March 27, Dear Ms. Syrek Jensen,
|
|
|
- Willa Dennis
- 8 years ago
- Views:
Transcription
1 !! March 27, 2016 Tamara Syrek Jensen, JD Director, Coverage and Analysis Group Centers for Medicare & Medicaid Services 7500 Security Blvd. Mailstop S Baltimore, MD Re: A Formal Request for a National Coverage Determination (NCD) on Percutaneous Image Guided Lumbar Decompression (PILD) for Lumbar Spinal Stenosis (LSS) Dear Ms. Syrek Jensen, I greatly appreciate the time you and your staff have spent considering the National Coverage Determination (NCD) for percutaneous image guided lumbar decompression (PILD) for lumbar spinal stenosis (LSS). I am also grateful for your guidance and advice on developing the Coverage with Evidence Development (CED) trial and assistance with timelines as the trial moved toward completion. Vertos Medical, Inc. is now prepared to formally request that the NCD on PILD be reopened. We are making this request based on the published 6-month results and are fully aware that 12- month results are needed to fulfill the requirement of the approved CED study. Background PILD for LSS is a posterior decompression of the lumbar spine performed under indirect image guidance without any direct visualization of the surgical area. PILD is a treatment for symptomatic LSS, specifically neurogenic claudication, that is unresponsive to conservative therapy. The procedure is minimally invasive, using specially designed instruments to percutaneously remove a portion of the lamina and debulk the ligamentum flavum through a 5.1 mm non-dilating canula. It is performed via an ipsilateral approach under x-ray guidance (e.g., fluoroscopic, CT) with the assistance of contrast media to identify and monitor the treatment area via epidurogram. On January 9, 2014, CMS determined that PILD for LSS is not reasonable and necessary under section 1862(a)(1)(A) of the Social Security Act. CMS went on to state that that PILD will be covered by Medicare when provided in a clinical study under section 1862(a)(1)(E) through CED for beneficiaries with LSS who are enrolled in an approved clinical study that meet certain criteria. 95!Enterprise,!Suite!325,!Aliso!Viejo,!CA!92656!!!!!!!!!!!!!!!T!! !!!!!F! !!!!!!!!!!!!!!!
2 !! In the NCD, CMS specified that any CED study must address several questions using a prospective, randomized, controlled design, with current validated and reliable measurement instruments and clinically appropriate comparator treatments, including appropriate medical or surgical interventions or a sham controlled arm, for patients randomized to the non-pild group. Also, the CED study protocol must specify a statistical analysis and a minimum length of patient follow up time that evaluates the effect of beneficiary characteristics on patient health outcomes as well as the duration of benefit. The following are study questions of interest to CMS: i. Does PILD provide a clinically meaningful improvement of function and/or quality of life in Medicare beneficiaries with LSS compared to other treatments? ii. iii. Does PILD provide clinically meaningful reduction in pain in Medicare beneficiaries with LSS compared to other treatments? Does PILD affect the overall clinical management of LSS and decision making, including use of other medical treatments or services, compared to other treatments? On May 6, 2014 CMS approved the following CED study that was determined to meet the requirements specified in the NCD: Study Title: MILD Percutaneous Image-Guided Lumbar Decompression versus Epidural Steroid Injections in Patients Diagnosed with Lumbar Spinal Stenosis Exhibiting Neurogenic Claudication. Sponsor: Vertos Medical Clinicaltrials.gov Number: NCT CMS Approval Date: 05/06/2014 MiDAS ENCORE Study Protocol The above CED approved study is colloquially known as the MiDAS ENCORE (Evidence-based Neurogenic Claudication Outcomes Research) Study. It is a prospective, multi-center, randomized controlled clinical trial. Twenty-six interventional pain management centers in the U.S. were enrolled to participate in the study. The objective was to compare patient outcomes following treatment with either minimally invasive lumbar decompression (MILD also known as PILD) (treatment group) or epidural steroid injections (ESIs) (active control group) in LSS patients with neurogenic claudication and verified ligamentum flavum hypertrophy. Inclusion criteria required patients to be Medicare beneficiaries (65 years or older) and experiencing neurogenic claudication symptoms for at least 3 months, which has failed to respond to physical therapy, home exercise programs, and oral analgesics. Additionally, lumbar spinal stenosis with neurogenic needed to be diagnosed via a symptomatic diagnosis and radiological evidence of LSS with ligamentum flavum thickness of greater than 2.5mm. Patients with comorbid conditions commonly associated with spinal stenosis were allowed to be included into the study unless the treating physician determined that the condition was too advanced. 95!Enterprise,!Suite!325,!Aliso!Viejo,!CA!92656!!!!!!!!!!!!!!!T!! !!!!!F! !!!!!!!!!!!!!!!
3 !! The study design called for a 6-month follow-up and 1-year follow-up conducted for patients in both study arms, and supplementary 2-year outcome data collected for patients in the MILD group only. Outcomes were assessed using the Oswestry Disability Index (ODI), numeric pain rating scale (NPRS) and the Zurich Claudication Questionnaire (ZCQ). The primary efficacy endpoint is the proportion of ODI responders, tested for statistical superiority of the MILD group versus the active control group. ODI responders are defined as patients achieving the validated minimal important change (MIC) of 10 point improvement in ODI from baseline to follow-up. Secondary efficacy includes proportion of NPRS and ZCQ responders using validated MIC thresholds. Primary safety is the incidence of device or procedure-related adverse events in each group. MiDAS ENCORE 6-Month Results Results at six months for the MiDAS ENCORE study have been published. 1 A total of 302 patients were enrolled, with 149 randomized to MILD and 153 to the active control. The mean age of randomized patients was 75.6 years in the MILD arm, and 75.0 in the ESI arm. Although there was a significant difference in gender between the two groups, a covariate effects analysis determined that this difference did not meaningfully impact the primary endpoint outcome. The most common cofactors were bulging discs, foraminal narrowing, facet hypertrophy, and facet arthropathy, each of which occurred in over 75% of patients in both arms of the study. For the primary efficacy endpoint, the proportion of ODI responders in the MILD group (62.2%) was statistically significantly higher than the proportion of ODI responders in the ESI group (35.7%) at 6-month follow-up (p<0.001). Secondary efficacy endpoints related to pain were also statistically significant higher in the MILD group than the ESI group as evidenced by the responder rates for NPRS (55.9% for MILD vs. 33.3% for ESI, p<0.001) and ZCQ Pain subdomain (58.0% for MILD vs. 42.6% for ESI, p=0.011). All other secondary efficacy endpoints also demonstrated statistical superiority in responder rates for the MILD group vs. the ESI group. There was no statistically significant difference in safety between the two study groups and neither cohort experienced any significant device related adverse events. The 6-month results from the study demonstrate that, the MILD procedure is statistically superior to ESIs in the treatment of these patients. The results of all primary and secondary efficacy outcome measures achieved statistically superior outcomes in the MILD group versus the ESI group. There were no statistically significant differences in the safety profile between study groups. This prospective, multi-center, randomized controlled clinical study therefore provides Level I evidence of the safety and effectiveness of MILD versus ESIs. 1 Staats PS, Benyamin RM. MiDAS ENCORE: Randomized Controlled Clinical Trial, Report of 6-Month Results. Pain Physician Feb;19(2): !Enterprise,!Suite!325,!Aliso!Viejo,!CA!92656!!!!!!!!!!!!!!!T!! !!!!!F! !!!!!!!!!!!!!!!
4 !! Conclusion Based on the published and highly favorable 6 months results of the MILD versus ESI CED study, we would like to formally request reconsideration of the PILD NCD. We understand that completion of the NCD depends on the publication of the 12-month results. We plan to submit the report on the 12-month results to you by the middle of May. We also plan to submit the 12- month data for publication at that time. I look forward to continuing to work with you on the PILD NCD. Please let me know if you have any questions. Sincerely, Eric Wichems President & CEO Vertos Medical, Inc. cc: Joseph Chin, MD Attachment: Staats PS, Benyamin RM. MiDAS ENCORE: Randomized Controlled Clinical Trial, Report of 6-Month Results. Pain Physician Feb;19(2):25-38! 95!Enterprise,!Suite!325,!Aliso!Viejo,!CA!92656!!!!!!!!!!!!!!!T!! !!!!!F! !!!!!!!!!!!!!!!
5 1BJO1IZTJDJBOt*44/ Randomized Trial MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results Peter S. Staats, MD 1, and Ramsin M. Benyamin, MD 2, for the MiDAS ENCORE Investigators* From: 1Premier Pain Centers, Shrewsbury, NJ and Johns Hopkins University School of Medicine, Baltimore, MD; 2 Millennium Pain Center, Bloomington, IL, and University of Illinois, Urbana Champaign, IL Address Correspondence: Peter S. Staats, MD Adjunct Associate Professor, Johns Hopkins University Partner, Premier Pain Centers Shrewsbury, NJ peterstaats@hotmail.com Disclaimer: Drs. Staats and Benyamin are Study Principal Investigators for the MiDAS ENCORE Study. *Investigators in the MiDAS ENCORE Study are listed in the Appendix. Conflict of interest: Each author certifies that he, or a member of his immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript. Manuscript received: Accepted for publication: Free full manuscript: Background: Patients suffering from neurogenic claudication due to lumbar spinal stenosis (LSS) often experience moderate to severe pain and significant functional disability. Neurogenic claudication results from progressive degenerative changes in the spine, and most often affects the elderly. Both the MILD procedure and epidural steroid injections (ESIs) offer interventional pain treatment options for LSS patients experiencing neurogenic claudication refractory to more conservative therapies. MILD provides an alternative to ESIs via minimally invasive lumbar decompression. Study Design: Prospective, multi-center, randomized controlled clinical trial. Setting: Twenty-six US interventional pain management centers. Objective: To compare patient outcomes following treatment with either MILD (treatment group) or ESIs (active control group) in LSS patients with neurogenic claudication and verified ligamentum flavum hypertrophy. Methods: This prospective, multi-center, randomized controlled clinical trial includes 2 study arms with a 1-to-1 randomization ratio. A total of 302 patients were enrolled, with 149 randomized to MILD and 153 to the active control. Six-month follow-up has been completed and is presented in this report. In addition, one year follow-up will be conducted for patients in both study arms, and supplementary 2 year outcome data will be collected for patients in the MILD group only. Outcome Measures: Outcomes are assessed using the Oswestry Disability Index (ODI), numeric pain rating scale (NPRS) and Zurich Claudication Questionnaire (ZCQ). Primary efficacy is the proportion of ODI responders, tested for statistical superiority of the MILD group versus the active control group. ODI responders are defined as patients achieving the validated Minimal Important Change (MIC) of 10 point improvement in ODI from baseline to follow-up. Similarly, secondary efficacy includes proportion of NPRS and ZCQ responders using validated MIC thresholds. Primary safety is the incidence of device or procedure-related adverse events in each group. Results: At 6 months, all primary and secondary efficacy results provided statistically significant evidence that MILD is superior to the active control. For primary efficacy, the proportion of ODI responders in the MILD group (62.2%) was statistically significantly higher than for the epidural steroid group (35.7%) (P < 0.001). Further, all secondary efficacy parameters demonstrated statistical superiority of MILD versus the active control. The primary safety endpoint was achieved, demonstrating that there is no difference in safety between MILD and ESIs (P = 1.00). Limitations: Limitations include lack of patient blinding due to considerable differences in treatment protocols, and a potentially higher non-responder rate for both groups versus standard-of-care due to study restrictions on adjunctive pain therapies. Conclusions: Six month follow-up data from this trial demonstrate that the MILD procedure is statistically superior to epidural steroids, a known active treatment for LSS patients with neurogenic claudication and verified central stenosis due to ligamentum flavum hypertrophy. The results of all primary and secondary efficacy outcome measures achieved statistically superior outcomes in the MILD group versus ESIs. Further, there were no statistically significant differences in the safety profile between study groups. This prospective, multi-center, randomized controlled clinical trial provides strong evidence of the effectiveness of MILD versus epidural steroids in this patient population.
6 Pain Physician: February 2016: 19:25-37 Clinical Trial Registration: NCT Key words: MILD, lumbar central spinal stenosis, minimally invasive lumbar decompression, interlaminar epidural steroid injection, neurogenic claudication, ligamentum flavum, Oswestry Disability Index, ODI, Numeric Pain Rating Scale, NPRS, Zurich Claudication Questionnaire, ZCQ Pain Physician 2016; 19:25-37 Lumbar spinal stenosis (LSS) is a common cause of moderate to severe low back and lower extremity pain, and often leads to significant functional disability. Especially prevalent in the elderly, lumbar central spinal stenosis is caused by progressive degenerative changes in the spine, which result in structural narrowing of the central vertebral canal. This narrowing, which can be caused by many factors including osteophyte formation, facet hypertrophy, disk herniation, and ligamentum flavum hypertrophy, compresses neural elements resulting in nerve root ischemia and leads to painful neurogenic claudication symptoms (1-3). Many therapeutic modalities have been advocated for the treatment of lumbar central spinal stenosis. Treatment strategies generally begin with conservative measures that may include physical therapy, oral analgesics, or many other non-invasive options. Once more conservative therapies fail, LSS patients are often treated with epidural injections which have been reported to provide significant short-term improvement for this patient population (4-9). More invasive interventions such as interspinous spacers and decompressive surgery, with or without fusion, are also options for these patients, but are associated with higher complication rates (10,11). The objective of MiDAS ENCORE (Evidence-based Neurogenic Claudication Outcomes Research) is to provide strong evidence of the effectiveness of MILD (treatment group) versus epidural steroid injections (ESIs) (the active control) in managing neurogenic claudication symptoms in LSS patients. This prospective, multicenter, randomized controlled clinical trial compares patient outcomes following treatment with either MILD or the active control in LSS patients with neurogenic claudication and having verified ligamentum flavum hypertrophy as a contributing factor. This study was designed to assess 2 minimally invasive therapies, with epidural steroids, an active control considered to be a standard treatment with a high level of evidence. MiDAS ENCORE has been approved by the Centers for Medicare & Medicaid Services (CMS) as a Coverage with Evidence Development (CED) study to provide high quality evidence supporting the clinical safety and effectiveness of the MILD procedure (12). MiDAS ENCORE is registered with the US Clinical Trial Registry (NCT ). This is a report of 6-month results for patients participating in this trial. METHODS MiDAS ENCORE is being conducted at 26 interventional pain management centers in the United States. The trial protocol was approved by Institutional Review Boards for all participating sites and Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed (13). The study design of MiDAS ENCORE has been previously described (14). Patients Study participants include 302 Medicare beneficiaries who met all study inclusion/exclusion criteria, as well as an additional symptomatic diagnosis screening assessment to confirm symptoms of neurogenic claudication (15) (Table 1). All patients provided written informed consent. Patients in both study arms are required to complete study follow-up evaluations at 6 months and one year. Supplementary safety and efficacy outcome data will be collected for patients in the MILD arm through 2 years. Randomization Patients were randomized in an allocation ratio of 1-to-1 to the MILD or ESI study cohorts. Randomization was implemented automatically through an online electronic data collection system. The treatment regimens were substantially different between the 2 groups, including the allowance of multiple ESI procedures for the active control group during the study period. As a result, neither investigator nor patient blinding was feasible. In order to minimize advance patient knowledge of study group, sites were advised to inform patients of their randomization group on the day of the procedure. Preoperative instructions and workup were the same for all patients. 26
7 MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results Table 1. Selection criteria and neurogenic claudication symptomatic diagnosis Inclusion Criteria years or older and a Medicare beneficiary. 2. Patients experiencing neurogenic claudication symptoms for at least 3 months duration which has failed to respond or poorly responded to physical therapy, home exercise programs, and oral analgesics. 3. Lumbar spinal stenosis with neurogenic claudication diagnosed via a. Symptomatic diagnosis (see below) and b. Radiologic evidence of LSS with unilateral or bilateral ligamentum flavum > 2.5 mm confirmed by pre-op MRI or CT performed within 12 months of baseline visit. 4. Patients with comorbid conditions commonly associated with spinal stenosis, such as osteophytes, facet hypertrophy, minor spondylolisthesis, foraminal stenosis, and/or disk protrusion may be included unless the treating physician has determined that the condition is too advanced. 5. Available to complete 6-month and 1-year follow-up visits. Exclusion Criteria 1. ODI Score < 31 (0-100 ODI Scale). 2. NPRS Score < 5 (0-10 NPRS Scale). 3. Prior surgery at any treatment level. 4. History of spinal fractures with current related pain symptoms. 5. Patients with Grade III or higher spondylolisthesis. 6. Motor deficit or disabling back and/or leg pain from causes other than LSS neurogenic claudication. 7. Unable to walk 10 feet unaided before being limited by pain. 8. Patients previously randomized and/or treated in this clinical study. 9. Patients that have previously received the MILD procedure. 10. ESI during 8 weeks prior to study enrollment. 11. Epidural lipomatosis (if deemed to be a significant contributor of canal narrowing by the physician). 12. On (or pending) Workman s Compensation or known to be considering litigation associated with back pain. Neurogenic Claudication Symptomatic Diagnosis 1. Pain/Discomfort in leg, buttocks, or lower back while walking or standing. 2. Pain relief experienced when bending forward or sitting down. 3. Flexion forward while walking. 4. Inability to stand unaided for more than 15 minutes without bending at the waist. 5. Inability to walk unaided for more than one quarter mile without bending at the waist. 6. History of symptoms 12 weeks. Interventions MILD percutaneous lumbar decompression uses a dorsal approach to decompress the spinal canal by selectively removing small portions of lamina and hypertrophic ligamentum flavum, while minimizing trauma to surrounding tissue. Generally conducted using local anesthetic and moderate sedation, this procedure is performed ipsilaterally through a small 6-gauge port and does not involve the use of implants. Contrastenhanced fluoroscopic guidance provides visualization throughout the MILD procedure. The MILD procedure has been previously described (14,16-24). In the active control group, ESIs are administered via an interlaminar approach, using intermittent fluoroscopy with contrast to guide needle placement. Patients assigned to ESIs may have up to 4 treatments per year, consistent with American Society of Interventional Pain Physicians (ASIPP) guidelines (25). The interval between ESIs was recommended to be 2 months or longer, provided that > 50% relief was obtained for 2 months (25). Patients received 80 mg of Kenalog or Depo-Medrol (40 mg for diabetics) during the initial ESI procedure, and between 40 mg and 80 mg during subsequent procedures. For both study arms, treatment could be unilateral or bilateral, and at multiple levels. Patients were discharged per institutional standard of care, and provided instructions regarding the use of any adjunctive conservative therapies. MILD patients are prohibited from receiving ESIs in the lumbar region during the study period. The use of opioid and non-opioid analgesics for neurogenic claudication pain are recorded. Outcome Measures Multiple validated outcome measures are used including the Oswestry Disability Index (ODI), Numeric Pain Rating Scale (NPRS), and Zurich Claudication Questionnaire (ZCQ). ODI is used to evaluate functional disability by providing an assessment of the effect of back pain on activities of daily living (26). ODI ranges from 0 to 100, with lower scores indicating less severe symptoms. NPRS measures the level of back and leg pain on a 0 to 10 scale, from no pain to the worst pain imaginable (27). ZCQ is an assessment tool specific to LSS that evaluates symptom severity, physical function characteristics, and patient satisfaction following treatment. ZCQ is comprised of symptom severity and physical 27
8 Pain Physician: February 2016: 19:25-37 function domains. The ZCQ symptom severity domain is divided into 2 subdomains pain and neuroischemic. For each of these domains, lower scores indicate better health status. The ZCQ patient satisfaction domain is recorded at follow-up only, and lower scores indicate higher patient satisfaction with the procedure (19,22). For all protocol-defined outcome measures, efficacy is determined by comparing the percentage of responders between the 2 study groups. The primary efficacy outcome measure is the proportion of ODI responders, tested for statistical superiority of MILD versus epidural steroids, a known active treatment. ODI responders are defined as patients who report a 10 point improvement in ODI score from baseline to follow-up. Published validation studies have indicated that a 10-point Minimal Important Change (MIC) improvement in ODI score represents a clinically significant efficacy threshold (28,29). Patients who did not experience a 10-point improvement in ODI at follow-up, or who received or intended to receive a disallowed treatment in the lumbar region, or who voluntarily withdrew because of poor response to the study procedure were considered non-responders. Secondary efficacy endpoints include evaluation of the proportion of NPRS and ZCQ responders in each of the 2 study groups using validated MIC thresholds. An improvement in NPRS of 2 points has been determined to be a MIC (28-32). An improvement of 0.5 in ZCQ domains denotes a MIC, and an absolute patient satisfaction score of 2.5 indicates that a patient is satisfied with the procedure (33-36). Statistical superiority is determined by comparing the proportion of responders between the study groups. All device or procedure-related adverse events, as well as all serious adverse events regardless of relationship, are reported. The primary safety outcome measure is the incidence of device or procedure-related adverse events. All reportable adverse events through 6-month follow-up have been evaluated and adjudicated by the study principal investigators, and adjudicated outcomes are used for reporting. For standardization of reporting, all adverse events have been coded by an outside agency into MedDRA System Organ Class and Preferred Term classifications. Sample Size and Power Sample size was calculated to obtain at least 80% power for testing the primary superiority hypothesis. The total sample size of 302 is sufficient to meet this objective under the assumption of a 2-sided hypothesis, type 1 error of 0.05, power (1-β) at least 80%, randomization ratio of 1:1, and accounting for dropouts. Statistical Methods Descriptive summaries are presented by randomized group for all baseline and outcome measures. Continuous data is summarized using means and standard deviations, while categorical variables are summarized using frequency counts and percentages. All P values presented are 2-sided, with values less than 0.05 considered statistically significant. The primary efficacy objective is to demonstrate statistical superiority of MILD to epidural steroids on the proportion of ODI responders. The hypothesis is tested by constructing the 2-sided 95% confidence interval around the difference between the population proportions (pmild pesi). If the lower bound of the 2-sided confidence interval is greater than 0, superiority is declared and the endpoint met. Secondary efficacy endpoints are also tested for superiority of MILD to epidural steroids. The primary safety endpoint is met if the device or procedure-related adverse event rate is not significantly greater with MILD than with epidural steroids. RESULTS Participant Flow MiDAS ENCORE patients were enrolled from June 2014 through April A total of 302 patients were included out of 320 patients assessed for eligibility. Eighteen patients did not meet the study selection criteria and were excluded. Group allocation included 149 patients randomized to MILD and 153 to epidural steroids. Following randomization, 6 MILD and 22 epidural steroid patients voluntarily withdrew prior to study treatment, leaving 143 and 131 patients in the MILD and ESI cohorts, respectively. Of these, 2 ESI patients missed their 6-month follow-up visit, resulting in 143 MILD and 129 ESI patients for 6-month data analysis. Fig. 1 presents the participant flow through 6 months. Patient Characteristics Patient characteristics and baseline clinical data are provided in Table 2. There was a significant difference in gender between the 2 groups, with a larger proportion of men in the MILD group. The most commonly reported lumbar spine presenting co-factors included bulging disc, foraminal narrowing, facet hypertrophy, and facet arthropathy, and were similar in incidence except that the epidural steroid group had significantly 28
9 MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results Fig. 1. Presentation of patient flow through 6-month follow-up. Table 2. Patient characteristics. Characteristic MILD N=149 ESI N=153 P-value Age (Years) 75.6 ± ± Gender Male Female 49.7% (74) 50.3% (75) 37.9% (58) 62.1% (95) Lumbar Spine Presenting Co-Factors Ligamentum flavum hypertrophy 100.0% (149) 100.0% (153) Bulging disc 89.9% (134) 91.5% (140) Foraminal narrowing 87.2% (130) 88.2% (135) Facet hypertrophy 86.6% (129) 81.0% (124) Facet arthropathy 76.5% (114) 86.3% (132) 0.029* Degenerative disc disease 67.8% (101) 74.5% (114) Disc space loss 59.1% (88) 63.4% (97) Lateral recess narrowing 57.0% (85) 53.6% (82) Osteophytes 47.7% (71) 47.7% (73) Spondylosis 47.0% (70) 54.9% (84) Spondylolisthesis 44.3% (66) 52.3% (80) Nerve root impingement 33.6% (50) 36.6% (56) Herniated disc 27.5% (41) 36.6% (56) Scoliosis 22.1% (33) 26.8% (41) Other 19.5% (29) 21.6% (33) Oswestry Disability Index (ODI) 53.0 ± ± Numeric Pain Rating Scale (NPRS) 7.7 ± ± Zurich Claudication Questionnaire (ZCQ) Pain subdomain 3.8 ± ± Neuroischemic subdomain 3.2 ± ± Physical function domain 2.9 ± ± *significant difference between groups Mean ± SD *
10 Pain Physician: February 2016: 19:25-37 more patients presenting with facet arthropathy than the MILD group. Baseline values for ODI, NPRS, and ZCQ domains are also presented in Table 2. There were no significant differences between the groups at baseline. Previous conservative therapies were documented (Table 3). All patients had a history of physical therapy and had undergone a program of home exercise, while approximately half had prior experience with chiropractic adjustment. Aquatic therapy was the only prior conservative treatment that was different between the groups with a significantly higher incidence in the epidural steroid group. Procedures Procedure data is provided in Table 4. Procedure times for MILD patients were significantly greater than for ESI (mean of 43.0 minutes versus 7.7 minutes, respectively). Significantly more ESI patients underwent the study procedure outside of an ambulatory surgery center (ASC) or hospital, with ESI procedures primarily occurring in an office setting. Anesthesia was most commonly delivered via monitored anesthesia care (MAC) sedation for MILD patients, and by MAC sedation or local anesthesia only for ESI. The administration of ESIs is almost always done at a single level with the expectation of steroid migration to other levels, whereas MILD requires treatment at each level. In the MILD group, 67.7% (97 patients) received treatment at one level, 29.4% (42 patients) received treatment at 2 levels, and the remaining 2.8% (4 patients) received treatment at 3 levels. Specific lumbar levels treated in the MILD group were as follows: 9.8% (14 patients) L2-L3, 39.9% (57 patients) L3-L4, 77.6% (111 patients) L4-L5, 7.7% (11 patients) L5-S1. Frequency of unilateral and bilateral treatments did not differ between the groups. All patients were discharged within 24 hours of the procedure. While Table 4 reflects initial procedure data, patients in the ESI arm are allowed up to 4 ESI treatments during the one year study period. The 131 ESI patients underwent a total of 217 ESI procedures during the first 6 months of the trial. On average, ESI patients received 1.7 ESI treatments during the first 6 months (including initial treatment) with a range of one to 4, and a median of one. Medications At baseline, 90.6% of MILD and 83.0% of ESI patients reported use of medication for neurogenic claudication, and there were no significant differences between the groups (P = 0.075). At 6 months, the percent of patients using these medications in the MILD arm decreased slightly to 89.5%, and in the ESI arm increased to 85.3%. None of these changes were significant, and there were no significant differences between the groups (P = 0.44) (Table 5). Function and Pain Outcomes For the primary efficacy endpoint, the proportion of ODI responders in the MILD group was statistically significantly higher than the proportion of ODI responders in the ESI group at 6-month follow-up. The ODI responder rate for the MILD arm was 62.2% versus 35.7% in the ESI arm (P < 0.001). In addition, for all secondary efficacy endpoints, the proportion of responders in the MILD group was statistically significantly higher than the proportion of responders in the ESI group. Results of primary and secondary efficacy outcome measures are presented in Table 6. Fig. 2 provides a graphic illustration of the proportion of responders at 6 months for all primary and secondary efficacy endpoints. Table 3. Lumbar spine history previous treatments. Physical therapy Home exercise program Back brace Bed rest Walking aids Aquatic therapy Acupuncture Chiropractic adjustment TENS unit Biofeedback Activity restriction Other *significant difference between groups Treatment MILD % (n/n) ESI % (n/n) P-value 100% (149/149) 100% (149/149) 33.8% (50/148) 6.0% (9/149) 46.3% (69/149) 13.4% (20/149) 20.8% (31/149) 50.3% (75/149) 28.2% (42/149) 1.3% (2/149) 18.1% (27/149) 6.8% (10/148) 100% (153/153) 100% (153/153) 27.6% (42/152) 10.5% (16/153) 41.8% (64/153) 24.8% (38/153) 13.7% (21/153) 46.4% (71/153) 26.8% (41/153) 0.0% (0/153) 19.0% (29/153) 7.2% (11/152) *
11 MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results Table 4. Initial procedure information. Metric MILD N=143 ESI N=131 P-value Procedure time (min) 43.0 ± 24.3 (142) 7.7 ± 6.7 (131) <0.001* Procedure setting Ambulatory Surgery Center (ASC) Hospital outpatient Hospital inpatient Other (includes office setting) Anesthesia type General only General and local Local only MAC sedation Local and other Other Unilateral Treatment Bilateral Treatment *significant difference between groups 65.7% (94) 33.6% (48) 0.0% (0) 0.7% (1) 0.7% (1) 0.7% (1) 1.4% (2) 86.0% (123) 4.2% (6) 7.0% (10) 6.3% (9) 93.7% (134) 62.6% (82) 21.4% (28) 0.8% (1) 15.3% (20) <0.001* 0.0% (0) 0.0% (0) 44.3% (58) 45.8% (60) 9.2% (12) 0.8% (1) <0.001* 13.7% (18) 86.3% (113) Table 5. Medication for neurogenic claudication at 6-month follow-up. Medication at 6 Months MILD N=133 % (n) [events] ESI N=109 % (n) [events] P-value ALL MEDICATIONS for Neurogenic Claudication 89.5% (119) [236] 85.3% (93) [190] 0.44 Acetaminophen And Hydrocodone 26.3% (35) [36] 23.9% (26) [31] 0.77 Gabapentin 20.3% (27) [28] 15.6% (17) [18] 0.44 Tramadol 18.0% (24) [26] 20.2% (22) [22] 0.80 Ibuprofen 15.0% (20) [20] 11.9% (13) [13] 0.61 Acetaminophen 14.3% (19) [19] 14.7% (16) [16] 1.00 Naproxen 13.5% (18) [19] 11.9% (13) [13] 0.86 Hydrocodone 9.0% (12) [12] 7.3% (8) [8] 0.81 Acetaminophen And Oxycodone 7.5% (10) [11] 9.2% (10) [10] 0.82 Celecoxib 4.5% (6) [6] 0.9% (1) [1] 0.20 Meloxicam 3.8% (5) [5] 3.7% (4) [4] 1.00 Oxycodone 3.8% (5) [5] 2.8% (3) [3] 0.94 Diclofenac Topical 3.0% (4) [4] 1.8% (2) [2] 0.87 Acetaminophen And Codeine 3.0% (4) [4] 0.9% (1) [2] 0.49 Lidocaine Topical 2.3% (3) [3] 5.5% (6) [6] 0.32 Pregabalin 2.3% (3) [3] 3.7% (4) [5] 0.80 Diclofenac 2.3% (3) [3] 2.8% (3) [3] 1.00 Note: An additional 33 medications were reported to be in use by less than 4% of patients at 6 months. Table 7 presents mean change in ODI, NPRS, and ZCQ domain scores from baseline to 6-month follow-up. A comparison of mean changes between the study groups shows statistically significantly greater improvement in the MILD arm versus the active control for all outcome measures. The mean (± SE) ZCQ Patient Satisfaction score for MILD was 2.3 ± 0.1 versus 3.0 ± 0.1 for the active control (P < 0.001). In addition, for both groups and for all efficacy endpoints, the within group change from baseline to follow-up was statistically significant. 31
12 Pain Physician: February 2016: 19:25-37 Table 6: Primary and secondary efficacy proportion of responders at 6 months. Outcome Measure: Responder Definition MILD % (n/n) ESI % (n/n) P-value (between groups) Primary Efficacy: ODI: 10 point improvement 62.2% (89/143) 35.7% (46/129) <0.001* Secondary Efficacy: NPRS: 2.0 point improvement ZCQ: 0.5 point improvement in each domain Pain subdomain Neuroischemic subdomain Physical function domain ZCQ: Patient satisfaction 2.5 *significant difference between groups Lower scores indicate a higher level of satisfaction with the procedure. 55.9% (80/143) 58.0% (83/143) 50.0% (71/142) 52.4% (75/143) 64.8% (92/142) 33.3% (43/129) 42.6% (55/129) 30.2% (39/129) 14.0% (18/129) 30.2% (39/129) <0.001* 0.011* 0.001* <0.001* <0.001* Fig. 2. Illustration of proportion of responders at 6 months for all efficacy endpoints. Safety As the primary safety analysis, Table 8 presents the incidence of device or procedure-related adverse events in each cohort. With the same percentage of patients in each study arm experiencing a device or procedure-related adverse event (1.3%), there was no significant difference in safety between the 2 study groups (P = 1.00). There were no serious device or procedure-related adverse events in either cohort. There also is no statistical difference between the groups in any of the MedDRA System Organ Class or Preferred Term classifications. During one MILD case, a procedural hemorrhage was reported. In this case, intraoperative oozing was observed at the decompression site and Gelfoam was administered through the cannula into the interlaminar space. This event was categorized by the site as mild, and this characterization was upheld with subsequent adjudication. The patient was discharged on the same day as the procedure with no complications. A further 32
13 MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results Table 7. Mean change in outcome measures at 6 months. Outcome Measure MILD ESI P-value (between groups) Oswestry Disability Index Mean ± SE ± 1.6 (143) -5.6 ± 1.3 (129) Median (min, max) (-77.1, 20.0) 0.0 (-45.7, 31.4) <0.001* P-value (within group) <0.001 <0.001 Numeric Pain Rating Scale Mean ± SE -2.9 ± 0.3 (143) -0.9 ± 0.2 (129) Median (min, max) -2.0 (-10.0, 3.0) 0.0 (-6.0, 3.0) <0.001* P-value (within group) <0.001 <0.001 Zurich Claudication Questionnaire Pain subdomain Mean ± SE -0.8 ± 0.1 (143) -0.4 ± 0.1 (129) <0.001* Median (min, max) -0.5 (-3.2, 0.8) -0.2 (-2.8, 1.0) P-value (within group) <0.001 <0.001 Neuroischemic subdomain Mean ± SE -0.7 ± 0.1 (142) -0.3 ± 0.1 (129) <0.001* Median (min, max) -0.5 (-3.3, 2.0) 0.0 (-2.7, 1.3) P-value (within group) < Physical function domain Mean ± SE -0.6 ± 0.1 (143) -0.1 ± 0.1 (129) <0.001* Median (min, max) -0.6 (-2.6, 1.0) 0.0 (-1.4, 0.8) P-value (within group) < *significant difference between groups significant difference with baseline values within the group Table 8. Adverse events. Adverse event MILD N=149 % (n) [events] ESI N=153 % (n) [events] P-value Total related AEs 1.3% (2) [2] 1.3% (2) [3] 1.00 Total related SAEs 0.0% (0) [0] 0.0% (0) [0] 1.00 MedDRA system organ class / preferred term Cardiac disorders 0.0% (0) [0] 0.7% (1) [1] 1.00 Sinus bradycardia 0.0% (0) [0] 0.7% (1) [1] 1.00 Injury, poisoning and procedural complications 1.3% (2) [2] 0.0% (0) [0] 0.47 Procedural haemorrhage 0.7% (1) [1] 0.0% (0) [0] 0.99 Procedural pain 0.7% (1) [1] 0.0% (0) [0] 0.99 Musculoskeletal and connective tissue disorders 0.0% (0) [0] 0.7% (1) [2] 1.00 Back pain 0.0% (0) [0] 0.7% (1) [1] 1.00 Pain in extremity 0.0% (0) [0] 0.7% (1) [1] 1.00 comparison of the incidence of all serious, non-related adverse events identified no statistically significant differences between groups (10.1% and 7.2% for MILD and the active control, respectively) (P = 0.49). DISCUSSION All primary and secondary efficacy endpoints of this randomized controlled clinical trial provided statiswww.painphysicianjournal.com 33
14 Pain Physician: February 2016: 19:25-37 tically significant evidence that MILD is superior to ESIs in the treatment of LSS patients suffering from neurogenic claudication and having verified ligamentum flavum hypertrophy. It is also important to highlight that the within group change was statistically significant for all efficacy endpoints for both study groups. This result supports the comparative design of this study, and reiterates the efficacy of the active control for these patients. Enrolled patients met precise study selection criteria, as well as symptomatic diagnosis screening criteria confirming neurogenic claudication. Twenty-eight patients voluntarily withdrew prior to study treatment, and of those, a disproportionate number were randomized to ESIs (22 patients) versus MILD (6 patients). Of the 22 ESI patients, 8 decided to have surgery or other non-study therapy, 8 withdrew for personal or insurance reasons, and 6 withdrew because of dissatisfaction with randomization results. In the MILD arm, 5 withdrew for personal or insurance reasons and one was unwilling to comply with study assessments. Ultimately, 143 MILD and 131 ESI patients underwent treatment in the trial. Following treatment, 10 MILD and 20 ESI patients withdrew prior to 6-month follow-up due to poor response to the study treatment or intention to receive an invasive non-study procedure. These patients are included in the analysis and are considered to be nonresponders in their study arm. Of 10 MILD patients in this category, 8 received ESIs and 2 received facet blocks with steroids. Of 20 ESI patients, 8 opted for surgical treatment, 4 chose to undergo MILD, 2 received medial branch blocks with steroids, 2 received transforaminal ESIs, and 4 stated that their symptoms did not improve and they chose to withdraw. The number of ESI patients withdrawn due to poor response to their study procedure is numerically although not significantly greater than for MILD. The safety and efficacy outcomes of patients treated with MILD in this trial are supported by numerous previous reports of MILD patient series. Mekhail and colleagues (37) reported one-year follow-up for 40 patients treated prospectively with MILD at a single center. Patients in this study experienced statistically significant improvement in function and neurogenic claudication symptoms at 3, 6, 9, and 12 months post procedure. Deer et al (38) reported one-year follow-up of 46 patients treated with MILD at a single center. In this prospective study, MILD patients experienced significant improvement in mobility and reduction of pain at 12-week, 6-month, and one-year follow-up. The longest follow-up was reported by Chopko (39) in a report of 45 MILD patients treated prospectively at 11 US sites. At 2 years, MILD patients in this study experienced statistically significant pain relief and improved functionality. This significant improvement was initially observed at one week post MILD, and proved to be durable through 2 years (39). These 3 studies with one and 2-year follow-up indicate that improvements in patient outcomes following MILD ligamentum flavum debulking remain stable over the long term. While there is no conclusive data regarding ligamentum flavum regrowth, physiologically the fibrous connective tissue of the ligamentum flavum does not have significant blood supply, and therefore regeneration is most likely slow. In the only other published randomized controlled trial comparing MILD with ESIs, Brown (17) reported significantly greater improvements in pain and function for MILD versus ESI patients at 6 weeks. No significant device or procedure-related adverse events were reported in any of these studies. It is common for LSS patients presenting with neurogenic claudication to also suffer from other pathophysiological causes of low back pain and reduced mobility. While all study patients presented with hypertrophic ligamentum flavum, there were numerous other lumbar spine co-factors frequently reported (Table 2). It is notable, that while the MILD procedure debulks the hypertrophic ligamentum flavum specifically, these patients with multiple lumbar spine co-factors experienced significant improvement in neurogenic claudication symptoms. While ESIs did not demonstrate the same level of efficacy as MILD in this trial, some published studies have reported successful outcomes using ESIs to treat discogenic and radicular pain (40-44). Given the range of pathophysiological causes of low back pain, including inflammation in many cases, ESIs may be an appropriate adjunct therapy for patients undergoing MILD. Three patients were treated with the alternate study therapy, instead of the therapy to which they were randomized. Per ITT methodology, these patients are included with their original randomization group, however a supplementary as treated analysis was conducted. In this analysis, the proportion of ODI responders still demonstrated statistically significant superiority of MILD versus ESIs. The ODI responder rate for MILD patients was 61.3% (87/142) compared to 36.9% (48/130) for ESI patients (P = 0.001). In addition, the proportion of responders for all secondary efficacy endpoints was still statistically significantly higher with MILD versus the active control, and there was no difference in safety between groups. 34
15 MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results A covariate effects analysis determined that the 3 significant baseline differences did not meaningfully impact the primary endpoint outcome. Additionally, covariates of clinical interest (gender, age, baseline ODI score, facet arthropathy, degenerative disc disease, lateral recess narrowing, and spondylolisthesis) were examined in subgroup analyses to evaluate their effect on the primary endpoint success. The superiority of the treatment effect of MILD is unaffected by the introduction of these potential predictors into the primary effectiveness model. There were certain limitations of this trial. In order to minimize confounding between the 2 study arms, adjunctive pain therapy within the lumbar region is restricted. As a result, responder rates may be lower for both groups within the trial compared to a non-study setting, as previously described (14). In addition, due to significant differences in treatment protocols between the 2 groups, including multiple ESI procedures during the study period, patient blinding was not possible. CONCLUSION The data from this trial demonstrate that at 6-month follow-up, the MILD procedure is statistically superior to epidural steroids, a known active treatment for LSS patients with neurogenic claudication and verified central stenosis due to ligamentum flavum hypertrophy. The results of all primary and secondary efficacy outcome measures achieved statistically superior outcomes in the MILD group versus ESIs. Further, there were no statistically significant differences in the safety profile between study groups. These results collectively confirm that the study s primary safety and efficacy hypotheses are met at 6 months. This prospective, multi-center, randomized controlled clinical trial provides strong evidence of the effectiveness of MILD versus epidural steroids in this patient population. Acknowledgments The authors wish to thank Scott Brown, PhD, of Altair Biostatistics LLC for statistical analysis, and Debbie Barber, MS, an independent contractor, for assistance in preparation of this manuscript. The authors would also like to thank the editorial board of Pain Physician for review and criticism in improving the manuscript. Appendix The following investigators enrolled patients in the study, with institutions listed in order from highest to lowest enrollment of patients: Deaconess Comprehensive Pain Center West, Evansville, IN: F. McDonnell, J. Waling; Michigan Interventional Pain Center, Brownstown Township, MI: R. Haladjian, N. Patel; Spine Intervention Medical Corp, Fresno, CA: W. Von Kaenel; Roanoke-Chowan Pain Management, Ahoskie, NC: B. Chafin; Newport Beach Headache and Pain, Newport Beach, CA: R. Paicius; Southeastern Spine Institute, Mt. Pleasant, SC: W.B. Richardson, M. Netherton; Premier Pain Centers, Shrewsbury, NJ: S. Li; Willow Creek Pain Center, Vincennes, IN: G. Chartier; Florida Pain Institute, Merritt Island, FL: S. Golovac; Millennium Pain Center, Bloomington, IL: R. Vallejo; Pain Consultants of San Diego, La Mesa, CA: M. Verdolin; Michigan Pain Specialists, Ypsilanti, MI: E. Washabaugh, J. Chatas, L. Bojrab; Regenerative Institute of Newport Beach, Newport Beach, CA: K. Zaffarkhan, H. Sata; Kramer Orthopedics, Newport Beach, CA: S. Kramer; SC Pain & Spine Specialists, Murrells Inlet, SC: J. Rosenberg; The Knox Surgical Center, Covington, GA: M. Hanowell; Frankfort Pain Clinic, Frankfort, KY: R. Lingreen; The Spine Institute, Murrieta, CA: V. Johnson; Montefiore Medical Center, Bronx, NY: S. Wahezi; Valley Pain Consultants, Scottsdale, AZ: D. Choi; The Center for Pain Relief, Charleston, WV: C. Kim, R. Bowman; Texas Spine and Joint Hospital, Tyler, TX; A. Calodney; Advanced Pain Management, Rancho Mirage, CA: R. Reinhart; Mayo Clinic, Rochester, MN: T. Lamer, B. Hoelzer; Comprehensive Center for Pain Management, Toledo, OH: N. Moghal, W. James. 35
16 Pain Physician: February 2016: 19:25-37 REFERENCES 1. Katz JN, Harris MB. Lumbar spinal stenosis. N Engl J Med 2008; 358: Simotas AC, Direy FJ, Hansraj KK, Cammisa F. Nonoperative treatment for lumbar spinal stenosis: Clinical and outcome results and a 3-year survivorship analysis. Spine 2000; 25: Porter RW. Spinal stenosis and neurogenic claudication. Spine 1996; 21: Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Sullivan SD, Bauer Z, Bresnahan BW, Avins AL, Nedeljkovic SS, Nerenz DR, Standaert C, Kessler L, Akuthota V, Annaswamy T, Chen A, Diehn F, Firtch W, Gerges FJ, Gilligan C, Goldberg H, Kennedy DJ, Mandel S, Tyburski M, Sanders W, Sibell D, Smuck M, Wasan A, Won L, Jarvik JG. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med 2014; 371: Manchikanti L, Candido KD, Kaye AD, Boswell MV, Benyamin RM, Falco FJ, Gharibo CG, Hirsch JA. Randomized trial of epidural injections for spinal stenosis published in the New England Journal of Medicine: Further confusion without clarification. Pain Physician 2014; 17:E475-E Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Results of 2-year follow-up of a randomized, doubleblind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician 2012; 15: Manchikanti L, Cash KA, McManus CD, Damron KS, Pampati V, Falco FJ. A randomized, double-blind controlled trial of lumbar interlaminar epidural injections in central spinal stenosis: 2-year follow-up. Pain Physician 2015; 18: Manchikanti L, Kaye AD, Manchikanti K, Boswell M, Pampati V, Hirsch J. Efficacy of epidural injections in the treatment of lumbar central spinal stenosis: A systematic review. Anesth Pain Med 2015; 5:e Manchikanti L, Falco FJ, Pampati V, Hirsch JA. Lumbar interlaminar epidural injections are superior to caudal epidural injections in managing lumbar central spinal stenosis. Pain Physician 2014; 17:E691-E Weinstein JN, Tosteson RD, Lurie JD, Tosteson ANA, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H, for the SPORT Investigators. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008; 358: Deyo RA, Martin BI, Ching A, Tosteson AN, Jarvik JG, Kreuter W, Mirza SK. Interspinous spacers compared with decompression or fusion for lumbar stenosis: Complications and repeat operations in the Medicare population. Spine (Phila Pa 1976) 2013; 38: Tunis SR, Stryer DB, Clancy CM. Practical clinical trials. Increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003; 290: Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CON SORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomized trials. BMJ 2010; 340:c Benyamin RM, Staats PS. MiDAS EN CORE: Randomized controlled study design and protocol. Pain Physician 2015; 18: Sandella DE, Haig AJ, Tomkins-Lane C, Yamakawa K. Defining the clinical syndrome of lumbar spinal stenosis: A recursive specialist survey process. PMR 2013; 5: Basu S. Mild procedure single-site prospective IRB study. Clin J Pain 2012; 28: Brown L. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract 2012; 12: Chen H, Kelling J. mild procedure for lumbar decompression: A review. Pain Pract 2013; 13: Chopko BW, Caraway D. MiDAS I (mild decompression alternative to open surgery): A preliminary report of a prospective, multi-center clinical study. Pain Physician 2010; 13: Deer T, Kapural L. New image-guided ultra-minimally invasive lumbar decompression method: The mild procedure. Pain Physician 2010; 13: Deer T, Mekhail N, Lopez G, Amirdelfan A. Minimally invasive lumbar decompression for spinal stenosis. JNR 2011; 1: Mekhail N, Vallejo R, Coleman MH, Benyamin RM. Long-term results of percutaneous lumbar decompression mild for spinal stenosis. Pain Pract 2012; 12: Schomer DF, Solsberg D, Wong W, Chopko BW. Mild Lumbar decompression for the treatment of lumbar spinal stenosis. Neuroradiology J 2011; 24: Wong WHM. mild interlaminar decompression for the treatment of lumbar spinal stenosis. Procedure description and case series with 1-year follow-up. Clin J Pain 2012; 28: Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, Bryce DA, Burks PA, Caraway DL, Calodney AK, Cash KA, Christo PJ, Cohen SP, Colson J, Conn A, Cordner H, Coubarous S, Datta S, Deer TR, Diwan S, Falco FJ, Fellows B, Geffert S, Grider JS, Gupta S, Hameed H, Hameed M, Hansen H, Helm S 2nd, Janata JW, Justiz R, Kaye AD, Lee M, Manchikanti KN, McManus CD, Onyewu O, Parr AT, Patel VB, Racz GB, Sehgal N, Sharma ML, Simopoulos TT, Singh V, Smith HS, Snook LT, Swicegood JR, Vallejo R, Ward SP, Wargo BW, Zhu J, Hirsch JA. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: Guidance and recommendations. Pain Physician 2013; 16:S49-S Fairbank NCT, Pynsent PB. The Oswestry Disability Index. Spine 2000; 25: Williamson A, Hoggart B. Pain: A review of three commonly used pain rating scales. J Clin Nurs 2005; 14: Hägg O, Fritzell P, Nordwall A; Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 2003; 12: Ostelo RWJG, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC. Interpreting change scores for pain and functional status in low back pain towards international consensus regarding minimal important change. Spine 2008; 33: Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005; 30: Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: Salaffi F, Stancati A, Silverstri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic muscuwww.painphysicianjournal.com
10801 Sixth St, Suite 120 Rancho Cucamonga, CA 91730 Tel (909) 890-2000 Fax (909) 890-2003 Visit our web site at: www.iehp.org.
 Percutaneous Image-Guided Lumbar Decompression for Lumbar Spinal Stenosis (PILD), Including the Commercial Procedure Known as Minimally Invasive Lumbar Decompression (mild -Vertos Medical) Policy: Based
Percutaneous Image-Guided Lumbar Decompression for Lumbar Spinal Stenosis (PILD), Including the Commercial Procedure Known as Minimally Invasive Lumbar Decompression (mild -Vertos Medical) Policy: Based
Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis
 Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis Policy Number: 7.01.126 Last Review: 6/2015 Origination: 5/2010 Next Review: 6/2016 Policy Blue Cross and Blue Shield of Kansas
Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis Policy Number: 7.01.126 Last Review: 6/2015 Origination: 5/2010 Next Review: 6/2016 Policy Blue Cross and Blue Shield of Kansas
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Imaged-Guided Minimally Invasive Lumbar Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Image-Guided Minimally Invasive Lumbar Professional Institutional
Imaged-Guided Minimally Invasive Lumbar Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Image-Guided Minimally Invasive Lumbar Professional Institutional
Effective Date: 01/01/2012 Revision Date: 07/24/2013 Comments: Policy Accepted during 2013 Annual Review with no changes.
 Health Plan Coverage Policy ARBenefits Approval: 01/01/2012 Effective Date: 01/01/2012 Revision Date: 07/24/2013 Comments: Policy Accepted during 2013 Annual Review with no changes. Title: Minimally Invasive,
Health Plan Coverage Policy ARBenefits Approval: 01/01/2012 Effective Date: 01/01/2012 Revision Date: 07/24/2013 Comments: Policy Accepted during 2013 Annual Review with no changes. Title: Minimally Invasive,
mild Lumbar Decompression for the Treatment of Lumbar Spinal Stenosis
 The Neuroradiology Journal 24: 620-626, 2011 www.centauro.it mild Lumbar Decompression for the Treatment of Lumbar Spinal Stenosis D.F. SCHOMER 1, D. SOLSBERg 1, W. WONg 2, B.W. CHOPkO 3 1 Radiology Imaging
The Neuroradiology Journal 24: 620-626, 2011 www.centauro.it mild Lumbar Decompression for the Treatment of Lumbar Spinal Stenosis D.F. SCHOMER 1, D. SOLSBERg 1, W. WONg 2, B.W. CHOPkO 3 1 Radiology Imaging
Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization
 Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
X Stop Spinal Stenosis Decompression
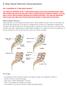 X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
Name of Policy: Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis
 Name of Policy: Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis Policy #: 419 Latest Review Date: April 2015 Category: Surgery Policy Grade: B Background/Definitions: As a general
Name of Policy: Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis Policy #: 419 Latest Review Date: April 2015 Category: Surgery Policy Grade: B Background/Definitions: As a general
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis:
 Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis: Presented By: Michelle Emsley Senior Spinal In-Patient Physiotherapist Learning objectives Indications Evidence Post operative
Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis: Presented By: Michelle Emsley Senior Spinal In-Patient Physiotherapist Learning objectives Indications Evidence Post operative
Medical Affairs Policy
 Medical Affairs Policy Service: Sacroiliac Joint Treatments and Coccydynia Injections: Sacroiliac Joint Injections, Sacroiliac Joint Ablation, Sacroiliac Neuroablation, Sacroiliac Fusion, Lateral Branch
Medical Affairs Policy Service: Sacroiliac Joint Treatments and Coccydynia Injections: Sacroiliac Joint Injections, Sacroiliac Joint Ablation, Sacroiliac Neuroablation, Sacroiliac Fusion, Lateral Branch
Title: Interspinous Process Decompression with the X-Stop Device for Lumbar Spinal Stenosis: A Retrospective Review. Authors: Jennifer R.
 Title: Interspinous Process Decompression with the X-Stop Device for Lumbar Spinal Stenosis: A Retrospective Review. Authors: Jennifer R. Madonia-Barr, MS, PA-C and David L. Kramer, MD Institution: Connecticut
Title: Interspinous Process Decompression with the X-Stop Device for Lumbar Spinal Stenosis: A Retrospective Review. Authors: Jennifer R. Madonia-Barr, MS, PA-C and David L. Kramer, MD Institution: Connecticut
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy
 Pain Physician 2007; 10:313-318 ISSN 1533-3159 Case Series Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy R. Allen Hooper
Pain Physician 2007; 10:313-318 ISSN 1533-3159 Case Series Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy R. Allen Hooper
Sample Treatment Protocol
 Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Efficacy of Epidural Injections in the Treatment of Lumbar Central Spinal Stenosis: A Systematic Review
 Efficacy of Epidural Injections in the Treatment of Lumbar Central Spinal Stenosis: A Systematic Review The Harvard community has made this article openly available. Please share how this access benefits
Efficacy of Epidural Injections in the Treatment of Lumbar Central Spinal Stenosis: A Systematic Review The Harvard community has made this article openly available. Please share how this access benefits
OUTLINE. Anatomy Approach to LBP Discogenic LBP. Treatment. Herniated Nucleus Pulposus Annular Tear. Non-Surgical Surgical
 DISCOGENIC PAIN OUTLINE Anatomy Approach to LBP Discogenic LBP Herniated Nucleus Pulposus Annular Tear Treatment Non-Surgical Surgical Facet Joints: bear 20% of weight Discs bear 80% of weight Neural Foramen
DISCOGENIC PAIN OUTLINE Anatomy Approach to LBP Discogenic LBP Herniated Nucleus Pulposus Annular Tear Treatment Non-Surgical Surgical Facet Joints: bear 20% of weight Discs bear 80% of weight Neural Foramen
Lumbar spinal stenosis (LSS) with intermittent neurogenic
 SPINE Volume 40, Number 5, pp 275-282 2015, Wolters Kluwer Health, Inc. All rights reserved. RANDOMIZED TRIAL Superion Interspinous Process Spacer for Intermittent Neurogenic Claudication Secondary to
SPINE Volume 40, Number 5, pp 275-282 2015, Wolters Kluwer Health, Inc. All rights reserved. RANDOMIZED TRIAL Superion Interspinous Process Spacer for Intermittent Neurogenic Claudication Secondary to
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy. Spine Volume 21(16) August 15, 1996, pp 1877-1883
 Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants
 White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
Image-guided Spine Procedures for Relief of Severe Lower Back Pain:
 Image-guided Spine Procedures for Relief of Severe Lower Back Pain: A Guide to Epidural Steroid Injection, Facet Joint Injection, and Selective Nerve Root Block. PETER H TAKEYAMA MD HENRY WANG MD PhD SVEN
Image-guided Spine Procedures for Relief of Severe Lower Back Pain: A Guide to Epidural Steroid Injection, Facet Joint Injection, and Selective Nerve Root Block. PETER H TAKEYAMA MD HENRY WANG MD PhD SVEN
Neck Pain Overview Causes, Diagnosis and Treatment Options
 Neck Pain Overview Causes, Diagnosis and Treatment Options Neck pain is one of the most common forms of pain for which people seek treatment. Most individuals experience neck pain at some point during
Neck Pain Overview Causes, Diagnosis and Treatment Options Neck pain is one of the most common forms of pain for which people seek treatment. Most individuals experience neck pain at some point during
Local Coverage Determination (LCD): Sacroiliac Joint Injections (L34443)
 Local Coverage Determination (LCD): Sacroiliac Joint Injections (L34443) Contractor Information Contractor Name Wisconsin Physicians Service Insurance Corporation LCD Information Document Information LCD
Local Coverage Determination (LCD): Sacroiliac Joint Injections (L34443) Contractor Information Contractor Name Wisconsin Physicians Service Insurance Corporation LCD Information Document Information LCD
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
https://www.laserspineinstitute.com/back_problems/foraminal_stenosis/e...
 Questions? Call toll free 1-866-249-1627 Contact us today. We're here for you seven days a week. MRI Review Consultation Live help Call 1-866-249-1627 Chat Live Home Laser Spine Institute Laser Spine Institute's
Questions? Call toll free 1-866-249-1627 Contact us today. We're here for you seven days a week. MRI Review Consultation Live help Call 1-866-249-1627 Chat Live Home Laser Spine Institute Laser Spine Institute's
Imaging degenerative disk disease in the lumbar spine. Elaine Besancon MS III Dr. Gillian Lieberman
 Imaging degenerative disk disease in the lumbar spine Elaine Besancon MS III Dr. Gillian Lieberman Learning Objectives Anatomy review Pathophysiology of degenerative disc disease Common sequelae of disk
Imaging degenerative disk disease in the lumbar spine Elaine Besancon MS III Dr. Gillian Lieberman Learning Objectives Anatomy review Pathophysiology of degenerative disc disease Common sequelae of disk
Low Back Pain (LBP) Prevalence. Low Back Pain (LBP) Prevalence. Lumbar Fusion: Where is the Evidence?
 15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
International Journal of Therapeutic Applications ISSN 2320-138X
 International Journal of Therapeutic Applications ISSN 2320-138X SURGERY OR CONSERVATIVE MANAGEMENT FOR LUMBAR SPINAL PAIN: WHAT DOES THE EVIDENCE SAY? Supreet Bindra Physiotherapist, ESIC Model Hospital,
International Journal of Therapeutic Applications ISSN 2320-138X SURGERY OR CONSERVATIVE MANAGEMENT FOR LUMBAR SPINAL PAIN: WHAT DOES THE EVIDENCE SAY? Supreet Bindra Physiotherapist, ESIC Model Hospital,
MEDICAL POLICY SUBJECT: SPINAL INJECTIONS (EPIDURAL AND FACET INJECTIONS) FOR PAIN MANAGEMENT
 MEDICAL POLICY SUBJECT: SPINAL INJECTIONS (EPIDURAL AND PAGE: 1 OF: 9 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: SPINAL INJECTIONS (EPIDURAL AND PAGE: 1 OF: 9 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
Spinal Surgery 2. Teaching Aims. Common Spinal Pathologies. Disc Degeneration. Disc Degeneration. Causes of LBP 8/2/13. Common Spinal Conditions
 Teaching Aims Spinal Surgery 2 Mr Mushtaque A. Ishaque BSc(Hons) BChir(Cantab) DM FRCS FRCS(Ed) FRCS(Orth) Hunterian Professor at The Royal College of Surgeons of England Consultant Orthopaedic Spinal
Teaching Aims Spinal Surgery 2 Mr Mushtaque A. Ishaque BSc(Hons) BChir(Cantab) DM FRCS FRCS(Ed) FRCS(Orth) Hunterian Professor at The Royal College of Surgeons of England Consultant Orthopaedic Spinal
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Patients with pain in the neck, arm, low back, or leg (sciatica) may benefit from ESI. Specifically, those with the following conditions:
 Overview An epidural steroid injection (ESI) is a minimally invasive procedure that can help relieve neck, arm, back, and leg pain caused by inflamed spinal nerves. ESI may be performed to relieve pain
Overview An epidural steroid injection (ESI) is a minimally invasive procedure that can help relieve neck, arm, back, and leg pain caused by inflamed spinal nerves. ESI may be performed to relieve pain
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA
 LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
Anatomical Flow Pattern of Contrast in Lumbar Epidural Space: A Human Study with a Midline vs. Parasagittal Interlaminar Approach under Fluoroscopy
 Pain Physician 2015; 18:317-324 ISSN 1533-3159 Randomized Trial Anatomical Flow Pattern of Contrast in Lumbar Epidural Space: A Human Study with a Midline vs. Parasagittal Interlaminar Approach under Fluoroscopy
Pain Physician 2015; 18:317-324 ISSN 1533-3159 Randomized Trial Anatomical Flow Pattern of Contrast in Lumbar Epidural Space: A Human Study with a Midline vs. Parasagittal Interlaminar Approach under Fluoroscopy
Surgical Treatment for Lumbar Spinal Stenosis Dynamic Interspinous Distraction Interlaminar Stabilization Implant - Coflex
 International 31st Course For Percutaneous Endoscopic Spinal Surgery And Complementary Minimal Invasive Techniques Zurich, Switzerland January 24-25, 2013 Surgical Treatment for Lumbar Spinal Stenosis
International 31st Course For Percutaneous Endoscopic Spinal Surgery And Complementary Minimal Invasive Techniques Zurich, Switzerland January 24-25, 2013 Surgical Treatment for Lumbar Spinal Stenosis
National Medical Policy
 National Medical Policy Subject: Epidural Steroid Injections for Treatment of Low Back and Cervical Pain Policy Number: NMP487 Effective Date*: July 2003 Updated: October 2015 This National Medical Policy
National Medical Policy Subject: Epidural Steroid Injections for Treatment of Low Back and Cervical Pain Policy Number: NMP487 Effective Date*: July 2003 Updated: October 2015 This National Medical Policy
Cervical Spine Radiculopathy: Convervative Treatment. Christos K. Yiannakopoulos, MD Orthopaedic Surgeon
 Cervical Spine Radiculopathy: Convervative Treatment Christos K. Yiannakopoulos, MD Orthopaedic Surgeon Laboratory for the Research of the Musculoskeletal System, University of Athens & IASO General Hospital,
Cervical Spine Radiculopathy: Convervative Treatment Christos K. Yiannakopoulos, MD Orthopaedic Surgeon Laboratory for the Research of the Musculoskeletal System, University of Athens & IASO General Hospital,
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Health Benchmarks Program Clinical Quality Indicator Specification 2013
 Health Benchmarks Program Clinical Quality Indicator Specification 2013 Measure Title USE OF IMAGING STUDIES FOR LOW BACK PAIN Disease State Musculoskeletal Indicator Classification Utilization Strength
Health Benchmarks Program Clinical Quality Indicator Specification 2013 Measure Title USE OF IMAGING STUDIES FOR LOW BACK PAIN Disease State Musculoskeletal Indicator Classification Utilization Strength
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
(Formerly Sacroiliac Joint Arthrography and Injection)
 Protocol Diagnosis and Treatment of Sacroiliac Joint Pain (Formerly Sacroiliac Joint Arthrography and Injection) Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* Yes Review
Protocol Diagnosis and Treatment of Sacroiliac Joint Pain (Formerly Sacroiliac Joint Arthrography and Injection) Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* Yes Review
An Algorithmic Approach for Clinical Management of Chronic Spinal Pain
 Pain Physician 2009; 12:E225-E264 ISSN 2150-1149 Evidence-Based Medicine An Algorithmic Approach for Clinical Management of Chronic Spinal Pain Laxmaiah Manchikanti, MD 1, Standiford Helm, MD 2, Vijay
Pain Physician 2009; 12:E225-E264 ISSN 2150-1149 Evidence-Based Medicine An Algorithmic Approach for Clinical Management of Chronic Spinal Pain Laxmaiah Manchikanti, MD 1, Standiford Helm, MD 2, Vijay
REVIEW DECISION. Review Reference #: R0103014 Board Decision under Review: March 3, 2009
 REVIEW DECISION Re: Review Reference #: R0103014 Board Decision under Review: March 3, 2009 Date: Review Officer: Lyall Zucko The worker requests a review of the decision of WorkSafeBC (the Board) dated
REVIEW DECISION Re: Review Reference #: R0103014 Board Decision under Review: March 3, 2009 Date: Review Officer: Lyall Zucko The worker requests a review of the decision of WorkSafeBC (the Board) dated
Evidence Based Medicine in Spinal Surgery
 Evidence Based Medicine in Spinal Surgery Roger Härtl, MD Associate Professor of Neurosurgery Chief of Spinal Surgery Neurosurgeon to the GIANTS Football team Brain & Spine Center Weill Cornell Medical
Evidence Based Medicine in Spinal Surgery Roger Härtl, MD Associate Professor of Neurosurgery Chief of Spinal Surgery Neurosurgeon to the GIANTS Football team Brain & Spine Center Weill Cornell Medical
Corporate Medical Policy
 Corporate Medical Policy Diagnosis and Treatment of Sacroiliac Joint Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: diagnosis_and_treatment_of_sacroiliac_joint_pain 8/2010
Corporate Medical Policy Diagnosis and Treatment of Sacroiliac Joint Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: diagnosis_and_treatment_of_sacroiliac_joint_pain 8/2010
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN. Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA
 DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA MEDICAL ALGORITHM OF REALITY LOWER BACK PAIN Yes Patient will never get better until case
DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA MEDICAL ALGORITHM OF REALITY LOWER BACK PAIN Yes Patient will never get better until case
1 REVISOR 5223.0070. (4) Pain associated with rigidity (loss of motion or postural abnormality) or
 1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
Case Studies Updated 10.24.11
 S O L U T I O N S Case Studies Updated 10.24.11 Hill DT Solutions Cervical Decompression Case Study An 18-year-old male involved in a motor vehicle accident in which his SUV was totaled suffering from
S O L U T I O N S Case Studies Updated 10.24.11 Hill DT Solutions Cervical Decompression Case Study An 18-year-old male involved in a motor vehicle accident in which his SUV was totaled suffering from
ISPI Newsletter Archive Lumbar Spine Surgery
 ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
STATE OF WEST VIRGINIA SUPREME COURT OF APPEALS MEMORANDUM DECISION
 STATE OF WEST VIRGINIA GARY E. GOSNELL, Claimant Below, Petitioner SUPREME COURT OF APPEALS FILED March 27, 2015 RORY L. PERRY II, CLERK SUPREME COURT OF APPEALS OF WEST VIRGINIA vs.) No. 14-0614 (BOR
STATE OF WEST VIRGINIA GARY E. GOSNELL, Claimant Below, Petitioner SUPREME COURT OF APPEALS FILED March 27, 2015 RORY L. PERRY II, CLERK SUPREME COURT OF APPEALS OF WEST VIRGINIA vs.) No. 14-0614 (BOR
Ms. Jackson is the Manager of Health Finance and Reimbursement, Division of Health Policy and Practice Services, Washington, DC.
 Electrodiagnostic Testing with Same Day Evaluation Management By: Shane J. Burr, MD; Scott I. Horn, DO; Jenny J. Jackson, MPH, CPC; Joseph P. Purcell, DO Dr. Burr practices general inpatient and outpatient
Electrodiagnostic Testing with Same Day Evaluation Management By: Shane J. Burr, MD; Scott I. Horn, DO; Jenny J. Jackson, MPH, CPC; Joseph P. Purcell, DO Dr. Burr practices general inpatient and outpatient
NON SURGICAL SPINAL DECOMPRESSION. Dr. Douglas A. VanderPloeg
 NON SURGICAL SPINAL DECOMPRESSION Dr. Douglas A. VanderPloeg CONTENTS I. Incidence of L.B.P. II. Anatomy Review III. IV. Disc Degeneration, Bulge, and Herniation Non-Surgical Spinal Decompression 1. History
NON SURGICAL SPINAL DECOMPRESSION Dr. Douglas A. VanderPloeg CONTENTS I. Incidence of L.B.P. II. Anatomy Review III. IV. Disc Degeneration, Bulge, and Herniation Non-Surgical Spinal Decompression 1. History
Clinical Guideline. Low Back Pain Orthopaedics. Princess Alexandra Hospital Emergency Department. 1 Purpose. 2 Background
 Princess Alexandra Hospital Emergency Department Clinical Guideline Orthopaedics Review Officer: Katherine Isoardi Version no: 1 Approval date: 18/03/2015 Review date: 18/03/2017 Approving Officer Dr James
Princess Alexandra Hospital Emergency Department Clinical Guideline Orthopaedics Review Officer: Katherine Isoardi Version no: 1 Approval date: 18/03/2015 Review date: 18/03/2017 Approving Officer Dr James
LOW BACK PAIN; MECHANICAL
 1 ORTHO 16 LOW BACK PAIN; MECHANICAL Background This case definition was developed by the Armed Forces Health Surveillance Center (AFHSC) for the purpose of epidemiological surveillance of a condition
1 ORTHO 16 LOW BACK PAIN; MECHANICAL Background This case definition was developed by the Armed Forces Health Surveillance Center (AFHSC) for the purpose of epidemiological surveillance of a condition
MEDICAL POLICY I. POLICY POLICY TITLE
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg
 Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
Degenerative Lumbar Scoliosis with Stenosis Successfully Treated with Cox Distraction Manipulation
 Cox Technic Case Report #92 (sent February 2011 2/8/11 ) 1 Degenerative Lumbar Scoliosis with Stenosis Successfully Treated with Cox Distraction Manipulation Presented By Robert E. Patterson Jr., D.C.
Cox Technic Case Report #92 (sent February 2011 2/8/11 ) 1 Degenerative Lumbar Scoliosis with Stenosis Successfully Treated with Cox Distraction Manipulation Presented By Robert E. Patterson Jr., D.C.
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
Treating Bulging Discs & Sciatica. Alexander Ching, MD
 Treating Bulging Discs & Sciatica Alexander Ching, MD Disclosures Depuy Spine Teaching and courses K2 Spine Complex Spine Study Group Disclosures Take 2 I am a spine surgeon I like spine surgery I believe
Treating Bulging Discs & Sciatica Alexander Ching, MD Disclosures Depuy Spine Teaching and courses K2 Spine Complex Spine Study Group Disclosures Take 2 I am a spine surgeon I like spine surgery I believe
visualized. The correct level is then identified again. With the use of a microscope and
 SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
Design System review of Medline, CINAHL, AMED, PEDro, and Cochrane databases till August 2010.
 The McKenzie Institute International 2013 Vol. 2, No. 2 LITERATURE REVIEWS Summary and Perspective of Recent Literature Stephen May, PhD, MA, FCSP, Dip. MDT, MSc (UK) May S, Comer C. Is surgery more effective
The McKenzie Institute International 2013 Vol. 2, No. 2 LITERATURE REVIEWS Summary and Perspective of Recent Literature Stephen May, PhD, MA, FCSP, Dip. MDT, MSc (UK) May S, Comer C. Is surgery more effective
Important Predictors of Outcome in Patients with Cervical Spondylotic Myelopathy and Radiculopathy undergoing Surgery
 Important Predictors of Outcome in Patients with Cervical Spondylotic Myelopathy and Radiculopathy undergoing Surgery Michael G. Fehlings Professor of Neurosurgery Vice Chair Research, Department of Surgery
Important Predictors of Outcome in Patients with Cervical Spondylotic Myelopathy and Radiculopathy undergoing Surgery Michael G. Fehlings Professor of Neurosurgery Vice Chair Research, Department of Surgery
Motion Preservation. Hansen Yuan, MD President, Spine Arthroplasty Society
 Motion Preservation Procedure Codes Hansen Yuan, MD President, Spine Arthroplasty Society Who are we? The Spine Arthroplasty Society (SAS) is a group of medical and associated specialists devoted to the
Motion Preservation Procedure Codes Hansen Yuan, MD President, Spine Arthroplasty Society Who are we? The Spine Arthroplasty Society (SAS) is a group of medical and associated specialists devoted to the
Physical Therapy Self-Referral ( Direct Access )
 Physical Therapy Self-Referral ( Direct Access ) Summary of Statutes and Regulations by State December 2007 The American Association of Orthopaedic Surgeons (AAOS) supports a patient-centered approach
Physical Therapy Self-Referral ( Direct Access ) Summary of Statutes and Regulations by State December 2007 The American Association of Orthopaedic Surgeons (AAOS) supports a patient-centered approach
.org. Herniated Disk in the Lower Back. Anatomy. Description
 Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
Lumbar Spinal Stenosis
 Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Non Surgical Management of Back pain. Dr Pankaj Wadhwa MBBS,MD(Anaesth),DNB,Dip.Acu, FIPP, Fellowship in Pain Management.
 Non Surgical Management of Back pain. Dr Pankaj Wadhwa MBBS,MD(Anaesth),DNB,Dip.Acu, FIPP, Fellowship in Pain Management. MAGNITUDE OF THE PROBLEM It is one of the commonest experiences of humankind. It
Non Surgical Management of Back pain. Dr Pankaj Wadhwa MBBS,MD(Anaesth),DNB,Dip.Acu, FIPP, Fellowship in Pain Management. MAGNITUDE OF THE PROBLEM It is one of the commonest experiences of humankind. It
Utilization and Cost of Surgery for Lumbar Spinal Stenosis in a Commercially Insured Population
 Utilization and Cost of Surgery for Lumbar Spinal Stenosis in a Commercially Insured Population Prepared by Milliman, Inc. Kathryn Fitch, RN, MEd Principal and Healthcare Management Consultant Helen Blumen,
Utilization and Cost of Surgery for Lumbar Spinal Stenosis in a Commercially Insured Population Prepared by Milliman, Inc. Kathryn Fitch, RN, MEd Principal and Healthcare Management Consultant Helen Blumen,
Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011
 1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
Vertebral Axial Decompression For Low Back Pain
 Vertebral Axial Decompression For Low Back Pain By WCB Evidence Based Practice Group Dr. Craig W. Martin, Senior Medical Advisor February 2005 Program Design Division TABLE OF CONTENTS Page Background...1
Vertebral Axial Decompression For Low Back Pain By WCB Evidence Based Practice Group Dr. Craig W. Martin, Senior Medical Advisor February 2005 Program Design Division TABLE OF CONTENTS Page Background...1
Contractor Number 11302. Oversight Region Region IV
 Local Coverage Determination (LCD): Spinal Cord Stimulators for Chronic Pain (L32549) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11302 Contractor Type MAC
Local Coverage Determination (LCD): Spinal Cord Stimulators for Chronic Pain (L32549) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11302 Contractor Type MAC
University of Toronto Lumbar Spinal Stenosis Study
 University of Toronto Lumbar Spinal Stenosis Study The Evaluation of Four Novel Self Management Strategies to Improve Walking Ability in Neurogenic Claudication due to Degenerative Lumbar Spinal Stenosis
University of Toronto Lumbar Spinal Stenosis Study The Evaluation of Four Novel Self Management Strategies to Improve Walking Ability in Neurogenic Claudication due to Degenerative Lumbar Spinal Stenosis
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
Frequent headache is defined as headaches 15 days/month and daily. Course of Frequent/Daily Headache in the General Population and in Medical Practice
 DISEASE STATE REVIEW Course of Frequent/Daily Headache in the General Population and in Medical Practice Egilius L.H. Spierings, MD, PhD, Willem K.P. Mutsaerts, MSc Department of Neurology, Brigham and
DISEASE STATE REVIEW Course of Frequent/Daily Headache in the General Population and in Medical Practice Egilius L.H. Spierings, MD, PhD, Willem K.P. Mutsaerts, MSc Department of Neurology, Brigham and
BEFORE THE ARKANSAS WORKERS' COMPENSATION COMMISSION WCC NO. G405820. LINDA BECKER, Employee. GOODWILL INDUSTRIES, Employer
 BEFORE THE ARKANSAS WORKERS' COMPENSATION COMMISSION WCC NO. G405820 LINDA BECKER, Employee GOODWILL INDUSTRIES, Employer RISK MANAGEMENT RESOURCES, Carrier CLAIMANT RESPONDENT RESPONDENT OPINION FILED
BEFORE THE ARKANSAS WORKERS' COMPENSATION COMMISSION WCC NO. G405820 LINDA BECKER, Employee GOODWILL INDUSTRIES, Employer RISK MANAGEMENT RESOURCES, Carrier CLAIMANT RESPONDENT RESPONDENT OPINION FILED
By James D. Gould, MD FACS
 By James D. Gould, MD FACS BACKGROUND Balloon devices enlarge narrowed sinus ostia and outflow tracts by remodeling the surrounding bone and paranasal sinus structures. Multiple studies have demonstrated
By James D. Gould, MD FACS BACKGROUND Balloon devices enlarge narrowed sinus ostia and outflow tracts by remodeling the surrounding bone and paranasal sinus structures. Multiple studies have demonstrated
Overview Anatomy of the spinal canal What is spinal stenosis? > 1
 Spinal Stenosis Overview Spinal stenosis is the narrowing of your spinal canal and nerve root canal along with the enlargement of your facet joints. Most commonly it is caused by osteoarthritis and your
Spinal Stenosis Overview Spinal stenosis is the narrowing of your spinal canal and nerve root canal along with the enlargement of your facet joints. Most commonly it is caused by osteoarthritis and your
Enrollment Snapshot of Radiography, Radiation Therapy and Nuclear Medicine Technology Programs 2015
 Enrollment Snapshot of, Radiation Therapy and Nuclear Medicine Technology Programs 2015 December 2015 2015 ASRT. All rights reserved. Reproduction in any form is forbidden without written permission from
Enrollment Snapshot of, Radiation Therapy and Nuclear Medicine Technology Programs 2015 December 2015 2015 ASRT. All rights reserved. Reproduction in any form is forbidden without written permission from
Diagnosis and Treatment of Lumbar Spinal Canal Stenosis
 Low Back Pains Diagnosis and Treatment of Lumbar Spinal Canal Stenosis JMAJ 46(10): 439 444, 2003 Katsuro TOMITA Department of Orthopedic Surgery, Kanazawa University Abstract: Lumbar spinal canal stenosis
Low Back Pains Diagnosis and Treatment of Lumbar Spinal Canal Stenosis JMAJ 46(10): 439 444, 2003 Katsuro TOMITA Department of Orthopedic Surgery, Kanazawa University Abstract: Lumbar spinal canal stenosis
Employees Compensation Appeals Board
 U. S. DEPARTMENT OF LABOR Employees Compensation Appeals Board In the Matter of DEBORAH R. EVANS and U.S. POSTAL SERVICE, POST OFFICE, Orlando, FL Docket No. 02-1888; Submitted on the Record; Issued December
U. S. DEPARTMENT OF LABOR Employees Compensation Appeals Board In the Matter of DEBORAH R. EVANS and U.S. POSTAL SERVICE, POST OFFICE, Orlando, FL Docket No. 02-1888; Submitted on the Record; Issued December
Ben Okafor FRCS FRCS.orth Consultant Orthopaedic & Spine Surgeon Whipps Cross University Hospital
 Ben Okafor FRCS FRCS.orth Consultant Orthopaedic & Spine Surgeon Whipps Cross University Hospital Classification Pathology Clinical features Imaging Treatment Options Outcomes Definition: 1. Narrowing
Ben Okafor FRCS FRCS.orth Consultant Orthopaedic & Spine Surgeon Whipps Cross University Hospital Classification Pathology Clinical features Imaging Treatment Options Outcomes Definition: 1. Narrowing
IN THE COURT OF APPEALS OF THE STATE OF MISSISSIPPI NO. 2012-WC-01407-COA MISSISSIPPI WORKERS COMPENSATION APPEALED:
 IN THE COURT OF APPEALS OF THE STATE OF MISSISSIPPI NO. 2012-WC-01407-COA FLOYD MAYFIELD APPELLANT v. ADVANCED DISPOSAL SERVICES MISSISSIPPI, LLC AND ARCH INSURANCE COMPANY APPELLEES DATE OF JUDGMENT:
IN THE COURT OF APPEALS OF THE STATE OF MISSISSIPPI NO. 2012-WC-01407-COA FLOYD MAYFIELD APPELLANT v. ADVANCED DISPOSAL SERVICES MISSISSIPPI, LLC AND ARCH INSURANCE COMPANY APPELLEES DATE OF JUDGMENT:
But My Back Hurts Only When I m Standing!
 But My Back Hurts Only When I m Standing! Axial Loading for Spinal Canal Stenosis Matthew Cham, MD; Akio Hiwatashi, MD; Per-Lennart Westesson, MD, PhD, DDS Division of Diagnostic and Interventional Neuroradiology,
But My Back Hurts Only When I m Standing! Axial Loading for Spinal Canal Stenosis Matthew Cham, MD; Akio Hiwatashi, MD; Per-Lennart Westesson, MD, PhD, DDS Division of Diagnostic and Interventional Neuroradiology,
UPPER LUMBAR DISC HERNIATION WITH CENTRAL AND FAR LATERAL STENOTIC CHANGES RESULTING IN ANTERIOR THIGH PAIN
 Cox Technic Case Report #60 sent 5/13/08 1 UPPER LUMBAR DISC HERNIATION WITH CENTRAL AND FAR LATERAL STENOTIC CHANGES RESULTING IN ANTERIOR THIGH PAIN History, Examination & Imaging Review: A 53-year-old
Cox Technic Case Report #60 sent 5/13/08 1 UPPER LUMBAR DISC HERNIATION WITH CENTRAL AND FAR LATERAL STENOTIC CHANGES RESULTING IN ANTERIOR THIGH PAIN History, Examination & Imaging Review: A 53-year-old
POST SURGICAL RETURN OF RIGHT LEG PAIN. TREATED SUCCESSFULLY WITH COX FLEXION DISTRACTION DECOMPRESSION ADJUSTING
 POST SURGICAL RETURN OF RIGHT LEG PAIN. TREATED SUCCESSFULLY WITH COX FLEXION DISTRACTION DECOMPRESSION ADJUSTING A 47 year old white married female was seen for the chief complaint of low back and right
POST SURGICAL RETURN OF RIGHT LEG PAIN. TREATED SUCCESSFULLY WITH COX FLEXION DISTRACTION DECOMPRESSION ADJUSTING A 47 year old white married female was seen for the chief complaint of low back and right
How To Treat Pain With Pain Management
 SUTTER PHYSICIANS ALLIANCE (SPA) 2800 L Street, 7 th Floor Sacramento, CA 95816 SPA PCP Treatment & Referral Guidelines Pain Management Developed June 1, 2003 Revised - (Format Revisions) November 13,
SUTTER PHYSICIANS ALLIANCE (SPA) 2800 L Street, 7 th Floor Sacramento, CA 95816 SPA PCP Treatment & Referral Guidelines Pain Management Developed June 1, 2003 Revised - (Format Revisions) November 13,
Practice Guidelines For Low Back Pain
 Consumers Guide Practice Guidelines For Low Back Pain Copyright 2008 American Chronic Pain Association Page 1 Written by: Penney Cowan Founder Executive Director American Chronic Pain Association Editors:
Consumers Guide Practice Guidelines For Low Back Pain Copyright 2008 American Chronic Pain Association Page 1 Written by: Penney Cowan Founder Executive Director American Chronic Pain Association Editors:
Workers Compensation Research
 Workers Compensation Research About WCRI Independent, not-for-profit research organization Founded in Cambridge MA in 1983 Diverse membership support Studies are peer-reviewed Resource for public officials
Workers Compensation Research About WCRI Independent, not-for-profit research organization Founded in Cambridge MA in 1983 Diverse membership support Studies are peer-reviewed Resource for public officials
Compression Fractures
 September 2006 Compression Fractures Eleanor Adams Harvard Medical School Year IV Overview Spine Anatomy Thoracolumbar Fractures Cases Compression Fractures, Ddx Radiologic Tests of Choice Treatment Options
September 2006 Compression Fractures Eleanor Adams Harvard Medical School Year IV Overview Spine Anatomy Thoracolumbar Fractures Cases Compression Fractures, Ddx Radiologic Tests of Choice Treatment Options
A Phase 2 Study of Interferon Beta-1a (Avonex ) in Ulcerative Colitis
 A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
October 3, 2005. RE: Appropriate use of CPT 76003 and 76005. Dear Ms. Kotowicz:
 October 3, 2005 Grace M. Kotowicz, Director CPT Editorial Research and Development American Medical Association 515 North State Street Chicago, IL 60610 RE: Appropriate use of CPT 76003 and 76005 Dear
October 3, 2005 Grace M. Kotowicz, Director CPT Editorial Research and Development American Medical Association 515 North State Street Chicago, IL 60610 RE: Appropriate use of CPT 76003 and 76005 Dear
Effects of vertebral axial decompression on intradiscal pressure
 This article is reprinted with the permission of the authors from the Journal of Neurosurgery, Volume 81. J Neurosurg 81:350-353, 1994 Effects of vertebral axial decompression on intradiscal pressure GUSTAVO
This article is reprinted with the permission of the authors from the Journal of Neurosurgery, Volume 81. J Neurosurg 81:350-353, 1994 Effects of vertebral axial decompression on intradiscal pressure GUSTAVO
EXPERIMENTAL AND THERAPEUTIC MEDICINE 5: 567-571, 2013
 EXPERIMENTAL AND THERAPEUTIC MEDICINE 5: 567-571, 2013 Treatment of multilevel degenerative lumbar spinal stenosis with spondylolisthesis using a combination of microendoscopic discectomy and minimally
EXPERIMENTAL AND THERAPEUTIC MEDICINE 5: 567-571, 2013 Treatment of multilevel degenerative lumbar spinal stenosis with spondylolisthesis using a combination of microendoscopic discectomy and minimally
LOW BACK PAIN. common of these conditions include: muscle strain ( pulled muscle ), weak core muscles
 LOW BACK PAIN Most episodes of low back pain are caused by relatively harmless conditions. The most common of these conditions include: muscle strain ( pulled muscle ), weak core muscles (abdominal and
LOW BACK PAIN Most episodes of low back pain are caused by relatively harmless conditions. The most common of these conditions include: muscle strain ( pulled muscle ), weak core muscles (abdominal and
Cervical Spondylosis (Arthritis of the Neck)
 Copyright 2009 American Academy of Orthopaedic Surgeons Cervical Spondylosis (Arthritis of the Neck) Neck pain is extremely common. It can be caused by many things, and is most often related to getting
Copyright 2009 American Academy of Orthopaedic Surgeons Cervical Spondylosis (Arthritis of the Neck) Neck pain is extremely common. It can be caused by many things, and is most often related to getting
EPIDURAL STEROID AND FACET INJECTIONS FOR SPINAL PAIN
 MEDICAL POLICY EPIDURAL STEROID AND FACET INJECTIONS FOR SPINAL PAIN Policy Number: 2015T0004W Effective Date: December 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE APPLICABLE CODES..
MEDICAL POLICY EPIDURAL STEROID AND FACET INJECTIONS FOR SPINAL PAIN Policy Number: 2015T0004W Effective Date: December 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE APPLICABLE CODES..
Lumbar Spinal Stenosis
 Copyright 2009 American Academy of Orthopaedic Surgeons Lumbar Spinal Stenosis Almost everyone will experience low back pain at some point in their lives. A common cause of low back pain is lumbar spinal
Copyright 2009 American Academy of Orthopaedic Surgeons Lumbar Spinal Stenosis Almost everyone will experience low back pain at some point in their lives. A common cause of low back pain is lumbar spinal
