Hepatitis C Virus Direct-Acting Antivirals Prior Authorization Request Form
|
|
|
- Damon Flowers
- 10 years ago
- Views:
Transcription
1 Hepatitis C Virus Direct-Acting Antivirals Prior Authorization Request Form For assistance, please call or fax completed form to Medical documentation may be requested. This form will be returned if not completed in full. Member Information Member Name: Prescriber Information Prescriber Name: Prescriber s Specialty: Member ID#: Address: NPI#: Address: City: State: City: State: Home Phone: Zip: Office Phone #: Office Fax #: Zip: Sex (circle): M F DOB: Contact Person: Diagnosis and Medical Information Medication: Strength and Route of Administration: New Prescription OR Expected Length of Therapy: Date Therapy Initiated: Height/Weight: Drug Allergies: Diagnosis: Frequency: Qty: Prescriber s Signature: Date: Criteria for Initial Prior Authorization FORM CANNOT BE PROCESSED UNLESS ALL INFORMATION BELOW IS COMPLETE Requested HCV treatment regimen (include dose, schedule and duration): Please indicate the member s hepatitis C genotype: Please indicate the member s liver staging (based on a Fibroscan, Fibrosure or liver biopsy test): Does the member have a limited life expectancy of less than 12 months due to non-liver related comorbid conditions? Yes No Have all drug interactions with the requested Hepatitis C regimen been addressed by the provider? What actions have been taken? Page 1 of 5
2 If requesting a peginterferon-free regimen, when peginterferon is indicated, please check reason for ineligibility: Hypersensitivity to peginterferon or any of its components Prior reaction to peginterferon requiring discontinuation History of depression with suicidality or resulting in hospital admission and the member is currently receiving antidepressant therapy Autoimmune hepatitis and other immune disorders A baseline neutrophil count below 1500/µL, a baseline platelet count below 90,000/µL or baseline hemoglobin below 10 g/dl Decompensated hepatic disease with a MELD (Model for End-Stage Liver Disease) score 15 Does member have cirrhosis Is the member co-infected with HIV/AIDS Does member have hepatocellular carcinoma (that meets Milan criteria) and is awaiting liver For Olysio requests ONLY: If the member has genotype 1a, was the member screened for Q80K polymorphism If yes, please list the date and result: If yes, please list previous treatment, dates, duration of therapy, and treatment response (partial responder, nonresponder, or relapser): Regimen Dates Duration of Therapy Treatment Response Was member compliant with previous Hepatitis C medication regimen? Have the following lab values been obtained within the last 3 months? (Please attach results) Hepatic Function Panel Complete Blood Count Basic Metabolic Panel Baseline HCV RNA Viral Load What is the member s glomerular filtration rate? ml/min/1.73m 2 If member is female of childbearing potential, and was prescribed ribavirin, please provide pregnancy test results and date: Date: Page 2 of 5
3 If concurrent ribavirin therapy is indicated and prescribed for male members, is their female partner pregnant or planning a pregnancy? Has the member been instructed to practice effective contraception during therapy and for 6 months following discontinuation of ribavirin treatment? Psychiatric Evaluation (for patients prescribed peginterferon): Does member have a history of the following? Prior suicide attempt Bipolar Disorder Major Depressive Disorder Schizophrenia Substance Dependency Disorder (within the past 3 years) Anxiety Disorders If the answer to any of the above questions is yes, and patient was prescribed peginterferon, was a psychiatric evaluation performed within 6 months Psychiatrist Was the member cleared to start hepatitis C treatment by the psychiatrist? If the member was prescribed peginterferon and does not have a history of any psychiatric disorder or substance dependency disorder listed above, was a mental health evaluation performed by the prescriber? Has the member completed at least 6 months of abstinence from alcohol and illegal controlled substances prior to initiation of treatment? If yes, is the m For ALL members: Has a blood alcohol level screen been completed over the past six months Yes If yes, please list all results below: Date Result (Negative or Positive) Page 3 of 5
4 Has a urine drug If yes, please list all results below: Date Result (Negative or Positive) Did the member receive pre-treatment readiness education about hepatitis C treatment expectations by a health Has the member committed in writing to the documented planned course of treatment including anticipated blood tests and visits, during and after treatment Is the member agreeable to counseling and monitoring by representatives from Geisinger Health Plan? Criteria for reauthorization Initial Prior Authorization ORM CANNOT BE PROCESSED UNLESS ALL ORMATION BELOW IS COMTE For members being treated with therapy that requires reauthorization, please provide the following lab data: Treatment Week Baseline Week 4 Week 8 Other Date of HCV RNA Viral Load Testing HCV RNA Viral Load Results For Health Plan internal use only: Date received Date reviewed Request approved: Y / N / NA Page 4 of 5
5 Instructions for Completing the Form 1. Submit a separate form for each medication. 2. Complete ALL information on the form. NOTE: The prescribing physician should, in most cases, complete the form. 3. Please be sure to provide the physician address in a legible format, as it is required for notification. 4. Once form is completed, mail or fax to: Geisinger Health Plan Attn: Pharmacy Department N. Academy Avenue Danville, PA Fax: Page 5 of 5
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Hepatitis C Agents that meet any of the following
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Hepatitis C Agents that meet any of the following
HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information. Diagnosis Acute Hep C Chronic Hep C Hepatocellular Carcinoma
 HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information Recipient: MA#: Date of Birth: Phone #: Body Weight: Treatment Plan Sovaldi (sofosbuvir) 400mg: Take once daily for weeks Olysio
HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information Recipient: MA#: Date of Birth: Phone #: Body Weight: Treatment Plan Sovaldi (sofosbuvir) 400mg: Take once daily for weeks Olysio
PHARMACY PRIOR AUTHORIZATION
 PHARMACY PRIOR AUTHORIZATION Hepatitis C Clinical Guideline Harvoni (sofosbuvir/ledipasvir), Sovaldi (sofosbuvir), Viekira PAK (ombitsavir, paritapravir/ritonavir, dasubavir), and Olysio (simeprevir) Authorization
PHARMACY PRIOR AUTHORIZATION Hepatitis C Clinical Guideline Harvoni (sofosbuvir/ledipasvir), Sovaldi (sofosbuvir), Viekira PAK (ombitsavir, paritapravir/ritonavir, dasubavir), and Olysio (simeprevir) Authorization
Sovaldi (sofosbuvir) Prior Authorization Criteria
 INITIAL REVIEW CRITERIA Sovaldi (sofosbuvir) Prior Authorization Criteria 1. Adult patient age 18 years old; AND 2. Prescribed by a hepatologist, gastroenterologist, infectious disease specialist, transplant
INITIAL REVIEW CRITERIA Sovaldi (sofosbuvir) Prior Authorization Criteria 1. Adult patient age 18 years old; AND 2. Prescribed by a hepatologist, gastroenterologist, infectious disease specialist, transplant
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Interferon,
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Interferon,
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT
 PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 5/21/2014 3/24/2016 3/24/2015 Policy Name Policy Number Hepatitis C Oral SRx-0003 Medical Policy Statements
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 5/21/2014 3/24/2016 3/24/2015 Policy Name Policy Number Hepatitis C Oral SRx-0003 Medical Policy Statements
HEPATITIS C TREATMENT GUIDELINES
 HEPATITIS C TREATMENT GUIDELINES Updated May 21, 2014 INSTRUCTIONS: 1. Review the posted Hepatitis C Treatment Guidelines document to validate that your patient meets the criteria for treatment. 2. Complete
HEPATITIS C TREATMENT GUIDELINES Updated May 21, 2014 INSTRUCTIONS: 1. Review the posted Hepatitis C Treatment Guidelines document to validate that your patient meets the criteria for treatment. 2. Complete
Clinical Criteria for Hepatitis C (HCV) Therapy
 Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL
 Pennsylvania PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Pennsylvania Hepatitis C Virus (HCV) in Pennsylvania Prevalence + + Although state-wide data were unavailable,
Pennsylvania PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Pennsylvania Hepatitis C Virus (HCV) in Pennsylvania Prevalence + + Although state-wide data were unavailable,
Ledipasvir/Sofosbuvir (Harvoni) for Treatment of Hepatitis C
 Ledipasvir/Sofosbuvir (Harvoni) for Treatment of Hepatitis C Policy Number: Original Effective Date: MM.04.034 12/1/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/1/2014
Ledipasvir/Sofosbuvir (Harvoni) for Treatment of Hepatitis C Policy Number: Original Effective Date: MM.04.034 12/1/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/1/2014
HIV and Hepatitis Co-infection. Martin Fisher Brighton and Sussex University Hospitals, UK
 HIV and Hepatitis Co-infection Martin Fisher Brighton and Sussex University Hospitals, UK Useful References British HIV Association 2010 http://www.bhiva.org/documents/guidelines/hepbc/2010/ hiv_781.pdf
HIV and Hepatitis Co-infection Martin Fisher Brighton and Sussex University Hospitals, UK Useful References British HIV Association 2010 http://www.bhiva.org/documents/guidelines/hepbc/2010/ hiv_781.pdf
PRIOR AUTHORIZATION POLICY
 PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
Prior Authorization Policy
 Prior Authorization Policy http://www.paramounthealthcare.com/providers Ribavirin Rebetol (ribavirin capsule or oral solution) Copegus (ribavirin tablet), Moderiba (ribavirin tablet), Ribasphere (ribavirin
Prior Authorization Policy http://www.paramounthealthcare.com/providers Ribavirin Rebetol (ribavirin capsule or oral solution) Copegus (ribavirin tablet), Moderiba (ribavirin tablet), Ribasphere (ribavirin
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Pegasys and non-preferred Hepatitis C Agents must
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Pegasys and non-preferred Hepatitis C Agents must
NEW DRUGS FOR THE TREATMENT OF HEPATITIS C. Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15)
 NEW DRUGS FOR THE TREATMENT OF HEPATITIS C Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15) Objectives Determine initial treatment options for patients with
NEW DRUGS FOR THE TREATMENT OF HEPATITIS C Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15) Objectives Determine initial treatment options for patients with
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP. Primary Care Provider:
 Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
New IDSA/AASLD Guidelines for Hepatitis C
 NORTHWEST AIDS EDUCATION AND TRAINING CENTER New IDSA/AASLD Guidelines for Hepatitis C John Scott, MD, MSc Associate Professor, UW SoM Asst Director, Liver Clinic, Harborview Medical Center Presentation
NORTHWEST AIDS EDUCATION AND TRAINING CENTER New IDSA/AASLD Guidelines for Hepatitis C John Scott, MD, MSc Associate Professor, UW SoM Asst Director, Liver Clinic, Harborview Medical Center Presentation
Update on Hepatitis C. Sally Williams MD
 Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
HCSP GUIDES A GUIDE TO: PREPARING FOR TREATMENT. A publication of the Hepatitis C Support Project
 HCSP GUIDES T R E AT M E N T I S S U E S A publication of the Hepatitis C Support Project The information in this guide is designed to help you understand and manage HCV and is not intended as medical
HCSP GUIDES T R E AT M E N T I S S U E S A publication of the Hepatitis C Support Project The information in this guide is designed to help you understand and manage HCV and is not intended as medical
Monitoring of Treatment of viral hepatitis C
 Monitoring of Treatment of viral hepatitis C J.Boubaker Department of Gastroenterology La Rabta hospital Tunis-Tunisia Monitoring of Hepatitis C Treatment Aims of Monitoring : Evaluate Efficacy. Detect
Monitoring of Treatment of viral hepatitis C J.Boubaker Department of Gastroenterology La Rabta hospital Tunis-Tunisia Monitoring of Hepatitis C Treatment Aims of Monitoring : Evaluate Efficacy. Detect
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Hepatitis C Second Generation Antivirals Page 1 of 22 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Hepatitis C Second Generation Antivirals Through Preferred
Hepatitis C Second Generation Antivirals Page 1 of 22 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Hepatitis C Second Generation Antivirals Through Preferred
Hepatitis C Class Review
 Hepatitis C Class Review Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Month/Year of Review: January
Hepatitis C Class Review Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Month/Year of Review: January
HCV Case Study. Optimizing Outcomes with Current Therapies
 HCV Case Study Optimizing Outcomes with Current Therapies This program is supported by educational grants from Kadmon and Merck Pharmaceuticals. Program Disclosure This activity has been planned and implemented
HCV Case Study Optimizing Outcomes with Current Therapies This program is supported by educational grants from Kadmon and Merck Pharmaceuticals. Program Disclosure This activity has been planned and implemented
July 2015 Preferred Drug List Review and Other Pharmacy Policy Changes
 Update June 2015 No. 2015-27 Affected Programs: BadgerCare Plus, Medicaid, SeniorCare To: Blood Banks, Dentists, Federally Qualified Health Centers, Hospital Providers, Nurse Practitioners, Nursing Homes,
Update June 2015 No. 2015-27 Affected Programs: BadgerCare Plus, Medicaid, SeniorCare To: Blood Banks, Dentists, Federally Qualified Health Centers, Hospital Providers, Nurse Practitioners, Nursing Homes,
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Cirrhosis and HCV. Jonathan Israel M.D.
 Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Review: How to work up your patient with Hepatitis C
 Review: How to work up your patient with Hepatitis C You screened your patient, and now the HCV antibody test is positive. What do you do next? The antibody test only means they have been exposed to HCV.
Review: How to work up your patient with Hepatitis C You screened your patient, and now the HCV antibody test is positive. What do you do next? The antibody test only means they have been exposed to HCV.
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Hepatitis C Second Generation Antivirals (2015) Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Hepatitis C Second Generation Antivirals Through
Hepatitis C Second Generation Antivirals (2015) Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Hepatitis C Second Generation Antivirals Through
Appendices to Interim Report on the Baltimore Buprenorphine Initiative. Managed Care Organization Information Pages
 Appendices to Interim Report on the Baltimore Buprenorphine Initiative Appendix A Managed Care Organization Information Pages Appendix B Buprenorphine Online Physician Training Information Packet Appendix
Appendices to Interim Report on the Baltimore Buprenorphine Initiative Appendix A Managed Care Organization Information Pages Appendix B Buprenorphine Online Physician Training Information Packet Appendix
Viral Hepatitis Case Report
 Page 1 of 9 Viral Hepatitis Case Report Perinatal Hepatitis B Virus Infection Michigan Department of Community Health Communicable Disease Division Investigation Information Investigation ID Onset Date
Page 1 of 9 Viral Hepatitis Case Report Perinatal Hepatitis B Virus Infection Michigan Department of Community Health Communicable Disease Division Investigation Information Investigation ID Onset Date
Kanawha Valley Fellowship Home
 Kanawha Valley Fellowship Home Client Assessment Form Date: Time: Assessment Taken Caller s Name: Agency (if applicable) Address: County: Relationship to Patient: Phone # Client s Name: Age: D.O.B.: Current
Kanawha Valley Fellowship Home Client Assessment Form Date: Time: Assessment Taken Caller s Name: Agency (if applicable) Address: County: Relationship to Patient: Phone # Client s Name: Age: D.O.B.: Current
MAT Disclosures & Consents 1 of 6. Authorization & Disclosure
 MAT Disclosures & Consents 1 of 6 Authorization & Disclosure ***YOUR INSURANCE MAY NOT PAY FOR ROUTINE SCREENING*** *** APPROPRIATE SCREENING DIAGNOSES MUST BE PROVIDED WHEN INDICATED*** Urine Drug Test
MAT Disclosures & Consents 1 of 6 Authorization & Disclosure ***YOUR INSURANCE MAY NOT PAY FOR ROUTINE SCREENING*** *** APPROPRIATE SCREENING DIAGNOSES MUST BE PROVIDED WHEN INDICATED*** Urine Drug Test
Transmission of HCV in the United States (CDC estimate)
 Transmission of HCV in the United States (CDC estimate) Past and Future US Incidence and Prevalence of HCV Infection Decline among IDUs Overall incidence Overall prevalence Infected 20+ years Armstrong
Transmission of HCV in the United States (CDC estimate) Past and Future US Incidence and Prevalence of HCV Infection Decline among IDUs Overall incidence Overall prevalence Infected 20+ years Armstrong
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance
 Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance Introduction Indication/Licensing information: Naltrexone is licensed for use as an additional therapy, within
Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance Introduction Indication/Licensing information: Naltrexone is licensed for use as an additional therapy, within
DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource
 E-Resource March, 2015 DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource Depression affects approximately 20% of the general population
E-Resource March, 2015 DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource Depression affects approximately 20% of the general population
Application Form. Executive MBA
 Department of Business Administration The International School Application Form Executive MBA Instructions All of the following materials must be submitted before your application will be processed: Application
Department of Business Administration The International School Application Form Executive MBA Instructions All of the following materials must be submitted before your application will be processed: Application
Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary
 Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary This program applies to Health Insurance Marketplace, FlexRx
Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary This program applies to Health Insurance Marketplace, FlexRx
SPOUSE / PARTNER ONE TO COMPLETE THIS SECTION SEPARATELY. Name: (Last) (First) (Middle Initial)
 Katherine E. Walker, PhD, LPC, NCC, BCIA-C Licensed Professional Counselor 8300 Health Park, Suite 201 Raleigh, NC 27615 Mobile: 919-760-3068 Fax: 919-676-9946 Email: [email protected] Couples
Katherine E. Walker, PhD, LPC, NCC, BCIA-C Licensed Professional Counselor 8300 Health Park, Suite 201 Raleigh, NC 27615 Mobile: 919-760-3068 Fax: 919-676-9946 Email: [email protected] Couples
Rekindling House Dual Diagnosis Specialist
 Rekindling House Dual Diagnosis Specialist Tel: 01582 456 556 APPLICATION FOR TREATMENT Application Form / Comprehensive Assessment Form Please provide as much detail as you can it will help us process
Rekindling House Dual Diagnosis Specialist Tel: 01582 456 556 APPLICATION FOR TREATMENT Application Form / Comprehensive Assessment Form Please provide as much detail as you can it will help us process
TREATMENT PLAN UNDER MHL SECTION 9.60 Article 28 Facilities
 TREATMENT PLAN UNDER MHL SECTION 9.60 Article 28 Facilities Subject of the Petition Name: Subject Also Known As : Subject Home/Residence Address D/O/B: Physician s Name:, M.D.. Board eligible: [ ] Board
TREATMENT PLAN UNDER MHL SECTION 9.60 Article 28 Facilities Subject of the Petition Name: Subject Also Known As : Subject Home/Residence Address D/O/B: Physician s Name:, M.D.. Board eligible: [ ] Board
Management of hepatitis C: pre- and post-liver transplantation. Piyawat Komolmit Bangkok
 Management of hepatitis C: pre- and post-liver transplantation Piyawat Komolmit Bangkok Liver transplantation and CHC Cirrhosis secondary to HCV is the leading cause of liver transplantation in the US
Management of hepatitis C: pre- and post-liver transplantation Piyawat Komolmit Bangkok Liver transplantation and CHC Cirrhosis secondary to HCV is the leading cause of liver transplantation in the US
New Port Centre. 5. DHQ Drug History Questionnaire 6. Adverse Consequences Questionnaire 7. Tracking Sheet With Scores of Other Provincial Assessments
 New Port Centre Page 1 of 2 NIAGARA HEALTH SYSTEM PORT COLBORNE GENERAL SITE 260 Sugarloaf Street, Port Colborne ON, L3K 2N7 Phone (905) 378-4647 Ext 32500 Fax: (905) 834-3002 E-mail: [email protected]
New Port Centre Page 1 of 2 NIAGARA HEALTH SYSTEM PORT COLBORNE GENERAL SITE 260 Sugarloaf Street, Port Colborne ON, L3K 2N7 Phone (905) 378-4647 Ext 32500 Fax: (905) 834-3002 E-mail: [email protected]
Psychiatric Residential Treatment Facility Referral
 Psychiatric Residential Treatment Facility Referral Date of referral: Psychiatric Residential Treatment Facility (PRTF) Referral Information Referral contact: Phone number: Referring facility/agency: Fax
Psychiatric Residential Treatment Facility Referral Date of referral: Psychiatric Residential Treatment Facility (PRTF) Referral Information Referral contact: Phone number: Referring facility/agency: Fax
AmeriHealth Caritas District of Columbia Psychiatric Residential Treatment Facility Referral
 AmeriHealth Caritas District of Columbia Psychiatric Residential Treatment Facility Referral Date of referral: Psychiatric Residential Treatment Facility (PRTF) Referral Information Referral contact: Phone
AmeriHealth Caritas District of Columbia Psychiatric Residential Treatment Facility Referral Date of referral: Psychiatric Residential Treatment Facility (PRTF) Referral Information Referral contact: Phone
When an occupational exposure occurs, the source patient should be evaluated for both hepatitis B and hepatitis C. (AII)
 XI. OCCUPATIONAL EXPOSURES TO HEPATITIS B AND C RECOMMENDATION: When an occupational exposure occurs, the source patient should be evaluated for both hepatitis B and hepatitis C. (AII) The risk of transmission
XI. OCCUPATIONAL EXPOSURES TO HEPATITIS B AND C RECOMMENDATION: When an occupational exposure occurs, the source patient should be evaluated for both hepatitis B and hepatitis C. (AII) The risk of transmission
Harvoni (Ledipasvir/Sofosbuvir) Treatment Agreement
 Harvoni (Ledipasvir/Sofosbuvir) Treatment Agreement Liver Disease & Hepatitis Program Providers: Brian McMahon, MD; Youssef Barbour, MD; Lisa Townshend-Bulson, FNP-C; Annette Hewitt, FNP-C; Prabhu Gounder,
Harvoni (Ledipasvir/Sofosbuvir) Treatment Agreement Liver Disease & Hepatitis Program Providers: Brian McMahon, MD; Youssef Barbour, MD; Lisa Townshend-Bulson, FNP-C; Annette Hewitt, FNP-C; Prabhu Gounder,
SUPPORT PATH PROGRAM INTAKE FORM PHONE: 1-855-769-7284 FAX: 1-855-298-8700
 SUPPORT PATH PROGRAM INTAKE FORM PHONE: 1-855-769-7284 FAX: 1-855-298-8700 1 REQUESTED SERVICE(S) (REQUIRED) CHECK ALL BOXES THAT APPLY Benefits Investigation Prior Authorization and Appeals Support Patient
SUPPORT PATH PROGRAM INTAKE FORM PHONE: 1-855-769-7284 FAX: 1-855-298-8700 1 REQUESTED SERVICE(S) (REQUIRED) CHECK ALL BOXES THAT APPLY Benefits Investigation Prior Authorization and Appeals Support Patient
SCIENTIFIC DISCUSSION
 London, 13 October 2005 Product name: PEGINTRON Procedure No. EMEA/H/C/280/II/54 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86
London, 13 October 2005 Product name: PEGINTRON Procedure No. EMEA/H/C/280/II/54 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86
Preconception Clinical Care for Women Medical Conditions
 Preconception Clinical Care for Women All women of reproductive age are candidates for preconception care; however, preconception care must be tailored to meet the needs of the individual. Given that preconception
Preconception Clinical Care for Women All women of reproductive age are candidates for preconception care; however, preconception care must be tailored to meet the needs of the individual. Given that preconception
Fax # s for CAMH programs and services
 INFORMATION AND INSTRUCTIONS STEP 1 BEFORE COMPLETING THE REFERRAL FORM CATS Program / General Psychiatry Memory Clinic, Geriatric Mental Health Program Go to www.camh.net for detailed information on each
INFORMATION AND INSTRUCTIONS STEP 1 BEFORE COMPLETING THE REFERRAL FORM CATS Program / General Psychiatry Memory Clinic, Geriatric Mental Health Program Go to www.camh.net for detailed information on each
GUIDELINES FOR THE SCREENING, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS C INFECTION POLICY BRIEF
 NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR THE SCREENING, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS C INFECTION POLICY BRIEF NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR
NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR THE SCREENING, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS C INFECTION POLICY BRIEF NEW RECOMMENDATIONS IN THE UPDATED WHO GUIDELINES FOR
Florida Medicaid: Mental Health and Substance Abuse Services
 Florida Medicaid: Mental Health and Substance Abuse Services Beth Kidder Assistant Deputy Secretary for Medicaid Operations Agency for Health Care Administration House Children, Families, and Seniors Subcommittee
Florida Medicaid: Mental Health and Substance Abuse Services Beth Kidder Assistant Deputy Secretary for Medicaid Operations Agency for Health Care Administration House Children, Families, and Seniors Subcommittee
EXAMINING HEPATITIS C VIRUS TREATMENT ACCESS:
 EXAMINING HEPATITIS C VIRUS TREATMENT ACCESS: PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL With special thanks to our Harvard Law School students, James Fullmer, Kellen
EXAMINING HEPATITIS C VIRUS TREATMENT ACCESS: PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL With special thanks to our Harvard Law School students, James Fullmer, Kellen
Hepatitis C Infections in Oregon September 2014
 Public Health Division Hepatitis C Infections in Oregon September 214 Chronic HCV in Oregon Since 25, when positive laboratory results for HCV infection became reportable in Oregon, 47,252 persons with
Public Health Division Hepatitis C Infections in Oregon September 214 Chronic HCV in Oregon Since 25, when positive laboratory results for HCV infection became reportable in Oregon, 47,252 persons with
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
HCV/HIVCo-infection A case study by. Dominic Côté, Nurse Clinician B.Sc Chronic Viral Illness Services McGill University Health Centre
 HCV/HIVCo-infection A case study by Dominic Côté, Nurse Clinician B.Sc Chronic Viral Illness Services McGill University Health Centre Objectives By sharing a case study of a patient co-infected with HIV/HCV
HCV/HIVCo-infection A case study by Dominic Côté, Nurse Clinician B.Sc Chronic Viral Illness Services McGill University Health Centre Objectives By sharing a case study of a patient co-infected with HIV/HCV
Orthopaedic Institute of Ohio Demographic Information Date:
 Orthopaedic Institute of Ohio Demographic Information Date: Patient Name Home Phone Cell Phone Employer Phone Mailing Address (include PO Box and Apt. #) Family Doctor Name and Phone Number City, State,
Orthopaedic Institute of Ohio Demographic Information Date: Patient Name Home Phone Cell Phone Employer Phone Mailing Address (include PO Box and Apt. #) Family Doctor Name and Phone Number City, State,
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
1960 Ogden St. Suite 120, Denver, CO 80218, 303-318-3840
 Dear Valued Patient, 1960 Ogden St. Suite 120, Denver, CO 80218, 303-318-3840 Thank you for choosing Denver Medical Associates as your healthcare provider. We strive to provide you with the best possible
Dear Valued Patient, 1960 Ogden St. Suite 120, Denver, CO 80218, 303-318-3840 Thank you for choosing Denver Medical Associates as your healthcare provider. We strive to provide you with the best possible
HCV in 2020: Any cases left? Rafael Esteban Hospital General Universitario Valle Hebron Barcelona. Spain
 HCV in 2020: Any cases left? Rafael Esteban Hospital General Universitario Valle Hebron Barcelona. Spain Yes, still too many Measures to eradicate an Infectious Disease Prevention: Vaccination Screening
HCV in 2020: Any cases left? Rafael Esteban Hospital General Universitario Valle Hebron Barcelona. Spain Yes, still too many Measures to eradicate an Infectious Disease Prevention: Vaccination Screening
HCV Treatment Failure
 بسم االله الرحمن الرحيم HCV Treatment Failure Gamal Esmat PROF.OF HEPATOLOGY&TROPICAL MEDICINE CAIRO UNIVERSITY Director of Viral Hepatitis Treatment Centers (VHTCs( VHTCs) MOH-EGYPT www.gamalesmat.com
بسم االله الرحمن الرحيم HCV Treatment Failure Gamal Esmat PROF.OF HEPATOLOGY&TROPICAL MEDICINE CAIRO UNIVERSITY Director of Viral Hepatitis Treatment Centers (VHTCs( VHTCs) MOH-EGYPT www.gamalesmat.com
Disclosures. Advisory Boards: Speaker Bureaus: Janssen, Gilead, AbbVie. Janssen, Gilead, AbbVie, Bristol-Myers Squibb, Entera Health, Salix
 Disclosures Advisory Boards: Janssen, Gilead, AbbVie Speaker Bureaus: Janssen, Gilead, AbbVie, Bristol-Myers Squibb, Entera Health, Salix Outline Assess patient readiness for treatment Examine various
Disclosures Advisory Boards: Janssen, Gilead, AbbVie Speaker Bureaus: Janssen, Gilead, AbbVie, Bristol-Myers Squibb, Entera Health, Salix Outline Assess patient readiness for treatment Examine various
Hepatitis C. Laboratory Tests and Hepatitis C
 Hepatitis C Laboratory Tests and Hepatitis C If you have hepatitis C, your doctor will use laboratory tests to check your health. This handout will help you understand what the major tests are and what
Hepatitis C Laboratory Tests and Hepatitis C If you have hepatitis C, your doctor will use laboratory tests to check your health. This handout will help you understand what the major tests are and what
NHS GRAMPIAN. Community Pharmacy Supply Of Hepatitis C Treatments. Pharmaceutical Care Information Pack
 NHS GRAMPIAN Community Pharmacy Supply Of Hepatitis C Treatments Pharmaceutical Care Information Pack NHS Grampian Hepatology Team April 2015 NHS GRAMPIAN Community Pharmacy Supply Of Hepatitis C Treatments
NHS GRAMPIAN Community Pharmacy Supply Of Hepatitis C Treatments Pharmaceutical Care Information Pack NHS Grampian Hepatology Team April 2015 NHS GRAMPIAN Community Pharmacy Supply Of Hepatitis C Treatments
Continued Dependent Life Insurance for a Disabled Child Instructions
 Continued Dependent Life Insurance Instructions Your application for consists of four forms. Every space should be filled in to avoid delay in processing your application. If a section does not apply,
Continued Dependent Life Insurance Instructions Your application for consists of four forms. Every space should be filled in to avoid delay in processing your application. If a section does not apply,
Treating Chronic Hepatitis C. A Review of the Research for Adults
 Treating Chronic Hepatitis C A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have chronic hepatitis C.
Treating Chronic Hepatitis C A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have chronic hepatitis C.
Understanding the Reimbursement Environment in Hepatitis C
 Understanding the Reimbursement Environment in Hepatitis C Camilla S. Graham, MD, MPH, Division of Infectious Diseases, Beth Israel Deaconess Medical Center Robert Greenwald, JD, Clinical Professor of
Understanding the Reimbursement Environment in Hepatitis C Camilla S. Graham, MD, MPH, Division of Infectious Diseases, Beth Israel Deaconess Medical Center Robert Greenwald, JD, Clinical Professor of
Revised April 1, 2015 Page 1 of 5
 Interview Date: Community Treatment Center 1215 Lake Drive Cocoa, Florida 32922 Phone: 321-632-5958 Fax: 321-632-2533 Do you have a substance abuse problem? Yes No Do you have a mental health diagnosis?
Interview Date: Community Treatment Center 1215 Lake Drive Cocoa, Florida 32922 Phone: 321-632-5958 Fax: 321-632-2533 Do you have a substance abuse problem? Yes No Do you have a mental health diagnosis?
North of Tyne Area Prescribing Committee
 North of Tyne Area Prescribing Committee ANTIPSYCHOTICS IN PSYCHOSIS, BIPOLAR DISORDER AND AUGMENTATION THERAPY IN TREATMENT RESISTANT DEPRESSION Information for Primary Care Updated November 2013 This
North of Tyne Area Prescribing Committee ANTIPSYCHOTICS IN PSYCHOSIS, BIPOLAR DISORDER AND AUGMENTATION THERAPY IN TREATMENT RESISTANT DEPRESSION Information for Primary Care Updated November 2013 This
Prior Authorization, Pharmacy and Health Case Management Information. Prior Authorization. Pharmacy Information. Health Case Management
 Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
Prior Authorization, Pharmacy and Health Case Management Information The purpose of this information sheet is to provide you with details on how Great-West Life will be assessing and managing your claim
HEALTH INFORMATION FORM FOR STUDY ABROAD PARTICIPANTS
 HEALTH INFORMATION FORM FOR STUDY ABROAD PARTICIPANTS Student: Last name: First Name: Middle Initial: Period of intended study abroad: Year(s): Fall Spring Academic Year Country Foreign Institution or
HEALTH INFORMATION FORM FOR STUDY ABROAD PARTICIPANTS Student: Last name: First Name: Middle Initial: Period of intended study abroad: Year(s): Fall Spring Academic Year Country Foreign Institution or
HEPATITIS C. The Facts. Get Tested. Get Cured! Health
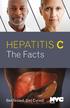 HEPATITIS C The Facts Get Tested. Get Cured! Health EVEN IF YOU FEEL HEALTHY, HEPATITIS C MAY BE DAMAGING YOUR LIVER. Your liver keeps you healthy in many ways, such as by removing toxins from your blood
HEPATITIS C The Facts Get Tested. Get Cured! Health EVEN IF YOU FEEL HEALTHY, HEPATITIS C MAY BE DAMAGING YOUR LIVER. Your liver keeps you healthy in many ways, such as by removing toxins from your blood
Considerations in Medication Assisted Treatment of Opiate Dependence. Stephen A. Wyatt, D.O. Dept. of Psychiatry Middlesex Hospital Middletown, CT
 Considerations in Medication Assisted Treatment of Opiate Dependence Stephen A. Wyatt, D.O. Dept. of Psychiatry Middlesex Hospital Middletown, CT Disclosures Speaker Panels- None Grant recipient - SAMHSA
Considerations in Medication Assisted Treatment of Opiate Dependence Stephen A. Wyatt, D.O. Dept. of Psychiatry Middlesex Hospital Middletown, CT Disclosures Speaker Panels- None Grant recipient - SAMHSA
Update on hepatitis C: treatment and care and future directions
 Update on hepatitis C: treatment and care and future directions Professor Greg Dore Viral Hepatitis Clinical Research Program, National Centre in HIV Epidemiology and Clinical Research, University of New
Update on hepatitis C: treatment and care and future directions Professor Greg Dore Viral Hepatitis Clinical Research Program, National Centre in HIV Epidemiology and Clinical Research, University of New
Hepatitis C Glossary of Terms
 Acute Hepatitis C A short-term illness that usually occurs within the first six months after someone is exposed to the hepatitis C virus (HCV). 1 Antibodies Proteins produced as part of the body s immune
Acute Hepatitis C A short-term illness that usually occurs within the first six months after someone is exposed to the hepatitis C virus (HCV). 1 Antibodies Proteins produced as part of the body s immune
PATIENT TREATMENT AGREEMENT
 PATIENT TREATMENT AGREEMENT Patient Name: : As a participant in buprenorphine treatment for opioid misuse and dependence, I freely and voluntarily agree to accept this treatment agreement as follows: I
PATIENT TREATMENT AGREEMENT Patient Name: : As a participant in buprenorphine treatment for opioid misuse and dependence, I freely and voluntarily agree to accept this treatment agreement as follows: I
A Proposal for Managing the Harvoni Wave June 22, 2015
 A Proposal for Managing the Harvoni Wave June 22, 2015 Clinical Background Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV) that damages the liver over time. The disease affects
A Proposal for Managing the Harvoni Wave June 22, 2015 Clinical Background Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV) that damages the liver over time. The disease affects
Appendix 3 Exposure Incident Report Form
 Appendix 3 Exposure Incident Report Form January, 2015 Page 1 of 6 Please see the following pages for the Exposure Incident Report Form. Guidelines for the Management of Exposure to Blood and Body Fluids
Appendix 3 Exposure Incident Report Form January, 2015 Page 1 of 6 Please see the following pages for the Exposure Incident Report Form. Guidelines for the Management of Exposure to Blood and Body Fluids
PARTNERS IN PEDIATRIC CARE. Intake and History for Mental Health Referral
 PARTNERS IN PEDIATRIC CARE Intake and History for Mental Health Referral This form is designed to give you an opportunity to provide us with background information that will help us help you. Please read
PARTNERS IN PEDIATRIC CARE Intake and History for Mental Health Referral This form is designed to give you an opportunity to provide us with background information that will help us help you. Please read
Wake Forest Mind and Health, PLLC 501 North Main Street Wake Forest, NC 27587
 Wake Forest Mind and Health, PLLC 501 rth Main Street Wake Forest, NC 27587 Katherine E. Walker, PhD, LPC, NCC, BCIA-C Jennifer Endries, MEd, LPC Licensed Professional Counselor Licensed Professional Counselor
Wake Forest Mind and Health, PLLC 501 rth Main Street Wake Forest, NC 27587 Katherine E. Walker, PhD, LPC, NCC, BCIA-C Jennifer Endries, MEd, LPC Licensed Professional Counselor Licensed Professional Counselor
ACCIDENTAL INJURY CLAIM FORM
 Washington National Insurance Company Home Office: 11825 N. Pennsylvania St., Carmel, IN 46032 Questions about your claim submission? CALL (800) 541-2254. ACCIDENTAL INJURY CLAIM FORM PLEASE SUBMIT THESE
Washington National Insurance Company Home Office: 11825 N. Pennsylvania St., Carmel, IN 46032 Questions about your claim submission? CALL (800) 541-2254. ACCIDENTAL INJURY CLAIM FORM PLEASE SUBMIT THESE
NP/PA Clinical Hepatology Fellowship Summary of Year-Long Curriculum
 OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
Scottish Medicines Consortium
 Scottish Medicines Consortium peginterferon alfa-2a, 135 microgram/ml and 180 microgram/ml pre-filled injections of solution for subcutaneous injection (Pegasys ) No. (561/09) Roche Products Limited 10
Scottish Medicines Consortium peginterferon alfa-2a, 135 microgram/ml and 180 microgram/ml pre-filled injections of solution for subcutaneous injection (Pegasys ) No. (561/09) Roche Products Limited 10
