In Vitro Proliferation and Differentiation of Human Neural Stem Cell and Progenitor Cells Using NeuroCult or NeuroCult -XF
|
|
|
- Solomon Rodney Ross
- 7 years ago
- Views:
Transcription
1 In Vitro Proliferation and Differentiation of Human Neural Stem Cell and Progenitor Cells Using NeuroCult or NeuroCult -XF
2
3 i Table of Contents 1.0 Materials and Reagents Storage of Basal Media and Supplements Additional Required Reagents and Cultureware For Proliferation of Neural Stem and Progenitor Cells For Differentiation of Neural Stem and Progenitor Cells For Immunolabelling Differentiated Cells Required Equipment Preparation of Materials and Reagents For Proliferation of Neural Stem and Progenitor Cells Preparation of 10 µg/ml rh EGF Stock Solution Preparation of 10 µg/ml rh bfgf Stock Solution Preparation of Complete NeuroCult NS-A Proliferation Medium Preparation of Complete NeuroCult -XF Proliferation Medium Preparation of Coated Cultureware for Adherent Monolayer Cultures For Differentiation of Neural Stem and Progenitor Cells Preparation of Complete NeuroCult NS-A Differentiation Medium Suggested Preparation for Xeno-Free Differentiation Medium Preparation of Coated Cultureware for Differentiation Cultures Expansion of Neural Stem and Progenitor Cells in Neurosphere Cultures Background Initial Plating of Primary Human CNS Cells in Neurosphere Cultures Passaging Cells from Neurosphere Cultures Expansion of Neural Stem and Progenitor Cells in Adherent Monolayer Cultures Background Initial Plating of Primary Human CNS Cells in Adherent Monolayer Cultures Passaging Cells from Adherent Monolayer Cultures Differentiation of Neural Stem and Progenitor Cells Harvesting Cells from Neurosphere Cultures for Differentiation Harvesting Cells from Adherent Monolayer Cultures for Differentiation Plating Cells for Differentiation Immunolabeling to Identify Differentiated Cell Types Fixation Permeabilization Blocking and Labeling with Primary Antibodies Secondary Labeling... 17
4 ii 6.5 Mounting Representative Images of Neural Stem and Progenitor Cell Cultures Neurosphere Cultures Adherent Monolayer Cultures Representative Images of Immunofluorescent Staining to Identify Differentiated Cells Obtained from Neurospheres * NeuroCult media is sold under license from StemCells California, Inc. US Patent Nos. 5,750,376; 5,851,832; 5,980,885; 5,968,829; 5,981,165; 6,071,889; 6,093,531; 6,103,530; 6,165,783; 6,238,922
5 1 1.0 Materials and Reagents 1.1 Storage of Basal Media and Supplements Table 1: NeuroCult NS-A Component Storage and Stability COMPONENT SIZE STORAGE AND STABILITY NOTES NeuroCult NS-A Basal Medium (Human; #05750) 450 ml Product stable at 2 8 C until expiry date (EXP) on label.* NeuroCult NS-A Basal Medium must be used with supplements suitable for the desired cell culture application: NeuroCult NS-A Proliferation Supplement and required cytokines or NeuroCult NS-A Differentiation Supplement. NeuroCult NS-A Proliferation Supplement (Human; #05753) 50 ml Product stable at -20 C until expiry date (EXP) on label.* Storage of aliquots at -20 C is possible; do not freeze/thaw more than twice. Storage at 2 8 C is not recommended. Components of NeuroCult NS-A Proliferation Supplement are pretested and selected to support the growth and expansion of human neural stem and progenitor cells when combined with NeuroCult NS-A Basal Medium. Required cytokines are not included. NeuroCult NS-A Differentiation Supplement (Human; #05754) 50 ml Product stable at -20 C until expiry date (EXP) on label.* Storage of aliquots at -20 C is possible; do not freeze/thaw more than twice. Storage at 2 8 C is not recommended. Components of NeuroCult NS-A Differentiation Supplement are pretested and selected to support the differentiation of human neural stem and progenitor cells into astrocytes, neurons and oligodendrocytes when combined with NeuroCult NS-A Basal Medium. *After NeuroCult NS-A Proliferation or Differentiation Supplements have been added to NeuroCult NS-A Basal Medium store at 2-8 C for up to 1 month. Table 2: NeuroCult -XF Component Storage and Stability COMPONENT SIZE STORAGE AND STABILITY NOTES NeuroCult -XF Basal Medium (#05760) 450 ml Product stable at 2 8 C until expiry date (EXP) on label.* NeuroCult -XF Basal Medium must be used with the NeuroCult -XF Proliferation Supplement and desired cytokines. NeuroCult -XF Proliferation Supplement (#05763) 50 ml Product stable at -20 C until expiry date (EXP) on label.* Storage of aliquots at -20 C is possible; do not freeze/thaw more than twice. Storage at 2 8 C is not recommended. Components of the NeuroCult -XF Proliferation Supplement are pretested and selected to support the growth and expansion of human neural stem and progenitor cells when combined with NeuroCult -XF Basal Medium. Required cytokines are not included. *After the NeuroCult -XF Proliferation Supplement has been added to NeuroCult -XF Basal Medium store at 2-8 C for up to 1 month.
6 2 1.2 Additional Required Reagents and Cultureware For Proliferation of Neural Stem and Progenitor Cells Recombinant Human Epidermal Growth Factor (rh EGF; Catalog #02633) or Carrier-Free rh EGF 1 (Catalog #02653) Recombinant Human Basic Fibroblast Growth Factor (rh bfgf; Catalog #02634) or Carrier-Free rh bfgf 1 (Catalog #02654) 0.2% Heparin Sodium Salt in PBS (Catalog #07980) 2 10 mm acetic acid (required for reconstitution of rh EGF) Bovine serum albumin (BSA) or human serum albumin (HSA; required for reconstitution of rh EGF and rh bfgf) Poly-D-Lysine (Sigma Catalog #P7280) [optional; only needed for adherent cultures] Laminin (Sigma Catalog #L2020) [optional; only needed for adherent cultures] ACCUTASE (Catalog #07920) [optional; for passaging cells] Trypan Blue (Catalog #07050) Table 3: Recommended Cultureware for Proliferation (Neurosphere and Adherent Monolayer Methods) TISSUE CULTURE VESSEL SUGGESTED SUPPLIERS 6-Well Plate Corning Costar 6-Well TC-Treated Plates (Corning Catalog #3516) T-25 cm 2 Flask Nunc EasYFlasks Nunclon (Nunc Catalog # or VWR Catalog # ) Corning Cell Culture Flask (Corning Catalog # or VWR Catalog # ) T-75 cm 2 Flask Corning Cell Culture Flask (Corning Catalog #3276 or Fisher Scientific Catalog # ) For Differentiation of Neural Stem and Progenitor Cells 12 mm round glass coverslips (Carolina Biological Catalog #633029) [optional] Corning Matrigel, Growth Factor Reduced (Corning Catalog # or #354230) [optional] Table 4: Recommended Cultureware for Differentiation TISSUE CULTURE VESSEL SUGGESTED SUPPLIERS 24-Well Plate Corning Costar 24-Well TC-Treated Plates (Corning Catalog #3526) Corning Matrigel - Coated 24-Well Plate Corning BioCoat Matrigel Matrix 24-Well Plates (Corning Catalog # Carrier-Free cytokines are supplied without carrier proteins, such as bovine serum albumin (BSA). To provide a complete xeno-free culture system, human serum albumin (HSA) can be added during preparation of the carrier-free cytokines, to increase stability. 2 Heparin solution contains animal-derived components. To provide a complete xeno-free system, the use of heparin can be omitted.
7 For Immunolabeling Differentiated Cells Dulbecco s phosphate-buffered saline (PBS) without Ca 2+ or Mg 2+ (Catalog #37350) 4% Paraformaldehyde (in PBS ph 7.2; Sigma Catalog #P6148) Triton X-100 (Sigma Catalog #T-9284) Mounting medium (e.g. FluorSave Reagent, EMD Millipore Catalog #345789) Neural lineage-specific primary antibodies and secondary detection antibodies (Catalog #05716) 1.3 Required Equipment Biohazard Safety Cabinet certified for Level II handling of biological materials 37 C incubator with humidity and gas control to maintain > 95% humidity and an atmosphere of 5% CO 2 in air Bench top centrifuge Vortex Pipette-aid Micropipettes: 10 µl, 200 µl and 1 ml with sterile disposable plastic pipette tips Tubes: 15 ml polypropylene (Falcon Corning Catalog #352096) or 17 x 100 mm polystyrene (Falcon Corning Catalog #352051) Tubes: 50 ml polypropylene (Falcon Corning Catalog #352070) Hemacytometer Forceps for use in moving coverslips during immunolabeling Routine light microscope for hemacytometer cell counts Inverted microscope with flatfield objectives and eye pieces to give object magnification of approximately 20-30X, 80X, and 125X
8 4 2.0 Preparation of Materials and Reagents Use sterile technique when preparing materials and reagents. 2.1 For Proliferation of Neural Stem and Progenitor Cells Preparation of 10 µg/ml rh EGF Stock Solution Note: If a xeno-free preparation of rh EGF is desired to prepare a completely xeno-free medium, use Carrier- Free rh EGF (Catalog #02653) and substitute HSA for BSA in step Dissolve rh EGF (200 µg/vial) in 0.1 ml sterile 10 mm acetic acid containing at least 0.1% BSA. 2. Add 19.9 ml NeuroCult NS-A Basal Medium (Human) containing NeuroCult NS-A Proliferation Supplement (Human). Refer to Section for preparation details. 3. Store aliquots ( ml) of the stock solution of 10 µg/ml rh EGF at -20 C. Do not freeze/thaw each vial more than 3 times Preparation of 10 µg/ml rh bfgf Stock Solution Note: If a xeno-free preparation of rh bfgf is desired to prepare a completely xeno-free medium, use Carrier- Free rh bfgf (Catalog #02654) and substitute HSA for BSA in step Dilute rh bfgf (25 µg/vial) in sterile PBS containing 0.1% BSA to a final concentration of 10 µg/ml rh bfgf. 2. Store aliquots ( ml) of the stock solution of 10 µg/ml rh bfgf at -20 C. Do not freeze/thaw each vial more than 3 times Preparation of Complete NeuroCult NS-A Proliferation Medium 1. Thaw bottles of NeuroCult NS-A Proliferation Supplement (Human) overnight at 2-8 C or for 1-2 hours at 37 C. NeuroCult NS-A Proliferation Supplement can be aliquoted into 10 ml volumes and stored at -20 C until required for use. Repeated thawing and freezing is not recommended. Note: If the supplement thaws during shipping, it should be aliquoted immediately. Providing the supplement remains cool (not more than ~10 C), performance should be unaffected. 2. Add the entire volume (50 ml) of thawed NeuroCult NS-A Proliferation Supplement (Human) to 1 bottle (450 ml) of NeuroCult NS-A Basal Medium (Human). OR Add 1 ml NeuroCult NS-A Proliferation Supplement (Human) to each 9 ml NeuroCult NS-A Basal Medium (Human). 3. Mix well. NeuroCult Proliferation Medium without cytokines is now ready for use. Note: NeuroCult Proliferation Medium without cytokines is only used in very specific steps in the procedure, e.g. when resuspending and washing cell suspensions. Do not use this cytokine-free medium for cell culture. After the NeuroCult NS-A Proliferation Supplement has been added to NeuroCult NS-A Basal Medium, store at 2-8 C for up to 1 month. Avoid repeated exposure of medium to room temperature and light.
9 5 4. To each 10 ml NeuroCult Proliferation Medium without cytokines (i.e. NeuroCult NS-A Basal Medium containing NeuroCult NS-A Proliferation Supplement), add the following: 20 µl of 10 µg/ml rh EGF (to give a final concentration of 20 ng/ml rh EGF) 10 µl of 10 µg/ml rh bfgf (to give a final concentration of 10 ng/ml rh bfgf) 10 µl of 0.2% Heparin (to give a final concentration of % Heparin (w/v) equals 2 µg/ml) This will be known hereafter as Complete NeuroCult Proliferation Medium. Complete NeuroCult Proliferation Medium (cytokine-containing) should be used within 1 week Preparation of Complete NeuroCult -XF Proliferation Medium 1. Thaw bottles of NeuroCult -XF Proliferation Supplement overnight at 2-8 C or for 1-2 hours at 37 C. NeuroCult -XF Proliferation Supplement can be aliquoted into 10 ml volumes and stored at -20 C until required for use. Repeated thawing and freezing is not recommended. Note: If the supplement thaws during shipping, it should be aliquoted immediately. Providing the supplement remains cool (not more than ~10 C), performance should be unaffected. 2. Add the entire volume (50 ml) of thawed NeuroCult -XF Proliferation Supplement to one bottle (450 ml) of NeuroCult -XF Basal Medium. OR Add 1 ml NeuroCult -XF Proliferation Supplement to each 9 ml NeuroCult -XF Basal Medium. 3. Mix well. NeuroCult -XF Proliferation Medium without cytokines is now ready for use. Note: NeuroCult -XF Proliferation Medium without cytokines is only used in very specific steps in the procedure, e.g. when resuspending and washing cell suspensions. Do not use this cytokine-free medium for cell culture. After the NeuroCult -XF Proliferation Supplement has been added to NeuroCult -XF Basal Medium, store at 2-8 C for up to 1 month. Avoid repeated exposure of medium to room temperature and light. 4. To each 10 ml NeuroCult -XF Proliferation Medium without cytokines (i.e. NeuroCult -XF Basal Medium containing NeuroCult -XF Proliferation Supplement), add the following: 20 µl of xeno-free 10 µg/ml rh EGF (to give a final concentration of 20 ng/ml rh EGF) 10 µl of xeno-free 10 µg/ml rh bfgf (to give a final concentration of 10 ng/ml rh bfgf) 10 µl of 0.2% Heparin* (to give a final concentration of % Heparin (w/v) equals 2 µg/ml) *The Heparin solution contains non-human animal-derived components and can be omitted if a complete xeno-free system is desired. This will be known hereafter as Complete NeuroCult -XF Proliferation Medium. Complete NeuroCult -XF Proliferation Medium (cytokine-containing) should be used within 1 week.
10 Preparation of Coated Cultureware for Adherent Monolayer Cultures To culture neural stem and progenitor cells as adherent monolayers, an appropriate substrate is required to coat the surface of the tissue culture vessel. By coating the surface of the culture vessel with poly-d-lysine (PDL), laminin, poly-l-ornithine, or combinations of these substrates, cell-to-substrate attachment is promoted, as opposed to the cell-to-cell attachment, which leads to aggregation and formation of neurospheres. Preparation of 100 µg/ml PDL Stock Solution 1. Dissolve 5 mg poly-d-lysine (Sigma Catalog #P7280) in 50 ml sterile water. 2. Aliquot solution in polypropylene vials and store at 2-8 C. Preparation of 10 µg/ml Laminin Stock Solution 1. Thaw laminin (Sigma Catalog #L2020) at 2-8 C, to prevent laminin from gelling. 2. Prepare a 10 µg/ml working solution of laminin by diluting the laminin in sterile PBS or sterile water (the amount prepared should correspond to the amount needed for immediate use). 3. Store the remaining laminin (which has not been diluted) in appropriately-sized working aliquots at -20 C. Preparation of PDL/Laminin-coated Tissue Cultureware 1. Dispense the appropriate volume of 100 µg/ml PDL stock solution for the chosen tissue culture vessel, as indicated in Table 5. Table 5: Recommended Substrate Volume for Coating Tissue Cultureware TISSUE CULTURE VESSEL 6-Well Plate VOLUME OF SUBSTRATE SOLUTION 1-2 ml/well T-25 cm 2 Flask 3 ml T-75 cm 2 Flask 6 ml 2. Incubate for 2 hours at 37 C or overnight (~20 hours) at 2-8 C. 3. Wash each well/flask with sterile PBS, according to the recommended volumes in Table 6. Remove as much of the PBS as possible. Table 6: Recommended PBS Wash Volume TISSUE CULTURE VESSEL 6-Well Plate VOLUME OF PBS WASH BUFFER 1-2 ml/well T-25 cm 2 Flask 5 ml T-75 cm 2 Flask 10 ml 4. Dispense 10 µg/ml laminin stock solution at the volume indicated in Table Incubate for 2 hours at 37 C or overnight at 2-8 C.
11 7 6. Wash each well/flask with sterile PBS, according to the recommended volumes in Table 6. Only remove the PBS when ready to plate the cells. Do not let the coated plates dry completely. 7. The substrate-coated tissue cultureware is ready for use and should be used within the same day as completing the coating procedure. 8. Proceed to Section 4.0 for plating cells for adherent monolayer cultures. Preparation of Laminin-Only Coated Tissue Cultureware 1. Dispense 10 µg/ml laminin stock solution at the volume indicated in Table Incubate for 2 hours at 37 C or overnight at 2-8 C. 3. Wash each well/flask with sterile PBS, according to the recommended volumes in Table 6. Only remove the PBS when ready to plate the cells. Do not let the coated plates dry completely. 4. The substrate-coated tissue cultureware is ready for use and should be used within the same day as completing the coating procedure. 5. Proceed to Section 4.0 for plating cells for adherent monolayer cultures. 2.2 For Differentiation of Neural Stem and Progenitor Cells Preparation of Complete NeuroCult NS-A Differentiation Medium 1. Thaw bottles of NeuroCult NS-A Differentiation Supplement (Human) overnight at 2-8 C or for 1-2 hours at 37 C. NeuroCult NS-A Differentiation Supplement can be aliquoted into 10 ml volumes and stored at -20 C until required for use. Repeated thawing and freezing is not recommended. 2. Add the entire volume (50 ml) of thawed NeuroCult NS-A Differentiation Supplement to one bottle (450 ml) of NeuroCult NS-A Basal Medium (Human). OR Add 1 ml NeuroCult NS-A Differentiation Supplement (Human) to every 9 ml NeuroCult NS-A Basal Medium (Human) (1/10 dilution). 3. Mix well. Complete NeuroCult NSC Differentiation Medium is now ready for use. Store Complete NeuroCult NSC Differentiation Medium at 2-8 C for up to 1 month Suggested Preparation for Xeno-Free Differentiation Medium 1. Add human serum to a final concentration of 2% to NeuroCult -XF Proliferation Medium without cytokines (refer to steps 1-3, Section 2.1.3). 2. Mix well. Complete Xeno-Free Differentiation Medium is now ready for use. Store Complete Xeno-Free Differentiation Medium at 2-8 C for up to 1 month.
12 Preparation of Coated Cultureware for Differentiation Cultures If using round glass coverslips for immunocytochemistry, ensure that the chosen coverslips can easily be added to and removed from the wells of the (e.g. 24-well) plate used for culturing the cells. Sterilize coverslips by autoclaving prior to coating with Matrigel. Preparation of Matrigel -Coated Glass Coverslips 1. Thaw Matrigel (Corning Catalog #354277) at 2-8 C until it liquefies. To prevent gelation, Matrigel must be kept cold (keep on ice or at 2-8 C). Aliquot the entire bottle of thawed Matrigel into 2 mg aliquots in 2 ml screw-cap tubes. 2. Keep aside a sufficient number of 2 mg aliquots for experimental use and store the remaining unused aliquots of Matrigel at -20 C. One 2 mg Matrigel aliquot generates enough solution to coat well plates (depending on whether 0.5 or 1 ml of solution is used to coat each well). The protein concentration of each lot of Matrigel varies. Refer to the supplier Certificate of Analysis to determine the appropriate volume of Matrigel required to prepare 2 mg aliquots. 3. Add 23 ml NeuroCult Proliferation Medium without cytokines (refer to steps 1-3, Section 2.1.3) into a 50 ml polypropylene tube. 4. Add 1 ml COLD NeuroCult Proliferation Medium without cytokines to the tube containing 2 mg Matrigel and pipette up and down to mix the solution. 5. Transfer the diluted Matrigel solution to the 50 ml tube containing 23 ml NeuroCult Proliferation Medium without cytokines and pipette up and down gently to mix. 6. Using sterile forceps, transfer one pre-sterilized glass coverslip (Carolina Biological Catalog #633029) per well of a 24-well plate. 7. Add ml diluted Matrigel into each well containing a coverslip. 8. Incubate at room temperature (15-25 C) for 2 hours or overnight at 2-8 C. 9. Remove the Matrigel solution when ready to use the coated glass coverslips. Cells can be plated directly on to the Matrigel -coated glass coverslips without the need for a wash step. 10. Proceed to Section 5.0 for plating cells for differentiation.
13 9 3.0 Expansion of Neural Stem and Progenitor Cells in Neurosphere Cultures 3.1 Background Neural stem cells (NSCs) can be isolated from different regions of the human embryonic and fetal CNS, including the striatum, cortex, spinal cord, thalamus, and ventral mesencephalon (Vescovi et al., 1999) at different stages of development. In the adult human brain NSCs reside within specific niches, such as the subventricular zone of the lateral ventricle (Sanai et al., 2004). Multipotent NSC-like cells, known as brain tumor stem cells (BTSCs) or cancer stem cells (CSCs), have been identified and isolated from different grades and types of brain cancers, including gliomas, medulloblastomas, astrocytomas, and ependymomas (Vescovi et al., 2006). For information on the isolation of primary human CNS tissues, refer to Cultures of Stem Cells from the Central Nervous System. In: Fedoroff and Richardson, Eds, Protocols for Neural Cell Culture, 3rd edition, pp 173. All procedures must be performed in accordance with rules outlined by local ethics committees. The protocol below describes a method for expansion of human NSCs from primary tissues (obtained using standard microdissection techniques) in neurosphere cultures. NeuroCult NS-A and NeuroCult -XF (xeno-free) Proliferation Media are optimized for the culture of human neural stem and progenitor cells as cellular aggregates called neurospheres. Important notes when culturing human neurospheres: 1. Overall, human NSCs have a slower growth rate than mouse NSCs; however, the growth rate is also highly dependent on the CNS region from which the cells are isolated. In optimized culture conditions human embryonic CNS cells (e.g. forebrain regions: telencephalon, diencephalon) exhibit a doubling time between 5-12 days, whereas mouse embryonic CNS cells only require 1-2 days. 2. Expansion of human embryonic CNS stem cells requires the presence of both epidermal growth factor (EGF) and basic fibroblast growth factor (bfgf). 3. Human neural stem cells are extremely sensitive to mechanical dissociation. To avoid excessive cell death, observe the following precautions and follow the Trituration procedure in Section 3.3, step 4. Do not allow neurospheres to overgrow as it is more difficult to dissociate larger neurospheres. Neurospheres should not exceed 100 µm in diameter. Always pre-wet the pipette tip with media before drawing cells up, to prevent the cells from sticking to the walls of the tip. 3.2 Initial Plating of Primary Human CNS Cells in Neurosphere Cultures 1. Once CNS tissue has been separated from other tissues, dissected and processed to create a single cell suspension, resuspend the cells in 1 ml Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium in a 15 ml polypropylene tube or 17 x 100 mm polystyrene tube. Note: Use of polypropylene tubes is a precautionary step to prevent cell loss due to cells sticking to the plasticware. 2. Using a pre-wet disposable pipette tip attached to a 1 ml micropipette set at ml, triturate the cell sample using a consistent rhythm approximately times, or until a single cell suspension is achieved. Note: It is extremely important not to expel the last volume of medium from the tip as this will introduce air bubbles into the cell suspension, which will damage or kill cells. 3. Add 1 ml Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium to the single cell suspension and mix carefully to avoid creating any bubbles. Leave for approximately 1 minute to allow any undispersed pieces of tissue to settle. 4. Transfer the supernatant containing the single cell suspension to a new sterile 15 ml tube.
14 10 5. Centrifuge at 192 x g for 10 minutes. Discard the supernatant. 6. Add 1 ml Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium and resuspend cells with a brief trituration (pipette up and down 2 times) using a 1 ml micropipette set at ml. 7. Measure the total volume of the cell suspension. Count viable cells using the Trypan Blue exclusion assay on a hemacytometer. 8. Seed cells at a density of 5 x 10 4 viable cells/cm 2 in Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium as indicated in Table 7. Table 7: Recommended Primary Human CNS Cell Plating Conditions for Neurosphere Cultures TISSUE CULTURE VESSEL VOLUME OF MEDIUM CELL DENSITY TOTAL CELLS 6-Well Plate 3 ml/well 5 x 10 4 viable cells/cm x 10 5 cells T-25 cm 2 Flask 6 ml 5 x 10 4 viable cells/cm x 10 6 cells T-75 cm 2 Flask 10 ml 5 x 10 4 viable cells/cm x 10 6 cells 9. Incubate cultures at 37 C in a 5% CO 2 humidified incubator. 10. Monitor the condition of the cultures daily (e.g. morphology of neurospheres, cell density, media depletion, etc.). Viable neurospheres generally appear semi-transparent and phase bright, with many of the cells on the outer surface displaying microspikes. Refer to Section 7.1 for representative images of cultured neurospheres. 11. The medium should be replenished every 2 days until the cells are ready for subculture. To replenish the medium, add 0.5 ml (T-25 cm 2 ) or 1 ml (T-75 cm 2 ) fresh Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium. Do not exceed the volume capacity of the flasks (i.e. 11 ml for T-25 cm 2 flask or 21 ml for T-75 cm 2 flask). 3.3 Passaging Cells from Neurosphere Cultures Neurospheres should be passaged when they reach approximately μm in diameter (typically occurs 7-14 days after plating, although in some cases it may take up to 21 days), to avoid generating hypoxic cells in the centre of the spheres. Passaging should also be performed before the cells reach high densities and prior to the growth medium becoming acidic (turns orange/yellow in color). If the medium does turn orange/yellow in color before the neurospheres have reached μm in diameter, it is recommended to do a half-medium change. Do not let the neurospheres grow too large (> 200 μm in diameter); the cells within the core of large neurospheres lack appropriate gas and nutrient/waste exchange, becoming necrotic. 1. Harvest and collect the entire cell suspension from the culture into a 50 ml tube (if using a T-75 cm 2 or T-162 cm 2 flask), or a 15 ml tube (if using a T-25 cm 2 flask), depending on the volume harvested. If neurospheres are attached to the culture flask, tap the culture flask or shoot a stream of medium across the attached cells to detach them. Pool all of the cells and medium into the same tube. Note: Use of polypropylene is precautionary, to prevent cell loss due to cells sticking to the plasticware. 2. Centrifuge at 192 x g for 10 minutes. 3. Remove supernatant, leaving behind approximately 0.2 ml (200 μl) medium. 4. Triturate neurospheres with a 200 μl micropipette as follows: a) Adjust the volume on the 200 μl micropipette to 180 μl, to avoid expelling the entire cell suspension and producing bubbles. Setting the pipette to a volume less than 180 μl will not provide efficient
15 11 mechanical force to dissociate all of the neurospheres. Resuspend cells in a maximum volume of 200 μl. b) Angle the tube and pipette tip at 45, however, do not press the tip against the side or bottom of the tube as this will create too much force on the cells during expulsion. Triturate the neurospheres by pipetting up and down times in a firm, but not too vigorous, consistent rhythm. c) Rinse the side of the tube during trituration to remove the remaining neurospheres that are attached to the side of the tube; however, do not rinse too high above the cell suspension volume. d) Once the cells appear to be in a single cell suspension (i.e. no obvious cell aggregates detected by eye), observe an aliquot of the cell suspension on a hemacytometer, to determine if any cellular aggregates remain. e) Repeat the trituration procedure an additional times, if needed, to dissociate cellular aggregates. A homogenous single cell suspension should be present at this point. 5. Measure the total volume of the cell suspension. Count viable cells using the Trypan Blue exclusion assay on a hemacytometer. 6. Seed cells at a density of 1 x 10 4 viable cells/cm 2 in Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium as indicated in Table 8. Table 8: Recommended Subculture Plating Conditions for Neurosphere Cultures TISSUE CULTURE VESSEL VOLUME OF MEDIUM CELL DENSITY TOTAL CELLS 6-Well Plate 3 ml/well 1 x 10 4 viable cells/cm x 10 4 cells T-25 cm 2 Flask 6 ml 1 x 10 4 viable cells/cm x 10 5 cells T-75 cm 2 Flask 10 ml 1 x 10 4 viable cells/cm x 10 5 cells 7. Incubate cultures at 37 C in a 5% CO 2 humidified incubator. 8. Monitor cultures daily. Medium should be replenished every 2 days after plating, as described in Section 3.2, step 11. Depending on the culture conditions and the CNS region the cells were obtained from, a 2-fold or greater expansion of total cells should be observed by the end of the culture period (Vescovi, AL, personal communication; Vescovi AL, Synder EY. Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo. Brain Pathol 9(3): , 1999).
16 Expansion of Neural Stem and Progenitor Cells in Adherent Monolayer Cultures 4.1 Background Neural stem cells (NSCs) can be isolated from different regions of the human embryonic and fetal CNS including the striatum, cortex, spinal cord, thalamus, and ventral mesencephalon (Vescovi et al., 1999) at different stages of development. In the adult human brain NSCs reside within specific niches, such as the subventricular zone of the lateral ventricle (Sanai et al., 2004). Multipotent NSC-like cells, known as brain tumor stem cells (BTSCs) or cancer stem cells (CSCs), have been identified and isolated from different grades and types of brain cancers, including gliomas, medulloblastomas, astrocytomas, and ependymomas (Vescovi et al., 2006). For information on the isolation of primary human CNS tissues refer to Cultures of Stem Cells from the Central Nervous System. In: Fedoroff and Richardson, Eds, Protocols for Neural Cell Culture, 3rd Edition, pp. 173, All procedures must be performed in accordance with rules outlined by local ethics committees. The protocol below describes a method for expansion of human NSCs from primary tissues (obtained using standard microdissection techniques) in neurosphere cultures. 4.2 Initial Plating of Primary Human CNS Cells in Adherent Monolayer Cultures 1. Once CNS tissue has been separated from other tissues, dissected and processed to create a single cell suspension, resuspend the cells in 1 ml Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium in a 15 ml polypropylene tube or 17 x 100 mm polystyrene tube. Note: Use of polypropylene is precautionary, to prevent cell loss due to cells sticking to the plasticware. 2. Using a pre-wet disposable pipette tip attached to a 1 ml micropipette set at ml, triturate the cell sample using a consistent rhythm approximately times, or until single cell suspension is achieved. Note: It is extremely important not to expel the last volume of medium from the tip as this will introduce air bubbles into the cell suspension, which will damage or kill cells. 3. Add 1 ml Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium to the single cell suspension and mix carefully to avoid creating any bubbles. Leave for approximately 1 minute to allow any undispersed pieces of tissue to settle. 4. Transfer supernatant containing the single cell suspension to a new sterile 15 ml tube. 5. Centrifuge at 192 x g for 10 minutes. Discard the supernatant. 6. Add 1 ml Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium and resuspend cells with a brief trituration (pipette up and down 2 times) using a 1 ml micropipette set at ml. 7. Measure the total volume of the cell suspension. Count viable cells using the Trypan Blue exclusion assay on a hemacytometer. 8. Seed cells at a density of 5 x 10 4 viable cells/cm 2 in Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium into tissue culture vessels coated with PDL/laminin or laminin as indicated in Table 9. For coating instruction see Section
17 13 Table 9: Recommended Primary Human CNS Cell Plating Conditions for Adherent Cultures TISSUE CULTURE VESSEL VOLUME OF MEDIUM CELL DENSITY TOTAL CELLS 6-Well Plate 3 ml/well 5 x 10 4 viable cells/cm x 10 5 cells T-25 cm 2 Flask 10 ml 5 x 10 4 viable cells/cm x 10 6 cells T-75 cm 2 Flask 10 ml 5 x 10 4 viable cells/cm x 10 6 cells 9. Incubate cultures at 37 C in a 5% CO 2 humidified incubator. In the presence of a substrate, neural stem and progenitor cells will adhere to the substrate-coated tissue culture vessel within 24 hours. The attached cells show a flattened morphology and are mostly bipolar. 4.3 Passaging Cells from Adherent Monolayer Cultures Cultures should be passaged when they reach 60-80% confluence. Note: Before harvesting the cells, it is important to prepare the required number of pre-coated wells or flasks needed for subculture. The procedure outlined below uses ACCUTASE to dissociate the cells. ACCUTASE dissociation results in high cell viability and dissociated cells with the ability to initiate new adherent cultures for multiple passages. 1. Remove the medium from the culture vessel using a pipette. 2. Add PBS to wash the cells as follows: To each well of a 6-well plate, add 3 ml PBS To each T-25 cm 2 flask, add 10 ml PBS 3. Swirl the culture plate or flask gently and then remove the PBS and discard. 4. Add ACCUTASE as follows: To each well of a 6-well plate, add 0.5 ml ACCUTASE To each T-25 cm 2 flask, add 1 ml ACCUTASE 5. Incubate for 5 minutes at 37 C. 6. Observe the culture to determine if the cells are starting to detach and detachment is complete. If cells have not completely detached after 5 minutes, shoot a stream of medium across the surface of the tissue culture vessel to detach the cells (refer to step 7 and 8 below). 7. Add Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium using a disposable pipette to the detached cells as follows: To each well of a 6-well plate, add 2 ml medium To each T-25 cm 2 flask, add 5 ml medium 8. Using the same pipette, resuspend the detached cells by pipetting the cell/medium suspension up and down 2-3 times. 9. Collect the cells and place in a new sterile 15 ml tube. If cells remain in the vessel, add a small volume of Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium and repeat the procedure to collect the remaining cells. Note: Use of polypropylene is precautionary, to prevent cell loss due to cells sticking to the plasticware.
18 Centrifuge at 110 x g for 5 minutes. 11. Remove the supernatant and resuspend cells in a maximum of 200 µl Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium with a plastic disposable pipette tip attached to a 200 µl micropipette set at 180 µl, pipetting until single cell suspension is achieved. 12. Resuspend cells in an appropriate (approximately ml) volume of Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium. 13. Measure the total volume of the cell suspension. Count viable cells using the Trypan Blue exclusion assay on a hemacytometer. 14. Seed cells at a density of 1 x 10 4 viable cells/cm 2 in Complete NeuroCult NS-A Proliferation Medium or Complete NeuroCult -XF Proliferation Medium into tissue culture vessels coated with PDL/laminin or laminin as indicated in Table 10. For coating instruction see Section Table 10: Recommended Subculture Plating Conditions for Adherent Cultures TISSUE CULTURE VESSEL VOLUME OF MEDIUM CELL DENSITY TOTAL CELLS 6-Well Plate 3 ml/well 1 x 10 4 viable cells/cm x 10 4 cells T-25 cm 2 Flask 10 ml 1 x 10 4 viable cells/cm x 10 5 cells T-75 cm 2 Flask 10 ml 1 x 10 4 viable cells/cm x 10 5 cells 15. Incubate cultures at 37 C in a 5% CO 2 humidified incubator. This procedure can be repeated for multiple passages.
19 Differentiation of Neural Stem and Progenitor Cells In the presence of cytokines (rh EGF and rh bfgf) neural stem cells remain in a relatively undifferentiated state. Upon removal of the cytokines and addition of a small amount of serum, neural stem and progenitor cells are induced to differentiate into neurons, astrocytes and oligodendrocytes. 5.1 Harvesting Cells from Neurosphere Cultures for Differentiation To initiate differentiation, single cells obtained from dissociated neurospheres should be cultured on Matrigel -coated round glass coverslips in 24-well plates Neurospheres should be harvested 7-10 days after plating. At this stage the neurospheres will be approximately 100 μm in diameter. 1. Collect neurospheres and place in a sterile 15 ml polypropylene tube or 17 x 100 mm polystyrene tube. Note: Use of polypropylene is precautionary, to prevent cell loss due to cells sticking to the plasticware. 2. Centrifuge at 192 x g for 10 minutes. 3. Remove supernatant and discard. 4. Wash the cells to remove the cytokines by gently resuspending the cell pellet in 10 ml Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium using a 10 ml disposable plastic pipette. 5. Centrifuge at 192 x g for 10 minutes. 6. Remove supernatant, leaving behind approximately 200 μl of the medium. Using a 200 μl micropipette set at 180 μl, triturate the neurosphere suspension (according to the instructions in Section 3.3, step 4) until a single cell suspension is achieved. 7. Measure the total volume of the cell suspension. Count viable cells using the Trypan Blue exclusion assay on a hemacytometer. 5.2 Harvesting Cells from Adherent Monolayer Cultures for Differentiation Cells should be harvested when the culture is 60-80% confluent. To initiate differentiation, single cells obtained from adherent monolayer cultures should be cultured on Matrigel -coated round glass coverslips in 24-well plates. Note: Before harvesting the cells, it is important to prepare the required number of pre-coated coverslips needed for subculture. 1. Remove the medium from the culture vessel using a pipette. 2. Add PBS to wash the cells as follows: To each well of a 6-well plate, add 3 ml PBS To each T-25 cm 2 flask, add 10 ml PBS 3. Swirl the culture plate or flask gently and then remove the PBS and discard. 4. Add ACCUTASE as follows: To each well of a 6-well plate, add 0.5 ml ACCUTASE To each T-25 cm 2 flask, add 1 ml ACCUTASE 5. Incubate for 5 minutes at 37 C.
20 16 6. Observe the culture to determine if detachment is complete. If cells have not completely detached after 5 minutes, shoot a stream of medium across the surface of the tissue culture vessel to detach the cells (refer to step 7 and 8 below). 7. Wash the cells to remove the cytokines by adding Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium. Using a disposable pipette add medium to the detached cells as follows: To each well of a 6-well plate, add 2 ml medium To each T-25 cm 2 flask, add 5 ml medium 8. Using the same pipette, resuspend the detached cells by pipetting the cell/medium suspension up and down 2-3 times. 9. Collect the cells and place in a new sterile 15 ml tube. If cells remain in the vessel, add a small volume of Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium and repeat the procedure to collect the remaining cells. Note: Use of polypropylene is precautionary, to prevent cell loss due to cells sticking to the plasticware. 10. Centrifuge at 110 x g for 5 minutes. 11. Remove the supernatant and resuspend cells in a maximum of 200 µl Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium with a plastic disposable pipette tip attached to a 200 µl micropipette set at 180 µl, pipetting until a single cell suspension is achieved. 12. Resuspend cells in an appropriate volume (approximately ml) of Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium. 13. Measure the total volume of the cell suspension. Count viable cells using the Trypan Blue exclusion assay on a hemacytometer. 5.3 Plating Cells for Differentiation 1. Resuspend cells in Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium to yield a plating cell density of x 10 4 cells/cm Seed cells onto Matrigel -coated round glass coverslips in 24-well plates or onto Matrigel -coated plates as indicated in Table 11. Table 11: Recommended Plating Conditions for Differentiation TISSUE CULTURE VESSEL VOLUME OF MEDIUM CELL DENSITY TOTAL CELLS 24-Well Plate with Matrigel - Coated Glass Coverslips Matrigel -Coated 24-Well Plate 1 ml x 10 4 cells/cm x 10 5 cells 1 ml x 10 4 cells/cm x 10 5 cells 3. Incubate cultures in a 5% CO 2 humidified incubator at 37 C. 4. Monitor cultures daily, to determine if the medium needs to be changed during the differentiation procedure. If the medium becomes acidic (turns yellow), perform a half-medium change by removing approximately half of the medium and replacing with fresh Complete NeuroCult NS-A Differentiation Medium or Complete Xeno-Free Differentiation Medium. 5. Cultures should be examined after 5-10 days with an inverted light microscope to determine if the cells have differentiated (attached). Viable cells appear phase contrast bright. If differentiated cells are present, cells can be processed for indirect immunofluorescence as described in Section 6.0.
21 Immunolabeling to Identify Differentiated Cell Types 6.1 Fixation 1. Remove approximately 90% of the culture medium from each well of a 24-well plate. Note: Do not remove all of the culture medium prior to fixation. The unfixed cells should not be exposed to air. 2. Working in a chemical fume hood, add 1 ml of 4% paraformaldehyde (in PBS, ph 7.2) to each well. 3. Incubate for 30 minutes at room temperature (15-25 C). 4. Working in a chemical fume hood, remove the paraformaldehyde solution. 5. Add PBS (ph 7.2) to the samples and incubate for 5 minutes at room temperature (15-25 C). 6. Repeat the PBS wash procedure two more times, for a total of three washes. 6.2 Permeabilization 1. Add 1 ml of 0.3% Triton X-100 (in PBS) to each well. 2. Incubate for 5-10 minutes at room temperature (15-25 C). 3. Remove Triton X-100/PBS. 4. Perform two additional 5 minute PBS washes. 6.3 Blocking and Labeling with Primary Antibodies 1. Prepare the blocking solution of 10% serum in PBS. The type of serum used should correspond to the animal in which the secondary antibody was generated. 2. Dilute the primary antibody in blocking solution (containing the appropriate serum) to give an appropriate dilution for immunolabeling. A minimum volume of 250 μl should be added to each well. 3 Note: See Table 12 for a list of available antibodies, or visit or techsupport@stemcell.com for a complete list. 3. Add diluted primary antibodies to the desired wells of a 24-well plate. 4. Incubate for 2 hours at 37 C or overnight at 2-8 C. 5. Wash off the primary antibodies with three 5 minute PBS washes. 6.4 Secondary Labeling 1. Dilute secondary antibodies 1:100 in PBS + 2% serum (the same serum used for preparing the blocking solution). Add a minimum volume of 250 µl to each well containing differentiated cells labeled with primary antibodies. 2. Incubate secondary antibodies for 30 minutes at 37 C. Note: Fluorophore-conjugated secondary antibodies are sensitive to light; keep samples in the dark to prevent bleaching. 3. Wash off the secondary antibody with three 5 minute PBS washes. 4. After the last wash, add distilled water to each well. 3 Alternatively, a small volume of antibody (approximately 50 µl) can be added directly on the coverslip containing the differentiated cells and a second clean coverslip can be placed directly on top.
22 18 Table 12: Recommended Primary Antibodies TARGET ANTIGEN CLONE ISOTYPE APPLICATIONS CATALOG # Neuron Markers Microtubule Associated Protein 2 (MAP2) AP20 Mouse IgG 1 IF TUJ1 Mouse IgG 2a IF Neuronal Class III β-tubulin 2G10-TB3 Mouse IgG 2a ICC, IF, WB AA10 Mouse IgG 2a ICC, IF, WB Tyrosine Hydroxylase TH2 Mouse IgG 1 IF Astrocyte Markers Glial Fibrillary Acidic Protein (GFAP) 2E1.E9 Mouse IgG 2b FC, ICC, IF, WB Rabbit Polyclonal IF Oligodendrocyte Markers Oligodendrocyte Marker O4 81 Mouse IgM IF Undifferentiated Neural Cell Markers Nestin 10C2 Mouse IgG 1 FC, ICC, IF, WB SOX2 - Rabbit Polyclonal FC, ICC, IF, WB FC - Flow cytometry; ICC - Immunocytochemistry; IF - Immunofluorescence microscopy; WB - Western blotting 6.5 Mounting 1. Add 10 µl mounting medium (e.g. FluorSave Reagent, EMD Millipore Catalog #345789) onto a clean glass coverslip. Remove immunolabeled coverslip from the 24-well plate and gently tap corner of the coverslip to remove excess water. 2. Place coverslip cells-side-down onto the mounting medium. Avoid trapping any air bubbles. 3. Visualize immunolabeling under a fluorescent microscope using the appropriate filters for each fluorophore. Refer to Section 8.0 for representative images of immunolabeled differentiated cells.
23 Representative Images of Neural Stem and Progenitor Cell Cultures 7.1 Neurosphere Cultures Figures A -B. Neurospheres derived from human CNS cells observed after being cultured for 10 days in Complete NeuroCult NS-A Proliferation Medium. Neurospheres measure approximately µm in diameter, are phase contrast bright, semi-transparent and have small microspikes on the periphery of the spheres (arrows). Magnification: 10X (A) and 40X (B). Note: Human CNS-derived neurospheres cultured in NeuroCult -XF Proliferation Medium display similar morphology. 7.2 Adherent Monolayer Cultures Figures A - B. Adherent monolayer cultures of human CNS-derived neural stem and progenitor cells cultured in Complete NeuroCult NS-A Proliferation Medium on tissue culture plates coated with 10 µg/ml laminin. A) Day 10 of in vitro culture of passage 10 cells (~80-85% confluent). B) Day 16 of in vitro culture of passage 11 cells (~60% confluent). Magnification: 10X.
24 Representative Images of Immunofluorescent Staining to Identify Differentiated Cells Obtained from Neurospheres A. Neurons (red) were detected with a mouse monoclonal β-tubulin III antibody. Magnification: 10X. B. Astrocytes (green) were detected with a rabbit polyclonal GFAP antibody. Magnification: 20X. C. Immature oligodendrocytes (purple) were detected with a rabbit monoclonal O4 Oligodendrocyte Marker antibody. Magnification: 20X. D. Mature oligodendrocytes (purple) were detected with a galactocerebroside antibody. Magnification: 20X. Figures A - D. Neurospheres derived from human CNS tissue were cultured in NeuroCult NS-A Proliferation Medium. Prior to initiating differentiation, neurospheres were dissociated and plated on coverslips. NeuroCult NS-A Differentiation Medium was used to promote differentiation of neural stem and progenitor cells to neurons, astrocytes and oligodendrocytes. Note: Cells obtained from neurospheres cultured in NeuroCult -XF Proliferation Medium and differentiated under xeno-free differentiation conditions display similar immunofluorescent staining patterns when treated with the same antibodies. Images courtesy of A. Vescovi. Copyright 2014 by STEMCELL Technologies Inc. All rights reserved including graphics and images. STEMCELL Technologies & Design, STEMCELL Shield Design, Scientists Helping Scientists, NeuroCult are trademarks of STEMCELL Technologies Inc. Biocoat, Corning, Costar, Falcon, and Matrigel are trademarks of Corning Incorporated. ACCUTASE is a trademark of Innovative Cell Technologies Inc. EasYFlask, Nunc, and Nunclon are trademarks of Thermo Fisher Scientific. All other trademarks are the property of their respective holders. While STEMCELL has made all reasonable efforts to ensure that the information provided by STEMCELL and its suppliers is correct, it makes no warranties or representations as to the accuracy or completeness of such information.
25
26
27
28 Scientists Helping Scientists TM TOLL FREE PHONE PHONE INFO@STEMCELL.COM TECHSUPPORT@STEMCELL.COM FOR GLOBAL CONTACT DETAILS VISIT OUR WEBSITE STEMCELL TECHNOLOGIES INC. S QUALITY MANAGEMENT SYSTEM IS CERTIFIED TO ISO MEDICAL DEVICE STANDARDS. DOCUMENT #28724 VERSION 3.1.0
Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System
 Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System i Table of Contents 1.0 Introduction... 1 2.0 Materials, Reagents and Equipment... 2 2.1 Materials Required for Neural
Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System i Table of Contents 1.0 Introduction... 1 2.0 Materials, Reagents and Equipment... 2 2.1 Materials Required for Neural
NEURAL STEM CELL PRODUCTS. Neural Stem Cells. Standardized Media and Reagents. Table of Contents
 NEURAL STEM CELL PRODUCTS Neural Stem Cells Standardized Media and Reagents Table of Contents Table of Contents 3 4 6 8 12 13 13 14 14 Superior Neural Stem Cell Culture with Consistent, High-Quality Media
NEURAL STEM CELL PRODUCTS Neural Stem Cells Standardized Media and Reagents Table of Contents Table of Contents 3 4 6 8 12 13 13 14 14 Superior Neural Stem Cell Culture with Consistent, High-Quality Media
Related topics: Application Note 27 Data Analysis of Tube Formation Assays.
 Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs)
 HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
PROTOCOL. Immunocytochemistry (ICC) MATERIALS AND EQUIPMENT REQUIRED
 PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
Human Adult Mesothelial Cell Manual
 Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Cellartis Protocol Culturing of hes Cells
 Cellartis Protocol Culturing of hes Cells This material was cultured and frozen using Cellartis protocols. WiCell recommends that stem cells should be thawed and established in the conditions in which
Cellartis Protocol Culturing of hes Cells This material was cultured and frozen using Cellartis protocols. WiCell recommends that stem cells should be thawed and established in the conditions in which
Poietics human mesenchymal stem cells Instructions for use
 Poietics human mesenchymal stem cells Instructions for use www.lonza.com U.S. Scientific Support: 800-521-0390 scientific.support@lonza.com EU/ROW Scientific Support: +49-221-99199-400 scientific.support.eu@lonza.com
Poietics human mesenchymal stem cells Instructions for use www.lonza.com U.S. Scientific Support: 800-521-0390 scientific.support@lonza.com EU/ROW Scientific Support: +49-221-99199-400 scientific.support.eu@lonza.com
MTT Cell Proliferation Assay
 ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
Mesenchymal Stem Cells
 Mesenchymal Stem Cells Instruction Manual Product Size Catalog Number from Bone Marrow (hmsc-bm) from Umbilical Cord Matrix (hmsc-uc) from Adipose Tissue (hmsc-at) 500,000 cryopreserved cells 500,000 proliferating
Mesenchymal Stem Cells Instruction Manual Product Size Catalog Number from Bone Marrow (hmsc-bm) from Umbilical Cord Matrix (hmsc-uc) from Adipose Tissue (hmsc-at) 500,000 cryopreserved cells 500,000 proliferating
STEMCELL Quality Control Kits
 STEMCELL Quality Control Kits i Table of Contents 1.0 Introduction...1 2.0 Thawing Cells, Plating and Colony Enumeration...2 2.1 Supplies and reagents included in the QC kit...2 2.2 Additional reagents
STEMCELL Quality Control Kits i Table of Contents 1.0 Introduction...1 2.0 Thawing Cells, Plating and Colony Enumeration...2 2.1 Supplies and reagents included in the QC kit...2 2.2 Additional reagents
BD PuraMatrix Peptide Hydrogel
 BD PuraMatrix Peptide Hydrogel Catalog No. 354250 Guidelines for Use FOR RESEARCH USE ONLY NOT FOR CLINICAL, DIAGNOSTIC OR THERAPEUTIC USE SPC-354250-G rev 2.0 1 TABLE OF CONTENTS Intended Use... 2 Materials
BD PuraMatrix Peptide Hydrogel Catalog No. 354250 Guidelines for Use FOR RESEARCH USE ONLY NOT FOR CLINICAL, DIAGNOSTIC OR THERAPEUTIC USE SPC-354250-G rev 2.0 1 TABLE OF CONTENTS Intended Use... 2 Materials
RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method)
 Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
Required Materials. Stemgent Reprogramming-Qualified NuFF Cells GSC-3002G 4 x 10 6 cells/vial Liquid Nitrogen
 Page 1 Overview This protocol for microrna-enhanced mrna reprogramming describes the combined use of the Stemgent mrna Reprogramming Kit, the Stemgent microrna Booster Kit, and the Stemgent Stemfect RNA
Page 1 Overview This protocol for microrna-enhanced mrna reprogramming describes the combined use of the Stemgent mrna Reprogramming Kit, the Stemgent microrna Booster Kit, and the Stemgent Stemfect RNA
LIVE/DEAD Fixable Dead Cell Stain Kits
 USER GUIDE LIVE/DEAD Fixable Dead Cell Stain Kits Pub. No. MAN0002416 (MP34955) Rev. A.0 Table 1. Contents and storage Material Amount Storage Stability Individual Kits: Blue, violet, aqua, yellow-, green,
USER GUIDE LIVE/DEAD Fixable Dead Cell Stain Kits Pub. No. MAN0002416 (MP34955) Rev. A.0 Table 1. Contents and storage Material Amount Storage Stability Individual Kits: Blue, violet, aqua, yellow-, green,
Dot Blot Analysis. Teacher s Guidebook. (Cat. # BE 502) think proteins! think G-Biosciences www.gbiosciences.com
 PR110 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Dot Blot Analysis Teacher s Guidebook (Cat. # BE 502) think proteins! think G-Biosciences
PR110 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Dot Blot Analysis Teacher s Guidebook (Cat. # BE 502) think proteins! think G-Biosciences
Intestinal Epithelial Organoid Culture with IntestiCult Organoid Growth Medium (Mouse)
 TECHNICL ULLETIN Intestinal Epithelial Organoid Culture with IntestiCult Organoid Growth Medium (Mouse) Introduction The use of three-dimensional (3D) culture systems continues to increase, due in large
TECHNICL ULLETIN Intestinal Epithelial Organoid Culture with IntestiCult Organoid Growth Medium (Mouse) Introduction The use of three-dimensional (3D) culture systems continues to increase, due in large
Optimized Protocol sirna Test Kit for Cell Lines and Adherent Primary Cells
 page 1 of 8 sirna Test Kit for Cell Lines and Adherent Primary Cells Kit principle Co-transfection of pmaxgfp, encoding the green fluorescent protein (GFP) from Pontellina p. with an sirna directed against
page 1 of 8 sirna Test Kit for Cell Lines and Adherent Primary Cells Kit principle Co-transfection of pmaxgfp, encoding the green fluorescent protein (GFP) from Pontellina p. with an sirna directed against
Human/Mouse/Rat Neural Progenitor Cell Marker Antibody Panel
 Human/Mouse/Rat Neural Progenitor Cell Marker Antibody Panel Catalog Number SC025 Reagents for the identification of human/mouse/rat neural progenitor cells. This package insert must be read in its entirety
Human/Mouse/Rat Neural Progenitor Cell Marker Antibody Panel Catalog Number SC025 Reagents for the identification of human/mouse/rat neural progenitor cells. This package insert must be read in its entirety
GIBCO Human Neural Stem Cells (H9 hesc-derived)
 GIBCO Human Neural Stem Cells (H9 hesc-derived) Catalog nos. N7800-100, N7800-200 Rev. date: 7 December 2009 Manual part no. A11592 MAN0001758 User Manual Table of Contents Contents and Storage... iv GIBCO
GIBCO Human Neural Stem Cells (H9 hesc-derived) Catalog nos. N7800-100, N7800-200 Rev. date: 7 December 2009 Manual part no. A11592 MAN0001758 User Manual Table of Contents Contents and Storage... iv GIBCO
Measuring Cell Viability/Cytotoxicity: Cell Counting Kit-F
 Introduction The Cell Counting Kit-F is a fluorometic assay for the determination of viable cell numbers. Calcein-AM in this kit passes through the cell membrane and is hydrolized by the esterase in the
Introduction The Cell Counting Kit-F is a fluorometic assay for the determination of viable cell numbers. Calcein-AM in this kit passes through the cell membrane and is hydrolized by the esterase in the
Follicle Dermal Papilla Cell
 Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Bovine Vitamin B12 (VB12) ELISA Kit
 Bovine Vitamin B12 (VB12) ELISA Kit Catalog Number. CSB-E14089B For the quantitative determination of endogenic bovine vitamin B12 (VB12) concentrations in serum, plasma, tissue homogenates. This package
Bovine Vitamin B12 (VB12) ELISA Kit Catalog Number. CSB-E14089B For the quantitative determination of endogenic bovine vitamin B12 (VB12) concentrations in serum, plasma, tissue homogenates. This package
Human Mesenchymal Stem Cell Functional Identification Kit
 Human Mesenchymal Stem Cell Functional Identification Kit Catalog Number SC006 Reagents for the identification of human bone marrow-derived stem cells (BMSCs)/mesenchymal stem cells (MSCs) by in vitro
Human Mesenchymal Stem Cell Functional Identification Kit Catalog Number SC006 Reagents for the identification of human bone marrow-derived stem cells (BMSCs)/mesenchymal stem cells (MSCs) by in vitro
EdU Flow Cytometry Kit. User Manual
 User Manual Ordering information: (for detailed kit content see Table 2) EdU Flow Cytometry Kits for 50 assays: Product number EdU Used fluorescent dye BCK-FC488-50 10 mg 6-FAM Azide BCK-FC555-50 10 mg
User Manual Ordering information: (for detailed kit content see Table 2) EdU Flow Cytometry Kits for 50 assays: Product number EdU Used fluorescent dye BCK-FC488-50 10 mg 6-FAM Azide BCK-FC555-50 10 mg
Instructions. Torpedo sirna. Material. Important Guidelines. Specifications. Quality Control
 is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
Radius 24-Well Cell Migration Assay (Laminin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Annexin V-EGFP Apoptosis Detection Kit
 ab14153 Annexin V-EGFP Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples This product is for research use only and is not intended
ab14153 Annexin V-EGFP Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples This product is for research use only and is not intended
GASTRIC ORGANOID CULTURE PROTOCOL
 GASTRIC ORGANOID CULTURE PROTOCOL THIS PROTOCOL PROVIDES THE PROCEDURE FOR SUBCULTURING NORMAL HUMAN GASTRIC ORGANOIDS WHICH WAS DERIVED FROM THE SUBMERGED METHOD AS DESCRIBED IN BARKER N, ET AL. LGR5+VE
GASTRIC ORGANOID CULTURE PROTOCOL THIS PROTOCOL PROVIDES THE PROCEDURE FOR SUBCULTURING NORMAL HUMAN GASTRIC ORGANOIDS WHICH WAS DERIVED FROM THE SUBMERGED METHOD AS DESCRIBED IN BARKER N, ET AL. LGR5+VE
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis
 ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
ArC Amine Reactive Compensation Bead Kit
 ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
Running protein gels and detection of proteins
 Running protein gels and detection of proteins 1. Protein concentration determination using the BIO RAD reagent This assay uses a colour change reaction to give a direct measurement of protein concentration.
Running protein gels and detection of proteins 1. Protein concentration determination using the BIO RAD reagent This assay uses a colour change reaction to give a direct measurement of protein concentration.
QuickZyme Soluble Collagen Assay
 QuickZyme Soluble Collagen Assay Version June 2012 FOR RESEARCH USE ONLY NOT FOR USE IN DIAGNOSTIC PROCEDURES This package insert must be read in its entirety before using this product. Introduction Collagen
QuickZyme Soluble Collagen Assay Version June 2012 FOR RESEARCH USE ONLY NOT FOR USE IN DIAGNOSTIC PROCEDURES This package insert must be read in its entirety before using this product. Introduction Collagen
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit
 Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma, tissue homogenates.
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma, tissue homogenates.
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit
 Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma and tissue
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma and tissue
Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit
 Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit Catalog No: E0479r 96 Tests Operating instructions www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit Catalog No: E0479r 96 Tests Operating instructions www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Canine creatine kinase MB isoenzyme (CK-MB)ELISA Kit
 Canine creatine kinase MB isoenzyme (CK-MB)ELISA Kit Catalog No. CSB-E15852c (96T) This immunoassay kit allows for the in vitro quantitative determination of canine CK-MB concentrations in serum and plasma.
Canine creatine kinase MB isoenzyme (CK-MB)ELISA Kit Catalog No. CSB-E15852c (96T) This immunoassay kit allows for the in vitro quantitative determination of canine CK-MB concentrations in serum and plasma.
Amaxa Mouse T Cell Nucleofector Kit
 Amaxa Mouse T Cell Nucleofector Kit For T cells isolated from C57BL/6 & BALB/c mice Evaluated for murine T cells isolated from C57BL/6 & BALB/c mice This protocol is designed for murine lymphocytes or
Amaxa Mouse T Cell Nucleofector Kit For T cells isolated from C57BL/6 & BALB/c mice Evaluated for murine T cells isolated from C57BL/6 & BALB/c mice This protocol is designed for murine lymphocytes or
Malondialdehyde (MDA) ELISA
 Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
IgM ELISA. For the quantitative determination of IgM in human serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
Osteoblast Differentiation and Mineralization
 Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
Mouse Keyhole Limpet Hemocyanin antibody(igm) ELISA Kit
 Mouse Keyhole Limpet Hemocyanin antibody(igm) ELISA Kit Catalog No. MBS702810 (96 tests) This immunoassay kit allows for the in vitro semi-quantitative determination of mouse KLH(IgM)antibody concentrations
Mouse Keyhole Limpet Hemocyanin antibody(igm) ELISA Kit Catalog No. MBS702810 (96 tests) This immunoassay kit allows for the in vitro semi-quantitative determination of mouse KLH(IgM)antibody concentrations
Annexin V-FITC Apoptosis Detection Kit
 ab14085 Annexin V-FITC Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Apoptosis in living cells (adherent and suspension). This product is for research
ab14085 Annexin V-FITC Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Apoptosis in living cells (adherent and suspension). This product is for research
protocol handbook 3D cell culture mimsys G hydrogel
 handbook 3D cell culture mimsys G hydrogel supporting real discovery handbook Index 01 Cell encapsulation in hydrogels 02 Cell viability by MTS assay 03 Live/Dead assay to assess cell viability 04 Fluorescent
handbook 3D cell culture mimsys G hydrogel supporting real discovery handbook Index 01 Cell encapsulation in hydrogels 02 Cell viability by MTS assay 03 Live/Dead assay to assess cell viability 04 Fluorescent
For the development of sandwich ELISAs to measure phosphorylated Epidermal Growth Factor Receptor (EGF R) in cell lysates.
 DuoSet IC Human Phospho-EGF R Catalog Number DYC1095-2 Catalog Number DYC1095-5 For the development of sandwich ELISAs to measure phosphorylated Epidermal Growth Factor Receptor (EGF R) in cell lysates.
DuoSet IC Human Phospho-EGF R Catalog Number DYC1095-2 Catalog Number DYC1095-5 For the development of sandwich ELISAs to measure phosphorylated Epidermal Growth Factor Receptor (EGF R) in cell lysates.
Glycolysis Cell-Based Assay Kit
 Glycolysis Cell-Based Assay Kit Item No. 600450 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Glycolysis Cell-Based Assay Kit Item No. 600450 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Islet Viability Assessment by Single Cell Flow Cytometry
 Islet Viability Assessment by Single Cell Flow Cytometry Page 1 of 8 Purpose: To comprehensively assess the viability of the islet cell preparation prior to transplantation. Tissue Samples: A sample containing
Islet Viability Assessment by Single Cell Flow Cytometry Page 1 of 8 Purpose: To comprehensively assess the viability of the islet cell preparation prior to transplantation. Tissue Samples: A sample containing
Human Free Testosterone(F-TESTO) ELISA Kit
 Human Free Testosterone(F-TESTO) ELISA Kit Catalog Number. MBS700040 For the quantitative determination of human free testosterone(f-testo) concentrations in serum, plasma. This package insert must be
Human Free Testosterone(F-TESTO) ELISA Kit Catalog Number. MBS700040 For the quantitative determination of human free testosterone(f-testo) concentrations in serum, plasma. This package insert must be
Cell Culture Protocol for Biogelx Peptide Hydrogel 2D and 3D Cell Culture PRO/BGX/001
 Protocol (PRO) Cell Culture Protocol for Hydrogel 2D and 3D Cell Culture PRO/BGX/001 Materials Provided Gel solution (stiffness of Gel will vary as per type of product requested), packaged in a glass vial.
Protocol (PRO) Cell Culture Protocol for Hydrogel 2D and 3D Cell Culture PRO/BGX/001 Materials Provided Gel solution (stiffness of Gel will vary as per type of product requested), packaged in a glass vial.
Mouse glycated hemoglobin A1c(GHbA1c) ELISA Kit
 Mouse glycated hemoglobin A1c(GHbA1c) ELISA Kit Catalog Number. CSB-E08141m For the quantitative determination of mouse glycated hemoglobin A1c(GHbA1c) concentrations in lysate for RBC. This package insert
Mouse glycated hemoglobin A1c(GHbA1c) ELISA Kit Catalog Number. CSB-E08141m For the quantitative determination of mouse glycated hemoglobin A1c(GHbA1c) concentrations in lysate for RBC. This package insert
Classic Immunoprecipitation
 292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
Chromatin Immunoprecipitation (ChIP)
 Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
BioResearch. RAFT 3D Cell Culture Kit Protocol
 BioResearch RAFT 3D Cell Culture Kit Protocol RAFT 3D Cell Culture Kit This protocol describes in detail how to produce RAFT 3D Cell Cultures in 96-well plates, 24-well plates and 24-well inserts. It is
BioResearch RAFT 3D Cell Culture Kit Protocol RAFT 3D Cell Culture Kit This protocol describes in detail how to produce RAFT 3D Cell Cultures in 96-well plates, 24-well plates and 24-well inserts. It is
Hydrogen Peroxide Cell-Based Assay Kit
 Hydrogen Peroxide Cell-Based Assay Kit Item No. 600050 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Hydrogen Peroxide Cell-Based Assay Kit Item No. 600050 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Annexin V-FITC Apoptosis Detection Kit
 ab14085 Annexin V-FITC Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Apoptosis in living cells (adherent and suspension). This product is for research
ab14085 Annexin V-FITC Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Apoptosis in living cells (adherent and suspension). This product is for research
Inc. Wuhan. Quantity Pre-coated, ready to use 96-well strip plate 1 Plate sealer for 96 wells 4 Standard (liquid) 2
 Uscn Life Science Inc. Wuhan Website: www.uscnk.com Phone: +86 27 84259552 Fax: +86 27 84259551 E-mail: uscnk@uscnk.com ELISA Kit for Human Prostaglandin E1(PG-E1) Instruction manual Cat. No.: E0904Hu
Uscn Life Science Inc. Wuhan Website: www.uscnk.com Phone: +86 27 84259552 Fax: +86 27 84259551 E-mail: uscnk@uscnk.com ELISA Kit for Human Prostaglandin E1(PG-E1) Instruction manual Cat. No.: E0904Hu
Human Hepatic Sinusoidal Endothelial Cell Manual
 Human Hepatic Sinusoidal Endothelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0093.00 Human Hepatic Sinusoidal Cells. Orders are delivered via Federal Express courier. All US and Canada orders
Human Hepatic Sinusoidal Endothelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0093.00 Human Hepatic Sinusoidal Cells. Orders are delivered via Federal Express courier. All US and Canada orders
NimbleGen DNA Methylation Microarrays and Services
 NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
Human IP-10 ELISA Kit, pink-one
 1. Catalog No. K0331210P 2. Quantity 96 tests 3. Storage 4 C Human IP-10 ELISA Kit, pink-one 4. Description Human IP-10 ELISA kit contains all the necessary reagents required for performing quantitative
1. Catalog No. K0331210P 2. Quantity 96 tests 3. Storage 4 C Human IP-10 ELISA Kit, pink-one 4. Description Human IP-10 ELISA kit contains all the necessary reagents required for performing quantitative
竞 争 性 分 析 Epitope Mapping 实 验 方 法
 竞 争 性 分 析 Epitope Mapping 实 验 方 法 ABSTRACT The simplest way to determine whether two monoclonal antibodies bind to distinct sites on a protein antigen is to carry out a competition assay. The assay can
竞 争 性 分 析 Epitope Mapping 实 验 方 法 ABSTRACT The simplest way to determine whether two monoclonal antibodies bind to distinct sites on a protein antigen is to carry out a competition assay. The assay can
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Handling Instructions
 Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Amaxa 4D-Nucleofector Protocol for Mouse Embryonic Stem [ES] Cells For 4D-Nucleofector X Unit Transfection in suspension
![Amaxa 4D-Nucleofector Protocol for Mouse Embryonic Stem [ES] Cells For 4D-Nucleofector X Unit Transfection in suspension Amaxa 4D-Nucleofector Protocol for Mouse Embryonic Stem [ES] Cells For 4D-Nucleofector X Unit Transfection in suspension](/thumbs/30/14702458.jpg) For 4D-Nucleofector X Unit Transfection in suspension Cells derived from mouse blastocysts; round cells, growing in clumps s 1. This protocol is meant to provide an outline for the handling and the Nucleofection
For 4D-Nucleofector X Unit Transfection in suspension Cells derived from mouse blastocysts; round cells, growing in clumps s 1. This protocol is meant to provide an outline for the handling and the Nucleofection
RiboZol RNA Extraction Reagents
 RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
ab83369 Alkaline Phosphatase Assay kit (Colorimetric)
 ab83369 Alkaline Phosphatase Assay kit (Colorimetric) Instructions for use: For the rapid, sensitive and accurate measurement of Alkaline Phosphatase in various samples. This product is for research use
ab83369 Alkaline Phosphatase Assay kit (Colorimetric) Instructions for use: For the rapid, sensitive and accurate measurement of Alkaline Phosphatase in various samples. This product is for research use
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION
 Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
MEF Starter Nucleofector Kit
 page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
INTERFERin in vitro sirna/mirna transfection reagent PROTOCOL. 1 Standard sirna transfection of adherent cells... 2
 INTERFERin in vitro sirna/mirna transfection reagent PROTOCOL DESCRIPTION INTERFERin is a powerful sirna/mirna transfection reagent that ensures efficient gene silencing and reproducible transfection in
INTERFERin in vitro sirna/mirna transfection reagent PROTOCOL DESCRIPTION INTERFERin is a powerful sirna/mirna transfection reagent that ensures efficient gene silencing and reproducible transfection in
Rat Creatine Kinase MB Isoenzyme (CKMB) ELISA
 Rat Creatine Kinase MB Isoenzyme (CKMB) ELISA For the quantitative determination of rat CKMB in serum, plasma, tissue homogenates and other biological fluids. Cat. No. KT-12247 For Research Use Only. Not
Rat Creatine Kinase MB Isoenzyme (CKMB) ELISA For the quantitative determination of rat CKMB in serum, plasma, tissue homogenates and other biological fluids. Cat. No. KT-12247 For Research Use Only. Not
ECL Western Blotting Substrate INSTRUCTIONS FOR USE OF PRODUCTS W1001 AND W1015.
 Technical Manual ECL Western Blotting Substrate INSTRUCTIONS FOR USE OF PRODUCTS W1001 AND W1015. PRINTED IN USA. 6/09 ECL Western Blotting Substrate All technical literature is available on the Internet
Technical Manual ECL Western Blotting Substrate INSTRUCTIONS FOR USE OF PRODUCTS W1001 AND W1015. PRINTED IN USA. 6/09 ECL Western Blotting Substrate All technical literature is available on the Internet
Mouse IgM ELISA. Cat. No. KT-407 K-ASSAY. For the quantitative determination of IgM in mouse biological samples. For Research Use Only. 1 Rev.
 K-ASSAY Mouse IgM ELISA For the quantitative determination of IgM in mouse biological samples Cat. No. KT-407 For Research Use Only. 1 Rev. 072309 K-ASSAY PRODUCT INFORMATION Mouse IgM ELISA Cat. No. KT-407
K-ASSAY Mouse IgM ELISA For the quantitative determination of IgM in mouse biological samples Cat. No. KT-407 For Research Use Only. 1 Rev. 072309 K-ASSAY PRODUCT INFORMATION Mouse IgM ELISA Cat. No. KT-407
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Aseptic Technique. A GMP/GTP Training Module
 Aseptic Technique A GMP/GTP Training Module Aseptic Technique The GMP Facility manufactures products for clinical use These products must meet a number of requirements, one of which is that they are sterile
Aseptic Technique A GMP/GTP Training Module Aseptic Technique The GMP Facility manufactures products for clinical use These products must meet a number of requirements, one of which is that they are sterile
Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum
 SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
Cell Migration, Chemotaxis and Invasion Assay Protocol
 Cell Migration, Chemotaxis and Invasion Assay Protocol Hilary Sherman, Pilar Pardo and Todd Upton Corning Life Sciences 900 Chelmsford Street Lowell, MA 01851 Introduction Cell migration, the movement
Cell Migration, Chemotaxis and Invasion Assay Protocol Hilary Sherman, Pilar Pardo and Todd Upton Corning Life Sciences 900 Chelmsford Street Lowell, MA 01851 Introduction Cell migration, the movement
Minimal residual disease detection in Acute Myeloid Leukaemia on a Becton Dickinson flow cytometer
 Minimal residual disease detection in Acute Myeloid Leukaemia on a Becton Dickinson flow cytometer Purpose This procedure gives instruction on minimal residual disease (MRD) detection in patients with
Minimal residual disease detection in Acute Myeloid Leukaemia on a Becton Dickinson flow cytometer Purpose This procedure gives instruction on minimal residual disease (MRD) detection in patients with
Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
Standard Operating Procedures (SOPs) for the production and testing of embryonic stem cell lines in Taiwan Stem Cell Bank
 Standard Operating Procedures (SOPs) for the production and testing of embryonic stem cell lines in Taiwan Stem Cell Bank Bioresource Collection and Research Centre (BCRC) at Food Industry Research and
Standard Operating Procedures (SOPs) for the production and testing of embryonic stem cell lines in Taiwan Stem Cell Bank Bioresource Collection and Research Centre (BCRC) at Food Industry Research and
TransIT -2020 Transfection Reagent
 Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Xpert TM Animal Tissue Culture Teaching Kit
 Xpert TM Animal Tissue Culture Teaching Kit Product Code: CCK005 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques
Xpert TM Animal Tissue Culture Teaching Kit Product Code: CCK005 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques
Mouse Insulin ELISA. For the quantitative determination of insulin in mouse serum and plasma
 Mouse Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use Only. Not For Use
Mouse Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use Only. Not For Use
ab185915 Protein Sumoylation Assay Ultra Kit
 ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
Modulating Glucose Uptake in Skeletal Myotubes:
 icell Skeletal Myoblasts Application Protocol Introduction Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis The skeletal muscle is one of the
icell Skeletal Myoblasts Application Protocol Introduction Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis The skeletal muscle is one of the
Effective Methods For Culturing Breast Cancer Cell Lines
 Abstract Effective Methods For Culturing Breast Cancer Cell Lines William H. Marshall II Life Science Team Cell culturing is one of the most useful and prolific techniques practiced in biological science
Abstract Effective Methods For Culturing Breast Cancer Cell Lines William H. Marshall II Life Science Team Cell culturing is one of the most useful and prolific techniques practiced in biological science
Xpert TM Karyotyping Teaching Kit
 Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Benchtop Mitochondria Isolation Protocol
 Benchtop Mitochondria Isolation Protocol Note: Specific protocols are available for the following products: MS850 Mitochondria Isolation Kit for Rodent Tissue MS851 Mitochondria Isolation Kit for Rodent
Benchtop Mitochondria Isolation Protocol Note: Specific protocols are available for the following products: MS850 Mitochondria Isolation Kit for Rodent Tissue MS851 Mitochondria Isolation Kit for Rodent
Creatine Kinase (CK) Enzymatic Assay Kit Manual Catalog #: 3460-07
 Creatine Kinase (CK) Enzymatic Assay Kit Manual Catalog #: 3460-07 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3
Creatine Kinase (CK) Enzymatic Assay Kit Manual Catalog #: 3460-07 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3
Fighting the Battles: Conducting a Clinical Assay
 Fighting the Battles: Conducting a Clinical Assay 6 Vocabulary: In Vitro: studies in biology that are conducted using components of an organism that have been isolated from their usual biological surroundings
Fighting the Battles: Conducting a Clinical Assay 6 Vocabulary: In Vitro: studies in biology that are conducted using components of an organism that have been isolated from their usual biological surroundings
LAB 14 ENZYME LINKED IMMUNOSORBENT ASSAY (ELISA)
 STUDENT GUIDE LAB 14 ENZYME LINKED IMMUNOSORBENT ASSAY (ELISA) GOAL The goal of this laboratory lesson is to explain the concepts and technique of enzyme linked immunosorbent assay (ELISA). OBJECTIVES
STUDENT GUIDE LAB 14 ENZYME LINKED IMMUNOSORBENT ASSAY (ELISA) GOAL The goal of this laboratory lesson is to explain the concepts and technique of enzyme linked immunosorbent assay (ELISA). OBJECTIVES
QIAGEN Supplementary Protocol
 QIAGEN Supplementary Protocol Isolation of Peripheral Blood Mononuclear Cells (PBMC) and Purification of Total RNA from PBMC Using the RNeasy Micro or Mini Kit This protocol describes how to isolate PBMC
QIAGEN Supplementary Protocol Isolation of Peripheral Blood Mononuclear Cells (PBMC) and Purification of Total RNA from PBMC Using the RNeasy Micro or Mini Kit This protocol describes how to isolate PBMC
RayBio Creatine Kinase (CK) Activity Colorimetric Assay Kit
 RayBio Creatine Kinase (CK) Activity Colorimetric Assay Kit User Manual Version 1.0 May 28, 2014 RayBio Creatine Kinase Activity Colorimetric Assay (Cat#: 68CL-CK-S100) RayBiotech, Inc. We Provide You
RayBio Creatine Kinase (CK) Activity Colorimetric Assay Kit User Manual Version 1.0 May 28, 2014 RayBio Creatine Kinase Activity Colorimetric Assay (Cat#: 68CL-CK-S100) RayBiotech, Inc. We Provide You
Data Sheet. PD-L1:B7-1[Biotinylated] Inhibitor Screening Assay Kit Catalog # 72026 Size: 96 reactions
![Data Sheet. PD-L1:B7-1[Biotinylated] Inhibitor Screening Assay Kit Catalog # 72026 Size: 96 reactions Data Sheet. PD-L1:B7-1[Biotinylated] Inhibitor Screening Assay Kit Catalog # 72026 Size: 96 reactions](/thumbs/40/21461210.jpg) Data Sheet PD-L1:B7-1[Biotinylated] Inhibitor Screening Assay Kit Catalog # 72026 Size: 96 reactions BACKGROUND: There have been a number of studies defining a role for PD-L1 in the regulation of immune
Data Sheet PD-L1:B7-1[Biotinylated] Inhibitor Screening Assay Kit Catalog # 72026 Size: 96 reactions BACKGROUND: There have been a number of studies defining a role for PD-L1 in the regulation of immune
Chromatin Immunoprecipitation
 Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
The fastest spin-column based procedure for purifying up to 10 mg of ultra-pure endotoxin-free transfection-grade plasmid DNA.
 INSTRUCTION MANUAL ZymoPURE Plasmid Gigaprep Kit Catalog Nos. D4204 (Patent Pending) Highlights The fastest spin-column based procedure for purifying up to 10 mg of ultra-pure endotoxin-free transfection-grade
INSTRUCTION MANUAL ZymoPURE Plasmid Gigaprep Kit Catalog Nos. D4204 (Patent Pending) Highlights The fastest spin-column based procedure for purifying up to 10 mg of ultra-pure endotoxin-free transfection-grade
PRODUCT INFORMATION...
 VIROMER RED In vitro plasmid DNA and mrna Standard Transfection PRODUCT INFORMATION... 2 GENERAL... 2 RED OR YELLOW?... 3 PROTOCOL GUIDELINES... 4 GENERAL REMARKS... 4 CELL CULTURE AND PLATING... 4 FORWARD/REVERSE
VIROMER RED In vitro plasmid DNA and mrna Standard Transfection PRODUCT INFORMATION... 2 GENERAL... 2 RED OR YELLOW?... 3 PROTOCOL GUIDELINES... 4 GENERAL REMARKS... 4 CELL CULTURE AND PLATING... 4 FORWARD/REVERSE
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY. STANDARD OPERATING PROCEDURE PROCEDURE NO: GLP 079 MOD: 1st Issue Page: 1 of 7
 THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY STANDARD OPERATING PROCEDURE PROCEDURE NO: GLP 079 MOD: 1st Issue Page: 1 of 7 Procedure Type: Title: General Laboratory Procedure Cell Counts
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY STANDARD OPERATING PROCEDURE PROCEDURE NO: GLP 079 MOD: 1st Issue Page: 1 of 7 Procedure Type: Title: General Laboratory Procedure Cell Counts
Automation of Cell Staining Technical Information Bulletin
 Automation of Cell Staining Technical Information Bulletin Automation of Cell Staining Using the Biomek 4000 Laboratory Automation Workstation Amy N. Yoder, Sr. Development Scientist and Li Liu, Sr. Development
Automation of Cell Staining Technical Information Bulletin Automation of Cell Staining Using the Biomek 4000 Laboratory Automation Workstation Amy N. Yoder, Sr. Development Scientist and Li Liu, Sr. Development
Amaxa Mouse/Rat Hepatocyte Nucleofector Kit
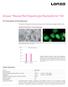 Amaxa Mouse/Rat Hepatocyte Nucleofector Kit For Primary Mouse and Rat Hepatocytes This protocol is designed for freshly isolated mouse and rat hepatocytes; polygonal, adherent cells Example for Nucleofection
Amaxa Mouse/Rat Hepatocyte Nucleofector Kit For Primary Mouse and Rat Hepatocytes This protocol is designed for freshly isolated mouse and rat hepatocytes; polygonal, adherent cells Example for Nucleofection
MEF Nucleofector Kit 1 and 2
 page 1 of 7 MEF Nucleofector Kit 1 and 2 for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the they are isolated
page 1 of 7 MEF Nucleofector Kit 1 and 2 for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the they are isolated
