Danish Multiple Sclerosis Center Annual Report 2009
|
|
|
- Rosaline Mills
- 8 years ago
- Views:
Transcription
1 Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark Danish Multiple Sclerosis Center Annual Report 2009 DANISH Multiple sclerosis CENTER annual report
2 DMSC staff seminar DANISH Multiple sclerosis CENTER annual report 2009
3 Table of contents Annual Report A short review of 2009 in the Danish Multiple Sclerosis Center (DMSC) DMSC missions and aims About the DMSC Organization Research activities 2009 Clinical research Neuroimaging Neurogenetics Neuroimmunology Neuropathology Routine analyses in Neuroimmunology Laboratory Scientific publications Prizes Honorary offices Scientific collaboration Acknowledgements DANISH Multiple sclerosis CENTER annual report
4 Publisher: Danish Multiple Sclerosis Center Authors: Per Soelberg Sørensen Morten Blinkenberg Finn Sellebjerg Annette Oturai Helle Bach Søndergaard Photos: Peter Fischer Concept, design, graphic production and print: Stibo Zone Njalsgade 19 D DK-2300 København S Tlf.: DANISH Multiple sclerosis CENTER annual report 2009
5 Professor Per Soelberg Sørensen, MD, DMSc Director of the Danish Multiple Sclerosis Center (DMSC) A short review of 2009 in the Danish Multiple Sclerosis Center In 2009 the number of patients in the Danish Multiple Sclerosis Center (DMSC) increased to To meet the challenge of providing care of an increasing number of MS patients referred for diagnosis and therapy, in particular for treatment with tysabri infusions, we employed an additional staff neurologist, MS nurse and secretary to the MS Clinic. By the end of 2009 approximately 300 patients were receiving monthly infusions of Tysabri and it has been a challenge to organize the treatments due to the limited space in the MS Clinic facilities. In 2009 we completed two multi-center, international trials organized and directed from DMSC. These trials showed that it is possible to achieve significant improvement in the efficacy of interferon-beta by giving monthly pulses of methylprednisolone as add-on therapy. We have expanded our international research collaboration in clinical research, neurogenetics, neuroimmunology and MS pathology. We have published a series of scientific papers on response of the immune system to various treatments. These papers have extended our understanding of the deleterious effects of antibodies to interferon-beta and a paper has reported the first series of patients treated with Tysabri outside a clinical trial setting. We have published papers together with Austrian collaborators showing that compartmentalized central nervous system inflammation is closely related to neurodegeneration giving us the hope that therapies able to eliminate the inflammation in the central nervous system will stop the degenerative processes in the brain and spinal cord. I hope that you will enjoy reading the annual report of DMSC. Per Soelberg Sørensen DANISH Multiple sclerosis CENTER annual report
6 DMSC missions and aims The mission of Rigs hospitalet is to be the leading hospital in Denmark for patients in need of highly specialized treatment The missions of the Danish Multiple Sclerosis Center (DMSC) are: The aims of the DMSC are: to be the leading multiple sclerosis (MS) center in Denmark to be at the forefront of highly specialized management of MS to carry out research and development in MS at an advanced international level to collaborate scientifically and exchange knowledge in MS research to educate staff to a highly specialized level in their relevant fields to contribute with professional advice on MS to the healthcare community to meet people with MS at their terms with openness and respect to provide the optimal interdisciplinary patient care to all MS patients in the region and to patients from other regions in need of highly specialized therapy to carryout high quality research in MS with focus on clinical research, new therapies, MS genetics, neuroimmunology and MS pathology to teach undergraduate students and PhDstudents and stimulate their interest in MS research to educate post docs, MS physicians, nurses, secretaries and other professionals to a high level of knowledge of MS in their relevant expert fields to lead the national research in Denmark in partnership with other Danish researchers and to establish a broad international collaboration with MS research groups in Europe and from overseas. 6 DANISH Multiple sclerosis CENTER annual report 2009
7 DANISH Multiple sclerosis CENTER annual report
8 About The Danish Multiple Sclerosis Center 8 DANISH Multiple sclerosis CENTER annual report 2009
9 DANISH Multiple sclerosis CENTER annual report
10 The Danish Multiple Sclerosis Center About The Danish Multiple Sclerosis Center The Danish Multiple Sclerosis Center (DMSC) is composed of the MS Clinic and the MS Research Unit. The MS Clinic is located on the 8th floor of the main complex of Rigshospitalet. The MS Clinic comprises offices of the professor and consultants, the reception desk and secretary office, the nurses offices, the consultation rooms as well as the facilities for intravenous therapy with Tysabri and rooms for invasive procedures. The MS Clinic provides a multi-disciplinary care for almost 1800 MS patients of whom the majority receive diseasemodifying therapy dispensed by the MS Clinic. Apart from disease modifying therapy patients are offered symptomatic therapy for spasticity, bladder and bowel disturbances, pain and cognitive symptoms. The medical staff comprises a professor, 4 consultants and 3 staff neurologists; there is one leading nurse and 6 MS specialist nurses, 3 secretaries, a neuropsychologist, a physiotherapist and a medical social counselor. Approximately half of the patients in the MS Clinic are from our local Copenhagen region and the other half are patients referred for highly specialized therapy from the neighbouring regions all over Zealand. The MS Clinic has been appointed by the National Board of Health to perform highly specialized therapy such as strong immunosuppression and Tysabri therapy. In addition patients are referred for experimental therapy with highly specific monoclonal antibodies. Further, the MS Clinic provides highly specialized treatment of spasticity with an intrathecal baclofen pump. In collaboration with pediatricians children and adolescents with MS from Eastern Denmark are also treated at the MS Clinic. It is the aim of the MS Clinic to provide high quality multi-disciplinary care for all our patients with openness and respect. The MS Research Unit is partly located in the proximity of the MS Clinic and partly in the Michaelsen Building 63. Clinical research has to be performed in close proximity to the MS Clinic and offices for 3 research nurses and 1 research secretary are embedded in the MS Clinic. The remaining part of the MS Research Unit with the Neuroimmunology Laboratory is located on the first floor in the Michaelsen building 63 and in the basement of building of 93. The facilities contain offices for the research staff and laboratories. The laboratories contain an 8-color flow cytometer, facilities for doing real-time polymerase chainreaction (PCR), a neurogenetics laboratory for DNA preparation and facilities for making routine laboratory tests. The focus of the research is clinical research including neuroimaging, neuroimmunology, neurogenetics and neuropathology of MS. ACtive patients in DMSC DANISH Multiple sclerosis CENTER annual report 2009
11 DANISH Multiple sclerosis CENTER annual report
12 Organization Director: Professor Per Soelberg Sørensen MS clinic MS Research Unit Neurologists Professor Per Soelberg Sørensen Consultant Morten Blinkenberg Consultant Finn Sellebjerg Consultant Annette Oturai Consultant Karen Schreiber Staff neurologist Ana Voldsgaard Staff neurologist Lars Pindborg Staff neurologist Melinda Magyari Staff neurologist Henrik Mathiesen PhD student Stephan Bramow PhD student Lars Börnsen PhD student Jeppe Romme Christensen PhD student Dan Hesse PhD student Rikke Ratzer Junior physician Morten Møller MS nurses Leading nurse Anne Hansen Dorte Stauning Rasmussen Anette Husted Pedersen Lene Almind Julie Yoon S. Moberg Louise Nathalie Christiansen Maj Daae Christensen Sidsel Nielsen Secretaries Annette Larsen Malene Møllesøe Maria Brændbjerg Helle Vilhhelmsen Neuropsychologist Agnete Jønsson Physiotherapist Lis Albrechtsen Medical social counselor Keld Nissen Clinical research Professor Per Soelberg Sørensen Consultant Karen Schreiber Staff neurologist Ana Voldsgaard Staff neurologist Melinda Magyari Neuropsycologist Agnete Jønsson Research nurses Vibeke Jespersen Joan Pietraszek Sidsel Nielsen Research secretary Annette Larsen Neuroimaging research Consultant Morten Blinkenberg Staff neurologist Henrik Mathiesen Genetic research Consultant Annette Oturai Senior research fellow Helle Bach Søndergaard Junior research fellow Morten Møller Neuroimmunology research Ass. professor Finn Sellebjerg Senior research fellow Helle Bach Søndergaard PhD students Dan Hesse Lars Börnsen Jeppe Romme Christensen Rikke Ratzer Junior research fellow Marianne Hansen Neuropathology research Professor Per Soelberg Sørensen Ph.d. student Stephan Bramow Neuroimmunology Laboratory Laborarory leader Poul Erik Hyldgaard Jensen Laboratory technicians Leading Laboratory technician Joy Mendel-Hartvig Marie Koefoed Anne Mette Hedegaard Nielsen Michael Jensen Vibeke Fuglholt 12 DANISH Multiple sclerosis CENTER annual report 2009
13 Professor Per Soelberg Sørensen Ass. professor Finn Sellebjerg Consultant Morten Blinkenberg Consultant Annette Oturai Consultant Karen Schreiber Staff neurologist Ana Voldsgaard Staff neurologist Henrik Mathiesen Staff neurologist Melinda Magyari Laboratory leader Poul Erik Hyldgaard Jensen Senior research fellow Helle Bach Søndergaard Leading laboratory technician Joy Mendel- Hartvig Leading nurse Anne Hansen DANISH Multiple sclerosis CENTER annual report
14 Research activities 2009 Clinical research Clinical research Neuroimaging Neurogenetics Neuroimmunology Neuropathology Routine analyses in Neuroimmunology Laboratory 14 DANISH Multiple sclerosis CENTER annual report 2009
15 DANISH Multiple sclerosis CENTER annual report
16 Research activities 2009 Clinical research Clinical research group: Per Soelberg Sørensen, Morten Blinkenberg, Finn Sellebjerg, Annette Oturai, Ana Voldsgaard, Karen Schreiber, Henrik Mathiesen, Melinda Magyari, Dan Hesse, Stephan Bramow, Lars Börnsen, Jeppe Romme Christensen, Agnete Jønsson, Vibeke Jespersen, Joan Pietraszek, Sidsel Walther Nielsen, Anne Hansen, Annette Larsen Therapeutic trials The results of two international multi-center clinical trials managed by DMSC using monthly pulse therapy of methylprednisolone tablets as add-on therapy to interferon-beta were presented at major MS congresses in The NORMIMS trial showed that in patients with breakthrough disease on interferon-beta 1a treatment add-on therapy with methylprednisolone tablets 5 days a month reduced the monthly relapse rate by 62% compared to add-on of placebo and reduced the accumulation of lesions on MRI. These results were corroborated in the MECOMBIN study of previously untreated patients showing that the combination of interferon-beta 1a and methylprednisolone tablets 3 days monthly reduced the relapse rate by 38% and showed beneficial effect in MRI outcomes compared to interferon-beta and placebo. Two other large combination trials are currently directed from DMSC. The SIMCOMBIN study is a Nordic multi-center study of Simvastatin as addon therapy to interferon-beta in de novo treated patients with relapsing-remitting MS. More than 300 patients have been recruited and the trial will be completed by the end of April The RECY- CLINE study is a European multi-center study exploring the effect of Minocycline as add-on therapy to interferon-beta in de novo treated patients. Recruitment of 320 patients has been completed and the results will be available in We are currently performing three single center studies: A study of erytropoietin (EPO) in patients with progressive MS; a small safety study of treatment with eggs of the pig whipworm (Trichuris suis); and we have as the first center initiated an exploratory trial of natalizumab (Tysabri) in patients with progressive MS. Neutralizing antibodies (NAb) against biological therapies In a study using affymetrix gene chips we showed that interferon-beta treated NAb-positive patients, in whom MxA could not be induced by interferonbeta, had no residual bioactivity present and were virtually untreated. DMSC collaborates with an a EU supported European study of neutralizing antibodies against interferon-beta (NABINMS study). We are currently analyzing data from population-based cohorts of patients from Denmark, Spain, The Netherlands and The Czech Republic. Further, the NABINMS study has introduced a new assay for neutralizing antibodies based on a Luciferase reporter gene under control of interferon-beta, and this assay has been adapted for routine measurements of neutralizing antibodies in DMSC. The MS Clinic is located on the 8th floor of the main complex of Rigshospitalet. 16 DANISH Multiple sclerosis CENTER annual report 2009
17 DANISH Multiple sclerosis CENTER annual report
18 Research activities 2009 Neuroimaging Neuroimaging research group: Morten Blinkenberg, Henrik Mathiesen, Per Soelberg Sørensen PET During the last decades, functional imaging have improved our understanding of the neurodegenerative processes in MS. Former positron emission tomography (PET) studies, carried out in the Danish Multiple Sclerosis Center, have shown that cerebral activation in MS patients, is severely reduced as a consequence of disease progression. Our current PET studies focuses on the early relapsing remitting phase of the disease and the corresponding changes in cerebral activation. Preliminary results show, that reductions in PET is associated with neuronal dysfunction or loss, measured by magnetic resonance spectroscopy (MRS). In this way there seem to be a close relationship between these two measures of cerebral neuronal integrity in MS. MRI In collaboration with Danish Research Centre for Magnetic Resonance (DRCMR), Hvidovre Hospital we have previously shown, that measurements of MRS correlate with cognitive dysfunction in MS. This cohort has now been reevaluated and analyzed for longitudinal changes in MRS. Preliminary data show, that MRS decrease in the early stage of MS, predicts disease progression after 5 years. MRS may therefore be used as a clinical tool, to determine disease course in MS. Another study focuses on the pathophysiological mechanisms underlying changes in cerebral activation in MS patients. Resting-state fmri is a new approach to assess functional brain connectivity by measuring the synchronous fluctuation in the blood-oxygen-level dependent signal between remote brain regions at rest. DRCMR uses this method to study, whether changes in functional connectivity of the motor network, reflect disease severity in patients with MS. Brain functional connectivity is investigated in a crosssectional study of 42 patients and furthermore in a longitudinal study of 12 patients evaluating the temporal dynamics during a relapse and clinical remission. Patient recruitment has been completed during year 2009 and elaborated statistical analysis is ongoing. 18 DANISH Multiple sclerosis CENTER annual report 2009
19 Neurogenetics Neurogenetics research group: Annette Bang Oturai, Helle Bach Søndergaard, Finn Sellebjerg, Julie Maria Hejgaard The ultimate cause of multiple sclerosis (MS) is still unknown. The probable cause is thought to be a combination of hereditary factors, an environmental trigger and a defect in the immune system. From family studies we have known for many years that genes plays a role. Identifying the involved genes is a complex challenge, because no single Mendelian locus causes MS. Through decades scientists have worked to find gene variations tied to MS. Genome linkage screens have failed finding major genes except genes of the HLA-complex, where linkage to DR2 (HLADRB1*15) is strongest in Northern Europeans. The HLA-DR2 association to MS was already identified back in A break-through took place in the summer 2007, when three new studies showed that the IL7 receptor and IL2receptor genes were important risk genes of MS. Both genes are important for the T cell regulation, and thus the immune system. Since then, several new genes have been confirmed. Presently, the Wellcome Trust Case Control Consortium is investigating the largest set of MS cases and controls (11.000/11.000) by a SNPs chips. Results are expected later this year. We are taking part in this large international genetic collaboration through our membership of the IMSGC (International Multiple Sclerosis Genetic Consortium). For more than 15 years the MS Genetic Group at Rigshospitalet have collected DNA, and today we have DNA from more than 1800 Danish MS patients and 1200 controls, all kept in the Danish Multiple Sclerosis Biobank located at our department at Rigshospitalet. In order to increase the sample size for genetic testing, we have participated in the Nordic MS Genetic Network since 1994, and today the Nordic material consists of 5000 MS cases and 5000 controls. Local research is focused on the candidate gene approaches and the genetic influence on the differences in treatment response. DANISH Multiple sclerosis CENTER annual report
20 Research activities 2009 Neuroimmunology Neuroimmunology research group: Finn Sellebjerg, Helle Bach Søndergaard, Poul Erik Hyldgaard Jensen, Dan Hesse, Jeppe Romme Christensen, Lars Börnsen, Rikke Ratzer, Per Soelberg Sørensen Immunological studies The interplay between the different types of immune cells in relapsing-remitting MS is only incompletely understood. Furthermore, the role of immune cells in the pathogenesis of the slowly developing deterioration in chronic progressive MS is a matter of debate. Some researchers consider immune activation to be of crucial importance in the pathogenesis of progressive MS, others consider progressive MS to result from slowly developing, neurodegenerative processes that are initiated by previous, inflammatory insults rather than by active inflammation. We are using a combination of immunological studies, addressing the reactivity of subsets of T cells to autoantigens expressed in the brain and spinal cord, studies addressing the role of other leukocytes in the activation of T cells, and studies of the effect of immunomodulatory treatment to further our understanding of the role of immune activation in different disease stages in MS. Initial studies using these techniques, conducted in collaboration with the Institute for Inflammation Research at the Copenhagen University Hospital Rigshospitalet, have provided evidence that active MS is associated with the activation of a recently discovered subtype of T cells termed Th17 cells. We are now studying the nature of these cells in more detail. In other studies we use flow cytometry, which is a method that allows the analysis of the phenotype of individual, circulating lymphocytes by measuring the binding of fluorochrome-coupled, monoclonal antibodies to distinct molecules. This technique is well suited for studying the activation status of subsets of lymphocytes. By combining the analysis of different molecules expressed on the cell surface, these cells can be divided into a large amount of functionally relevant subtypes, which can be quantitated both in relative and absolute terms. We have previously reported that flow cytometry can detect changes in the activation of circulating T cells and monocytes that differ in relapsing-remitting and secondary progressive MS. We are now exploring the extent to which such changes can be observed in other lymphocyte subsets, and whether they are associated with changes in the expression of cytokines and intrathecal immune activation that may reflect disease activity in progressive MS. Molecular biology The functional activation of immune cells requires complex interactions between different immune cells and coordinated, tightly regulated expression of numerous different molecules within single cells. DNA arrays provide a platform for unbiased studies of gene expression in different disease states and for studying the effect of treatment on gene expression. Using Affymetrix GeneChips, we have found that even in clinical remission, circulating immune cells from MS patients show profound changes in gene expression compared to healthy control subjects. Furthermore, we have identified a gene expression pattern that appears to reflect the activity of endogenous interferon-beta, and is associated with the expression of factors that control disease activity in MS. Gene expression is controlled by the activity of transcription factors that are either constitutively expressed in active forms, expressed in forms that require activation by other signals, or are inducible on cellular activation. The transcription 20 DANISH Multiple sclerosis CENTER annual report 2009
21 factors direct the expression of messenger RNA (mrna) from the genes encoded by the DNA sequence, and after export from the nucleus the mrna provides a template for protein synthesis on ribosomes in the cytosol. Within the last decade it has become apparent that endogenous, small RNA molecules termed microrna (mirna) have a key role in regulating the translation of protein from mrna in the cytosol. MiRNA can either induce the degradation of mrna, or can inhibit the translation of mrna into protein by binding to the 3 untranslated region of mrna molecules. Recent studies have identified mirna molecules that are differentially expressed in MS patients and healthy controls, and it has been suggested that pathogenic immune activation in MS may, indeed, reflect changes in mirna expression. We have studied mirna expression in MS, and have found that even patients with MS in clinical remission show clear changes in the expression of mirna compared to healthy control subjects. Furthermore, we have identified mirna molecules that are specifically regulated by treatment with interferon-beta. As mirna have profound effects on the translation of protein within the cell, studies of mirna molecules that are differentially expressed in MS, may provide the key to understanding the regulatory mechanisms involved in MS pathogenesis. Indeed, as mirna can now be therapeutically introduced into specific human cells, treatment with mirna or with molecules blocking the effect of individual molecules may by a feasible avenue for the future treatment of MS. In ongoing studies, we are analysing the functional relevance of the changes in mirna expression observed in our previous studies. Biomarker studies The term biomarker is used for, e.g., a gene product or protein, that can be measured in blood or other body fluids, and reflects a pathogenetically or therapeutically relevant in vivo process. Immunological and molecular biology studies provide the platform for the development of biomarkers, but the validation of these also requires access to collections of well characterized samples from MS patients with well characterized disease subtypes, who are followed prospectively. Such biomarker studies are important both for improving our understanding of the pathogenesis of MS and for understanding the therapeutic effects of immunomodulatory and immunosuppressive treatment strategies. Our group has been active in studying the immunological effects of treatment with interferon-beta. These studies have resulted in the identification of novel markers that may reflect the therapeutic effect of treatment better than the currently used biomarkers. Furthermore, we have used systematic DNA array biomarkers studies to show that the development of neutralizing anti-interferon-beta antibodies results in complete abolishment of the biologic effects of treatment. These studies have also resulted in the identification of a gene expression pattern that appears to be associated with disease protection, and may identify patients that are more likely to remain in clinical remission on treatment with interferon-beta. Cerebrospinal fluid is well suited for studying biomarkers that reflect pathogenic processes within the intrathecal compartment. Although cerebrospinal fluid is more difficult to obtain on a routine basis than blood samples, studies of such biomarkers have yielded important information about the dynamics of immune cell infiltration, immune regulation and tissue damage in MS. Most recently, we have studied changes in cerebrospinal fluid in patients treated with natalizumab (Tysabri), and have identified an apparently central role for the chemotactic factor (chemokine) CXCL13 in controlling the recruitment of immune cells to the brain and spinal cord in MS. The development of biomarkers that may serve as surrogate outcomes in clinical trials is an important aim of the ongoing biomarker research at our center. We assess changes in cerebrospinal fluid biomarkers in patients with progressive MS that participate in ongoing treatment trials, and analyse the relationship between the different types of biomarkers and clinical and magnetic resonance imaging measures of disease activity and disease severity. These studies provide the platform for the design of future phase 2 studies of new MS treatments that may be conducted more rapidly, requiring a lower number of patients than the current protocols for phase 2 studies in MS. DANISH Multiple sclerosis CENTER annual report
22 Research activities 2009 Neuropathology Neuropathology research group: Stephan Bramow, Per Soelberg Sørensen (Henning Laursen, Laboratory of Neuropathology) In close collaboration with Dr. Henning Laursen, Laboratory of Neuropathology, Rigshospitalet and Professor Hans Lassmann, Director of Institute for Brain Research in Vienna, we have studied the pathological correlates in brain and spinal cord of patients with long-standing MS disease. We have been able to show that inflammation and neurodegeneration are closely associated. We identified a group of patients without any active or slowly expanding demyelination in the brain, and these patients did not show any signs of acute axonal injury apart from confounding pathology unrelated to MS such as Alzheimer disease. Planimetric analysis of brain and spinal cords from patients with clinically well characterized primary progressive or secondary progressive MS showed that at total demyelination was more profound in brains of secondary progressive patients compared to primary progressive patients, whereas demyelination in the spinal cord was similar. Active demyelination correlated with shorter survival in patients with secondary progressive MS. We also demonstrated that remyelinated areas had increased vulnerability and recurrent demyelination in the remyelinated areas were more frequent than appearance of new demyelinating plaques. This could explain why patients often develop recurrence of symptoms in new relapses. We also showed that diffuse inflammation in the white-matter outside plaques correlated with the load of active demyelination elsewhere in the brain. The correlation between diffuse new inflammation and active focal demyelination emphasizes the necessity of an action on both sides of the bloodbrain-barrier for new treatments of progressive MS. 22 DANISH Multiple sclerosis CENTER annual report 2009
23 Routine analysis in neuroimmunology laboratory Neuroimmunology Laboratory research group: Poul Erik H. Jensen, laboratory technicians: Joy Mendel-Hartvig, Marie Koefoed, Michael Kolbjørn Jensen, Vibeke Fuglholt Diagnostic evaluation The presence in CSF of oligoclonal IgG-bands is of interest in the diagnosis of Multiple Sclerosis (MS). In 2009 we have analyzed 624 patient samples, using isoelectric focusing of CSF and corresponding plasma samples for the characterization of IgG bands. In the autoimmune disease Myasthenia gravis (MG), autoantibodies against the acetylcholine receptor (AChR) may cause a diminished binding of ACh on muscular surfaces and thereby a reduced impulse transmission to the postsynaptic membrane of the neuromuscular endplate occurs. For diagnostic and therapeutic purposes, we measure the concentrations of these autoantibodies from patient serum samples, using a radio-immunoassay kit, and in year 2009 we analyzed 1233 patient samples. Measurement of neutralizing antibodies Subgroups of MS patients, treated with interferonbeta or Tysabri, generate neutralizing antibodies, which diminish the therapeutic effects. Interferonbeta molecules bind to leucocytes and a specific up-regulation of MxA mrna in the cells occur. Neutralizing antibodies may abolish this effect, and therefore we measure the neutralization of interferon-beta by antibodies in new cell-culture assay based on luciferase-induced expression, and further by the MxA mrna-expression as a biological response to treatment with interferon-beta. In 2009 we have analyzed 244 patient samples for neutralizing antibodies, and 131 patient samples for MxA mrna expression. Furthermore, we have screened 36 patient samples for interferon-beta binding antibodies using an ELISA-assay. The action of Tysabri differs from interferonbeta, since it blocks mononuclear cell binding to endothelial cells. In this way Tysabri inhibits mononuclear cells from entering the central nervous system. The generation of neutralizing antibodies to Tysabri in MS patients block the biological effects of Tysabri. In 2009 we analyzed 634 blood samples for the presence of antibodies to Tysabri by ELISA. DANISH Multiple sclerosis CENTER annual report
24 Scientific publications, prizes, collaboration, acknowledgements Scientific publications Prizes Honorary offices Scientific collaboration Acknowledgements 24 DANISH Multiple sclerosis CENTER annual report 2009
25 DANISH Multiple sclerosis CENTER annual report
26 Scientific publications Publications 2008 Peer reviewed original papers Krakauer M, Sørensen PS, Khademi M, Olsson T, Sellebjerg F. Increased IL-10 mrna and IL-23 mrna expression in multiple sclerosis - interferon-beta treatment increases IL-10 mrna expression while reducing IL-23 mrna expression. Mult Scler 2008;14: Sellebjerg F, Datta P, Larsen J, Rieneck K, Alsing I, Oturai A, Svejgaard A, Sørensen PS, Ryder LP. Gene expression analysis of interferon-beta treatment in multiple sclerosis. Mult Scler 2008;14: Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 2008;125: International Multiple Sclerosis Genetics Consortium. Refining novel associations in multiple sclerosis. Lancet Neurol 2008; 7: Hedegaard CJ, Krakauer M, Bendtzen K, Sorensen PS, Sellebjerg F, Nielsen CH. The effect of beta-interferon therapy on myelin basic protein-elicited CD4+ T cell proliferation and cytokine production in multiple sclerosis. Clin Immunol 2008;129: Lorentzen Å, Smestad C, Lie B, Oturai A, Åkesson E, Saarela J, Myhr K-M, Vartdal F, Celius E, Sørensen PS, Hillert J, Spurkland A, Harbo HF. The SH2D2A gene and susceptibility to multiple sclerosis. J Neuroimmunol 2008;197(2): International Multiple Sclerosis Genetic Consortium (IMSGC): Booth D, Heard R, Stewart G, Goris A, Dobosi R, Dubois B, Oturai A, Soendergaard HB, Sellebjerg F, Saarela J, Leppä V, Palotie A, Peltonen L, Fontaine B, Cournu-Rebeix I, Clerget-Darpoux F, Babron MC, Weber F, Holsboer F, Müller-Myhsok B, Rieckmann P, Kroner A, Graham C, Vandenbroeck K, Hawkins S, D Alfonso S, Bergamaschi L, Naldi P, Guerini FR, Salvetti M, Galimberti D, Hintzen R, van Duijn C, Lorentzen AR, Celius EG, Harbo HF, Spurkland A, Cucca F, Marrosu MG, Comabella M, Montalban X, Villoslada P, Olsson T, Kockum I, Hillert J, Ban M, Walton A, Sawcer S, Compston A, Hawkins C, Mihalova T, Robertson N, Ingram G, Hafler DA, Rioux J, Daly M, Barcellos L, Ivinson A, Pericak-Vance M, Oksenberg J, Hauser SL, McCauley J, Sexton D, Haines J. Refining novel associations in multiple sclerosis. Lancet Neurol 2008;7(7): Sorensen PS. Intravenous polyclonal human immunoglobulins in multiple sclerosis. Neurodegener Dis 2008;5(1):8-15. Sorensen PS, Koch-Henriksen N, Flachs EM, Bendtzen K. Is the treatment effect of IFN-beta restored after the disappearance of neutralizing antibodies? Mult Scler 2008;14(6): Bramow S, Faber-Rod JC, Jacobsen C, Kutzelnigg A, Patrikios P, Sorensen PS, Lassmann H, Laursen H. Fatal neurogenic pulmonary edema in a patient with progressive multiple sclerosis. Mult Scler 2008;14(5): Fazekas F, Lublin FD, Li D, Freedman MS, Hartung HP, Rieckmann P, Sørensen PS, Maas-Enriquez M, Sommerauer B, Hanna K; PRIVIG Study Group; UBC MS/ MRI Research Group. Intravenous immunoglobulin in relapsing-remitting multiple sclerosis: a dose-finding trial. Neurology 2008;71(4): Giovannoni G, Barbarash O, Casset-Semanaz F, King J, Metz L, Pardo G, Simsarian J, Sørensen PS, Stubinski B. Safety and immunogenicity of a new formulation of interferon beta-1a (Rebif(R) New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler Elovaara I, Apostolski S, van Doorn P, Gilhus NE, Hietaharju A, Honkaniemi J, van Schaik IN, Scolding N, Sørensen PS, Udd B. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol 2008;15(9): Ravnborg M, Bendtzen K, Christensen O, Jensen P, Hesse D, Tovey M, Sørensen PS. Treatment with azathioprine and cyclic methylprednisolone has little or no effect on bioactivity in anti-interferon beta antibody-positive patients with multiple sclerosis. Mult Scler Other publications Sellebjerg F, Oturai A, Sørensen PS; Dansk Neurologisk Selskab. Multipel sklerose genernes betydning. Ugeskr Læger 2008;170: Sørensen PS, Sellebjerg F. Biologisk behandling af multipel sklerose. Ugeskr Læger 2008;170: DANISH Multiple sclerosis CENTER annual report 2009
27 Mathiesen HK, Ravnborg M, Sørensen PS. Magnetic resonance imaging in the diagnosis and follow-up of multiple sclerosis. Ugeskr Laeger 2008;170(34): Sørensen PS. REGARD: what can we learn from randomised, open-label, head-to-head studies? Lancet Neurol 2008;7(10): Sorensen PS, Sellebjerg F. Oral fumarate for relapsing-remitting multiple sclerosis. Lancet 2008;372(9648): Sorensen PS. Can we spot the IFN-beta nonresponders? Neurology 2008;71(24): Publications 2009 Peer reviewed original papers Kemppinen A, Suvela M, Tienari PJ, Elovaara I, Koivisto K, Pirttilä T, Reunanen M, Baumann P, Hillert J, Lundmark F, Oturai A, Ryder L1, Harbo H, Celius EG, Palotie A, Daly M, Peltonen L, Saarela J. MYO9B polymorphisms in multiple sclerosis. Eur J Hum Genet 2009;17(6): Khademi M, Börnsen L, Rafatnia F, Andersson M, Brundin L, Piehl F, Sellebjerg F, Olsson T. The effects of natalizumab on inflammatory mediators in multiple sclerosis: prospects for treatment-sensitive biomarkers. Eur J Neurol 2009;16: Sellebjerg F, Börnsen L, Khademi M, Krakauer M, Olsson T, Frederiksen JL, Sorensen PS. B cell chemokine CXCL13 in cerebrospinal fluid in active MS. Neurology 2009;73: Mero I-L, ÅR Lorentzen, Ban M, Smestad C, Celius EG, Aarseth J, Myhr K-M, Link J, Hillert J, Olsson T, Kockum I, Masterman T, Oturai AB, Søndergaard HB, Sellebjerg F, Saarela J, Kemppinen A, Elovaara I, Spurkland A, Dudbridge F, Lie BA, Harbo HF. A rare variant of the TYK2 gene is confirmed to be associated with multiple sclerosis. Eur J Hum Genet 2010;18(4): Sellebjerg F, Börnsen L, Khademi M, Krakauer M, Olsson T, Frederiksen JL, Sørensen PS. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology 2009;73(23): Ockinger J, Stridh P, Beyeen AD, Lundmark F, Seddighzadeh M, Oturai A, Sørensen PS, Lorentzen AR, Celius EG, Leppä V, Koivisto K, Tienari PJ, Alfredsson L, Padyukov L, Hillert J, Kockum I, Jagodic M, Olsson T. Genetic variants of CC chemokine genes in experimental autoimmune encephalomyelitis, multiple sclerosis and rheumatoid arthritis. Genes Immun 2010;11(2): Hesse D, Sellebjerg F, Sorensen PS. Absence of MxA induction by interferon beta in patients with MS reflects complete loss of bioactivity. Neurology 2009;73(5): Sellebjerg F, Krakauer M, Hesse D, Ryder LP, Alsing I, Jensen PE, Koch-Henriksen N, Svejgaard A, Soelberg Sørensen P. Identification of new sensitive biomarkers for the in vivo response to interferon-beta treatment in multiple sclerosis using DNA-array evaluation. Eur J Neurol 2009;16(12): Enevold C, Oturai AB, Sørensen PS, Ryder LP, Koch- Henriksen N, Bendtzen K. Multiple sclerosis and polymorphisms of innate pattern recognition receptors TLR1-10, NOD1-2, DDX58, and IFIH1. J Neuroimmunol 2009;212(1-2): Sorensen PS, Mellgren SI, Svenningsson A, Elovaara I, Frederiksen JL, Beiske AG, Myhr KM, Søgaard LV, Olsen IC, Sandberg-Wollheim M. NORdic trial of oral Methylprednisolone as add-on therapy to Interferon beta-1a for treatment of relapsing-remitting Multiple Sclerosis (NORMIMS study): a randomised, placebocontrolled trial. Lancet Neurol 2009: Oturai AB, Koch-Henriksen N, Petersen T, Jensen PE, Sellebjerg F, Sorensen PS. Efficacy of natalizumab in multiple sclerosis patients with high disease activity: a Danish nationwide study. Eur J Neurol 2009;16(3): Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009;132(Pt5): Koch-Henriksen N, Sorensen PS, Bendtzen K, Flachs EM. The clinical effect of neutralizing antibodies against interferon-beta is independent of the type of inter- DANISH Multiple sclerosis CENTER annual report
28 Scientific publications 2009 feron-beta used for patients with relapsing-remitting multiple sclerosis. Mult Scler 2009;15(5): Hedegaard CJ, Chen N, Sellebjerg F, Sørensen PS, Leslie RG, Bendtzen K, Nielsen CH. Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: a role in regulating cytokine responses to MBP. Immunology 2009;128(1 Suppl):e Nielsen TR, Rostgaard K, Askling J, Steffensen R, Oturai A, Jersild C, Koch-Henriksen N, Sørensen PS, Hjalgrim H. Effects of infectious mononucleosis and HLA-DRB1*15 in multiple sclerosis. Mult Scler 2009;15(4): O Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L; FTY720 D2201 Study Group. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 2009;72(1):73-9. Other publications Waldemar G, Boysen GM, Høgenhaven H, Knudsen GM, Sørensen PS, Vissing J. Neurologi. I: Schaffalitzky de Muckadell OB; Haunsø S, Vilstrup H (red.). Medicinsk Kompendium 17. Udgave, Nyt Nordisk Forlag Arnold Busck, København 2009; pp Sorensen PS. How effective is natalizumab as secondline treatment for multiple sclerosis in daily clinical praxis? Eur J Neurol 2009;16(3): Sorensen PS. Management of patients with neutralizing antibodies against interferon-beta: stop IFN-beta therapy or wait for the antibodies to go away? Eur J Neurol 2009;16(1):1-2. Hesse D, Frederiksen JL, Koch-Henriksen N, Schreiber K, Stenager E, Heltberg A, Ravnborg M, Bendtzen K, Sellebjerg F, Sorensen PS. Methylprednisolone does not restore biological response in multiple sclerosis patients with neutralizing antibodies against interferon-beta. Eur J Neurol 2009;16(1):43-7. Ravnborg M, Bendtzen K, Christensen O, Jensen PE, Hesse D, Tovey MG, Sørensen PS. Treatment with azathioprine and cyclic methylprednisolone has little or no effect on bioactivity in anti-interferon beta antibody-positive patients with multiple sclerosis. Mult Scler 2009;15(3): Giovannoni G, Barbarash O, Casset-Semanaz F, King J, Metz L, Pardo G, Simsarian J, Sørensen PS, Stubinski B; Rebif New Formulation Study Group. Safety and immunogenicity of a new formulation of interferon beta-1a (Rebif New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler 2009;15(2): Jagodic M, Colacios C, Nohra R, Dejean AS, Beyeen AD, Khademi M, Casemayou A, Lamouroux L, Duthoit C, Papapietro O, Sjöholm L, Bernard I, Lagrange D, Dahlman I, Lundmark F, Oturai AB, Soendergaard HB, Kemppinen A, Saarela J, Tienari PJ, Harbo HF, Spurkland A, Ramagopalan SV, Sadovnick DA, Ebers GC, Seddighzadeh M, Klareskog L, Alfredsson L, Padyukov L, Hillert J, Clanet M, Edan G, Fontaine B, Fournié GJ, Kockum I, Saoudi A, Olsson T. A role for VAV1 in experimental autoimmune encephalomyelitis and multiple sclerosis. Sci Transl Med 2009;1(10):10ra DANISH Multiple sclerosis CENTER annual report 2009
29 Prizes and honorary offices Prizes Annette Oturai Henry Hansen and wifes grant : kr, donated for many years of scientific working within multiple sclerosis, Honorary offices Annette Bang Oturai has held the following honorary offices: A: National Board member of the Danish Sociaty for Multiple Sclerosis (DAREMUS) Finn Sellebjerg has held the following honorary offices: A: National chairman of the Danish Society for Research in Multiple Sclerosis (MS) and chairman of the research board of the Danish MS Society. B: International chairman of the task force on treatment of MS relapses and member of the scientist panel on demyelinating disease of the European Federation of Neurological Societies. Per Soelberg Sorensen has held the following honorary offices: A: National Chairman of the Foundation for Research in Neurology, Chairman of the Scientific Advisory Committee of the Danish MS Society, Chairman of the Danish Multiple Sclerosis Group, B: International executive Board Member of the European Charcot Foundation for Research in Multiple Sclerosis, Member, Medical Advisory Board of the International Federation of Multiple Sclerosis Societies, Member, US National Multiple Sclerosis Society Advisory Committee on Clinical Trials, chairman, Scientist Panel on Multiple Sclerosis, European Federation of Neurological Societies, Chairman, Publication Committee, European Federation of Neurological Societies, executive Board Member of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), DANISH Multiple sclerosis CENTER annual report
30 Scientific collaboration Scientific collaboration National Danish Research Centre for Magnetic Resonance, Copenhagen University Hospital, Hvidovre, Copenhagen, Denmark The Danish Multiple Sclerosis Register, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark (Nils Koch-Henriksen, MD) Department of Clinical Immunology, Copenhagen University Hospital, Rigshospitalet, Denmark (Jacob Larsen MD, Lars Ryder, Klaus Rieneck, MD, Hans O. Madsen, MD) Institute for Inflammatory Research, Copenhagen University Hospital, Rigshospitalet, Denmark (Christian Enevold-Johansen MD, Professor Klaus Bendtzen) Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark (Trine Rasmussen Nielsen, MD, Henrik Hjalgrim, MD, professor Mads Melbye, Peter Michael Bager, ph.d.) Department of Human Genetics, Aarhus University, Denmark (Bjørn Andersen Nexø) Laboratory of Neuropathology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark (Henning Laursen, MD) International Nordic MS Genetic Network: Collaboration between the Nordic countries: Sweden (Huddinge, Lund, Gothenburg, Stockholm), Norway (Oslo, Bergen), Finland (Helsinki) and Denmark (Copenhagen) Institute of Immunology, Rikshospitalet, University Hospital, Oslo, Norway (Anne Spurkland, MD, Hanne F. Harbo, MD, professor Frode Vartdal) Department of Neurology, Ullevål University Hospital, Oslo, Norway (Elisabeth G Celius, MD) Department of Neurology, Haukeland Hospital, Bergen, Norway (Professor Kjell-Morten Myhr) Department of Neurology, Huddinge University Hospital, Karolinska Institute, Huddinge, Sweden (Professor Jan Hillert, Eva Åkesson, MD, Helena Modin, MD) Department of Clinical Neuroscience and Centrum for Molecular Medicine Karolinska Insitutet at Karolinska University Hospial, Solna Stockholm, Sweden (Professor Tomas Olsson, associate Professor Ingrid Kockum) Department of Neurology, Lund University Hospital, Lund, Sweden (Professor Magnhild Sandberg-Wollheim) Department of Neurology, Gothenburg University Hospital, Gothenburg, Sweden (Professor Oluf Andersen) Institute for Molecular Medicine Finland, University of Helsinki, Finland (Janna Saarela) University of Cambridge, Neurology Unit, Addenbrooke s Hospital, Cambridge, United Kingdom (Stephen Sawcer, MD, Professor Alastair Compston) International Multiple Sclerosis Genetic Consortium (IMSG): collaboration between 20 countries from Europe, USA, Canada and Australia Department of Immunopathology, Brain Research Center, Medical University of Vienna, Vienna, Austria. (Professor Hans Lassmann and Josa Frischer, MD) Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA (Professor Claudia F. Lucchinetti) VU Medical Centre, Amsterdam, The Netherlands (Professor Chris Polman) Utrecht University, Utrecht, The Netherlands (Professor Hub Schellekens) Heinrich-Heine-University, Düsseldorf, Germany (Professor Hans-Peter Hartung) Ospedale Universitario San Luigi, Torino, Italy (Professor Auturio Bertolotto) Queen Square, London, The United Kingdom (Professor Gavin Giovannoni) Innsbruck Medical University, Innsbruck, Austria (Professor Florian Deisenhammer) General Charles University, Prague, Czech Republic (Professor Eva Havrdova) 30 DANISH Multiple sclerosis CENTER annual report 2009
31 Collaboration with pharmaceutical companies on clinical trials Novartis, Denmark Merck Serono Nordic, Denmark, Norway and Sweden Biogen idec, Denmark and USA Teva/Aventis, Israel and Denmark Sanofi-aventis, Denmark Bayer Schering, Germany Octapharma, Austria Genmab, Denmark Genzymes, Holland BioMS Glaxo Smith-Kline Acknowledgements Danish Multiple Sclerosis Research Center has received generous support from a number of public and private research funds: Danish Medical Research Council Danish MS Society Warwara Larsen Foundation Rigshospitalets Scientific Board EU Sixth Framework Programme Brdr. Røjne Holding Jeppe Juel Memorial Legacy RoFar Foundation Roche Denmark The Danish Strategic Research Council The Johnsen Memorial Foundation The Lounkær Foundation Biogen idec Sanofi-aventis Merck Serono Ejner Jonasson og Hustrus mindelegat Fondsbørsvekselerer Henry Hansen og Hustrus Legat DANISH Multiple sclerosis CENTER annual report
32 32 DANISH Multiple sclerosis CENTER annual report 2009
A short review of 2008 in the Danish Multiple Sclerosis Research Center... Facilities, organization and staff...
 TABLE OF CONTENTS A short review of 2008 in the Danish Multiple Sclerosis Research Center... Facilities, organization and staff... Research activities 2008 Clinical research... Neuroimmunology... Routine
TABLE OF CONTENTS A short review of 2008 in the Danish Multiple Sclerosis Research Center... Facilities, organization and staff... Research activities 2008 Clinical research... Neuroimmunology... Routine
Danish Multiple Sclerosis center Annual report 2010
 DepArtMeNt of Neurology the NeuroScIeNce centre copenhagen university HoSpItAl rigshospitalet copenhagen, DeNMArk www.ms-research.dk Danish Multiple Sclerosis center Annual report 2010 DANISH MultIple
DepArtMeNt of Neurology the NeuroScIeNce centre copenhagen university HoSpItAl rigshospitalet copenhagen, DeNMArk www.ms-research.dk Danish Multiple Sclerosis center Annual report 2010 DANISH MultIple
Danish Multiple Sclerosis Center Annual Report 2011
 Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark www.ms-research.dk Danish Multiple Sclerosis Center Annual Report 2011 DMSC staff seminar
Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark www.ms-research.dk Danish Multiple Sclerosis Center Annual Report 2011 DMSC staff seminar
Danish Multiple Sclerosis Center Annual Report 2012
 Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark www.ms-research.dk Danish Multiple Sclerosis Center Annual Report 2012 DMSC staff seminar
Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark www.ms-research.dk Danish Multiple Sclerosis Center Annual Report 2012 DMSC staff seminar
TABLE OF CONTENTS INTRODUCTION
 INTRODUCTION It is a pleasure to present the annual report from Copenhagen Multiple Sclerosis Centre, Department of Neurology, Neuroscience Centre, Copenhagen University Hospital, Rigshospitalet. Copenhagen
INTRODUCTION It is a pleasure to present the annual report from Copenhagen Multiple Sclerosis Centre, Department of Neurology, Neuroscience Centre, Copenhagen University Hospital, Rigshospitalet. Copenhagen
We hope that you will enjoy reading the annual report for the year 2004. On behalf of the Danish Multiple Sclerosis Research Centre
 INTRODUCTION It is a pleasure to present the annual report from The Danish Multiple Sclerosis Research Centre, Department of Neurology, Neuroscience Center Copenhagen University Hospital, Rigshospitalet.
INTRODUCTION It is a pleasure to present the annual report from The Danish Multiple Sclerosis Research Centre, Department of Neurology, Neuroscience Center Copenhagen University Hospital, Rigshospitalet.
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Danish Multiple Sclerosis Center Annual Report 2013
 Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark www.ms-research.dk Danish Multiple Sclerosis Center Annual Report 2013 DMSC missions and
Department of Neurology THE Neuroscience Centre Copenhagen University Hospital Rigshospitalet Copenhagen, Denmark www.ms-research.dk Danish Multiple Sclerosis Center Annual Report 2013 DMSC missions and
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study
 Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study Nele Claes 1 *; Tessa Dhaeze 1 *; Judith Fraussen PhD 1 ; Bieke Broux
Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study Nele Claes 1 *; Tessa Dhaeze 1 *; Judith Fraussen PhD 1 ; Bieke Broux
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Media Release. Basel, 8 October 2015
 Media Release Basel, 8 October 2015 Roche s ocrelizumab first investigational medicine to show positive pivotal study results in both relapsing and primary progressive forms of multiple sclerosis Ocrelizumab
Media Release Basel, 8 October 2015 Roche s ocrelizumab first investigational medicine to show positive pivotal study results in both relapsing and primary progressive forms of multiple sclerosis Ocrelizumab
ß-interferon and. ABN Guidelines for 2007 Treatment of Multiple Sclerosis with. Glatiramer Acetate
 ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
The Immunopathogenesis of Relapsing MS
 The Immunopathogenesis of Relapsing MS Olaf Stüve, M.D., Ph.D. Neurology Section VA North Texas Health Care System Dallas VA Medical Center Departments of Neurology and Neurotherapeutics University of
The Immunopathogenesis of Relapsing MS Olaf Stüve, M.D., Ph.D. Neurology Section VA North Texas Health Care System Dallas VA Medical Center Departments of Neurology and Neurotherapeutics University of
14. E. Vilella #, G. Bengtsson-Olivecrona*, T. Stigbrand*, and P. E. H. Jensen*, Biochemistry and Biophysics, University of Umeå, Umeå, Sweden.
 February 204 PUBLICATIONS. Poul Erik Hyldgaard Jensen. P. E. H. Jensen and L. Sottrup-Jensen, Dept. of Molecular Biology and Plant Physiology, Aarhus University, Aarhus, Denmark. Primary Structure of Human
February 204 PUBLICATIONS. Poul Erik Hyldgaard Jensen. P. E. H. Jensen and L. Sottrup-Jensen, Dept. of Molecular Biology and Plant Physiology, Aarhus University, Aarhus, Denmark. Primary Structure of Human
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
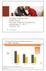 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Clinical Trials of Disease Modifying Treatments
 MS CENTER CLINICAL RESEARCH The UCSF MS Center is an internationally recognized leader in multiple sclerosis clinical research. We conduct clinical trials involving the use of experimental treatments,
MS CENTER CLINICAL RESEARCH The UCSF MS Center is an internationally recognized leader in multiple sclerosis clinical research. We conduct clinical trials involving the use of experimental treatments,
Accuracy in Space and Time: Diagnosing Multiple Sclerosis. 2012 Genzyme Corporation, a Sanofi company.
 Accuracy in Space and Time: Diagnosing Multiple Sclerosis 2012 Genzyme Corporation, a Sanofi company. Brought All rights to reserved. you by www.msatrium.com, MS.US.PO876.1012 your gateway to MS knowledge.
Accuracy in Space and Time: Diagnosing Multiple Sclerosis 2012 Genzyme Corporation, a Sanofi company. Brought All rights to reserved. you by www.msatrium.com, MS.US.PO876.1012 your gateway to MS knowledge.
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW EFFICACY IN PEOPLE WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS IN LARGE PHASE III STUDY
 NEWS RELEASE Media Contact: Tara Iannuccillo (650) 467-6800 Investor Contacts: Stefan Foser Karl Mahler (650) 467-2016 011 41 61 687 8503 GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW
NEWS RELEASE Media Contact: Tara Iannuccillo (650) 467-6800 Investor Contacts: Stefan Foser Karl Mahler (650) 467-2016 011 41 61 687 8503 GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Autoimmunity. Autoimmunity. Genetic Contributions to Autoimmunity. Targets of Autoimmunity
 Autoimmunity Factors predisposing an individual to autoimmune disease Mechanisms of initiation of autoimmunity Pathogenesis of particular autoimmune disease Animal models of autoimmune disease Treatment
Autoimmunity Factors predisposing an individual to autoimmune disease Mechanisms of initiation of autoimmunity Pathogenesis of particular autoimmune disease Animal models of autoimmune disease Treatment
13 September 2016 - London, UK
 INTERNATIONAL WORKSHOP PRELIMINARY PROGRAMME MS Academia: Multiple sclerosis advanced course 13 September 2016 - London, UK MS Academia - Multiple sclerosis advanced course Overview Multiple sclerosis
INTERNATIONAL WORKSHOP PRELIMINARY PROGRAMME MS Academia: Multiple sclerosis advanced course 13 September 2016 - London, UK MS Academia - Multiple sclerosis advanced course Overview Multiple sclerosis
Chapter 10. Summary & Future perspectives
 Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS.
![PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS. PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS.](/thumbs/33/16408576.jpg) Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM Condition Name Multiple Sclerosis (MS) Multiple
Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM Condition Name Multiple Sclerosis (MS) Multiple
Clinical Department of Neurology, Innsbruck Medical University, Austria 1
 CHAPTER 6 LEVEL S OF SERUM IGM ANTIBODY AGAINST NATIVE REFOLDED MOG PREDICT RESPONSIVENESS TO CORTICOSTEROIDS FOR TRE ATMENT OF ACUTE MULTIPLE SCLEROSIS REL APSES Michael Khalil 1, Bettina Kuenz, Andreas
CHAPTER 6 LEVEL S OF SERUM IGM ANTIBODY AGAINST NATIVE REFOLDED MOG PREDICT RESPONSIVENESS TO CORTICOSTEROIDS FOR TRE ATMENT OF ACUTE MULTIPLE SCLEROSIS REL APSES Michael Khalil 1, Bettina Kuenz, Andreas
Treatments for MS: Immunotherapy. Gilenya (fingolimod) Glatiramer acetate (Copaxone )
 Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
The Norwegian Multiple Sclerosis Registry and Biobank
 Acta Neurol Scand 2015: 132 (Suppl. 199): 24 28 DOI: 10.1111/ane.12427 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd ACTA NEUROLOGICA SCANDINAVICA Review Article The Norwegian Multiple
Acta Neurol Scand 2015: 132 (Suppl. 199): 24 28 DOI: 10.1111/ane.12427 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd ACTA NEUROLOGICA SCANDINAVICA Review Article The Norwegian Multiple
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
EMA and Progressive Multifocal Leukoencephalopathy.
 EMA and Progressive Multifocal Leukoencephalopathy. ENCePP Plenary, London 23 November 2011 Presented by: Henry Fitt Head of Coordination & Networking, Pharmacovigilance & Risk Management An agency of
EMA and Progressive Multifocal Leukoencephalopathy. ENCePP Plenary, London 23 November 2011 Presented by: Henry Fitt Head of Coordination & Networking, Pharmacovigilance & Risk Management An agency of
Update: MRI in Multiple sclerosis
 Nyt indenfor MS ved MR Update: MRI in Multiple sclerosis Hartwig Roman Siebner Danish Research Centre for Magnetic Resonance (DRCMR) Copenhagen University Hospital Hvidovre Dansk Radiologisk Selskabs 10.
Nyt indenfor MS ved MR Update: MRI in Multiple sclerosis Hartwig Roman Siebner Danish Research Centre for Magnetic Resonance (DRCMR) Copenhagen University Hospital Hvidovre Dansk Radiologisk Selskabs 10.
ALLIANCE FOR LUPUS RESEARCH AND PFIZER S CENTERS FOR THERAPEUTIC INNOVATION CHALLENGE GRANT PROGRAM PROGRAM GUIDELINES
 ALLIANCE FOR LUPUS RESEARCH AND PFIZER S CENTERS FOR THERAPEUTIC INNOVATION CHALLENGE GRANT PROGRAM PROGRAM GUIDELINES DESCRIPTION OF GRANT MECHANISM The Alliance for Lupus Research (ALR) is an independent,
ALLIANCE FOR LUPUS RESEARCH AND PFIZER S CENTERS FOR THERAPEUTIC INNOVATION CHALLENGE GRANT PROGRAM PROGRAM GUIDELINES DESCRIPTION OF GRANT MECHANISM The Alliance for Lupus Research (ALR) is an independent,
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549. Report of Foreign Private Issuer
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
Conflict of Interest Declaration. Overview of New Medications for Multiple Sclerosis. Assessment Question. Objectives 4/1/2011
 Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
Personalised Medicine in MS
 Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that new data from three Phase III studies of the
 Media Release Basel, 12 April 2016 Roche to present new data at AAN showing superior efficacy of investigational medicine ocrelizumab versus comparators on disease activity and progression in two forms
Media Release Basel, 12 April 2016 Roche to present new data at AAN showing superior efficacy of investigational medicine ocrelizumab versus comparators on disease activity and progression in two forms
AUBMC Multiple Sclerosis Center
 AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
Clinically isolated syndrome (CIS)
 Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA
 Media Release Basel, 28 June 2016 Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA OCREVUS is the first investigational medicine
Media Release Basel, 28 June 2016 Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA OCREVUS is the first investigational medicine
Autoimmunity and immunemediated. FOCiS. Lecture outline
 1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
IF YOU ARE RECEIVING TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS (NATALIZUMAB)
 IF YOU ARE RECEIVING (NATALIZUMAB) TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS Read the patient information leaflet that accompanies the medicine carefully. 1 This brochure is a supplement to the
IF YOU ARE RECEIVING (NATALIZUMAB) TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS Read the patient information leaflet that accompanies the medicine carefully. 1 This brochure is a supplement to the
The Prospect of Stem Cell Therapy in Multiple Sclerosis. Multiple sclerosis is a multifocal inflammatory disease of the central
 The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
Monday, August 25, 2014
 Danish Dementia Research Centre, Copenhagen University Kristian Steen Frederiksen, MD, PhD; Danish Dementia Research Centre, Copenhagen University Danish Dementia Research Centre, Copenhagen University
Danish Dementia Research Centre, Copenhagen University Kristian Steen Frederiksen, MD, PhD; Danish Dementia Research Centre, Copenhagen University Danish Dementia Research Centre, Copenhagen University
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
Life with MS: Striving for Maximal Independence & Fulfillment
 Life with MS: Striving for Maximal Independence & Fulfillment St. Louis, May 7, 2005 Florian P. Thomas, MA, MD, PhD MS Center, Department of Neurology Associate Professor, Saint Louis University Brain
Life with MS: Striving for Maximal Independence & Fulfillment St. Louis, May 7, 2005 Florian P. Thomas, MA, MD, PhD MS Center, Department of Neurology Associate Professor, Saint Louis University Brain
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
What is MS? 1. disease that affects the central nervous. Is a disease that affects both white and gray matter
 What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine to Drive Growth
 Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
Multiple Sclerosis. Matt Hulvey BL A - 615
 Multiple Sclerosis Matt Hulvey BL A - 615 Multiple Sclerosis Multiple Sclerosis (MS) is an idiopathic inflammatory disease of the central nervous system (CNS) MS is characterized by demyelination (lesions)
Multiple Sclerosis Matt Hulvey BL A - 615 Multiple Sclerosis Multiple Sclerosis (MS) is an idiopathic inflammatory disease of the central nervous system (CNS) MS is characterized by demyelination (lesions)
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Gene Therapy. The use of DNA as a drug. Edited by Gavin Brooks. BPharm, PhD, MRPharmS (PP) Pharmaceutical Press
 Gene Therapy The use of DNA as a drug Edited by Gavin Brooks BPharm, PhD, MRPharmS (PP) Pharmaceutical Press Contents Preface xiii Acknowledgements xv About the editor xvi Contributors xvii An introduction
Gene Therapy The use of DNA as a drug Edited by Gavin Brooks BPharm, PhD, MRPharmS (PP) Pharmaceutical Press Contents Preface xiii Acknowledgements xv About the editor xvi Contributors xvii An introduction
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Collaborative Network Award Planning Grants Project Summaries
 Collaborative Network Award Planning Grants Project Summaries PRIORITY AREA: Projects that will drive development of one or more pre-clinical drug candidates through identification and validation of molecular
Collaborative Network Award Planning Grants Project Summaries PRIORITY AREA: Projects that will drive development of one or more pre-clinical drug candidates through identification and validation of molecular
Ulf Kallweit, MD; Ilijas Jelcic, MD; Nathalie Braun, MD, PhD; Heike Fischer, MD; Björn Zörner,
 Sustained efficacy of Natalizumab in the treatment of relapsing-remitting multiple sclerosis independent of disease activity and disability at baseline: real-life data from a Swiss cohort Ulf Kallweit,
Sustained efficacy of Natalizumab in the treatment of relapsing-remitting multiple sclerosis independent of disease activity and disability at baseline: real-life data from a Swiss cohort Ulf Kallweit,
Understanding. Multiple Sclerosis. Tim, diagnosed in 2004.
 Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Programa Cooperación Farma-Biotech Neurociencias NT-KO-003
 Programa Cooperación Farma-Biotech Neurociencias NT-KO-003 A new oral treatment for Multiple Sclerosis based on a novel mechanism of action Barcelona, 15 de febrero 2011 Programa Cooperación Farma-Biotech
Programa Cooperación Farma-Biotech Neurociencias NT-KO-003 A new oral treatment for Multiple Sclerosis based on a novel mechanism of action Barcelona, 15 de febrero 2011 Programa Cooperación Farma-Biotech
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
43. MULTIPLE SCLEROSIS
 Guidelines to be followed by centres, services and units in order to be designated as Reference Centres, Services and Units of the National Health System, as agreed by the Interterritorial Board 43. MULTIPLE
Guidelines to be followed by centres, services and units in order to be designated as Reference Centres, Services and Units of the National Health System, as agreed by the Interterritorial Board 43. MULTIPLE
B Cells and Antibodies
 B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
The Nuts and Bolts of Multiple Sclerosis. Rebecca Milholland, M.D., Ph.D. Center for Neurosciences
 The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
March 30, 2007. I. IVIg IS AN ACCEPTED THERAPY FOR RRMS, AND IS PRESCRIBED ALONG WITH A DISEASE-MODIFYING AGENT SUCH AS COPAXONE
 18 Timberline Drive Farmington, CT 06032 (860) 674-1370 (phone) (860) 674-1378 (fax) (860) 305-9835 (cell) www.advocacyforpatients.org patient_advocate@sbcglobal.net AZ Benefit Options ATTN: Appeals PO
18 Timberline Drive Farmington, CT 06032 (860) 674-1370 (phone) (860) 674-1378 (fax) (860) 305-9835 (cell) www.advocacyforpatients.org patient_advocate@sbcglobal.net AZ Benefit Options ATTN: Appeals PO
Sessions. Workshops. Lectures, discussions and workshops. PhD students, members of the PhD Network of Diabetes and Metabolism, Danish Diabetes Academy
 Sessions PHD COURSE PROGRAMME BASAL METABOLISM AND MOLECULAR MECHANISMS IN THE METABOLIC SYNDROME I II III IV V Basal metabolism The metabolic syndrome (MS), epidemiology and fat cells Inflammation, exercise
Sessions PHD COURSE PROGRAMME BASAL METABOLISM AND MOLECULAR MECHANISMS IN THE METABOLIC SYNDROME I II III IV V Basal metabolism The metabolic syndrome (MS), epidemiology and fat cells Inflammation, exercise
Natalizumab (Tysabri) and PMLthe current figures. Paul-Ehrlich-Institut Brigitte Keller Stanislawski Paul-Ehrlich-Str. 51-59 63225 Langen GERMANY
 Natalizumab (Tysabri) and PMLthe current figures Paul-Ehrlich-Institut Brigitte Keller Stanislawski Paul-Ehrlich-Str. 51-59 63225 Langen GERMANY Humanised MAB Natalizumab Specific binding to a4-integrin
Natalizumab (Tysabri) and PMLthe current figures Paul-Ehrlich-Institut Brigitte Keller Stanislawski Paul-Ehrlich-Str. 51-59 63225 Langen GERMANY Humanised MAB Natalizumab Specific binding to a4-integrin
Guidance for evaluation of new neurological symptoms in patients receiving TYSABRI
 Guidance for evaluation of new neurological symptoms in patients receiving TYSABRI Background information Progressive multifocal leukoencephalopathy (PML) PML is a demyelinating disease that attacks the
Guidance for evaluation of new neurological symptoms in patients receiving TYSABRI Background information Progressive multifocal leukoencephalopathy (PML) PML is a demyelinating disease that attacks the
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
Using the MS Clinical Course Descriptions in Clinical Practice
 Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Case Report Early Bilateral Cystoid Macular Oedema Secondary to Fingolimod in Multiple Sclerosis
 Case Reports in Medicine Volume 2012, Article ID 134636, 4 pages doi:10.1155/2012/134636 Case Report Early Bilateral Cystoid Macular Oedema Secondary to Fingolimod in Multiple Sclerosis Lei Liu and Fiona
Case Reports in Medicine Volume 2012, Article ID 134636, 4 pages doi:10.1155/2012/134636 Case Report Early Bilateral Cystoid Macular Oedema Secondary to Fingolimod in Multiple Sclerosis Lei Liu and Fiona
MS Learn Online Feature Presentation Understanding MS Research Robert Lisak, MD. Tom>> And I'm Tom Kimball. Welcome to MS Learn Online.
 Tracey>> Hi I'm Tracey Kimball MS Learn Online Feature Presentation Understanding MS Research Robert Lisak, MD Tom>> And I'm Tom Kimball. Welcome to MS Learn Online. Tracey>> There's so much to learn when
Tracey>> Hi I'm Tracey Kimball MS Learn Online Feature Presentation Understanding MS Research Robert Lisak, MD Tom>> And I'm Tom Kimball. Welcome to MS Learn Online. Tracey>> There's so much to learn when
- Patients treated with alemtuzumab in CARE-MS II were more than twice as likely to experience disability improvement compared to Rebif -
 PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Issues Regarding Use of Placebo in MS Drug Trials. Peter Scott Chin, MD Novartis Pharmaceuticals Corporation
 Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
New research in primary and secondary progressive multiple sclerosis. Dr Claire McCarthy MRCP PhD Neurology SpR Addenbrooke s Hospital
 New research in primary and secondary progressive multiple sclerosis Huntingdon MS Society Annual Meeting Sat 22nd March 2014 Dr Claire McCarthy MRCP PhD Neurology SpR Addenbrooke s Hospital 1 Overview
New research in primary and secondary progressive multiple sclerosis Huntingdon MS Society Annual Meeting Sat 22nd March 2014 Dr Claire McCarthy MRCP PhD Neurology SpR Addenbrooke s Hospital 1 Overview
Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis
 May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines
 TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines DATE: 13 March 2013 CONTEXT AND POLICY ISSUES Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central
TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines DATE: 13 March 2013 CONTEXT AND POLICY ISSUES Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
Future therapies in multiple sclerosis
 Neurology Asia 2008; 13 : 189 193 Future therapies in multiple sclerosis David Bates Department of Neurology, University of Newcastle upon Tyne, UK Abstract It is now 15 years since the first disease modifying
Neurology Asia 2008; 13 : 189 193 Future therapies in multiple sclerosis David Bates Department of Neurology, University of Newcastle upon Tyne, UK Abstract It is now 15 years since the first disease modifying
Multiple Sclerosis - Relapsing and Remissioning
 DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
MULTIMODAL THERAPY FOR MS- ASSOCIATED COGNITIVE DYSFUNCTION
 MULTIMODAL THERAPY FOR MS- ASSOCIATED COGNITIVE DYSFUNCTION Michael K. Racke, MD Professor and Chairman in Neurology The Helen C. Kurtz Chair in Neurology Department of Neurology Ohio State University
MULTIMODAL THERAPY FOR MS- ASSOCIATED COGNITIVE DYSFUNCTION Michael K. Racke, MD Professor and Chairman in Neurology The Helen C. Kurtz Chair in Neurology Department of Neurology Ohio State University
Genzyme s Multiple Sclerosis Franchise Featured at AAN
 PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
