Indication: Posology: Indication: Posology:
|
|
|
- David Lester
- 10 years ago
- Views:
Transcription
1 Maklumat tambahan indikasi untuk upload pada laman web Year 2014 Products Approved For Additional Indication (DCA Mei 2014) NO PRODUCT (ACTIVE INGREDIENT) Trajenta Duo 2.5mg/500mg Film-Coated Tablets [Linagliptin 2.5mg & metformin hydrochloride 500mg] 1.2 Trajenta Duo 2.5mg/850mg Film-Coated Tablets [ Linagliptin 2.5mg & metformin hydrochloride 850mg ] 1.3 Trajenta Duo 2.5mg/1000mg Film-Coated Tablets [Linagliptin 2.5mg & metformin hydrochloride 1000mg] ADDITIONAL INDICATION Trajenta Duo is indicated as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus when treatment with both linagliptin and metformin is appropriate. For patients currently not treated with metformin In patients currently not treated with metformin, initiate treatment with 2.5mg linagliptin/500mg metformin hydrochloride twice daily. MARKETING AUTHORIZATION HOLDER BOEHRINGER INGELHEIM Suite 15-5, Level 15 Wisma UOA Damansara II No.6, Jalan Changkat Semantan, Damansara Heights, Kuala Lumpur Afinitor 2.5mg Tablet [Everolimus 2.5mg] 2.2 Afinitor 5mg Tablet [ Everolimus 5mg ] 2.3 Afinitor 10mg Tablet [Everolimus 10mg] Treatment of adult and paediatric patients, 1 year of age and older, with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. Pediatric Use Paediatric use of Afinitor is recommended for patients 1 year of age and older with TSC for the treatment of SEGA that requires therapeutic intervention but cannot be curatively resected. The safety and effectiveness of Afinitor have not been established in paediatric patients with renal angiomyolipoma with TSC in the absence of SEGA. The effectiveness of AFINITOR in paediatric patients with SEGA was demonstrated in two NOVARTIS CORPORATION Level 22, Tower B, Plaza 33
2 clinical trials based on demonstration of durable objective response, as evidenced by reduction in SEGA tumour volume. Improvement in diseaserelated symptoms and overall survival in paediatric patients with SEGA has not been demonstrated. The long term effects of Afinitor on growth and pubertal development are unknown. Study 1 was a randomized, double-blind, multicenter trial comparing Afinitor (n=78) to placebo (n=39) in paediatric and adult patients. The median age was 9.5 years (range 0.8 to 26 years). At the time of randomization, a total of 20 patients were < 3 years of age, 54 patients were 3 to < 12 years of age, 27 patients were 12 to < 18 years of age, and 16 patients were 18 years of age. The overall nature, type, and frequency of adverse reactions across the age groups evaluated were similar, with the exception of a higher per patient incidence of infectious serious adverse events in patients < 3 years of age. A total of 6 of 13 patients (46%) < 3 years of age had at least one serious adverse event due to infection, compared to 2 of 7 patients (29%) treated with placebo. No patient in any age group discontinued Afinitor due to infection. Subgroup analyses showed reduction in SEGA volume with Afinitor treatment in all paediatric age subgroups. Study 2 was an open-label, single-arm, singlecenter trial of Afinitor (N=28) in patients aged 3 years; median age was 11 years (range 3 to 34 years). A total of 16 patients were 3 to < 12 years, 6 patients were 12 to < 18 years, and 6 patients were 18 years. The frequency of adverse reactions across the age groups was generally similar. Subgroup analyses showed reductions in SEGA volume with Afinitor treatment in all paediatric age subgroups. Everolimus clearance normalized to body surface area was higher in paediatric patients than in adults
3 with SEGA. The recommended starting dose and subsequent requirement for therapeutic drug monitoring to achieve and maintain trough concentrations of 5 to 15 ng/ml are the same for adult and paediatric patients with SEGA Eylea 40mg/ml Solution For Injection In Vial [Aflibercept 40mg/mL] 3.2 Eylea 40mg/ml Solution For Injection In Prefilled Syringe [Aflibercept 40mg/mL] Eylea is indicated for the treatment of visual impairment due to macular oedema secondary to central retinal vein occlusion (CRVO). The recommended dose for Eylea is 2 mg aflibercept, equivalent to 0.05mL (50 μl). After the initial injection, treatment is given monthly. The interval between two doses should not be shorter than one month. If there is no improvement in visual and anatomic outcomes over the course of the first three injections, continued treatment is not recommended. Monthly treatment continues until visual and anatomic outcomes are stable for three monthly assessments. Thereafter the need for continued treatment should be reconsidered. If necessary, treatment may be continued with gradually increasing treatment intervals to maintain a stable visual and anatomic outcome. If treatment has been discontinued, visual and anatomic outcomes should be monitored and treatment should be resumed if these deteriorate. Usually, monitoring should be done at the injection visits. During treatment interval extension through to completion of therapy, the monitoring schedule should be determined by the treating physician based on the individual patient s response and may be more frequent than the schedule of injections. BAYER CO. (MALAYSIA) SDN. BHD. T1-14 Jaya 33, No.3, Jalan Semangat, Seksyen 13,
4 Ilaris 150mg Powder For Solution For Injection [Canakinumab 150mg/ml] Ilaris is indicated for the treatment of active Systemic Juvenile Idiopathic Arthritis (SJIA) in patients aged 2 years and older who have responded inadequately to previous therapy with non-steroidal anti-inflammatory drugs (NSAIDs) and systemic corticosteroids. Ilaris can be given as monotherapy or in combination with methotrexate. The recommended dose of Ilaris for SJIA patients with body weight 7.5 kg is 4 mg/kg (up to a maximum of 300 mg) administered every four weeks via subcutaneous injection. NOVARTIS CORPORATION Level 22, Tower B, Plaza 33 No. 1, Jalan Kemajuan, Petaling Jaya, Apidra 100 U/ml- Solution For Injection In Vial [Insulin glulisine 100U/ml] Posology The potency of this preparation is stated in units. These units are exclusive to Apidra and are not the same as IU or the units used to express the potency of other insulin analogues. Apidra should be used in regimens that include an intermediate or long acting insulin or basal insulin analogue and can be used with oral hypoglycaemic agents. The dose of Apidra should be individually adjusted. Method of administration Intravenous use Apidra can be administered intravenously under medical supervision for glycemic control with close monitoring of blood glucose and serum potassium to avoid hypoglycemia and hypokalemia.. Apidra must not be mixed with glucose or Ringer s solution or with any other insulin. Subcutaneous use Apidra should be given by subcutaneous injection shortly (0-15 min) before or soon after meals or by continuous subcutaneous pump infusion. SANOFI-AVENTIS Level TB-18-1, Tower B, Plaza 33
5 Apidra should be administered subcutaneously in the abdominal wall, thigh or deltoid or by continuous infusion in the abdominal wall. Injection sites and infusion sites within an injection area (abdomen, thigh or deltoid) should be rotated from one injection to the next. The rate of absorption, and consequently the onset and duration of action, may be affected by the injection site, exercise and other variables. Subcutaneous injection in the abdominal wall ensures a slightly faster absorption than other injection sites. Care should be taken to ensure that a blood vessel has not been entered. After injection, the site of injection should not be massaged. Patients must be educated to use proper injection techniques. Mixing with insulins When administered as a subcutaneous injection, Apidra must not be mixed with other medicinal products except NPH human insulin. Continuous subcutaneous insulin infusion Apidra may be used for Continuous Subcutaneous Insulin Infusion (CSII) in pump systems suitable for insulin infusion with the appropriate catheters and reservoirs. Patients using CSII should be comprehensively instructed on the use of the pump system. The infusion set and reservoir used with Apidra must be changed at least every 48 hours using aseptic technique. These instructions may differ from general pump manual instructions. It is important that patients follow the Apidra specific instructions when using Apidra. Failure to follow Apidra specific instructions may lead to serious adverse events. When used with a subcutaneous insulin infusion pump, Apidra must not be mixed with diluents or any other insulin. Patients administering Apidra by CSII must have an alternative insulin delivery system available
6 LANTUS SOLOSTAR 100IU/ML SOLUTION FOR INJECTION IN A PRE-FILLED PEN 6.2 LANTUS FOR OPTIPEN 100 UNITS/ML,3ML CARTRIDGES 6.3 LANTUS 100 UNITS/ML,10ML VIALS 6.4 LANTUS 100 UNITS/ML OPTISET Treatment of diabetes mellitus in adults, adolescents and children aged 2 years and above. Lantus contains insulin glargine, an insulin analogue, and has a prolonged duration of action. Lantus should be administered once daily at any time but at the same time each day. The Lantus dose regimen (dose and timing) should be individually adjusted. In patients with type 2 diabetes mellitus, Lantus can also be given together with orally active antidiabetic medicinal products. The potency of this medicinal product is stated in units. These units are exclusive to Lantus and are not the same as IU of the units used to express the potency of other insulin analogues. Special Population Elderly population In the elderly, progressive deterioration of renal function may lead to a steady decrease in insulin requirements. Renal Impairment In patients with renal impairment, insulin requirements may be diminished due to reduced insulin metabolism. Hepatic Impairment In patients with hepatic impairment, insulin requirements may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism. Paediatric Population Safety and efficacy of Lantus have been established in adolescents and children aged 2 years and older. Lantus has not been studied in children below the age of 2 years. SANOFI-AVENTIS Level TB-18-1, Tower B, Plaza 33
7 MYOZYME POWDER FOR SOLUTION FOR INJECTION [Alglucosidase alfa 52.5mg/vial] Myozyme is indicated for long-term enzyme replacement therapy (ERT) in patients with a confirmed diagnosis of Pompe disease (acid α- glucosidase deficiency). Myozyme is indicated in adults and paediatric patients of all ages SANOFI-AVENTIS Level TB-18-1, Tower B, Plaza 33
Indication: Indication: Protaxos is indicated in adults.
 Maklumat tambahan indikasi untuk upload pada laman web Year 2013 Products Approved For Additional Indication (DCA 262 28 Mac 2013) NO PRODUCT (ACTIVE INGREDIENT) 1. 1.1 EVISTA TABLET 60MG [ Raloxifene
Maklumat tambahan indikasi untuk upload pada laman web Year 2013 Products Approved For Additional Indication (DCA 262 28 Mac 2013) NO PRODUCT (ACTIVE INGREDIENT) 1. 1.1 EVISTA TABLET 60MG [ Raloxifene
Scottish Medicines Consortium
 Scottish Medicines Consortium insulin glulisine for subcutaneous injection 100 units/ml (Apidra ) No. (298/06) Sanofi Aventis 4 August 2006 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium insulin glulisine for subcutaneous injection 100 units/ml (Apidra ) No. (298/06) Sanofi Aventis 4 August 2006 The Scottish Medicines Consortium (SMC) has completed its assessment
Intensive Insulin Therapy in Diabetes Management
 Intensive Insulin Therapy in Diabetes Management Lillian F. Lien, MD Medical Director, Duke Inpatient Diabetes Management Assistant Professor of Medicine Division of Endocrinology, Metabolism, & Nutrition
Intensive Insulin Therapy in Diabetes Management Lillian F. Lien, MD Medical Director, Duke Inpatient Diabetes Management Assistant Professor of Medicine Division of Endocrinology, Metabolism, & Nutrition
Harmony Clinical Trial Medical Media Factsheet
 Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PC - Apidra, Levemir Therapeutic Class: Hormones and Synthetic Substitutes Therapeutic Sub-Class: Antidiabetic Agents Client: CA, CO, NV, OK, OR, WA and AZ Approval
Prior Authorization Guideline Guideline: PC - Apidra, Levemir Therapeutic Class: Hormones and Synthetic Substitutes Therapeutic Sub-Class: Antidiabetic Agents Client: CA, CO, NV, OK, OR, WA and AZ Approval
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PC (CO) - Insulin Delivery Systems Therapeutic Class: Hormones and Synthetic Substitutes Therapeutic Sub-Class: Insulin Delivery Systems Client: CO Approval Date:
Prior Authorization Guideline Guideline: PC (CO) - Insulin Delivery Systems Therapeutic Class: Hormones and Synthetic Substitutes Therapeutic Sub-Class: Insulin Delivery Systems Client: CO Approval Date:
10 to 30 minutes ½ to 3 hours 3 to 5 hours. 30 60 minutes 1 to 5 hours 8 hours. 1 to 4 hours
 Insulin Action There are several types of insulin. They are classified by how long they act: very fast, fast, slow and very slow acting. Each type of insulin has a certain time period in which it works.
Insulin Action There are several types of insulin. They are classified by how long they act: very fast, fast, slow and very slow acting. Each type of insulin has a certain time period in which it works.
Abdulaziz Al-Subaie. Anfal Al-Shalwi
 Abdulaziz Al-Subaie Anfal Al-Shalwi Introduction what is diabetes mellitus? A chronic metabolic disorder characterized by high blood glucose level caused by insulin deficiency and sometimes accompanied
Abdulaziz Al-Subaie Anfal Al-Shalwi Introduction what is diabetes mellitus? A chronic metabolic disorder characterized by high blood glucose level caused by insulin deficiency and sometimes accompanied
at The Valley Hospital (TVH) for Nursing Students/Nursing Instructors 2012
 at The Valley Hospital (TVH) for Nursing Students/Nursing Instructors 2012 Subject - Insulin Safety Background Insulin known to be high risk medication Can promote serious hypoglycemia if given incorrectly
at The Valley Hospital (TVH) for Nursing Students/Nursing Instructors 2012 Subject - Insulin Safety Background Insulin known to be high risk medication Can promote serious hypoglycemia if given incorrectly
Diabetes: When To Treat With Insulin and Treatment Goals
 Diabetes: When To Treat With Insulin and Treatment Goals Lanita. S. White, Pharm.D. Director, UAMS 12 th Street Health and Wellness Center Assistant Professor of Pharmacy Practice, UAMS College of Pharmacy
Diabetes: When To Treat With Insulin and Treatment Goals Lanita. S. White, Pharm.D. Director, UAMS 12 th Street Health and Wellness Center Assistant Professor of Pharmacy Practice, UAMS College of Pharmacy
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Afrezza Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Afrezza (human insulin) Prime Therapeutics will review Prior Authorization requests Prior Authorization
Afrezza Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Afrezza (human insulin) Prime Therapeutics will review Prior Authorization requests Prior Authorization
For Educational Use Only - Not for Detailing or Distribution
 This document is intended for healthcare professionals practicing in the United States and may contain information that has not been approved by the FDA. It is supplied to you as a professional courtesy
This document is intended for healthcare professionals practicing in the United States and may contain information that has not been approved by the FDA. It is supplied to you as a professional courtesy
Therapy Insulin Practical guide to Health Care Providers Quick Reference F Diabetes Mellitus in Type 2
 Ministry of Health, Malaysia 2010 First published March 2011 Perkhidmatan Diabetes dan Endokrinologi Kementerian Kesihatan Malaysia Practical guide to Insulin Therapy in Type 2 Diabetes Mellitus Quick
Ministry of Health, Malaysia 2010 First published March 2011 Perkhidmatan Diabetes dan Endokrinologi Kementerian Kesihatan Malaysia Practical guide to Insulin Therapy in Type 2 Diabetes Mellitus Quick
INPATIENT DIABETES MANAGEMENT Robert J. Rushakoff, MD Professor of Medicine Director, Inpatient Diabetes University of California, San Francisco
 INPATIENT DIABETES MANAGEMENT Robert J. Rushakoff, MD Professor of Medicine Director, Inpatient Diabetes University of California, San Francisco CLINICAL RECOGNITION Background: Appropriate inpatient glycemic
INPATIENT DIABETES MANAGEMENT Robert J. Rushakoff, MD Professor of Medicine Director, Inpatient Diabetes University of California, San Francisco CLINICAL RECOGNITION Background: Appropriate inpatient glycemic
IMPROVED METABOLIC CONTROL WITH A FAVORABLE WEIGHT PROFILE IN PATIENTS WITH TYPE 2 DIABETES TREATED WITH INSULIN GLARGINE (LANTUS ) IN CLINICAL
 464 IMPROVED METABOLIC CONTROL WITH A FAVORABLE WEIGHT PROFILE IN PATIENTS WITH TYPE 2 DIABETES TREATED WITH INSULIN GLARGINE (LANTUS ) IN CLINICAL PRACTICE STEPHAN A SCHREIBER AND ANIKA RUßMAN ABSTRACT
464 IMPROVED METABOLIC CONTROL WITH A FAVORABLE WEIGHT PROFILE IN PATIENTS WITH TYPE 2 DIABETES TREATED WITH INSULIN GLARGINE (LANTUS ) IN CLINICAL PRACTICE STEPHAN A SCHREIBER AND ANIKA RUßMAN ABSTRACT
Humulin R (U500) insulin: Prescribing Guidance
 Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
INSULIN PRODUCTS. Jack DeRuiter
 INSULIN PRODUCTS Jack DeRuiter The number and types of insulin preparations available in the United States is constantly changing, thus students should refer to recent drug resources for a current list
INSULIN PRODUCTS Jack DeRuiter The number and types of insulin preparations available in the United States is constantly changing, thus students should refer to recent drug resources for a current list
Types of insulin and How to Use Them
 Diabetes and Insulin Pumps Amy S. Pullen Pharm.D ISHP Spring Meeting April 2012 Objectives Describe the different types of insulin used in diabetes Identify the types of insulin that are compatible with
Diabetes and Insulin Pumps Amy S. Pullen Pharm.D ISHP Spring Meeting April 2012 Objectives Describe the different types of insulin used in diabetes Identify the types of insulin that are compatible with
Safe use of insulin e- learning module
 Safe use of insulin e- learning module Page 1 Introduction Insulin is a hormone produced by the beta cells in the pancreas, it is released when blood glucose levels are raised for example after a meal.
Safe use of insulin e- learning module Page 1 Introduction Insulin is a hormone produced by the beta cells in the pancreas, it is released when blood glucose levels are raised for example after a meal.
DIABETES MEDICATION INSULIN
 Section Three DIABETES MEDICATION INSULIN This section will tell you: About insulin. How to care and store your insulin. When to take your insulin. Different ways of taking insulin. WHAT IS INSULIN? Insulin
Section Three DIABETES MEDICATION INSULIN This section will tell you: About insulin. How to care and store your insulin. When to take your insulin. Different ways of taking insulin. WHAT IS INSULIN? Insulin
SHORT CLINICAL GUIDELINE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SHORT CLINICAL GUIDELINE SCOPE 1 Guideline title Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes 1.1 Short title Type 2
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SHORT CLINICAL GUIDELINE SCOPE 1 Guideline title Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes 1.1 Short title Type 2
linagliptin, 5mg film-coated tablet (Trajenta ) SMC No. (746/11) Boehringer Ingelheim / Eli Lilly and Company Ltd
 linagliptin, 5mg film-coated tablet (Trajenta ) SMC No. (746/11) Boehringer Ingelheim / Eli Lilly and Company Ltd 09 December 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of
linagliptin, 5mg film-coated tablet (Trajenta ) SMC No. (746/11) Boehringer Ingelheim / Eli Lilly and Company Ltd 09 December 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
Inpatient Treatment of Diabetes
 Inpatient Treatment of Diabetes Alan J. Conrad, MD Medical Director Diabetes Services EVP, Physician Alignment Diabetes Symposium November 12, 2015 Objectives Explain Palomar Health goals for inpatient
Inpatient Treatment of Diabetes Alan J. Conrad, MD Medical Director Diabetes Services EVP, Physician Alignment Diabetes Symposium November 12, 2015 Objectives Explain Palomar Health goals for inpatient
Diabetes and the Elimination of Sliding Scale Insulin. Date: April 30 th 2013. Presenter: Derek Sanders, D.Ph.
 Diabetes and the Elimination of Sliding Scale Insulin Date: April 30 th 2013 Presenter: Derek Sanders, D.Ph. Background Information Epidemiology and Risk Factors Diabetes Its Definition and Its Impact
Diabetes and the Elimination of Sliding Scale Insulin Date: April 30 th 2013 Presenter: Derek Sanders, D.Ph. Background Information Epidemiology and Risk Factors Diabetes Its Definition and Its Impact
Sponsor / Company: Sanofi Drug substance(s): HOE901 (insulin glargine)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
A new insulin order form should be completed for subsequent changes to type of insulin and/or frequency of administration
 of nurse A new insulin order form should be completed for subsequent changes to type of insulin and/or frequency of administration 1. Check times for point of care meter blood glucose testing. Pre-Breakfast
of nurse A new insulin order form should be completed for subsequent changes to type of insulin and/or frequency of administration 1. Check times for point of care meter blood glucose testing. Pre-Breakfast
Diabetes mellitus. Lecture Outline
 Diabetes mellitus Lecture Outline I. Diagnosis II. Epidemiology III. Causes of diabetes IV. Health Problems and Diabetes V. Treating Diabetes VI. Physical activity and diabetes 1 Diabetes Disorder characterized
Diabetes mellitus Lecture Outline I. Diagnosis II. Epidemiology III. Causes of diabetes IV. Health Problems and Diabetes V. Treating Diabetes VI. Physical activity and diabetes 1 Diabetes Disorder characterized
Diabetes Medications: Insulin Therapy
 Diabetes Medications: Insulin Therapy Courtesy Univ Texas San Antonio Eric L. Johnson, M.D. Department of Family and Community Medicine Diabetes and Insulin Type 1 Diabetes Autoimmune destruction of beta
Diabetes Medications: Insulin Therapy Courtesy Univ Texas San Antonio Eric L. Johnson, M.D. Department of Family and Community Medicine Diabetes and Insulin Type 1 Diabetes Autoimmune destruction of beta
Why is Insulin so Important?
 Insulin Therapy Why is Insulin so Important? If the glucose stays in your blood it doesn t do your cells (body) any good The glucose has to get inside the cells for the body to use it What Does Insulin
Insulin Therapy Why is Insulin so Important? If the glucose stays in your blood it doesn t do your cells (body) any good The glucose has to get inside the cells for the body to use it What Does Insulin
Insulin and Diabetes
 Insulin What is Insulin? Insulin is a hormone produced by special cells in the pancreas These cells that are produced are called beta cells Insulin allows the glucose from food we eat to enter the cells
Insulin What is Insulin? Insulin is a hormone produced by special cells in the pancreas These cells that are produced are called beta cells Insulin allows the glucose from food we eat to enter the cells
Insulin pen start checklist
 Insulin pen start checklist Topic Instruction Date & Initials 1. Cognitive Assessment 2. Insulin Delivery loading appropriate mixing priming shot dialing up dose delivery of insulin 3. Insulin type/action
Insulin pen start checklist Topic Instruction Date & Initials 1. Cognitive Assessment 2. Insulin Delivery loading appropriate mixing priming shot dialing up dose delivery of insulin 3. Insulin type/action
PRODUCT MONOGRAPH APIDRA. insulin glulisine (rdna origin) Solution for injection 100 U/mL. ATC code: A10AB. Antidiabetic Agent
 PRODUCT MONOGRAPH APIDRA insulin glulisine (rdna origin) Solution for injection 100 U/mL ATC code: A10AB Antidiabetic Agent Short-acting Recombinant Human Insulin Analogue sanofi-aventis Canada Inc. 2905
PRODUCT MONOGRAPH APIDRA insulin glulisine (rdna origin) Solution for injection 100 U/mL ATC code: A10AB Antidiabetic Agent Short-acting Recombinant Human Insulin Analogue sanofi-aventis Canada Inc. 2905
NCT00272090. sanofi-aventis HOE901_3507. insulin glargine
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
INSULINThere are. T y p e 1 T y p e 2. many different insulins for
 T y p e 1 T y p e 2 INSULINThere are many different insulins for Characteristics The three characteristics of insulin are: Onset. The length of time before insulin reaches the bloodstream and begins lowering
T y p e 1 T y p e 2 INSULINThere are many different insulins for Characteristics The three characteristics of insulin are: Onset. The length of time before insulin reaches the bloodstream and begins lowering
A list of all medications you are taking also include any vitamins, supplements, over-the-counter medicines, or herbal products
 This is your customized insulin discussion guide to bring to your next doctor s visit. WHAT TO BRING TO YOUR NEXT CHECK UP A record of your recent blood sugar readings A list of all medications you are
This is your customized insulin discussion guide to bring to your next doctor s visit. WHAT TO BRING TO YOUR NEXT CHECK UP A record of your recent blood sugar readings A list of all medications you are
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
P A T I E N T I N F O R M A T I O N. Apidra
 P A T I E N T I N F O R M A T I O N Apidra We have written this leaflet for those of you with diabetes who have been prescribed Apidra by your doctor. The primary goal of all diabetes treatment is to achieve
P A T I E N T I N F O R M A T I O N Apidra We have written this leaflet for those of you with diabetes who have been prescribed Apidra by your doctor. The primary goal of all diabetes treatment is to achieve
Diabetes Management Tube Feeding/Parenteral Nutrition Order Set (Adult)
 Review Due Date: 2016 May PATIENT CARE ORDERS Weight (kg) Known Adverse Reactions or Intolerances DRUG No Yes (list) FOOD No Yes (list) LATEX No Yes ***See Suggestions for Management (on reverse)*** ***If
Review Due Date: 2016 May PATIENT CARE ORDERS Weight (kg) Known Adverse Reactions or Intolerances DRUG No Yes (list) FOOD No Yes (list) LATEX No Yes ***See Suggestions for Management (on reverse)*** ***If
Insulin therapy in various type 1 diabetes patients workshop
 Insulin therapy in various type 1 diabetes patients workshop Bruce H.R. Wolffenbuttel, MD PhD Dept of Endocrinology, UMC Groningen website: www.umcg.net & www.gmed.nl Twitter: @bhrw Case no. 1 Male of
Insulin therapy in various type 1 diabetes patients workshop Bruce H.R. Wolffenbuttel, MD PhD Dept of Endocrinology, UMC Groningen website: www.umcg.net & www.gmed.nl Twitter: @bhrw Case no. 1 Male of
CASE A1 Hypoglycemia in an Elderly T2DM Patient with Heart Failure
 Hypoglycemia in an Elderly T2DM Patient with Heart Failure 1 I would like to introduce you to Sophie, an elderly patient with long-standing type 2 diabetes, who has a history of heart failure, a common
Hypoglycemia in an Elderly T2DM Patient with Heart Failure 1 I would like to introduce you to Sophie, an elderly patient with long-standing type 2 diabetes, who has a history of heart failure, a common
UW MEDICINE PATIENT EDUCATION. Using Insulin. Basic facts about insulin and self-injection. What is insulin? How does diabetes affect the body?
 UW MEDICINE PATIENT EDUCATION Using Insulin Basic facts about insulin and self-injection This handout explains what insulin is, the different types of insulin, how to store it, how to give an injection
UW MEDICINE PATIENT EDUCATION Using Insulin Basic facts about insulin and self-injection This handout explains what insulin is, the different types of insulin, how to store it, how to give an injection
Initiate Atorvastatin 20mg daily
 Type 2 Diabetes Patient Objectives Stopping Smoking BMI > 25 kg m² Control BP to
Type 2 Diabetes Patient Objectives Stopping Smoking BMI > 25 kg m² Control BP to
Insulin Pens & Improving Patient Adherence
 Insulin Pens & Improving Patient Adherence Bonnie Pepon, RN, BSN, CDE Certified Diabetes Educator Conemaugh Diabetes Institute Kip Benko, MD FACEP Asst Clinical Professor University of Pittsburgh School
Insulin Pens & Improving Patient Adherence Bonnie Pepon, RN, BSN, CDE Certified Diabetes Educator Conemaugh Diabetes Institute Kip Benko, MD FACEP Asst Clinical Professor University of Pittsburgh School
Novel Trial Designs in T2D to Satisfy Regulatory Requirements for CV Safety
 Novel Trial Designs in T2D to Satisfy Regulatory Requirements for CV Safety Anders Svensson MD, PhD Head of Global Clinical Development Metabolism, F Hoffmann LaRoche Ltd. Basel, Switzerland Overview of
Novel Trial Designs in T2D to Satisfy Regulatory Requirements for CV Safety Anders Svensson MD, PhD Head of Global Clinical Development Metabolism, F Hoffmann LaRoche Ltd. Basel, Switzerland Overview of
An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION
 Science of CRVO www.scienceofcrvo.org An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION This brochure will guide you in understanding CRVO and the treatment options available to prevent vision loss.
Science of CRVO www.scienceofcrvo.org An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION This brochure will guide you in understanding CRVO and the treatment options available to prevent vision loss.
A Simplified Approach to Initiating Insulin. 4. Not meeting glycemic goals with oral hypoglycemic agents or
 A Simplified Approach to Initiating Insulin When to Start Insulin: 1. Fasting plasma glucose (FPG) levels >250 mg/dl or 2. Glycated hemoglobin (A1C) >10% or 3. Random plasma glucose consistently >300 mg/dl
A Simplified Approach to Initiating Insulin When to Start Insulin: 1. Fasting plasma glucose (FPG) levels >250 mg/dl or 2. Glycated hemoglobin (A1C) >10% or 3. Random plasma glucose consistently >300 mg/dl
Continuous Subcutaneous Insulin Infusion (CSII)
 IMPORTANCE OF FOCUS CSII (Insulin pumps) have been used for more than 35 years. In the U.S. in 2005, the level of insulin pump penetration was estimated at 20 to 30% in patients with type 1 diabetes mellitus
IMPORTANCE OF FOCUS CSII (Insulin pumps) have been used for more than 35 years. In the U.S. in 2005, the level of insulin pump penetration was estimated at 20 to 30% in patients with type 1 diabetes mellitus
CLASS OBJECTIVES. Describe the history of insulin discovery List types of insulin Define indications and dosages Review case studies
 Insulins CLASS OBJECTIVES Describe the history of insulin discovery List types of insulin Define indications and dosages Review case studies INVENTION OF INSULIN 1921 The first stills used to make insulin
Insulins CLASS OBJECTIVES Describe the history of insulin discovery List types of insulin Define indications and dosages Review case studies INVENTION OF INSULIN 1921 The first stills used to make insulin
Approximate Cost Reference List i for Antihyperglycemic Agents
 Alpha Glucosidase Inhibitor Acarbose (Glucobay ) Biguanides Metformin (Glucophage, generic) Metformin ER (Glumetza ) Approximate Cost Reference List i for Antihyperglycemic Agents Incretin Agents - DPP-4
Alpha Glucosidase Inhibitor Acarbose (Glucobay ) Biguanides Metformin (Glucophage, generic) Metformin ER (Glumetza ) Approximate Cost Reference List i for Antihyperglycemic Agents Incretin Agents - DPP-4
Initiation and Adjustment of Insulin Regimens for Type 2 Diabetes
 PL Detail-Document #300128 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER January 2014 Initiation and Adjustment of Insulin Regimens for Type
PL Detail-Document #300128 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER January 2014 Initiation and Adjustment of Insulin Regimens for Type
Shared Care Agreement Insulin Degludec (Tresiba )
 Licensed Indication Shared Care Agreement Insulin Degludec (Tresiba ) Insulin Degludec is licensed for the treatment of diabetes mellitus in adults. Countess of Chester prescribing guidelines Restricting
Licensed Indication Shared Care Agreement Insulin Degludec (Tresiba ) Insulin Degludec is licensed for the treatment of diabetes mellitus in adults. Countess of Chester prescribing guidelines Restricting
Resident s Guide to Inpatient Diabetes
 Resident s Guide to Inpatient Diabetes 1. All patients with diabetes of ANY TYPE, regardless of reason for admission, must have a Hemoglobin A1C documented in the medical record within 24 hours of admission
Resident s Guide to Inpatient Diabetes 1. All patients with diabetes of ANY TYPE, regardless of reason for admission, must have a Hemoglobin A1C documented in the medical record within 24 hours of admission
INSULIN TREATMENT FOR TYPE 2 DIABETES MANAGEMENT
 INSULIN TREATMENT FOR TYPE 2 DIABETES MANAGEMENT APIRADEE SRIWIJITKAMOL DIVISION OF ENDOCRINOLOGY AND METABOLISM DEPARTMENT OF MEDICINE FACULTY OF MEDICINE SIRIRAJ HOSPITOL QUESTION 1 1. ท านเคยเป นแพทย
INSULIN TREATMENT FOR TYPE 2 DIABETES MANAGEMENT APIRADEE SRIWIJITKAMOL DIVISION OF ENDOCRINOLOGY AND METABOLISM DEPARTMENT OF MEDICINE FACULTY OF MEDICINE SIRIRAJ HOSPITOL QUESTION 1 1. ท านเคยเป นแพทย
PRODUCT INFORMATION APIDRA
 PRODUCT INFORMATION APIDRA NAME OF THE DRUG Insulin glulisine DESCRIPTION APIDRA [insulin glulisine injection {rdna origin}] is a recombinant human insulin analogue produced by recombinant DNA technology.
PRODUCT INFORMATION APIDRA NAME OF THE DRUG Insulin glulisine DESCRIPTION APIDRA [insulin glulisine injection {rdna origin}] is a recombinant human insulin analogue produced by recombinant DNA technology.
Presented By: Dr. Nadira Husein
 Presented By: Dr. Nadira Husein I have no conflict of interest Disclosures I have received honoraria/educational grants from the following: Novo Nordisk, Eli Lilly, sanofi-aventis, Novartis, Astra Zeneca,
Presented By: Dr. Nadira Husein I have no conflict of interest Disclosures I have received honoraria/educational grants from the following: Novo Nordisk, Eli Lilly, sanofi-aventis, Novartis, Astra Zeneca,
INJEX Self Study Program Part 1
 INJEX Self Study Program Part 1 What is Diabetes? Diabetes is a disease in which the body does not produce or properly use insulin. Diabetes is a disorder of metabolism -- the way our bodies use digested
INJEX Self Study Program Part 1 What is Diabetes? Diabetes is a disease in which the body does not produce or properly use insulin. Diabetes is a disorder of metabolism -- the way our bodies use digested
Cochrane Quality and Productivity topics
 Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus NICE has developed the Cochrane Quality and Productivity (QP) topics to help the NHS identify practices
Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus NICE has developed the Cochrane Quality and Productivity (QP) topics to help the NHS identify practices
Glycaemic Control in Adults with Type 1 Diabetes
 Glycaemic Control in Adults with Type 1 Diabetes Aim(s) and objective(s) This document aims to provide guidance on good clinical practice in managing glycaemic control in adult patients with Type 1 Diabetes
Glycaemic Control in Adults with Type 1 Diabetes Aim(s) and objective(s) This document aims to provide guidance on good clinical practice in managing glycaemic control in adult patients with Type 1 Diabetes
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
insulin & diabetes What is insulin? Why must it be injected? What if I have to go on to insulin? Are there different types of insulin?
 Talking diabetes No.24 insulin & diabetes Insulin injections are required when the body produces little or no insulin, as with type 1 diabetes. They are also required for some people with type 2 diabetes
Talking diabetes No.24 insulin & diabetes Insulin injections are required when the body produces little or no insulin, as with type 1 diabetes. They are also required for some people with type 2 diabetes
There seem to be inconsistencies regarding diabetic management in
 Society of Ambulatory Anesthesia (SAMBA) Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery Review of the consensus statement and additional
Society of Ambulatory Anesthesia (SAMBA) Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery Review of the consensus statement and additional
Tuberculosis And Diabetes. Dr. hanan abuelrus Prof.of internal medicine Assiut University
 Tuberculosis And Diabetes Dr. hanan abuelrus Prof.of internal medicine Assiut University TUBERCULOSIS FACTS More than 9 million people fall sick with tuberculosis (TB) every year. Over 1.5 million die
Tuberculosis And Diabetes Dr. hanan abuelrus Prof.of internal medicine Assiut University TUBERCULOSIS FACTS More than 9 million people fall sick with tuberculosis (TB) every year. Over 1.5 million die
tips Insulin Pump Users 1 Early detection of insulin deprivation in continuous subcutaneous 2 Population Study of Pediatric Ketoacidosis in Sweden:
 tips Top International Publications Selection Insulin Pump Users Early detection of insulin deprivation in continuous subcutaneous insulin infusion-treated Patients with TD Population Study of Pediatric
tips Top International Publications Selection Insulin Pump Users Early detection of insulin deprivation in continuous subcutaneous insulin infusion-treated Patients with TD Population Study of Pediatric
Chapter 15. Reconstitution and Dosages Measured in Units. Copyright 2012, 2007 Mosby, Inc., an affiliate of Elsevier Inc. All rights reserved.
 Chapter 15 Reconstitution and Dosages Measured in Units 1 Objectives Calculating drug dosage problems that first require reconstitution of a powdered drug into a liquid form Using a proportion to solve
Chapter 15 Reconstitution and Dosages Measured in Units 1 Objectives Calculating drug dosage problems that first require reconstitution of a powdered drug into a liquid form Using a proportion to solve
Insulin T Y P E 1 T Y P E 2
 T Y P E 1 T Y P E 2 INSULIN There are many different insulins for many different situations and lifestyles. This section should help you and your doctor decide which insulin or insulins are best for you.
T Y P E 1 T Y P E 2 INSULIN There are many different insulins for many different situations and lifestyles. This section should help you and your doctor decide which insulin or insulins are best for you.
INSULIN AND INCRETIN THERAPIES: WHAT COMBINATIONS ARE RIGHT FOR YOUR PATIENT?
 INSULIN AND INCRETIN THERAPIES: WHAT COMBINATIONS ARE RIGHT FOR YOUR PATIENT? MARTHA M. BRINSKO, MSN, ANP-BC CHARLOTTE COMMUNITY HEALTH CLINIC CHARLOTTE, NC Diagnosed and undiagnosed diabetes in the United
INSULIN AND INCRETIN THERAPIES: WHAT COMBINATIONS ARE RIGHT FOR YOUR PATIENT? MARTHA M. BRINSKO, MSN, ANP-BC CHARLOTTE COMMUNITY HEALTH CLINIC CHARLOTTE, NC Diagnosed and undiagnosed diabetes in the United
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
Guideline for Insulin Therapeutic Review in patients with Type 2 Diabetes
 Diabetes Sans Frontières Guideline for Insulin Therapeutic Review in patients with Type 2 Diabetes 1. Introduction This guideline has been developed in order to support practices to undertake insulin therapeutic
Diabetes Sans Frontières Guideline for Insulin Therapeutic Review in patients with Type 2 Diabetes 1. Introduction This guideline has been developed in order to support practices to undertake insulin therapeutic
INSULIN INTENSIFICATION: Taking Care to the Next Level
 INSULIN INTENSIFICATION: Taking Care to the Next Level By J. Robin Conway M.D., Diabetes Clinic, Smiths Falls, ON www.diabetesclinic.ca Type 2 Diabetes is an increasing problem in our society, due largely
INSULIN INTENSIFICATION: Taking Care to the Next Level By J. Robin Conway M.D., Diabetes Clinic, Smiths Falls, ON www.diabetesclinic.ca Type 2 Diabetes is an increasing problem in our society, due largely
THE INS AND OUTS OF INSULIN. Mary Beth Wald, RN,BSN,CDE
 THE INS AND OUTS OF INSULIN Mary Beth Wald, RN,BSN,CDE WHAT HAPPENS IN MY BODY? When we eat, the food gets changed into glucose, a type of sugar. Glucose travels in the blood to all the cells in your body
THE INS AND OUTS OF INSULIN Mary Beth Wald, RN,BSN,CDE WHAT HAPPENS IN MY BODY? When we eat, the food gets changed into glucose, a type of sugar. Glucose travels in the blood to all the cells in your body
Insulin Treatment. J A O Hare. www.3bv.org. Bones, Brains & Blood Vessels
 Insulin Treatment J A O Hare www.3bv.org Bones, Brains & Blood Vessels Indications for Insulin Treatment Diabetic Ketoacidosis Diabetics with unstable acute illness ICU Gestational Diabetes: diet failure
Insulin Treatment J A O Hare www.3bv.org Bones, Brains & Blood Vessels Indications for Insulin Treatment Diabetic Ketoacidosis Diabetics with unstable acute illness ICU Gestational Diabetes: diet failure
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Diabetes mellitus 1 عبد هللا الزعبي. pharmacology. Shatha Khalil Shahwan. 1 P a g e
 Diabetes mellitus 1 pharmacology عبد هللا الزعبي 1 P a g e 4 Shatha Khalil Shahwan Diabetes mellitus The goals of the treatment of diabetes 1. Treating symptoms 2. Treating and Preventing acute complications
Diabetes mellitus 1 pharmacology عبد هللا الزعبي 1 P a g e 4 Shatha Khalil Shahwan Diabetes mellitus The goals of the treatment of diabetes 1. Treating symptoms 2. Treating and Preventing acute complications
Onset Peak Duration Comments
 Rapid- Acting 5-15 minutes 0.5-3 hours 3-5 hours Meal should be available before administering, ideally taking within 10 minutes of eating). Good in refrigerator (36-46 F) until expiration date. Protect
Rapid- Acting 5-15 minutes 0.5-3 hours 3-5 hours Meal should be available before administering, ideally taking within 10 minutes of eating). Good in refrigerator (36-46 F) until expiration date. Protect
PHARMACOTHERAPY HOW TO INJECT INSULIN. Living your life as normal as possible. www.lilly-pharma.de www.lilly-diabetes.de
 PHARMACOTHERAPY HOW TO INJECT INSULIN Living your life as normal as possible www.lilly-pharma.de www.lilly-diabetes.de In Germany about 1.9 million people with diabetes are being treated with insulin.
PHARMACOTHERAPY HOW TO INJECT INSULIN Living your life as normal as possible www.lilly-pharma.de www.lilly-diabetes.de In Germany about 1.9 million people with diabetes are being treated with insulin.
Clinical Guideline Diabetes management during surgery (adults)
 Clinical Guideline Diabetes management during surgery (adults) Standard 8 of the National Service Framework for Diabetes states that all children, young people and adults with diabetes admitted to hospital,
Clinical Guideline Diabetes management during surgery (adults) Standard 8 of the National Service Framework for Diabetes states that all children, young people and adults with diabetes admitted to hospital,
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
Inpatient Guidelines: Insulin Infusion Pump Management
 Inpatient Guidelines: Insulin Infusion Pump Management Developed by the Statewide Diabetes Clinical Network Steering Committee July 2012 Clinical Access and Redesign Unit Table of Contents Purpose...4
Inpatient Guidelines: Insulin Infusion Pump Management Developed by the Statewide Diabetes Clinical Network Steering Committee July 2012 Clinical Access and Redesign Unit Table of Contents Purpose...4
Insulin. and diabetes. What is insulin? Who needs to inject insulin? Why must it be injected? What if I have to go on to insulin?
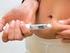 Insulin What is insulin? and diabetes Insulin is a hormone made by special cells, called beta cells, in the pancreas. When we eat, insulin is released into the blood stream where it helps to move glucose
Insulin What is insulin? and diabetes Insulin is a hormone made by special cells, called beta cells, in the pancreas. When we eat, insulin is released into the blood stream where it helps to move glucose
10/30/2012. Anita King, DNP, RN, FNP, CDE, FAADE Clinical Associate Professor University of South Alabama Mobile, Alabama
 Faculty Medications for Diabetes Satellite Conference and Live Webcast Wednesday, November 7, 2012 2:00 4:00 p.m. Central Time Anita King, DNP, RN, FNP, CDE, FAADE Clinical Associate Professor University
Faculty Medications for Diabetes Satellite Conference and Live Webcast Wednesday, November 7, 2012 2:00 4:00 p.m. Central Time Anita King, DNP, RN, FNP, CDE, FAADE Clinical Associate Professor University
Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Managing the Hospitalized Patient on Insulin: Care Transition. Catie Prinzing MSN, APRN, CNS
 Managing the Hospitalized Patient on Insulin: Care Transition Catie Prinzing MSN, APRN, CNS Diabetes and Hospitalization People with DM are hospitalized 3x more frequently than patients without diabetes
Managing the Hospitalized Patient on Insulin: Care Transition Catie Prinzing MSN, APRN, CNS Diabetes and Hospitalization People with DM are hospitalized 3x more frequently than patients without diabetes
Injectable Insulin During Pregnancy
 Injectable Insulin During Pregnancy What is insulin? Insulin is a hormone made by the pancreas. The pancreas is a small organ that lies behind and below the stomach. Insulin allows the food you eat to
Injectable Insulin During Pregnancy What is insulin? Insulin is a hormone made by the pancreas. The pancreas is a small organ that lies behind and below the stomach. Insulin allows the food you eat to
User guide Basal-bolus Insulin Dosing Chart: Adult
 Contacts and further information Local contact Clinical pharmacy or visiting pharmacy Diabetes education service Director of Medical Services Visiting or local endocrinologist or diabetes physician For
Contacts and further information Local contact Clinical pharmacy or visiting pharmacy Diabetes education service Director of Medical Services Visiting or local endocrinologist or diabetes physician For
NSW Adult Subcutaneous Insulin Prescribing Chart User Guide
 NSW Adult Subcutaneous Insulin Prescribing Chart User Guide Adult Subcutaneous Insulin Prescribing Chart User Guide - 13 August 2013 Contents Target audience:... 1 Exceptions:... 1 1. Introduction... 2
NSW Adult Subcutaneous Insulin Prescribing Chart User Guide Adult Subcutaneous Insulin Prescribing Chart User Guide - 13 August 2013 Contents Target audience:... 1 Exceptions:... 1 1. Introduction... 2
BASAL BOLUS INSULIN FOR MEDICAL- SURGICAL INPATIENTS
 BASAL BOLUS INSULIN FOR MEDICAL- SURGICAL INPATIENTS C O N T A C T D I A B E T E S S E R V I C E S F O R M O R E I N F O R M A T I O N 8 4 7-9 1 7-6 9 0 7 THIS SLIDE PRESENTATION WAS PREPARED BY SUE DROGOS,
BASAL BOLUS INSULIN FOR MEDICAL- SURGICAL INPATIENTS C O N T A C T D I A B E T E S S E R V I C E S F O R M O R E I N F O R M A T I O N 8 4 7-9 1 7-6 9 0 7 THIS SLIDE PRESENTATION WAS PREPARED BY SUE DROGOS,
Criteria: CWQI HCS-123 (This criteria is consistent with CMS guidelines for External Infusion Insulin Pumps)
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Origination Date: 05/15 Revision Date(s): 05/2015 Developed By: Medical Criteria Committee 06/24/2015 External Infusion Insulin Pumps Page 1 of
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Origination Date: 05/15 Revision Date(s): 05/2015 Developed By: Medical Criteria Committee 06/24/2015 External Infusion Insulin Pumps Page 1 of
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
