CONSULTATION ON AVAILABILITY OF DICLOFENAC AS A PHARMACY MEDICINE
|
|
|
- Jeffery Hamilton
- 7 years ago
- Views:
Transcription
1 To: All interested organisations 5 August 2013 Our reference: MLX 382 CONSULTATION ON AVAILABILITY OF DICLOFENAC AS A PHARMACY MEDICINE Introduction 1. We are writing to consult you on the continued availability of diclofenac as a Pharmacy (P) medicine and in particular on risk minimisation measures advised by the Commission on Human Medicines. Application to England, Wales, Scotland and Northern Ireland 2. This consultation is being made available in England, Wales, Scotland and Northern Ireland. The proposed changes would apply throughout the United Kingdom. Background 3. As a result of an EU-wide review of the cardiovascular (CV) risks of the non-steroidal analgesic diclofenac, the European Medicines Agency s Pharmacovigilance Risk Assessment Committee (PRAC) has recently recommended new contraindications, warnings and precautions to all marketing authorisation for diclofenac. detail_ jsp&mid=wc0b01ac058004d5c Diclofenac is a non-selective non-steroidal anti-inflammatory (NSAID) available in a variety of indications, forms, dosages and pack size quantities for relieving pain and inflammation. It is available as injections; tablets and capsules for oral use; and as gels and creams for topical use. A summary of UK products containing diclofenac appears in Appendix Studies have consistently shown that when given systemically (by means such as capsules, tablets or injections) there is an increased risk with diclofenac of cardiovascular side effects, such as heart attack or stroke compared to other similar painkillers, such as ibuprofen and naproxen. The risks are similar to those of selective COX -2 inhibitors, another group of painkillers. 6. The evidence of additional risk is clear when diclofenac is used at a high dose (150mg) daily and for long term treatment. The findings could not rule out the possibility that this
2 increased risk could be associated with the low doses and short lengths of treatment of nonprescription products. 7. The new advice is that people who have serious underlying heart or circulatory conditions, such as heart failure, heart disease, circulatory problems or a previous heart attack or stroke, should not use diclofenac. Patients with certain cardiovascular risk factors (such as high blood pressure, raised blood cholesterol, diabetes or smoking) should only use diclofenac after careful risk assessment. Healthcare professionals have also been advised to periodically reassess the need for patients to continue taking the medicine. The product information recommends that NSAIDs be used at the lowest effective dose for the shortest period of time necessary to control pain symptoms. 8. The new advice does not apply to topical (ie, gel or cream) formulations of diclofenac. Availability of diclofenac without prescription 9. Diclofenac is available in the UK without prescription in tablet form as a P medicine, supplied from pharmacies by or under the supervision of a pharmacist, under the following circumstances: Indication: For the short term relief of headache, dental pain, period pain, rheumatic and muscular pain, backache and the symptoms of colds and flu, including fever, in adults and children aged 14 years and over. Maximum strength: 25mg Maximum dose: 25mg Maximum daily dose: 75mg Maximum duration of treatment: 3 days Maximum pack size: 225mg of product (9 x 25mg tablets) Recommendations of the Commission on Human Medicines (CHM) 11. In June 2013 the CHM considered the implications of the PRAC recommendations for the current circumstances for non-prescription classification in the UK of medicinal products containing diclofenac in oral dosage forms. 12. The CHM noted that it was difficult to identify people with high cardiovascular risk although the risk would be higher with age. The CHM also considered that the wide availability of other NSAIDs without prescription as P and GSL medicines presented a risk that the products could be used together and that indications for OTC diclofenac were wide ranging, and included colds, flu and fever. 13. The CHM advised that a number of measures should be taken to strengthen the risk minimisation of oral diclofenac as a P medicine, including: Removing from the indications, the symptoms of colds and flu, including fever, Strengthening the warnings on the pack about use by those with cardiovascular disease or with significant risk factors for cardiovascular events
3 Strengthening warnings on the pack and about not taking diclofenac with other NSAIDs. Engaging with the pharmacy profession to support the implementation of further measures to minimise the risk of OTC diclofenac Requesting that the Marketing Authorisation Holders prepare a Risk Management Plan to strengthen the risk minimisation of oral diclofenac as a P medicine. Options for risk minimisation 14. The MHRA wishes to take into account all stakeholders views when reaching a final position on regulatory action, and therefore seeks views on the following options for risk minimisation. Option No new risk minimisation measures Option Implement all the measures identified by CHM: Remove from the indications, the symptoms of colds and flu, including fever, Strengthen the warnings on the pack about use by those with cardiovascular disease or with significant risk factors for cardiovascular events Strengthen warnings on the pack and about not taking diclofenac with other NSAIDs. Engage with the pharmacy profession to support the implementation of further measures to minimise the risk of OTC diclofenac Request that the Marketing Authorisation holders prepare a Risk Management Plan to strengthen the risk minimisation of oral diclofenac as a P medicine. Option Amend the current conditions for supply of diclofenac as a P medicine so that preparations for oral use can no longer be supplied without prescription. This would result in diclofenac tablets currently authorised as P medicines being required to be reclassified from P to POM (Prescription Only). Topical formulations would continue to be available without prescription. Summary of comments sought 15. We would welcome views on the merits of the proposals and options outlined in the paragraphs above and to receive any further views that responders would wish to make. We particularly wish to receive the views of: Marketing Authorisation holders for medicinal products containing diclofenac Medicines users and patient organisations, particularly those with experience of self management of pain Health professionals and professional organisations
4 Impact assessment 16. We think that the proposals contained in this consultation document will have little substantive adverse impact on industry or on patients. Overall, we consider that the proposal has no new significant effect on the sector and will not impose any additional costs or have any effect on issues of equality. 17. Similarly, the proposal does not impose any additional regulatory or financial burdens on the public sector but we would welcome views on whether an impact assessment would be useful and if so, any data to support the preparation of one. 18. We do not believe that the proposal in this consultation will have any adverse effect on any equality issue. We would welcome information on any instances where you believe that there will - or could be - any adverse affect on equality issues under any of the following: Competition Assessment Small Firms Impact Test Legal Aid Sustainable Development Carbon Assessment Other Environment Health Impact Assessment Race Equality Disability Equality Gender Equality Human Rights Rural Proofing How to respond 19. We would be grateful if any comments in response to this letter could be ed to: reclassification@mhra.gsi.gov.uk alternatively they may be addressed to: Colette McCreedy, Self Medication Specialist and Unit Manager, Medicines and Healthcare products Regulatory Agency, 3rd Floor, 151 Buckingham Palace Road, London SW1W 9SZ. Comments must arrive no later than 28 October Comments received after this date will not be taken into account. Circulation of proposals 20. This consultation letter is being brought to the attention of those organisations listed at Annex A. Copies of the consultation are also available from our website - and replies are welcome from all interested parties. 21. This consultation abides by consultation criteria set out in the revised Code of Practice on Consultation published by the Department for Business Innovation & Skills and viewable in full via
5 Responses: Confidentiality and Disclaimer 22. The information you send us may be passed to colleagues within the Government or related agencies. Furthermore, information provided in response to this consultation, including personal information, may be published or disclosed in accordance with the access to information regimes. These are primarily the Freedom of Information Act 2000 (FOIA), the Data Protection Act 1998 (DPA) and the Environmental Information Regulations If you want the information that you provide to be treated as confidential, please be aware that, under the FOIA, there is a statutory Code of Practice with which public authorities must comply and which deals, amongst other things, with obligations of confidence. In view of this it would be helpful if you could explain why you regard the information you have provided as confidential. If we receive a request for disclosure of the information we will take full account of your explanation, but we cannot give an assurance that confidentiality can be maintained in all circumstances. An automatic confidentiality disclaimer generated by your IT system will not, of itself, be regarded as binding on us. 24. Please ensure that your response is marked clearly, if you wish your response (whole or in part) and name to be kept confidential. Confidential responses will be included in any statistical summary of numbers of comments received and summary of views expressed. 25. The Agency s Information Centre at 151 Buckingham Palace Road will supply copies on request. An administrative charge, to cover the cost of photocopying and postage, may be applied. Alternatively, personal callers can inspect replies at the Information Centre by prior appointment (telephone ). Colette McCreedy MHRA VRMM
6 APPENDIX 1 Diclofenac Products authorised as Pharmacy only (P) in the UK Boots Joint Pain Relief 12.5mg Film-Coated Tablets Double Action Pain Relief 12.5mg Tablets (Actavis) Voltarol Pain-eze Tablets Voltarol Joint Pain 12.5mg Tablets Voltarol Pain-eze extra strength 25mg tablets
7 To: Colette McCreedy Self Medication Specialist and Unit Lead, Medicines and Healthcare products Regulatory Agency, 3rd Floor, 151 Buckingham Palace Road, London SW1W 9SZ. From: CONSULTATION LETTER MLX 382 CONSULTATION ON AVAILABILITY OF DICLOFENAC AS A PHARMACY MEDICINE * 1. I support Option 1 * 2. I support Option 2 * 3. I support Option 3 * 4. I have no comment to make on the proposals in the MLX *5. My comments on the proposals in the MLX are below/attached. * My reply may be made freely available. * My reply is confidential. * My reply is partially confidential (indicate clearly in the text any confidential elements) Signed: * Delete as appropriate
8 APPENDIX 2 MLX 382 N:B THE ATTENTION OF AT LEAST THE FOLLOWING HAS BEEN DRAWN TO THIS CONSULTATION. HOWEVER, THIS LISTIS NOT EXHAUSTIVE AND REPLIES ARE WELCOME FROM ALL INTERESTED PARTIES ABPI ACTAVIS ARTHRITIS CARE ASSOC OF ANAESTHETISTS OF GB AND IRELAND ASTHMA & ALLERGY RESEARCH ASSOC OF INDEPENDENT MULTIPLE PHARMACIES BOOTS BRIT ASSOC OF PHARMACEUTICAL WHOLESALERS BRITISH DIABETIC ASSOCIATION BRITISH HEART FOUNDATION BRITISH PAIN SOCIETY BRITISH RETAIL CONSORTIUM BRITISH SOCIETY OF GASTROENTEROLOGY CHEMIST AND DRUGGIST COMMUNITY PHARMACY MAGAZINE COMPANY CHEMIST ASSOCIATION LTD CONSUMERS ASSOCIATION DEPARTMENT OF AGRICULTURE DISPENSING DOCTORS ASSOCIATION DOCTOR MAGAZINE GENERAL MEDICAL COUNCIL GENERAL PRACTITIONERS COMMITTEE HEALTH SERVICE COMMISSIONER IMPERIAL CANCER RESEARCH FUND MEDICAL PROTECTION SOCIETY LTD MEDICAL WOMENS FEDERATION NATIONAL AIDS TRUST NATIONAL ASSOCIATION OF GP CO-OPERATIVES NATIONAL FARMERS UNION ENGLAND & WALES NATIONAL FEDERATION OF RETAIL NEWSAGENTS NATIONAL PHARMACY ASSOCIATION NEUROLOGICAL ALLIANCE NOVARTIS CONSUMER HEALTH OPTHALMIC GROUP COMMITTEE OTC BULLETIN PHARMACEUTICAL SERVICES NEGOTIATING COMMITTEE BRITISH INTERNATIONAL DOCTORS ASSOC PATIENTS ASSOCIATION ROYAL COLLEGE OF GENERAL PRACTITIONERS ROYAL COLLEGE OF NURSING
9 ROYAL COLLEGE OF NURSING (N IRELAND) ROYAL COLLEGE OF PATHOLOGISTS ROYAL COLLEGE OF PHYSICIANS & SURGEONS ROYAL COLLEGE OF PHYSICIANS (LONDON) ROYAL COLLEGE OF PSYCHIATRISTS ROYAL COLLEGE OF RADIOLOGISTS ROYAL COLLEGE OF SURGEONS (EDINBURGH) SCOTTISH GENERAL PRACTITIONERS COMMITTEE SCOTTISH WHOLESALE DRUGGISTS ASSOCIATION TERRANCE HIGGINS TRUST VETERINARY MEDICINES DIRECTORATE (VMD) ROYAL COLLEGE OF SURGEONS CENTRAL MEDICAL ADVISORY COMMITTEE COMMITTEE FOR PRACTITIONERS & GENERAL PRACTITIONERS ASSOCIATION (NI) BRIT ASSOC OF EUROPEAN PHARM DISTRIBUTOR BRITISH GENERIC MANUFACTURERS ASSOC NURSING & MIDWIFERY COUNCIL BRIT CARDIAC PATIENTS ASSOCIATION BRITISH MEDICAL ASSOCIATION ASSOCIATION OF PHARMACEUTICAL IMPORTERS DRUG INFORMATION PHARMACISTS GROUP NORTHERN IRELAND CONSUMER COUNCIL DHSSPS BMA (WALES) THE BRITISH SOCIETY FOR ALLERGY BRITISH ASSOCIATION OF EUROPEAN PHARMACEUTICAL DISTRIBUTORS BRITISH GERIATRIC SOCIETY UK INTERPROFESSIONAL GROUP ASSOCIATION OF BRITISH CARDIAC NURSES HEALTH WHICH UK HOMOEOPATHIC MEDICAL ASSOCIATION SURGICAL DRESSINGS MANUFACTURERS ASSN INTERNATIONAL RESEARCH CONSULTANTS NATIONAL BACK PAIN ASSOCIATION
PROPOSALS FOR AMENDMENTS TO THE HUMAN MEDICINES REGULATIONS 2012 TO ALLOW WIDER ACCESS TO NALOXONE FOR USE IN EMERGENCIES
 To: Interested Organisations Our reference: MLX MLX 383 Date: 19 November 2013 Dear Sir/Madam PROPOSALS FOR AMENDMENTS TO THE HUMAN MEDICINES REGULATIONS 2012 TO ALLOW WIDER ACCESS TO NALOXONE FOR USE
To: Interested Organisations Our reference: MLX MLX 383 Date: 19 November 2013 Dear Sir/Madam PROPOSALS FOR AMENDMENTS TO THE HUMAN MEDICINES REGULATIONS 2012 TO ALLOW WIDER ACCESS TO NALOXONE FOR USE
To: Interested Parties. Our reference: MLX 310 Date: 2 August 2004. Dear Sir/Madam
 To: Interested Parties 020 7084 2642 020 7084 2121 anne.thyer@mhra.gsi.gov.uk Our reference: MLX 310 Date: 2 August 2004 Dear Sir/Madam PROPOSALS TO ENABLE THE USE OF ELECTRONIC SIGNATURES ON PRESCRIPTIONS
To: Interested Parties 020 7084 2642 020 7084 2121 anne.thyer@mhra.gsi.gov.uk Our reference: MLX 310 Date: 2 August 2004 Dear Sir/Madam PROPOSALS TO ENABLE THE USE OF ELECTRONIC SIGNATURES ON PRESCRIPTIONS
CONSULTATION DOCUMENT: ARM 86; NUROFEN EXPRESS 400MG ORAL POWDER
 Dear Sir/Madam CONSULTATION DOCUMENT: ARM 86; NUROFEN EXPRESS 400MG ORAL POWDER REQUEST TO RECLASSIFY A PRODUCT FROM P TO GSL I am writing to inform you that consultation document ARM 86 which includes
Dear Sir/Madam CONSULTATION DOCUMENT: ARM 86; NUROFEN EXPRESS 400MG ORAL POWDER REQUEST TO RECLASSIFY A PRODUCT FROM P TO GSL I am writing to inform you that consultation document ARM 86 which includes
Using big data to identify opportunity and drive informed change in secondary care
 Using big data to identify opportunity and drive informed change in secondary care Exploring the changing patterns of Non-Steroidal Anti-Inflammatory Drug dispensing in UK hospitals ABOUT IMS HEALTH IMS
Using big data to identify opportunity and drive informed change in secondary care Exploring the changing patterns of Non-Steroidal Anti-Inflammatory Drug dispensing in UK hospitals ABOUT IMS HEALTH IMS
Public Assessment Report. Pharmacy to General Sales List Reclassification. Pirinase Hayfever Relief for Adults 0.05% Nasal Spray.
 Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
LEMSIP MAX DAY & NIGHT COLD & FLU RELIEF CAPSULES. (paracetamol, caffeine and phenylephrine hydrochloride)
 LEMSIP MAX DAY & NIGHT COLD & FLU RELIEF CAPSULES (paracetamol, caffeine and phenylephrine hydrochloride) PL 00063/0529 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Summary of
LEMSIP MAX DAY & NIGHT COLD & FLU RELIEF CAPSULES (paracetamol, caffeine and phenylephrine hydrochloride) PL 00063/0529 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Summary of
SUMMARY OF RESPONSES TO CONSULTATION MLX310
 SUMMARY OF RESPONSES TO CONSULTATION MLX310 1. Consultation MLX 310 was issued with a deadline for comments of 29 October. It was circulated to a range of interested organisations throughout the UK and
SUMMARY OF RESPONSES TO CONSULTATION MLX310 1. Consultation MLX 310 was issued with a deadline for comments of 29 October. It was circulated to a range of interested organisations throughout the UK and
Guidance for registered pharmacies providing pharmacy services at a distance, including on the internet
 Guidance for registered pharmacies providing pharmacy services at a distance, including on the internet April 2015 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians
Guidance for registered pharmacies providing pharmacy services at a distance, including on the internet April 2015 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians
Patient Group Directions. Guidance and information for nurses
 Patient Group Directions Guidance and information for nurses Patient Group Directions Guidance and information for nurses Contents Introduction 4 What is a patient group direction (PGD)? 4 When can PGDs
Patient Group Directions Guidance and information for nurses Patient Group Directions Guidance and information for nurses Contents Introduction 4 What is a patient group direction (PGD)? 4 When can PGDs
HOSPITAL PHARMACY PRACTICE IN THE UK AND THE RESPONSIBLE PHARMACIST REQUIREMENTS
 HOSPITAL PHARMACY PRACTICE IN THE UK AND THE RESPONSIBLE PHARMACIST REQUIREMENTS ROYAL PHARMACEUTICAL SOCIETY OF GREAT BRITAIN AND THE PHARMACEUTICAL SOCIETY OF NORTHERN IRELAND STATUS OF THIS DOCUMENT
HOSPITAL PHARMACY PRACTICE IN THE UK AND THE RESPONSIBLE PHARMACIST REQUIREMENTS ROYAL PHARMACEUTICAL SOCIETY OF GREAT BRITAIN AND THE PHARMACEUTICAL SOCIETY OF NORTHERN IRELAND STATUS OF THIS DOCUMENT
ARRANGEMENTS FOR THE FUTURE SUPPLY AND REIMBURSEMENT OF GENERIC MEDICINES FOR NHS SCOTLAND. Consultation Document
 ARRANGEMENTS FOR THE FUTURE SUPPLY AND REIMBURSEMENT OF GENERIC MEDICINES FOR NHS SCOTLAND Consultation Document Scottish Executive Health Department October 2003 Arrangements For The Future Supply And
ARRANGEMENTS FOR THE FUTURE SUPPLY AND REIMBURSEMENT OF GENERIC MEDICINES FOR NHS SCOTLAND Consultation Document Scottish Executive Health Department October 2003 Arrangements For The Future Supply And
2000 No. 1917 MEDICINES. The Prescription Only Medicines (Human Use) Amendment Order 2000
 5310291001 28-07-00 13:19:26 Pag Table: STATIN PPSysB Unit: pag1 STATUTORY INSTRUMENTS 2000 No. 1917 MEDICINES The Prescription Only Medicines (Human Use) Amendment Order 2000 Made - - - - - 17th July
5310291001 28-07-00 13:19:26 Pag Table: STATIN PPSysB Unit: pag1 STATUTORY INSTRUMENTS 2000 No. 1917 MEDICINES The Prescription Only Medicines (Human Use) Amendment Order 2000 Made - - - - - 17th July
Food supplements. Summary information on legislation relating to the sale of food supplements
 Food supplements Summary information on legislation relating to the sale of food supplements 1 This guidance applies to the whole of the UK and was prepared by the Department of Health in association with
Food supplements Summary information on legislation relating to the sale of food supplements 1 This guidance applies to the whole of the UK and was prepared by the Department of Health in association with
Ibuprofen 200mg Soft Capsules PL 03105/0105 UKPAR
 Ibuprofen 200mg Soft Capsules PL 03105/0105 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps taken for assessment Page 13 Summary of Product Characteristics Page 14 Patient
Ibuprofen 200mg Soft Capsules PL 03105/0105 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps taken for assessment Page 13 Summary of Product Characteristics Page 14 Patient
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
STANDARDS AND GUIDELINES TITLE: CIRCULATION DATE: March June 2013 REVISED: June 2013 APPROVAL DATE: July 29, 2013
 College of Homeopaths of Ontario 163 Queen Street East, 4 th Floor, Toronto, Ontario, M5A 1S1 TEL 416-862-4780 OR 1-844-862-4780 FAX 416-874-4077 www.collegeofhomeopaths.on.ca STANDARDS AND GUIDELINES
College of Homeopaths of Ontario 163 Queen Street East, 4 th Floor, Toronto, Ontario, M5A 1S1 TEL 416-862-4780 OR 1-844-862-4780 FAX 416-874-4077 www.collegeofhomeopaths.on.ca STANDARDS AND GUIDELINES
Montelukast 10mg film-coated tablets PL 17907/0474
 Montelukast 10mg film-coated tablets PL 17907/0474 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 4 Steps Taken for Assessment Page 11 Steps Taken After Initial Authorisation Page
Montelukast 10mg film-coated tablets PL 17907/0474 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 4 Steps Taken for Assessment Page 11 Steps Taken After Initial Authorisation Page
Memantine hydrochloride 20 mg film-coated tablets PL 17907/0291
 Memantine hydrochloride 20 mg film-coated tablets PL 17907/0291 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 13 Summary of Product Characteristics
Memantine hydrochloride 20 mg film-coated tablets PL 17907/0291 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 13 Summary of Product Characteristics
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
St Bernard s Catholic School. Administration of Medicine Policy
 St Bernard s Catholic School Page 1 of 10 The St Bernard s administration of medicine policy has been developed to ensure that children with medical needs have the same access to education as their peers
St Bernard s Catholic School Page 1 of 10 The St Bernard s administration of medicine policy has been developed to ensure that children with medical needs have the same access to education as their peers
Identifying and treating long-term kidney problems (chronic kidney disease)
 Understanding NICE guidance Information for people who use NHS services Identifying and treating long-term kidney problems (chronic kidney disease) NICE clinical guidelines advise the NHS on caring for
Understanding NICE guidance Information for people who use NHS services Identifying and treating long-term kidney problems (chronic kidney disease) NICE clinical guidelines advise the NHS on caring for
BVA. British Trout Association
 CODE OF PRACTICE ON THE RESPONSIBLE USE OF ANIMAL MEDICINES ON THE FARM THIS CODE IS SUPPORTED BY THE FOLLOWING ORGANISATIONS: BVA British Trout Association LAST UPDATED: SEPTEMBER 2008 TABLE OF CONTENTS
CODE OF PRACTICE ON THE RESPONSIBLE USE OF ANIMAL MEDICINES ON THE FARM THIS CODE IS SUPPORTED BY THE FOLLOWING ORGANISATIONS: BVA British Trout Association LAST UPDATED: SEPTEMBER 2008 TABLE OF CONTENTS
Evidence on the quality of medical note keeping: Guidance for use at appraisal and revalidation
 Health Informatics Unit Evidence on the quality of medical note keeping: Guidance for use at appraisal and revalidation April 2011 Funded by: Acknowledgements This project was funded by the Academy of
Health Informatics Unit Evidence on the quality of medical note keeping: Guidance for use at appraisal and revalidation April 2011 Funded by: Acknowledgements This project was funded by the Academy of
Medical Device Alert. Device Peristeen anal irrigation system manufactured by Coloplast Limited. Action by
 Medical Device Alert Issued: 26 February 2014 at 16:00 Device Peristeen anal irrigation system manufactured by Coloplast Limited. Problem Risk of using transanal irrigation inappropriately. The manufacturer
Medical Device Alert Issued: 26 February 2014 at 16:00 Device Peristeen anal irrigation system manufactured by Coloplast Limited. Problem Risk of using transanal irrigation inappropriately. The manufacturer
Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator,
 Reprinted from FDA s website by Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator, Guidance And Antiasthmatic for Drug Industry Products for Over-the-Counter Labeling
Reprinted from FDA s website by Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator, Guidance And Antiasthmatic for Drug Industry Products for Over-the-Counter Labeling
Draft guidance for registered pharmacies providing internet and distance sale, supply or service provision
 Draft guidance for registered pharmacies providing internet and distance sale, supply or service provision September 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy
Draft guidance for registered pharmacies providing internet and distance sale, supply or service provision September 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy
Faster, better, safer communications
 Faster, better, safer communications Using email in health and social care (in England) For patients and healthcare professionals March 2015 Acknowledgements This project was funded by the NHSmail team
Faster, better, safer communications Using email in health and social care (in England) For patients and healthcare professionals March 2015 Acknowledgements This project was funded by the NHSmail team
Local Enhanced Service Specification for the Supply of Pharmaceutical Services to Care Homes through Community Pharmacy
 Local Enhanced Service Specification for the Supply of Pharmaceutical Services to Care Homes through Community Pharmacy Contents: 1. Introduction and purpose 2. Period of Service 3. Aim of the Service
Local Enhanced Service Specification for the Supply of Pharmaceutical Services to Care Homes through Community Pharmacy Contents: 1. Introduction and purpose 2. Period of Service 3. Aim of the Service
Donepezil hydrochloride 10 mg film-coated tablets PL 19156/0130
 Donepezil hydrochloride 10 mg film-coated tablets PL 19156/0130 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 4 Steps Taken for Assessment Page 12 Summary of Product Characteristics
Donepezil hydrochloride 10 mg film-coated tablets PL 19156/0130 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 4 Steps Taken for Assessment Page 12 Summary of Product Characteristics
YOUR MEDICAL RECORDS AN UPDATE PROVIDED BY THE OTFORD PATIENT PARTICIPATION GROUP (PPG)
 YOUR MEDICAL RECORDS AN UPDATE PROVIDED BY THE OTFORD PATIENT PARTICIPATION GROUP (PPG) Background to this Update There are a lot of articles in the press and on television and radio about care.data and
YOUR MEDICAL RECORDS AN UPDATE PROVIDED BY THE OTFORD PATIENT PARTICIPATION GROUP (PPG) Background to this Update There are a lot of articles in the press and on television and radio about care.data and
SWINE FLU: FROM CONTAINMENT TO TREATMENT
 SWINE FLU: FROM CONTAINMENT TO TREATMENT SWINE FLU: FROM CONTAINMENT TO TREATMENT INTRODUCTION As Swine Flu spreads and more people start to catch it, it makes sense to move from intensive efforts to contain
SWINE FLU: FROM CONTAINMENT TO TREATMENT SWINE FLU: FROM CONTAINMENT TO TREATMENT INTRODUCTION As Swine Flu spreads and more people start to catch it, it makes sense to move from intensive efforts to contain
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
Proposals to simplify the reimbursement arrangements for NHS dispensing contractors: A consultation
 Proposals to simplify the reimbursement arrangements for NHS dispensing contractors: A consultation 0 CONSULTATION PAPER PROPOSALS TO SIMPLIFY THE REIMBURSEMENT ARRANGEMENTS FOR NHS DISPENSING CONTRACTORS
Proposals to simplify the reimbursement arrangements for NHS dispensing contractors: A consultation 0 CONSULTATION PAPER PROPOSALS TO SIMPLIFY THE REIMBURSEMENT ARRANGEMENTS FOR NHS DISPENSING CONTRACTORS
US Health Statistics: Americans Most Over-Prescribed Country in the World
 US Health Statistics: Americans Most Over-Prescribed Country in the World A vitally important story reported in the April 15, 1998, issue of the Journal of the American Medical Association (JAMA), sums
US Health Statistics: Americans Most Over-Prescribed Country in the World A vitally important story reported in the April 15, 1998, issue of the Journal of the American Medical Association (JAMA), sums
Safer recruitment scheme for the issue of alert notices for healthcare professionals in England
 Safer recruitment scheme for the issue of alert notices for healthcare professionals in England November 2006 The issue of alert notices for healthcare professionals Summary 1. NHS Employers and the Department
Safer recruitment scheme for the issue of alert notices for healthcare professionals in England November 2006 The issue of alert notices for healthcare professionals Summary 1. NHS Employers and the Department
THE USE OF DRUGS BEYOND (OFF-LABEL) AND WITHOUT (UNLICENSED) MARKETING AUTHORIZATION
 THE USE OF DRUGS BEYOND (OFF-LABEL) AND WITHOUT (UNLICENSED) MARKETING AUTHORIZATION The use of drugs for off-label purposes is widespread. Surveys suggest that up to 1/4 of all prescriptions in palliative
THE USE OF DRUGS BEYOND (OFF-LABEL) AND WITHOUT (UNLICENSED) MARKETING AUTHORIZATION The use of drugs for off-label purposes is widespread. Surveys suggest that up to 1/4 of all prescriptions in palliative
Paracetamol apollo +9191 46 950 950. Paracetamol apollo +9191 46 950 950. Paracetamol
 Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
A competency framework for all prescribers updated draft for consultation
 A competency framework for all prescribers updated draft for consultation Consultation closes 15 April 2016 Contents 1 Introduction... 3 2 Uses of the framework... 4 3 Scope of the competency framework...
A competency framework for all prescribers updated draft for consultation Consultation closes 15 April 2016 Contents 1 Introduction... 3 2 Uses of the framework... 4 3 Scope of the competency framework...
Pain relief at home. Information for adult patients
 Pain relief at home Information for adult patients 3 Contents Page 3 Page 3 Page 5 Page 7 Page 8 Introduction to pain relief Common pain relief medicines and their side effects More information about pain
Pain relief at home Information for adult patients 3 Contents Page 3 Page 3 Page 5 Page 7 Page 8 Introduction to pain relief Common pain relief medicines and their side effects More information about pain
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
National Chlamydia Screening Programme September 2012 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS
 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
HMRC Digital Strategy legislative changes to enable Paperless Self Assessment
 HMRC Digital Strategy legislative changes to enable Paperless Self Assessment Consultation document Publication date: 27 November 2013 Closing date for comments: 27 December 2013 Subject of this consultation:
HMRC Digital Strategy legislative changes to enable Paperless Self Assessment Consultation document Publication date: 27 November 2013 Closing date for comments: 27 December 2013 Subject of this consultation:
GUIDELINES FOR THE CONTROL AND ADMINISTRATION OF MEDICINES DOMICILIARY CARE AGENCIES
 GUIDELINES FOR THE CONTROL AND ADMINISTRATION OF MEDICINES DOMICILIARY CARE AGENCIES January 2009 Contents Page Number 1.0 Introduction 3 2.0 Background 4 3.0 Criteria 5 3.1 Referral 5 3.2 Levels of assistance/consent
GUIDELINES FOR THE CONTROL AND ADMINISTRATION OF MEDICINES DOMICILIARY CARE AGENCIES January 2009 Contents Page Number 1.0 Introduction 3 2.0 Background 4 3.0 Criteria 5 3.1 Referral 5 3.2 Levels of assistance/consent
Summary of the role and operation of NHS Research Management Offices in England
 Summary of the role and operation of NHS Research Management Offices in England The purpose of this document is to clearly explain, at the operational level, the activities undertaken by NHS R&D Offices
Summary of the role and operation of NHS Research Management Offices in England The purpose of this document is to clearly explain, at the operational level, the activities undertaken by NHS R&D Offices
IQ Level 2 Certificate in Understanding the Safe Handling of Medicines (QCF) Specification
 IQ Level 2 Certificate in Understanding the Safe Handling of Medicines (QCF) Specification Regulation No: 601/2010/1 Page 1 of 25 IQB/0.2/211 Version 1.0 13/01/2014 Author AW Contents Page Industry Qualifications...
IQ Level 2 Certificate in Understanding the Safe Handling of Medicines (QCF) Specification Regulation No: 601/2010/1 Page 1 of 25 IQB/0.2/211 Version 1.0 13/01/2014 Author AW Contents Page Industry Qualifications...
Fexofenadine Hydrochloride 120 mg Film-coated Tablets Fexofenadine Hydrochloride 180 mg Film-coated Tablets PL 36390/0053-4 UKPAR TABLE OF CONTENTS
 Fexofenadine Hydrochloride 120 mg Film-coated Tablets Fexofenadine Hydrochloride 180 mg Film-coated Tablets PL 36390/0053-4 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 5 Steps
Fexofenadine Hydrochloride 120 mg Film-coated Tablets Fexofenadine Hydrochloride 180 mg Film-coated Tablets PL 36390/0053-4 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 5 Steps
Information for you Abortion care
 Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Consultation on the Road Traffic (Drink Driving) (Amendment) Bill and additional measures to tackle drink and drug driving in Northern Ireland
 ANNEX A: Reply Form Consultation on the Road Traffic (Drink Driving) (Amendment) Bill and additional measures to tackle drink and drug driving in Northern Ireland Title: Dr.. Name: Peter Rice Organisation
ANNEX A: Reply Form Consultation on the Road Traffic (Drink Driving) (Amendment) Bill and additional measures to tackle drink and drug driving in Northern Ireland Title: Dr.. Name: Peter Rice Organisation
1. JOB PURPOSE 2. KEY ACCOUNTABILITIES PRINCIPAL DUTIES:
 Job Title: Location/Base: Dept.: Reporting to: Pharmacy Technician Claremont Hospital Pharmacy Pharmacy Manager 1. JOB PURPOSE The Pharmacy Technician, as part of a dedicated team, plays a key role in
Job Title: Location/Base: Dept.: Reporting to: Pharmacy Technician Claremont Hospital Pharmacy Pharmacy Manager 1. JOB PURPOSE The Pharmacy Technician, as part of a dedicated team, plays a key role in
IN-USE PRODUCT SAFETY ASSESSMENT REPORT BENEPALI (ETANERCEPT BIOSIMILAR)
 IN-USE PRODUCT SAFETY ASSESSMENT REPORT BENEPALI (ETANERCEPT BIOSIMILAR) SUMMARY OF ASSESSMENT AND ITS FINDINGS BACKGROUND A UK marketing authorisation has been granted to an etanercept biosimilar (SB4,
IN-USE PRODUCT SAFETY ASSESSMENT REPORT BENEPALI (ETANERCEPT BIOSIMILAR) SUMMARY OF ASSESSMENT AND ITS FINDINGS BACKGROUND A UK marketing authorisation has been granted to an etanercept biosimilar (SB4,
Pharmacy Policy (General)
 WORKSAFE VICTORIA Pharmacy Policy (General) WorkSafe can pay the reasonable costs of medications and other pharmacy items required as a result of a work-related injury or illness in accordance with Victorian
WORKSAFE VICTORIA Pharmacy Policy (General) WorkSafe can pay the reasonable costs of medications and other pharmacy items required as a result of a work-related injury or illness in accordance with Victorian
Part surrenders and part assignments of life insurance policies
 Part surrenders and part assignments of life insurance policies Consultation document Publication date: 20 April 2016 Closing date for comments: 13 July 2016 Subject of this consultation: Scope of this
Part surrenders and part assignments of life insurance policies Consultation document Publication date: 20 April 2016 Closing date for comments: 13 July 2016 Subject of this consultation: Scope of this
8. To ensure the accurate use of all pharmacy computer systems and to record all issues, receipts and returns of medicines.
 JOB DESCRIPTION JOB TITLE PAY BAND DIRECTORATE / DIVISION DEPARTMENT BASE RESPONSIBLE TO ACCOUNTABLE TO RESPONSIBLE FOR Student Pharmacy Technician Band 4 (1st year 70% of top point on band 4, 2 nd year
JOB DESCRIPTION JOB TITLE PAY BAND DIRECTORATE / DIVISION DEPARTMENT BASE RESPONSIBLE TO ACCOUNTABLE TO RESPONSIBLE FOR Student Pharmacy Technician Band 4 (1st year 70% of top point on band 4, 2 nd year
REVIEW OF POLICE LEADERSHIP AND TRAINING. Ministerial foreword
 REVIEW OF POLICE LEADERSHIP AND TRAINING Ministerial foreword The Government has set out a clear vision for 21st century policing: rebalancing accountability through Police and Crime Commissioners at force
REVIEW OF POLICE LEADERSHIP AND TRAINING Ministerial foreword The Government has set out a clear vision for 21st century policing: rebalancing accountability through Police and Crime Commissioners at force
Public Assessment Report. (Budesonide) PL 17901/0254. AstraZeneca UK Ltd
 Public Assessment Report Rhinocort Hay Fever 64 micrograms, Nasal Spray (Budesonide) PL 17901/0254 AstraZeneca UK Ltd 1 LAY SUMMARY Rhinocort Hay Fever 64 micrograms, Nasal Spray (Budesonide) This is a
Public Assessment Report Rhinocort Hay Fever 64 micrograms, Nasal Spray (Budesonide) PL 17901/0254 AstraZeneca UK Ltd 1 LAY SUMMARY Rhinocort Hay Fever 64 micrograms, Nasal Spray (Budesonide) This is a
Changes to VAT zerorating of exports from the UK
 Changes to VAT zerorating of exports from the UK Consultation document Publication date: 13 May 2013 Closing date for comments: 5 July 2013 Subject of this consultation: Scope of this consultation: Changes
Changes to VAT zerorating of exports from the UK Consultation document Publication date: 13 May 2013 Closing date for comments: 5 July 2013 Subject of this consultation: Scope of this consultation: Changes
medicineupdate Tramadol for pain Asking the right questions about new medicines Page Section 1: What tramadol is 1 Section 2: What tramadol is for 1
 medicineupdate Asking the right questions about new medicines Tramadol for pain Page Section 1: What tramadol is 1 Section 2: What tramadol is for 1 Section 3: Who can take tramadol 2 Section 4: What does
medicineupdate Asking the right questions about new medicines Tramadol for pain Page Section 1: What tramadol is 1 Section 2: What tramadol is for 1 Section 3: Who can take tramadol 2 Section 4: What does
DESCRIPTION OF THE MEDICINES FOR HUMAN USE (CLINICAL TRIALS) REGULATIONS 2004
 DESCRIPTION OF THE MEDICINES FOR HUMAN USE (CLINICAL TRIALS) REGULATIONS 2004 Page 1 of 17 TABLE OF CONTENTS INTRODUCTION 4 EXECUTIVE SUMMARY 4 PUBLIC HEALTH BENEFITS 5 Good clinical practice 5 Good manufacturing
DESCRIPTION OF THE MEDICINES FOR HUMAN USE (CLINICAL TRIALS) REGULATIONS 2004 Page 1 of 17 TABLE OF CONTENTS INTRODUCTION 4 EXECUTIVE SUMMARY 4 PUBLIC HEALTH BENEFITS 5 Good clinical practice 5 Good manufacturing
ANASTROZOLE 1 MG FILM-COATED TABLETS. (Anastrozole) PL 40378/0123 UKPAR TABLE OF CONTENTS
 ANASTROZOLE 1 MG FILM-COATED TABLETS (Anastrozole) PL 40378/0123 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
ANASTROZOLE 1 MG FILM-COATED TABLETS (Anastrozole) PL 40378/0123 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Triennial Review of NHS Blood and Transplant (NHSBT) A Review of NHS Blood and Transplant (NHSBT) Call for Evidence
 Triennial Review of NHS Blood and Transplant (NHSBT) A Review of NHS Blood and Transplant (NHSBT) Call for Evidence August 2015 Title: Triennial Review of NHS Blood and Transplant Call for Evidence Author:
Triennial Review of NHS Blood and Transplant (NHSBT) A Review of NHS Blood and Transplant (NHSBT) Call for Evidence August 2015 Title: Triennial Review of NHS Blood and Transplant Call for Evidence Author:
Amendments to the Controlled Drugs (Supervision of Management and Use) Regulations (Northern Ireland) 2009. Consultation Response Report
 Amendments to the Controlled Drugs (Supervision of Management and Use) Regulations (Northern Ireland) 2009 Consultation Response Report June 2015 INDEX 1. Summary 2. Background 3. Consultation feedback
Amendments to the Controlled Drugs (Supervision of Management and Use) Regulations (Northern Ireland) 2009 Consultation Response Report June 2015 INDEX 1. Summary 2. Background 3. Consultation feedback
Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine
 The British Pain Society Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine This is a consensus document prepared on behalf of the British Pain Society
The British Pain Society Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine This is a consensus document prepared on behalf of the British Pain Society
Omeprazole 20 mg gastro-resistant tablets PL 14017/0277
 Omeprazole 20 mg gastro-resistant tablets PL 14017/0277 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 4 Steps Taken for Assessment Page 11 Steps Taken After Initial Authorisation
Omeprazole 20 mg gastro-resistant tablets PL 14017/0277 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 4 Steps Taken for Assessment Page 11 Steps Taken After Initial Authorisation
MULTI AGENCY POLICY FOR THE ADMINISTRATION OF MEDICATION AND HEALTH CARE PROCEDURES:
 MULTI AGENCY POLICY FOR THE ADMINISTRATION OF MEDICATION AND HEALTH CARE PROCEDURES: Early years provision, Educational Establishments and Voluntary Services Document reference number: C O R P O L O 0
MULTI AGENCY POLICY FOR THE ADMINISTRATION OF MEDICATION AND HEALTH CARE PROCEDURES: Early years provision, Educational Establishments and Voluntary Services Document reference number: C O R P O L O 0
2. Characteristics of staff Qualifications required. Additional requirements. Continued education & training requirements
 Patient Group Direction The supply of Azithromycin 1g as a single dose by accredited Community Pharmacists to patients in receipt of a positive test result to Chlamydia trachomatis, and treatment of their
Patient Group Direction The supply of Azithromycin 1g as a single dose by accredited Community Pharmacists to patients in receipt of a positive test result to Chlamydia trachomatis, and treatment of their
Medical Device Alert. Device All metal-on-metal (MoM) hip replacements. Action by. CAS deadlines. Ref: MDA/2012/036 Issued: 25 June 2012 at 11:00
 Medical Device Alert Issued: 25 June 2012 at 11:00 Device All metal-on-metal (MoM) hip replacements Problem The MHRA is issuing updated information and advice about the follow-up of implanted with metal-on-metal
Medical Device Alert Issued: 25 June 2012 at 11:00 Device All metal-on-metal (MoM) hip replacements Problem The MHRA is issuing updated information and advice about the follow-up of implanted with metal-on-metal
Following minor gynaecological surgery
 Following minor gynaecological surgery Exceptional healthcare, personally delivered n Following your operation you should have an adult to take you home and remain with you overnight. Transport home should
Following minor gynaecological surgery Exceptional healthcare, personally delivered n Following your operation you should have an adult to take you home and remain with you overnight. Transport home should
Pharmacists improving care in care homes
 The Royal Pharmaceutical Society believes that better utilisation of pharmacists skills in care homes will bring significant benefits to care home residents, care homes providers and the NHS. Introduction
The Royal Pharmaceutical Society believes that better utilisation of pharmacists skills in care homes will bring significant benefits to care home residents, care homes providers and the NHS. Introduction
EU DIRECTIVE ON GOOD CLINICAL PRACTICE IN CLINICAL TRIALS DH & MHRA BRIEFING NOTE
 EU DIRECTIVE ON GOOD CLINICAL PRACTICE IN CLINICAL TRIALS DH & MHRA BRIEFING NOTE Purpose 1. The Clinical Trials Directive 2001/20/EC heralds certain additional responsibilities for the Medicines and Healthcare
EU DIRECTIVE ON GOOD CLINICAL PRACTICE IN CLINICAL TRIALS DH & MHRA BRIEFING NOTE Purpose 1. The Clinical Trials Directive 2001/20/EC heralds certain additional responsibilities for the Medicines and Healthcare
A Guide to Patient Services. Cedars-Sinai Health Associates
 A Guide to Patient Services Cedars-Sinai Health Associates Welcome Welcome to Cedars-Sinai Health Associates. We appreciate the trust you have placed in us by joining our dedicated network of independent-practice
A Guide to Patient Services Cedars-Sinai Health Associates Welcome Welcome to Cedars-Sinai Health Associates. We appreciate the trust you have placed in us by joining our dedicated network of independent-practice
Hiring agency staff during strike action: reforming regulation
 Recruitment Sector Hiring agency staff during strike action: reforming regulation JULY 2015 Contents Contents... 2 Hiring agency staff during strike action: reforming regulation... 3 1. Executive Summary...
Recruitment Sector Hiring agency staff during strike action: reforming regulation JULY 2015 Contents Contents... 2 Hiring agency staff during strike action: reforming regulation... 3 1. Executive Summary...
Legal and governance framework
 Annex A Legal and governance framework This annex is a brief guide to the legal and governance framework relevant to research in the UK. It is not intended to be a comprehensive statement of the law or
Annex A Legal and governance framework This annex is a brief guide to the legal and governance framework relevant to research in the UK. It is not intended to be a comprehensive statement of the law or
Cash, tax evasion and the hidden economy. Call for evidence Publication date: 25 th November 2015 Closing date for comments: 27 th January 2016
 Cash, tax evasion and the hidden economy Call for evidence Publication date: 25 th November 2015 Closing date for comments: 27 th January 2016 Contents 1 Introduction 3 2 What do changing trends in payment
Cash, tax evasion and the hidden economy Call for evidence Publication date: 25 th November 2015 Closing date for comments: 27 th January 2016 Contents 1 Introduction 3 2 What do changing trends in payment
Emerging Device Topics for Regulatory Consideration.. Janine Jamieson May 2015
 Emerging Device Topics for Regulatory Consideration. Janine Jamieson May 2015 Disclaimer These are my personal views and not necessarily those of MHRA as an organisation. 2 European regulation of combination
Emerging Device Topics for Regulatory Consideration. Janine Jamieson May 2015 Disclaimer These are my personal views and not necessarily those of MHRA as an organisation. 2 European regulation of combination
Consultation on Regulation of Independent Healthcare in Scotland. Consultation Report
 Consultation on Regulation of Independent Healthcare in Scotland Consultation Report Consultation on Regulation of Independent Healthcare in Scotland Consultation Report The Scottish Government, Edinburgh
Consultation on Regulation of Independent Healthcare in Scotland Consultation Report Consultation on Regulation of Independent Healthcare in Scotland Consultation Report The Scottish Government, Edinburgh
Choosing Pain Medicine for Osteoarthritis. A Guide for Consumers
 Choosing Pain Medicine for Osteoarthritis A Guide for Consumers Fast Facts on Pain Relievers Acetaminophen (Tylenol ) works on mild pain and has fewer risks than other pain pills. Prescription (Rx) pain
Choosing Pain Medicine for Osteoarthritis A Guide for Consumers Fast Facts on Pain Relievers Acetaminophen (Tylenol ) works on mild pain and has fewer risks than other pain pills. Prescription (Rx) pain
UNIVERSITY OF BIRMINGHAM AND UNIVERSITY OF YORK HEALTH ECONOMICS CONSORTIUM (NICE EXTERNAL CONTRACTOR) Health economic report on piloted indicator(s)
 UNIVERSITY OF BIRMINGHAM AND UNIVERSITY OF YORK HEALTH ECONOMICS CONSORTIUM (NICE EXTERNAL CONTRACTOR) Health economic report on piloted indicator(s) Pilot QOF indicator: The percentage of patients 79
UNIVERSITY OF BIRMINGHAM AND UNIVERSITY OF YORK HEALTH ECONOMICS CONSORTIUM (NICE EXTERNAL CONTRACTOR) Health economic report on piloted indicator(s) Pilot QOF indicator: The percentage of patients 79
SCHEDULE OF THIRD PARTIES WITH WHOM THE TRUST HAS A DUTY OF CO-OPERATION
 Directorate of Clinical and Quality Assurance & Trust Secretary SCHEDULE OF THIRD PARTIES WITH WHOM THE TRUST HAS A DUTY OF CO-OPERATION Reference: CEM004 Version: 1.4 This version issued: 14/12/12 Result
Directorate of Clinical and Quality Assurance & Trust Secretary SCHEDULE OF THIRD PARTIES WITH WHOM THE TRUST HAS A DUTY OF CO-OPERATION Reference: CEM004 Version: 1.4 This version issued: 14/12/12 Result
New medicines in Scotland
 NHS SCOTLAND New medicines in Scotland who decides what the NHS can provide? What is this factsheet about? This factsheet explains the process that medicines go through before NHS doctors in Scotland can
NHS SCOTLAND New medicines in Scotland who decides what the NHS can provide? What is this factsheet about? This factsheet explains the process that medicines go through before NHS doctors in Scotland can
Tobacco levy: consultation
 Tobacco levy: consultation December 2014 Tobacco levy: consultation December 2014 Crown copyright 2014 This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise
Tobacco levy: consultation December 2014 Tobacco levy: consultation December 2014 Crown copyright 2014 This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise
Pharmacovigilance and the Internet: A Call for Change
 Pharmacovigilance and the Internet: A Call for Change A white paper from the ABPI Pharmacovigilance Expert Network 13 June 2011 Page 2 of 6 Pharmacovigilance and the Internet: A Call for Change (ABPI)
Pharmacovigilance and the Internet: A Call for Change A white paper from the ABPI Pharmacovigilance Expert Network 13 June 2011 Page 2 of 6 Pharmacovigilance and the Internet: A Call for Change (ABPI)
Medication Management Guidelines for Nurses and Midwives
 Medication Management Guidelines for Nurses and Midwives 1. Introduction As the statutory body responsible for the regulation of nursing and midwifery practice in Western Australia (WA), the Nurses & Midwives
Medication Management Guidelines for Nurses and Midwives 1. Introduction As the statutory body responsible for the regulation of nursing and midwifery practice in Western Australia (WA), the Nurses & Midwives
Getting the most from blood pressure medicines
 P R E S S U R E P O I N T S S E R I E S : NO. 4 Getting the most from blood pressure medicines B L O O D P R E S S U R E A S S O C I AT I O N Pressure Points series Pressure Points is a series of booklets
P R E S S U R E P O I N T S S E R I E S : NO. 4 Getting the most from blood pressure medicines B L O O D P R E S S U R E A S S O C I AT I O N Pressure Points series Pressure Points is a series of booklets
Introduction. Background to this event. Raising awareness 09/11/2015
 Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Management of Pupils with Health Care Needs in Schools Policy
 Management of Pupils with Health Care Needs in Schools Policy Date: January 2013 Version number: 1 Author: Sheila Fraser, PHN Development Manager Review date: January 2016 If you would like this document
Management of Pupils with Health Care Needs in Schools Policy Date: January 2013 Version number: 1 Author: Sheila Fraser, PHN Development Manager Review date: January 2016 If you would like this document
CONSENT FOR MEDICAL TREATMENT
 CONSENT FOR MEDICAL TREATMENT Patient Name DOB Date I, the patient or authorized representative, consent to any examination, evaluation and treatment regarding any illness, injury or other health concern
CONSENT FOR MEDICAL TREATMENT Patient Name DOB Date I, the patient or authorized representative, consent to any examination, evaluation and treatment regarding any illness, injury or other health concern
swine flu vaccination:
 swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
UNDERGOING TERMINATION OF PREGNANCY AFTER 12 WEEKS
 UNDERGOING TERMINATION OF PREGNANCY AFTER 12 WEEKS Information Leaflet Your Health. Our Priority. Page 2 of 5 The staff at Stepping Hill Hospital will support you as much as we can during this sad time.
UNDERGOING TERMINATION OF PREGNANCY AFTER 12 WEEKS Information Leaflet Your Health. Our Priority. Page 2 of 5 The staff at Stepping Hill Hospital will support you as much as we can during this sad time.
Health Administration Regulation 2015
 New South Wales Health Administration Regulation 2015 under the Health Administration Act 1982 His Excellency the Governor, with the advice of the Executive Council, has made the following Regulation under
New South Wales Health Administration Regulation 2015 under the Health Administration Act 1982 His Excellency the Governor, with the advice of the Executive Council, has made the following Regulation under
Draft guidance for registered pharmacies preparing unlicensed medicines
 Draft guidance for registered pharmacies preparing unlicensed medicines January 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians and registered pharmacies
Draft guidance for registered pharmacies preparing unlicensed medicines January 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians and registered pharmacies
Including Me, Managing Complex Health Needs in Schools and Early Years Settings
 Model Policy for The Administration of Medicines in Schools Evelyn Green Steph King Moira Young Jo Howcroft Scott Lisa Maidment Jenny Tapply John Studley Liz Foster Sarah Fletcher Sally Anne Clark Manager
Model Policy for The Administration of Medicines in Schools Evelyn Green Steph King Moira Young Jo Howcroft Scott Lisa Maidment Jenny Tapply John Studley Liz Foster Sarah Fletcher Sally Anne Clark Manager
DIRECTOR OF PUBLIC HEALTH ROLE PROFILE
 Appendix A DIRECTOR OF PUBLIC HEALTH ROLE PROFILE Title: Employing Organisation: Accountable to: Hours: Work base: Key Relationships Director of Public Health London Borough of Tower Hamlets Professionally
Appendix A DIRECTOR OF PUBLIC HEALTH ROLE PROFILE Title: Employing Organisation: Accountable to: Hours: Work base: Key Relationships Director of Public Health London Borough of Tower Hamlets Professionally
Memantine (Ebixa) Drug treatment for Alzheimer s disease
 IS 20 October 2011 Information sheet Memantine (Ebixa) Drug treatment for Alzheimer s disease Introduction... 1 How does Ebixa work?... 1 Who might benefit?... 2 What effect might Ebixa have?... 2 How
IS 20 October 2011 Information sheet Memantine (Ebixa) Drug treatment for Alzheimer s disease Introduction... 1 How does Ebixa work?... 1 Who might benefit?... 2 What effect might Ebixa have?... 2 How
Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery
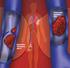 medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
Proposals for the reform of the regulation of unlicensed herbal remedies in the United Kingdom made up to meet the needs of individual patients
 Proposals for the reform of the regulation of unlicensed herbal remedies in the United Kingdom made up to meet the needs of individual patients Summary of responses to consultation document MLX299 January
Proposals for the reform of the regulation of unlicensed herbal remedies in the United Kingdom made up to meet the needs of individual patients Summary of responses to consultation document MLX299 January
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust.
 Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
A Guide to pain relief medicines For patients receiving Palliative Care
 A Guide to pain relief medicines For patients receiving Palliative Care 1 Which pain medicines are you taking? Contents Page No. Amitriptyline 8 Codeine 9 Co-codamol 10 Co-dydramol 11 Diclofenac (Voltarol
A Guide to pain relief medicines For patients receiving Palliative Care 1 Which pain medicines are you taking? Contents Page No. Amitriptyline 8 Codeine 9 Co-codamol 10 Co-dydramol 11 Diclofenac (Voltarol
Pharmacist, Mr C A Pharmacy Company. A Report by the Health and Disability Commissioner. (Case 04HDC10718)
 Pharmacist, Mr C A Pharmacy Company A Report by the Health and Disability Commissioner (Case 04HDC10718) Opinion/04HDC10718 Parties involved Mrs A Ms B Mr C A Pharmacy Company Dr D Dr E Ms F Consumer
Pharmacist, Mr C A Pharmacy Company A Report by the Health and Disability Commissioner (Case 04HDC10718) Opinion/04HDC10718 Parties involved Mrs A Ms B Mr C A Pharmacy Company Dr D Dr E Ms F Consumer
TRANSPARENCY C OMMITTEE
 The legally binding text is the original French version TRANSPARENCY C OMMITTEE Opinion 20 November 2013 VOLTAFLEX 625 mg, film-coated tablet B/60 tablets (CIP: 34009 384 573 2) Applicant: NOVARTIS SANTÉ
The legally binding text is the original French version TRANSPARENCY C OMMITTEE Opinion 20 November 2013 VOLTAFLEX 625 mg, film-coated tablet B/60 tablets (CIP: 34009 384 573 2) Applicant: NOVARTIS SANTÉ
