During photosynthesis, plants use light energy (sunlight) from the sun, carbon dioxide (CO 2. ) and water (H 2
|
|
|
- Gordon Higgins
- 7 years ago
- Views:
Transcription
1 Scientist Guide The Spice of Life Introduction Plants make the oxygen we breathe and the food we consume. They do this through a process known as photosynthesis. Photosynthesis is the single most important chemical process on the planet because all living creatures depend on it to survive. Without plants, photosynthesis would not occur and life on Earth would not exist. During photosynthesis, plants use light energy (sunlight) from the sun, carbon dioxide (CO 2 ) and water (H 2 O) to produce oxygen (O 2 ) and energy in the form of glucose (C 6 H 12 O 6 ). As we breathe, we inhale oxygen that is produced by the plants and exhale carbon dioxide that the plants use to make more oxygen. Plants convert the glucose that they produce into energy that they need to grow. When we eat plants or plant-derived food, we also use the glucose as energy. In fact, every living creature on the planet uses the glucose produced by plants. Photosynthesis is a chemical process that involves compounds made up of elements found in the periodic table. With a little knowledge about chemistry, the elements and how they interact, you will be able to build the compounds involved in photosynthesis. Chemistry is the study of the composition, properties and behavior of matter. Matter is anything that has mass and takes up space. The smallest known unit of matter is the atom. E Atoms consist of three particles: protons, neutrons and electrons. Protons contain a positive electrical charge and are located in the nucleus of atoms. Neutrons are also located in the nucleus and are electrically neutral, meaning they have no electrical charge. Electrons have a negative electrical charge and move around the nucleus in what is called the atom s electron cloud, a spherical cloud of varying density surrounding the nucleus that is often depicted as shells or orbits around the nucleus (Figure 1). Electron Proton Neutron E P N N N P P Nucleus Figure 1. E An element is matter made of only one kind of atom. Each element is different and has unique properties. So far, 118 elements have been identified; 98 of them occur naturally. These elements make up gases in the air, minerals in rocks and liquids such as water. Examples of naturally occurring elements include oxygen and nitrogen in the air you breathe, and metals such as iron, silver and gold. Elements fall into three categories: metals, metalloids and nonmetals. The elements in each category have similar properties.
2 To help organize and display the elements, chemists have created a chart called the periodic table of elements. The periodic table is neatly arranged in rows (left to right) and columns (top to bottom). The rows are called periods. Elements in a period have the same number of electron shells. The columns are called groups, and elements in each group have the same number of valence electrons or electrons in the outermost electron shell. The periodic table also contains letters and numbers that describe each element. Each element of the periodic table is abbreviated with one or two letters known as the element s symbol. For example, the symbol for oxygen is O and the symbol for sodium is Na. Numbers for each element represent the element s atomic number and atomic mass. The number above the symbol is the atomic number which Figure 2. describes how many protons are in the nucleus of each atom of that element. The number found below the element symbol is the atomic mass, the weighted average mass of an element (Figure 2). This number can vary because of isotopes or atoms of the same element that contain different numbers of neutrons. Elements are capable of combining to form a compound. A compound is a substance whose smallest unit is made up of atoms of more than one element bonded together. Compounds are expressed as chemical formulas; the formula tells you which elements make up a compound as well as how many atoms (indicated by a subscript number written below and to the right of each element s symbol) of each element exist in one unit of that compound. For example, the chemical formula for water is H 2 O, and it is made up of two atoms of hydrogen (H 2 ) and one atom of oxygen (O). Activity Overview In this activity, you will learn about the periodic table and use spice drops to construct the lifesustaining molecules involved in photosynthesis and a variety of other molecules. Materials Spice drops (different colors) Toothpicks Safety Precautions Do not eat or drink in the laboratory. Please use caution when handling the toothpicks as their ends are sharp.
3 Procedure To assist you in building your molecules, please fill out the information below. Element Symbol Color Number of Bonds Hydrogen H 1 Nitrogen Sodium Orange 1 Carbon Chlorine Cl Oxygen Red 2 Using the information in the table above, construct the following molecules (can you identify the molecules involved in photosynthesis?): Gases: O 2 (Oxygen) N 2 (Nitrogen) CO (Carbon monoxide) CO 2 (Carbon dioxide) Liquids: H 2 O (Water) H 2 O 2 (Hydrogen peroxide) C 2 H 6 O (Ethanol) CH 3 COOH (Acetic acid, vinegar) Solids: NaCl (Table salt) NaHCO 3 (Baking soda) C 6 H 10 O 5 (Wheat flour) C 6 H 12 O 6 (Glucose) Try-on-your-own Activities Using the information you have learned in this activity, try constructing more challenging molecules such as: C 8 H 10 N 4 O 2 (Caffeine) C 12 H 22 O 11 (Table sugar) To learn more about the periodic table and its elements, try some of these online resources at home:
4 Student Worksheet Name 1. What is the smallest known unit of matter? 2. Atoms are made up of what three particles? 3. A substance that is made up of only one specific type of atom is called an. 4. A group of two or more elements bonded together is called a. 5. What three pieces of information for each element are contained in one square of the periodic table?
5 Teacher Resource The Spice of Life Follow-up Name 1. What is an atom? 2. What is the electrical charge for a proton, neutron and electron? 3. What is an element? 4. A compound is made up of how many elements? 5. What does the atomic number describe for an element?
6 Careers in Agriculture
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck. atoms- the smallest particle of an element that can be identified with that element
 Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Name Block Date Ch 17 Atomic Nature of Matter Notes Mrs. Peck atoms- the smallest particle of an element that can be identified with that element are the building blocks of matter consists of protons and
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
7-5.5. Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including:
 7-5.5 Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including: NaCl [salt], H 2 O [water], C 6 H 12 O 6 [simple sugar], O 2 [oxygen
7-5.5 Translate chemical symbols and the chemical formulas of common substances to show the component parts of the substances including: NaCl [salt], H 2 O [water], C 6 H 12 O 6 [simple sugar], O 2 [oxygen
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Atoms and Molecules. Preparation. Objectives. Standards. Materials. Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4
 Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
Atoms and Molecules Preparation Grade Level: 5-8 Group Size: 20-30 Time: 60 90 Minutes Presenters: 2-4 Objectives This lesson will enable students to: Describe how atoms are the building blocks of matter
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
******* KEY ******* Atomic Structure & Periodic Table Test Study Guide
 Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
Atomic Structure & Periodic Table Test Study Guide VOCABULARY: Write a brief definition of each term in the space provided. 1. Atoms: smallest unit of an element that has all of the properties of that
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
19.1 Bonding and Molecules
 Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Most of the matter around you and inside of you is in the form of compounds. For example, your body is about 80 percent water. You learned in the last unit that water, H 2 O, is made up of hydrogen and
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Chapter Outline. 3 Elements and Compounds. Elements and Atoms. Elements. Elements. Elements 9/4/2013
 3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
3 Elements and Compounds Chapter Outline 3.1 Elements A. Distribution of Elements Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright This reclining Buddha in Thailand is
About the course GENERAL CHEMISTRY. Recommended literature: Chemistry: science of the matter. Responsible for the course: Dr.
 About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
About the course GENERAL CHEMISTRY University of Pécs Medical School Academic year 2009-2010. Responsible for the course: Dr. Attila AGÓCS Optional course for 2 credit points. To have grade at the and
7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
 7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
Elements, Atoms & Ions
 Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Introductory Chemistry: A Foundation FOURTH EDITION by Steven S. Zumdahl University of Illinois Elements, Atoms & Ions Chapter 4 1 2 Elements Aims: To learn about the relative abundances of the elements,
Directions: T. Trimpe 2005 http://sciencespot.net/
 Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
CHEMICAL FORMULAS AND EQUATIONS
 reflect Imagine that you and three other classmates had enough supplies and the recipe to make one pepperoni pizza. The recipe might include a ball of dough, a cup of pizza sauce, a cup of cheese, and
reflect Imagine that you and three other classmates had enough supplies and the recipe to make one pepperoni pizza. The recipe might include a ball of dough, a cup of pizza sauce, a cup of cheese, and
Element of same atomic number, but different atomic mass o Example: Hydrogen
 Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document.
 Name: Period: Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document. 1. Which of the following is a NOT a physical property of hydrogen? A. It is gas C. It is
Name: Period: Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document. 1. Which of the following is a NOT a physical property of hydrogen? A. It is gas C. It is
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
ATOMS. Multiple Choice Questions
 Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Chemical Calculations: The Mole Concept and Chemical Formulas. AW Atomic weight (mass of the atom of an element) was determined by relative weights.
 1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
1 Introduction to Chemistry Atomic Weights (Definitions) Chemical Calculations: The Mole Concept and Chemical Formulas AW Atomic weight (mass of the atom of an element) was determined by relative weights.
20.2 Chemical Equations
 All of the chemical changes you observed in the last Investigation were the result of chemical reactions. A chemical reaction involves a rearrangement of atoms in one or more reactants to form one or more
All of the chemical changes you observed in the last Investigation were the result of chemical reactions. A chemical reaction involves a rearrangement of atoms in one or more reactants to form one or more
Trends of the Periodic Table Diary
 Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Trends of the Periodic Table Diary Trends are patterns of behaviors that atoms on the periodic table of elements follow. Trends hold true most of the time, but there are exceptions, or blips, where the
Mole Notes.notebook. October 29, 2014
 1 2 How do chemists count atoms/formula units/molecules? How do we go from the atomic scale to the scale of everyday measurements (macroscopic scale)? The gateway is the mole! But before we get to the
1 2 How do chemists count atoms/formula units/molecules? How do we go from the atomic scale to the scale of everyday measurements (macroscopic scale)? The gateway is the mole! But before we get to the
Chapter 2 Atoms and Molecules
 Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Chapter 2 Atoms and Molecules 2-1 Elements and their symbols Most of the chemicals you find in everyday life can be broken down into simper substances Key Concepts: A substance that cannot be broken down
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Chemistry Worksheet: Matter #1
 Chemistry Worksheet: Matter #1 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up
Chemistry Worksheet: Matter #1 1. A mixture (is/is not) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements that make up
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
neutrons are present?
 AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
AP Chem Summer Assignment Worksheet #1 Atomic Structure 1. a) For the ion 39 K +, state how many electrons, how many protons, and how many 19 neutrons are present? b) Which of these particles has the smallest
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Unit 2: Quantities in Chemistry
 Mass, Moles, & Molar Mass Relative quantities of isotopes in a natural occurring element (%) E.g. Carbon has 2 isotopes C-12 and C-13. Of Carbon s two isotopes, there is 98.9% C-12 and 11.1% C-13. Find
Mass, Moles, & Molar Mass Relative quantities of isotopes in a natural occurring element (%) E.g. Carbon has 2 isotopes C-12 and C-13. Of Carbon s two isotopes, there is 98.9% C-12 and 11.1% C-13. Find
KS3 Science: Chemistry Contents
 summary KS3 Science MyWorks Guide Chemistry KS3 Science: Chemistry Mini zes: 40 Super zes: 5 Extension zes: 4 Skills zes: 6 TOTAL 54 What are MyWorks zes? MyWorks zes are short individual learning tasks
summary KS3 Science MyWorks Guide Chemistry KS3 Science: Chemistry Mini zes: 40 Super zes: 5 Extension zes: 4 Skills zes: 6 TOTAL 54 What are MyWorks zes? MyWorks zes are short individual learning tasks
FIRST GRADE CHEMISTRY
 FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS
 3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining
Chapter 1: Moles and equations. Learning outcomes. you should be able to:
 Chapter 1: Moles and equations 1 Learning outcomes you should be able to: define and use the terms: relative atomic mass, isotopic mass and formula mass based on the 12 C scale perform calculations, including
Chapter 1: Moles and equations 1 Learning outcomes you should be able to: define and use the terms: relative atomic mass, isotopic mass and formula mass based on the 12 C scale perform calculations, including
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Study Guide For Chapter 7
 Name: Class: Date: ID: A Study Guide For Chapter 7 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The number of atoms in a mole of any pure substance
Name: Class: Date: ID: A Study Guide For Chapter 7 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The number of atoms in a mole of any pure substance
Unit 3 Study Guide: Electron Configuration & The Periodic Table
 Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Name: Teacher s Name: Class: Block: Date: Unit 3 Study Guide: Electron Configuration & The Periodic Table 1. For each of the following elements, state whether the element is radioactive, synthetic or both.
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Chemistry Diagnostic Questions
 Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
Unit 9 Compounds Molecules
 Unit 9 Compounds Molecules INTRODUCTION Compounds are the results of combinations of elements. These new substances have unique properties compared to the elements that make them up. Compounds are by far
Unit 9 Compounds Molecules INTRODUCTION Compounds are the results of combinations of elements. These new substances have unique properties compared to the elements that make them up. Compounds are by far
PERIODIC TABLE. reflect
 reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
reflect Suppose you wanted to organize your locker at school. How could you separate and arrange everything in an organized way? You could place the books, notebooks, and folders on a shelf that is separate
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance
 Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
Lewis Dot Notation Ionic Bonds Covalent Bonds Polar Covalent Bonds Lewis Dot Notation Revisited Resonance Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an
What s in a Mole? Molar Mass
 LESSON 10 What s in a Mole? Molar Mass OVERVIEW Key Ideas Lesson Type Lab: Groups of 4 Chemists compare moles of substances rather than masses because moles are a way of counting atoms. When considering
LESSON 10 What s in a Mole? Molar Mass OVERVIEW Key Ideas Lesson Type Lab: Groups of 4 Chemists compare moles of substances rather than masses because moles are a way of counting atoms. When considering
Matter. Atomic weight, Molecular weight and Mole
 Matter Atomic weight, Molecular weight and Mole Atomic Mass Unit Chemists of the nineteenth century realized that, in order to measure the mass of an atomic particle, it was useless to use the standard
Matter Atomic weight, Molecular weight and Mole Atomic Mass Unit Chemists of the nineteenth century realized that, in order to measure the mass of an atomic particle, it was useless to use the standard
Periodic Table. 1. In the modern Periodic Table, the elements are arranged in order of increasing. A. atomic number B. mass number
 Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Name: ate: 1. In the modern, the elements are arranged in order of increasing. atomic number. mass number. oxidation number. valence number 5. s the elements in Group I are considered in order of increasing
Name: Teacher: Pd. Date:
 Name: Teacher: Pd. Date: STAAR Tutorial : Energy and Matter: Elements, Compounds, and Chemical Equations: 6.5C Differentiate between elements and compounds on the most basic level. 8.5F Recognize whether
Name: Teacher: Pd. Date: STAAR Tutorial : Energy and Matter: Elements, Compounds, and Chemical Equations: 6.5C Differentiate between elements and compounds on the most basic level. 8.5F Recognize whether
Exam 2 Chemistry 65 Summer 2015. Score:
 Name: Exam 2 Chemistry 65 Summer 2015 Score: Instructions: Clearly circle the one best answer 1. Valence electrons are electrons located A) in the outermost energy level of an atom. B) in the nucleus of
Name: Exam 2 Chemistry 65 Summer 2015 Score: Instructions: Clearly circle the one best answer 1. Valence electrons are electrons located A) in the outermost energy level of an atom. B) in the nucleus of
Atoms, Elements, and the Periodic Table (Chapter 2)
 Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
The Mole Concept and Atoms
 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 4 24 September 2013 Calculations and the Chemical Equation The Mole Concept and Atoms Atoms are exceedingly
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 4 24 September 2013 Calculations and the Chemical Equation The Mole Concept and Atoms Atoms are exceedingly
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
Science 20. Unit A: Chemical Change. Assignment Booklet A1
 Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Science 20 Unit A: Chemical Change Assignment Booklet A FOR TEACHER S USE ONLY Summary Teacher s Comments Chapter Assignment Total Possible Marks 79 Your Mark Science 20 Unit A: Chemical Change Assignment
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Bonding Practice Problems
 NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
The Empirical Formula of a Compound
 The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More
 KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More The Modern Periodic Table The Periodic Law - when elements are arranged according
KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More The Modern Periodic Table The Periodic Law - when elements are arranged according
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Chemical Calculations: Formula Masses, Moles, and Chemical Equations
 Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
Chemical Calculations: Formula Masses, Moles, and Chemical Equations Atomic Mass & Formula Mass Recall from Chapter Three that the average mass of an atom of a given element can be found on the periodic
Chemistry Post-Enrolment Worksheet
 Name: Chemistry Post-Enrolment Worksheet The purpose of this worksheet is to get you to recap some of the fundamental concepts that you studied at GCSE and introduce some of the concepts that will be part
Name: Chemistry Post-Enrolment Worksheet The purpose of this worksheet is to get you to recap some of the fundamental concepts that you studied at GCSE and introduce some of the concepts that will be part
Chapter 2 Atoms, Molecules, and Ions
 Chapter 2 Atoms, Molecules, and Ions 1. Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ration of 3:1 by mass. In ethane,
Chapter 2 Atoms, Molecules, and Ions 1. Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ration of 3:1 by mass. In ethane,
APS Science Curriculum Unit Planner
 APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
APS Science Curriculum Unit Planner Grade Level/Subject Chemistry Stage 1: Desired Results Enduring Understanding Topic 1: Elements and the Periodic Table: The placement of elements on the periodic table
Chemistry 65 Chapter 6 THE MOLE CONCEPT
 THE MOLE CONCEPT Chemists find it more convenient to use mass relationships in the laboratory, while chemical reactions depend on the number of atoms present. In order to relate the mass and number of
THE MOLE CONCEPT Chemists find it more convenient to use mass relationships in the laboratory, while chemical reactions depend on the number of atoms present. In order to relate the mass and number of
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Section 1: Arranging the Elements Pages 106-112
 Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test
 Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test NAME Section 7.1 The Mole: A Measurement of Matter A. What is a mole? 1. Chemistry is a quantitative science. What does this term mean?
Unit 3 Notepack Chapter 7 Chemical Quantities Qualifier for Test NAME Section 7.1 The Mole: A Measurement of Matter A. What is a mole? 1. Chemistry is a quantitative science. What does this term mean?
Chapter Test. Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a.
 Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
Assessment Chapter Test A Teacher Notes and Answers 5 The Periodic Law TEST A 1. b 2. d 3. b 4. b 5. d 6. a 7. b 8. b 9. b 10. a 11. c 12. a 13. c 14. d 15. c 16. b 17. d 18. a 19. d 20. c 21. d 22. a
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
U3-LM2B-WS Molar Mass and Conversions
 U3-LM2B-WS Molar Mass and Conversions Name: KEY 1. The molar mass of chlorine is: 2 x 35.45 g/mol Cl = 70.90 g/mol Cl 2 (Remember that chlorine exists as a diatomic molecule in nature) 2. The molar mass
U3-LM2B-WS Molar Mass and Conversions Name: KEY 1. The molar mass of chlorine is: 2 x 35.45 g/mol Cl = 70.90 g/mol Cl 2 (Remember that chlorine exists as a diatomic molecule in nature) 2. The molar mass
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
Bohr s Model of the Atom
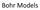 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
(1) e.g. H hydrogen that has lost 1 electron c. anion - negatively charged atoms that gain electrons 16-2. (1) e.g. HCO 3 bicarbonate anion
 GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
Chapter NP-1. Nuclear Physics. Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES
 Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Chapter NP-1 Nuclear Physics Atomic Nature of Matter TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 PROPERTIES OF SUBSTANCES 1.1 CHEMICAL AND PHYSICAL PROPERTIES 2.0 COMPOSITION OF ATOMS 2.1 ATOMIC STRUCTURE
Balancing Chemical Equations Worksheet
 Balancing Chemical Equations Worksheet Student Instructions 1. Identify the reactants and products and write a word equation. 2. Write the correct chemical formula for each of the reactants and the products.
Balancing Chemical Equations Worksheet Student Instructions 1. Identify the reactants and products and write a word equation. 2. Write the correct chemical formula for each of the reactants and the products.
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* CHEMISTRY 0620/03 Paper 3 Theory (Core) For Examination from 2016 SPECIMEN PAPER 1 hour
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* CHEMISTRY 0620/03 Paper 3 Theory (Core) For Examination from 2016 SPECIMEN PAPER 1 hour
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Composition of nucleus. Priority Vocabulary: Electron, Proton, Neutron, Nucleus, Isotopes, Atomic Number, Atomic Mass, Element, Electron Shell,
 Lake County, Lakeview, 9 th grade, Physical Science, Brent Starr Standard: H1P1 Explain how atomic structure is related to the properties of elements and their position in the Periodic Table. Explain how
Lake County, Lakeview, 9 th grade, Physical Science, Brent Starr Standard: H1P1 Explain how atomic structure is related to the properties of elements and their position in the Periodic Table. Explain how
19.2 Chemical Formulas
 In the previous section, you learned how and why atoms form chemical bonds with one another. You also know that atoms combine in certain ratios with other atoms. These ratios determine the chemical formula
In the previous section, you learned how and why atoms form chemical bonds with one another. You also know that atoms combine in certain ratios with other atoms. These ratios determine the chemical formula
AS1 MOLES. oxygen molecules have the formula O 2 the relative mass will be 2 x 16 = 32 so the molar mass will be 32g mol -1
 Moles 1 MOLES The mole the standard unit of amount of a substance the number of particles in a mole is known as Avogadro s constant (L) Avogadro s constant has a value of 6.023 x 10 23 mol -1. Example
Moles 1 MOLES The mole the standard unit of amount of a substance the number of particles in a mole is known as Avogadro s constant (L) Avogadro s constant has a value of 6.023 x 10 23 mol -1. Example
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name Date Class CHAPTER 1 REVIEW. Answer the following questions in the space provided.
 CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
