Chronic Hepatitis C Virus (Chronic HCV)
|
|
|
- Camron Cobb
- 8 years ago
- Views:
Transcription
1 Chronic Hepatitis C Virus (Chronic HCV) Amie Baumgartner PA-C Fall 2015 Purpose of Lecture! Educate Gastroenterology nurses about Hepatitis C and its treatment in order to improve their knowledge and confidence in caring for patients with Hepatitis C. Objectives! Discuss epidemiology of HCV (Hepatitis C Virus).! Discuss symptoms of HCV.! Discuss testing related to HCV.! Discuss treatments for HCV.! Discuss obstacles to treatment.
2 Hepatitis Viruses! Hepatitis A and E Fecal-oral transmission! Hepatitis B and D Blood and some body fluid transmission! Hepatitis C Blood transmission Chronic Hepatis C! 1.6% of population has HCV 5x increased risk if born (CDC rec screen)! Most common cause of chronic liver disease (40% of chronic liver disease)! Most common cause of liver transplant in the U.S.! Hepatitis C Virus (HCV) is 10 times more common in the alcoholic population than in the general population Genotypes! Type 1 through 6! Type 1, 2, and 3 most common in U.S.! Type 1 most common (about 70% of patients)
3 Transmission of HCV! Blood to blood! Rarely by mucosal tissue exposed to body fluids Not transmitted in breast milk! NOT transmitted by causal contact, kissing, drinking from same glass, etc! Should not share razors or tooth brushes Risk Factors for HCV! IVDU! Intranasal drug users! Blood or blood component transfusion or organ transplant prior to 1992! If received clotting factor concentrate (eg for Hemophilia) prior to 1987! Hemodialysis! Children of HCV mothers! Sexual transmission (rare if monogamous); increase with anal or oral intercourse! Exposure to blood as health care worker! Tattoos, piercings! Also test if elevated liver enzymes without known cause or if infected with HIV or HBV. Symptoms! Fatigue & Malaise! RUQ pain! Arthralgias! Rash (Cutanea Porphyria Tarda)! Cryoglobulinemia! Often no symptoms
4 Extrahepatic Manifestations! Skin lesions / rash! Arthralgias / Myalgias! Neuropathy! Hypertension! Diabetes! Ocular disease (dry eyes, uveitis)! Autoimmune disease! Cardiac disease (myocarditis, cardiomyopathy)! Hematologic disorders Cryoglobulinemia, lymphoma, Diagnosis! Hepatitis C antibody! HCV quantitative RNA levels! Genotyping! Check for concomitant Hepatitis B infection and HIV Determining degree of fibrosis! Lab tests Eg, APRI score, FibroTest, FibroSure, ActiTest, Hepascore, FibroSpect II, SHASTA, European Liver Fibrosis Panel! Imaging tests US elastography (eg, Fibroscan) MR elastography Acoustic radiation force impulse Imaging Real-time shear wave elastography (SWE)! Liver biopsy (eg, METAVIR score) F0: No fibrosis F1: Portal fibrosis without septa F2: Few septa F3: Numerous septa without cirrhosis F4: Cirrhosis
5 HCV Progression! 20-30% will develop cirrhosis over 20 years! Alcohol causes more severe and more rapidly progressive disease! HIV or HBV co-infection increases risk of cirrhosis and HCC! Once have cirrhosis, risk for HCC is 2-4% per year. Thus screen with every 6 month RUQ US. Used to also screen with AFP every 6 months. Now not considered cost effective. Risk factors for progression to cirrhosis! Alcohol use! Longer duration of HCV! Steatosis! Other co-infections (HBV, HIV)! Male gender! Extent of current histologic damage! Genotype 3 Treatment! Treatment medication selection, dosing, and duration depends on several variables: Genotype Viral load Treatment experienced vs naïve Presence of cirrhosis Degree of cirrhosis Renal function Co-infection with Hepatitis B Virus Co-infection with HIV.
6 Treatment! Medications must be taken religiously. No missed doses!! It must be a priority for the patients. They need to do whatever it takes to remember (setting alarms, having other people help them remember, etc.).! Each medicine is unique and has its own side effect profile, drug interactions, etc. Treatment! Pegylated Interferon alpha-2a and alpha-2b! Ribavirin (Copegus, Moderiba, Rebetol, Ribasphere, Ribasphere RibaPak, Virazole))! Direct Acting Antivirals Daklinza (Daclatasvir) Sovaldi (Sofosbuvir) Olysio (Simepravir) Harvoni (Sofosbuvir/Ledipasvir) Viekira Pak (Ombitasvir, Paritaprevir with Ritonavir, Dasabuvir) Treatment Options Genotype 1a! Alphabetical Order! Harvoni weeks! Daklinza plus Sovaldi +/- Ribavirin x weeks! Olysio plus Sovaldi x weeks! Viekira Pak plus Ribavirin x weeks
7 Treatment Options Genotype 1b! Alphabetical Order! Harvoni weeks! Daklinza plus Sovaldi +/- Ribavirin x weeks! Olysio plus Sovaldi x weeks! Viekira Pak +/- Ribavirin x weeks Treatment Options Genotype 2! Alphabetical Order! Daklinza plus Sovaldi +/- Ribavirin x weeks! Solvaldi plus Ribavirin x weeks Treatment Options Genotype 3! Alphabetical Order! Daklinza plus Sovaldi +/_ Ribavirin x weeks.! Sovaldi plus Ribavirin plus Interferon x 12 weeks
8 Pegylated Interferon alpha-2a/2b! Mechanism of Action: Inhibits viral replication! Dose: injection once weekly! Contraindications: Dose adjustments based upon renal function.! Side effects: MULTITUDE!!! Terrible fatigue, bone marrow suppression, elevated liver tests (including liver failure), hypersensitivity reactions, depression (including suicidal ideation), Headaches, Rash (including potentially life-threatening), pulmonary disease, thyroid disease, ophthalmic disease. Pegylated Interferon alpha-2a/2b! Lab monitoring: Every 2 weeks to start with then every 4.! Drug Interactions: MULTITUDE!!! Cost Estimate for 4 week supply: $4,560 Ribavirin! Mechanism of Action: Inhibits viral replication.! Dose: 1000 mg daily if < 75 kg (400 mg am, 600 mg pm) OR 1200 mg daily if >=75 kg (600 mg BID). Administer with food.! Drug Interactions: Several. See package insert.! Cost Estimate per 4 week supply: $3,927
9 Ribavirin! Potential side effects Teratogenic must absolutely not become pregnant during tx and for 6 mo afterward. Anemia usually hgb decreases by 2-3 grams. Neutropenia, thrombocytopenia Fatigue and malaise Depression / mood changes GI symptoms Rash Blurred vision Itching Insomnia Ribavirin! Contraindications / Cautions:! Contraindicated in women who are pregnant and in the male partners of women who are pregnant; FDA Pregnancy Category X.! Male patients: Condoms every time and female partners also on a reliable method of birth control for duration of treatment AND for 6 months after completion of treatment.! Female patients: Two forms of birth control and male partners also must use condoms, for duration of treatment AND for 6 months after completion of treatment.! Renal dose adjustments Ribavirin! Monitor:! AST, ALT, alk phos, bili, CBC, lytes, creatinine, uric acid (weeks 0, 2, 4, 6, 8, 12, 16, 20, 24)! HCV quantitative level (weeks 0, 4, 12, 24)! TSH (weeks 0, 12, 24)! Pregnancy test (weeks 0, 4, 8, 12, 16, 20, 24)
10 Sovaldi (Sofosbuvir)! Mechanism of Action: Directly kills the Hepatitis C virus. It can be used for genotypes 1, 2, 3, and 4.! Dose: one tablet daily with or without food. Must be combined with other medications. Cannot be used as mono-therapy.! Contraindications: Cannot be used in severe renal impairment (GFR < 30) or ESRD (end-stage renal disease) Avoid use if pregnant or breast feeding Sovaldi (Sofosbuvir)! Common side effects: headache, fatigue, nausea, insomnia, diarrhea. Severity is usually mild.! Can cause elevations of Total Bilirubin, Lipase, CPK usually transient and asymptomatic.! Some Drug Interactions: Amiodarone, St John s Wort! Cost Estimate per 4 week supply: $33,600 Harvoni (Ledipasvir / Sofosbuvir)! Mechanism of Action: Directly kills the Hepatitis C virus.! Dose: one tablet daily with or without food. It can be used for genotypes 1 and potentially 4.! Contraindications: Cannot be used in severe renal impairment (GFR < 30) or ESRD (end-stage renal disease) Avoid use if pregnant or breast feeding
11 Harvoni (Ledipasvir / Sofosbuvir)! Common side effects: headache, fatigue, nausea, insomnia, diarrhea. Severity is usually mild.! Can cause elevations of Total Bilirubin, Lipase, CPK usually transient and asymptomatic.! Some Drug Interactions: Antacids such Maalox or Rolaids can be taken between 4 hours after and 4 hours before Harvoni, H2-blocker or a PPI (low dose only) must be given on an empty stomach, at the same time as the Harvoni. St John s Wort, Amiodarone Harvoni (Ledipasvir / Sofosbuvir! Estimate of cost for 4 week supply: $37,800 Viekira Pak (Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir)! Mechanism of Action: Directly kills the Hepatitis C virus.! Dose: Three tablets am, and one tablet pm. It can be used for genotypes 1 and potentially 4. It usually, but not always, requires the use of Ribavirin as well.! Contraindications: Cannot be used in ESRD (end-stage renal disease) on hemodialysis. Avoid use if pregnant or breast feeding
12 Viekira Pak (Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir)! Common side effects: nausea, pruritus, insomnia. Severity is usually mild. Can cause elevations of AST, ALT and Total Bilirubin. These are usually asymptomatic.! Some Drug Interactions: It cannot be used if the patient is taking certain birth control pills. Note that if Ribavirin is used, 2 non-hormonal birth control methods must be used. Amiodarone, some narcotics, St John s Wort Many drug interactions, not all of them significant! Cost Estimate per 4 week supply: $33,328 Olysio (Simeprevir)! Mechanism of Action: Directly kills the Hepatitis C virus.! Dose: one 150 mg tablet daily with food. Must be combined with other medications. Cannot be used as monotherapy.! Contraindications / Cautions: Cannot be used in pregnancy or breast feeding. Pregnancy class C. The patient must use birth control throughout duration of treatment and for 6 months after treatment. Patients with East Asia ancestry have an increased risk of side effects. Use with caution. If genotype 1a, check for the NS3Q80K polymorphism. If present, the sustained viral response with the use of Simeprevir is markedly reduced. Contains a sulfa moiety. Use with caution in patients with a sulfa allergy. Olysio (Simeprevir)! Common side effects: photo (sun) sensitivity, rash, fatigue, headache, dizziness, insomnia, nausea, diarrhea, elevated bilirubin, myalgia, dyspnea, elevated alk phos.! Monitor: bili, alk phos, AST, ALT, UA, HCV at weeks 4, 12, 24! Some drug interactions: St John s Wort, statins! Estimated cost of 4 week supply: $28,544
13 Daklinza (Daclatasvir)! Mechanism of Action: Directly kills the virus. Pangenotypic.! Dose: one tablet daily. Not to be used as monotherapy. Dose may be changed based upon drug interactions.! Contraindications / Cautions No issues with renal function! Common side effects Fatigue, headache, nausea, diarrhea, elevated lipase Daklinza (Daclatasvir)! Estimated cost of 4 week supply: $25,200! Some drug interactions St John s Wort, Amiodarone, statins Many drugs change dosing on Daklinza Cure Rates Overall! Type 3: about 84-88%! Type 2: about 93%! Type 1: about 95%! Treatment duration is weeks! (Note: This does not cover decompensated cirrhosis (Child-Pugh class B or C))
14 Child-Pugh Class! Scoring system based upon T bili, albumin, INR, ascites, encephalopathy! Class A: Compensated cirrhosis! Class B: Decompensated cirrhosis! Class C: Severely decompensated cirrhosis (End Stage Liver Disease (ESLD)) Barriers to Treatment! Cost is very expensive! Limitations by some insurers Must meet certain criteria (eg, degree of fibrosis)! Limitations by pharmaceutical companies Some will not provide free drug if treatment not covered by insurance. Do offer co-pay assistance for commercial insurers What if you are exposed?! Get baseline HCV antibody test! 4 weeks after exposure get HCV antibody test and a quantitative HCV RNA test
15 References! AASLD, IDSA, IAS-USA Recommendations for Testing, Managing, and Treating Hepatitis C. 12/ Mayo Clinic Gastroenterology & Hepatology Board Review, 4 th Edition.! Up-To-Date! GI / Liver Secrets Plus, 4 th Edition. Peter R McNally. Questions? Thank You!!
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT
 PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
Request for Prior Authorization HEPATITIS C TREATMENTS
 IA Medicaid Member ID # Patient name DOB FAX Completed Form To 1 (800) 574-2515 Provider Help Desk 1 (877) 776-1567 Patient address Patient phone Provider NPI Prescriber name Phone Prescriber address Fax
IA Medicaid Member ID # Patient name DOB FAX Completed Form To 1 (800) 574-2515 Provider Help Desk 1 (877) 776-1567 Patient address Patient phone Provider NPI Prescriber name Phone Prescriber address Fax
NEW DRUGS FOR THE TREATMENT OF HEPATITIS C. Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15)
 NEW DRUGS FOR THE TREATMENT OF HEPATITIS C Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15) Objectives Determine initial treatment options for patients with
NEW DRUGS FOR THE TREATMENT OF HEPATITIS C Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15) Objectives Determine initial treatment options for patients with
Clinical Criteria for Hepatitis C (HCV) Therapy
 Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
An Approach to the Diagnosis and Treatment of Hepatitis C Virus Infection in 2015. Matthew McMahon, MD
 An Approach to the Diagnosis and Treatment of Hepatitis C Virus Infection in 2015 Matthew McMahon, MD In the United States, 2.2-3.2 million people are infected with the hepatitis C virus (HCV) Half unaware
An Approach to the Diagnosis and Treatment of Hepatitis C Virus Infection in 2015 Matthew McMahon, MD In the United States, 2.2-3.2 million people are infected with the hepatitis C virus (HCV) Half unaware
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Applicable Coding: GCN: Solvaldi - 35708; Harvoni 037179; Viekira Pak 037614; Daklinza 037073, 037074; Technivie - 037844
 Policy Subject: Policy Number: Classification: Hepatitis C Agents (new) SHS PBD38 Anti-virals: Hepatitis C Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS
Policy Subject: Policy Number: Classification: Hepatitis C Agents (new) SHS PBD38 Anti-virals: Hepatitis C Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS
Review: How to work up your patient with Hepatitis C
 Review: How to work up your patient with Hepatitis C You screened your patient, and now the HCV antibody test is positive. What do you do next? The antibody test only means they have been exposed to HCV.
Review: How to work up your patient with Hepatitis C You screened your patient, and now the HCV antibody test is positive. What do you do next? The antibody test only means they have been exposed to HCV.
Update on Hepatitis C. Sally Williams MD
 Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
Sovaldi (sofosbuvir) Prior Authorization Criteria
 INITIAL REVIEW CRITERIA Sovaldi (sofosbuvir) Prior Authorization Criteria 1. Adult patient age 18 years old; AND 2. Prescribed by a hepatologist, gastroenterologist, infectious disease specialist, transplant
INITIAL REVIEW CRITERIA Sovaldi (sofosbuvir) Prior Authorization Criteria 1. Adult patient age 18 years old; AND 2. Prescribed by a hepatologist, gastroenterologist, infectious disease specialist, transplant
HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information. Diagnosis Acute Hep C Chronic Hep C Hepatocellular Carcinoma
 HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information Recipient: MA#: Date of Birth: Phone #: Body Weight: Treatment Plan Sovaldi (sofosbuvir) 400mg: Take once daily for weeks Olysio
HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information Recipient: MA#: Date of Birth: Phone #: Body Weight: Treatment Plan Sovaldi (sofosbuvir) 400mg: Take once daily for weeks Olysio
New IDSA/AASLD Guidelines for Hepatitis C
 NORTHWEST AIDS EDUCATION AND TRAINING CENTER New IDSA/AASLD Guidelines for Hepatitis C John Scott, MD, MSc Associate Professor, UW SoM Asst Director, Liver Clinic, Harborview Medical Center Presentation
NORTHWEST AIDS EDUCATION AND TRAINING CENTER New IDSA/AASLD Guidelines for Hepatitis C John Scott, MD, MSc Associate Professor, UW SoM Asst Director, Liver Clinic, Harborview Medical Center Presentation
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Hepatitis C Agents that meet any of the following
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Hepatitis C Agents that meet any of the following
Hepatitis C Treatment Criteria Commercial & Minnesota Health Care Programs
 Last update: February 23, 2015 Hepatitis C Treatment Criteria Commercial & Minnesota Health Care Programs Please see healthpartners.com for Medicare coverage criteria. Table of Contents 1. Harvoni 2. Sovaldi
Last update: February 23, 2015 Hepatitis C Treatment Criteria Commercial & Minnesota Health Care Programs Please see healthpartners.com for Medicare coverage criteria. Table of Contents 1. Harvoni 2. Sovaldi
PHARMACY PRIOR AUTHORIZATION
 PHARMACY PRIOR AUTHORIZATION Hepatitis C Clinical Guideline Harvoni (sofosbuvir/ledipasvir), Sovaldi (sofosbuvir), Viekira PAK (ombitsavir, paritapravir/ritonavir, dasubavir), and Olysio (simeprevir) Authorization
PHARMACY PRIOR AUTHORIZATION Hepatitis C Clinical Guideline Harvoni (sofosbuvir/ledipasvir), Sovaldi (sofosbuvir), Viekira PAK (ombitsavir, paritapravir/ritonavir, dasubavir), and Olysio (simeprevir) Authorization
Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary
 Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary This program applies to Health Insurance Marketplace, FlexRx
Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary This program applies to Health Insurance Marketplace, FlexRx
Transmission of HCV in the United States (CDC estimate)
 Transmission of HCV in the United States (CDC estimate) Past and Future US Incidence and Prevalence of HCV Infection Decline among IDUs Overall incidence Overall prevalence Infected 20+ years Armstrong
Transmission of HCV in the United States (CDC estimate) Past and Future US Incidence and Prevalence of HCV Infection Decline among IDUs Overall incidence Overall prevalence Infected 20+ years Armstrong
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Hepatitis C Second Generation Antivirals Page 1 of 22 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Hepatitis C Second Generation Antivirals Through Preferred
Hepatitis C Second Generation Antivirals Page 1 of 22 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Hepatitis C Second Generation Antivirals Through Preferred
Hepatitis Update. HCV Cure As A Paradigm for Convergence of Interests. Evidence Based Nuts and Bolts For the Family Doc 11/5/2014
 Evidence Based Nuts and Bolts For the Family Doc Hepatitis Update William Carey MD MACG, FAASLD Oct 25, 2014 HCV Cure As A Paradigm for Convergence of Interests Hepatitis C Cure 1 Get Ready Get SET Go
Evidence Based Nuts and Bolts For the Family Doc Hepatitis Update William Carey MD MACG, FAASLD Oct 25, 2014 HCV Cure As A Paradigm for Convergence of Interests Hepatitis C Cure 1 Get Ready Get SET Go
Ledipasvir/Sofosbuvir (Harvoni) for Treatment of Hepatitis C
 Ledipasvir/Sofosbuvir (Harvoni) for Treatment of Hepatitis C Policy Number: Original Effective Date: MM.04.034 12/1/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/1/2014
Ledipasvir/Sofosbuvir (Harvoni) for Treatment of Hepatitis C Policy Number: Original Effective Date: MM.04.034 12/1/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/1/2014
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Hepatitis C Second Generation Antivirals (2015) Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Hepatitis C Second Generation Antivirals Through
Hepatitis C Second Generation Antivirals (2015) Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Hepatitis C Second Generation Antivirals Through
Hepatitis C in 2015. Primary Care Providers: Linkage to Care
 Hepatitis C in 2015 Primary Care Providers: Linkage to Care Michael D Voigt Medical Director, Liver Failure and Transplantation University of Iowa, Hospitals and Clinics Disclosures I have NO disclosures
Hepatitis C in 2015 Primary Care Providers: Linkage to Care Michael D Voigt Medical Director, Liver Failure and Transplantation University of Iowa, Hospitals and Clinics Disclosures I have NO disclosures
HEPATITIS C TREATMENT GUIDELINES
 HEPATITIS C TREATMENT GUIDELINES Updated May 21, 2014 INSTRUCTIONS: 1. Review the posted Hepatitis C Treatment Guidelines document to validate that your patient meets the criteria for treatment. 2. Complete
HEPATITIS C TREATMENT GUIDELINES Updated May 21, 2014 INSTRUCTIONS: 1. Review the posted Hepatitis C Treatment Guidelines document to validate that your patient meets the criteria for treatment. 2. Complete
Clinical Criteria for Hepatitis C (HCV) Therapy
 Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C, genotype and sub-genotype specified to determine the length of therapy; Liver biopsy or other accepted test demonstrating
Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C, genotype and sub-genotype specified to determine the length of therapy; Liver biopsy or other accepted test demonstrating
A Cure is Within Reach:
 A Cure is Within Reach: A Review of the New HCV Medications Misty Miller, Pharm.D., BCPS, AAHIVP Clinical Assistant Professor University of Oklahoma College of Pharmacy September 11, 2015 Pre Assessment
A Cure is Within Reach: A Review of the New HCV Medications Misty Miller, Pharm.D., BCPS, AAHIVP Clinical Assistant Professor University of Oklahoma College of Pharmacy September 11, 2015 Pre Assessment
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 5/21/2014 3/24/2016 3/24/2015 Policy Name Policy Number Hepatitis C Oral SRx-0003 Medical Policy Statements
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 5/21/2014 3/24/2016 3/24/2015 Policy Name Policy Number Hepatitis C Oral SRx-0003 Medical Policy Statements
HEPATITIS C - STATE OF THE ART -
 HEPATITIS C - STATE OF THE ART - Steven J. Ayres, M.D. January 23, 2015 11 th Annual Northcentral Montana Rural Physicians and Advanced Practitioners Conference Disclosures Dr. Ayres has no disclosures
HEPATITIS C - STATE OF THE ART - Steven J. Ayres, M.D. January 23, 2015 11 th Annual Northcentral Montana Rural Physicians and Advanced Practitioners Conference Disclosures Dr. Ayres has no disclosures
INFORMATION ABOUT HEPATITIS C
 INFORMATION ABOUT HEPATITIS C PATIENT BOOKLET Index 1 About Hepatitis C About Hepatitis C Page 1 Hepatitis C Transmission Page 3 Hepatitis C Disease Progression Page 4 Hepatitis C Diagnosis and Treatment
INFORMATION ABOUT HEPATITIS C PATIENT BOOKLET Index 1 About Hepatitis C About Hepatitis C Page 1 Hepatitis C Transmission Page 3 Hepatitis C Disease Progression Page 4 Hepatitis C Diagnosis and Treatment
Disclosure. Pharmacy technician objectives. Pharmacist objectives. Epidemiology. Terminology 3/3/2015. Treatment of Hepatitis C
 Disclosure Treatment of Hepatitis C Katerina Tagaras PGY-1 Resident Palmetto General Hospital I do not have a vested interest in or affiliation with any corporate organization offering financial support
Disclosure Treatment of Hepatitis C Katerina Tagaras PGY-1 Resident Palmetto General Hospital I do not have a vested interest in or affiliation with any corporate organization offering financial support
The following should be current within the past 6 months:
 EVALUATION Baseline Labs Obtain at time or prior to initial evaluation CBC with diff PT/INR CMP HCV Genotype (obtained PRIOR TO consult visit) HCV RNA (obtained PRIOR TO consult visit) Hep A IgG Hep BsAg,
EVALUATION Baseline Labs Obtain at time or prior to initial evaluation CBC with diff PT/INR CMP HCV Genotype (obtained PRIOR TO consult visit) HCV RNA (obtained PRIOR TO consult visit) Hep A IgG Hep BsAg,
Hepatitis C Infection In Singapore
 Hepatitis C Infection In Singapore Richard Guan MBBS, MRCP(UK), FAMS(Gastro), FRCP(Edin), FRCP(Lond) Gastroenterologist & Hepatologist, Mt Elizabeth Medical Centre, Singapore Summary of HCV treatment practice
Hepatitis C Infection In Singapore Richard Guan MBBS, MRCP(UK), FAMS(Gastro), FRCP(Edin), FRCP(Lond) Gastroenterologist & Hepatologist, Mt Elizabeth Medical Centre, Singapore Summary of HCV treatment practice
1.How did I get Hepatitis C?
 1.How did I get Hepatitis C? Sharing needles and syringes ( iv drug use ) or using contaminated straws to snort cocaine Reused needles or medications in a health care setting- (LV endoscopy outbreak, glucometers
1.How did I get Hepatitis C? Sharing needles and syringes ( iv drug use ) or using contaminated straws to snort cocaine Reused needles or medications in a health care setting- (LV endoscopy outbreak, glucometers
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Interferon,
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Interferon,
UPDATE ON NEW HEPATITIS C MEDICINES
 UPDATE ON NEW HEPATITIS C MEDICINES Diana Sylvestre, MD 2014 AASLD/IDSA Guidelines: Gt 1 Treatment naïve Interferon eligible: sofosuvir + ribavirin + PEG-IFN x 12 weeks Interferon ineligible: sofosbuvir
UPDATE ON NEW HEPATITIS C MEDICINES Diana Sylvestre, MD 2014 AASLD/IDSA Guidelines: Gt 1 Treatment naïve Interferon eligible: sofosuvir + ribavirin + PEG-IFN x 12 weeks Interferon ineligible: sofosbuvir
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP. Primary Care Provider:
 Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
Objectives. Disclosures 1/26/2016. Which of These Drugs is FDA Approved for the Treatment of Genotype 1 Infection?
 New Horizons: Navigating the Changing Tides of Hepatitis C Therapy Jennifer Andres, PharmD, BCPS Clinical Assistant Professor of Pharmacy Practice Temple University School of Pharmacy Objectives Review
New Horizons: Navigating the Changing Tides of Hepatitis C Therapy Jennifer Andres, PharmD, BCPS Clinical Assistant Professor of Pharmacy Practice Temple University School of Pharmacy Objectives Review
The question and answer session is not available after the live webinar.
 1 Read verbatim. 2 The Infectious Diseases Society of America (IDSA) Hepatitis C Knowledge Network offers monthly, 1 hour webinars to educate IDSA members on current recommended practices and treatments
1 Read verbatim. 2 The Infectious Diseases Society of America (IDSA) Hepatitis C Knowledge Network offers monthly, 1 hour webinars to educate IDSA members on current recommended practices and treatments
Hepatitis B: Objectives. Hepatitis C: Objectives. Hepatitis B: Natural History. Hepatitis B is a DNA virus and is NOT curable.
 Two Silent Epidemics - Chronic Hepatitis B and Hepatitis C: A Primer for Primary Care Practitioners Rebekah Hamner, MA, MSN, APRN, AGCNS Austin Hepatitis Center Dr. Imtiaz Alam 1/25/15 Hepatitis B: Objectives
Two Silent Epidemics - Chronic Hepatitis B and Hepatitis C: A Primer for Primary Care Practitioners Rebekah Hamner, MA, MSN, APRN, AGCNS Austin Hepatitis Center Dr. Imtiaz Alam 1/25/15 Hepatitis B: Objectives
Hepatitis C Virus Direct-Acting Antivirals Prior Authorization Request Form
 Hepatitis C Virus Direct-Acting Antivirals Prior Authorization Request Form For assistance, please call 1-855-552-6028 or fax completed form to 570-271-5610. Medical documentation may be requested. This
Hepatitis C Virus Direct-Acting Antivirals Prior Authorization Request Form For assistance, please call 1-855-552-6028 or fax completed form to 570-271-5610. Medical documentation may be requested. This
PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL
 Pennsylvania PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Pennsylvania Hepatitis C Virus (HCV) in Pennsylvania Prevalence + + Although state-wide data were unavailable,
Pennsylvania PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Pennsylvania Hepatitis C Virus (HCV) in Pennsylvania Prevalence + + Although state-wide data were unavailable,
Newly Diagnosed: HEPATITIS C. American Liver Foundation Support Guide
 Newly Diagnosed: HEPATITIS C American Liver Foundation Support Guide T he American Liver Foundation's mission is to facilitate, advocate, and promote education, support, and research for the prevention,
Newly Diagnosed: HEPATITIS C American Liver Foundation Support Guide T he American Liver Foundation's mission is to facilitate, advocate, and promote education, support, and research for the prevention,
Albumin. Prothrombin time. Total protein
 Hepatitis C Fact Sheet February 2016 www.hepatitis.va.gov Laboratory Tests and Hepatitis If you have hepatitis C, your doctor will use laboratory tests to about learn more about your individual hepatitis
Hepatitis C Fact Sheet February 2016 www.hepatitis.va.gov Laboratory Tests and Hepatitis If you have hepatitis C, your doctor will use laboratory tests to about learn more about your individual hepatitis
HEPATITIS C (HCV) CME ACCREDITED INTERACTIVE TRAINING SESSION: @DDW 2015
 CME ACCREDITED INTERACTIVE TRAINING SESSION: HEPATITIS C (HCV) @DDW 2015 LIVER DISEASE 905 891 1900 www.careeducation.ca info@careeducation.ca Community Academic Research Education @wearecare CME ACCREDITED
CME ACCREDITED INTERACTIVE TRAINING SESSION: HEPATITIS C (HCV) @DDW 2015 LIVER DISEASE 905 891 1900 www.careeducation.ca info@careeducation.ca Community Academic Research Education @wearecare CME ACCREDITED
Prior Authorization Policy
 Prior Authorization Policy http://www.paramounthealthcare.com/providers Ribavirin Rebetol (ribavirin capsule or oral solution) Copegus (ribavirin tablet), Moderiba (ribavirin tablet), Ribasphere (ribavirin
Prior Authorization Policy http://www.paramounthealthcare.com/providers Ribavirin Rebetol (ribavirin capsule or oral solution) Copegus (ribavirin tablet), Moderiba (ribavirin tablet), Ribasphere (ribavirin
Hepatitis C treatment What to expect.
 Hepatitis C treatment What to expect. Anne Glass Hepatitis Clinical Nurse Consultant Liver Clinic SSWAHS Bankstown and Liverpool Hospital Contact 87384074 Monday -Friday What s hepatitis? Hepatitis means
Hepatitis C treatment What to expect. Anne Glass Hepatitis Clinical Nurse Consultant Liver Clinic SSWAHS Bankstown and Liverpool Hospital Contact 87384074 Monday -Friday What s hepatitis? Hepatitis means
Treating Chronic Hepatitis C. A Review of the Research for Adults
 Treating Chronic Hepatitis C A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have chronic hepatitis C.
Treating Chronic Hepatitis C A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have chronic hepatitis C.
HEPATITIS C. The Facts. Get Tested. Get Cured! Health
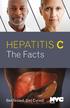 HEPATITIS C The Facts Get Tested. Get Cured! Health EVEN IF YOU FEEL HEALTHY, HEPATITIS C MAY BE DAMAGING YOUR LIVER. Your liver keeps you healthy in many ways, such as by removing toxins from your blood
HEPATITIS C The Facts Get Tested. Get Cured! Health EVEN IF YOU FEEL HEALTHY, HEPATITIS C MAY BE DAMAGING YOUR LIVER. Your liver keeps you healthy in many ways, such as by removing toxins from your blood
GUIDELINES FOR THE SCREENING, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS C INFECTION POLICY BRIEF
 NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR THE SCREENING, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS C INFECTION POLICY BRIEF NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR
NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR THE SCREENING, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS C INFECTION POLICY BRIEF NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR
Hepatitis C: Increasing Access and Improving Cure Rates
 Hepatitis C: Increasing Access and Improving Cure Rates David Hachey, Pharm.D., AAHIVP Professor Idaho State University Department of Family Medicine 1 Disclosures The planners and presenter have disclosed
Hepatitis C: Increasing Access and Improving Cure Rates David Hachey, Pharm.D., AAHIVP Professor Idaho State University Department of Family Medicine 1 Disclosures The planners and presenter have disclosed
HIV and Hepatitis Co-infection. Martin Fisher Brighton and Sussex University Hospitals, UK
 HIV and Hepatitis Co-infection Martin Fisher Brighton and Sussex University Hospitals, UK Useful References British HIV Association 2010 http://www.bhiva.org/documents/guidelines/hepbc/2010/ hiv_781.pdf
HIV and Hepatitis Co-infection Martin Fisher Brighton and Sussex University Hospitals, UK Useful References British HIV Association 2010 http://www.bhiva.org/documents/guidelines/hepbc/2010/ hiv_781.pdf
Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection Federal Bureau of Prisons Clinical Practice Guidelines July 2015
 Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection Federal Bureau of Prisons Clinical Practice Guidelines July 2015 Clinical guidelines are made available to the public for informational
Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection Federal Bureau of Prisons Clinical Practice Guidelines July 2015 Clinical guidelines are made available to the public for informational
Hepatitis C Virus (HCV)
 HFS Report to Legislative Task Force Hepatitis C Arvind Goyal MD, MPH, MBA Medical Director Illinois Department of Healthcare and Family Services Hepatitis C Virus (HCV) Slowly progressive disease 40 years-median
HFS Report to Legislative Task Force Hepatitis C Arvind Goyal MD, MPH, MBA Medical Director Illinois Department of Healthcare and Family Services Hepatitis C Virus (HCV) Slowly progressive disease 40 years-median
BASIC INFORMATION ABOUT HIV, HEPATITIS B and C, and TUBERCULOSIS Adapted from the CDC
 BASIC INFORMATION ABOUT HIV, HEPATITIS B and C, and TUBERCULOSIS Adapted from the CDC HIV What are HIV and AIDS? HIV stands for Human Immunodeficiency Virus. This is the virus that causes AIDS. HIV is
BASIC INFORMATION ABOUT HIV, HEPATITIS B and C, and TUBERCULOSIS Adapted from the CDC HIV What are HIV and AIDS? HIV stands for Human Immunodeficiency Virus. This is the virus that causes AIDS. HIV is
Patterns of abnormal LFTs and their differential diagnosis
 Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Summary liver function / liver function
Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Summary liver function / liver function
Hepatitis C Treatment Expansion Initiative Multi-Site Conference Call. March 16, 2011
 Hepatitis C Treatment Expansion Initiative Multi-Site Conference Call March 16, 2011 Case Presentations Kansas City Free Health Clinic Carilion Clinic Didactic Session Challenges in Determining HCV Treatment
Hepatitis C Treatment Expansion Initiative Multi-Site Conference Call March 16, 2011 Case Presentations Kansas City Free Health Clinic Carilion Clinic Didactic Session Challenges in Determining HCV Treatment
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Hepatitis C - genotype 2/3
 Hepatitis C - genotype 2/3 Information for you Follow us on Twitter @NHSaaa Find us on Facebook at www.facebook.com/nhsaaa Visit our website: www.nhsaaa.net All our publications are available in other
Hepatitis C - genotype 2/3 Information for you Follow us on Twitter @NHSaaa Find us on Facebook at www.facebook.com/nhsaaa Visit our website: www.nhsaaa.net All our publications are available in other
A 55 year old man with cirrhosis due to chronic hepatitis C (CHC) genotype 3a is referred for liver transplantation.
 A 55 year old man with cirrhosis due to chronic hepatitis C (CHC) genotype 3a is referred for liver transplantation. Three years ago he was treated with 24 weeks of peginterferon alfa-2a (180 µg/wk, PEGIFN)
A 55 year old man with cirrhosis due to chronic hepatitis C (CHC) genotype 3a is referred for liver transplantation. Three years ago he was treated with 24 weeks of peginterferon alfa-2a (180 µg/wk, PEGIFN)
Recommendations 8/14/2014. Hepatitis C Clinical Approach Primary Care. Purpose of Presentation. HCV Prevalence Year of Birth
 Hepatitis C Clinical Approach Primary Care Dr. Vicki L. MIt McIntyre, FNP Tucson Gastroenterology Specialists Tucson, Arizona University of Phoenix Lead Faculty, Department of Nursing Tucson, Arizona Purpose
Hepatitis C Clinical Approach Primary Care Dr. Vicki L. MIt McIntyre, FNP Tucson Gastroenterology Specialists Tucson, Arizona University of Phoenix Lead Faculty, Department of Nursing Tucson, Arizona Purpose
CPE Session 4. Recent Advances in the Journey to Cure Hepatitis C
 CPE Session 4 Recent Advances in the Journey to Cure Hepatitis C Friday, April 24, 2015 ACPE UAN 0128-0000-15-024-L01-P 1.0 CPE Hour Jennifer Hickman, PharmD, BCACP Dr. Jennifer Hickman is an ambulatory
CPE Session 4 Recent Advances in the Journey to Cure Hepatitis C Friday, April 24, 2015 ACPE UAN 0128-0000-15-024-L01-P 1.0 CPE Hour Jennifer Hickman, PharmD, BCACP Dr. Jennifer Hickman is an ambulatory
Treatment of Hepatitis C in Patients with Renal Insufficiency
 HEPATITIS WEB STUDY HEPATITIS C ONLINE Treatment of Hepatitis C in Patients with Renal Insufficiency Robert G. Gish MD Professor Consultant, Stanford University Medical Center Senior Medical Director,
HEPATITIS WEB STUDY HEPATITIS C ONLINE Treatment of Hepatitis C in Patients with Renal Insufficiency Robert G. Gish MD Professor Consultant, Stanford University Medical Center Senior Medical Director,
PRACTICE GUIDANCE Hepatitis C Guidance: AASLD-IDSA Recommendations for Testing, Managing, and Treating Adults Infected With Hepatitis C Virus
 PRACTICE GUIDANCE Hepatitis C Guidance: AASLD-IDSA s for Testing, Managing, and Treating Adults Infected With Hepatitis C Virus AASLD/IDSA HCV Guidance Panel* Preamble The pace of hepatitis C virus (HCV)
PRACTICE GUIDANCE Hepatitis C Guidance: AASLD-IDSA s for Testing, Managing, and Treating Adults Infected With Hepatitis C Virus AASLD/IDSA HCV Guidance Panel* Preamble The pace of hepatitis C virus (HCV)
12/2/2015 HEPATITIS B AND HEPATITIS C BLOOD EXPOSURE OBJECTIVES VIRAL HEPATITIS
 HEPATITIS B AND HEPATITIS C BLOOD EXPOSURE DISEASE 101 ONLINE CONFERENCE SARAH WENINGER, MPH VIRAL HEPATITIS.STD.HIV PREVENTION COORDINATOR DECEMBER 3, 2015 OBJECTIVES Describe the populations that should
HEPATITIS B AND HEPATITIS C BLOOD EXPOSURE DISEASE 101 ONLINE CONFERENCE SARAH WENINGER, MPH VIRAL HEPATITIS.STD.HIV PREVENTION COORDINATOR DECEMBER 3, 2015 OBJECTIVES Describe the populations that should
Objectives. Hepatitis C: The new era of screening and treatment. Distribution of HCV genotypes 11/1/2014. History of HCV diagnosis and screening
 Objectives Hepatitis C: The new era of screening and treatment David Crabb, M.D. Department of Medicine, Indiana University and Eskenazi Health Describe the natural history of HCV infection Be able to
Objectives Hepatitis C: The new era of screening and treatment David Crabb, M.D. Department of Medicine, Indiana University and Eskenazi Health Describe the natural history of HCV infection Be able to
Disclosure of Conflicts of Interest Learner Assurance Statement:
 Raj Reddy, MD Ruimy Family President's Distinguished Professor of Medicine Professor of Medicine in Surgery Director of Hepatology Director, Viral Hepatitis Center Medical Director, Liver Transplantation
Raj Reddy, MD Ruimy Family President's Distinguished Professor of Medicine Professor of Medicine in Surgery Director of Hepatology Director, Viral Hepatitis Center Medical Director, Liver Transplantation
A Proposal for Managing the Harvoni Wave June 22, 2015
 A Proposal for Managing the Harvoni Wave June 22, 2015 Clinical Background Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV) that damages the liver over time. The disease affects
A Proposal for Managing the Harvoni Wave June 22, 2015 Clinical Background Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV) that damages the liver over time. The disease affects
HEPATITIS C DISCUSSION GUIDE:
 HEPATITIS C DISCUSSION GUIDE: INFORMATION AND ANSWERS TO AID YOUR COUNSELING OF PATIENTS INDICATION HARVONI is indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection in adults. Warnings
HEPATITIS C DISCUSSION GUIDE: INFORMATION AND ANSWERS TO AID YOUR COUNSELING OF PATIENTS INDICATION HARVONI is indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection in adults. Warnings
Hepatitis C. Laboratory Tests and Hepatitis C
 Hepatitis C Laboratory Tests and Hepatitis C If you have hepatitis C, your doctor will use laboratory tests to check your health. This handout will help you understand what the major tests are and what
Hepatitis C Laboratory Tests and Hepatitis C If you have hepatitis C, your doctor will use laboratory tests to check your health. This handout will help you understand what the major tests are and what
The most serious symptoms of this stage are:
 The Natural Progression of Hepatitis C The natural history of hepatitis C looks at the likely outcomes for people infected with the virus if there is no medical intervention. However, the process of trying
The Natural Progression of Hepatitis C The natural history of hepatitis C looks at the likely outcomes for people infected with the virus if there is no medical intervention. However, the process of trying
New Era in HCV Management: Primary Care Innovations
 New Era in HCV Management: Primary Care Innovations Marwan Haddad MD, MPH, AAHIVS Medical Director of HIV, HCV, and Buprenorphine Services Community Health Center, Inc., Connecticut September 24, 2015
New Era in HCV Management: Primary Care Innovations Marwan Haddad MD, MPH, AAHIVS Medical Director of HIV, HCV, and Buprenorphine Services Community Health Center, Inc., Connecticut September 24, 2015
The Hepatitis B virus (HBV)
 The Hepatitis B virus (HBV) There are 400 million people in the world who live with chronic hepatitis B, including France. Most people don t even know they are infected. But there are several important
The Hepatitis B virus (HBV) There are 400 million people in the world who live with chronic hepatitis B, including France. Most people don t even know they are infected. But there are several important
The Comparative Clinical Effectiveness and Value of Simeprevir and Sofosbuvir in the Treatment of Chronic Hepatitis C Infection
 The Comparative Clinical Effectiveness and Value of Simeprevir and Sofosbuvir in the Treatment of Chronic Hepatitis C Infection A Technology Assessment Final Report April 15, 2014 Completed by: Institute
The Comparative Clinical Effectiveness and Value of Simeprevir and Sofosbuvir in the Treatment of Chronic Hepatitis C Infection A Technology Assessment Final Report April 15, 2014 Completed by: Institute
HCV/HIVCo-infection A case study by. Dominic Côté, Nurse Clinician B.Sc Chronic Viral Illness Services McGill University Health Centre
 HCV/HIVCo-infection A case study by Dominic Côté, Nurse Clinician B.Sc Chronic Viral Illness Services McGill University Health Centre Objectives By sharing a case study of a patient co-infected with HIV/HCV
HCV/HIVCo-infection A case study by Dominic Côté, Nurse Clinician B.Sc Chronic Viral Illness Services McGill University Health Centre Objectives By sharing a case study of a patient co-infected with HIV/HCV
Introduction. Background
 INFORMATION DRIVES SOUND ANALYSIS, INSIGHT PHARMACY BENEFIT ADVISORY Introduction According to the Centers for Disease Control and Prevention (CDC), the rate of new hepatitis C virus (HCV) infections in
INFORMATION DRIVES SOUND ANALYSIS, INSIGHT PHARMACY BENEFIT ADVISORY Introduction According to the Centers for Disease Control and Prevention (CDC), the rate of new hepatitis C virus (HCV) infections in
HIV/HCV Co-infection. HIV/HCV Co-infection. Epidemiology. Dr Ranjababu Kulasegaram Guy s & St Thomas Hospital London. Extrahepatic manifestations
 HIV/HCV Co-infection Dr Ranjababu Kulasegaram Guy s & St Thomas Hospital London HIV/HCV Co-infection Epidemiology Impact of HIV on HCV Epidemiology Impact of HCV on HIV Management issues Future Extrahepatic
HIV/HCV Co-infection Dr Ranjababu Kulasegaram Guy s & St Thomas Hospital London HIV/HCV Co-infection Epidemiology Impact of HIV on HCV Epidemiology Impact of HCV on HIV Management issues Future Extrahepatic
Hepatitis C. Pedro Jose Greer, MD
 Hepatitis C Pedro Jose Greer, MD T he hepatitis C virus (HCV) was identified in 1989 and found to account for the majority of those patients with non-a, non-b hepatitis. HCV is now the most common blood-borne
Hepatitis C Pedro Jose Greer, MD T he hepatitis C virus (HCV) was identified in 1989 and found to account for the majority of those patients with non-a, non-b hepatitis. HCV is now the most common blood-borne
Preface. TTY: (888) 232-6348 or cdcinfo@cdc.gov. Hepatitis C Counseling and Testing, contact: 800-CDC-INFO (800-232-4636)
 Preface The purpose of this CDC Hepatitis C Counseling and Testing manual is to provide guidance for hepatitis C counseling and testing of individuals born during 1945 1965. The guide was used in draft
Preface The purpose of this CDC Hepatitis C Counseling and Testing manual is to provide guidance for hepatitis C counseling and testing of individuals born during 1945 1965. The guide was used in draft
PRIOR AUTHORIZATION POLICY
 PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
Technology appraisal guidance Published: 25 November 2015 nice.org.uk/guidance/ta365
 Ombitasvir paritaprevir ritonavir with or without dasabuvir for treating chronic hepatitis C Technology appraisal guidance Published: 25 November 2015 nice.org.uk/guidance/ta365 NICE 2015. All rights reserved.
Ombitasvir paritaprevir ritonavir with or without dasabuvir for treating chronic hepatitis C Technology appraisal guidance Published: 25 November 2015 nice.org.uk/guidance/ta365 NICE 2015. All rights reserved.
Hepatitis C: Update on Testing and Treatment. Karla Thornton, MD, MPH. Karla Thornton:
 Hepatitis C: Update on Testing and Treatment Karla Thornton, MD, MPH Karla Thornton: Thank you all for inviting me. I m very excited to be here today. My name is Karla Thornton. I m a professor in the
Hepatitis C: Update on Testing and Treatment Karla Thornton, MD, MPH Karla Thornton: Thank you all for inviting me. I m very excited to be here today. My name is Karla Thornton. I m a professor in the
Epidemiology of Hepatitis C Infection. Pablo Barreiro Service of Infectious Diseases Hospital Carlos III, Madrid
 Epidemiology of Hepatitis C Infection Pablo Barreiro Service of Infectious Diseases Hospital Carlos III, Madrid Worldwide Prevalence of Hepatitis C 10% No data available WHO.
Epidemiology of Hepatitis C Infection Pablo Barreiro Service of Infectious Diseases Hospital Carlos III, Madrid Worldwide Prevalence of Hepatitis C 10% No data available WHO.
Emerging Direct-Acting Antivirals for Treatment of Chronic Hepatitis C
 Emerging Direct-Acting Antivirals for Treatment of Chronic Hepatitis C Debra Birnkrant, MD Director, DAVP DILI Conference March 20, 2013 1 HCV in the United States 3 to 4 million people have chronic HCV
Emerging Direct-Acting Antivirals for Treatment of Chronic Hepatitis C Debra Birnkrant, MD Director, DAVP DILI Conference March 20, 2013 1 HCV in the United States 3 to 4 million people have chronic HCV
Learning about Hepatitis C and Chronic Kidney Disease
 Learning about Hepatitis C and Chronic Kidney Disease Hepatitis C and Chronic Kidney Disease If you have chronic kidney disease (CKD), you want to learn all you can about your disease and what you can
Learning about Hepatitis C and Chronic Kidney Disease Hepatitis C and Chronic Kidney Disease If you have chronic kidney disease (CKD), you want to learn all you can about your disease and what you can
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
HEPATITIS WEB STUDY Acute Hepatitis C Virus Infection: Epidemiology, Clinical Features, and Diagnosis
 HEPATITIS WEB STUDY Acute C Virus Infection: Epidemiology, Clinical Features, and Diagnosis H. Nina Kim, MD Assistant Professor of Medicine Division of Infectious Diseases University of Washington School
HEPATITIS WEB STUDY Acute C Virus Infection: Epidemiology, Clinical Features, and Diagnosis H. Nina Kim, MD Assistant Professor of Medicine Division of Infectious Diseases University of Washington School
Hepatitis B and C Co-infection. Mark Hull MHSc, FRCPC Clinical Assistant Professor Division of AIDS
 Hepatitis B and C Co-infection Mark Hull MHSc, FRCPC Clinical Assistant Professor Division of AIDS Objectives Review natural history of hepatitis coinfection Brief overview of treatment indications for
Hepatitis B and C Co-infection Mark Hull MHSc, FRCPC Clinical Assistant Professor Division of AIDS Objectives Review natural history of hepatitis coinfection Brief overview of treatment indications for
Sofosbuvir, Pegylated Interferon and Ribavirin for the Treatment of Hepatitis C
 Sofosbuvir, Pegylated Interferon and Ribavirin for the Treatment of Hepatitis C Department of Hepatology Digestive Diseases Centre Patient Information This leaflet is designed to give you important information
Sofosbuvir, Pegylated Interferon and Ribavirin for the Treatment of Hepatitis C Department of Hepatology Digestive Diseases Centre Patient Information This leaflet is designed to give you important information
TOH Viral Hepatitis Multidisciplinary Team
 TOH Viral Hepatitis Multidisciplinary Team Jan Pappas RN Ottawa Hospital-General Campus February 2011 1 The scope of hepatitis C Worldwide, approximately 170 million people are living with Hepatitis C
TOH Viral Hepatitis Multidisciplinary Team Jan Pappas RN Ottawa Hospital-General Campus February 2011 1 The scope of hepatitis C Worldwide, approximately 170 million people are living with Hepatitis C
HCV Treatment Failure
 بسم االله الرحمن الرحيم HCV Treatment Failure Gamal Esmat PROF.OF HEPATOLOGY&TROPICAL MEDICINE CAIRO UNIVERSITY Director of Viral Hepatitis Treatment Centers (VHTCs( VHTCs) MOH-EGYPT www.gamalesmat.com
بسم االله الرحمن الرحيم HCV Treatment Failure Gamal Esmat PROF.OF HEPATOLOGY&TROPICAL MEDICINE CAIRO UNIVERSITY Director of Viral Hepatitis Treatment Centers (VHTCs( VHTCs) MOH-EGYPT www.gamalesmat.com
The Epidemiology of Hepatitis A, B, and C
 The Epidemiology of Hepatitis A, B, and C Jamie Berkes M.D. University of Illinois at Chicago jberkes@uic.edu Epidemiology: Definitions The study of the incidence and prevalence of diseases in large populations
The Epidemiology of Hepatitis A, B, and C Jamie Berkes M.D. University of Illinois at Chicago jberkes@uic.edu Epidemiology: Definitions The study of the incidence and prevalence of diseases in large populations
Treatment of Chronic Hepatitis C - September 2015 Update
 Treatment of Chronic Hepatitis C - September 2015 Update Swiss Association for the Study of the Liver and Swiss Society for Infectious Diseases Written by: Beat Müllhaupt, Andri Rauch, Jan Fehr and Darius
Treatment of Chronic Hepatitis C - September 2015 Update Swiss Association for the Study of the Liver and Swiss Society for Infectious Diseases Written by: Beat Müllhaupt, Andri Rauch, Jan Fehr and Darius
Feds to Medicaid: Stop HCV Treatment Restrictions
 Get Tested Get Treated Get Cured NOVEMBER 2015 Vol. 18, Issue 11.5 Feds to Medicaid: Stop HCV Treatment Restrictions On November 05, 2015, the Centers for Medicare and Medicaid Services (CMS) issued a
Get Tested Get Treated Get Cured NOVEMBER 2015 Vol. 18, Issue 11.5 Feds to Medicaid: Stop HCV Treatment Restrictions On November 05, 2015, the Centers for Medicare and Medicaid Services (CMS) issued a
Harvoni (Ledipasvir/Sofosbuvir) Treatment Agreement
 Harvoni (Ledipasvir/Sofosbuvir) Treatment Agreement Liver Disease & Hepatitis Program Providers: Brian McMahon, MD; Youssef Barbour, MD; Lisa Townshend-Bulson, FNP-C; Annette Hewitt, FNP-C; Prabhu Gounder,
Harvoni (Ledipasvir/Sofosbuvir) Treatment Agreement Liver Disease & Hepatitis Program Providers: Brian McMahon, MD; Youssef Barbour, MD; Lisa Townshend-Bulson, FNP-C; Annette Hewitt, FNP-C; Prabhu Gounder,
Stepwise Approach for the Prevention and Treatment of Hepatitis C and Cirrhosis Federal Bureau of Prisons Clinical Practice Guidelines March 2011
 Stepwise Approach for the Prevention and Treatment of Hepatitis C and Cirrhosis Clinical Practice Guidelines March 2011 Clinical guidelines are made available to the public for informational purposes only.
Stepwise Approach for the Prevention and Treatment of Hepatitis C and Cirrhosis Clinical Practice Guidelines March 2011 Clinical guidelines are made available to the public for informational purposes only.
Hepatitis C Virus Infection in Massachusetts: A tale of two epidemics
 Hepatitis C Virus Infection in Massachusetts: A tale of two epidemics Daniel Church, MPH Massachusetts Department of Public Health Bureau of Infectious Disease 1 Goals of presentation Provide an overview
Hepatitis C Virus Infection in Massachusetts: A tale of two epidemics Daniel Church, MPH Massachusetts Department of Public Health Bureau of Infectious Disease 1 Goals of presentation Provide an overview
We are glad to hear you are interested in treatment for hepatitis C!
 Liver Disease & Hepatitis Program 4315 Diplomacy Drive, Anchorage, AK 99508 Phone: 907-729-1560 Fax: 907-729-1570 Website: http://www.anthctoday.org/community/hep/index.html We are glad to hear you are
Liver Disease & Hepatitis Program 4315 Diplomacy Drive, Anchorage, AK 99508 Phone: 907-729-1560 Fax: 907-729-1570 Website: http://www.anthctoday.org/community/hep/index.html We are glad to hear you are
HIV/AIDS: General Information & Testing in the Emergency Department
 What Is HIV? HIV/AIDS: General Information & Testing in the Emergency Department HIV is the common name for the Human Immunodeficiency Virus. HIV is a retrovirus. This means it can enter the body s own
What Is HIV? HIV/AIDS: General Information & Testing in the Emergency Department HIV is the common name for the Human Immunodeficiency Virus. HIV is a retrovirus. This means it can enter the body s own
Hepatitis C. Screening, Diagnosis and Linkage to Care
 Hepatitis C Screening, Diagnosis and Linkage to Care Diagnosis If your hepatitis C antibody test is reactive, a second test will be needed to diagnose and determine if you are currently infected. Screening
Hepatitis C Screening, Diagnosis and Linkage to Care Diagnosis If your hepatitis C antibody test is reactive, a second test will be needed to diagnose and determine if you are currently infected. Screening
Hepatitis C Class Review
 Hepatitis C Class Review Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Month/Year of Review: January
Hepatitis C Class Review Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Month/Year of Review: January
