PROTONS, NEUTRONS, ELECTRONS
|
|
|
- Brittney Hines
- 7 years ago
- Views:
Transcription
1 PROTONS, NEUTRONS, ELECTRONS NAME DATE PERIOD SC.A determines the mass number and atomic number of an atom from the number of protons and neutrons MA.A solves multi-step real-world problems involving whole numbers, fractions or decimals using appropriate methods of computation, such as mental computation, paper and pencil, and calculator MATERIALS: Periodic Table BACKGROUND INFORMATION: 1. Element atoms with the same number of protons are of the same element 2. Isotope atoms of the same element with different numbers of neutrons are different isotopes of that element 3. Atomic Number = Number of Protons 4. Number of Protons = Number of Electrons 5. Atomic Mass Number of Protons = Number of Neutrons COMPLETE THE FOLLOWING CHART: NOTE: Masses have been rounded to the nearest whole number. ELEMENT PROTONS NEUTRONS MASS ELECTRONS Hydrogen 1 0 Nitrogen 7 7 Potassium Gold Isotopes 1
2 PROTONS, NEUTRONS, ELECTRONS Page 2 ISOTOPE OR DIFFERENT ATOM: Determine if the following atoms are isotopes or different atoms. 1. Atom A has 8 protons and 9 neutrons. Atom D has 8 protons and 10 neutrons. 2. Atom E has 10 protons and 9 neutrons. Atom G has 12 protons and 9 neutrons. 3. Atom J has an atomic number of 4 and 6 neutrons. Atom L has 4 protons and 9 neutrons. Isotopes 2
3 PROTONS, NEUTRONS, ELECTRONS NAME TEACHER COPY DATE PERIOD SC.A determines the mass number and atomic number of an atom from the number of protons and neutrons MA.A solves multi-step real-world problems involving whole numbers, fractions or decimals using appropriate methods of computation, such as mental computation, paper and pencil, and calculator MATERIALS: Periodic Table BACKGROUND INFORMATION: 1. Element atoms with the same number of protons are of the same element 2. Isotope atoms of the same element with different numbers of neutrons are different isotopes of that element 3. Atomic Number = Number of Protons 4. Number of Protons = Number of Electrons 5. Atomic Mass Number of Protons = Number of Neutrons COMPLETE THE FOLLOWING CHART: NOTE: Masses have been rounded to the nearest whole number. ELEMENT PROTONS NEUTRONS MASS ELECTRONS Hydrogen Nitrogen Potassium Gold Isotopes 3
4 PROTONS, NEUTRONS, ELECTRONS Teacher Copy Page 2 ISOTOPE OR DIFFERENT ATOM: Determine if the following atoms are isotopes or different atoms. 1. Atom A has 8 protons and 9 neutrons. Atom D has 8 protons and 10 neutrons. 2. Atom E has 10 protons and 9 neutrons. Atom G has 12 protons and 9 neutrons. 3. Atom J has an atomic number of 4 and 6 neutrons. Atom L has 4 protons and 9 neutrons. ISOTOPE DIFFERENT ISOTOPE Isotopes 4
5 ATOMS KWL CHART NAME DATE PERIOD SC.A determines the mass number and atomic number of an atom from the number of protons and neutrons K What do you KNOW? W What do you WANT to know? 1. From the periodic table what number equals the number of protons? L What did you LEARN? 2. From the periodic table how is the mass of a regular atom determined? 3. Define isotope. 4. Why are the masses on the periodic table decimal numbers? Isotopes 5
6 ATOMS KWL CHART NAME TEACHER COPY DATE PERIOD SC.A determines the mass number and atomic number of an atom from the number of protons and neutrons K What do you KNOW? W What do you WANT to know? 1. From the periodic table what number equals the number of protons? ATOMIC NUMBER L What did you LEARN? 2. From the periodic table how is the mass of a regular atom determined? PROTONS+ NEUTRONS = ATOMIC MASS 3. Define isotope. ATOM OF AN ELEMENT THAT HAS THE SAME NUMBER OF PROTONS AS ANOTHER ATOM OF THE SAME ELEMENT BUT A DIFFERENT NUMBER OF NEUTRONS. 4. Why are the masses on the periodic table decimal numbers? AVERAGE OF ALL THE ISOTOPES OF THAT ELEMENT Isotopes 6
7 ISOTOPES RUBRIC NAME DATE PERIOD SC.A determines the mass number and atomic number of an atom from the number of protons and neutrons SC.H uses appropriate procedures for safety in the classroom, home and community MA.B measures accurately with the measurement tools to the specified degree of accuracy for the task and in keeping with the precision of the measurement tool Category ATOMIC NUMBER MASS NUMBER USE OF LAB EQUIPMENT LAB SAFETY MEASUREMENT GOAL 3: #3 GOAL 3: #8 Identifies the atomic number as being the same number as the number of protons. Identifies the mass number as being the number protons + the number of neutrons. Lab equipment used with little or no direction from teacher. All safety rules in the lab are followed. Measurements made with little or no direction from teacher. The student accurately uses numeric operations to complete mass averages. The student completes all tasks and works/communicates effectively with other class members. Lab equipment used effectively with some extra direction from teacher. Measurements made with some extra direction from teacher. The student completes most important tasks and works/communicates effectively with other class members. Lab equipment used effectively, but with guidance from teacher. Measurements made effectively, but with guidance from teacher. The student completes some of the important tasks and works/communicates effectively with other class members. With help identifies the atomic number as being the same number as the number of protons. With help identifies the mass number as being the number protons + the number of neutrons. Struggles with directions for using lab equipment. One or more safety rules are not followed. Struggles with directions for completing measurements. The student shows minimal accuracy in the use of numeric operations and is unable to complete the mass averages. The student shows minimal understanding of the tasks and is unable to work/communicate effectively with other class members. Isotopes 7
8 ISOTOPES MODEL LAB NAME DATE PERIOD SC.H uses appropriate procedures for safety in the classroom, home and community MA.B measures accurately with the measurement tools to the specified degree of accuracy for the task and in keeping with the precision of the measurement tool MA.A solves multi-step real-world problems involving whole numbers, fractions or decimals using appropriate methods of computation, such as mental computation, paper and pencil, and calculator OBJECTIVE: To construct an isotope model. MATERIALS: 60 dry lima beans (20 small, 20 medium, 20 large) sandwich-size Ziploc bag balance (triple beam or electronic) PROCEDURE: 1. Count out 50 whole lima beans from the Ziploc bag. Place the others back in the bag. 2. Place the 50 lima beans on the platform of the balance and determine the mass to the nearest tenth of a gram. 3. Record this mass on your Data Chart. (See below) 4. Now find the average mass of these 50 lima beans and record on the Data Chart. Average mass = mass of beans divided by number of beans. 5. Remove the 50 lima beans from the platform and divide into three piles. Small, medium, large. 6. Select one small bean and place on the platform of the balance. Determine the mass and record on the Data Chart. 7. Repeat procedure #6 with a medium size bean and then a large bean. 8. Answer Questions # Look at the three piles of beans and the individual masses for each size bean. Now answer Question # Place all the lima beans in the Ziploc bag and clean up. DATA CHART: 50 Beans TOTAL MASS = AVG. MASS = 1 small bean MASS = 1 medium bean MASS = 1 large bean MASS = Isotopes 8
9 ISOTOPES MODEL LAB Page 2 QUESTIONS: Questions 1 3 circle the correct answer. 1. How does the mass of the smaller bean compare with the average mass of the 50 beans? 2. How does the mass of the medium bean compare with the average mass of the 50 beans? 3. How does the mass of the larger bean compare with the average mass of the 50 beans? 4. How are the lima beans in this experiment like the isotopes of an element? Isotopes 9
10 ISOTOPES MODEL LAB Teacher Copy Page 2 QUESTIONS: Questions 1 3 circle the correct answer. ANSWERS TO #2 CAN VARY. 1. How does the mass of the smaller bean compare with the average mass of the 50 beans? 2. How does the mass of the medium bean compare with the average mass of the 50 beans? 3. How does the mass of the larger bean compare with the average mass of the 50 beans? 4. How are the lima beans in this experiment like the isotopes of an element? The size and mass vary but they are still lima beans. (Isotopes of an element will vary in size and mass, but will still be called by that element name.) Isotopes 10
Element of same atomic number, but different atomic mass o Example: Hydrogen
 Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
Atomic mass: p + = protons; e - = electrons; n 0 = neutrons p + + n 0 = atomic mass o For carbon-12, 6p + + 6n 0 = atomic mass of 12.0 o For chlorine-35, 17p + + 18n 0 = atomic mass of 35.0 atomic mass
3. ROUNDING OFF DECIMAL NUMBERS TO THE NEAREST TENTH
 3. ROUNDING OFF DECIMAL NUMBERS TO THE NEAREST TENTH Material: Decimal Board and materials Small gold circle Prepared problems Paper and pencil Presentation: 1. Form a number on the board with discs: 0.68.
3. ROUNDING OFF DECIMAL NUMBERS TO THE NEAREST TENTH Material: Decimal Board and materials Small gold circle Prepared problems Paper and pencil Presentation: 1. Form a number on the board with discs: 0.68.
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
6.7: Explaining the Periodic Table pg. 234
 Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Unit C: Atoms, elements, and Compounds 6.7: Explaining the Periodic Table pg. 234 Key Concepts: 3. Elements are organized according to their atomic number and electron arrangement on the periodic table.
Creating Graphs. Learning Objective-To create graphs that show data.
 Creating Graphs Summary- Key Words- Students will be able to identify graphs, components of graphs, interpret graphs, and construct various types of graphs. Pictograph Bar Graph Circle Graph Background
Creating Graphs Summary- Key Words- Students will be able to identify graphs, components of graphs, interpret graphs, and construct various types of graphs. Pictograph Bar Graph Circle Graph Background
Math. Rounding Decimals. Answers. 1) Round to the nearest tenth. 8.54 8.5. 2) Round to the nearest whole number. 99.59 100
 1) Round to the nearest tenth. 8.54 8.5 2) Round to the nearest whole number. 99.59 100 3) Round to the nearest tenth. 310.286 310.3 4) Round to the nearest whole number. 6.4 6 5) Round to the nearest
1) Round to the nearest tenth. 8.54 8.5 2) Round to the nearest whole number. 99.59 100 3) Round to the nearest tenth. 310.286 310.3 4) Round to the nearest whole number. 6.4 6 5) Round to the nearest
Chemical Composition Review Mole Calculations Percent Composition. Copyright Cengage Learning. All rights reserved. 8 1
 Chemical Composition Review Mole Calculations Percent Composition Copyright Cengage Learning. All rights reserved. 8 1 QUESTION Suppose you work in a hardware store and a customer wants to purchase 500
Chemical Composition Review Mole Calculations Percent Composition Copyright Cengage Learning. All rights reserved. 8 1 QUESTION Suppose you work in a hardware store and a customer wants to purchase 500
Atomic Theory Part 1
 Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Atomic Theory Part 1 Reading: Ch 2 sections 1 6, 8 Homework: Chapter 2: 39, 47, 43, 49, 51*, 53, 55, 57, 71, 73, 77, 99, 103 (optional) * = important homework question The Atomic Theory (John Dalton, 1803)
Chapter Five: Atomic Theory and Structure
 Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Chapter Five: Atomic Theory and Structure Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
( + and - ) ( - and - ) ( + and + ) Atoms are mostly empty space. = the # of protons in the nucleus. = the # of protons in the nucleus
 Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Atoms are mostly empty space Atomic Structure Two regions of every atom: Nucleus - is made of protons and neutrons - is small and dense Electron cloud -is a region where you might find an electron -is
Name: Worksheet: Electron Configurations. I Heart Chemistry!
 1. Which electron configuration represents an atom in an excited state? 1s 2 2s 2 2p 6 3p 1 1s 2 2s 2 2p 6 3s 2 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name:
1. Which electron configuration represents an atom in an excited state? 1s 2 2s 2 2p 6 3p 1 1s 2 2s 2 2p 6 3s 2 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name:
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Directions: T. Trimpe 2005 http://sciencespot.net/
 Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
2 The Structure of Atoms
 CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
CHAPTER 4 2 The Structure of Atoms SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What do atoms of the same element have in common? What are isotopes? How is an element
Welcome to Physics 40!
 Welcome to Physics 40! Physics for Scientists and Engineers Lab 1: Introduction to Measurement SI Quantities & Units In mechanics, three basic quantities are used Length, Mass, Time Will also use derived
Welcome to Physics 40! Physics for Scientists and Engineers Lab 1: Introduction to Measurement SI Quantities & Units In mechanics, three basic quantities are used Length, Mass, Time Will also use derived
Teacher Information Lesson Title: Density labs
 Teacher Information Lesson Title: Density labs Lesson Description: These labs are hands on exercises that will allow the students to measure and calculate the densities of different types of objects. The
Teacher Information Lesson Title: Density labs Lesson Description: These labs are hands on exercises that will allow the students to measure and calculate the densities of different types of objects. The
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS
 Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
Woods Chem-1 Lec-02 10-1 Atoms, Ions, Mole (std) Page 1 ATOMIC THEORY, MOLECULES, & IONS Proton: A positively charged particle in the nucleus Atomic Number: We differentiate all elements by their number
Particle Soup: Big Bang Nucleosynthesis
 Name: Partner(s): Lab #7 Particle Soup: Big Bang Nucleosynthesis Purpose The student explores how helium was made in the Big Bang. Introduction Very little helium is made in stars. Yet the universe is
Name: Partner(s): Lab #7 Particle Soup: Big Bang Nucleosynthesis Purpose The student explores how helium was made in the Big Bang. Introduction Very little helium is made in stars. Yet the universe is
Plumbing and Pipe-Fitting Challenges
 Plumbing and Pipe-Fitting Challenges Students often wonder when they will use the math they learn in school. These activities answer that question as it relates to measuring, working with fractions and
Plumbing and Pipe-Fitting Challenges Students often wonder when they will use the math they learn in school. These activities answer that question as it relates to measuring, working with fractions and
The Cell Cycle: A series of modeling activities
 The Cell Cycle: A series of modeling activities Cancer Education Project University of Rochester Premise: Students learn best when exposed to a variety of activities Overview 1. Information Gathering:
The Cell Cycle: A series of modeling activities Cancer Education Project University of Rochester Premise: Students learn best when exposed to a variety of activities Overview 1. Information Gathering:
hundred thousands one thousands ten thousands one millions hundreds
 Title: Rounding Numbers Grade(s): 4 Subject(s): Mathematics, Technology Education Author: ICAC Team Overview: After reviewing place value and rounding, students will create place value tables and solve
Title: Rounding Numbers Grade(s): 4 Subject(s): Mathematics, Technology Education Author: ICAC Team Overview: After reviewing place value and rounding, students will create place value tables and solve
 Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
Questions Q1. Lithium, sodium and potassium are metals in group 1 of the periodic table. They are good conductors of heat and electricity. The freshly-cut metals are shiny. (a) (i) Give another physical
INTRODUCTION TO FRACTIONS
 Tallahassee Community College 16 INTRODUCTION TO FRACTIONS Figure A (Use for 1 5) 1. How many parts are there in this circle?. How many parts of the circle are shaded?. What fractional part of the circle
Tallahassee Community College 16 INTRODUCTION TO FRACTIONS Figure A (Use for 1 5) 1. How many parts are there in this circle?. How many parts of the circle are shaded?. What fractional part of the circle
DIVISION OF DECIMALS. 1503 9. We then we multiply by the
 Tallahassee Community College 0 DIVISION OF DECIMALS To divide 9, we write these fractions: reciprocal of the divisor 0 9. We then we multiply by the 0 67 67 = = 9 67 67 The decimal equivalent of is. 67.
Tallahassee Community College 0 DIVISION OF DECIMALS To divide 9, we write these fractions: reciprocal of the divisor 0 9. We then we multiply by the 0 67 67 = = 9 67 67 The decimal equivalent of is. 67.
Matter. Atomic weight, Molecular weight and Mole
 Matter Atomic weight, Molecular weight and Mole Atomic Mass Unit Chemists of the nineteenth century realized that, in order to measure the mass of an atomic particle, it was useless to use the standard
Matter Atomic weight, Molecular weight and Mole Atomic Mass Unit Chemists of the nineteenth century realized that, in order to measure the mass of an atomic particle, it was useless to use the standard
Guidance paper - The use of calculators in the teaching and learning of mathematics
 Guidance paper - The use of calculators in the teaching and learning of mathematics Background and context In mathematics, the calculator can be an effective teaching and learning resource in the primary
Guidance paper - The use of calculators in the teaching and learning of mathematics Background and context In mathematics, the calculator can be an effective teaching and learning resource in the primary
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1. A chemical equation. (C-4.4)
 Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
Honors Chemistry: Unit 6 Test Stoichiometry PRACTICE TEST ANSWER KEY Page 1 1. 2. 3. 4. 5. 6. Question What is a symbolic representation of a chemical reaction? What 3 things (values) is a mole of a chemical
ATOMS A T O M S, I S O T O P E S, A N D I O N S. The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39)
 ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
ATOMS A T O M S, I S O T O P E S, A N D I O N S The Academic Support Center @ Daytona State College (Science 120, Page 1 of 39) THE ATOM All elements listed on the periodic table are made up of atoms.
Atomic Structure OBJECTIVES SCHEDULE PREPARATION VOCABULARY MATERIALS. For each team of four. The students. For the class.
 activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
activity 4 Atomic Structure OBJECTIVES Students are introduced to the structure of the atom and the nature of subatomic particles. The students are introduced to the properties of protons, neutrons, and
MathSphere MATHEMATICS. Equipment. Y6 Fractions 6365 Round decimals. Equivalence between decimals and fractions
 MATHEMATICS Y6 Fractions 6365 Round decimals. Equivalence between decimals and fractions Paper, pencil, ruler Fraction cards Calculator Equipment MathSphere 6365 Round decimals. Equivalence between fractions
MATHEMATICS Y6 Fractions 6365 Round decimals. Equivalence between decimals and fractions Paper, pencil, ruler Fraction cards Calculator Equipment MathSphere 6365 Round decimals. Equivalence between fractions
THE PERIODIC TABLE O F T H E E L E M E N T S. The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27)
 THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
THE PERIODIC TABLE O F T H E E L E M E N T S The Academic Support Center @ Daytona State College (Science 117, Page 1 of 27) THE PERIODIC TABLE In 1872, Dmitri Mendeleev created the periodic table arranged
PIZZA! PIZZA! TEACHER S GUIDE and ANSWER KEY
 PIZZA! PIZZA! TEACHER S GUIDE and ANSWER KEY The Student Handout is page 11. Give this page to students as a separate sheet. Area of Circles and Squares Circumference and Perimeters Volume of Cylinders
PIZZA! PIZZA! TEACHER S GUIDE and ANSWER KEY The Student Handout is page 11. Give this page to students as a separate sheet. Area of Circles and Squares Circumference and Perimeters Volume of Cylinders
Rational Number Project
 Rational Number Project Fraction Operations and Initial Decimal Ideas Lesson 12: Overview Students review ordering and equivalence and practice adding and subtracting decimals in problem solving contexts.
Rational Number Project Fraction Operations and Initial Decimal Ideas Lesson 12: Overview Students review ordering and equivalence and practice adding and subtracting decimals in problem solving contexts.
Converting Units of Measure Measurement
 Converting Units of Measure Measurement Outcome (lesson objective) Given a unit of measurement, students will be able to convert it to other units of measurement and will be able to use it to solve contextual
Converting Units of Measure Measurement Outcome (lesson objective) Given a unit of measurement, students will be able to convert it to other units of measurement and will be able to use it to solve contextual
2014 Spring CHEM101 Ch1-2 Review Worksheet Modified by Dr. Cheng-Yu Lai,
 Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Ch1 1) Which of the following underlined items is not an intensive property? A) A chemical reaction requires 3.00 g of oxygen. B) The density of helium at 25 C is 1.64 10-4 g/cm3. C) The melting point
Performance Based Learning and Assessment Task Pizza Sector Task I. ASSESSSMENT TASK OVERVIEW & PURPOSE: Students will be introduced to the concept
 Performance Based Learning and Assessment Task Pizza Sector Task I. ASSESSSMENT TASK OVERVIEW & PURPOSE: Students will be introduced to the concept of a sector of a circle and will learn the formula used
Performance Based Learning and Assessment Task Pizza Sector Task I. ASSESSSMENT TASK OVERVIEW & PURPOSE: Students will be introduced to the concept of a sector of a circle and will learn the formula used
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Star of the Solar System-The Sun
 Star of the Solar System-The Sun Lesson Concept Link The solar system is comprised of the Sun, our closest star, and eight planets. The sun is at the center and is the primary energy source for Earth.
Star of the Solar System-The Sun Lesson Concept Link The solar system is comprised of the Sun, our closest star, and eight planets. The sun is at the center and is the primary energy source for Earth.
Study Guide For Chapter 7
 Name: Class: Date: ID: A Study Guide For Chapter 7 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The number of atoms in a mole of any pure substance
Name: Class: Date: ID: A Study Guide For Chapter 7 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The number of atoms in a mole of any pure substance
100 cm 1 m. = 614 cm. 6.14 m. 2.54 cm. 1 m 1 in. 1 m. 2.54 cm 1ft. 1 in = 242 in. 614 cm. 242 in 1 ft. 1 in. 100 cm = 123 m
 Units and Unit Conversions 6. Define the problem: If the nucleus were scaled to a diameter of 4 cm, determine the diameter of the atom. Develop a plan: Find the accepted relationship between the size of
Units and Unit Conversions 6. Define the problem: If the nucleus were scaled to a diameter of 4 cm, determine the diameter of the atom. Develop a plan: Find the accepted relationship between the size of
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
CHEM 101/105 Numbers and mass / Counting and weighing Lect-03
 CHEM 101/105 Numbers and mass / Counting and weighing Lect-03 Interpretation of Elemental Chemical Symbols, Chemical Formulas, and Chemical Equations Interpretation of an element's chemical symbol depends
CHEM 101/105 Numbers and mass / Counting and weighing Lect-03 Interpretation of Elemental Chemical Symbols, Chemical Formulas, and Chemical Equations Interpretation of an element's chemical symbol depends
Department of Physics and Geology The Elements and the Periodic Table
 Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Department of Physics and Geology The Elements and the Periodic Table Physical Science 1422 Equipment Needed Qty Periodic Table 1 Part 1: Background In 1869 a Russian chemistry professor named Dmitri Mendeleev
Name Date Class CHEMICAL QUANTITIES. SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296)
 Name Date Class 10 CHEMICAL QUANTITIES SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296) This section defines the mole and explains how the mole is used to measure matter. It also teaches
Name Date Class 10 CHEMICAL QUANTITIES SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296) This section defines the mole and explains how the mole is used to measure matter. It also teaches
Lesson 6: Earth and the Moon
 Lesson 6: Earth and the Moon Reading Assignment Chapter 7.1: Overall Structure of Planet Earth Chapter 7.3: Earth s Interior More Precisely 7-2: Radioactive Dating Chapter 7.5: Earth s Magnetosphere Chapter
Lesson 6: Earth and the Moon Reading Assignment Chapter 7.1: Overall Structure of Planet Earth Chapter 7.3: Earth s Interior More Precisely 7-2: Radioactive Dating Chapter 7.5: Earth s Magnetosphere Chapter
NOTES ON The Structure of the Atom
 NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
NOTES ON The Structure of the Atom Chemistry is the study of matter and its properties. Those properties can be explained by examining the atoms that compose the matter. An atom is the smallest particle
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004. Name (print) SSN
 CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
CHM 1311: General Chemistry 1, Fall 2004 Exam #1, September 8, 2004 Name (print) SSN Pledge: I have neither given nor received aid on this exam: Signature For ALL problems: SHOW ALL WORK TO GET FULL CREDIT
1. Get to know the SCRATCH Work Space:
 1. Get to know the SCRATCH Work Space: iquest Lesson http://www.csusm.edu/iquest - NSF Project Award Number: 0833753 Scratch 1 2. Click Control Drag When green flag is clicked. Always start every new script
1. Get to know the SCRATCH Work Space: iquest Lesson http://www.csusm.edu/iquest - NSF Project Award Number: 0833753 Scratch 1 2. Click Control Drag When green flag is clicked. Always start every new script
High Flying Balloons
 Second Grade Science Design Brief High Flying Balloons Background: In our study of science we have been investigating the three stages of matter: solids, liquids and gases. You will use your knowledge
Second Grade Science Design Brief High Flying Balloons Background: In our study of science we have been investigating the three stages of matter: solids, liquids and gases. You will use your knowledge
From the Webisode: Math Meets Culinary Arts
 lesson Connections to the Core Scaling Up From the Webisode: Math Meets Culinary Arts In this lesson, s solve a multi-step problem by using ratio and rate reasoning to scale for large quantities and compare
lesson Connections to the Core Scaling Up From the Webisode: Math Meets Culinary Arts In this lesson, s solve a multi-step problem by using ratio and rate reasoning to scale for large quantities and compare
How To Teach The Atom And Bonding
 An Instructional Unit Resource Guide for the Ninth Grade Curriculum Based on Principles of Universal Design and Differentiated Instruction Structure of Atoms and Bonding Designed By Elaine Marshall Rebecca
An Instructional Unit Resource Guide for the Ninth Grade Curriculum Based on Principles of Universal Design and Differentiated Instruction Structure of Atoms and Bonding Designed By Elaine Marshall Rebecca
Chapter 4, Lesson 2: The Periodic Table
 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of
Figure 1. A typical Laboratory Thermometer graduated in C.
 SIGNIFICANT FIGURES, EXPONENTS, AND SCIENTIFIC NOTATION 2004, 1990 by David A. Katz. All rights reserved. Permission for classroom use as long as the original copyright is included. 1. SIGNIFICANT FIGURES
SIGNIFICANT FIGURES, EXPONENTS, AND SCIENTIFIC NOTATION 2004, 1990 by David A. Katz. All rights reserved. Permission for classroom use as long as the original copyright is included. 1. SIGNIFICANT FIGURES
Autumn - 12 Weeks. Spring 11 Weeks. Summer 12 Weeks. Not As We Know It Limited 2014
 A Year 5 Mathematician Planning of coverage and resources. Autumn - 12 Weeks Spring 11 Weeks Summer 12 Weeks TARGETS NHM YR 5 Collins 5 Abacus 5 Abacus 6 LA Prior Step NHM 4 CPM 4 Ginn 4 Number, place
A Year 5 Mathematician Planning of coverage and resources. Autumn - 12 Weeks Spring 11 Weeks Summer 12 Weeks TARGETS NHM YR 5 Collins 5 Abacus 5 Abacus 6 LA Prior Step NHM 4 CPM 4 Ginn 4 Number, place
In mathematics, there are four attainment targets: using and applying mathematics; number and algebra; shape, space and measures, and handling data.
 MATHEMATICS: THE LEVEL DESCRIPTIONS In mathematics, there are four attainment targets: using and applying mathematics; number and algebra; shape, space and measures, and handling data. Attainment target
MATHEMATICS: THE LEVEL DESCRIPTIONS In mathematics, there are four attainment targets: using and applying mathematics; number and algebra; shape, space and measures, and handling data. Attainment target
Mass Spectrometry. Overview
 Mass Spectrometry Overview Mass Spectrometry is an analytic technique that utilizes the degree of deflection of charged particles by a magnetic field to find the relative masses of molecular ions and fragments.2
Mass Spectrometry Overview Mass Spectrometry is an analytic technique that utilizes the degree of deflection of charged particles by a magnetic field to find the relative masses of molecular ions and fragments.2
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
Materials: Student-paper, pencil, circle manipulatives, white boards, markers Teacher- paper, pencil, circle manipulatives, worksheet
 Topic: Creating Mixed Numbers and Improper Fractions SOL: 5.7- The student will add and subtract with fractions and mixed numbers, with and without regrouping, and express answers in simplest form. Problems
Topic: Creating Mixed Numbers and Improper Fractions SOL: 5.7- The student will add and subtract with fractions and mixed numbers, with and without regrouping, and express answers in simplest form. Problems
FOR TEACHERS ONLY The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION
 PS C FOR TEACERS ONLY The University of the State of New York REGENTS IG SCOOL EXAMINATION PYSICAL SETTING/CEMISTRY Wednesday, June, 0 :5 to :5 p.m., only SCORING KEY AND RATING GUIDE Directions to the
PS C FOR TEACERS ONLY The University of the State of New York REGENTS IG SCOOL EXAMINATION PYSICAL SETTING/CEMISTRY Wednesday, June, 0 :5 to :5 p.m., only SCORING KEY AND RATING GUIDE Directions to the
Atoms and Elements [6th grade]
![Atoms and Elements [6th grade] Atoms and Elements [6th grade]](/thumbs/40/21183873.jpg) Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, jwray@alum.trinity.edu
Trinity University Digital Commons @ Trinity Understanding by Design: Complete Collection Understanding by Design Summer 6-11-2015 Atoms and Elements [6th grade] Jennifer J. Wray Trinity University, jwray@alum.trinity.edu
Law of Conservation of Matter
 Law of onservation of Matter Type of Lesson: ontent with Process: Focus on constructing knowledge though active learning. IP ontent TEKS: 8 Investigate and identify the law of conservation of mass. Learning
Law of onservation of Matter Type of Lesson: ontent with Process: Focus on constructing knowledge though active learning. IP ontent TEKS: 8 Investigate and identify the law of conservation of mass. Learning
5th Grade Unit 1: Whole Number and Decimal Fraction Place Value to the One Thousandths (4 Weeks)
 5th Grade Unit : Whole Number and Decimal Fraction Place Value to the One Thousandths (4 Weeks) Stage Desired Results Established Goals Unit Description Students continue to extend and apply their understanding
5th Grade Unit : Whole Number and Decimal Fraction Place Value to the One Thousandths (4 Weeks) Stage Desired Results Established Goals Unit Description Students continue to extend and apply their understanding
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* CHEMISTRY 0620/03 Paper 3 Theory (Core) For Examination from 2016 SPECIMEN PAPER 1 hour
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0123456789* CHEMISTRY 0620/03 Paper 3 Theory (Core) For Examination from 2016 SPECIMEN PAPER 1 hour
LESSON 7 Don t Be A Square by Michael Torres
 CONCEPT AREA GRADE LEVEL Measurement 5-6 TIME ALLOTMENT Two 60-minute sessions LESSON OVERVIEW LESSON ACTIVITIES OVERVIEW LEARNING OBJECTIVES STANDARDS (TEKS) Students will learn the relationship between
CONCEPT AREA GRADE LEVEL Measurement 5-6 TIME ALLOTMENT Two 60-minute sessions LESSON OVERVIEW LESSON ACTIVITIES OVERVIEW LEARNING OBJECTIVES STANDARDS (TEKS) Students will learn the relationship between
3 Atomic Structure 15
 3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
3 Atomic Structure 15 3.1 Atoms You need to be familiar with the terms in italics The diameter of the nucleus is approximately 10-15 m and an atom 10-10 m. All matter consists of atoms. An atom can be
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Rational Number Project
 Rational Number Project Fraction Operations and Initial Decimal Ideas Lesson : Overview Students estimate sums and differences using mental images of the 0 x 0 grid. Students develop strategies for adding
Rational Number Project Fraction Operations and Initial Decimal Ideas Lesson : Overview Students estimate sums and differences using mental images of the 0 x 0 grid. Students develop strategies for adding
The Mole. Chapter 2. Solutions for Practice Problems
 Chapter 2 The Mole Note to teacher: You will notice that there are two different formats for the Sample Problems in the student textbook. Where appropriate, the Sample Problem contains the full set of
Chapter 2 The Mole Note to teacher: You will notice that there are two different formats for the Sample Problems in the student textbook. Where appropriate, the Sample Problem contains the full set of
LAB 5 - PLANT NUTRITION. Chemical Ionic forms Approximate dry Element symbol Atomic weight Absorbed by plants tissue concentration
 LAB 5 PLANT NUTRITION I. General Introduction All living organisms require certain elements for their survival. Plants are known to require carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus
LAB 5 PLANT NUTRITION I. General Introduction All living organisms require certain elements for their survival. Plants are known to require carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus
The Mole. 6.022 x 10 23
 The Mole 6.022 x 10 23 Background: atomic masses Look at the atomic masses on the periodic table. What do these represent? E.g. the atomic mass of Carbon is 12.01 (atomic # is 6) We know there are 6 protons
The Mole 6.022 x 10 23 Background: atomic masses Look at the atomic masses on the periodic table. What do these represent? E.g. the atomic mass of Carbon is 12.01 (atomic # is 6) We know there are 6 protons
MACMILLAN/McGRAW-HILL. MATH CONNECTS and IMPACT MATHEMATICS WASHINGTON STATE MATHEMATICS STANDARDS. ESSENTIAL ACADEMIC LEARNING REQUIREMENTS (EALRs)
 MACMILLAN/McGRAW-HILL MATH CONNECTS and IMPACT MATHEMATICS TO WASHINGTON STATE MATHEMATICS STANDARDS ESSENTIAL ACADEMIC LEARNING REQUIREMENTS (EALRs) And GRADE LEVEL EXPECTATIONS (GLEs) / Edition, Copyright
MACMILLAN/McGRAW-HILL MATH CONNECTS and IMPACT MATHEMATICS TO WASHINGTON STATE MATHEMATICS STANDARDS ESSENTIAL ACADEMIC LEARNING REQUIREMENTS (EALRs) And GRADE LEVEL EXPECTATIONS (GLEs) / Edition, Copyright
Key. Name: OBJECTIVES
 Name: Key OBJECTIVES Correctly define: observation, inference, classification, percent deviation, density, rate of change, cyclic change, dynamic equilibrium, interface, mass, volume GRAPHICAL RELATIONSHIPS
Name: Key OBJECTIVES Correctly define: observation, inference, classification, percent deviation, density, rate of change, cyclic change, dynamic equilibrium, interface, mass, volume GRAPHICAL RELATIONSHIPS
9/13/2013. However, Dalton thought that an atom was just a tiny sphere with no internal parts. This is sometimes referred to as the cannonball model.
 John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
John Dalton was an English scientist who lived in the early 1800s. Dalton s atomic theory served as a model for how matter worked. The principles of Dalton s atomic theory are: 1. Elements are made of
Addition Methods. Methods Jottings Expanded Compact Examples 8 + 7 = 15
 Addition Methods Methods Jottings Expanded Compact Examples 8 + 7 = 15 48 + 36 = 84 or: Write the numbers in columns. Adding the tens first: 47 + 76 110 13 123 Adding the units first: 47 + 76 13 110 123
Addition Methods Methods Jottings Expanded Compact Examples 8 + 7 = 15 48 + 36 = 84 or: Write the numbers in columns. Adding the tens first: 47 + 76 110 13 123 Adding the units first: 47 + 76 13 110 123
10.2 45-45 -90 Triangles
 Page of 6 0. --0 Triangles Goal Find the side lengths of --0 triangles. Key Words --0 triangle isosceles triangle p. 7 leg of a right triangle p. hypotenuse p. Geo-Activity Eploring an Isosceles Right
Page of 6 0. --0 Triangles Goal Find the side lengths of --0 triangles. Key Words --0 triangle isosceles triangle p. 7 leg of a right triangle p. hypotenuse p. Geo-Activity Eploring an Isosceles Right
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
Atomic Structure Chapter 5 Assignment & Problem Set
 Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
Atomic Structure Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Atomic Structure 2 Study Guide: Things You Must Know Vocabulary (know the definition
The Empirical Formula of a Compound
 The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
The Empirical Formula of a Compound Lab #5 Introduction A look at the mass relationships in chemistry reveals little order or sense. The ratio of the masses of the elements in a compound, while constant,
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Charlesworth School Year Group Maths Targets
 Charlesworth School Year Group Maths Targets Year One Maths Target Sheet Key Statement KS1 Maths Targets (Expected) These skills must be secure to move beyond expected. I can compare, describe and solve
Charlesworth School Year Group Maths Targets Year One Maths Target Sheet Key Statement KS1 Maths Targets (Expected) These skills must be secure to move beyond expected. I can compare, describe and solve
BPS Math Year at a Glance (Adapted from A Story Of Units Curriculum Maps in Mathematics K-5) 1
 Grade 4 Key Areas of Focus for Grades 3-5: Multiplication and division of whole numbers and fractions-concepts, skills and problem solving Expected Fluency: Add and subtract within 1,000,000 Module M1:
Grade 4 Key Areas of Focus for Grades 3-5: Multiplication and division of whole numbers and fractions-concepts, skills and problem solving Expected Fluency: Add and subtract within 1,000,000 Module M1:
Medium term Plans for Spring Year 5
 Medium term Plans for Spring Year 5 Help these children be in a better position to achieve good results in the Y6 Sats in 2015. Although these tests will officially be based on the old curriculum, it is
Medium term Plans for Spring Year 5 Help these children be in a better position to achieve good results in the Y6 Sats in 2015. Although these tests will officially be based on the old curriculum, it is
Introduce Decimals with an Art Project Criteria Charts, Rubrics, Standards By Susan Ferdman
 Introduce Decimals with an Art Project Criteria Charts, Rubrics, Standards By Susan Ferdman hundredths tenths ones tens Decimal Art An Introduction to Decimals Directions: Part 1: Coloring Have children
Introduce Decimals with an Art Project Criteria Charts, Rubrics, Standards By Susan Ferdman hundredths tenths ones tens Decimal Art An Introduction to Decimals Directions: Part 1: Coloring Have children
Instructors Guide: Atoms and Their Isotopes
 Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
Instructors Guide: Atoms and Their Isotopes Standards Connections Connections to NSTA Standards for Science Teacher Preparation C.3.a.1 Fundamental structures of atoms and molecules. C.3.b.27 Applications
For example: (Example is from page 50 of the Thinkbook)
 SOLVING COMBINED SPECTROSCOPY PROBLEMS: Lecture Supplement: page 50-53 in Thinkbook CFQ s and PP s: page 216 241 in Thinkbook Introduction: The structure of an unknown molecule can be determined using
SOLVING COMBINED SPECTROSCOPY PROBLEMS: Lecture Supplement: page 50-53 in Thinkbook CFQ s and PP s: page 216 241 in Thinkbook Introduction: The structure of an unknown molecule can be determined using
O o. Thomas Jefferson National Accelerator Facility - Office of Science Education http://education.jlab.org/
 O o b l ekk c What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for
O o b l ekk c What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for
Bohr s Model of the Atom
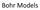 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Rational Number Project
 Rational Number Project Fraction Operations and Initial Decimal Ideas Lesson 1: Overview Students review how to model fractions with fraction circles by ordering unit fractions, using 1-half as a benchmark
Rational Number Project Fraction Operations and Initial Decimal Ideas Lesson 1: Overview Students review how to model fractions with fraction circles by ordering unit fractions, using 1-half as a benchmark
Science Notebooks in the Classroom. Notebook Criteria
 presentapresents Presents Science Notebooks in the Classroom kdkrifr Notebook Criteria This document was developed by Bay Area Schools for Excellence in Education (BASEE) a local systemic change project
presentapresents Presents Science Notebooks in the Classroom kdkrifr Notebook Criteria This document was developed by Bay Area Schools for Excellence in Education (BASEE) a local systemic change project
FIZIKA ANGOL NYELVEN
 ÉRETTSÉGI VIZSGA 2011. május 17. FIZIKA ANGOL NYELVEN KÖZÉPSZINTŰ ÍRÁSBELI VIZSGA 2011. május 17. 8:00 Az írásbeli vizsga időtartama: 120 perc Pótlapok száma Tisztázati Piszkozati NEMZETI ERŐFORRÁS MINISZTÉRIUM
ÉRETTSÉGI VIZSGA 2011. május 17. FIZIKA ANGOL NYELVEN KÖZÉPSZINTŰ ÍRÁSBELI VIZSGA 2011. május 17. 8:00 Az írásbeli vizsga időtartama: 120 perc Pótlapok száma Tisztázati Piszkozati NEMZETI ERŐFORRÁS MINISZTÉRIUM
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Changing a Decimal or Fraction to a Percent
 6. Changing a Decimal or Fraction to a Percent 6. OBJECTIVES. Change a decimal to a percent. Change a fraction to a percent. Change a mixed number to a percent Changing a decimal to a percent is the opposite
6. Changing a Decimal or Fraction to a Percent 6. OBJECTIVES. Change a decimal to a percent. Change a fraction to a percent. Change a mixed number to a percent Changing a decimal to a percent is the opposite
10 The Mole. Section 10.1 Measuring Matter
 Name Date Class The Mole Section.1 Measuring Matter In your textbook, read about counting particles. In Column B, rank the quantities from Column A from smallest to largest. Column A Column B 0.5 mol 1.
Name Date Class The Mole Section.1 Measuring Matter In your textbook, read about counting particles. In Column B, rank the quantities from Column A from smallest to largest. Column A Column B 0.5 mol 1.
