Sugar Transport and Fructose Metabolism in Human Intestine In Vitro
|
|
|
- Lucy Henderson
- 2 years ago
- Views:
Transcription
1 Sugar Transport and Fructose Metabolism in Human Intestine In Vitro Lawrence W. White, Bernard R. Landau J lin Invest. 1965;44(7): Research Article Find the latest version:
2 Journal of linical Investigation Vol. 44, No. 7, 1965 Sugar Transport and Fructose Metabolism in Human Intestine In Vitro * LAWRENE W. WHITE AND BERNARD R. LANDAU t (From the Departments of Medicine and Biochemistry, Western Reserve University, leveland, Ohio) Present knowledge regarding sugar transport by human intestine has been derived primarily by extrapolation of data from other species. Previous investigations, performed chiefly with everted sacs of hamster intestine, show that a sugar requires a specific structure before it can be transported by intestine against a concentration gradient, a process referred to as "active transport" (1, 2). Thus, in the hamster, the necessary requirements for a monosaccharide to be actively transported are a pyranose ring, a D-configuration, a hydroxyl group in the glucose configuration at carbon 2, and a methyl or substituted methyl group at carbon 5. Sugars that lack one of these features, or possess a large substituent on some part of their structure, are not actively transported. Fructose, lacking these requirements, is therefore not transported against a gradient. Nonetheless, fructose disappears from the rat intestinal lumen at a rate intermediate between that for sugars such as glucose or galactose, which are actively transported, and sugars such as mannose or pentoses, which are not (3). Since passive diffusion of a sugar through the intestinal wall is dependent upon the difference between its intracellular and extracellular concentration (2), the enhanced rate of fructose disappearance as compared to mannose or pentoses may be accounted *Submitted for publication January 8, 1965; accepted March 18, Supported by grants from the National Institute of Arthritis and Metabolic Diseases (AM-06810), the American Heart Association, and the Lee Fund of Western Reserve University for the Study of Diabetes and Related Diseases. t Address requests for reprints to Dr. Bernard R. Landau, Dept. of Medicine, Western Reserve University School of Medicine, leveland, Ohio This study was performed during the tenure of an Established Investigatorship of the American Heart Association. for by its partial conversion to another substance, such as lactate (4) or glucose (4-8). onversion of fructose to glucose by the intestine was first suggested by Bollman and Mann (5) and since then has been demonstrated in the guinea pig (4, 6), hamster (7), rat (4), and dog (8). onversion of fructose to glucose by human intestine has been assumed, but never established. Miller, raig, Drucker, and Woodward (9), in a patient with cirrhosis of the liver given fructose orally, observed a greater glucose concentration in portal anastomotic vein blood than femoral arterial blood, by 5 mg per 100 ml, suggesting a conversion of fructose to glucose. Their observation that oral fructose administration produced effects other than those of parenteral fructose supported the possibility of intestinal conversion, but different rates and sites of initial presentation could explain this finding. The mechanism of conversion of fructose to glucose in animals has been examined. The possible pathways are outlined in Figure 1. Fructose can either be converted to glucose via initial phosphorylation to fructose-l-phosphate, catalyzed by fructokinase, or to fructose-6-phosphate, catalyzed by hexokinase. Kjerulf-Jensen (10) isolated fructose-l-phosphate from guinea pig intestine and suggested that it was an intermediate in fructose absorption. Subsequent reactions in the metabolism of fructose-i-phosphate are cleavage to dihydroxyacetone phosphate and glyceraldehyde, isomerization of the dihydroxyacetone phosphate to glyceraldehyde phosphate, phosphorylation of the glyceraldehyde, condensation of triose phosphates to form fructose-6-phosphate via fructose-1,6-diphosphate, isomerization to glucose-6-phosphate, and hydrolysis to free glucose by the action of glucose-6-phosphatase. Figure 1 traces the pathway followed by carbon 1 of fructose-i-'4. If metabolism is via fructose-i-phos- 1200
3 TRANSPORT AND FRUTOSE IN INTESTINE lglyogen 1201 Q GLUOSE hexokinase 0 -_ -, -_ - P-fructo FRUTOSE kinase fructokinase VQ P / FRUTOSE- 1,6- DIP aldolaseir =O -P triose-p- -P DIHYDROXY- isomerase GLYERALDE- P 0 AETONE-P HYDE-3-P FRUTOSE-I-P aldolase O GLYERALDEHYDE PYRUVATE. 02, ET. FIG. 1. ONVERSION OF FRUTOSE TO GLUOSE. The labeling pattern of intermediates is for the metabolism of fructose-l-' via fructose-l-phosphate, where a is traced by boldface type. phate, glucose-1,6-14 is formed. Via fructose-6- phosphate, glucose-i-"4 would be expected. Ginsberg and Hers (11) and Salomon and Johnson (12) provided good evidence for conversion via fructose-l-phosphate by isolating glucose-1,6- "' after incubation of intestine from guinea pig with fructose-i-"4 or fructose-6-"4. To form glucose from fructose, an essential reaction is the hydrolysis of glucose-6-phosphate to glucose. The failure to demonstrate glucose-6- phosphatase in rat intestine presumably accounts for the inability of rat intestine to convert fructose to glucose (11), although a recent abstract reports the presence of glucose-6-phosphatase activity in rat mucosa, accompanied by an endogenous inhibitor preventing its action (13). This contrasts with the demonstration in the guinea pig of a preferential hydrolysis of glucose-6-phosphate as compared with glucose-l-phosphate, consistent with the conversion of fructose to glucose (11). In a single study on human intestine, no glucose- 6-phosphatase activity was found (11). However, other groups have recently demonstrated glucose-6-phosphatase activity in human intestine (14, 15). In the present communication, in vitro techniques have been applied to the study of sugar transport and fructose metabolism in human intestine. Methods Source of tissue. Studies were performed on human intestine removed at the time of surgery. The source of tissue and the pathological diagnoses are outlined in Table I. Areas adjacent to the resection site, grossly free of pathological processes, were used. The mucosa was quickly dissected from the other layers, cut into strips, weighed, and incubated with appropriate solutions as outlined below. The time from surgical removal to the beginning of incubation was 15 to 30 minutes, during which period the tissue was kept cold. Histological examination of unincubated tissues verified the adequacy of the dissections. Mucosa was normal in all tissues except that in one area of the sample of tissue no. 4, a small ulcer was seen. Sugar specificity. To determine the structural requirements for active transport, the ability of a tissue to accumulate monosaccharides of different structures
4 1202 LAWRENE W. WHITE AND BERNARD R. LANDAU TABLE I Source of intestinal mucosa Site of tissue Tissues linical features excised Surgery employed Pathological diagnosis 1 Abdominal High Resection Lymphosarcoma pain, rectal jejunum of jejunum with focal ulcerbleeding, mass ation and acute inflammation of small intestine 2 Diarrhea, Terminal Total colec- Acute ulcerweight loss ileum tomy and ative enteroileostomy colitis 3 Rectal bleeding High Resection Leiomyoma of jejunum of jejunum small intestine 4 Diarrhea, Terminal Resection of Regional weight loss, ileum terminal ileum enteritis debility and ascending colon 5 Melena, insidi- Middle Resection of Adenomatous ous development jejunum jejunum polyp of jejunum of anemia 6 Abdominal pain, High Resection of Fibromyoma rectal bleeding, jejunum jejunum of jejunum mass 7 Abdominal pain, Duodenum Resection of Malignant obstructive duodenal carcinoid jaundice tumor tumor 8 Abdominal pain, Terminal Ileocolectomy Regional enteritis intractable ileum diarrhea against a concentration gradient was examined (16). Segments of mucosa weighing 0.8 to 1.2 g were added to flasks containing one of several monosaccharides in 3 ml of Krebs-Ringer bicarbonate buffer modified to a 1.3 mm a++ concentration. The sugars were a-methyl-dglucose-u-", prepared as previously described (17), D-xylose-1-", and D-galactose-2-"; 1 D-fructose-U-"; 2 D-mannose-U-'; 8 3-O-methyl-"-D-glucose; 4 and 6- deoxy-d-glucose, prepared as previously described (18). Incubation was performed at 370 with shaking for 30 minutes under an atmosphere of 95% 02 and 5% 02. After incubation, the segments were removed from each flask, blotted, and homogenized in 4 ml of cold water. Samples of initial and final media and tissue homogenate were deproteinized with Ba(OH)2-ZnSO4 (19), and the supernatants were used for determination of " activity where the sugar was labeled and in some cases for measurement of sugar content. Tissue water was determined by the difference in tissue weight before and after 1 Purchased from the National Bureau of Standards, Washington, D.. 2 New England Nuclear orp., Boston, Mass. 8 Schwarz Laboratories, Mount Vernon, N. Y. 4 Isotope Specialties o., Burbank, alif. drying in vacuo at 85 for 24 hours. Samples were plated on aluminum planchets and assayed in a thin window gas flow proportional counter. The results are expressed as counts per minute per milliliter initial or final medium, or counts per minute per milliliter of tissue water. Fructose was determined by the method of Roe, Epstein, and Goldstein (20), 6-deoxyglucose by the method of Dische and Shettles (21), and galactose using "galactostat." 5 After incubation with a-methylglucose, 3-O-methylglucose, and xylose, samples of initial medium and deproteinized tissue homogenate were chromatographed on paper in a butanol: pyridine: water (6: 4: 3) system (22). To localize the " activity, the paper was cut into small segments, which were placed in scintillation vials containing 0.5 ml of water to which was added 10 ml of a solution of 125 g naphthalene, 7.5 g PPO (2,5-diphenyloxazole) and g dimethyl POPOP [1,4-bis-2- (4-methyl-5-phenyloxazolyl) benzene] per liter of p-dioxane (23), and counted in a liquid scintillation spectrometer.6 A single ' peak, corresponding to the peak in the initial medium, was found for the samples from the tissue homogenate, indicating that intracellu- 5Worthington Biochemical orp., Freehold, N. J. 6 Nuclear hicago orp., hicago, Ill.
5 TRANSPORT AND FRUTOSE IN INTESTINE 1203 lar accumulation of a-methylglucose, 3-0-methylglucose, and xylose occurred without hydrolysis or other structural alteration. Pathways and quantitation of fructose conversion to glucose. Segments of mucosa, weighing 0.6 to 2 g were incubated in 5 ml of the Krebs-Ringer bicarbonate buffer, containing either 10 mg or 15 mg of unlabeled glucose and either 1 mg or 3.5 mg of fructose-l-' or fructose-6-'. In one experiment, no glucose was present. Incubation lasted 20 minutes to 75 minutes and was performed at 370 with shaking under 95% 02 and 5% 02. Variations in tissue weight and incubation time depended on the amount of available tissue, but were kept constant for each experiment. Glucose and fructose uptakes, i.e., the difference between sugar content in initial and final media, were determined by incubating samples of media with and without glucose oxidase, followed by determination of reducing content by the Somogyi-Nelson procedure (19). The difference between total reducing content and nonglucose reducing content was the measure of true glucose. Fructose content was considered to equal the nonglucose reducing content. Fructose was also determined by the method of Roe and co-workers (20), and the two methods were in agreement. Disappearance of fructose-" was determined by making the fructosazones from samples of initial and final medium after treatment with glucose oxidase. These were plated on stainless steel planchets and assayed in a thin, end-window, gas-flow, proportional counter. At the completion of incubation, tissue glycogen was isolated, purified, and hydrolyzed to glucose. arrier glucose was added. A sample was combusted to 02 for assay for ". This allowed an estimate of the recovery of " in the subsequent degradation as well as a measure of the total incorporation of " into glycogen. The remainder of the glucose was degraded using Leuconostoc mesenteroides as previously described (24, 25). This procedure yields carbon 1 as 02, carbons 2,3 as acetate via ethyl alcohol, and carbons 4,5,6 as lactate; the acetate and lactate were combusted to O2. Where indicated, all six carbons were isolated individually as O2 (24, 25). To examine the glucose in the medium, deproteinization with Ba(OH)a-ZnSO4 was performed (19); the supernatant was purified by passage through a mixed bed column of Amberlite IR in the H+ form and Duolite A-4 8 in the OH- form, and the eluate was lyophilized. To remove radioactive fructose from the solution, two consecutive procedures were employed. The glucose and fructose were initially separated by descending paper chromatography on Whatman 3 MM paper using an n-butanol: acetic acid: water solvent (4: 1: 5 by volume) with fructose and glucose as guide spots (26). The spots were visualized by means of a periodate-permanganate spray. Good separation was obtained, and the area 7 Rohm and Haas o., Philadelphia, Pa. 8 hemical Process o., Redwood ity, alif. paralleling the glucose guide spots was eluted with water. The glucose was then oxidized to gluconic acid by incubation with 1 mg of glucose oxidase (type III, 40,000 U per g) 9 in 1 M phosphate buffer ph 5.6 under O. with shaking at 37 for 2i hours. After incubation, the solution was deproteinized with Ba(OH)2- ZnSO,, potassium gluconate was added as carrier, and the gluconate was separated from any residual fructose by passage through Dowex-1 10 in the l- form. This retained the gluconate, but not the fructose, when the column was thoroughly washed with water. Gluconate was eluted in fractions with 0.05 N Hl. Presence of gluconate in the various fractions was determined by oxidizing samples with periodic acid and testing for the presence of formaldehyde colorimetrically with chromotropic acid reagent (27). Fractions that contained gluconate were combined. A sample was combusted to O2 to determine the adequacy of recovery of " in the subsequent degradation, as well as to measure the total incorporation of " into glucose. Gluconate was degraded by oxidation with periodate (28) to yield carbon 1 as O, carbons 2,3,4,5 as formic acid, and carbon 6 as formaldehyde. Formaldehyde was separated from formic acid by distillation and was oxidized to 02 with potassium permanganate (29). Formic acid was steam distilled and oxidized to O2 with mercuric acetate (30). O2 obtained from the individual carbons of glucose and glycogen was evolved into ethanolamine and methyl cellosolve (1: 2 by volume), which was dissolved in scintillation fluid consisting of toluene, methyl cellosolve, and ethanol (110: 88:13 by volume), containing PPO, and the samples were counted in the liquid scintillation spectrometer (31). The preparation of fructose-6-" has been described previously (32). Fructose-l-" 11 was treated with glucose oxidase and isolated after paper chromatography to assure removal of any glucose contaminant. To confirm the position of label, osazones of fructose-l-" and 6-" were prepared, and their radioactivity was compared to that of the bis-phenylhydrazones of mesoxalaldehyde prepared by the method of Topper and Hastings (29). With fructose-i-", over 90% of the activity was recovered in the phenylhydrazone, containing carbons 1,2,3 of glucose. With fructose-6-", only 0.1% of the activity was in this fraction. Incorporation of " of D-glyceraldehyde-3-" into glucose. Further information was obtained regarding the fate of D-glyceraldehyde in intestinal mucosa by incubating tissue with 1.6 mg of D-glyceraldehyde-3-' in the presence of 10 or 15 mg of unlabeled glucose. The procedure was identical to that outlined above for fructose. The D-glyceraldehyde-3-" was prepared as previously described (32). Pentose cycle estimation. The contribution of the pentose cycle to over-all glucose metabolism was estimated by the method of Katz and Wood (33, 34). Incubation of 9 Sigma hemical o., St. Louis, Mo. 0 Dow hemical o., Midland, Mich. 11 Purchased from Tracerlab, Inc., Boston, Mass.
6 LAWRENE W. WHITE AND BERNARD R. LANDAU ~~0~~~) cd c 00(-~~~~~~0 * IOW O t > 0 0) 000'.0%00t- ~~~0 0~ 0 0) - z 00000z0 0 I= in I 0) ~~~~~~~ )~~~~~~~ 0% -14 t~~~- 0% ~~~~~~~1I)4 oo r6 Th UIf) ~~~~~~~%0~~~~'f) ~~~~~ -e..ioo ~ ~ ~~(Ud 0~ ' ~~~~~~~~~~~~~~~~~~4- -a '-4 i ; 4-' 0V 0 ~~~ ~~~-) ~~~~~-0 q0 ~~~~~~~ 0* 1~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ tl-00 0\0\0) - 0 Ob '-4 ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ - 0~~~~~~~~~)0 S t - - \.0 *4 8.~~~~~~~~~~~~~. 40j. 4. cd 0%'~~~004-~~~of)'0 e~~~o ci- -~~~~ 0%~~~~44( ) co14-4 0U4o c) oc.40 0 cd~~~~~~~~~~~~~~~~~~c 1e w 9b (a
7 TRANSPORT AND FRUTOSE IN INTESTINE 1205 TABLE III Distribution of 14 in the glucose unit of glycogen from human intestinal mucosa incubated with fructose-i-14 and 6-14 Specific activity* Substrate Tissue -1-2,3 4,5,6-6t Recoveryl Fructose-i-14 I ** Fructose-6-'4 I * * Relative specific activity in -1, -2,3, or 4,5,6 with the sum of activities of ,3 + -4,5,6 set equal to 100. t Specific activity in carbon 6 was obtained by calculation. There is very little 14 in carbons 2 and 3, and based on previous experience, carbons 4 and 5 should also contain minimal activity. Since carbons 1,2,3 and 4,5,6 remain intact in the formation of hexose from triose phosphate, 14 activity in carbon 1 to carbon 6 should be proportional to the 14 activity in carbons 1,2,3 to that in carbons 4,5,6 so that -6 = [-1 (-4,5,6)]/[ ,3]. To verify the reliability of this calculation, lactate, representing -4,5,6 of glycogen in tissue No. 3, with fructose-6-14 as substrate, was degraded and each carbon was assayed individually. Relative activities in carbons 4,5, and 6 were 1.6, 0.8, and $Recovery represents the sum of the activities of ,2 + -4,5,6 obtained by degradation expressed as a per cent of the total activity in the intact glucose molecule as determined by combustion. Tissue No. 1: 2 g mucosa incubated 25 minutes with 10 mg nonlabeled glucose and 1 mg 14-fructose. Tissue No. 2: 0.6 g mucosa incubated 75 minutes with 10 mg nonlabeled glucose and 1 mg 14-fructose. Tissue No. 3: 1.1 g mucosa incubated 20 minutes with 15 mg nonlabeled glucose and 3.5 mg 14-fructose. $* Tissue No. 6: 0.9 g mucosa incubated 20Qminutes with 15 mg "4-fructose in the absence of glucose. three tissues, weighing 0.89 g, 1.0 g, and 0.35 g, respectively, with 30 mg of glucose-2-",' was performed for 45 minutes, and the degree of randomization of 1' in glucose from glycogen was measured. The larger quantity of glucose was used in this experiment to approach steady state conditions throughout the incubation. Over 60%o of the glucose remained at the end of incubation. Enzyme studies. Fructokinase was assayed by the method of Ginsberg and Hers (11). A homogenate of intestinal mucosa was incubated with fructose in the presence and absence of glucose. The disappearance of fructose in the presence of ATP, as measured by the method of Roe and associates (20) on a Ba(OH)2- ZnSO4 filtrate, was taken as evidence of phosphorylation. To determine whether the phosphorylation product was fructose-l-phosphate or fructose-6-phosphate, the fructose phosphate was isolated as a barium salt and tested for lability, i.e., the liberation of fructose by 1 N Hl at To measure the liberation, samples were neutralized, the remaining fructose phosphate was precipitated by Ba(OH)2 and ZnSO4 addition, and free fructose was determined. Glucose-6-phosphatase activity was determined by measuring for 60 minutes over a ph range the hydrolysis of glucose-6-phosphate as compared to glucose-lphosphate by a microsomal fraction of intestinal mucosa (11). Parallel studies were performed on rat, hamster, and human intestinal mucosa. A control experiment, performed in the absence of mucosal homogenate, demonstrated no hydrolysis of glucose-6-phosphate or glucose-iphosphate at the various ph values. Results Sugar specificity. The studies of structural specificity with respect to accumulation of monosaccharides against a gradient are outlined in Table II. As measured by 14, recovery was essentially complete; with colorimetric determinations, however, recovery was more variable. Tissue concentrations following incubation of jejunal mucosa with galactose and nonmetabolizable glucose derivatives, a-methylglucose, 3-0-methylglucose and 6-deoxyglucose, were markedly higher than the initial medium concentration. At the completion of incubation, a definite concentration gradient for these sugars existed between medium and tissue (as indicated in Table II by the T/M ratios). No definite "' concentration gradient was produced with xylose and mannose. An accumulation of "4 was observed in tissue with fructose as with galactose; calorimetrically, however, fructose was shown not to be concentrated, and the "' accumulation is presumably attributable to metabolic products. Incubation with ileum (tissue no. 4) differed in that only galactose and a-methylglucose were concentrated, and their gradients were less than that seen in the two experiments with jejunum. With ileum (tissue no.
8 1206 LAWRENE W. WHITE AND BERNARD R. LANDAU TABLE IV Distribution of 14 in medium glucose after incubation with fructose-1-'4 and 6-14* Specific activity Substrate Tissue -1-2,3,4,5-6 Recoveryt Fructose--" Fructose * Experiments of Table III. t Recovery represents the sum of the activities of all six carbons obtained by degradation expressed as a per cent of the total activity in the intact glucose molecule as determined by combustion. 8) definite concentration gradients were found as with jejunum, but except for a-methylglucose the gradients were less. Incorporation of 14 of fructose-1-14 and 6-14 into glycogen and glucose. The experiments with fructose-i-14 and 6-14 outlined in Tables III and IV demonstrate the occurrence of marked randomization of 14 between carbons 1 and 6 in the glucose unit derived from tissue glycogen, as well as the glucose formed in the medium. In most tissues, incubation with fructose-i-14 resulted in higher 14 activity in carbon 6 than 1. This can be accounted for by metabolism via the transaldolase reactions that are discussed subsequently. The similar pattern in tissue glycogen and medium glucose is consistent with their derivation from a single pool of glucose-6-phosphate. Incorporation of 14 of D-glyceraldehyde-3-"4 into glucose. Most of the 14 of D-glyceraldehyde (Table V) appeared in carbons 1 and 6 of the glucose unit of glycogen. Much more activity was in carbon 6 than 1. TABLE V Distribution of " in glucose unit of glycogen after incubation with glyceraldehyde-3-"4 Specific activity* Tissue Recovery 2t $ * Relative specific activity in individual carbons with the sum of activities in all six carbons set equal to 100. t Tissue No g mucosa incubated 75 minutes with 10 mg glucose and 1.6 mg glyceraldehyde-3-'4. I Sum of activities in Sum of activities in Tissue No g mucosa incubated 20 minutes with 15 mg glucose and 1.6 mg glyceraldehyde-3-"4. Pentose cycle. 14 of glucose-2-"4 was randomized into carbons 1 and 3 of the glucose from glycogen (Table VI). The ratios of the specific activities of the top 3 carbons in a hexose-6-phosphate derivative (in this case glycogen) may be used to calculate the %o of over-all metabolism occurring via the pentose cycle (34). The data suggest a pentose cycle contribution of 5 to 10%o. A small percentage of the 14 activity was found in carbons 4, 5, and 6. Enzyme studies. The results of one assay for fructokinase are recorded in Table VII. Disappearance of fructose to the extent of 58%o only occurred in the presence of ATP, presumptive evidence for phosphorylation. The presence of glucose did not inhibit the disappearance. The product of the reaction was identified as fructose-i-phosphate by testing its rate of hydrolysis in acid (Figure 2). Eighty per cent of the fructose in the phosphorylated product was liberated in 40 minutes. Under these conditions fructose-1- TABLE VI Distribution of 14 in glucose unit of glycogen after incubation with glucose-2-"4 Specific activity* Pentose Tissue -i S -6 Recovery cyclet 3t * Relative specific activity in individual carbons with the activity of carbon 2 set equal to 100. t alculations of pentose cycle are based on the ratio of 14 in carbons 1:2 and carbons 3:2, as described by Katz and Wood (34). This assumes complete equilibration of hexose-6-phosphates, and is supported by the similar ratio in carbons 1:2 compared to 6:5 with tissue No. 3 (33). With tissue No. 7, however, the ratio of 14 in carbons 6:5 is higher than in carbons 1:2, suggesting that fructose-6-phosphate and glucose-6-phosphate are not completely equilibrated. alculation based on nonequilibration gives a contribution of 15% (33). t 0.89 g mucosa (tissue No. 3), 1.0 g mucosa (tissue No. 6), or 0.35 g mucosa (tissue No. 7) incubated 45 minutes with 30 mg glucose-2-"4. j
9 TRANSPORT AND FRUTOSE IN INTESTINE 1207 TABLE VII Fructokinase assay in human intestinal mucosa* Glucose Fructose present ATP disappearance pm % * Fructokinase was assayed by the method of Ginsberg and Hers (11). The reaction mixture contained 2.2 Mmoles fructose, 8 umoles Mg12, 900 pmoles K1, 20 Emoles cacodylate buffer ph 7.0, 100 &moles KF, 1 ml 20% homogenate of intestinal mucosa in water (tissue No. 3), and 8 jumoles ATP when present, with a final volume of 2 ml. Incubation was for 40 minutes, followed by deproteinization and measurement of fructose remaining in the proteinfree filtrate F no MAN R AT // // / /S _Wo0 // / G-6-P / G-I-P o----o / I phosphate is very labile as compared to fructose- 6-phosphate (35). In an assay of a second tissue, 45% of the fructose disappeared in the presence of ATP; the disappearance again was E A _~~~~~0 HAMSTER _ O.- ~ ~ ~ ~~~ 75 _ 0.5 _ 0- /- n 50 - UL Time (min) FIG. 2. LABILITY OF FRUTOSE PHOSPHATE FORMED BY HOMOGENATES FROM HUMAN INTESTINAL MUOSA. Fructose phosphate was prepared as outlined in Table VII, deproteinized with perchloric acid, filtered, neutralized with KOH, and refiltered. Phosphate esters were precipitated by adding 25% Bal2 and 2 vol of ethanol. The precipitate was washed with 60% ethanol, dissolved in 1 N H1, and placed in a water bath at 1000 (11). Samples were removed at the intervals shown, neutralized, treated with Ba(OH)2 and ZnSO4, and free fructose was determined in the supernatant by the method of Roe and associates (20). Total fructose was determined on a sample not treated with Ba(OH)2 and ZnSO. Free fructose is plotted as a per cent of the total fructose. 0 r 56 FIG. 3. GLUOSE-6-PHOSPHATASE ATIVITY IN A) MAN, B) RAT, AND ) HAMSTER INTESTINAL MUOSA. Mucosas were homogenized in cold 025 M sucrose. A microsomal fraction was prepared by centrifuging the homogenates for 5 minutes at 25,000 rpm and then centrifuging the supernatants at 38,000 rpm for 30 minutes (11). Assay was essentially as described by Ginsberg and Hers (11) with activity determined by the release of free phosphorus from glucose-6-phosphate compared to glucose-l-phosphate. unaffected by the presence of glucose. Acid hydrolysis was 75% at 40 minutes. A microsomal fraction from human jejunal mucosa hydrolyzed glucose-6-phosphate at a rate considerably in excess of that of glucose-l-phosphate in the ph range of 5.0 to 7.6 (Figure 3A). At _ ph 8
10 1208 LAWRENE W. WHITE AND BERNARD R. LANDAU ph 6.0, which is the ph optimum for liver microsomes (11), 4.1 umoles of glucose-6-phosphate, as compared with 0.2 /hmoles of glucose-i-phosphate, was hydrolyzed per g of mucosa per 60 minutes. In a second jejunal tissue, 2.7 jmoles of glucose-6-phosphate, compared with 0.4 fnmole of glucose-i-phosphate, was hydrolyzed per g of mucosa. Parallel assays of rat and hamster intestinal mucosa, presented in Figure 3B and 3, confirmed the findings (11) that microsomes from rat intestinal mucosa hydrolyze glucose-6-phosphate and glucose-i-phosphate at the same rate, whereas microsomes from hamster intestinal mucosa hydrolyze glucose-6-phosphate more rapidly than glucose-i-phosphate in the range of ph 5 to 7. Discussion Sugar specificity. The mechanism of active transport of sugars by intestinal mucosa is unknown. Historical concepts regarding the transport mechanism, such as phosphorylation-dephosphorylation, or other chemical modifications, have not succeeded in accounting for the known phenomena. The current consensus favors adsorption to an unidentified membrane carrier on the luminal border of the cell membrane, and this interpretation is supported by kinetic data (1, 2). Movement across a layer of cells such as the intestinal epithelium (transcellular transport) is closely related to movement into and out of cells (transmembranous transport) (36). Thus, in the hamster (16), accumulation within cells accompanies transcellular transport against a gradient. Any tenable hypothesis regarding the mechanism of this transport must account for certain recently established facts. First, experiments with a variety of natural and synthetic sugars have shown that active transport has molecular specificity, i.e., the transported molecule has certain minimal structural requirements (1, 2, 17). Secondly, studies on sugars known to be actively transported have demonstrated mutual inhibition, indicating competition for a common pathway (18). These features are analogous to the specificity and competitive inhibition of enzyme action by different substrates and suggest that sugars which are actively transported share a common carrier (1, 2). The specificity of sugar transport by human intestinal mucosa (Table II) parallels data for hamster intestine (1, 2, 17). Pentoses, or sugars lacking a hydroxyl group of the glucose configuration in position 2, are not concentrated against a gradient. However, there is recent evidence that D-xylose will move against a gradient, if a counterflow with glucose is induced in the opposite direction (37), suggesting the facilitation of diffusion by mediation of a carrier shared with glucose. More recently, saky and Lassen (38), by using a very small initial concentration of D-xylose, demonstrated uphill transport with a higher flux from lumen to serosa than the reverse. In keeping with data reported in other species (18, 39), 6-deoxyglucose is concentrated against a gradient by human intestinal mucosa, suggesting that in man, as well as in other species, active transport does not involve phosphorylation of glucose in the 6 position. Pathways of fructose conversion to glucose. As noted in the introduction (see Figure 1), the conversion of fructose to glucose can occur by two major pathways. The first involves phosphorylation of fructose in the 6 position, with subsequent isomerization to glucose-6-phosphate, and hydrolysis to free glucose. When tested with specifically labeled fructose, conversion to glucose by this pathway should not result in randomization of the label.12 The second is via fructose-1- phosphate, cleavage of the ester to triose phosphates, and condensation to fructose-1,6-diphosphate. By this pathway, 14 would be randomized during conversion to glucose, as a result of isotopic equilibration of triose phosphates. 12 Isolation of glucose-1,6-", after incubation with either fructose-l-" or fructose-6-", but not glucose-i- ' or glucose-6-", indicates metabolism via fructose-iphosphate rather than fructose-6-phosphate (40). A major assumption required for this conclusion is that the phosphohexose isomerase catalyzed reaction is relatively rapid. If the conversion of fructose-6-phosphate to dihydroxyacetone phosphate and glyceraldehyde phosphate, their equilibration, and reconversion to fructose-6- phosphate were rapid relative to the action of phosphohexose isomerase, fructose-6-p-1,6-" would be formed after the phosphorylation in the 6 position of fructose-i- " or fructose-6-". This is considered unlikely in intestine because of a) the relatively high activity of phosphohexose isomerase in tissues in which it has been assayed (41) and b) the large percentage of fructose-iphosphate in the barium precipitate demonstrable after incubation with fructose.
11 TRANSPORT AND FRUTOSE IN INTESTINE Isolation of glucose-1,6-" (Tables III and IV), after incubation with fructose-i-"4 or fructose-6-"4, indicates that the second pathway exists in human intestinal mucosa. The degree of randomization between carbon 1 and carbon 6, similar to results reported for guinea pig intestine (11), indicates that triose phosphate isomerase is active. The possibility that the first pathway also operates to some extent cannot be excluded by this study, but its presence would not be necessary to account for the data. The demonstration that fructose-l-phosphate is at least the major fraction of the fructose phosphate formed (Figure 2) supports the conclusion that the major proportion of fructose metabolism is via phosphorylation to fructose-l-phosphate. Sols has shown that fructose is phosphorylated to fructose-6-phosphate by hexokinase at a high rate in rat intestinal mucosa, but that this reaction is strongly inhibited in the presence of glucose (42). It might be expected that fructose would preferentially be phosphorylated via hexokinase in human intestine in the absence of glucose, whereas in the presence of glucose (for which hexokinase has a higher affinity), fructose would preferentially follow the pathway via fructokinase. However, the labeling pattern obtained with fructose-i-"4 in the one experiment performed in the absence of glucose (Table III, tissue no. 6) was similar to that observed in the presence of glucose. This suggests either that hexokinase of the human intestine does not phosphorylate fructose preferentially in the absence of glucose to an extent sufficient to alter the pattern, or that endogenous glucose present in the tissue was sufficient to compete successfully with fructose for the hexokinase. Attempts have been made to quantitate the conversion of fructose to glucose in man and in other species. In their patient, Miller and associates (9) estimated a conversion of fructose to glucose of 18%o during transfer through the cell wall. Kiyasu and haikoff (4) collected mesenteric vein blood from jejunal loops of guinea pig intestine, after administering labeled fructose, and found a 70%o conversion to glucose. However, in the rat, only about 10o% of the absorbed fructose was recovered as glucose, possibly due to the absence of glucose-6-phosphatase. Recently, Shoemaker, Yanof, Turk, and Wilson (8) found, 1209 in the triply cannulated dog fed fructose, that 42%o of the fructose was absorbed unchanged, whereas 58%o was converted to glucose. For tissues no. 2 and no. 6, 14 to 16%o of the 14 of the fructose utilized was incorporated into glucose present in the final medium and 2 to 10%o was incorporated into tissue glycogen. Since glucose is utilized continuously during the incubation, this represents a minimal value. In tissue no. 2, with only 0.2 mg per ml fructose present in the medium, 0.57 mg per g tissue was taken up in 75 minutes. In tissue no. 6, with 1 mg per ml fructose present, disappearance was 1.12 mg per g in 20 minutes. Although a significant per cent of the fructose carbon utilized can be converted to glucose carbon, the equating of incorporation with net synthesis is not possible (43). As will be shown subsequently, there is good evidence that transaldolase reactions are partially responsible for the incorporation observed in these experiments. ontribution of the pentose cycle. The presence of the pentose cycle has been established in hamster intestine by the greater 1402 production from glucose-i-'4 than glucose-6-l" (44). The present report (Table VI) gives a quantitative measure of the presence of the pentose cycle in human intestinal mucosa. Participation of the transaldolase reactions. Introduction via the Embden-Meyerhof pathway of 14 from fructose-i-"4 into carbon 6 of glucose necessitates a cleavage to triose phosphates, isotopic equilibration, and condensation to form glucose-6-phosphate. If there is failure to achieve isotopic equilibration of the triose phosphates, there will be unequal labeling of the top and bottom halves of the glucose molecule with greater activity in carbon 1 than carbon 6. As the rate of isomerization of triose phosphates becomes more rapid, the 14 activity in carbon 1 and carbon 6 will approach each other, but the activity of carbon 6 cannot be greater than that of carbon 1. Therefore, in three experiments with fructose-i-"4 (Table III), the finding that 55 to 70%o of the 14 was in the bottom half of the molecule requires another mechanism. In the nonoxidative portion of the pentose cycle, a series of group transfers, catalyzed by transaldolase and transketolase, results in the conversion of three molecules of pentose-5-phosphate to two
12 1210 LAWRENE W. WHITE AND BERNARD R. LANDAU molecules of fructose-6-phosphate and one mole- is the transfer of a dihydroxyacetone moiety from cule of glyceraldehyde-3-phosphate. One of the sedoheptulose-7-phosphate to glyceraldehyde-3- intermediate reactions catalyzed by transaldolase phosphate to form fructose-6-phosphate: ==O -P Sedoheptulose-7- phosphate =O + Transaldolase - 14-P I Glyceraldehyde-3- phosphate =O 14-P Fructose-6- phosphate ==o + -P Erythrose-4- p osphate If fructose-i-phosphate-i-"4 is converted to triose phosphates, which then undergo isotopic equilibration, the glyceraldehyde-3-phosphate-3-l4 will equilibrate with the glyceraldehyde-3-phosphate pool derived from pentose cycle intermediates (provided there is a single pool of glyceraldehyde- 3-phosphate). The glyceraldehyde-3-phosphate can then be incorporated into fructose-6- phosphate, with 14 exclusively in the 6 position. The transaldolase reaction occurs in intestinal mucosa, since it is a part of the pentose cycle, and the randomization of glucose-2-"4 indicates that the cycle is present in the intestine. By this mechanism, 14 is incorporated only into carbon 6, regardless of whether the initial substrate is fructose-1-0" or fructose-6-l". onsequently the finding of a significant quantity of activity in position 1 after incubation with fructose-6-"4 indicates that the condensation of triose phosphates via the Embden-Meyerhof pathway must also occur. An additional mechanism for the incorporation of 14 from fructose-i-"4 into carbon 6 of glucose is by means of a transaldolase exchange reaction, between labeled glyceraldehyde-3-phosphate from fructose-i-phosphate-i_-14, and unlabeled and carbon i-labeled fructose-6-phosphate (45): ==O + 14-eP Glyceraldehyde-3-P =O Transaldolase ==O -' + -P -P Fructose-6-P Glyceraldehyde-3-P c=o Fructose-6-P The unlabeled fructose-6-p participating in the reaction could arise from sources such as glycogen present in the tissue at the beginning of incubation and the fructose-6-p-i-"4 from triose phosphates via aldolase condensation and possibly by phosphorylation of fructose-i-"4 via hexokinase. The unlabeled glyceraldehyde-3-phosphate formed would be expected to enter the glyceraldehyde-3- phosphate pool and be equilibrated at least partially with dihydroxyacetone-p, as evidenced by the formation of glucose-1,6-14 after incubation with fructose-6-"4. Although the transaldolase exchange reaction results in the incorporation of label into position 6 of glucose, it does not result in a net synthesis of glucose. Incorporation can also occur after reversal of the reactions of the pentose cycle involving transaldolase and transketolase (46). onversion of D-glyceraldehyde-3-"4 to glucose. The initial metabolism of D-glyceraldehyde, when presented to the cell, may differ from that of D-glyceraldehyde derived from fructose by cell metabolism (32). D-Glyceraldehyde may enter the Embden-Meyerhof scheme by formation of D-glyceraldehyde phosphate. If tested with D-glyceraldehyde-3-14, this would give rise to hexose- 1,6-4, via condensation of triose phosphates, and hexose-6-" via the transaldolase reactions. D-Glyceraldehyde may also be reduced to glycerol with subsequent conversion to dihydroxyacetone phosphate to yield hexose-3,4-14. In human intestinal mucosa, the finding of 83.8%o of the 14
13 in carbons 1 and 6 (Table V) indicates that metabolism is primarily by conversion to D-glyceraldehyde-3-phosphate. The much greater incorporation of 14 into carbon 6 than carbon 1 indicates either hexose formation by condensation of triose phosphates with incomplete isotopic equilibration or a contribution by transaldolase-transketolase reactions. Enzyme studies. The conversion of fructose to glucose via fructose-l-phosphate requires the presence of fructokinase and glucose-6-phosphatase, known to have a limited tissue distribution, as well as the more ubiquitous enzymes of the Embden-Meyerhof pathway. The action of fructokinase has been differentiated from that of hexokinase by the effect of glucose on the uptake of fructose (47). The demonstration of a phosphorylated product having the hydrolysis properties of fructose-l-phosphate, and the failure of glucose to inhibit its formation, as well as the labeling pattern found in glycogen, indicate the action of fructokinase. Glucose-6-phosphatase is required as the final step in the conversion of fructose to glucose, and the absence of this enzyme (11) presumably accounts for the failure of rat intestine to convert fructose to glucose (Figure 3B). Ginsberg and Hers (11) compared the phosphatase activity of microsomes from the intestines of several species with respect to hydrolysis of glucose-6-phosphate and glucose-l-phosphate. In guinea pigs and rabbits, a peak of glucose-6-phosphatase activity occurs at ph 6.0. They found, and we have confirmed, that no peak occurs for hamster intestine at ph 6.0 (Figure 3). However, hydrolysis of glucose-6-phosphate proceeds 3 to 6 times more rapidly than that of glucose-l-phosphate in the ph range of 5.0 to 7.0. In the case of human intestinal mucosa, Ginsberg and Hers state that no evidence for a glucose-6-phosphatase peak at ph 6.0 could be obtained, and they question the conversion of fructose to glucose by human intestinal mucosa on this basis. Although our findings corroborate the absence of a peak at ph 6.0, they also show that hydrolysis of glucose-6-phosphate occurs at 6 to 20 times the rate of glucosei-phosphate at ph 6.0, similar to the findings in the hamster, and indicating the presence of glucose-6-phosphatase activity in human intestinal mucosa. Ockerman found that fractionated hu- TRANSPORT AND FRUTOSE IN INTESTINE man intestinal mucosa was able to hydrolyze glucose-6-phosphate at ph 6.0, but the specificity of the hydrolysis as compared to other phosphates was not established (14). Field, Epstein, and Egan recently demonstrated glucose-6-phosphatase activity in human intestine biopsy samples at ph 6.5 after correction for nonspecific phosphatases as measured with glycerol phosphate (15). Summary Sugar transport and fructose metabolism have been studied in specimens of human intestinal mucosa removed at surgery, and the following has been found: 1) Transport of sugars in man conforms to structural requirements in the hamster; some sugars are transported actively as shown by intracellular accumulation, whereas other sugars, such as fructose, are not. 2) Fructose is converted to glucose as measured by incorporation of 14 from fructose--"4 and fructose-6-"4 into glucose and glycogen. 3) Initial metabolism of fructose is primarily via fructose-l-phosphate, as evidenced by the labeling pattern obtained with fructose-l-"4 and fructose-6-4 and by the hydrolysis characteristic of the phosphate formed from fructose. 4) Glucose-6-phosphatase activity is present, as evidenced by the preferential hydrolysis of glucose-6-phosphate over glucose-lphosphate at acid ph.- 5) The pentose cycle is active in human intestine, as measured by randomization of glucose-2-"4; the transaldolase catalyzed reactions, which are associated with the cycle, play a part in the incorporation of 14 from fructose-"4 into glucose. 6) Glyceraldehyde is initially metabolized via glyceraldehyde-3-phosphate, as determined by the labeling pattern obtained with glyceraldehyde-3-"4. Acknowledgments 121 We were fortunate to have the expert technical assistance of Mr. Hollis R. Williams. We thank the members of the Department of Surgery, Western Reserve University, for assistance in obtaining the samples of intestine. References 1. rane, R. K. Intestinal absorption of sugars. Physiol. Rev. 1960, 40, Wilson, T. H. Intestinal Absorption. Philadelphia, W. B. Saunders, 1962, p. 69.
14 1212 LAWRENE W. WHITE AND BERNARD R. LANDAU 3. ori,. F. The fate of sugar in the animal body. I. The rate of absorption of hexoses and pentoses from the intestinal tract. J. biol. hem. 1925, 66, Kiyasu, J. Y., and I. L. haikoff. On the manner of transport of absorbed fructose. J. biol. hem. 1957, 224, Bollman, J. L., and F.. Mann. The physiology of the liver. XIX. The utilization of fructose following complete removal of the liver. Amer. J. Physiol. 1963, 96, Darlington, W. A., and J. H. Quastel. Absorption of sugars from isolated surviving intestine. Arch. Biochem. 1953, 43, Wilson, T. H., and T. N. Vincent. Absorption of sugars in vitro by the intestine of the golden hamster. J. biol. hem. 1955, 216, Shoemaker, W.., H. M. Yanof, L. N. Turk, and T. H. Wilson. Glucose and fructose absorption in the unanesthetized dog. Gastroenterology 1963, 44, Miller, M., J. W. raig, W. R. Drucker, and H. Woodward, Jr. The metabolism of fructose in man. Yale J. Biol. Med. 1956, 29, Kjerulf-Jensen, K. The hexosemonophosphoric acids formed within the intestinal mucosa during absorption of fructose, glucose and galactose. Acta physiol. scand. 1942, 4, Ginsberg, V., and H. G. Hers. On the conversion of fructose to glucose by guinea pig intestine. Biochim. biophys. Acta (Amst.) 1960, 38, Salomon, L. L., and J. E. Johnson. Transfer of fructose and glucose across surviving guinea pig intestine. Arch. Biochem. 1959, 82, James, J., and L. L. Salomon. Intestinal glucose-6- phosphatase. Fed. Proc. 1964, 23, Ockerman, P. A. Glucose-6-phosphatase in human jejunal mucosa. Lack of activity in glycogenesis of ori's type I. lin. chim. Acta 1964, 9, Field, J. B., S. M. Epstein, and T. Egan. Intestinal glucose-6-phosphatase in patients with von Gierke's disease and their relatives (abstract). Program of the Forty-sixth Meeting of the Endocrine Society, June 1964, p rane, R. K., and P. Mandelstam. The active transport of sugars by various preparations of hamster intestine. Biochim. biophys. Acta (Amst.) 1960, 45, Landau, B. R., L. Bernstein, and T. H. Wilson. Hexose transport by hamster intestine in vitro. Amer. J. Physiol. 1962, 203, Jorgensen,. R., B. R. Landau, and T. H. Wilson. A common pathway for sugar transport in hamster intestine. Amer. J. Physiol. 1961, 200, Somogyi, M. Notes on sugar determination. J. biol. hem. 1952, 195, Roe, J. H., J. H. Epstein, and N. P. Goldstein. A photometric method for the determination of inulin in plasma and urine. J. biol. hem. 1949, 178, Dische, Z., and L. B. Shettles. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. biol. hem. 1948, 175, Jeanes, A.,. S. Wise, and R. J. Dimler. Improved techniques in paper chromatography of carbohydrates. Analyt. hem. 1951, 23, Werbin, H., I. L. haikoff, and M. R. Imada. Rapid sensitive method for determining H8-water in body fluids by liquid scintillation spectrometry. Proc. Soc. exp. Biol. (N. Y.) 1959, 102, Merlevede, W., G. Weaver, and B. R. Landau. Effects of thyrotropic hormone on carbohydrate metabolism in thyroid slices. J. clin. Invest. 1963, 42, Sakami, W., Ed. Degradation of glycogen in Handbook of Isotope Tracer Methods. leveland, Western Reserve University, 1955, p Partridge, S. M. Filter-paper partition chromatography of sugars. I. General description and application to the qualitative analysis of sugars in apple juice, egg white, and foetal blood of sheep. Biochem. J., 1948, 42, Burton, R. M. The determination of glycerol and dihydroxyacetone in Methods in Enzymology, S. P. olowick and N. 0. Kaplan, Eds. New York, Academic Press, 1957, vol. 3, p Eisenberg, F., Jr. Degradation of isotopicallylabeled glucose via periodate oxidation of gluconate. J. Amer. chem. Soc. 1954, 76, Topper, Y. J., and A. B. Hastings. A study of the chemical origins of glycogen by use of '4-labeled carbon dioxide, acetate, and pyruvate. J. biol. hem. 1949, 179, Pirie, N. W. The manometric determination of formic acid. Biochem. J. 1946, 40, Kornblatt, J. A., P. Bernath, and J. Katz. The determination of specific activity of Ba'403 by liquid scintillation assay. Int. J. appl. Radiat. 1964, 15, Landau, B. R., and W. Merlevede. Initial reactions in the metabolism of D- and L-glyceraldehyde by rat liver. J. biol. hem. 1963, 238, Landau, B. R., G. E. Bartsch, J. Katz, and H. G. Wood. Estimation of pathway contributions to glucose metabolism and of the rate of isomerization of hexose-6-phosphate. J. biol. hem. 1964, 239, Katz, J., and H. G. Wood. The use of glucose-' for the evaluation of the pathways of glucose metabolism. J. biol. hem. 1960, 235, ori, G. T., S. Ochoa, M. W. Slein, and. F. ori. The metabolism of fructose in liver. Isolation of fructose-l-phosphate and inorganic pyrophosphate. Biochim. biophys. Acta (Amst.) 1951, 7, Wilbrandt, W., and T. Rosenberg. The concept of
The diagram below summarizes the effects of the compounds that cells use to regulate their own metabolism.
 Regulation of carbohydrate metabolism Intracellular metabolic regulators Each of the control point steps in the carbohydrate metabolic pathways in effect regulates itself by responding to molecules that
Regulation of carbohydrate metabolism Intracellular metabolic regulators Each of the control point steps in the carbohydrate metabolic pathways in effect regulates itself by responding to molecules that
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Chapter 14 Glycolysis. Glucose. 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) TCA Cycle
 Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
Creatine Kinase Activity Colorimetric Assay Kit ABE5487 100 assays; Store at -20 C
 Creatine Kinase Activity Colorimetric Assay Kit ABE5487 100 assays; Store at -20 C I. Introduction: Creatine Kinase (CK) also known as creatine phosphokinase (CPK) and ATP: creatine N- phosphotransferase
Creatine Kinase Activity Colorimetric Assay Kit ABE5487 100 assays; Store at -20 C I. Introduction: Creatine Kinase (CK) also known as creatine phosphokinase (CPK) and ATP: creatine N- phosphotransferase
Copyright 2010 Pearson Education, Inc. Chapter Twenty Three 1
 23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
Chapter 25: Metabolism and Nutrition
 Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND
 #3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
#3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Overview of Glycolysis Under anaerobic conditions, the glycolytic pathway present in most species results in a balanced reaction:
 Glycolysis Glucose is a valuable molecule. It can be used to generate energy (in red blood cells and in brain under normal conditions, glucose is the sole energy source), and it can be used to generate
Glycolysis Glucose is a valuable molecule. It can be used to generate energy (in red blood cells and in brain under normal conditions, glucose is the sole energy source), and it can be used to generate
I. ACID-BASE NEUTRALIZATION, TITRATION
 LABORATORY 3 I. ACID-BASE NEUTRALIZATION, TITRATION Acid-base neutralization is a process in which acid reacts with base to produce water and salt. The driving force of this reaction is formation of a
LABORATORY 3 I. ACID-BASE NEUTRALIZATION, TITRATION Acid-base neutralization is a process in which acid reacts with base to produce water and salt. The driving force of this reaction is formation of a
HiPer Ion Exchange Chromatography Teaching Kit
 HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
Methods of Grading S/N Style of grading Percentage Score 1 Attendance, class work and assignment 10 2 Test 20 3 Examination 70 Total 100
 COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
RayBio Creatine Kinase (CK) Activity Colorimetric Assay Kit
 RayBio Creatine Kinase (CK) Activity Colorimetric Assay Kit User Manual Version 1.0 May 28, 2014 RayBio Creatine Kinase Activity Colorimetric Assay (Cat#: 68CL-CK-S100) RayBiotech, Inc. We Provide You
RayBio Creatine Kinase (CK) Activity Colorimetric Assay Kit User Manual Version 1.0 May 28, 2014 RayBio Creatine Kinase Activity Colorimetric Assay (Cat#: 68CL-CK-S100) RayBiotech, Inc. We Provide You
Quality. Now Certified to ISO 9001:2008
 Quality Now Certified to ISO 90012008 Quality Policy It is Peptides International's goal is to achieve complete customer satisfaction by addressing customer needs and delivering what we promise. The company
Quality Now Certified to ISO 90012008 Quality Policy It is Peptides International's goal is to achieve complete customer satisfaction by addressing customer needs and delivering what we promise. The company
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Marmara Üniversitesi Fen-Edebiyat Fakültesi Kimya Bölümü / Biyokimya Anabilim Dalı PURIFICATION AND CHARACTERIZATION OF PROTEINS
 EXPERIMENT VI PURIFICATION AND CHARACTERIZATION OF PROTEINS I- Protein isolation and dialysis In order to investigate its structure and properties a protein must be obtained in pure form. Since proteins
EXPERIMENT VI PURIFICATION AND CHARACTERIZATION OF PROTEINS I- Protein isolation and dialysis In order to investigate its structure and properties a protein must be obtained in pure form. Since proteins
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
TOTAL PROTEIN FIBRINOGEN
 UNIT: Proteins 16tproteins.wpd Task Determination of Total Protein, Albumin and Globulins Objectives Upon completion of this exercise, the student will be able to: 1. Explain the ratio of albumin and globulin
UNIT: Proteins 16tproteins.wpd Task Determination of Total Protein, Albumin and Globulins Objectives Upon completion of this exercise, the student will be able to: 1. Explain the ratio of albumin and globulin
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
General Properties Protein Nature of Enzymes Folded Shape of Enzymes H-bonds complementary
 Proteins that function as biological catalysts are called enzymes. Enzymes speed up specific metabolic reactions. Low contamination, low temperature and fast metabolism are only possible with enzymes.
Proteins that function as biological catalysts are called enzymes. Enzymes speed up specific metabolic reactions. Low contamination, low temperature and fast metabolism are only possible with enzymes.
experiment5 Understanding and applying the concept of limiting reagents. Learning how to perform a vacuum filtration.
 81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
105 Adopted: 27.07.95
 105 Adopted: 27.07.95 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Adopted by the Council on 27 th July 1995 Water Solubility INTRODUCTION 1. This guideline is a revised version of the original Guideline
105 Adopted: 27.07.95 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Adopted by the Council on 27 th July 1995 Water Solubility INTRODUCTION 1. This guideline is a revised version of the original Guideline
1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain.
 Lipid Metabolism 1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain. 2. How can excess acetyl CoA trapped in the mitochondria, be utilized as a substrate for fatty
Lipid Metabolism 1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain. 2. How can excess acetyl CoA trapped in the mitochondria, be utilized as a substrate for fatty
Experiment 7 (Lab Period 8) Quantitative Determination of Phosphatase Activity
 Experiment 7 (Lab Period 8) Quantitative Determination of Phosphatase Activity Phosphatases are enzymes that catalyze the hydrolysis of organic-phosphate compounds, releasing inorganic phosphate from the
Experiment 7 (Lab Period 8) Quantitative Determination of Phosphatase Activity Phosphatases are enzymes that catalyze the hydrolysis of organic-phosphate compounds, releasing inorganic phosphate from the
TECHNICAL BULLETIN. HIS-Select Nickel Affinity Gel. Catalog Number P6611 Storage Temperature 2 8 C
 HIS-Select Nickel Affinity Gel Catalog Number P6611 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description HIS-Select Nickel Affinity Gel is an immobilized metalion affinity chromatography (IMAC)
HIS-Select Nickel Affinity Gel Catalog Number P6611 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description HIS-Select Nickel Affinity Gel is an immobilized metalion affinity chromatography (IMAC)
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Transmembrane proteins span the bilayer. α-helix transmembrane domain. Multiple transmembrane helices in one polypeptide
 Transmembrane proteins span the bilayer α-helix transmembrane domain Hydrophobic R groups of a.a. interact with fatty acid chains Multiple transmembrane helices in one polypeptide Polar a.a. Hydrophilic
Transmembrane proteins span the bilayer α-helix transmembrane domain Hydrophobic R groups of a.a. interact with fatty acid chains Multiple transmembrane helices in one polypeptide Polar a.a. Hydrophilic
Creatine Kinase Activity Assay Kit (Colorimetric)
 ab155901 Creatine Kinase Activity Assay Kit (Colorimetric) Instructions for Use For the sensitive and accurate measurement of Creatine Kinase activity in various samples. This product is for research use
ab155901 Creatine Kinase Activity Assay Kit (Colorimetric) Instructions for Use For the sensitive and accurate measurement of Creatine Kinase activity in various samples. This product is for research use
Biology for Science Majors
 Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Figure 5. Energy of activation with and without an enzyme.
 Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Creatine Kinase Microplate Assay Kit User Manual
 Creatine Kinase Microplate Assay Kit User Manual Catalog # CAK1045 Detection and Quantification of Creatine Kinase (CK) Activity in Urine, Serum, Plasma, Tissue extracts, Cell lysate, Cell culture media
Creatine Kinase Microplate Assay Kit User Manual Catalog # CAK1045 Detection and Quantification of Creatine Kinase (CK) Activity in Urine, Serum, Plasma, Tissue extracts, Cell lysate, Cell culture media
PRACTICE SET 1. Free energy changes and equilibrium constants
 PRACTICE SET 1 Free energy changes and equilibrium constants 1. Calculate the standard free-energy changes of the following metabolically important enzyme-catalyzed reactions at 25 C and ph 7.0 from the
PRACTICE SET 1 Free energy changes and equilibrium constants 1. Calculate the standard free-energy changes of the following metabolically important enzyme-catalyzed reactions at 25 C and ph 7.0 from the
LECTURE 1 RENAL FUNCTION
 LECTURE 1 RENAL FUNCTION Components of the Urinary System 2 Kidneys 2 Ureters Bladder Urethra Refer to Renal System Vocabulary in your notes Figure 2-1,page10 Kidney Composition Cortex Outer region Contains
LECTURE 1 RENAL FUNCTION Components of the Urinary System 2 Kidneys 2 Ureters Bladder Urethra Refer to Renal System Vocabulary in your notes Figure 2-1,page10 Kidney Composition Cortex Outer region Contains
n-heptane and /i-hexane Enhance in a Dose-Dependent Manner Insulin Binding to Erythrocytes and Its Degradation
 Gen. Physiol. Biophys. (1987). 6, 197 201 197 Short communication n-heptane and /i-hexane Enhance in a Dose-Dependent Manner Insulin Binding to Erythrocytes and Its Degradation Š. ZÓRAD, I. K.L1MEŠ, E.
Gen. Physiol. Biophys. (1987). 6, 197 201 197 Short communication n-heptane and /i-hexane Enhance in a Dose-Dependent Manner Insulin Binding to Erythrocytes and Its Degradation Š. ZÓRAD, I. K.L1MEŠ, E.
PRE-LAB FOR YEAST RESPIRATION AND FERMENTATION
 PRE-LAB FOR YEAST RESPIRATION AND FERMENTATION PURPOSE: To identify the products of yeast cultures grown under aerobic and anaerobic conditions STUDENTS' ENTERING COMPETENCIES: Before doing this lab, students
PRE-LAB FOR YEAST RESPIRATION AND FERMENTATION PURPOSE: To identify the products of yeast cultures grown under aerobic and anaerobic conditions STUDENTS' ENTERING COMPETENCIES: Before doing this lab, students
THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT*
 THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT* BY JAMES M. ORTEN AND HENRY B. DEVLINt (From the Deparkment of Physiological Chemistry, Wayne University College of Medicine,
THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT* BY JAMES M. ORTEN AND HENRY B. DEVLINt (From the Deparkment of Physiological Chemistry, Wayne University College of Medicine,
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Work and Energy in Muscles
 Work and Energy in Muscles Why can't I sprint forever? I'll start this section with that silly question. What lies behind the undisputable observation that we must reduce speed if we want to run longer
Work and Energy in Muscles Why can't I sprint forever? I'll start this section with that silly question. What lies behind the undisputable observation that we must reduce speed if we want to run longer
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Creatine Kinase Assay Kit
 Creatine Kinase Assay Kit Catalog Number KA1665 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
Creatine Kinase Assay Kit Catalog Number KA1665 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because:
 Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
EXPERIMENT # 3 ELECTROLYTES AND NON-ELECTROLYTES
 EXPERIMENT # 3 ELECTROLYTES AND NON-ELECTROLYTES Purpose: 1. To investigate the phenomenon of solution conductance. 2. To distinguish between compounds that form conducting solutions and compounds that
EXPERIMENT # 3 ELECTROLYTES AND NON-ELECTROLYTES Purpose: 1. To investigate the phenomenon of solution conductance. 2. To distinguish between compounds that form conducting solutions and compounds that
Chapter 14 Solutions
 Chapter 14 Solutions 1 14.1 General properties of solutions solution a system in which one or more substances are homogeneously mixed or dissolved in another substance two components in a solution: solute
Chapter 14 Solutions 1 14.1 General properties of solutions solution a system in which one or more substances are homogeneously mixed or dissolved in another substance two components in a solution: solute
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Saturated NaCl solution rubber tubing (2) Glass adaptor (2) thermometer adaptor heating mantle
 EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif Dehydration of Alcohols - Dehydration of Cyclohexanol Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination
EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif Dehydration of Alcohols - Dehydration of Cyclohexanol Purpose - The purpose of this lab is to produce cyclohexene through the acid catalyzed elimination
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT
 BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
Digestive System Functions
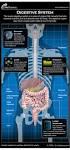 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
ph: Measurement and Uses
 ph: Measurement and Uses One of the most important properties of aqueous solutions is the concentration of hydrogen ion. The concentration of H + (or H 3 O + ) affects the solubility of inorganic and organic
ph: Measurement and Uses One of the most important properties of aqueous solutions is the concentration of hydrogen ion. The concentration of H + (or H 3 O + ) affects the solubility of inorganic and organic
PHOTOSYNTHESIS AND CELLULAR RESPIRATION
 reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
reflect Wind turbines shown in the photo on the right are large structures with blades that move in response to air movement. When the wind blows, the blades rotate. This motion generates energy that is
Name Section Lab 5 Photosynthesis, Respiration and Fermentation
 Name Section Lab 5 Photosynthesis, Respiration and Fermentation Plants are photosynthetic, which means that they produce their own food from atmospheric CO 2 using light energy from the sun. This process
Name Section Lab 5 Photosynthesis, Respiration and Fermentation Plants are photosynthetic, which means that they produce their own food from atmospheric CO 2 using light energy from the sun. This process
Biological Membranes. Impermeable lipid bilayer membrane. Protein Channels and Pores
 Biological Membranes Impermeable lipid bilayer membrane Protein Channels and Pores 1 Biological Membranes Are Barriers for Ions and Large Polar Molecules The Cell. A Molecular Approach. G.M. Cooper, R.E.
Biological Membranes Impermeable lipid bilayer membrane Protein Channels and Pores 1 Biological Membranes Are Barriers for Ions and Large Polar Molecules The Cell. A Molecular Approach. G.M. Cooper, R.E.
ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS. II. Ultrastructure of the Beta Granule
 ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS II Ultrastructure of the Beta Granule MARIE H GREIDER, S L HOWELL, and P E LACY From the Department of Pathology, Washington
ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS II Ultrastructure of the Beta Granule MARIE H GREIDER, S L HOWELL, and P E LACY From the Department of Pathology, Washington
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
TANNIC ACID. SYNONYMS Tannins (food grade), gallotannic acid, INS No. 181 DEFINITION DESCRIPTION
 TANNIC ACID Prepared at the 39th JECFA (1992), published in FNP Add 1 (1992) superseding specifications prepared at the 35th JECFA (1989), published in FNP 49 (1990) and in FNP 52 (1992). Metals and arsenic
TANNIC ACID Prepared at the 39th JECFA (1992), published in FNP Add 1 (1992) superseding specifications prepared at the 35th JECFA (1989), published in FNP 49 (1990) and in FNP 52 (1992). Metals and arsenic
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Separation of Amino Acids by Paper Chromatography
 Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Absorption and Transport of Nutrients
 Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
Page1 Digestion Food travels from mouth esophagus stomach small intestine colon rectum anus. Food mixes with digestive juices, moving it through the digestive tract Large molecules of food are broken into
ENZYME KINETICS ENZYME-SUBSTRATE PRODUCTS
 ENZYME KINETICS INTRODUCTION The study of reaction rates catalyzed by enzymes and the factors affecting them is generally referred to as enzyme kinetics. The basic components of an enzyme catalyzed reaction
ENZYME KINETICS INTRODUCTION The study of reaction rates catalyzed by enzymes and the factors affecting them is generally referred to as enzyme kinetics. The basic components of an enzyme catalyzed reaction
The 3 stages of Glycolysis
 The Glycolytic pathway describes the oxidation of glucose to pyruvate with the generation of ATP and NADH It is also called as the Embden-Meyerhof Pathway is a universal pathway; present in all organisms:
The Glycolytic pathway describes the oxidation of glucose to pyruvate with the generation of ATP and NADH It is also called as the Embden-Meyerhof Pathway is a universal pathway; present in all organisms:
HighPure Maxi Plasmid Kit
 HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
lung cancer targeted photodynamic therapy and imaging
 99m Tc-Hematoporphyrin linked albumin nanoparticles for lung cancer targeted photodynamic therapy and imaging Su-Geun Yang, Ji-Eun Chang, Byungchul Shin, Sanghyun Park, Kun Na and Chang-Koo Shim* *Corresponding
99m Tc-Hematoporphyrin linked albumin nanoparticles for lung cancer targeted photodynamic therapy and imaging Su-Geun Yang, Ji-Eun Chang, Byungchul Shin, Sanghyun Park, Kun Na and Chang-Koo Shim* *Corresponding
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Creatine Kinase (CK) Enzymatic Assay Kit Manual Catalog #: 3460-07
 Creatine Kinase (CK) Enzymatic Assay Kit Manual Catalog #: 3460-07 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3
Creatine Kinase (CK) Enzymatic Assay Kit Manual Catalog #: 3460-07 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3
α-cyclodextrin SYNONYMS α-schardinger dextrin, α-dextrin, cyclohexaamylose, cyclomaltohexaose, α- cycloamylase
 α-cyclodextrin New specifications prepared at the 57th JECFA (2001) and published in FNP 52 Add 9 (2001). An ADI not specified was established at the 57th JECFA (2001). SYNONYMS α-schardinger dextrin,
α-cyclodextrin New specifications prepared at the 57th JECFA (2001) and published in FNP 52 Add 9 (2001). An ADI not specified was established at the 57th JECFA (2001). SYNONYMS α-schardinger dextrin,
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Gastrointestinal Bleeding
 Gastrointestinal Bleeding Introduction Gastrointestinal bleeding is a symptom of many diseases rather than a disease itself. A number of different conditions can cause gastrointestinal bleeding. Some causes
Gastrointestinal Bleeding Introduction Gastrointestinal bleeding is a symptom of many diseases rather than a disease itself. A number of different conditions can cause gastrointestinal bleeding. Some causes
Regulation of enzyme activity
 1 Regulation of enzyme activity Regulation of enzyme activity is important to coordinate the different metabolic processes. It is also important for homeostasis i.e. to maintain the internal environment
1 Regulation of enzyme activity Regulation of enzyme activity is important to coordinate the different metabolic processes. It is also important for homeostasis i.e. to maintain the internal environment
Table of Content. Enzymes and Their Functions Teacher Version 1
 Enzymes and Their Functions Jeisa Pelet, Cornell University Carolyn Wilczynski, Binghamton High School Cornell Learning Initiative in Medicine and Bioengineering (CLIMB) Table of Content Title Page Abstract..
Enzymes and Their Functions Jeisa Pelet, Cornell University Carolyn Wilczynski, Binghamton High School Cornell Learning Initiative in Medicine and Bioengineering (CLIMB) Table of Content Title Page Abstract..
How To Incorporate Leucine Into Protein
 THE INCORPORATION OF LEUCINE-C'4 INTO PROTEIN BY A CELL-FREE PREPARATION FROM MAIZE KERNELS BY ROBERT RABSON AND G. DAVID NOVELLI BIOLOGY DIVISION, OAK RIDGE NATIONAL LABORATORY,* OAK RIDGE, TENNESSEE
THE INCORPORATION OF LEUCINE-C'4 INTO PROTEIN BY A CELL-FREE PREPARATION FROM MAIZE KERNELS BY ROBERT RABSON AND G. DAVID NOVELLI BIOLOGY DIVISION, OAK RIDGE NATIONAL LABORATORY,* OAK RIDGE, TENNESSEE
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Metabolism: Cellular Respiration, Fermentation and Photosynthesis
 Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Enzymes: Amylase Activity in Starch-degrading Soil Isolates
 Enzymes: Amylase Activity in Starch-degrading Soil Isolates Introduction This week you will continue our theme of industrial microbiologist by characterizing the enzyme activity we selected for (starch
Enzymes: Amylase Activity in Starch-degrading Soil Isolates Introduction This week you will continue our theme of industrial microbiologist by characterizing the enzyme activity we selected for (starch
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Factors Affecting Enzyme Activity
 INTRODUCTION Factors Affecting Enzyme Activity The chemical reactions occurring in living things are controlled by enzymes. An enzyme is a protein in the cell which lowers the activation energy of a catalyzed
INTRODUCTION Factors Affecting Enzyme Activity The chemical reactions occurring in living things are controlled by enzymes. An enzyme is a protein in the cell which lowers the activation energy of a catalyzed
BCOR 011 Exam 2, 2004
 BCOR 011 Exam 2, 2004 Name: Section: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. According to the first law of thermodynamics, A. the universe
BCOR 011 Exam 2, 2004 Name: Section: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. According to the first law of thermodynamics, A. the universe
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
serum protein and A/ G ratio
 serum protein and A/ G ratio Blood plasma contains at least 125 individual proteins. Serum ( as contrasted with plasma) is deficient in those coagulation protein which are consumed during the process of
serum protein and A/ G ratio Blood plasma contains at least 125 individual proteins. Serum ( as contrasted with plasma) is deficient in those coagulation protein which are consumed during the process of
ASPARTAME The Study of a Peptide Bond
 ASPARTAME The Study of a Peptide Bond INTRODUCTION Aspartame, commonly known as NutraSweet or Equal, is the most popular artificial, low-calorie sweetener available to consumers today. Chemically, aspartame
ASPARTAME The Study of a Peptide Bond INTRODUCTION Aspartame, commonly known as NutraSweet or Equal, is the most popular artificial, low-calorie sweetener available to consumers today. Chemically, aspartame
Oasis HLB Cartridges and 96-Well Plates
 CONTENTS I. INTRODUCTION II. SAMPLE PRE-TREATMENT a. Biological Samples b. Solid Samples: Soil, Whole Foods, Tissue c. Aqueous Samples: Water, Beverages d. Non-Aqueous Liquid III. SOLID PHASE EXTRACTION
CONTENTS I. INTRODUCTION II. SAMPLE PRE-TREATMENT a. Biological Samples b. Solid Samples: Soil, Whole Foods, Tissue c. Aqueous Samples: Water, Beverages d. Non-Aqueous Liquid III. SOLID PHASE EXTRACTION
Chapter 14- RESPIRATION IN PLANTS
 Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Thermodynamics of glycolysis. Chemistry 433. The role of enzymes. Phosphorylation of glucose. Question. Question
 hemistry 433 Lecture 15 pplications of Free Energy N State University hermodynamics of glycolysis Reaction kj/mol D-glucose P D-glucose-6-phosphate DP Δ o = -16.7 D-glucose-6-phosphate D-fructose-6-phosphate
hemistry 433 Lecture 15 pplications of Free Energy N State University hermodynamics of glycolysis Reaction kj/mol D-glucose P D-glucose-6-phosphate DP Δ o = -16.7 D-glucose-6-phosphate D-fructose-6-phosphate
Affi-Prep Protein A Matrix Instruction Manual
 Affi-Prep Protein A Matrix Instruction Manual Catalog Numbers 156-0005 156-0006 Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547 LIT-230 Rev B Table of Contents Section 1 Introduction...1
Affi-Prep Protein A Matrix Instruction Manual Catalog Numbers 156-0005 156-0006 Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547 LIT-230 Rev B Table of Contents Section 1 Introduction...1
The Digestive System. You are what you eat!
 The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
The Digestive System You are what you eat! Try to label the diagram (PENCIL!!) What is Digestion? Digestion: the breakdown of large macromolecules (proteins, fats, carbohydrates) into smaller molecules
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Chapter 19a Oxidative Phosphorylation and Photophosphorylation. Multiple Choice Questions
 Chapter 19a Oxidative Phosphorylation and Photophosphorylation Multiple Choice Questions 1. Electron-transfer reactions in mitochondria Page: 707 Difficulty: 1 Ans: E Almost all of the oxygen (O 2 ) one
Chapter 19a Oxidative Phosphorylation and Photophosphorylation Multiple Choice Questions 1. Electron-transfer reactions in mitochondria Page: 707 Difficulty: 1 Ans: E Almost all of the oxygen (O 2 ) one
EXPERIMENT 9 (Organic Chemistry II) Pahlavan - Cherif Synthesis of Aspirin - Esterification
 EXPERIMENT 9 (rganic hemistry II) Pahlavan - herif Materials Hot plate 125-mL Erlenmeyer flask Melting point capillaries Melting point apparatus Büchner funnel 400-mL beaker Stirring rod hemicals Salicylic
EXPERIMENT 9 (rganic hemistry II) Pahlavan - herif Materials Hot plate 125-mL Erlenmeyer flask Melting point capillaries Melting point apparatus Büchner funnel 400-mL beaker Stirring rod hemicals Salicylic
Membrane Structure and Function
 Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
CHEMICAL SENSORS 1. DEFINITION
 CHEMICAL SENSORS 1. DEFINITION A chemical sensor is a device that transforms chemical information (composition, presence of a particular element or ion, concentration, chemical activity, partial pressure
CHEMICAL SENSORS 1. DEFINITION A chemical sensor is a device that transforms chemical information (composition, presence of a particular element or ion, concentration, chemical activity, partial pressure
Introduction, Noncovalent Bonds, and Properties of Water
 Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Calculation of Molar Masses. Molar Mass. Solutions. Solutions
 Molar Mass Molar mass = Mass in grams of one mole of any element, numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements
Molar Mass Molar mass = Mass in grams of one mole of any element, numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements
