OUT OF MIND,*BUT ALWAYS THERE.
|
|
|
- Carol Booker
- 7 years ago
- Views:
Transcription
1 BIRTH CONTROL THAT S OUT OF SIGHT, OUT OF MIND,*BUT ALWAYS THERE. Paragard is indicated for intrauterine contraception for up to 10 years. Wouldn t it be nice if there were a birth control that didn t require daily or weekly disruptive routines? Or better yet, something you rarely had to think about? With Paragard (intrauterine copper contraceptive), there is.* Paragard is an intrauterine contraceptive (IUC) that is more than 99% effective. An IUC, sometimes called an IUD, is a small, flexible, T _ shaped object that is inserted into the uterus by your healthcare professional. Paragard is designed to fit comfortably inside your body, even if you haven t had a child, and can last as long as you want _ 2,, even up to 10 years. *With Paragard, there s just a simple monthly string check. Paragard is reversible If you change your mind, your healthcare professional can remove Paragard at any time You can start trying to get pregnant the same day it s removed Paragard is Hormone Free May be appropriate for women who want to avoid hormones Won t interfere with your natural menstrual cycle Bleeding or spotting may increase at first but should decrease in 2 to 3 months As your body adjusts to Paragard, you may experience heavier or longer periods or spotting between periods. This is common. Most of these side effects should diminish after 2 to 3 months. Not actual size. Please see Important Safety Information on the next page and accompanying full Prescribing Information.
2 Paragard (intrauterine copper contraceptive) may fit comfortably in your life. Once it has been placed, you can start to enjoy effective birth control on your own terms. Paragard is one of the most effective forms of birth control 1 1% 70% to 8% 90% to 9% CHANCE CONDOMS, WITHDRAWAL, RHYTHM METHOD, SPERMICIDE How do I get it? Your healthcare professional can typically insert Paragard in just minutes during a routine visit. Once in place, all you ll have to do is a simple monthly string check. What to expect During insertion, you may experience some pinching and cramping, or may feel dizzy, nauseous, or faint, but you should be able to return to normal daily activities after placement. Your healthcare professional may suggest a medication for pain prior to insertion. It s reliable Paragard has been FDA approved for more than 2 years. It has been used by over 1.8 million women. Visit Paragard.com to learn how Paragard is helping women like you experience effective birth control on their own terms. Ask your healthcare professional about Paragard today. It s affordable Under the Affordable Care Act (ACA), Paragard may now be available to insured women without a co _ pay, coinsurance costs, or deductibles. Talk to your doctor or call your insurance provider for information specific to your plan. PILL, PATCH, INJECTION, RING >99% NON-HORMONAL HORMONAL IUD, MALE AND FEMALE STERILIZATION, HORMONAL IMPLANTS It just adds up Paragard is a truly flexible birth control. You won t have to worry about taking a pill or changing a patch.* And if you should decide to start a family, you can return to your own level of fertility as soon as your healthcare professional removes it for you. * With Paragard, there s just a simple monthly string check. Paragard is indicated for intrauterine contraception for up to 10 years. Important Safety Information Do not use Paragard (intrauterine copper contraceptive) if you have a pelvic infection, get infections easily or have certain cancers. Less than 1% of users get a serious infection called pelvic inflammatory disease. If you have persistent pelvic or stomach pain, or if Paragard comes out tell your healthcare provider. If it comes out, use back-up birth control. In rare cases, Paragard may attach to or go through the uterine wall and cause other problems. Although uncommon, pregnancy while using Paragard can be life threatening and may result in loss of pregnancy or fertility. Bleeding or spotting may increase at first but should decrease in 2 to 3 months. Paragard does not protect against HIV or STDs. You are encouraged to report negative side effects of prescription drugs to the FDA at or call FDA Please see accompanying full Prescribing Information. Reference 1. Trussell J, Guthrie KA. Choosing a contraceptive: efficacy, safety, and personal considerations. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS, eds. Contraceptive Technology. th ed. New York, NY: Ardent Media; 11:4 _ Paragard ( ) Paragard.com Paragard is a registered trademark of Teva Women s Health, Inc. 14 Teva Women s Health, Inc. PAR February 14
3 ParaGard T 380A INTRAUTERINE COPPER CONTRACEPTIVE P/N Rev. 6/13 Rx only PRESCRIBING INFORMATION Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. should be placed and removed only by healthcare professionals who are experienced with these procedures. DESCRIPTION (ParaGard ) is a T-shaped intrauterine device (IUD), measuring 32 mm horizontally and 36 mm vertically, with a 3 mm diameter bulb at the tip of the vertical stem. A monofilament polyethylene thread is tied through the tip, resulting in two white threads, each at least 10. cm in length, to aid in detection and removal of the device. The T-frame is made of polyethylene with barium sulfate to aid in detecting the device under x-ray. ParaGard also contains copper: approximately 176 mg of wire coiled along the vertical stem and a 68.7 mg collar on each side of the horizontal arm. The total exposed copper surface area is 380 ± 23 mm². One ParaGard weighs less than one (1) gram. No component of ParaGard or its packaging contains latex. ParaGard is packaged together with an insertion tube and solid white rod in a Tyvek polyethylene pouch that is then sterilized. A moveable flange on the insertion tube aids in gauging the depth of insertion through the cervical canal and into the uterine cavity. CLINICAL PHARMACOLOGY The contraceptive effectiveness of ParaGard is enhanced by copper continuously released into the uterine cavity. Mechanism(s) by which copper enhances contraceptive efficacy include interference with sperm transport and fertilization of an egg, and possibly prevention of implantation. INDICATIONS AND USAGE ParaGard is indicated for intrauterine contraception for up to 10 years. The pregnancy rate in clinical studies has been less than 1 pregnancy per 100 women each year. Table 1: Percentage of women experiencing an unintended pregnancy during the first year of typical use and first year of perfect use of contraception and the percentage continuing use at the end of the first year: United States % of Women Experiencing an Accidental Pregnancy within the First Year of Use % of Women Continuing Use at One Year 3 Method (1) Typical Use 1 (2) Perfect Use 2 (3) (4) Chance Spermicides Periodic Abstinence Calendar Ovulation Method Sympto-thermal 6 Post-ovulation Cap 7 Parous women Nulliparous women Sponge Parous women Nulliparous women Diaphragm Withdrawal 19 4 Condom 8 Female (Reality) Male Pill Progestin only Combined IUD Progesterone T Copper T 380A LNg % of Women Experiencing % of Women an Accidental Pregnancy within Continuing the First Year of Use Use at One Year 3 Method (1) Typical Use 1 (2) Perfect Use 2 (3) (4) Depo Provera 70 Norplant 88 and Norplant-2 Female sterilization Male sterilization Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 7%. 9 Lactational Amenorrhea Method: LAM is a highly effective temporary method of contraception. 10 Footnotes to Table 1 Source: Trussell J, Contraceptive efficacy. In Hatcher RA, Trussell J, Stewart F, Cates W, Stewart GK, Kowal D, Guest F, Contraceptive Technology: Seventeenth Revised Edition. New York NY: Irvington Publishers, Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. 2. Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any reason. 3. Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year. 4. The percents becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 8%) to represent the percentage who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether.. Foams, creams, gels, vaginal suppositories, and vaginal film. 6. Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases. 7. With spermicidal cream or jelly. 8. Without spermicides. 9. The treatment schedule is one dose within 72 hours after unprotected intercourse, and a second dose 12 hours after the first dose. Preven is the only dedicated product specifically marketed for emergency contraception. The Food and Drug Administration has also declared the following brands of oral contraceptive to be safe and effective for emergency contraception: Ovral (1 dose is 2 white pills), Alesse (1 dose is pink pills), Nordette or Levlen (1 dose is 4 light-orange pills), Lo/Ovral (1 dose is 4 white pills), Triphasil or Tri-Levlen (1 dose is 4 yellow pills). 10. However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced or the baby reaches 6 months of age. CONTRAINDICATIONS ParaGard should not be placed when one or more of the following conditions exist: 1. Pregnancy or suspicion of pregnancy 2. Abnormalities of the uterus resulting in distortion of the uterine cavity 3. Acute pelvic inflammatory disease, or current behavior suggesting a high risk for pelvic inflammatory disease 4. Postpartum endometritis or postabortal endometritis in the past 3 months. Known or suspected uterine or cervical malignancy 6. Genital bleeding of unknown etiology 7. Mucopurulent cervicitis 8. Wilson s disease 9. Allergy to any component of ParaGard 10. A previously placed IUD that has not been removed WARNINGS 1. Intrauterine Pregnancy If intrauterine pregnancy occurs with ParaGard in place and the string is visible, ParaGard should be removed because of the risk of spontaneous abortion, premature delivery, sepsis, septic shock, and, rarely, death. Removal may be followed by pregnancy loss. If the string is not visible, and the woman decides to continue her pregnancy, check if the ParaGard is in her uterus (for example, by ultrasound). If ParaGard is in her uterus, warn her that there is an increased risk of spontaneous abortion and sepsis, septic shock, and rarely, death. 1 In addition, the risk of premature labor and delivery is increased. 1 Human data about risk of birth defects from copper exposure are limited. However, studies have not detected a pattern of abnormalities, and published reports do not suggest a risk that is higher than the baseline risk for birth defects. 1
4 2. Ectopic Pregnancy Women who become pregnant while using ParaGard should be evaluated for ectopic pregnancy. A pregnancy that occurs with ParaGard in place is more likely to be ectopic than a pregnancy in the general population. However, because ParaGard prevents most pregnancies, women who use ParaGard have a lower risk of an ectopic pregnancy than sexually active women who do not use any contraception Pelvic Infection Although pelvic inflammatory disease (PID) in women using IUDs is uncommon, IUDs may be associated with an increased relative risk of PID compared to other forms of contraception and to no contraception. The highest incidence of PID occurs within days following insertion. Therefore, the visit following the first post-insertion menstrual period is an opportunity to assess the patient for infection, as well as to check that the IUD is in place. (See INSTRUCTIONS FOR USE, Continuing Care.) Since pelvic infection is most frequently associated with sexually transmitted organisms, IUDs are not recommended for women at high risk for sexual infection. Prophylactic antibiotics at the time of insertion do not appear to lower the incidence of PID. 4 PID can have serious consequences, such as tubal damage (leading to ectopic pregnancy or infertility), hysterectomy, sepsis, and, rarely, death. It is therefore important to promptly assess and treat any woman who develops signs or symptoms of PID. Guidelines for treatment of PID are available from the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia at or Antibiotics are the mainstay of therapy. Most healthcare professionals also remove the IUD. The significance of actinomyces-like organisms on Papanicolaou smear in an asymptomatic IUD user is unknown, -6 and so this finding alone does not always require IUD removal and treatment. However, because pelvic actinomycosis is a serious infection, a woman who has symptoms of pelvic infection possibly due to actinomyces should be treated and have her IUD removed. 4. Immunocompromise Women with AIDS should not have IUDs inserted unless they are clinically stable on antiretroviral therapy. Limited data suggest that asymptomatic women infected with human immunodeficiency virus may use intrauterine devices. Little is known about the use of IUDs in women who have illnesses causing serious immunocompromise. Therefore these women should be carefully monitored for infection if they choose to use an IUD. The risk of pregnancy should be weighed against the theoretical risk of infection.. Embedment Partial penetration or embedment of ParaGard in the myometrium can make removal difficult. In some cases, surgical removal may be necessary. 6. Perforation Partial or total perforation of the uterine wall or cervix may occur rarely during placement, although it may not be detected until later. Spontaneous migration has also been reported. If perforation does occur, remove ParaGard promptly, since the copper can lead to intraperitoneal adhesions. Intestinal penetration, intestinal obstruction, and/or damage to adjacent organs may result if an IUD is left in the peritoneal cavity. Pre-operative imaging followed by laparoscopy or laparotomy is often required to remove an IUD from the peritoneal cavity. 7. Expulsion Expulsion can occur, usually during the menses and usually in the first few months after insertion. There is an increased risk of expulsion in the nulliparous patient. If unnoticed, an unintended pregnancy could occur. 8. Wilson s Disease Theoretically, ParaGard can exacerbate Wilson s disease, a rare genetic disease affecting copper excretion. PRECAUTIONS Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. 1. Information for patients Before inserting ParaGard discuss the Patient Package Insert with the patient, and give her time to read the information. Discuss any questions she may have concerning ParaGard as well as other methods of contraception. Instruct her to promptly report symptoms of infection, pregnancy, or missing strings. 2. Insertion precautions, continuing care, and removal. (See INSTRUCTIONS FOR USE.) 3. Vaginal bleeding In the 2 largest clinical trials with ParaGard (see ADVERSE REACTIONS, Table 2), menstrual changes were the most common medical reason for discontinuation of ParaGard. Discontinuation rates for pain and bleeding combined are highest in the first year of use and diminish thereafter. The percentage of women who discontinued ParaGard because of bleeding problems or pain during these studies ranged from 11.9% in the first year to 2.2 % in year 9. Women complaining of heavy vaginal bleeding should be evaluated and treated, and may need to discontinue ParaGard. (See ADVERSE REACTIONS.) 4. Vasovagal reactions, including fainting Some women have vasovagal reactions immediately after insertion. Hence, patients should remain supine until feeling well and should be cautious when getting up.. Expulsion following placement after a birth or abortion ParaGard has been placed immediately after delivery, although risk of expulsion may be higher than when ParaGard is placed at times unrelated to delivery. 7 However, unless done immediately postpartum, insertion should be delayed to the second postpartum month because insertion during the first postpartum month (except for immediately after delivery) has been associated with increased risk of perforation. 8 ParaGard can be placed immediately after abortion, although immediate placement has a slightly higher risk of expulsion than placement at other times. 9 Placement after second trimester abortion is associated with a higher risk of expulsion than placement after the first trimester abortion Magnetic resonance imaging (MRI) Limited data suggest that MRI at the level of 1. Tesla is acceptable in women using ParaGard. One study examined the effect of MRI on the CU-7 Intrauterine Copper Contraceptive and Lippes Loop TM intrauterine devices. Neither device moved under the influence of the magnetic field or heated during the spin-echo sequences usually employed for pelvic imaging. 10 An in vitro study did not detect movement or temperature change when ParaGard was subjected to MRI Medical diathermy Theoretically, medical (non-surgical) diathermy (short-wave and microwave heat therapy) in a patient with a metal-containing IUD may cause heat injury to the surrounding tissue. However, a small study of eight women did not detect a significant elevation of intrauterine temperature when diathermy was performed in the presence of a copper IUD Pregnancy ParaGard is contraindicated during pregnancy. (See CONTRAINDICATIONS and WARNINGS.) 9. Nursing mothers Nursing mothers may use ParaGard. No difference has been detected in concentration of copper in human milk before and after insertion of copper IUDs. The literature is conflicting, but limited data suggest that there may be an increased risk of perforation and expulsion if a woman is lactating Pediatric use ParaGard is not indicated before menarche. Safety and efficacy have been established in women over 16 years old. ADVERSE REACTIONS The most serious adverse events associated with intrauterine contraception are discussed in WARNINGS and PRECAUTIONS. These include: Intrauterine pregnancy Septic abortion Ectopic pregnancy Pelvic infection Perforation Embedment Table 2 shows discontinuation rates from two clinical studies by adverse event and year. Table 2. Summary of Rates (No. per 100 Subjects) by Year for Adverse Events Causing Discontinuation Adverse Event Pregnancy Expulsion Bleeding/Pain Other Medical Event No. of Women at Start of Year Year *Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the Population Council (3,36 subjects) and the World Health Organization (1,396 subjects) trials. The following adverse events have also been observed. These are listed alphabetically and not by order of frequency or severity. Anemia Backache Dysmenorrhea Dyspareunia Expulsion, complete or partial Leukorrhea Menstrual flow, prolonged Menstrual spotting Pain and cramping Urticarial allergic skin reaction Vaginitis INSTRUCTIONS FOR USE The placement technique for ParaGard is different from that used for other IUDs. Therefore, the clinician should be familiar with the following instructions. ParaGard may be placed at any time during the cycle when the clinician is reasonably certain the patient is not pregnant. For information about timing of postpartum and postabortion insertions, see PRECAUTIONS. A single ParaGard should be placed at the fundus of the uterine cavity. ParaGard should be removed on or before 10 years from the date of insertion. Before Placement: 1. Make sure that the patient is an appropriate candidate for ParaGard and that she has read the Patient Package Insert. 2. Use of an analgesic before insertion is at the discretion of the patient and the clinician. 3. Establish the size and position of the uterus by pelvic examination. 4. Insert a speculum and cleanse the vagina and cervix with an antiseptic solution.. Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity. 6. Gently insert a sterile sound to measure the depth of the uterine cavity. 32 2
5 7. The uterus should sound to a depth of 6 to 9 cm except when inserting ParaGard immediately post-abortion or post-partum. Insertion of ParaGard into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation. If you encounter cervical stenosis, avoid undue force. Dilators may be helpful in this situation. How to Load and Place ParaGard : Do not bend the arms of ParaGard earlier than minutes before it is to be placed in the uterus. Use aseptic technique when handling ParaGard and the part of the insertion tube that will enter the uterus. STEP 1 Load ParaGard into the insertion tube by folding the two horizontal arms of ParaGard against the stem and push the tips of the arms securely into the inserter tube. If you do not have sterile gloves, you can do STEPS 1 and 2 while ParaGard is in the sterile package. First, place the package face up on a clean surface. Next, open at the bottom end (where arrow says OPEN). Pull the solid white rod partially from the package so it will not interfere with assembly. Place thumb and index finger on top of package on ends of the horizontal arms. Use other hand to push insertion tube against arms of ParaGard (shown by arrow in Fig. 1). This will start bending the T arms. STEP Gently and carefully move the insertion tube upward toward the top of the uterus, until slight resistance is felt. This will ensure placement of the T at the highest possible position within the uterus (Fig. ). Fig. HOLD SOLID WHITE ROD STEP 6 Hold the insertion tube steady and withdraw the solid white rod (Fig. 6). Fig. 6 PUSH TUBE UP Fig. 1 PUSH TUBE STEP 2 Bring the thumb and index finger closer together to continue bending the arms until they are alongside the stem. Use the other hand to withdraw the insertion tube just enough so that the insertion tube can be pushed and rotated onto the tips of the arms. Your goal is to secure the tips of the arms inside the tube (Fig. 2). Insert the arms no further than necessary to insure retention. Introduce the solid white rod into the insertion tube from the bottom, alongside the threads, until it touches the bottom of the ParaGard. STEP 7 Gently and slowly withdraw the insertion tube from the cervical canal. Only the threads should be visible protruding from the cervix. (Fig. 7). Trim the threads so that 3 to 4 cm protrude into the vagina. Note the length of the threads in the patient s records. Fig. 7 Fig. 2 ROTATE AND PUSH TUBE STEP 3 Grasp the insertion tube at the open end of the package; adjust the blue flange so that the distance from the top of the ParaGard (where it protrudes from the inserter) to the blue flange is the same as the uterine depth that you measured with the sound. Rotate the insertion tube so that the horizontal arms of the T and the long axis of the blue flange lie in the same horizontal plane (Fig. 3). Now pass the loaded insertion tube through the cervical canal until ParaGard just touches the fundus of the uterus. The blue flange should be at the cervix in the horizontal plane. Fig. 3 STEP 4 To release the arms of ParaGard, hold the solid white rod steady and withdraw the insertion tube no more than one centimeter. This releases the arms of ParaGard high in the uterine fundus (Fig. 4). Fig. 4 If you suspect that ParaGard is not in the correct position, check placement (with ultrasound, if necessary). If ParaGard is not positioned completely within the uterus, remove it and replace it with a new ParaGard. Do not reinsert an expelled or partially expelled ParaGard. CAUTION Instrumentation of the cervical os may result in vasovagal reactions, including fainting. Have the patient remain supine until she feels well, and have her get up with caution. Continuing Care: Following placement, examine the patient after her first menses to confirm that ParaGard is still in place. You should be able to see or feel only the threads. If ParaGard has been partially or completely expelled, remove it. You can place a new ParaGard if the patient desires and if she is not pregnant. Do not reinsert a used ParaGard. Evaluate the patient promptly if she complains of any of the following: Abdominal or pelvic pain, cramping, or tenderness; malodorous discharge; bleeding; fever A missed period (See WARNINGS, Pelvic Infection, Intrauterine Pregnancy and Ectopic Pregnancy.) The length of the visible threads may change with time. However, no action is needed unless you suspect partial expulsion, perforation, or pregnancy. If you cannot find the threads in the vagina, check that ParaGard is still in the uterus. The threads can retract into the uterus or break, or ParaGard can break, perforate the uterus, or be expelled. Gentle probing of the cavity, radiography, or sonography may be required to locate the IUD. If there is evidence of partial expulsion, perforation, or breakage, remove ParaGard. How to Remove ParaGard Remove ParaGard with forceps, pulling gently on the exposed threads. The arms of ParaGard will fold upwards as it is withdrawn from the uterus. You may immediately insert a new ParaGard if the patient requests it and has no contraindications. Embedment or breakage of ParaGard in the myometrium can make removal difficult. Analgesia, paracervical anesthesia, and cervical dilation may assist in removing an embedded ParaGard. An alligator forceps or other grasping instrument may be helpful. Hysteroscopy may also be helpful. HOW SUPPLIED ParaGard is available in cartons of 1 (one) sterile unit (NDC ). Each ParaGard is packaged together with an insertion tube and solid white rod in a Tyvek polyethylene pouch. 3
6 REFERENCES 1. Tatum HJ, Schmidt FH, Jain AK. Management and outcome of pregnancies associated with the Copper T intrauterine contraceptive device. Am J Obstet Gynecol. 1976;126: Sivin I. Dose- and age-dependent ectopic pregnancy risks with intrauterine contraception. Obstet Gynecol. 1991;78: Franks AL, Beral V, Cates W Jr, Hogue CJR. Contraception and ectopic pregnancy risk. Am J Obstet Gynecol. 1990;163: Grimes DA, Schulz KF. Prophylactic antibiotics for intrauterine device insertion: a meta-analysis of the randomized controlled trials. Contraception. 1999;60: Lippes J. Pelvic actinomycosis: a review and preliminary look at prevalence. Am J Obstet Gynecol. 1999;180: Petitti DB, Yamamoto D, Morgenstern N. Factors associated with actinomyceslike organisms on Papanicolaou smear in users of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983;14: Grimes D, Schulz K, van Vliet H, Stanwood N. Immediate post-partum insertion of intrauterine devices: a Cochrane review. Hum Reprod. 02;17: Cole LP, Edelman DA, Potts DM, Wheeler RG, Laufe LE. Postpartum insertion of modified intrauterine devices. J Reprod Med. 1984;29: Grimes DA, Schulz KF, Stanwood N. Immediate post-abortal insertion of intrauterine devices. (Cochrane Review). In: The Cochrane Library, Issue 2, 03. Oxford: Update Software. 10. Hess T, Stepanow B, Knopp MV. Magnetic resonance imaging: safety of intrauterine contraceptive devices during MR imaging. Eur Radiol. 1996;6: Mark AS, Hricak H. Intrauterine contraceptive devices: MR imaging. Radiology. 1987; 162: Heick A., Espersen T., Pedersen HL, Raahauge J: Is diathermy safe in women with copper-bearing IUDs? Acta Obstet Gynecol Scand. 1991;70(2): Rodrigues da Cunha AC, Dorea JG, Cantuaria AA. Intrauterine device and maternal copper metabolism during lactation. Contraception 01;63:37-9. INFORMATION FOR PATIENTS ParaGard T 380A Intrauterine Copper Contraceptive is used to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases. It is important for you to understand this brochure and discuss it with your healthcare provider before choosing (ParaGard ). You should also learn about other birth control methods that may be an option for you. What is ParaGard? ParaGard is a copper-releasing device that is placed in your uterus to prevent pregnancy for up to 10 years. ParaGard is made of white plastic in the shape of a T. Copper is wrapped around the stem and arms of the T. Two white threads are attached to the stem of the T. The threads are the only part of ParaGard that you can feel when ParaGard is in your uterus. ParaGard and its components do not contain latex. Number of women out of 100 women who are likely to get pregnant over one year Method of birth control No Method Spermicides Periodic abstinence Cap with Spermicides Vaginal Sponge Diaphragm with Spermicides Withdrawal Condom without spermicides (female) Condom without spermicides (male) Oral Contraceptives IUDs, Depo-Provera, implants, sterilization Pregnancies per 100 women over one year to less than 1 Who might use ParaGard? You might choose ParaGard if you need birth control that is very effective need birth control that stops working when you stop using it need birth control that is easy to use Who should not use ParaGard? You should not use ParaGard if you Might be pregnant Have a uterus that is abnormally shaped inside Have a pelvic infection called pelvic inflammatory disease (PID) or have current behavior that puts you at high risk of PID (for example, because you are having sex with several men, or your partner is having sex with other women) Have had an infection in your uterus after a pregnancy or abortion in the past 3 months Have cancer of the uterus or cervix Have unexplained bleeding from your vagina Have an infection in your cervix Have Wilson s disease (a disorder in how the body handles copper) Are allergic to anything in ParaGard Already have an intrauterine contraceptive in your uterus How is ParaGard placed in the uterus? ParaGard is placed in your uterus during an office visit. Your healthcare provider first examines you to find the position of your uterus. Next, he or she will cleanse your vagina and cervix, measure your uterus, and then slide a plastic tube containing ParaGard into your uterus. The tube is removed, leaving ParaGard inside your uterus. Two white threads extend into your vagina. The threads are trimmed so they are just long enough for you to feel with your fingers when doing a self-check. As ParaGard goes in, you may feel cramping or pinching. Some women feel faint, nauseated, or dizzy for a few minutes afterwards. Your healthcare provider may ask you to lie down for a while and to get up slowly. How long can I keep ParaGard in place? You can keep ParaGard in your uterus for up to 10 years. After 10 years, you should have ParaGard removed by your healthcare provider. If you wish and if it is still right for you, you may get a new ParaGard during the same visit. What if I change my mind and want to become pregnant? Your healthcare provider can remove ParaGard at any time. After discontinuation of ParaGard, its contraceptive effect is reversed. How does ParaGard work? Ideas about how ParaGard works include preventing sperm from reaching the egg, preventing sperm from fertilizing the egg, and possibly preventing the egg from attaching (implanting) in the uterus. ParaGard does not stop your ovaries from making an egg (ovulating) each month. How well does ParaGard work? Fewer than 1 in 100 women become pregnant each year while using ParaGard. The table below shows the chance of getting pregnant using different types of birth control. The numbers show typical use, which includes people who don t always use birth control correctly. How do I check that ParaGard is in my uterus? Visit your healthcare provider for a check-up about one month after placement to make sure ParaGard is still in your uterus. You can also check to make sure that ParaGard is still in your uterus by reaching up to the top of your vagina with clean fingers to feel the two threads. Do not pull on the threads. If you cannot feel the threads, ask your healthcare provider to check if ParaGard is in the right place. If you can feel more of ParaGard than just the threads, ParaGard is not in the right place. If you can t see your healthcare provider right away, use an additional birth control method. If ParaGard is in the wrong place, your chances of getting pregnant are increased. It is a good habit for you to check that ParaGard is in place once a month. You may use tampons when you are using ParaGard. 4
7 What if I become pregnant while using ParaGard? If you think you are pregnant, contact your healthcare professional right away. If you are pregnant and ParaGard is in your uterus, you may get a severe infection or shock, have a miscarriage or premature labor and delivery, or even die. Because of these risks, your healthcare provider will recommend that you have ParaGard removed, even though removal may cause miscarriage. If you continue a pregnancy with ParaGard in place, see your healthcare provider regularly. Contact your healthcare provider right away if you get fever, chills, cramping, pain, bleeding, flu-like symptoms, or an unusual, bad smelling vaginal discharge. A pregnancy with ParaGard in place has a greater than usual chance of being ectopic (outside your uterus). Ectopic pregnancy is an emergency that may require surgery. An ectopic pregnancy can cause internal bleeding, infertility, and death. Unusual vaginal bleeding or abdominal pain may be signs of an ectopic pregnancy. Copper in ParaGard does not seem to cause birth defects. What side effects can I expect with ParaGard? The most common side effects of ParaGard are heavier, longer periods and spotting between periods; most of these side effects diminish after 2-3 months. However, if your menstrual flow continues to be heavy or long, or spotting continues, contact your healthcare provider. Infrequently, serious side effects may occur: Pelvic inflammatory disease (PID): Uncommonly, ParaGard and other IUDs are associated with PID. PID is an infection of the uterus, tubes, and nearby organs. PID is most likely to occur in the first days after placement. You have a higher chance of getting PID if you or your partner have sex with more than one person. PID is treated with antibiotics. However, PID can cause serious problems such as infertility, ectopic pregnancy, and chronic pelvic pain. Rarely, PID may even cause death. More serious cases of PID require surgery or a hysterectomy (removal of the uterus). Contact your healthcare provider right away if you have any of the signs of PID: abdominal or pelvic pain, painful sex, unusual or bad smelling vaginal discharge, chills, heavy bleeding, or fever. Difficult removals: Occasionally ParaGard may be hard to remove because it is stuck in the uterus. Surgery may sometimes be needed to remove ParaGard. Perforation: Rarely, ParaGard goes through the wall of the uterus, especially during placement. This is called perforation. If ParaGard perforates the uterus, it should be removed. Surgery may be needed. Perforation can cause infection, scarring, or damage to other organs. If ParaGard perforates the uterus, you are not protected from pregnancy. Expulsion: ParaGard may partially or completely fall out of the uterus. This is called expulsion. Women who have never been pregnant may be more likely to expel ParaGard than women who have been pregnant before. If you think that ParaGard has partly or completely fallen out, use an additional birth control method, such as a condom and call your healthcare provider. You may have other side effects with ParaGard. For example, you may have anemia (low blood count), backache, pain during sex, menstrual cramps, allergic reaction, vaginal infection, vaginal discharge, faintness, or pain. This is not a complete list of possible side effects. If you have questions about a side effect, check with your healthcare provider. When should I call my healthcare provider? Call your healthcare provider if you have any concerns about ParaGard. Be sure to call if you Think you are pregnant Have pelvic pain or pain during sex Have unusual vaginal discharge or genital sores Have unexplained fever Might be exposed to sexually transmitted diseases (STDs) Cannot feel ParaGard s threads or can feel the threads are much longer Can feel any other part of the ParaGard besides the threads Become HIV positive or your partner becomes HIV positive Have severe or prolonged vaginal bleeding Miss a menstrual period General advice about prescription medicines This brochure summarizes the most important information about ParaGard. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider for information about ParaGard that is written for healthcare professionals. Checklist This checklist will help you and your healthcare provider discuss the pros and cons of ParaGard for you. Do you have any of the following conditions? Abnormal Pap smear Abnormalities of the uterus Allergy to copper Anemia or blood clotting problems Bleeding between periods Cancer of the uterus or cervix Fainting attacks Yes No Don t know continued Yes No Don t know Genital sores Heavy menstrual flow HIV or AIDS Infection of the uterus or cervix IUD in place now or in the past More than one sexual partner Pelvic infection (PID) Possible pregnancy Repeated episodes of pelvic infection (PID) Serious infection following a pregnancy or abortion in the past 3 months Severe menstrual cramps Sexual partner who has more than one sexual partner Sexually transmitted disease (STD) such as gonorrhea or chlamydia Wilson s disease Teva Women s Health, Inc. A Subsidiary of Teva Pharmaceuticals USA, Inc. Sellersville, PA Rev. 6/13 P/N PAR-40232
% of Women Experiencing an Accidental Pregnancy within. Depo Provera 0.3 0.3 70 Norplant
 ParaGard T 80A INTRAUTERINE COPPER CONTRACEPTIVE P/N 101700 Rev. /14 Rx only PRESCRIBING INFORMATION Patients should be counseled that this product does not protect against HIV infection (AIDS) and other
ParaGard T 80A INTRAUTERINE COPPER CONTRACEPTIVE P/N 101700 Rev. /14 Rx only PRESCRIBING INFORMATION Patients should be counseled that this product does not protect against HIV infection (AIDS) and other
LIPPES LOOP TRADEMARK. your intrauterine contraceptive
 LIPPES LOOP TRADEMARK your intrauterine contraceptive LIPPES LOOP Patient Information This brochure provides information on the use of In trauterine Contraceptive Devices (lud s). There are other birth
LIPPES LOOP TRADEMARK your intrauterine contraceptive LIPPES LOOP Patient Information This brochure provides information on the use of In trauterine Contraceptive Devices (lud s). There are other birth
Effective long-lasting strategy to prevent unintended pregnancy. The intrauterine system for contraception after abortion.
 Effective long-lasting strategy to prevent unintended pregnancy. The intrauterine system for contraception after abortion. After the abortion I started re-thinking my birth control method. I am looking
Effective long-lasting strategy to prevent unintended pregnancy. The intrauterine system for contraception after abortion. After the abortion I started re-thinking my birth control method. I am looking
Birth Control Options
 1 of 5 6/2/2014 9:46 AM Return to Web version Birth Control Options What is contraception? Contraception means preventing pregnancy, also called birth control. Most people know about options such as birth
1 of 5 6/2/2014 9:46 AM Return to Web version Birth Control Options What is contraception? Contraception means preventing pregnancy, also called birth control. Most people know about options such as birth
Copper intra-uterine device (IUD)
 Oxford University Hospitals NHS Trust Copper intra-uterine device (IUD) Page What is an inter-uterine device? 3 How does it work? 4 Would an IUD be suitable for me? 5 Are there any risks or complications?
Oxford University Hospitals NHS Trust Copper intra-uterine device (IUD) Page What is an inter-uterine device? 3 How does it work? 4 Would an IUD be suitable for me? 5 Are there any risks or complications?
Step-by-Step Insertion Instructions
 Step-by-Step Insertion Instructions Please read these instructions carefully and please visit LILETTAHCP.com for the full Prescribing Information. You may also visit LILETTAhcp.com/video for a video demonstration.
Step-by-Step Insertion Instructions Please read these instructions carefully and please visit LILETTAHCP.com for the full Prescribing Information. You may also visit LILETTAhcp.com/video for a video demonstration.
This is Jaydess. Patient Information. What is Jaydess? How does Jaydess work?
 , Patient Information This is Jaydess We hope that this brochure will answer your questions and concerns about Jaydess. What is Jaydess? Jaydess is an intrauterine device consisting of a hormone capsule
, Patient Information This is Jaydess We hope that this brochure will answer your questions and concerns about Jaydess. What is Jaydess? Jaydess is an intrauterine device consisting of a hormone capsule
All methods of birth control are MUCH SAFER than being pregnant! If 100 women use each method for a year, how many of them get pregnant?
 The Correct Use of Birth Control: In order for any method of birth control to be effective, it must be used correctly ALL THE TIME. This means: One condom every time you have sex One pill every day One
The Correct Use of Birth Control: In order for any method of birth control to be effective, it must be used correctly ALL THE TIME. This means: One condom every time you have sex One pill every day One
Safe & Unsafe. abortion
 Safe & Unsafe Facts About abortion WHAT IS THE DIFFERENCE BETWEEN UNSAFE AND SAFE ABORTION? What is unsafe abortion? Unsafe abortion is a procedure for terminating an unplanned pregnancy either by a person
Safe & Unsafe Facts About abortion WHAT IS THE DIFFERENCE BETWEEN UNSAFE AND SAFE ABORTION? What is unsafe abortion? Unsafe abortion is a procedure for terminating an unplanned pregnancy either by a person
High quality follow up support and care
 High quality follow up support and care How do you think the benefits of high quality follow up care? Client satisfaction Safe and effective continuation of the method What are the tasks involved in routine
High quality follow up support and care How do you think the benefits of high quality follow up care? Client satisfaction Safe and effective continuation of the method What are the tasks involved in routine
Abnormal Uterine Bleeding
 Abnormal Uterine Bleeding WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Abnormal uterine bleeding is one of the most common reasons women see their doctors. It can occur at any age and has
Abnormal Uterine Bleeding WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Abnormal uterine bleeding is one of the most common reasons women see their doctors. It can occur at any age and has
How to Deal with Rumors and Misconceptions about IUDs
 How to Deal with Rumors and Misconceptions about IUDs Rumors are unconfirmed stories that are transferred from one person to another by word of mouth. In general, rumors arise when: an issue or information
How to Deal with Rumors and Misconceptions about IUDs Rumors are unconfirmed stories that are transferred from one person to another by word of mouth. In general, rumors arise when: an issue or information
Uterine fibroids (Leiomyoma)
 Uterine fibroids (Leiomyoma) What are uterine fibroids? Uterine fibroids are fairly common benign (not cancer) growths in the uterus. They occur in about 25 50% of all women. Many women who have fibroids
Uterine fibroids (Leiomyoma) What are uterine fibroids? Uterine fibroids are fairly common benign (not cancer) growths in the uterus. They occur in about 25 50% of all women. Many women who have fibroids
STANDARD APRN PROTOCOL FOR IUD INSERTION: Levonorgestrel (LNG) Releasing Intrauterine System
 STANDARD APRN PROTOCOL FOR IUD INSERTION: Levonorgestrel (LNG) Releasing Intrauterine System DEFINITION The LNG-releasing intrauterine systems (Mirena, Liletta and Skyla ) are on the market. The LNG-releasing
STANDARD APRN PROTOCOL FOR IUD INSERTION: Levonorgestrel (LNG) Releasing Intrauterine System DEFINITION The LNG-releasing intrauterine systems (Mirena, Liletta and Skyla ) are on the market. The LNG-releasing
CONTRACEPTION TYPES CONTRACEPTION LARA SANDERS, RN CHAPTER 7 PAGES 159-174
 CONTRACEPTION LARA SANDERS, RN CHAPTER 7 PAGES 159-174 CONTRACEPTION Voluntary prevention of pregnancy More than half of pregnancies every year are unintended in women younger than 20 years of age May
CONTRACEPTION LARA SANDERS, RN CHAPTER 7 PAGES 159-174 CONTRACEPTION Voluntary prevention of pregnancy More than half of pregnancies every year are unintended in women younger than 20 years of age May
Young Women and Long-Acting Reversible Contraception. Safe, Reliable, and Cost-Effective Birth Control
 ISSUES AT A GLANCE Young Women and Long-Acting Reversible Contraception Safe, Reliable, and Cost-Effective Birth Control In 2012, the American College of Obstetricians and Gynecologists (ACOG) revised
ISSUES AT A GLANCE Young Women and Long-Acting Reversible Contraception Safe, Reliable, and Cost-Effective Birth Control In 2012, the American College of Obstetricians and Gynecologists (ACOG) revised
MHRI IUD Protocol. Migraine with aura Current DVT or PE History of or current breast cancer Active viral hepatitis Severe cirrhosis or liver tumors
 Table of Contents A. Indications B. Contraindications C. Prior to Insertion D. Insertion E. Follow-Up Visit F. Removal G. Re-Insertion H. Complications/Side Effects I. Appendices MHRI IUD Protocol A. Indications
Table of Contents A. Indications B. Contraindications C. Prior to Insertion D. Insertion E. Follow-Up Visit F. Removal G. Re-Insertion H. Complications/Side Effects I. Appendices MHRI IUD Protocol A. Indications
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Birth Control Pills
 Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
Plan B (Levonorgestrel) Tablets, 0.75 mg
 Plan B (Levonorgestrel) Tablets, 0.75 mg Rx only for women age 17 and younger For women age 17 and younger, Plan B is a prescription only emergency contraceptive. Plan B is intended to prevent pregnancy
Plan B (Levonorgestrel) Tablets, 0.75 mg Rx only for women age 17 and younger For women age 17 and younger, Plan B is a prescription only emergency contraceptive. Plan B is intended to prevent pregnancy
Heavy menstrual bleeding and what you can do about it!
 Heavy menstrual bleeding and what you can do about it! The intrauterine system as an alternative to hysterectomy. What is heavy menstrual bleeding? Do I have it? A woman s menstrual periods are considered
Heavy menstrual bleeding and what you can do about it! The intrauterine system as an alternative to hysterectomy. What is heavy menstrual bleeding? Do I have it? A woman s menstrual periods are considered
Copper-Bearing Intrauterine Device
 CHAPTER 9 Copper-Bearing Intrauterine Device This chapter describes primarily the TCu-380A intrauterine device (for the Levonorgestrel Intrauterine Device, see p. 157). Key Points for Providers and Clients
CHAPTER 9 Copper-Bearing Intrauterine Device This chapter describes primarily the TCu-380A intrauterine device (for the Levonorgestrel Intrauterine Device, see p. 157). Key Points for Providers and Clients
BACKGROUNDER CONTRACEPTION
 BACKGROUNDER CONTRACEPTION DID YOU KNOW?» Approximately 85 out of 100 sexually active women who are not using any contraceptive method will get pregnant within one year. 1» Worldwide 38% of women who become
BACKGROUNDER CONTRACEPTION DID YOU KNOW?» Approximately 85 out of 100 sexually active women who are not using any contraceptive method will get pregnant within one year. 1» Worldwide 38% of women who become
Anatomy and Physiology of Human Reproduction. Module 10a
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
Intrauterine Device (IUD) THE FACTS
 Intrauterine Device (IUD) Quick Facts Effectiveness in Preventing Pregnancy: Use: Of 100 women using IUDs for a year, about one may become pregnant. IUDs are better at preventing pregnancy than condoms,
Intrauterine Device (IUD) Quick Facts Effectiveness in Preventing Pregnancy: Use: Of 100 women using IUDs for a year, about one may become pregnant. IUDs are better at preventing pregnancy than condoms,
FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use
 FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use IMPLANON does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this
FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use IMPLANON does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this
MULTILOAD cu 250 / cu 375
 MULTILOAD cu 250 / cu 375 CE 0336 Read all of this leaflet carefully before you decide to have Multiload Radiopaque inserted. This leaflet provides information that may help you in your decision to start
MULTILOAD cu 250 / cu 375 CE 0336 Read all of this leaflet carefully before you decide to have Multiload Radiopaque inserted. This leaflet provides information that may help you in your decision to start
Abnormal Uterine Bleeding FAQ Sheet
 Abnormal Uterine Bleeding FAQ Sheet What is abnormal uterine bleeding? Under normal circumstances, a woman's uterus sheds a limited amount of blood during each menstrual period. Bleeding that occurs between
Abnormal Uterine Bleeding FAQ Sheet What is abnormal uterine bleeding? Under normal circumstances, a woman's uterus sheds a limited amount of blood during each menstrual period. Bleeding that occurs between
the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD your guide to
 your guide to Helping you choose the method of contraception that is best for you IUD IUD the e IUD IU IUD the IUD 2 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put
your guide to Helping you choose the method of contraception that is best for you IUD IUD the e IUD IU IUD the IUD 2 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put
IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. your guide to
 your guide to Helping you choose the method of contraception that is best for you IUD he the the the 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your
your guide to Helping you choose the method of contraception that is best for you IUD he the the the 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your
Clinical Interruption of Pregnancy (Medical/Surgical Abortion)
 Clinical Interruption of Pregnancy (Medical/Surgical Abortion) Approximately one fifth of all pregnancies in the United States end in abortion (Ventura et al., 2009). According to the CDC (2011a), there
Clinical Interruption of Pregnancy (Medical/Surgical Abortion) Approximately one fifth of all pregnancies in the United States end in abortion (Ventura et al., 2009). According to the CDC (2011a), there
IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD the IUD. the IUD. the the IUD. the IUD. the IUD. the IUD. the IUD. the IUD.
 your guide to Helping you choose the method of contraception that is best for you I the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your uterus (womb).
your guide to Helping you choose the method of contraception that is best for you I the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your uterus (womb).
Objective. Indications for IUDs. IUDs 3 types. ParaGard IUD. Mirena IUD. Sonographic Evaluation of Intrauterine Devices (IUDs) Inert
 Sonographic Evaluation of Intrauterine Devices (IUDs) Anna S. Lev-Toaff, MD FACR Department of Radiology Hospital of the University of Pennsylvania Philadelphia, Pennsylvania Leading Edge in Diagnostic
Sonographic Evaluation of Intrauterine Devices (IUDs) Anna S. Lev-Toaff, MD FACR Department of Radiology Hospital of the University of Pennsylvania Philadelphia, Pennsylvania Leading Edge in Diagnostic
For many years, the intrauterine
 Insertion and Removal of Intrauterine Devices BRETT ANDREW JOHNSON, M.D., Methodist Charlton Medical Center, Dallas, Texas The intrauterine device (IUD) is an effective contraceptive for many women. The
Insertion and Removal of Intrauterine Devices BRETT ANDREW JOHNSON, M.D., Methodist Charlton Medical Center, Dallas, Texas The intrauterine device (IUD) is an effective contraceptive for many women. The
A Guide to Hysteroscopy. Patient Education
 A Guide to Hysteroscopy Patient Education QUESTIONS AND ANSWERS ABOUT HYSTEROSCOPY Your doctor has recommended that you have a procedure called a hysteroscopy. Naturally, you may have questions about
A Guide to Hysteroscopy Patient Education QUESTIONS AND ANSWERS ABOUT HYSTEROSCOPY Your doctor has recommended that you have a procedure called a hysteroscopy. Naturally, you may have questions about
implant contraceptiv contraceptive contraceptive raceptiv contraceptive implant contraceptive contraceptive ontraceptive implant ontraceptive im
 your guide to the contraceptive implant Helping you choose the method of contraception that is best for you contra ontraceptive im contraceptive implant mpl ceptive contraceptive contracepti ntraceptive
your guide to the contraceptive implant Helping you choose the method of contraception that is best for you contra ontraceptive im contraceptive implant mpl ceptive contraceptive contracepti ntraceptive
Medical criteria for IUCD s Based on the WHO MEC (2004- Annexure 3) system a woman s eligibility for IUCD insertion falls in 4 categories. These categ
 CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
THE WELL. Intrauterine Contraceptive Devices WOMAN CENTRE
 THE WELL WOMAN CENTRE Intrauterine Contraceptive Devices INTRAUTERINE CONTRACEPTIVE DEVICES How does the Mirena work? How effective is the Mirena? What are the advantages of the Mirena? What are the disadvantages
THE WELL WOMAN CENTRE Intrauterine Contraceptive Devices INTRAUTERINE CONTRACEPTIVE DEVICES How does the Mirena work? How effective is the Mirena? What are the advantages of the Mirena? What are the disadvantages
Birth Control Methods
 page 1 Birth Control Methods Q: What is the best method of birth control (or contraception)? A: There is no best method of birth control. Each method has its pros and cons. All women and men can have control
page 1 Birth Control Methods Q: What is the best method of birth control (or contraception)? A: There is no best method of birth control. Each method has its pros and cons. All women and men can have control
Sterilisation for women and men: what you need to know
 Sterilisation for women and men: what you need to know Published January 2004 by the RCOG Contents Page number Key points 1 About this information 2 What are tubal occlusion and vasectomy? 2 What do I
Sterilisation for women and men: what you need to know Published January 2004 by the RCOG Contents Page number Key points 1 About this information 2 What are tubal occlusion and vasectomy? 2 What do I
Hysterosalpingography
 Scan for mobile link. Hysterosalpingography Hysterosalpingography uses a real-time form of x-ray called fluoroscopy to examine the uterus and fallopian tubes of a woman who is having difficulty becoming
Scan for mobile link. Hysterosalpingography Hysterosalpingography uses a real-time form of x-ray called fluoroscopy to examine the uterus and fallopian tubes of a woman who is having difficulty becoming
CODING GUIDELINES FOR CONTRACEPTIVES. Updated for ICD-10 CM (post October 1, 2015)
 CODING GUIDELINES FOR CONTRACEPTIVES Updated for ICD-10 CM (post October 1, 2015) TABLE OF CONTENTS ICD-10 CM Diagnosis Codes: Encounter for contraception page 2 LARC: Coding for IUD Insertion and Removal
CODING GUIDELINES FOR CONTRACEPTIVES Updated for ICD-10 CM (post October 1, 2015) TABLE OF CONTENTS ICD-10 CM Diagnosis Codes: Encounter for contraception page 2 LARC: Coding for IUD Insertion and Removal
What Are Fertility Awareness Methods?
 CHAPTER 17 Fertility Awareness Methods Key Points for Providers and Clients Fertility awareness methods require partners' cooperation. Couple must be committed to abstaining or using another method on
CHAPTER 17 Fertility Awareness Methods Key Points for Providers and Clients Fertility awareness methods require partners' cooperation. Couple must be committed to abstaining or using another method on
LILETTA CODING UPDATE
 Considerations before submitting claims Considerations after submitting claims LILETTA CODING UPDATE BEGINNING JANUARY 1, 2016, THE HCPCS* CODE (J-CODE) APPROPRIATE FOR LILETTA IS CHANGING FROM J7302 TO
Considerations before submitting claims Considerations after submitting claims LILETTA CODING UPDATE BEGINNING JANUARY 1, 2016, THE HCPCS* CODE (J-CODE) APPROPRIATE FOR LILETTA IS CHANGING FROM J7302 TO
PILLS & RING INFORMATION AND INSTRUCTIONS ON COMBINED HORMONAL CONTRACEPTION INCLUDING BIRTH CONTROL PILLS & NUVA RING
 PILLS & RING INFORMATION AND INSTRUCTIONS ON COMBINED HORMONAL CONTRACEPTION INCLUDING BIRTH CONTROL PILLS & NUVA RING What is combined hormonal contraception? Birth control which contains two hormones
PILLS & RING INFORMATION AND INSTRUCTIONS ON COMBINED HORMONAL CONTRACEPTION INCLUDING BIRTH CONTROL PILLS & NUVA RING What is combined hormonal contraception? Birth control which contains two hormones
CONTRACEPTION LONG-ACTING REVERSIBLE CONTRACEPTIVES LARCS
 6 SAND HILL ROAD SUITE 102 FLEMINGTON, NJ 08822 PHONE 908-782-6700 FAX 908-788-5861 CONTRACEPTION LONG-ACTING REVERSIBLE CONTRACEPTIVES LARCS In 2014 the Academy of Pediatrics recommended that long-acting
6 SAND HILL ROAD SUITE 102 FLEMINGTON, NJ 08822 PHONE 908-782-6700 FAX 908-788-5861 CONTRACEPTION LONG-ACTING REVERSIBLE CONTRACEPTIVES LARCS In 2014 the Academy of Pediatrics recommended that long-acting
Acute pelvic inflammatory disease: tests and treatment
 Acute pelvic inflammatory disease: tests and treatment Information for you Information for you Published August 2010 Published in August 2010 (next review date: 2014) Acute What is pelvic inflammatory
Acute pelvic inflammatory disease: tests and treatment Information for you Information for you Published August 2010 Published in August 2010 (next review date: 2014) Acute What is pelvic inflammatory
EARLY PREGNANCY LOSS A Patient Guide to Treatment
 EARLY PREGNANCY LOSS A Patient Guide to Treatment You have a pregnancy that has stopped growing, or you have started to miscarry and the process has not completed. If so, there are four ways to manage
EARLY PREGNANCY LOSS A Patient Guide to Treatment You have a pregnancy that has stopped growing, or you have started to miscarry and the process has not completed. If so, there are four ways to manage
This Protocol is adapted from the University of Colorado Protocol dated August 26, 2009.
 Protocol for Post-Placental IUD insertion July 14, 2010 This Protocol is adapted from the University of Colorado Protocol dated August 26, 2009. Background Post-placental intrauterine device (IUD) insertion
Protocol for Post-Placental IUD insertion July 14, 2010 This Protocol is adapted from the University of Colorado Protocol dated August 26, 2009. Background Post-placental intrauterine device (IUD) insertion
injections injections injections injections injections injection injections injections injections tions njections injections injections injections
 your guide to contraceptive Helping you choose the method of contraception that is best for you ions ections injection njections injection tions 2 Contraceptive Contraceptive contain a progestogen hormone
your guide to contraceptive Helping you choose the method of contraception that is best for you ions ections injection njections injection tions 2 Contraceptive Contraceptive contain a progestogen hormone
Post-Coital Hormonal Contraception Instructions for Use of Plan B, Plan B One-Step, Next Choice One Dose, My Way, Generic Levonorgestrel and Ella
 Post-Coital Hormonal Contraception Instructions for Use of Plan B, Plan B One-Step, Next Choice One Dose, My Way, Generic Levonorgestrel and Ella Several options for emergency birth control exist for women
Post-Coital Hormonal Contraception Instructions for Use of Plan B, Plan B One-Step, Next Choice One Dose, My Way, Generic Levonorgestrel and Ella Several options for emergency birth control exist for women
progestog progestogen stogen-only pill progestogen progestogen-only pill he progestogen-only pill progestogen-onl progestogen-o the progestogenonly
 your guide to the progestogenonly pill Helping you choose the method of contraception that is best for you the progestogen rogestogen-only the progestogen-only pill progestogen-only pill stogen-only progestoge
your guide to the progestogenonly pill Helping you choose the method of contraception that is best for you the progestogen rogestogen-only the progestogen-only pill progestogen-only pill stogen-only progestoge
Chlamydia THE FACTS. How do people get Chlamydia?
 What is Chlamydia? Chlamydia is a common bacterial infection that is sexually transmitted and often causes no symptoms. If not treated, chlamydia can damage reproductive organs and make it difficult for
What is Chlamydia? Chlamydia is a common bacterial infection that is sexually transmitted and often causes no symptoms. If not treated, chlamydia can damage reproductive organs and make it difficult for
MECHANISM OF ACTION. Fertility Awareness-Based Methods. Victoria H. Jennings, PhD Marcos Arevalo, MD, MPH Deborah Kowal, MA, PA
 Fertility Awareness-Based Methods Victoria H. Jennings, PhD Marcos Arevalo, MD, MPH Deborah Kowal, MA, PA Fertility awareness helps couples understand how to avoid pregnancy or how to become pregnant.
Fertility Awareness-Based Methods Victoria H. Jennings, PhD Marcos Arevalo, MD, MPH Deborah Kowal, MA, PA Fertility awareness helps couples understand how to avoid pregnancy or how to become pregnant.
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Menstruation
 Menstruation WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Menstruation is a normal and healthy part of growing up. The discharge of blood and tissue from the lining of your uterus each
Menstruation WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Menstruation is a normal and healthy part of growing up. The discharge of blood and tissue from the lining of your uterus each
Combination Birth Control Pills - FAQ
 Combination Birth Control Pills - FAQ How does the birth control pill work? prevents ovulation thickens cervical mucus, which makes it hard for sperm to enter the uterus thins the lining of the uterus,
Combination Birth Control Pills - FAQ How does the birth control pill work? prevents ovulation thickens cervical mucus, which makes it hard for sperm to enter the uterus thins the lining of the uterus,
Information for you Abortion care
 Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
About the Uterus. Hysterectomy may be done to treat conditions that affect the uterus. Some reasons a hysterectomy may be needed include:
 Hysterectomy removal of the uterus is a way of treating problems that affect the uterus. Many conditions can be cured with hysterectomy. Because it is major surgery, your doctor may suggest trying other
Hysterectomy removal of the uterus is a way of treating problems that affect the uterus. Many conditions can be cured with hysterectomy. Because it is major surgery, your doctor may suggest trying other
Understanding Fertility
 Understanding Fertility 6 Introduction The word fertile means the ability to become pregnant or to cause pregnancy. Basic knowledge of both the male and female reproductive systems is important for understanding
Understanding Fertility 6 Introduction The word fertile means the ability to become pregnant or to cause pregnancy. Basic knowledge of both the male and female reproductive systems is important for understanding
Gonorrhoea. Looking after your sexual health
 Gonorrhoea Looking after your sexual health 2 Gonorrhoea Gonorrhoea is a bacterial sexually transmitted infection (STI). It can be painful and can cause serious health problems such as infertility in both
Gonorrhoea Looking after your sexual health 2 Gonorrhoea Gonorrhoea is a bacterial sexually transmitted infection (STI). It can be painful and can cause serious health problems such as infertility in both
Menstruation and the Menstrual Cycle
 Menstruation and the Menstrual Cycle Q: What is menstruation? A: Menstruation is a woman s monthly bleeding, also called a period. When you menstruate, your body is shedding the lining of the uterus (womb).
Menstruation and the Menstrual Cycle Q: What is menstruation? A: Menstruation is a woman s monthly bleeding, also called a period. When you menstruate, your body is shedding the lining of the uterus (womb).
PATIENTS SHOULD BE COUNSELED THAT THIS PRODUCT DOES NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES
 Mirena (levonorgestrel-releasing intrauterine system) PATIENTS SHOULD BE COUNSELED THAT THIS PRODUCT DOES NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES Rx only DESCRIPTION
Mirena (levonorgestrel-releasing intrauterine system) PATIENTS SHOULD BE COUNSELED THAT THIS PRODUCT DOES NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES Rx only DESCRIPTION
LILETTA (levonorgestrel-releasing intrauterine system) Initial U.S. Approval: 2015
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LILETTA safely and effectively. See full prescribing information for LILETTA. LILETTA (levonorgestrel-releasing
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LILETTA safely and effectively. See full prescribing information for LILETTA. LILETTA (levonorgestrel-releasing
ency emergency contra-
 your guide to emergency contraception Helping you choose the method of contraception that is best for you emergency cont gency contrace emergency contraception ency emergency contra- emergen mergency contraception
your guide to emergency contraception Helping you choose the method of contraception that is best for you emergency cont gency contrace emergency contraception ency emergency contra- emergen mergency contraception
OVARIAN CYSTS. Types of Ovarian Cysts There are many types of ovarian cysts and these can be categorized into functional and nonfunctional
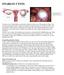 OVARIAN CYSTS Follicular Cyst Ovarian cysts are fluid-filled sacs that form within or on the ovary. The majority of these cysts are functional meaning they usually form during a normal menstrual cycle.
OVARIAN CYSTS Follicular Cyst Ovarian cysts are fluid-filled sacs that form within or on the ovary. The majority of these cysts are functional meaning they usually form during a normal menstrual cycle.
Birth Control Comparison Chart HORMONAL METHODS
 Emergency Contraception Morning After Pill What is it? Emergency Contraception Pills prevent pregnancy after sex. Also called Morning After Pill. Brand name is Plan B. Contains a short burst of a high
Emergency Contraception Morning After Pill What is it? Emergency Contraception Pills prevent pregnancy after sex. Also called Morning After Pill. Brand name is Plan B. Contains a short burst of a high
F REQUENTLY A SKED Q UESTIONS. Birth Control. A: There are many methods of birth con- ents. Making decisions about birth
 F REQUENTLY A SKED Q UESTIONS Birth Control Bear in mind that NO method of birth control prevents pregnancy all of the time. Birth control methods can fail, Methods but you can greatly increase a method
F REQUENTLY A SKED Q UESTIONS Birth Control Bear in mind that NO method of birth control prevents pregnancy all of the time. Birth control methods can fail, Methods but you can greatly increase a method
the abortion pill by David Hager, M.D.
 the abortion pill by David Hager, M.D. A positive pregnancy test is one of the most life-changing moments for a woman. Never is it more important to base your decisions on accurate information. Try to
the abortion pill by David Hager, M.D. A positive pregnancy test is one of the most life-changing moments for a woman. Never is it more important to base your decisions on accurate information. Try to
COMMUNITY HEALTH INFORMATION CARDS. Taking Action for Our Health
 COMMUNITY HEALTH INFORMATION CARDS Taking Action for Our Health COMMUNITY HEALTH INFORMATION CARDS The overall objective of these cards is to: Increase awareness about family planning and postabortion
COMMUNITY HEALTH INFORMATION CARDS Taking Action for Our Health COMMUNITY HEALTH INFORMATION CARDS The overall objective of these cards is to: Increase awareness about family planning and postabortion
Specimen collection and transport for Chlamydia trachomatis and Neisseria gonorrhoeae testing
 Specimen collection and transport for Chlamydia trachomatis and Neisseria gonorrhoeae testing Overview Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections are two of the most common sexually
Specimen collection and transport for Chlamydia trachomatis and Neisseria gonorrhoeae testing Overview Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections are two of the most common sexually
Ask us about LARC. LARC stands for Long Acting Reversible Contraception. Types of LARC are: Contraceptive implant IUS IUD Contraceptive injection
 Ask us about LARC LARC stands for Long Acting Reversible Contraception. Types of LARC are: Contraceptive implant IUS IUD Contraceptive injection visit our website Long Acting Reversible Contraception (LARC)
Ask us about LARC LARC stands for Long Acting Reversible Contraception. Types of LARC are: Contraceptive implant IUS IUD Contraceptive injection visit our website Long Acting Reversible Contraception (LARC)
What s New in Contraception? Evelyn Kieltyka, Maine Family Planning, ekieltyka@mainefamilyplanning.org
 What s New in Contraception? Evelyn Kieltyka, Maine Family Planning, ekieltyka@mainefamilyplanning.org WHAT S NEW IN CONTRACEPTION? EVELYN KIELTYKA, MSN, MS, FNP 1 2 CONTRACEPTIVES FOR TEENS: THE NEW PARADIGM
What s New in Contraception? Evelyn Kieltyka, Maine Family Planning, ekieltyka@mainefamilyplanning.org WHAT S NEW IN CONTRACEPTION? EVELYN KIELTYKA, MSN, MS, FNP 1 2 CONTRACEPTIVES FOR TEENS: THE NEW PARADIGM
IUD training principles 2013
 IUD training principles 2013 Introduction These principles were developed in 2011 2012 by representatives and members of the following organisations: Sexual Health and Family Planning Australia (SHFPA)
IUD training principles 2013 Introduction These principles were developed in 2011 2012 by representatives and members of the following organisations: Sexual Health and Family Planning Australia (SHFPA)
FDA-Approved Patient Labeling
 FDA-Approved Patient Labeling Guide for Using Lo Loestrin Fe WARNING TO WOMEN WHO SMOKE Do not use Lo Loestrin Fe if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious
FDA-Approved Patient Labeling Guide for Using Lo Loestrin Fe WARNING TO WOMEN WHO SMOKE Do not use Lo Loestrin Fe if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious
Glossary. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty.
 Glossary amenorrhea - absence or cessation of menstrual periods. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty. A amenorrhea, secondary - due to some physical
Glossary amenorrhea - absence or cessation of menstrual periods. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty. A amenorrhea, secondary - due to some physical
Treating heavy menstrual bleeding caused by fibroids or polyps
 Treating heavy menstrual bleeding caused by fibroids or polyps With today s medical advances the outlook for successful treatment of fibroids and polyps has never been better. You don t have to live with
Treating heavy menstrual bleeding caused by fibroids or polyps With today s medical advances the outlook for successful treatment of fibroids and polyps has never been better. You don t have to live with
Where to get services How to use this brochure Questions to ask These choices don t work These choices might work Emergency Contraceptive Pills
 Where to get services How to use this brochure Questions to ask These choices don t work These choices might work Emergency Contraceptive Pills Abstinence Birth Control Pills Cervical Barriers Condoms
Where to get services How to use this brochure Questions to ask These choices don t work These choices might work Emergency Contraceptive Pills Abstinence Birth Control Pills Cervical Barriers Condoms
Frequently Asked Questions
 What does the product do? The Stork by Rinovum Women s Health bridges the gap between natural intercourse and more aggressive, costly treatments, like IUI or IVF. The Stork is a home conception kit that
What does the product do? The Stork by Rinovum Women s Health bridges the gap between natural intercourse and more aggressive, costly treatments, like IUI or IVF. The Stork is a home conception kit that
Department of Gynaecology Early medically induced termination of pregnancy. Information for patients
 Department of Gynaecology Early medically induced termination of pregnancy Information for patients Medically induced termination of pregnancy In this procedure the termination of pregnancy is brought
Department of Gynaecology Early medically induced termination of pregnancy Information for patients Medically induced termination of pregnancy In this procedure the termination of pregnancy is brought
Family Planning for Women and Couples following Fistula Repair
 Family Planning for Women and Couples following Fistula Repair Fistula Care at EngenderHealth 440 Ninth Avenue, 13th Floor New York, NY, USA 10001 Tel: 212-561-8000 E-mail: fistulacare@engenderhealth.org
Family Planning for Women and Couples following Fistula Repair Fistula Care at EngenderHealth 440 Ninth Avenue, 13th Floor New York, NY, USA 10001 Tel: 212-561-8000 E-mail: fistulacare@engenderhealth.org
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Endometriosis
 Endometriosis WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 The lining of the uterus is called the endometrium. Sometimes, endometrial tissue grows elsewhere in the body. When this happens
Endometriosis WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 The lining of the uterus is called the endometrium. Sometimes, endometrial tissue grows elsewhere in the body. When this happens
abortion abortion abortion abortion abortion abortion abortion on abortio abortion ortion abortion abortion abortion abortion abortio
 Abortion Your questions answered abortio bortion ion ortion on abortio 2 Are you pregnant but not sure you want to have the baby? Do you need more information about your pregnancy choices? Unplanned pregnancy
Abortion Your questions answered abortio bortion ion ortion on abortio 2 Are you pregnant but not sure you want to have the baby? Do you need more information about your pregnancy choices? Unplanned pregnancy
TERMINATION OF PREGNANCY- MEDICAL
 TERMINATION OF PREGNANCY- MEDICAL Information Leaflet Your Health. Our Priority. Page 2 of 8 You have been offered a medical termination of pregnancy using mifepristone. You will have been given some verbal
TERMINATION OF PREGNANCY- MEDICAL Information Leaflet Your Health. Our Priority. Page 2 of 8 You have been offered a medical termination of pregnancy using mifepristone. You will have been given some verbal
Out-patient hysteroscopy. Information for patients
 Out-patient hysteroscopy Information for patients Important If there is any chance you may be pregnant please tell a member of the team immediately. We will not be able to perform a hysteroscopy if you
Out-patient hysteroscopy Information for patients Important If there is any chance you may be pregnant please tell a member of the team immediately. We will not be able to perform a hysteroscopy if you
after you ve had you after you ve had your baby after you ve after you ve had your baby fter you ve had your baby after contraceptive choices
 your guide to contraceptive choices after you ve had your baby Helping you choose the method of contraception that is best for you after you ve had you ve had your bab after you ve had your baby after
your guide to contraceptive choices after you ve had your baby Helping you choose the method of contraception that is best for you after you ve had you ve had your bab after you ve had your baby after
Emergency Contraceptive Pills
 There are many different forms of birth control available in Canada, many of which we will discuss in this booklet. There is no way to determine which is the best form of birth control, because each person
There are many different forms of birth control available in Canada, many of which we will discuss in this booklet. There is no way to determine which is the best form of birth control, because each person
Let s Learn the Basics about Cervical Cancer
 A Publication of the National Center for Farmworker Health October-December 2014 Let s Learn the Basics about Cervical Cancer What is cancer? The body is made up of millions and millions of living cells.
A Publication of the National Center for Farmworker Health October-December 2014 Let s Learn the Basics about Cervical Cancer What is cancer? The body is made up of millions and millions of living cells.
abortion your questions answered
 abortion your questions answered About Marie Stopes International Marie Stopes International is a specialist reproductive healthcare organisation and a registered charity working in both the UK and overseas.
abortion your questions answered About Marie Stopes International Marie Stopes International is a specialist reproductive healthcare organisation and a registered charity working in both the UK and overseas.
Lippes Loop intrauterine device left in the uterus for 50 years. Case report
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Lippes Loop intrauterine device left in the uterus for 50 years Case report Background.The first Lippes Loop intrauterine device was distributed in 1962. It was a
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Lippes Loop intrauterine device left in the uterus for 50 years Case report Background.The first Lippes Loop intrauterine device was distributed in 1962. It was a
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MIRENA safely and effectively. See full prescribing information for MIRENA. MIRENA (levonorgestrel-releasing
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MIRENA safely and effectively. See full prescribing information for MIRENA. MIRENA (levonorgestrel-releasing
Summa Health System. A Woman s Guide to Hysterectomy
 Summa Health System A Woman s Guide to Hysterectomy Hysterectomy A hysterectomy is a surgical procedure to remove a woman s uterus (womb). The uterus is the organ which shelters and nourishes a baby during
Summa Health System A Woman s Guide to Hysterectomy Hysterectomy A hysterectomy is a surgical procedure to remove a woman s uterus (womb). The uterus is the organ which shelters and nourishes a baby during
Migration of an intrauterine contraceptive device to the sigmoid colon: a case report
 The European Journal of Contraception and Reproductive Health Care 2003;8:229 232 Case Report Migration of an intrauterine contraceptive device to the sigmoid colon: a case report Ü. S. nceboz, H. T. Özçakir,
The European Journal of Contraception and Reproductive Health Care 2003;8:229 232 Case Report Migration of an intrauterine contraceptive device to the sigmoid colon: a case report Ü. S. nceboz, H. T. Özçakir,
Ovarian Cyst. Homoeopathy Clinic. Introduction. Types of Ovarian Cysts. Contents. Case Reports. 21 August 2002
 Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Leader's Resource. Note: Both men and women can have an STD without physical symptoms.
 Leader's Resource Information on Sexually Transmitted Diseases (STDs) Signs and Symptoms of STDs Note: Both men and women can have an STD without physical symptoms. Any of the following can indicate to
Leader's Resource Information on Sexually Transmitted Diseases (STDs) Signs and Symptoms of STDs Note: Both men and women can have an STD without physical symptoms. Any of the following can indicate to
Trichomonas vaginalis. Looking after your sexual health
 Trichomonas vaginalis Looking after your sexual health 2 3 Trichomonas vaginalis Trichomonas vaginalis is a sexually transmitted infection (STI). It is sometimes referred to as trichomonas or trichomoniasis,
Trichomonas vaginalis Looking after your sexual health 2 3 Trichomonas vaginalis Trichomonas vaginalis is a sexually transmitted infection (STI). It is sometimes referred to as trichomonas or trichomoniasis,
Abigail R. Proffer, M.D. October 4, 2013
 Abigail R. Proffer, M.D. October 4, 2013 Topics Human Papillomavirus (HPV) Vaccines Pap smears Colposcopy Contraception Polycystic Ovary Syndrome (PCOS) Can I get pregnant? Miscarriage Abnormal Uterine
Abigail R. Proffer, M.D. October 4, 2013 Topics Human Papillomavirus (HPV) Vaccines Pap smears Colposcopy Contraception Polycystic Ovary Syndrome (PCOS) Can I get pregnant? Miscarriage Abnormal Uterine
contraception contraception contraception contracepti contraception contraception contraception aception contraception contraception contraception
 your guide to Helping you choose the method of that is best for you raception aception contracept contracepti contracep contracepti Your guide to This leaflet shows the available contraceptive methods,
your guide to Helping you choose the method of that is best for you raception aception contracept contracepti contracep contracepti Your guide to This leaflet shows the available contraceptive methods,
WHAT YOU SHOULD KNOW ABOUT ABORTION
 WHAT YOU SHOULD KNOW ABOUT ABORTION It is the public policy of the state of Idaho to prefer live childbirth over abortion: "The Supreme Court of the United States having held that the states have a "profound
WHAT YOU SHOULD KNOW ABOUT ABORTION It is the public policy of the state of Idaho to prefer live childbirth over abortion: "The Supreme Court of the United States having held that the states have a "profound
Facts for Women Termination of pregnancy, abortion, or miscarriage management
 Patient Education Facts for Women Termination of pregnancy, abortion, or miscarriage management This handout answers common questions about miscarriage management and the termination of a pregnancy, also
Patient Education Facts for Women Termination of pregnancy, abortion, or miscarriage management This handout answers common questions about miscarriage management and the termination of a pregnancy, also
Diseases that can be spread during sex
 Diseases that can be spread during sex Did you know... over 65 million people in the United States have a chronic, incurable sexually transmitted disease (STD)? and that every year another 19 million persons
Diseases that can be spread during sex Did you know... over 65 million people in the United States have a chronic, incurable sexually transmitted disease (STD)? and that every year another 19 million persons
Immunization Healthcare Branch. Human Papillomavirus Vaccination Program Questions and Answers. Prepared by
 Immunization Healthcare Branch Human Papillomavirus Vaccination Program Questions and Answers Prepared by Immunization Healthcare Branch (IHB), Defense Health Agency Last Updated: 02 Jan 14 www.vaccines.mil
Immunization Healthcare Branch Human Papillomavirus Vaccination Program Questions and Answers Prepared by Immunization Healthcare Branch (IHB), Defense Health Agency Last Updated: 02 Jan 14 www.vaccines.mil
