National Medical Policy
|
|
|
- Melvyn Cunningham
- 10 years ago
- Views:
Transcription
1 National Medical Policy Subject: Policy Number: Balloon Sinuplasty for Treatment of Chronic Sinusitis NMP307 Effective Date*: December 2006 Updated: August 2015 This National Medical Policy is subject to the terms in the IMPORTANT NOTICE at the end of this document For Medicaid Plans: Please refer to the appropriate Medicaid Manuals for coverage guidelines prior to applying Health Net Medical Policies The Centers for Medicare & Medicaid Services (CMS) For Medicare Advantage members please refer to the following for coverage guidelines first: Use Source Reference/Website Link National Coverage Determination (NCD) National Coverage Manual Citation Local Coverage Determination (LCD)* Article (Local)* Other X None Use Health Net Policy Instructions Medicare NCDs and National Coverage Manuals apply to ALL Medicare members in ALL regions. Medicare LCDs and Articles apply to members in specific regions. To access your specific region, select the link provided under Reference/Website and follow the search instructions. Enter the topic and your specific state to find the coverage determinations for your region. *Note: Health Net must follow local coverage determinations (LCDs) of Medicare Administration Contractors (MACs) located outside their service area when those MACs have exclusive coverage of an item or service. (CMS Manual Chapter 4 Section 90.2) If more than one source is checked, you need to access all sources as, on occasion, an LCD or article contains additional coverage information than contained in the NCD or National Coverage Manual. Balloon Sinuplasty Aug 15 1
2 If there is no NCD, National Coverage Manual or region specific LCD/Article, follow the Health Net Hierarchy of Medical Resources for guidance. Current Policy Statement Health Net, Inc. considers balloon sinuplasty (e.g. Relieva Sinus Balloon Dilation Catheter) medically necessary to relieve obstruction of the maxillary, sphenoid, and frontal sinus ostia, either alone or in combination with standard endoscopic sinus surgery techniques, for patients with chronic rhinosinusitis (CRS) when all of the following are met: 1. Documentation that the inflammation of the paranasal sinuses has persisted for 12 weeks or longer 2. Patient has at least one of the following symptoms/signs: Anterior or posterior mucopurulent nasal discharge Nasal obstruction Facial-pain-pressure-fullness Headache 3. Patient has at least one finding of chronic sinusitis by CT scan: Air fluid levels Mucosal thickening > 2 mm Opacification 4. Continued symptoms/findings after antibiotic therapy for 3 wks, meeting either one of the following: Antibiotic therapy guided by C & S Beta-lactamase resistant antibiotic (e.g., trimethoprim-sulfisoxazole, amoxicillin clavulanate, cefuroxime) Health Net, Inc, considers balloon sinuplasty contraindicated and therefore not medically necessary in any of the following situations: 1. For the treatment of ethmoid disease; 2. Patient has sinonasal polyps; 3. Extensive previous surgery with significant osteoneogenesis. Codes Related To This Policy NOTE: The codes listed in this policy are for reference purposes only. Listing of a code in this policy does not imply that the service described by this code is a covered or noncovered health service. Coverage is determined by the benefit documents and medical necessity criteria. This list of codes may not be all inclusive. On October 1, 2015, the ICD-9 code sets used to report medical diagnoses and inpatient procedures will be replaced by ICD-10 code sets. Health Net National Balloon Sinuplasty Aug 15 2
3 Medical Policies will now include the preliminary ICD-10 codes in preparation for this transition. Please note that these may not be the final versions of the codes and that will not be accepted for billing or payment purposes until the October 1, 2015 implementation date. ICD-9 Codes Chronic sinusitis, maxillary Chronic sinusitis, frontal Chronic sinusitis, ethmoidal Chronic sinusitis, sphenoidal Other chronic sinusitis Unspecified sinusitis (chronic) ICD-10 Codes J32.0-J32.8 Chronic sinusitis CPT Code Unlisted procedure, accessory sinuses 2011 CPT Codes Nasal/sinus endoscopy, surgical; with dilation of maxillary sinus ostium (e.g., balloon dilation), transnasal or via canine fossa Nasal/sinus endoscopy, surgical; with dilation of frontal sinus ostium (e.g., balloon dilation) Nasal/sinus endoscopy, surgical; with dilation of sphenoid sinus ostium (e.g., balloon dilation) HCPCS Codes S2344 Nasal/sinus endoscopy, surgical; with enlargement of sinus ostium opening using inflatable device (i.e., balloon sinuplasty) (Deleted in 2012) Scientific Rationale Update August 2015 A position statement (revised ) endorsed by the American Rhinologic Society and the Academy of Otolaryngology - Head and Neck Surgery states, Sinus ostial dilation (e.g. balloon ostial dilation) is an appropriate therapeutic option for selected patients with sinusitis. This approach may be used alone to dilate a sinus ostium (frontal, maxillary, or sphenoid) or in conjunction with other instruments (eg, microdebrider, forceps). The final decision regarding use of techniques or instrumentation for sinus surgery is the responsibility of the attending surgeon. Bizaki et al (2014) conducted the first prospective randomized controlled trial that evaluated and compared the clinical outcome and impact of balloon sinuplasty and endoscopic sinus surgery (ESS) on the quality of life of patients suffering from chronic or recurrent rhinosinusitis (CRS) of the maxillary sinus. Adult patients with symptomatic chronic or recurrent rhinosinusitis without severe findings in the sinuses, as documented in the sinus Computer Tomography scan and clinical exam, were randomized in 2 groups: ESS and Balloon Sinuplasty. The main variable in our study is the Sinonasal Outcome Test-22 (SNOT 22) and its parameters. These parameters were analyzed preoperatively and at 3 months, postoperatively. There was a subjective improvement in symptoms after surgery. The authors also noticed an objective improvement in the quality of life of our patients seen as a decrease in Balloon Sinuplasty Aug 15 3
4 the total SNOT 22 score. Both balloon sinuplasty and ESS significantly improved almost all the parameters of SNOT22, with no significant difference being found between these two groups. The authors concluded both balloon sinuplasty and endoscopic sinus surgery improved the quality of life of patients with mild chronic or recurrent rhinosinusitis. However, the remarkably higher material cost of balloon sinuplasty compared to ESS sets limits on its broad use. There is an obvious need for further study to find out if, as an office procedure, balloon sinuplasty could deliver cost-savings high enough to cover the higher material cost of balloon sinuplasty. They also noted the study was, however, too small to enable firm conclusions to be drawn Abreu et al (2014) evaluated the effectiveness of balloon sinuplasty in patients with chronic rhinosinusitis. This was a prospective cohort study comprising 18 patients with chronic rhinosinusitis without polyposis who underwent balloon sinuplasty. Patients were evaluated for clinical criteria, quality of life (Sino-Nasal Outcome Questionnaire Test-20 [SNOT-20]), and computed tomography of the sinuses (Lund- Mackay staging) preoperatively and three to six months after the procedure. Out of 18 patients assessed, 13 were included, with a mean age of 39.9±15.6 years. Ostia sinuplasty was performed in 24 ostia (four sphenoid, ten frontal, and ten maxillary sinus). At the follow-up, 22 (92%) ostia were patent and there was no major complication. There was symptomatic improvement (SNOT-20), with Cronbach coefficients for consistency of the questionnaire items of 0.86 (95% CI: ) preoperatively and of 0.88 (95% CI: ) postoperatively, the difference being statistically significant (p<0.001). In addition, there was marked reduction of the computed tomography signs, an average of 4.2 point score (p<0.001). The authors concluded sinuplasty is effective in reducing symptoms and improving quality of life as a treatment option for chronic rhinosinusitis in selected patients. ElBadaway et al (2014) assessed the QOL of patients after one of three frontal sinus procedures, using the Glasgow Benefit Inventory (GBI) and the 22-item Sino-Nasal Outcome Test (SNOT-22). The authors designed an observational study with two arms. The first arm was a cross-sectional retrospective study recruiting all patients (with rhinosinusitis or mucocele) who had balloon sinuplasty, frontal recess clearance, or endoscopic modified Lothrop procedure in our tertiary referral unit at Newcastle upon Tyne Hospitals, between April 2010 and April The second arm was a prospective cohort study recruiting all patients having frontal sinus procedures between April 2012 and September The QOL was measured primarily by the GBI and SNOT-22 questionnaires. A total of 45 patients were recruited. Retrospectively, we identified 27 patients, of whom 19 (70%) returned the questionnaires. Eighteen patients were recruited in the prospective cohort and 14 (77.7%) of them completed the questionnaires 3 months postoperatively. The total benefit of frontal sinus surgery was found to be +31 for the retrospective group and for the prospective arm. The three domains of GBI showed a positive impact after surgery. The general domain scored in the retrospective study and for the prospective one. The social domain scored retrospectively and prospectively. The physical domain scores were retrospectively and +13 prospectively. The SNOT-22 preoperative score was and this significantly improved to (p = 0.017). The authors concluded the study was the first report of QOL benefit after all three frontal sinus procedures using the validated GBI, showing benefit in all aspects of health domains. The physical symptoms and QOL assessed by SNOT-22 significantly improved after all three procedures. Balloon Sinuplasty Aug 15 4
5 Scientific Rationale Update August 2013 Tomazic et al (2013) evaluated the feasibility of Balloon sinuplasty (BSP) in routine treatment of patients suffering from chronic rhinosinusitis (CRS). Patients with CRS refractory to medical therapy who had been scheduled for endoscopic sinus surgery between 2009 and 2011 were included in this study. Forty-five consecutive patients were included in this study, in whom 112 sinuses were approached by BSP. Of the 112 sinuses, 68 (60%) were planned as a "Balloon-Only" procedure and 44 (40%) were planned as a "Hybrid" procedure. Of the 68 sinuses in the "Balloon-Only" group, in 44 sinuses BSP failed, equating to a failure rate of 65%. Forty-four sinuses were planned for "Hybrid" procedures. In 29 of these sinuses BSP failed, giving a failure rate of 66%. Investigators concluded that according to literature, BSP can be a useful adjunct technique to standard FESS. In their experience, however, a failure rate of 65% for "Balloon-Only" and of 66% for "Hybrid" procedures occurred, which was regarded as unacceptable by the study group. Therefore, the study initially scheduled for 200 consecutive patients, was abandoned. Koskinen et al (2013) compared the symptom outcomes after maxillary sinus surgery with either the endoscopic sinus surgery (ESS) or the balloon sinuplasty technique in a retrospective study. No previous or additional sinonasal operations were accepted. Two hundred eight patients with chronic rhinosinusitis (CRS) without nasal polyps underwent either balloon sinuplasty or ESS. The patients who met with the inclusion criteria (n = 45 in ESS group and n = 40 in balloon group) replied to a questionnaire of history factors, exacerbations, and a visual analog scale (VAS) scoring of the change in symptoms, on average 28 ± 6 (mean ± SD) months postoperatively. The groups were identical in the response rate (64%), patient characteristics, and the improvement in all of the asked symptoms. Patients with CRS-related comorbidity and/or present occupational exposure had a statistically significantly better symptom reduction after ESS than after balloon sinusotomy. Moreover, the balloon sinusotomy group reported a statistically significant higher number of maxillary sinus punctures and antibiotic courses during the last 12 months. Investigators concluded ESS might be superior to balloon sinuplasty, especially in patients with risk factors. There is a need to perform more controlled studies on the treatment choices of CRS. Scientific Rationale Update September 2011 The ethmoid sinuses lack ostia that can be penetrated with the balloon, and therefore cannot be treated with balloon sinuplasty, and require conventional sinus surgery for management. Individuals with ethmoid sinus disease, nasal polyps, history of mucoceles, prior nasal & sinus surgeries, or abnormal nasal anatomy are not candidates for a balloon sinuplasty. Scientific Rationale Update December 2010 Ramadan (2009) Balloon sinuplasty is a fairly new technique that was recently introduced for the treatment of chronic rhinosinusitis (CRS). The initial experience in adults has been promising. The technique allows for restoring ventilation to the sinuses with minimal risk and trauma to the tissues. I present our initial experience of its use for treatment of CRS in children. The author performed a prospective study of 30 children in whom medical therapy failed and who were scheduled for surgery. They were offered treatment with balloon sinuplasty of selected sinuses. The data collected included age, computed tomography score, and co-morbid conditions. The primary outcome was the intraoperative success of dilation of the sinuses and the rate of adverse events due to the procedure. RESULTS: The procedure was Balloon Sinuplasty Aug 15 5
6 successful in 51 of 56 sinuses (91%) in 30 children. Five sinuses, of which 4 were hypoplastic maxillary sinuses and 1 was a frontal sinus, were not amenable to dilation. No complications or side effects were noted. The initial experience with balloon sinuplasty in children seems to be very encouraging. Because there is no bone or tissue removal, the procedure seems to be suitable for use in children. A hypoplastic sinus may not be amenable to balloon sinuplasty. Kim et al. (2009) Chronic sinusitis affects millions of patients. Balloon technology is a tool that has enhanced the surgeon's ability to treat patients suffering from chronic sinusitis. Three companies have developed products to dilate sinus ostia. An extensive literature review reveals that balloon catheters have an impressive safety profile and seem to be an effective tool for ostial dilatation. These tools are particularly effective in frontal recess dilatation and have great potential for office use under local anesthesia. Scientific Rationale Update June 2009 (2007) Per the American Rhinological Society (ARS): Based on currently available scientific medical evidence, endoscopic balloon dilation technology is acceptable and safe for use in the management of sinus disease and that endoscopic balloon catheter dilation as a tool for dilating the opening of the maxillary, sphenoid, and frontal sinuses is not investigational or experimental and should not be viewed as such. The ARS also stated that, although balloon catheter technology may eliminate the need for other surgical approaches in some patients, other patients may need balloon sinusotomy combined with FESS, especially for disorders of the ethmoid sinuses. (2007) Per the American Academy of Otolaryngology, Head and Neck Surgery: The current body of evidence regarding safety as it relates to balloon catheter dilation of the paranasal sinuses has been supportive. Evidence regarding indications for use, efficacy, and long-term results remain pending. While this technology may hold potential for future expanded uses, at the present time sinus balloon catheterization may be indicated for selected cases of rhinosinusitis without polyposis involving the frontal, sphenoid or maxillary sinuses either in conjunction with or in place of conventional instrumentation. Further, inflammatory disease of the ethmoid sinuses, which constitutes the most commonly involved site of paranasal sinus disease, still requires the use of conventional surgical instrumentation when surgery is indicated. Scientific Rationale Update June 2007 In September 2006, Friedman et al at the Annual Meeting of the American Rhinologic Society presented their paper on functional endoscopic dilatation of the sinuses: Safety, feasibility, patient satisfaction and cost. This was a retrospective study of 70 patients undergoing FESS (35 treated with balloon catheter devices). The conclusions of the analysis were that FESS procedures performed using balloon catheter devices could be performed safely and resulted in significant quality of life improvements and patient satisfaction. In a September 2006 Annual Meeting of the American Academy of Otolaryngology-Head Neck Surgery, Wynn et al compared 11 patients who had FESS with the balloon sinuplasty device to 10 patients who had FESS with conventional instrumentation to assess post-operative pain, use of analgesics, epistaxis and length of time to return to regular activity. They discovered that Balloon Sinuplasty Aug 15 6
7 patients undergoing sinus surgery with balloon sinuplasty devices reported less postoperative pain, less pain medication usage and shorter times to full activity. In a paper accepted for publication by the journal Otolaryngology-Head & Neck Surgery sometime in 2007, Bolger et al (2007) sought to further evaluate the effectiveness of balloon catheter devices in relieving sinus ostial obstruction and maintaining sinus ostia patency, to confirm the safety of sinusotomy using balloon catheters in a larger patient group and to gain insight into the ability of sinusotomy with balloon catheters to relieve sinus symptoms, either alone or in combination with standard endoscopic sinus surgery techniques. Through a prospective, multi-center evaluation, safety was assessed by rate of adverse events, patency was determined by endoscopic examination and sinus symptoms by the Sino-Nasal Outcome Test (SNOT 20). At the conclusion of the 24-week analysis, endoscopy determined the sinusotomy was patent in (80.5%) 247/307 sinuses, non-patent in 1.6% (5/307) and could not determine ostial patency status in 17.9% (55/307). Of the ostia visualized on endoscopy, 98% were patent (247/252), while 2% (5/252) were considered non-patent. SNOT-20 scores showed consistent symptomatic improvement over baseline. Revision treatment was required in 3 sinuses (3/307 sinuses, 0.98%) in 3 patients (3/109 patients, 2.75%). These were impressive findings to support the conclusion that inflation of the balloon catheter to gently remodel the ostium appears safe and effective in relieving ostial obstruction. Patients were pleased and indicated they experienced symptomatic improvement. The American Academy of Otolaryngology - Head and Neck Surgery (AAO-HNS) March 2007 position statement regarding endoscopic balloon catheter dilation reads:.the evidence regarding the safety of sinus balloon catheterization has been supportive, and that balloon catheterization is a promising technique for the treatment of selected cases of rhinosinusitis. These include those without polyposis involving the frontal, sphenoid or maxillary sinuses either in conjunction with or in place of conventional instrumentation. With the above statement in mind, in May 2007, The American Rhinologic Society (ARS) issued their revised position statement on endoscopic balloon catheter sinus dilation technology. They stated that: Based on currently available scientific medical evidence, endoscopic balloon dilation technology is acceptable and safe for use in the management of sinus disease..patients who are treated with this technology may require concurrent conventional endoscopic sinus surgery especially in the ethmoid sinuses much like any surgical instrument that may be used in some parts of the sinus and not others or in combination with other technologies. In a group of selected patients, the use of balloon catheter dilation technology alone may eliminate the need for other surgical techniques. As a tool for dilating the sphenoid, maxillary, and frontal sinuses, balloon catheter technology is not investigational or experimental and should not be viewed as such. As with all surgical interventions, continuing outcome and safety data is monitored to appropriately evaluate the long-term success of balloon catheter sinus dilation technology. Balloon Sinuplasty Aug 15 7
8 Scientific Rationale - Initial Chronic sinusitis is defined as a sinus infection (i.e. inflammation of the mucosa of the nose and paranasal sinuses) persisting for more than 3 months and is one of the most commonly diagnosed chronic illnesses in the United States. Symptoms of chronic sinusitis include nasal congestion or obstruction, postnasal drip, facial pressure or headache, and malaise. Most cases are continuations of unresolved acute sinusitis. The goal of medical therapy (e.g. antibiotics, nasal irrigation, topical corticosteroids) is directed toward facilitating the drainage of sinus secretions and treatment to eradicate the offending pathogens. Surgical intervention may be indicated when the patient requires more than three courses of antibiotics for sinusitis within a 12-month period along with evidence of abnormalities of the sinuses or ostiomeatal complex (OMC) on nasal endoscopy or CT imaging. The goal of functional endoscopic sinus surgery or FESS is to restore physiologic sinus ventilation and drainage, which allows for the gradual resolution of mucosal disease. Balloon dilation is a new technology currently being investigated as a less invasive alternative to endoscopic sinus surgery in the management of chronic sinusitis. The Relieva Balloon Sinuplasty System by Acclarant Inc. received FDA approval in April of Per the FDA, the Relieva Sinus Balloon Dilation Catheter is intended to provide a means to dilate the sinus ostia and spaces within the paranasal sinus cavities for diagnostic and therapeutic procedures to open passages and to restore normal drainage. Balloon sinuplasty is proposed to treat patients with chronic sinusitis who have exhausted less aggressive treatment options. The Relieva Balloon Sinuplasty System is a set of single-use, endoscopic, catheter-based instruments for minimally invasive sinus surgery. The goal of balloon sinuplasty is to restore normal sinus drainage by enlarging passages of the sinus ostia and spaces within the paranasal sinus cavities, without cutting bone or removing tissue. Per the manufacturer, the procedure is performed under fluoroscopic guidance using endoscopic technique, by an otolaryngologist trained in the use of the Balloon Sinuplasty System. The initial sinus access is achieved by the introduction of a guide catheter into the target sinus. A flexible guidewire is then introduced through the guide catheter and gently advanced into the target sinus. The balloon catheter tracks smoothly over the guidewire and positioned across the blocked ostium. After the position of the balloon catheter is confirmed, it is gradually inflated to gently restructure the blocked ostium. The system is removed leaving the ostium open and allowing the return of normal sinus drainage and function with little to no disruption to mucosal lining. Balloon sinuplasty may be performed in conjunction with endoscopic sinus surgery, and used as an assistive procedure for sinus tissue biopsy or culturing, sinus lavage, drainage, or antibiotic irrigation. Per the American Rhinologic Society (ARS) Position Statement On Balloon Sinuplasty, September 2006, the ARS stated the scientific literature has no data on the technology for long-term safety, indications, efficacy and outcomes. While balloon sinus dilatation may be a potentially helpful adjunct to medical and surgical management of rhinosinusitis, additional studies are needed. They concluded that: Balloon dilation technology may have some potential application where surgical management of sinus disease is required. This technology has limited surgical indications at this time. Balloon Sinuplasty Aug 15 8
9 The majority of patients who are treated with this technology may still require conventional endoscopic sinus surgery. In a small group of very selected patients, the use of balloon dilation technology alone may eliminate the need for other surgical techniques. Brown et al. (2006) investigated the safety and feasibility of balloon catheter dilation of paranasal sinus ostia. A nonrandomized prospective cohort of 10 endoscopic sinus surgery (ESS) candidates was offered treatment with a new technique of balloon catheter dilation of targeted sinus ostia. The frontal, maxillary, and sphenoid sinuses were considered appropriate for this innovative catheter-based technology. The primary study end points were intraoperative procedural success and absence of adverse events. The investigator reported that a total of 18 sinus ostial regions were successfully catheterized and dilated, including 10 maxillary, 5 sphenoid, and 3 frontal recesses. No adverse events occurred. Mucosal trauma and bleeding appeared to be less with catheter dilation than is typically observed with ESS techniques. The investigator concluded that dilation of sinus ostial regions via balloon catheter-based technology appears to be relatively safe and feasible. Larger multicenter clinical trials are now warranted to further establish safety and to determine the role of this new technique. Clinical trial data on balloon sinuplasty is limited. There is a lack of any large longterm studies, as well as a lack of comparison studies regarding the safety and efficacy of this technology as compared to currently accepted treatment for chronic sinusitis, such as medical therapy or endoscopic sinus surgery. It remains unclear if sinuplasty obviates or postpones the need for more invasive surgery. A phase III multi-center, non-randomized, open label, uncontrolled trial sponsored by the manufacturer of the Relieva Balloon Sinuplasty System, Acclarent Inc. is ongoing. The rationale of the Clinical Evaluation to Confirm Safety and Efficacy of Sinuplasty in the Paranasal Sinuses (CLEAR) study is to collect data on the effectiveness and safety of balloon dilation of the Frontal, Maxillary and Sphenoid sinuses by treating patients and observing them for a six month period of time following the procedure. Interim data is to be presented in late September 2006 at the American Academy of Otolaryngology meeting in Toronto, Canada. Review History December 2006 March 2007 June 2007 June 2009 December 2010 September 2011 August 2012 August 2013 August 2014 August 2015 Medical Advisory Council, initial approval Code update Revised as medically necessary if criteria met Update. No revisions. Codes reviewed. Update. Added Medicare Table. Added new 2011 CPT codes 31295, 31296, and No other revisions. Update. Added Revised Medicare Table. No revisions. Update. No revisions. Update no revisions. Code updates. Update no revisions. Code updates. Update no revisions This policy is based on the following evidenced-based guidelines: 1. Hayes Medical Technology Outlook. Endoscopic, catheter-based balloon dilation with the Relieva Balloon Sinuplasty System for the treatment of chronic sinusitis. September 2006 Balloon Sinuplasty Aug 15 9
10 2. U.S. Food and Drug Administration (FDA). 510(K) Summary. Relieva Sinus Balloon Dilation Catheter. 3. The American Academy of Otolaryngology-Head and Neck Surgery. Sinus Balloon Catheterization Position Statement American Rhinologic Society (ARS). Revised Position Statement on Endoscopic Balloon Catheter Sinus Dilation Technology, May, Hayes. Health Technology Brief. Relieva Balloon Sinuplasty (Acclarent Inc.) for Treatment of Chronic Sinusitis in Adults. November 11, Update Search July 14, Updated November 4, Update Aug American Rhinologic Society (ARS). Ostial Balloon Dilation Positon Statement. Revised 1/8/2015. Available at: References Update August Abreu CB, Balsalobre L, Pascoto GR, et al. Effectiveness of balloon sinuplasty in patients with chronic rhinosinusitis without polyposis. Braz J Otorhinolaryngol Nov-Dec;80(6): Bizaki AJ, Taulu R, Numminen J, Rautiainen M. Quality of life after endoscopic sinus surgery or balloon sinuplasty: a randomized clinical study. Rhinology Dec;52(4): ElBadawey MR, Alwaa A, ElTaher M, Carrie S. Quality of life benefit after endoscopic frontal sinus surgery. Am J Rhinol Allergy Sep-Oct;28(5): Holy CE, Ellison JM, Schneider C, Levine HL. The impact of balloon catheter dilation on frequency of sinus surgery in the United States. Med Devices (Auckl) Apr 28;7: Isaacson G. Surgical treatment of pediatric rhinosinusitis. Minerva Pediatr Aug;67(4): References Update August Gould J, Alexander I, Tomkin E, et al. In-office, multisinus balloon dilation: 1- Year outcomes from a prospective, multicenter, open label trial. Am J Rhinol Allergy. 2014; 28(2): Hamilos DL. Management of chronic rhinosinusitis. UpToDate. May 8, Hughes N. Bewick J. Van Der Most R, et al. A previously unreported serious adverse event during balloon sinuplasty. BMJ Case Reports Karanfilov B, Silvers S, Pasha R, et al. Office-based balloon sinus dilation: A prospective, multicenter study of 203 patients. International Forum of Allergy and Rhinology. 3 (5) (pp ), Marzetti A, Tedaldi M, Passali FM. The role of balloon sinuplasty in the treatment of sinus headache. Otolaryngol Pol. 2014; 68(1): References Update August Akkari M, Dupret-Bories A, Louvrier C, et al. Balloon catheter dilatation for frontal sinus ostium stenosis: surgical technique and preliminary report. Rev Laryngol Otol Rhinol (Bord). 2012;133(2): Koskinen A, Penttilä M, Myller J, et al. Endoscopic sinus surgery might reduce exacerbations and symptoms more than balloon sinuplasty. Am J Rhinol Allergy Nov-Dec;26(6):e Steffen A, Linke R, Wollenberg B. Treatment of chronic rhinosinusitis using balloon sinuplasty : A quality of life analysis. HNO Jul;61(7): Balloon Sinuplasty Aug 15 10
11 4. Thottam PJ, Haupert M, Saraiya S, et al. Functional endoscopic sinus surgery (FESS) alone versus balloon catheter sinuplasty (BCS) and ethmoidectomy: a comparative outcome analysis in pediatric chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol Sep;76(9): Tomazic PV, Stammberger H, Braun H, et al. Feasibility of balloon sinuplasty in patients with chronic rhinosinusitis: the Graz experience. Rhinology Jun;51(2): References Update August Ahmed J, Pal S, Hopkins C, et al. Functional endoscopic balloon dilation of sinus ostia for chronic rhinosinusitis. Clin Otolaryngol Jun;37(3): Bedrosian JC, Garcia-Navarro V, McCoul ED, et al. Endoscopic balloon dilation as an adjunct to extended endoscopic approaches to the skull base. Neurosurg Jun;116(6): References Update September Stankiewicz J, Truitt T, Atkins J Jr. One-year results: Transantral balloon dilation of the ethmoid infundibulum. Ear Nose Throat J Feb;89(2): Ramadan HH, McLaughlin K, Josephson G, et al. Balloon catheter sinuplasty in young children. Am J Rhinol Allergy Jan-Feb;24(1):e54-6. References Update December Coding & Documentation Update. Healthcare Services Group, October Flint: Cummings Otolaryngology: Head & Neck Surgery, 5 th Edition Mosby, An of Elsevier. Endoscopic Sinus Surgery for Rinosinusitis. 3. Stewart AE, Vaughan WC. Balloon sinuplasty versus surgical management of chronic rhinosinusitis. Curr Allergy Asthma Rep May;10(3): Ramadan HH. Safety and feasibility of balloon sinuplasty for treatment of chronic rhinosinusitis in children. Ann Otol Rhinol Laryngol, 01-MAR-2009; 118(3): Kim E, Cutler JL. Balloon Dilatation of the Paranasal Sinuses: A Tool in Sinus Surgery. Otolaryngologic Clinics of North America - Volume 42, Issue 5 (October 2009). 6. Catalano PJ, Payne SC. Balloon Dilation of the Frontal Recess in Patients With Chronic Frontal Sinusitis and Advanced Sinus Disease: An Initial Report. Ann Otol. Rhinol Laryngol 2009;118(2): Wittkopf ML, Becker SS, Duncavage JA, et al. Balloon sinuplasty for the surgical management of immunocompromised and critically ill patients with acute rhinosinusitis. Otolaryngol Head Neck Surg 2009;140(4): References Update June Levine HL, Sertich AP, Hoisington DR, et al. Multicenter registry of balloon catheter sinusotomy outcomes for 1,036 patients. Ann Otol Rhinol Laryngol. 2008;117(4): Vaughan WC. Review of balloon sinuplasty. Curr Opin Otolaryngol Head Neck Surg. 2008;16: Kuhn FA, Church CA, Goldberg AN et al. Balloon catheter sinusotomy One-year follow-up Outcomes and role in functional endoscopic sinus surgery. Otolaryngol Head Neck Surg 2008;139 (Suppl): Balloon Sinuplasty Aug 15 11
12 4. Weiss RL, Church DA, Kuhn FA et al. Long-term outcomes analysis of balloon catheter sinusotomy Two-year follow-up. Otolaryngol Head Neck Surg 2008; 139 (Suppl): References Update June Friedman, M, Schalch P., et al. Functional endoscopic dilatation of the sinuses: Safety, feasibility, patient satisfaction and cost Annual Meeting of the American Rhinologic Society. September Wynn, R, Vaughan, W. Postoperative recovery: FESS with balloon sinuplasty device. Poster Annual Meeting of the American Academy of Otolaryngology-Head Neck Surgery. September Brown CL, Bolger WE, Safety and feasibility of balloon catheter dilation of paranasal sinus ostia: a preliminary investigation. Annals of Otology, Rhinology & Laryngology. 115(4):293-9; discussion 300-1, 2006, April. 4. Bolger WE, Vaughn WC, Catheter based dilation of the sinus ostia: initial safety and feasibility analysis in a cadaver model. American Journal of Rhinology, 20(3):290-4, 2006, May-June. 5. Bolger WE, et al.: Safety & Outcomes of Balloon Catheter Sinusotomy: A Multi- Center 24 week Analysis in 115 Patients (The CLEAR Study). Otolaryngology- Head & Neck Surgery. Accepted for publication, References Initial 1. Brown CL, Bolger WE. Safety and feasibility of balloon catheter dilation of paranasal sinus ostia: a preliminary investigation. Ann Otol Rhinol Laryngol Apr;115(4):293-9; discussion Centers for Medicare and Medicaid Services. Medicare Coverage Database. Draft LCD for Balloon Sinuplasty (DL24048) Available at: all 3. Bajracharya H, Hinthorn D. Sinusitis, Chronic. emedicine Last Updated January Available at: 4. Subramanian, HN, Schechtman, KB, Hamilos, DL. A retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for chronic sinusitis. Am J Rhinol 2002; 16: Acclarent Inc. Balloon Sinuplasty Technology. Available at: 6. U.S. National Institute of Health. Clinical Trials.gov. Clinical Evaluation to Confirm Safety and Efficacy of Sinuplasty in the Paranasal Sinuses (CLEAR). Available at: Important Notice General Purpose. Health Net's National Medical Policies (the "Policies") are developed to assist Health Net in administering plan benefits and determining whether a particular procedure, drug, service or supply is medically necessary. The Policies are based upon a review of the available clinical information including clinical outcome studies in the peer-reviewed published medical literature, regulatory status of the drug or device, evidence-based guidelines of governmental bodies, and evidence-based guidelines and positions of select national health professional organizations. Coverage determinations are made on a case-by-case basis and are subject to all of the terms, conditions, limitations, and exclusions of the member's contract, including medical necessity requirements. Health Net may use the Policies to determine whether under the facts and circumstances of a particular case, the proposed procedure, drug, service or supply is medically necessary. The conclusion that a procedure, drug, service or supply is medically necessary does not constitute coverage. The member's contract defines which procedure, drug, service or supply is covered, excluded, limited, or subject to dollar caps. The policy provides for clearly written, reasonable and current Balloon Sinuplasty Aug 15 12
13 criteria that have been approved by Health Net s National Medical Advisory Council (MAC). The clinical criteria and medical policies provide guidelines for determining the medical necessity criteria for specific procedures, equipment, and services. In order to be eligible, all services must be medically necessary and otherwise defined in the member's benefits contract as described this "Important Notice" disclaimer. In all cases, final benefit determinations are based on the applicable contract language. To the extent there are any conflicts between medical policy guidelines and applicable contract language, the contract language prevails. Medical policy is not intended to override the policy that defines the member s benefits, nor is it intended to dictate to providers how to practice medicine. Policy Effective Date and Defined Terms. The date of posting is not the effective date of the Policy. The Policy is effective as of the date determined by Health Net. All policies are subject to applicable legal and regulatory mandates and requirements for prior notification. If there is a discrepancy between the policy effective date and legal mandates and regulatory requirements, the requirements of law and regulation shall govern. * In some states, prior notice or posting on the website is required before a policy is deemed effective. For information regarding the effective dates of Policies, contact your provider representative. The Policies do not include definitions. All terms are defined by Health Net. For information regarding the definitions of terms used in the Policies, contact your provider representative. Policy Amendment without Notice. Health Net reserves the right to amend the Policies without notice to providers or Members. In some states, prior notice or website posting is required before an amendment is deemed effective. No Medical Advice. The Policies do not constitute medical advice. Health Net does not provide or recommend treatment to members. Members should consult with their treating physician in connection with diagnosis and treatment decisions. No Authorization or Guarantee of Coverage. The Policies do not constitute authorization or guarantee of coverage of particular procedure, drug, service or supply. Members and providers should refer to the Member contract to determine if exclusions, limitations, and dollar caps apply to a particular procedure, drug, service or supply. Policy Limitation: Member s Contract Controls Coverage Determinations. Statutory Notice to Members: The materials provided to you are guidelines used by this plan to authorize, modify, or deny care for persons with similar illnesses or conditions. Specific care and treatment may vary depending on individual need and the benefits covered under your contract. The determination of coverage for a particular procedure, drug, service or supply is not based upon the Policies, but rather is subject to the facts of the individual clinical case, terms and conditions of the member s contract, and requirements of applicable laws and regulations. The contract language contains specific terms and conditions, including pre-existing conditions, limitations, exclusions, benefit maximums, eligibility, and other relevant terms and conditions of coverage. In the event the Member s contract (also known as the benefit contract, coverage document, or evidence of coverage) conflicts with the Policies, the Member s contract shall govern. The Policies do not replace or amend the Member s contract. Policy Limitation: Legal and Regulatory Mandates and Requirements The determinations of coverage for a particular procedure, drug, service or supply is subject to applicable legal and regulatory mandates and requirements. If there is a discrepancy between the Policies and legal mandates and regulatory requirements, the requirements of law and regulation shall govern. Reconstructive Surgery CA Health and Safety Code requires health care service plans to cover reconstructive surgery. Reconstructive surgery means surgery performed to correct or repair abnormal structures of the body caused by congenital defects, developmental abnormalities, trauma, infection, tumors, or disease to do either of the following: (1) To improve function or (2) To create a normal appearance, to the extent possible. Reconstructive surgery does not mean cosmetic surgery," which is surgery performed to alter or reshape normal structures of the body in order to improve appearance. Requests for reconstructive surgery may be denied, if the proposed procedure offers only a minimal improvement in the appearance of the enrollee, in accordance with the standard of care as practiced by physicians specializing in reconstructive surgery. Balloon Sinuplasty Aug 15 13
14 Reconstructive Surgery after Mastectomy California Health and Safety Code requires treatment for breast cancer to cover prosthetic devices or reconstructive surgery to restore and achieve symmetry for the patient incident to a mastectomy. Coverage for prosthetic devices and reconstructive surgery shall be subject to the co-payment, or deductible and coinsurance conditions, that are applicable to the mastectomy and all other terms and conditions applicable to other benefits. "Mastectomy" means the removal of all or part of the breast for medically necessary reasons, as determined by a licensed physician and surgeon. Policy Limitations: Medicare and Medicaid Policies specifically developed to assist Health Net in administering Medicare or Medicaid plan benefits and determining coverage for a particular procedure, drug, service or supply for Medicare or Medicaid members shall not be construed to apply to any other Health Net plans and members. The Policies shall not be interpreted to limit the benefits afforded Medicare and Medicaid members by law and regulation. Balloon Sinuplasty Aug 15 14
New treatment options for chronic sinusitis
 New treatment options for chronic sinusitis Balloon Sinuplasty Technology Vishram Jalukar, MD Mason City Clinic ENT & Allergy MKT01014 Rev. D Sinusitis Overview Inflammation of the sinus lining caused
New treatment options for chronic sinusitis Balloon Sinuplasty Technology Vishram Jalukar, MD Mason City Clinic ENT & Allergy MKT01014 Rev. D Sinusitis Overview Inflammation of the sinus lining caused
Introduction. 31 million Americans suffer from chronic sinusitis Surgical treatment for chronic sinusitis has evolved tremendously since its inception
 Balloon Sinuplasty Ki-Hong Kevin Ho, MD Faculty Advisor: Patricia Maeso, MD Department of Otolaryngology The University of Texas Medical Branch Grand Rounds Presentation December 16, 2009 Introduction
Balloon Sinuplasty Ki-Hong Kevin Ho, MD Faculty Advisor: Patricia Maeso, MD Department of Otolaryngology The University of Texas Medical Branch Grand Rounds Presentation December 16, 2009 Introduction
BALLOON SINUS OSTIAL DILATION
 BALLOON SINUS OSTIAL DILATION Effective Date: March 11, 2014 Review Dates: 12/11, 12/12, 2/13, 2/14, 2/15 Date Of Origin: December 14, 2011 Status: Current I. POLICY/CRITERIA A. The use of balloon sinus
BALLOON SINUS OSTIAL DILATION Effective Date: March 11, 2014 Review Dates: 12/11, 12/12, 2/13, 2/14, 2/15 Date Of Origin: December 14, 2011 Status: Current I. POLICY/CRITERIA A. The use of balloon sinus
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Balloon Sinuplasty for Treatment of Chronic Sinusitis Page 1 of 11 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Balloon Sinuplasty for Treatment of Chronic
Balloon Sinuplasty for Treatment of Chronic Sinusitis Page 1 of 11 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Balloon Sinuplasty for Treatment of Chronic
Patency of Maxillary Sinus Ostia Following Dilation with a Novel Osmotic Expansion Device
 WHITE PAPER Patency of Maxillary Sinus Ostia Following Dilation with a Novel Osmotic Expansion Device Twelve-Month Results from a Multi-Center Prospective Study Jerome Hester, MD California Sleep Institute,
WHITE PAPER Patency of Maxillary Sinus Ostia Following Dilation with a Novel Osmotic Expansion Device Twelve-Month Results from a Multi-Center Prospective Study Jerome Hester, MD California Sleep Institute,
Balloon Catheter Dilation for Chronic Rhinosinusitis
 1 (Note: A mini HTA report consists of two parts. The first is completed by the applicant at the time the new technology is requested. The second consists of a commentary and possibly additional evidence
1 (Note: A mini HTA report consists of two parts. The first is completed by the applicant at the time the new technology is requested. The second consists of a commentary and possibly additional evidence
Surgical Treatment of Chronic Rhinosinusitis in. Children
 Surgical Treatment of Chronic Rhinosinusitis in Children Fuad M. Baroody, M.D., F.A.C.S. Professor of Otolaryngology-Head and Neck Surgery and Pediatrics The University of Chicago Medicine and Biological
Surgical Treatment of Chronic Rhinosinusitis in Children Fuad M. Baroody, M.D., F.A.C.S. Professor of Otolaryngology-Head and Neck Surgery and Pediatrics The University of Chicago Medicine and Biological
What is Balloon Sinuplasty?
 What is Balloon Sinuplasty? The painful symptoms associated with chronic sinusitis can be overwhelming. If symptoms are difficult to control with medications alone, your primary doctor may refer you to
What is Balloon Sinuplasty? The painful symptoms associated with chronic sinusitis can be overwhelming. If symptoms are difficult to control with medications alone, your primary doctor may refer you to
Balloon Sinuplasty for Chronic Sinusitis: The Latest Recommendations
 Balloon Sinuplasty for Chronic Sinusitis: The Latest Recommendations Shannon Hunter, MD Board-Certified Otolaryngologist Thank you for joining us today We are recording this webinar and will be sending
Balloon Sinuplasty for Chronic Sinusitis: The Latest Recommendations Shannon Hunter, MD Board-Certified Otolaryngologist Thank you for joining us today We are recording this webinar and will be sending
By James D. Gould, MD FACS
 By James D. Gould, MD FACS BACKGROUND Balloon devices enlarge narrowed sinus ostia and outflow tracts by remodeling the surrounding bone and paranasal sinus structures. Multiple studies have demonstrated
By James D. Gould, MD FACS BACKGROUND Balloon devices enlarge narrowed sinus ostia and outflow tracts by remodeling the surrounding bone and paranasal sinus structures. Multiple studies have demonstrated
Balloon Ostial Dilation for Treatment of Chronic Sinusitis
 Balloon Ostial Dilation for Treatment of Chronic Sinusitis Policy Number: 7.01.105 Last Review: 3/2016 Origination: 3/2007 Next Review: 9/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Balloon Ostial Dilation for Treatment of Chronic Sinusitis Policy Number: 7.01.105 Last Review: 3/2016 Origination: 3/2007 Next Review: 9/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Tired of Sinusitis Pain and Pressure?
 Tired of Sinusitis Pain and Pressure? Instant relief that lasts Quick recovery Sinusitis, Balloon Sinus Dilation, and You 1 What are the Sinuses? How do Healthy Sinuses Work? Paranasal sinuses are air
Tired of Sinusitis Pain and Pressure? Instant relief that lasts Quick recovery Sinusitis, Balloon Sinus Dilation, and You 1 What are the Sinuses? How do Healthy Sinuses Work? Paranasal sinuses are air
HealthPACT. Health Policy Advisory Committee on Technology Australia and New Zealand. Technology Brief
 HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief Balloon Sinuplasty for Chronic Rhinosinusitis February 2012 State of Queensland (Queensland Health)
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief Balloon Sinuplasty for Chronic Rhinosinusitis February 2012 State of Queensland (Queensland Health)
Chronic sinusitis is a symptomatic inflammation of the. The Role of Balloon Sinuplasty in the Treatment of Chronic Sinusitis. balloon sinuplasty
 The Role of Balloon Sinuplasty in the Treatment of Chronic Sinusitis Joseph P. Cummings, PhD, Hoyee Leong, PhD, and Karl A. Matuszewski, MS, PharmD Abstract Objective: To evaluate the status of balloon
The Role of Balloon Sinuplasty in the Treatment of Chronic Sinusitis Joseph P. Cummings, PhD, Hoyee Leong, PhD, and Karl A. Matuszewski, MS, PharmD Abstract Objective: To evaluate the status of balloon
Balloon catheter sinusotomy: One-year follow-up Outcomes and role in functional endoscopic sinus surgery
 Otolaryngology Head and Neck Surgery (2008) 139, S27-S37 ORIGINAL RESEARCH Balloon catheter sinusotomy: One-year follow-up Outcomes and role in functional endoscopic sinus surgery Frederick A. Kuhn, MD,
Otolaryngology Head and Neck Surgery (2008) 139, S27-S37 ORIGINAL RESEARCH Balloon catheter sinusotomy: One-year follow-up Outcomes and role in functional endoscopic sinus surgery Frederick A. Kuhn, MD,
Coding Endoscopic Sinus Surgery
 Coding Endoscopic Sinus Surgery Audio Seminar/Webinar July 31, 2008 Practical Tools for Seminar Learning Copyright 2008 American Health Information Management Association. All rights reserved. Disclaimer
Coding Endoscopic Sinus Surgery Audio Seminar/Webinar July 31, 2008 Practical Tools for Seminar Learning Copyright 2008 American Health Information Management Association. All rights reserved. Disclaimer
Sinusitis is a highly prevalent condition, affecting approximately
 ORIGINAL ARTICLE Office-based balloon sinus dilation: a prospective, multicenter study of 203 patients Boris Karanfilov, MD 1, Stacey Silvers, MD 2, Raza Pasha, MD 3, Ashley Sikand, MD 4, Alan Shikani,
ORIGINAL ARTICLE Office-based balloon sinus dilation: a prospective, multicenter study of 203 patients Boris Karanfilov, MD 1, Stacey Silvers, MD 2, Raza Pasha, MD 3, Ashley Sikand, MD 4, Alan Shikani,
Prevention and Management of Complications in Maxillary Sinus Surgery
 Prevention and Management of Complications in Maxillary Sinus Surgery Esther Kim, MD, James A. Duncavage, MD* KEYWORDS Maxillary antrostomy Management of complications Prevention of complications Caldwell-Luc
Prevention and Management of Complications in Maxillary Sinus Surgery Esther Kim, MD, James A. Duncavage, MD* KEYWORDS Maxillary antrostomy Management of complications Prevention of complications Caldwell-Luc
Treatment of Maxillary Sinusitis Using the SinuSys Vent-Os Sinus Dilation System
 White PAPer Treatment of Maxillary Sinusitis Using the SinuSys Vent-Os Sinus Dilation System Jerome Hester, MD Chief Medical Officer SinuSys Corporation Palo Alto, California USA introduction Establishment
White PAPer Treatment of Maxillary Sinusitis Using the SinuSys Vent-Os Sinus Dilation System Jerome Hester, MD Chief Medical Officer SinuSys Corporation Palo Alto, California USA introduction Establishment
Standalone balloon dilation versus sinus surgery for chronic rhinosinusitis: A prospective, multicenter, randomized, controlled trial DO NOT COPY
 Standalone balloon dilation versus sinus surgery for chronic rhinosinusitis: A prospective, multicenter, randomized, controlled trial Jeffrey Cutler, M.D., 1 Nadim Bikhazi, M.D., 2 Joshua Light, M.D.,
Standalone balloon dilation versus sinus surgery for chronic rhinosinusitis: A prospective, multicenter, randomized, controlled trial Jeffrey Cutler, M.D., 1 Nadim Bikhazi, M.D., 2 Joshua Light, M.D.,
GUIDELINE Sinusitis. David M. Poetker MD, MA Associate Professor. Division of Rhinology and Sinus Surgery
 GUIDELINE Sinusitis David M. Poetker MD, MA Associate Professor Division of Rhinology and Sinus Surgery Guideline Fokkens et al. The European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology.
GUIDELINE Sinusitis David M. Poetker MD, MA Associate Professor Division of Rhinology and Sinus Surgery Guideline Fokkens et al. The European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology.
Sinus Headache vs. Migraine
 Sinus Headache vs. Migraine John M. DelGaudio, MD, FACS Professor and Vice Chair Chief of Rhinology and Sinus Surgery Department of Otolaryngology Emory University School of Medicine 1 Sinus Headache Problems
Sinus Headache vs. Migraine John M. DelGaudio, MD, FACS Professor and Vice Chair Chief of Rhinology and Sinus Surgery Department of Otolaryngology Emory University School of Medicine 1 Sinus Headache Problems
National Medical Policy
 National Medical Policy Subject: Policy Number: Vertebral Axial Decompression (VAX-D) NMP42 Effective Date*: October 2003 Updated: November 2015 This National Medical Policy is subject to the terms in
National Medical Policy Subject: Policy Number: Vertebral Axial Decompression (VAX-D) NMP42 Effective Date*: October 2003 Updated: November 2015 This National Medical Policy is subject to the terms in
YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY
 YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY What is functional endoscopic sinus surgery (FESS)? Functional endoscopic sinus surgery
YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY What is functional endoscopic sinus surgery (FESS)? Functional endoscopic sinus surgery
Surgery of the Frontal Sinus
 Surgery of the Frontal Sinus Steven D. Pletcher MD Assistant Professor Department of Otolaryngology Head and Neck Surgery University of California, San Francisco Disclosures Co-author patent application
Surgery of the Frontal Sinus Steven D. Pletcher MD Assistant Professor Department of Otolaryngology Head and Neck Surgery University of California, San Francisco Disclosures Co-author patent application
Coverage Analysis: The Cornerstone of Clinical Research Billing Presented by: Mary L. Veazie, CPA, MBA, CHC, CHRC Executive Director, Clinical
 Coverage Analysis: The Cornerstone of Clinical Research Billing Presented by: Mary L. Veazie, CPA, MBA, CHC, CHRC Executive Director, Clinical Research Finance The University of Texas MD Anderson Cancer
Coverage Analysis: The Cornerstone of Clinical Research Billing Presented by: Mary L. Veazie, CPA, MBA, CHC, CHRC Executive Director, Clinical Research Finance The University of Texas MD Anderson Cancer
Nasal polyps differ in terms of origin, as well
 Focus on CME at the University of Saskatchewan Clearing the Air on Nasal Polyps By Gordon Franke, MD, BSc, FRCS(C) Presented at the University of Saskatchewan Practical Otolaryngology Conference 2003,
Focus on CME at the University of Saskatchewan Clearing the Air on Nasal Polyps By Gordon Franke, MD, BSc, FRCS(C) Presented at the University of Saskatchewan Practical Otolaryngology Conference 2003,
PROVIDING PATIENTS A HIGHLY EFFECTIVE ALTERNATIVE TO TRADITIONAL SINUS SURGERY 2014 ANNUAL REPORT
 PROVIDING PATIENTS A HIGHLY EFFECTIVE ALTERNATIVE TO TRADITIONAL SINUS SURGERY 2014 ANNUAL REPORT DEAR ENTELLUS MEDICAL STOCKHOLDER: Entellus Medical was successful in 2014 at completing several major
PROVIDING PATIENTS A HIGHLY EFFECTIVE ALTERNATIVE TO TRADITIONAL SINUS SURGERY 2014 ANNUAL REPORT DEAR ENTELLUS MEDICAL STOCKHOLDER: Entellus Medical was successful in 2014 at completing several major
Functional endoscopic balloon dilation of sinus ostia for chronic rhinosinusitis (Review)
 Functional endoscopic balloon dilation of sinus ostia for chronic rhinosinusitis (Review) Ahmed J, Pal S, Hopkins C, Jayaraj S This is a reprint of a Cochrane review, prepared and maintained by The Cochrane
Functional endoscopic balloon dilation of sinus ostia for chronic rhinosinusitis (Review) Ahmed J, Pal S, Hopkins C, Jayaraj S This is a reprint of a Cochrane review, prepared and maintained by The Cochrane
Nasal and Sinus Disorders
 Nasal and Sinus Disorders Chronic Nasal Congestion When nasal obstruction occurs without other symptoms (such as sneezing, facial pressure, postnasal drip etc.) then a physical obstruction might be the
Nasal and Sinus Disorders Chronic Nasal Congestion When nasal obstruction occurs without other symptoms (such as sneezing, facial pressure, postnasal drip etc.) then a physical obstruction might be the
Pulsating Aerosol. The New Wave in SINUSitis Therapy. For the precise, effective and gentle treatment of sinusitis
 The New Wave in SINUSitis Therapy Pulsating Aerosol For the precise, effective and gentle treatment of sinusitis PARI SINUS Inhalation treatment for acute and chronic diseases of the upper airways www.parimedical.co.uk
The New Wave in SINUSitis Therapy Pulsating Aerosol For the precise, effective and gentle treatment of sinusitis PARI SINUS Inhalation treatment for acute and chronic diseases of the upper airways www.parimedical.co.uk
Corporate Medical Policy Septoplasty
 Corporate Medical Policy Septoplasty File Name: Origination: Last CAP Review: Next CAP Review: Last Review: septoplasty 4/1999 8/2015 8/2016 8/2015 Description of Procedure or Service There are many potential
Corporate Medical Policy Septoplasty File Name: Origination: Last CAP Review: Next CAP Review: Last Review: septoplasty 4/1999 8/2015 8/2016 8/2015 Description of Procedure or Service There are many potential
Chapter 10. All chapters, full text, free download, available at http://www.divingmedicine.info SINUS BAROTRAUMA ANATOMY OF THE SINUSES
 Chapter 10 All chapters, full text, free download, available at http://www.divingmedicine.info SINUS BAROTRAUMA ANATOMY OF THE SINUSES The sinuses are air filled cavities contained within the bones of
Chapter 10 All chapters, full text, free download, available at http://www.divingmedicine.info SINUS BAROTRAUMA ANATOMY OF THE SINUSES The sinuses are air filled cavities contained within the bones of
istent Trabecular Micro-Bypass Stent Reimbursement Guide
 istent Trabecular Micro-Bypass Stent Reimbursement Guide Table of Contents Overview Coding 3 4 Coding Overview Procedure Coding Device Coding Additional Coding Information Coverage Payment 10 11 Payment
istent Trabecular Micro-Bypass Stent Reimbursement Guide Table of Contents Overview Coding 3 4 Coding Overview Procedure Coding Device Coding Additional Coding Information Coverage Payment 10 11 Payment
Illinois Insurance Facts Illinois Department of Insurance Coverage for the Diagnosis and Treatment of Breast Conditions
 Illinois Insurance Facts Illinois Department of Insurance Coverage for the Diagnosis and Treatment of Breast Conditions Revised May 2015 Note: This information was developed to provide consumers with general
Illinois Insurance Facts Illinois Department of Insurance Coverage for the Diagnosis and Treatment of Breast Conditions Revised May 2015 Note: This information was developed to provide consumers with general
fig.1: CT scan of sinuses: Right isolated sphenoid sinus opacification
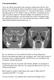 Case presentation: An 8 year old boy presented to the emergency department with few days history of severe headache. Pain is described as fronto-occipital, continuous and not relieved by pain medications.
Case presentation: An 8 year old boy presented to the emergency department with few days history of severe headache. Pain is described as fronto-occipital, continuous and not relieved by pain medications.
Name of Policy: Reconstructive versus Cosmetic Surgery
 Name of Policy: Reconstructive versus Cosmetic Surgery Policy #: 106 Latest Review Date: February 2010 Category: Administrative Policy Grade: Background/Definitions: As a general rule, benefits are payable
Name of Policy: Reconstructive versus Cosmetic Surgery Policy #: 106 Latest Review Date: February 2010 Category: Administrative Policy Grade: Background/Definitions: As a general rule, benefits are payable
UNIFORM HEALTH CARRIER EXTERNAL REVIEW MODEL ACT
 Model Regulation Service April 2010 UNIFORM HEALTH CARRIER EXTERNAL REVIEW MODEL ACT Table of Contents Section 1. Title Section 2. Purpose and Intent Section 3. Definitions Section 4. Applicability and
Model Regulation Service April 2010 UNIFORM HEALTH CARRIER EXTERNAL REVIEW MODEL ACT Table of Contents Section 1. Title Section 2. Purpose and Intent Section 3. Definitions Section 4. Applicability and
SURGICAL TREATMENT OF NASAL CONGESTION AND RHINORRHEA
 SURGICAL TREATMENT OF NASAL CONGESTION AND RHINORRHEA 2010 Allergy Symposium November 6, 2010 David Keschner, MD, JD HNS Orange County General Introduction Overview of material Anatomy and Clinical Features
SURGICAL TREATMENT OF NASAL CONGESTION AND RHINORRHEA 2010 Allergy Symposium November 6, 2010 David Keschner, MD, JD HNS Orange County General Introduction Overview of material Anatomy and Clinical Features
istent Trabecular Micro-Bypass Stent Reimbursement Guide
 istent Trabecular Micro-Bypass Stent Reimbursement Guide Table of Contents Overview Coding 2 3 Coding Overview Procedure Coding Device Coding Additional Coding Information Coverage Payment 8 9 Payment
istent Trabecular Micro-Bypass Stent Reimbursement Guide Table of Contents Overview Coding 2 3 Coding Overview Procedure Coding Device Coding Additional Coding Information Coverage Payment 8 9 Payment
BOTOX Injection (Onabotulinumtoxin A) for Migraine Headaches [Preauthorization Required]
![BOTOX Injection (Onabotulinumtoxin A) for Migraine Headaches [Preauthorization Required] BOTOX Injection (Onabotulinumtoxin A) for Migraine Headaches [Preauthorization Required]](/thumbs/29/13417512.jpg) BOTOX Injection (Onabotulinumtoxin A) for Migraine Headaches [Preauthorization Required] Medical Policy: MP-RX-01-11 Original Effective Date: March 24, 2011 Reviewed: Revised: This policy applies to products
BOTOX Injection (Onabotulinumtoxin A) for Migraine Headaches [Preauthorization Required] Medical Policy: MP-RX-01-11 Original Effective Date: March 24, 2011 Reviewed: Revised: This policy applies to products
MEDICAL COVERAGE POLICY. SERVICE: Varicose Veins of the Lower Extremities. PRIOR AUTHORIZATION: Required.
 Page 1 of 5 MEDICAL COVERAGE POLICY Important note Even though this policy may indicate that a particular service or supply may be considered covered, this conclusion is not based upon the terms of your
Page 1 of 5 MEDICAL COVERAGE POLICY Important note Even though this policy may indicate that a particular service or supply may be considered covered, this conclusion is not based upon the terms of your
Chapter 4 Health Care Management Unit 1: Care Management
 Chapter 4 Health Care Unit 1: Care In This Unit Topic See Page Unit 1: Care Care 2 6 Emergency 7 4.1 Care Healthcare Healthcare (HMS), Highmark Blue Shield s medical management division, is responsible
Chapter 4 Health Care Unit 1: Care In This Unit Topic See Page Unit 1: Care Care 2 6 Emergency 7 4.1 Care Healthcare Healthcare (HMS), Highmark Blue Shield s medical management division, is responsible
Underestimation of Rhinogenic Causes in Patients Presenting to the Emergency Department with Acute Headache
 Original Article 37 Underestimation of Rhinogenic Causes in Patients Presenting to the Emergency Department with Acute Headache Jae-Heon Lee, Hyun-Jik Kim, Young-Ho Hong, Kyung-Soo Kim Abstract- Objectives:
Original Article 37 Underestimation of Rhinogenic Causes in Patients Presenting to the Emergency Department with Acute Headache Jae-Heon Lee, Hyun-Jik Kim, Young-Ho Hong, Kyung-Soo Kim Abstract- Objectives:
Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Hearing Aids (BAHA) Amended Date: October 1, 2015.
 Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Conductive Hearing Loss... 1 1.2 Sensorineural Hearing Loss...
Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Conductive Hearing Loss... 1 1.2 Sensorineural Hearing Loss...
CODE AUDITING RULES. SAMPLE Medical Policy Rationale
 CODE AUDITING RULES As part of Coventry Health Care of Missouri, Inc s commitment to improve business processes, we are implemented a new payment policy program that applies to claims processed on August
CODE AUDITING RULES As part of Coventry Health Care of Missouri, Inc s commitment to improve business processes, we are implemented a new payment policy program that applies to claims processed on August
How To Cover Occupational Therapy
 Guidelines for Medical Necessity Determination for Occupational Therapy These Guidelines for Medical Necessity Determination (Guidelines) identify the clinical information MassHealth needs to determine
Guidelines for Medical Necessity Determination for Occupational Therapy These Guidelines for Medical Necessity Determination (Guidelines) identify the clinical information MassHealth needs to determine
Get Your Head In The Game. Matthew Voorman, MD Hutchinson Clinic March 21, 2016
 Get Your Head In The Game Matthew Voorman, MD Hutchinson Clinic March 21, 2016 About Me Otolaryngology Head & Neck Surgery Geisinger Medical Center General Surgery University of California San Francisco
Get Your Head In The Game Matthew Voorman, MD Hutchinson Clinic March 21, 2016 About Me Otolaryngology Head & Neck Surgery Geisinger Medical Center General Surgery University of California San Francisco
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION
 LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
Global Surgery Fact Sheet
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services R Global Surgery Fact Sheet Fact Sheet Definition of a Global Surgical Package Medicare established a national definition
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services R Global Surgery Fact Sheet Fact Sheet Definition of a Global Surgical Package Medicare established a national definition
Nasal Polyps B. Todd Schaeffer, MD, FACS Lake Success, Long Island, NY
 Advanced Sinus /Skull Base Center ENT & Allergy Associates, LLP Fall 2014 Nasal Polyps B. Todd Schaeffer, MD, FACS Lake Success, Long Island, NY www.schaeffermd.com New Treatments for Nasal Polyps Dr Schaeffer
Advanced Sinus /Skull Base Center ENT & Allergy Associates, LLP Fall 2014 Nasal Polyps B. Todd Schaeffer, MD, FACS Lake Success, Long Island, NY www.schaeffermd.com New Treatments for Nasal Polyps Dr Schaeffer
Endoscopic Surgical Treatment of Chronic Maxillary Sinusitis of Dental Origin
 Endoscopic Surgical Treatment of Chronic Maxillary Sinusitis of Dental Origin Fabio Costa, MD,* Enzo Emanuelli, MD, Massimo Robiony, MD, FEBOMS, Nicoletta Zerman, MD, Francesco Polini, MD, and Massimo
Endoscopic Surgical Treatment of Chronic Maxillary Sinusitis of Dental Origin Fabio Costa, MD,* Enzo Emanuelli, MD, Massimo Robiony, MD, FEBOMS, Nicoletta Zerman, MD, Francesco Polini, MD, and Massimo
MEDICAL COVERAGE POLICY. SERVICE: Bariatric (Weight Loss) Surgery Policy Number: 053 Effective Date: 5/27/2014 Last Review: 4/24/2014
 Page 1 of 6 MEDICAL COVERAGE POLICY Important note Even though this policy may indicate that a particular service or supply is considered covered, this conclusion is not necessarily based upon the terms
Page 1 of 6 MEDICAL COVERAGE POLICY Important note Even though this policy may indicate that a particular service or supply is considered covered, this conclusion is not necessarily based upon the terms
AMBULANCE SERVICES. Page
 AMBULANCE SERVICES COVERAGE DETERMINATION GUIDELINE Guideline Number: CDG.001.03 Effective Date: June 1, 2015 Table of Contents COVERAGE RATIONALE... DEFINITIONS. APPLICABLE CODES... REFERENCES... HISTORY/REVISION
AMBULANCE SERVICES COVERAGE DETERMINATION GUIDELINE Guideline Number: CDG.001.03 Effective Date: June 1, 2015 Table of Contents COVERAGE RATIONALE... DEFINITIONS. APPLICABLE CODES... REFERENCES... HISTORY/REVISION
Royal National Throat, Nose and Ear Hospital. Functional endoscopic sinus surgery (FESS) Ear, Nose and Throat Surgery
 Royal National Throat, Nose and Ear Hospital Functional endoscopic sinus surgery (FESS) Ear, Nose and Throat Surgery 31 If you need a large print, audio or translated copy of the document, please contact
Royal National Throat, Nose and Ear Hospital Functional endoscopic sinus surgery (FESS) Ear, Nose and Throat Surgery 31 If you need a large print, audio or translated copy of the document, please contact
ICD-10 Coding for Audiology
 ICD-10 Coding for Audiology Mary Sue Fino-Szumski, Ph.D., M.B.A. Vanderbilt University School of Medicine Vanderbilt Bill Wilkerson Center Department of Hearing and Speech Sciences Disclosure Financial
ICD-10 Coding for Audiology Mary Sue Fino-Szumski, Ph.D., M.B.A. Vanderbilt University School of Medicine Vanderbilt Bill Wilkerson Center Department of Hearing and Speech Sciences Disclosure Financial
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION. POLICY NUMBER: 8.01.19 CATEGORY: Therapy/Rehabilitation
 MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
X-Plain Sinus Surgery Reference Summary
 X-Plain Sinus Surgery Reference Summary Introduction Sinus surgery is a very common and safe operation. Your doctor may recommend that you have sinus surgery. The decision whether or not to have sinus
X-Plain Sinus Surgery Reference Summary Introduction Sinus surgery is a very common and safe operation. Your doctor may recommend that you have sinus surgery. The decision whether or not to have sinus
SURGICAL PREAMBLE SPECIFIC ELEMENTS SURGICAL SERVICES WHICH ARE NOT LISTED AS A "Z" CODE
 Surgical PreambleApril 1, 2015 PREAMBLE SPECIFIC ELEMENTS In addition to the common elements, all surgical services include the following specific elements. A. Supervising the preparation of and/or preparing
Surgical PreambleApril 1, 2015 PREAMBLE SPECIFIC ELEMENTS In addition to the common elements, all surgical services include the following specific elements. A. Supervising the preparation of and/or preparing
WELLCARE CLAIM PAYMENT POLICIES
 WellCare and Harmony Health Plan s claim payment policies are based on publicly distributed guidelines from established industry sources such as the Centers for Medicare and Medicaid Services (CMS), the
WellCare and Harmony Health Plan s claim payment policies are based on publicly distributed guidelines from established industry sources such as the Centers for Medicare and Medicaid Services (CMS), the
Glossary of Health Coverage and Medical Terms
 Glossary of Health Coverage and Medical Terms This glossary defines many commonly used terms, but isn t a full list. These glossary terms and definitions are intended to be educational and may be different
Glossary of Health Coverage and Medical Terms This glossary defines many commonly used terms, but isn t a full list. These glossary terms and definitions are intended to be educational and may be different
Physician Fee Schedule BCBSRI follows CMS Physician Fee Schedule (PFS) Relative Value Units (RVU) for details relating to
 Policy Coding and Guidelines EFFECTIVE DATE: 09 01 2015 POLICY LAST UPDATED: 09 02 2015 OVERVIEW This Policy provides an overview of coding and guidelines as they pertain to claims submitted to Blue Cross
Policy Coding and Guidelines EFFECTIVE DATE: 09 01 2015 POLICY LAST UPDATED: 09 02 2015 OVERVIEW This Policy provides an overview of coding and guidelines as they pertain to claims submitted to Blue Cross
1) There are 0 indicator edits, which are never correctly reported together;
 Medical Coverage Policy Coding and Guidelines sad EFFECTIVE DATE: 11/15/2011 POLICY LAST UPDATED: 11/1/2013 OVERVIEW This Policy provides an overview of coding and guidelines as they pertain to claims
Medical Coverage Policy Coding and Guidelines sad EFFECTIVE DATE: 11/15/2011 POLICY LAST UPDATED: 11/1/2013 OVERVIEW This Policy provides an overview of coding and guidelines as they pertain to claims
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
EXHIBIT COORDINATING PROVISIONS-STATE/FEDERAL LAW, ACCREDITATION STANDARDS AND GEOGRAPHIC EXCEPTIONS MARYLAND
 EXHIBIT COORDINATING PROVISIONS-STATE/FEDERAL LAW, ACCREDITATION STANDARDS AND GEOGRAPHIC EXCEPTIONS MARYLAND I. INTRODUCTION: Scope. To the extent of any conflict between the Agreement and this State
EXHIBIT COORDINATING PROVISIONS-STATE/FEDERAL LAW, ACCREDITATION STANDARDS AND GEOGRAPHIC EXCEPTIONS MARYLAND I. INTRODUCTION: Scope. To the extent of any conflict between the Agreement and this State
Corporate Medical Policy
 File Name: anesthesia_services Origination: 8/2007 Last CAP Review: 1/2016 Next CAP Review: 1/2017 Last Review: 1/2016 Corporate Medical Policy Description of Procedure or Service There are three main
File Name: anesthesia_services Origination: 8/2007 Last CAP Review: 1/2016 Next CAP Review: 1/2017 Last Review: 1/2016 Corporate Medical Policy Description of Procedure or Service There are three main
100.1 - Payment for Physician Services in Teaching Settings Under the MPFS. 100.1.1 - Evaluation and Management (E/M) Services
 MEDICARE CLAIMS PROCESSING MANUAL Accessed September 25, 2005 100.1 - Payment for Physician Services in Teaching Settings Under the MPFS Payment is made for physician services furnished in teaching settings
MEDICARE CLAIMS PROCESSING MANUAL Accessed September 25, 2005 100.1 - Payment for Physician Services in Teaching Settings Under the MPFS Payment is made for physician services furnished in teaching settings
AMBULANCE SERVICES. Page
 AMBULANCE SERVICES COVERAGE DETERMINATION GUIDELINE Guideline Number: CS003.C Effective Date: July 1, 2015 Table of Contents COVERAGE RATIONALE... DEFINITIONS APPLICABLE CODES... REFERENCES... HISTORY/REVISION
AMBULANCE SERVICES COVERAGE DETERMINATION GUIDELINE Guideline Number: CS003.C Effective Date: July 1, 2015 Table of Contents COVERAGE RATIONALE... DEFINITIONS APPLICABLE CODES... REFERENCES... HISTORY/REVISION
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998
 Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
PANNICULECTOMY & BODY CONTOURING PROCEDURES
 COVERAGE DETERMINATION GUIDELINE PANNICULECTOMY & BODY CONTOURING PROCEDURES Guideline Number: CDG.014.05 Effective Date: December 1, 2015 Table of Contents COVERAGE RATIONALE... DEFINITIONS. APPLICABLE
COVERAGE DETERMINATION GUIDELINE PANNICULECTOMY & BODY CONTOURING PROCEDURES Guideline Number: CDG.014.05 Effective Date: December 1, 2015 Table of Contents COVERAGE RATIONALE... DEFINITIONS. APPLICABLE
Pulsating Aerosol. The New Wave in SINUSitis Therapy. Cystic Fibrosis. Focus on. For the precise, effective and gentle treatment of sinusitis
 The New Wave in SINUSitis Therapy Focus on Cystic Fibrosis Pulsating Aerosol For the precise, effective and gentle treatment of sinusitis PARI SINUS Inhalation treatment for acute and chronic diseases
The New Wave in SINUSitis Therapy Focus on Cystic Fibrosis Pulsating Aerosol For the precise, effective and gentle treatment of sinusitis PARI SINUS Inhalation treatment for acute and chronic diseases
Test Request Tip Sheet
 With/Without Contrast CT, MRI Studies should NOT be ordered simultaneously as dual studies (i.e., with and without contrast). Radiation exposure is doubled and both views are rarely necessary. The study
With/Without Contrast CT, MRI Studies should NOT be ordered simultaneously as dual studies (i.e., with and without contrast). Radiation exposure is doubled and both views are rarely necessary. The study
SUBJECT: MANAGEMENT OF BREAST EFFECTIVE DATE: 12/16/99 IMPLANTS REVISED DATE:
 MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
American Chiropractic Association. Commentary on Centers for Medicare and Medicaid Services (CMS)/PART. Clinical Documentation Guidelines
 American Chiropractic Association Commentary on Centers for Medicare and Medicaid Services (CMS)/PART Clinical Documentation Guidelines DISCLAIMER The American Chiropractic Association provides this commentary
American Chiropractic Association Commentary on Centers for Medicare and Medicaid Services (CMS)/PART Clinical Documentation Guidelines DISCLAIMER The American Chiropractic Association provides this commentary
PSYCHOLOGICAL AND NEUROPSYCHOLOGICAL TESTING
 Status Active Medical and Behavioral Health Policy Section: Behavioral Health Policy Number: X-45 Effective Date: 01/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Status Active Medical and Behavioral Health Policy Section: Behavioral Health Policy Number: X-45 Effective Date: 01/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Mississippi Medicaid. Provider Reference Guide. For Part 203. Physician Services
 Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
MEDICAL ASSOCIATES HEALTH PLANS HEALTH CARE SERVI CES POLICY AND PROCEDURE MANUAL POLICY NUMBER: PP 60
 POLICY TITLE: CRITERIA UTILIZED IN DETERMINATION OF MEDICAL NECESSITY. POLICY STATEMENT: MAHP utilizes evidence based medicine in its decision making process. This policy is to establish and maintain medical
POLICY TITLE: CRITERIA UTILIZED IN DETERMINATION OF MEDICAL NECESSITY. POLICY STATEMENT: MAHP utilizes evidence based medicine in its decision making process. This policy is to establish and maintain medical
The Lewin Group undertook the following steps to identify the guidelines relevant to the 11 targeted procedures:
 Guidelines The following is a list of proposed medical specialty guidelines that have been found for the 11 targeted procedures to be included in the Medicare Imaging Demonstration. The list includes only
Guidelines The following is a list of proposed medical specialty guidelines that have been found for the 11 targeted procedures to be included in the Medicare Imaging Demonstration. The list includes only
THE MEDICAL TREATMENT GUIDELINES
 THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
Florida Medicaid. Anesthesia Services Coverage Policy. Agency for Health Care Administration. Draft Rule
 Florida Medicaid Agency for Health Care Administration Draft Rule Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2
Florida Medicaid Agency for Health Care Administration Draft Rule Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2
Department of Anesthesia & Perioperative Medicine 5-Year Strategic Plan FY 2012-2016. Contents
 Anesthesia & Perioperative Medicine 167 Ashley Avenue, Suite 301 MSC 912 Charleston, SC 29425-9120 Tel 843 792 2322 Fax 843 792 9314 Department of Anesthesia & Perioperative Medicine 5-Year Strategic Plan
Anesthesia & Perioperative Medicine 167 Ashley Avenue, Suite 301 MSC 912 Charleston, SC 29425-9120 Tel 843 792 2322 Fax 843 792 9314 Department of Anesthesia & Perioperative Medicine 5-Year Strategic Plan
CODING. Neighborhood Health Plan 1 Provider Payment Guidelines
 CODING Policy The terms of this policy set forth the guidelines for reporting the provision of care rendered by NHP participating providers, including but not limited to use of standard diagnosis and procedure
CODING Policy The terms of this policy set forth the guidelines for reporting the provision of care rendered by NHP participating providers, including but not limited to use of standard diagnosis and procedure
LEGISLATURE OF THE STATE OF IDAHO Sixtieth Legislature First Regular Session 2009 IN THE HOUSE OF REPRESENTATIVES HOUSE BILL NO.
 LEGISLATURE OF THE STATE OF IDAHO Sixtieth Legislature First Regular Session 0 IN THE HOUSE OF REPRESENTATIVES HOUSE BILL NO. BY BUSINESS COMMITTEE 0 AN ACT RELATING TO HEALTH INSURANCE; AMENDING TITLE,
LEGISLATURE OF THE STATE OF IDAHO Sixtieth Legislature First Regular Session 0 IN THE HOUSE OF REPRESENTATIVES HOUSE BILL NO. BY BUSINESS COMMITTEE 0 AN ACT RELATING TO HEALTH INSURANCE; AMENDING TITLE,
Zimmer Payer Coverage Approval Process Guide
 Zimmer Payer Coverage Approval Process Guide Market Access You ve Got Questions. We ve Got Answers. INSURANCE VERIFICATION PROCESS ELIGIBILITY AND BENEFITS VERIFICATION Understanding and verifying a patient
Zimmer Payer Coverage Approval Process Guide Market Access You ve Got Questions. We ve Got Answers. INSURANCE VERIFICATION PROCESS ELIGIBILITY AND BENEFITS VERIFICATION Understanding and verifying a patient
Modifier -25 Significant, Separately Identifiable E/M Service
 Manual: Policy Title: Reimbursement Policy Modifier -25 Significant, Separately Identifiable E/M Service Section: Modifiers Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM028 Last Updated:
Manual: Policy Title: Reimbursement Policy Modifier -25 Significant, Separately Identifiable E/M Service Section: Modifiers Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM028 Last Updated:
Intra-operative Nerve Monitoring Coding Guide. March 1, 2011
 Intra-operative Nerve Monitoring Coding Guide March 1, 2011 Please direct any questions to: Patty Telgener, RN Vice President, Reimbursement Services Emerson Consultants (303) 526-7604 (office) (303) 570-2159
Intra-operative Nerve Monitoring Coding Guide March 1, 2011 Please direct any questions to: Patty Telgener, RN Vice President, Reimbursement Services Emerson Consultants (303) 526-7604 (office) (303) 570-2159
Reimbursement for Medical Products: Ensuring Marketplace
 Reimbursement for Medical Products: Ensuring Marketplace Success by Securing Coverage and Payment Christopher J. Panarites, Ph.D. Director, Endovascular Products Health Economics and Outcomes Research
Reimbursement for Medical Products: Ensuring Marketplace Success by Securing Coverage and Payment Christopher J. Panarites, Ph.D. Director, Endovascular Products Health Economics and Outcomes Research
MEDICAL POLICY No. 91608-R1 MENTAL HEALTH RESIDENTIAL TREATMENT: ADULT
 MENTAL HEALTH RESIDENTIAL TREATMENT: ADULT Effective Date: June 4, 2015 Review Dates: 5/14, 5/15 Date Of Origin: May 12, 2014 Status: Current Summary of Changes Clarifications: Pg 4, Description, updated
MENTAL HEALTH RESIDENTIAL TREATMENT: ADULT Effective Date: June 4, 2015 Review Dates: 5/14, 5/15 Date Of Origin: May 12, 2014 Status: Current Summary of Changes Clarifications: Pg 4, Description, updated
