OECD GUIDELINE FOR THE TESTING OF CHEMICALS
|
|
|
- Scarlett Dean
- 7 years ago
- Views:
Transcription
1 OECD GUIDELINE FOR THE TESTING OF CHEMICALS PROPOSAL FOR UPDATING GUIDELINE 473 In Vitro Mammalian Chromosome Aberration Test INTRODUCTION 1. The purpose of the in vitro chromosome aberration test is to identify agents that cause structural chromosome aberrations in cultured mammalian cells (1)(2)(3). Structural aberrations may be of two types, chromosome or chromatid. With the majority of chemical mutagens, induced aberrations are of the chromatid type, but chromosome-type aberrations also occur. An increase in polyploidy may indicate that a chemical has the potential to induce numerical aberrations. However, this guideline is not designed to measure numerical aberrations and is not routinely used for that purpose. Chromosome aberrations and related events are the cause of many human genetic diseases and there is substantial evidence that chromosome mutations and related events causing alterations in oncogenes and tumour suppressor genes of somatic cells are involved in cancer induction in humans and experimental animals. 2. The in vitro chromosome aberration test may employ cultures of cell lines or primary cell cultures. The cells used are selected on the basis of growth ability in culture, stability of the karyotype, chromosome number, chromosome diversity and spontaneous frequency of chromosome aberrations. At the present time, the available data suggest that it is important to consider the p53 status, genetic (karyotype) stability, DNA repair capacity and origin (rodent versus human) of the cells chosen for testing (Pfuhler et al., 2011). These characteristics may be considered relevant for demonstration of chemical safety in human population. 3. Definitions used are set out in the Annex. INITIAL CONSIDERATIONS 4. Tests conducted in vitro generally require the use of an exogenous source of metabolic activation unless the cells are metabolically competent with respect to the substances being tested. The exogenous metabolic activation system does not entirely mimic in vivo conditions. Care should also be taken to avoid conditions that would lead to artifactual positive results which do not reflect intrinsic mutagenicity, and may arise from such factors as marked changes in ph or osmolality, or by high levels of cytotoxicity (4) (5) (A41). 5. This test is used to detect chromosomal aberrations which may result from clastogenic events. To analyse the induction of chromosomal aberrations, it is essential that mitosis has occurred in both treated and untreated cultures. PRINCIPLE OF THE TEST METHOD 6. Cell cultures are exposed to the test substance both with and without an exogenous source of metabolic activation unless cells with an adequate metabolizing capability are used. At predetermined intervals after the start of exposure of cell cultures to the test substance, they are treated with a metaphase-arresting substance (e.g. Colcemid or colchicine), harvested, stained and metaphase cells are analysed microscopically for the presence of chromosome aberrations.
2 DESCRIPTION OF THE METHOD Preparations Cells 7. A variety of cell lines, strains or primary cell cultures, including human cells, may be used. Human peripheral blood lymphocytes should be obtained from young (approximately years of age), healthy, non-smoking individuals with no known recent exposures to genotoxic chemicals or radiation. [If cells from more than one donor are pooled for use, the number of donors should be specified. The baseline incidence of chromosome aberrations increases with age and this trend is more marked in females than in males (B4)] [Alternative: It should be considered in the selection of donor cells for pooling that the baseline incidence of chromosome aberrations increases with age and that this trend is more marked in females than in males (44) ]. Cell cultures are maintained in an exponential cell growth and no more synchronized during exposure to the test substance Media and culture conditions 8. Appropriate culture medium and incubation conditions (culture vessels, CO 2 concentration, temperature, and humidity) should be used for maintaining cultures. Cell lines should be checked routinely for the stability of the modal chromosome number and the absence of Mycoplasma contamination, and should not be used if contaminated or if the modal chromosome number has changed. The normal cell cycle time for the culture conditions used in the testing laboratory should be established and appropriate to the cell line. Preparation of cultures 9. Cell lines: cells are propagated from stock cultures, seeded in culture medium at a density such that the cultures will not reach confluency in monolayers, and suspension cultures will not reach excessive density before the time of harvest, and incubated at 37 C. 10. Lymphocytes: whole blood treated with an anti-coagulant (e.g. heparin) or separated lymphocytes are cultured at 37 C in the presence of a mitogen e.g. phytohaemagglutinin (PHA). Metabolic activation 11. Exogenous metabolising systems should be used when employing cells which have inadequate endogenous metabolic capacity. The most commonly used system is a co-factorsupplemented post-mitochondrial fraction (S9) prepared from the livers of rodents treated with enzyme-inducing agents such as Aroclor 1254 (6) (7) (8) (9) (A46) or a combination of phenobarbitone and β-naphthoflavone (A46) (10) (11) (12) (A49). The latter combination does not conflict with the Stockholm Convention on Persistent Organic Pollutants (A50) and has been shown to be as effective as Aroclor 1254 for inducing mixed-function oxidases (A46) (10) (11) (A49). The S9 fraction typically is used at concentrations ranging from 1-10% (v/v) in the final test medium. The choice of type and concentration of exogenous metabolic activation system or metabolic inducer employed may be influenced by the class of chemical being tested. For a more detailed discussion on this, please see Section of the Introduction chapter. Test substance/preparation 12. Solid test substances should be dissolved in appropriate solvents or vehicles and diluted, if appropriate, prior to treatment of the cells. Where this is not possible with compatible solvents, suspensions may need to be used. Liquid test substances may be added directly to the test systems and/or diluted prior to treatment. Gaseous or volatile substances should be tested by appropriate
3 methods, such as in sealed culture vessels (15) (16). Fresh preparations of the test substance should be used unless stability data demonstrate the acceptability of storage. Test conditions Solvents/vehicles 13. The solvent/vehicle should be chosen to optimize the solubility of the test agent without adversely impacting the assay conduct, i.e., cell growth, integrity of the test material, reaction with culture vessels, metabolic activation system, etc. (Ref. to add). It is recommended that, wherever possible, the use of an aqueous solvent should be considered first. Well established solvents/vehicles are for example water, cell culture medium, dimethyl sulfoxide). Generally organic solvents should not exceed 1% (v/v) and aqueous solvents should not exceed 10% (v/v) in the final treatment medium. If other than well established solvents are used, their use should be supported by data indicating their compatibility with the test substance and their lack of genetic toxicity. In the absence of that supporting data, it is important to include untreated controls (see Glossary) to demonstrate that no deleterious or mutagenic effects are induced by the chosen solvent. Measuring cell proliferation and cytotoxicity and choosing exposure concentrations 14. When determining the highest test substance concentration to be tested, concentrations that have the capability of producing artifactual positive responses, such as those producing excessive cytotoxicity (see paragraph 20), precipitation (see paragraph 21) in the culture medium, and marked changes in ph or osmolality (see paragraph 4), should be avoided. If the test chemical causes a marked change in the ph of the medium at the time of addition, the ph might be adjusted by buffering the final treatment medium so as to avoid artifactual positive results and to maintain good cell growth. 15. Cytotoxicity should be determined with and without metabolic activation in the main experiment using an appropriate indication of cell integrity and growth. 16. For cell lines, it is necessary to demonstrate that the cells scored in the culture have undergone division during or following treatment with the test substance, or else false negative responses may be produced. Relative Population Doubling (RPD) or Relative Increase in Cell Count (RICC) are appropriate methods for the assessment of cytotoxicity in cytogenetics (A60) (B1) (B2) (see Annex 2 for formulas). In case of treatment and sampling times longer than 1.5 normal cell cycle, RPD might underestimate cytotoxicity (B7). Under these circumstances RICC could be a better measure. Alternatively, the evaluation of cytotoxicity after a 1.5 normal cell cycles would be a helpful estimate. 17. For lymphocytes in primary cultures, while the mitotic index is only an indirect measure of cytotoxic/cytostatic effects and depends on the time after treatment, the mitotic index is acceptable because other toxicity measurements may be cumbersome and impractical. 18. Assessment of other indicators of cytotoxicity (e.g. confluency, cell number, cell integrity, viable cell counts, apoptosis, necrosis, metaphase counting) can also provide useful information for assessing cytotoxicity. It may be useful to determine cytotoxicity and solubility in a preliminary experiment. 19. At least three analysable test concentrations from duplicate cultures should be evaluated. For substances demonstrating little or no toxicity, concentration intervals of approximately 2 to 3 fold will usually be appropriate. However, many substances exhibit steep concentration response curves and in order to obtain data at low and moderate toxicity, it will be necessary to use more closely spaced concentrations. When it is desirable to study the dose response relationship in detail, more than three concentrations will be needed. In these cases a larger number of concentrations (single cultures or duplicates) will be necessary. If single cultures are used then the negative control should be in
4 duplicate. Where cytotoxicity occurs, the test concentrations selected should cover a range from that producing cytotoxicity as described in paragraph 20 and including concentrations at which there is moderate and little or no cytotoxicity. 20. If the maximum concentration is based on cytotoxicity, the highest concentration should aim to achieve a 50% reduction in cell proliferation. Care should be taken not to markedly exceed 50% cytotoxicity because higher levels may induce chromosome damage as a secondary effect of cytotoxicity (60). 21. For poorly soluble compounds that are not cytotoxic at concentrations lower than the lowest insoluble concentration, the highest concentration should produce turbidity or a precipitate visible by eye or with the aid of an inverted microscope at the end of the treatment. Even if cytotoxicity occurs above the lowest insoluble concentration, it is advisable to test at only one concentration producing turbidity or with a visible precipitate because artifactual effects may result from the precipitate. For suspension cultures, care should be taken to assure that the precipitate does not interfere with the conduct of the assay (e.g. staining or scoring). 22. If no cytotoxicity or precipitate is observed, the highest test concentration should correspond to [0.01 M, 5 mg/ml or 5 l/ml, whichever is the lowest]. For mixtures (no one component is more than 50% of the total by weight or volume), the top concentration should be at least 5 mg/ml. In some circumstances, for mixtures, higher concentrations might be advisable. Controls 23. Concurrent negative vehicle controls should be included in each experiment conducted either with or without metabolic activation. 24. Positive controls are needed to demonstrate the ability of the cells to identify clastogens and/or aneugens under the conditions of the test protocol used. A clastogen that requires metabolic activation (see table in Annex 3) should be used to affirm the metabolic capability of the metabolic activation system preparation. Positive controls should be used at concentrations expected to give a reproducible and detectable increase over background which demonstrates the sensitivity of the test system i.e. the effects are clear but do not immediately reveal the identity of the coded slides to the reader. 25. Because in vitro mammalian cell tests for genetic toxicity are sufficiently standardized the use of positive controls may be confined to a chemical requiring metabolic activation (provided it is done concurrently with the non-activated test using the same treatment duration) to demonstrate the activity of the metabolic activation system and the responsiveness of the test system.
5 PROCEDURE Treatment with test substance 26. Proliferating cells are treated with the test substance in the presence and absence of a metabolic activation system. Treatment of lymphocytes should commence at about 48 hours after mitogenic stimulation. 27. Duplicate cultures should normally be used for each test substance concentration and for the negative (vehicle or untreated) control cultures. Where single cultures are used, e.g. for study of the shape of the dose response relationship (see on number of test concentrations), an increased number of concentrations has to be analysed but negative controls should be done in duplicate. Culture harvest time 28. In the first experiment, cells should be exposed to the test substance both with and without metabolic activation for 3-6 hours, and sampled at a time equivalent to about 1.5 normal cell cycle length after the beginning of treatment (12). If this protocol gives negative results both with and without activation, an additional experiment without activation should be done, with continuous treatment until sampling at a time equivalent to about 1.5 normal cell cycle lengths. Certain chemicals may be more readily detected by treatment/sampling times longer than 1.5 normal cell cycle lengths. Chromosome preparation 29. Cell cultures are treated with Colcemid or colchicine usually for one to three hours prior to harvesting. Each cell culture is harvested and processed separately for the preparation of chromosomes. Chromosome preparation involves hypotonic treatment of the cells, fixation and staining. Analysis 30. All slides, including those of positive and negative controls, should be independently coded before microscopic analysis. Since fixation procedures often result in the breakage of a proportion of metaphase cells with loss of chromosomes, the cells scored should therefore contain a number of centromeres equal to the modal number +2 for all cell types. At least 200 well-spread metaphases should be scored per concentration and control equally divided amongst the duplicates, if applicable. This number can be reduced when high numbers of aberrations are observed. 31. Though the purpose of the test is to detect structural chromosome aberrations, it is important to record polyploidy and endoreduplication when these events are seen. Proficiency of the laboratory 32. In order to demonstrate proficiency, the laboratory should perform a series of experiments with reference positive chemicals acting via different mechanisms (Annex 3) and various solvents. These positive and negative control responses should be consistent with the published literature. During the course of these investigations, the laboratory should establish: - A historical positive control range and distribution, - A historical negative (untreated, vehicle) control range and distribution. Re-evaluation of laboratory proficiency is recommended if major changes to the experimental conditions (e.g. use of a new cell type) are proposed for the assay.
6 DATA AND REPORTING Treatment of results 33. The percentage of cells with structural chromosome aberration(s) should be evaluated. Chromatid- and chromosome-type aberrations should be listed separately with their numbers and frequencies for experimental and control cultures. Gaps are recorded and reported separately but not included in the total aberration frequency. 34. Concurrent measures of cytotoxicity for all treated and negative control cultures in the main aberration experiment(s) should be recorded. 35. Individual culture data should be provided. Additionally, all data should be summarised in tabular form. Evaluation and interpretation of results [this section needs to be further discussed by the Expert group] 36. There is no requirement for verification of a clear positive response. Equivocal results should be clarified by further testing preferably using modification of experimental conditions. The need to confirm negative results has been discussed in paragraph 28. Modification of study parameters to extend the range of conditions assessed should be considered in follow-up experiments. Study parameters that might be modified include the concentration spacing and the metabolic activation conditions. 37. Although there are several criteria for a positive result, biological relevance of the results should be considered first. Appropriate statistical methods may be used as an aid in evaluating the test results. However, the results of statistical testing should be assessed with respect to dose-response relationship and a statistically significant increase alone is not sufficient for the determination of a positive result. A result can be considered clearly biologically relevant if the following criteria are all satisfied: (1) the increase is dose-related, (2) at least one of the measure points is statistically significant higher than the concurrent negative control, (3) the positive result is reproducible (e.g. between duplicates or between experiments), (4) the positive result is outside the range of the historical negative control data. The positive and negative controls are within the historical positive range for the test within the laboratory. 38. An increase in the number of polyploid cells may indicate that the test substance has the potential to inhibit mitotic processes and to induce numerical chromosome aberrations. An increase in the number of cells with endoreduplicated chromosomes may indicate that the test substance has the potential to inhibit cell cycle progression (17)(18). 39. A test substance for which the results do not meet the above criteria is considered nonmutagenic in this system. 40. Although most experiments will give clearly positive or negative results, in rare cases the data set will preclude making a definite judgement about the activity of the test substance.. These equivocal or questionable responses may occur regardless of the number of times the experiment is repeated.
7 41. Positive results from the in vitro chromosome aberration test indicate that the test substance induces structural chromosome aberrations in cultured mammalian somatic cells. Negative results indicate that, under the test conditions, the test substance does not induce chromosome aberrations in cultured mammalian somatic cells. Test report 42. The test report must include the following information: Test substance: Solvent/Vehicle: Cells: Test conditions: - identification data and CAS no., if known; - physical nature and purity; - physicochemical properties relevant to the conduct of the study; - stability of the test substance, if known. - justification for choice of solvent/vehicle. - solubility and stability of the test substance in solvent/vehicle, if known. - type and source of cells - karyotype features and suitability of the cell type used; - absence of mycoplasma, if applicable; - for cell lines, information on cell cycle length, doubling time or proliferation index; - sex of blood donors, whole blood or separated lymphocytes, mitogen used; - number of passages, if applicable; - methods for maintenance of cell cultures if applicable; - modal number of chromosomes. - identity of metaphase arresting substance, its concentration and duration of cell exposure; - rationale for selection of concentrations and number of cultures including, e.g. cytotoxicity data and solubility limitations, if available; - composition of media, CO 2 concentration if applicable; - concentration of test substance; - volume of vehicle and test substance added; - incubation temperature; - incubation time; - duration of treatment; - cell density at seeding, if appropriate; - type and composition of metabolic activation system, including acceptability criteria; - positive and negative controls; - methods of slide preparation; - criteria for scoring aberrations; - number of metaphases analyzed; - methods for the measurements of toxicity; - criteria for considering studies as positive, negative or equivocal. Results (individual data): - the number of cells plated (or treated) and the number of cells harvested for each culture
8 - cytotoxicity measurements and indications, e.g. RPD, RICC, mitotic index, degree of confluency, cell cycle data, cell counts,; - information on cell cycle length, doubling time or proliferation index; - signs of precipitation; - data on ph and osmolality of the treatment medium, if determined; - definition for aberrations, including gaps; - number of cells with chromosome aberrations and type of chromosome aberrations given separately for each treated and control culture; - changes in ploidy if seen; - concentration-response relationship, where possible; - statistical analyses, if any; - concurrent negative (solvent/vehicle) and positive control data; - historical negative (solvent/vehicle) and positive control data, with ranges, means and standard deviations. Discussion of the results. Conclusion.
9 LITERATURE (1) Evans, H.J. (1976). Cytological Methods for Detecting Chemical Mutagens. In: Chemical Mutagens, Principles and Methods for their Detection, Vol. 4, Hollaender, A. (ed) Plenum Press, New York and London, pp (2) Ishidate, M. Jr. and Sofuni, T. (1985). The In Vitro Chromosomal Aberration Test Using Chinese Hamster Lung (CHL) Fibroblast Cells in Culture. In: Progress in Mutation Research, Vol. 5, Ashby, J. et al., (Eds) Elsevier Science Publishers, Amsterdam-New York- Oxford, pp (3) Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B., Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., Rimpo, J., Margolin, G. H., Resnick, M. A., Anderson, G. and Zeiger, E. (1987). Chromosome aberration and sister chromatid exchanges in Chinese hamster ovary cells: Evaluation of 108 chemicals. Environ. Molec. Mutagen 10 (suppl. 10), (4) Scott, D., Galloway, S.M., Marshall, R.R., Ishidate, M. Jr, Brusick, D., Ashby, J. and Myhr, B.C., (1991). Genotoxicity under Extreme Culture Conditions. A report from ICPEMC Task Group 9. Mutation Res., 257, (5) Morita, T., Nagaki, T., Fukuda, I. and Okumura, K., (1992). Clastogenicity of Low ph to Various Cultured Mammalian Cells. Mutation Res., 268, (6) Ames, B.N., McCann, J. and Yamasaki, E. (1975). Methods for Detecting Carcinogens and Mutagens with the Salmonella/Mammalian Microsome Mutagenicity Test. Mutation Res., 31, (7) Maron, D.M. and Ames, B.N. (1983). Revised Methods for the Salmonella Mutagenicity Test. Mutation Res., 113, (8) Natarajan, A.T., Tates, A.D, van Buul, P.P.W., Meijers, M. and de Vogel, N. (1976). Cytogenetic Effects of Mutagens/Carcinogens after Activation in a Microsomal System In Vitro, I. Induction of Chromosome Aberrations and Sister Chromatid Exchanges by Diethylnitrosamine (DEN) and Dimethylnitrosamine (DMN) in CHO Cells in the Presence of Rat-Liver Microsomes. Mutat. Res., 37, (9) Matsuoka, A., Hayashi, M. and Ishidate, M., Jr. (1979). Chromosomal Aberration Tests on 29 Chemicals Combined with S9 Mix In vitro. Mutation. Res., 66, (10) Elliot, B.M., Combes, R.D., Elcombe, C.R., Gatehouse, D.G., Gibson, G.G., Mackay, J.M. and Wolf, R.C. (1992). Report of UK Environmental Mutagen Society Working Party. Alternatives to Aroclor 1254-induced S9 in In Vitro Genotoxicity Assays. Mutagenesis, 7, (11) Matsushima, T., Sawamura, M., Hara, K. and Sugimura, T. (1976). A Safe Substitute for Polychlorinated Biphenyls as an Inducer of Metabolic Activation Systems. In: de Serres, F.J., Fouts, J.R., Bend, J.R. and Philpot, R.M. (eds) In Vitro Metabolic Activation in Mutagenesis Testing, Elsevier, North-Holland, pp (12) Galloway, S.M., Aardema, M.J., Ishidate, M.,Jr., Ivett., J.L., Kirkland, D.J., Morita, T., Mosesso, P., Sofuni, T. (1994). Report from Working Group on in Vitro Tests for Chromosomal Aberrations. Mutation Res., 312,
10 (13) Richardson, C., Williams, D.A., Allen, J.A., Amphlett, G., Chanter, D.O., and Phillips, B. (1989). Analysis of Data from In Vitro Cytogenetic Assays. In: Statistical Evaluation of Mutagenicity Test Data. Kirkland, D.J., (ed) Cambridge University Press, Cambridge, pp (14) Soper, K.A. and Galloway S.M. (1994). Replicate Flasks are not Necessary for In Vitro Chromosome Aberration Assays in CHO Cells. Mutation Res., 312, (15) Krahn, D.F., Barsky, F.C. and McCooey, K.T. (1982). CHO/HGPRT Mutation Assay: Evaluation of Gases and Volatile Liquids. In: Tice, R.R., Costa, D.L., Schaich, K.M. (eds.) Genotoxic Effects of Airborne Agents. New York, Plenum, pp (16) Zamora, P.O., Benson, J.M., Li, A.P. and Brooks, A.L. (1983). Evaluation of an Exposure System Using Cells Grown on Collagen Gels for Detecting Highly Volatile Mutagens in the CHO/HGPRT Mutation Assay. Environmental Mutagenesis, 5, (17) Locke-Huhle, C. (1983) Endoreduplication in Chinese hamster cells during alpha-radiation induced G2 arrest. Mutation Res. 119, (18) Huang, Y., Change, C. and Trosko, J.E. (1983) Aphidicolin - induced endoreduplication in Chinese hamster cells. Cancer Res., 43, (A41) Brusick, D. (1986), Genotoxic effects in cultured mammalian cells produced by low ph treatment conditions and increased ion concentrations, Environ. Mutagen., 8, (A46) Ong, T.-m., Mukhtar, M., Wolf, C.R. and Zeiger, E. (1980), Differential effects of cytochrome P450-inducers on promutagen activation capabilities and enzymatic activities of S-9 from rat liver, J. Environ. Pathol. Toxicol., 4, (A49) Johnson, T.E., Umbenhauer, D.R. and Galloway, S.M. (1996), Human liver S-9 metabolic activation: proficiency in cytogenetic assays and comparison with phenobarbital/beta-naphthoflavone or Aroclor 1254 induced rat S-9, Environ. Mol. Mutagen., 28, (A50) UNEP (2001), Stockholm Convention on Persistent Organic Pollutants, United Nations Environment Programme (UNEP). Available at: [ (A60) Galloway, S. (2000), Cytotoxicity and chromosome aberrations in vitro: Experience in industry and the case for an upper limit on toxicity in the aberration assay, Environ. Molec. Mutagenesis 35, (B1) Lorge, E., Hayashi, M., Albertini, S. and Kirkland, D. (2008), Comparison of different methods for an accurate assessment of cytotoxicity in the in vitro micronucleus test. I. Theoretical aspects, Mutation Res., 655, 1-3. (B2) Sheila Galloway, Elisabeth Lorge, Marilyn J. Aardema, David Eastmond, Mick Fellows, Robert Heflich, David Kirkland, Dan D. Levy, Anthony M. Lynch, Daniel Marzin, Takeshi Morita, Maik Schuler, Günter Speit. (2011). Workshop summary: Top concentration for in vitro mammalian cell genotoxicity assays; and Report from working group on toxicity measures and top concentration for in vitro cytogenetics assays (chromosome aberrations and micronucleus). Mutat. Res, 723, (B3) M. Hayashi, K. Dearfield, P. Kasper, D. Lovell, HJ. Martus, V. Thybaud. Compilation and use of genetic toxicity historical control Data Mutat.Res.:Genet.Toxicol. Environ. Mutagen.(2010),doi: /j.mrgentox
11 (B4) Hedner K., Högstedt B., Kolnig A.-M., Mark-Vendel E., Strömbeck B. and Mitelman F. (1982), Sister chromatid exchanges and structural chromosome aberrations in relation to age and sex, Hum. Genet., 62, , 1982 (B5) Ramsey M.J., Moore II D.H., Briner J.F., Lee D.A., Olsen L.A., Senft J.R. and Tucker J.D. (1995), The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutation Res., 338, (B6) Kirkland culture medium (B7) Honma M. (2011), Cytotoxicity measurement in in vitro chromosome aberration test and micronucleus test, Mutation Res., 724, Pfuhler S., Fellows M., van Benthem J., Corvi R., Curren R., Dearfield K., Fowler P., Frötschl R., Elhajouji A., Le Hégarat L., Kasamatsu T., Kojima H., Ouédraogo G., Scott A., Speit G. (2011), In vitro genotoxicity test approaches with better predictivity: Summary of an IWGT workshop, Mutation Res., 723,
12 Annex 1 DEFINITIONS Aneugen: any substance that, by interacting with the components of the mitotic and meiotic cell division cycle, leads to aneuploidy in cells or organisms. Aneuploidy: any deviation from the normal diploid (or haploid) number of chromosomes by a single chromosome or more than one, but not by entire set(s) of chromosomes (polyploidy). Chromatid-type aberration: structural chromosome damage expressed as breakage of single chromatids or breakage and reunion between chromatids. Chromosome-type aberration: structural chromosome damage expressed as breakage, or breakage and reunion, of both chromatids at an identical site. Clastogen: any substance which causes structural chromosomal aberrations in populations of cells or organisms. Endoreduplication: a process in which after an S period of DNA replication, the nucleus does not go into mitosis but starts another S period. The result is chromosomes with 4, 8, 16,...chromatids. Gap: an achromatic lesion smaller than the width of one chromatid, and with minimum misalignment of the chromatids. Mitotic index: the ratio of cells in metaphase divided by the total number of cells observed in a population of cells; an indication of the degree of proliferation of that population. Numerical aberration: a change in the number of chromosomes from the normal number characteristic of the cells utilised. Polyploidy: numerical chromosome aberrations in cells or organisms involving entire set(s) of chromosomes, as opposed to an individual chromosome or chromosomes (aneuploidy). Relative Increase in Cell Counts (RICC): the increase in the number of cells in chemically-exposed cultures versus increase in non-treated cultures, a ratio expressed as a percentage. Relative Population Doubling (RPD): the increase in the number of population doublings in chemically-exposed cultures versus increase in non-treated cultures, a ratio expressed as a percentage Solvent/vehicle: Structural aberration: a change in chromosome structure detectable by microscopic examination of the metaphase stage of cell division, observed as deletions and fragments, intrachanges or interchanges. Untreated controls:
13 Annex 2 FORMULAS FOR CYTOTOXICITY ASSESSMENT Mitotic index (MI): Number of mitotic cells MI = Total number of cells scored where: Relative Increase in Cell Counts (RICC) or on Relative Population Doubling (RPD) is recommended, as both take into account the proportion of the cell population which has divided. (Increase in number of cells in treated cultures (final starting)) RICC = (Increase in number of cells in control cultures (final starting)) (No. of Population doublings in treated cultures) RPD = (No. of Population doublings in control cultures) Population Doubling = [log (Post-treatment cell number Initial cell number)] log 2 As an example, a RICC, or a RPD of 53% indicates 47% cytotoxicity/cytostasis.
14 Annex 3 REFERENCE CHEMICALS RECOMMENDED FOR ASSESSING LABORATORY PROFICIENCY AND FOR SELECTION OF POSITIVE CONTROLS Category Chemical CASRN 1. Clastogens active without metabolic activation 2. Clastogens requiring metabolic activation Methyl methanesulphonate Mitomycin C Nitroquinoline-N-Oxide Benzo(a)pyrene Cyclophosphamide Positive control chemicals should be able to provide appropriate demonstration of metabolic activation and detection of relevant endpoints or mechanisms covered by the test (clastogenicity).
ICH guideline S2 (R1) on genotoxicity testing and data interpretation for pharmaceuticals intended for human use
 June 2012 EMA/CHMP/ICH/126642/2008 ICH guideline S2 (R1) on genotoxicity testing and data interpretation for pharmaceuticals intended for human use Step 5 Transmission to CHMP March 2008 Adoption by CHMP
June 2012 EMA/CHMP/ICH/126642/2008 ICH guideline S2 (R1) on genotoxicity testing and data interpretation for pharmaceuticals intended for human use Step 5 Transmission to CHMP March 2008 Adoption by CHMP
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S2(R1)
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDANCE ON GENOTOXICITY TESTING AND DATA INTERPRETATION
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDANCE ON GENOTOXICITY TESTING AND DATA INTERPRETATION
July 17, 2014 version OECD GUIDELINE FOR THE TESTING OF CHEMICALS. New Test Guideline;
 July 17, 2014 version OECD GUIDELINE FOR THE TESTING OF CHEMICALS New Test Guideline; In Vitro Mammalian Cell Gene Mutation Assays Using the Thymidine Kinase Gene Comment [MM1]: Paragraphs highlighted
July 17, 2014 version OECD GUIDELINE FOR THE TESTING OF CHEMICALS New Test Guideline; In Vitro Mammalian Cell Gene Mutation Assays Using the Thymidine Kinase Gene Comment [MM1]: Paragraphs highlighted
485 Adopted: 23 Oct 1986
 OECD GUIDELINE FOR TESTING OF CHEMICALS 485 Adopted: 23 Oct 1986 "Genetic Toxicology: Mouse Heritable 1. I N T R O D U C T O R Y I N F O R M A T I O N P r e r e q u i s i t e s Solid, liquid, vapour or
OECD GUIDELINE FOR TESTING OF CHEMICALS 485 Adopted: 23 Oct 1986 "Genetic Toxicology: Mouse Heritable 1. I N T R O D U C T O R Y I N F O R M A T I O N P r e r e q u i s i t e s Solid, liquid, vapour or
AMES TEST: Bacterial Reverse Mutation Assay
 AMES TEST: Bacterial Reverse Mutation Assay 1. Introduction The bacteria reversed mutation assay (Ames Test) is used to evaluate the mutagenic properties of test articles. The test uses amino acid-dependent
AMES TEST: Bacterial Reverse Mutation Assay 1. Introduction The bacteria reversed mutation assay (Ames Test) is used to evaluate the mutagenic properties of test articles. The test uses amino acid-dependent
474 Adopted: 21st July 1997
 474 Adopted: 21st July 1997 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Mammalian Erythrocyte Micronucleus Test INTRODUCTION 1. The mammalian in vivo micronucleus test is used for the detection of damage
474 Adopted: 21st July 1997 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Mammalian Erythrocyte Micronucleus Test INTRODUCTION 1. The mammalian in vivo micronucleus test is used for the detection of damage
1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Conclusion No cytotoxicity can be detected at concentrations up to 100%.
 Endotoxicity and Cytotoxicity Studies 1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Chinese Hamster Ovary (CHO) cells were grown in monolayer with and without metabolic activation.
Endotoxicity and Cytotoxicity Studies 1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Chinese Hamster Ovary (CHO) cells were grown in monolayer with and without metabolic activation.
6 TH INTERNATIONAL WORKSHOP ON GENOTOXICITY TESTING. Bourbon Cataratas Hotel, Foz do Iguaçu, Brazil
 International Workshops on Genotoxicity Testing (IWGT) 6 TH INTERNATIONAL WORKSHOP ON GENOTOXICITY TESTING Bourbon Cataratas Hotel, Foz do Iguaçu, Brazil October 31 November 2, 2013 IWGT 2013 detailed
International Workshops on Genotoxicity Testing (IWGT) 6 TH INTERNATIONAL WORKSHOP ON GENOTOXICITY TESTING Bourbon Cataratas Hotel, Foz do Iguaçu, Brazil October 31 November 2, 2013 IWGT 2013 detailed
HP8 herbal formula selectively inhibits prostate cancer cell line proliferation by inducing G2/M cell cycle arrest.
 HP8 herbal formula selectively inhibits prostate cancer cell line proliferation by inducing G2/M cell cycle arrest. Flowers A, Banbury L, Leach D and Waterman P. Centre for Phytochemistry and Pharmacology,
HP8 herbal formula selectively inhibits prostate cancer cell line proliferation by inducing G2/M cell cycle arrest. Flowers A, Banbury L, Leach D and Waterman P. Centre for Phytochemistry and Pharmacology,
471 Adopted: 21st July 1997
 471 Adopted: 21st July 1997 OECD GUIDELINE FOR TESTING OF CHEMICALS Bacterial Reverse Mutation Test INTRODUCTION 1. The bacterial reverse mutation test uses amino-acid requiring strains of Salmonella typhimurium
471 Adopted: 21st July 1997 OECD GUIDELINE FOR TESTING OF CHEMICALS Bacterial Reverse Mutation Test INTRODUCTION 1. The bacterial reverse mutation test uses amino-acid requiring strains of Salmonella typhimurium
GUIDANCE ON A STRATEGY FOR GENOTOXICITY TESTING OF CHEMICAL SUBSTANCES
 GUIDANCE ON A STRATEGY FOR GENOTOXICITY TESTING OF CHEMICAL SUBSTANCES CONTENTS PARAGRAPH I. Preface 1-5 II. Introduction 6-10 III. Significance of Chemical Induced Mutation for Human Health 11-12 IV.
GUIDANCE ON A STRATEGY FOR GENOTOXICITY TESTING OF CHEMICAL SUBSTANCES CONTENTS PARAGRAPH I. Preface 1-5 II. Introduction 6-10 III. Significance of Chemical Induced Mutation for Human Health 11-12 IV.
Instructions. Torpedo sirna. Material. Important Guidelines. Specifications. Quality Control
 is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
Cell Phone Radiation and Genomic Damage: In Vitro Exposure and Assessment
 Cell Phone Radiation and Genomic Damage: In Vitro Exposure and Assessment Chinar Shah 1, Anu Nair 1, Mehul Naik 2, Sonal Bakshi 3 Institute of Science, Nirma University, Ahmedabad, Gujarat, India 1 Electronics
Cell Phone Radiation and Genomic Damage: In Vitro Exposure and Assessment Chinar Shah 1, Anu Nair 1, Mehul Naik 2, Sonal Bakshi 3 Institute of Science, Nirma University, Ahmedabad, Gujarat, India 1 Electronics
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B
 PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
MTT Cell Proliferation Assay
 ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
What is Cancer? Cancer is a genetic disease: Cancer typically involves a change in gene expression/function:
 Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Cell Division CELL DIVISION. Mitosis. Designation of Number of Chromosomes. Homologous Chromosomes. Meiosis
 Cell Division CELL DIVISION Anatomy and Physiology Text and Laboratory Workbook, Stephen G. Davenport, Copyright 2006, All Rights Reserved, no part of this publication can be used for any commercial purpose.
Cell Division CELL DIVISION Anatomy and Physiology Text and Laboratory Workbook, Stephen G. Davenport, Copyright 2006, All Rights Reserved, no part of this publication can be used for any commercial purpose.
Chapter 13: Meiosis and Sexual Life Cycles
 Name Period Chapter 13: Meiosis and Sexual Life Cycles Concept 13.1 Offspring acquire genes from parents by inheriting chromosomes 1. Let s begin with a review of several terms that you may already know.
Name Period Chapter 13: Meiosis and Sexual Life Cycles Concept 13.1 Offspring acquire genes from parents by inheriting chromosomes 1. Let s begin with a review of several terms that you may already know.
Fighting the Battles: Conducting a Clinical Assay
 Fighting the Battles: Conducting a Clinical Assay 6 Vocabulary: In Vitro: studies in biology that are conducted using components of an organism that have been isolated from their usual biological surroundings
Fighting the Battles: Conducting a Clinical Assay 6 Vocabulary: In Vitro: studies in biology that are conducted using components of an organism that have been isolated from their usual biological surroundings
EVALUATION OF THE EFFECT OF THE COMPOUND RIBOXYL ON THE ATP LEVEL AND CELLULAR RESPIRATION FROM HUMAN DERMAL FIBROBLASTS
 EVALUATION OF THE EFFECT OF THE COMPOUND RIBOXYL ON THE ATP LEVEL AND CELLULAR RESPIRATION FROM HUMAN DERMAL FIBROBLASTS FINAL REPORT OF THE STUDY GT050923 Study promoter: LUCAS MEYER COSMETICS ZA Les
EVALUATION OF THE EFFECT OF THE COMPOUND RIBOXYL ON THE ATP LEVEL AND CELLULAR RESPIRATION FROM HUMAN DERMAL FIBROBLASTS FINAL REPORT OF THE STUDY GT050923 Study promoter: LUCAS MEYER COSMETICS ZA Les
3.4 TEST FOR BACTERIAL ENDOTOXINS. Final text for revision of The International Pharmacopoeia
 Document QAS/11.5 FINAL July 01 3. TEST FOR BACTERIAL ENDOTOXINS Final text for revision of The International Pharmacopoeia This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
Document QAS/11.5 FINAL July 01 3. TEST FOR BACTERIAL ENDOTOXINS Final text for revision of The International Pharmacopoeia This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
SISTER CHROMATID EXCHANGES: A STUDY IN FLUOROTIC INDIVIDUALS OF NORTH GUJARAT
 Fluoride Vol.27 No.4 215-219 1994 Research Report 215 SISTER CHROMATID EXCHANGES: A STUDY IN FLUOROTIC INDIVIDUALS OF NORTH GUJARAT F J Sheth, A S Multani and N J Chinoy Ahmedabad, India SUMMARY: The purpose
Fluoride Vol.27 No.4 215-219 1994 Research Report 215 SISTER CHROMATID EXCHANGES: A STUDY IN FLUOROTIC INDIVIDUALS OF NORTH GUJARAT F J Sheth, A S Multani and N J Chinoy Ahmedabad, India SUMMARY: The purpose
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing
 Environmental and Molecular Mutagenesis 35:206 221 (2000) Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing R. R. Tice, 1 * E. Agurell, 2 D. Anderson, 3 B. Burlinson,
Environmental and Molecular Mutagenesis 35:206 221 (2000) Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing R. R. Tice, 1 * E. Agurell, 2 D. Anderson, 3 B. Burlinson,
Transgenic Rodent Gene Mutation Assays: Current State of Validation. George R. Douglas
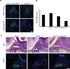 Transgenic Rodent Gene Mutation Assays: Current State of Validation Prepared for Organization for Economic Cooperation and Development (OECD) by George R. Douglas Mechanistic Studies Division Environmental
Transgenic Rodent Gene Mutation Assays: Current State of Validation Prepared for Organization for Economic Cooperation and Development (OECD) by George R. Douglas Mechanistic Studies Division Environmental
www.njctl.org PSI Biology Mitosis & Meiosis
 Mitosis and Meiosis Mitosis Classwork 1. Identify two differences between meiosis and mitosis. 2. Provide an example of a type of cell in the human body that would undergo mitosis. 3. Does cell division
Mitosis and Meiosis Mitosis Classwork 1. Identify two differences between meiosis and mitosis. 2. Provide an example of a type of cell in the human body that would undergo mitosis. 3. Does cell division
TransIT -2020 Transfection Reagent
 Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) GUIDANCE ON MODULE 3 OF THE HOMEOPATHIC MEDICINAL PRODUCTS DOSSIER
 HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) GUIDANCE ON MODULE 3 OF THE HOMEOPATHIC MEDICINAL PRODUCTS DOSSIER DISCUSSION IN THE HMPWG 2003-2005 RELEASE FOR CONSULTATION December 2005 DEADLINE
HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) GUIDANCE ON MODULE 3 OF THE HOMEOPATHIC MEDICINAL PRODUCTS DOSSIER DISCUSSION IN THE HMPWG 2003-2005 RELEASE FOR CONSULTATION December 2005 DEADLINE
Steatosis Colorimetric Assay Kit
 Steatosis Colorimetric Assay Kit Item No. 10012643 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Steatosis Colorimetric Assay Kit Item No. 10012643 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Cell Cycle Phase Determination Kit
 Cell Cycle Phase Determination Kit Item No. 10009349 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Safety
Cell Cycle Phase Determination Kit Item No. 10009349 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Safety
Human Chromosomes lab 5
 Human Chromosomes lab 5 Objectives Upon completion of this activity, you should be able to: describe the structure of human chromosomes with reference to size, centromere position, and presence or absence
Human Chromosomes lab 5 Objectives Upon completion of this activity, you should be able to: describe the structure of human chromosomes with reference to size, centromere position, and presence or absence
1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells
 Cell Growth and Reproduction 1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells A. is half of that of the parent cell. B. remains the same as in the
Cell Growth and Reproduction 1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells A. is half of that of the parent cell. B. remains the same as in the
Chapter 13: Meiosis and Sexual Life Cycles
 Name Period Concept 13.1 Offspring acquire genes from parents by inheriting chromosomes 1. Let s begin with a review of several terms that you may already know. Define: gene locus gamete male gamete female
Name Period Concept 13.1 Offspring acquire genes from parents by inheriting chromosomes 1. Let s begin with a review of several terms that you may already know. Define: gene locus gamete male gamete female
OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE XXX: Transgenic Rodent Gene Mutation Assay
 OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE XXX: Transgenic Rodent Gene Mutation Assay INTRODUCTION 1. OECD guidelines are available for a wide range of in vitro mutation
OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE XXX: Transgenic Rodent Gene Mutation Assay INTRODUCTION 1. OECD guidelines are available for a wide range of in vitro mutation
Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines
 Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines TABLE OF CONTENTS GUIDANCE DOCUMENT ON REVISIONS TO OECD GENETIC TOXICOLOGY TEST GUIDELINES... 1 1 GENERAL INTRODUCTION (PREAMBLE)...
Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines TABLE OF CONTENTS GUIDANCE DOCUMENT ON REVISIONS TO OECD GENETIC TOXICOLOGY TEST GUIDELINES... 1 1 GENERAL INTRODUCTION (PREAMBLE)...
Animal Cell Culture. Third Edition. A Practical Approach OXJORD VNIVVRSITY 1'RVSS
 Animal Cell Culture Third Edition A Practical Approach Edited by John R. W. Masters 3rd Floor Research Laboratories, University College London OXJORD VNIVVRSITY 1'RVSS Contents List of protocols page xiii
Animal Cell Culture Third Edition A Practical Approach Edited by John R. W. Masters 3rd Floor Research Laboratories, University College London OXJORD VNIVVRSITY 1'RVSS Contents List of protocols page xiii
5. The cells of a multicellular organism, other than gametes and the germ cells from which it develops, are known as
 1. True or false? The chi square statistical test is used to determine how well the observed genetic data agree with the expectations derived from a hypothesis. True 2. True or false? Chromosomes in prokaryotic
1. True or false? The chi square statistical test is used to determine how well the observed genetic data agree with the expectations derived from a hypothesis. True 2. True or false? Chromosomes in prokaryotic
Sexual Reproduction. and Meiosis. Sexual Reproduction
 Sexual Reproduction and Meiosis Describe the stages of meiosis and how sex cells are produced. Explain why meiosis is needed for sexual reproduction. Name the cells that are involved in fertilization.
Sexual Reproduction and Meiosis Describe the stages of meiosis and how sex cells are produced. Explain why meiosis is needed for sexual reproduction. Name the cells that are involved in fertilization.
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
Biology 3A Laboratory MITOSIS Asexual Reproduction
 Biology 3A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Biology 3A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Working Party on Control of Medicines and Inspections. Final Version of Annex 15 to the EU Guide to Good Manufacturing Practice
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals and cosmetics Brussels, July 2001 Working Party on Control
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals and cosmetics Brussels, July 2001 Working Party on Control
GENOTOXICITY TESTING, A REGULATORY REQUIREMENT FOR DRUG DISCOVERY AND DEVELOPMENT: IMPACT OF ICH GUIDELINES
 Indian Journal of Pharmacology 2002; 34: 86-99 EDUCATIONAL FORUM GENOTOXICITY TESTING IN DRUG SAFETY EVALUATION GENOTOXICITY TESTING, A REGULATORY REQUIREMENT FOR DRUG DISCOVERY AND DEVELOPMENT: IMPACT
Indian Journal of Pharmacology 2002; 34: 86-99 EDUCATIONAL FORUM GENOTOXICITY TESTING IN DRUG SAFETY EVALUATION GENOTOXICITY TESTING, A REGULATORY REQUIREMENT FOR DRUG DISCOVERY AND DEVELOPMENT: IMPACT
Biological importance of metabolites. Safety and efficacy aspects
 Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
CCR Biology - Chapter 5 Practice Test - Summer 2012
 Name: Class: Date: CCR Biology - Chapter 5 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. If a cell cannot move enough material
Name: Class: Date: CCR Biology - Chapter 5 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. If a cell cannot move enough material
Unit 1 Higher Human Biology Summary Notes
 Unit 1 Higher Human Biology Summary Notes a. Cells tissues organs body systems Division of labour occurs in multicellular organisms (rather than each cell carrying out every function) Most cells become
Unit 1 Higher Human Biology Summary Notes a. Cells tissues organs body systems Division of labour occurs in multicellular organisms (rather than each cell carrying out every function) Most cells become
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL
 EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate C - Scientific Opinions C2 - Management of scientific committees II; scientific co-operation and networks Revision of the
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate C - Scientific Opinions C2 - Management of scientific committees II; scientific co-operation and networks Revision of the
CHROMOSOME STRUCTURE CHROMOSOME NUMBERS
 CHROMOSOME STRUCTURE 1. During nuclear division, the DNA (as chromatin) in a Eukaryotic cell's nucleus is coiled into very tight compact structures called chromosomes. These are rod-shaped structures made
CHROMOSOME STRUCTURE 1. During nuclear division, the DNA (as chromatin) in a Eukaryotic cell's nucleus is coiled into very tight compact structures called chromosomes. These are rod-shaped structures made
Cell Division Mitosis and the Cell Cycle
 Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Lecture 11 The Cell Cycle and Mitosis
 Lecture 11 The Cell Cycle and Mitosis In this lecture Cell division Chromosomes The cell cycle Mitosis PPMAT Apoptosis What is cell division? Cells divide in order to reproduce themselves The cell cycle
Lecture 11 The Cell Cycle and Mitosis In this lecture Cell division Chromosomes The cell cycle Mitosis PPMAT Apoptosis What is cell division? Cells divide in order to reproduce themselves The cell cycle
1. Why is mitosis alone insufficient for the life cycle of sexually reproducing eukaryotes?
 Chapter 13: Meiosis and Sexual Life Cycles 1. Why is mitosis alone insufficient for the life cycle of sexually reproducing eukaryotes? 2. Define: gamete zygote meiosis homologous chromosomes diploid haploid
Chapter 13: Meiosis and Sexual Life Cycles 1. Why is mitosis alone insufficient for the life cycle of sexually reproducing eukaryotes? 2. Define: gamete zygote meiosis homologous chromosomes diploid haploid
Chapter 8: Variation in Chromosome Structure and Number
 Chapter 8: Variation in Chromosome Structure and Number Student Learning Objectives Upon completion of this chapter you should be able to: 1. Know the principles and terminology associated with variations
Chapter 8: Variation in Chromosome Structure and Number Student Learning Objectives Upon completion of this chapter you should be able to: 1. Know the principles and terminology associated with variations
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
Combined effects of carcinogens
 Combined effects of carcinogens Institute of Environmental Medicine ulla.stenius@ki.se National Cancer Institute Ulla Stenius, IMM, KI 1 477 chemicals tested in NTP cancer test analyzed 278 rat carcinogens
Combined effects of carcinogens Institute of Environmental Medicine ulla.stenius@ki.se National Cancer Institute Ulla Stenius, IMM, KI 1 477 chemicals tested in NTP cancer test analyzed 278 rat carcinogens
The Somatic Cell Cycle
 The Somatic Cell Cycle Maternal chromosome Diploid Zygote Diploid Zygote Paternal chromosome MITOSIS MITOSIS Maternal chromosome Diploid organism Diploid organism Paternal chromosome Int terpha ase The
The Somatic Cell Cycle Maternal chromosome Diploid Zygote Diploid Zygote Paternal chromosome MITOSIS MITOSIS Maternal chromosome Diploid organism Diploid organism Paternal chromosome Int terpha ase The
Fast, easy and effective transfection reagent for mammalian cells
 METAFECTENE EASY + Fast, easy and effective transfection reagent for mammalian cells For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
METAFECTENE EASY + Fast, easy and effective transfection reagent for mammalian cells For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
Cell, Tissue and Tumor Kinetics
 Cell, issue and umor Kinetics Proliferation Kinetics: rate of growth of a population, change in total cell number. Adult tissues are in homeostasis. Children (and tumors) grow. I. Quantitative Assessment
Cell, issue and umor Kinetics Proliferation Kinetics: rate of growth of a population, change in total cell number. Adult tissues are in homeostasis. Children (and tumors) grow. I. Quantitative Assessment
Classify chromosomes in a karyotype according to size and centromere position. Identify metacentric, submetacentric and acrocentric chromosomes
 Mitosis, Meiosis and the Cell Cycle Prof. Alfred Cuschieri University of Malta Department of Anatomy Objectives By the end of the session the student shoud be able to: Define the meaning of chromosomes
Mitosis, Meiosis and the Cell Cycle Prof. Alfred Cuschieri University of Malta Department of Anatomy Objectives By the end of the session the student shoud be able to: Define the meaning of chromosomes
WHEY PROTEIN IMPORTANCE. Dan Phillips
 WHEY PROTEIN IMPORTANCE Dan Phillips Studies on whey demonstrate it's an even better protein supplement than previously thought. Although whey protein's health benefits have only recently been elucidated,
WHEY PROTEIN IMPORTANCE Dan Phillips Studies on whey demonstrate it's an even better protein supplement than previously thought. Although whey protein's health benefits have only recently been elucidated,
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
Chromosomes, Karyotyping, and Abnormalities (Learning Objectives) Learn the components and parts of a metaphase chromosome.
 Chromosomes, Karyotyping, and Abnormalities (Learning Objectives) Learn the components and parts of a metaphase chromosome. Define the terms karyotype, autosomal and sex chromosomes. Explain how many of
Chromosomes, Karyotyping, and Abnormalities (Learning Objectives) Learn the components and parts of a metaphase chromosome. Define the terms karyotype, autosomal and sex chromosomes. Explain how many of
Overview on EFSA data requirements for the safety evaluation of food enzymes applications
 Overview on EFSA data requirements for the safety evaluation of food enzymes applications Fidel Toldrá and Klaus-Dieter Jany EFSA CEF Panel Info session on Food Enzymes applications Parma, 27 May 2014
Overview on EFSA data requirements for the safety evaluation of food enzymes applications Fidel Toldrá and Klaus-Dieter Jany EFSA CEF Panel Info session on Food Enzymes applications Parma, 27 May 2014
Safety Report of GelRed and GelGreen
 Safety Report of GelRed and GelGreen A Summary of Mutagenicity and Environmental Safety Test Results from Three Independent Laboratories Last updated: October 16, 2013 Overview Ethidium bromide (EB) has
Safety Report of GelRed and GelGreen A Summary of Mutagenicity and Environmental Safety Test Results from Three Independent Laboratories Last updated: October 16, 2013 Overview Ethidium bromide (EB) has
IMPURITIES IN NEW DRUG PRODUCTS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE IMPURITIES IN NEW DRUG PRODUCTS Q3B(R2) Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE IMPURITIES IN NEW DRUG PRODUCTS Q3B(R2) Current
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
The Science of Chemical Safety Essential Toxicology - 4. Hazard and Risk. John Duffus & Howard Worth. IUPAC Educators Resource Material IUPAC
 The Science of Chemical Safety Essential Toxicology - 4 Hazard and Risk John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Hazard and Risk - 1 Hazard is the potential of a substance to
The Science of Chemical Safety Essential Toxicology - 4 Hazard and Risk John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Hazard and Risk - 1 Hazard is the potential of a substance to
Cell Growth and Reproduction Module B, Anchor 1
 Cell Growth and Reproduction Module B, Anchor 1 Key Concepts: - The larger a cell becomes, the more demands the cell places on its DNA. In addition, a larger cell is less efficient in moving nutrients
Cell Growth and Reproduction Module B, Anchor 1 Key Concepts: - The larger a cell becomes, the more demands the cell places on its DNA. In addition, a larger cell is less efficient in moving nutrients
BioSci 2200 General Genetics Problem Set 1 Answer Key Introduction and Mitosis/ Meiosis
 BioSci 2200 General Genetics Problem Set 1 Answer Key Introduction and Mitosis/ Meiosis Introduction - Fields of Genetics To answer the following question, review the three traditional subdivisions of
BioSci 2200 General Genetics Problem Set 1 Answer Key Introduction and Mitosis/ Meiosis Introduction - Fields of Genetics To answer the following question, review the three traditional subdivisions of
Uses of Flow Cytometry
 Uses of Flow Cytometry 1. Multicolour analysis... 2 2. Cell Cycle and Proliferation... 3 a. Analysis of Cellular DNA Content... 4 b. Cell Proliferation Assays... 5 3. Immunology... 6 4. Apoptosis... 7
Uses of Flow Cytometry 1. Multicolour analysis... 2 2. Cell Cycle and Proliferation... 3 a. Analysis of Cellular DNA Content... 4 b. Cell Proliferation Assays... 5 3. Immunology... 6 4. Apoptosis... 7
List, describe, diagram, and identify the stages of meiosis.
 Meiosis and Sexual Life Cycles In this topic we will examine a second type of cell division used by eukaryotic cells: meiosis. In addition, we will see how the 2 types of eukaryotic cell division, mitosis
Meiosis and Sexual Life Cycles In this topic we will examine a second type of cell division used by eukaryotic cells: meiosis. In addition, we will see how the 2 types of eukaryotic cell division, mitosis
402 Adopted: 24 Feb 1987
 OECD GUIDELINE FOR TESTING OF CHEMICALS Adopted: 24 Feb 1987 1. I N T R O D U C T O R Y I N F O R M A T I O N P r e r e q u i s i t e s Solid or liquid test substance Chemical identification of test substance
OECD GUIDELINE FOR TESTING OF CHEMICALS Adopted: 24 Feb 1987 1. I N T R O D U C T O R Y I N F O R M A T I O N P r e r e q u i s i t e s Solid or liquid test substance Chemical identification of test substance
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
Biopharmaceutical Process Evaluated for Viral Clearance
 Authored by S. Steve Zhou, Ph.D. Microbac Laboratories, Inc., Microbiotest Division The purpose of Viral Clearance evaluation is to assess the capability of a manufacturing production process to inactivate
Authored by S. Steve Zhou, Ph.D. Microbac Laboratories, Inc., Microbiotest Division The purpose of Viral Clearance evaluation is to assess the capability of a manufacturing production process to inactivate
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE Q5B
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
Biodiesel by-products: A Case Study in BiodieselGlycerol and Cancer
 Journal of Proactive Medicine Vol 1. No 2. (2012) Journal of Proactive Medicinee The International Journal of cancer prevention, chemoprevec ention andd epidemiology Journal homepage: www.jproactive.hu
Journal of Proactive Medicine Vol 1. No 2. (2012) Journal of Proactive Medicinee The International Journal of cancer prevention, chemoprevec ention andd epidemiology Journal homepage: www.jproactive.hu
Especially developed for the transfection of mammalian cells with sirna or mirna
 METAFECTENE SI Especially developed for the transfection of mammalian cells with sirna or mirna For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No.
METAFECTENE SI Especially developed for the transfection of mammalian cells with sirna or mirna For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No.
Materials and Methods. Ribonuclease.--Removal of ribonucleic acid from meristematic onion cells did not show any qualitative differences
 B~U~ Noz~s 129 Alterations in Nuclear Ribonucleic Acid Metabolism Induced by l~inetin.* BY RUTH GUNMAN. From the Department of Genetics, University of California, Be, kezey.~ Kinetin, a substance recently
B~U~ Noz~s 129 Alterations in Nuclear Ribonucleic Acid Metabolism Induced by l~inetin.* BY RUTH GUNMAN. From the Department of Genetics, University of California, Be, kezey.~ Kinetin, a substance recently
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
 GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
EFSA explains the carcinogenicity assessment of glyphosate
 PESTICIDES UNIT EFSA explains the carcinogenicity assessment of glyphosate Background 12 November 2015 During the EFSA peer-review process for the renewal of the approval of the pesticide active substance
PESTICIDES UNIT EFSA explains the carcinogenicity assessment of glyphosate Background 12 November 2015 During the EFSA peer-review process for the renewal of the approval of the pesticide active substance
Manual for: sirna-trans Maximo Reagent
 Manual for: sirna-trans Maximo Reagent Contact: info@geneon.net; www.geneon.net GeneON GmbH Germany GeneON GmbH / Protocol sirna-trans reagent vs. 1.3 / www.geneon.net / - 0 - sirna-trans reagent Instruction
Manual for: sirna-trans Maximo Reagent Contact: info@geneon.net; www.geneon.net GeneON GmbH Germany GeneON GmbH / Protocol sirna-trans reagent vs. 1.3 / www.geneon.net / - 0 - sirna-trans reagent Instruction
Animal welfare, etológia és tartástechnológia
 Animal welfare, etológia és tartástechnológia Animal welfare, ethology and housing systems Volume 9 Issue 3 Különszám/Special Issue Gödöllő 2013 USE OF SISTER CHROMATID EXCHANGES AND COMET ASSAY IN GENETIC
Animal welfare, etológia és tartástechnológia Animal welfare, ethology and housing systems Volume 9 Issue 3 Különszám/Special Issue Gödöllő 2013 USE OF SISTER CHROMATID EXCHANGES AND COMET ASSAY IN GENETIC
Appendix C DNA Replication & Mitosis
 K.Muma Bio 6 Appendix C DNA Replication & Mitosis Study Objectives: Appendix C: DNA replication and Mitosis 1. Describe the structure of DNA and where it is found. 2. Explain complimentary base pairing:
K.Muma Bio 6 Appendix C DNA Replication & Mitosis Study Objectives: Appendix C: DNA replication and Mitosis 1. Describe the structure of DNA and where it is found. 2. Explain complimentary base pairing:
The Impact of Polyphenolic Compounds on Apoptosis in Epithelial Cells
 The Impact of Polyphenolic Compounds on Apoptosis in Epithelial Cells Mayson Husband, 12 th grade, Latta High School Abstract The purpose of this investigation was to determine whether polyphenolic compounds
The Impact of Polyphenolic Compounds on Apoptosis in Epithelial Cells Mayson Husband, 12 th grade, Latta High School Abstract The purpose of this investigation was to determine whether polyphenolic compounds
SCANTIBODIES Laboratory, Inc. Contract Monoclonal Antibody Production
 A Technical Publication of SCANTIBODIES Laboratory, Inc. Volume 1 Number 4 9336 Abraham Way Santee, CA 92071 USA (619) 258-9300 fax (619) 258-9366 www.scantibodies.com SCANTIBODIES Laboratory, Inc. Contract
A Technical Publication of SCANTIBODIES Laboratory, Inc. Volume 1 Number 4 9336 Abraham Way Santee, CA 92071 USA (619) 258-9300 fax (619) 258-9366 www.scantibodies.com SCANTIBODIES Laboratory, Inc. Contract
Overview: Human Health Effects of Environmental Contaminants
 Overview: Human Health Effects of Environmental Contaminants 1 1 OVERVIEW: HUMAN HEALTH EFFECTS OF ENVIRONMENTAL CONTAMINANTS Human activity has led to the production and release into the environment of
Overview: Human Health Effects of Environmental Contaminants 1 1 OVERVIEW: HUMAN HEALTH EFFECTS OF ENVIRONMENTAL CONTAMINANTS Human activity has led to the production and release into the environment of
Introduction. MassDEP, Office of Research and Standards 1 Tetrachloroethylene
 Summary of the Basis of Cancer Risk Values for Massachusetts Department of Environmental Protection (MassDEP) Office of Research and Standards January 22, 2014 Introduction In February 2012 the United
Summary of the Basis of Cancer Risk Values for Massachusetts Department of Environmental Protection (MassDEP) Office of Research and Standards January 22, 2014 Introduction In February 2012 the United
Lecture 2: Mitosis and meiosis
 Lecture 2: Mitosis and meiosis 1. Chromosomes 2. Diploid life cycle 3. Cell cycle 4. Mitosis 5. Meiosis 6. Parallel behavior of genes and chromosomes Basic morphology of chromosomes telomere short arm
Lecture 2: Mitosis and meiosis 1. Chromosomes 2. Diploid life cycle 3. Cell cycle 4. Mitosis 5. Meiosis 6. Parallel behavior of genes and chromosomes Basic morphology of chromosomes telomere short arm
LESSON 3.5 WORKBOOK. How do cancer cells evolve? Workbook Lesson 3.5
 LESSON 3.5 WORKBOOK How do cancer cells evolve? In this unit we have learned how normal cells can be transformed so that they stop behaving as part of a tissue community and become unresponsive to regulation.
LESSON 3.5 WORKBOOK How do cancer cells evolve? In this unit we have learned how normal cells can be transformed so that they stop behaving as part of a tissue community and become unresponsive to regulation.
Cleaning Validation in Active pharmaceutical Ingredient manufacturing plants
 Cleaning Validation in Active pharmaceutical Ingredient manufacturing plants September 1999 Table of contents 1. Foreword...... 2 2. Objective...... 3 3. Scope........ 4 4. Potential residues... 5 5. Current
Cleaning Validation in Active pharmaceutical Ingredient manufacturing plants September 1999 Table of contents 1. Foreword...... 2 2. Objective...... 3 3. Scope........ 4 4. Potential residues... 5 5. Current
Xpert TM Karyotyping Teaching Kit
 Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
European Medicines Agency
 European Medicines Agency July 1996 CPMP/ICH/139/95 ICH Topic Q 5 B Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of r-dna Derived Protein
European Medicines Agency July 1996 CPMP/ICH/139/95 ICH Topic Q 5 B Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of r-dna Derived Protein
Module Three. Risk Assessment
 Module Three Risk Assessment 136 Module Three Introduction to Risk Assessment Time Allotted: 90 Minutes Objectives: Upon completion of this module, the learner will be able to # Define and understand the
Module Three Risk Assessment 136 Module Three Introduction to Risk Assessment Time Allotted: 90 Minutes Objectives: Upon completion of this module, the learner will be able to # Define and understand the
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Chemistry 51 Chapter 8 TYPES OF SOLUTIONS. A solution is a homogeneous mixture of two substances: a solute and a solvent.
 TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present
TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present
High deleterious genomic mutation rate in stationary phase of Escherichia coli
 Loewe, L et al. (2003) "High deleterious genomic mutation rate in stationary phase of Escherichia coli" 1 High deleterious genomic mutation rate in stationary phase of Escherichia coli Laurence Loewe,
Loewe, L et al. (2003) "High deleterious genomic mutation rate in stationary phase of Escherichia coli" 1 High deleterious genomic mutation rate in stationary phase of Escherichia coli Laurence Loewe,
CHAPTER 9 CELLULAR REPRODUCTION P. 243-257
 CHAPTER 9 CELLULAR REPRODUCTION P. 243-257 SECTION 9-1 CELLULAR GROWTH Page 244 ESSENTIAL QUESTION Why is it beneficial for cells to remain small? MAIN IDEA Cells grow until they reach their size limit,
CHAPTER 9 CELLULAR REPRODUCTION P. 243-257 SECTION 9-1 CELLULAR GROWTH Page 244 ESSENTIAL QUESTION Why is it beneficial for cells to remain small? MAIN IDEA Cells grow until they reach their size limit,
NATIONAL HEALTH COUNCIL RESOLUTION Nº 251, DATED 7 AUGUST 1997
 NATIONAL HEALTH COUNCIL RESOLUTION Nº 251, DATED 7 AUGUST 1997 Plenary of the National Health Council in its 15 th Special Meeting, held on 5 August 1997, in the exercise of its competencies, as set forth
NATIONAL HEALTH COUNCIL RESOLUTION Nº 251, DATED 7 AUGUST 1997 Plenary of the National Health Council in its 15 th Special Meeting, held on 5 August 1997, in the exercise of its competencies, as set forth
Cytotoxic and Biotherapies Credentialing Programme Module 2
 Cytotoxic and Biotherapies Credentialing Programme Module 2 1. The Cell Cycle 2. Cancer Therapies 3. Adjunctive Therapies On completion of this module the RN will State the difference between a normal
Cytotoxic and Biotherapies Credentialing Programme Module 2 1. The Cell Cycle 2. Cancer Therapies 3. Adjunctive Therapies On completion of this module the RN will State the difference between a normal
Cellular Reproduction
 9 Cellular Reproduction section 1 Cellular Growth Before You Read Think about the life cycle of a human. On the lines below, write some of the stages that occur in the life cycle of a human. In this section,
9 Cellular Reproduction section 1 Cellular Growth Before You Read Think about the life cycle of a human. On the lines below, write some of the stages that occur in the life cycle of a human. In this section,
MLX BCG Buccal Cell Genomic DNA Extraction Kit. Performance Characteristics
 MLX BCG Buccal Cell Genomic DNA Extraction Kit Performance Characteristics Monolythix, Inc. 4720 Calle Carga Camarillo, CA 93012 Tel: (805) 484-8478 monolythix.com Page 2 of 9 MLX BCG Buccal Cell Genomic
MLX BCG Buccal Cell Genomic DNA Extraction Kit Performance Characteristics Monolythix, Inc. 4720 Calle Carga Camarillo, CA 93012 Tel: (805) 484-8478 monolythix.com Page 2 of 9 MLX BCG Buccal Cell Genomic
