Treatment of Hepatitis B
|
|
|
- Ashlyn Harvey
- 10 years ago
- Views:
Transcription
1 Treatment of Hepatitis B Paul Y Kwo, MD Professor of Medicine Gastroenterology/Hepatology Division Medical Director, Liver Transplantation Indiana University Health Indiana University School of Medicine 975 W. Walnut, IB 327 Indianapolis, IN phone fax [email protected] Hepatitis B Virus 42 nm 22 nm Nucleic Acid: 3.2 kb DNA Classification: Hepadnaviridae Multiple serotypes and genotypes A-H Enveloped In vitro model: primary hepatocyte culture and transfection of cloned HBV DNA In vivo replication: in cytoplasm, cccdna in nucleus; hepatocyte and other tissues, human and other primates 1
2 Hepatitis B Virus 42 nm 42 nm 22 nm HBsAg HBV DNA HBcAg Nucleic Acid: 3.2 kb DNA Classification: Hepadnaviridae Multiple serotypes and genotypes A-H Enveloped In vitro model: primary hepatocyte culture and transfection of cloned HBV DNA In vivo replication: in cytoplasm, cccdna in nucleus; hepatocyte and other tissues, human and other primates Hepatitis B Virus 42 nm 22 nm 22 nm HBsAg Nucleic Acid: 3.2 kb DNA Classification: Hepadnaviridae Multiple serotypes and genotypes A-H Enveloped In vitro model: primary hepatocyte culture and transfection of cloned HBV DNA In vivo replication: in cytoplasm, cccdna in nucleus; hepatocyte and other tissues, human and other primates 2
3 Paul Y. Kwo, MD, FACG Prevalence of HBV: Global Estimates 350 million With Chronic HBV HBsAg Positive (%) Taiwan Vietnam China Africa 5-19 Philippines 5-16 Thailand Japan Indonesia HBsAg Prevalence High (>8%) Intermediate (2%-7%) Low (<2%) South Korea India Russia United States Weinbaum CM, et al. MMWR Recomm Rep. 2008;57(RR-8):1-20. Custer B, et al. J Clin Gastroenterol. 2004;38(10 suppl):s158-s168. Inciden nce (per 100,00 00) New HBV Infections by Year: United States ( ) Vaccine Licensed HBsAg Screening of Pregnant Women Recommended Infant Immunization Recommended OSHA Rule Enacted Adolescent Immunization Recommended Year Wasley A, et al. MMWR Surveill Summ 2008;57:
4 HBV and Hepatocellular cancer (HCC) Globally, commonest underlying cause of HCC In Asia, up to 40% of HCC in HBV in noncirrhotics Western countries show significantly less risk in HBV carriers Annual incidence: 0.2% to 2.5% 4
5 Natural History of Chronic HBV Infection HBeAg HBV DNA Integration Yim HJ and Lok AS. Hepatology 2006;43:S Hepatitis B: Natural History If it is not treated, in 1/3 of patients, hepatitis B can cause liver damage leading to cirrhosis and liver cancer 1 Hepatitis B is responsible for 80% of primary liver cancer globally, which is almost always fatal 2 Liver cancer is the 2 nd highest cause of death by cancer 3 Without appropriate treatment or monitoring, 1 in 4 persons with chronic hepatitis B will die of liver cancer or liver disease 1. WHO. Available at: 2. Hepatitis B Foundation. Hepatitis B and Primary Liver Cancer. Available at Accessed 4 February 2010; 3. WHO. Cancer Fact Sheet. Available at 5
6 Natural History of Chronic HBV Infection Childhood >95% Immune Tolerance Adulthood <5% HBeAg+ CHB HBeAg- CHB Inactive carrier <15-30% of HCC associated with HBV occurs in the absence of cirrhosis or advanced fibrosis HCC And or cirrhosis Pungpapong S, et al. Mayo Clin Proc 2007;82:967-5; Chen DS. J Gastroenterol Hepatol 1993;8:470-5; Seeff LB, et al. N Engl J Med 1987;316:965-70; Lok ASF, McMahon BJ. Hepatology 2009;50:1-36. HBV DNA vs. Liver Cirrhosis : REVEAL data 130:
7 HBV DNA vs. HCC : REVEAL Data Aiming for True Inactive Carrier Status Milestone 1: Start of decline of HBV DNA Milestone 2: HBeAg/ anti- HBe sero- conversion Milestone 3: HBV DNA decreased to undetectable Milestone 4: Clearance of HBsAg Milestone 5: Clearance of cccdna HBV DNA >10 9 copies/ml HBV DNA level This is where we would like our patients to be HBeAg/ anti-hbe status Undetectable level of HBV DNA HBeAg(+), anti-hbe(-) HBeAg(-), anti-hbe(+) Low HBV DNA (<2000 IU/mL) for reduced progression risk HBsAg status HBsAg+ HBsAg- ALT level Immune tolerance Immune clearance Inactive carrier state Functional cure>>>cure Immune control 7
8 Goals of therapy for Hepatitis B Liver histology Improves Serum HBV DNA declines Prevention of Death, Cirrhosis, and HCC Seroconversion (loss of HBeAg, production of anti-hbe, loss of HBsAg) ALT normalization U.S. FDA dates of Approved Therapies for CHB Nucleosides/Nucleotides Tenofovir* VIREAD Gilead Sciences 2008 Telbivudine TYZEKA Idenix / Novartis 2006 Entecavir* BARACLUDE Bristol-Myers Squibb 2005 Adefovir dipivoxil HEPSERA Gilead Sciences 2002 Lamivudine EPIVIR-HBV GlaxoSmithKline 1998 Interferons Peginterferon alfa-2a* PEGASYS Roche Laboratories Interferon alfa-2b, recombinant 2005 INTRON A Schering / Merck 1992 Preferred therapies AASLD Guidelines 8
9 HBV DNA threshold (IU/L) HBeAg positive HBeAg negative ALT: Normal range Candidates for HBV Treatment APASL (2008) 20, EASL (2012) Keeffe et al (2008) 20, Use revised, lower range (M: 30 U/L; F: 19 U/L) AASLD (2009) 20, ,000 Use revised, lower range (M: 30 U/L; F: 19 U/L) When to treat: HBV DNA HBV DNA HBV DNA HBV DNA key factors and ALT and ALT and ALT and ALT Biopsy Consider in certain groups Consider in certain groups Consider in certain groups Consider in certain groups Lok AS, et al. Hepatology 2009;50: Available at: Keeffe EB, et al. Clin Gastroenterol Hepatol 2008;6: EASL. J Hepatol 2012 vol. 57 j Liaw Y-F, et al. Hepatol Int 2008;2: Treatment Guidelines: Recommendations for First-Line Therapy in Patients Without Cirrhosis HBeAg Positive or Negative Chronic HBV Preferred Alternative Not Preferred Tenofovir DF Adefovir Lamivudine Entecavir Peg-IFN alfa-2a Telbivudine* *HBV DNA must be undetectable at 24 weeks to continue (Keeffe). AASLD guidelines: lamivudine and telbivudine not preferred due to relatively high rate of resistance. Adefovir not preferred due to weak antiviral activity and relatively high rate of resistance in HBeAg-negative studies. Lok AS, et al. Hepatology 2009;50: Available at: Keeffe EB, et al. Clin Gastroenterol Hepatol 2008;6:
10 Treatment Guidelines: Recommendations for Patients With Cirrhosis Compensated Cirrhosis Preferred Tenofovir DF Potential Peg-IFN alfa-2a* Not Preferred Lamivudine Entecavir Adefovir Telbivudine Decompensated Cirrhosis Preferred Tenofovir DF plus lamivudine Tenofovir DF Entecavir Not Preferred Peg-IFN alfa-2a and alfa-2b Note: therapies are approved for monotherapy only. *Early cirrhosis only. Contraindicated. Keeffe EB, et al. Clin Gastroenterol Hepatol 2008;6: A tool to minimize resistance: HBV Roadmap: Definitions of Virologic Primary Non- at Week 12 (HBV DNA <1 log 10 IU/mL decrease from baseline) Early Predictors of Efficacy at Week 24 Complete (PCR Negative) Partial (HBV DNA >60 to <2000 IU/mL) Inadequate (HBV DNA >2000 IU/mL) Keeffe EB, et al. Clin Gastroenterol Hepatol 2008;6:
11 HBV Roadmap: Treatment to HBV DNA Early Predictors of Efficacy at Week 24 Complete (PCR Negative) Partial (HBV DNA >60 to <2000 IU/mL) Inadequate (HBV DNA >2000 IU/mL) No Change in Treatment Keeffe EB, et al. Clin Gastroenterol Hepatol. 2008;6: HBV Roadmap: Treatment to HBV DNA Early Predictors of Efficacy at Week 24 Complete (PCR Negative) Partial (HBV DNA >60 to <2000 IU/mL) Inadequate (HBV DNA >2000 IU/mL) Low Genetic Barrier Drug Switch or add alternative drug More frequent monitoring (every 3 months) High Genetic Barrier Drug No change in treatment More frequent monitoring (every 3 months) Keeffe EB, et al. Clin Gastroenterol Hepatol. 2008;6:
12 HBV Roadmap: Treatment to HBV DNA Early Predictors of Efficacy at tweek k24 Complete (PCR Negative) Partial (HBV DNA >60 to <2000 IU/mL) Inadequate (HBV DNA >2000 IU/mL) Switch or Add Alternative Therapy Narrow Monitoring Frequency Keeffe EB, et al. Clin Gastroenterol Hepatol. 2008;6: Cirrhosis Reversal Following Lamivudine Rx in HBV Courtesy of Ian Wanless, MD. 12
13 Effect of LAM on Incidence of HCC in CHB and Advanced Fibrosis nosis of HCC (%) Diag 10 0 P =.047 Placebo Lamivudine Months Liaw YF, et al. N Engl J Med 2004;351: Types of Virological HBV DN NA (Log10 IU/ml) On Treatment LLOD 0 Months Relapse Sustained LLOD On Continuous Treatment Primary non-response Breakthrough Months Breakthrough Maintained 13
14 Implications of Resistance to HBV Therapies Loss of clinical benefits Loss of initial HBV DNA response with rebound ALT increase and eventual reversion of histologic improvement Progressive liver disease In patients with cirrhosis, decompensation Development of multidrug resistance Cross resistance New resistance mutations Transmission of resistant virus Keeffe EB, et al. Clin Gastroenterol Hepatol 2006;4: Lok AS, et al. Hepatology 2009;50: Available at: Antiviral Resistance: Nomenclature Genotypic resistance Phenotypic resistance Virologic breakthrough Biochemical breakthrough Detection of HBV polymerase mutation(s) associated with resistance Decreased in vitro susceptibility to an antiviral agent Increase in HBV DNA by >1 log 10 over nadir on treatment Increase in ALT on treatment Lok AS, et al. Hepatology 2009;50: Available at: 14
15 Factors associated with antiviral resistance VIRUS Daily production Replication fidelity Pre-existent mutations HOST DRUG Potency Genetic barrier to resistance Prior Rx Compliance Immune Status Pharmacogenetics Body Size What causes antiviral-resistant HBV mutants to become dominant? Survival of the fittest: selection of virus with survival advantage in the presence of antiviral therapy S S R S S S R S R S R S R R R Antiviral Therapy S 15
16 Manifestations of Antiviral Resistance 8 HBV DNA HBV DNA (log 10 IU/mL) ALT (U/L) ALT Upper Limit of Normal Virologic Breakthrough Genotypic Resistance Virologic Rebound Hepatitis Flare Biochemical Breakthrough Years on Treatment Lok AS, et al. Hepatology 2009;50: Available at: Consequences of Antiviral Resistance Loss of initial virologic, biochemical and histological response Hepatitis flares, hepatic decompensation and death Increased risk of HBV recurrence post-liver transplant Limit future treatment options Transmission to treatment-naïve persons public health problem 16
17 Patients with resistanc ce (%) Differences in Development of Resistance with Long-term Treatment in Nuc-naïve Patients Not head to head trials Different patient populations and trial designs Lamivudine 1 Adefovir 2 Entecavir 3-6 Telbivudine 7,8 Tenofovir 9,10 1. Lok ASF, et al. Gastroenterology 2003;125: ; 2. Hadziyannis SJ, et al. Gastroenterology 2006;131: ; 3. Colonno RJ, et al. Hepatology 2006;44: ; 4. Colonno RJ, et al, Hepatology 2006, 44 (Suppl 1):229; 5. Colonno RJ, et al. J Hepatol. 2007;46(Suppl 1):S294; 6. Tenney DJ et al. Gastroenterology 2009;136(Suppl 1):A-865; 7. Telbivudine (Tyzeka ) prescribing information; May 2009; Novartis Pharmaceuticals, East Hanover, NJ; 8. Lai CL, Hepatology 2006;44(Suppl 1):222A. 9. Tenofovir (Viread ) prescribing information; May. 2009; Gilead Sciences, Foster City, CA; 10. Snow-Lampart A et al. Hepatology 2008;48(Suppl 1):745A. The hepatitis B virus (HBV) polymerase open reading frame Lok et al Hepatology 2007;46:
18 In Vitro HBV Cross-resistance LAM ETV LdT ADV Selection of mutations in YMDD motif may affect future treatment options Thus lamivudine should not be first line treatment Locarnini S, et al. Antivir Ther 2004;9: Diagnosis of Antiviral Resistance Determination of virologic breakthrough Increase in serum HBV DNA by > 1.0 log as compared with nadir Rule out non-hbv-related causes of treatment failure Adherence Confirm resistance with HBV mutant detection Characterization of mutations will help guide future therapy (cross-resistance) Note that clinical resistance (biochemical breakthrough) lags behind viral resistance Rescue therapy should be considered in patients with viral resistance to prevent hepatitis flares Rescue therapy is more effective when initiated at the time of viral resistance, prior to clinical resistance* Adapted from Lok ASF, McMahon BJ. Hepatology 2007;45: *Lampertico P, Hepatology 2005; 2:
19 Summary: Guidelines for Management of Antiviral-Resistant HBV Resistance Lamivudine Rescue Therapy Add adefovir or tenofovir DF Stop lamivudine, switch to emtricitabine/tenofovir DF Adefovir Entecavir Telbivudine Adefovir/ Lamivudine Lamivudine Entecavir Add lamivudine Stop adefovir, switch to: Emtricitabine/tenofovir DF Switch to or add entecavir (if no prior lamivudine resistance) Switch to tenofovir DF or emtricitabine/tenofovir DF Add adefovir or tenofovir DF Stop telbivudine, switch to emtricitabine/tenofovir DF Consider tenofovir emtricitabine DF, or tenofovir+ entecavir Consider tenofovir or tenofovir DF/emtricitabine Lok AS, et al. Hepatology 2009;50: Lok et al. Hepatology 2007;46: Tenofovir + Entecavir for Multidrug resistant HBV infection 57 subjects received ed ETV 0.5 or 1 mg with TDF 300 mg Peterson Journal of Hepatology 2012 vol. 56 j
20 Tenofovir + Entecavir for Multidrug resistant HBV infection 51/57 (90%) of patients achieving HBV-DNA undetectability (LLoD 80IU/ml) Peterson Journal of Hepatology 2012 vol. 56 j Prevention Summary Avoid unnecessary treatment Initiate treatment with potent antiviral that has low rate of drug resistance (tenofovir or entecavir) or with combination therapy Switch to alternative therapy in patients with primary non-response Monitoring Test for serum HBV DNA (PCR assay) every 3-6 months during treatment Check for medication compliance in patients with virologic breakthrough Confirm antiviral resistance with genotype testing 20
21 Summary Treatment Guided by genotypic assays Add on therapy or switch therapy per guidelines Rescue therapies for multi-drug resistance tenofovir + entecavir tenofovir DF/emtricitabine 21
PURPOSE: To define the criteria to be used to determine the medical necessity of antiviral therapy in the treatment of Chronic Hepatitis B.
 COVENTRY Health Care Guidelines for Hepatitis B Therapy SUBJECT: Chronic Hepatitis B Therapy: a. Interferons - Intron A (interferon alfa-2b) and Pegasys (peginterferon alfa-2a) b. Nucleoside analogues
COVENTRY Health Care Guidelines for Hepatitis B Therapy SUBJECT: Chronic Hepatitis B Therapy: a. Interferons - Intron A (interferon alfa-2b) and Pegasys (peginterferon alfa-2a) b. Nucleoside analogues
Virology. Behandlung der Hepatitis B. HBV Genome. HBV life cycle. HBV Genotypes. Natural History. 8 genotypes: A, B, C, D, E, F, G, H
 Virology Behandlung der Hepatitis B Markus Heim Universitätsspital Basel 1 2 HBV Genome HBV life cycle 3 4 HBV Genotypes Natural History 8 genotypes: A, B, C, D, E, F, G, H genotypes A and D are prevalent
Virology Behandlung der Hepatitis B Markus Heim Universitätsspital Basel 1 2 HBV Genome HBV life cycle 3 4 HBV Genotypes Natural History 8 genotypes: A, B, C, D, E, F, G, H genotypes A and D are prevalent
Safety of Nucleos(t)ide Analogues during Long-Term Treatment of Chronic Hepatitis B
 Safety of Nucleos(t)ide Analogues during Long-Term Treatment of Chronic Hepatitis B Anna S. F. Lok, MD Alice Lohrman Andrews Research Professor in Hepatology Director of Clinical Hepatology University
Safety of Nucleos(t)ide Analogues during Long-Term Treatment of Chronic Hepatitis B Anna S. F. Lok, MD Alice Lohrman Andrews Research Professor in Hepatology Director of Clinical Hepatology University
Optimising therapy in chronic hepatitis B: Switch or add treatment
 Optimising therapy in chronic hepatitis B: Switch or add treatment Teerha Piratvisuth NKC Institute of Gastroenterology and Hepatology Prince of Songkla University,Thailand NA + NA Percent with resistance
Optimising therapy in chronic hepatitis B: Switch or add treatment Teerha Piratvisuth NKC Institute of Gastroenterology and Hepatology Prince of Songkla University,Thailand NA + NA Percent with resistance
HBV Treatment Guidelines. By: Prof.Dr. Abdelfatah Hanno Professor of Tropical Medicine Alexandria Faculty of Medicine
 HBV Treatment Guidelines By: Prof.Dr. Abdelfatah Hanno Professor of Tropical Medicine Alexandria Faculty of Medicine A 29 Y old lady diagnosed as chronic HBV 3 years ago during her pregnancy, no treatment
HBV Treatment Guidelines By: Prof.Dr. Abdelfatah Hanno Professor of Tropical Medicine Alexandria Faculty of Medicine A 29 Y old lady diagnosed as chronic HBV 3 years ago during her pregnancy, no treatment
Treatment Strategies of Hepatitis B in China
 Treatment Strategies of Hepatitis B in China Guangbi Yao MD Shanghai Jing-An Qu Central Hospital 200040 Characteristic features of CHB in China Huge amount of patients Infection during early life Maternal
Treatment Strategies of Hepatitis B in China Guangbi Yao MD Shanghai Jing-An Qu Central Hospital 200040 Characteristic features of CHB in China Huge amount of patients Infection during early life Maternal
Long-term Results of Pegylated Interferon alfa-2a and Tenofovir for Hepatitis B
 Long-term Results of Pegylated Interferon alfa-2a and Tenofovir for Hepatitis B Patrick Marcellin Viral Hepatitis Research Center Hôpital Beaujon, University of Paris France OBJECTIVES OF THERAPY IN CHRONIC
Long-term Results of Pegylated Interferon alfa-2a and Tenofovir for Hepatitis B Patrick Marcellin Viral Hepatitis Research Center Hôpital Beaujon, University of Paris France OBJECTIVES OF THERAPY IN CHRONIC
The availability of newer antiviral agents, as
 TREATMENT OF HEPATITIS B VIRUS INFECTION * Norman L. Sussman, MD ABSTRACT In treating chronic hepatitis B virus (HBV) infection, the primary goal of therapy is to achieve sustained suppression of HBV replication,
TREATMENT OF HEPATITIS B VIRUS INFECTION * Norman L. Sussman, MD ABSTRACT In treating chronic hepatitis B virus (HBV) infection, the primary goal of therapy is to achieve sustained suppression of HBV replication,
Therapy of decompensated cirrhosis Pre-transplant for HBV and HCV
 Therapy of decompensated cirrhosis Pre-transplant for HBV and HCV Universitätsklinikum Leipzig Thomas Berg Sektion Hepatologie Klinik und Poliklinik für Gastroenterologie und Rheumatologie Leber- und Studienzentrum
Therapy of decompensated cirrhosis Pre-transplant for HBV and HCV Universitätsklinikum Leipzig Thomas Berg Sektion Hepatologie Klinik und Poliklinik für Gastroenterologie und Rheumatologie Leber- und Studienzentrum
The Natural History of Chronic Hepatitis B Virus Infection
 The Natural History of Chronic Hepatitis B Virus Infection Brian J. McMahon, M.D. 1 ABSTRACT Three stages of chronic hepatitis B virus (HBV) infection are recognized: the immune tolerant phase, the chronic
The Natural History of Chronic Hepatitis B Virus Infection Brian J. McMahon, M.D. 1 ABSTRACT Three stages of chronic hepatitis B virus (HBV) infection are recognized: the immune tolerant phase, the chronic
EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection
 EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection European Association for the Study of the Liver Introduction Our understanding of the natural history of hepatitis B
EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection European Association for the Study of the Liver Introduction Our understanding of the natural history of hepatitis B
In the last decade, important advances have been made
 REVIEW Management of Hepatitis B: Summary of a Clinical Research Workshop Jay H. Hoofnagle, 1 Edward Doo, 1 T. Jake Liang, 2 Russell Fleischer, 3 and Anna S.F. Lok 4 Chronic hepatitis B is caused by persistent
REVIEW Management of Hepatitis B: Summary of a Clinical Research Workshop Jay H. Hoofnagle, 1 Edward Doo, 1 T. Jake Liang, 2 Russell Fleischer, 3 and Anna S.F. Lok 4 Chronic hepatitis B is caused by persistent
Clinical Application of HBs quantification
 Clinical Application of HBs quantification Hepatology on the Nile 2 Advances in Liver Disease 2014, "World Expert Review» Wednesday, September 24, 2014 Pr Tarik Asselah MD, PhD; Service d Hépatologie &
Clinical Application of HBs quantification Hepatology on the Nile 2 Advances in Liver Disease 2014, "World Expert Review» Wednesday, September 24, 2014 Pr Tarik Asselah MD, PhD; Service d Hépatologie &
Lamivudine for Patients with hronic Hepatitis B and Advanced Liver Disease. From : New England Journal of Medicine
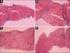 Lamivudine for Patients with hronic Hepatitis B and Advanced Liver Disease From : New England Journal of Medicine Volume 351:1521-1531, Number 15, Oct 7, 2004 馬 偕 紀 念 醫 院 一 般 內 科, 肝 膽 腸 胃 科 新 竹 分 院 陳 重
Lamivudine for Patients with hronic Hepatitis B and Advanced Liver Disease From : New England Journal of Medicine Volume 351:1521-1531, Number 15, Oct 7, 2004 馬 偕 紀 念 醫 院 一 般 內 科, 肝 膽 腸 胃 科 新 竹 分 院 陳 重
AASLD PRACTICE GUIDELINES Chronic Hepatitis B: Update 2009
 AASLD PRACTICE GUIDELINES Chronic Hepatitis B: Update 2009 Anna S. F. Lok 1 and Brian J. McMahon 2 This guideline has been approved by the American Association for the Study of Liver Diseases and represents
AASLD PRACTICE GUIDELINES Chronic Hepatitis B: Update 2009 Anna S. F. Lok 1 and Brian J. McMahon 2 This guideline has been approved by the American Association for the Study of Liver Diseases and represents
Molecular Diagnosis of Hepatitis B and Hepatitis D infections
 Molecular Diagnosis of Hepatitis B and Hepatitis D infections Acute infection Detection of HBsAg in serum is a fundamental diagnostic marker of HBV infection HBsAg shows a strong correlation with HBV replication
Molecular Diagnosis of Hepatitis B and Hepatitis D infections Acute infection Detection of HBsAg in serum is a fundamental diagnostic marker of HBV infection HBsAg shows a strong correlation with HBV replication
HEPATITIS COINFECTIONS
 HEPATITIS COINFECTIONS Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Disclosures (Activity w/i 12 months)
HEPATITIS COINFECTIONS Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Disclosures (Activity w/i 12 months)
AASLD PRACTICE GUIDELINES Chronic Hepatitis B
 AASLD PRACTICE GUIDELINES Chronic Hepatitis B Anna S. F. Lok 1 and Brian J. McMahon 2 This guideline has been approved by the American Association for the Study of Liver Diseases and represents the position
AASLD PRACTICE GUIDELINES Chronic Hepatitis B Anna S. F. Lok 1 and Brian J. McMahon 2 This guideline has been approved by the American Association for the Study of Liver Diseases and represents the position
Chronic hepatitis B (CHB) remains an important public SPECIAL REPORT
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1315 1341 SPECIAL REPORT A Treatment Algorithm for the Management of Chronic Hepatitis B Virus Infection in the United States: 2008 Update EMMET B. KEEFFE,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1315 1341 SPECIAL REPORT A Treatment Algorithm for the Management of Chronic Hepatitis B Virus Infection in the United States: 2008 Update EMMET B. KEEFFE,*
EASL Clinical Practice Guidelines: Management of chronic hepatitis B
 Journal of Hepatology 50 (2009) 227 242 www.elsevier.com/locate/jhep EASL Clinical Practice Guidelines: Management of chronic hepatitis B European Association for the Study of the Liver * Keywords: Hepatitis
Journal of Hepatology 50 (2009) 227 242 www.elsevier.com/locate/jhep EASL Clinical Practice Guidelines: Management of chronic hepatitis B European Association for the Study of the Liver * Keywords: Hepatitis
CASG HBV Study Richard B. Pollard, MD David M. Asmuth, MD Division of Infectious Diseases University of California Davis Medical Center Sacramento, CA
 CASG HBV Study Richard B. Pollard, MD David M. Asmuth, MD Division of Infectious Diseases University of California Davis Medical Center Sacramento, CA Characteristics of HBV DNA Hepadnavirus Reverse transcriptase
CASG HBV Study Richard B. Pollard, MD David M. Asmuth, MD Division of Infectious Diseases University of California Davis Medical Center Sacramento, CA Characteristics of HBV DNA Hepadnavirus Reverse transcriptase
Update on Pharmacotherapy of Chronic Hepatitis B and C
 Update on Pharmacotherapy of Chronic Hepatitis B and C Rebecca L. Corey, Pharm.D., BCPS Reviewed by Paulina Deming, B.S., Pharm.D., PhC; Lisa C. Hutchison, Pharm.D., MPH, FCCP, BCPS; and Hannah R. Howell,
Update on Pharmacotherapy of Chronic Hepatitis B and C Rebecca L. Corey, Pharm.D., BCPS Reviewed by Paulina Deming, B.S., Pharm.D., PhC; Lisa C. Hutchison, Pharm.D., MPH, FCCP, BCPS; and Hannah R. Howell,
HBV screening and management in HIV-infected children and adolescents
 HBV screening and management in HIV-infected children and adolescents Linda Aurpibul M.D. Research Institute for Health Sciences, Chiang Mai University 8% HIV and Hepatitis B Co-infection Among Perinatally
HBV screening and management in HIV-infected children and adolescents Linda Aurpibul M.D. Research Institute for Health Sciences, Chiang Mai University 8% HIV and Hepatitis B Co-infection Among Perinatally
Hepatitis C Glossary of Terms
 Acute Hepatitis C A short-term illness that usually occurs within the first six months after someone is exposed to the hepatitis C virus (HCV). 1 Antibodies Proteins produced as part of the body s immune
Acute Hepatitis C A short-term illness that usually occurs within the first six months after someone is exposed to the hepatitis C virus (HCV). 1 Antibodies Proteins produced as part of the body s immune
HBV DNA < monitoring interferon Rx
 Hepatitis B Virus Suspected acute hepatitis >>Order: Acute Unknown hepatitis screen Suspected chronic hepatitis >>Order: Chronic unknown hepatitis screen Acute HBV or Delayed Anti HBs response after acute
Hepatitis B Virus Suspected acute hepatitis >>Order: Acute Unknown hepatitis screen Suspected chronic hepatitis >>Order: Chronic unknown hepatitis screen Acute HBV or Delayed Anti HBs response after acute
Hepatitis C Class Review
 Hepatitis C Class Review Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Month/Year of Review: January
Hepatitis C Class Review Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Month/Year of Review: January
EASL INTERNATIONAL CONSENSUS CONFERENCE ON HEPATITIS B 13 14 September, 2002 Geneva, Switzerland Consensus statement (Short version)
 Journal of Hepatology 38 (2003) 533 540 Special article EASL INTERNATIONAL CONSENSUS CONFERENCE ON HEPATITIS B 13 14 September, 2002 Geneva, Switzerland Consensus statement (Short version) The EASL Jury*
Journal of Hepatology 38 (2003) 533 540 Special article EASL INTERNATIONAL CONSENSUS CONFERENCE ON HEPATITIS B 13 14 September, 2002 Geneva, Switzerland Consensus statement (Short version) The EASL Jury*
Medical publications on HBV and HCV Coinfection
 Recent advances of HBV and HCV co-infection 台 中 榮 總 內 科 部 胃 腸 肝 膽 科 呂 宜 達 醫 師 2013.03.28 Outline Epidemiology of HBV and HCV coinfection Clinical significance of HBV and HCV coinfection Interplay between
Recent advances of HBV and HCV co-infection 台 中 榮 總 內 科 部 胃 腸 肝 膽 科 呂 宜 達 醫 師 2013.03.28 Outline Epidemiology of HBV and HCV coinfection Clinical significance of HBV and HCV coinfection Interplay between
Focus on Transplantation: Treatment Post-transplant for HBV and HCV
 Focus on Transplantation: Treatment Post-transplant for HBV and HCV The Viral Hepatitis Congress, Frankfurt, 09. September 2012 Christoph Sarrazin J. W. Goethe-University Hospital Medizinische Klinik I
Focus on Transplantation: Treatment Post-transplant for HBV and HCV The Viral Hepatitis Congress, Frankfurt, 09. September 2012 Christoph Sarrazin J. W. Goethe-University Hospital Medizinische Klinik I
HIV and Hepatitis Co-infection. Martin Fisher Brighton and Sussex University Hospitals, UK
 HIV and Hepatitis Co-infection Martin Fisher Brighton and Sussex University Hospitals, UK Useful References British HIV Association 2010 http://www.bhiva.org/documents/guidelines/hepbc/2010/ hiv_781.pdf
HIV and Hepatitis Co-infection Martin Fisher Brighton and Sussex University Hospitals, UK Useful References British HIV Association 2010 http://www.bhiva.org/documents/guidelines/hepbc/2010/ hiv_781.pdf
Management of Chronic Hepatitis B: Consensus Guidelines
 Management of Chronic Hepatitis B: Consensus Guidelines Morris Sherman MD PhD 1, Stephen Shafran MD 2, Kelly Burak MD 3, Karen Doucette MD 2, Winnie Wong MD 2, Nigel Girgrah MD 1, Eric Yoshida MD 4, Eberhard
Management of Chronic Hepatitis B: Consensus Guidelines Morris Sherman MD PhD 1, Stephen Shafran MD 2, Kelly Burak MD 3, Karen Doucette MD 2, Winnie Wong MD 2, Nigel Girgrah MD 1, Eric Yoshida MD 4, Eberhard
Stepwise Approach for Detecting, Evaluating, and Treating Chronic Hepatitis B Virus Infection
 Stepwise Approach for Detecting, Evaluating, and Treating Chronic Hepatitis B Virus Infection Federal Bureau of Prisons Clinical Practice Guidelines January 20, 2011 Clinical guidelines are made available
Stepwise Approach for Detecting, Evaluating, and Treating Chronic Hepatitis B Virus Infection Federal Bureau of Prisons Clinical Practice Guidelines January 20, 2011 Clinical guidelines are made available
Cirrhosis and HCV. Jonathan Israel M.D.
 Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
2015 Outpatient Chronic Hepatitis B Management
 2015 Outpatient Chronic Hepatitis B Management Hepatitis B Hepatitis B Info 70% of acute infections are subclinical More severe symptoms when in addition to other liver disease Fulminant Hepatitis
2015 Outpatient Chronic Hepatitis B Management Hepatitis B Hepatitis B Info 70% of acute infections are subclinical More severe symptoms when in addition to other liver disease Fulminant Hepatitis
Liver Disease and Therapy of Hepatitis B Virus Infections
 Liver Disease and Therapy of Hepatitis B Virus Infections University of Adelaide Catherine Scougall Arend Grosse Huey-Chi Low Allison Jilbert Fox Chase Cancer Center Chunxiao Xu Carol Aldrich Sam Litwin
Liver Disease and Therapy of Hepatitis B Virus Infections University of Adelaide Catherine Scougall Arend Grosse Huey-Chi Low Allison Jilbert Fox Chase Cancer Center Chunxiao Xu Carol Aldrich Sam Litwin
Phase: IV. Study Period: 20 Jan. 2006-17 Sep. 2008
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Prior Authorization Policy
 Prior Authorization Policy http://www.paramounthealthcare.com/providers Ribavirin Rebetol (ribavirin capsule or oral solution) Copegus (ribavirin tablet), Moderiba (ribavirin tablet), Ribasphere (ribavirin
Prior Authorization Policy http://www.paramounthealthcare.com/providers Ribavirin Rebetol (ribavirin capsule or oral solution) Copegus (ribavirin tablet), Moderiba (ribavirin tablet), Ribasphere (ribavirin
Hepatitis B Virus Program
 Hepatitis B Virus Program Abstract Hepatitis B is a viral hepatitis representing a major global health problem. According to the World Health Organization (WHO), some 360 million people, or 5% of the world
Hepatitis B Virus Program Abstract Hepatitis B is a viral hepatitis representing a major global health problem. According to the World Health Organization (WHO), some 360 million people, or 5% of the world
Peg-IFN and ribavirin: what sustained virologic response can be achieved by using HCV genotyping and viral kinetics?
 Peg-IFN and ribavirin: what sustained virologic response can be achieved by using HCV genotyping and viral kinetics? Prof. I. Bakulin Gastroenterology Department Key Questions Background Worldwide prevalence
Peg-IFN and ribavirin: what sustained virologic response can be achieved by using HCV genotyping and viral kinetics? Prof. I. Bakulin Gastroenterology Department Key Questions Background Worldwide prevalence
Future Challenges in Hepatitis B Treatment. Maria Buti Hospital Universitario Valle Hebron Barcelona
 Future Challenges in Hepatitis B Treatment Maria Buti Hospital Universitario Valle Hebron Barcelona Disclosures Advisor: AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck
Future Challenges in Hepatitis B Treatment Maria Buti Hospital Universitario Valle Hebron Barcelona Disclosures Advisor: AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck
How To Treat Hepatitis B With Entecavir
 Management of Patients with Viral Hepatitis, Paris, 2004 New Nucleoside Analogs for the Treatment of Chronic Hepatitis B Maria Buti, Rafael Esteban Three agents have been approved for the treatment of
Management of Patients with Viral Hepatitis, Paris, 2004 New Nucleoside Analogs for the Treatment of Chronic Hepatitis B Maria Buti, Rafael Esteban Three agents have been approved for the treatment of
HBsAg level and hepatitis B viral load correlation with focus on pregnancy
 ORIGINAL ARTICLE Annals of Gastroenterology (2015) 28, 1-6 HBsAg level and hepatitis B viral load correlation with focus on pregnancy Maria Belopolskaya a, Viktor Avrutin b, Sergey Firsov a, Alexey Yakovlev
ORIGINAL ARTICLE Annals of Gastroenterology (2015) 28, 1-6 HBsAg level and hepatitis B viral load correlation with focus on pregnancy Maria Belopolskaya a, Viktor Avrutin b, Sergey Firsov a, Alexey Yakovlev
CASL Symposium Hepatitis B Co-chairs: Carla Coffin and Mang Ma
 CASL Symposium Hepatitis B Co-chairs: Carla Coffin and Mang Ma Occult HBV Infection: Assessment and Clinical Significance D. Lorne Tyrrell Director, Li Ka Shing Institute of Virology University of Alberta
CASL Symposium Hepatitis B Co-chairs: Carla Coffin and Mang Ma Occult HBV Infection: Assessment and Clinical Significance D. Lorne Tyrrell Director, Li Ka Shing Institute of Virology University of Alberta
HCV in 2020: Any cases left? Rafael Esteban Hospital General Universitario Valle Hebron Barcelona. Spain
 HCV in 2020: Any cases left? Rafael Esteban Hospital General Universitario Valle Hebron Barcelona. Spain Yes, still too many Measures to eradicate an Infectious Disease Prevention: Vaccination Screening
HCV in 2020: Any cases left? Rafael Esteban Hospital General Universitario Valle Hebron Barcelona. Spain Yes, still too many Measures to eradicate an Infectious Disease Prevention: Vaccination Screening
Chronic Hepatitis B (CHB) Recommendations
 Clinical update Australian and New Zealand Chronic Hepatitis B (CHB) Recommendations 1st Edition 2008 Digestive Health Foundation 2008 Digestive Health Foundation Table of contents CHAPTER PAGE 1. Chronic
Clinical update Australian and New Zealand Chronic Hepatitis B (CHB) Recommendations 1st Edition 2008 Digestive Health Foundation 2008 Digestive Health Foundation Table of contents CHAPTER PAGE 1. Chronic
GUIDELINE. South African guideline for the management of chronic hepatitis B: 2013
 South African guideline for the management of chronic hepatitis B: 2013 C W N Spearman, 1 MB ChB, FCP (SA), MMed, PhD; M W Sonderup, 1,2 MB ChB, BPharm, FCP (SA); J F Botha, 2,3 MB ChB, FCP (SA); S W van
South African guideline for the management of chronic hepatitis B: 2013 C W N Spearman, 1 MB ChB, FCP (SA), MMed, PhD; M W Sonderup, 1,2 MB ChB, BPharm, FCP (SA); J F Botha, 2,3 MB ChB, FCP (SA); S W van
PRIOR AUTHORIZATION POLICY
 PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
1.1.2 Amend the that the Special Authority relating to tenofovir for use in pregnancy for postpartum care;
 Anti-Infective Subcommittee of PTAC Meeting held 1 March 2012 (minutes for web publishing) Anti-Infective Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Anti-Infective Subcommittee of PTAC Meeting held 1 March 2012 (minutes for web publishing) Anti-Infective Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
PERINATAL AND CHILDHOOD HEPATITIS.. WHAT ABOUT THE CHILDREN?
 PERINATAL AND CHILDHOOD HEPATITIS.. WHAT ABOUT THE CHILDREN? John T. Stutts, MD, MPH University of Louisville School of Medicine Department of Pediatrics Division of Pediatric Gastroenterology, Hepatology
PERINATAL AND CHILDHOOD HEPATITIS.. WHAT ABOUT THE CHILDREN? John T. Stutts, MD, MPH University of Louisville School of Medicine Department of Pediatrics Division of Pediatric Gastroenterology, Hepatology
The natural history of chronic HBV infection
 Hepatitis B Viral Factors in HBeAg-Negative Carriers with Persistently Normal Serum Alanine Aminotransferase Levels Chih-Lin Lin, 1* Li-Ying Liao, 1* Chun-Jen Liu, 2 Ming-Whei Yu, 3 Pei-Jer Chen, 2,4 Ming-Yang
Hepatitis B Viral Factors in HBeAg-Negative Carriers with Persistently Normal Serum Alanine Aminotransferase Levels Chih-Lin Lin, 1* Li-Ying Liao, 1* Chun-Jen Liu, 2 Ming-Whei Yu, 3 Pei-Jer Chen, 2,4 Ming-Yang
IT IS ESTIMATED that there are 400 million patients of
 bs_bs_banner Hepatology Research 2014; 44 (Suppl. 1): 1 58 doi: 10.1111/hepr.12269 Special Report JSH Guidelines for the Management of Hepatitis B Virus Infection Drafting Committee for Hepatitis Management
bs_bs_banner Hepatology Research 2014; 44 (Suppl. 1): 1 58 doi: 10.1111/hepr.12269 Special Report JSH Guidelines for the Management of Hepatitis B Virus Infection Drafting Committee for Hepatitis Management
HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years
 HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years Massimo Fasano 1, Pietro Lampertico 2, Alfredo Marzano 3, Vito Di Marco 4,
HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years Massimo Fasano 1, Pietro Lampertico 2, Alfredo Marzano 3, Vito Di Marco 4,
Comprehensive analysis of hepatitis B virus antiviral drug resistance mutations based on a new high-throughput phenotypic assay
 Fakultät für Medizin Institut für virologie Comprehensive analysis of hepatitis B virus antiviral drug resistance mutations based on a new high-throughput phenotypic assay Ke Zhang Vollständiger Abdruck
Fakultät für Medizin Institut für virologie Comprehensive analysis of hepatitis B virus antiviral drug resistance mutations based on a new high-throughput phenotypic assay Ke Zhang Vollständiger Abdruck
New treatment options for HCV: implications for the Optimal Use of HCV Assays
 New treatment options for HCV: implications for the Optimal Use of HCV Assays Hans Orlent Dept. of Gastroenterology & Hepatology AZ Sint Jan Brugge-Oostende, Brugge This program is supported by educational
New treatment options for HCV: implications for the Optimal Use of HCV Assays Hans Orlent Dept. of Gastroenterology & Hepatology AZ Sint Jan Brugge-Oostende, Brugge This program is supported by educational
Management of hepatitis C: pre- and post-liver transplantation. Piyawat Komolmit Bangkok
 Management of hepatitis C: pre- and post-liver transplantation Piyawat Komolmit Bangkok Liver transplantation and CHC Cirrhosis secondary to HCV is the leading cause of liver transplantation in the US
Management of hepatitis C: pre- and post-liver transplantation Piyawat Komolmit Bangkok Liver transplantation and CHC Cirrhosis secondary to HCV is the leading cause of liver transplantation in the US
Dr. Abonyi Margit PhD SE 1st Medical Clinic Associate Professor. Hepatology-2014
 Dr. Abonyi Margit PhD SE 1st Medical Clinic Associate Professor Hepatology-2014 Other virus hepatitis Epstein-Barr vírus Cytomegalo vírus Herpes simplex vírus Coxsackie vírus Hepatitis B vírus Hepadnavírus,
Dr. Abonyi Margit PhD SE 1st Medical Clinic Associate Professor Hepatology-2014 Other virus hepatitis Epstein-Barr vírus Cytomegalo vírus Herpes simplex vírus Coxsackie vírus Hepatitis B vírus Hepadnavírus,
Hepatitis B and C Co-infection. Mark Hull MHSc, FRCPC Clinical Assistant Professor Division of AIDS
 Hepatitis B and C Co-infection Mark Hull MHSc, FRCPC Clinical Assistant Professor Division of AIDS Objectives Review natural history of hepatitis coinfection Brief overview of treatment indications for
Hepatitis B and C Co-infection Mark Hull MHSc, FRCPC Clinical Assistant Professor Division of AIDS Objectives Review natural history of hepatitis coinfection Brief overview of treatment indications for
Disclosure of Conflicts of Interest Learner Assurance Statement:
 Raj Reddy, MD Ruimy Family President's Distinguished Professor of Medicine Professor of Medicine in Surgery Director of Hepatology Director, Viral Hepatitis Center Medical Director, Liver Transplantation
Raj Reddy, MD Ruimy Family President's Distinguished Professor of Medicine Professor of Medicine in Surgery Director of Hepatology Director, Viral Hepatitis Center Medical Director, Liver Transplantation
CANADIAN CONSENSUS GUIDELINES. Management of chronic hepatitis B: Consensus guidelines
 CANADIAN CONSENSUS GUIDELINES Management of chronic hepatitis B: Consensus guidelines Morris Sherman MD PhD 1, Stephen Shafran MD 2, Kelly Burak MD 3, Karen Doucette MD 2, Winnie Wong MD 2, Nigel Girgrah
CANADIAN CONSENSUS GUIDELINES Management of chronic hepatitis B: Consensus guidelines Morris Sherman MD PhD 1, Stephen Shafran MD 2, Kelly Burak MD 3, Karen Doucette MD 2, Winnie Wong MD 2, Nigel Girgrah
Acute viral hepatitis B (AVH-B) is successfully. A Randomized Controlled Trial of Lamivudine to Treat Acute Hepatitis B. Patients and Methods
 A Randomized Controlled Trial of Lamivudine to Treat Acute Hepatitis B M. Kumar, S. Satapathy, R. Monga, K. Das, S. Hissar, C. Pande, B. C. Sharma, and S. K. Sarin The role of antivirals in patients with
A Randomized Controlled Trial of Lamivudine to Treat Acute Hepatitis B M. Kumar, S. Satapathy, R. Monga, K. Das, S. Hissar, C. Pande, B. C. Sharma, and S. K. Sarin The role of antivirals in patients with
Surveillance for Hepatocellular Carcinoma
 Surveillance for Hepatocellular Carcinoma Marion G. Peters, MD John V. Carbone, MD, Endowed Chair Professor of Medicine Chief of Hepatology Research University of California San Francisco Recorded on April
Surveillance for Hepatocellular Carcinoma Marion G. Peters, MD John V. Carbone, MD, Endowed Chair Professor of Medicine Chief of Hepatology Research University of California San Francisco Recorded on April
Case Finding for Hepatitis B and Hepatitis C
 Case Finding for Hepatitis B and Hepatitis C John W. Ward, M.D. Division of Viral Hepatitis Centers for Disease Control and Prevention Atlanta, Georgia, USA Division of Viral Hepatitis National Center
Case Finding for Hepatitis B and Hepatitis C John W. Ward, M.D. Division of Viral Hepatitis Centers for Disease Control and Prevention Atlanta, Georgia, USA Division of Viral Hepatitis National Center
Hepatitis C Monitoring and Complications (and Treatment!) Dr Mark Douglas
 Hepatitis C Monitoring and Complications (and Treatment!) Dr Mark Douglas Hepatitis C Virus Shimizu et al., 1996 Positive single strand RNA virus Flaviviridae family, Hepacivirus genus 9.6 kbp genome ~3000
Hepatitis C Monitoring and Complications (and Treatment!) Dr Mark Douglas Hepatitis C Virus Shimizu et al., 1996 Positive single strand RNA virus Flaviviridae family, Hepacivirus genus 9.6 kbp genome ~3000
HIV and Hepatitis B CoInfection
 HIV and Hepatitis B CoInfection Douglas G. Fish, MD June 3, 2014 44 yo male with AIDS who had fallen out of care and returned in October 2013 Last seen in November 2012 CD4 at that time 340 cells/cmm HIV
HIV and Hepatitis B CoInfection Douglas G. Fish, MD June 3, 2014 44 yo male with AIDS who had fallen out of care and returned in October 2013 Last seen in November 2012 CD4 at that time 340 cells/cmm HIV
Hepatitis B reactivation. Geoffrey Dusheiko UCL Institute for Liver and Digestive Health Royal Free Hospital London, UK
 Hepatitis B reactivation Geoffrey Dusheiko UCL Institute for Liver and Digestive Health Royal Free Hospital London, UK HBV reactivation Defined as abrupt rise in HBV replication (> 1 log) Followed by lab
Hepatitis B reactivation Geoffrey Dusheiko UCL Institute for Liver and Digestive Health Royal Free Hospital London, UK HBV reactivation Defined as abrupt rise in HBV replication (> 1 log) Followed by lab
Quantitative HBV DNA measurements and the management of infected health care workers
 Quantitative HBV DNA measurements and the management of infected health care workers A.A. van der Eijk Department of Virology, Erasmus MC, Rotterdam, the Netherlands Introduction Worldwide since 1970s,
Quantitative HBV DNA measurements and the management of infected health care workers A.A. van der Eijk Department of Virology, Erasmus MC, Rotterdam, the Netherlands Introduction Worldwide since 1970s,
LA TERAPIA PER HBV ed HCV Differenze di Genere? Alfredo Alberti. Dipartimento di Medicina Molecolare UOC Medicina Generale VIMM Università di Padova
 LA TERAPIA PER HBV ed HCV Differenze di Genere? Alfredo Alberti Dipartimento di Medicina Molecolare UOC Medicina Generale VIMM Università di Padova HBV ed HCV Due virus Diversi ma con molte Cose in Comune
LA TERAPIA PER HBV ed HCV Differenze di Genere? Alfredo Alberti Dipartimento di Medicina Molecolare UOC Medicina Generale VIMM Università di Padova HBV ed HCV Due virus Diversi ma con molte Cose in Comune
Clinical Criteria for Hepatitis C (HCV) Therapy
 Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
HEPATITIS C TREATMENT GUIDELINES
 HEPATITIS C TREATMENT GUIDELINES Updated May 21, 2014 INSTRUCTIONS: 1. Review the posted Hepatitis C Treatment Guidelines document to validate that your patient meets the criteria for treatment. 2. Complete
HEPATITIS C TREATMENT GUIDELINES Updated May 21, 2014 INSTRUCTIONS: 1. Review the posted Hepatitis C Treatment Guidelines document to validate that your patient meets the criteria for treatment. 2. Complete
Prolonged therapy of hepatitis delta for 96 weeks with PEG-IFNa-2a plus tenofovir or placebo does not prevent HDV RNA relapse: The HIDIT-2 study.
 Prolonged therapy of hepatitis delta for 96 weeks with PEG-IFNa-2a plus tenofovir or placebo does not prevent HDV RNA relapse: The HIDIT-2 study. Heiner Wedemeyer*, Cihan Yurdaydın*, Stefanie Ernst, Florin
Prolonged therapy of hepatitis delta for 96 weeks with PEG-IFNa-2a plus tenofovir or placebo does not prevent HDV RNA relapse: The HIDIT-2 study. Heiner Wedemeyer*, Cihan Yurdaydın*, Stefanie Ernst, Florin
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT
 PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
J Clin Oncol 26:177-182. 2008 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 26 NUMBER 2 JANUARY 10 2008 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T High Viral Load and Hepatitis B Virus Subgenotype Ce Are Associated With Increased Risk of Hepatocellular Carcinoma
VOLUME 26 NUMBER 2 JANUARY 10 2008 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T High Viral Load and Hepatitis B Virus Subgenotype Ce Are Associated With Increased Risk of Hepatocellular Carcinoma
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Pegasys and non-preferred Hepatitis C Agents must
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Pegasys and non-preferred Hepatitis C Agents must
TESTING AND MANAGEMENT. Dr Nicole Allard GP Cohealth, Joslin Clinic, West Footscray PhD student, Epidemiology Unit VIDRL
 TESTING AND MANAGEMENT Dr Nicole Allard GP Cohealth, Joslin Clinic, West Footscray PhD student, Epidemiology Unit VIDRL Disclosure and acknowledgments No conflicts of interest Board Member of Hepatitis
TESTING AND MANAGEMENT Dr Nicole Allard GP Cohealth, Joslin Clinic, West Footscray PhD student, Epidemiology Unit VIDRL Disclosure and acknowledgments No conflicts of interest Board Member of Hepatitis
Real Time PCR Usage in the Quantification of Hepatitis B Virus DNA-Clinical Applications in Disease Management
 Review Article Real Time PCR Usage in the Quantification of Hepatitis B Virus DNA-Clinical Applications in Disease Management *Narotam Sharma 1, Jagdish Kandpal 1, Satish C Nautiyal 1, Shweta Rawat 2,
Review Article Real Time PCR Usage in the Quantification of Hepatitis B Virus DNA-Clinical Applications in Disease Management *Narotam Sharma 1, Jagdish Kandpal 1, Satish C Nautiyal 1, Shweta Rawat 2,
Co-infected health-care workers
 Co-infected health-care workers Y.Yazdanpanah Service Universitaire des Maladies Infectieuses et du Voyageur C.H. Tourcoing, Faculté de Médecine de Lille CNRS U362, Lille, France Co-infected health-care
Co-infected health-care workers Y.Yazdanpanah Service Universitaire des Maladies Infectieuses et du Voyageur C.H. Tourcoing, Faculté de Médecine de Lille CNRS U362, Lille, France Co-infected health-care
Transmission of HCV in the United States (CDC estimate)
 Transmission of HCV in the United States (CDC estimate) Past and Future US Incidence and Prevalence of HCV Infection Decline among IDUs Overall incidence Overall prevalence Infected 20+ years Armstrong
Transmission of HCV in the United States (CDC estimate) Past and Future US Incidence and Prevalence of HCV Infection Decline among IDUs Overall incidence Overall prevalence Infected 20+ years Armstrong
1. A 19 year old Caucasian male is referred to you because he recently developed acute HBV.
 VIRAL HEPATITIS TEST QUESTIONS AND ANSWERS 1. A 19 year old Caucasian male is referred to you because he recently developed acute HBV. At the time of diagnosis he was HB surface antigen positive, anti-hb
VIRAL HEPATITIS TEST QUESTIONS AND ANSWERS 1. A 19 year old Caucasian male is referred to you because he recently developed acute HBV. At the time of diagnosis he was HB surface antigen positive, anti-hb
Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: A systematic review
 Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: A systematic review George V. Papatheodoridis 1,, Spilios Manolakopoulos 1,
Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: A systematic review George V. Papatheodoridis 1,, Spilios Manolakopoulos 1,
Viral Hepatitis. 2009 APHL survey report
 Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
In this study, the DCV+ASV regimen had low rates of discontinuation (5%) due to adverse events, and low rates of serious adverse events (5.
 BMS Submits First All-Oral, Interferon-Free and Ribavirin-Free Treatment Regimen for Regulatory Review in Japan for Patients with Chronic Hepatitis C Infection An overall SVR 24 rate of 84.7 percent was
BMS Submits First All-Oral, Interferon-Free and Ribavirin-Free Treatment Regimen for Regulatory Review in Japan for Patients with Chronic Hepatitis C Infection An overall SVR 24 rate of 84.7 percent was
