Safety. matters. CryoMACS Products
|
|
|
- Amy Cannon
- 9 years ago
- Views:
Transcription
1 Safety matters CryoMACS Products
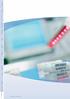
2 CryoMACS Freezing Bags The right step towards safe more cryopreservation safety in cryopreserv Overwrap bag protects both sample and freezing bag during cryopreservation. Made of the same resistant tubular ethylene vinyl acetate (EVA) material as the freezing bag. Provided with each freezing bag. Space-saving shape enables compatibility with existing freezing cassettes. Ribbed structured material facilitates opening of overwrap bag and introduction of freezing bag. Integrated filling assembly with Injection port, two female Luer-Lock connectors, one male Luer-Lock connector, and roller clamps for high flexibility. The extra-long PVC tubing is compatible with sterile connecting devices. Optimized Y-adaptor with combined Luer-Lock injection port.
3 ation Hanger holes for easy handling of the bag. Advanced bag design enables full drainage of the bag. Freezing bag made of a resistant tubular EVA film with fewer welds for more durability and less storage space. Label pouch for written sample information and to ensure label integrity throughout the freeze-thaw cycle. Two secure spike ports for removal of the thawed cell product. The single-component ports are secured with sealed, twist-off protective caps. Internal protection tubes inhibit perforation of the freezing bag during removal of the cell product. Extra-long EVA tubing enables sampling for quality control by simple heat-sealing of sample-filled tubing sections.
4 CryoMACS Freezing Bags Safety to rely on Make assurance double sure CryoMACS Freezing Bags consist of a freezing bag and a corresponding overwrap bag. Both bags are made of EVA The overwrap bag provides extra security by protecting the freezing bag and the stored cellular product, while maintaining sterility and preventing contamination risks during storage. 1, 2 Single sterile packaging for convenient handling Each CryoMACS Bag, including freezing bag and corresponding overwrap bag, is individually packed and sterilized by electron-beam irradiation. The outer packaging can be disinfected for easy clean -room handling. This helps to maintain a sterile workflow from cell collection to storage and subsequent thawing. Advanced bag design The CryoMACS Freezing Bags are made of an EVA tubular film with few welds for high durability. The design is optimized for complete drainage of the bags. The extralong EVA tubing of the integrated filling assembly allows the user to prepare small samples for quality controls: simple heat sealing can separate filled tube sections. The spike ports are secured with sealed twist-off protective caps to prevent cross-contamination. The spike ports internal protection tube inhibits perforation of the freezing bag during removal of the thawed cell product. References 1. Tedder, R et al. (1995) Lancet 346: Fountain, D et al. (1997) Transfusion 37:
5 Make Designed assurance for safer double cryopreservation sure (some more text please CryoMACS Unique bag Freezing concept Bags with consist few welds of a freezing making bag it highly and a corresponding durable overwrap bag. Both bags are made of EVA (ethylene vinyl acetate). The overwrap bag provides extra Corresponding overwrap bag to protect freezing bag security by protecting the freezing bag and the stored and stored product cellular product, while maintaining sterility and preventing contamination Optimized shape risks and during spike storage.1, ports for 2 complete some more text please drainage of the freezing bag Single, sterile packaging helps to maintain aseptic workflow More protection in the same storage space
6 CryoMACS Freezing Bags Developed by specialists, used by experts Robustness of the CryoMACS Freezing Bags for routine clinical use has been shown in a number of internal and external tests. The integrity of CryoMACS Freezing Bags after filling and exposure to ten cycles of freezing and thawing has been demonstrated in a series of laboratory experiments. Results of the freeze-thaw bag integrity test Bag designation Fill volume Number of test failures Failure at indicated cycle number CryoMACS Freezing 120 ml 2/90 9, 9 Bag 750 CryoMACS Freezing 150 ml 0/10 Bag 750 Manufacturer A ml 3/10 3, 4, 6 CryoMACS Freezing 20 ml 0/100 Bag 50 Manufacturer A ml 0/20 One hundred CryoMACS Freezing Bags 750 and ten 750 ml reference bags were filled and exposed to ten consecutive cycles of freezing and thawing. One hundred CryoMACS Freezing Bags 50 and twenty 50 ml reference bags were also tested. The results of all freezing tests are summarized here: 2 out of 100 (2%) of the 750 ml CryoMACS Freezing Bags developed leaks at very late stages 3 out of 10 (30%) of the reference bags failed the integrity test at early stages all 50 ml freezing bags passed the integrity test
7 CryoMACS Freezing Bags have been shown to be impermeable to microorganisms in accordance with the requirements of ISO ISO * The advanced bag design of the CryoMACS Freezing Bags (e.g. secured spike ports, appropriate overwrap bags) helps avoid viral contamination during use.* Several external test series concluded that CryoMACS Freezing Bags are suitable for introduction into routine clinical use and clean-room processing. 1 A comparative study showed that the CryoMACS Freezing Bags fulfill the requirements of a cryogenic freezing bag for the cryopreservation of human progenitor cells for transplantation. 2 *Reprints of these technical reports of tests performed in-house are available on request. References 1. Ings, S.J. et al. (2009) Cytotherapy 11: Sputtek, A. et al. (2011) Cytotherapy 13:
8 CryoMACS Freezing Bags Safety matters The CryoMACS Freezing Bags are intended for a single cycle of freezing, storage (down to 196 C), and subsequent thawing (at +37 C) of hematopoietic progenitor cells. Developed with the customer CryoMACS Freezing Bags have been developed in cooperation with our clients and showed excellent standards for cryopreservation. Proven quality The freezing bags and their corresponding overwrap bags are made of EVA, a durable material that has been used for 30 years in liquid nitrogen cryopreservation procedures. Different applications, different sizes A useful range of CryoMACS Freezing Bags with nominal volumes ranging from 50 to 1000 ml (recommended fill volumes from 10 to 270 ml) is available and offers flexibility for various applications.
9 Order no. Product Nominal volume CryoMACS Freezing Bag CryoMACS Freezing Bag CryoMACS Freezing Bag CryoMACS Freezing Bag CryoMACS Freezing Bag 1000 Recommended fill volume 50 ml ml 250 ml ml 500 ml ml 750 ml ml 1000 ml ml The CryoMACS Freezing Bags are manufactured by Miltenyi Biotec GmbH and controlled under an ISO13485 certified quality system. These products are available in Europe as CE-marked medical devices and are marketed in the USA under FDA 510(k) clearance. For availability in your country, please contact your local sales representative.
10 CryoMACS DMSO 10 (EP, USP) Tested and certified quality CryoMACS DMSO 10 (EP, USP) is a high quality, pure and sterile dimethyl sulfoxide, supplied in glass vials. DMSO is a well established and commonly used cryoprotective agent. It protects cells during controlled freezing and storage at cryogenic temperatures. CryoMACS DMSO is manufactured and tested under a certified ISO 9001 quality system and in compliance with relevant GMP guidelines. Each batch of CryoMACS DMSO 10 (EP, USP) is tested according to the European Pharmacopoeia (EP) and to the United States Pharmacopoeia (USP) and comes with a detailed batch-specific certificate. CryoMACS DMSO 10 (EP, USP) is for research use and ex vivo cell culture processing only, and is not intended for human in vivo applications.
11 Order no. Product Components CryoMACS DMSO 10 (EP, USP) ml glass vials CryoMACS DMSO is manufactured and tested under a certified ISO 9001 quality system and in compliance with relevant GMP guidelines. CryoMACS DMSO is for research use and ex vivo cell culture processing only, and is not intended for human in vivo applications. For regulatory status in the USA, please contact your local representative.
12 cryomacs.com Miltenyi Biotec GmbH Friedrich-Ebert-Straße Bergisch Gladbach Germany Phone Fax Miltenyi Biotec provides products and services worldwide. Visit to find your nearest Miltenyi Biotec contact
Cord Blood Collection, Processing and Freezing Bag Sets for the Cryopreservation of Blood and Cellular Components
 Cord Blood Collection, Processing and Freezing Bag Sets for the Cryopreservation of Blood and Cellular Components Facilitate each step in the collection, processing, freezing and transfusion process for
Cord Blood Collection, Processing and Freezing Bag Sets for the Cryopreservation of Blood and Cellular Components Facilitate each step in the collection, processing, freezing and transfusion process for
14.0 Stem Cell Laboratory Services
 Laboratory Services Contact Information: To inquire about assisting with surgical harvesting of bone marrow, cellular therapy (CT) product processing, cryopreservation, storage, or any other lab services,
Laboratory Services Contact Information: To inquire about assisting with surgical harvesting of bone marrow, cellular therapy (CT) product processing, cryopreservation, storage, or any other lab services,
HEALTHCARE Single - Use Systems CONTRACT MANUFACTURING SOLUTIONS PARTNER
 HEALTHCARE Single - Use Systems CONTRACT MANUFACTURING SOLUTIONS PARTNER ASI (A Part of Thermo Fisher Scientific) is dedicated to providing innovative and cost effective solutions to the Life Sciences
HEALTHCARE Single - Use Systems CONTRACT MANUFACTURING SOLUTIONS PARTNER ASI (A Part of Thermo Fisher Scientific) is dedicated to providing innovative and cost effective solutions to the Life Sciences
Guidance for Industry
 Guidance for Industry Class II Special Controls Guidance Document: Cord Blood Processing System and Storage Container This guidance is for immediate implementation. FDA is issuing this guidance for immediate
Guidance for Industry Class II Special Controls Guidance Document: Cord Blood Processing System and Storage Container This guidance is for immediate implementation. FDA is issuing this guidance for immediate
Processing Cord Blood With the SynGenX -1000 System
 Processing Cord Blood With the SynGenX -1000 System üadvanced ü Technology for Cell Separation and Cryopreservation ühigh ü CD34, TNC, MNC Recovery and Post Thaw Viability ücritical ü Process Control with
Processing Cord Blood With the SynGenX -1000 System üadvanced ü Technology for Cell Separation and Cryopreservation ühigh ü CD34, TNC, MNC Recovery and Post Thaw Viability ücritical ü Process Control with
Corning Flexible Packaging Systems. Bioprocess Containers, Tank Liners, Tubing Sets and Custom Assembly Services
 Corning Flexible Packaging Systems Bioprocess Containers, Tank Liners, Tubing Sets and Custom Assembly Services Flexible Packaging Systems Overview Corning flexible packaging systems are available in multiple
Corning Flexible Packaging Systems Bioprocess Containers, Tank Liners, Tubing Sets and Custom Assembly Services Flexible Packaging Systems Overview Corning flexible packaging systems are available in multiple
3039878000or8009926372
 3039878000or8009926372 bi s a l e s @me s a l a bs. c om Regulatory officials and sterilization experts have voiced concerns regarding the appropriateness of using a Biological Indicator (BI) Ampoule interchangeably
3039878000or8009926372 bi s a l e s @me s a l a bs. c om Regulatory officials and sterilization experts have voiced concerns regarding the appropriateness of using a Biological Indicator (BI) Ampoule interchangeably
Thermo Scientific Nunc Products for InVitro Fertilization. security, safety and reproducibility. for your valuable. samples
 Thermo Scientific Nunc Products for InVitro Fertilization security, safety and reproducibility for your valuable samples The criteria for an IVF product is that it is reliable and does not offer any threat
Thermo Scientific Nunc Products for InVitro Fertilization security, safety and reproducibility for your valuable samples The criteria for an IVF product is that it is reliable and does not offer any threat
Thermo Scientific Capitol Vial Drugs of Abuse Products. The tools to create and maintain. a safe and drug free environment
 Thermo Scientific Capitol Vial Drugs of Abuse Products The tools to create and maintain a safe and drug free environment Drugs of Abuse Specimen Collection We are a world leading manufacturer of Drugs
Thermo Scientific Capitol Vial Drugs of Abuse Products The tools to create and maintain a safe and drug free environment Drugs of Abuse Specimen Collection We are a world leading manufacturer of Drugs
www.gbo.com/bioscience Tissue Culture 1 Cell/ Microplates 2 HTS- 3 Immunology/ HLA 4 Microbiology/ Bacteriology Purpose Beakers 5 Tubes/Multi-
 14 I 2 Polypropylene 14 I 2 Polyamide 14 I 2 Mailing Container 14 I 3 Media Bottles 14 I 3 Barcode Service 14 l 4 Linear Barcode Labelling of 14 l 4 Linear Barcode Labelling of Cryo.s TM 14 l 4 Ideal for
14 I 2 Polypropylene 14 I 2 Polyamide 14 I 2 Mailing Container 14 I 3 Media Bottles 14 I 3 Barcode Service 14 l 4 Linear Barcode Labelling of 14 l 4 Linear Barcode Labelling of Cryo.s TM 14 l 4 Ideal for
+852 2956 7500 +852 2956 2110 1225 120 77008 80, 38F,
 Product Catalogue Table of contents Sepax 2...4 Sepax 2 Standard traceability kit Advanced traceability kit Sepax 2 protocol software...5 Stem cell banking protocols Regenerative medicine protocols Stem
Product Catalogue Table of contents Sepax 2...4 Sepax 2 Standard traceability kit Advanced traceability kit Sepax 2 protocol software...5 Stem cell banking protocols Regenerative medicine protocols Stem
Family Cord Blood Stem Cells Bank
 Family Cord Blood Stem Cells Bank Hope for life......evolves from life itself WHAT ARE STEM CELLS AND WHY ARE THEY PRESERVED? Cord blood stem cells are in the greatest percentage haematopoetic stem cells
Family Cord Blood Stem Cells Bank Hope for life......evolves from life itself WHAT ARE STEM CELLS AND WHY ARE THEY PRESERVED? Cord blood stem cells are in the greatest percentage haematopoetic stem cells
Packaging Validation according to ISO 11607
 Synergy makes sence Packaging Validation according to ISO 11607 MG-FSI72-104 Last revision: March 2011 5, Chemin du Catupolan - 69120 Vaulx en Velin - France - Tel. 33 (0)4 72 81 22 62 - Fax : 33 (0)4
Synergy makes sence Packaging Validation according to ISO 11607 MG-FSI72-104 Last revision: March 2011 5, Chemin du Catupolan - 69120 Vaulx en Velin - France - Tel. 33 (0)4 72 81 22 62 - Fax : 33 (0)4
Screw Cap Micro Tubes
 Screw Cap Micro Tubes For transport, storage and sample preparation Reliable quality and versatility Sicherheit Uncompromising... quality and purity Sicherheit... Over 30 years ago, Sarstedt introduced
Screw Cap Micro Tubes For transport, storage and sample preparation Reliable quality and versatility Sicherheit Uncompromising... quality and purity Sicherheit... Over 30 years ago, Sarstedt introduced
EU GMP Annex 1 Update 2008: Airborne Particle Counting
 EU GMP Annex 1 Update 2008: Airborne Particle Counting Airborne Particle Counting for Pharmaceutical Facilities: Update 2008, EU GMP Annex 1 Morgan Polen - VP of Applications Technology, Lighthouse Worldwide
EU GMP Annex 1 Update 2008: Airborne Particle Counting Airborne Particle Counting for Pharmaceutical Facilities: Update 2008, EU GMP Annex 1 Morgan Polen - VP of Applications Technology, Lighthouse Worldwide
The essential choice in cell processing
 The essential choice in cell processing Established in 1997 The Biosafe Group is an internationally active medical technology company with its roots in the Lake Geneva region of Switzerland. Since it was
The essential choice in cell processing Established in 1997 The Biosafe Group is an internationally active medical technology company with its roots in the Lake Geneva region of Switzerland. Since it was
Quick guide: using CTE security straws for cryopreservation of pronuclear stages, embryos and sperm
 Quick guide: using CTE security straws for cryopreservation of pronuclear stages, embryos and sperm 1. How to prepare straws for pronuclear stages and embryos (use straws with plug, 66.5 mm) Straws are
Quick guide: using CTE security straws for cryopreservation of pronuclear stages, embryos and sperm 1. How to prepare straws for pronuclear stages and embryos (use straws with plug, 66.5 mm) Straws are
Precision injection moulding
 Extrusion Moulding Assembly Precision injection moulding Customised polymer solutions Your development partner and system supplier for medical and pharmaceutical assemblies and systems CERTIFICATE The
Extrusion Moulding Assembly Precision injection moulding Customised polymer solutions Your development partner and system supplier for medical and pharmaceutical assemblies and systems CERTIFICATE The
Bio Deutschland CEO & CFO-Meeting 29. November 2012. Unternehmertum in den Life Sciences am Beispiel der Geschichte von Miltenyi Biotec
 Bio Deutschland CEO & CFO-Meeting 29. November 2012 Unternehmertum in den Life Sciences am Beispiel der Geschichte von Miltenyi Biotec Major locations Köln Our Passion SHARING OUR PASSION FOR CELLS We
Bio Deutschland CEO & CFO-Meeting 29. November 2012 Unternehmertum in den Life Sciences am Beispiel der Geschichte von Miltenyi Biotec Major locations Köln Our Passion SHARING OUR PASSION FOR CELLS We
CHAPTER 3 COLLECTION PROCEDURES
 CHAPTER 3 COLLECTION PROCEDURES Chapter 3 COLLECTION PROCEDURES 3.1 COLLECTION KIT Principle The cord blood collection kit will be carefully assembled by the Cord Blood Bank (CBB) supervisor or his/her
CHAPTER 3 COLLECTION PROCEDURES Chapter 3 COLLECTION PROCEDURES 3.1 COLLECTION KIT Principle The cord blood collection kit will be carefully assembled by the Cord Blood Bank (CBB) supervisor or his/her
Sampling Solutions. Fuel and Lube Oil sampling Equipment. Convenient, Representative, Approved.
 Sampling Solutions Fuel and Lube Oil sampling Equipment. Convenient, Representative, Approved. Oil Samples are used to obtain a clear indication of the operating status of your machinery. One of the most
Sampling Solutions Fuel and Lube Oil sampling Equipment. Convenient, Representative, Approved. Oil Samples are used to obtain a clear indication of the operating status of your machinery. One of the most
Liquids Suspensions Gels
 Liquids Suspensions Gels EMCM: your product development and manufacturing partner Centre of excellence European Medical Contract Manufacturing (EMCM) is the centre of excellence in developing and manufacturing
Liquids Suspensions Gels EMCM: your product development and manufacturing partner Centre of excellence European Medical Contract Manufacturing (EMCM) is the centre of excellence in developing and manufacturing
How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs
 WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
Assembly and Installation Procedures
 Assembly and Installation Procedures for Pall Pharmaceutical Grade Capsule Assemblies 1. Introduction The following procedures must be followed for the installation of Pall pharmaceutical grade capsule
Assembly and Installation Procedures for Pall Pharmaceutical Grade Capsule Assemblies 1. Introduction The following procedures must be followed for the installation of Pall pharmaceutical grade capsule
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Handling Instructions
 Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
BioArchive Freezing Curve for Cord Blood
 BioArchive Freezing Curve for Cord Blood Determination of the Specific Freezing in the BioArchive System to Provide the Highest Viability of Cord Blood MNC as Measured by -C Assays Introduction Freezing
BioArchive Freezing Curve for Cord Blood Determination of the Specific Freezing in the BioArchive System to Provide the Highest Viability of Cord Blood MNC as Measured by -C Assays Introduction Freezing
Cleaning validation of cleanrooms and preparation equipments
 Cleaning validation of cleanrooms and preparation equipments Head of production Central Pharmacy, Geneva University Hospitals EAHP Foundation Seminar: Patient Safety; More About Compounding" 23-25 May,
Cleaning validation of cleanrooms and preparation equipments Head of production Central Pharmacy, Geneva University Hospitals EAHP Foundation Seminar: Patient Safety; More About Compounding" 23-25 May,
Sterile Cleanroom Management
 Sterile Cleanroom Management Lynn Stanard, Sr. Quality Manager, Berkshire Corporation When manufacturing in an aseptic environment, it is critical to ensure that the various cleanroom consumables, such
Sterile Cleanroom Management Lynn Stanard, Sr. Quality Manager, Berkshire Corporation When manufacturing in an aseptic environment, it is critical to ensure that the various cleanroom consumables, such
TEMPERATURE SENSORS AND PROBES FOR BIO-MEDICAL APPLICATIONS
 TEMPERATURE SENSORS AND PROBES FOR BIO-MEDICAL APPLICATIONS www.meas-spec.com Introduction Measurement Specialties is the market leader in critical temperature measurement and is a leading provider of
TEMPERATURE SENSORS AND PROBES FOR BIO-MEDICAL APPLICATIONS www.meas-spec.com Introduction Measurement Specialties is the market leader in critical temperature measurement and is a leading provider of
THE BASICS OF PACKAGING
 THE BASICS OF PACKAGING Objectives: Review the regulatory requirements for packaging Present the purpose, function and essential characteristics of packaging Describe packaging options, their use, and
THE BASICS OF PACKAGING Objectives: Review the regulatory requirements for packaging Present the purpose, function and essential characteristics of packaging Describe packaging options, their use, and
Barcode Labeling in the Lab Closing the Loop of Patient Safety
 Barcode Labeling in the Lab Closing the Loop of Patient Safety INTRODUCTION Detecting and preventing errors that threaten patient safety is a closed-loop process that begins at the point of care, extends
Barcode Labeling in the Lab Closing the Loop of Patient Safety INTRODUCTION Detecting and preventing errors that threaten patient safety is a closed-loop process that begins at the point of care, extends
GMP ANNEX 1 REVISION 2008, INTERPRETATION OF MOST IMPORTANT CHANGES FOR THE MANUFACTURE OF STERILE MEDICINAL PRODUCTS
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 032-2 8 January 2010 RECOMMENDATION GMP ANNEX 1 REVISION 2008, INTERPRETATION OF MOST IMPORTANT CHANGES FOR THE MANUFACTURE
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 032-2 8 January 2010 RECOMMENDATION GMP ANNEX 1 REVISION 2008, INTERPRETATION OF MOST IMPORTANT CHANGES FOR THE MANUFACTURE
Particle testing in cleanroom high-pressure gas lines to ISO 14644 made easy with the MET ONE 3400 gas calibrations
 Particle testing in cleanroom high-pressure gas lines to ISO 14644 made easy with the MET ONE 3400 gas calibrations B e c k m a n C o u l t e r L i f e S c i e n c e s 2 5 0 S. K r a e m e r B o u l e
Particle testing in cleanroom high-pressure gas lines to ISO 14644 made easy with the MET ONE 3400 gas calibrations B e c k m a n C o u l t e r L i f e S c i e n c e s 2 5 0 S. K r a e m e r B o u l e
Caring for a Tenckhoff Catheter
 Caring for a Tenckhoff Catheter UHN A Patient s Guide What is a Pleural Effusion? There is a small space between the outside of your lung and the chest wall (ribs). This space is called the pleural space.
Caring for a Tenckhoff Catheter UHN A Patient s Guide What is a Pleural Effusion? There is a small space between the outside of your lung and the chest wall (ribs). This space is called the pleural space.
Climate chamber with illumination
 KBWF series (E5.2) Climate chambers with illumination Climate chamber with illumination The BINDER climate chamber with illumination of the KBW series achieves homogeneous light distribution with its natural
KBWF series (E5.2) Climate chambers with illumination Climate chamber with illumination The BINDER climate chamber with illumination of the KBW series achieves homogeneous light distribution with its natural
NALCO WT-1000 Closed System Corrosion Inhibitor
 PRODUCT NAME NALCO WT-1000 Closed System Corrosion Inhibitor PRODUCT DESCRIPTION AND APPLICATION Nalco WT-1000 is a liquid corrosion inhibitor for use in closed cooling and hot water heating systems. Nalco
PRODUCT NAME NALCO WT-1000 Closed System Corrosion Inhibitor PRODUCT DESCRIPTION AND APPLICATION Nalco WT-1000 is a liquid corrosion inhibitor for use in closed cooling and hot water heating systems. Nalco
Liquid II Cell Culture Media Manufacturing Plant. Overview Facilities Water for Injection Sterile Environment Media Handling Cleanroom Interior
 Liquid II Cell Culture Media Manufacturing Plant Overview Facilities Water for Injection Sterile Environment Media Handling Cleanroom Interior Overview History of BioConcept and Amimed BioConcept has close
Liquid II Cell Culture Media Manufacturing Plant Overview Facilities Water for Injection Sterile Environment Media Handling Cleanroom Interior Overview History of BioConcept and Amimed BioConcept has close
EUROTUBO DELTALAB 4. PETRI DISHES AND LOOPS
 77 78 90 x 14 mm Petri Dish Made in polystyrene. Vented. Supplied in groups of 20 units, packaged in heat sealed bags. Code 200200 is ASEPTIC. Code 200209 is sterile by gamma radiation. Suitable for automatic
77 78 90 x 14 mm Petri Dish Made in polystyrene. Vented. Supplied in groups of 20 units, packaged in heat sealed bags. Code 200200 is ASEPTIC. Code 200209 is sterile by gamma radiation. Suitable for automatic
laboratory glass bottles and screw caps
 LABWARE SCHOTT DURAN E SCHOTT DURAN laboratory glass bottles and screw caps - Rely on the original! - SCHOTT DURAN laboratory glass bottles Fields of application and properties The outstanding properties
LABWARE SCHOTT DURAN E SCHOTT DURAN laboratory glass bottles and screw caps - Rely on the original! - SCHOTT DURAN laboratory glass bottles Fields of application and properties The outstanding properties
Cord Blood: Thawing & Infusion Practical Methods
 Cord Blood: Thawing & Infusion Practical Methods Donna M Regan, MT(ASCP)SBB St. Louis Cord Blood Bank @ SSM Cardinal Glennon Children s s Medical Center Objectives Present variety of product configurations
Cord Blood: Thawing & Infusion Practical Methods Donna M Regan, MT(ASCP)SBB St. Louis Cord Blood Bank @ SSM Cardinal Glennon Children s s Medical Center Objectives Present variety of product configurations
Single-Use Flow-Through Cell ph FTC-SU-HP8
 Single-Use Flow-Through Cell ph FTC-SU-HP8 SENSOR PROBES Instruction Manual Single-Use Flow-Through Cell ph FTC-SU-HP8 Specification: ph sensor stick integrated in a single-use flow-through cell Document
Single-Use Flow-Through Cell ph FTC-SU-HP8 SENSOR PROBES Instruction Manual Single-Use Flow-Through Cell ph FTC-SU-HP8 Specification: ph sensor stick integrated in a single-use flow-through cell Document
Hematopoiesis i starts from. HPC differentiates t to sequentially more mature. HPC circulate in very low
 Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Design, Operation and Management of GTP/GMP Cell Engineering Facilities
 Design, Operation and Management of GTP/GMP Cell Engineering Facilities Scott R. Burger, MD Advanced Cell & Gene Therapy BFDA 2007 International Symposium on Regulation of Human Cell and Tissue- Based
Design, Operation and Management of GTP/GMP Cell Engineering Facilities Scott R. Burger, MD Advanced Cell & Gene Therapy BFDA 2007 International Symposium on Regulation of Human Cell and Tissue- Based
Gravity Bag Cord Blood Collection Method Training Manual
 Gravity Bag Cord Blood Collection Method Training Manual CordBank Ltd. Training Manual Page 1 of 13 Table of Contents Chapter Page Introduction 3 Requirements for Collection of Cord Blood 3 Collector s
Gravity Bag Cord Blood Collection Method Training Manual CordBank Ltd. Training Manual Page 1 of 13 Table of Contents Chapter Page Introduction 3 Requirements for Collection of Cord Blood 3 Collector s
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs)
 HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
Helping to protect you from exposure to hazardous drugs
 Helping to protect you from exposure to hazardous drugs Closed system solution: Texium closed male luer and SmartSite needle-free valve products What you can t see can hurt you The risks are well documented
Helping to protect you from exposure to hazardous drugs Closed system solution: Texium closed male luer and SmartSite needle-free valve products What you can t see can hurt you The risks are well documented
Cellartis Protocol Culturing of hes Cells
 Cellartis Protocol Culturing of hes Cells This material was cultured and frozen using Cellartis protocols. WiCell recommends that stem cells should be thawed and established in the conditions in which
Cellartis Protocol Culturing of hes Cells This material was cultured and frozen using Cellartis protocols. WiCell recommends that stem cells should be thawed and established in the conditions in which
Overcoming Challenges in the Manufacturing of Pre-Filled Syringes. Farvea, a new comer in the PFS world PFS Tech 2016. June 2016 pharma@fareva.
 Overcoming Challenges in the Manufacturing of Pre-Filled Syringes Farvea, a new comer in the PFS world PFS Tech 2016 June 2016 [email protected] Our presence around the world 11 countries 35 sites 12
Overcoming Challenges in the Manufacturing of Pre-Filled Syringes Farvea, a new comer in the PFS world PFS Tech 2016 June 2016 [email protected] Our presence around the world 11 countries 35 sites 12
Microbiological Evaluation of the STI Series 2000 Medical Waste Treatment Process
 WNWN International,Inc. WNWN International Phone: 860-675-1217 Fax 860-675-1311 PO Box 1164 Burlington, CT. 06013 USA Microbiological Evaluation of the STI Series 2000 Medical Waste Treatment Process January
WNWN International,Inc. WNWN International Phone: 860-675-1217 Fax 860-675-1311 PO Box 1164 Burlington, CT. 06013 USA Microbiological Evaluation of the STI Series 2000 Medical Waste Treatment Process January
USP CLASS VI GASKETS PRODUCTS OF INTEGRITY...FROM PEOPLE OF INTEGRITY
 USP CLASS VI GASKETS PRODUCTS OF INTEGRITY...FROM PEOPLE OF INTEGRITY USP Class VI Compliance Means Substantial Savings for You Established in 1973 David Newman set out to create the only company in the
USP CLASS VI GASKETS PRODUCTS OF INTEGRITY...FROM PEOPLE OF INTEGRITY USP Class VI Compliance Means Substantial Savings for You Established in 1973 David Newman set out to create the only company in the
Sterile ReadyToProcess Hollow Fiber Cartridges Instructions for Use
 GE Healthcare Instructions 28-9226-84 AD ReadyToProcess Sterile ReadyToProcess Hollow Fiber Cartridges Instructions for Use 1 Introduction... 1 2 Inspect the Cartridge... 1 3 General Description... 2 4
GE Healthcare Instructions 28-9226-84 AD ReadyToProcess Sterile ReadyToProcess Hollow Fiber Cartridges Instructions for Use 1 Introduction... 1 2 Inspect the Cartridge... 1 3 General Description... 2 4
Standard Operating Procedure (SOP) Work Package 8. Sample Collection and Storage
 Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
www.gbo.com/bioscience Tissue Culture 1 Cell/ Microplates 2 HTS- 3 Immunology/ HLA 4 Microbiology/ Bacteriology Purpose Beakers 5 Tubes/Multi-
 www.gbo.com/bioscience 11 Cryotechnics Technical Information 11 I 2 Cryo.s TM 11 I 3 Cryo.s TM 1 ml 11 I 3 Cryo.s TM 2 ml 11 I 3 Cryo.s TM 4 ml 11 I 4 Cryo.s TM 5 ml 11 I 4 Support Rack 11 I 5 Cryo Storage
www.gbo.com/bioscience 11 Cryotechnics Technical Information 11 I 2 Cryo.s TM 11 I 3 Cryo.s TM 1 ml 11 I 3 Cryo.s TM 2 ml 11 I 3 Cryo.s TM 4 ml 11 I 4 Cryo.s TM 5 ml 11 I 4 Support Rack 11 I 5 Cryo Storage
 THERMAL CHARACTERISTICS Nominal operating cell temperature(noct) [ C] 48,2±2 48 Temperature coefficient P MPP [%K] -0,41-0,47 Temperature coefficient I SC [%K] 0,05 0,04 Temperature coefficient U OC [%K]
THERMAL CHARACTERISTICS Nominal operating cell temperature(noct) [ C] 48,2±2 48 Temperature coefficient P MPP [%K] -0,41-0,47 Temperature coefficient I SC [%K] 0,05 0,04 Temperature coefficient U OC [%K]
C 5. chemical development contract research custom synthesis cgmp API manufacturing commercial production. Welcome to
 C 5 chemical development contract research custom synthesis cgmp API manufacturing commercial production Welcome to ChemCon Company profile Company profile ChemCon offers outstanding chemical services
C 5 chemical development contract research custom synthesis cgmp API manufacturing commercial production Welcome to ChemCon Company profile Company profile ChemCon offers outstanding chemical services
Particle Monitoring Requirements in Pharmaceutical Cleanrooms
 Particle Monitoring Requirements in Pharmaceutical Cleanrooms All drugs must be manufactured in accordance with the current Good Manufacturing Practice (cgmp) regulations. Pharmaceutical manufacturers
Particle Monitoring Requirements in Pharmaceutical Cleanrooms All drugs must be manufactured in accordance with the current Good Manufacturing Practice (cgmp) regulations. Pharmaceutical manufacturers
Public Cord Blood Banking at the National Cord Blood Program (NCBP)
 Public Cord Blood Banking at the National Cord Blood Program (NCBP) A. Scaradavou, MD Medical Director, National Cord Blood Program, New York Blood Center Associate Attending, Pediatric BMT Memorial Sloan-Kettering
Public Cord Blood Banking at the National Cord Blood Program (NCBP) A. Scaradavou, MD Medical Director, National Cord Blood Program, New York Blood Center Associate Attending, Pediatric BMT Memorial Sloan-Kettering
WHITE PAPER April 18, 2016
 WHITE PAPER April 18, 2016 Nitrogen Dioxide Biodecontamination: A New, Effective and Cost-saving Option for Biodecontaminating Syringe Tubs Prior to the Filling Line Prepared by: David Opie, PhD Maura
WHITE PAPER April 18, 2016 Nitrogen Dioxide Biodecontamination: A New, Effective and Cost-saving Option for Biodecontaminating Syringe Tubs Prior to the Filling Line Prepared by: David Opie, PhD Maura
(800) 786-7235 Cryo-Cell.com
 HEALTHCARE PROVIDER Instructions Keep these instructions in the kit [ When handling blood products, use personal protective measures, maintain aseptic techniques and adequate room for collection processes.
HEALTHCARE PROVIDER Instructions Keep these instructions in the kit [ When handling blood products, use personal protective measures, maintain aseptic techniques and adequate room for collection processes.
CORD BLOOD BANKING FAQ
 CORD BLOOD BANKING FAQ Cord Blood & Stem Cells Q: What is umbilical cord blood (UCB)? A: Bone marrow, peripheral blood and UCB constitute the three primary sources of stem cells. Cord blood, which, until
CORD BLOOD BANKING FAQ Cord Blood & Stem Cells Q: What is umbilical cord blood (UCB)? A: Bone marrow, peripheral blood and UCB constitute the three primary sources of stem cells. Cord blood, which, until
PQS performance specification
 PQS performance specification WHO/PQS/E08/JI01.1 Original: English Distribution: General TITLE: Single-use auto-disable needle-free syringe injectors Specification reference: E08/JI01.1 Product verification
PQS performance specification WHO/PQS/E08/JI01.1 Original: English Distribution: General TITLE: Single-use auto-disable needle-free syringe injectors Specification reference: E08/JI01.1 Product verification
Disposables the Original by B. Braun
 Disposables the Original by B. Braun Flexibility and Accuracy for All Pumps Automated Infusion Systems Infusomat Space Lines A System Dedicated to Flexibility for All Therapies and All Wards Easy Start
Disposables the Original by B. Braun Flexibility and Accuracy for All Pumps Automated Infusion Systems Infusomat Space Lines A System Dedicated to Flexibility for All Therapies and All Wards Easy Start
www.sampling.com World Class Equipment worldwide Edition J
 www.sampling.com World Class Equipment worldwide Edition J Welcome Welcome to the latest edition of our Range Brochure Firstly, we would like to thank you our customers for helping us create the new range
www.sampling.com World Class Equipment worldwide Edition J Welcome Welcome to the latest edition of our Range Brochure Firstly, we would like to thank you our customers for helping us create the new range
Federal Wage System Job Grading Standard For Laboratory Working, 3511. Table of Contents
 Federal Wage System Job Grading Standard For Laboratory Working, 3511 Table of Contents WORK COVERED... 2 WORK NOT COVERED...2 TITLES... 2 GRADE LEVELS... 2 NOTE TO USERS... 3 LABORATORY WORKER, GRADE
Federal Wage System Job Grading Standard For Laboratory Working, 3511 Table of Contents WORK COVERED... 2 WORK NOT COVERED...2 TITLES... 2 GRADE LEVELS... 2 NOTE TO USERS... 3 LABORATORY WORKER, GRADE
Recommendations to Transplant Centres Performing Cord Blood Transplants. Why Choosing the Right Thaw Method Could Save a Patient s Life
 Recommendations to Transplant Centres Performing Cord Blood Transplants Vicki Antonenas Why Choosing the Right Thaw Method Could Save a Patient s Life Lynn O Donnell (USA) 1 The process of receiving, testing
Recommendations to Transplant Centres Performing Cord Blood Transplants Vicki Antonenas Why Choosing the Right Thaw Method Could Save a Patient s Life Lynn O Donnell (USA) 1 The process of receiving, testing
Cryopreservation of Umbilical Cord Tissue for Stem Cell Harvesting Executive Summary Figure 1. Cross section of normal umbilical cord.
 Cryopreservation of Umbilical Cord Tissue for Stem Cell Harvesting By Steven Buchwald, Nicole Chiu, Melvin Chu, Hesed Kim, Calvin Yeang BEE 453 5/2/03 Executive Summary Stem cell transplantation has become
Cryopreservation of Umbilical Cord Tissue for Stem Cell Harvesting By Steven Buchwald, Nicole Chiu, Melvin Chu, Hesed Kim, Calvin Yeang BEE 453 5/2/03 Executive Summary Stem cell transplantation has become
Total Aerobic Bacteria, Yeasts and Molds
 Total Aerobic Bacteria, Yeasts and Molds DOC316.53.01223 Total Aerobic Bacteria (Amber), Yeast and Mold (Red), Total Coliform (Red) and Disinfection Control (Purple) Paddle Testers Scope and application:
Total Aerobic Bacteria, Yeasts and Molds DOC316.53.01223 Total Aerobic Bacteria (Amber), Yeast and Mold (Red), Total Coliform (Red) and Disinfection Control (Purple) Paddle Testers Scope and application:
Medical aids for 1 colon diagnostics PRODUCT CATALOG
 Medical aids for 1 colon diagnostics PRODUCT CATALOG PRIMEDISTOM TRACHEALKANÜLEN OHNE CUFF 2 PRIMEDISTOM TRACHEALKANÜLEN OHNE CUFF 3 Medizintechnik GmbH is a highly experienced manufacturer of medical
Medical aids for 1 colon diagnostics PRODUCT CATALOG PRIMEDISTOM TRACHEALKANÜLEN OHNE CUFF 2 PRIMEDISTOM TRACHEALKANÜLEN OHNE CUFF 3 Medizintechnik GmbH is a highly experienced manufacturer of medical
Cleanroom. For. Sterile Manufacturing Facilities
 Cleanroom For Sterile Manufacturing Facilities Praphon Angtrakool Food and Drug Administration 1 WHO TRS No. 823 Annex 1, 1992 (1) General 17.1 The production of sterile preparations should be carried
Cleanroom For Sterile Manufacturing Facilities Praphon Angtrakool Food and Drug Administration 1 WHO TRS No. 823 Annex 1, 1992 (1) General 17.1 The production of sterile preparations should be carried
VALIDATION AND QUALIFICATION
 VALIDATION AND QUALIFICATION Lizette Caballero, B.S., M.T. (ASCP) Laboratory Manager Cellular Therapy Laboratory Florida Hospital Cancer Institute Orlando, FL 1 Learning Objectives Validation of Equipment
VALIDATION AND QUALIFICATION Lizette Caballero, B.S., M.T. (ASCP) Laboratory Manager Cellular Therapy Laboratory Florida Hospital Cancer Institute Orlando, FL 1 Learning Objectives Validation of Equipment
KGI MEDICAL WASTE MANAGEMENT PLAN
 1revised 2012 I. Facility Information KGI MEDICAL WASTE MANAGEMENT PLAN a. Contact Person: Barbara Erwin Director Research Operations/Chemical and Biological Laboratory Safety/Chair. 909-607-0160 i. Facility:
1revised 2012 I. Facility Information KGI MEDICAL WASTE MANAGEMENT PLAN a. Contact Person: Barbara Erwin Director Research Operations/Chemical and Biological Laboratory Safety/Chair. 909-607-0160 i. Facility:
Sterilization methods and equipment Lab 1-2
 Sterilization methods and equipment Lab 1-2 PHT 434 Sterilization Sterilization a process that by which all viable M.O are removed or destroyed, based on a probability function. Sterilization concept Sterilization
Sterilization methods and equipment Lab 1-2 PHT 434 Sterilization Sterilization a process that by which all viable M.O are removed or destroyed, based on a probability function. Sterilization concept Sterilization
Cyto-Set. Safer cytostatic preparation and application. IV Administration
 Cyto-Set Safer cytostatic preparation and application IV Administration Always a closed system Protect yourself and your environment The number of cytostatic infusions that have to be prepared is growing
Cyto-Set Safer cytostatic preparation and application IV Administration Always a closed system Protect yourself and your environment The number of cytostatic infusions that have to be prepared is growing
How To Safely Infuse Aupbsc
 The Process of Infusing Cryopreserved Stem Cells by Registered Nurses Presenters: Kristen Brazel, RN BScN Kate Duke, RN BScN 5 West Haematology Unit, The Ottawa General Hospital, Ottawa, Ontario, Canada
The Process of Infusing Cryopreserved Stem Cells by Registered Nurses Presenters: Kristen Brazel, RN BScN Kate Duke, RN BScN 5 West Haematology Unit, The Ottawa General Hospital, Ottawa, Ontario, Canada
Aseptic preparations, including TPN, for a limited number of patients
 Aseptic preparations, including TPN, for a limited number of patients Group F (TPN) 1 Objective Presentation of our business case: setting up an aseptic TPN production in the Hilton Pharmacy instead of
Aseptic preparations, including TPN, for a limited number of patients Group F (TPN) 1 Objective Presentation of our business case: setting up an aseptic TPN production in the Hilton Pharmacy instead of
USER MANUAL. 10 GPM Small Single Line Cryogenic Coupling
 JC Carter LLC LNG Couplings USER MANUAL 10 GPM Small Single Line Cryogenic Coupling Models 50E722 Nozzle and 50E721 Receptacle User s Manual and Installation Guide! Warning: Read the User s Manual and
JC Carter LLC LNG Couplings USER MANUAL 10 GPM Small Single Line Cryogenic Coupling Models 50E722 Nozzle and 50E721 Receptacle User s Manual and Installation Guide! Warning: Read the User s Manual and
Case Study Neubau einer Parenteralia Fabrik 3. GMP-Forum, Kirchzarten 28. September 2012, Basel. Philip Schneider, F.
 3. GMP-Forum, Kirchzarten 28. September 2012, Basel Philip Schneider, F. Hoffmann-La Roche Content Introduction Decontamination cycle Set-up and change over Aseptic connections Glove handling and testing
3. GMP-Forum, Kirchzarten 28. September 2012, Basel Philip Schneider, F. Hoffmann-La Roche Content Introduction Decontamination cycle Set-up and change over Aseptic connections Glove handling and testing
ISO 21501 A Standard Methodology to Optical Particle Counter Calibration and What It Means to Cleanroom Owners
 ISO 21501 A Standard Methodology to Optical Particle Counter Calibration and What It Means to Cleanroom Owners Tony Harrison and Bob Latimer ISO 21501- A Standard Methodology to Optical Particle Counter
ISO 21501 A Standard Methodology to Optical Particle Counter Calibration and What It Means to Cleanroom Owners Tony Harrison and Bob Latimer ISO 21501- A Standard Methodology to Optical Particle Counter
Guidance on safe use of Autoclaves
 Safety Office Reviewed: Dec 2013 Reviewed: DD Month Year Guidance on safe use of Autoclaves 1. Hazards & Uses There are several different types of hazard associated with the use of autoclaves. The main
Safety Office Reviewed: Dec 2013 Reviewed: DD Month Year Guidance on safe use of Autoclaves 1. Hazards & Uses There are several different types of hazard associated with the use of autoclaves. The main
syriq Glass Prefillable Syringes
 syriq Glass Prefillable Syringes 2 SCHOTT is a leading international technology group in the areas of specialty glass and glass-ceramics. With more than 130 years of outstanding development, materials
syriq Glass Prefillable Syringes 2 SCHOTT is a leading international technology group in the areas of specialty glass and glass-ceramics. With more than 130 years of outstanding development, materials
Transport and Mailing Systems
 Transport and Mailing Systems For transportation of diagnostic samples and blood products The complete product range for mailing and transport requirements Safe and compliant with transportation guidelines
Transport and Mailing Systems For transportation of diagnostic samples and blood products The complete product range for mailing and transport requirements Safe and compliant with transportation guidelines
Guidelines for Collection, Processing, and Storage of Cord Blood Stem Cells Second Edition March 2003
 Guidelines for Collection, Processing, and Storage of Cord Blood Stem Cells Second Edition March 2003 New York State Council on Human Blood and Transfusion Services New York State Department of Health
Guidelines for Collection, Processing, and Storage of Cord Blood Stem Cells Second Edition March 2003 New York State Council on Human Blood and Transfusion Services New York State Department of Health
Kinematic viscometers in accordance with ISO 3105, DIN 51562, ASTM D445 For automatic and manual viscosity measuring Greatest precision and durability
 T h e r i g h t t e m p e r a t u r e w o r l d w i d e Kinematic viscometers in accordance with ISO 3105, DIN 51562, ASTM D445 For automatic and manual viscosity measuring Greatest precision and durability
T h e r i g h t t e m p e r a t u r e w o r l d w i d e Kinematic viscometers in accordance with ISO 3105, DIN 51562, ASTM D445 For automatic and manual viscosity measuring Greatest precision and durability
Washing. Sterilising. Filling. Closing. Inspecting. Labelling
 Washing. Sterilising. Filling. Closing. Inspecting. Labelling Washing Page 4/5 Sterilising Page 6/7 Filling Page 8/9, 10/11 Closing Page 8/9, 10/11 Inspecting Page 12/13 Labelling Page 14/15 Syringe processing
Washing. Sterilising. Filling. Closing. Inspecting. Labelling Washing Page 4/5 Sterilising Page 6/7 Filling Page 8/9, 10/11 Closing Page 8/9, 10/11 Inspecting Page 12/13 Labelling Page 14/15 Syringe processing
Tablet Packaging Safety
 Tablet Packaging Safety Tablet Packaging Safety Gossen Metrawatt supplies controllers which assure minimal temperature deviation even in critical applications. TLT 2600 Packaging Machine at Bayer HealthCare
Tablet Packaging Safety Tablet Packaging Safety Gossen Metrawatt supplies controllers which assure minimal temperature deviation even in critical applications. TLT 2600 Packaging Machine at Bayer HealthCare
CHAPTER 5 SHIPPING PROCEDURES
 CHAPTER 5 SHIPPING PROCEDURES Chapter 5 SHIPPING PROCEDURES 5.1 SHIPPING CBUS FROM COLLECTION CENTERS TO CORD BLOOD BANKS Principle Collected umbilical cord blood has been found to maintain cell viability
CHAPTER 5 SHIPPING PROCEDURES Chapter 5 SHIPPING PROCEDURES 5.1 SHIPPING CBUS FROM COLLECTION CENTERS TO CORD BLOOD BANKS Principle Collected umbilical cord blood has been found to maintain cell viability
Mycoplasma Testing Products & Services. M-175 CELLshipper Mycoplasma Detection Kit (In-house sample preparation and slide fixation)
 Cell Culture Testing M-1500 Real-Time PCR with Broth Enrichment A specific and sensitive method for the detection of mycoplasma using Real-Time PCR coupled with a pre-enrichment procedure to enhance method
Cell Culture Testing M-1500 Real-Time PCR with Broth Enrichment A specific and sensitive method for the detection of mycoplasma using Real-Time PCR coupled with a pre-enrichment procedure to enhance method
James M. Pleasants Company
 James M. Pleasants Company SUBMITTAL DATA GAINESVILLE MECHANICAL DECEMBER 20, 2013 PROJECT: GSU: J-183 HUMANITIES LAW BLDG. QUOTE NO:12116 ENGINEER: STEVENS & WILKINSON GASKETED PLATE HEAT EXCHANGER Tag:
James M. Pleasants Company SUBMITTAL DATA GAINESVILLE MECHANICAL DECEMBER 20, 2013 PROJECT: GSU: J-183 HUMANITIES LAW BLDG. QUOTE NO:12116 ENGINEER: STEVENS & WILKINSON GASKETED PLATE HEAT EXCHANGER Tag:
FILTRATION SOLUTIONS PhARmAceUTIcAL manufacturing FILTRATION SPecIALISTS
 FILTRATION SOLUTIONS Pharmaceutical Manufacturing filtration specialists Delivering quality filtration products As one of Europe s leading manufacturers of process filters, Amazon Filters is able to offer
FILTRATION SOLUTIONS Pharmaceutical Manufacturing filtration specialists Delivering quality filtration products As one of Europe s leading manufacturers of process filters, Amazon Filters is able to offer
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
AATB/ICCBBA Interim Guidance Document. For use of ISBT 128 by North American Tissue Banks
 AATB/ICCBBA Interim Guidance Document For use of ISBT 128 by North American Tissue Banks Version 1.2.0 September 2012 Published by: ICCBBA PO Box 11309, San Bernardino, California, USA 1.909.793.6516 [email protected]
AATB/ICCBBA Interim Guidance Document For use of ISBT 128 by North American Tissue Banks Version 1.2.0 September 2012 Published by: ICCBBA PO Box 11309, San Bernardino, California, USA 1.909.793.6516 [email protected]
Tabletop Line. Automatic and Semi-Automatic Autoclave Series
 Tabletop Line Automatic and Semi-Automatic Autoclave Series Tuttnauer Your Sterilization & Infection Control Partner Company Profile For over 80 years, Tuttnauer s sterilization and infection control products
Tabletop Line Automatic and Semi-Automatic Autoclave Series Tuttnauer Your Sterilization & Infection Control Partner Company Profile For over 80 years, Tuttnauer s sterilization and infection control products
Building Owners Guide to a Duro-Last System!
 Building Owners Guide to a Duro-Last System Table of Contents Section 1: Introduction to Duro-Last Roofing Systems Section 2: Benefits of a Duro-Last Roofing System Section 3: Comparing a Duro-Last Roof
Building Owners Guide to a Duro-Last System Table of Contents Section 1: Introduction to Duro-Last Roofing Systems Section 2: Benefits of a Duro-Last Roofing System Section 3: Comparing a Duro-Last Roof
