DICOM Conformance Statement. CR Console. (Standard) Copyright FUJIFILM Corporation, Japan. June th Edition 897N0547D ( )
|
|
|
- Peregrine Short
- 7 years ago
- Views:
Transcription
1 DICOM Conformance Statement CR Console (Standard) June th Edition Copyright FUJIFILM Corporation, Japan 897N0547D ( )
2 Revision History History Date Revision Description 1st Edition April, nd Edition October, rd Edition January, th Edition May, th Edition April, th Edition July, th Edition June, th Edition October, 2006 The corporate name was changed. 9th Edition March, th Edition June,
3 CONTENTS 1. Introduction Implementation Mode Data Flow Diagram Functional Definitions of Application Entities Sequencing of Real World Activities AE Specification CR Console AE Specification Association Establishment Policies General Number of Associations Asynchronous Nature Implementation ID information Association Initiation Policy Send Image(s) to Remote AE Associated Real-World Activity Proposed Presentation Context SOP Specific Conformance Print Image(s) Associated Real-World Activity Proposed Presentation Context SOP Specific Conformance Query/Retrieve Image(s) Associated Real-World Activity Proposed Presentation Context SOP Specific Conformance Get Worklist Associated Real-World Activity Proposed Presentation Context SOP Specific Conformance Inform Procedure State Associated Real-World Activity Proposed Presentation Context SOP Specific Conformance Storage Commitment Associated Real-World Activity Proposed Presentation Context
4 SOP Specific Conformance Verification Associated Real-World Activity Proposed Presentation Context SOP Specific Conformance Association Acceptance Policy Verification Request from Remote AE Associated Real-World Activity Presentation Context SOP Specific Conformance Communication Profiles Supported Communication Stacks TCP/IP Stack Physical Media Support Standard Extended / Specialized / Privatization Configuration Support of Extended Character Sets CR IOD Overview CR Image IOD Module Table Information Module Definitions Patient IE Module Patient Module Study IE Module General Study Module Patient Study Module Series IE Module General Series Module Equipment IE Module General Equipment Module Common Image IE Module General Image Module Image Type Image Pixel Module Contrast/Bolus Module Computed Radiography Image CR Series Module CR Image Module Modality LUT Module
5 Study Classification Module VOI LUT Module General Module SOP Common Module Private Module Private Control Information Module Private Exposure Information Module Private Print Information Module Private Image Information Module Private Creator List Digital Mammography X-ray Image Information Overview Digital Mammography X-ray Image Information IOD Module Table Information Module Definitions Series IE Module General Series Module Equipment IE Module General Equipment Module Common Image IE Module X-ray Module X-ray Acquisition Dose Module X-ray Generation Module X-ray Grid Module DX Module DX Series Module DX Image Module Image Type DX Detector Module DX Positioning Module Mammography Module Mammography Series Module Mammography Image Module View Code Sequence General Module Acquisition Context SOP Common Module Modality Worklist Query/Retrieve Attribute Overview Matching Key Attributes Return Key Attributes
6 11. Modality Performed Procedure Step IOD Attribute Overview Modality Worklist for Patient Information (MWM PI) Query/Retrieve Attribute Overview Matching Key Attributes Return Key Attributes Storage Commitment Attribute Overview DIMSE-Service and Attributes in the Basic Grayscale Print Management DIMSE-Service Basic Film Session SOP Class Basic Film Box SOP Class Basic Grayscale Image Box SOP Class Printer SOP Class
7 1. Introduction This document provides the DICOM conformance statement for the CR Console. 2. Implementation Mode Connected to the Fuji Computed Radiography, the CR Console constitutes an X-ray imaging generating modality and contains the following service classes. Modality Worklist Management Modality Performed Procedure Step CR Storage and Storage Commitment Basic Grayscale Print Management Digital Mammography Image Storage Query/Retrieve 5
8 2.1 Data Flow Diagram Dicom Standard Interface Get Worklist Modality Worklist DICOM Modality Worklist SCP Create New Performed Procedure Step Performed Procedure Step DICOM Performed Procedure Step SCP Image Import C-STORE DICOM Image Storage SCU CR Console Query/ Retrieve C-FIND / C-MOVE DICOM Query/ Retrieve SCP Image Export C-STORE Storage Commitment DICOM Image Storage SCP Image Print Print Request DICOM Basic Grayscale Print Management SCP Local Remote 6
9 2.2 Functional Definitions of Application Entities The CR Console has a DICOM interface with the HIS/RIS, archives, and printers. The CR Console retrieves the actual worklist from the HIS/RIS. The CR Console informs the HIS/RIS that a particular procedure step has started/completed. The CR Console transmits acquired image data to the Archive. The CR Console prints acquired image data with the Printer. The CR Console retrieves the requested images from the Image Server. 2.3 Sequencing of Real World Activities Not applicable to Real World Activities. 7
10 3. AE Specification 3.1 CR Console AE Specification The CR Console Application Entity provides Standard Conformance to the following DICOM SOP classes. SOP Class Name SOP Class UID Role Verification SOP Class SCU / SCP Modality Worklist Information Model FIND SOP Class SCU Modality Performed Procedure Step SOP Class SCU Computed Radiography Image Storage SOP Class SCU / SCP Storage Commitment Push Model SOP Class SCU Basic Grayscale Print Management Meta SOP Class SCU Digital Mammography Image Storage-for Presentation SCU / SCP Digital Mammography Image Storage-for Processing SCU / SCP Study Root Query/Retrieve Information Model FIND SOP Class SCU Study Root Query/Retrieve Information Model MOVE SOP Class SCU Association Establishment Policies General N-CREATE and N-SET of Modality Performed Procedure Step will be issued through different associations established as time elapses. N-EVENT-REPORT of Storage Commitment can be received either through the association that has issued N-ACTION or any other associations. The CR Console accepts a request for establishing an association so that it is available for the latter case and functions as the SCU in SCP/SCU ROLE SELECTION NEGOTIATION. The maximum PDU size is 32K Bytes Number of Associations The CR Console will establish the following associations at a time. Three associations as Computed Radiography Image Storage SOP Class SCU. One association as MWM and MPPS SCU. One association as Basic Grayscale Print Management Meta SOP Class SCU. Four associations as Study Root Query/Retrieve Information Model FIND SOP Class SCU. One association as Study Root Query/Retrieve Information Model MOVE SOP Class SCU. One association as Computed Radiography Image Storage SOP Class SCP Asynchronous Nature Does not support negotiation of multiple outstanding transactions Implementation ID information Implementation Class UID is
11 3.1.2 Association Initiation Policy The CR Console initiates associations as a result of the following local Real-World activities. a) Transmission of acquired images to the remote host. b) Confirmation that images thus sent to the remote host have been stored successfully. c) Printing of acquired images. d) Request for a remote Worklist. d) Notice informing that a particular procedure step has been started or completed. e) Querying/Retrieving images from the remote host Send Image(s) to Remote AE Associated Real-World Activity The CR Console will acquire images and send those images automatically to the pre-set remote host or select images from the list of images thus stored and send them to the specified destination Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation CR Image Storage See next table. SCU None MG Image Storage For Presentation SCU None MG Image Storage For Processing SCU None Transfer Syntax Name UID Implicit VR Little Endian Explicit VR Little Endian JPEG Lossless Hierarchical First-Order Prediction SOP Specific Conformance This implementation tries to send all images that belong to a single study over a single association. If some of the images could not be sent successfully, this implementation will terminate the association and try to resend all images over another association Print Image(s) Associated Real-World Activity The CR Console acquires images and prints those images automatically with a pre-set printer or selects images from the list of images thus stored and prints them by specifying the destination. 9
12 Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Basic Grayscale Print Management See next table. SCU None Meta SOP Class Printer SOP Class SCU None Transfer Syntax Name UID Implicit VR Little Endian SOP Specific Conformance The CR Console uses the Basic Grayscale Print Management Meta SOP Class for image printing. Absolutely asynchronously with this, the CR Console will use only the Printer SOP Class periodically for monitoring status of the printer Query/Retrieve Image(s) Associated Real-World Activity The CR Console queries images stored in the Image Server and display the Study List. It retrieves images in the studies selected from the Study List Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Study Root Query/Retrieve See next table. SCU None Information Model Find Study Root Query/Retrieve Information Model Move SCU None Transfer Syntax Name UID Implicit VR Little Endian SOP Specific Conformance The CR Console supports following attributes as query keys. Description Tag Type Specific Character Set (0008,0005) NONE Query/Retrieve Level (0008,0052) NONE Retrieve AE Title (0008,0054) NONE 10
13 Study Level Keys Description Tag Type Patient s Name (0010,0010) R Patient ID (0010,0020) R Patient Birth Date (0010,0030) O Patient Sex (0010,0040) O Study Date (0008,0020) R Study Time (0008,0030) R Accession Number (0008,0050) R Study ID (0020,0010) R (Not used) Study Instance UID (0020,000D) U Series Level Keys Description Tag Type Modality (0008,0060) R Series Number (0020,0011) R Series Instance UID (0020,000E) U Image Level Keys Description Tag Type SOP Instance UID (0008,0018) U Instance Number (0020,0013) R Acquisition Device Processing Description (0018,1400) O Get Worklist Associated Real-World Activity The CR Console regularly acquires a worklist stored in the HIS/RIS. The CR Console also acquires it as instructed manually Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Modality Worklist Information Model See next table. SCU None FIND Transfer Syntax Name UID Implicit VR Little Endian SOP Specific Conformance The CR Console can use both Procedure Step and Patient Information or only Patient Information. 11
14 Inform Procedure State Associated Real-World Activity When a procedure is started, the CR Console informs the HIS/RIS of it. Also when it is completed (at time of completion of image acquisition), the CR Console informs of it to the HIS/RIS Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Modality Performed Procedure Step See next table. SCU None SOP Class Transfer Syntax Name UID Implicit VR Little Endian SOP Specific Conformance None Storage Commitment Associated Real-World Activity When all images that belong to a certain study have been completely recorded, the CR Console sends those images to the pre-specified image archive. Once all the images are transferred, the commitment request will be sent to the archive on a separate association Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Storage Commitment Push Model SOP Class See next table. SCU None Transfer Syntax Name UID Implicit VR Little Endian
15 SOP Specific Conformance When all images that belong to a study have been completely acquired, those images will be marked Undeletable. The CR Console will then send them to the pre-specified image archive. Once all of the images are transferred, the commitment request will be sent to the archive on a separate association. The CR Console waits for the response from the archive on the same association for a configurable amount of time. If it does not receive the response during this time, it will close the association. The CR Console can, however, accept a response from the archive at any time on another association. Once the N-EVENT-REPORT response is received, the following actions will be taken depending on the status of response. Complete success: The images in the study will be marked Deletable and deleted automatically as necessary. Other cases: The images in the study will remain marked Undeletable. The images will be deleted manually and not will be deleted automatically. Image retransmission for a storage commitment that was unsuccessful and reissuance of the storage commitment will both be instructed manually Verification Associated Real-World Activity The C-ECHO message will be issued if the operator selects remote DICOM AE and issues a verification message Proposed Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Verification See next table. SCU None Transfer Syntax Name UID Implicit VR Little Endian SOP Specific Conformance The CR Console provides standard conformance to the DICOM Verification Service Class. 13
16 3.1.3 Association Acceptance Policy A single association will be accepted at any time to receive Storage Commitment responses. A single association will be accepted at any time to verify application level communication by using the C-EHO service Verification Request from Remote AE Associated Real-World Activity The CR Console is indefinitely listening for associations. No operator action is required to respond to a verification message Presentation Context Presentation Context Abstract Syntax Extended Transfer Syntax Role Name UID Negotiation Verification See next table. SCP None Transfer Syntax Name UID Implicit VR Little Endian SOP Specific Conformance The CR Console provides standard conformance to the DICOM Verification Service Class. 14
17 4. Communication Profiles 4.1 Supported Communication Stacks DICOM Upper Layer is supported using TCP/IP. 4.2 TCP/IP Stack The TCP/IP stack is inherited from the Windows 2000/XP Operating System. 4.3 Physical Media Support IEEE (10BASE-T) / IEEE 802.3U (100BASE-TX) 5. Standard Extended / Specialized / Privatization The CR Console uses some private IOD modules. The CR Console optionally allows Pixel Spacing (0028,0030) attribute to be used. If this field is used, the actual size of any objects displayed on the workstation may not be accurate. See 8. Information Object Definitions. 6. Configuration The CR Console can be configured on the DICOM characteristics specified below. Local IP Address Host name AE Title Port number Remote IP Address Host name AE Title Port number 7. Support of Extended Character Sets ISO-IR 100 (Latin Alphabet #1) ISO-IR 101 (Latin Alphabet #2) ISO-IR 13/14 (Japanese Katakana: JIS X 0201) ISO-IR 87 (Japanese Kanji: JIS X 0208) ISO-IR 192 (Unicode: UTF-8) GB
18 8. CR IOD Overview This section describes the CR IOD that the CR Console handles. 8.1 CR Image IOD Module Table Following is a list of the modules used for the CR image storage SOP class. Information Entity Module Usage Method Reference Patient Patient M Study General Study M Patient Study U Series General Series M CR Series M Equipment General Equipment M Image General Image M Image Pixels M Contrast/Bolus C CR Image M Overlay U Not supported. Curve U Not supported. Modality LUT U VOI LUT U Common SOP M DX Positioning U X-ray Acquisition Dose U X-ray Generation U X-ray Grid U Other Study Classification U Private Control Information U Private Exposure Information U Private Print Information U Private Image Information U Information Module Definitions Tags not specifically mentioned in notes are handled in the same way as DICOM definitions Patient IE Module Patient Module Attribute Name Tag Type DICOM Definition Implementation on CR Console Patient s Name (0010,0010) 2 Patient s name Multi-byte base Patient ID (0010,0020) 2 Main hospital ID no. or code for patient Patient s Birth Date (0010,0030) 2 Patient s date of birth Patient s Sex (0010,0040) 2 Patient s sex. Enumerated values: M = Male F = Female O = Other If not set, Length = 0. 16
19 Other Patient IDs (0010,1000) 3 Other identification numbers or codes are used to identify the patient. Ethnic Group (0010,2160) 3 Ethnic group or race of the patient. Patient Comments (0010,4000) 3 User-defined additional information about the patient Study IE Module General Study Module Attribute Name Tag Type DICOM Definition Implementation on CR Console Study Instance UID (0020,000D) 1 Identifier unique to study An HIS/RIS-generated / IDT-generated / IR-generated number is set. When not obtained from HIS/RIS, CR Console will generate this information by one of the following methods. 1. Generate by Accession Number. 2. Generate by Accession Number & Study Date. 3. Generate by Study Date & Patient ID & Modality. 4. Generate by Study Date & Patient ID & Requesting Service. 5. Generate UID by each image. Study Date (0008,0020) 2 Date study began. Date compilation of study information began Study Time (0008,0030) 2 Time study began. Time compilation of study information began Referring Physician s Name (0008,0090) 2 Physician making referral Due to the current lack of means of input, Length = 0 at the modality. Values received from another company s modalities will be stored. Study ID (0020,0010) 2 Study identifier issued by user or equipment Accession Number (0008,0050) 2 HIS/RIS-issued number for identifying order of study. Study Description (0008,1030) 3 Institution-issued description or classification of study (component element) conducted Physician(s) of Record (0008,1048) 3 Physician(s) who are responsible for overall patient care at time of Study. Information is set so that modalities can identify test types. An HIS/RIS-issued study number is set. When not obtained from HIS/RIS, Length = Patient Study Module Attribute Name Tag Type DICOM Definition Implementation on CR Console Additional Patient s History (0010,21B0) 3 Additional information about the Patient's medical history. 17
20 8.2.3 Series IE Module General Series Module Attribute Name Tag Type DICOM Definition Modality (0008,0060) 1 Modality CR Operator s Name (0008,1070) 3 Technologist supporting the Series Implementation on CR Console Series Instance UID (0020,000E) 1 Identifier unique to series Generate UID by each image. Series Number (0020,0011) 2 Series ID number Laterality (0020,0060) 2 Whether right or left of body part is to be examined. Necessary when part to be examined is pair-structured. Enumerated values: R = Right L = Left Series Date (0008,0021) 3 Date series began Series Time (0008,0031) 3 Time series began Series Description (0008,103E) 3 Description provided by series user Protocol Name (0018,1030) 3 User-defined description of the conditions under which the Series was performed. Note: This attribute conveys series specific protocol identification and may or may not be identical to the one presented in the Performed Protocol Code Sequence (0040,0260). Referenced Study Component Sequence >Referenced SOP Class UID >Referenced SOP Instance UID (0008,1111) 3 Uniquely identifies the Study Component SOP Instance or Modality Performed Procedure step SOP Instance to which the series is related.the Sequence shall have one item. (0008,1150) 1C Uniquely identifies the referenced SOP Class. Required if Referenced Study Component Sequence (0008,1111) is sent. (0008,1155) 1C Uniquely identifies the referenced SOP Instance. Required if Referenced Study Component Sequence (0008,1111) is sent. Body Part Examined (0018,0015) 3 Description of test of body part to be examined. Definitions: SKULL, CSPINE, TSPINE, LSPINE, SSPINE, COCCYX, CHEST, CLAVICLE, BREAST, ABDOMEN, PELVIS, HIP, SHOULDER, ELBOW, KNEE, ANKLE, HAND, FOOT, EXTREMITY Performed Procedure Step ID (0040,0253) 3 Identification of that part of a Procedure that has been carried out within this step. Length = 0 Protocol Name Referenced Study Component Sequence >Referenced SOP Class UID >Referenced SOP Instance UID Values shown below are used for CR. Body part definitions not existing in the DICOM definitions will be added. HEAD, NECK, CHEST, BREAST, ABDOMEN, PELVIS, UP_EXM, LOW_EXM, TEST 18
21 Performed Procedure Step Start Date Performed Procedure Step Start Time Performed Procedure Step Description Performed Protocol Code Sequence (0040,0244) 3 Date on which the Performed Procedure Step started. (0040,0245) 3 Time on which the Performed Procedure Step started. (0040,0254) 3 Institution-generated description or classification of the Procedure Step that was performed. (0040,0260) 3 Sequence describing the Protocol performed for this Procedure Step. One or more Items may be included in this Sequence. >Code Value (0008,0100) 1C Required if a sequence item is present. >Coding Scheme Designator >Coding Scheme Version (0008,0102) 1C Required if a sequence item is present. (0008,0103) 1C Required if a sequence item is present. >Code Meaning (0008,0104) 1C Required if a sequence item is present. Request Attributes Sequence >Requested Procedure ID >Scheduled Procedure Step ID >Scheduled Procedure Step Description >Scheduled Protocol Code Sequence (0040,0275) 3 Sequence that contains attributes from the Imaging Service Request. The sequence may have one or more Items. (0040,1001) 1C Identifier which identifies the Requested Procedure in the Imaging Service Request. Required if Sequence item is present. (0040,0009) 1C Identifier which identifies the Scheduled Procedure Step. Required if Sequence item is present. (0040,0007) 3 Institution-generated description or classification of the Scheduled Procedure Step to be performed. (0040,0008) 3 Sequence describing the Scheduled Protocol following a specific coding scheme. This sequence contains one or more items. >Code Value (0008,0100) 1C Required if a sequence item is present. >Coding Scheme Designator >Coding Scheme Version (0008,0102) 1C Required if a sequence item is present. (0008,0103) 1C Required if a sequence item is present. >Code Meaning (0008,0104) 1C Required if a sequence item is present. 19
22 8.2.4 Equipment IE Module General Equipment Module Attribute Name Tag Type DICOM Definition Manufacturer (0008,0070) 2 Name of manufacturer of equipment that generated the digital image. Institution Name (0008,0080) 3 Institution at which equipment that generated the digital image was installed. Station Name (0008,1010) 3 User-defined name for identifying the equipment that generated the digital image. Institutional Department Name Manufacturer s Model Name (0008,1040) 3 Name of department at institution at which equipment that generated the digital image was installed. (0008,1090) 3 Manufacturer s model name for equipment that generated the digital image. Software Version(s) (0018,1020) 3 Manufacturer s name of software version for the equipment that generated the digital image. Implementation on CR Console FUJIFILM Corporation Sets the host name of the equipment that generated the image (Image Reader s host name or the device name). Model name given to the equipment by the manufacturer. Sets exposure model character strings. Sets the software version for the equipment (Image Reader) that generated the image Common Image IE Module General Image Module Attribute Name Tag Type DICOM Definition Implementation on CR Console Image Number (0020,0013) 2 Number that identifies the image The Image number that generated by CR Image Reader. Patient Orientation (0020,0020) 2C Direction patient faced for line or row of image. Necessary for series in which image does not require an image module. Content Date (0008,0023) 2C Date on which image pixel data generation began. Necessary when image is part of a time-related series. Content Time (0008,0033) 2C Time at which image pixel data generation began. Necessary when image is part of a time-related series. Image Type (0008,0008) 3 Image identification characteristic. For details, see DICOM PS3.3 C Acquisition Number (0020,0012) 3 Number that identifies one continuous acquisition of data over a certain period of time that formed the image. Assumes Length = 0. Stores values received from other modalities. Sets the date of start of image generation. Sets the time of start of image generation. See Issues numbers sequentially in units of IPs read at the Image Reader. The same number will be assigned to multiple images generated from one IP
23 Acquisition Date (0008,0022) 3 Date on which acquisition of data that formed the image began. Acquisition Time (0008,0032) 3 Time at which acquisition of data that formed the image began. Derivation Description (0008,2111) 3 Text description of image derivation method. Source Image Sequence >Referenced SOP Class UID >Referenced SOP Instance UID Lossy Image Compression (0008,2112) 3 Sequence that identifies the set of image class/instance of the image used for deriving the image. Encoded as a sequence for the item (0008, 1150), (0008, 1155). (0008,1150) 1C Uniquely identifies the referenced SOP class. Necessary when (0008, 2112) will be transmitted. (0008,1155) 1C Uniquely identifies the referenced SOP class. Necessary when (0008, 2112) will be transmitted. (0028,2110) 3 Clarifies whether image has been subjected to irreversible compression. Enumerated values: 00: Not subjected to irreversible compression 01: Subjected to irreversible compression Image Comments (0020,4000) 3 User-defined comments about the image Pixel Spacing (0028,0030) 3 Physical distance, within the patient, between the centers of each pixel. Expressed as a numerical set: space between adjoining rows (delimiter), space between adjoining columns. Unit: mm. Sets date of start of IP reading. Sets time of start of IP reading. See Note 1 below. Note 1: The CR image is a projected image and Imager Pixel Spacing (0018,1164) must be used for the distance between the centers of each pixel. However, because some workstations perform necessary processing based on Pixel Spacing (0028,0030), such distance on the IP surface is determined for it. According to DICOM definitions, the Pixel Spacing (0028,0030) is to represent the distance between the centers of each pixel in the patient s body. Because the CR image is a projected image, it is not possible to calculate the distance between the centers of each pixel in the patient s body. Therefore, the value determined here is not correct in the light of the DICOM definitions. Note that even if the distance, area or dimensions are calculated based on the value presented here, the resultant values thus calculated do not precisely reflect an object in the patient s body. (Remember that the CR image is a projected image, which disables measurements of the dimensions of the object precisely reflected by actual dimensions in the patient s body.) 21
24 Image Type The Image Type consists of the following elements as per DICOM definitions. Value 1: Pixel data Characteristics Value 2: Patient Examination Characteristics Value 3: Modality Specific Characteristics Value 4 or after: Other Value n ( n represents a numeric value.) On the FCR system, the above values should be interpreted as described below. Note that each of the elements may be omitted (only delimiters exist). When a portion after a certain element is fully omitted, even delimiters do not exist. Because Value 1 and Value 2 have DICOM-defined meanings, they comply with the DICOM definitions. If omitted, they will be considered to be ORIGINAL or PRIMARY. Value 3 determines image data types such as pre-normalized image, normalized image or processed image. If omitted, it will be considered to be NORMALIZED. Value 4 (Other Value 1) represents processing purpose type of an image. When omitted, it will be considered to be RT. Value 5 (Other Value 2) determines a types of change processing performed on an original image. Value 5 will not be determined if no changes have been made. Value 6 (Other Value 3) determines a date of the change made on an image with Value 5 (Other Value 2) above. Value 6 will not be determined if no changes have been made. Value 7 (Other Value 4) determines a type of special processing performed on an image, which will not be determined if no special image processing has been performed. Value 8 (Other Value 5) determines a date of the special image processing performed with Value 7 (Other Value 4) above. Value 8 will not be determined if no special image processing has been performed. Value 9 (Other Value 6) determines the distance (nm) between the centers of each pixel when an IP is read. Each of the values mentioned above will represent the following specific meaning. Value 1: (as per DICOM definitions) ORIGINAL DERIVED An image whose pixel size is based on the original image (pre-normalized image or normalized image). An image derived from pixel size of one or more images according to a specific method. (processed image). Value 2: (as per DICOM definitions) PRIMARY An image generated as a direct result from a patient study. SECONDARY An image generated after the first patient study. 22
25 Value 3: PRE_NORMALIZED NORMALIZED POST_PROCESSED A pre-normalized image. A normalized image. An already processed image. Value 4: RT ES_L ES_H Routine exposure image Low-pressure image for energy subtraction processing. High-pressure image for energy subtraction processing. Value 5: RENORMALIZED MODIFIED_PARAM A re-normalized image. An image on which image processing parameters have been modified. Value 6 and Value 8: Determine in the YYYYMMDDhhmmss format a date when image processing was performed. Value 7: STICHED BONE SOFT_TISSUE Image composition processing that generates one image from multiple images. A bone image based on the energy subtraction processing. Soft tissue image based on the energy subtraction processing. Value 9: The distance (nm) between the centers of each pixel when an IP is read. When RENORMALIZED has been determined for Value 5, what is determined will not be changed even if parameters were modified. 23
26 Image Pixel Module Attribute Name Tag Type DICOM Definition Samples per Pixel (0028,0002) 1 Number of sample surfaces an image has. Photometric Interpretation (0028,0004) 1 Specifies the intended interpretation of image data. MONOCHROME1 Indicates that pixel data has a single monochrome image surface. Minimum sample value is to be displayed in white following VOI gray scale conversion. MONOCHROME2 Indicates that pixel data has a single monochrome surface. Minimum sample value is to be displayed in black following VOI gray scale conversion. The following definitions also exist: PALETTE COLOR, RGB, HSV, ARCB, CMYK Rows (0028,0010) 1 Number of rows in an image Columns (0028,0011) 1 Number of columns in an image Bits Allocated (0028,0100) 1 Number of bits allocated to each pixel sample. Each sample has the same number of bits allocated. Bit Stored (0028,0101) 1 Number of bits to be stored for each pixel sample. Each sample will have the same number of bits stored. High Bit (0028,0102) 1 High bit for each pixel sample. Each sample will have the same number of high bits. Pixel Representation (0028,0103) 1 Data representation for pixel sample. Each sample will have the same pixel representation. Enumerated values: 0000H: Integer with no encoding 0001H: Complement of 2. Pixel Data (7FE0,0010) 1 Stream of pixel samples that compose the image. Fixed at 1. Implementation on CR Console Fixed at MONOCHROME H Contrast/Bolus Module This is necessary when a contrast medium or bolus has been used (and is not necessary when they have not). As there is no means for determining whether they have been used, a tag will be attached but no information set. Attribute Name Tag Type DICOM Definition Implementation on CR Console Contrast/Bolus Agent (0018,0010) 2 Contrast or bolus agent Length = 0 24
27 8.2.6 Computed Radiography Image CR Series Module Attribute Name Tag Type DICOM Definition Body Part Examined (0018,0015) 2 Text description of body part examined. Definitions follow. SKULL, CSPINE, TSPINE, LSPINE, SSPINE, COCCYX, CHEST, CLAVICLE, BREAST, ABDOMEN, PELVIS, HIP, SHOULDER, ELBOW, KNEE, ANKLE, HAND, FOOT, EXTREMITY View Position (0018,5101) 2 Visual field of X-ray related to patient s position. Definitions follow. AP = Anterior/Posterior PA = Posterior/Anterior LL = Left Lateral RL = Right Lateral RLD = Right Lateral Decubitus LLD = Left Lateral Decubit RLD = Right Lateral Oblique LLD = Left Lateral Oblique Implementation on CR Console Values shown below are used for CR. Body part definitions not existing in the DICOM definitions will be added. HEAD, NECK, CHEST, BREAST, ABDOMEN, PELVIS, UP_EXM, LOW_EXM, TEST Length = CR Image Module Attribute Name Tag Type DICOM Definition KVP (0018,0060) 3 Peak KVP output of the X-ray-generator used. Plate ID (0018,1004) 3 ID or serial no. of the sensing plate on which the image was collected. Exposure Time (0018,1150) 3 X-ray exposure time. Unit: msec. X-ray Tube Current (0018,1151) 3 X-ray tube current. Unit: ma. Exposure (0018,1152) 3 The exposure in mas, for example calculated from Exposure Time and X-ray Tube Current. Exposure in µas (0018,1153) 3 The exposure expressed in µas, for example calculated from Exposure Time and X-ray Tube Current. Imager Pixel Spacing (0018,1164) 3 Physical distance measured at the front of the detector housing between the center of each image pixel specified by a numeric pair row spacing value (delimiter) column spacing value in mm. Acquisition Device Processing Description (0018,1400) 3 Processing descriptions particular to image-related equipment. (Ex.: description of internal organ.) Implementation on CR Console Sets the IP bar code no. Format: a********c See Note 1 below. Sets menu name. Exposure menu name. 25
28 Acquisition Device Processing Code (0018,1401) 3 Code indicating processing particular to image-related equipment. (Ex.: CR internal organ filter code.) Sensitivity (0018,6000) 3 Reading sensitivity Sets menu code. Codifies the body part, exposure method and exposure menu. Taken to be FFFF if no value exists. Note 1: Note that the implementation differs depending on the used CR Console software version. Software Ver. All the A versions and V1.0 (B) to V1.2 (B) (HotFix and service pack for those versions are included.) Implementation on CR Console The distance between the centers of each pixel when an IP is read. Variations in the distance between the centers of each pixel, due to the change made in the pixel density after an IP has been read accordingly, will not be reflected. Therefore, it is not possible to calculate the distance between the two points using this data. V2.0 (B) or later When the pixel density was subjected to change after an IP has been read, it was adjusted appropriately so that the influence of such a change is reflected accordingly. It will always be the distance between the centers of each pixel on the IP surface of the pixel data determined to be the PixelData (7FE0,0010) Modality LUT Module Attribute Name Tag Type DICOM Definition Rescale Intercept (0028,1052) 1C Output unit specified within Storage Value (SV) and Rescale Type (0028,1054) is m *SV + b. Necessary when Modality LUT Sequence (0028,3000) does not exist. Rescale Slope (0028,1053) 1C This is the m within the formula given in terms of Rescale Intercept (0028,1052). Necessary when Rescale Intercept exists. Rescale Type (0028,1054) 1C Specifies the output values for Rescale Slope (0028,1053) and Rescale Intercept (0028,1052). Necessary when Rescale Intercept exists. Modality LUT Sequence (0028,3000) 1C Defines a sequence of Modality LUTs. >LUT Descriptor (0028,3002) 1C Specifies the format of the LUT Data in this Sequence. See C for further explanation. Required if the Modality LUT Sequence (0028,3000) is sent. >LUT Explanation (0028,3003) 3 Free form text explanation of the meaning of the LUT. >Modality LUT Type (0028,3004) 1C Specifies the output values of this Modality LUT. See C for further explanation. Required if the Modality LUT Sequence (0028,3000) is sent. 0 1 US n 2 \0\16 Implementation on CR Console n :Bit Depth of image CRTLUT US
29 >LUT Data (0028,3006) 1C LUT Data in this Sequence. Required if the Modality LUT Sequence (0028,3000) is sent Study Classification Module Attribute Name Tag Type DICOM Definition Study Comments (0032,4000) 3 User-defined comments about the study Implementation on CR Console VOI LUT Module Attribute Name Tag Type DICOM Definition VOI LUT Sequence (0028,3010) 3 Defines a sequence of VOI LUTs. >LUT Descriptor (0028,3002) 1C Specifies the format of the LUT Data in this Sequence. See PS3.3 C for further explanation. Required if the VOI LUT Sequence (0028,3010) is sent. >LUT Explanation (0028,3003) 3 Free from text explanation of the meaning of the LUT. >LUT Data (0028,3006) 1C LUT Data in this Sequence. Required if the VOI LUT Sequence (0028,3010) is sent. Implementation on CR Console General Module SOP Common Module Attribute Name Tag Type DICOM Definition SOP Class UID (0008,0016) 1 Uniquely identifies the SOP class. SOP Instance UID (0008,0018) 1 Uniquely identifies the SOP instance. Specific Character Set (0008,0005) 1C Used to expand the basic figure set or when using a substitute character set. Necessary in expansion or when using a substitute character set. Implementation on CR Console Alphanumerics: No tag European languages: ISO_IR 100 \ ISO_IR 101 Japanese (backslash is half-size) Half-size kana only: ISO_IR 13 Half-size kana + kanji: ISO 2022 IR 13 \ ISO 2022 IR 87 Unicode (UTF-8): ISO_IR 192 Chinese Simplified: GB
30 8.2.8 Private Module Private Control Information Module Attribute Name Tag VR Type Implementation on CR Console Image Control Unit (0009,xx04) SH 3 Host name of the image control unit (archiver). Image UID (0009,xx05) OW 3 Fuji s specific number that identifies an image uniquely. Route Image UID (0009,xx06) OW 3 Original image UID that is changed when an image is subjected to correction. Image Display Information Version No. (0009,xx08) UL 3 Image parameter version number. Patient Information Version No. (0009,xx09) UL 3 Patient ID information version number. Film UID (0009,xx0C) OW 3 ID assigned to each film. Exposure Unit Type Code (0009,xx10) CS 3 Code that identifies an exposure unit used. Kanji Hospital Name (0009,xx80) LO 3 Kanji hospital name (0008,0080). Distribution Code (0009,xx90) ST 3 Code used to determine a destination for image distribution. Kanji Department Name (0009,xx92) SH 3 Kanji requesting department. Blackening Process Flag (0009,xxF0) CS 3 A flag that identifies an image subjected to blackening process. 00: Not subjected to blackening process. 01: Subjected to blackening process. Processing Information Flag (0009,xxF1) ST 1C A flag that determines contents of the information on image data, which are described in the ABCD format. A: Whether LUT correction processing is applied or not. (0=Not applied/1=already applied, Other=Not applied) B: Whether GPR processing is applied or not. (0=OFF/1=ON1/2=ON2, Other=OFF) C: Whether FNC processing is applied or not. (0=OFF/1=ON, Other=OFF) D: Whether chest wall paint-out processing is applied or not. (0=OFF/1=ON, Other=OFF) FCR Image ID (0021,xx10) CS 3 ID of an image generated by the Fuji system, consisting of the four characters. For a CR image, the first one character constitutes an ID that identifies each device, and the remaining three characters constitute a serial number for an image generated by a certain device. Numbers from 000 to 999 are used cyclically. Image Processing Modification Flag (50F1,xx20) CS 3 Information that indicates whether image processing parameters have been modified or not. First byte : Left-side image parameter Second byte : Right-side image parameter Data Compression Code (0029,xx50) CS 3 Code that indicates to what extent an image data piece has been compressed previously. 00: Not compressed. 21: 10-bit data 2/5 lossy compression. 8-bit data 1/2 lossless compression. 22: 1/2 lossless compression. 23: 1/12 lossy compression. 25: New 1/2 lossless compression. 31: 1/20 lossy compression. 41: 1/5 lossy compression. Requesting Physician (0032,1032) PN 3 A physician who requested a study
31 Requesting Service (0032,1033) LO 3 A hospital department that issued a request for service Private Exposure Information Module Attribute Name Tag VR Type Implementation on CR Console Relative Light Emission Amount Sk Term of Correction for Each IP Type St (0025,xx10) US 3 EDR normalization conditions. A relative light emission amount when 2.00 is presumed to be an IP light emission amount, with no object for exposures at 80KVP0.5mR. Set a 100 value. The range of values is from 0 to 600. (0025,xx11) US 3 EDR normalization conditions. Timing correction value for each IP quality type. Set a 100 value. The range of values is from 0 to 400. Reading Gain Gp (0025,xx12) US 3 EDR normalization conditions. Dynamic range to be read as image signals out of X rays that have been recorded. Set a 100 value. The range of values is from 100 to Kanji Body Part for Exposure (0019,xx15) LO 3 Kanji body part to be exposed for study. Convert it from the body part menu code and set accordingly. Kanji Menu Name (0019,xx32) LO 3 Kanji exposure menu name. Image Processing Type (0019,xx40) CS 3 Indicates what type of function processing was performed on an image. EDR Mode (0019,xx50) CS 3 Code that indicates EDR mode. Defined values: 0: AUTO MODE 1: SEMI AUTO MODE 2: FIX MODE 3: MANUAL (AUTO) MODE 4: MANUAL (SEMI AUTO) MODE 5: MANUAL (FIX) MODE Numbers from 6 thereafter are reserved for SEMI-XMODE. Radiographer s Code (0019,xx60) SH 3 Code that identifies a radiographer. Split Exposure Format (0019,xx70) IS 3 Split exposure formats. Defined values: 0: Front 1: Right-and-left two divisions 2: Top-and-bottom two divisions 3: Three divisions 4: Four divisions No. of Split Exposure Frames Reading Position Specification Reading Sensitivity Center (0019,xx71) IS 3 The number of frames exposed on one IP. When there is no such element, handle it to be one frame. (0019,xx80) IS 3 Reference position for starting image reading process on an IP when it is read by an Image Reader. 00: Right top reference 01: Center top reference 02: Center reference 03: Bottom center reference (0019,xx81) IS 3 Controls the sensitivity center when reading an IP on an Image Reader. 0: Standard sensitivity 1: Semi-high sensitivity 2: High sensitivity
32 Set No. (0021,xx30) CS 3 A number that identifies function processing in the FCR system. Note, however, that the available range is from 1 to ZZ. Image No. in the Set (0021,xx40) IS 3 Serial numbers from 01 to 99 within the set number (0021, xx30). Pair Processing Information Equipment Type-Specific Information Energy Subtraction Param. Subtraction Registration Result Energy Subtraction Param. 2 Afin Conversion Coefficient (0021,xx50) CS 3 Information available when an operation is performed between images. (0021,xx80) OB 3 Image generator-specific information. (50F1,xx06) CS 3 The four coefficient table types, Ta, Tb, Tc and Td, used for energy subtraction processing. Each parameter is represented by one character from A to T. Characters other than A to T are illegal. Note that each parameter is represented by one byte, four bytes in totality. (50F1,xx07) CS 3 Indicates the result of image positioning by energy subtraction processing. 0: OK 1: NG (50F1,xx08) CS 1C Parameters to be used by new energy subtraction processing. These parameters are indispensable for images generated by new energy subtraction processing and for original images that will be subjected to new energy subtraction processing. For the former, the images imply to have been subjected to processing using these parameters, and for the latter, the images imply that they are to be subjected to processing using these parameters. (50F1,xx09) SL 1C The parameter that controls the afin conversion coefficient information for the purpose of image positioning during new energy subtraction processing. This parameter is stored to be VM=4 and is indispensable for images generated by new energy subtraction processing and for original images that will be subjected to new energy subtraction processing. For the former, the images imply to have been subjected to processing using this parameter, and for the latter, the images imply that they are to be subjected to processing using this parameter. FNC Parameters (50F1,xx0A) SH 1C FNC parameters to be determined as character strings in order of FNL (A to Z), FRB (A to Z), FRT (A to Z) and FRE (0.0 to 1.0). Example: ABC0.7 When FNC is applied (when FNC is ON according to the processing information flag), this tag will be required. When FNC is OFF, this tag will not be output Private Print Information Module Attribute Name Tag VR Type Implementation on CR Console Film Annotation (0019,xx90) SH 3 Film annotation character string 1. Character String 1 Film Annotation (0019,xx91) SH 3 Film annotation character string 2. Character String 2 Image Display Format (2010,0010) ST 3 Image display format type. A format based on which an image is output on film can be stored only when the four-frame format is used
33 Annotation Display Format ID (2010,0030) CS 3 Identifies an annotation display format, which is described as follows. CR +A+B CR : A character string that identifies a modality. A: Date of birth 0: Date of birth 1: Age B: Film annotation character format (for identification of frontal/lateral) 0: Other than frontal/lateral 1: Frontal/lateral Film Orientation (2010,0040) CS 3 Film orientation. Enumerated values are as follows. PORTRAIT and LANDSCAPE Border Density (2010,0100) CS 3 The density between an image and its surroundings on film. Shown below are the densities defined by DICOM. BLACK WHITE Trim (2010,0140) CS 3 Specifies whether to print images with trimming frame or not. Enumerated values: YES or NO. Image Position Specifying Flag (2011,xx11) CS 3 Specifies an image display position within an area assigned for an image. The default value determined for each device prevails when no such information is set up. 00: Default position 01: Right-justified position 02: Left-justified position Image Position (2020,0010) US 3 Image position on film according to a specified image display format. Film Output Format (50F1,xx10) CS 3 Pre-set parameters used for CR image output format and image processing. Either A or B format is available. A 0: For previous models. Single for the 8 10 format. LR for others. 1: Single 2: L/R B 0: Parameters for the left-side image are used. 1: Parameters for ht right-side image are used. Other : Parameters for the left-side image are used Private Image Information Module Attribute Name Tag VR Type Implementation on CR Console Image Scanning Direction Extended Reading Size Value (0029,xx20) CS 3 Information that indicates a position where image data starts and to which direction the image data is scanned, assuming to be a reference a scanning direction where an image is scanned from top right to the left, when an exposed image is seen as a non-reverse image. This four-byte information has the following formats. First byte : 180 image rotation Second byte : Right-and-left image reversal in the main scanning direction Third byte : 90 image rotation Fourth byte : Space (0029,xx30) CS 3 Image reading size
HDI 4000 Ultrasound System
 HDI 4000 Ultrasound System DICOM Conformance Statement Rev 1.0 Philips Medical Systems HDI 4000 DICOM Conformance Rev. 1.0 12/17/2001 Philips Medical Systems Table of Contents 0. Introduction...3 0.1 DICOM
HDI 4000 Ultrasound System DICOM Conformance Statement Rev 1.0 Philips Medical Systems HDI 4000 DICOM Conformance Rev. 1.0 12/17/2001 Philips Medical Systems Table of Contents 0. Introduction...3 0.1 DICOM
Dx MM DICOM 3.0 Conformance Statement
 Dx MM DICOM 3.0 Conformance Statement NAME FONCTION VISA AUTHOR Alain Battistini Senior Engineer CONFORM TO ORIGINAL REVIEW Jean-François Hoh Senior Engineer CONFORM TO ORIGINAL APPROVAL Pascal Gilbert-Jeantet
Dx MM DICOM 3.0 Conformance Statement NAME FONCTION VISA AUTHOR Alain Battistini Senior Engineer CONFORM TO ORIGINAL REVIEW Jean-François Hoh Senior Engineer CONFORM TO ORIGINAL APPROVAL Pascal Gilbert-Jeantet
Version 8 DICOM Conformance Statement. Version 3.04, September 2014
 Version 8 DICOM Conformance Statement Version 3.04, September 2014 1 Conformance Statement Overview The application described in this Conformance Statement VEPRO EMR Manager PACS is a collection of processes
Version 8 DICOM Conformance Statement Version 3.04, September 2014 1 Conformance Statement Overview The application described in this Conformance Statement VEPRO EMR Manager PACS is a collection of processes
Printlink5-ID_IV. DICOM 3.0 Conformance Statement PRINT MANAGEMENT SYSTEM CODE NO. 1118. Manufacturer:
 PRINT MANAGEMENT SYSTEM Printlink5-ID_IV DICOM 3.0 Conformance Statement CODE NO. 1118 Manufacturer: 2970 Ishikawa-machi, Hachioji-shi Tokyo 192-8505 Japan DICOM Conformance Statement Important Notes Konica
PRINT MANAGEMENT SYSTEM Printlink5-ID_IV DICOM 3.0 Conformance Statement CODE NO. 1118 Manufacturer: 2970 Ishikawa-machi, Hachioji-shi Tokyo 192-8505 Japan DICOM Conformance Statement Important Notes Konica
DICOM 3.0 Conformance Statement
 Picture Archiving and Communication System DICOM 3.0 Conformance Statement CODE NO.1245 Manufacturer: 1 Sakura-machi, Hino-shi Tokyo 191-8511, Japan Important Notes Konica Minolta Medical & Graphic, Inc.
Picture Archiving and Communication System DICOM 3.0 Conformance Statement CODE NO.1245 Manufacturer: 1 Sakura-machi, Hino-shi Tokyo 191-8511, Japan Important Notes Konica Minolta Medical & Graphic, Inc.
Hologic Physician s Viewer 7.0 DICOM Conformance Statement
 Hologic Physician s Viewer 7.0 DICOM Conformance Statement MAN-02386 REV 001 Page 1 of 32 1. Conformance Statement Overview This document is the DICOM Conformance Statement for Hologic Physician s Viewer,
Hologic Physician s Viewer 7.0 DICOM Conformance Statement MAN-02386 REV 001 Page 1 of 32 1. Conformance Statement Overview This document is the DICOM Conformance Statement for Hologic Physician s Viewer,
DICOM CONFORMANCE STATEMENT FOR ZIOSTATION 2.0
 DICOM CONFORMANCE STATEMENT FOR ZIOSTATION 2.0 DICOM Conformance Statement, Ziostation 2.0 1 Copyright 2006-2011, Ziosoft, Inc. 0 DICOM CONFORMANCE STATEMENT OVERVIEW 4 0.1 APPLICATION DATA FLOW... 5 0.2
DICOM CONFORMANCE STATEMENT FOR ZIOSTATION 2.0 DICOM Conformance Statement, Ziostation 2.0 1 Copyright 2006-2011, Ziosoft, Inc. 0 DICOM CONFORMANCE STATEMENT OVERVIEW 4 0.1 APPLICATION DATA FLOW... 5 0.2
Technical Publication. DICOM Conformance Statement. DICOM Proxy 2.0. Document Revision 3. October 20, 2010. 2010 Copyright Brainlab AG
 Technical Publication DICOM Conformance Statement Document Revision 3 October 20, 2010 2010 Copyright Brainlab AG 1 Conformance Statement Overview This is a conformance statement for the Brainlab software
Technical Publication DICOM Conformance Statement Document Revision 3 October 20, 2010 2010 Copyright Brainlab AG 1 Conformance Statement Overview This is a conformance statement for the Brainlab software
DICOM Conformance Statement
 DICOM Conformance Statement Page 1 of 14 1 Introduction... 3 2 Quick Summary... 4 3 Storing Studies... 6 3.1 Ideal Store scenario...6 3.2 Non-ideal scenarios...6 3.2.1 Accession number...7 3.2.2 No indication
DICOM Conformance Statement Page 1 of 14 1 Introduction... 3 2 Quick Summary... 4 3 Storing Studies... 6 3.1 Ideal Store scenario...6 3.2 Non-ideal scenarios...6 3.2.1 Accession number...7 3.2.2 No indication
DICOM CONFORMANCE STATEMENT STORAGE SCU, Q/R SCP, PRINT SCU & STORAGE COMMITMENT SCU FOR TOSHIBA SUPERCONDUCTING MRI SYSTEMS (MIIMR0001EAB)
 DICOM CONFORMANCE STATEMENT STORAGE SCU, Q/R SCP, PRINT SCU & STORAGE COMMITMENT SCU FOR TOSHIBA SUPERCONDUCTING MRI SYSTEMS FLEXART-series/VISART-series/ EXCELART-series/SECOND CONSOLE (MIIMR0001EAB)
DICOM CONFORMANCE STATEMENT STORAGE SCU, Q/R SCP, PRINT SCU & STORAGE COMMITMENT SCU FOR TOSHIBA SUPERCONDUCTING MRI SYSTEMS FLEXART-series/VISART-series/ EXCELART-series/SECOND CONSOLE (MIIMR0001EAB)
DigitizingStation. DICOM V3.0 Conformance Statement
 DigitizingStation DICOM V3.0 Conformance Statement Copyright 2004 by image diagnost GmbH Page 1 of 17 Image Diagnost International GmbH Reproduction of this document without prior written approval is prohibited.
DigitizingStation DICOM V3.0 Conformance Statement Copyright 2004 by image diagnost GmbH Page 1 of 17 Image Diagnost International GmbH Reproduction of this document without prior written approval is prohibited.
DICOM Conformance Statement FORUM
 DICOM Conformance Statement FORUM Version 3.1 Carl Zeiss Meditec AG Goeschwitzerstraße 51-52 07745 Jena Germany www.meditec.zeiss.com Document: DICOM Conformance Statement_FORUM_3.1.doc Page 1 of 25 1
DICOM Conformance Statement FORUM Version 3.1 Carl Zeiss Meditec AG Goeschwitzerstraße 51-52 07745 Jena Germany www.meditec.zeiss.com Document: DICOM Conformance Statement_FORUM_3.1.doc Page 1 of 25 1
ONIS 2.0 DICOM CLIENT. DICOM 3 Conformance statement
 ONIS 2.0 DICOM CLIENT DICOM 3 Conformance statement Table of Contents 0 Introduction...4 0.1 Scope...4 0.2 Standards...4 0.3 Intended Audience...4 0.4 Important Remarks...4 0.5 Definitions...4 1 Implementation
ONIS 2.0 DICOM CLIENT DICOM 3 Conformance statement Table of Contents 0 Introduction...4 0.1 Scope...4 0.2 Standards...4 0.3 Intended Audience...4 0.4 Important Remarks...4 0.5 Definitions...4 1 Implementation
DICOM CONFORMANCE STATEMENT FOR ZIOCUBE 1.0
 DICOM CONFORMANCE STATEMENT FOR ZIOCUBE 1.0 DICOM Conformance Statement, ZioCube 1.0 1 Ziosoft, Inc. 0 DICOM CONFORMANCE STATEMENT OVERVIEW 4 0.1 APPLICATION DATA FLOW...5 0.2 FUNCTIONAL DEFINITION OF
DICOM CONFORMANCE STATEMENT FOR ZIOCUBE 1.0 DICOM Conformance Statement, ZioCube 1.0 1 Ziosoft, Inc. 0 DICOM CONFORMANCE STATEMENT OVERVIEW 4 0.1 APPLICATION DATA FLOW...5 0.2 FUNCTIONAL DEFINITION OF
HP Medical Archive Solutions DICOM Conformance Statement. January 2007 (Third Edition) Part Number T4434-96003
 HP Medical Archive Solutions DICOM Conformance Statement January 2007 (Third Edition) Part Number Copyright 2007, 2007 Hewlett-Packard Development Company, L.P. Confidential computer software. Valid license
HP Medical Archive Solutions DICOM Conformance Statement January 2007 (Third Edition) Part Number Copyright 2007, 2007 Hewlett-Packard Development Company, L.P. Confidential computer software. Valid license
Infinity Medical Image Server
 Infinity Medical Image Server DICOM Server Conformance Statement Part Number: 900-420-002 Rev. 02 Document/Software Version: v1.3.0 Date: May 12, 2009 Copyright 2008-2009 Codonics, Inc. All Rights Reserved.
Infinity Medical Image Server DICOM Server Conformance Statement Part Number: 900-420-002 Rev. 02 Document/Software Version: v1.3.0 Date: May 12, 2009 Copyright 2008-2009 Codonics, Inc. All Rights Reserved.
DICOM Conformance Statement
 Philips Medical Systems DICOM Conformance Statement Integris Systems with High Speed DICOM Image Interface MCV 2974 MCV 3621 Cardiac DICOM XA MCV 3761 Vascular DICOM XA MCV 3031 DICOM RIS Interface and
Philips Medical Systems DICOM Conformance Statement Integris Systems with High Speed DICOM Image Interface MCV 2974 MCV 3621 Cardiac DICOM XA MCV 3761 Vascular DICOM XA MCV 3031 DICOM RIS Interface and
GE PACS Conformance Statement for DICOM v3.0
 g GE Medical Systems Technical Publications IIS P/N 4361668 Revision 01 GE PACS Conformance Statement for DICOM v3.0 Copyright 1998 By General Electric Company g GE Medical Systems GE Medical Systems,
g GE Medical Systems Technical Publications IIS P/N 4361668 Revision 01 GE PACS Conformance Statement for DICOM v3.0 Copyright 1998 By General Electric Company g GE Medical Systems GE Medical Systems,
DICOM Conformance Statement
 Inter Medical Medizintechnik GmbH Daimlerstr. 34-36 D-32312 Lübbecke Tel. (0 57 41) 23 59-0 Fax (0 57 41) 23 59-10 E-Mail Contact @Intmed.de www.intmed.de Doc. Rev. 1 GammaXP DICOM Conformance Statement
Inter Medical Medizintechnik GmbH Daimlerstr. 34-36 D-32312 Lübbecke Tel. (0 57 41) 23 59-0 Fax (0 57 41) 23 59-10 E-Mail Contact @Intmed.de www.intmed.de Doc. Rev. 1 GammaXP DICOM Conformance Statement
DICOM Conformance Statement For Diagnostic Review Workstation Software Version 5-x MAN-00546
 DICOM Conformance Statement For Diagnostic Review Workstation Software Version 5-x MAN-00546 SecurView DX DICOM Conformance Statement Part Number MAN-00546 Revision 001 For Software version 5-X Service
DICOM Conformance Statement For Diagnostic Review Workstation Software Version 5-x MAN-00546 SecurView DX DICOM Conformance Statement Part Number MAN-00546 Revision 001 For Software version 5-X Service
DICOM 3.0 Conformance Statement
 Version 2.7 DICOM 3.0 Conformance Statement 1 1. Conformance Statement Overview SOP Classes Query and Retrieve Study Root Query/Retrieve Information Model Find Study Root Query/Retrieve Information Model
Version 2.7 DICOM 3.0 Conformance Statement 1 1. Conformance Statement Overview SOP Classes Query and Retrieve Study Root Query/Retrieve Information Model Find Study Root Query/Retrieve Information Model
g GE Medical Systems Advantage Cluster Storage / Archive System Release 1 v1.1.5.2 Conformance Statement Direction number: 2160628 Revision: 2
 g GE Medical Systems Advantage Cluster Storage / Archive System Release 1 v1.1.5.2 Conformance Direction number: 2160628 Revision: 2 Date: September 20, 1996 Revision History Revision Date Author Reason
g GE Medical Systems Advantage Cluster Storage / Archive System Release 1 v1.1.5.2 Conformance Direction number: 2160628 Revision: 2 Date: September 20, 1996 Revision History Revision Date Author Reason
MiPACS Storage Server Conformance Statement Version 1.02.0.000
 MiPACS Storage Server Conformance Statement Version 1.02.0.000 December 2009 Version 1.00.4.001 Conformance Statement Document Information Issued by: Medicor Imaging 1927 S. Tryon St., Suite 200 Charlotte,
MiPACS Storage Server Conformance Statement Version 1.02.0.000 December 2009 Version 1.00.4.001 Conformance Statement Document Information Issued by: Medicor Imaging 1927 S. Tryon St., Suite 200 Charlotte,
DICOM Conformance Statement. Version: 1.0
 DICOM Conformance Statement Version: 1.0 Table of Contents 1 Preface...4 2 Overview...5 3 Introduction...5 3.1 Revision History...5 3.2 Audience...5 3.3 Remarks...6 3.4 Definitions, Terms and Abbreviations...6
DICOM Conformance Statement Version: 1.0 Table of Contents 1 Preface...4 2 Overview...5 3 Introduction...5 3.1 Revision History...5 3.2 Audience...5 3.3 Remarks...6 3.4 Definitions, Terms and Abbreviations...6
Candelis, Inc. DICOM Conformance Statement. ImageGrid Storage Server
 18821 Bardeen Ave. Irvine, CA 92612 Phone: 800.800.8600 Fax: 949.752.7317 Candelis, Inc. DICOM Conformance Statement ImageGrid Storage Server 0 INTRODUCTION ImageGrid Storage Server is a complete hardware/software,
18821 Bardeen Ave. Irvine, CA 92612 Phone: 800.800.8600 Fax: 949.752.7317 Candelis, Inc. DICOM Conformance Statement ImageGrid Storage Server 0 INTRODUCTION ImageGrid Storage Server is a complete hardware/software,
Technical Publications
 g GE Healthcare Technical Publications Direction DOC0855383 Revision 2 Centricity TM RIS-i MedoraCom 4.2 Plus Copyright 2011 by General Electric Co. Do not duplicate REVISION HISTORY REV DATE REASON FOR
g GE Healthcare Technical Publications Direction DOC0855383 Revision 2 Centricity TM RIS-i MedoraCom 4.2 Plus Copyright 2011 by General Electric Co. Do not duplicate REVISION HISTORY REV DATE REASON FOR
MammoView 1.5. DICOM Conformance Statement 1.0. medigration GmbH All rights reserved
 All rights reserved medigration GmbH, Schuhstr. 30, 91052 Erlangen Tel: +49 (0) 9131/69087-40, Fax: +49 (0) 9131/69087-50 www.medigration.de Copyright. All rights reserved. Table of Contens 1 Introduction...
All rights reserved medigration GmbH, Schuhstr. 30, 91052 Erlangen Tel: +49 (0) 9131/69087-40, Fax: +49 (0) 9131/69087-50 www.medigration.de Copyright. All rights reserved. Table of Contens 1 Introduction...
CARESTREAM PACS Suite (Client, Server, and CD Direct) Version 11.0. DICOM Conformance Statement
 CARESTREAM PACS Suite (Client, Server, and CD Direct) Version 11.0 DICOM Conformance Statement Publication # 8G8913 22 April 2009 Table of Contents Table of Contents...2 1 Introduction...5 1.1 Abbreviations
CARESTREAM PACS Suite (Client, Server, and CD Direct) Version 11.0 DICOM Conformance Statement Publication # 8G8913 22 April 2009 Table of Contents Table of Contents...2 1 Introduction...5 1.1 Abbreviations
Medflow Imaging DICOM Server
 DICOM Server version 7.6.2 Revision 2.0 Copyright 2011 Medflow, Inc. Revision History Date June 14, 2011 August 30, 2011 Rev Description 7.6.1 First version. 7.6.2 Revised to reflect version 7.6.2 changes
DICOM Server version 7.6.2 Revision 2.0 Copyright 2011 Medflow, Inc. Revision History Date June 14, 2011 August 30, 2011 Rev Description 7.6.1 First version. 7.6.2 Revised to reflect version 7.6.2 changes
DICOM Conformance Statement
 Date: 2010-10-7 DICOM Conformance Statement Stratus OCT Instrument and Review Software Version 7.0 Carl Zeiss Meditec, Inc. 5160 Hacienda Drive Dublin, CA 94568 USA www.meditec.zeiss.com Document: DICOM_Conformance_Statement_Stratus_7.0.doc
Date: 2010-10-7 DICOM Conformance Statement Stratus OCT Instrument and Review Software Version 7.0 Carl Zeiss Meditec, Inc. 5160 Hacienda Drive Dublin, CA 94568 USA www.meditec.zeiss.com Document: DICOM_Conformance_Statement_Stratus_7.0.doc
Centricity TM RISi DICOM Conformance Statement
 Technical Publications Direction No 2118780 Release 3.12 and 4.2 - General Electric Company - All rights reserved. Table of Contents 1 Introduction 4 1.1 Overview 4 1.2 OVERALL DICOM CONFORMANCE STATEMENT
Technical Publications Direction No 2118780 Release 3.12 and 4.2 - General Electric Company - All rights reserved. Table of Contents 1 Introduction 4 1.1 Overview 4 1.2 OVERALL DICOM CONFORMANCE STATEMENT
DICOM Conformance Statement
 DICOM Conformance Statement CARESTREAM Vue PACS v11.3.2.4 Lesion Management and Bookmark Applications Part # 6K4188 2012-07-02 PAGE 1 of 142 Table of Contents 1 INTRODUCTION... 4 1.1 ABBREVIATIONS AND
DICOM Conformance Statement CARESTREAM Vue PACS v11.3.2.4 Lesion Management and Bookmark Applications Part # 6K4188 2012-07-02 PAGE 1 of 142 Table of Contents 1 INTRODUCTION... 4 1.1 ABBREVIATIONS AND
DOLPHIN DICOM IMAGING DICOM CONFORMANCE STATEMENT
 DOLPHIN DICOM IMAGING DICOM CONFORMANCE STATEMENT NOTICE The software described in this document has been validated in accordance with the governing DICOM standard at the time of this document's release.
DOLPHIN DICOM IMAGING DICOM CONFORMANCE STATEMENT NOTICE The software described in this document has been validated in accordance with the governing DICOM standard at the time of this document's release.
DICOM Conformance Statement
 This printed copy is not subject to any change control XXXXXXXXXXXXxxXXXXXXXXXXXXXXXXXXX MR 2013-03-14 Siemens AG 2013 All rights reserved Siemens AG Healthcare Sector, Henkestr. 127, D-91050 Erlangen,
This printed copy is not subject to any change control XXXXXXXXXXXXxxXXXXXXXXXXXXXXXXXXX MR 2013-03-14 Siemens AG 2013 All rights reserved Siemens AG Healthcare Sector, Henkestr. 127, D-91050 Erlangen,
DICOM Conformance Statement CBS Images and Worklist Version 2.01
 DICOM Conformance Statement CBS Images and Worklist Version 2.01 Compulink Inc. 1100 Business Center Circle Thousand Oaks, CA 91320 Internal document number: 4226-000-000-001 rev 1 Creation Date: 20131101
DICOM Conformance Statement CBS Images and Worklist Version 2.01 Compulink Inc. 1100 Business Center Circle Thousand Oaks, CA 91320 Internal document number: 4226-000-000-001 rev 1 Creation Date: 20131101
AGFA HEALTHCARE DICOM Conformance Statement
 Page 1 of 79 AGFA HEALTHCARE DICOM Conformance Statement IMPAX 6.5 Solution Document No. 001310 Revision: 1.1 When printed, this is NOT a controlled document. Page 2 of 79 Document Information Service-related
Page 1 of 79 AGFA HEALTHCARE DICOM Conformance Statement IMPAX 6.5 Solution Document No. 001310 Revision: 1.1 When printed, this is NOT a controlled document. Page 2 of 79 Document Information Service-related
DICOM Conformance Statement Merge Eye Care PACS v. 4.0
 DICOM Conformance Statement Merge Eye Care PACS v. 4.0 Merge Healthcare 900 Walnut Ridge Drive Hartland, WI 53029 USA 877.44.MERGE 2012 Merge Healthcare. The information contained herein is confidential
DICOM Conformance Statement Merge Eye Care PACS v. 4.0 Merge Healthcare 900 Walnut Ridge Drive Hartland, WI 53029 USA 877.44.MERGE 2012 Merge Healthcare. The information contained herein is confidential
Philips Medical Systems DICOM Conformance Statement
 Philips Medical Systems DICOM Conformance Statement DSI Pro 1.0 Document Number 4522 164 07221 20 February 2001 Copyright Philips Medical Systems Nederland B.V. 2001 Page ii DICOM Conformance Statement
Philips Medical Systems DICOM Conformance Statement DSI Pro 1.0 Document Number 4522 164 07221 20 February 2001 Copyright Philips Medical Systems Nederland B.V. 2001 Page ii DICOM Conformance Statement
AquariusNET DICOM Conformance Statement. DICOM Conformance Statement. AquariusNET 4.4. Rev. 12.03.2015 B
 DICOM Conformance AquariusNET 4.4 Rev. 12.03.2015 B 1 1 Conformance Overview The AquariusNET server is a 2D/ 3D enterprise thin client-server solution. This central 3D processing server streams real-time
DICOM Conformance AquariusNET 4.4 Rev. 12.03.2015 B 1 1 Conformance Overview The AquariusNET server is a 2D/ 3D enterprise thin client-server solution. This central 3D processing server streams real-time
Table of Contents. Version History
 Table of Contents 1. Introduction... 1 2. Implementation Model... 1 2.1 Application Data Flow Diagram... 1 2.2 Functional Definition of AE s... 2 2.3 Sequencing of Real World Activities... 2 3. AE Specifications...
Table of Contents 1. Introduction... 1 2. Implementation Model... 1 2.1 Application Data Flow Diagram... 1 2.2 Functional Definition of AE s... 2 2.3 Sequencing of Real World Activities... 2 3. AE Specifications...
Technical Publications
 g GE Healthcare Technical Publications Direction DOC1486636 Revision 2 Optima CT Dicom Conformance Statement for DICOM * Refer to section 1 for a list of products in the Optima CT families to which this
g GE Healthcare Technical Publications Direction DOC1486636 Revision 2 Optima CT Dicom Conformance Statement for DICOM * Refer to section 1 for a list of products in the Optima CT families to which this
GENIE Acquisition R3.1 Conformance Statement for DICOM v3.0
 Technical Publications Direction 2358711-100 Revision 0 GENIE Acquisition R3.1 Conformance Statement for DICOM v3.0 This document applies to the following systems: Millennium MPR/MPS, MG and MC Optima
Technical Publications Direction 2358711-100 Revision 0 GENIE Acquisition R3.1 Conformance Statement for DICOM v3.0 This document applies to the following systems: Millennium MPR/MPS, MG and MC Optima
DICOM 3.0 CONFORMANCE STATEMENT
 DICOM 3.0 CONFORMANCE STATEMENT JETStream Workspace Version 1.0 9705-0067, Rev A October, 004 Copyright Statement Philips Medical Systems has taken care to ensure the accuracy of this document. However,
DICOM 3.0 CONFORMANCE STATEMENT JETStream Workspace Version 1.0 9705-0067, Rev A October, 004 Copyright Statement Philips Medical Systems has taken care to ensure the accuracy of this document. However,
DICOM Conformance Statement
 Document Number: 67923 Revision: B Date: 2009-01-15 Author: Sonia Yu DICOM Conformance Statement Cirrus HD-OCT Instrument and Review Software Version 4.0 Carl Zeiss Meditec, Inc. 5160 Hacienda Drive Dublin,
Document Number: 67923 Revision: B Date: 2009-01-15 Author: Sonia Yu DICOM Conformance Statement Cirrus HD-OCT Instrument and Review Software Version 4.0 Carl Zeiss Meditec, Inc. 5160 Hacienda Drive Dublin,
DICOM Conformance Statement. GDxPRO
 Document Number: 2660021133317 Revision: D Date: 01/20/2010 Author: Vidya Kulkarni DICOM Conformance Statement GDxPRO GDxPRO Instrument Software Version 1.1 GDxPRO Review Software Version 1.0 Carl Zeiss
Document Number: 2660021133317 Revision: D Date: 01/20/2010 Author: Vidya Kulkarni DICOM Conformance Statement GDxPRO GDxPRO Instrument Software Version 1.1 GDxPRO Review Software Version 1.0 Carl Zeiss
Carestream DirectView CR System Software Version 5.6. DICOM Conformance Statement. March 2, 2012 Document Number 9J6333 Ver 1.0
 Carestream DirectView CR System Software Version 5.6 DICOM Conformance Statement March 2, 2012 Document Number 9J6333 Ver 1.0 Table of Contents 1 INTRODUCTION...4 1.1 EXECUTIVE OVERVIEW...4 1.2 SCOPE AND
Carestream DirectView CR System Software Version 5.6 DICOM Conformance Statement March 2, 2012 Document Number 9J6333 Ver 1.0 Table of Contents 1 INTRODUCTION...4 1.1 EXECUTIVE OVERVIEW...4 1.2 SCOPE AND
Technical Publications
 g GE Healthcare Technical Publications Direction 5357330-1EN (DOC1498472) Revision 3 Xeleris 3.0 and Xeleris 3.1 DICOM CONFORMANCE STATEMENT Copyright 2014 by General Electric Co. Do not duplicate g GE
g GE Healthcare Technical Publications Direction 5357330-1EN (DOC1498472) Revision 3 Xeleris 3.0 and Xeleris 3.1 DICOM CONFORMANCE STATEMENT Copyright 2014 by General Electric Co. Do not duplicate g GE
DICOM Conformance Statement Print Management Service Class FUJI NETWORK PRINT SERVER FN-PS551
 DICOM Conformance Statement Print Management Service Class FUJI NETWORK PRINT SERVER FN-PS55 October 006 5th Edition Copyright FUJIFILM Corporation, Japan 897N00094A (006-03-40) 006.0 Revision History
DICOM Conformance Statement Print Management Service Class FUJI NETWORK PRINT SERVER FN-PS55 October 006 5th Edition Copyright FUJIFILM Corporation, Japan 897N00094A (006-03-40) 006.0 Revision History
Xeleris 2.0 Conformance Statement for DICOM V3.0
 g GE Healthcare Technical Publications DIRECTION 5168972-100 Revision 2 Xeleris 2.0 Conformance Statement for DICOM V3.0 Document Structure Information: The DICOM Print Services for Xeleris are defined
g GE Healthcare Technical Publications DIRECTION 5168972-100 Revision 2 Xeleris 2.0 Conformance Statement for DICOM V3.0 Document Structure Information: The DICOM Print Services for Xeleris are defined
DICOM Conformance Statement
 Carestream DryView 5700 Laser Imager DICOM Conformance Statement June 15, 2011 Document # 8J5397 PAGE 1 of 40 This publication is protected by Federal Copyright law, with all rights reserved. Note: Publication
Carestream DryView 5700 Laser Imager DICOM Conformance Statement June 15, 2011 Document # 8J5397 PAGE 1 of 40 This publication is protected by Federal Copyright law, with all rights reserved. Note: Publication
DICOM. Conformance Statement. Envisor Software Version C.0
 DICOM Conformance Statement Envisor Software Version C.0 COPYRIGHT KONINKLIJKE PHILIPS ELECTRONICS N.V. 2005. ALL RIGHTS RESERVED. NO PART OF THIS PUBLICATION MAY BE REPRODUCED OR TRANSMITTED IN ANY FORM
DICOM Conformance Statement Envisor Software Version C.0 COPYRIGHT KONINKLIJKE PHILIPS ELECTRONICS N.V. 2005. ALL RIGHTS RESERVED. NO PART OF THIS PUBLICATION MAY BE REPRODUCED OR TRANSMITTED IN ANY FORM
DICOM Conformance Statement
 Date: 2014-12-18 DICOM Conformance Statement CIRRUS TM HD-OCT 5000 and 500 Instrument Version 7.5/8.0 Carl Zeiss Meditec, Inc. 5160 Hacienda Drive Dublin, CA 94568 USA www.meditec.zeiss.com Document: DICOM_Conformance_Statement_CIRRUS_7.5_8.0.Docx
Date: 2014-12-18 DICOM Conformance Statement CIRRUS TM HD-OCT 5000 and 500 Instrument Version 7.5/8.0 Carl Zeiss Meditec, Inc. 5160 Hacienda Drive Dublin, CA 94568 USA www.meditec.zeiss.com Document: DICOM_Conformance_Statement_CIRRUS_7.5_8.0.Docx
AGFA MEDICAL IMAGING DICOM Conformance Statement
 MED/HB/000405 Page 1 of 19 AGFA MEDICAL IMAGING DICOM Conformance Statement ADC PRID - Software Version 1.1.09 SCU Worklist Management Page 2 of 19 MED/HB/000405 18 September, 2001 Agfa Medical Imaging
MED/HB/000405 Page 1 of 19 AGFA MEDICAL IMAGING DICOM Conformance Statement ADC PRID - Software Version 1.1.09 SCU Worklist Management Page 2 of 19 MED/HB/000405 18 September, 2001 Agfa Medical Imaging
DICOM Conformance Statement. CT Tomoscan M/EG/EG Compact Release 4.0. Document Number 4522 220 59691 30 March 2000
 Philips Medical Systems DICOM Conformance Statement CT Tomoscan M/EG/EG Compact Release 4.0 Document Number 4522 220 59691 30 March 2000 Copyright Philips Medical Systems Nederland B.V. 2000 Philips Medical
Philips Medical Systems DICOM Conformance Statement CT Tomoscan M/EG/EG Compact Release 4.0 Document Number 4522 220 59691 30 March 2000 Copyright Philips Medical Systems Nederland B.V. 2000 Philips Medical
Technical Publications
 GE Healthcare Technical Publications Direction 5720690-1EN Revision 1 AW Server 3.2 CONFORMANCE STATEMENT for DICOM do not duplicate Copyright 2015 By General Electric Co. THIS PAGE LEFT INTENTIONALLY
GE Healthcare Technical Publications Direction 5720690-1EN Revision 1 AW Server 3.2 CONFORMANCE STATEMENT for DICOM do not duplicate Copyright 2015 By General Electric Co. THIS PAGE LEFT INTENTIONALLY
DV5950 DICOM Conformance Statement. Product: DryView 5950 Laser Imaging System
 DV5950 DICOM Conformance Statement Product: DryView 5950 Laser Imaging System Document # 9J5331 Author: Shobha Murthy This publication is protected by Federal Copyright law, with all rights reserved. Note:
DV5950 DICOM Conformance Statement Product: DryView 5950 Laser Imaging System Document # 9J5331 Author: Shobha Murthy This publication is protected by Federal Copyright law, with all rights reserved. Note:
ClearCanvas ImageServer DICOM Conformance Statement
 ClearCanvas ImageServer DICOM Conformance Statement Document Version: 2.2 Product Name(s): ClearCanvas ImageServer Release: 11.0 Date: January 12, 2014 ClearCanvas Inc. 1920 439 University Ave. Toronto,
ClearCanvas ImageServer DICOM Conformance Statement Document Version: 2.2 Product Name(s): ClearCanvas ImageServer Release: 11.0 Date: January 12, 2014 ClearCanvas Inc. 1920 439 University Ave. Toronto,
PARCA Certified PACS Interface Analyst (CPIA) Requirements
 PARCA Certified PACS Interface Analyst (CPIA) Requirements Copyright notice: Copyright 2005 PACS Administrators in Radiology Certification Association (PARCA). All rights reserved. All rights reserved.
PARCA Certified PACS Interface Analyst (CPIA) Requirements Copyright notice: Copyright 2005 PACS Administrators in Radiology Certification Association (PARCA). All rights reserved. All rights reserved.
Technical Publications
 g GE Healthcare Technical Publications Direction 1007228 Revision 0 Navigation Plus System Version 5.2 For DICOM Copyright 2004 by General Electric Co. Do not duplicate THIS PAGE LEFT INTENTIONALLY BLANK
g GE Healthcare Technical Publications Direction 1007228 Revision 0 Navigation Plus System Version 5.2 For DICOM Copyright 2004 by General Electric Co. Do not duplicate THIS PAGE LEFT INTENTIONALLY BLANK
How To Write A Dicom Dicoma Dicomm Test Article
 Technical Publications Direction 2183325 100 Revision 0, March 27, 1997 GENIE Processing & Review System, R1.7 DICOM 3.0 Conformance Statement Document Structure Information: The DICOM Print Services for
Technical Publications Direction 2183325 100 Revision 0, March 27, 1997 GENIE Processing & Review System, R1.7 DICOM 3.0 Conformance Statement Document Structure Information: The DICOM Print Services for
Varian System Server. DICOM Conformance Statement
 Varian System Server DICOM Conformance Statement P/N VA8005D3CS July 2007 General Information Abstract This document provides information about the DICOM Conformance of the Varian System Server product
Varian System Server DICOM Conformance Statement P/N VA8005D3CS July 2007 General Information Abstract This document provides information about the DICOM Conformance of the Varian System Server product
Kodak PACS Link Medical Image Manager 200 Kodak PACS Link 25 Print Server as Print Server (SCP) Software Version 6.1.1
 Kodak PACS Link Medical Image Manager 200 Kodak PACS Link 25 Print Server as Print Server (SCP) Software Version 6.1.1 DICOM Conformance Statement June 30, 2005 Document # 3E5252, Rev. J Revision History
Kodak PACS Link Medical Image Manager 200 Kodak PACS Link 25 Print Server as Print Server (SCP) Software Version 6.1.1 DICOM Conformance Statement June 30, 2005 Document # 3E5252, Rev. J Revision History
CT RADIATION DOSE REPORT FROM DICOM. Frank Dong, PhD, DABR Diagnostic Physicist Imaging Institute Cleveland Clinic Foundation Cleveland, OH
 CT RADIATION DOSE REPORT FROM DICOM Frank Dong, PhD, DABR Diagnostic Physicist Imaging Institute Cleveland Clinic Foundation Cleveland, OH CT Patient comes out... Patient goes in... Big Black Box Radiology
CT RADIATION DOSE REPORT FROM DICOM Frank Dong, PhD, DABR Diagnostic Physicist Imaging Institute Cleveland Clinic Foundation Cleveland, OH CT Patient comes out... Patient goes in... Big Black Box Radiology
Digital Imaging and Communications in Medicine (DICOM) Part 4: Service Class Specifications
 Digital Imaging and Communications in Medicine (DICOM) Part 4: Service Class Specifications Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Digital Imaging and Communications in Medicine (DICOM) Part 4: Service Class Specifications Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
DICOM Conformance Statement
 Document Number: CG-S2F-MS-039 Revision: 1.0 Date: 2011-05-31 DICOM Conformance Statement FORUM LINK net Version 1.0 Carl Zeiss Meditec AG Göschwitzer Strasse 51-52 07745 Jena Germany www.meditec.zeiss.com
Document Number: CG-S2F-MS-039 Revision: 1.0 Date: 2011-05-31 DICOM Conformance Statement FORUM LINK net Version 1.0 Carl Zeiss Meditec AG Göschwitzer Strasse 51-52 07745 Jena Germany www.meditec.zeiss.com
Technical Publications
 Technical Publications Direction DOC0708777 Revision 3 Centricity Enterprise Archive V4.0 DICOM CONFORMANCE STATEMENT Copyright 2011 by General Electric Company 1 CONFORMANCE STATEMENT OVERVIEW The Enterprise
Technical Publications Direction DOC0708777 Revision 3 Centricity Enterprise Archive V4.0 DICOM CONFORMANCE STATEMENT Copyright 2011 by General Electric Company 1 CONFORMANCE STATEMENT OVERVIEW The Enterprise
DICOM Conformance Statement. DICOMscope 3.5. Software developed by: M. Eichelberg 1, K. Kleber 2, J. Riesmeier 1, A. Schröter 2, A.
 DICOM Conformance Statement DICOMscope 3.5 Software developed by: M. Eichelberg 1, K. Kleber 2, J. Riesmeier 1, A. Schröter 2, A. Thiel 1 Organizational support: J. Holstein 2, H. Oosterwijk 3 1) Kuratorium
DICOM Conformance Statement DICOMscope 3.5 Software developed by: M. Eichelberg 1, K. Kleber 2, J. Riesmeier 1, A. Schröter 2, A. Thiel 1 Organizational support: J. Holstein 2, H. Oosterwijk 3 1) Kuratorium
Digital Imaging and Communications in Medicine (DICOM) Supplement 119: Frame Level Retrieve SOP Classes
 Page i 5 Digital Imaging and Communications in Medicine (DICOM) Supplement 119: Frame Level Retrieve SOP Classes 10 15 20 Prepared by: Working Group 6 DICOM Standards Committee, Working Group 6 1300 N.
Page i 5 Digital Imaging and Communications in Medicine (DICOM) Supplement 119: Frame Level Retrieve SOP Classes 10 15 20 Prepared by: Working Group 6 DICOM Standards Committee, Working Group 6 1300 N.
DICOM Correction Proposal
 DICOM Correction Proposal STATUS Assigned Date of Last Update 20165/01/08 Person Assigned Submitter Name Harry Solomon Harry Solomon Submission Date 2015/09/21 Correction Number CP-1550 Log Summary: Refactor
DICOM Correction Proposal STATUS Assigned Date of Last Update 20165/01/08 Person Assigned Submitter Name Harry Solomon Harry Solomon Submission Date 2015/09/21 Correction Number CP-1550 Log Summary: Refactor
Understanding Digital Modalities: System Integration and Use
 Understanding Digital Modalities: System Integration and Use Donald J. Peck, PhD Henry Ford Health System Detroit, MI donaldp@rad.hfh.edu Dicom Digital Imaging and Communications in Medicine Part 3: Information
Understanding Digital Modalities: System Integration and Use Donald J. Peck, PhD Henry Ford Health System Detroit, MI donaldp@rad.hfh.edu Dicom Digital Imaging and Communications in Medicine Part 3: Information
DICOM Conformance Statement. Veradius Unity
 DICOM Conformance Statement Veradius Unity Koninklijke Philips Electronics N.V. 2014 All rights are reserved. Document Number: ICAP-PF.0008674 Issued by: Philips Healthcare Philips Nederlands Best P.O.
DICOM Conformance Statement Veradius Unity Koninklijke Philips Electronics N.V. 2014 All rights are reserved. Document Number: ICAP-PF.0008674 Issued by: Philips Healthcare Philips Nederlands Best P.O.
DICOM Conformance Statement
 DICO Conformance Statement IDS5 11.4; IDS7, Sectra Healthcare Server, WISE, ImageServer/s, ImageServer/xd Version 12.2.3, June 2010 About this document Sectra Imtec AB, Sweden, 2010 Disclaimer Sectra Imtec
DICO Conformance Statement IDS5 11.4; IDS7, Sectra Healthcare Server, WISE, ImageServer/s, ImageServer/xd Version 12.2.3, June 2010 About this document Sectra Imtec AB, Sweden, 2010 Disclaimer Sectra Imtec
Centricity Enterprise Web 3.0 DICOM Conformance Memo DOC0094970
 DOC0094970 CONTENTS 1 Introduction... 3 1.1 Scope and Purpose... 3 1.2 Intended Audience... 3 1.3 Scope and Field of Application... 3 1.4 References... 4 1.5 Definitions... 4 1.6 Symbols and Abbreviations...
DOC0094970 CONTENTS 1 Introduction... 3 1.1 Scope and Purpose... 3 1.2 Intended Audience... 3 1.3 Scope and Field of Application... 3 1.4 References... 4 1.5 Definitions... 4 1.6 Symbols and Abbreviations...
Extracting, Storing And Viewing The Data From Dicom Files
 Extracting, Storing And Viewing The Data From Dicom Files L. Stanescu, D.D Burdescu, A. Ion, A. Caldare, E. Georgescu University of Kraiova, Romania Faculty of Control Computers and Electronics www.software.ucv.ro/en.
Extracting, Storing And Viewing The Data From Dicom Files L. Stanescu, D.D Burdescu, A. Ion, A. Caldare, E. Georgescu University of Kraiova, Romania Faculty of Control Computers and Electronics www.software.ucv.ro/en.
DICOM Conformance Statement
 DICOM Conformance Statement IntelliSpace Breast R3.1 Multi-modality features powered by IntelliSpace Clinical Applications R9.1V7 Koninklijke Philips Electronics N.V. 2013 All rights are reserved. Issued
DICOM Conformance Statement IntelliSpace Breast R3.1 Multi-modality features powered by IntelliSpace Clinical Applications R9.1V7 Koninklijke Philips Electronics N.V. 2013 All rights are reserved. Issued
DICOM CONFORMANCE STATEMENT
 Technical Publications Revision 3 Reporting Tool 2.7 DICOM CONFORMANCE STATEMENT Copyright 2005-2010 by General Electric Co. Do not duplicate Page 1 of 34 REVISION HISTORY Revision Date Reason for Change
Technical Publications Revision 3 Reporting Tool 2.7 DICOM CONFORMANCE STATEMENT Copyright 2005-2010 by General Electric Co. Do not duplicate Page 1 of 34 REVISION HISTORY Revision Date Reason for Change
DICOM Conformance Statement
 Revision: 1.0 Date: Sep, 15th 2015 DICOM Conformance Statement VISUCAM 224 and VISUCAM 524 Version 6.0 Carl Zeiss Meditec AG Goeschwitzer Strasse 51-52 07745 Jena Germany www.zeiss.com/med Document: DICOM_Conformance_Statement_VISUCAM_224_524_6.0.doc
Revision: 1.0 Date: Sep, 15th 2015 DICOM Conformance Statement VISUCAM 224 and VISUCAM 524 Version 6.0 Carl Zeiss Meditec AG Goeschwitzer Strasse 51-52 07745 Jena Germany www.zeiss.com/med Document: DICOM_Conformance_Statement_VISUCAM_224_524_6.0.doc
DICOM: Definitions and Testing
 DICOM: Definitions and Testing Bruce Curran University of Michigan (AAPM Representative, DICOM WG-7 Walter R. Bosch Advanced Technology Consortium Washington University of St. Louis Acknowledgements Sam
DICOM: Definitions and Testing Bruce Curran University of Michigan (AAPM Representative, DICOM WG-7 Walter R. Bosch Advanced Technology Consortium Washington University of St. Louis Acknowledgements Sam
A PACS-Aware DICOM Image Object
 Designing and Implementing A PACS-Aware DICOM Image Object For Digital X-ray, Mammography and Intraoral Applications David A. Clunie Quintiles Intelligent Imaging Clear Vision for the Healthcare Industry
Designing and Implementing A PACS-Aware DICOM Image Object For Digital X-ray, Mammography and Intraoral Applications David A. Clunie Quintiles Intelligent Imaging Clear Vision for the Healthcare Industry
DICOM CONFORMANCE STATEMENT
 Technical Publications Revision 2 Reporting Tool DICOM CONFORMANCE STATEMENT Copyright 2005-2009 by General Electric Co. Do not duplicate Page 1 of 31 REVISION HISTORY Revision Date Reason for Change A
Technical Publications Revision 2 Reporting Tool DICOM CONFORMANCE STATEMENT Copyright 2005-2009 by General Electric Co. Do not duplicate Page 1 of 31 REVISION HISTORY Revision Date Reason for Change A
Cenova Image Analytics Server DICOM Conformance Statement
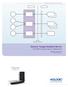 breast imaging solutions DICOM Standard Interface Cenova Image Analytics Server DICOM Conformance Statement Software Version 2.3 MAN-03715 rev 001 Cenova Image Analytics Server 2.3 DICOM Conformance Statement
breast imaging solutions DICOM Standard Interface Cenova Image Analytics Server DICOM Conformance Statement Software Version 2.3 MAN-03715 rev 001 Cenova Image Analytics Server 2.3 DICOM Conformance Statement
DICOM Correction Item
 Correction Number DICOM Correction Item CP-626 Log Summary: Type of Modification Clarification Rationale for Correction Name of Standard PS 3.3 2004 + Sup 83 The description of pixel spacing related attributes
Correction Number DICOM Correction Item CP-626 Log Summary: Type of Modification Clarification Rationale for Correction Name of Standard PS 3.3 2004 + Sup 83 The description of pixel spacing related attributes
DICOM CONFORMANCE STATEMENT
 g GE Healthcare Technical Publications Direction 2067192-001 Revision 4 Universal Viewer* Web Client Version 5.0 SP5 * Trademark of General Electric Company Copyright 2014 by General Electric Co. Do not
g GE Healthcare Technical Publications Direction 2067192-001 Revision 4 Universal Viewer* Web Client Version 5.0 SP5 * Trademark of General Electric Company Copyright 2014 by General Electric Co. Do not
Workstation, Server Hosted
 Workstation, Server Hosted With so many PACS out there, Why just make another? So, we didn't. Distribute, Burn, Print, Import, Archive The Internet DICOM Eye Web Web Viewer PACStoGo DICOM Distribution
Workstation, Server Hosted With so many PACS out there, Why just make another? So, we didn't. Distribute, Burn, Print, Import, Archive The Internet DICOM Eye Web Web Viewer PACStoGo DICOM Distribution
Philips Medical Systems DICOM Conformance Statement
 Philips Medical Systems DICOM Conformance Statement Gyroscan Intera R 7.5.1, R 8.1 and R 9.1 Document Number 4522 131 88661 24 May 2002 Copyright Philips Medical Systems Nederland B.V. 2002 DICOM Conformance
Philips Medical Systems DICOM Conformance Statement Gyroscan Intera R 7.5.1, R 8.1 and R 9.1 Document Number 4522 131 88661 24 May 2002 Copyright Philips Medical Systems Nederland B.V. 2002 DICOM Conformance
ConnectPro for CT/i 4.0 Systems Conformance Statement for DICOM v3.0
 Technical Publications Direction 2210573-100 Revision 0 ConnectPro for CT/i 4.0 Systems Conformance Statement for DICOM v3.0 Copyright 1998 by General Electric Company GE Medical Systems: Telex 3797371
Technical Publications Direction 2210573-100 Revision 0 ConnectPro for CT/i 4.0 Systems Conformance Statement for DICOM v3.0 Copyright 1998 by General Electric Company GE Medical Systems: Telex 3797371
SwissVision TR4000. DICOM Conformance Statement File Media Storage Services. Program Version 9.3 or later Document Revision 1.2.
 SwissVision TR4000 DICOM Conformance Statement File Media Storage Services Program Version 9.3 or later Document Revision 1.2 Date: 22-Mar-06 SHD_15_121_415_12_E Page I Document Revision History Version
SwissVision TR4000 DICOM Conformance Statement File Media Storage Services Program Version 9.3 or later Document Revision 1.2 Date: 22-Mar-06 SHD_15_121_415_12_E Page I Document Revision History Version
What s New in DICOM. DICOM Workshop @ SPIE MEDICAL IMAGING February 2010. Bas Revet, Philips Healthcare (Chair WG 6)
 What s New in DICOM DICOM Workshop @ SPIE MEDICAL IMAGING February 2010 Bas Revet, Philips Healthcare (Chair WG 6) DICOM is the International Standard for Medical Imaging and related information: images,
What s New in DICOM DICOM Workshop @ SPIE MEDICAL IMAGING February 2010 Bas Revet, Philips Healthcare (Chair WG 6) DICOM is the International Standard for Medical Imaging and related information: images,
COST EFFECTIVE FLAT PANEL DIGITAL RADIOGRAPHY UPGRADE SOLUTIONS
 COST EFFECTIVE FLAT PANEL DIGITAL RADIOGRAPHY UPGRADE SOLUTIONS DRive is a digital imaging DR hardware & Software solution designed for General Radiography of anatomy. It is intended to replace film/screen
COST EFFECTIVE FLAT PANEL DIGITAL RADIOGRAPHY UPGRADE SOLUTIONS DRive is a digital imaging DR hardware & Software solution designed for General Radiography of anatomy. It is intended to replace film/screen
SIGNA PROFILE CONFORMANCE STATEMENT
 Technical Publications Direction 2174981 Revision 1.0 SIGNA PROFILE CONFORMANCE STATEMENT for DICOM v3.0 (ID/Net v3.0) sm - Service Manual do not duplicate Copyright 1996 by General Electric Company blank
Technical Publications Direction 2174981 Revision 1.0 SIGNA PROFILE CONFORMANCE STATEMENT for DICOM v3.0 (ID/Net v3.0) sm - Service Manual do not duplicate Copyright 1996 by General Electric Company blank
FUJIFILM MEDICAL SYSTEMS
 FUJIFILM MEDICAL SYSTEMS Product Data Synapse Release Version 4.2 Server Modules and Interfaces Application Synapse server is a collection of software modules built on Microsoft Windows Server, which together
FUJIFILM MEDICAL SYSTEMS Product Data Synapse Release Version 4.2 Server Modules and Interfaces Application Synapse server is a collection of software modules built on Microsoft Windows Server, which together
NEMA Standards Publication PS 3 Supplement 41. Digital Imaging and Communications in Medicine (DICOM) Digital Signatures
 NEMA Standards Publication PS 3 Supplement 1 Digital Imaging and Communications in Medicine (DICOM) Digital Signatures Status: Final Text Sep 001 Prepared by DICOM Standards Committee, Working Group 1
NEMA Standards Publication PS 3 Supplement 1 Digital Imaging and Communications in Medicine (DICOM) Digital Signatures Status: Final Text Sep 001 Prepared by DICOM Standards Committee, Working Group 1
Liberate Your Image Data
 Liberate Your Image Data Does Adherence to the DICOM Standard Guarantee Interoperability? 2009-2014 Mach7 Technologies, Inc. Page 1 of 9 Table of Contents Abstract...3 The DICOM Standard Does Not Guarantee
Liberate Your Image Data Does Adherence to the DICOM Standard Guarantee Interoperability? 2009-2014 Mach7 Technologies, Inc. Page 1 of 9 Table of Contents Abstract...3 The DICOM Standard Does Not Guarantee
Supplement 113 Email Transport
 Digital Imaging and Communications in Medicine (DICOM) Supplement 113 Email Transport Prepared by: DICOM Standards Committee, Working Group 1300 N. 17th Street Suite 175 Rosslyn, Virginia 09 USA VERSION:
Digital Imaging and Communications in Medicine (DICOM) Supplement 113 Email Transport Prepared by: DICOM Standards Committee, Working Group 1300 N. 17th Street Suite 175 Rosslyn, Virginia 09 USA VERSION:
Digital Imaging and Communications in Medicine (DICOM) Part 5: Data Structures and Encoding
 Digital Imaging and Communications in Medicine (DICOM) Part 5: Data Structures and Encoding Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Digital Imaging and Communications in Medicine (DICOM) Part 5: Data Structures and Encoding Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Page: 1 of 6 Page: 1 of 6
 Page: 1 of 6 Page: 1 of 6 CR Basics and FAQ Overview Computed Radiography is a term used to describe a system that electronically records a radiographic image. Computed Radiographic systems use unique
Page: 1 of 6 Page: 1 of 6 CR Basics and FAQ Overview Computed Radiography is a term used to describe a system that electronically records a radiographic image. Computed Radiographic systems use unique
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview
 Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Interfacing Fuji CR in a DICOM Network Environment
 Interfacing Fuji CR in a DICOM Network Environment Wayne T. DeJarnette, Ph.D., Valmore Albrecht, RT, Alan Orth, RT, Jon Sayer, M.S. DeJarnette Research Systems, Inc. 401 Washington Avenue Towson, MD 21204
Interfacing Fuji CR in a DICOM Network Environment Wayne T. DeJarnette, Ph.D., Valmore Albrecht, RT, Alan Orth, RT, Jon Sayer, M.S. DeJarnette Research Systems, Inc. 401 Washington Avenue Towson, MD 21204
IHE Radiology: Mammography User s Handbook
 Integrating the Healthcare Enterprise IHE Radiology: Mammography User s Handbook Rev. 1.0 March 27, 2007 Copyright 2007: ACC/HIMSS/RSNA Contributors Editors: Christoph Dickmann, Nikolaos A. Gkanatsios
Integrating the Healthcare Enterprise IHE Radiology: Mammography User s Handbook Rev. 1.0 March 27, 2007 Copyright 2007: ACC/HIMSS/RSNA Contributors Editors: Christoph Dickmann, Nikolaos A. Gkanatsios
Patient Database and PACS Communication Manual
 Patient Database and PACS Communication Manual January 28, 2016 Software platform v2.0 R4870 MEDVISO AB http://www.medviso.com Griffelvägen 3 SE-224 67 Lund Sweden Tel: +46-76-183 6442 ii Contents 1 Conventions
Patient Database and PACS Communication Manual January 28, 2016 Software platform v2.0 R4870 MEDVISO AB http://www.medviso.com Griffelvägen 3 SE-224 67 Lund Sweden Tel: +46-76-183 6442 ii Contents 1 Conventions
