideration of an adalimumab Special Authority approval where: the patient was taking infliximab, adalimumab or etanercept at the date of this notification letter had been doing so for a period of at least 1 month; the patient would have met the criteria for adalimumab funding at the time they were initiated on the hospital-funded treatment; supporting evidence for both of the above points is provided. A Page 1 of 9
2 Details of the decision Adalimumab (Humira HumiraPen) Access to adalimumab in Section B of the Pharmaceutical Schedule will be widened to include last-line treatment of ankylosing spondylitis, Crohn s disease, chronic severe plaque psoriasis psoriatic arthritis from 1 August The Special Authority criteria are attached at the end of this consultation letter. Note that several changes to the originally proposed Special Authority criteria have been made as a result of points raised during consultation. Adalimumab will be subject to a confidential rebate, reducing its net price. Adalimumab will have protection from delisting subsidy reduction until 1 August Leuprorelin (Lucrin Depot, Lucrin Depot PDS, Eligard) The Special Authority criteria will be removed from leuprorelin from 1 August A new, prefilled syringe presentation of leuprorelin (Lucrin Depot PDS), including a 6- month (30 mg) preparation, will be listed in Section B in Part II of Section H of the Pharmaceutical Schedule from 1 August 2009 at the following prices subsidies (ex-manufacturer, excluding GST): Chemical Br Form Strength Strength Pack Size Price subsidy Leuprorelin Lucrin Depot PDS Solution for injection (prefilled syringe) 3.75 mg (for monthly administration) 1 $ Leuprorelin Lucrin Depot PDS Solution for injection (prefilled syringe) mg (for 3-monthly administration) 1 $ Leuprorelin Lucrin Depot PDS Solution for injection (prefilled syringe) 30 mg (for 6-monthly administration) 1 $1, Lucrin Depot Lucrin Depot PDS will have protection from delisting subsidy reduction until 1 August A rebate will apply to Lucrin Depot Lucrin Depot PDS which will reduce the net price by 25%. The subsidy for Eligard will be reduced to the net effective price of Lucrin Depot Lucrin Depot PDS through the application of reference pricing as outlined in the following table (prices/subsidies expressed ex-manufacturer, excluding GST) from 1 October 2009: A T Page 2 of 9
3 Chemical Br Presentation Pack Size Current price subsidy New subsidy Leuprorelin Eligard Inj 7.5 mg 1 $ $ Leuprorelin Eligard Inj 22.5 mg 1 $ $ Leuprorelin Eligard Inj 30 mg 1 $ $ Leuprorelin Eligard Inj 45 mg 1 $1, $ This means that if Hospira New Zeal Limited, the supplier of Eligard, does not decrease the price to match the new subsidy, a manufacturer s surcharge would apply to Eligard patients would need to take the Lucrin Depot or Lucrin Depot PDS brs to remain on a fully funded br of leuprorelin. Levothyroxine (Synthroid) Levothyroxine (Synthroid) tablets will be listed in Section B of the Pharmaceutical Schedule from 1 August 2009 as follows (prices/subsidies expressed ex-manufacturer, excluding GST): Pharmaceutical Br Form Strength Pack Size Price subsidy Levothyroxine Synthroid Tablet 25 μg 1,000 $43.24 Levothyroxine Synthroid Tablet 50 μg 1,000 $45.00 Levothyroxine Synthroid Tablet 100 μg 1,000 $46.75 Feedback received during consultation We appreciate all the feedback we received acknowledge the time people took to respond. All consultation responses received by 6 July 2009 were considered in their entirety in making a decision on the proposed changes. The majority of responses were supportive of the proposal. Several responders proposed changes to the adalimumab Special Authority criteria, many of which were incorporated. Common themes that emerged during consultation, that we are able to publicise, are outlined in the table on the following pages, along with our response. A T Page 3 of 9
4 Theme Response Responses relating to autoimmune biologics A number of consultation responders requested changes to the proposed Special Authority criteria for adalimumab. Some responders were concerned that the proposal would restrict the ability of DHB hospitals to provide funding for the hospital administered treatments (infliximab, rituximab, abatacept), or that it would restrict access to etanercept for juvenile idiopathic arthritis. Several consultation responders considered that there was a need for a second funded biologic treatment for use in patients in whom adalimumab was not effective. Many of the suggested changes have been incorporated into the approved Special Authority. These changes are too numerous to detail in this notification letter, so please refer to the consultation document (which can be accessed on for comparison. We would welcome applications for further alterations to the criteria. We note that we intend to monitor the working of the current criteria for at least 6 months before considering any changes. Where requested changes would result in additional patients accessing treatment, applicants should refer to the application guidelines on PHARMAC s website, which can be found at: The decision will not prevent DHB hospitals from choosing to provide funding for infliximab, abatacept or rituximab for use in the treatment of autoimmune disorders within DHB hospitals. This decision also will not affect the current community funding of etanercept for juvenile idiopathic arthritis, which will continue to be available. PHARMAC welcomes funding applications for autoimmune biologics in their registered indication(s) for use as a second-line treatment following adalimumab failure. Guidelines on how to make funding applications can be found on PHARMAC s website at: Responses relating to levothyroxine Several consultation responders called for PHARMAC to stop funding the current Eltroxin formulation /or re-fund the original Eltroxin formulation. It is not possible to re-fund the original Eltroxin formulation because it is no longer available. This is because the manufacturer, GlaxoSmithKline, has stopped making it. We note that the new Eltroxin formulation is currently used by around 80% of patients talking levothyroxine, we are not aware of any information to indicate that it is unsafe. A T Page 4 of 9
5 Several consultation responders requested funding of whole thyroid extract as another alternative treatment for patients who have difficulties with Synthroid /or the other funded brs of levothyroxine. Whole thyroid extract is not registered by Medsafe for use as a therapeutic product in New Zeal. We underst that it is currently being purchased by patients under Section 29 of the Medicines Act 1981 on a named patient basis. We have not received an application for funding from the manufacturer nor, as far as we can tell, has an application for registration been submitted to Medsafe. If whole thyroid extract gains Medsafe registration, PHARMAC would welcome a funding application. Guidelines on how to make funding applications can be found on PHARMAC s website at Responses relating to leuprorelin Some responders were concerned that the proposal would result in no fully funded 6- month preparation of leuprorelin. Some responders were concerned that the proposal would further restrict access to goserelin (Zoladex) in some way. There will be no break in full funding for a 6-month preparation of leuprorelin as a result of this decision. The 6-month leuprorelin preparation of Lucrin Depot PDS will be available fully funded from 1 August From 1 October 2009 the subsidy for the 6-month preparation for Eligard will be reduced, so a manufacturer s surcharge could apply if the supplier does not decrease its price. The 6-month Lucrin Depot PDS formulation will continue to be available fully funded from 1 October This decision does not restrict access to goserelin (Zoladex), which will continue to be available, subject to Special Authority criteria, as it is currently. We note that there are differences between the registered indications for leuprorelin goserelin, clinicians should refer to the registered datasheets for more information. These can be found at A T Page 5 of 9
6 New Special Authority Criteria for Adalimumab (Humira HumiraPen) Initial application (rheumatoid arthritis) only from a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria: 1 Patient has had severe active erosive rheumatoid arthritis for six months duration or longer; 2 Treatment is to be used as an adjunct to methotrexate therapy or monotherapy where use of methotrexate is limited by toxicity or intolerance; 3 Patient has tried not responded to at least three months of oral or parenteral methotrexate at a dose of at least 20 mg weekly or a maximum tolerated dose; 4 Patient has tried not responded to at least three months of oral or parenteral methotrexate in combination with at least two of the following (triple therapy): sulphasalazine, prednisone at a dose of at least 7.5 mg per day, azathioprine, intramuscular gold, or hydroxychloroquine sulphate (at maximum tolerated doses); 5 Either: 5.1 Patient has tried not responded to at least three months therapy at the maximum tolerated dose of cyclosporin alone or in combination with another agent; or 5.2 Patient has tried not responded to at least three months therapy at the maximum tolerated dose of leflunomide alone or in combination with another agent; 6 Either: 6.1 Patient has persistent symptoms of poorly controlled active disease in at least 20 active, swollen, tender joints; or 6.2 Patient has persistent symptoms of poorly controlled active disease in at least four active joints from the following: wrist, elbow, knee, ankle, either shoulder or hip; 7 Either: 7.1 Patient has a C-reactive protein level greater than 15 mg/l measured no more than one month prior to the date of this application; or 7.2 C-reactive protein levels not measured as patient is currently receiving prednisone therapy at a dose of greater than 5 mg per day has done so for more than three months. Initial application (Crohn s disease) only from a gastroenterologist. Approvals valid for 3 months for applications meeting the following criteria: 1 Patient has severe active Crohn s disease; 2 Any of the following: 2.1 Patient has a Crohn s Disease Activity Index (CDAI) score of greater than or equal to 300; or 2.2 Patient has extensive small intestine disease affecting more than 50 cm of the small intestine; or 2.3 Patient has evidence of short gut syndrome or would be at risk of short gut syndrome with further bowel resection; or 2.4 Patient has an ileostomy or colostomy has intestinal inflammation; 3 Patient has tried but had an inadequate response to, or has experienced intolerable side effects from, prior systemic therapy with immunomodulators at maximum tolerated doses (unless contraindicated) corticosteroids; 4 Surgery (or further surgery) is considered to be clinically inappropriate. Initial application (severe chronic plaque psoriasis) only from a dermatologist. Approvals valid for 4 months for applications meeting the following criteria: 1.1 Patient has whole body severe chronic plaque psoriasis with a Psoriasis Area Severity Index (PASI) score of greater than 15, where lesions have been present for at least 6 months from the time of initial diagnosis; or A T Page 6 of 9
7 1.2 Patient has severe chronic plaque psoriasis of the face, or palm of a h or sole of a foot, where the plaque or plaques have been present for at least 6 months from the time of initial diagnosis; 2 Patient has tried, but had an inadequate response (see Note) to, or has experienced intolerable side effects from, at least three of the following (at maximum tolerated doses unless contraindicated): phototherapy, methotrexate, cyclosporin, or acitretin; 3 A PASI assessment has been completed for at least the most recent prior treatment course (but preferably all prior treatment courses), preferably while still on treatment but no longer than 1 month following cessation of each prior treatment course; 4 The most recent PASI assessment is no more than 1 month old at the time of application. Note: Inadequate response is defined as: for whole body severe chronic plaque psoriasis, a PASI score of greater than 15, as assessed preferably while still on treatment but no longer than 1 month following cessation of the most recent prior treatment; for severe chronic plaque psoriasis of the face, h or foot, at least 2 of the 3 PASI symptom subscores for erythema, thickness scaling are rated as severe or very severe, the skin area affected is 30% or more of the face, palm of a h or sole of a foot, as assessed preferably while still on treatment but no longer than 1 month following cessation of the most recent prior treatment. Initial application (ankylosing spondylitis) only from a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria: 1 Patient has a confirmed diagnosis of ankylosing spondylitis present for more than six months; 2 Patient has low back pain stiffness that is relieved by exercise but not by rest; 3 Patient has bilateral sacroiliitis demonstrated by plain radiographs, CT or MRI scan; 4 Patient s ankylosing spondylitis has not responded adequately to treatment with two or more non-steroidal anti-inflammatory drugs (NSAIDs), in combination with anti-ulcer therapy if indicated, while patient was undergoing at least 3 months of an exercise regime supervised by a physiotherapist; 5 Either: 5.1 Patient has limitation of motion of the lumbar spine in the sagittal the frontal planes as determined by a score of at least 1 on each of the lumbar flexion lumbar side flexion measurements of the Bath Ankylosing Spondylitis Metrology Index (BASMI); or 5.2 Patient has limitation of chest expansion by at least 2.5 cm below the average normal values corrected for age gender (see Notes); 6 A Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) of at least 6 on a 0-10 scale; 7 Either: 7.1 An elevated erythrocyte sedimentation rate (ESR) greater than 25 mm per hour; or 7.2 A C-reactive protein (CRP) level greater than 15 mg per litre. Notes: The BASDAI must have been determined at the completion of the 3 month exercise trial, but prior to ceasing NSAID treatment. The BASDAI, ESR CRP measures must be no more than 1 month old at the time of initial application. Average normal chest expansion corrected for age gender: years Male: 7.0 cm; Female: 5.5 cm years Male: 7.5 cm; Female: 5.5 cm years Male: 6.5 cm; Female: 4.5 cm years Male: 6.0 cm; Female: 5.0 cm years Male: 5.5 cm; Female: 4.0 cm years Male: 4.0 cm; Female: 4.0 cm 75+ years Male: 3.0 cm; Female: 2.5 cm Initial application (psoriatic arthritis) only from a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria: 1 Patient has had severe active psoriatic arthritis for six months duration or longer; A T Page 7 of 9
8 2 Patient has tried not responded to at least three months of oral or parenteral methotrexate at a dose of at least 20 mg weekly or a maximum tolerated dose; 3 Patient has tried not responded to at least three months of sulphasalazine at a dose of at least 2 g per day or leflunomide at a dose of up to 20 mg daily (or maximum tolerated doses); 4 Either: 4.1 Patient has persistent symptoms of poorly controlled active disease in at least 20 active, swollen, tender joints; or 4.2 Patient has persistent symptoms of poorly controlled active disease in at least four active joints from the following: wrist, elbow, knee, ankle, either shoulder or hip; 5 Any of the following: 5.1 Patient has a C-reactive protein level greater than 15 mg/l measured no more than one month prior to the date of this application; or 5.2 Patient has an elevated erythrocyte sedimentation rate (ESR) greater than 25 mm per hour; or 5.3 ESR CRP not measured as patient is currently receiving prednisone therapy at a dose of greater than 5 mg per day has done so for more than three months. Renewal (rheumatoid arthritis) only from a rheumatologist or Practitioner on the recommendation of a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria: 1.1 Applicant is a rheumatologist; or 1.2 Applicant is a Practitioner confirms that a rheumatologist has provided a letter, or fax recommending that the patient continues with adalimumab treatment; 2 Treatment is to be used as an adjunct to methotrexate therapy or monotherapy where use of methotrexate is limited by toxicity or intolerance; 3 Either: 3.1 Following 4 months initial treatment, the patient has at least a 50% decrease in active joint count from baseline a clinically significant response to treatment in the opinion of the physician; or 3.2 On subsequent reapplications, the patient demonstrates at least a continuing 30% improvement in active joint count from baseline a clinically significant response to treatment in the opinion of the physician. Renewal (Crohn s disease) only from a gastroenterologist or Practitioner on the recommendation of a gastroenterologist. Approvals valid for 6 months for applications meeting the following criteria: Both: 1.1 Applicant is a gastroenterologist; or 1.2 Applicant is a Practitioner confirms that a gastroenterologist has provided a letter, or fax recommending that the patient continues with adalimumab treatment; 2 The treatment remains appropriate the patient is benefiting from treatment. Renewal (severe chronic plaque psoriasis) only from a dermatologist or Practitioner on the recommendation of a dermatologist. Approvals valid for 6 months for applications meeting the following criteria: Both: 1.1 Applicant is a dermatologist; or 1.2 Applicant is a Practitioner confirms that a dermatologist has provided a letter, or fax recommending that the patient continues with adalimumab treatment; 2 Either: 2.1 Both: Patient has whole body severe chronic plaque psoriasis; A T Page 8 of 9
9 2.1.2 Following each prior adalimumab treatment course the patient has a PASI score which is reduced by 75% or more, or is sustained at this level, when compared with the pre-adalimumab treatment baseline value; or 2.2 Both: Patient has severe chronic plaque psoriasis of the face, or palm of a h or sole of a foot; Either: Following each prior adalimumab treatment course the patient has a reduction in the PASI symptom subscores for all 3 of erythema, thickness scaling, to slight or better, or sustained at this level, as compared to the treatment course baseline values; or Following each prior adalimumab treatment course the patient has a reduction of 75% or more in the skin area affected, or sustained at this level, as compared to the pre-adalimumab treatment baseline value. Note: An adalimumab treatment course is defined as a minimum of 12 weeks of adalimumab treatment. Renewal (ankylosing spondylitis) only from a rheumatologist or Practitioner on the recommendation of a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria: 1.1 Applicant is a rheumatologist; or 1.2 Applicant is a Practitioner confirms that a rheumatologist has provided a letter, or fax recommending that the patient continues with adalimumab treatment; 2 Following 12 weeks of adalimumab treatment, BASDAI has improved by 4 or more points from pre-adalimumab baseline on a 10 point scale, or by 50%, whichever is less; 3 Either: 3.1 ESR or CRP is within the normal range; or 3.2 ESR CRP not measured as patient is currently receiving prednisone therapy at a dose of greater than 5 mg per day has done so for more than three months; 4 Physician considers that the patient has benefited from treatment that continued treatment is appropriate. Renewal (psoriatic arthritis) only from a rheumatologist or Practitioner on the recommendation of a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria: Both: 1.1 Applicant is a rheumatologist; or 1.2 Applicant is a Practitioner confirms that a rheumatologist has provided a letter, or fax recommending that the patient continues with adalimumab treatment; 2 Either: 2.1 Following 4 months initial treatment, the patient has at least a 50% decrease in active joint count from baseline a clinically significant response to treatment in the opinion of the treating physician; or 2.2 The patient demonstrates at least a continuing 50% improvement in active joint count from baseline a clinically significant response to prior adalimumab treatment in the opinion of the treating physician. A T Page 9 of 9
SECTION 3. Criteria for Special Authorization of Select Drug Products. Section 3 Criteria for Special Authorization of Select Drug Products
 SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
Rheumatoid Arthritis
 Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Psoriatic Arthritis www.arthritis.org.nz
 Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Arthritis and Rheumatology Clinics of Kansas Patient Education. Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis
 Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Drug Therapy Guidelines: Humira (adalimumab)
 Drug Therapy Guidelines: Humira (adalimumab) Effective Date: 5/1/08 Committee Review Date: 1/6/01, 9/18/01, 1/15/02, 1/7/03, 1/20/04, 1/18/05, 12/7/05, 10/15/06, 7/2/07, 11/5/07, 3/25/08 Policy Statements:
Drug Therapy Guidelines: Humira (adalimumab) Effective Date: 5/1/08 Committee Review Date: 1/6/01, 9/18/01, 1/15/02, 1/7/03, 1/20/04, 1/18/05, 12/7/05, 10/15/06, 7/2/07, 11/5/07, 3/25/08 Policy Statements:
DISEASE-MODIFYING ANTIRHEUMATIC DRUG THERAPY FOR RHEUMATOID ARTHRITIS
 DISEASE-MODIFYING ANTIRHEUMATIC DRUG THERAPY FOR RHEUMATOID ARTHRITIS APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA APPROVED CODES HEDIS
DISEASE-MODIFYING ANTIRHEUMATIC DRUG THERAPY FOR RHEUMATOID ARTHRITIS APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA APPROVED CODES HEDIS
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists
 MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
Rheumatoid Arthritis. Outline. Treatment Goal 4/10/2013. Clinical evaluation New treatment options Future research Discussion
 Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
Rheumatoid Arthritis www.arthritis.org.nz
 Rheumatoid Arthritis www.arthritis.org.nz Did you know? Rheumatoid arthritis (RA) is the third most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most
Rheumatoid Arthritis www.arthritis.org.nz Did you know? Rheumatoid arthritis (RA) is the third most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most
Immune modulation in rheumatology. Geoff McColl University of Melbourne/Australian Rheumatology Association
 Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
subcutaneous initially every 4 weeks then every 12 weeks Coverage Criteria: Express Scripts, Inc. monograph dated 02/24/2010
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Humira (adalimumab subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Humira (adalimumab subcutaneous injection) Commercial HMO/PPO/CDHP
Rheumatoid Arthritis. Disease RA Final.indd 2 15. 6. 10. 11:23
 Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists
 Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Rheumatoid Arthritis Information
 Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
How To Choose A Biologic Drug
 North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital
 Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
Patient Input Information Clinical Trials Outcomes Common Drug Review
 CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
Understanding Rheumatoid Arthritis
 Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Response to Public Comments From the Core Leadership Team for the ACR/SPARTAN/SAA Axial SpA Guideline Development Project July 2013
 We thank members of the rheumatology community for their interest in the proposed treatment recommendations for patients with axial spondyloarthritis (axspa) including ankylosing spondylitis (AS). In general,
We thank members of the rheumatology community for their interest in the proposed treatment recommendations for patients with axial spondyloarthritis (axspa) including ankylosing spondylitis (AS). In general,
Psoriatic arthritis FACTSHEET
 1 What is psoriatic arthritis? Psoriatic arthritis (PsA) is a disease where joints around the body become inflamed and sore. It can make moving about difficult and painful. People who have PsA also have
1 What is psoriatic arthritis? Psoriatic arthritis (PsA) is a disease where joints around the body become inflamed and sore. It can make moving about difficult and painful. People who have PsA also have
Immune Modulating Drugs Prior Authorization Request Form
 Patient: HPHC member ID #: Requesting provider: Phone: Servicing provider: Diagnosis: Contact for questions (name and phone #): Projected start and end date for requested Requesting provider NPI: Fax:
Patient: HPHC member ID #: Requesting provider: Phone: Servicing provider: Diagnosis: Contact for questions (name and phone #): Projected start and end date for requested Requesting provider NPI: Fax:
Evidence-based Management of Rheumatoid Arthritis (2009)
 CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary
 November 1, 2014 Bulletin #150 ISSN 1923-0761 SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary New Exception Drug Status (EDS) Listings Effective November 1, 2014
November 1, 2014 Bulletin #150 ISSN 1923-0761 SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary New Exception Drug Status (EDS) Listings Effective November 1, 2014
Rheumatic Diseases, Psoriasis, and Crohn s Disease
 Rheumatic Diseases, Psoriasis, and Crohn s Disease What does this handout cover? This handout has information about rheumatic disease, psoriasis, and Crohn s disease. It also has information on how these
Rheumatic Diseases, Psoriasis, and Crohn s Disease What does this handout cover? This handout has information about rheumatic disease, psoriasis, and Crohn s disease. It also has information on how these
Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd
 ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
Medicines for Psoriatic Arthritis. A Review of the Research for Adults
 Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
Guideline for the use of Biological Therapies in the Treatment of Psoriasis
 1 Date of Production: March1 st 2011 Date of 1 st review: July 10 th 2015 Date for next review: March1 st 2018 Local Contact Dermatology Consultant Shanti Ayob Patient group to which this applies: Patients
1 Date of Production: March1 st 2011 Date of 1 st review: July 10 th 2015 Date for next review: March1 st 2018 Local Contact Dermatology Consultant Shanti Ayob Patient group to which this applies: Patients
Rheumatoid Arthritis www.arthritis.org.nz
 Rheumatoid Arthritis www.arthritis.org.nz Did you know? RA is the second most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most often appears between
Rheumatoid Arthritis www.arthritis.org.nz Did you know? RA is the second most common form of arthritis Approximately 40,000 New Zealanders have RA RA can occur at any age, but most often appears between
Rheumatoid Arthritis monitoring of DMARDs
 www.bpac.org.nz keyword: DMARDS Rheumatoid Arthritis monitoring of DMARDs Key reviewers: Professor John Highton, Head of Section, Department of Medical and Surgical Sciences, Dunedin School of Medicine,
www.bpac.org.nz keyword: DMARDS Rheumatoid Arthritis monitoring of DMARDs Key reviewers: Professor John Highton, Head of Section, Department of Medical and Surgical Sciences, Dunedin School of Medicine,
Methods for Measuring Dose Escalation in TNF Antagonists for Rheumatoid Arthritis Patients Treated in Routine Clinical Practice
 Methods for Measuring Dose Escalation in TNF Antagonists for Rheumatoid Arthritis Patients Treated in Routine Clinical Practice Gu NY 1, Huang XY 2, Globe D 2, Fox KM 3 1 University of Southern California,
Methods for Measuring Dose Escalation in TNF Antagonists for Rheumatoid Arthritis Patients Treated in Routine Clinical Practice Gu NY 1, Huang XY 2, Globe D 2, Fox KM 3 1 University of Southern California,
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
X-Plain Rheumatoid Arthritis Reference Summary
 X-Plain Rheumatoid Arthritis Reference Summary Introduction Rheumatoid arthritis is a fairly common joint disease that affects up to 2 million Americans. Rheumatoid arthritis is one of the most debilitating
X-Plain Rheumatoid Arthritis Reference Summary Introduction Rheumatoid arthritis is a fairly common joint disease that affects up to 2 million Americans. Rheumatoid arthritis is one of the most debilitating
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis
 Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Medicines for Rheumatoid. Arthritis. A Review of the Research for Adults
 Medicines for Rheumatoid Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this summary is for you if: Your doctor* has told you that you have rheumatoid (pronounced
Medicines for Rheumatoid Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this summary is for you if: Your doctor* has told you that you have rheumatoid (pronounced
Once the immune system is triggered, cells migrate from the blood into the joints and produce substances that cause inflammation.
 HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
Decisions relating to Multiple Sclerosis treatments
 10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
Is Monotherapy Treatment of Etanercept Effective Against Plaque Psoriasis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Information on Rheumatoid Arthritis
 Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Critical Issues in School Health Arthritis in the School Setting. Lawrence Zemel MD Tegan Willard RN Connecticut Children s
 Critical Issues in School Health Arthritis in the School Setting Lawrence Zemel MD Tegan Willard RN Connecticut Children s Juvenile Rheumatoid Idiopathic Arthritis Definition of JRA (JIA) Persistent arthritis
Critical Issues in School Health Arthritis in the School Setting Lawrence Zemel MD Tegan Willard RN Connecticut Children s Juvenile Rheumatoid Idiopathic Arthritis Definition of JRA (JIA) Persistent arthritis
GENETIC ANALYSIS OF PSORIASIS AND PSORIATIC ARTHRITIS Department of Dermatology, University of Michigan
 GENETIC ANALYSIS OF PSORIASIS AND PSORIATIC ARTHRITIS Department of Dermatology, University of Michigan SELF ASSESSMENT FORM FOR STUDY SUBJECTS AND CONTROLS Accession Number (will be filled in by lab)
GENETIC ANALYSIS OF PSORIASIS AND PSORIATIC ARTHRITIS Department of Dermatology, University of Michigan SELF ASSESSMENT FORM FOR STUDY SUBJECTS AND CONTROLS Accession Number (will be filled in by lab)
Rheumatoid Arthritis
 What is Rheumatoid Arthritis? Rheumatoid Arthritis (RA) is a chronic and systemic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints
What is Rheumatoid Arthritis? Rheumatoid Arthritis (RA) is a chronic and systemic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints
A Survey of Barriers to Treatment Access in Rheumatoid Arthritis. Country Annex Report: UK
 A Survey of Barriers to Treatment Access in Rheumatoid Arthritis Country Annex Report: UK October 2009 1 Interviews In the UK, five rheumatologists and one patient representative were interviewed. The
A Survey of Barriers to Treatment Access in Rheumatoid Arthritis Country Annex Report: UK October 2009 1 Interviews In the UK, five rheumatologists and one patient representative were interviewed. The
PRACTICAL HELP FROM THE ARTHRITIS FOUNDATION. www.arthritis.org 800-283-7800. Psoriatic Arthritis
 Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Psoriatic Arthritis. having psoriatic arthritis.
 Psoriatic Arthritis having psoriatic arthritis. Psoriatic arthritis is a chronic disease characterized by a form of inflammation of the skin (psoriasis) and joints (inflammatory arthritis). Psoriasis is
Psoriatic Arthritis having psoriatic arthritis. Psoriatic arthritis is a chronic disease characterized by a form of inflammation of the skin (psoriasis) and joints (inflammatory arthritis). Psoriasis is
I. INCLUSION AND EXCLUSION CRITERIA
 Page 1 of 5 I. INCLUSION AND EXCLUSION CRITERIA INCLUSION CRITERIA Yes No 1. Patient is diagnosed with according to the American College of Rheumatology 1987 revised criteria. 2. All 4 grandparents are
Page 1 of 5 I. INCLUSION AND EXCLUSION CRITERIA INCLUSION CRITERIA Yes No 1. Patient is diagnosed with according to the American College of Rheumatology 1987 revised criteria. 2. All 4 grandparents are
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
Rheumatology. Rheumatoid Arthritis
 Rheumatology Rheumatoid Arthritis The Rheumatology service specialises in the diagnosis and treatment of diseases affecting the musculoskeletal system. Other than providing inpatient and outpatient consultation,
Rheumatology Rheumatoid Arthritis The Rheumatology service specialises in the diagnosis and treatment of diseases affecting the musculoskeletal system. Other than providing inpatient and outpatient consultation,
X-Plain Psoriasis Reference Summary
 X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis
 Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
(Intro to Arthritis with a. Arthritis) Manager of Education & Services for the Vancouver Island Region of The Arthritis Society
 Arthritis 101 (Intro to Arthritis with a Focus on Rheumatoid Arthritis) by Cari Taylor by Cari Taylor Manager of Education & Services for the Vancouver Island Region of The Arthritis Society What You Will
Arthritis 101 (Intro to Arthritis with a Focus on Rheumatoid Arthritis) by Cari Taylor by Cari Taylor Manager of Education & Services for the Vancouver Island Region of The Arthritis Society What You Will
Do I need a physician referral? Yes, we see patients on referral from a health care provider.
 FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
Infl ectra for rheumatoid arthritis
 Infl ectra for rheumatoid arthritis Some important information to get you started with your treatment This booklet is intended only for use by patients who have been prescribed Inflectra. Introduction
Infl ectra for rheumatoid arthritis Some important information to get you started with your treatment This booklet is intended only for use by patients who have been prescribed Inflectra. Introduction
The management of rheumatoid arthritis
 Medical management of rheumatoid arthritis Peter Jones Correspondence to: peter.jones@qehealth.co.nz The management of rheumatoid arthritis has changed in the last 10 years. Back then, a patient who awoke
Medical management of rheumatoid arthritis Peter Jones Correspondence to: peter.jones@qehealth.co.nz The management of rheumatoid arthritis has changed in the last 10 years. Back then, a patient who awoke
Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011
 Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011 Working party: Eva Baecklund, Helena Forsblad d Elia, Carl Turesson Background Our purpose
Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011 Working party: Eva Baecklund, Helena Forsblad d Elia, Carl Turesson Background Our purpose
Arthritis in Children: Juvenile Rheumatoid Arthritis By Kerry V. Cooke
 Reading Comprehension Read the following essay on juvenile rheumatoid arthritis. Then use the information in the text to answer the questions that follow. Arthritis in Children: Juvenile Rheumatoid Arthritis
Reading Comprehension Read the following essay on juvenile rheumatoid arthritis. Then use the information in the text to answer the questions that follow. Arthritis in Children: Juvenile Rheumatoid Arthritis
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: Enbrel Therapeutic Class: Miscellaneous Therapeutic Agents Therapeutic Sub-Class: Disease-modifying Antirheumatic Drugs Client: 2007 AARP Medicare Rx Inj, 2007
Prior Authorization Guideline Guideline: Enbrel Therapeutic Class: Miscellaneous Therapeutic Agents Therapeutic Sub-Class: Disease-modifying Antirheumatic Drugs Client: 2007 AARP Medicare Rx Inj, 2007
Cytokine and CAM Antagonists
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
(THE CHANGING LANDSCAPE)
 Updating the therapeutic strategy in RA What is effective, what is changing in daily practice regarding the use of DMARDs and biological agents in the Balkan countries The Greek experience (THE CHANGING
Updating the therapeutic strategy in RA What is effective, what is changing in daily practice regarding the use of DMARDs and biological agents in the Balkan countries The Greek experience (THE CHANGING
Proposal for rivaroxaban, moxifloxacin, interferon beta-1-beta and other funded treatments for multiple sclerosis
 30 September 2010 Proposal for rivaroxaban, moxifloxacin, interferon beta-1-beta and other funded treatments for multiple sclerosis PHARMAC is seeking feedback on a provisional agreement with Bayer New
30 September 2010 Proposal for rivaroxaban, moxifloxacin, interferon beta-1-beta and other funded treatments for multiple sclerosis PHARMAC is seeking feedback on a provisional agreement with Bayer New
Methotrexate Dose For Juvenile Rheumatoid Arthritis
 Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
It is most common between the ages of 40 and 70, but can affect people of any age.
 Rheumatoid arthritis Introduction Rheumatoid arthritis is a condition that causes pain and swelling in the joints. Hands, feet and wrists are commonly affected, but it can also damage other parts of the
Rheumatoid arthritis Introduction Rheumatoid arthritis is a condition that causes pain and swelling in the joints. Hands, feet and wrists are commonly affected, but it can also damage other parts of the
RHEUMATOID ARTHRITIS. Dr Bruce Kirkham Rheumatology Clinical Lead
 RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
Psoriatic Arthritis. What is psoriatic arthritis? Understanding joints. Who gets psoriatic arthritis? Page 1 of 5
 Page 1 of 5 Psoriatic Arthritis Psoriatic arthritis causes inflammation, pain, and swelling of joints in some people who have psoriasis. Other parts of the body may also be affected. For example, in many
Page 1 of 5 Psoriatic Arthritis Psoriatic arthritis causes inflammation, pain, and swelling of joints in some people who have psoriasis. Other parts of the body may also be affected. For example, in many
The exacerbation of cutaneous psoriasis induced by anti - TNF therapy - case report
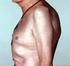
 The exacerbation of cutaneous psoriasis induced by anti - TNF therapy - case report Anca Costache 1, Mădălina Ionela Chiriac 2, Elena Rezuş*,1,2 1 Rheumatology Department, Rehabilitation Hospital Iasi,
The exacerbation of cutaneous psoriasis induced by anti - TNF therapy - case report Anca Costache 1, Mădălina Ionela Chiriac 2, Elena Rezuş*,1,2 1 Rheumatology Department, Rehabilitation Hospital Iasi,
Rheumatoid Arthritis. How are joints in the body designed?
 Rheumatoid Arthritis How are joints in the body designed? There are more than 100 joints that connect the body s 206 bones. These joints allow us to move the way we do when we walk to work, throw a ball
Rheumatoid Arthritis How are joints in the body designed? There are more than 100 joints that connect the body s 206 bones. These joints allow us to move the way we do when we walk to work, throw a ball
Rheumatoid Arthritis. Nicole Klett,, M.D.
 Rheumatoid Arthritis Nicole Klett,, M.D. Rheumatoid Arthritis Systemic Chronic Inflammatory Primarily targets the synovium of diarthrodial joints Etiology likely combination genetic and environmental Diarthrodial
Rheumatoid Arthritis Nicole Klett,, M.D. Rheumatoid Arthritis Systemic Chronic Inflammatory Primarily targets the synovium of diarthrodial joints Etiology likely combination genetic and environmental Diarthrodial
It is worth noting that people with psoriasis can also develop other forms of arthritis such as rheumatoid arthritis and osteoarthritis.
 Psoriatic Arthritis Main Colour - pantone 2597u Research - pantone 206u Children - pantone 123 4 What is psoriatic arthritis? Psoriatic arthritis is an inflammatory joint disease associated with psoriasis.
Psoriatic Arthritis Main Colour - pantone 2597u Research - pantone 206u Children - pantone 123 4 What is psoriatic arthritis? Psoriatic arthritis is an inflammatory joint disease associated with psoriasis.
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy
 Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
PSORIATIC ARTHRITIS. » Diagnosis» Symptoms» Treatments» + more
 PSORIATIC ARTHRITIS» Diagnosis» Symptoms» Treatments» + more WHAT IS PSORIASIS? PSORIASIS is pronounced sore-eye-ah-sis. It is an autoimmune disease, meaning that certain triggers cause the immune system
PSORIATIC ARTHRITIS» Diagnosis» Symptoms» Treatments» + more WHAT IS PSORIASIS? PSORIASIS is pronounced sore-eye-ah-sis. It is an autoimmune disease, meaning that certain triggers cause the immune system
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH)
 A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
Psoriasis, Incidence, Quality of Life, Psoriatic Arthritis, Prevalence
 1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
Arthritis www.patientedu.org
 written by Harvard Medical School Arthritis www.patientedu.org Arthritis is the most common chronic disease in the world, and it s the leading cause of disability in the United States. There are more than
written by Harvard Medical School Arthritis www.patientedu.org Arthritis is the most common chronic disease in the world, and it s the leading cause of disability in the United States. There are more than
1. Title 2. Background
 1. Title EARLY PsA Effectiveness of early Adalimumab therapy in psoriatic arthritis patients from Reuma.pt, the Rheumatic Diseases Portuguese Register, Portuguese RheumatoLogy SocietY (SPR) 2. Background
1. Title EARLY PsA Effectiveness of early Adalimumab therapy in psoriatic arthritis patients from Reuma.pt, the Rheumatic Diseases Portuguese Register, Portuguese RheumatoLogy SocietY (SPR) 2. Background
What s new in clinical assesment of ankylosing spondylitis?
 What s new in clinical assesment of ankylosing spondylitis? Désirée van der Heijde Professor of Rheumatology Leiden University Medical Center, the Netherlands Diakonhjemmet Hospital, Oslo, Norway Content
What s new in clinical assesment of ankylosing spondylitis? Désirée van der Heijde Professor of Rheumatology Leiden University Medical Center, the Netherlands Diakonhjemmet Hospital, Oslo, Norway Content
to Part of Dossier: Name of Active Ingredient: Title of Study: Quality of life study with adalimumab in rheumatoid arthritis. ESCALAR.
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
Rheumatoid Arthritis. What is rheumatoid arthritis? Understanding joints. What causes rheumatoid arthritis?
 Page 1 of 6 Rheumatoid Arthritis Rheumatoid arthritis causes inflammation, pain, and swelling of joints. In time, affected joints typically become damaged. The severity can vary from mild to severe. Treatments
Page 1 of 6 Rheumatoid Arthritis Rheumatoid arthritis causes inflammation, pain, and swelling of joints. In time, affected joints typically become damaged. The severity can vary from mild to severe. Treatments
Issue date: August 2010
 Issue date: August 2010 Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor Part review of NICE technology appraisal
Issue date: August 2010 Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor Part review of NICE technology appraisal
Development and Validation of a Screening Questionnaire for Psoriatic Arthritis
 Development and Validation of a Screening Questionnaire for Psoriatic Arthritis Dafna D. Gladman 1, Catherine T. Schentag 1, Brian D. Tom 2, Vinod Chandran 1, Cheryl F. Rosen 1 Vernon T. Farewell 2 1 University
Development and Validation of a Screening Questionnaire for Psoriatic Arthritis Dafna D. Gladman 1, Catherine T. Schentag 1, Brian D. Tom 2, Vinod Chandran 1, Cheryl F. Rosen 1 Vernon T. Farewell 2 1 University
Rheumatoid Arthritis. GP workshop 15 January 2011
 Rheumatoid Arthritis GP workshop 15 January 2011 Case 1 A 72 year old Malay woman with RA comes for routine follow up. She feels generally unwell in the last 5 days. Appetite is fair. Her joints are fine.
Rheumatoid Arthritis GP workshop 15 January 2011 Case 1 A 72 year old Malay woman with RA comes for routine follow up. She feels generally unwell in the last 5 days. Appetite is fair. Her joints are fine.
RHEUMATOLOGY ICD-10 CROSSWALK
 RHEUMATOLOGY ICD-10 CROSSWALK ICD is revised periodically and is currently in its tenth edition and will be implemented in the United States on October 1, 2015. There is an annual minor update and three-yearly
RHEUMATOLOGY ICD-10 CROSSWALK ICD is revised periodically and is currently in its tenth edition and will be implemented in the United States on October 1, 2015. There is an annual minor update and three-yearly
SPECIAL AUTHORIZATION GUIDELINES
 91B ALBERTA DRUG BENEFIT LIST SPECIAL AUTHORIZATION GUIDELINES 37BSpecial Authorization Policy 90BDrug Products Eligible for Consideration by Special Authorization Drug Products may be considered for coverage
91B ALBERTA DRUG BENEFIT LIST SPECIAL AUTHORIZATION GUIDELINES 37BSpecial Authorization Policy 90BDrug Products Eligible for Consideration by Special Authorization Drug Products may be considered for coverage
PSORIATIC ARTHRITIS. Chryssanthie Kafkala, M.D. INTRODUCTION:
 PSORIATIC ARTHRITIS Chryssanthie Kafkala, M.D. INTRODUCTION: Psoriatic arthritis is a disease with generally good prognosis. Both ocular and systemic involvement is usually benign, however, the following
PSORIATIC ARTHRITIS Chryssanthie Kafkala, M.D. INTRODUCTION: Psoriatic arthritis is a disease with generally good prognosis. Both ocular and systemic involvement is usually benign, however, the following
Axial Spondyloarthritis
 NATIONAL ANKYLOSING SPONDYLITIS SOCIETY UK Axial Spondyloarthritis The National Ankylosing Spondylitis Society (Axial SpA) In this leaflet we aim give you an understanding of axial spondyloarthritis and
NATIONAL ANKYLOSING SPONDYLITIS SOCIETY UK Axial Spondyloarthritis The National Ankylosing Spondylitis Society (Axial SpA) In this leaflet we aim give you an understanding of axial spondyloarthritis and
Patient Assistance Application for HUMIRA (adalimumab)
 The AbbVie Patient Assistance Foundation provides AbbVie medicines at no cost to patients experiencing financial difficulties. Eligible patients typically have no healthcare coverage for the requested
The AbbVie Patient Assistance Foundation provides AbbVie medicines at no cost to patients experiencing financial difficulties. Eligible patients typically have no healthcare coverage for the requested
Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon
 Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon Rheumatology Specialist Registrar & PhD Research Fellow 2 Overview Back ground on psoriatic arthritis (PsA) Epidemiology
Profile of Psoriatic Arthritis: What to expect as a typical patient Dr Deepak Jadon Rheumatology Specialist Registrar & PhD Research Fellow 2 Overview Back ground on psoriatic arthritis (PsA) Epidemiology
How To Treat Ankylosing Spondylitis
 Inflammatory Arthritis and Biologic therapy Ministry of Health(MOH) Recommendations Biologic Therapy for Inflammatory Arthritis Ministry of Health Recommendations Introduction Chronic inflammatory arthropathies
Inflammatory Arthritis and Biologic therapy Ministry of Health(MOH) Recommendations Biologic Therapy for Inflammatory Arthritis Ministry of Health Recommendations Introduction Chronic inflammatory arthropathies
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Multiple Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Psoriatic Arthritis. Psoriatic Arthritis 3/05
 Psoriatic Arthritis 3/05 Psoriatic Arthritis Arthritis Ireland, 1 Clanwilliam Square, Grand Canal Quay, Dublin 2 T: (01) 661 8188 F: (01) 661 8261 www.arthritisireland.ie What is in this booklet? This
Psoriatic Arthritis 3/05 Psoriatic Arthritis Arthritis Ireland, 1 Clanwilliam Square, Grand Canal Quay, Dublin 2 T: (01) 661 8188 F: (01) 661 8261 www.arthritisireland.ie What is in this booklet? This
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Elbow Injuries and Disorders
 Elbow Injuries and Disorders Introduction Your elbow joint is made up of bone, cartilage, ligaments and fluid. Muscles and tendons help the elbow joint move. There are many injuries and disorders that
Elbow Injuries and Disorders Introduction Your elbow joint is made up of bone, cartilage, ligaments and fluid. Muscles and tendons help the elbow joint move. There are many injuries and disorders that
Cytokine and CAM Antagonists
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS
 TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
