Measure Applications Partnership. Clinician Workgroup. December 15-16, 2014
|
|
|
- Bartholomew Thompson
- 10 years ago
- Views:
Transcription
1 Measure Applications Partnership Clinician Workgroup December 15-16, 2014
2 Clinician Workgroup Membership Workgroup Chair: Mark McClellan, MD, PhD Organizational Members American Academy of Family Physicians American Association of Nurse Practitioners American Academy of Pediatrics American College of Cardiology American College of Emergency Physicians American College of Radiology Association of American Medical Colleges Center for Patient Partnerships Consumers CHECKBOOK Kaiser Permanente March of Dimes Minnesota Community Measurement National Business Coalition on Health National Center for Interprofessional Practice and Education Pacific Business Group on Health Patient-Centered Primary Care Collaborative Physician Consortium for Performance Improvement The Alliance WellPoint Amy Mullins, MD, FAAFP Diane Padden, PhD, CRNP, FAANP Terry Adirim, MD, MPH Paul Casale, MD, FACC Jay Schuur, MD, MHS David Seidenwurm, MD Janis Orlowski, MD Rachel Grob, PhD Robert Krughoff, JD Amy Compton-Phillips, MD Cynthia Pellegrini Beth Averbeck, MD Bruce Sherman, MD, FCCP, FACOEM James Pacala, MD, MS David Hopkins, PhD Marci Nielsen, PhD, MPH Mark Metersky, MD Amy Moyer, MS, PMP 2
3 Clinician Workgroup Membership Subject Matter Experts Disparities Palliative Care Surgical Care Luther Clark, MD Constance M. Dahlin, MSN, ANP-BC, ACHPN, FPCN, FAAN Eric B. Whitacre, MD, FACS Federal Government Members Centers for Disease Control and Prevention (CDC) Centers for Medicare & Medicaid Services (CMS) Health Resources and Services Administration (HRSA) Peter Briss, MD, MPH Kate Goodrich, MD Girma Alemu, MD, MPH Duals Workgroup Liaison Humana George Andrews, MD, MBA, CPE, FACP, FACC, FCCP 3
4 Meeting Objectives Review and provide input on measures under consideration for federal programs applicable to clinicians and other eligible professionals Finalize input to the MAP Coordinating Committee on measures for use in federal programs Identify gaps in measures for federal clinician quality programs 4
5 Follow-up from October web meeting Draft programmatic deliverable summarizes issues identified by Workgroup Include more high-value measures in federal programs» Scorecard (next slide)» Measures under consideration: 27 of 96 measures for PQRS are composites, outcomes, PROs, appropriate use/efficiency measures Alignment across federal programs» AMA: need to synchronize and simplify» Alignment of PQRS-based programs and the EHR Incentive programs» Alignment with the Medicare Shared Savings Program Participation and incentives» 36% participation of EPs in PQRS in 2012» PQRS measures for public reporting and payment going forward» PQRS non-participation penalties begin in 2015» No differential incentives for reporting high-value measures 5
6 6
7 Significant issues for PQRS Encourage greater participation by including measures that allow more EPs to participate by reporting measures that are meaningful to their practice. All PQRS measures will be used for public reporting on Physician Compare and for payment adjustment in the Valuebased Payment Modifier. The effect of measure turnover in PQRS (20 measures added and 50 measures removed from PQRS for 2015); disruption of participating EPs quality reporting and creating new gaps in measures for EPs. 7
8 CMS Presentation: Discussion of emeasures & Measures of Cost Minet Javellan, CMS Camille Chicklis, Acumen Jennifer Podulka, Acumen 8
9 Developing Electronic Clinical Quality Measures (ecqms) for use in CMS Programs Minet Javellana, RN Eligible Professionals ecqm Project Lead Center for Clinical Standards and Quality Centers for Medicare and Medicaid Services December, 2014
10 What is an ecqm? Electronic clinical quality measures (ecqms) are standardized performance measures derived solely for use in EHRs. Current CMS policy classifies ecqms into the National Quality Strategy domains: Clinical Processes / Effectiveness Care Coordination Patient and Family Engagement Population and Public Health Patient Safety Efficient Use of Healthcare Resources The Meaningful Use Program provides financial incentives for Eligible Professional (EPs), Eligible Hospitals (EHs), and Critical Access Hospitals (CAHs) to report ecqms. Note: ecqms are not the only requirement to receive a financial incentive. *ecqms are also referred to as emeasures or electronic measures* 10
11 Key Stakeholders and ecqm Tools Developing an electronic measure requires the involvement of many stakeholders and use of many measure development tools and resources* Stakeholders: Healthcare Providers Centers for Medicare & Medicaid Services (CMS) Electronic Measures Issues Group (emig) Federal Regulators HL7 Measures Application Partnership (MAP) Measure Developers National Quality Forum (NQF) National Library of Medicine (NLM) Office of the National Coordinator for Health Information Technology (ONC) Patients and the general public Technical Expert Panel (TEP) the Appendix 11 Tools and Resources Cypress Certification Tool ecqm Library CMS Measures Inventory CMS Measures Management System (MMS) Blueprint Health Quality Measures Format (HQMF) Standard Measure Authoring Tool (MAT) NQF Quality Positioning System NQF Quality Data Model (QDM) Quality Reporting Document Architecture (QRDA) Standard Value Set Authority Center (VSAC) Bonnie for test driven development *Definitions of these tools and resources and stakeholders are provided in 11
12 Paper-Based vs. ecqm Measure Development Paper-Based Measure Development ecqm Measure Development Measure developers Develop measure narrative, numerator/ denominator in line with existing administrative data and/or data typically found in patient medical records (these can be paper or electronic charts). Create a list of code sets, data elements and abstraction definitions to represent the concepts within the measure. Solicit public comment on the measures. Measure developers conduct complete feasibility, reliability and validity testing. Measure developers Develop measure narrative, numerator/ denominator, workflow and logic, in line with existing standards (e.g., CMS Measures Management System (MMS) Blueprint Create value sets, collaborating with the Value Set Authority Center and clinical terminology (e.g. SNOMED-CT, LOINC) stakeholders as needed. Use the Measure Authoring Tool (MAT) Conduct complete feasibility, reliability and validity testing which can include working with EHR vendors to understand data element availability and implementation in the field. Testing, Testing, Testing - for certification, implementation and new standards Utilize industry standards Healthcare Quality Measures Format (HQMF) and Quality Reporting Data Architecture (QRDA) based on the Quality Data Model (QDM) Solicit public comment on the measures 12
13 Output of the MAT In order to report ecqms, electronic specifications must be developed in the Measure Authoring Tool (MAT). Each component helps to accurately capture, calculate and report ecqms: XML Description: A CQM written in Health Quality Measures Format (HQMF) syntax. HQMF is the industry (HL7) standard for representing a CQM as an electronic document. Likely User: EHR system developers and administrators, analysts. Use: To enable the automated creation of queries against an EHR or other operational data store for quality reporting. Human-Readable Description: The human-readable HTML equivalent of the XML file content. Likely User: EHR users [suggest saying EPs, EHs)] Use: To identify the details of the CQM in a human-readable format, so that the user can understand both how the elements are defined and the underlying logic of the measure calculation. 13
14 ecqm Development Lifecycle 1 Measure Conceptualization / Selection 5 Measure Use and Evaluation 2 Measure Specification 4 Measure Implementation 3 Measure Testing 14
15 ecqm Components: Visual Basics 15
16 Value Sets: What You See 16
17 Vocabularies Used in Building Value Sets There are specific vocabularies or terminologies that are used to identify clinical concepts identified by the data elements within an ecqm. These vocabulary requirements are based on the ONC Health Information Technology Standards Committee (HITSC) recommendations for standard and transition vocabularies. ecqms include both standard and transition vocabularies to convey the intended clinical intent: Standard- are primarily clinical vocabularies (as opposed to billing) and can serve more needs and for a longer period of time; however are not widely used. Transition- allow for immediate use and least burdensome for ecqm reporting purposes while standard vocabulary use is not yet widespread. Standard Transition SNOMED CT LOINC RxNorm ICF CVX PHIN/VADS ICD-9-CM ICD-10-CM ICD-10-PCS CPT HCPCS (refer to appendices for details on each specific code set) 17
18 Vocabularies in Relation to Data Elements Each data element type has a required vocabulary from either the standard or transitional and in many instances both. Measure specifications MUST use all that apply from the standard and transitional vocabularies. Quality Data Model Category Condition/ Diagnosis/ Problem Encounter (any provider interaction) Laboratory test (names) Laboratory test (results) Diagnostic study test names Diagnostic study test results Quality Data Model Data type Condition/ Diagnosis/ Problem Quality Data Model Attribute Clinical Vocabulary Standards Transition Vocabulary N/A SNOMED CT ICD-9-CM, ICD-10-CM Encounter N/A SNOMED CT CPT, HCPCS, ICD-9-CM Procedures, ICD-10-PCS Laboratory Test N/A LOINC N/A Laboratory Test Result SNOMED CT N/A Diagnostic Study Diagnostic Study N/A LOINC HCPCS Result SNOMED CT N/A Procedure Procedure N/A SNOMED CT CPT, HCPCS, ICD-9-CM Procedures, ICD-10-PCS 18 18
19 Updates to Vocabularies and Standards Vocabularies and Standards are updated by the healthcare industry CMS updates and utilizes vocabularies and standards for ecqm based on the industry Vocabularies are updated: Annually SnoMed, Loinc, CPT Monthly RX Norm Standard HQMF International standards for developing quality measures Stage 2 ecqm HQMF R1 Future ecqm HQMF R2.1 Updating has to go through balloting and voting by HL7, the standard development organization 19
20 The ecqm Development Lifecycle 20
21 ecqm Development Lifecycle 1 Measure Conceptualization / Selection 5 Measure Use and Evaluation 2 Measure Specification 4 Measure Implementation 3 Measure Testing 21
22 Intersection of QDM, HQMF and QRDA To view ecqm packages: 22
23 ecqm Development Lifecycle* * Timelines are notional, not actual, and intended for the purposes of discussion. Measure development timelines vary based on the measure. Measure Conceptualization/ Selection Measure Specification Measure Testing Measure Implementation Measure Use and Evaluation Generate a list of measure concepts to be developed Draft measure specifications and conduct initial feasibility Develop and implement comprehensive measure testing plan Support measure rollout including Federal rulemaking, business process and education and outreach Assess how measure performs in the field and conduct measure maintenance Month 1 Month 5 Month 12 Month 21 Month 27 23
24 Clinical Episode-Based Payment Measures MAP Clinician Workgroup December 2014
25 CMS s Clinical Episode-Based Payment Measures Introduction CMS s physician-based episode measures assess the efficiency of clinically-related services provided for the treatment for an episode of care. The measures are payment standardized to allow comparison of Medicare payment for clinically cohesive episodes related to a given condition, across the nation. They are risk adjusted for beneficiary clinical presentation and their construction generally parallels that of the NQF-endorsed Medicare spending per beneficiary measure. Developed for use in conjunction with measures of quality in value-based purchasing programs, the measures enable assessment of efficiency as the relative cost of clinical resources used to achieve a measured level of quality The six clinical episode-based payment measures include: 1. Lumbar spine fusion/refusion 2. Kidney/urinary tract infection (UTI) 3. Cellulitis 4. Gastrointestinal (GI) hemorrhage 5. Hip replacement/revision 6. Knee replacement/revision 25
26 Episodes of Care An episode of care (or episode ) includes the set of discrete medical services typically involved in managing a particular health event or condition Episodes allow related medical services delivered for management, treatment, and follow-up of a health event or condition and its complications to be assessed and valued using a single unit that informs all managing providers about the efficiency of their practice patterns 26
27 Goals of Episode Cost Reporting The principal goal of episode-based payment measures is to encourage efficient patterns of care Inclusion of only services that are clinically related to the episode trigger responds to stakeholder request for clinically cohesive measures Reporting episode-based payment measures provides actionable, transparent information to support medical group practices' efforts to gauge and improve the efficiency of care provided to patients with certain medical conditions Finally, reporting of episode-based measures can assist medical group practices in identifying opportunities for improvements in care coordination 27
28 Basic Model of an Episode 1. An episode begins with a clinical trigger event, such as: An inpatient hospital admission A claim with diagnosis/procedure information indicating the presence of the index condition/procedure 2. During the episode, services and procedures are grouped that: Are clinically relevant Occur during the episode time period May occur a few days prior to the trigger event, for some episodes 3. An episode ends: When there is a break in service, or At a fixed time period after the trigger event 28
29 Purpose of the Measures The clinical episode-based measures fulfill, in part, CMS s quality strategy to improve beneficiary health and quality of care while lowering medical costs The measures were constructed as part of CMS response to the mandate in Section 3003 of the Affordable Care Act (ACA) of 2010 that the Secretary of the Department of Health and Human Services (HHS) develop an episode grouper to improve care efficiency and quality The measures are designed to encourage care coordination between multiple physicians caring for a patient within an episode The six conditions chosen: can be linked to near-term outcomes; have high variation in post-treatment expenditures; account for a large share of total Medicare spending; and have a large share of expenditures attributable to post-acute care 29
30 Measure Vetting History All six clinical episode-based measures were reported in the 2012 Supplemental Quality and Resource Use Reports (QRURs) The 2012 Supplemental QRURs are confidential feedback reports provided to medical group practices with 100 or more eligible professionals (EPs) with information on the management of their Medicare fee-for-service (FFS) patients The 2012 Supplemental QRURs are for informational purposes only and complement the per capita cost and quality information provided in the 2012 QRURs CMS sought public comment on the measures in both the FY 2015 Physician Fee Schedule (PFS) Proposed Rule and in the FY 2015 Inpatient Prospective Payment System (IPPS)/Long-Term Care Hospital (LTCH) Proposed Rule 30
31 Measures Under Consideration (MUCs) CMS s Open Call for Measures Final MUC list: 96 measures same measures for PQRS, Physician Compare, Physician Feedback/Quality and Resource Utilization Reports; Physician Value-based Payment Modifier» 43 are fully developed, tested and ready for implementation» 53 are still in development» 7 measures are NQF-endorsed; most MUCs have not been submitted to NQF 32 emeasures for the EHR Incentive Programs» All are in development 6 episode based payment measures for the VBPM 107 measures for the Medicare Shared Savings Program» 75 also under consideration for PQRS 31
32 Approach to Pre-Rulemaking decision-making Supporting deliberations with preliminary analysis Standardized approach across all workgroups: The measures under consideration were divided into related groups for the purposes of discussion and voting A preliminary analysis by staff based on a standard decision algorithm applying the MAP measure selection criteria was performed for each measure under consideration Discussion guide notes the result of the preliminary analysis and provide rationale to support how that conclusion was reached 32
33 MAP Measure Selection Criteria 1. NQF-endorsed measures are required for program measure sets, unless no relevant endorsed measures are available to achieve a critical program objective 2. Program measure set adequately addresses each of the National Quality Strategy s three aims 3. Program measure set is responsive to specific program goals and requirements 4. Program measure set includes an appropriate mix of measure types 5. Program measure set enables measurement of person- and familycentered care and services 6. Program measure set includes considerations for healthcare disparities and cultural competency 33
34 Preliminary analysis of measures with MAP measure selection criteria 34
35 Preliminary analysis of measures under development 35
36 MAP Process Improvements Preliminary Analysis Results are a strawman to facilitate review of many MUCs Focus discussion on areas of major issues or disagreement Discussion Guide Easily navigated electronic document with more detail on measures Grouping of measures by consent calendars Voting on individual MUCs pulled out for a different recommendation than the preliminary analysis result En bloc voting on measures in a group when WG agrees with preliminary analysis result At least 60% approval to finalize a WG recommendation Pre-meeting public comments 36
Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment
 Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment 1 Conflict of Interest Disclosure Deborah Krauss, MS, BSN, RN Maria Michaels, MBA, CCRP, PMP Maria Harr, MBA, RHIA Have
Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment 1 Conflict of Interest Disclosure Deborah Krauss, MS, BSN, RN Maria Michaels, MBA, CCRP, PMP Maria Harr, MBA, RHIA Have
Open Source Software Tools for Electronic Clinical Quality Measures
 Open Source Software Tools for Electronic Clinical Quality Measures Beth Halley, MBA, RN, FHIMSS Renee Rookwood, MS, RN Sharon Sebastian, MS, RN-BC, PMP, CPHIMS Objectives Define electronic clinical quality
Open Source Software Tools for Electronic Clinical Quality Measures Beth Halley, MBA, RN, FHIMSS Renee Rookwood, MS, RN Sharon Sebastian, MS, RN-BC, PMP, CPHIMS Objectives Define electronic clinical quality
A Blueprint for the CMS Measures Management System
 A Blueprint for the CMS Measures Management System Version 9 Volume 1 of 2 August 2012 Prepared by: 3133 E Camelback Road, Ste 300 Phoenix, AZ 85016 Phone: 602.264.6382 Fax: 602.241.0757 Web: www.hsag.com
A Blueprint for the CMS Measures Management System Version 9 Volume 1 of 2 August 2012 Prepared by: 3133 E Camelback Road, Ste 300 Phoenix, AZ 85016 Phone: 602.264.6382 Fax: 602.241.0757 Web: www.hsag.com
Health IT Enabled Quality Measurement and Improvement: The HL7 Clinical Quality Information Workgroup
 Health IT Enabled Quality Measurement and Improvement: The HL7 Clinical Quality Information Workgroup Walter G. Suarez, MD, MPH Executive Director, Health IT Strategy and Policy Kaiser Permanente Co-Chair,
Health IT Enabled Quality Measurement and Improvement: The HL7 Clinical Quality Information Workgroup Walter G. Suarez, MD, MPH Executive Director, Health IT Strategy and Policy Kaiser Permanente Co-Chair,
CCO Incentive Metrics: Requirements for Reporting on EHR- Based Measures in 2015 GUIDANCE DOCUMENTATION
 CCO Incentive Metrics: Requirements for Reporting on EHR- Based Measures in 2015 GUIDANCE DOCUMENTATION Oregon Health Authority Page 1 of 22 Contents Section 1: Executive Summary... 4 1.1 Objective...
CCO Incentive Metrics: Requirements for Reporting on EHR- Based Measures in 2015 GUIDANCE DOCUMENTATION Oregon Health Authority Page 1 of 22 Contents Section 1: Executive Summary... 4 1.1 Objective...
Welcome to the Data Analytics Toolkit PowerPoint presentation on clinical quality measures, meaningful use, and data analytics.
 Welcome to the Data Analytics Toolkit PowerPoint presentation on clinical quality measures, meaningful use, and data analytics. According to the Centers for Medicare and Medicaid Services, Clinical Quality
Welcome to the Data Analytics Toolkit PowerPoint presentation on clinical quality measures, meaningful use, and data analytics. According to the Centers for Medicare and Medicaid Services, Clinical Quality
Navigating CMS Incentive Programs for Eligible Professionals Why It Matters and What You Need to Know. Dr. Paul Mulhausen, CMO
 Navigating CMS Incentive Programs for Eligible Professionals Why It Matters and What You Need to Know Dr. Paul Mulhausen, CMO Objectives Better understand CMS Incentive Programs and payment adjustments
Navigating CMS Incentive Programs for Eligible Professionals Why It Matters and What You Need to Know Dr. Paul Mulhausen, CMO Objectives Better understand CMS Incentive Programs and payment adjustments
Meaningful Use Stage 2 Certification: A Guide for EHR Product Managers
 Meaningful Use Stage 2 Certification: A Guide for EHR Product Managers Terminology Management is a foundational element to satisfying the Meaningful Use Stage 2 criteria and due to its complexity, and
Meaningful Use Stage 2 Certification: A Guide for EHR Product Managers Terminology Management is a foundational element to satisfying the Meaningful Use Stage 2 criteria and due to its complexity, and
Performance Measurement in CMS Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS
 Performance Measurement in CMS Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS Mind the Gap: Improving Quality Measures in Accountable Care Systems October
Performance Measurement in CMS Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS Mind the Gap: Improving Quality Measures in Accountable Care Systems October
CMS Physician Quality Reporting Programs Strategic Vision
 CMS Physician Quality Reporting Programs Strategic Vision FINAL DRAFT March 2015 1 EXECUTIVE SUMMARY As the largest payer of healthcare services in the United States, the Centers for Medicare & Medicaid
CMS Physician Quality Reporting Programs Strategic Vision FINAL DRAFT March 2015 1 EXECUTIVE SUMMARY As the largest payer of healthcare services in the United States, the Centers for Medicare & Medicaid
CMS s framework for Value Modifier
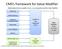 CMS s framework for Value Modifier Relationship between quality of care, cost composites and the Value Modifier Clinical Care Patient Experience Population/ Community Health Patient Safety Care Coordination
CMS s framework for Value Modifier Relationship between quality of care, cost composites and the Value Modifier Clinical Care Patient Experience Population/ Community Health Patient Safety Care Coordination
In the context of Meaningful Use, the Center for Medicaid and Medicare Services defines CQMs as follows on www.cms.gov:
 Use Case Summary NAME OF UC: EXCHANGE/ACCESS CLINICAL QUALITY MEASURES Sponsor(s): Jason Werner, MDHHS Date: 9/22/15 The purpose of this Use Case Summary is to allow Sponsors, Participants, and other readers
Use Case Summary NAME OF UC: EXCHANGE/ACCESS CLINICAL QUALITY MEASURES Sponsor(s): Jason Werner, MDHHS Date: 9/22/15 The purpose of this Use Case Summary is to allow Sponsors, Participants, and other readers
CMS is requesting information to aid in the planning and implementation of the MIPS in the following areas:
 Summary of Medicare s Request for Information on the Provisions in MACRA which Allow for Implementation of Alternative Payment Models and a Merit-Based Incentive Payment System On September 28, 2015, the
Summary of Medicare s Request for Information on the Provisions in MACRA which Allow for Implementation of Alternative Payment Models and a Merit-Based Incentive Payment System On September 28, 2015, the
CMS QCDR (Qualified Clinical Data Registry) and Other Ways PPRNet Can Help with Value-Based Payment
 CMS QCDR (Qualified Clinical Data Registry) and Other Ways PPRNet Can Help with Value-Based Payment Cara Litvin MD, MS Assistant Professor MUSC Department of Medicine Agenda Provide an update of the current
CMS QCDR (Qualified Clinical Data Registry) and Other Ways PPRNet Can Help with Value-Based Payment Cara Litvin MD, MS Assistant Professor MUSC Department of Medicine Agenda Provide an update of the current
Re: CMS 3323 NC, Request for Information (RFI): Certification Frequency and Requirements for the Reporting of Quality Measures Under CMS Programs
 February 5, 2016 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, DC 20201 Re: CMS 3323
February 5, 2016 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, DC 20201 Re: CMS 3323
Physician Quality Reporting System (PQRS)
 Physician Quality Reporting System (PQRS) and the Value-Based Payment Modifier Implementation guide for registry-based reporting for the Hepatitis C (HCV) Measures Group 2015 1 Overview of PQRS 1,2 What
Physician Quality Reporting System (PQRS) and the Value-Based Payment Modifier Implementation guide for registry-based reporting for the Hepatitis C (HCV) Measures Group 2015 1 Overview of PQRS 1,2 What
CMS Quality Measurement and Value Based Purchasing Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS
 CMS Quality Measurement and Value Based Purchasing Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS American Urological Association Quality Improvement Summit
CMS Quality Measurement and Value Based Purchasing Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS American Urological Association Quality Improvement Summit
Value Based Care and Healthcare Reform
 Value Based Care and Healthcare Reform Dimensions in Cardiac Care November, 2014 Jacqueline Matthews, RN, MS Senior Director, Quality Reporting & Reform Quality and Patient Safety Institute Cleveland Clinic
Value Based Care and Healthcare Reform Dimensions in Cardiac Care November, 2014 Jacqueline Matthews, RN, MS Senior Director, Quality Reporting & Reform Quality and Patient Safety Institute Cleveland Clinic
Aligning Meaningful Use CQM and PQRS Reporting for 2015
 Aligning Meaningful Use CQM and PQRS Reporting for 2015 August 19, 2015 Introductions Marni Anderson Project Specialist, MetaStar [email protected] 608-441-8253 Laura Sawyer Clinical Application Coordinator,
Aligning Meaningful Use CQM and PQRS Reporting for 2015 August 19, 2015 Introductions Marni Anderson Project Specialist, MetaStar [email protected] 608-441-8253 Laura Sawyer Clinical Application Coordinator,
Clinical Quality Measures (CSI) Electronic Copy of Health Information
 Clinical Quality Measures (CSI) Electronic Copy of Health Information Presenters David Taylor, MHS, RPh, PA-C, RN Deborah Alcorn, MSN, RN, CPC Chris Lamer, PharmD, MHS, BCPS, CDE Electronic Copy of Health
Clinical Quality Measures (CSI) Electronic Copy of Health Information Presenters David Taylor, MHS, RPh, PA-C, RN Deborah Alcorn, MSN, RN, CPC Chris Lamer, PharmD, MHS, BCPS, CDE Electronic Copy of Health
TEST INSTRUCTIONS FOR CROSS VENDOR EXCHANGE TABLE OF CONTENTS
 TEST INSTRUCTIONS FOR CROSS VENDOR EXCHANGE TABLE OF CONTENTS Introduction...2 Meaningful Use Objectives and Measures... 3 Eligible Professionals 495.6(j)(14)... 3 Eligible Hospitals and Critical Access
TEST INSTRUCTIONS FOR CROSS VENDOR EXCHANGE TABLE OF CONTENTS Introduction...2 Meaningful Use Objectives and Measures... 3 Eligible Professionals 495.6(j)(14)... 3 Eligible Hospitals and Critical Access
May 7, 2012. Submitted Electronically
 May 7, 2012 Submitted Electronically Secretary Kathleen Sebelius Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 edition EHR
May 7, 2012 Submitted Electronically Secretary Kathleen Sebelius Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 edition EHR
Medicare Physician Reporting: Beyond PQRS. Mary Patton Wheatley Senior Specialist, AAMC August 17, 2011
 Medicare Physician Reporting: Beyond PQRS Mary Patton Wheatley Senior Specialist, AAMC August 17, 2011 Who is the AAMC? The Association of American Medical Colleges (AAMC) serves and leads the academic
Medicare Physician Reporting: Beyond PQRS Mary Patton Wheatley Senior Specialist, AAMC August 17, 2011 Who is the AAMC? The Association of American Medical Colleges (AAMC) serves and leads the academic
Minnesota EHR Incentive Program (MEIP) 2015 2017 Program Year Timeline for EPs, EHs and CAHs. Updated November 2015
 Minnesota EHR Incentive Program (MEIP) 2015 2017 Program Year Timeline for EPs, EHs and CAHs Updated November 2015 Glossary CAH Critical access hospitals CEHRT Certified electronic health record technology
Minnesota EHR Incentive Program (MEIP) 2015 2017 Program Year Timeline for EPs, EHs and CAHs Updated November 2015 Glossary CAH Critical access hospitals CEHRT Certified electronic health record technology
HL7 & Meaningful Use. Charles Jaffe, MD, PhD CEO Health Level Seven International. HIMSS 11 Orlando February 23, 2011
 HL7 & Meaningful Use Charles Jaffe, MD, PhD CEO Health Level Seven International HIMSS 11 Orlando February 23, 2011 Overview Overview of Meaningful Use HIT Standards and Meaningful Use Overview HL7 Standards
HL7 & Meaningful Use Charles Jaffe, MD, PhD CEO Health Level Seven International HIMSS 11 Orlando February 23, 2011 Overview Overview of Meaningful Use HIT Standards and Meaningful Use Overview HL7 Standards
Prospective Attribution as a Single-Step Assignment Process
 Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS 1461 P P.O. Box 8013 Baltimore, MD 21244 8013 Dear Administrator Tavenner:
Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS 1461 P P.O. Box 8013 Baltimore, MD 21244 8013 Dear Administrator Tavenner:
Crosswalk: CMS Shared Savings Rules & NCQA ACO Accreditation Standards 12/1/2011
 Crosswalk: CMS Shared Savings Rules & NCQA ACO Accreditation Standards 12/1/2011 The table below details areas where NCQA s ACO Accreditation standards overlap with the CMS Final Rule CMS Pioneer ACO CMS
Crosswalk: CMS Shared Savings Rules & NCQA ACO Accreditation Standards 12/1/2011 The table below details areas where NCQA s ACO Accreditation standards overlap with the CMS Final Rule CMS Pioneer ACO CMS
Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation
 Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation Proposal 113 th Congress - - H.R.4015/S.2000 114 th Congress - - H.R.1470 SGR Repeal and Annual Updates General
Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation Proposal 113 th Congress - - H.R.4015/S.2000 114 th Congress - - H.R.1470 SGR Repeal and Annual Updates General
HIMSS Public Policy Initiatives in 2015: Using Health IT to Enable Healthcare Transformation Jeff Coughlin Senior Director Federal & State Affairs
 HIMSS Public Policy Initiatives in 2015: Using Health IT to Enable Healthcare Transformation Jeff Coughlin Senior Director Federal & State Affairs March 26, 2015 Agenda Meaningful Use Stage 3 NPRM 2015
HIMSS Public Policy Initiatives in 2015: Using Health IT to Enable Healthcare Transformation Jeff Coughlin Senior Director Federal & State Affairs March 26, 2015 Agenda Meaningful Use Stage 3 NPRM 2015
Re: Comments on 2015 Interoperability Standards Advisory Best Available Standards and Implementation Specifications
 April 29, 2015 Karen DeSalvo, MD, MPH, MSc National Coordinator Office of National Coordinator for Health IT Department of Health and Human Services 200 Independence Ave, SW Washington, DC 20201 Re: Comments
April 29, 2015 Karen DeSalvo, MD, MPH, MSc National Coordinator Office of National Coordinator for Health IT Department of Health and Human Services 200 Independence Ave, SW Washington, DC 20201 Re: Comments
2015 Physician Quality Reporting System (PQRS): Implementation Guide
 2015 Physician Quality Reporting System (PQRS): Implementation Guide 1/15/2015; Revised Table of Contents Introduction... 3 PQRS Measure Selection Considerations... 6 Satisfactorily Report Measures...
2015 Physician Quality Reporting System (PQRS): Implementation Guide 1/15/2015; Revised Table of Contents Introduction... 3 PQRS Measure Selection Considerations... 6 Satisfactorily Report Measures...
HEDIS/CAHPS 101. August 13, 2012 Minnesota Measurement and Reporting Workgroup
 HEDIS/CAHPS 101 Minnesota Measurement and Reporting Workgroup Objectives Provide introduction to NCQA Identify HEDIS/CAHPS basics Discuss various components related to HEDIS/CAHPS usage, including State
HEDIS/CAHPS 101 Minnesota Measurement and Reporting Workgroup Objectives Provide introduction to NCQA Identify HEDIS/CAHPS basics Discuss various components related to HEDIS/CAHPS usage, including State
IMPACT Act: Connecting Post-Acute Care Across the Care Continuum
 IMPACT Act: Connecting Post-Acute Care Across the Care Continuum Jennie Harvell, M.Ed, Senior Technical Adviser, Centers for Medicare & Medicaid Services Terrence A. O Malley, MD, Physician, Massachusetts
IMPACT Act: Connecting Post-Acute Care Across the Care Continuum Jennie Harvell, M.Ed, Senior Technical Adviser, Centers for Medicare & Medicaid Services Terrence A. O Malley, MD, Physician, Massachusetts
Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and payment changes (CMS-1632-P)
 Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and payment changes (CMS-1632-P) Date 2015-04-17 Title Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and
Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and payment changes (CMS-1632-P) Date 2015-04-17 Title Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and
1900 K St. NW Washington, DC 20006 c/o McKenna Long
 1900 K St. NW Washington, DC 20006 c/o McKenna Long Centers for Medicare & Medicaid Services U. S. Department of Health and Human Services Attention CMS 1345 P P.O. Box 8013, Baltimore, MD 21244 8013 Re:
1900 K St. NW Washington, DC 20006 c/o McKenna Long Centers for Medicare & Medicaid Services U. S. Department of Health and Human Services Attention CMS 1345 P P.O. Box 8013, Baltimore, MD 21244 8013 Re:
Clinical Quality Measures (CQMs) What are CQMs?
 Clinical Quality Measures (CQMs) What are CQMs? What are CQMs? Clinical quality measures, or CQMs, are tools that help eligible providers (EPs) measure and track the quality of health care services provided
Clinical Quality Measures (CQMs) What are CQMs? What are CQMs? Clinical quality measures, or CQMs, are tools that help eligible providers (EPs) measure and track the quality of health care services provided
Problem-Centered Care Delivery
 HOW INTERFACE TERMINOLOGY MAKES STANDARDIZED HEALTH INFORMATION POSSIBLE Terminologies ensure that the languages of medicine can be understood by both humans and machines. by June Bronnert, RHIA, CCS,
HOW INTERFACE TERMINOLOGY MAKES STANDARDIZED HEALTH INFORMATION POSSIBLE Terminologies ensure that the languages of medicine can be understood by both humans and machines. by June Bronnert, RHIA, CCS,
Repeal the Sustainable Growth Rate (SGR), avoiding annual double digit payment cuts;
 Background Summary of H.R. 2: The Medicare Access and CHIP Reauthorization Act of 2015 SGR Reform Law Enacts Payment Reforms to Improve Quality, Outcomes, and Cost On April 16, 2015, the President signed
Background Summary of H.R. 2: The Medicare Access and CHIP Reauthorization Act of 2015 SGR Reform Law Enacts Payment Reforms to Improve Quality, Outcomes, and Cost On April 16, 2015, the President signed
Extreme Makeover - ICD-10 Code Edition: Demystifying the Conversion Toolkit
 Extreme Makeover - ICD-10 Code Edition: Demystifying the Conversion Toolkit Deborah Kohn, MPH, RHIA, FACHE, CPHIMS, FHIMSS Principal - Dak Systems Consulting, San Mateo CA DISCLAIMER: The views and opinions
Extreme Makeover - ICD-10 Code Edition: Demystifying the Conversion Toolkit Deborah Kohn, MPH, RHIA, FACHE, CPHIMS, FHIMSS Principal - Dak Systems Consulting, San Mateo CA DISCLAIMER: The views and opinions
An Open Source Clinical Quality Measure Testing and Certification Tool
 An Open Source Clinical Quality Measure Testing and Certification Tool Goals Proposed to be the rigorous and repeatable testing tool of Electronic Health Records (EHRs) and EHR modules in calculating Meaningful
An Open Source Clinical Quality Measure Testing and Certification Tool Goals Proposed to be the rigorous and repeatable testing tool of Electronic Health Records (EHRs) and EHR modules in calculating Meaningful
FY 2016 Inpatient PPS Proposed Rule Quality Issues May 21, 2015
 FY 2016 Inpatient PPS Proposed Rule Quality Issues May 21, 2015 AAMC Staff: Scott Wetzel, [email protected] Mary Wheatley, [email protected] Agenda Summary of key quality and payment IPPS provisions Cross-cutting
FY 2016 Inpatient PPS Proposed Rule Quality Issues May 21, 2015 AAMC Staff: Scott Wetzel, [email protected] Mary Wheatley, [email protected] Agenda Summary of key quality and payment IPPS provisions Cross-cutting
Accountable Care Organizations: Importance to Physicians in Value Based Payment June 19, 2014 12:00-1:00pm EST
 Accountable Care Organizations: Importance to Physicians in Value Based Payment June 19, 2014 12:00-1:00pm EST Ahmed Haque, Director of Care Transformation Health IT U.S. Department of Health & Human Services
Accountable Care Organizations: Importance to Physicians in Value Based Payment June 19, 2014 12:00-1:00pm EST Ahmed Haque, Director of Care Transformation Health IT U.S. Department of Health & Human Services
Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview. Program Year 2014
 Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview Program Year 2014 Disclaimers This presentation was current at the time it was published or uploaded
Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview Program Year 2014 Disclaimers This presentation was current at the time it was published or uploaded
Meaningful Use in 2015 and Beyond Changes for Stage 2
 Meaningful Use in 2015 and Beyond Changes for Stage 2 Jennifer Boaz Transformation Support Specialist Proprietary 1 Definitions AIU = Adopt, Implement or Upgrade EP = Eligible Professional API = Application
Meaningful Use in 2015 and Beyond Changes for Stage 2 Jennifer Boaz Transformation Support Specialist Proprietary 1 Definitions AIU = Adopt, Implement or Upgrade EP = Eligible Professional API = Application
Stage 2 Overview Tipsheet Last Updated: August, 2012
 Stage 2 Overview Tipsheet Last Updated: August, 2012 Overview CMS recently published a final rule that specifies the Stage 2 criteria that eligible professionals (EPs), eligible hospitals, and critical
Stage 2 Overview Tipsheet Last Updated: August, 2012 Overview CMS recently published a final rule that specifies the Stage 2 criteria that eligible professionals (EPs), eligible hospitals, and critical
Health Level Seven International Unlocking the Power of Health Information
 Health Level Seven International Unlocking the Power of Health Information An ANSI accredited standards developer March 15, 2010 Centers for Medicare and Medicaid Services Department of Health and Human
Health Level Seven International Unlocking the Power of Health Information An ANSI accredited standards developer March 15, 2010 Centers for Medicare and Medicaid Services Department of Health and Human
Overview of MU Stage 2 Joel White, Health IT Now
 Overview of MU Stage 2 Joel White, Health IT Now 1 Agenda 1. Introduction 2. Context 3. Adoption Rates of HIT 4. Overview of Stage 2 Rules 5. Overview of Issues 6. Trend in Standards: Recommendations v.
Overview of MU Stage 2 Joel White, Health IT Now 1 Agenda 1. Introduction 2. Context 3. Adoption Rates of HIT 4. Overview of Stage 2 Rules 5. Overview of Issues 6. Trend in Standards: Recommendations v.
STATEMENT OF ACHIEVING THE PROMISE OF HEALTH INFORMATION TECHNOLOGY BEFORE THE UNITED STATES SENATE COMMITTEE ON HEALTH, EDUCATION, LABOR & PENSIONS
 STATEMENT OF PATRICK CONWAY, MD, MSc ACTING PRINCIPAL DEPUTY ADMINISTRATOR, DEPUTY ADMINISTRATOR FOR INNOVATION AND QUALITY, AND CHIEF MEDICAL OFFICER, CENTERS FOR MEDICARE & MEDICAID SERVICES ON ACHIEVING
STATEMENT OF PATRICK CONWAY, MD, MSc ACTING PRINCIPAL DEPUTY ADMINISTRATOR, DEPUTY ADMINISTRATOR FOR INNOVATION AND QUALITY, AND CHIEF MEDICAL OFFICER, CENTERS FOR MEDICARE & MEDICAID SERVICES ON ACHIEVING
CPDP Strategy Session on Stage 2 Meaningful Use
 CPDP Strategy Session on Stage 2 Meaningful Use March 29, 2012 Christine Bechtel, Vice President National Partnership for Women & Families David Lansky,President and Chief Executive Officer Pacific Business
CPDP Strategy Session on Stage 2 Meaningful Use March 29, 2012 Christine Bechtel, Vice President National Partnership for Women & Families David Lansky,President and Chief Executive Officer Pacific Business
Meaningful Use: Terms & Timelines, Changes to Stage 1, and Stage 2 Overview
 Meaningful Use: Terms & Timelines, Changes to Stage 1, and Stage 2 Overview NYC REACH Primary Care Information Project NYC Department of Health & Mental Hygiene 1 Agenda Terms & Timelines of Meaningful
Meaningful Use: Terms & Timelines, Changes to Stage 1, and Stage 2 Overview NYC REACH Primary Care Information Project NYC Department of Health & Mental Hygiene 1 Agenda Terms & Timelines of Meaningful
This proposed rule clarifies and makes updates to details regarding this program that were finalized in
 2014 Ambulatory Surgery Center (ASC) and Outpatient Prospective Payment System (OPPS) A Summary of the Quality Provisions of the Proposed Rule Overview On July 8, 2013, the Centers for Medicare and Medicaid
2014 Ambulatory Surgery Center (ASC) and Outpatient Prospective Payment System (OPPS) A Summary of the Quality Provisions of the Proposed Rule Overview On July 8, 2013, the Centers for Medicare and Medicaid
DETAILED SUMMARY--MEDCIARE SHARED SAVINGS/ACCOUNTABLE CARE ORGANIZATION (ACO) PROGRAM
 1 DETAILED SUMMARY--MEDCIARE SHARED SAVINGS/ACCOUNTABLE CARE ORGANIZATION (ACO) PROGRAM Definition of ACO General Concept An ACO refers to a group of physician and other healthcare providers and suppliers
1 DETAILED SUMMARY--MEDCIARE SHARED SAVINGS/ACCOUNTABLE CARE ORGANIZATION (ACO) PROGRAM Definition of ACO General Concept An ACO refers to a group of physician and other healthcare providers and suppliers
Eligible Professionals please see the document: MEDITECH Prepares You for Stage 2 of Meaningful Use: Eligible Professionals.
 s Preparing for Meaningful Use in 2014 MEDITECH (Updated December 2013) Professionals please see the document: MEDITECH Prepares You for Stage 2 of Meaningful Use: Professionals. Congratulations to our
s Preparing for Meaningful Use in 2014 MEDITECH (Updated December 2013) Professionals please see the document: MEDITECH Prepares You for Stage 2 of Meaningful Use: Professionals. Congratulations to our
Standardized Terminologies Used in the Learning Health System
 Standardized Terminologies Used in the Learning Health System Judith J. Warren, PhD, RN, BC, FAAN, FACMI Christine A. Hartley Centennial Professor University of Kansas School of Nursing 1 Learning Objectives
Standardized Terminologies Used in the Learning Health System Judith J. Warren, PhD, RN, BC, FAAN, FACMI Christine A. Hartley Centennial Professor University of Kansas School of Nursing 1 Learning Objectives
THE STIMULUS AND STANDARDS. John D. Halamka MD
 THE STIMULUS AND STANDARDS John D. Halamka MD THE ONC STRATEGY Grants - Accelerating Adoption Standards - Interim Final Rule Meaningful Use - Notice of Proposed Rulemaking Certification - Notice of Proposed
THE STIMULUS AND STANDARDS John D. Halamka MD THE ONC STRATEGY Grants - Accelerating Adoption Standards - Interim Final Rule Meaningful Use - Notice of Proposed Rulemaking Certification - Notice of Proposed
DEPARTMENT OF HEALTH AND HUMAN SERVICES & 42 CFR 412 45 CFR
 1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 412 Office of the Secretary 45 CFR Part 170[CMS-1632-P] RIN-0938-AS41 Medicare Program; Hospital Inpatient
1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 412 Office of the Secretary 45 CFR Part 170[CMS-1632-P] RIN-0938-AS41 Medicare Program; Hospital Inpatient
T he Health Information Technology for Economic
 A BNA, INC. HEALTH IT! LAW & INDUSTRY REPORT Reproduced with permission from Health IT Law & Industry Report, 2 HITR 23, 01/18/2010. Copyright 2010 by The Bureau of National Affairs, Inc. (800-372- 1033)
A BNA, INC. HEALTH IT! LAW & INDUSTRY REPORT Reproduced with permission from Health IT Law & Industry Report, 2 HITR 23, 01/18/2010. Copyright 2010 by The Bureau of National Affairs, Inc. (800-372- 1033)
