Health Policy Advisory Committee on Technology Technology Brief
|
|
|
- Brenda Powell
- 9 years ago
- Views:
Transcription
1 Health Policy Advisory Committee on Technology Technology Brief LifeStent vascular stent for symptomatic lesions of the superficial femoral or proximal popliteal artery August 2012
2 State of Queensland (Queensland Health) 2012 This work is licensed under a Creative Commons Attribution Non-Commercial No Derivatives 2.5 Australia licence. In essence, you are free to copy and communicate the work in its current form for non-commercial purposes, as long as you attribute the authors and abide by the licence terms. You may not alter or adapt the work in any way. To view a copy of this licence, visit For further information, contact the HealthPACT Secretariat at: HealthPACT Secretariat c/o Access Improvement Service, Centre for Healthcare Improvement, Queensland Health Lobby 2, Level 2, Citilink Business Centre 153 Campbell Street, Bowen Hills QLD 4006 Postal Address: GPO Box 48, Brisbane Qld [email protected] Telephone: (07) For permissions beyond the scope of this licence contact: Intellectual Property Officer, Queensland Health, GPO Box 48, Brisbane Qld 4001, [email protected], phone (07) Electronic copies can be obtained from: DISCLAIMER: This brief is published with the intention of providing information of interest. It is based on information available at the time of research and cannot be expected to cover any developments arising from subsequent improvements to health technologies. This brief is based on a limited literature search and is not a definitive statement on the safety, effectiveness or costeffectiveness of the health technology covered. The State of Queensland acting through Queensland Health ( Queensland Health ) does not guarantee the accuracy, currency or completeness of the information in this brief. Information may contain or summarise the views of others, and not necessarily reflect the views of Queensland Health. This brief is not intended to be used as medical advice and it is not intended to be used to diagnose, treat, cure or prevent any disease, nor should it be used for therapeutic purposes or as a substitute for a health professional's advice. It must not be relied upon without verification from authoritative sources. Queensland Health does not accept any liability, including for any injury, loss or damage, incurred by use of or reliance on the information. This brief was commissioned by Queensland Health, in its role as the Secretariat of the Health Policy Advisory Committee on Technology (HealthPACT). The production of this brief was overseen by HealthPACT. HealthPACT comprises representatives from health departments in all States and Territories, the Australian and New Zealand governments and MSAC. It is a subcommittee of the Australian Health Ministers Advisory Council (AHMAC), reporting to AHMAC s Hospital Principal Committee (HPC). AHMAC supports HealthPACT through funding. This brief was prepared by Ms Robyn Lambert and Ms Stefanie Gurgacz from the Australian Safety and Efficacy Register for New Interventional Procedures Surgical (ASERNIP-S).
3 TECHNOLOGY BRIEF Register ID Name of technology Purpose and target group WP109 Bard peripheral vascular LifeStent Endovascular treatment for symptomatic stenotic, occlusive or restenotic lesions of the superficial femoral or popliteal artery Stage of development in Australia Yet to emerge Established Experimental Established but changed indication or modification of technique Investigational Should be taken out of use Nearly established Australian Therapeutic Goods Administration approval Yes ARTG number No Not applicable International utilisation Country Trials underway or completed Level of use Limited use USA Europe (Austria & German) Korea Widely diffused Impact summary The LifeStent and LifeStent XL vascular stent (Bard Peripheral Vascular, Tempe, AZ, USA) have been assessed for the treatment of symptomatic de novo lesions of the superficial femoral artery (SFA) and/or the proximal popliteal artery. Placement of the stent occurs at the time of, or immediately after, angioplasty of the affected vessel. Stent placement is a minimally-invasive, image-guided therapy that aims to relieve stenosis or occlusion of the vessel and increase perfusion to the lower limb. The stent is placed by an interventional radiologist or vascular surgeon under local anaesthesia and/or sedation. The LifeStent may offer patients lower rates of intermediate and long-term target lesion restenosis as compared to percutaneous transluminal balloon angioplasty alone (PTA). 1
4 Background The arteries involved in providing perfusion to the lower leg are depicted in Figure 1. The superficial femoral artery (SFA), which becomes the popliteal artery above the knee, is the dominant artery supplying the calf and foot. The SFA passes through the muscular adductor canal where it becomes the popliteal artery; it is also the longest artery in the body. 2 The SFA is highly mobile and subject to unique mechanical forces not observed in other arteries. It is also characterised by high resistance and disturbed flow, factors implicated in the development of arteriosclerosis. 2 Figure 1 Arteries of the leg 1 Peripheral arterial disease (PAD), a condition in which atherosclerosis leads to stenosis or occlusion of the SFA, is associated with the intimal thickening of arterial vessels due to plaques caused by accumulation of fatty acids and cholesterol. 2, 3 Patients with SFA stenosis or occlusion may be asymptomatic; however, they may present with symptoms that negatively impact quality of life, including: 3 Intermittent claudication ischemic pain caused by decreased flow capacity during exercise; Muscle weakness and fatigue; and Critical limb ischemia (CLI) chronic ischemic rest pain, ulcers or gangrene of the lower leg or foot. The Rutherford claudication classification system is most commonly used to classify the severity of peripheral arterial disease. 2
5 Table 1 Rutherford claudication classification system 4 Fontaine grade Rutherford category Clinical 0 0 Asymptomatic I 1 Mild claudication I 2 Moderate claudication I 3 Severe claudication II 4 Ischemic rest pain III 5 Minor tissue loss III 6 Major tissue loss PAD is associated with elevated mortality, risk of myocardial infarction and stroke compared with age-matched peers. 4, 5 Left untreated, stenosis or occlusion of the SFA may progress to CLI, requiring possible amputation of the affected limb. Studies involving large patient cohorts followed for eight to 10 years suggest that amputation may occur in between and per cent of patients with intermittent claudication. Treatments for intermittent claudication and CLI can be pharmacological, endovascular and surgical. Pharmacological therapies target processes implicated in PAD and aim to control hypertension, diabetes and lipid profiles. 9 Bypass surgery, the traditional treatment for CLI, is considered the most durable revascularisation technique; however, it is associated with significant morbidity and mortality compared to endovascular, pharmacological and lifestyle interventions. 9 Endovascular procedures are aimed at revascularisation of the affected limb and associated relief of symptoms as well as minimisation of tissue loss and healing of ulcers or gangrene. Endovascular procedures are minimally-invasive, performed under local anaesthesia, have a lower risk of morbidity, and may be repeated, potentially without compromising future surgical options. 8, 21 For stenosis or occlusion of the SFA, endovascular procedures include PTA, primary stenting or provisional stenting. Provisional stenting refers to the placement of a stent in patients treated with PTA alone, with unsatisfactory post-angioplasty results at the time of angiography. 21 PTA alone has been associated with high initial technical success; however, restenosis has been reported to occur in the majority of patients at one to two year follow-up. 12 Particularly poor outcomes are seen in patients with long lesions (greater than 100 mm) with rates of restenosis at one year exceeding 70 per cent. 3
6 Randomised controlled trials comparing first generation stainless steel stents to angioplasty did not find benefit of stenting over PTA, with high rates of restenosis. 9, 21 This is thought to be due to the inability of the stents to withstand the mechanical strain of the SFA, resulting in stent compression and subsequent re-occlusion. 9 High rates of neointimal hyperplasia are also reported with stainless steel stents. 21 Second generation self-expandable nitinol stents such as the LifeStent offer increased flexibility and exertion of outward radial pressure enabling them to better withstand the mechanical strain on the SFA. Whilst second generation stents may provide more durable outcomes compared to first generation stents or PTA alone, data beyond three years are not currently available. The LifeStent Vascular Stent is a nitinol self-expanding stent introduced via a sheathed delivery system. The stent is a flexible, fine tubular mesh prosthesis with a helical design. Upon deployment into the target vessel, the stent expands to achieve its unconstrained diameter, establishing patency. The stent has 12 radiopaque tantalum markers located on its ends. 14 The LifeStent Vascular Stent systems, Lifestent and Lifestent XL, received USA Food and Drug Administration (FDA) approval for use in the biliary tree in 2005 and 2006, and were approved for use in lesions up to 160 mm in the SFA and proximal popliteal artery in They have been available in the European Union since Currently, they have not received Therapeutic Goods Administration (TGA) approval. The LifeStent is intended to improve luminal diameter in the treatment of symptomatic de novo or restenotic lesions up to 150 mm in length in native SFA and/or proximal popliteal arteries with reference vessel diameters ranging from 4.0 to 6.5 mm. Contraindications include: patients with known hypersensitivity to nitinol (nickel, titanium) and/or tantalum, patients who cannot receive recommended antiplatelet and/or anti-coagulation therapy, and patients who are judged to have a lesion which prevents complete inflation of an angioplasty balloon or correct placement of the stent or stent delivery system. Bard Peripheral Vascular Inc. also provides the LifeStent XL which is FDA approved for lesions up to 240 mm in length. 13 Clinical need and burden of disease A cross-sectional survey 15 of males aged 65 to 85 in Western Australia reported that the age-standardised prevalence of PAD is 15.6 per cent (95% Confidence Intervals: %), whilst AIHW principle diagnosis data indicate that there were 14,589 separations for arteriosclerosis of arteries of extremities in The prevalence of PAD is higher among males and increases with age. Rates of PAD in persons over the age of 65 are reported to be between 12 and 15 per cent. 17 Risk 4
7 factors include: diabetes, smoking, hypertension, high cholesterol, and renal 4, 18 insufficiency. Diffusion of technology in Australia There is no evidence to suggest that the LifeStent is currently being used within Australia. A search of current controlled trials in Australia and New Zealand did not yield any results. Comparators Comparators to the LifeStent include other self-expanding vascular stents, drugeluting stents and PTA without stenting. PTA mechanically expands a stenotic lesion by crushing fatty deposits. It involves insertion of a balloon catheter that can be inflated under pressure within the vessel to achieve dilation. Initial procedural success rates are high; however, one-year restenosis rates are around 40 to 60 per cent. 11 Drug-eluting vascular stents are designed to slowly release pharmacological agents which prevent re-occlusion due to atherosclerotic plaques. These drugs are classed as antiproliferative agents, and include paclitaxel, sirolimus and everolimus. Two recent clinical trials (the SIROCCO and Zilver PTX trials; NCT and NCT ) have been completed, and 24 (SIROCCO) and 12-month (Zilver PTX ) results indicate that the safety and effectiveness profiles of these drug-eluting vascular stents appear to be similar to the equivalent bare stents available in the market. Currently the Zilver PXT vascular stent, which is comprised of the mitotic inhibitor paclitaxel, is approved by the TGA. Table 2 summarises the self-expandable nitinol stents and the Zilver PXT drug-eluting stent with ARTG approval for use in the SFA and/or popliteal arteries. 19 Table 2 ARTG approved stents ARTG approved stent and manufacturer, ARTG number Approval date Approved indication William A Cook Australia Pty Ltd - Zilver Flex Vascular Stent - Multiple peripheral arteries stent Boston Scientific Pty Ltd - Epic Nitinol Vascular Stent System - Prosthesis, internal, stent, vascular 24/05/2011 The device is intended for use in the iliac, superficial femoral artery and above-the-knee popliteal artery for the treatment of arteriosclerotic stenosis, and total occlusions that have been recanalised. The device provides mechanical support to maintain constant blood flow of the vessel 13/02/2009 The Epic Nitinol Vascular Stent System is indicated for the treatment of peripheral vascular lesions and obstructions Bard Australia Pty Ltd - Fluency Plus Vascular Stent Graft 22/08/2007 Indicated to maintain the patency of iliac and femoral arteries
8 Bard Australia Pty Ltd - Bard E- LUMINEXX Vascular Stent 23/07/2007 The e-luminexx Vascular stent is indicated for use in iliac and femoral arteries William A Cook Australia Pty Ltd - Zilver PTX Drug-Eluting Peripheral Stent /12/2011 Indicated for use in the treatment of symptomatic vascular disease of the above-the-knee femoropopliteal arteries having a reference vessel diameter from 4 mm to 9 mm. ARTG: Australian Register of Therapeutic Goods Safety and effectiveness Table 3 summarises the clinical trials underway or recently completed for LifeStent. For inclusion in this brief, two publications on the Edwards Lifesciences Self- Expanding Stent Peripheral Vascular Disease Study (RESILIENT) were identified (level II evidence). 6
9 Table 3 Summary of clinical trials involving the LifeStent Study Study population Status and endpoints Edwards Lifesciences Self-Expanding Stent Peripheral Vascular Disease Study (RESILIENT) (USA and Europe) NCT Bard LifeStent Vascular Stent Delivery System Study (Austria and Germany) NCT CONTINuous Infra-Inguinal Stenting Using the Bard LifeStent VascUlar Stent SysteMs (CONTINUUM) (USA) NCT Efficacy of Self-Expanding Nitinol S.M.A.R.T-CONTROL Stent Versus LifeStent for the Atherosclerotic Femoro-Popliteal Arterial Disease (SENS-FP-1) (Korea) NCT Endovascular Treatment of Popliteal Artery - Balloon Angioplasty Versus Primary Stenting (ETAP) (Germany) NCT patients from 24 centres enrolled in a prospective, multi-centre, randomised study. Patients randomised in a 2:1 ratio to either PTA or self-expandable nitinol stent after predilation 76 patients enrolled in a single-arm, non-randomised, prospective, multicentre study using the Bard LifeStent Vascular Stent Delivery System. Subjects will be treated with PTA followed by implantation of the Bard LifeStent Vascular Stent Prospective, multi-centre, single-arm, non-randomised study enrolling up to 170 subjects. All subjects enrolled in the study will receive PTA and stenting Prospective, multi-centre, randomised, controlled trial comparing two self-expandable nitinol stent types. Estimated enrolment of 170 participants Randomised controlled trial comparing PTA and stenting with the Bard Lifestent Complete Endpoints: 30-day primary safety endpoint, 6-month primary effectiveness endpoint. Patients were also evaluated at 36 months Ongoing (Not yet complete) Endpoints: Effectiveness; prior to hospital discharge. Safety; prior to hospital discharge, 30-days, and 12-, 24-, and 36-months post-index procedure Enrolling (by invitation) Effectiveness: intra-procedure, 30 days and 12 months post procedure. Safety: intra-procedure, 30 days and 12 months post procedure Not yet recruiting 12 month safety and efficacy endpoints Unknown (has not been verified in over two years) Source: Clinical Trials Database (US) accessed May Additionally, a retrospective analysis of long lesions presented in an FDA Summary of Safety and Effectiveness Data (SSED) of the LifeStent and LifeStent XL was located. The FDA SSED summary indicated that a confirmatory clinical study (a case-series, n=84; level IV evidence) was conducted in addition to the RESILIENT trial; however, these data were not available in the peer-reviewed literature. 7
10 Laird et al (2010) 21 - RESILIENT trial Study description The Edwards Lifesciences Self-Expanding Stent Peripheral Vascular Disease Study (RESILIENT) was a prospective, multi-centre randomised controlled trial comparing primary stenting with LifeStent to PTA alone in the treatment of obstructive lesions of the SFA and proximal popliteal artery over 20 months in The study included 206 adults (234 lesions) with symptoms of intermittent claudication (Rutherford categories 1 to 3) who were candidates for angioplasty or stenting at 24 study centres across the USA and Europe. Enrolled patients suffered from either de novo stenotic, occlusive, or restenotic lesions in the SFA or popliteal artery or both, and at least one patent infrapopliteal drainage vessel to the foot. Patients were not significantly different between study groups except that the PTAalone group had a higher rate of hypertension (94% versus 84%; p=0.03). Stenosis or restenosis of at least 50 per cent and lesion length of 150 mm or less was verified by angiographic examination of the target lesion. Appropriate stent sizing required the reference vessel to have a diameter between 4 mm and 6.5 mm. Patients who had been treated for a restenosed or re-occluded lesion were included only if the intervention was more than six months prior to study enrolment and did not include stenting. Only one limb per patient was included in the study. The primary effectiveness outcome was rate of target lesion revascularisation (TLR) at 12 months post procedure. Secondary effectiveness outcomes were primary and secondary patency at six and 12 months using duplex ultrasound, TLR at six months, acute lesion and haemodynamic success, target vessel revascularisation (TVR) and clinical success at 6 and 12 months, and quality-of-life (QOL). Acute lesion success was defined as 30 per cent stenosis of the treatment postintervention. Acute haemodynamic success was defined by more than a 0.10 improvement in the ankle brachial index from pre-procedure to discharge. Clinical success was an improvement of baseline symptoms by at least one Rutherford category, sustained through follow-up with no additional intervention. Safety outcomes included death within 30 days (primary safety outcome) and freedom from major adverse clinical events (MACE) at six and 12 months post procedure. MACE is a composite end point consisting of 30-day death, stroke, myocardial infarction, emergent surgical revascularisation, significant embolism in the target limb, thrombosis of the target vessel, and worsening of at least one Rutherford category of CLI. 8
11 After predilation, patients were randomly assigned 2:1 in blocks of six to LifeStent (n=134) or PTA alone (n=72) via computer-generated randomisation. All patients underwent PTA and were then assessed by angiography for suboptimal results. At the 12-month endpoint, three patients were lost to follow-up, seven had died and nine withdrew consent. Follow-up data were available for: LifeStent group: 88% of patients at six months and 87% at 12 months PTA group: 91% of patients at six months and 87% at 12 months. Safety No patients died within 30 days of the procedure; however, two unplanned single toe amputations occurred in the PTA-alone group. MACE rates were not significantly different: LifeStent group: 93% at six months, 86% at 12 months PTA-alone group: 93% at six months, 87% at 12 months Radiographic analysis of stents showed nine fractures at 12 months, although no fractures resulted in loss of primary patency or required revascularisation. Effectiveness Suboptimal results were detected in 29 of the 72 patients (40%) in the PTA-alone group due to flow limiting dissection (11 patients) or residual stenosis of 30 per cent or greater (18 patients). A supplementary analysis was carried out to compare patients receiving a provisional stent with those who did not receive a stent. In the supplementary analysis, provisional stenting was not considered a TLR. Patients in the LifeStent group experienced improved acute effectiveness with outcomes across all measures reaching statistical significance (Table 4). Table 4 Acute effectiveness LifeStent PTA Percentage difference Procedural success * Acute lesion success * Acute haemodynamic success ^ *p < 0.01, ^ p < 0.05, PTA: percutaneous transluminal balloon angioplasty All effectiveness measures were significantly improved in patients receiving a LifeStent as compared to those treated by PTA alone, except for secondary patency which was not significantly different (Table 5). 9
12 Table 5 Effectiveness outcomes LifeStent (n=134) PTA alone (n=74) Percentage difference 6-month effectiveness measures Freedom from TLR Freedom from TLR/TVR Primary patency Clinical success month effectiveness measures Freedom from TLR Freedom from TLR/TVR Primary patency Clinical success All p values < , PTA: percutaneous transluminal balloon angioplasty; TLR: target lesion revascularisation; TVR: target vessel revascularisation Both treatment groups experienced significant improvement across all QOL measures compared to baseline. Patients in the PTA-alone group reported more claudication pain at 12 months compared to patients in the LifeStent group (Walking Impairment Questionnaire evaluation, p<0.009); however, no other QOL measures were significantly different between groups (Table 6). Table 6 Quality of life measures Baseline Increase at 12 months Short Form 8 Question Health Survey LifeStent 41.4 ± ± 11.2 PTA 41.0 ± ± 11.2 Walking Impairment Questionnaire (walking distance score) LifeStent 26.1 ± ± 34.6 PTA 20.3 ± ± 37.4 *From 12 months compared to baseline; all p values <0.001, PTA: percutaneous transluminal balloon angioplasty Supplementary analysis of patients receiving a provisional stent Of the lesions in the PTA-alone group requiring a provisional stent, lesion lengths were significantly greater than those not requiring a provisional stent (70.3 ± 38.8 mm versus 47.7 ± 32.6 mm; p=0.0058). Moderate or severe calcification was more prevalent amongst the lesions requiring a provisional stent compared to those treated successfully with PTA (48% versus 25%; p=0.0072). Patients that required a provisional stent were younger than those who successfully received PTA (64.0 versus 69.3 years; p=0.014); had less severe claudication (41.9% versus 65.5% class 3; 10
13 p=0.058); and were more likely to have a history of myocardial infarction (34.9% versus 13.8%; p=0.059). Laird et al (2012) 22 - RESILIENT trial 24 and 36 month follow-up Study design The RESILIENT investigators recently published three-year follow-up results. 22 Of the initial 206 patients, 161 were available for follow-up at 36 months (78%), 106 in the LifeStent group and 55 in the PTA-alone group. Of the remaining patients, 15 had died, 20 had withdrawn consent, and 10 were lost to follow-up. A supplementary analysis was also conducted to compare patients experiencing stent fracture with those that did not. Safety Survival rate at 36 months was similar, 90 per cent for patients in the LifeStent group and 92 per cent for the PTA group (p=0.71). No deaths were deemed to be related to the procedure (the most common causes of death were cancer, myocardial infarction and other cardiovascular complications). Freedom from MACE at 36 months was 75 per cent for both groups. Radiographic evaluation for stent fracture analysis showed three new fractures from the 12 to 18 month evaluation. The supplementary evaluation of patients with stent fracture compared to those without did not reveal any relationship between fracture and clinical outcome. Efficacy The absolute difference in the freedom from TLR was consistently higher for the LifeStent group compared to PTA (78% versus 42% at 24 months, 76% versus 42% at 36 months; both p<0.0001), as was clinical success (69% versus 25% at 24 months, 63% versus 18% at 36 months; both p<0.0001; Table 7). Table 7 Effectiveness outcomes at 24 and 36 months LifeStent PTA Percentage difference % (95% CI) 24 months Freedom from TLR (%) (22.2 to 49.9) Clinical success (%) months Freedom from TLR (%) (19.6 to 47.8) Clinical success (%) p < for all measures, CI: confidence interval; PTA: percutaneous transluminal balloon angioplasty; TLR: target lesion revascularisation 11
14 QOL assessments indicated that both groups experienced a significant improvement across all QOL measures relative to baseline, while no significant differences between groups were detected (Table 8). Table 8 Quality of life outcomes at 24 and 36 months Baseline Increase at 36 months Short Form 8 Question Health Survey LifeStent 41.4 ± ± 11.3 PTA 41.0 ± ± 12.2 Walking Impairment Questionnaire LifeStent 26.1 ± ± 32.9 PTA 20.3 ± ± 36.1 p < for all measures; measures were at 36 months compared to baseline, PTA: percutaneous transluminal balloon angioplasty A supplementary analysis, comparing the strategy of primary stent implantation versus PTA plus provisional stent implantation, did not yield statistically significant results. Freedom from TLR at 36 months was 76 per cent for the LifeStent group compared to 70 per cent for the PTA plus provisional stent group. Clinical success at 36 months was 63 per cent for the primary stent group and 48 per cent for the PTA plus provisional stent group (p=ns). Expanded indication for the LifeStent and Lifestent XL 23 Study design In order to expand the indication for the LifeStent and LifeStent XL to lesions up to 240 mm (original FDA approval was limited to lesions up to 160 mm), a retrospective analysis of long lesions was conducted and reported in the FDA SSED 23 for the LifeStent and LifeStent XL. The applicant presented data from a post hoc caseseries analysis (level IV evidence) of: the RESILIENT trial; a multi-centre, nonrandomised, observational study conducted in Europe (ELODIE I); the routine clinical practice of a USA physician; and the routine clinical practice of a European physician. Of 285 patients with one or more implanted stents, 46 lesion segments longer than 160 mm were included in the analysis. Inclusion criteria, follow-up and outcomes were limited to the criteria specified in the primary clinical data. The primary safety endpoints of the retrospective review were 30-day and 12-month freedom from death and amputation (Kaplan-Meier analysis). The primary efficacy endpoint was defined as freedom from TVR/TLR at 12 months post-procedure. 12
15 Safety The 30-day freedom from death, amputation and TVR rate for patients with long lesions was 99.6 per cent with a standard error of 0.34 (95% CI: 97.59% %). Table 9 summarises the 12 month safety endpoint data stratified by lesion length. Table 9 Safety of LifeStent in long lesions Lesion length 12 month freedom from death and amputation, % (n/n) All 93.8% (17/291) < 50 mm 100% (0/72) mm 94.5% (6/112) mm 91.4% (5/61) mm 63.6% (4/13) mm 90.9% (1/11) All numbers are reported as number of lesions Efficacy The results for the primary effectiveness endpoint are summarised in Table 10. Table 10 Long segment lesion freedom from TLR/TVR Lesion length (mm) Freedom from TLR/TVR at 12 months Weibull/Kaplan-Meier analysis (n/n) Freedom from TLR/TVR at 24 months Average of all (total) lesion lengths (= mm) < 50 mm lesions (Weibull: 50 mm; n=72) 50 - <100 mm lesions (Weibull: 100 mm; n=112) <160 mm lesions (Weibull: 160 mm; n=61) <200 mm lesions (Weibull: 200 mm; n=13) < 240 mm lesions (Weibull: 240 mm; n=11) 82.4% / 79.2% (54/291) 63.3% / 62.5% (29/170) 85.4% / 83.4% (11/72) 69.0% / 68.1% (7/48) 81.9%/87.9% (12/112) 62.5% / 74.3% (9173) 76.7% / 76.5% (13/61) 53.6% / 55.2% (9/35) 72.6% / 38.9% (7/13) 47.0% / 38.9% (0/2 67.9% / 67.5% (3/11) 40.2% / NA (1/5) > 240 mm lesions (n=22) NA / 55.9% (8/22) NA / 23.9% (3/7) TLR: target lesion revascularisation; TVR: target vessel revascularisation The analysis was not adequately powered to detect statistically significant differences in the performance of the device for long lesions as compared to lesions less than 160 mm. The safety and efficacy results were deemed to be clinically acceptable for the FDA to expand the indication for the device. 13
16 Cost impact No studies evaluating the cost-effectiveness or cost impact of the LifeStent were identified from the retrieved material; however, Table 11 provides an overview of existing MBS items for the endovascular treatment of peripheral arteries by angioplasty and stenting. 25, 26 A USA-based trial, comparing the IntraCoil nitinol stent and PTA, assessed the costs of PTA with primary stent placement and reported that the use of the IntraCoil stent was associated with increased duration of procedure, equipment costs and physician services. Initial hospital costs were $3,455 higher for patients randomised to the IntraCoil stent compared with PTA ($8,435 versus $4,980, p<0.001). 27 Table 11 MBS items related to endovascular treatment of PAD Category Item number Benefit ($) Number of claims (July 2010 to June 2011) TRANSLUMINAL BALLOON ANGIOPLASTY of 1 peripheral artery or vein of 1 limb, percutaneous or by open exposure, excluding associated radiological services or preparation, and excluding aftercare Fee: $ Benefit: 75% = $ % = $ ,466 TRANSLUMINAL BALLOON ANGIOPLASTY of aortic arch branches, aortic visceral branches, or more than 1 peripheral artery or vein of 1 limb, percutaneous or by open exposure, excluding associated radiological services or preparation, and excluding aftercare TRANSLUMINAL STENT INSERTION including associated balloon dilatation for 1 peripheral artery or vein of 1 limb, percutaneous or by open exposure, excluding associated radiological services or preparation, and excluding aftercare TRANSLUMINAL STENT INSERTION including associated balloon dilatation for visceral arteries or veins, or more than 1 peripheral artery or vein of 1 limb, percutaneous or by open exposure, excluding associated radiological services or preparation, and excluding aftercare Fee: $ Benefit: 75% = $ % = $ Fee: $ Benefit: 75% = $ % = $ Fee: $ Benefit: 75% = $ % = $ ,205 1,893 2,622 PAD: peripheral arterial disease; MBS: Medicare Benefits Schedule Ethical, cultural or religious considerations No issues were identified from the retrieved material Other issues Five of the 13 RESILIENT investigators, including the lead investigator, are paid consultants, advisory board members or receive research support from Bard Vascular or Edwards Lifesciences. 14
17 Summary of findings The evidence indicates that primary stenting with the LifeStent is a safe and effective alternative to PTA alone in the SFA and popliteal arteries in lesions of up to 240 mm. Primary stenting appears to offer patients similar clinical performance compared to PTA at up to three years post-procedure. The comparative performance of primary stenting as compared to provisional stent implantation is unclear as is the durability of primary stent patency for more than three years. HealthPACT assessment: Based on the evidence from one randomised controlled trial and a low level caseseries, and given that the potential uptake of the technology is high, HealthPACT recommend that further research on this technology is not warranted. Number of studies included All evidence included for assessment in this Technology Brief has been assessed according to the revised NHMRC levels of evidence. A document summarising these levels may be accessed via the HealthPACT web site. Total number of studies 3 Total number of level II studies 2 Total number of level IV studies 1 References 1. Peron, S. (2010). Anatomy- arteries of the leg [internet]. Vascular Ultrasound. Available from: [Accessed 22 August 2012]. 2. Davies, M. G., Waldman, D. L. & Pearson, T. A. (2005). Comprehensive endovascular therapy for femoropopliteal arterial atherosclerotic occlusive disease, J Am Coll Surg, 201 (2), The Merck Manuals (2010). Peripheral artery disease [Internet]. The Merck Manual for Healthcare Professionals. Available from: /mmpe/print/sec07/ch080/ch080f.html [Accessed 2 May 2012]. 4. Norgren, L., Hiatt, W. R., Dormandy, J. A. et al (2007). Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II), Eur J Vasc Endovasc Surg, 33 Suppl 1, S Scottish Intercollegiate Guidelines Network (2006). Diagnosis and management of peripheral arterial disease: A national clinical guideline. Scottish Intercollegiate Guidelines Network, Edinburgh. Guideline Available from: /pdf/sign89.pdf. 6. Hirsch, A. T., et al (2006). ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease, J Am Coll Cardiol, 47, Kannel, W. B., Skinner, J. J. et al (1970). Intermittent claudication. Incidence in the Framingham Study, Circulation, 41 (5), Boyd, A. M. (1960). The natural course of arteriosclerosis of the lower extremities, Angiology, 11,
18 9. White, C. J. & Gray, W. A. (2007). Endovascular therapies for peripheral arterial disease an evidence-based review, Circulation, 116, Laird, J. R. (2006). Limitations of percutaneous transluminal angioplasty and stenting for the treatment of disease of the superficial femoral and popliteal arteries, J Endovasc Ther, 13 Suppl II, II-30 II Ansel, G. M. & Lumsden A. B. (2009). Evolving modalities for Femoropopliteal interventions, J Endovasc Ther, 16 Suppl II, Schillinger, M., Sabeti, S. et al (2006). Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery, New Engl J Med, 354 (18), Jaff, M. R. (2012). Advances in the management of patients with vascular disease, Expert Rev Cardiovasc Ther, 10 (2), Rocha-Singh, K., Jaff, M. R. et al (2007). Performance goals and end point assessments for clinical trials of femoropopliteal bare Nitinol stents in patients with symptomatic peripheral arterial disease, Catheter Cardiovasc Interv, 69, Bard Peripheral Vascular (2012) LifeStent Vascular Stent: Instructions for use [Internet]. Bard Peripheral Vascular. Available from: _vascular/in-prod-lifestent-ifu-wave.php [Accessed 2 May 2012]. 16. Fowler, B. Jamrozik, K. et al (2007). Prevalence of peripheral arterial disease: persistence of excess risk in former smokers, ANZ J Public Health, 26 (3), Australian Institute of Health and Welfare (2012). Principal diagnosis data cubes [Internet]. Available from: [Accessed 2 May 2012]. 18. Hirsch, A. T., Hartman, L. et al (2008). National health care costs of peripheral arterial disease in the Medicare population, Vasc Med, 13, National Heart, Lung, and Blood Institute (2010). Peripheral artery disease [Internet]. National Heart, Lung, and Blood Institute. Available from: [Accessed 2 May 2012]. 20. Australian Register of Therapeutic Goods (2012). Australian Register of Therapeutic Goods [Internet]. The Department of Health and Ageing. Available from: [Accessed 2 May 2012]. 21. U.S. National Institutes of Health (2012). Clinical trials.gov [Internet]. U.S. National Institutes of Health. Available from: [Accessed 2 May 2012]. 22. Laird, J. R., Katzen, B. T. et al (2010). Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery, Circ Cardiovasc Interv, 3, Laird, J. R., Katzen, B. T. et al (2012). Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial, J Endovasc Ther, 19, The Food and Drug Administration (2010). Summary of Safety and Effectiveness [Internet].FDA. Available from: 16
19 P070014b.pdf [Accessed 2 May 2012]. 25. The Department of Health and Ageing (2012). MBS online [Internet]. The Department of Health and Ageing. Available from: gov.au/ [Accessed 2 May 2012]. 26. Medicare Australia (2012). Statistics-Item reports [Internet]. Medicare Australia. Available from: item.shtml [Accessed 2 May 2012]. 27. Greenberg, D., Rosenfield, K. et al (2004). In-hospital costs of self-expanding nitinol stent implantation versus balloon angioplasty in the femoropopliteal artery (the vascucoil trial), J Vasc Interv Radiol, 15, Search criteria to be used (MeSH terms) Intermittent Claudication, Peripheral Artery Disease, Peripheral Vascular Diseases, Peripheral Occlusive Diseases, Percutaneous Transluminal Angioplasty, Stents 17
Majestic Trial 12 Month Results
 Majestic Trial 12 Month Results S.Müller-Hülsbeck, MD, EBIR, FCIRSE, FICA ACADEMIC HOSPITALS Flensburg of Kiel University Ev.-Luth. Diakonissenanstalt zu Flensburg Knuthstraße 1, 24939 FLENSBURG Dept.
Majestic Trial 12 Month Results S.Müller-Hülsbeck, MD, EBIR, FCIRSE, FICA ACADEMIC HOSPITALS Flensburg of Kiel University Ev.-Luth. Diakonissenanstalt zu Flensburg Knuthstraße 1, 24939 FLENSBURG Dept.
ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease. Erich Minar Medical University Vienna
 ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease Erich Minar Medical University Vienna for the Task Force on the Diagnosis and Treatment of Peripheral
ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease Erich Minar Medical University Vienna for the Task Force on the Diagnosis and Treatment of Peripheral
Popliteal artery: to stent or not to stent?
 Popliteal artery: to stent or not to stent? Karl-Ludwig Schulte Vascular Center Berlin Ev. Hospital Königin Elisabeth St. Gertrauden Hospital University Hospital Charité, CC11 Humboldt-University Berlin
Popliteal artery: to stent or not to stent? Karl-Ludwig Schulte Vascular Center Berlin Ev. Hospital Königin Elisabeth St. Gertrauden Hospital University Hospital Charité, CC11 Humboldt-University Berlin
Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty
 Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
MEDICAL POLICY No. 91580-R1 DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE
 DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE Effective Date: October 1, 2015 Review Dates: 10/11, 10/12, 10/13, 8/14, 8/15 Date Of Origin: October 12, 2011 Status: Current Summary of Changes Clarifications:
DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE Effective Date: October 1, 2015 Review Dates: 10/11, 10/12, 10/13, 8/14, 8/15 Date Of Origin: October 12, 2011 Status: Current Summary of Changes Clarifications:
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Supera Peripheral Stent System Instructions for Use
 Supera Peripheral Stent System Instructions for Use Table of Contents 1.0 DEVICE DESCRIPTION 2.0 HOW SUPPLIED 3.0 INDICATIONS 4.0 CONTRAINDICATIONS 5.0 WARNINGS 6.0 PRECAUTIONS 6.1 Stent Delivery System
Supera Peripheral Stent System Instructions for Use Table of Contents 1.0 DEVICE DESCRIPTION 2.0 HOW SUPPLIED 3.0 INDICATIONS 4.0 CONTRAINDICATIONS 5.0 WARNINGS 6.0 PRECAUTIONS 6.1 Stent Delivery System
Cilostazol versus Clopidogrel after Coronary Stenting
 Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Atherosclerosis of the aorta. Artur Evangelista
 Atherosclerosis of the aorta Artur Evangelista Atherosclerosis of the aorta Diagnosis Classification Prevalence Risk factors Marker of generalized atherosclerosis Risk of embolism Therapy Diagnosis Atherosclerosis
Atherosclerosis of the aorta Artur Evangelista Atherosclerosis of the aorta Diagnosis Classification Prevalence Risk factors Marker of generalized atherosclerosis Risk of embolism Therapy Diagnosis Atherosclerosis
How To Determine Pad
 Process Representation #1 : The PAD algorithm as a sequential flow thru all sections An exploded version of the above scoped section flow is shown below. Notes: The flow presupposes existing services (
Process Representation #1 : The PAD algorithm as a sequential flow thru all sections An exploded version of the above scoped section flow is shown below. Notes: The flow presupposes existing services (
WHY DO MY LEGS HURT? Veins, arteries, and other stuff.
 WHY DO MY LEGS HURT? Veins, arteries, and other stuff. Karl A. Illig, MD Professor of Surgery Chief, Division of Vascular Surgery Mitzi Ekers, ARNP April 2013 Why do my legs hurt? CONFLICTS OF INTEREST
WHY DO MY LEGS HURT? Veins, arteries, and other stuff. Karl A. Illig, MD Professor of Surgery Chief, Division of Vascular Surgery Mitzi Ekers, ARNP April 2013 Why do my legs hurt? CONFLICTS OF INTEREST
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
Facts About Peripheral Arterial Disease (P.A.D.)
 Facts About Peripheral Arterial Disease (P.A.D.) One in every 20 Americans over the age of 50 has P.A.D., a condition that raises the risk for heart attack and stroke. Peripheral arterial disease, or P.A.D.,
Facts About Peripheral Arterial Disease (P.A.D.) One in every 20 Americans over the age of 50 has P.A.D., a condition that raises the risk for heart attack and stroke. Peripheral arterial disease, or P.A.D.,
PRECOMBAT Trial. Seung-Whan Lee, MD, PhD On behalf of the PRECOMBAT Investigators
 Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease PRECOMBAT Trial Seung-Whan Lee, MD, PhD On behalf
Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease PRECOMBAT Trial Seung-Whan Lee, MD, PhD On behalf
Patients suffering from critical limb ischemia (CLI)
 Building a Successful Amputation Prevention Program Our single-center experience implementing an amputation prevention algorithm and how it has led to a trend in reduced amputation rates. By Jihad A. Mustapha,
Building a Successful Amputation Prevention Program Our single-center experience implementing an amputation prevention algorithm and how it has led to a trend in reduced amputation rates. By Jihad A. Mustapha,
REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES
 REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES Effective January 1, 2015, there was a change in CPT that affects reporting specific endovascular services provided in the
REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES Effective January 1, 2015, there was a change in CPT that affects reporting specific endovascular services provided in the
Duration of Dual Antiplatelet Therapy After Coronary Stenting
 Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
The largest clinical study of Bayer's Xarelto (rivaroxaban) Wednesday, 14 November 2012 07:38
 Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY
 STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
Iliac Artery Disease: A Case-Based Approach To Stent Selection
 Society for Vascular Medicine Iliac Artery Disease: A Case-Based Approach To Stent Selection Annotated Cited Reference Material (2002). "MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin
Society for Vascular Medicine Iliac Artery Disease: A Case-Based Approach To Stent Selection Annotated Cited Reference Material (2002). "MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin
SAMPLE. Asia-Pacific Interventional Cardiology Procedures Outlook to 2020. Reference Code: GDMECR0061PDB. Publication Date: May 2014
 Asia-Pacific Interventional Cardiology Procedures Outlook to 2020 Reference Code: GDMECR0061PDB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
Asia-Pacific Interventional Cardiology Procedures Outlook to 2020 Reference Code: GDMECR0061PDB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
Non-surgical treatment of severe varicose veins
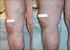 Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
PATIENT INFORMATION BOOKLET
 PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
Credentials for Peripheral Angioplasty: Comments on Society of Cardiac Angiography and Intervention Revisions
 Credentials for Peripheral Angioplasty: Comments on Society of Cardiac Angiography and Intervention Revisions David Sacks, MD, Gary J. Becker, MD, and Terence A.S. Matalon, MD J Vasc Interv Radiol 2003;
Credentials for Peripheral Angioplasty: Comments on Society of Cardiac Angiography and Intervention Revisions David Sacks, MD, Gary J. Becker, MD, and Terence A.S. Matalon, MD J Vasc Interv Radiol 2003;
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
Steven J. Yakubov, MD FACC For the CoreValve US Clinical Investigators
 Long-Term Outcomes Using a Self- Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Deemed Extreme Risk for Surgery: Two-Year Results From the CoreValve US Pivotal Trial Steven J. Yakubov,
Long-Term Outcomes Using a Self- Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Deemed Extreme Risk for Surgery: Two-Year Results From the CoreValve US Pivotal Trial Steven J. Yakubov,
A Patient s Guide to Primary and Secondary Prevention of Cardiovascular Disease Using Blood-Thinning (Anticoagulant) Drugs
 A Patient s Guide to Primary and Secondary Prevention of PATIENT EDUCATION GUIDE What Is Cardiovascular Disease? Cardiovascular disease (CVD) is a broad term that covers any disease of the heart and circulatory
A Patient s Guide to Primary and Secondary Prevention of PATIENT EDUCATION GUIDE What Is Cardiovascular Disease? Cardiovascular disease (CVD) is a broad term that covers any disease of the heart and circulatory
A Post-market Study to Assess the STENTYS Self-exPanding COronary Stent In AcuTe myocardial InfarctiON in Real Life APPOSITION III
 A Post-market Study to Assess the STENTYS Self-exPanding COronary Stent In AcuTe myocardial InfarctiON in Real Life APPOSITION III Gilles Montalescot, MD, PhD Pitié-Salpêtrière Hospital, Paris, France
A Post-market Study to Assess the STENTYS Self-exPanding COronary Stent In AcuTe myocardial InfarctiON in Real Life APPOSITION III Gilles Montalescot, MD, PhD Pitié-Salpêtrière Hospital, Paris, France
EFSUMB EUROPEAN FEDERATION OF SOCIETIES FOR ULTRASOUND IN MEDICINE AND BIOLOGY Building a European Ultrasound Community
 MINIMUM TRAINING REQUIREMENTS FOR THE PRACTICE OF MEDICAL ULTRASOUND IN EUROPE Appendix 8: Vascular Ultrasound Level 1 Training and Practice Practical training should involve at least two half day ultrasound
MINIMUM TRAINING REQUIREMENTS FOR THE PRACTICE OF MEDICAL ULTRASOUND IN EUROPE Appendix 8: Vascular Ultrasound Level 1 Training and Practice Practical training should involve at least two half day ultrasound
Renovascular Disease. Renal Artery and Arteriosclerosis
 Other names: Renal Artery Stenosis (RAS) Renal Vascular Hypertension (RVH) Renal Artery Aneurysm (RAA) How does the normal kidney work? The blood passes through the kidneys to remove the body s waste.
Other names: Renal Artery Stenosis (RAS) Renal Vascular Hypertension (RVH) Renal Artery Aneurysm (RAA) How does the normal kidney work? The blood passes through the kidneys to remove the body s waste.
Main Effect of Screening for Coronary Artery Disease Using CT
 Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up
 Hellenic J Cardiol 45: 379-383, 2004 Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up PETROS S. DARDAS, DIMITRIS
Hellenic J Cardiol 45: 379-383, 2004 Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up PETROS S. DARDAS, DIMITRIS
Ostial LAD: Single stent approach is the best. Antonio A. Pocoví, MD, FSCAI, MTSAC, Advisory Council Member, CACI
 Ostial LAD: Single stent approach is the best Antonio A. Pocoví, MD, FSCAI, MTSAC, Advisory Council Member, CACI Chair, Interventional Cardiology Sanatorio San Lucas Instituto Alexander Fleming Buenos
Ostial LAD: Single stent approach is the best Antonio A. Pocoví, MD, FSCAI, MTSAC, Advisory Council Member, CACI Chair, Interventional Cardiology Sanatorio San Lucas Instituto Alexander Fleming Buenos
The Cardiac Society of Australia and New Zealand
 The Cardiac Society of Australia and New Zealand Guidelines on Support Facilities for Coronary Angiography and Percutaneous Coronary Intervention (PCI) including Guidelines on the Performance of Procedures
The Cardiac Society of Australia and New Zealand Guidelines on Support Facilities for Coronary Angiography and Percutaneous Coronary Intervention (PCI) including Guidelines on the Performance of Procedures
Drug-Eluting Balloons. Klaus Bonaventura Department of Cardiology and Angiology Heart Thorax Vascular Center, Klinikum Ernst von Bergmann, Potsdam
 Drug-Eluting Balloons Klaus Bonaventura Department of Cardiology and Angiology Heart Thorax Vascular Center, Klinikum Ernst von Bergmann, Potsdam Potential conflicts of interest Speaker s name: Klaus Bonaventura
Drug-Eluting Balloons Klaus Bonaventura Department of Cardiology and Angiology Heart Thorax Vascular Center, Klinikum Ernst von Bergmann, Potsdam Potential conflicts of interest Speaker s name: Klaus Bonaventura
Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease
 Home SVCC Area: English - Español - Português Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease Martial G. Bourassa, MD Research Center, Montreal Heart Institute, Montreal, Quebec,
Home SVCC Area: English - Español - Português Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease Martial G. Bourassa, MD Research Center, Montreal Heart Institute, Montreal, Quebec,
GENERAL HEART DISEASE KNOW THE FACTS
 GENERAL HEART DISEASE KNOW THE FACTS WHAT IS Heart disease is a broad term meaning any disease affecting the heart. It is commonly used to refer to coronary heart disease (CHD), a more specific term to
GENERAL HEART DISEASE KNOW THE FACTS WHAT IS Heart disease is a broad term meaning any disease affecting the heart. It is commonly used to refer to coronary heart disease (CHD), a more specific term to
Local Coverage Article: Endovascular Repair of Aortic Aneurysms (A53124)
 Local Coverage Article: Endovascular Repair of Aortic Aneurysms (A53124) Contractor Information Contractor Name Novitas Solutions, Inc. Article Information General Information Article ID A53124 Original
Local Coverage Article: Endovascular Repair of Aortic Aneurysms (A53124) Contractor Information Contractor Name Novitas Solutions, Inc. Article Information General Information Article ID A53124 Original
Copenhagen University Hospital Rigshospitalet Aarhus University Hospital Skejby Denmark
 Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-elevation myocardial infarction 3 year follow-up of the randomised trial Peter Clemmensen, Henning Kelbæk,
Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-elevation myocardial infarction 3 year follow-up of the randomised trial Peter Clemmensen, Henning Kelbæk,
Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options. A Guide for Patients
 Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options A Guide for Patients Coronary Artery Disease If you or a member of your family has been diagnosed with coronary artery
Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options A Guide for Patients Coronary Artery Disease If you or a member of your family has been diagnosed with coronary artery
California Health and Safety Code, Section 1256.01
 California Health and Safety Code, Section 1256.01 1256.01. (a) The Elective Percutaneous Coronary Intervention (PCI) Pilot Program is hereby established in the department. The purpose of the pilot program
California Health and Safety Code, Section 1256.01 1256.01. (a) The Elective Percutaneous Coronary Intervention (PCI) Pilot Program is hereby established in the department. The purpose of the pilot program
Transcatheter Mitral Valve-in-Valve and Valve-in-Ring Implantations. Danny Dvir, MD On behalf of VIVID registry investigators
 Transcatheter Mitral Valve-in-Valve and Valve-in-Ring Implantations Danny Dvir, MD On behalf of VIVID registry investigators Introduction Bioprosthetic valves are increasingly implanted in open-heart surgeries.
Transcatheter Mitral Valve-in-Valve and Valve-in-Ring Implantations Danny Dvir, MD On behalf of VIVID registry investigators Introduction Bioprosthetic valves are increasingly implanted in open-heart surgeries.
Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page)
 Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page) The Kidney and the Renal Arteries... 1 Renal Artery Disease... 2 Diagnosis of Renal.Artery Disease...
Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page) The Kidney and the Renal Arteries... 1 Renal Artery Disease... 2 Diagnosis of Renal.Artery Disease...
Bayer Extends Clinical Investigation of Rivaroxaban into Important Areas of Unmet Medical Need in Arterial Thromboembolism
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer Extends Clinical Investigation of Rivaroxaban into Important Areas of
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer Extends Clinical Investigation of Rivaroxaban into Important Areas of
GUIDELINES ON MEDICAL DEVICES EVALUATION OF CLINICAL DATA - A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES -
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Cosmetics and Medical Devices MEDDEV 2.7.1 Appendix 1 December 2008 GUIDELINES ON MEDICAL DEVICES EVALUATION OF CLINICAL DATA
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Cosmetics and Medical Devices MEDDEV 2.7.1 Appendix 1 December 2008 GUIDELINES ON MEDICAL DEVICES EVALUATION OF CLINICAL DATA
Vascular Stent System (6F, 20 100mm stents)
 100000000922.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System (6F, 20 100mm stents) Cordis S.M.A.R.T. Vascular Stent System (6F, 120 150mm stents) Symbols Catalog Number LOT NonPyrogenic
100000000922.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System (6F, 20 100mm stents) Cordis S.M.A.R.T. Vascular Stent System (6F, 120 150mm stents) Symbols Catalog Number LOT NonPyrogenic
Ultrasound in Vascular Surgery. Torbjørn Dahl
 Ultrasound in Vascular Surgery Torbjørn Dahl 1 The field of vascular surgery Veins dilatation and obstruction (varicose veins and valve dysfunction) Arteries dilatation and narrowing (aneurysms and atherosclerosis)
Ultrasound in Vascular Surgery Torbjørn Dahl 1 The field of vascular surgery Veins dilatation and obstruction (varicose veins and valve dysfunction) Arteries dilatation and narrowing (aneurysms and atherosclerosis)
Imaging of Thoracic Endovascular Stent-Grafts
 Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
DISCLOSURES RISK ASSESSMENT. Stroke and Heart Disease -Is there a Link Beyond Risk Factors? Daniel Lackland, MD
 STROKE AND HEART DISEASE IS THERE A LINK BEYOND RISK FACTORS? D AN IE L T. L AC K L AN D DISCLOSURES Member of NHLBI Risk Assessment Workgroup RISK ASSESSMENT Count major risk factors For patients with
STROKE AND HEART DISEASE IS THERE A LINK BEYOND RISK FACTORS? D AN IE L T. L AC K L AN D DISCLOSURES Member of NHLBI Risk Assessment Workgroup RISK ASSESSMENT Count major risk factors For patients with
COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR DISEASE PREVENTION
 European Medicines Agency London, 19 July 2007 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR
European Medicines Agency London, 19 July 2007 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR
Interventional Cardiology Peripheral Interventions Rhythm Management
 FY2016 Hospital Inpatient Rule (IPPS) Interventional Cardiology Peripheral Interventions Rhythm Management On April 17, 2015 the Centers for Medicare and Medicaid Services (CMS) released the Hospital Inpatient
FY2016 Hospital Inpatient Rule (IPPS) Interventional Cardiology Peripheral Interventions Rhythm Management On April 17, 2015 the Centers for Medicare and Medicaid Services (CMS) released the Hospital Inpatient
CARDIA 288 MONTH FOLLOW-UP SUPPLEMENTAL FORM (FORM B) HOSPITALIZATION CASE #: INTERVIEWER ID FY288BIVID2. Page 1 of 6 FY288BH4CN
 HOSPITALIZATION CASE #: 2 8 8 0 H FY288BH4CN Has the participant indicated any of the following reasons for being admitted overnight for this case? 1. Suspected or confirmed problems with the heart, circulation,
HOSPITALIZATION CASE #: 2 8 8 0 H FY288BH4CN Has the participant indicated any of the following reasons for being admitted overnight for this case? 1. Suspected or confirmed problems with the heart, circulation,
Patient Information Booklet. Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms
 Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
ESC/EASD Pocket Guidelines Diabetes, pre-diabetes and cardiovascular disease
 Diabetes, prediabetes and cardiovascular disease Classes of recommendations Levels of evidence Recommended treatment targets for patients with diabetes and CAD Definition, classification and screening
Diabetes, prediabetes and cardiovascular disease Classes of recommendations Levels of evidence Recommended treatment targets for patients with diabetes and CAD Definition, classification and screening
The Pantera Lux Paclitaxel DEB Device Description and Clinical Studies. Christoph Hehrlein, University Clinic Freiburg i.br.
 The Pantera Lux Paclitaxel DEB Device Description and Clinical Studies Christoph Hehrlein, University Clinic Freiburg i.br. Germany Disclosure Statement of Financial Interest Within the past 12 months,
The Pantera Lux Paclitaxel DEB Device Description and Clinical Studies Christoph Hehrlein, University Clinic Freiburg i.br. Germany Disclosure Statement of Financial Interest Within the past 12 months,
Stroke: Major Public Health Burden. Stroke: Major Public Health Burden. Stroke: Major Public Health Burden 5/21/2012
 Faculty Prevention Sharon Ewer, RN, BSN, CNRN Stroke Program Coordinator Baptist Health Montgomery, Alabama Satellite Conference and Live Webcast Monday, May 21, 2012 2:00 4:00 p.m. Central Time Produced
Faculty Prevention Sharon Ewer, RN, BSN, CNRN Stroke Program Coordinator Baptist Health Montgomery, Alabama Satellite Conference and Live Webcast Monday, May 21, 2012 2:00 4:00 p.m. Central Time Produced
INTRODUCTION TO EECP THERAPY
 INTRODUCTION TO EECP THERAPY is an FDA cleared, Medicare approved, non-invasive medical therapy for the treatment of stable and unstable angina, congestive heart failure, acute myocardial infarction, and
INTRODUCTION TO EECP THERAPY is an FDA cleared, Medicare approved, non-invasive medical therapy for the treatment of stable and unstable angina, congestive heart failure, acute myocardial infarction, and
Experience of Direct Coronary Stenting at National Institute of Cardiovascular Diseases
 Experience of Direct Coronary Stenting at National Institute of Cardiovascular Diseases T. Masood,T. Sagheer,D. Jan,N. Qamar,A.M.A. Faruqui ( National Institute of Cardiovascular Diseases (NICVD), Karachi.
Experience of Direct Coronary Stenting at National Institute of Cardiovascular Diseases T. Masood,T. Sagheer,D. Jan,N. Qamar,A.M.A. Faruqui ( National Institute of Cardiovascular Diseases (NICVD), Karachi.
NOVOSTE BETA-CATH SYSTEM
 HOSPITAL INPATIENT AND OUTPATIENT BILLING GUIDE FOR THE NOVOSTE BETA-CATH SYSTEM INTRAVASCULAR BRACHYTHERAPY DEVICE This guide is intended solely for use as a tool to help hospital billing staff resolve
HOSPITAL INPATIENT AND OUTPATIENT BILLING GUIDE FOR THE NOVOSTE BETA-CATH SYSTEM INTRAVASCULAR BRACHYTHERAPY DEVICE This guide is intended solely for use as a tool to help hospital billing staff resolve
Endovascular Repair of an Axillary Artery Aneurysm: A Novel Approach
 Endovascular Repair of an Axillary Artery Aneurysm: A Novel Approach Bao- Thuy D. Hoang, MD 1, Jonathan- Hien Vu, MD 2, Jerry Matteo, MD 3 1 Department of Surgery, University of Florida College of Medicine,
Endovascular Repair of an Axillary Artery Aneurysm: A Novel Approach Bao- Thuy D. Hoang, MD 1, Jonathan- Hien Vu, MD 2, Jerry Matteo, MD 3 1 Department of Surgery, University of Florida College of Medicine,
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair
 A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
Talent Thoracic Stent Graft with THE Xcelerant Delivery System. Expanding the Indications for TEVAR
 Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM
 INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM NAME: DATE: 1. PURPOSE AND EXPLANATION OF PROCEDURE I hereby consent to voluntarily engage in an acceptable
INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM NAME: DATE: 1. PURPOSE AND EXPLANATION OF PROCEDURE I hereby consent to voluntarily engage in an acceptable
CHAPTER V DISCUSSION. normal life provided they keep their diabetes under control. Life style modifications
 CHAPTER V DISCUSSION Background Diabetes mellitus is a chronic condition but people with diabetes can lead a normal life provided they keep their diabetes under control. Life style modifications (LSM)
CHAPTER V DISCUSSION Background Diabetes mellitus is a chronic condition but people with diabetes can lead a normal life provided they keep their diabetes under control. Life style modifications (LSM)
Is Stenting or Coronary Artery By-pass Grafting the Better Treatment for This Patient?
 Is Stenting or Coronary Artery By-pass Grafting the Better Treatment for This Patient? --- NIRS-IVUS TVC Imaging Adds Additional Information for the Heart Team Dr. Luis Tami Memorial Regional Hospital
Is Stenting or Coronary Artery By-pass Grafting the Better Treatment for This Patient? --- NIRS-IVUS TVC Imaging Adds Additional Information for the Heart Team Dr. Luis Tami Memorial Regional Hospital
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR DISEASE PREVENTION
 European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 25 September 2008 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 25 September 2008 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
Dual Antiplatelet Therapy. Stephen Monroe, MD FACC Chattanooga Heart Institute
 Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Mechanical Properties of Nitinol Stents and Stent-grafts. Comparison of 6 mm Diameter Devices
 Mechanical Properties of Nitinol Stents and Stent-grafts Comparison of 6 mm Diameter Devices P E R F O R M A N C E b y d e s i g n Purpose The association of target vessel restenosis with stent fractures
Mechanical Properties of Nitinol Stents and Stent-grafts Comparison of 6 mm Diameter Devices P E R F O R M A N C E b y d e s i g n Purpose The association of target vessel restenosis with stent fractures
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief
 HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief Percutaneous venoplasty for the treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief Percutaneous venoplasty for the treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple
LEADERS: 5-Year Follow-up
 LEADERS: -Year Follow-up from a Prospective, Randomized Trial of Biolimus A9-eluting Stents with a Biodegradable Polymer vs. Sirolimus-eluting Stents with a Durable Polymer : Final Report of the LEADERS
LEADERS: -Year Follow-up from a Prospective, Randomized Trial of Biolimus A9-eluting Stents with a Biodegradable Polymer vs. Sirolimus-eluting Stents with a Durable Polymer : Final Report of the LEADERS
Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence
 Guidelines Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence Stroke/TIA Nearly 700,000 ischemic strokes and 240,000 TIAs every year in the United States Currently, the risk for
Guidelines Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence Stroke/TIA Nearly 700,000 ischemic strokes and 240,000 TIAs every year in the United States Currently, the risk for
Summary Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency (CCSVI) in People with Multiple Sclerosis (MS)
 ETMIS 2012; Vol. 8: N o 7 Summary Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency (CCSVI) in People with Multiple Sclerosis (MS) March 2012 A production of the Institut national d
ETMIS 2012; Vol. 8: N o 7 Summary Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency (CCSVI) in People with Multiple Sclerosis (MS) March 2012 A production of the Institut national d
Overview of Newer Stent Devices for Aneurysm Treatment
 Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Col league. SMMC Vascular Center Opens A PUBLICATION FOR SOUTHERN MAINE PHYSICIANS
 A PUBLICATION FOR SOUTHERN MAINE PHYSICIANS Col league 8 2012 SMMC Vascular Center Opens By Frank Lavoie, MD, Executive Vice President and Chief Operating Officer During the last year, Southern Maine Medical
A PUBLICATION FOR SOUTHERN MAINE PHYSICIANS Col league 8 2012 SMMC Vascular Center Opens By Frank Lavoie, MD, Executive Vice President and Chief Operating Officer During the last year, Southern Maine Medical
ECG may be indicated for patients with cardiovascular risk factors
 eappendix A. Summary for Preoperative ECG American College of Cardiology/ American Heart Association, 2007 A1 2002 A2 European Society of Cardiology and European Society of Anaesthesiology, 2009 A3 Improvement,
eappendix A. Summary for Preoperative ECG American College of Cardiology/ American Heart Association, 2007 A1 2002 A2 European Society of Cardiology and European Society of Anaesthesiology, 2009 A3 Improvement,
LifeStent XL Biliary Stent System
 B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
Rivaroxaban for acute coronary syndromes
 Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study
 DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study National Diabetes Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study National Diabetes Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What
Innova Vascular OVER-THE-WIRE
 POTENTIAL ADVERSE EVENTS... HOW SUPPLIED...6 909580-0 Innova Vascular OVER-THE-WIRE Self-Expanding Stent System TABLE OF CONTENTS 05-07 < en > WARNING... DEVICE DESCRIPTION... Contents... INTENDED USE
POTENTIAL ADVERSE EVENTS... HOW SUPPLIED...6 909580-0 Innova Vascular OVER-THE-WIRE Self-Expanding Stent System TABLE OF CONTENTS 05-07 < en > WARNING... DEVICE DESCRIPTION... Contents... INTENDED USE
The treatment of peripheral vascular disease has
 Covered s in Peripheral Vascular Aneurysms and Emergencies Reviewing current covered stent selection and use through case study and discussion. By Alberto Posabella, MD; Raffaele Rosso, MD, FACS, FRCS;
Covered s in Peripheral Vascular Aneurysms and Emergencies Reviewing current covered stent selection and use through case study and discussion. By Alberto Posabella, MD; Raffaele Rosso, MD, FACS, FRCS;
Renovascular Hypertension
 Renovascular Hypertension Philip Stockwell, MD Assistant Professor of Medicine (Clinical) Warren Alpert School of Medicine Cardiology for the Primary Care Provider September 28, 201 Renovascular Hypertension
Renovascular Hypertension Philip Stockwell, MD Assistant Professor of Medicine (Clinical) Warren Alpert School of Medicine Cardiology for the Primary Care Provider September 28, 201 Renovascular Hypertension
CHAPTER 9 DISEASES OF THE CIRCULATORY SYSTEM (I00-I99)
 CHAPTER 9 DISEASES OF THE CIRCULATORY SYSTEM (I00-I99) March 2014 2014 MVP Health Care, Inc. CHAPTER 9 CHAPTER SPECIFIC CATEGORY CODE BLOCKS I00-I02 Acute rheumatic fever I05-I09 Chronic rheumatic heart
CHAPTER 9 DISEASES OF THE CIRCULATORY SYSTEM (I00-I99) March 2014 2014 MVP Health Care, Inc. CHAPTER 9 CHAPTER SPECIFIC CATEGORY CODE BLOCKS I00-I02 Acute rheumatic fever I05-I09 Chronic rheumatic heart
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Adult Cardiology. Diagnosis of Arterial Disease of the Lower Extremities With Duplex Scanning: A Validation Study
 Adult Cardiology Diagnosis of Arterial Disease of the Lower Extremities With Duplex Scanning: A Validation Study Rosella S. Arellano, MD; Ma. Teresa B. Abola, MD. Background --- While standard x-ray arteriography
Adult Cardiology Diagnosis of Arterial Disease of the Lower Extremities With Duplex Scanning: A Validation Study Rosella S. Arellano, MD; Ma. Teresa B. Abola, MD. Background --- While standard x-ray arteriography
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
Antonio Colombo MD on behalf of the SECURITY Investigators
 Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve-Month - Dual Antiplatelet Therapy - The SECURITY Randomized Clinical Trial Antonio Colombo MD on behalf of the SECURITY
Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve-Month - Dual Antiplatelet Therapy - The SECURITY Randomized Clinical Trial Antonio Colombo MD on behalf of the SECURITY
Use of Stents and Stent Grafts to Salvage Angioplasty Failures in Patients with Hemodialysis Grafts
 Use of Stents and Stent Grafts to Salvage Angioplasty Failures in Patients with Hemodialysis Grafts Thomas M. Vesely, Mohammed Zaheer Amin, and Thomas Pilgram St. Louis, Missouri ABSTRACT To determine
Use of Stents and Stent Grafts to Salvage Angioplasty Failures in Patients with Hemodialysis Grafts Thomas M. Vesely, Mohammed Zaheer Amin, and Thomas Pilgram St. Louis, Missouri ABSTRACT To determine
CASE B1. Newly Diagnosed T2DM in Patient with Prior MI
 Newly Diagnosed T2DM in Patient with Prior MI 1 Our case involves a gentleman with acute myocardial infarction who is newly discovered to have type 2 diabetes. 2 One question is whether anti-hyperglycemic
Newly Diagnosed T2DM in Patient with Prior MI 1 Our case involves a gentleman with acute myocardial infarction who is newly discovered to have type 2 diabetes. 2 One question is whether anti-hyperglycemic
Reimbursement for Medical Products: Ensuring Marketplace
 Reimbursement for Medical Products: Ensuring Marketplace Success by Securing Coverage and Payment Christopher J. Panarites, Ph.D. Director, Endovascular Products Health Economics and Outcomes Research
Reimbursement for Medical Products: Ensuring Marketplace Success by Securing Coverage and Payment Christopher J. Panarites, Ph.D. Director, Endovascular Products Health Economics and Outcomes Research
JUL 2 2008. Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular Cardiac Therapies 3200 Lakeside Drive Santa Clara, CA 95054-2807
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
Lifecheque Basic Critical Illness Insurance
 Lifecheque Basic Critical Illness Insurance Strong. Reliable. Trustworthy. Forward-thinking. Extra help on the road to recovery Surviving a critical illness can be very challenging financially Few of us
Lifecheque Basic Critical Illness Insurance Strong. Reliable. Trustworthy. Forward-thinking. Extra help on the road to recovery Surviving a critical illness can be very challenging financially Few of us
Bard LifeStar Vascular Stent System
 Bard LifeStar Vascular Stent System Instructions For Use (IFU) Bard LifeStar Vascular Stent System Delivery System Diagram B05685 Vers.1/08-11 1 Instructions for use Read the Bard LifeStar Vascular Stent
Bard LifeStar Vascular Stent System Instructions For Use (IFU) Bard LifeStar Vascular Stent System Delivery System Diagram B05685 Vers.1/08-11 1 Instructions for use Read the Bard LifeStar Vascular Stent
