Health Policy Advisory Committee on Technology
|
|
|
- Branden Lawson
- 10 years ago
- Views:
Transcription
1 Health Policy Advisory Committee on Technology Technology Brief AIGISRx Antibacterial Envelope for preventing infection in implanted cardiac devices August 2013
2 State of Queensland (Queensland Department of Health) 2013 This work is licensed under a Creative Commons Attribution Non-Commercial No Derivatives 2.5 Australia licence. In essence, you are free to copy and communicate the work in its current form for non-commercial purposes, as long as you attribute the authors and abide by the licence terms. You may not alter or adapt the work in any way. To view a copy of this licence, visit For further information, contact the HealthPACT Secretariat at: HealthPACT Secretariat c/o Clinical Access and Redesign Unit, Health Service and Clinical Innovation Division Department of Health, Queensland Level 13, Block 7 Royal Brisbane and Women s Hospital HERSTON QLD 4029 Postal Address: GPO Box 48, Brisbane Qld [email protected] Telephone: For permissions beyond the scope of this licence contact: Intellectual Property Officer, Queensland Health, GPO Box 48, Brisbane Qld 4001, [email protected], phone (07) Electronic copies can be obtained from: DISCLAIMER: This brief is published with the intention of providing information of interest. It is based on information available at the time of research and cannot be expected to cover any developments arising from subsequent improvements to health technologies. This brief is based on a limited literature search and is not a definitive statement on the safety, effectiveness or costeffectiveness of the health technology covered. The State of Queensland acting through Queensland Health ( Queensland Health ) does not guarantee the accuracy, currency or completeness of the information in this brief. Information may contain or summarise the views of others, and not necessarily reflect the views of Queensland Health. This brief is not intended to be used as medical advice and it is not intended to be used to diagnose, treat, cure or prevent any disease, nor should it be used for therapeutic purposes or as a substitute for a health professional's advice. It must not be relied upon without verification from authoritative sources. Queensland Health does not accept any liability, including for any injury, loss or damage, incurred by use of or reliance on the information. This brief was commissioned by Queensland Health, in its role as the Secretariat of the Health Policy Advisory Committee on Technology (HealthPACT). The production of this brief was overseen by HealthPACT. HealthPACT comprises representatives from health departments in all States and Territories, the Australian and New Zealand governments and MSAC. It is a sub-committee of the Australian Health Ministers Advisory Council (AHMAC), reporting to AHMAC s Hospitals Principal Committee (HPC). AHMAC supports HealthPACT through funding. This brief was prepared by Dr Meegan Vandepeer from the Australian Safety and Efficacy Register of New Interventional Procedures Surgical (ASERNIP-S).
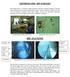
3 Technology, Company and Licensing Register ID Technology name Patient indication WP165 AIGISRx Antibacterial Envelope For use in patients who are being implanted with either a pacemaker or implantable cardioverter-defibrillator (ICD) Description of the technology The AIGISRX Antibacterial Envelope is intended to securely enclose a cardiac implantable electronic device (CIED), that is a pacemaker or implantable cardioverter defibrillator (ICD), in order to create a stable environment around the device and reduce the risk of postsurgery infections associated with the implanted pulse generator (Figure 1). The envelope is made from a non-resorbable mesh comprising a knitted polypropylene substrate that is coated with a bioresorbable polymer containing two antimicrobial agents (minocycline and rifampicin). The envelopes come in two sizes one for pacemakers (medium, 6.3 cm x 6.9 cm) and one for defibrillators (large, 7.4 cm x 8.5 cm) and may be used for a patient s first implant or when the pacemaker or ICD needs to be replaced. The envelopes are not reusable. 1 Figure 1 The AIGISRx Antibacterial Envelope 1 The two antibiotics are released continuously over a 7- to 10-day period by the antibacterial envelope (locally, at the surgical site), following implantation of the pacemaker or ICD. According to the manufacturers of AIGISRX, minocycline has been shown to be effective against Staphylococcus aureus, Streptococcus pneumonia, Escherichia coli, Enterobacter aerogenes, Haemophilus influenzae and Acinetobacter baumannii, whilst rifampicin has been shown to be effective against Staphylococcus aureus, Staphylococcus epidermidis and Haemophilus influenzae. The mesh contains 86 µg/cm 2 of each antibiotic. 2 Within the first 24 hours of device implantation, the amount of antibiotics is approximately 5,000 times the minimum lethal concentration of antibiotics that would kill Staphylococcus AIGISRx Antibacterial Envelope for implanted cardiac devices: August
4 aureus (including methicillin-resistant Staphylococcus aureus), Staphylococcus epidermis, Escherichia coli, Acinetobacter. baumannii and Staphylococcus capitis. 2 The resorbable polymer that contains the two antibiotics takes approximately 140 days to be absorbed by the body, leaving the non-resorbable mesh behind to hold and stabilise the device. 1 Prior to implantation, the skin at the site where the CIED is to be placed is shaved/clipped and cleaned and the patient is given a local anaesthetic and sedative. An incision is made in the chest where the leads and device are to be inserted. The initial surgical incision needs to be somewhat larger than what is needed for placing the same pacemaker or ICD without an antibiotic envelope, especially for subpectoral implantations. After the generator is attached to the leads, the AIGISRx is activated by briefly immersing it in saline solution. Next the pacemaker or ICD generator is placed in the AIGISRx Envelope. This may require expanding the opening of the envelope by tearing or cutting it. The generator, and as much of the looped leads as possible, are placed inside. The AIGISRx Envelope, containing either the pacemaker or ICD, is placed into the pocket underneath the skin, which is then closed following normal procedure. Removal of the AIGISRx Envelope during a pulse generator replacement is not required except in the case of an infection. In this situation, it is common practice to perform a capsulectomy, a procedure in which the entire capsule of scar tissue surrounding the implant is removed, and/or a debridement. 1 Company or developer TYRX, Inc., New Jersey, United States of America (USA). Reason for assessment The AIGISRx Antibacterial Envelope may reduce infections that can occur when implanting pacemakers and ICDs, particularly in high-risk patients, with potential cost benefits to the healthcare system. Stage of development in Australia Yet to emerge Experimental Investigational Nearly established Established Established but changed indication or modification of technique Should be taken out of use Licensing, reimbursement and other approval TYRX, Inc. s AIGISRx Antibacterial Envelope was granted United States Food and Drug Administration (FDA) 510 (k) clearance in January 2008 (application number: K063091). 3 In June 2012, TYRX, Inc. received a licence from Health Canada to market the AIGISRx Antibacterial Envelope with implantable electronic devices. 4 The AIGISRx Antibacterial AIGISrx Antibacterial Envelope for implanted cardiac devices: August
5 Envelope does not appear to have received a CE mark, nor is it listed on the Australian Register of Therapeutic Goods (ARTG). Australian Therapeutic Goods Administration approval Yes ARTG number (s) Not applicable No Not applicable Technology type Technology use Device Preventative Patient Indication and Setting Disease description and associated mortality and morbidity Surgical site infections (SSI) can develop in anyone who receives an implantable pacemaker or ICD. However, there are a number of patient-specific and procedure-based factors that increase the risk of developing a SSI. Patient-related risk factors include the use of oral anticoagulants and steroids, the presence of a fever within the previous 24 hours and the presence of comorbidities such as diabetes and chronic renal disease. Procedure-related factors that can increase the risk of infection include not using antibiotic prophylaxis, the number of leads implanted, the procedure type, the operator s experience, the performance of a device revision or replacement versus a new implant and the need for early re-intervention. 5 There are two main types of CIED infections. A. Local SSIs at the generator pocket and the subcutaneous segment of the leads. Symptoms of local infections include erythema, warmth, wound dehiscence, tenderness, purulent drainage, erosion of the generator or leads through the skin. These typically occur within the first several months after implantation or revision, although late-onset infections can occur years after device manipulation. B. Trans-venous lead infections or lead-related endocarditis. These infections are less common and are deeper than SSIs. These patients commonly present with systemic symptoms, such as fever or sepsis, and often produce positive blood cultures. Transoesophageal echocardiography may be useful in detecting CIED-related endocarditis. 6 Complications resulting from a CIED infection may occur at the site of implantation or at an anatomically remote site. Complications that can occur at the site of implantation include chest wall abscess, septic thrombophlebitis and right-sided endocarditis. Complications that occur more remotely include skeletal complications, both local (clavicular osteomyelitis and AIGISrx Antibacterial Envelope for implanted cardiac devices: August
6 sternoclavicular arthritis) and remote (metastatic osteomyelitis, discitis and septic arthritis); cardiopulmonary complications (septic pulmonary emboli, mycotic pulmonary artery aneurysm and left-sided endocarditis); and metastatic complications, including soft tissue and organ or muscle abscess formation and sepsis. 7 Evidence-based recommendations for the management of CIED infections have been provided by the American Heart Association. 7 It recommends that all infections should be treated with antibiotic therapy, and in cases of severe infection, the device should be removed. Number of patients According to public hospital reports from the Australian Institute of Health and Welfare (AIHW), between 2009 and 2010 there were: 12,842 procedures for insertion of cardiac pacemaker generators 5,083 procedures for adjustment, replacement or removal of cardiac pacemaker generators 3,198 procedures for insertion of cardiac defibrillator generators 1,644 procedures for adjustment, replacement or removal of cardiac defibrillator generators. 8 Medical Benefits Schedule (MBS) statistics for similar procedures within the private hospital system revealed that in 2012 there were: 8,342 services for permanent cardiac pacemaker insertion, removal or replacement (MSB item 38353) 742 services for automatic defibrillator generator insertion or replacement (MBS item 38387) 1,065 services for automatic defibrillator generator insertion or replacement not for patients with heart failure or as primary prevention for tachycardia arrhythmias (MBS item 38393). 9 CIED infection rates vary between medical centres but tend to occur in one to two per cent of patients receiving an implantable pacemaker or ICD. 10, 11 One Australian study investigating cases of pacemaker and ICD-related CIED infections in three hospitals in Geelong between June 1994 and December 2004 was identified. In this study, a total of 24 infections were recorded at the primary centre, where 1,481 operations were performed (infection rate of 1.6%). 12 All patients had received perioperative prophylactic antibiotics. There were no New Zealand data identified for the number of patients undergoing pacemaker or ICD implantation/removal, or for the incidence of CIED infection in this population. AIGISrx Antibacterial Envelope for implanted cardiac devices: August
7 Speciality Technology setting Cardiovascular disease and vascular surgery General hospital Impact Alternative and/or complementary technology As the AIGISRx Antibacterial Envelope works locally, at the site where the device is implanted, it is designed to complement, not replace, the current conventional treatment of prophylactic intravenous or oral systemic antibiotics for patients receiving an ICD or pacemaker implant. Current technology The current recommendation by the American Heart Association to prevent CIED infection is to use prophylactic antibiotics which have in vitro activity against Staphylococcus. 7 Cefazolin is the recommended antibiotic and should be administered intravenously one hour prior to surgery. In patients who are allergic to cephalosporins and in centres where oxacillin resistance among staphylococci is high, vancomycin is used as an alternative to cefazolin. It should be administered two hours prior to surgery. 7 The AIGISRX Antibacterial Envelope is the first antibacterial device for preventing infections associated with the implantation of cardiac devices. The purported advantages of using local antibacterial prophylaxis are as follows: higher local concentration of antibiotics than with systemic administration, thus reducing the potential for antibacterial resistance; ability to manipulate the duration of antibacterial activity; and more effective inhibition of bacterial colonisation of the implanted device. 2 Diffusion of technology in Australia The AIGISRx Antibacterial Envelope is yet to emerge in Australia. International utilisation Country Level of Use Trials underway or Limited use Widely diffused completed Canada USA a a A representative from TYRX Inc. has indicated that the AIGISRx Antibacterial Envelope is widely diffused in the USA however, as no evidence could be found to support this in the literature its level of use is marked as trials underway or completed. AIGISrx Antibacterial Envelope for implanted cardiac devices: August
8 Cost infrastructure and economic consequences The AIGISRx Antibacterial Envelope costs US $895 (A$ ) for either the medium pacemaker size (6.3 cm x 6.9 cm) or large (ICD) sizes (7.4 cm x 8.5 cm). No additional costs are anticipated for use of this device. 13 Ethical, cultural or religious considerations No ethical, cultural or religious considerations were identified in the published literature. Evidence and Policy Safety and effectiveness One non-randomised comparative study with a historical control (level III-3 Intervention evidence) and one retrospective case series study (level IV Intervention evidence) were included in this Technology Brief. The historical control group in the comparative study was a cohort of patients with matched risk factors and a CIED implanted prior to the use of the antibacterial envelope. The safety and effectiveness of the AIGISRx was assessed in a total of 884 patients across the two studies. Relevant details regarding the studies are presented in Table 1. Table 1 Details of the included studies Study Kolek et al, Bloom et al, Level of evidence III-3 IV Number of patients AIGISRx n = 260, Control n = Patient details Intervention Comparative treatment Patients with 2 risk factors for CIED infection who had a CIED implanted (types of CIED not stated) Implantation with AIGISRx plus systemic prophylactic antibiotics Implantation with systemic prophylactic antibiotics Patients with a first-time PM, ICD or CRT-D or revision/replacement implantation utilizing the AIGISRx AIGISRx NA Outcomes CIED infection, deaths Implantation success, CIED infection Randomisation NA NA Mean follow-up (SD) Conflict of interest 19 months (SD 7.7) (intervention group) 42 months (SD 5.2) (control group) One author received honorarium/speakers fees from TYRX, Inc. 2 months (SD 2.4) Authors received research grant to conduct study from TYRX, Inc., who defined the budget and scope of the research. One author is the Chief Medical Officer at TYRX, Inc. CRT-D: cardiac resynchronisation therapy device with defibrillator; ICD: implantable cardioverter-defibrillator; PM: pacemaker; NA: not applicable; SD: standard deviation. 1 1 US = AUD (source: currency-converter.com, 16 th July 2013) AIGISrx Antibacterial Envelope for implanted cardiac devices: August
9 Kolek et al, A non-randomised comparative study with a historical control (Level III-3 Intervention Evidence) was conducted in the USA by Kolek et al. (2013) 14 The intervention group included 260 consecutive adults who had a CIED implanted with an AIGISRx Envelope between November 1, 2009 and April 30, The patients had at least two of the following risk factors present within two weeks of original implantation: diabetes, renal insufficiency (creatinine 1.5 mg/dl 24 hours prior to implantation), systemic anticoagulation (treatmentdose heparin or warfarin), chronic daily corticosteroid use, fever (temperature 38.1 C) or leucocytosis ( 11,000 white blood cells/µl 24 hours prior to implantation), prior documented CIED infection, or at least three trans-venous leads (3-lead cardiac resynchronisation therapy system or at least one abandoned lead, pacemaker dependence, or early pocket re-entry). The historical control group (n = 639) was derived from a patient database. The database is a de-identified, time-shifted and previously validated clone of the electronic medical record. Adult patients who had a CIED implanted between August 2007 and February 2009 (18 months prior to the institutional use of the AIGISRx envelope) and who had at least two risk factors for CIED infection (as defined above) were included. Selection was not consecutive. The types of CIED devices implanted were not described. All patients in the intervention and control cohorts received peri-procedural antibiotic prophylaxis infused from 0 to 15 minutes prior to skin incision. Outpatients received 1 g of intravenous cefazolin unless they were allergic to penicillin, in which case they received 1 g of vancomycin. The mean duration of follow-up was 19 months (standard deviation [SD] 7.7 months) for the intervention group and 42 months (SD 5.2) for the control group. No losses to follow-up were reported. The mean age of the intervention group was 67 years (SD 12.5) and 30 per cent were women. The mean age of the control group was 68 years (SD 13.0) and 37 per cent were female. The number of risk factors did not differ between the two groups, with both having a mean of 3 (SD 1.2) (p > 0.05), however, significant differences were noted between the intervention and control groups with respect to the type of risk factors. Significantly more patients in the intervention group had at least three leads (62.7% versus 26.5%), experienced early pocket re-entry (15% versus 3%) or were undergoing either a generator change, device upgrade, or lead or other revision (47.3% versus 34%). In comparison, significantly more patients in the control group had diabetes (54.1% versus 40.8%; p <0.001). Safety Death was only reported for patients who developed an infection. In the control group (n = 639), four of the 19 patients who developed an infection died (21%). CIED infection was a cause of, or major contributing factor to, mortality in three of the four patients. The cause of death in the other control patient was not mentioned. No deaths occurred in the AIGISrx Antibacterial Envelope for implanted cardiac devices: August
10 intervention group. No mention was made in the study as to whether any deaths occurred in patients without infections. Major and minor complications were not reported. Effectiveness The study s primary outcome was CIED infection after a minimum of 90 days follow-up. CIED infection was defined as local or systemic infection (e.g., sepsis, bacteraemia or endocarditis) requiring systemic antibiotics and/or explantation of the CIED system. The incidence of infection was significantly lower in the intervention, with 0.4 per cent (1/260 patients) of the patients developing an infection compared with three per cent (19/639 patients) in the control group (p = 0.04). The one patient who developed an infection in the intervention group was a 77-year old man with a history of atrial fibrillation, chronic anticoagulation, heart failure and left bundle branch block. He had previously undergone relocation of a left-sided cardiac resynchronisation therapy device with defibrillator (CRT-D) to his right chest to facilitate local radiation therapy for lung cancer. Forty-five days after this procedure, he presented with poor wound healing, which necessitated relocation of the device to the left chest. Two months later he developed a skin erosion that required pocket revision. During this procedure an AIGISRx Antibacterial Envelope was used. One week after treatment he presented with fever, pneumonia, purulent discharge from the incision and a positive blood culture (Pseudomonas aeruginosa). He was treated with systemic antibiotics and the CIED was explanted. Later, he underwent implantation of a new CRT-D with the AIGISRx Envelope. No further infection was observed during nine months of follow-up. Of the 19 patients in the control group who developed infections, 13 were considered systemic infections whilst the other six appeared to be localised to the device. The mean time from CIED implantation until diagnosis of infection was 5.1 months (SD 4.1). Treatment included complete system removal in addition to parenteral antibiotics (13 patients) or antibiotics alone (6 patients). In order to account for the effect of significant differences in individual CIED infection risk factors on CIED infection incidence between the intervention and control groups, the authors re-evaluated the data using multivariate logistic regression, after adjustment for the variable of having an antibacterial envelope, using a propensity score. In addition, they further analysed the data by univariate logistic regression on propensity score matched data. The significant difference in CIED infection rate between the intervention and control groups persisted, even after adjustment using the propensity score (odds ratio 0.09, 95% CI [0.01, 0.73], p = 0.024) and propensity score matching (odds ratio 0.11, 95% CI[0.01, 0.85], p = 0.04). AIGISrx Antibacterial Envelope for implanted cardiac devices: August
11 Bloom et al, A case series (level IV Intervention evidence) was conducted at 10 medical centres (academic, community and Veterans Affairs) in the USA by Bloom et al. (2011) with data reported retrospectively. 15 A total of 642 consecutive CIED patients who had undergone initial implantation or revision/replacement procedures utilising the AIGISRx Antibacterial Envelope between June 2008 and June 2009 were enrolled. Of the included patients, 35 per cent received pacemakers, 29 per cent received an ICD and 36 per cent received CRT-Ds. Revision/replacement procedures accounted for 62 per cent of the pacemaker procedures, 73 per cent of the ICD procedures and 69 per cent of the CRT-D procedures. The patients had an average of three (SD 1.4) predefined risk factors for infection based on nine predefined risk factors the authors identified from published literature. Forty-nine per cent had three or more predefined risk factors. At least one method of antimicrobial prophylaxis (oral, intravenous, or generator pocket irrigation), in addition to the antibacterial envelope, was administered in 99.5 per cent of the procedures. Antibiotics were administered systemically prior to or during the procedure (88%), locally to the generator pocket during the procedure (87%) and/or systemically after the procedure (70%). Of the 642 patients, 17 were excluded from analyses following device removal for a prior CIED infection and one was excluded from analysis because the antibacterial envelope had been used for an offlabel indication. Seven of the patients died, although the authors did not state when the deaths occurred; these patients were included in the analyses of implantation success rate and incidence of infection. The mean duration of follow-up was two months (SD 2.4). Of the included patients, 32 per cent were women. The mean age of the entire cohort was 70 years (SD 13.0). Safety There were seven deaths. The authors stated that none of these were attributable to the antibacterial envelope or the CIED procedure. Major and minor complications were not reported. Effectiveness The primary effectiveness endpoints of the study included successful CIED implantation (defined as implantation without removal in the subsequent 24 hours) and the occurrence of major and minor CIED infections. Minor infection was regarded as superficial incisional SSI, while major infection was defined as deep incisional or organ/space (generator pocket) SSI or endocarditis. A replacement/revision procedure was defined as any CIED procedure performed between the time of implantation and hospital discharge. A CIED implantation success rate of 99.5 per cent was reported (621 out of 624 procedures). The three unsuccessful implantations were all CRT-Ds. One of these was attributed to the antibacterial envelope, the distal end of which folded upon itself and made it difficult to AIGISrx Antibacterial Envelope for implanted cardiac devices: August
12 implant the CRT-D. The other two unsuccessful implantations were caused by a stenosed innominate vein and the need for lead repositioning. Overall, the incidence of infection was 0.48 per cent (0% for initial procedures and 0.7% for replacement/revision procedures). The highest rate of infection occurred for CRT-Ds (0.89%), followed by ICDS (0.56%). There were no CIED infections in patients receiving pacemakers. All three infections which occurred during the study were considered major (one in the ICD group and two in the CRT-D group) and resulted in the CIEDS being explanted. In two of the three patients, the diagnosis was made within 12 days of the procedure. In the third patient, a chronic, non-healing incision at the implantation site was monitored until the CIED was finally removed 146 days after the procedure. No minor infections were reported. Economic evaluation The potential cost effectiveness of an AIGISRx ICD Antibacterial Envelope has been estimated in a USA setting. 16 The estimated absolute risk reduction associated with the use of the device was 2.5 per cent, with a corresponding 40 patients needed to treat to prevent one infection. Using data from Sohail et al 17, Ellis and Kolek 16 estimate that USA hospitals could expect to recoup US$17,549 (A$19, ) for each ICD-related infection that could be prevented. Ongoing research Two clinical trials that are currently underway were identified from searches of and the Australian and New Zealand Clinical Trials Register (Table 2). Table 2 Registered clinical trials underway for AIGISRx Trial Identifier Trial Status N Study location Study Design Interventions Outcome measures Estimated completion date NCT Recruitment suspended. Safety data collection in progress 465 United States (multicentre) Non-randomised comparative study AIGISRX vs no AIGISRX CRMD mechanical complicatio n July 2013 NCT Recruitment suspended. Safety data analysis continues 4000 * United States (multicentre) Non-randomised comparative study AIGISRx vs no AIGISRX Major CRMD infection July 2013 *Estimated enrolment; CRMD: cardiac rhythm management device 2 1 US = AUD (source: currency-converter.com.au, July 16 th 2013) AIGISrx Antibacterial Envelope for implanted cardiac devices: August
13 Both trials are sponsored by TYRX, Inc., the company that manufactures the AIGISRx Antibacterial Envelope. The study population in one of the trials is patients who have undergone cardiac rhythm management device (CRMD) replacement with an ICD (NCT ), whilst the study population in the other trial is patients who have undergone CRMD replacement with a CRT (NCT ). Other issues Bloom et al. (2011) 15 acknowledged several limitations to their study, including the retrospective design and its associated biases, the short follow-up, the lack of a control group, and the small study sample size. They suggested that a prospective randomised controlled trial is required to further evaluate the antibacterial envelope. The study reported that data were analysed on an intent-to-treat basis; however, the authors stated that 18 of the enrolled patients were excluded. These patients were not included in the analyses. No mention was made in the methods regarding pre-defined exclusion criteria. The manufacturers of the AIGISRx Antibacterial Envelope state that it is indicated for holding a pacemaker or ICD. In the study by Bloom et al. (2011) 15, patients with a CRT-D were included as well, so the results may not reflect the population for which the device is intended. The authors stated that, in addition to the antibacterial envelope, at least one method of antimicrobial prophylaxis (oral, intravenous or generator pocket irrigation) was administered in 99.5 per cent of procedures. They were administered systemically prior to or during the procedure, locally to the generator pocket during the procedure and/or systemically after the procedure. It is possible that the method and timing of antimicrobial prophylaxis may affect the results achieved with the AIGISRx Antibacterial Envelope. Ideally, it should be assessed in patients who have all received the same prophylactic antibiotic treatment with respect to method and timing of administration and type of antibiotic used. Conflicts of interest associated with this study are that it was funded by the manufacturer of the AIGISRx Antibacterial Envelope, which prospectively defined the budget and scope of the research. In addition, one of the authors is the Chief Medical Officer at TYRX, Inc. Kolek et al. (2013) 14 also reported several limitations to their study, including that it was a single-centre study and, therefore, the results may not be generalisable to a broader clinician or patient population. Furthermore, it was retrospective in design and used a historical control group. They stated that differences in clinical practice between the intervention and control groups may have resulted in the observed differences in CIED infection incidence. Another potentially confounding variable was the difference in prevalence of various CIED infection risk factors between the treatment groups. They further stated that women were under-represented in the study and that there might be differences between men and women in their risk of CIED infection. The types of CIED devices that were implanted in the study were not stated, so it is unknown whether the AIGISrx Antibacterial Envelope for implanted cardiac devices: August
14 patient population was representative of those for whom the device is indicated. A conflict of interest associated with this study is that one of the authors is a recipient of honorarium/speakers fees from TYRX, Inc. It should be noted that a newer generation fully-resorbable AIGISRx Antibacterial Envelope has been developed. The fully resorbable AIGISRx Antibacterial Envelope is constructed from a bioresorbable multifilament knitted mesh substrate (polymer made of glycolide, caprolactone, and trimethylene carbonate) that is coated with a bioresorbable polyarylate polymer. It comes in the same sizes as the non-resorbable envelope. This newer device was given FDA 510 (k) approval in May 2013 (K130943) and in January 2013 TYRX received a licence from Health Canada to market the fully resorbable antibacterial envelope with implantable electronic devices. At a cost of US $ (A$1, ) for both the medium and large sizes it is slightly more expensive than the non-resorbable antibacterial envelope. Currently no peer reviewed studies have been published on this newer fully resorbable version of the antibacterial envelope and thus its benefits, if any, over the non-resorbable envelope are unknown. Summary of findings Two studies were eligible for inclusion in this Technology Brief: one retrospective case series study and one non-randomised comparative study with a historical control cohort. In total, the AIGISRx Antibacterial Envelope was evaluated in 884 patients who were implanted with CIEDs for either primary or replacement/revision procedures and who had two or more risk factors for CIED infection. Only the case series study reported the type of devices that were implanted, which included pacemakers, ICDs and CRT-Ds. Neither study reported on complications related to the implant procedure. No deaths were associated with the use of the antibacterial envelope. The incidence of infection was low in both studies (<0.5%) considering that the selected patients had a high risk for CIED infection. In addition, the patients implanted with the AIGISRx Antibacterial Envelope in the comparative study had a significantly lower incidence of infection compared with the historical control group. Only the case series reported on CIED implantation success rate, which was very high (99.5%). Only one of the three unsuccessful implants was attributed to the AIGISRx Antibacterial Envelope. Several limitations/issues were identified with both studies, many of them highlighted by the authors, which compromised the validity of their results. The comparative study was limited by its retrospective design, used an historical control and had significant differences in the prevalence of various CIED infection risk factors between the treatment groups that may have affected treatment outcomes. With respect to the case series, it was retrospective in design, had a very short follow-up period and the patients varied in the timing, type and 3 1 US = AUD (source: currency-converter.com, 16 th July 2013) AIGISrx Antibacterial Envelope for implanted cardiac devices: August
15 method of administration of the prophylactic antibiotic treatment they received. The study reported that data were analysed on an intention-to-treat basis; however, 18 of the enrolled patients were excluded from analysis. Both studies had conflicts of interest in that they had connections to the company that manufactures the AIGISRx Antibacterial Envelope. It was not clear whether the patient population in either study was entirely representative of that for which the device is indicated by the manufacturers. HealthPACT assessment Positive results have been reported for the AIGISRx Antibacterial Envelope with respect to CIED implantation success and incidence of infection. However, this has come from two lowquality studies and data from randomised trials demonstrating a clear reduction in device pocket infections are required to reliably inform its clinical use. It is, therefore, recommended that the AIGISRx Antibacterial Envelope be monitored for a period of 24 months. During this time, results from two non-randomised comparative trials that are currently underway should become available in the peer-reviewed literature and will add to the current evidence base for the technology. Number of studies included All evidence included for assessment in this Technology Brief has been assessed according to the revised NHMRC levels of evidence. A document summarising these levels may be accessed via the HealthPACT web site. Total number of studies 2 Total number of Level III-3 studies 1 Total number of Level IV studies 1 References 1. TYRX (2013). AIGISRx Overview [Internet]. TYRX. Available from: [Accessed 3rd June 2013]. 2. Darouiche, R. O. & Voigt, J. (2010). Antibacterial prophylaxis in cardiac implantable electronic devices, EP Lab Digest [Internet]. December 2010 (Supplement), Available from: US/TYRX%20EPLD%20Supplement%2012%2010%2010.pdf [Accessed 3 June 2013]. 3. Food and Drug Administration (2013). 510(k) Premarket Notification. [Internet]. U.S. Department of Health and Human Services. Available from: [Accessed 1st July 2013]. 4. Health Canada (2009). Medical Devices Active License Listing [Internet]. Health Canada. Available from: [Accessed 16th July 2013]. 5. Padeletti, L., Mascioli, G. et al (2011). 'Critical appraisal of cardiac implantable electronic devices: complications and management'. Med Devices (Auckl), 4, AIGISrx Antibacterial Envelope for implanted cardiac devices: August
16 6. Uslan, D. Z. (2009). Overview of infections of cardiac rhythm management devices, EP Lab Digest [Internet], May 2009 (Supplement), 2-5. Available from: US/EP_TYRX_FinalPDF.pdf [Accessed 3 June 2013]. 7. Baddour, L. M., Epstein, A. E. et al (2010). 'Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association'. Circulation, 121 (3), Australian Institute of Health and Welfare (2013). Procedures data cubes [Internet]. AIHW. Available from: [Accessed 4 June 2013]. 9. Australian Government, Department of Health and Ageing (2013). MBS Online: Medicare Benefits Schedule [Internet]. Australian Government Department of Health and Ageing. Available from: [Accessed 4 June 2013]. 10. Greenspon, A. J., Patel, J. D. et al (2011). '16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008'. J Am Coll Cardiol, 58 (10), Nery, P. B., Fernandes, R. et al (2010). 'Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences'. J Cardiovasc Electrophysiol, 21 (7), Catanchin, A., Murdock, C. J.&Athan, E. (2007). 'Pacemaker infections: a 10-year experience'. Heart Lung Circ, 16 (6), National Institute for Health Research (2012). AIGISRx Antibacterial Envelope for preventing infection in implanted cardiac devices [Internet]. NIHR Horizon Scanning Centre, University of Birmingham. Available from: [Accessed 19 July 2013]. 14. Kolek, M. J., Dresen, W. F. et al (2013). 'Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients'. Pacing Clin Electrophysiol, 36 (3), Bloom, H. L., Constantin, L. et al (2011). 'Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope'. Pacing Clin Electrophysiol, 34 (2), Ellis, C. R. & Kolek, M. J. (2011). 'Rising infection rate in cardiac electronic device implantation; the role of the AIGISRx Antibacterial Envelope in prophylaxis'. Combination Products in Therapy, 1 (1), Sohail, M. R., Henrikson, C. A. et al (2011). 'Mortality and cost associated with cardiovascular implantable electronic device infections'. Arch Intern Med, 171 (20), Search criteria to be used (MeSH terms) Text words were: AIGISrx Antibacterial Envelope for implanted cardiac devices: August
17 AIGISRx, antibacterial envelope, cardiovascular implantable electronic device* AND antimicrobial, cardiovascular implantable electronic device* AND antibiotic*, CIED and antimicrobial*, CIED and antibiotic*, CIED and antibacterial, cardiac pacemaker and antibiotic*, cardiac pacemaker and antimicrobial*, cardiac pacemaker and antibacterial, defibrillator AND antimicrobial*, defibrillator AND antibiotic*, defibrillator AND antibacterial. AIGISrx Antibacterial Envelope for implanted cardiac devices: August
Hemodialysis catheter infection
 Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Health Policy Advisory Committee on Technology
 Health Policy Advisory Committee on Technology Technology Brief Microbial sealant to reduce surgical site infections following coronary artery bypass graft August 2012 State of Queensland (Queensland Health)
Health Policy Advisory Committee on Technology Technology Brief Microbial sealant to reduce surgical site infections following coronary artery bypass graft August 2012 State of Queensland (Queensland Health)
Antibiotic Prophylaxis for the Prevention of Infective Endocarditis and Prosthetic Joint Infections for Dentists
 PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
Fungal Infection in Total Joint Arthroplasty. Dr.Wismer Dr.Al-Sahan
 Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
Fungal Infection in Total Joint Arthroplasty Dr.Wismer Dr.Al-Sahan Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature David M.
Surgical Site Infection Prevention
 Surgical Site Infection Prevention 1 Objectives 1. Discuss risk factors for SSI 2. Describe evidence-based best practices for SSI prevention 3. State principles of antibiotic prophylaxis 4. Discuss novel
Surgical Site Infection Prevention 1 Objectives 1. Discuss risk factors for SSI 2. Describe evidence-based best practices for SSI prevention 3. State principles of antibiotic prophylaxis 4. Discuss novel
Post-surgical V.A.C. VeraFlo Therapy with Prontosan Instillation on Inpatient Infected Wounds * COLLECTION OF CASE STUDIES
 COLLECTION OF CASE STUDIES Post-surgical V.A.C. VeraFlo Therapy with Prontosan Instillation on Inpatient Infected Wounds * *All patients were treated with systemic antibiotics Post-surgical V.A.C. VeraFlo
COLLECTION OF CASE STUDIES Post-surgical V.A.C. VeraFlo Therapy with Prontosan Instillation on Inpatient Infected Wounds * *All patients were treated with systemic antibiotics Post-surgical V.A.C. VeraFlo
Atrial Fibrillation and Cardiac Device Therapy RAKESH LATCHAMSETTY, MD DIVISION OF ELECTROPHYSIOLOGY UNIVERSITY OF MICHIGAN HOSPITAL ANN ARBOR, MI
 Atrial Fibrillation and Cardiac Device Therapy RAKESH LATCHAMSETTY, MD DIVISION OF ELECTROPHYSIOLOGY UNIVERSITY OF MICHIGAN HOSPITAL ANN ARBOR, MI Outline Atrial Fibrillation What is it? What are the associated
Atrial Fibrillation and Cardiac Device Therapy RAKESH LATCHAMSETTY, MD DIVISION OF ELECTROPHYSIOLOGY UNIVERSITY OF MICHIGAN HOSPITAL ANN ARBOR, MI Outline Atrial Fibrillation What is it? What are the associated
Staphyloccus aureus sepsis: follow- up practice guidelines
 Staphyloccus aureus sepsis: follow- up practice guidelines March 17, 2012 National Study Day Hospital Antibiotic Stewardship prof. dr. Dirk Vogelaers, Ghent University Hospital apr. Franky Buyle, Ghent
Staphyloccus aureus sepsis: follow- up practice guidelines March 17, 2012 National Study Day Hospital Antibiotic Stewardship prof. dr. Dirk Vogelaers, Ghent University Hospital apr. Franky Buyle, Ghent
Health Policy Advisory Committee on Technology
 Health Policy Advisory Committee on Technology Technology Brief ZIO XT Patch for diagnosis of cardiac arrhythmia March 2015 State of Queensland (Queensland Department of Health) 2015 This work is licensed
Health Policy Advisory Committee on Technology Technology Brief ZIO XT Patch for diagnosis of cardiac arrhythmia March 2015 State of Queensland (Queensland Department of Health) 2015 This work is licensed
SPONTANEOUS PNEUMOTHORAX AS A COMPLICATION OF SEPTIC PULMONARY EMBOLISM IN AN INTRAVENOUS DRUG USER: A CASE REPORT
 Spontaneous pneumothorax in an IV drug user SPONTANEOUS PNEUMOTHORAX AS A COMPLICATION OF SEPTIC PULMONARY EMBOLISM IN AN INTRAVENOUS DRUG USER: A CASE REPORT Chau-Chyun Sheu, Jhi-Jhu Hwang, Jong-Rung
Spontaneous pneumothorax in an IV drug user SPONTANEOUS PNEUMOTHORAX AS A COMPLICATION OF SEPTIC PULMONARY EMBOLISM IN AN INTRAVENOUS DRUG USER: A CASE REPORT Chau-Chyun Sheu, Jhi-Jhu Hwang, Jong-Rung
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Remote Monitoring of Cardiac Implantable Electrical Devices (CIEDs)
 Remote Monitoring of Cardiac Implantable Electrical Devices (CIEDs) Changing the face of enhanced self-management and improved coordinated healthcare K Fan, CKC Tsui, KL Au, RTC Ng, CYS Chung, KW Lai,
Remote Monitoring of Cardiac Implantable Electrical Devices (CIEDs) Changing the face of enhanced self-management and improved coordinated healthcare K Fan, CKC Tsui, KL Au, RTC Ng, CYS Chung, KW Lai,
CLINICAL QUALITY MEASURES FINALIZED FOR ELIGIBLE HOSPITALS AND CRITICAL ACCESS HOSPITALS BEGINNING WITH FY 2014
 CLINICAL QUALITY MEASURES FINALIZED FOR ELIGIBLE HOSPITALS AND CRITICAL ACCESS HOSPITALS BEGINNING WITH FY 2014 e 55 0495 2 Emergency Department (ED)- 1 Emergency Department Throughput Median time from
CLINICAL QUALITY MEASURES FINALIZED FOR ELIGIBLE HOSPITALS AND CRITICAL ACCESS HOSPITALS BEGINNING WITH FY 2014 e 55 0495 2 Emergency Department (ED)- 1 Emergency Department Throughput Median time from
healthcare associated infection 1.2
 healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet
 Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
PSA Screening for Prostate Cancer Information for Care Providers
 All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
What Are Arrhythmias?
 What Are Arrhythmias? Many people have questions about what the word arrhythmia means, and arrhythmias can be a difficult subject to understand. The text below should give you a better understanding of
What Are Arrhythmias? Many people have questions about what the word arrhythmia means, and arrhythmias can be a difficult subject to understand. The text below should give you a better understanding of
Atrial Fibrillation An update on diagnosis and management
 Dr Arvind Vasudeva Consultant Cardiologist Atrial Fibrillation An update on diagnosis and management Atrial fibrillation (AF) remains the commonest disturbance of cardiac rhythm seen in clinical practice.
Dr Arvind Vasudeva Consultant Cardiologist Atrial Fibrillation An update on diagnosis and management Atrial fibrillation (AF) remains the commonest disturbance of cardiac rhythm seen in clinical practice.
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Treating AF: The Newest Recommendations. CardioCase presentation. Ethel s Case. Wayne Warnica, MD, FACC, FACP, FRCPC
 Treating AF: The Newest Recommendations Wayne Warnica, MD, FACC, FACP, FRCPC CardioCase presentation Ethel s Case Ethel, 73, presents with rapid heart beating and mild chest discomfort. In the ED, ECG
Treating AF: The Newest Recommendations Wayne Warnica, MD, FACC, FACP, FRCPC CardioCase presentation Ethel s Case Ethel, 73, presents with rapid heart beating and mild chest discomfort. In the ED, ECG
CARDIO/PULMONARY MEDICINE FOR PRIMARY CARE. Las Vegas, Nevada Bellagio March 4 6, 2016. Participating Faculty
 CARDIO/PULMONARY MEDICINE FOR PRIMARY CARE Las Vegas, Nevada Bellagio March 4 6, 2016 Participating Faculty Friday, March 4th: 7:30 am - 8:00 am Registration and Hot Breakfast 8:00 am - 9:00 am Pulmonary
CARDIO/PULMONARY MEDICINE FOR PRIMARY CARE Las Vegas, Nevada Bellagio March 4 6, 2016 Participating Faculty Friday, March 4th: 7:30 am - 8:00 am Registration and Hot Breakfast 8:00 am - 9:00 am Pulmonary
Treatments to Restore Normal Rhythm
 Treatments to Restore Normal Rhythm In many instances when AF causes significant symptoms or is negatively impacting a patient's health, the major goal of treatment is to restore normal rhythm and prevent
Treatments to Restore Normal Rhythm In many instances when AF causes significant symptoms or is negatively impacting a patient's health, the major goal of treatment is to restore normal rhythm and prevent
Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013
 Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013 Family physician with Rivergrove Medical Clinic Practice in the north end since 1985 Medical Director of the Wellness
Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013 Family physician with Rivergrove Medical Clinic Practice in the north end since 1985 Medical Director of the Wellness
Management of Symptomatic Atrial Fibrillation
 Management of Symptomatic Atrial Fibrillation John F. MacGregor, MD, FHRS Associate Medical Director, Cardiac Electrophysiology PeaceHealth St. Joseph Medical Center, Bellingham, WA September 18, 2015
Management of Symptomatic Atrial Fibrillation John F. MacGregor, MD, FHRS Associate Medical Director, Cardiac Electrophysiology PeaceHealth St. Joseph Medical Center, Bellingham, WA September 18, 2015
Electronic Health Record (EHR) Data Analysis Capabilities
 Electronic Health Record (EHR) Data Analysis Capabilities January 2014 Boston Strategic Partners, Inc. 4 Wellington St. Suite 3 Boston, MA 02118 www.bostonsp.com Boston Strategic Partners is uniquely positioned
Electronic Health Record (EHR) Data Analysis Capabilities January 2014 Boston Strategic Partners, Inc. 4 Wellington St. Suite 3 Boston, MA 02118 www.bostonsp.com Boston Strategic Partners is uniquely positioned
RATE VERSUS RHYTHM CONTROL OF ATRIAL FIBRILLATION: SPECIAL CONSIDERATION IN ELDERLY. Charles Jazra
 RATE VERSUS RHYTHM CONTROL OF ATRIAL FIBRILLATION: SPECIAL CONSIDERATION IN ELDERLY Charles Jazra NO CONFLICT OF INTEREST TO DECLARE Relationship Between Atrial Fibrillation and Age Prevalence, percent
RATE VERSUS RHYTHM CONTROL OF ATRIAL FIBRILLATION: SPECIAL CONSIDERATION IN ELDERLY Charles Jazra NO CONFLICT OF INTEREST TO DECLARE Relationship Between Atrial Fibrillation and Age Prevalence, percent
How To Improve Health Care For Remote Workers
 CRM Devices and Telemonitoring Where the industry stands today Annette Brüls VP CRDM Marketing CareLink Status worldwide More than 450.000000 patients in > 4000 clinics > 30 countries 9 years of experience
CRM Devices and Telemonitoring Where the industry stands today Annette Brüls VP CRDM Marketing CareLink Status worldwide More than 450.000000 patients in > 4000 clinics > 30 countries 9 years of experience
National Patient Safety Goals Effective January 1, 2015
 National Patient Safety Goals Effective January 1, 2015 Goal 1 Improve the accuracy of resident identification. NPSG.01.01.01 Long Term are ccreditation Program Medicare/Medicaid ertification-based Option
National Patient Safety Goals Effective January 1, 2015 Goal 1 Improve the accuracy of resident identification. NPSG.01.01.01 Long Term are ccreditation Program Medicare/Medicaid ertification-based Option
Establishing a Remote Monitoring Program. Martha Ferrara, FNP
 Establishing a Remote Monitoring Program Martha Ferrara, FNP Establishing a Remote Monitoring Program What is Remote Monitoring? Martha Ferrara, FNP, CCDS November 2012 CIED Timeline: Cardiovascular Implantable
Establishing a Remote Monitoring Program Martha Ferrara, FNP Establishing a Remote Monitoring Program What is Remote Monitoring? Martha Ferrara, FNP, CCDS November 2012 CIED Timeline: Cardiovascular Implantable
ORTHOPAEDIC INFECTION PREVENTION AND CONTROL: AN EMERGING NEW PARADIGM
 ORTHOPAEDIC INFECTION PREVENTION AND CONTROL: AN EMERGING NEW PARADIGM AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77th Annual Meeting March 9-12, 2010 New Orleans, Louisiana COMMITTEE ON PATIENT SAFETY PREPARED
ORTHOPAEDIC INFECTION PREVENTION AND CONTROL: AN EMERGING NEW PARADIGM AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77th Annual Meeting March 9-12, 2010 New Orleans, Louisiana COMMITTEE ON PATIENT SAFETY PREPARED
Right-sided infective endocarditis:tunisian experience
 Right-sided infective endocarditis:tunisian experience L. Ammari, A. Ghoubontini, A. Berriche, R. Abdelmalek, S.Aissa, F.Kanoun, B.Kilani, H.Tiouiri Benaissa, T.Ben chaabane Department of Infectious diseases,
Right-sided infective endocarditis:tunisian experience L. Ammari, A. Ghoubontini, A. Berriche, R. Abdelmalek, S.Aissa, F.Kanoun, B.Kilani, H.Tiouiri Benaissa, T.Ben chaabane Department of Infectious diseases,
Hong Kong Joint Venture Agreements
 Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness,
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness,
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT of Atrial Fibrillation (AF)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT of Atrial Fibrillation (AF) Key priorities Identification and diagnosis Treatment for persistent AF Treatment for permanent AF Antithrombotic
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT of Atrial Fibrillation (AF) Key priorities Identification and diagnosis Treatment for persistent AF Treatment for permanent AF Antithrombotic
Disclosures. Anesthesia and Lead Extractions. Lead Extractions: Objectives. Lead Removal Techniques. None
 Anesthesia and Lead Extractions Disclosures None Bryan Ahlgren DO Assistant Professor University of Colorado Dept of Anesthesiology Objectives Define lead extraction procedures and why Anesthesiologists
Anesthesia and Lead Extractions Disclosures None Bryan Ahlgren DO Assistant Professor University of Colorado Dept of Anesthesiology Objectives Define lead extraction procedures and why Anesthesiologists
Appendix. Costing Case Samples for OOHCA
 Appendix Costing Case Samples for OOHCA The patient (ICD-1) Treatment Codes (OPCS 4) Patient 27 Admitted to ICU following percutaneous cardiac intervention (PCI) with 2 drugeluting stents following a VF
Appendix Costing Case Samples for OOHCA The patient (ICD-1) Treatment Codes (OPCS 4) Patient 27 Admitted to ICU following percutaneous cardiac intervention (PCI) with 2 drugeluting stents following a VF
A case of concurrent deep venous thrombosis, pseudoaneurysm, and extremity abscess in an intravenous methamphetamine abuser
 A case of concurrent deep venous thrombosis, pseudoaneurysm, and extremity abscess in an intravenous methamphetamine abuser BY MATTHEW L HARRISON Abstract Introduction. Intravenous drug abuse is a global
A case of concurrent deep venous thrombosis, pseudoaneurysm, and extremity abscess in an intravenous methamphetamine abuser BY MATTHEW L HARRISON Abstract Introduction. Intravenous drug abuse is a global
National Patient Safety Goals Effective January 1, 2015
 National Patient Safety Goals Goal 1 Nursing are enter ccreditation Program Improve the accuracy of patient and resident identification. NPSG.01.01.01 Use at least two patient or resident identifiers when
National Patient Safety Goals Goal 1 Nursing are enter ccreditation Program Improve the accuracy of patient and resident identification. NPSG.01.01.01 Use at least two patient or resident identifiers when
ECG may be indicated for patients with cardiovascular risk factors
 eappendix A. Summary for Preoperative ECG American College of Cardiology/ American Heart Association, 2007 A1 2002 A2 European Society of Cardiology and European Society of Anaesthesiology, 2009 A3 Improvement,
eappendix A. Summary for Preoperative ECG American College of Cardiology/ American Heart Association, 2007 A1 2002 A2 European Society of Cardiology and European Society of Anaesthesiology, 2009 A3 Improvement,
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT
 THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
Are venous catheters safe in terms of blood tream infection? What should I know?
 Are venous catheters safe in terms of blood tream infection? What should I know? DIAGNOSIS, PREVENTION AND TREATMENT OF HAEMODIALYSIS CATHETER-RELATED BLOOD STREAM INFECTIONS (CRBSI): A POSITION STATEMENT
Are venous catheters safe in terms of blood tream infection? What should I know? DIAGNOSIS, PREVENTION AND TREATMENT OF HAEMODIALYSIS CATHETER-RELATED BLOOD STREAM INFECTIONS (CRBSI): A POSITION STATEMENT
Lung Cancer. Know how to stay strong
 Lung Cancer Know how to stay strong What is cancer? 2 Cancer is a disease when some cells in the body grow out of control Normal cells Your body has many tiny cells and keeps making new cells to keep you
Lung Cancer Know how to stay strong What is cancer? 2 Cancer is a disease when some cells in the body grow out of control Normal cells Your body has many tiny cells and keeps making new cells to keep you
Nursing college, Second stage Microbiology Dr.Nada Khazal K. Hendi L14: Hospital acquired infection, nosocomial infection
 L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
Interventional Cardiology Peripheral Interventions Rhythm Management
 FY2016 Hospital Inpatient Rule (IPPS) Interventional Cardiology Peripheral Interventions Rhythm Management On April 17, 2015 the Centers for Medicare and Medicaid Services (CMS) released the Hospital Inpatient
FY2016 Hospital Inpatient Rule (IPPS) Interventional Cardiology Peripheral Interventions Rhythm Management On April 17, 2015 the Centers for Medicare and Medicaid Services (CMS) released the Hospital Inpatient
STROKE PREVENTION IN ATRIAL FIBRILLATION. TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: ABBREVIATIONS: BACKGROUND:
 STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
Open Ventral Hernia Repair
 Ventral Hernias Open Ventral Hernia Repair UCSF Postgraduate Course in General Surgery Maui, HI March 21, 2011 Hobart W. Harris, MD, MPH Ventral Hernias: National Experience Occur following 11-23% of laparotomies,
Ventral Hernias Open Ventral Hernia Repair UCSF Postgraduate Course in General Surgery Maui, HI March 21, 2011 Hobart W. Harris, MD, MPH Ventral Hernias: National Experience Occur following 11-23% of laparotomies,
Introduction. Planned surgical procedures
 Guidelines for the perioperative management of patients with implantable pacemakers or implantable cardioverter defibrillators, where the use of surgical diathermy/electrocautery is anticipated. Introduction
Guidelines for the perioperative management of patients with implantable pacemakers or implantable cardioverter defibrillators, where the use of surgical diathermy/electrocautery is anticipated. Introduction
INFORMED CONSENT FOR SLEEVE GASTRECTOMY
 INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
C-Difficile Infection Control and Prevention Strategies
 C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer [email protected] 1/18/2016 1 Disclosure This educational activity does not have commercial
C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer [email protected] 1/18/2016 1 Disclosure This educational activity does not have commercial
How should we treat atrial fibrillation in heart failure
 Advances in Cardiac Arrhhythmias and Great Innovations in Cardiology Torino, 23/24 Ottobre 2015 How should we treat atrial fibrillation in heart failure Matteo Anselmino Dipartimento Scienze Mediche Città
Advances in Cardiac Arrhhythmias and Great Innovations in Cardiology Torino, 23/24 Ottobre 2015 How should we treat atrial fibrillation in heart failure Matteo Anselmino Dipartimento Scienze Mediche Città
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
296 cohort patient study. May 2015. Spirometry-monitored deep breathing technique to increase the accuracy of radiotherapy treatment
 breath-hold radiotherapy for breast cancer: Cancer Partners UK s approach to improving outcomes in left-sided breast cancer radiotherapy - an evidence - based review 296 cohort patient study May 2015 Overview
breath-hold radiotherapy for breast cancer: Cancer Partners UK s approach to improving outcomes in left-sided breast cancer radiotherapy - an evidence - based review 296 cohort patient study May 2015 Overview
MN Community Measurement Total Knee Replacement Impact and Recommendation Document June 2010
 MN Community Measurement Total Knee Replacement Impact and Recommendation Document June 2010 Degree of Impact Relevance to Consumers, Employers and Payers Annually there are over 500,000 total knee replacement
MN Community Measurement Total Knee Replacement Impact and Recommendation Document June 2010 Degree of Impact Relevance to Consumers, Employers and Payers Annually there are over 500,000 total knee replacement
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal. Drugs for the treatment of pulmonary arterial hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Remit / Appraisal objective: Final scope To appraise the clinical and cost effectiveness of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Remit / Appraisal objective: Final scope To appraise the clinical and cost effectiveness of
ESCMID Online Lecture Library. by author
 Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
CCAD Training Manual. Cardiac Rhythm Management (CRM)
 CCAD Training Manual Cardiac Rhythm Management (CRM) Version 1.0 A D Cunningham 19/3/2008 Introduction This manual is intended to assist users of the Notes Client version of the CCAD Cardiac Rhythm Management
CCAD Training Manual Cardiac Rhythm Management (CRM) Version 1.0 A D Cunningham 19/3/2008 Introduction This manual is intended to assist users of the Notes Client version of the CCAD Cardiac Rhythm Management
Errors in the Operating Room. Patrick E. Voight RN BSN MSA CNOR President Association of perioperative Registered Nurses (AORN)
 Errors in the Operating Room Patrick E. Voight RN BSN MSA CNOR President Association of perioperative Registered Nurses (AORN) What What We All We Strive All Strive For: For: Patient Patient Safety Safety
Errors in the Operating Room Patrick E. Voight RN BSN MSA CNOR President Association of perioperative Registered Nurses (AORN) What What We All We Strive All Strive For: For: Patient Patient Safety Safety
ARTHROSCOPIC HIP SURGERY
 ARTHROSCOPIC HIP SURGERY Hip Arthroscopy is a relatively simple procedure whereby common disorders of the hip can be diagnosed and treated using keyhole surgery. Some conditions, which previously were
ARTHROSCOPIC HIP SURGERY Hip Arthroscopy is a relatively simple procedure whereby common disorders of the hip can be diagnosed and treated using keyhole surgery. Some conditions, which previously were
STANDARDS FOR IMPLANTATION AND FOLLOW-UP OF CARDIAC RHYTHM MANAGEMENT DEVICES IN ADULTS January 2013
 1. INTRODUCTION STANDARDS FOR IMPLANTATION AND FOLLOW-UP OF CARDIAC RHYTHM MANAGEMENT DEVICES IN ADULTS January 2013 This document replaces the previous Heart Rhythm UK documents Clinical Guidance by Consensus
1. INTRODUCTION STANDARDS FOR IMPLANTATION AND FOLLOW-UP OF CARDIAC RHYTHM MANAGEMENT DEVICES IN ADULTS January 2013 This document replaces the previous Heart Rhythm UK documents Clinical Guidance by Consensus
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry
 National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
Weight Loss before Hernia Repair Surgery
 Weight Loss before Hernia Repair Surgery What is an abdominal wall hernia? The abdomen (commonly called the belly) holds many of your internal organs. In the front, the abdomen is protected by a tough
Weight Loss before Hernia Repair Surgery What is an abdominal wall hernia? The abdomen (commonly called the belly) holds many of your internal organs. In the front, the abdomen is protected by a tough
Inpatient Anticoagulation Safety. To provide safe and effective anticoagulation therapy through a collaborative approach.
 Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
INFORMED CONSENT DERMABRASION
 INFORMED CONSENT DERMABRASION INSTRUCTION This is an informed-consent document which has been prepared to help your plastic surgeon inform you about dermabrasion, its risks, and alternative treatments.
INFORMED CONSENT DERMABRASION INSTRUCTION This is an informed-consent document which has been prepared to help your plastic surgeon inform you about dermabrasion, its risks, and alternative treatments.
NSQHS Standard 1 Governance
 NSQHS Standard 1 Governance Definitions sheet Governance Audit Tools Definitions Contents 1. Open Disclosure Program Page 1 2. ACUTE Clinical Record Audit Tools Page 2 -----------------------------------------------------------------------------------
NSQHS Standard 1 Governance Definitions sheet Governance Audit Tools Definitions Contents 1. Open Disclosure Program Page 1 2. ACUTE Clinical Record Audit Tools Page 2 -----------------------------------------------------------------------------------
Reimbursement Information For Electrophysiology and Arrhythmia Service Procedures 1
 GE Healthcare Information For Electrophysiology and Arrhythmia Procedures 1 2011 Update www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for electrophysiology procedures
GE Healthcare Information For Electrophysiology and Arrhythmia Procedures 1 2011 Update www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for electrophysiology procedures
Christopher M. Wright, MD, MBA Pioneer Cardiovascular Consultants Tempe, Arizona
 Christopher M. Wright, MD, MBA Pioneer Cardiovascular Consultants Tempe, Arizona Areas to be covered Historical, current, and future treatments for various cardiovascular disease: Atherosclerosis (Coronary
Christopher M. Wright, MD, MBA Pioneer Cardiovascular Consultants Tempe, Arizona Areas to be covered Historical, current, and future treatments for various cardiovascular disease: Atherosclerosis (Coronary
10. Treatment of peritoneal dialysis associated fungal peritonitis
 10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
It is important that you read this information carefully and completely.
 Placement of Permanent Breast Implant Following Tissue Expansion 1. I hereby authorize Dr. John P. Stratis and such assistants as may be selected to perform the following procedure or treatment INFORMED-
Placement of Permanent Breast Implant Following Tissue Expansion 1. I hereby authorize Dr. John P. Stratis and such assistants as may be selected to perform the following procedure or treatment INFORMED-
2013 Medicare Physician Coding and Reimbursement Changes
 2013 Medicare Physician Coding and Reimbursement Changes Disclaimer This presentation is intended for educational use. Any duplication is prohibited without written consent of Medtronic s Economic Strategies
2013 Medicare Physician Coding and Reimbursement Changes Disclaimer This presentation is intended for educational use. Any duplication is prohibited without written consent of Medtronic s Economic Strategies
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants
 Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
FY2015 Final Hospital Inpatient Rule Summary
 FY2015 Final Hospital Inpatient Rule Summary Interventional Cardiology (IC) Peripheral Interventions (PI) Rhythm Management (RM) On August 4, 2014, the Centers for Medicare & Medicaid Services (CMS) released
FY2015 Final Hospital Inpatient Rule Summary Interventional Cardiology (IC) Peripheral Interventions (PI) Rhythm Management (RM) On August 4, 2014, the Centers for Medicare & Medicaid Services (CMS) released
2014: nowadays the one shot technologies and the injectable monitor allow a wide and complete AF patient management. Why shouldn t we use them?
 2014: nowadays the one shot technologies and the injectable monitor allow a wide and complete AF patient management. Why shouldn t we use them? Gaetano Senatore DIVISION OF CARDIOLOGY HOSPITAL OF CIRIE
2014: nowadays the one shot technologies and the injectable monitor allow a wide and complete AF patient management. Why shouldn t we use them? Gaetano Senatore DIVISION OF CARDIOLOGY HOSPITAL OF CIRIE
2015 Subcutaneous Implantable Defibrillator (the S-ICD TM System) Coding Guide Rhythm Management
 2015 Subcutaneous Implantable Defibrillator (the S-ICD TM System) Coding Guide Rhythm Management Contents Physician Coding... 2 Hospital Outpatient Coding and Payment... 3 Ambulatory Surgery Center (ASC)
2015 Subcutaneous Implantable Defibrillator (the S-ICD TM System) Coding Guide Rhythm Management Contents Physician Coding... 2 Hospital Outpatient Coding and Payment... 3 Ambulatory Surgery Center (ASC)
Antimicrobial Prophylaxis for Transrectal Prostate Biopsy: Organizational Recommendations. J. Stuart Wolf, Jr., M.D.
 Antimicrobial Prophylaxis for Transrectal Prostate Biopsy: Organizational Recommendations J. Stuart Wolf, Jr., M.D. Department of Urology University of Michigan Ann Arbor, MI Official Recommendations for
Antimicrobial Prophylaxis for Transrectal Prostate Biopsy: Organizational Recommendations J. Stuart Wolf, Jr., M.D. Department of Urology University of Michigan Ann Arbor, MI Official Recommendations for
Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty
 Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Inferior Vena Cava filter and removal
 Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Failure or significant adverse effects to all of the alternatives: Eliquis and Xarelto
 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutics
This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutics
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
STROKE PREVENTION IN ATRIAL FIBRILLATION
 STROKE PREVENTION IN ATRIAL FIBRILLATION OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention of ischemic stroke and arterial thromboembolism in patients
STROKE PREVENTION IN ATRIAL FIBRILLATION OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention of ischemic stroke and arterial thromboembolism in patients
September 12, 2011. Dear Dr. Corrigan:
 September 12, 2011 Janet M. Corrigan, PhD, MBA President and Chief Executive Officer National Quality Forum 601 13th Street, NW Suite 500 North Washington, D.C. 20005 Re: Measure Applications Partnership
September 12, 2011 Janet M. Corrigan, PhD, MBA President and Chief Executive Officer National Quality Forum 601 13th Street, NW Suite 500 North Washington, D.C. 20005 Re: Measure Applications Partnership
Department of Pharmacy, Kaiser Permanente San Francisco Medical Center, San Francisco 94115, California, USA
 Journal of Pharmacy and Pharmacology 3 (2015) 33-38 doi: 10.17265/2328-2150/2015.01.005 D DAVID PUBLISHING Evaluation of Glycemic Control with a Pharmacist-Managed Post-Cardiothoracic Surgery Insulin Protocol
Journal of Pharmacy and Pharmacology 3 (2015) 33-38 doi: 10.17265/2328-2150/2015.01.005 D DAVID PUBLISHING Evaluation of Glycemic Control with a Pharmacist-Managed Post-Cardiothoracic Surgery Insulin Protocol
Thrombosis and Hemostasis
 Thrombosis and Hemostasis Wendy Lim, MD, MSc, FRCPC Associate Professor, Department of Medicine McMaster University, Hamilton, ON Overview To review the important developments in venous thromboembolism
Thrombosis and Hemostasis Wendy Lim, MD, MSc, FRCPC Associate Professor, Department of Medicine McMaster University, Hamilton, ON Overview To review the important developments in venous thromboembolism
With Big Data Comes Big Responsibility
 With Big Data Comes Big Responsibility Using health care data to emulate randomized trials when randomized trials are not available Miguel A. Hernán Departments of Epidemiology and Biostatistics Harvard
With Big Data Comes Big Responsibility Using health care data to emulate randomized trials when randomized trials are not available Miguel A. Hernán Departments of Epidemiology and Biostatistics Harvard
Atrial Fibrillation 2014 How to Treat How to Anticoagulate. Allan Anderson, MD, FACC, FAHA Division of Cardiology
 Atrial Fibrillation 2014 How to Treat How to Anticoagulate Allan Anderson, MD, FACC, FAHA Division of Cardiology Projection for Prevalence of Atrial Fibrillation: 5.6 Million by 2050 Projected number of
Atrial Fibrillation 2014 How to Treat How to Anticoagulate Allan Anderson, MD, FACC, FAHA Division of Cardiology Projection for Prevalence of Atrial Fibrillation: 5.6 Million by 2050 Projected number of
Atrioventricular (AV) node ablation
 Patient information factsheet Atrioventricular (AV) node ablation The normal electrical system of the heart The heart has its own electrical conduction system. The conduction system sends signals throughout
Patient information factsheet Atrioventricular (AV) node ablation The normal electrical system of the heart The heart has its own electrical conduction system. The conduction system sends signals throughout
Measures for the Australian health system. Belinda Emms Health Care Safety and Quality Unit Australian Institute of Health and Welfare
 Measures for the Australian health system Belinda Emms Health Care Safety and Quality Unit Australian Institute of Health and Welfare Two sets of indicators The National Safety and Quality Indicators Performance
Measures for the Australian health system Belinda Emms Health Care Safety and Quality Unit Australian Institute of Health and Welfare Two sets of indicators The National Safety and Quality Indicators Performance
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
OFFICE OF GROUP BENEFITS 2014 OFFICE OF GROUP BENEFITS CDHP PLAN FOR STATE OF LOUISIANA EMPLOYEES AND RETIREES PLAN AMENDMENT
 OFFICE OF GROUP BENEFITS 2014 OFFICE OF GROUP BENEFITS CDHP PLAN FOR STATE OF LOUISIANA EMPLOYEES AND RETIREES PLAN AMENDMENT This Amendment is issued by the Plan Administrator for the Plan documents listed
OFFICE OF GROUP BENEFITS 2014 OFFICE OF GROUP BENEFITS CDHP PLAN FOR STATE OF LOUISIANA EMPLOYEES AND RETIREES PLAN AMENDMENT This Amendment is issued by the Plan Administrator for the Plan documents listed
