BOVINE IN-VITRO EMBRYO PRODUCTION AND ITS CONTRIBUTION TOWARDS IMPROVED FOOD SECURITY IN KENYA. Henry Mutembei
|
|
|
- Karin Stephens
- 8 years ago
- Views:
Transcription
1 BOVINE IN-VITRO EMBRYO PRODUCTION AND ITS CONTRIBUTION TOWARDS IMPROVED FOOD SECURITY IN KENYA Mutembei HM 1*, Tsuma VT 1, Muasa BT 1, Muraya J 1 and RM Erastus 1 Henry Mutembei *Corresponding author hmutembei@uonbi.ac.ke 1 Clinical Studies Department, University of Nairobi, P.O. Box , Nairobi, Kenya. 9722
2 ABSTRACT The Boran breed is mainly kept by pastoralist communities as a source of both milk and beef, and by commercial beef ranches mainly for beef production. Although this breed Boran may seemingly be low valued, it can be raised to higher reproductive potential using current reproductive technologies such as in-vitro embryo production and embryo transfer. In this author s literature search there was lack of locally optimized procedures for boosting the reproductive potential of the Boran cow using such technologies. This paper discusses simplified methods used for in-vitro embryo production and embryo transfer that have been tested and optimized for the Kenyan Boran cows as described in text. This article provides the reader with technical procedures, the outcomes and the challenges experienced during use of the technologies. The results of the reported in vitro embryo production obtained an oocyte maturation rate of over 90%, over 70% cleavage rate and an embryo output of 30-50% blastocyst rate. Embryo transfer had a conception rate of 45-50%. All the 17 calves born out of this work have since attained puberty without any abnormalities. This research was the first to produce a test tube calf in Kenya and built the capacity of 57 staff on these technical procedures within East Africa. Utilization of the in vitro embryo production technology in Kenya can be used to do value addition to indigenous cows and improve household incomes and food security. This avenue can be used to raise household revenues and attract many more farmers to engage in farming leading to improved national economic and food security. Kenya can utilize reproductive technologies in the dairy sector due to the massive potential benefits to revolutionize the sector for enhanced food security by increasing cattle productivity. The paper highlights practical ways of applying the technologies discussed therein to help raise the economy of the Kenyan livestock farmers and boost their food security. Key words: Reproductive Technologies, Boran cattle, Kenya 9723
3 INTRODUCTION The world food crisis and rise in demand for food of animal origin, competitive global markets for such foods and the negative effects of climatic change, especially in the marginal tropical areas [1], demand that innovative strategies and interventions be urgently employed [2]. The dairy sector in developing countries, especially in low input production systems, has the potential of providing cost effective animal protein requirements. To do this, such units need to be efficient, and the starting point is using appropriate genotypes. Many attempts aimed at improving the productivity of indigenous dairy cattle in developing countries through crossbreeding with temperate breeds have generally not been successful [3]. Many studies have shown that temperate dairy breeds best combine with local tropical breeds at 50% of each breed composition (F1 cross). The F1 is well adapted and produces reasonable amount of milk under the local conditions [3, 4]. Aggressive promotion of a few exotic cattle breeds in sub-saharan Africa (SSA) has resulted in cattle genotypes that are not only poorly adapted to the production systems but are also commercially inefficient [5]. In order to meet the increasing demand for appropriate improved dairy cattle genotypes in the marginal dairy systems in the Eastern African region, more efficient production and delivery of improved germplasm is needed. Breeding strategies that utilize a combination of emerging reproductive technologies, especially the in-vitro embryo production (IVEP), ovum pick up (OPU) and semen sexing, offer prudent possible options [6]. For example, indigenous breeds such as the Boran that are inferior commercial milk producers would be valuable as oocyte donors as well as surrogates for crossbred embryos under IVEP-based systems. Up to 80% of Kenya s landmass is arid or semi-arid (ASAL). The pastoralists who live in the ASAL depend heavily on livestock as their most viable source of livelihood. Among the Zebu breeds of livestock kept in these areas, the most commercially viable breed in Kenya is the Boran. Conventional embryo transfer technology is based on the superovulation of a highquality donor animal and the subsequent recovery of embryos by flushing the uterus a week after breeding to the bull of choice [7, 8, 9]. In cattle, this technology is well established. The technology can be advanced following further understanding of the reproductive physiology of the cow by the practitioners of the Embryo Transfer (ET) in Kenya so as to meet the demand for better bovine genetic improvement within Eastern African countries. An alternative to avoid the more expensive conventional superovulation procedures during ET is in-vitro embryo production (IVEP) [10]. This technology allows for cheaper production of a predictable supply of embryos from ovaries of live or slaughtered cows. From live selected animals, repeated recovery of primary oocytes using ovum pick up procedures (OPU) is possible [7, 8]. To date in Kenya, our team 9724
4 has shown that IVEP and OPU technique have given considerable success in cattle, achieving a success rate ranging from 30-50% for development of pre-implantation stage embryo in-vitro and conception rates of up to 50%. The IVEP technology does not only offer optimization of high-quality dams (mothers), but also allows the preservation and rapid multiplication of genetically superior cows by making embryos available for sexing, cloning and nuclear transfer [11]. Use of such advances in technologies can be utilized to turn around the economic gains of farmers. Utilization of the IVEP technology in Kenya can be used t undertake value addition to indigenous cows and improve household incomes and food security. Farmers keeping low-grade cows can enter into commercial contracts to provide these cows as surrogate recipients for production of heifer calves. In the process, owners of such cows will have a valued product to sell for income and be left with milk for use by family members. This avenue can be used to raise household revenues and attract many more farmers to engage in farming, leading to improved national economic and food security. Kenya can utilize reproductive technologies in the dairy sector due to their massive potential benefits to revolutionize the sector for enhanced food security by increasing cattle productivity. This paper highlights the practical ways of applying IVEP technologies to help turn around the economy of the Kenyan livestock farmers and boost food security. It discusses the technical aspects of the procedures involved in the in-vitro production of bovine embryos and embryo transfer, with special reference to the application of the techniques in Kenya. MATERIALS AND METHODS Production of embryos in the laboratory involved four major steps: 1) Ovary collection, 2; Oocyte maturation, 3) in vitro fertilization and 4) Embryo culture. In brief, ova were collected from live Boran cows in Kapiti Ranch using ovum pick-up (OPU) technology and transported to the laboratory in a warm saline solution (supplemented with 0.1g/L streptomycin). In some cases, ovaries from slaughtered animals were used. Pre-slaughter body condition scores (BCS) were assessed prior to slaughter at the holding area. Ova collected from cows were delivered to the laboratory within 4hrs of collection. Cumulus oocyte complexes (COCs) were obtained by aspiration of 3 to 8 mm follicles using a 21-gauge needle attached to a 10 ml syringe and manipulated in Tyrodes Albumin Pyruvate (TALP) Hepes medium supplemented with 0.4% bovine serum albumin. The COCs were graded morphologically according to oocyte cytoplasm aspect and morphology of overlying zona pelucida cumulus cell layers; only grade A and B COCs were used for the work. These were COCs with compact cumulus cell layers and oocytes with homogenous (grade A) or slightly heterogeneous (grade B) cytoplasm. Standard in vtro oocyte maturation, fertilization and embryo culture procedures were conducted. Synchronization of the recipient cows was achieved by 9725
5 knocking off the corpous luteum using cloprostenol 500ug (2ml of lutealyase) and embryo transfer was done at day seven using in vitro produced blastocysts. All chemicals used were from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise stated. RESULTS Outputs of IVEP From this IVEP work, data collected on the donor cows and results obtained in the laboratory at various levels are shown in Table 1-2. The BCS of the sampled animals ranged from 1 to 5. Seventy four (74) % of them had BCS of (Figure 1) Body condition score Number of cows Figure 1: Distribution of pre-slaughter body condition scores of the sampled animals Forty two (42) % of the cows sampled at slaughter were pregnant at different stages of gestation. Overall, 163 cows were sampled from the abattoirs and ovum pick up conducted on 26 cows. At slaughter 308 ovaries containing follicles were used for the study. Some ovaries were discarded on the basis of absence of follicles and presence of cysts. Three thousand, one hundred and ten (3,110) follicles were counted from the ovaries and 2,368 COC s were graded into grade A and B while 476 COC s were graded into grade C or discards. Two hundred sixty six (8.55%) of COC s were lost during aspiration and 1658 COCs were taken through the IVEP process. The process had conception rate of 46.5% and produced 17 healthy calves. Fifty seven technical staff members have been trained on the same technologies across East Africa - mainly from Kenya, Uganda and Ethiopia. 9726
6 DISCUSSION Review of the Technical aspect of the Technology In-vitro Embryo Production (IVEP) Media and Stock Solutions Media and stock solutions for in vitro embryo production need to be sterile, accurately done. The use of a Laminar flow Cabinet for sterility is indispensable to avoid any contamination. Using analytical balance with readability of at least 10 µg and accuracy of ±0.1 µg is very important. All components of the medium must be of the highest grade possible. To achieve good standards on specifications of media and stock solutions, the following important factors need consideration. i) Water A Millipore-Q system or its equivalent should be used to produce high quality pyrogenfree water with a resistance of >18 megahms-cm. This study utilized this kind of water. ii) Glassware Glassware for media preparation should be designated solely for this purpose. Glassware to be used must be sterile and pass through the standard tissue culture cleaning procedure. This study used glassware that was double cleaned and sterilized through a glassware autoclaving system. iii) Disposable Materials Disposable supplies such as Petri dishes, acrodisc filters, centrifuge tubes for media storage and pipette tips used during IVEP make the IVEP work seem expensive but the use of disposable materials is more beneficial and convenient in terms of eliminating contamination and ensuring purity and safety of the prepared media and the produced embryos. This study used disposable materials purchased from Nutricell Company in Brazil and all were stored within clean cabinets of the laboratory. 9727
7 iv) Filtration The use of a 0.22µm filter is recommended, considering that the size of the smallest bacteria is 0.3µm. In this study, use of 0.22µm filter used and the first few drops were discarded to remove any contaminants from the filter and the container. The remaining volume was filtered again and stored in a refrigerator. v) Embryo Culture and Working Area The culture media used for the growth of oocytes and embryos is highly prone to contamination. The % humidity and 38.5 o C temperature conditions for growth within the incubator are some of the factors that enhance the growth of any contaminating organisms present within the culture media. Sources of contamination could be air borne and/or from surfaces of the working area, or from any materials used in the preparation of the culture media. The following measures were implemented in this study to avoid contamination of the cultured oocytes and embryos. a) Working space was separated, with various designated areas; dirty benches for aspirations, clean benches for media preparation and sterile spaces for oocytes and embryo handling and manipulations. The interior laboratory space containing the incubators was out-of-bounds for all external people, who were only allowed to view the procedures through a transparent glass wall. b) Workers covered their hair, mouth and nose, and used disposable hoods whenever conducting oocyte and embryo culture techniques. c) Disinfectant such as 70% alcohol was applied to the working surfaces before the preparation of the culture media and prior to any embryo production techniques. d) Hands had to be washed first with clean water and sterilized with 70% alcohol before handling the materials for media preparation and embryo production. vi) Storage and Maintenance of Media and Reagents In order to improve on IVEP results, this study ensured the expiry date of all reagents and media were noted and cross-checked at all times prior to use. Stock solutions and the media were kept in their manufacturers prescribed storage conditions. To maintain the viability and efficacy of media and reagents, preparation of well labeled aliquots was done to avoid frequent open access to the stock solutions to eliminate any possibility of contamination. Storage containers were sealed securely to avoid any moisture contamination that tends to alter the ph or osmolality of the media. In our system, a folder/ file with all standard preparation procedures and also with documents showing important information on preparation and expiry dates for all media and reagents was available within the laboratory. Collection of Ovaries Pre-slaughter record parameters were obtained prior to slaughter and or OPU to track genetics of embryos produced. Records were entered into a well-designed data collection form. Ovaries should be collected within 10 minutes after the slaughter of the animals, and kept in a sealed container containing physiological saline (0.9% 9728
8 sodium chloride with either 1ml/L Gentamycin or 100 µm/ml Streptomycin and 100 IU/ml Penicillin), at a temperature ranging from C. Careful observation of the temperature of the saline is important to maintain the viability of the ovaries during the period of collection. In our system, the prepared saline was left to stand at 38 C overnight and the gentamycin was added in the morning of collection before transfer to a thermos flask. Temperature check-ups were done regularly at the slaughterhouse. The time interval between animal slaughter and oocyte recovery from the ovaries, and the temperature of the holding medium is also important. In general, the time spent between ovary collection and oocyte recovery should be within 3-4 hours and a C temperature range of the physiological saline should be maintained. However, in cases where the slaughterhouse is far distant from the laboratory, a decrease in the temperature is recommended, although the temperature should still be kept above 28 C to maintain the development potential of the oocytes. Prolonging the period of ovary collection may significantly affect the viability of the oocytes for IVEP. It is recommended that oocytes should be collected from the ovaries at most within a 6-hour period. Beyond six hours, development competence of the oocytes is greatly compromised. In our system, the slaughterhouses were located 10kms from the laboratory and the time taken between ovary collection and delivery to the laboratory was 3-4 hrs. Oocyte Recovery Before oocyte recovery, it is necessary to wash the ovaries in fresh sterile physiological saline (without antibiotics) to remove any contaminants. Thereafter, briefly rinse the ovary in 70% ethyl alcohol to eliminate surface organisms before beginning the oocyte recovery procedure. The ovaries are then dried lightly with sterile paper towels and primary oocytes are recovered from 1-6 mm vesicular follicles. It was important to avoid recovery of oocytes from big follicles (beyond 6 mm) because they contain secretions that tend to cause jell formation in the aspirates. In such cases the retrieval of oocytes during searching is negatively affected. Recovery of the oocytes from the ovaries may be done by either of the following techniques: Oocyte aspiration using needles Slicing the ovaries using scalpel blade Follicle dissection from ovaries Ovum pick-up from live animals For the ovaries collected from slaughtered animals, the aspiration method was commonly employed because of the convenience associated with it. Aspiration of oocytes was done using a needle attached to a 10-ml syringe. To avoid disruption of the surrounding cumulus cells, an 18-gauge needle was used. Possible toxicity associated with syringes containing rubber plungers and siloxane lubricants was avoided by using only sterilized syringes with plastic plungers. One of the difficulties associated with the aspiration approach is the fact that oocytes may only be retrieved from about 60% of the punctured follicles. To minimize this 9729
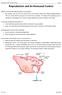
9 effect, it was necessary to prime the needle and syringe with approximately ml of aspiration medium. This is to provide the initial fluid that helps ensure the retrieved oocytes are within the fluid dispended from the syringe. The aspiration medium used was Tyrosine-albumin-pyruvate (TALP)-HEPES (4-(2- hydroxyethyl)-1-piperazineethanesulfonic acid) but also modified phosphate-buffered saline was used occasionally, especially, during training sessions. Aspiration medium can be prepared once, stored in a refrigerator and made available for one week's activity. However, it is very important to pre-warm the aspiration medium up to 38 C before use. After aspirations are made from follicles of each ovary, the contents in the aspirating syringe are slowly transferred into a sterile centrifuge tube that is pre-moistened with TALP-HEPES and the oocytes are allowed to settle at the bottom of the tube that is placed into a rack within a warm water bath set at 38 o C. The transfer of fluids containing the oocytes into the tube is done slowly with minimal disruption of the cumulus-oocyte complex (COC). When this is done from the last ovary of the day, the precipitate containing the oocytes that had settled at the bottom of the tube are selectively picked using a sterilized Pasteur pipette and transferred into sterile Petri dishes containing TALP-HEPES for subsequent searching, grading and counting of recovered COCs. The recovery work of COCs should be done in a sterile environment within room temperature of 25 C. Sometimes slicing of the ovaries is done in order to enhance retrieval of numbers of COCs. However, the method is more tedious and requires longer period of retrieval and is thus not recommended whenever manipulating a large number of ovaries because the extended period of oocyte retrieval would negatively affect oocyte competence. In this case, the method was used only when few ovaries were collected. Follicle dissection is also a method that can be used. This method is effective in retrieving good-quality oocytes as reviewed extensively by Gordon [10]. However, it also increases the time needed for the retrieval process. In this study, this was only used during research. Ovum pick up can also be done from live cows using ultrasound guided follicular aspiration through a needle passed trans-rectally as shown demonstrated in Fig
10 Figure 2: Photographic demonstration of the OPU procedure; donor cows selected (A), then ultrasound device inserted into the vagina (B), needle put into the device (C), the expert picks the ovary towards the intravaginal ultrasound device (D), the follicles are viewed through the ultrasound monitor (E), the follicles are aspirated into a tube (F), the follicles are poured into a petri dish (G), the follicles are searched (H), the follicles are recovered into another petri-dish (I), grade A COC (J), grade A COCs are loaded into thermos flask (K), the COCs are delivered to the laboratory (L) This technique is used for repeated collection of COCs from top grade cows. The method was also used within the IVEP system reported here. Selection of Oocytes for in Vitro Maturation Once the COCSs are located within the Petri dish during searching under a microscope at X10 objective, the COCs were picked using micropipettes with a bore diameter of about 200 µm and transferred into a dish of fresh pre-warmed TALP-Hepes washing medium. The plastic tip used in picking the COCs must have a bore diameter wide enough to avoid disruption of the cumulus cells surrounding the oocytes. The presence of cumulus cells surrounding the immature oocytes is a prerequisite both for successful maturation of the oocyte and for embryo competence. 9731
11 Only oocytes with compact multilayered cumulus investment and evenly granulated cytoplasm should be selected for in-vitro maturation. It should be noted, however, that the aspirated COCs may have a differences in their appearances [11]. Some will be denuded (with no cumulus cells attached to the zona pelucida), others being partly denuded (with one sided swollen cumulus cells, and others with an already expanded or spider-like cumulus cells. Others will also have uneven cytoplasm. Not all oocytes should be used for IVEP because they lack the potential to undergo normal maturation and will eventually end up as degenerated COCs after in-vitro fertilization. Further details on IVEP of Boran cows were described by Muasa in 2010, which contains a chapter providing guidelines on COC grading for IVEP [11]. In Vitro Maturation of COCs Prior to transfer to an in vitro maturation (IVM) medium, selected COCs must be washed 2-4 times in fresh pre-warmed TALP-Hepes medium and finally washed in invitro maturation medium. The medium used for in-vitro maturation (IVM) of oocytes varies among laboratories. It should be considered that the culture medium employed in IVM may affect the proportion of oocytes that reach metaphase II and become capable of undergoing fertilization. It also influences subsequent embryo development [12]. The culture media employed in maturation of oocytes can be broadly divided into simple and complex. Simple media are usually bicarbonate-buffered containing basic physiological saline with pyruvate, lactate and glucose. The main difference between various types of simple media is in their ion concentration and energy sources. The media are generally supplemented with serum or albumin, with trace amounts of antibiotics added (penicillin, streptomycin, gentamicin). Complex media, on the other hand, contain, in addition to the components of the simple media, amino acids, vitamins, purines and other substances, mainly at levels they are found in serum. Fixed nitrogen is present as free amino acids. This study used a widely-used complex media for IVM of cattle oocytes: Tissue Culture Medium 199 (TAC-199) with Earle's salt, L-Glutamine and 25 mm HEPES supplemented with 10% heat inactivated fetal calf serum (this can also be calf serum, steer serum or serum from cow in estrus), and supplemented with 1ug/ml of follicle stimulating hormone (FSH), Luteinizing Hormone (LH) and estradiol. Other IVM media can be used such as Synthetic Bovine Oviductal Fluid medium and others. All others, however, require further supplements in order to promote maturation. During IVM, extensive redistribution of intracellular organelles occurs. In addition to extensive cumulus expansion that occurs during maturation of oocytes during IVM, the mitochondria migrate to occupy a perinuclear location, and the cortical granules migrate outward to lie just beneath the vitelline membrane of the oocyte. At all times, the critical requirement of successful oocyte maturation is the capability to undergo normal embryonic development after sperm penetration and fertilization. An extensive description on how to evaluate and detect matured oocytes after IVM has been 9732
12 described in depth by Muasa [11]. However, the following are the important considerations one must take into account during IVM procedure: a) Water quality Water is the major constituent of any IVM medium and due to the presence of several basic contaminants such as ionized and non-ionized solids and gases, particulate matter, microbials and pyrogens, the use of ultrapure water is highly recommended. b) Buffering Systems and Osmolality The ph of the IVM media changes drastically whenever exposed to non-co2 controlled atmosphere. Hepes or phosphate-buffered media for short-term work with oocytes and embryos does not require a carbon-dioxide controlled gas phase to maintain a relatively constant ph. For this reason, these media should be commonly used for washing, and for storage of oocytes and embryos outside the incubator. The optimum range of osmolality of the IVM medium should range between 275 and 285 mosmol. If the osmolality of the IVM medium is not within this range, it should be discarded and a new one prepared. In some instances, the measured osmolality of a medium is less than the calculated value. This can be attributed to the incomplete dissociation of ions once they reach mm concentrations. In our system we utilized TALP-Hepes as washing media and osmolality of IVM made was 280 mosmol. c) Flux Culture This involves the regular, gentle agitation of the culture dishes to prevent the attachment and subsequent differentiation of cumulus cells within maturation tubes. Micro droplets of IVM media covered by oil offer advantages including preventing or reducing the evaporation of water, protection from microbial contamination, attenuation of temperature and gas fluctuations, and ease of examination during culture period. This practice is advisable. d) Maturation Time Some discrepancies exist in the length of time needed for IVM of cattle oocytes. The culture medium and the supplements used, as well as the quality of oocytes, influence the length of time required before oocytes attain the maturation stage (Metaphase II). This study showed that while there was no significant difference between maturation period in terms of maturation and cleavage rates, there was a significant difference in final blastocyst yield in favor of the 24 h maturation period. Thus, the 24 hr maturation period for IVM of oocytes is recommended. e) Other considerations i) Antibiotic cover This provides cover against growth and proliferation of microorganisms during the period of IVM culture. The concentration of antibiotics must be non-toxic and their inclusion in the medium needs to be combined with rigorous standards of hygiene in the IVM laboratory. 9733
13 ii) Light environment As part of routine maturation, fertilization and culture, the cattle embryos are inevitably exposed to varying amounts of light. The lesions caused by prolonged exposure of high light intensity under the microscope may contribute to embryo development failure. In principle, oocytes and embryos should not be over exposed to light longer than is necessary. Maturation and other events normally occur under conditions of darkness in the animal's reproductive organ and in our case, the laboratory windows were covered with opaque curtains. iii) Temperature and Gas phase The success of IVEP is temperature-dependent. Changes in temperature exposure to oocytes can lead to temperature shocks that can induce chromosomal abnormalities. The temperature range of C is found to be ideal for cattle oocytes during IVEP. The incubation temperature was set at 38.5 C in all IVEP procedures. Gas phase was also observed to have a very big influence on the success of Boran IVEP. Development to the morula and blastocyst stage of the embryo was found to be highly dependent on the gaseous environment of the incubator during IVEP. The best is an environment of 5% CO2, 5% O2 and % humidity. iv) Culture supplements The supplements usually added to the culture medium are the following: v) Proteins The most commonly used protein source is serum added in a concentration of 5 to 10%. Proteins in serum have macromolecules attached such as hormones, vitamins and fatty acids, as well as chelated metal ions and pyrogenes. Bovine serum albumin was used for protein supplement. The role of protein in the culture media may not only be a fixed nitrogen source, but also as a chelator of toxic metal ion. In this system we used 10% fetal calf serum. vi) Hormones and Growth factors Some of the hormones used as supplements include FSH, LH, oestradiol and prolactin. The direct action of these hormones on oocyte maturation and early development of embryo are not well established, but their addition to the culture media improves development of pre-implantation stage embryos. Growth factors added include IGF (insulin-like growth factor) and EGF (epidermal growth factor). These growth factors have mitogenic effects and also stimulate RNA and protein synthesis. In our? case we only used 1µg/ml of FSH, LH and estradiol. vii) Culture Apparatus In thsese experiments, it was observed that the type of culture apparatus used for in vitro culture of oocytes and embryos affected the outputs of in vitro maturation and also development of pre-implantation stages of the embryos. The use of ordinary Petri dishes was not as beneficial as the use of the Nunc 500um deep holed petri dishes, which was found to greatly enhance embryo development. 9734
14 Sperm Treatment and Capacitation To ensure a successful fertilization of the oocytes during IVEP, the sperm cells that are used must be viable, motile, capacitated and capable of expressing acrosome reaction. Thus, the cells must bind to the zona pellucida of the oocyte and pass through its vitelline membrane. This is only possible if the cells are able to fuse with the oolemma and be incorporated into the oocyte. This ability is normally acquired by the sperm cells as they pass through the epididymis through maturation process and within the female genitalia through the process of capacitation. The process of capacitation is critical for fertilization. Only capacitated cells can undergo acrosomal reaction, a vital step that aids the cell to penetrate the ova to achieve fertilization. Capacitation is a process that involves complex biochemical and physiological changes within the sperm cell. During this process, gradual removal or alteration of the acrosomal protective coat from the sperm surface occurs. This then permits exposure of acrosomal enzymes needed for effective penetration of the ova and also exposure of sperm cell receptors that aid in binding onto the zona pellucida receptor sites. In the laboratory, capacitation procedures are aimed at stimulating the sequence of events that normally occur in the female reproductive tract. The seminal proteins and any dead cells within the ejaculated and/ frozen semen are removed thorough either a process of swim-up through capacitation medium or by centrifuge process through elevated ionic strength. The following are the components of an effective capacitation medium. a) Bovine Serum Albumin (BSA) Bovine serum albumin (BSA) plays a key role in removing cholesterol and/or zinc ion from the sperm cell. Bovine serum albumin protein has considerable binding capacity for both cholesterol and zinc molecules. Cholesterol and zinc usually stabilize sperm cell membrane and must be removed during capacitation to enable acrosomal reaction and penetration of the ova. b) Heparin Heparin is a glucoseaminoglycan molecule added to the medium to mimic in-vivo heparin-binding proteins present usually present in female genitalia during capacitation. Heparin proteins play a role in fertilization by inducing the process of capacitation. c) Caffeine Caffeine is a cyclic nucleotide phosphodiesterase inhibitor used as a motilitystimulating agent in bull sperm. When caffeine inhibits phospodiesterase within the sperm cell the intracellular accumulation of cyclic adenosine monophosphate (camp) occurs resulting into activated sperm motility. d) Calcium Ionophore Calcium is added to increase calcium ion (Ca 2+ ) content of the medium to facilitate sperm motility and ova penetration during fertilization. 9735
15 Swim-up technique of sperm capacitation In-vivo sperm capacitation usually takes about 6 h within the female reproductive system. In the laboratory, we conducted a swim-up technique for this process. Prior to swim up, the semen is evaluated by placing a drop on a pre-warmed glass slide and checked under a microscope for vigour and motility. Only semen that attained over 50% motility was used. For swim-up, 0.25 ml of the semen was then pipetted and gently released at an angle of 45 at the bottom of the 10ml sterile centrifuge tube containing 1.0 ml of swim up -TALP medium as shown in figure 2 above. The swim up was done in a 5% CO2 buffered incubator for 60 minutes at 38.5 C to allow the motile sperm to swim up through the medium as they also at the same time undergo capacitation [13]. After one hour, about 0.8 ml of the upper layer of the medium (supernatant) with the vital sperm, was pipetted into a 15ml-apirogenic tube and centrifuged at 10,000 rotations per minute (RPM) for 5 minutes at room temperature. The supernatant was then discarded and the sperm cell pellet located at the bottom of the tube was re-suspended with 100ul of fertilization (Fert-TALP) medium. In Vitro Fertilization (IVF) Successful IVF can only take place with appropriate preparation of sperm and oocyte, and also in culturing conditions that are favorable to the metabolic activity of the male and female gametes. In cattle, IVF of in vitro matured oocytes is usually carried out hours within an incubator set at 38.5 o C, at % humidity and 5%CO2 [14]. In vitro fertilization (IVF) is carried out in a 100 µl micro droplets of IVF medium containing sperm cells at 1 x 10 6 sperm/ml concentrations, to fertilize oocytes. In order to avoid going into the laboratory at odd hours, it is recommended, as we practiced, to conduct IVF for 24hrs [15]. The oocytes were processed by washing twice in pre-warmed IVF medium. Sperm preparation for IVF The right concentration of the sperm was determined by use of a Neubauer chamber using a 1:20 dilution factor as previously described [16, 17, 18]. Five micro litre of the capacitated semen after swim up was mixed with 95ul of water in order to kill the sperm and also achieve a 1:20 dilution factor. Then this was mixed thoroughly and loaded into both sides of Neubauer chamber and the numbers of sperm cells in both fields of the Neubauer chambers counted under a microscope. The total number of sperm cells counted was used to determine the spermatozoa concentration (sperm cells/ml). Experience indicated that whenever using a dilution factor of 1:20 in Neubauer chamber to calculate sperm concentration for IVF, a final concentration of 1 X 10 6 sperm/ml can be obtained by using semen volume equivalent to the volume of IVF droplet divided by the number of counted cells after taking into account the percentage of the post-thaw motility [16, 17]. Thus Volume of semen to use = volume of IVF droplet / (no. of counted cells /10 x % post thaw semen motility). 9736
16 The calculated final volume of semen was then drawn and added to a droplet of IVF to be part of the 100ul of the final IVF droplet. Then the oocytes are added in the subsequent volume of IVF remaining to make up the required final 100ul IVF droplet. In-Vitro Culture (IVC) of Embryos In this system the zygotes were co-cultured with a monolayer of their cumulus cells. The presence of a somatic cell monolayer in the culture medium during in-vitro culture of the developing embryo enhances its developmental potential. The layer supports and provides the developing zygotes with a comfortable environment by secreting some growth factors to enhance their development. It is important to use culture medium that has been pre-conditioned in the incubator at least 1-2hrs prior to in vitro culture (IVC). In vitro culture (IVC) is usually carried out for seven days. At the third day, in order to remove any metabolic waste resultant during cumulus-oocyte complex maturation and provide the embryos with a fresh medium that could sustain their metabolic requirements, half of the medium is drawn out and another fresh one added [17,18,19]. Before IVC, oocytes are washed 2-4 times in a washing medium (culture medium) to remove the excess sperm cells attached to the zona pellucida. It is important to remove excess sperm cells to avoid the presence of dead and denaturing cells that may contaminate the IVC medium [20]. In vitro embryo culture, like other procedures of IVEP is carried out inside a water-jacketed incubator with 5% CO2 at 38.5 C. It is advisable to check for cleavage rate three days (72 hrs) after the initiation of IVC whenever changing the IVC medium. At that time, most of the zygotes will be over 5- cell stage. Blastocysts used during embryo transfer are expected to be formed at day seven of IVC. During the evaluation times, gentle shaking of the culture dish is advised to allow for a uniform environment among embryos, and to allow for unified distribution of any autocrine growth factors that the embryo may have secreted. Also recommend that oocytes that have not cleaved are removed from the culture droplets at those times. Other Important Considerations for Boran IVEP a) Cleaning of Glassware Cleanliness of the glassware is of utmost importance. Used glassware is rinsed thoroughly with tap water and soaked in detergent overnight. The following day, glassware is rinsed thoroughly at least times in tap water, and then submerged in a 3-5% hydrochloric acid (HCl) for a minimum of 2 hours. It is then rinsed in running water for about 15 minutes, and rinsed again times in tap water. Thereafter, glassware is rinsed with distilled water and placed upside-down to dry by heating it at 120 C for 2-4 hours. Glassware is then packed separately in aluminum foil and sterilized by heating at 120 C for 4 hours. An ultrasonic cleaner is also used for heavily soiled and difficult to wash glassware such as volumetric flash, test tube and volumetric pipettes. Cleaning by sonication takes around 15 minutes in hot water. After sonication, glassware is rinsed times with 9737
17 tap water and finally rinsed with distilled water. Thereafter, the drying and sterilization protocol described above is practiced. b) Availability of Back-up Equipment and Equipment Repair Kit In some cases, equipment needs repair when it malfunctions or becomes defective. Back-up equipment must be available, to avoid compromising the IVEP activity. Staff working at the laboratory must be familiar with any repair measures that may need to be implemented. Prompt repairs are a requirement. It is important to have spare gas cylinders (CO2), back-up power sources and a spare incubator. Discussion of the Basic Data An average BCS of 2.0 for the slaughtered Boran cows is reported in this study. Some authors recommend that ovaries be collected from donor cows of superior body condition score that exceeds 2.0 [10]. This study also demonstrates that Boran cows with a body condition of were able to produce competent COC s and viable embryos. However, in order to produce optimal results in IVEP for the Boran breed, further studies may be necessary to shed more light on influence of nutrients and BCS of the cow on its embryo production in vitro. Estrous synchronization Estrous synchronization is an old biotechnology that has been used widely in Kenya. The technology ensures that all cows treated together come on heat around the same time. This process can be used to control follicular development during ovum pick-up sessions and time heat occurrences so as to allow for timed embryo transfer (TET). The study utilized the technology involving use of hormones to knock-off corpus luteum (CL) from cycling animals at the same time so as to initiate similar patterns of follicular development in all treated animals. Given that hormonal control of the estrous cycle eases reproductive management and improves efficiency of cattle operations, the plan was to provide practical applications of the manipulations of estrous cycle of Boran cows so as to plan ovum pick-up sessions from donor cows and/or achieve mass embryo transfers to recipient cows. Ovum Pick UP (OPU) in Live Animals Ovum pick up is a technology used to harvest ova from live animals. This technique requires a special ultrasound scanning machine that is used to retrieve good numbers of quality ova from a donor cow per harvesting session. The plan is to utilize this technology and harvest ova from farmers keeping cows of known top genetics. It is known that in cows two to four waves of follicles (source of ova) develop in a given estrous cycle. Every wave has recruitment, selection and dominant phases. When ova harvesting is done either during recruitment or selection phase, over 70 ova per ovary per cow can be obtained. Protocol used for Field Estrus synchronization to knock-off CL Prostaglandin (PGF) is a naturally occurring hormone. During the normal estrous cycle of a non-pregnant animal, PGF is released from the uterus 16 to 18 days after the cow 9738
18 was in heat. This release of PGF functions to destroy the corpus luteum (CL). The CL is a structure in the ovary that produces progesterone and prevents the animal from returning to estrus. The release of PGF from the uterus is the triggering mechanism that results in the animal returning to estrus every 21 days. Commercially available PGF (Lutalyse, Estrumate, Prostamate) can be used to knock-off the CL from any cycling cow at a predetermined time convenient for heat detection and breeding. The major limitation of PGF is that it is not effective on animals that do not possess a CL. This includes animals within 6 to 7 days of a previous heat, prepubertal heifers and postpartum anestrous cows. Despite these limitations, prostaglandins are the simplest method to synchronize estrus in cows. In our system we used 500 µg cloprostenol (2ml of Lutalyse) injected intramuscularly. Heat detection was observed keenly after 48hrs twice a day (7-730 h and h) when animals were in the farm house. Also, heat detection was also done throughout the day as the animals grazed in the fields. Embryo Transfer Embryo transfer is a technology of placing a seven day old embryo in the uterus of a recipient cow. The transfer embryos can be flushed from the uterus of a donor cow or be obtained from an in-vitro embryo production (IVEP) laboratory. Obtaining embryos from donor cows requires that such animals are treated beforehand to enable superovulation to occur in them. In addition, this process requires that recipient cows are also hormonally treated to achieve uterine synchrony with the donors for effective transfers. By contrast, hormonal treatment steps are eliminated whenever embryos are produced in an IVEP setup, making this method of obtaining embryos a better option. Thus, as an alternative source of embryos to the already existing conventional embryo transfer in Kenya [4], IVEP is technically feasible and can allow for a predictable supply of embryos from ovaries of slaughtered or live selected cows. Embryo transfer is carried out after the cow is prepared by giving it 3-5 ml of lignocaine epidural anaesthetic at the sacro-coccigeal space to allow for easy rectal manipulations. Then the perineum is washed with water, and disinfected. The embryos were then loaded into the embryo transfer (ET) gun as the embryo transfer expert examines the ovaries of the cow so as to ascertain into which uterine horn to transfer the embryo. CONCLUSION Utilization of the IVEP technology in Kenya can be used to carry out value addition to indigenous cows. There can be situations where farmers keeping low-grade cows can enter into commercial contracts to provide these cows as surrogate recipients for production of heifer calves. In the process, owners of such cows will have a valued product to sell for income earnings and be left with milk for use by family members. This avenue can be used to raise household revenues and attract many more farmers to engage in farming, leading to improved national economic and food security. When IVEP and OPU technologies are combined, OPU enables a cow to provide over 100 ova per ovary per month for sale. This innovation can be applied to exploit this 9739
19 special potential of the cow to equip owners of such cows with an opportunity to obtain and sell ova/cow/month. Countries such as Brazil have embraced innovations coming out of IVEP and OPU to turn around the livestock sector recent years. In the applied context, the farmers, the private industry and the embryo transfer practitioners in Brazil entered into publicprivate partnership on mutual benefit relationships and revolutionized the dairy sector [22]. In the same way, the authors hope to see this technology widely utilized in Kenya, due to its massive potential benefits to the dairy sector. It is noted that although ovarian follicular dynamics has been documented in some Zebu cows, literature on the same for the Boran cow is scarce. Thus, further studies are recommended on ovarian follicular dynamics of the Boran cow so as to provide this information in order to rapidly optimize capacity of this breed through utilization of in vitro embryo production and ovum pick reproductive technologies. Also cost-benefit analysis studies are recommended before the establishment of this technology in Kenya. The authors recommend that IVEP is a process that requires optimization of any described protocols to suit the methodology and materials involved in any set ups. Thus caution should be taken wherever adopting protocols developed elsewhere in another laboratory. ACKNOWLEDGEMENT The authors acknowledge Heifer Project International for funding this work and International Livestock Research Institute (ILRI) for providing space for laboratory facilities. Authors also appreciate University of Nairobi for providing time for the technical experts and recipient cows and Kapiti Plains for providing donor cows. Abattoirs that allowed harvesting of ovaries are also appreciated. All support staff are appreciated for heat detections and herding the animals. 9740
20 Table 1: Summary of data from In-Vitro Embryo Production process* Follicle Size group Grade Aspirated Matured Cleaved Blastocyst 1-3mm A mm B mm Discards** >3-6mm A >3-6mm B >3-6mm Discards** >6mm A >6mm B >6mm Discards** *11 runs were carried out with each run signifying a different week ** Number of oocytes removed from the study after the aspiration stage Table 2: Distribution of follicles obtained from the various follicle group sizes Follicle Size Number of follicles Percent% 1-3mm >3-6mm >6mm
21 REFERENCES 1. Thornton PK, Jones PG, Owiyo T, Kruska RL, Herrero P, Kristjanson A, Notenbaert H, Bekele N and A Omolo Report to the Biotechnology Department for International Livestock Research Institute (ILRI), Seré C, van de Zijpp A, Persley G and E Rege Animal Genetic Resources, Information Bulletin 2008; 42: Cunningham EP and O Syrstad Crossbreeding Bos indicus and Bos taurus for milk production in the tropics. FAO Animal Production and Health Paper 1987, 68. Food and Agriculture Organization of the United Nations, Rome. 4. King JM, Pasons DJ, Turnpenny JR, Nyangaga J, Bakari P and CM Wathes Animal Science 2006; 82(5): Kahi AK, Nitter G, Thorpe W and CF Gall Cattle breeding in the tropics. Liv. Prod. Sci. 2000; 63: McClintock SE, Ouma R, Baltenweck I, Okeyo AM, McClintock AE and E Rege Proceedings of Association for the Advancement of Animal Breeding and Genetics 2007, Australia. 7. Wildt DT Potential applications of IVF technology for species conservation. In: Fertilization in Mammals, B.D. Bavister, E. Roldan, and J. Cummins, (Eds.). Serono Symposium, U.S.A., 1990: Mutembei HM, Muasa BS Origa R, Jimbo S, Ojango JK, Tsuma VT, Mutiga ER and AM Okeyo in-vitro Fertilization in cattle and its potential practical application in Kenya. Proced 6th FVM Biennial Scientific Conference, Sept 17 th -19 th 2008, Mutembei HM, Muasa BS Origa R, Jimbo S, Ojango JK, Tsuma VT, Mutiga ER and AM Okeyo Delivery of appropriate cattle genotypes to Eastern African smallholder farmers through in-vitro embryo production technologiesthe technical procedures, prospects and challenges. Aspects of African Biodiversity. Proceedings of the Pan Africa Chemistry Network Biodiversity Conference, RSC Publishing 321, 2009: 84-90, ISBN: Gordon I Laboratory Production of Cattle Embryos. CAB International, Wallingford, Oxon OX10 8DE, UK ; ISBN Muasa BS Effects of follicle size and cumulus oocyte complex grade on in vitro embryo developmental competence for Boran cows. Unpub. M.Sc. Thesis, University of Nairobi, Kenya 2010:
22 12. Aman RR and JE Parks Effects of cooling and rewarming on the meiotic spindle and chromosome of in vitro matured bovine oocytes. Biol. Reprod. 1994; 50: Parrish JJ, Susko-Parrish J, Leibfried-Rutledge ML, Critser ES, Evestone WH and NF First Bovine in vitro fertilization with frozen-thawed semen. Theriogenology ; 1986; 25: Bracket BG and G Oliphant Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975; 12: Chuangsoongneon U and M Kamonpatana Oocyte maturation, in vitro fertilization and culture system for developing preimplantation swamp buffalo embryos using frozen thawed semen. Buffalo Journal 1991; 2: Rogers BJ Factors affecting mammals in vitro fertilization. In: Bioregulators of reproduction. Academic Press, New York, 1981: Camargo LSA, Sá WFD, Ferreira ADM, Viana JHM and MCCD Araújo Effect of sperm concentration and oocyte-spermatozoa incubation period on in vitro fertilization of Gyr breed. Pesquisa Agropecuária Brasileira, Brasília, 2002; 37 (5): Camargo LSA, Viana JHM, Sá WF, Ferreira AM, Ramos AA and VR Vale Filho Factors influencing in vitro embryo production. Animal Reproduction 2006; 3 (1): Duran DH Studies related to the effects of Dimethyl Sulphoxide (DMSO) on the culture systems of bovine oocytes in vitro. Unpub. M.Sc. Thesis, Miyazaki University, Japan. 1996; 164: Duran DH Tsuzuki Y Ashizawa K and N Fujihara Enhanced bovine embryo development from cultured cells and conditioned medium treated with Dimethyl Sulfoxide (DMSO). In: Congress Programme of the 13 th International Congress on Animal Reproduction (Darling Harbour, Sydney, Australia) June 30 - July 4, 1996; 3: Gardner DK and M Lane Embryo Culture Systems. In: Handbook of In Vitro Fertilization, A. Trounson and D.K. Lane, (Eds.). CRC Press Inc., 1993: Viana JHM and LSA Camargo Bovine embryo production in Brazil: a new scenario. Acta Sci. Vet. 35,
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION
 Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
A POWERFUL IN VITRO FERTILIZATION
 A POWERFUL During the past 50 years technological advances in the field of bovine reproduction have led to some dramatic changes in the way cattle look, reproduce, perform, and even taste. Artificial Insemination
A POWERFUL During the past 50 years technological advances in the field of bovine reproduction have led to some dramatic changes in the way cattle look, reproduce, perform, and even taste. Artificial Insemination
ANS 3319C Reproductive Physiology and Endocrinology Techniques for In-Vitro Embryo Production
 ANS 3319C Reproductive Physiology and Endocrinology Techniques for In-Vitro Embryo Production Objectives 1) To gain an understanding of the process of in-vitro embryo production in cattle and other domestic
ANS 3319C Reproductive Physiology and Endocrinology Techniques for In-Vitro Embryo Production Objectives 1) To gain an understanding of the process of in-vitro embryo production in cattle and other domestic
Assisted Reproductive Technologies at IGO
 9339 Genesee Avenue, Suite 220 San Diego, CA 92121 858 455 7520 Assisted Reproductive Technologies at IGO Although IGO no longer operates an IVF laboratory or program as such, we work closely with area
9339 Genesee Avenue, Suite 220 San Diego, CA 92121 858 455 7520 Assisted Reproductive Technologies at IGO Although IGO no longer operates an IVF laboratory or program as such, we work closely with area
Effect of honeybee royal jelly on the nuclear maturation of bovine oocytes in vitro
 CPh6.8 Effect of honeybee royal jelly on the nuclear maturation of bovine oocytes in vitro M Kuran 1*, E Sirin 1,E Soydan 1, AG Onal 2 Department of Animal Science, Universities of 1 Ondokuz Mayis, Samsun,
CPh6.8 Effect of honeybee royal jelly on the nuclear maturation of bovine oocytes in vitro M Kuran 1*, E Sirin 1,E Soydan 1, AG Onal 2 Department of Animal Science, Universities of 1 Ondokuz Mayis, Samsun,
In Vitro Fertilization as a tool for the Genetics Improvement. History and Future Prospects.
 In Vitro Fertilization as a tool for the Genetics Improvement. History and Future Prospects. Ovum pick up followed by embryo production procedure (OPU-IVP) is strongly driven by the need of the breeding
In Vitro Fertilization as a tool for the Genetics Improvement. History and Future Prospects. Ovum pick up followed by embryo production procedure (OPU-IVP) is strongly driven by the need of the breeding
Artificial Insemination in Cattle
 Artificial Insemination in Cattle Introduction This slide show is designed to introduce students to artificial insemination in cattle. However, it is only a brief overview and further training is necessary
Artificial Insemination in Cattle Introduction This slide show is designed to introduce students to artificial insemination in cattle. However, it is only a brief overview and further training is necessary
EVERY LIVING THING has a number of
 Anatomy and Physiology of Animal Reproductive Systems EVERY LIVING THING has a number of organ systems operating to perform specific functions. If you were to examine one of these systems, you would observe
Anatomy and Physiology of Animal Reproductive Systems EVERY LIVING THING has a number of organ systems operating to perform specific functions. If you were to examine one of these systems, you would observe
SYNCHRONIZATION OF CATTLE
 UNDER ESTRUS SYNCHRONIZATION OF CATTLE FS921C Robin Salverson, Extension Livestock Educator, Harding County, and George Perry, Extension Beef Reproduction and Management Specialist Reproductive failure
UNDER ESTRUS SYNCHRONIZATION OF CATTLE FS921C Robin Salverson, Extension Livestock Educator, Harding County, and George Perry, Extension Beef Reproduction and Management Specialist Reproductive failure
Artificial Reproductive Technologies I: insemination
 Artificial Reproductive Technologies I: insemination Cinzia Allegrucci LO s List the main advantages and disadvantages of artificial insemination (AI) Explain methods for evaluating volume and concentration,
Artificial Reproductive Technologies I: insemination Cinzia Allegrucci LO s List the main advantages and disadvantages of artificial insemination (AI) Explain methods for evaluating volume and concentration,
Four Systematic Breeding Programs with Timed Artificial Insemination for Lactating Dairy Cows: A Revisit
 Four Systematic Breeding Programs with Timed Artificial Insemination for Lactating Dairy Cows: A Revisit Amin Ahmadzadeh Animal and Veterinary Science Department University of Idaho Why Should We Consider
Four Systematic Breeding Programs with Timed Artificial Insemination for Lactating Dairy Cows: A Revisit Amin Ahmadzadeh Animal and Veterinary Science Department University of Idaho Why Should We Consider
BOER GOAT EMBRYO TRANSFER
 BOER GOAT EMBRYO TRANSFER Good management No shortcuts PLAN AHEAD AVOID STRESS Some examples of how stress is induced are: Mixing groups or individual animals together that have not previously been together.
BOER GOAT EMBRYO TRANSFER Good management No shortcuts PLAN AHEAD AVOID STRESS Some examples of how stress is induced are: Mixing groups or individual animals together that have not previously been together.
MTT Cell Proliferation Assay
 ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs)
 HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
PICSI Sperm Selection Device Instructions for Use
 PICSI Sperm Selection Device Instructions for Use Manufacturer: Biocoat, Inc., 211 Witmer Rd., Horsham, PA 19044 USA Distributor: MidAtlantic Diagnostics, Inc., Telephone: 856-762-2000, www.midatlanticdiagnostics.com
PICSI Sperm Selection Device Instructions for Use Manufacturer: Biocoat, Inc., 211 Witmer Rd., Horsham, PA 19044 USA Distributor: MidAtlantic Diagnostics, Inc., Telephone: 856-762-2000, www.midatlanticdiagnostics.com
Headquarters in Sioux Center, IA 1
 Nicholas Lemmel Cornfields, soybeans, and cows, I had finally arrived at Trans Ova Genetics Headquarters in Sioux Center, Iowa. I pulled into the drive of the intern house located on the corner of the
Nicholas Lemmel Cornfields, soybeans, and cows, I had finally arrived at Trans Ova Genetics Headquarters in Sioux Center, Iowa. I pulled into the drive of the intern house located on the corner of the
Guide to IVF Laboratory Results
 Guide to IVF Laboratory Results PACIFIC CENTRE FOR REPRODUCTIVE MEDICINE 500-4601 Canada Way, Burnaby BC V5G 4X7 LAB-416-20140122-1 The following information will guide you through what results to expect
Guide to IVF Laboratory Results PACIFIC CENTRE FOR REPRODUCTIVE MEDICINE 500-4601 Canada Way, Burnaby BC V5G 4X7 LAB-416-20140122-1 The following information will guide you through what results to expect
ASSISTED REPRODUCTIVE TECHNOLOGIES (ART)
 ASSISTED REPRODUCTIVE TECHNOLOGIES (ART) Dr. Herve Lucas, MD, PhD, Biologist, Andrologist Dr. Taher Elbarbary, MD Gynecologist-Obstetrician Definitions of Assisted Reproductive Technologies Techniques
ASSISTED REPRODUCTIVE TECHNOLOGIES (ART) Dr. Herve Lucas, MD, PhD, Biologist, Andrologist Dr. Taher Elbarbary, MD Gynecologist-Obstetrician Definitions of Assisted Reproductive Technologies Techniques
Reproduction and its Hormonal Control
 Reproduction and its Hormonal Control Page 1 Reproduction and its Hormonal Control Different mammals have different patterns of reproduction Eg mammals, rats and mice can breed all year round, whereas
Reproduction and its Hormonal Control Page 1 Reproduction and its Hormonal Control Different mammals have different patterns of reproduction Eg mammals, rats and mice can breed all year round, whereas
Artificial insemination
 Artificial insemination What is involved? Artificial insemination is an assisted reproduction technique that consists of inserting laboratory-treated spermatozoa into the woman s uterus or cervical canal.
Artificial insemination What is involved? Artificial insemination is an assisted reproduction technique that consists of inserting laboratory-treated spermatozoa into the woman s uterus or cervical canal.
Welcome to chapter 8. The following chapter is called "Monitoring IVF Cycle & Oocyte Retrieval". The author is Professor Jie Qiao.
 Welcome to chapter 8. The following chapter is called "Monitoring IVF Cycle & Oocyte Retrieval". The author is Professor Jie Qiao. The learning objectives of this chapter are 2 fold. The first section
Welcome to chapter 8. The following chapter is called "Monitoring IVF Cycle & Oocyte Retrieval". The author is Professor Jie Qiao. The learning objectives of this chapter are 2 fold. The first section
Proceedings, Applied Reproductive Strategies in Beef Cattle September 11 and 12, 2007, Billings, Montana NEW TECHNOLOGIES FOR REPRODUCTION IN CATTLE
 Proceedings, Applied Reproductive Strategies in Beef Cattle September 11 and 12, 2007, Billings, Montana NEW TECHNOLOGIES FOR REPRODUCTION IN CATTLE George E. Seidel, Jr. Animal Reproduction and Biotechnology
Proceedings, Applied Reproductive Strategies in Beef Cattle September 11 and 12, 2007, Billings, Montana NEW TECHNOLOGIES FOR REPRODUCTION IN CATTLE George E. Seidel, Jr. Animal Reproduction and Biotechnology
Revised minimum standards for in vitro fertilization, gamete intrafallopian transfer, and related procedures
 Revised minimum standards for in vitro fertilization, gamete intrafallopian transfer, and related procedures A PRACTICE COMMITTEE REPORT Guidelines and Minimum Standards I. Introduction Treatment of the
Revised minimum standards for in vitro fertilization, gamete intrafallopian transfer, and related procedures A PRACTICE COMMITTEE REPORT Guidelines and Minimum Standards I. Introduction Treatment of the
Lab Exercise 3: Media, incubation, and aseptic technique
 Lab Exercise 3: Media, incubation, and aseptic technique Objectives 1. Compare the different types of media. 2. Describe the different formats of media, plate, tube etc. 3. Explain how to sterilize it,
Lab Exercise 3: Media, incubation, and aseptic technique Objectives 1. Compare the different types of media. 2. Describe the different formats of media, plate, tube etc. 3. Explain how to sterilize it,
Welcome to chapter 10. The following chapter is called Equipment maintenance and monitoring. The author is Ronny Janssens.
 Welcome to chapter 10. The following chapter is called Equipment maintenance and monitoring. The author is Ronny Janssens. 1 After finishing this chapter, the student should be familiar with - the requirements
Welcome to chapter 10. The following chapter is called Equipment maintenance and monitoring. The author is Ronny Janssens. 1 After finishing this chapter, the student should be familiar with - the requirements
Understanding Animal Reproduction Technology
 Lesson 251c Understanding Animal Reproduction Technology Core Area. Animal Science Unit 250. Genetics and Breeding Topic 251. Fertilization California Academic Standard. Science Grades 9 through 12 Biology/Life
Lesson 251c Understanding Animal Reproduction Technology Core Area. Animal Science Unit 250. Genetics and Breeding Topic 251. Fertilization California Academic Standard. Science Grades 9 through 12 Biology/Life
Measuring Cell Viability/Cytotoxicity: Cell Counting Kit-F
 Introduction The Cell Counting Kit-F is a fluorometic assay for the determination of viable cell numbers. Calcein-AM in this kit passes through the cell membrane and is hydrolized by the esterase in the
Introduction The Cell Counting Kit-F is a fluorometic assay for the determination of viable cell numbers. Calcein-AM in this kit passes through the cell membrane and is hydrolized by the esterase in the
Bacterial Transformation with Green Fluorescent Protein. Table of Contents Fall 2012
 Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Xpert TM Karyotyping Teaching Kit
 Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Instructions. Torpedo sirna. Material. Important Guidelines. Specifications. Quality Control
 is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
Related topics: Application Note 27 Data Analysis of Tube Formation Assays.
 Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Reproduction Multiple Choice questions
 Reproduction Multiple Choice questions 1. In mammals that are seasonal breeders, females are receptive only once a year. This is called A) a follicular cycle B) an estrous cycle C) a menstrual cycle D)
Reproduction Multiple Choice questions 1. In mammals that are seasonal breeders, females are receptive only once a year. This is called A) a follicular cycle B) an estrous cycle C) a menstrual cycle D)
Dot Blot Analysis. Teacher s Guidebook. (Cat. # BE 502) think proteins! think G-Biosciences www.gbiosciences.com
 PR110 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Dot Blot Analysis Teacher s Guidebook (Cat. # BE 502) think proteins! think G-Biosciences
PR110 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Dot Blot Analysis Teacher s Guidebook (Cat. # BE 502) think proteins! think G-Biosciences
Transformation Protocol
 To make Glycerol Stocks of Plasmids ** To be done in the hood and use RNase/DNase free tips** 1. In a 10 ml sterile tube add 3 ml autoclaved LB broth and 1.5 ul antibiotic (@ 100 ug/ul) or 3 ul antibiotic
To make Glycerol Stocks of Plasmids ** To be done in the hood and use RNase/DNase free tips** 1. In a 10 ml sterile tube add 3 ml autoclaved LB broth and 1.5 ul antibiotic (@ 100 ug/ul) or 3 ul antibiotic
Human Adult Mesothelial Cell Manual
 Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
ANS 3319C Reproductive Physiology and Endocrinology Artificial Insemination in Cattle. Objectives. What are the advantages and disadvantages of AI?
 ANS 3319C Reproductive Physiology and Endocrinology Artificial Insemination in Cattle Objectives 1) To provide an overview of the process of artificial insemination (AI) in cattle. 2) To gain an understanding
ANS 3319C Reproductive Physiology and Endocrinology Artificial Insemination in Cattle Objectives 1) To provide an overview of the process of artificial insemination (AI) in cattle. 2) To gain an understanding
PRODUCERS can choose to use natural
 Artificial Insemination PRODUCERS can choose to use natural or artificial means of breeding their animals. Technology has advanced in the last 30 to 40 years to allow animal producers to use means other
Artificial Insemination PRODUCERS can choose to use natural or artificial means of breeding their animals. Technology has advanced in the last 30 to 40 years to allow animal producers to use means other
Standard Operating Procedure IVF and ICSI procedures
 Pages: 1 of 6 PRINCIPLES Oocytes are aspirated from mature Graafian follicles after the administration of ovarian hyperstimulation pharmacological regimes designed to produce multiple follicular development.
Pages: 1 of 6 PRINCIPLES Oocytes are aspirated from mature Graafian follicles after the administration of ovarian hyperstimulation pharmacological regimes designed to produce multiple follicular development.
Reproductive System & Development: Practice Questions #1
 Reproductive System & Development: Practice Questions #1 1. Which two glands in the diagram produce gametes? A. glands A and B B. glands B and E C. glands C and F D. glands E and F 2. Base your answer
Reproductive System & Development: Practice Questions #1 1. Which two glands in the diagram produce gametes? A. glands A and B B. glands B and E C. glands C and F D. glands E and F 2. Base your answer
Protocol for Disinfection of Cell Culture and Tissue Culture in Media:
 Protocol for Disinfection of Cell Culture and Tissue Culture in Media: Location: Hickory Hall 001 Director: Dr. Guido Verbeck DECONTAMINATION OF CELL CULTURE WASTE Cell culture has become a common laboratory
Protocol for Disinfection of Cell Culture and Tissue Culture in Media: Location: Hickory Hall 001 Director: Dr. Guido Verbeck DECONTAMINATION OF CELL CULTURE WASTE Cell culture has become a common laboratory
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION
 DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
In - Vitro Fertilization Handbook
 In - Vitro Fertilization Handbook William F. Ziegler, D.O. Medical Director Scott Kratka, ELD, TS Embryology Laboratory Director Lauren F. Lucas, P.A.-C, M.S. Physician Assistant Frances Cerniak, R.N.
In - Vitro Fertilization Handbook William F. Ziegler, D.O. Medical Director Scott Kratka, ELD, TS Embryology Laboratory Director Lauren F. Lucas, P.A.-C, M.S. Physician Assistant Frances Cerniak, R.N.
Kevin Bogart and Justen Andrews. Extraction of Total RNA from Drosophila. CGB Technical Report 2006-10 doi:10.2506/cgbtr-200610
 Kevin Bogart and Justen Andrews Extraction of Total RNA from Drosophila CGB Technical Report 2006-10 doi:10.2506/cgbtr-200610 Bogart K and Andrews J. 2006. Extraction of Total RNA from Drosophila. CGB
Kevin Bogart and Justen Andrews Extraction of Total RNA from Drosophila CGB Technical Report 2006-10 doi:10.2506/cgbtr-200610 Bogart K and Andrews J. 2006. Extraction of Total RNA from Drosophila. CGB
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Direct Antiglobulin Test (DAT)
 Exercise 8 Exercise 9 Direct Antiglobulin Test (DAT) Elution Study Task Aim Introduction To perform the DAT and elution procedure with correct interpretation of results. To perform with 100% accuracy the
Exercise 8 Exercise 9 Direct Antiglobulin Test (DAT) Elution Study Task Aim Introduction To perform the DAT and elution procedure with correct interpretation of results. To perform with 100% accuracy the
THE WHY, HOW-TO, AND COST OF PROGRAMED AI BREEDING OF DAIRY COWS. J. S. Stevenson
 Dairy Day 1998 THE WHY, HOW-TO, AND COST OF PROGRAMED AI BREEDING OF DAIRY COWS J. S. Stevenson Summary Management of the estrous cycle is now more practical than it was a decade ago because of our understanding
Dairy Day 1998 THE WHY, HOW-TO, AND COST OF PROGRAMED AI BREEDING OF DAIRY COWS J. S. Stevenson Summary Management of the estrous cycle is now more practical than it was a decade ago because of our understanding
EXTRACTION OF DNA FROM CALF THYMUS CELLS Revised 2/1/96 Introduction
 Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
HighPure Maxi Plasmid Kit
 HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
Page 1. 1. The production of monoploid cells by spermatogenesis occurs in (1) zygotes (3) ovaries (2) testes (4) meristems
 1. The production of monoploid cells by spermatogenesis occurs in (1) zygotes (3) ovaries (2) testes (4) meristems Base your answers to questions 2 and 3 on the diagram below of the female reproductive
1. The production of monoploid cells by spermatogenesis occurs in (1) zygotes (3) ovaries (2) testes (4) meristems Base your answers to questions 2 and 3 on the diagram below of the female reproductive
Managing the Heat-Stressed Cow to Improve Reproduction
 Managing the Heat-Stressed Cow to Improve Reproduction Peter J. Hansen Department of Animal Sciences University of Florida, Gainesville Florida 32611-0910 Ph: 352-392-5590 Fax: 352-392-5595 The Growing
Managing the Heat-Stressed Cow to Improve Reproduction Peter J. Hansen Department of Animal Sciences University of Florida, Gainesville Florida 32611-0910 Ph: 352-392-5590 Fax: 352-392-5595 The Growing
ART - - - - - IVF - NO.1 IVF - IVF
 The success of any ART laboratory depends on its IVF laboratory. The primary function of an ART laboratory is to provide an optimal environment for gametes and embryos. To set up an ART laboratory, the
The success of any ART laboratory depends on its IVF laboratory. The primary function of an ART laboratory is to provide an optimal environment for gametes and embryos. To set up an ART laboratory, the
Standard Operating Procedure (SOP) Work Package 8. Sample Collection and Storage
 Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
Unit B: Understanding Animal Reproduction. Lesson 3: Understanding Animal Reproduction Technology
 Unit B: Understanding Animal Reproduction Lesson 3: Understanding Animal Reproduction Technology Student Learning Objectives: Instruction in this lesson should result in students achieving the following
Unit B: Understanding Animal Reproduction Lesson 3: Understanding Animal Reproduction Technology Student Learning Objectives: Instruction in this lesson should result in students achieving the following
Xpert TM Animal Tissue Culture Teaching Kit
 Xpert TM Animal Tissue Culture Teaching Kit Product Code: CCK005 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques
Xpert TM Animal Tissue Culture Teaching Kit Product Code: CCK005 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques
UltraClean Soil DNA Isolation Kit
 PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
Symposium on RECENT ADVANCES IN ASSISTED REPRODUCTIVE TECHNOLOGY
 Symposium on RECENT ADVANCES IN ASSISTED REPRODUCTIVE TECHNOLOGY Dr Niel Senewirathne Senior Consultant of Obstetrician & Gynaecologist De zoyza Maternity Hospita 1 ART - IVF & ICSI 2 Infertility No pregnancy
Symposium on RECENT ADVANCES IN ASSISTED REPRODUCTIVE TECHNOLOGY Dr Niel Senewirathne Senior Consultant of Obstetrician & Gynaecologist De zoyza Maternity Hospita 1 ART - IVF & ICSI 2 Infertility No pregnancy
Cell Cycle in Onion Root Tip Cells (IB)
 Cell Cycle in Onion Root Tip Cells (IB) A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules,
Cell Cycle in Onion Root Tip Cells (IB) A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules,
UltraClean PCR Clean-Up Kit
 UltraClean PCR Clean-Up Kit Catalog No. Quantity 12500-50 50 Preps 12500-100 100 Preps 12500-250 250 Preps Instruction Manual Please recycle Version: 02212013 1 Table of Contents Introduction... 3 Protocol
UltraClean PCR Clean-Up Kit Catalog No. Quantity 12500-50 50 Preps 12500-100 100 Preps 12500-250 250 Preps Instruction Manual Please recycle Version: 02212013 1 Table of Contents Introduction... 3 Protocol
Running protein gels and detection of proteins
 Running protein gels and detection of proteins 1. Protein concentration determination using the BIO RAD reagent This assay uses a colour change reaction to give a direct measurement of protein concentration.
Running protein gels and detection of proteins 1. Protein concentration determination using the BIO RAD reagent This assay uses a colour change reaction to give a direct measurement of protein concentration.
PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System) 50 preps Product #60200
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System)
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System)
Artificial Insemination Training Program
 Artificial Insemination Training Program HISTORY First AI research reports 200 years ago. A long ago, Arabs obtained semen from mated mares belonging to rival groups during night hours to inseminate their
Artificial Insemination Training Program HISTORY First AI research reports 200 years ago. A long ago, Arabs obtained semen from mated mares belonging to rival groups during night hours to inseminate their
Mixtures and Pure Substances
 Unit 2 Mixtures and Pure Substances Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances. They
Unit 2 Mixtures and Pure Substances Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances. They
Recommended Resources: The following resources may be useful in teaching this
 Unit B: Anatomy and Physiology of Poultry Lesson 4: Artificial Poultry Reproduction Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives:
Unit B: Anatomy and Physiology of Poultry Lesson 4: Artificial Poultry Reproduction Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives:
ANIMAL GENETICS SOLUTIONS
 THE NATIONAL ANIMAL GENETIC RESOURCES CENTRE AND DATABANK NAGRC & DB) COLLABORATION WITH IAEA AND OTHER STAKEHOLDERS IN ANGR DEVELOPMENT INTERLAKEN, SWITZERLAND SEPT. 2007 ANIMAL GENETICS SOLUTIONS Vision
THE NATIONAL ANIMAL GENETIC RESOURCES CENTRE AND DATABANK NAGRC & DB) COLLABORATION WITH IAEA AND OTHER STAKEHOLDERS IN ANGR DEVELOPMENT INTERLAKEN, SWITZERLAND SEPT. 2007 ANIMAL GENETICS SOLUTIONS Vision
Overview of Artificial Insemination of Kentucky Meat and Dairy Goats Terry Hutchens, Extension Associate University of Kentucky (G10307)
 Overview of Artificial Insemination of Kentucky Meat and Dairy Goats Terry Hutchens, Extension Associate University of Kentucky (G10307) General Prospective Kentucky goat producers can make great strides
Overview of Artificial Insemination of Kentucky Meat and Dairy Goats Terry Hutchens, Extension Associate University of Kentucky (G10307) General Prospective Kentucky goat producers can make great strides
General Properties Protein Nature of Enzymes Folded Shape of Enzymes H-bonds complementary
 Proteins that function as biological catalysts are called enzymes. Enzymes speed up specific metabolic reactions. Low contamination, low temperature and fast metabolism are only possible with enzymes.
Proteins that function as biological catalysts are called enzymes. Enzymes speed up specific metabolic reactions. Low contamination, low temperature and fast metabolism are only possible with enzymes.
Animal Sciences. Timed-Artificial Insemination in Beef Cows: What are the Options?
 Purdue Extension Animal Sciences AS-575-W Timed-Artificial Insemination in Beef Cows: What are the Options? Allen Bridges, Scott Lake, Ron Lemenager, and Matt Claeys, Purdue Beef Team, Department of Animal
Purdue Extension Animal Sciences AS-575-W Timed-Artificial Insemination in Beef Cows: What are the Options? Allen Bridges, Scott Lake, Ron Lemenager, and Matt Claeys, Purdue Beef Team, Department of Animal
2. What muscle pulls the testis down into the scrotum during development?
 Anatomy & Physiology Reproductive System Worksheet Male 1. Put the following structures in order from testis to urethra: ductus deferens, rete testis, epididymus, seminiferous tubules 1) 2) 3) 4) 2. What
Anatomy & Physiology Reproductive System Worksheet Male 1. Put the following structures in order from testis to urethra: ductus deferens, rete testis, epididymus, seminiferous tubules 1) 2) 3) 4) 2. What
Follicle Dermal Papilla Cell
 Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Assignment Discovery Online Curriculum
 Assignment Discovery Online Curriculum Lesson title: In Vitro Fertilization Grade level: 9-12, with adaptation for younger students Subject area: Life Science Duration: Two class periods Objectives: Students
Assignment Discovery Online Curriculum Lesson title: In Vitro Fertilization Grade level: 9-12, with adaptation for younger students Subject area: Life Science Duration: Two class periods Objectives: Students
2016 Protocols for Synchronization of Estrus and Ovulation in Beef Cows and Heifers
 2016 Protocols for Synchronization of Estrus and Ovulation in Beef Cows and Heifers Biotechnology presents beef producers with an unprecedented opportunity to improve herd genetics. Producers have more
2016 Protocols for Synchronization of Estrus and Ovulation in Beef Cows and Heifers Biotechnology presents beef producers with an unprecedented opportunity to improve herd genetics. Producers have more
How Does a Doctor Test for AIDS?
 Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Germ cell formation / gametogenesis And Fertilisation
 Developmental Biology BY1101 P. Murphy Lecture 3 The first steps to forming a new organism Descriptive embryology I Germ cell formation / gametogenesis And Fertilisation Why bother with sex? In terms of
Developmental Biology BY1101 P. Murphy Lecture 3 The first steps to forming a new organism Descriptive embryology I Germ cell formation / gametogenesis And Fertilisation Why bother with sex? In terms of
Transfection reagent for visualizing lipofection with DNA. For ordering information, MSDS, publications and application notes see www.biontex.
 METAFECTENE FluoR Transfection reagent for visualizing lipofection with DNA For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
METAFECTENE FluoR Transfection reagent for visualizing lipofection with DNA For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum
 SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
Blood Collection and Processing SOP
 Brisbane Breast Bank Blood Collection and Processing SOP Breast Pathology Laboratory University of Queensland Centre for Clinical Research Blood Collection We collect 30ml of blood from patients who have
Brisbane Breast Bank Blood Collection and Processing SOP Breast Pathology Laboratory University of Queensland Centre for Clinical Research Blood Collection We collect 30ml of blood from patients who have
Experiment 12- Classification of Matter Experiment
 Experiment 12- Classification of Matter Experiment Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances.
Experiment 12- Classification of Matter Experiment Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances.
ANS 431 - Reproductive Physiology of Domestic Animals (Spring 2015)
 1 ANS 431 - Reproductive Physiology of Domestic Animals (Spring 2015) Instructor: Dr. Eduardo L. Gastal, DVM, MS, PhD Room: AG 129; Phone: 453-1774; E-mail: egastal@siu.edu Office hours: MWF 11-12 a.m.;
1 ANS 431 - Reproductive Physiology of Domestic Animals (Spring 2015) Instructor: Dr. Eduardo L. Gastal, DVM, MS, PhD Room: AG 129; Phone: 453-1774; E-mail: egastal@siu.edu Office hours: MWF 11-12 a.m.;
Modulating Glucose Uptake in Skeletal Myotubes:
 icell Skeletal Myoblasts Application Protocol Introduction Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis The skeletal muscle is one of the
icell Skeletal Myoblasts Application Protocol Introduction Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis The skeletal muscle is one of the
Agrobacterium tumefaciens-mediated transformation of Colletotrichum graminicola and Colletotrichum sublineolum
 Agrobacterium tumefaciens-mediated transformation of Colletotrichum graminicola and Colletotrichum sublineolum Flowers and Vaillancourt, 2005. Current Genetics 48: 380-388 NOTE added by L. Vaillancourt:
Agrobacterium tumefaciens-mediated transformation of Colletotrichum graminicola and Colletotrichum sublineolum Flowers and Vaillancourt, 2005. Current Genetics 48: 380-388 NOTE added by L. Vaillancourt:
6 Characterization of Casein and Bovine Serum Albumin
 6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
Plant Genomic DNA Extraction using CTAB
 Plant Genomic DNA Extraction using CTAB Introduction The search for a more efficient means of extracting DNA of both higher quality and yield has lead to the development of a variety of protocols, however
Plant Genomic DNA Extraction using CTAB Introduction The search for a more efficient means of extracting DNA of both higher quality and yield has lead to the development of a variety of protocols, however
GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps)
 1 GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps) (FOR RESEARCH ONLY) Sample : Expected Yield : Endotoxin: Format : Operation Time : Elution Volume : 50-400 ml of cultured bacterial
1 GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps) (FOR RESEARCH ONLY) Sample : Expected Yield : Endotoxin: Format : Operation Time : Elution Volume : 50-400 ml of cultured bacterial
THE ACTIVITY OF LACTASE
 THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
Successful Timed AI Programs: Using Timed AI to Improve Reproductive Efficiency in High Producing Dairy Cattle
 Successful Timed Programs: Using Timed to Improve Reproductive Efficiency in High Producing Dairy Cattle Milo C. Wiltbank, Ph.D. Department of Dairy Science University of Wisconsin, Madison Introduction
Successful Timed Programs: Using Timed to Improve Reproductive Efficiency in High Producing Dairy Cattle Milo C. Wiltbank, Ph.D. Department of Dairy Science University of Wisconsin, Madison Introduction
105 Adopted: 27.07.95
 105 Adopted: 27.07.95 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Adopted by the Council on 27 th July 1995 Water Solubility INTRODUCTION 1. This guideline is a revised version of the original Guideline
105 Adopted: 27.07.95 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Adopted by the Council on 27 th July 1995 Water Solubility INTRODUCTION 1. This guideline is a revised version of the original Guideline
The IUI procedure Who should consider an IUI IUI success rates IUI cost What to consider if IUI is unsuccessful. The IUI procedure:
 A Complete Guide to understanding IUI (intrauterine insemination) and artificial insemination (Eric Daiter, MD Board Certified in Reproductive Endocrinology and Infertility) The IUI procedure Who should
A Complete Guide to understanding IUI (intrauterine insemination) and artificial insemination (Eric Daiter, MD Board Certified in Reproductive Endocrinology and Infertility) The IUI procedure Who should
Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides
 Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides Polyacrylamide gel electrophoresis (PAGE) and subsequent analyses are common tools in biochemistry and molecular biology. This Application
Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides Polyacrylamide gel electrophoresis (PAGE) and subsequent analyses are common tools in biochemistry and molecular biology. This Application
Seahorse XF Cell Mito Stress Test Kit
 Seahorse XF Cell Mito Stress Test Kit Part # 103015-100 User Guide For use with Seahorse XF e and XF Extracellular Flux Analyzers For Research Use Only 1 Table of Contents Product Description... 2 Introduction...
Seahorse XF Cell Mito Stress Test Kit Part # 103015-100 User Guide For use with Seahorse XF e and XF Extracellular Flux Analyzers For Research Use Only 1 Table of Contents Product Description... 2 Introduction...
GASTRIC ORGANOID CULTURE PROTOCOL
 GASTRIC ORGANOID CULTURE PROTOCOL THIS PROTOCOL PROVIDES THE PROCEDURE FOR SUBCULTURING NORMAL HUMAN GASTRIC ORGANOIDS WHICH WAS DERIVED FROM THE SUBMERGED METHOD AS DESCRIBED IN BARKER N, ET AL. LGR5+VE
GASTRIC ORGANOID CULTURE PROTOCOL THIS PROTOCOL PROVIDES THE PROCEDURE FOR SUBCULTURING NORMAL HUMAN GASTRIC ORGANOIDS WHICH WAS DERIVED FROM THE SUBMERGED METHOD AS DESCRIBED IN BARKER N, ET AL. LGR5+VE
Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
HBV Quantitative Real Time PCR Kit
 Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
TransIT -2020 Transfection Reagent
 Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
RiboZol RNA Extraction Reagents
 RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
Chromatin Immunoprecipitation
 Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein
 Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
In this experiment, we will use three properties to identify a liquid substance: solubility, density and boiling point..
 Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Environmental Water Testing: Surface Water, Groundwater, Hard Water, Wastewater, & Seawater
 Document: AND Sol Env 08 2013 Environmental Water Testing: Surface Water, Groundwater, Hard Water, Wastewater, & Seawater Matrix specific sample preparation and testing methods for environmental waters
Document: AND Sol Env 08 2013 Environmental Water Testing: Surface Water, Groundwater, Hard Water, Wastewater, & Seawater Matrix specific sample preparation and testing methods for environmental waters
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity
 Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
