3-MONTH FINANCIAL REPORT 2015
|
|
|
- Simon Joshua Gaines
- 8 years ago
- Views:
Transcription
1 3-MONTH FINANCIAL REPORT 2015 Sales revenue and earnings up; costs reduced substantially Subsidiary Heidelberg Pharma awarded grants to support ADC technology Rights issue successfully completed
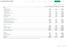
2 WILEX Group 3-month financial report 2015 KEY group FIGURES Key Group figures Q Q Earnings Sales revenue Other income Operating expenses (1,972) (3,616) of which research and development costs (813) (1,999) Operating result (1,074) (2,868) Earnings before tax (1,074) (2,884) Net loss for the period (1,074) (2,884) Earnings per share in (0.14) (0.37) 4 Balance sheet as of the end of the period Total assets 13,994 18,969 Cash and cash equivalents 1,369 5,546 Equity 10,813 12,085 Equity ratio 2 in % Cash flow statement Cash flow from operating activities (823) (3,380) Cash flow from investing activities (6) (45) Cash flow from financing activities (11) (42) Employees (number) Employees as of the end of the period Full-time equivalents as of the end of the period The reporting period begins on 1 December and ends on 28 February. 2 Equity/total assets 3 Including members of the Executive Management Board 4 In order to facilitate comparison, the earnings per share in the previous period (Q1 2014: 0.09) were adjusted to the current number of shares in a ratio of 4:1 in accordance with IAS For more information, see note 29 in the notes to the consolidated financial statements in the 2014 annual report. Rounding of exact figures may result in differences.
3 WILEX Group 3-month financial report 2015 LETTER TO THE SHAREHOLDERS 1 Letter to the shareholders We are satisfied with our performance in the first quarter of the 2015 financial year. We were able to boost our sales revenue and other income compared to the same quarter of 2014, and we also continued to lower our costs. The restructuring measures are bearing fruit and we managed to eliminate some of our rental costs. Development activities using our ADC technology are advancing at our subsidiary Heidelberg Pharma. We received funding commitments from Germany s Federal Ministry of Education and Research as well as from the European Union. This will enable us to pursue our own research strategy with a PSMA antibody Amanitin conjugate in the fight against prostate cancer and, as part of a consortium, apply our toxin linker technology to peptides. Heidelberg Pharma will present preclinical data at a number of scientific conferences. The cooperation with Roche is proceeding as planned and we hope that combining our unique toxin with antibodies from Roche will generate preclinical data for various target molecules. Other earlierstage partnerships also confirms the view that we are working on an extremely interesting approach. After the end of the reporting period we announced a rights issue, which has since been successfully completed. All shares were placed with existing shareholders through exercise of their subscription rights and the allocation of additional subscriptions. We are delighted to report that not only did our main shareholder dievini avail itself of this offer, but around 36 % of the shares were taken up by other shareholders from the free float. Prior to the transaction, dievini had stated that it was prepared to make funds of up to 5 million available. The gross proceeds from the rights issue of 4.16 million will be used to finance the further development of the ADC technology, particularly to establish drug production in accordance with good manufacturing practice (GMP). The cash will bolster our balance sheet and enable us to continue working on marketing our Phase III product candidates. An exciting year lies ahead of us. We expect instructive data from our own ADC studies in addition to those being conducted by our cooperation partners. We would like to thank our shareholders and business partners for their continuing support. Yours sincerely, Munich, 14 April 2015 Dr Jan Schmidt-Brand Spokesman for the Executive Management Board and Chief Financial Officer
4 2 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT Interim management report Reporting period from 1 December 2014 to 28 February 2015 Introduction WILEX is a biopharmaceutical company with a portfolio of antibody-based diagnostic and therapeutic products for the detection and targeted treatment of various types of cancer. WILEX AG ceased all clinical development activities at the Munich site in 2014 as part of an extensive restructuring programme. Since then it has mainly carried out activities relating to the Group parent s position as a holding company and has continued to work on marketing the RENCAREX and REDECTANE clinical antibody programmes. Research and development activities now focus on the operations of its subsidiary Heidelberg Pharma GmbH in Ladenburg, which primarily advances the development of the innovative platform technology for antibody drug conjugates (ADC technology) and offers preclinical services. WILEX reported on three segments in previous years applying IFRS 8 Operating Segments: Customer Specific Research (Cx), Diagnostics (Dx) and Therapeutics (Rx). Since the beginning of the 2015 financial year, in accordance with its existing internal reporting structures, WILEX no longer reports segment information because its business activities are centred on the ADC technology and the preclinical service business and are therefore performed almost exclusively in the Customer Specific Research segment. Business performance and research and development activities Clinical portfolio MESUPRON MESUPRON (INN: Upamostat) is an oral upa/serine protease inhibitor designed to block the activity of tumour-relevant serine proteases such as upa, plasmin and thrombin. This aims to prevent tumour growth and metastasis. In 2014, the worldwide rights to the development and commercialisation of MESUPRON were out-licensed to Link Health Co., Guangzhou, China, for the region comprising China, Hong Kong, Taiwan and Macau, and to RedHill Biopharma Ltd., Tel Aviv, Israel, for the rest of the world. All further development and marketing activities for this product candidate will be carried out by the partners. To the extent possible, WILEX will report on the development successes. RENCAREX RENCAREX (INN: Girentuximab) is a monoclonal antibody that binds to a tumour-specific antigen (carbonic anhydrase IX or CAIX ). This antigen is expressed in several types of cancer (kidney and colon cancer as well as head and neck tumours) but is generally not present in healthy tissue. The fact that the antibody binds to the antigen makes the tumour visible to the endogenous immune system such that natural killer cells can bind to destroy the tumour. In 2013, a Phase III trial with RENCAREX was completed that did not show a significant improvement in adjuvant therapy of clear cell renal cell cancer. Positive, but retrospective subgroup data could provide the basis for out-licensing the antibody. Talks are being held with different partners but have not yet resulted in a satisfactory outcome. REDECTANE REDECTANE (INN: 124I-Girentuximab) is a radiolabelled form of the antibody Girentuximab, which binds to the tumourspecific antigen CAIX on clear cell renal cell carcinoma. The antibody-based imaging diagnostic REDECTANE with PET/CT could support physicians in diagnosing kidney tumours. This could fundamentally change therapy planning for renal cancer patients. Furthermore, REDECTANE may also prove suitable for monitoring response to treatment and for diagnosing other kinds of tumours.
5 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT 3 WILEX is engaged in talks with potential new partners for the external development, financing, production and marketing of REDECTANE. Customer Specific Research Heidelberg Pharma is developing a technology platform for antibody drug conjugates and enhancing this with technolo gical support from its partners. The company also provides preclinical services for other areas of oncology and inflammatory diseases. ADC technology (antibody drug conjugates) The core of this technology consists in using a chemical compound (linker) to crosslink a suitable antibody to a specific toxin (= ADC). The role of the antibody is to transport the crosslinked toxin specifically to and then into the cancer cell. After binding to the tumour cell, the ADC is taken up and releases the toxin within the cell. The released toxin then destroys the tumour cell without affecting healthy tissue. Heidelberg Pharma works with the toxin Amanitin, a member of the amatoxin group of natural poisons occurring in the death cap (Amanita phalloides), among others. Second-generation ADCs, known as ATACs (Antibody Targeted Amanitin Conjugates) will be developed on the basis of the related innovative mode of action (inhibition of RNA polymerase II). The ATACs are characterised by improved efficacy, also as regards quiescent tumour cells, which are scarcely reached with existing standard therapies and contribute to tumour recurrence and resistance formation. These ATACs will also be used to treat therapy-resistant tumours that no longer respond to standard chemotherapy or anti-tumour antibodies. Selective treatment of tumours using cytotoxins via specific antibody drug conjugates could thus enable much more effective treatment of tumours with acceptable side effects. The business model is currently focused on a business-to-business activity in which the compound linker technology developed by Heidelberg Pharma is licensed by pharmaceutical and biotechnology companies to make their antibodies more therapeutically effective in the treatment of tumour diseases. Within this framework and under licence agreements, Heidelberg Pharma gives the cooperation partners not only the licensing rights but also technological support in the manufacture and purification of the conjugates, the production and delivery of the compound, and selected preclinical investigations. The company also pursues proprietary approaches to test in-licensed or third-party antibodies with the toxin linker technology and to conduct further research and development activities with them if appropriate. PSMA-ATAC project In early January 2015, our first proprietary ATAC project received a research grant to continue the development of PSMA antibody drug conjugates for the treatment of prostate cancer. The new research project estimated at 1.8 million runs for 30 months and will receive grants from the Federal Ministry of Education and Research (BMBF) totalling 0.9 million. PSMA is overexpressed in prostate cancer specifically and is an attractive target for an ADC approach, as it shows very low expression in normal tissues and sufficient internalisation after antibody binding. In pilot studies, Heidelberg Pharma investigated the anti-tumour potency of several monoclonal antibodies targeting the prostate-specific membrane antigen (PSMA) conjugated to small molecules from the amatoxin family. The BMBF funds will be used to further develop PSMA antibody targeted Amanitin conjugates (ATACs). The preclinical project covers the humanisation and de-immunisation of the selected anti-psma antibody which will be coupled via several linker combinations to α-amanitin based on Heidelberg Pharma s patented technology. These human anti-psma Amanitin conjugates will be tested preclinically for safety, tolerability and efficacy.
6 4 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT European MAGICBULLET training network The European Union supports promising research projects within the Horizon 2020 Framework Programme for Research and Innovation. In February 2015 ETN MAGICBULLET was granted a total of 3.75 million for the period from 2015 to 2018 for the development of new chemistry-driven concepts for anti-tumour therapies. Heidelberg Pharma is part of the ETN MAGICBULLET consortium which consists of seven academic research groups from Germany, Italy, Hungary and Finland, and two pharmaceutical companies (Heidelberg Pharma and Exiris in Italy). The aim of the consortium is to develop and validate an array of new peptide-drug conjugates combining tumour-specific peptides with potent cytotoxic drugs. Heidelberg Pharma s task is to identify, modify and validate tumour-specific peptide-drug conjugates based on its expertise in linker technology as well as to investigate the biological activity in vitro and in vivo. Preclinical service business Heidelberg Pharma also has the expertise and required infrastructure for in vivo pharmacology, cell biology, bioanalytics, molecular biology and chemistry, and offers preclinical research services in the field of cancer as well as inflammatory and autoimmune diseases. In its research process, the company concentrates on early substances (for example, lead structures to be optimised) up to the profiling of preclinical candidates. Here, both standard models and innovative developments for selected customers are offered in the specified indications. Finally, Heidelberg Pharma develops customerspecific efficacy models on request to support customers individual research activities. Market environment See pages 18 to 22 of the 2014 annual report for further information on the market environment for WILEX s products and product candidates. In the Company s view, there have been no significant changes since then. Results of operations, financial position and net assets The WILEX Group comprising WILEX AG and the subsidiary Heidelberg Pharma GmbH reports consolidated figures. The reporting period referred to below concerns the period from 1 December 2014 to 28 February 2015 (Q1 2015). The comparative figures relate to the period from 1 December 2013 to 28 February 2014 (Q1 2014). As a consequence of last year s restructuring measures, which led to the discontinuation of research and development activities at the Munich site, no further business activities are conducted that differ materially in their risk/reward profiles. R&D activities have since focused on the operations of WILEX s subsidiary Heidelberg Pharma in Ladenburg. As a result, from now on WILEX will no longer report on segments. Due to rounding, it is possible that individual figures in this report may not add up exactly to the totals and that percentages given may not precisely reflect the absolute figures to which they relate.
7 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT 5 Sales revenue and other income In the first three months of the 2015 financial year, the WILEX Group generated sales revenue and income totalling 0.9 million, up 29 % on the previous year ( 0.7 million). This figure includes sales revenue of 0.4 million (previous year: 0.4 million), which comprises components from the licence agreement with Roche and from the services business in roughly equal proportions. Income in million Q Q Sales revenue Other income 1 rounded At 0.5 million, other income was up on the previous year ( 0.3 million) due, among other things, to income from exchange rate differences ( 0.3 million), which is mainly attributable to a US dollar loan receivable. This item also included income from the reversal through profit and loss of provisions that were not needed in the projected amount ( 0.1 million) and income from sub-letting the office and laboratory space in Munich ( 0.1 million). Furthermore, grants from the Federal Ministry of Education and Research (BMBF) were received for research projects. Operating expenses Operating expenses including depreciation, amortisation and impairment losses amounted to 2.0 million in the reporting period, down 44 % compared with the previous year ( 3.6 million). This can be attributed to the discontinuation of clinical research activities at WILEX AG and to savings in the wake of the restructuring. Operating expenses in million Q Q Cost of sales Research and development costs Administrative costs Other expenses 1 rounded The cost of sales concerns the Group s costs directly related to sales revenue. They were incurred for customer-specific research in the reporting period and mounted to 0.4 million (previous year: 0.5 million), accounting for 19 % of operating expenses. Research and development costs, which were 2.0 million in the previous year, fell by 1.2 million to 0.8 million due to the discontinuation of R&D activities at the Munich site. However, at 41 % of operating expenses, these were still the largest cost item.
8 6 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT Administrative costs were reduced to 0.7 million in the first three months of 2015 in connection with the cost-cutting measures (previous year: 0.9 million). They accounted for 36 % of operating expenses and, among others, included legal consulting costs and all rental expenses at the Munich site. Other expenses for activities in the areas of business development, marketing and commercial market supply amounted to 0.1 million in the current reporting period (previous year: 0.2 million) and accounted for 4 % of operating expenses. Financial result At 0.2 k (previous year: 16 k), the WILEX Group almost broke even in terms of its financial result. While no further finance income was recorded (previous year: 24 k), finance costs were reduced to 0.2 k (previous year: 39 k). The expenses incurred in the previous year mainly comprised interest expense for the UCB shareholder loan, which on account of UCB s waiver of its claim for repayment later in the year was no longer incurred. Profit/loss for the period The WILEX Group posted a loss of 1.1 million for the first three months of the current financial year. The loss was substantially smaller than in the same period the previous year ( 2.9 million) and was mainly due to reduced costs. Reflecting the net loss for the period, earnings per share improved by 62 % to 0.14 (previous year: 0.37). In order to facilitate comparison, the earnings per share in the previous period ( 0.09) were adjusted to the current number of shares in a ratio of 4 :1 in accordance with IAS Assets Total assets as of 28 February 2015 amounted to 14.0 million, down from the figure of 15.0 million shown as of the 30 November 2014 reporting date. Balance sheet structure assets in million Non-current assets Other current assets Cash and cash equivalents 1 rounded Non-current assets at the end of the reporting period amounted to 12.1 million, which was on a par with the previous year (30 November 2014: 12.1 million). These included property, plant and equipment ( 1.0 million), intangible assets ( 2.9 million), the goodwill of Heidelberg Pharma ( 6.1 million) and the loan receivable from Nuclea ( 1.9 million), as well as rent deposits ( 0.2 million). Current assets totalled 1.9 million (30 November 2014: 2.9 million). The decline is due to the use of cash and cash equivalents for the Company s operations, amounting to 1.4 million as of 28 February 2015 (30 November 2014: 2.2 million).
9 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT 7 Equity Equity as of the end of the reporting period was 10.8 million (30 November 2014: 11.9 million). This corresponded to an equity ratio of 77.3 % (30 November 2014: 79.0 %, 28 February 2014: 63.7 %). Further information regarding the development of equity can be found in the notes to this quarterly report. Balance sheet structure equity and liabilities in million Equity Non-current liabilities Current liabilities * rounded Liabilities There were no non-current liabilities to show at the end of the reporting period (30 November 2014: 3 k). Current liabilities increased marginally to 3.2 million as of the end of the period (30 November 2014: 3.1 million). While trade payables ( 0.2 million), lease liabilities ( 13 k) and provisions ( 0.6 million) all decreased compared with the figures for 30 November 2014, other current liabilities rose to 2.4 million (30 November 2014: 2.1 million) on the back of additional deferred income. They comprise the following: Other current liabilities in million Obligation for holidays not taken Other deferred income Social security and other taxes Accrued liabilities 1 rounded Cash flow statement As a result of the restructuring and the associated cost savings, the net cash outflow from operating activities of 0.8 million after three months was substantially lower than in the same period the previous year (cash outflow of 3.4 million). The outflow of funds for investing activities was just 6 k (previous year: 45 k). A cash outflow from financing activities of 11 k that, similar to in the first quarter of the preceding year ( 42 k), was used exclusively to repay finance leases, was recorded in the reporting period.
10 8 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT In spite of the positive influence from exchange rate effects of 11 k on cash (previous year: 93 k), the net change in cash and cash equivalents therefore amounted to 0.8 million (previous year: 3.4 million). WILEX s average monthly funding requirement in the first three months of the financial year was 0.3 million (previous year: 1.1 million). The anticipated reduction as a result of the restructuring was achieved. Cash flow Q in million (0.82) (0.01) (0.01) 0, Cash as of 1 December 2014 Net change in cash from operating activities Net change in cash from investing activities Net change in cash from financing activities Exchange rate effects Cash as of 28 February rounded Employees and compensation system Including the members of its Executive Management Board, the WILEX Group had 51 employees (45 FTEs) at the close of the reporting period (30 November 2014: 52 employees/46 FTEs; 28 February 2014: 88 employees/81 FTEs). The reduction of the workforce was to a large extent a result of the restructuring measures at the Munich site. Page 16 The Company has a performance-related compensation system for its employees comprising a fixed annual salary and a variable salary component. In addition, the 2005 and 2011 stock option programmes give employees a stake in the Company s performance. For more information, see section D. Issue and measurement of stock options of the notes. Report on risks and opportunities WILEX is exposed to the risks typical for a biotechnology company, namely those arising from the development and production of potential drugs and diagnostic agents used in cancer therapies. The time between the commencement of drug develop ment and marketing approval usually spans many years. As a result of the focus on ADC technology, activities in the value chain were shifted forwards from clinical to preclinical development. This will lead to higher development risks yet lower costs. The Company is still unable to finance itself independently from product sales or licence revenue and is dependent on funding from equity providers or licensees. Risks and opportunities in connection with the WILEX Group s business are described in detail on pages 51 to 60 of the 2014 annual report. They remain unchanged unless noted otherwise.
11 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT 9 Report on post-balance sheet date events On 18 March 2015, WILEX AG announced the implementation of a rights issue from authorised capital. The subscription period ran from 20 March 2015 to 7 April The shareholders of WILEX AG exercised their subscription and additional subscription rights for all 1,486,732 new no par value bearer shares at a price of 2.80 per share by the end of the subscription period. Accordingly, the Executive Management Board resolved on 7 April 2015, with the approval of the Supervisory Board, to set the final scope of the rights issue at 1,486,732 new shares. Through the exercise of subscription rights, 582,240 new shares were subscribed. A total of 904,492 new shares were available for additional subscription by shareholders which were fully allocated to the shareholders through their custodian banks in connection with the rights issue. The main shareholder dievini Hopp BioTech holding GmbH & Co. KG, Walldorf, exercised all of its subscription rights and also subscribed shares as part of the additional subscription. WILEX AG plans to use the gross proceeds from the rights issue of 4.16 million to finance the further development of the ADC technology in particular the GMP transfer of the drug production as well as to enhance its equity. Given the difference in participation rights, the new shares will be traded separately under the ISIN DE000A14KND2/ WKN A14KND until the planned inclusion in the company s current listing (after the Annual General Meeting on 30 July 2015). Baader Bank AG, Unterschleißheim, was the sole lead manager of the capital measure. At the Supervisory Board meeting on 24 March 2015, the appointment of Dr Paul Bevan as Head of Research and Development was unanimously extended until 31 March After the end of the reporting period, no other significant events occurred which have a direct influence on the business activities of the WILEX Group. Outlook WILEX will concentrate on the further development and marketing of the ADC technology and the preclinical service business at Heidelberg Pharma and push ahead with the marketing activities for the WILEX product portfolio. It is expected that Heidelberg Pharma will not only continue its collaboration with Roche in the area of ADC technology but will also be able to expand the number of existing cooperations with pharmaceutical and biotech companies. The ATAC technology platform will be continuously refined, thereby extending the therapeutic window for ATACs. Efforts will additionally be made to identify proprietary ATAC candidates (antibody + toxin) for further development. One of the important next steps is initiating the transfer of Amanitin production to a GMP-compliant process. Moreover, some of Heidelberg Pharma s own research approaches for further improving the ADC technology will supply trend-setting data that will go beyond the existing toxin linker approaches and involve optimising antibodies for use in ADC technology. In the services business, the range of services on offer and sales revenue are to be expanded. Heidelberg Pharma will increasingly position itself as a specialist provider of comprehensive ADC research services comprising ADC synthesis and analytical quality control, as well as in vitro and in vivo testing. This explicitly includes also the work with alternative toxins used by customers and is not limited to Heidelberg Pharma s ATAC technology.
12 10 WILEX Group 3-month financial report 2015 INTERIM MANAGEMENT REPORT WILEX AG will continue to search for new licensing partners for the Phase III product candidates RENCAREX and REDECTANE. At the same time, it will assist its partners Link Health and RedHill in pushing ahead with the further development of MESUPRON. The guidance for the WILEX Group for the current financial year issued at the end of March 2015 remains unchanged. Financial outlook Guidance (03/2015) million Actual 2014 million Sales revenue and other income Operating expenses (7.0) (10.0) (10.6) Operating result (2.0) (5.0) (5.6) Total funding requirement (3.0) (5.0) (6.7) Funds required per month (0.3) (0.4) (0.6)
13 WILEX Group 3-month financial report 2015 FINANCIAL STATEMENTS 11 Consolidated statement of comprehensive income (IFRS) Reporting period from 1 December 2014 to 28 February 2015 Q Q Revenue 427, ,690 Other income 470, ,808 Income 897, ,498 Cost of sales (367,951) (539,631) Research and development costs (813,260) (1,998,618) Administrative costs (710,091) (918,000) Other expenses (80,884) (160,129) Operating expenses (1,972,186) (3,616,378) Operating result (1,074,247) (2,867,880) Finance income 0 23,614 Finance costs (227) (39,438) Financial result (227) (15,825) Earnings before tax (1,074,473) (2,883,705) Income tax 0 0 Net loss for the period (1,074,473) (2,883,705) Net currency gain/loss from consolidation 0 0 Other comprehensive income 0 0 Comprehensive income (1,074,473) (2,883,705) Earnings per share Basic and diluted earnings per share (0.14) (0.37) Average number of shares issued 7,818,876 7,818,876 Quarterly comparison Q Q Q Q Q Revenue , Other income 471 (1,873) 2, Operating expenses (1,972) (2,751) (1,861) (2,358) (3,616) Operating result (1,074) (3,864) 2,598 (1,442) (2,868) Financial result (0) 20 (18) (18) (16) Earnings before tax (1,074) (3,844) 2,580 (1,460) (2,884) Net loss for the period (1,074) (3,890) 2,580 (1,507) (2,884) Net currency gain/loss from consolidation Comprehensive income (1,074) (3,890) 2,580 (1,507) (2,884) Basic and diluted earnings per share in (0.14) (0.50) 0.33 (0.19) (0.37) Average number of shares issued 7,819 7,819 7,819 7,819 7,819 In order to facilitate comparison, the earnings per share in the previous period were adjusted to the current number of shares in a ratio of 4:1 in accordance with IAS For more information, see note 29 in the notes to the consolidated financial statements in the 2014 annual report. Rounding of exact figures may result in differences.
14 12 WILEX Group 3-month financial report 2015 FINANCIAL STATEMENTS Consolidated balance sheet (IFRS) as of 28 February 2015 and as of 30 November 2014 Assets Property, plant and equipment 1,003,715 1,052,891 Intangible assets 2,927,970 2,948,199 Goodwill 6,111,166 6,111,166 Financial assets 1,875,409 1,777,083 Other non-current assets 226, ,277 Non-current assets 12,145,051 12,119,616 Inventories 134, ,710 Prepayments 50,179 74,334 Trade receivables 182, ,359 Other receivables 112, ,033 Cash and cash equivalents 1,368,924 2,196,808 Current assets 1,848,656 2,910,244 Total assets 13,993,707 15,029,860 Equity and liabilities Subscribed capital 7,818,876 7,818,876 Capital reserve 185,376, ,364,837 Accumulated losses (182,382,146) (181,307,673) Equity 10,813,247 11,876,040 Other non-current liabilities 0 3,048 Non-current liabilities 0 3,048 Trade payables 151, ,618 Liabilities arising from leases 13,332 77,482 Provisions 630, ,509 Other current liabilities 2,385,257 2,066,162 Current liabilities 3,180,459 3,150,771 Total equity and liabilities 13,993,707 15,029,860 Rounding of exact figures may result in differences.
15 WILEX Group 3-month financial report 2015 FINANCIAL STATEMENTS 13 Consolidated cash flow statement (IFRS) Reporting period from 1 December 2014 to 28 February 2015 Q Q Net loss for the period (1,074,473) (2,883,705) Adjustment for items in the statement of comprehensive income Measurement of stock options 11,681 18,325 Depreciation/amortisation 75, ,621 Finance costs ,453 Finance income 0 (23,614) Tax expense , ,786 Changes in net working capital Inventories 55,341 27,819 Trade receivables (8,519) (43,391) Other receivables (690,374) (547,755) Prepayments 24,155 (3,481) Financial assets 98,325 0 Other non-current assets (98,377) 17,239 Trade payables (120,992) 339,499 Provisions (99,684) 0 Other liabilities 1,005,242 (274,728) 165,117 (484,797) Cash flow from operating activities (822,343) (3,251,716) Finance costs paid (372) (151,995) Finance income received ,671 Net cash flow from operating activities (822,570) (3,380,041) Cash flow from investing activities Purchase of property, plant and equipment (5,702) (44,843) Net cash flow from investing activities (5,702) (44,843) Cash flow from financing activities Repayment of finance leases (10,534) (42,201) Net cash flow from financing activities (10,534) (42,201) Influence of foreign exchange effects on cash and cash equivalents 10,921 92,570 Net change in cash and cash equivalents (827,884) (3,374,515) Cash and cash equivalents at beginning of period 2,196,808 8,920,064 at end of period 1,368,924 5,545,549 Rounding of exact figures may result in differences.
16 14 WILEX Group 3-month financial report 2015 FINANCIAL STATEMENTS Consolidated statement of changes in equity (IFRS) Reporting period from 1 December 2014 to 28 February 2015 Shares Subscribed capital Capital measures/ premium Capital reserve Measurement of stock options Currency translation differences Accumulated losses Total As of 1 December ,892,571 3,388,697 31,275,507 31,275, ,281,268 0 (175,606,823) 14,949,952 Measurement of stock options 18,325 18,325 Net currency gain/loss from consolidation 0 0 Net loss for the period (2,883,705) (2,883,705) Net change in equity (2,865,380) As of 28 February ,892,571 3,407,022 31,275,507 31,275, ,299,593 0 (178,490,528) 12,084,572 As of 1 December ,949,202 3,415,635 7,818,876 7,818, ,364,837 0 (181,307,673) 11,876,040 Measurement of stock options 11,681 11,681 Net loss for the period (1,074,473) (1,074,473) Net change in equity (1,062,793) As of 28 February ,949,202 3,427,316 7,818,876 7,818, ,376,518 0 (182,382,146) 10,813,247 Rounding of exact figures may result in differences.
17 WILEX Group 3-month financial report 2015 NOTES 15 Selected notes A. General disclosures This 3-month financial report as of 28 February 2015 was prepared in accordance with the same accounting policies as the consolidated financial statements as of 30 November The interim consolidated financial statements include the Group s parent, WILEX AG, Munich, Germany, as well as its subsidiary Heidelberg Pharma GmbH, Ladenburg, Germany jointly the Group. The Company s results of operations, financial position and net assets as well as essential items of these financial statements are explained in detail in the interim management report. The Company s business activities are not subject to seasonal or macroeconomic influences. The interim consolidated financial statements on the first quarter of the 2015 financial year reproduced in this report were prepared in accordance with the International Financial Reporting Standards (IFRS) endorsed and adopted by the European Union, specifically in accordance with IAS 34 Interim Financial Reporting issued by the International Accounting Standards Board (IASB) and in compliance with the Interpretations of the Standing Interpretations Committee (SIC) and the International Financial Reporting Interpretations Committee (IFRIC). These interim financial statements are abbreviated, do not include all the information and disclosures required for consolidated financial statements as of the end of a financial year, and must be read in the context of the IFRS consolidated financial statements as of 30 November 2014 published for the 2014 financial year. These interim consolidated financial statements were not subjected to a review by an auditor. Pursuant to our Declaration of Conformity issued in February 2015 concerning Section of the German Corporate Governance Code, both the interim financial statements and the interim management report for the Group were made available to the Supervisory Board s Audit Committee before being published. This interim report was approved for publication by the Executive Management Board of WILEX AG on 14 April B. Segment reporting As the research and development activities at the Munich site were discontinued in 2014, there is no longer any need for segment reporting. Starting with the 2015 financial year, in accordance with the existing internal reporting structures, WILEX will not report segment information because its business activities will be centred on ADC technology and customerspecific research and will therefore be performed almost exclusively in the former Customer Specific Research segment. This means that no business activities are conducted that differ materially in their risk/reward profiles. In first quarter of 2014, the WILEX Group still reported on three segments, and there was no change in the segmentation during the year: Customer Specific Research (Cx), Diagnostics (Dx) and Therapeutics (Rx).
18 16 WILEX Group 3-month financial report 2015 NOTES C. Change in equity The capital reduction resolved by the Annual General Meeting in the 2014 financial year and entered in the commercial regis ter in July 2014 reduced the number of outstanding no par value shares by 23,456,628 to 7,818,876 through a reverse split in the ratio of 4 :1. The share capital of WILEX AG amounted to 7,818, on 28 February The equity of the WILEX Group at the end of the reporting period was 10.8 million (30 November 2014: 11.9 million). The capital reserve remained at million (30 November 2014: million) and the losses accumulated since WILEX s foundation totalled million (30 November 2014: million). The equity ratio of the WILEX Group was 77.3 % (30 November 2014: 79.0 %). D. Issue and measurement of stock options On 18 May 2011 the Company s Annual General Meeting resolved the WILEX Stock Option Plan This resolution authorises the Company to issue a total of up to 1,156,412 stock options, of which up to 346,924 stock options (approx. 30 %) may be issued to members of the Company s Executive Management Board, up to 173,462 stock options (approx. 15 %) to executives of affiliated companies, up to 346,923 stock options (approx. 30 %) to employees of the Company and up to 289,103 stock options (approx. 25 %) to employees of the Company s affiliates. Similar to the approach described in the annual report as of 30 November 2014, WILEX s liabilities to employees resulting from the issue of stock options were reported pursuant to IFRS 2 in the reporting period just ended. These liabilities are calculated using a binomial model at the time the options are granted. The fair value of the work provided by the employees in return for the options granted to them is charged against the capital reserve, i. e. recognised in equity. The total expense to be recognised until the time at which the options become vested is determined by the fair value of the options granted, excluding effects of exercise thresholds that are not based on capital market parameters (e. g. profit and sales growth targets). Such non-capital-market exercise thresholds are considered in the assumptions regarding the number of options that are expected to become exercisable. Settlement is carried out in equity securities. The estimate of the number of options expected to become exercisable is reviewed on every reporting date. The effects of any adjustments that have to be considered with regard to initial estimates are recognised in the statement of comprehensive income as well as by adjusting equity accordingly. The measurement of the stock options in the first three months of the 2015 financial year entailed staff costs of 12 k, all of which was attributable to the measurement of the stock options issued in 2012 under the 2011 Stock Option Plan. No expenses were incurred from the 2005 Stock Option Plan, under which no more new options can be issued and whose options have all vested. No stock options were issued and no existing stock options were exercised in the 2015 financial year. No stock options were returned because Executive Management Board members and/or employees left the Company. Furthermore, no options held by members of the Executive Management Board and/or employees under the two relevant plans expired or were forfeited for other reasons. WILEX issued a total of 1,431,931 subscription rights to employees and members of the Executive Management Board under the 2005 and 2011 plans, of which 1,145,288 options (814,835 for current or former Executive Management Board members and 330,453 for current or former employees) were outstanding and of which 1,107,789 options had vested as of the end of the reporting period (797,835 for current or former Executive Management Board members and 309,954 for current or former employees).
19 WILEX Group 3-month financial report 2015 NOTES 17 A total of 4,250 options of the Executive Management Board and 5,125 options of employees have vested as of the reporting date compared with the 2014 balance sheet date. All outstanding options issued under the Stock Option Plan 2005 can now be exercised theoretically because the waiting period has expired and the options have vested. E. Related party transactions In the reporting period, the Company s executives reported no transactions (Directors dealings) subject to disclosure in accordance with Section 15a German Securities Trading Act (Wertpapierhandelsgesetz). F. Key events after the interim reporting period (report on post-balance sheet date events) All significant events that occurred after the end of the reporting period are explained in the report on post-balance sheet events that is part of the interim management report. Page 9
20 18 WILEX Group 3-month financial report 2015 Responsibility statement of the Executive Management Board To the best of our knowledge, and in accordance with the applicable reporting principles, the financial statements for the first three months give a true and fair view of the assets, liabilities, financial position and profit or loss of the WILEX Group, and the interim management report includes a fair review of the development and performance of the business and the position of the WILEX Group, together with a description of the material opportunities and risks associated with the expected development of the WILEX Group. Munich, 14 April 2015 The Executive Management Board of WILEX AG Dr Jan Schmidt-Brand Spokesman and CFO Dr Paul Bevan Head of Research and Development
WILEX AG: Interim management statement on the first quarter of 2016
 WILEX AG: Interim management statement on the first quarter of 2016 Munich, Germany, 14 April 2016. WILEX AG (ISIN DE000A11QVV0 / WL6 / FSE) today reported on the first three months of the 2016 financial
WILEX AG: Interim management statement on the first quarter of 2016 Munich, Germany, 14 April 2016. WILEX AG (ISIN DE000A11QVV0 / WL6 / FSE) today reported on the first three months of the 2016 financial
9-Month financial report 2014
 9-Month financial report 2014 Licence agreement signed with RedHill Biopharma for MESUPRON Reverse share split completed UCB waives repayment of shareholder loan Significant extension of the ADC licence
9-Month financial report 2014 Licence agreement signed with RedHill Biopharma for MESUPRON Reverse share split completed UCB waives repayment of shareholder loan Significant extension of the ADC licence
3-MoNTH FINANCIAL report 2011
 3-MoNTH FINANCIAL report 2011 Financials in line with expectations Funding through shareholder loan Interim analysis for efficacy of RENCAREX commenced Acquisition of Heidelberg Pharma AG successfully
3-MoNTH FINANCIAL report 2011 Financials in line with expectations Funding through shareholder loan Interim analysis for efficacy of RENCAREX commenced Acquisition of Heidelberg Pharma AG successfully
Employees Employees as at 31 August 53 44 20.5 Employees average during the reporting period 52 44 18.2
 9M 2007 9M 2006 Change in % Earnings key figures in EUR 000 Other operating income 2,158 966 123.4 Operating expenses (19,486) (13,149) 48.2 of which research and development (16,606) (10,527) 57.8 Operating
9M 2007 9M 2006 Change in % Earnings key figures in EUR 000 Other operating income 2,158 966 123.4 Operating expenses (19,486) (13,149) 48.2 of which research and development (16,606) (10,527) 57.8 Operating
9-MoNth financial report 2013
 9-MoNth financial report 2013 UCB acquires rights to an antibody programme for non-oncology indications Cooperation and licence agreement for ADC signed with Roche Nuclea acquires WILEX Inc. and will develop
9-MoNth financial report 2013 UCB acquires rights to an antibody programme for non-oncology indications Cooperation and licence agreement for ADC signed with Roche Nuclea acquires WILEX Inc. and will develop
ANNUAL REPORT 2015. Amanitin. Amanitin. Antibody Targeted Amanitin Conjugates. Innovative cancer therapies
 ANNUAL REPORT 2015 Amanitin Amanitin Antibody Targeted Amanitin Conjugates Innovative cancer therapies Key figures 2015 1 000 2014 1 000 Change in % Earnings Sales revenue 2,284 3,597 (36 %) Other income
ANNUAL REPORT 2015 Amanitin Amanitin Antibody Targeted Amanitin Conjugates Innovative cancer therapies Key figures 2015 1 000 2014 1 000 Change in % Earnings Sales revenue 2,284 3,597 (36 %) Other income
9-MONTH FINANCIAL REPORT 2010
 9-MONTH FINANCIAL REPORT 2010 Highlights Q3: Mandatory offer from dievini Hopp BioTech closed Rights issue successfully executed GMP certification renewed Financials in line with expectations WILEX AG
9-MONTH FINANCIAL REPORT 2010 Highlights Q3: Mandatory offer from dievini Hopp BioTech closed Rights issue successfully executed GMP certification renewed Financials in line with expectations WILEX AG
ANNUAL REPORT 2014. Amanitin. Amanitin. Next Generation ADCs
 ANNUAL REPORT 2014 Amanitin Amanitin Next Generation ADCs Key figures 2014 1 million 2013 1 million 2012 1 million Earnings Sales revenue 3.6 13.3 16.1 Other income 1.4 5.8 1.7 Operating expenses (10.6)
ANNUAL REPORT 2014 Amanitin Amanitin Next Generation ADCs Key figures 2014 1 million 2013 1 million 2012 1 million Earnings Sales revenue 3.6 13.3 16.1 Other income 1.4 5.8 1.7 Operating expenses (10.6)
2013 1 million. Sales revenue 13.3 16.1 9.9. Other income 5.8 1.7 1.8. Operating expenses (24.1) (26.8) (25.1)
 Annual Report 2013 New start 2014 Key figures 2013 1 million 2012 1 million 2011 million Earnings Sales revenue 13.3 16.1 9.9 Other income 5.8 1.7 1.8 Operating expenses (24.1) (26.8) (25.1) of which research
Annual Report 2013 New start 2014 Key figures 2013 1 million 2012 1 million 2011 million Earnings Sales revenue 13.3 16.1 9.9 Other income 5.8 1.7 1.8 Operating expenses (24.1) (26.8) (25.1) of which research
Overview of the key figures for the first half of the year
 Half-Year Report 2015 Q2 Revenues increase in the first half of the year by 23% EBIT increased by 1.5 million euros compared to the previous year Order book is growing Overall annual forecast remains unchanged
Half-Year Report 2015 Q2 Revenues increase in the first half of the year by 23% EBIT increased by 1.5 million euros compared to the previous year Order book is growing Overall annual forecast remains unchanged
HALF YEAR REPORT AS OF JUNE 30
 2 0 1 4 HALF YEAR REPORT AS OF JUNE 30 T O O U R S H A R E H O L D E R S Dear shareholders, ladies and gentlemen, The Nemetschek Group continued its successful development in the second quarter of 2014
2 0 1 4 HALF YEAR REPORT AS OF JUNE 30 T O O U R S H A R E H O L D E R S Dear shareholders, ladies and gentlemen, The Nemetschek Group continued its successful development in the second quarter of 2014
TO OUR SHAREHOLDERS DYNAMIC FIRST HALF YEAR
 HALF YEAR REPORT AS OF JUNE 30, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group maintained its dynamic development from the first quarter of 2015
HALF YEAR REPORT AS OF JUNE 30, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group maintained its dynamic development from the first quarter of 2015
TO OUR SHAREHOLDERS PROFITABLE GROWTH COURSE INTERNATIONALIZATION FURTHER EXTENDED US MARKET IN FOCUS
 QUARTERLY STATEMENT AS OF MARCH 31, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group has made a dynamic start in the 2015 financial year and continues
QUARTERLY STATEMENT AS OF MARCH 31, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group has made a dynamic start in the 2015 financial year and continues
Overview of the key figures for the first nine months
 Continued revenue growth: up 12% on previous year Results impacted by revenue structure and one-off effects High volume of orders: outlook remains optimistic Q3 Overview of the key figures for the first
Continued revenue growth: up 12% on previous year Results impacted by revenue structure and one-off effects High volume of orders: outlook remains optimistic Q3 Overview of the key figures for the first
Interim consolidated financial statements as of September 30, 2007
 1 Interim consolidated financial statements as of September 30, 2007 January 1 through September 30, 2007 MeVis Medical Solutions AG laying the foundation for further dynamic growth: Sales plus other operating
1 Interim consolidated financial statements as of September 30, 2007 January 1 through September 30, 2007 MeVis Medical Solutions AG laying the foundation for further dynamic growth: Sales plus other operating
3-month report January - March 2007 Published on August 10, 2007
 3-month report January - March 2007 Published on August 10, 2007 3-month report January March 2007 1. Group management report for the first quarter of 2007 Overview of the first quarter in 2007 Continued
3-month report January - March 2007 Published on August 10, 2007 3-month report January March 2007 1. Group management report for the first quarter of 2007 Overview of the first quarter in 2007 Continued
2014/2015 The IndusTrIal Group
 Q2 2014/2015 Half-Year Interim Report 2014/2015 1 April to 30 September 2014 The Industrial Group The first six months of financial year 2014/2015 at a glance Incoming orders increased in the first half
Q2 2014/2015 Half-Year Interim Report 2014/2015 1 April to 30 September 2014 The Industrial Group The first six months of financial year 2014/2015 at a glance Incoming orders increased in the first half
PROMETIC LIFE SCIENCES INC.
 PROMETIC LIFE SCIENCES INC. Investor Update July to September 2002 This present release must be considered as the quarterly report to shareholders. 1. Letter to Shareholders Dated November 21, 2002 2.
PROMETIC LIFE SCIENCES INC. Investor Update July to September 2002 This present release must be considered as the quarterly report to shareholders. 1. Letter to Shareholders Dated November 21, 2002 2.
9-MONTHS REPORT. Stable development of business in Q3 Lila Logistik confirms full-year forecast
 /08 9-MONTHS REPORT Stable development of business in Q3 Lila Logistik confirms full-year forecast Key figures for the first three quarters of 2008 in accordance with IFRS 01.01. 01.01. Change in Change
/08 9-MONTHS REPORT Stable development of business in Q3 Lila Logistik confirms full-year forecast Key figures for the first three quarters of 2008 in accordance with IFRS 01.01. 01.01. Change in Change
Consolidated Statement of Financial Position
 INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS 30 JUNE 2014 Consolidated Statement of Financial Position in CHF 1,000 Note 30 June 2014 31 December 2013 (unaudited) (audited) Assets Non-current assets
INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS 30 JUNE 2014 Consolidated Statement of Financial Position in CHF 1,000 Note 30 June 2014 31 December 2013 (unaudited) (audited) Assets Non-current assets
ico Therapeutics Inc. Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 (in Canadian dollars)
 Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 Balance Sheet (Unaudited) Assets Note September 30, 2012 December 31, 2011 Current assets Cash and cash equivalents 1,478,167
Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 Balance Sheet (Unaudited) Assets Note September 30, 2012 December 31, 2011 Current assets Cash and cash equivalents 1,478,167
Company update. German Equity Forum, Frankfurt November 2011
 Company update German Equity Forum, Frankfurt November 2011 At a glance Oncology-focused biopharmaceutical company Transition from R&D to commercially driven business Product candidates with strong competitive
Company update German Equity Forum, Frankfurt November 2011 At a glance Oncology-focused biopharmaceutical company Transition from R&D to commercially driven business Product candidates with strong competitive
2015 Quarterly Report II
 2015 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2015 01 06/2014 Change Sales million 69.0 61.9 + 11 % Return on revenue before tax % 16 % 9 % + 87 % EBITDA million 15.6 9.7 + 61 % EBIT million
2015 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2015 01 06/2014 Change Sales million 69.0 61.9 + 11 % Return on revenue before tax % 16 % 9 % + 87 % EBITDA million 15.6 9.7 + 61 % EBIT million
Letter from the Management Board 3. Key Financial Figures 4. Management Report 5. Consolidated Income Statement (IFRS) 9
 3-Months Report 2015 Content Letter from the Management Board 3 Key Financial Figures 4 Management Report 5 Consolidated Income Statement (IFRS) 9 Consolidated Statement of Comprehensive Income (IFRS)
3-Months Report 2015 Content Letter from the Management Board 3 Key Financial Figures 4 Management Report 5 Consolidated Income Statement (IFRS) 9 Consolidated Statement of Comprehensive Income (IFRS)
Travel24.com AG. Quarterly Report Q1 2015
 Travel24.com AG Quarterly Report Q1 2015 2 Selected Key Group Data January 1 - March 31 Change In thousands of euro 2015 2014 % Revenue 4,494 7,810-42 % EBIT 806 1,231-35 % Net profit 66 518-87 % Earnings
Travel24.com AG Quarterly Report Q1 2015 2 Selected Key Group Data January 1 - March 31 Change In thousands of euro 2015 2014 % Revenue 4,494 7,810-42 % EBIT 806 1,231-35 % Net profit 66 518-87 % Earnings
CONSOLIDATED INTERIM FINANCIAL STATEMENTS
 CONSOLIDATED INTERIM FINANCIAL STATEMENTS AND GROUP INTERIM MANAGEMENT REPORT SECOND QUARTER OF 2008 JUNE 30, 2008 FRANCONOFURT AG FRANKFURT AM MAIN FRANCONOFURT AG, FRANKFURT AM MAIN CONSOLIDATED INTERIM
CONSOLIDATED INTERIM FINANCIAL STATEMENTS AND GROUP INTERIM MANAGEMENT REPORT SECOND QUARTER OF 2008 JUNE 30, 2008 FRANCONOFURT AG FRANKFURT AM MAIN FRANCONOFURT AG, FRANKFURT AM MAIN CONSOLIDATED INTERIM
FINANCIAL REPORT H1 2014
 FINANCIAL REPORT H1 2014 HIGH SPEED BY PASSION 02_Key Figures 03_Group Status Report 05_Consolidated Financial Statements 10_Notes 11_Declaration of the Legal Representatives 02 PANKL KEY FIGURES EARNING
FINANCIAL REPORT H1 2014 HIGH SPEED BY PASSION 02_Key Figures 03_Group Status Report 05_Consolidated Financial Statements 10_Notes 11_Declaration of the Legal Representatives 02 PANKL KEY FIGURES EARNING
Global City Holdings N.V. Interim Financial Report as at 30 June 2015
 Interim Financial Report as at 30 June 2015 Interim Financial Report for the six months ended 30 June 2015 CONTENTS Page Directors' report 1 Interim Condensed Consolidated Financial Statements as at 30
Interim Financial Report as at 30 June 2015 Interim Financial Report for the six months ended 30 June 2015 CONTENTS Page Directors' report 1 Interim Condensed Consolidated Financial Statements as at 30
INTERIM REPORT ON FIRST QUARTER OF 2015 201fehlungBild austauschen) Q1
 Interim Report Q1 2015 INTERIM REPORT ON FIRST QUARTER OF 2015 201fehlungBild austauschen) Q1 1 Interim Report Q1 2015 2 Letter from the Management Board Dear Shareholders, Ladies and Gentlemen, These
Interim Report Q1 2015 INTERIM REPORT ON FIRST QUARTER OF 2015 201fehlungBild austauschen) Q1 1 Interim Report Q1 2015 2 Letter from the Management Board Dear Shareholders, Ladies and Gentlemen, These
For personal use only
 APPENDIX 4D INTERIM FINANCIAL REPORT RESULTS FOR ANNOUNCEMENT TO THE MARKET Appendix 4D item 2.1 Revenue from ordinary activities. Appendix 4D item 2.2 Profit (loss) from ordinary activities after tax
APPENDIX 4D INTERIM FINANCIAL REPORT RESULTS FOR ANNOUNCEMENT TO THE MARKET Appendix 4D item 2.1 Revenue from ordinary activities. Appendix 4D item 2.2 Profit (loss) from ordinary activities after tax
Condensed Interim Financial Statements Fiscal 2013 First Quarter (Unaudited) For the three months ended July 31, 2012 and 2011
 Condensed Interim Financial Statements Fiscal 2013 First Quarter (Unaudited) CRITICAL OUTCOME TECHNOLOGIES INC Page 2 Condensed Interim Financial Statements Table of Contents Notice of No Audit or Review
Condensed Interim Financial Statements Fiscal 2013 First Quarter (Unaudited) CRITICAL OUTCOME TECHNOLOGIES INC Page 2 Condensed Interim Financial Statements Table of Contents Notice of No Audit or Review
PONSSE PLC, STOCK EXCHANGE RELEASE, 26 OCTOBER 2010, 9:00 a.m. PONSSE S INTERIM REPORT FOR 1 JANUARY 30 SEPTEMBER 2010
 PONSSE PLC, STOCK EXCHANGE RELEASE, 26 OCTOBER 2010, 9:00 a.m. PONSSE S INTERIM REPORT FOR 1 JANUARY 30 SEPTEMBER 2010 - Net sales were EUR 171.8 (Q1-Q3/2009 EUR 98.9) million. - Q3 net sales were EUR
PONSSE PLC, STOCK EXCHANGE RELEASE, 26 OCTOBER 2010, 9:00 a.m. PONSSE S INTERIM REPORT FOR 1 JANUARY 30 SEPTEMBER 2010 - Net sales were EUR 171.8 (Q1-Q3/2009 EUR 98.9) million. - Q3 net sales were EUR
Addresses. Corporate Equity Partners AG. Subsidiaries. Company s Registered Head Office: Obmoos 4 CH 6301 Zug Switzerland. The Fantastic IP GmbH
 Corporate Equity Partners Group 9 Month Financial Statements 2009 Addresses Corporate Equity Partners AG Company s Registered Head Office: Obmoos 4 CH 6301 Zug Switzerland Subsidiaries The Fantastic IP
Corporate Equity Partners Group 9 Month Financial Statements 2009 Addresses Corporate Equity Partners AG Company s Registered Head Office: Obmoos 4 CH 6301 Zug Switzerland Subsidiaries The Fantastic IP
2014/2015 The IndusTrIal Group
 Q1 2014/2015 Interim Report 1 April to 30 june 2014 The Industrial Group The essentials at a glance in the first quarter Big increase in incoming orders, sales on par with previous year, earnings considerably
Q1 2014/2015 Interim Report 1 April to 30 june 2014 The Industrial Group The essentials at a glance in the first quarter Big increase in incoming orders, sales on par with previous year, earnings considerably
condensed consolidated interim financial statements 2015
 January march 2015 condensed consolidated interim financial statements 2015 (unaudited) contents 1. Income Statement 1 2. Statement of Comprehensive Income 2 3. Balance Sheet 3 4. Statement of Changes
January march 2015 condensed consolidated interim financial statements 2015 (unaudited) contents 1. Income Statement 1 2. Statement of Comprehensive Income 2 3. Balance Sheet 3 4. Statement of Changes
INTERIM REPORT, 1 January - 30 June 2003
 PRESS RELEASE, 9 July INTERIM REPORT, 1 January - 30 June In May, Medivir reached a licensing agreement on MIV-210 with GlaxoSmithKline; EUR 6 m was received upon signing and up to EUR 86 m on the achievement
PRESS RELEASE, 9 July INTERIM REPORT, 1 January - 30 June In May, Medivir reached a licensing agreement on MIV-210 with GlaxoSmithKline; EUR 6 m was received upon signing and up to EUR 86 m on the achievement
2014 Quarterly Report II
 2014 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2014 01 06/2013 Change Sales million 61.9 55.3 12% Return on revenue before tax % 9 % 12 % 26 % EBITDA million 9.7 10.2 5 % EBIT million 6.2 6.9
2014 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2014 01 06/2013 Change Sales million 61.9 55.3 12% Return on revenue before tax % 9 % 12 % 26 % EBITDA million 9.7 10.2 5 % EBIT million 6.2 6.9
Interim Report 201. Celesio AG. report as of 30 September 2015
 Interim Report 201 Celesio AG H1 Half-year financial report as of 30 September 2015 The Celesio Group Celesio is a leading international wholesale and retail company and provider of logistics and services
Interim Report 201 Celesio AG H1 Half-year financial report as of 30 September 2015 The Celesio Group Celesio is a leading international wholesale and retail company and provider of logistics and services
BIOMARK DIAGNOSTICS INC. CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS. June 30, 2015. (Stated in Canadian Dollars)
 CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (Unaudited Prepared by Management) NOTICE OF NO AUDITOR REVIEW OF CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (Unaudited Prepared by Management) NOTICE OF NO AUDITOR REVIEW OF CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
Interim Report HORNBACH HOLDING AG GROUP. 1st QUARTER 2004/2005 (March 1 to May 31, 2004)
 Interim Report HORNBACH HOLDING AG GROUP 1st QUARTER 2004/2005 (March 1 to May 31, 2004) page 2 HORNBACH HOLDING AG Group Interim Report (IFRS) for the First Quarter of 2004/2005 (March 1 to May 31, 2004)
Interim Report HORNBACH HOLDING AG GROUP 1st QUARTER 2004/2005 (March 1 to May 31, 2004) page 2 HORNBACH HOLDING AG Group Interim Report (IFRS) for the First Quarter of 2004/2005 (March 1 to May 31, 2004)
Contact 6-Month Report 2005
 Contact 6-Month Report 2005 Security Networks AG Kronprinzenstrasse 30 45128 ssen Germany Phone: +49 (0) 201 54 54-0 Fax: +49 (0) 201 54 54-456 Internet: www..com -mail: investor.relations@.com Key figures
Contact 6-Month Report 2005 Security Networks AG Kronprinzenstrasse 30 45128 ssen Germany Phone: +49 (0) 201 54 54-0 Fax: +49 (0) 201 54 54-456 Internet: www..com -mail: investor.relations@.com Key figures
3. CONSOLIDATED QUARTERLY FINANCIAL STATEMENTS
 3. CONSOLIDATED QUARTERLY FINANCIAL STATEMENTS (1) Consolidated Quarterly Balance Sheets September 30, 2014 and March 31, 2014 Supplementary Information 2Q FY March 2015 March 31, 2014 September 30, 2014
3. CONSOLIDATED QUARTERLY FINANCIAL STATEMENTS (1) Consolidated Quarterly Balance Sheets September 30, 2014 and March 31, 2014 Supplementary Information 2Q FY March 2015 March 31, 2014 September 30, 2014
ATS AUTOMATION TOOLING SYSTEMS INC.
 Interim Consolidated Financial Statements For the period ended June 29, 2014 (Unaudited) (Condensed) Interim Consolidated Statements of Financial Position (in thousands of Canadian dollars unaudited) June
Interim Consolidated Financial Statements For the period ended June 29, 2014 (Unaudited) (Condensed) Interim Consolidated Statements of Financial Position (in thousands of Canadian dollars unaudited) June
Interim Report. January - September
 Interim Report January - September LETTER TO THE SHAREHOLDERS RIB SOFTWARE AG LETTER TO THE SHAREHOLDERS Dear Shareholders, With two strategic acquisitions in the third quarter of, we have taken a further
Interim Report January - September LETTER TO THE SHAREHOLDERS RIB SOFTWARE AG LETTER TO THE SHAREHOLDERS Dear Shareholders, With two strategic acquisitions in the third quarter of, we have taken a further
Half Year 2015 Results
 Half Year 2015 Results Letter to shareholders LifeWatch First Half Highlights Revenue growth of 9.1% to USD 52.5 million Above-market growth of over 12% in core monitoring services resulting in market
Half Year 2015 Results Letter to shareholders LifeWatch First Half Highlights Revenue growth of 9.1% to USD 52.5 million Above-market growth of over 12% in core monitoring services resulting in market
UNAUDITED CONDENSED INTERIM CONSOLIDATED FINANCIAL STATEMENTS FOR THE SIX MONTHS ENDED JUNE 30, 2015
 BE SEMICONDUCTOR INDUSTRIES N.V. DUIVEN, THE NETHERLANDS UNAUDITED CONDENSED INTERIM CONSOLIDATED FINANCIAL STATEMENTS FOR THE SIX MONTHS ENDED JUNE 30, 2015 Contents Unaudited Condensed Interim Consolidated
BE SEMICONDUCTOR INDUSTRIES N.V. DUIVEN, THE NETHERLANDS UNAUDITED CONDENSED INTERIM CONSOLIDATED FINANCIAL STATEMENTS FOR THE SIX MONTHS ENDED JUNE 30, 2015 Contents Unaudited Condensed Interim Consolidated
Sonic Healthcare Limited ABN 24 004 196 909. PRELIMINARY FINAL REPORT FOR YEAR ENDED 30 JUNE 2007 Lodged with the ASX under Listing Rule 4.
 ABN 24 004 196 909 PRELIMINARY FINAL REPORT FOR YEAR ENDED 30 JUNE Lodged with the ASX under Listing Rule 4.3A RESULTS FOR ANNOUNCEMENT TO THE MARKET For the year ended Financial Results Revenue from ordinary
ABN 24 004 196 909 PRELIMINARY FINAL REPORT FOR YEAR ENDED 30 JUNE Lodged with the ASX under Listing Rule 4.3A RESULTS FOR ANNOUNCEMENT TO THE MARKET For the year ended Financial Results Revenue from ordinary
Consolidated Balance Sheets
 Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
Unaudited Financial Report
 RECRUITING SERVICES Amadeus FiRe AG Unaudited Financial Report Quarter I - 2015 Temporary Staffing. Permanent Placement Interim Management. Training www.amadeus-fire.de Unaudited Amadeus FiRe Group Financial
RECRUITING SERVICES Amadeus FiRe AG Unaudited Financial Report Quarter I - 2015 Temporary Staffing. Permanent Placement Interim Management. Training www.amadeus-fire.de Unaudited Amadeus FiRe Group Financial
How To Calculate Earnings In Euro
 3 MONTH REPORT AS AT 31 DECEMBER 2014 KEY FIGURES IFRS in KEUR 10/2014 12/2014 10/2013 12/2013 Difference in % Earnings situation Sales revenues 61,403 56,296 5,107 9% EBITDA 7,159 5,901 1,258 21% EBITDA
3 MONTH REPORT AS AT 31 DECEMBER 2014 KEY FIGURES IFRS in KEUR 10/2014 12/2014 10/2013 12/2013 Difference in % Earnings situation Sales revenues 61,403 56,296 5,107 9% EBITDA 7,159 5,901 1,258 21% EBITDA
Opening doors to new ideas. Interim Report 2007/08
 Opening doors to new ideas Interim Report 2007/08 SPG Media Group Plc Interim Report 2007/08 Contents 2 Chairman s Statement 4 Consolidated Interim Income Statement 5 Consolidated Interim Balance Sheet
Opening doors to new ideas Interim Report 2007/08 SPG Media Group Plc Interim Report 2007/08 Contents 2 Chairman s Statement 4 Consolidated Interim Income Statement 5 Consolidated Interim Balance Sheet
IMMEDIA GROUP PLC. ( Immedia or the Company ) INTERIM RESULTS
 IMMEDIA GROUP PLC ( Immedia or the Company ) INTERIM RESULTS Immedia Group Plc (AIM: IME), which provides bespoke digital networks, music strategies and brand conversation, today announces its interim
IMMEDIA GROUP PLC ( Immedia or the Company ) INTERIM RESULTS Immedia Group Plc (AIM: IME), which provides bespoke digital networks, music strategies and brand conversation, today announces its interim
Oxford Asymmetry International plc 151 Milton Park Abingdon Oxon OX14 4SD United Kingdom Tel +44 (0)1235 861561 Fax +44 (0)1235 863139 www.oai.co.
 Oxford Asymmetry International plc 151 Milton Park Abingdon Oxon OX14 4SD United Kingdom Tel +44 (0)1235 861561 Fax +44 (0)1235 863139 www.oai.co.uk 2000 Interim Statements Oxford Asymmetry International
Oxford Asymmetry International plc 151 Milton Park Abingdon Oxon OX14 4SD United Kingdom Tel +44 (0)1235 861561 Fax +44 (0)1235 863139 www.oai.co.uk 2000 Interim Statements Oxford Asymmetry International
Transition to International Financial Reporting Standards
 Transition to International Financial Reporting Standards Topps Tiles Plc In accordance with IFRS 1, First-time adoption of International Financial Reporting Standards ( IFRS ), Topps Tiles Plc, ( Topps
Transition to International Financial Reporting Standards Topps Tiles Plc In accordance with IFRS 1, First-time adoption of International Financial Reporting Standards ( IFRS ), Topps Tiles Plc, ( Topps
Ahlers AG, Herford. ISIN DE0005009708 and DE0005009732 INTERIM REPORT
 Ahlers AG, Herford ISIN DE0005009708 and DE0005009732 I N T E R I M R E P O R T for the first six months of the 2006/07 financial year (December 1, 2006 to May 31, 2007) BUSINESS DEVELOPMENT IN THE FIRST
Ahlers AG, Herford ISIN DE0005009708 and DE0005009732 I N T E R I M R E P O R T for the first six months of the 2006/07 financial year (December 1, 2006 to May 31, 2007) BUSINESS DEVELOPMENT IN THE FIRST
Volex Group plc. Transition to International Financial Reporting Standards Supporting document for 2 October 2005 Interim Statement. 1.
 Volex Group plc Transition to International Financial Reporting Standards Supporting document for 2 October 2005 Interim Statement 1. Introduction The consolidated financial statements of Volex Group plc
Volex Group plc Transition to International Financial Reporting Standards Supporting document for 2 October 2005 Interim Statement 1. Introduction The consolidated financial statements of Volex Group plc
Medivir s financial reports are available from its Website, www.medivir.se from these dates, under the Financial Information heading.
 PRESS RELEASE, 23 October INTERIM REPORT, 1 January - 30 September In July, Medivir entered a licensing agreement with Boehringer Ingelheim (BI) on MIV-310. CCS group divested to the Segulah II L.P. investment
PRESS RELEASE, 23 October INTERIM REPORT, 1 January - 30 September In July, Medivir entered a licensing agreement with Boehringer Ingelheim (BI) on MIV-310. CCS group divested to the Segulah II L.P. investment
NedSense enterprises n.v. Condensed consolidated Interim financial statements
 NED NEDSENSE enterprises n.v. NedSense enterprises n.v. Condensed consolidated Interim financial statements 30 June 2012 NedSense enterprises n.v. Half-year Report 2012 (unaudited) Report of the Board
NED NEDSENSE enterprises n.v. NedSense enterprises n.v. Condensed consolidated Interim financial statements 30 June 2012 NedSense enterprises n.v. Half-year Report 2012 (unaudited) Report of the Board
Quarterly Financial Report. as of March 31, 2010. Qarterly Financial Report. as of March 31, 2010
 Quarterly Financial Report as of March 31, 2010 Qarterly Financial Report as of March 31, 2010 PULSION Quarterly Financial Report as at March 31, 2010 1 PULSION at a glance PULSION (Group) according to
Quarterly Financial Report as of March 31, 2010 Qarterly Financial Report as of March 31, 2010 PULSION Quarterly Financial Report as at March 31, 2010 1 PULSION at a glance PULSION (Group) according to
Half Year Financial Statement And Announcement for the Period Ended 31/12/2010
 AUSSINO GROUP LTD Company Registration No.: 199100323H Half Year Financial Statement And Announcement for the Period Ended 31/12/2010 PART I - INFORMATION REQUIRED FOR ANNOUNCEMENTS OF QUARTERLY (Q1, Q2
AUSSINO GROUP LTD Company Registration No.: 199100323H Half Year Financial Statement And Announcement for the Period Ended 31/12/2010 PART I - INFORMATION REQUIRED FOR ANNOUNCEMENTS OF QUARTERLY (Q1, Q2
eqube Gaming Limited Condensed Interim Consolidated Financial Statements For the Three and Nine Months Ended November 30, 2015 (Unaudited)
 Condensed Interim Consolidated Financial Statements For the Three and Nine Months Ended November 30, 2015 Notice to Reader The following interim consolidated financial statements and notes have not been
Condensed Interim Consolidated Financial Statements For the Three and Nine Months Ended November 30, 2015 Notice to Reader The following interim consolidated financial statements and notes have not been
CONSOLIDATED PROFIT AND LOSS ACCOUNT For the six months ended June 30, 2002
 CONSOLIDATED PROFIT AND LOSS ACCOUNT For the six months ended June 30, 2002 Unaudited Unaudited Note Turnover 2 5,576 5,803 Other net losses (1) (39) 5,575 5,764 Direct costs and operating expenses (1,910)
CONSOLIDATED PROFIT AND LOSS ACCOUNT For the six months ended June 30, 2002 Unaudited Unaudited Note Turnover 2 5,576 5,803 Other net losses (1) (39) 5,575 5,764 Direct costs and operating expenses (1,910)
In addition, Outokumpu has adopted the following amended standards as of January 1, 2009:
 1. Corporate information Outokumpu Oyj is a Finnish public limited liability company organised under the laws of Finland and domiciled in Espoo. The parent company, Outokumpu Oyj, has been listed on the
1. Corporate information Outokumpu Oyj is a Finnish public limited liability company organised under the laws of Finland and domiciled in Espoo. The parent company, Outokumpu Oyj, has been listed on the
IV. UNAUDITED CONSOLIDATED STATEMENT OF CASH FLOWS. 6 VII. STATUTORY AUDITOR'S LIMITED REVIEW REPORT... 10
 2015 INTERIM REPORT TABLE OF CONTENTS I. INTERIM MANAGEMENT REPORT.. 2 II. III. CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION.. 4 CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE
2015 INTERIM REPORT TABLE OF CONTENTS I. INTERIM MANAGEMENT REPORT.. 2 II. III. CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION.. 4 CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE
DATA GROUP LTD. ANNOUNCES SECOND QUARTER FINANCIAL RESULTS FOR 2015
 For Immediate Release DATA GROUP LTD. ANNOUNCES SECOND QUARTER FINANCIAL RESULTS FOR 2015 SECOND QUARTER HIGHLIGHTS Second quarter 2015 ( Q2 ) Revenues of $73.4 million, a decrease of 4.3% year over year
For Immediate Release DATA GROUP LTD. ANNOUNCES SECOND QUARTER FINANCIAL RESULTS FOR 2015 SECOND QUARTER HIGHLIGHTS Second quarter 2015 ( Q2 ) Revenues of $73.4 million, a decrease of 4.3% year over year
2 Quarterly Report 02 Ratios. Jan Jun/2012 Jan Jun/2011 Change. Sales Million EUR 57.9 55.8 4% Return on revenue before tax % 16% 20% 23%
 Quarterly Report II 2 Quarterly Report 02 Ratios Jan Jun/ Jan Jun/ Change Sales Million EUR 57.9 55.8 4% Return on revenue before tax % 16% 20% 23% EBITDA Million EUR 13.8 15.4 11% EBIT Million EUR 10.0
Quarterly Report II 2 Quarterly Report 02 Ratios Jan Jun/ Jan Jun/ Change Sales Million EUR 57.9 55.8 4% Return on revenue before tax % 16% 20% 23% EBITDA Million EUR 13.8 15.4 11% EBIT Million EUR 10.0
15 September 2011 VOLEX PLC ( Volex or the Group ) Transition to US Dollar reporting Restatement of historical financial information in US Dollars
 15 September VOLEX PLC ( Volex or the Group ) Transition to US Dollar reporting Restatement of historical financial information in US Dollars As communicated in our annual financial statements for the
15 September VOLEX PLC ( Volex or the Group ) Transition to US Dollar reporting Restatement of historical financial information in US Dollars As communicated in our annual financial statements for the
An exploration stage company. Condensed Interim Consolidated Financial Statements
 An exploration stage company Condensed Interim Consolidated Financial Statements (Expressed in US Dollars - unaudited) Condensed interim consolidated statements of financial position (Expressed in United
An exploration stage company Condensed Interim Consolidated Financial Statements (Expressed in US Dollars - unaudited) Condensed interim consolidated statements of financial position (Expressed in United
China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results
 China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results HAIKOU CITY, China, August 10, 2010 China Pharma Holdings, Inc. (NYSE AMEX: CPHI) ( China Pharma or the Company ), a leading fully
China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results HAIKOU CITY, China, August 10, 2010 China Pharma Holdings, Inc. (NYSE AMEX: CPHI) ( China Pharma or the Company ), a leading fully
Unaudited Nine Months Financial Report
 RECRUITING SERVICES Amadeus FiRe AG Unaudited Nine Months Financial Report January to September 2015 Temporary Staffing. Permanent Placement Interim Management. Training www.amadeus-fire.de Unaudited Nine
RECRUITING SERVICES Amadeus FiRe AG Unaudited Nine Months Financial Report January to September 2015 Temporary Staffing. Permanent Placement Interim Management. Training www.amadeus-fire.de Unaudited Nine
Significant Accounting Policies
 Apart from the accounting policies presented within the corresponding notes to the financial statements, other significant accounting policies are set out below. These policies have been consistently applied
Apart from the accounting policies presented within the corresponding notes to the financial statements, other significant accounting policies are set out below. These policies have been consistently applied
Capcon Holdings plc. Interim Report 2011. Unaudited interim results for the six months ended 31 March 2011
 Capcon Holdings plc Interim Report 2011 Unaudited interim results for the six months ended 31 March 2011 Capcon Holdings plc ("Capcon" or the "Group"), the AIM listed investigations and risk management
Capcon Holdings plc Interim Report 2011 Unaudited interim results for the six months ended 31 March 2011 Capcon Holdings plc ("Capcon" or the "Group"), the AIM listed investigations and risk management
PRELIMINARY RESULTS FOR HALF YEAR ENDED 30 SEPTEMBER 2015
 Page 1 PRELIMINARY RESULTS FOR HALF YEAR ENDED 30 SEPTEMBER 2015 Reporting Period 6 months to 30 September 2015 Reporting Period 6 months to 30 September 2014 Amount NZ$ 000 Percentage Change % Revenue
Page 1 PRELIMINARY RESULTS FOR HALF YEAR ENDED 30 SEPTEMBER 2015 Reporting Period 6 months to 30 September 2015 Reporting Period 6 months to 30 September 2014 Amount NZ$ 000 Percentage Change % Revenue
Note 2 SIGNIFICANT ACCOUNTING
 Note 2 SIGNIFICANT ACCOUNTING POLICIES BASIS FOR THE PREPARATION OF THE FINANCIAL STATEMENTS The consolidated financial statements have been prepared in accordance with International Financial Reporting
Note 2 SIGNIFICANT ACCOUNTING POLICIES BASIS FOR THE PREPARATION OF THE FINANCIAL STATEMENTS The consolidated financial statements have been prepared in accordance with International Financial Reporting
Interim Financial Statements
 [Type text] Interim Financial Statements KCA Deutag Alpha Limited For the twelve months ended 31 December 2015 Page 1 of 11 Table of Contents Consolidated income statement... 3 Consolidated statement of
[Type text] Interim Financial Statements KCA Deutag Alpha Limited For the twelve months ended 31 December 2015 Page 1 of 11 Table of Contents Consolidated income statement... 3 Consolidated statement of
Amerigo Resources Ltd. Condensed Consolidated Interim Financial Statements For the quarter ended March 31, 2012 Unaudited (expressed in U.S.
 Condensed Consolidated Interim Financial Statements For the quarter Unaudited Condensed Consolidated Interim Statements of Financial Position - Unaudited December 31, Notes Assets Current assets Cash and
Condensed Consolidated Interim Financial Statements For the quarter Unaudited Condensed Consolidated Interim Statements of Financial Position - Unaudited December 31, Notes Assets Current assets Cash and
DEUFOL SE JOHANNES-GUTENBERG-STR. 3 5 65719 HOFHEIM (WALLAU), GERMANY PHONE: + 49 (61 22) 50-00 FAX: + 49 (61 22) 50-13 00 WWW.
 SEMI-ANNUAL REPORT 5 Key Figures for the Deufol Group figures in thousand 6M 2015 6M 2014 Results of operations Revenue (total) 152,088 141,450 Germany 83,770 77,730 Rest of the World 68,318 63,720 International
SEMI-ANNUAL REPORT 5 Key Figures for the Deufol Group figures in thousand 6M 2015 6M 2014 Results of operations Revenue (total) 152,088 141,450 Germany 83,770 77,730 Rest of the World 68,318 63,720 International
UNAUDITED PRO FORMA STATEMENT OF FINANCIAL POSITION AND STATEMENT OF COMPREHENSIVE INCOME OF HOLDSPORT
 Holdsport Limited (incorporated in the Republic of South Africa) (Registration number 2006/022562/06) JSE share code: HSP ISIN: ZAE000157046 ("Holdsport" or the "Company") UNAUDITED PRO FORMA STATEMENT
Holdsport Limited (incorporated in the Republic of South Africa) (Registration number 2006/022562/06) JSE share code: HSP ISIN: ZAE000157046 ("Holdsport" or the "Company") UNAUDITED PRO FORMA STATEMENT
Summary of Consolidated Financial Results for the Six Months Ended September 30, 2013
 November 6, 2013 Summary of Consolidated Financial Results for the Six Months Ended Name of Company Listed: Stock Exchange Listings: Nippon Paper Industries Co., Ltd. Tokyo Code Number: 3863 URL: Representative:
November 6, 2013 Summary of Consolidated Financial Results for the Six Months Ended Name of Company Listed: Stock Exchange Listings: Nippon Paper Industries Co., Ltd. Tokyo Code Number: 3863 URL: Representative:
PART I - INFORMATION REQUIRED FOR ANNOUNCEMENTS OF QUARTERLY (Q1, Q2 & Q3), HALF-YEAR AND FULL YEAR RESULTS
 EU YAN SANG INTERNATIONAL LTD (Company Registration No. : 199302179H) Unaudited Financial Statements And Dividend Announcement PART I - INFORMATION REQUIRED FOR ANNOUNCEMENTS OF QUARTERLY (Q1, Q2 & Q3),
EU YAN SANG INTERNATIONAL LTD (Company Registration No. : 199302179H) Unaudited Financial Statements And Dividend Announcement PART I - INFORMATION REQUIRED FOR ANNOUNCEMENTS OF QUARTERLY (Q1, Q2 & Q3),
Quarter Report 2014 ESSANELLE HAIR GROUP AG
 Quarter Report 2014 ESSANELLE HAIR GROUP AG Q1 2 Q1/2014 ESSANELLE HAIR GROUP KEY FIGURES for 1 January to 31 March 2014/2013 (IFRS) million 2014 2013 Change* Consolidated sales 32.3 30.8 +4.7% essanelle
Quarter Report 2014 ESSANELLE HAIR GROUP AG Q1 2 Q1/2014 ESSANELLE HAIR GROUP KEY FIGURES for 1 January to 31 March 2014/2013 (IFRS) million 2014 2013 Change* Consolidated sales 32.3 30.8 +4.7% essanelle
Consolidated Financial Results for the nine months of Fiscal Year 2010
 Consolidated Financial Results for the nine months of Fiscal Year 2010 (Fiscal Year 2010: Year ending March 31, 2010) Noritake Co., Limited Company Name Stock Exchange Listings Tokyo, Nagoya Code Number
Consolidated Financial Results for the nine months of Fiscal Year 2010 (Fiscal Year 2010: Year ending March 31, 2010) Noritake Co., Limited Company Name Stock Exchange Listings Tokyo, Nagoya Code Number
July September 2013. July September 2014
 Interim Report Interim Report Sales in the quarter increased to SEK 225.1 (216.9) million. In local currencies the decrease was 1.1 per cent. Operating profit for the quarter was SEK 12.9 (5.7) million.
Interim Report Interim Report Sales in the quarter increased to SEK 225.1 (216.9) million. In local currencies the decrease was 1.1 per cent. Operating profit for the quarter was SEK 12.9 (5.7) million.
Consolidated Settlement of Accounts for the First 3 Quarters Ended December 31, 2011 [Japanese Standards]
![Consolidated Settlement of Accounts for the First 3 Quarters Ended December 31, 2011 [Japanese Standards] Consolidated Settlement of Accounts for the First 3 Quarters Ended December 31, 2011 [Japanese Standards]](/thumbs/37/17704792.jpg) The figures for these Financial Statements are prepared in accordance with the accounting principles based on Japanese law. Accordingly, they do not necessarily match the figures in the Annual Report issued
The figures for these Financial Statements are prepared in accordance with the accounting principles based on Japanese law. Accordingly, they do not necessarily match the figures in the Annual Report issued
ASM INTERNATIONAL N.V. REPORTS THIRD QUARTER 2015 RESULTS
 Almere, The Netherlands October 28, 2015 ASM INTERNATIONAL N.V. REPORTS THIRD QUARTER 2015 RESULTS ASM International N.V. (Euronext Amsterdam: ASM) today reports its third quarter 2015 operating results
Almere, The Netherlands October 28, 2015 ASM INTERNATIONAL N.V. REPORTS THIRD QUARTER 2015 RESULTS ASM International N.V. (Euronext Amsterdam: ASM) today reports its third quarter 2015 operating results
Scancell Holdings plc ( Scancell Holdings or the Company )
 Release Date: 29 January 2010 GB00B39J5N63 Scancell Holdings plc ( Scancell Holdings or the Company ) Interim Results for the six month period to 31 Scancell (SCLP.PL), the developer of therapeutic cancer
Release Date: 29 January 2010 GB00B39J5N63 Scancell Holdings plc ( Scancell Holdings or the Company ) Interim Results for the six month period to 31 Scancell (SCLP.PL), the developer of therapeutic cancer
CROSSWORD CYBERSECURITY PLC
 Registered number: 08927013 CROSSWORD CYBERSECURITY PLC AUDITED CONSOLIDATED FINANCIAL STATEMENTS FOR THE PERIOD ENDED 31 DECEMBER 2014 COMPANY INFORMATION DIRECTORS T Ilube J Bottomley Professor D Secher
Registered number: 08927013 CROSSWORD CYBERSECURITY PLC AUDITED CONSOLIDATED FINANCIAL STATEMENTS FOR THE PERIOD ENDED 31 DECEMBER 2014 COMPANY INFORMATION DIRECTORS T Ilube J Bottomley Professor D Secher
Amadeus Global Travel Distribution, S.A.
 Amadeus Global Travel Distribution, S.A. Consolidated Interim Financial Statements as of June 30, 2002, prepared in accordance with International Accounting Standard 34 and Review Report of Independent
Amadeus Global Travel Distribution, S.A. Consolidated Interim Financial Statements as of June 30, 2002, prepared in accordance with International Accounting Standard 34 and Review Report of Independent
Interim Report 2014 January - June
 Interim Report 2014 January - June Letter to the shareholders Interim Report Jan Jun 2014 RIB Software AG Dear Shareholders, The Chinese Year of the Horse has met the high expectations placed on it within
Interim Report 2014 January - June Letter to the shareholders Interim Report Jan Jun 2014 RIB Software AG Dear Shareholders, The Chinese Year of the Horse has met the high expectations placed on it within
FOR IMMEDIATE RELEASE 28 September 2015 BOND INTERNATIONAL SOFTWARE PLC UNAUDITED INTERIM RESULTS
 FOR IMMEDIATE RELEASE 28 September 2015 BOND INTERNATIONAL SOFTWARE PLC UNAUDITED INTERIM RESULTS Bond International Software Plc ( the Group ), the specialist provider of software for the international
FOR IMMEDIATE RELEASE 28 September 2015 BOND INTERNATIONAL SOFTWARE PLC UNAUDITED INTERIM RESULTS Bond International Software Plc ( the Group ), the specialist provider of software for the international
Summary of Consolidated Financial Statements for the Second Quarter of Fiscal Year Ending March 31, 2012 (Japanese GAAP)
 This document is a translation of the Japanese financial statements and is not in conformity with accounting principles of the United States. Summary of Consolidated Financial Statements for the Second
This document is a translation of the Japanese financial statements and is not in conformity with accounting principles of the United States. Summary of Consolidated Financial Statements for the Second
Audited Financial Statements (IFRS) of SFC Smart Fuel Cell AG as of and for the Year Ended December 31, 2006
 Audited Financial Statements (IFRS) of SFC Smart Fuel Cell AG as of and for the Year Ended December 31, 2006 F-13 SFC Smart Fuel Cell AG Brunnthal, Munich Financial statement In accordance with International
Audited Financial Statements (IFRS) of SFC Smart Fuel Cell AG as of and for the Year Ended December 31, 2006 F-13 SFC Smart Fuel Cell AG Brunnthal, Munich Financial statement In accordance with International
MOLOGEN. Our research - for you. Interim Report as of March 31, 2013
 MOLOGEN. Our research - for you Interim Report as of March 31, 2013 MOLOGEN AG interim report as of March 31, 2013 Key data 2 KEY DATA of MOLOGEN AG according to IFRS as of March 31, 2013 Statement of
MOLOGEN. Our research - for you Interim Report as of March 31, 2013 MOLOGEN AG interim report as of March 31, 2013 Key data 2 KEY DATA of MOLOGEN AG according to IFRS as of March 31, 2013 Statement of
Group 9-month report Bastei Lübbe AG 1 Apr - 31 Dec 2015
 Group 9-month report Bastei Lübbe AG 1 Apr - 31 Dec 2015 At a Glance Key figures (IFRS) 01/04/2015-2015 01/04/- Change in % Business development in million Group turnover 79.4 86.8-8.5 % EBITDA 14.0 11.5
Group 9-month report Bastei Lübbe AG 1 Apr - 31 Dec 2015 At a Glance Key figures (IFRS) 01/04/2015-2015 01/04/- Change in % Business development in million Group turnover 79.4 86.8-8.5 % EBITDA 14.0 11.5
Symbility Solutions Inc. Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended June 30, 2015
 Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended Interim Consolidated Statements of Financial Position (Unaudited - In thousands of Canadian dollars) 2015 As at December 31,
Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended Interim Consolidated Statements of Financial Position (Unaudited - In thousands of Canadian dollars) 2015 As at December 31,
Quarterly Report II/2004
 Quarterly Report II/ Foreword Group management report Dear Shareholders, Result of operations Stagnating share prices and declining trading volumes dominated the German stock exchanges in Q2. The hike
Quarterly Report II/ Foreword Group management report Dear Shareholders, Result of operations Stagnating share prices and declining trading volumes dominated the German stock exchanges in Q2. The hike
July 23, 2015 FIRST HALF-YEAR REPORT 2015
 July 23, 2015 FIRST HALF-YEAR REPORT 2015 as of June 30, 2015 Financial summary YTD* 2015 YTD* 2014 Revenue CHF million 4.5 0.5 Net operating costs CHF million (2.3) (16.5) Net income/(loss) CHF million
July 23, 2015 FIRST HALF-YEAR REPORT 2015 as of June 30, 2015 Financial summary YTD* 2015 YTD* 2014 Revenue CHF million 4.5 0.5 Net operating costs CHF million (2.3) (16.5) Net income/(loss) CHF million
Quarterly Financial Report March 31, 2009. MBB Industries AG. Berlin
 Quarterly Financial Report March 31, 2009 MBB Industries AG. Berlin Quarterly Financial Report March 31, 2009 MBB Industries AG MBB Industries in Numbers 03 MBB Industries in Numbers Three Month (Jan.
Quarterly Financial Report March 31, 2009 MBB Industries AG. Berlin Quarterly Financial Report March 31, 2009 MBB Industries AG MBB Industries in Numbers 03 MBB Industries in Numbers Three Month (Jan.
Consolidated Interim Report
 Consolidated Interim Report as of 31 March 2015 UNIWHEELS AG CONTENTS 1. Key performance data 2. Condensed group management report as of 31 March 2015 3. Condensed consolidated financial statements as
Consolidated Interim Report as of 31 March 2015 UNIWHEELS AG CONTENTS 1. Key performance data 2. Condensed group management report as of 31 March 2015 3. Condensed consolidated financial statements as
