HUNTER TENDON IMPLANTS
|
|
|
- Claude Boone
- 9 years ago
- Views:
Transcription
1 HUNTER TENDON IMPLANTS The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe (tk) For additional languages, visit our website Then click on the Prescribing Information option. For additional information and translations please contact the manufacturer or local distributor. M C 0086* P Wright Medical Technology, Inc. Wright Medical EMEA 5677 Airline Rd. Krijgsman 11 Arlington, TN DM Amstelveen U.S.A. The Netherlands * The CE-Marking of Conformity is applied per catalog number and appears on the outer label, if applicable. January 2010 Printed in U.S.A
2
3 Attention Operating Surgeon IMPORTANT MEDICAL INFORMATION HUNTER TENDON IMPLANTS ( ) EN OUTLINE: I. GENERAL PRODUCT INFORMATION A. INDICATIONS B. CONTRAINDICATIONS C. WARNINGS D. PRECAUTIONS E. STERILIZATION PROCEDURES F. STORAGE CONDITIONS II. SPECIFIC PRODUCT INFORMATION A. HUNTER TENDON ROD & PASSIVE TENDON IMPLANTS B. HUNTER ACTIVE TENDON IMPLANTS C. HUNTER ACTIVE TENDON IMPLANT BC (BI-CORDAL) D. HUNTER ACTIVE TENDON IMPLANT DC (DISTAL CORD) E. HUNTER ACTIVE TENDON IMPLANT PC (PROXIMAL CORD) DEFINITIONS Symbols and abbreviations may be used on the package label. The following table provides the definition of these symbols and abbreviations.
4 Table 1. Definitions of Symbols and Abbreviations Symbol g h D Y i H l p N M P[]\ I Definition Batch code Catalog number Do not re-use Caution, consult accompanying documents Consult operating instructions Use by Temperature limitation Keep dry Keep away from sunlight Date of manufacture Manufacturer Authorized EC Representative in the European Community Sterilized using ethylene oxide
5 K STERILE GAS J Sterilized using radiation Sterilized using gas plasma Sterilized using aseptic processing techniques For prescription use only Abbreviation Ti Ti6Al4V CoCr SS UHMWPE Material Titanium Titanium Alloy Cobalt Chrome Alloy Stainless Steel Ultra High Molecular Weight Polyethylene I. GENERAL PRODUCT INFORMATION Through the advancement of partial and total joint replacement, the surgeon has been provided a means of restoring mobility, correcting deformity, and reducing pain for many patients. While the prostheses used are largely successful in attaining these goals, it must be recognized that they are manufactured from silicone and metal. In addition, the system will not be as strong, reliable, or durable as a natural human tendon.
6 The surgeon should be aware of the following: A. All products are manufactured from a woven polyester core covered with bariumimpregnated silicone elastomer. B. The judgement by a surgeon to implant silicone elastomer implants is a risk/benefit decision which must take into account the patient s needs and desire in addition to the surgeon s knowledge of expected results and complications as well as therapeutic alternatives. Wright Medical Technology, Inc., can provide a bibliography of articles on the use and complications of silicone elastomer implants to any physician. Please write or call Wright Medical Technology, Inc. C. The correct selection and sizing of the implant is extremely important. Selection of the proper size, shape, and design of the implant increases the potential for success. D. Wright Medical Technology, Inc., does not recommend a particular surgical technique when using the implant. Proper surgical procedures and techniques are necessarily the responsibility of the medical professional. Each surgeon must evaluate the appropriateness of the surgical technique used based on personal medical training and experience. E. Reshaping of the implant should be avoided because it can compromise or destroy the structural integrity and the functionality of the implant. F. In selecting patients for tendon replacements, the following factors can be critical to the eventual success of the procedure: 1. Patient s occupation or activity. If the patient is involved in an occupation or activity which includes substantial lifting or muscle strain, the resultant forces can cause failure of the fixation, the device, or both. The prosthesis will not restore function to the level expected with normal healthy tendon, and the patient should not have unrealistic functional expectations.
7 2. Condition of senility, mental illness, or alcoholism. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the prosthesis, leading to failure or other complications. 3. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests should be made prior to material selection or implantation. A. INDICATIONS This device is indicated for use in stage one of the two-stage procedure developed by Dr. James M. Hunter for the reconstruction of flexor and extensor tendons in individuals having significant hand tendon injury. This device is intended to be implanted temporarily in order to encourage the formation of a pseudosynovial sheath, which will later nourish and lubricate an autogenous tendon graft. See Section II for specific product information. B. CONTRAINDICATIONS This device is not intended for any use other than that indicated. Residual antecedent infection is a contraindication for the use of this device. Appropriate surgical and antimicrobial treatment and subsequent wound healing will allow the procedure to be carried out at a later date. A digit that has a scarred tendon bed, borderline nutrition, nerve deficit and severe joint stiffness could possibly be salvaged; however, this should probably be undertaken only in the patient with very special requirements. See Section II for specific product information.
8 C. WARNINGS Synovitis, adhesion, and wound infection have been reported as complications of reconstructive procedures which are similar to the HUNTER Tendon Implants, and therefore, must be assumed to be potential complications of the use of this device. Device migration has also been reported in the use of similar devices, but it is thought to be unlikely in the use of this device. In any surgical procedure, the potential for complications exists. The risks and complications with silicone implants include: Infection or painful, swollen or inflamed implant site Fracture of the implant Loosening or dislocation of the prosthesis requiring revision surgery Bone restoration or over-production Allergic reaction(s) to prosthesis material(s) Untoward histological responses possibly involving macrophages and/or fibroblasts Migration of particle wear debris possibly resulting in a bodily response Embolism Some degree of particle formation is inevitable with all implants including those made of silicone elastomer. The amount will vary with factors such as patient activity, joint stability or instability post-implantation, implant position and the amount of soft tissue support. The patient s biological response to these particles is variable, but can include local synovitis and bone lysis in contiguous bones. See Section II for specific product information.
9 D. PRECAUTIONS Following the instructions for use provided in product literature can minimize the potential for complications or adverse reactions with any implant. It is imperative that this device not be handled with bare fingers or come in contact with lint. Silicone elastomers are very electrostatic and therefore susceptible to contamination by airborne or surface particles. The presence of these contaminates could cause adverse tissue reaction. In the event that the implant is exposed to such contaminants, rinse the device thoroughly with sterile distilled water. This device is intended for SINGLE USE ONLY. Patient input and motivation are important to the success of this procedure because of the amount of participation required of the patient during the postoperative program of hand rehabilitation. Federal Law (USA) restricts this device to sale by or on the order of a physician. Resterilization of these devices is not recommended because resterilization of these devices may cause distortion of the device. It is the responsibility of each surgeon using implants to consider the clinical and medical status of each patient and to be knowledgeable about all aspects of implant procedure and the potential complications that may occur. The benefits derived from implant surgery may not meet the patient s expectations or may deteriorate with time, necessitating revision surgery to replace the implant or to carry out alternative procedures. Revision surgeries with implants are common. The patient s mental status must also be considered. Willingness and/or ability to follow post-operative instructions may also impact the surgical outcome. Surgeons must balance many considerations to achieve the best result in individual patients.
10 Clinical results depend on surgeon and technique, pre-operative and post-operative care, the implant, patient pathology and daily activity. It is important that surgeons obtain appropriate informed consent and discuss the potential for complications with each patient prior to surgery. This may include a review of alternative, non-implant procedures such as soft tissue reconstruction or arthrodesis. Recommendations Regarding Device Fragments 1. Use medical devices in accordance with their labeled indications and the manufacturer s instructions for use, especially during insertion and removal. 2. Inspect devices prior to use for damage during shipment or storage or any out-of-box defects that might increase the likelihood of fragmentation during a procedure. 3. Inspect devices immediately upon removal from the patient for any signs of breakage or fragmentation. 4. If the device is damaged, retain it to assist with the manufacturer s analysis of the event. 5. Carefully consider and discuss with the patient (if possible) the risks and benefits of retrieving vs. leaving the fragment in the patient. 6. Advise the patient of the nature and safety of unretrieved device fragments including the following information: a. The material composition of the fragment (if known); b. The size of the fragment (if known); c. The location of the fragment; d. The potential mechanisms for injury, e.g., migration, infection;
11 e. Procedures or treatments that should be avoided such as MRI exams in the case of metallic fragments. This may help to reduce the possibility of a serious injury from the fragment. Concerning Magnetic Resonance Environments The devices described in this package insert have not been evaluated for safety and compatibility in the MR environment. The devices described in this package insert have not been tested for heating or migration in the MR environment. See Section II for specific product information. E. STERILIZATION PROCEDURES Implants in sterile packaging should be inspected to ensure that the packaging has not been damaged or previously opened. If the inner package integrity has been compromised, contact the manufacturer for further instructions. The implants should be opened using aseptic OR technique; they should only be opened after the correct size has been determined. Handling of the implant should be done with blunt instruments to avoid surface trauma or contamination with foreign bodies. Rinse the implant thoroughly with sterile saline solution before insertion. This product is for single use only. An implant should never be re-sterilized after contact with body tissues or fluids. Devices labeled for single-use only should never be reused. Reuse of these devices may potentially result in serious patient harm. Examples of hazards related to the reuse of these devices include, but are not limited to: significant degradation in device performance, cross-infection, and contamination.
12 F. STORAGE CONDITIONS All implants must be stored in a clean, dry environment and be protected from sunlight and extremes in temperature. II. SPECIFIC PRODUCT INFORMATION A. HUNTER TENDON ROD & PASSIVE TENDON IMPLANTS DESCRIPTION The HUNTER Tendon Rod Implant consists of a woven polyester core covered with silicone elastomer (barium-impregnated for radiopacity). This design provides the necessary combination of inert qualities, firmness and flexibility as well as the smooth surface required to induce pseudosynovial sheath formation and insure ease of insertion and gliding through the finger, palm, and forearm. The HUNTER Passive Tendon Implant consists primarily of a woven polyester core covered with silicone elastomer (barium-impregnated for radiopacity). At the distal end of the device is a fixation component made of type 316 stainless steel. A hole in the fixation component accommodates a 2.0 mm stainless steel bone screw (not provided) which is used to secure the device to the phalanx. (See surgical technique for screw specifications). Both the HUNTER Tendon Rod Implants and HUNTER Passive Tendon Implants are double-pouched and are STERILE unless the inner package is opened and damaged. WARNINGS Stage I is not contraindicated in patients under 5 years of age. However, tendon reconstruction in children requires the use of a 2 to 4 mm reinforced tendon rod without distal fixation component.
13 B. HUNTER ACTIVE TENDON IMPLANTS DESCRIPTION The HUNTER Active Tendon Implant consists primarily of a woven polyester core covered with silicone elastomer (barium-impregnated for radiopacity) that provides the necessary combination of inert qualities, firmness and flexibility as well as the smooth surface required to induce pseudo-sheath formation and insure ease of insertion and gliding through the finger, palm, and forearm. At the distal end of the device is a fixation component made of type 316 stainless steel. A hole in the fixation component accommodates a 2.0 mm stainless steel bone screw (not provided) which is used to secure the device to the bone. The hole is angled to help direct the screw into cortical bone. The proximal end is terminated in a loop, permitting surgical attachment of the motor tendon to the device. The HUNTER Active Tendon Implant is STERILE unless the inner package is opened or damaged. CONTRAINDICATIONS The current range of device sizes limits this device to use in the mature adult hand. Tendon reconstruction in children requires the use of a 2 mm, 3 mm, or 4 mm reinforced Tendon Rod implant without distal fixation components or proximal loop. C. HUNTER ACTIVE TENDON IMPLANT BC (BI-CORDAL) DESCRIPTION The HUNTER Active Tendon Implant BC consists of a woven polyester core covered with silicone elastomer (barium-impregnated for radiopacity). The rod portion of the device is
14 4 mm wide and 2 mm thick and varies in length. At the proximal and distal ends of the device are two polyester cords, continuations of the rod s inner core, which provide the means for weave anastomosis to the motor tendon and for attachment to the phalanx, respectively. The cords extend 15 cm beyond the ends of the rod. The HUNTER Active Tendon Implant BC is double-pouched and is STERILE unless the inner pouch is opened or damaged. The interval between Stage One (implantation of the device and formation of the pseudosynovial sheath) and Stage Two (removal of the device and autogenous tendon grafting) should be two to six months to permit maturation of the tendon bed to the point where it can nourish and lubricate a tendon graft. The surgeon must determine, on the basis of the findings in the hand, the appropriate time within that period at which to commence Stage Two of the procedure. INDICATIONS The HUNTER Active Tendon Implant BC is specifically indicated in cases in which a bone screw distal fixation may be compromised due to the small size and poor quality of the phalanx. It is also indicated for use in patients having small pulleys, which unless opened surgically will not permit insertion of a standard HUNTER Active Tendon Implant. WARNINGS The patient should be monitored frequently by clinical examination and radiographs after Stage One surgery to assess progress and adjust treatment program as necessary.
15 D. HUNTER ACTIVE TENDON IMPLANT DC (DISTAL CORD) DESCRIPTION The HUNTER Active Tendon Implant DC consists of a woven polyester core covered with silicone elastomer (barium-impregnated for radiopacity). The rod portion of the device is 4 mm wide and 2 mm thick and varies in length. The proximal end is terminated in a loop, also comprised of a polyester core covered with barium-impregnated silicone elastomer. This loop permits anastomosis of the motor tendon to the device. At the distal end of the device are two polyester cords, continuations of the rod s inner core, which provide the means for attachment to the phalanx. The cords extend 15 cm beyond the distal end of the rod. The HUNTER Active Tendon Implant DC is double-pouched and is STERILE unless the inner pouch is opened or damaged. The interval between Stage One (implantation of the device and formation of the pseudosynovial sheath) and Stage Two (removal of the device and autogenous tendon grafting) should be two to six months to permit maturation of the tendon bed to the point where it can nourish and lubricate a tendon graft. The surgeon must determine, on the basis of the findings in the hand, the appropriate time within that period at which to commence Stage Two of the procedure. INDICATIONS The HUNTER Active Tendon Implant DC is specifically indicated in cases in which a bone screw distal fixation may be compromised due to the small size and poor quality of the phalanx. It may also be used, at the surgeon s option, in patients having small pulleys, which unless opened surgically will not permit insertion of a standard HUNTER Active Tendon Implant.
16 WARNINGS The patient should be monitored frequently by clinical examination and radiographs after Stage One surgery to assess progress and adjust treatment program as necessary. E. HUNTER ACTIVE TENDON IMPLANT PC (PROXIMAL CORD) DESCRIPTION The HUNTER Active Tendon Implant PC consists of a woven polyester core covered with silicone elastomer (barium-impregnated for radiopacity). The rod portion of the device is 4 mm wide and 2 mm thick and varies in length. At the proximal end of the device are two polyester cords, continuations of the rod s inner core, which provide the means for weave anastomosis to the motor tendon. The cords extend 15 cm beyond the proximal end of the rod. At the distal end of the device is a fixation component made of type 316 stainless steel. A hole in the fixation component accommodates a 2.0 mm stainless steel bone screw (not provided) which is used to secure the device to the bone. The hole is angled to help direct the screw into cortical bone. The HUNTER Active Tendon Implant PC is double-pouched and is STERILE unless the inner pouch is opened or damaged. The interval between Stage One (implantation of the device and formation of the pseudosynovial sheath) and Stage Two (removal of the device and autogenous tendon grafting) should be two to six months to permit maturation of the tendon bed to the point where it can nourish and lubricate a tendon graft. The surgeon must determine, on the basis of the findings in the hand, the appropriate time within that period at which to commence Stage Two of the procedure.
17 INDICATIONS The HUNTER Active Tendon Implant PC is specifically indicated in cases in which proximal anastomosis must be executed deep in the proximal forearm. It may also be used, at the surgeon s option, in patients having small pulleys, which unless opened surgically will not permit insertion of a standard HUNTER Active Tendon Implant. WARNINGS The patient should be monitored frequently by clinical examination and radiographs after Stage One surgery to assess progress and adjust treatment program as necessary. Trademarks and Registered Trademarks are owned or licensed by Wright Medical Technology, Inc.
SWANSON. hammertoe IMPLANT. surgical technique presented by LOWELL SCOTT WEIL, DPM
 hammertoe IMPLANT surgical technique presented by LOWELL SCOTT WEIL, DPM SWANSON hammertoe IMPLANT as described by Lowell Scott Weil, DPM general PRECAUTIONS The potential for complications or adverse
hammertoe IMPLANT surgical technique presented by LOWELL SCOTT WEIL, DPM SWANSON hammertoe IMPLANT as described by Lowell Scott Weil, DPM general PRECAUTIONS The potential for complications or adverse
Implant materials. Learning outcomes. Implant materials in trauma. How to use this handout? Functions of implants. Types of materials
 Implant materials How to use this handout? The left column is the information as given during the lecture. The column at the right gives you space to make personal notes. Learning outcomes At the end of
Implant materials How to use this handout? The left column is the information as given during the lecture. The column at the right gives you space to make personal notes. Learning outcomes At the end of
FUSEFORCE. Hand Fixation System SURGIC A L T ECHNIQUE
 FUSEFORCE Hand Fixation System SURGIC A L T ECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
FUSEFORCE Hand Fixation System SURGIC A L T ECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
(English) NEXUS SPINE SPACER SYSTEM
 (English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
(English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
VERILAST Technology for Hip Replacement Implants
 VERILAST Technology for Hip Replacement Implants Surgeon Name Clinic Name Clinic Address Clinic i Address Phone Number Web Address Total Hip Replacement What Is VERILAST Hip Technology? OXINIUM Oxidized
VERILAST Technology for Hip Replacement Implants Surgeon Name Clinic Name Clinic Address Clinic i Address Phone Number Web Address Total Hip Replacement What Is VERILAST Hip Technology? OXINIUM Oxidized
Lentur Cable System. Surgical Technique
 Lentur Cable System Surgical Technique Contents Introduction... Page 1 System Design Features And Benefits... Page 2 Implants... Page 3 Instrumentation... Page 4 Surgical Technique... Page 5 Single Cable...
Lentur Cable System Surgical Technique Contents Introduction... Page 1 System Design Features And Benefits... Page 2 Implants... Page 3 Instrumentation... Page 4 Surgical Technique... Page 5 Single Cable...
SWANSON. Titanium Radial Head Implant
 S U R G I C A L T E C H N I Q U E SWANSON Titanium Radial Head Implant titanium RADIAL HEAD IMPLANT regular and narrow stem surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS, MICHIGAN.
S U R G I C A L T E C H N I Q U E SWANSON Titanium Radial Head Implant titanium RADIAL HEAD IMPLANT regular and narrow stem surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS, MICHIGAN.
Total Hip Joint Replacement. A Patient s Guide
 Total Hip Joint Replacement A Patient s Guide Don t Let Hip Pain Slow You Down What is a Hip Joint? Your joints are involved in almost every activity you do. Simple movements such as walking, bending,
Total Hip Joint Replacement A Patient s Guide Don t Let Hip Pain Slow You Down What is a Hip Joint? Your joints are involved in almost every activity you do. Simple movements such as walking, bending,
TABLE OF CONTENTS. Surgical Technique 2. Indications 4. Product Information 5. 1. Patient Positioning and Approach 2
 Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery.
 Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Wrist and Hand. Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Fractures of the Wrist and Hand: Carpal bones
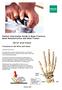 Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Wrist and Hand Fractures of the Wrist and Hand: Fractures of the wrist The wrist joint is made up of the two bones in your
Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Wrist and Hand Fractures of the Wrist and Hand: Fractures of the wrist The wrist joint is made up of the two bones in your
Contraindications: Malign or benign strictures in the upper part of esophagus close to the cricopharyngeal muscle.
 Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: [email protected] Instructions for Use FerX-ELLA Esophageal
Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: [email protected] Instructions for Use FerX-ELLA Esophageal
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Y O U R S U R G E O N S. choice of. implants F O R Y O U R S U R G E R Y
 Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Your Surgeon Has Chosen the C 2 a-taper Acetabular System The
Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Your Surgeon Has Chosen the C 2 a-taper Acetabular System The
The Total Ankle Replacement
 The Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please consult your physician
The Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please consult your physician
Patient Labeling Information System Description
 Patient Labeling Information System Description The Trident Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The
Patient Labeling Information System Description The Trident Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The
Arthroscopy of the Hand and Wrist
 Arthroscopy of the Hand and Wrist Arthroscopy is a minimally invasive procedure whereby a small camera is inserted through small incisions of a few millimeters each around a joint to view the joint directly.
Arthroscopy of the Hand and Wrist Arthroscopy is a minimally invasive procedure whereby a small camera is inserted through small incisions of a few millimeters each around a joint to view the joint directly.
Integra. MCP Joint Replacement PATIENT INFORMATION
 Integra MCP Joint Replacement PATIENT INFORMATION Integra MCP Patient Information This brochure summarizes information about the use, risks, and benefits of the Integra MCP finger implant. Be sure to discuss
Integra MCP Joint Replacement PATIENT INFORMATION Integra MCP Patient Information This brochure summarizes information about the use, risks, and benefits of the Integra MCP finger implant. Be sure to discuss
Instructions for Use
 Pleural Effusion Shunt with External Pump Chamber Catalog No. 42-9005 Instructions for Use Denver Biomedical, Inc. Table of Contents Description 2 Indications 2 Contraindications 2 Warnings 4 Cautions
Pleural Effusion Shunt with External Pump Chamber Catalog No. 42-9005 Instructions for Use Denver Biomedical, Inc. Table of Contents Description 2 Indications 2 Contraindications 2 Warnings 4 Cautions
Zimmer Small Fragment Universal Locking System. Surgical Technique
 Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Adult Forearm Fractures
 Adult Forearm Fractures Your forearm is made up of two bones, the radius and ulna. In most cases of adult forearm fractures, both bones are broken. Fractures of the forearm can occur near the wrist at
Adult Forearm Fractures Your forearm is made up of two bones, the radius and ulna. In most cases of adult forearm fractures, both bones are broken. Fractures of the forearm can occur near the wrist at
OVER 45 YEARS TEXTILE GRAFT TECHNOLOGY EXPERIENCE MAQUET THE GOLD STANDARD
 OVER 45 YEARS TEXTILE GRAFT TECHNOLOGY EXPERIENCE MAQUET THE GOLD STANDARD A comprehensive, proven vascular graft portfolio and exceptional professional support make MAQUET Cardiovascular a valuable asset
OVER 45 YEARS TEXTILE GRAFT TECHNOLOGY EXPERIENCE MAQUET THE GOLD STANDARD A comprehensive, proven vascular graft portfolio and exceptional professional support make MAQUET Cardiovascular a valuable asset
IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS?
 IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS? Dental implants are a very successful and accepted treatment option to replace lost or missing teeth. A dental implant is essentially an artificial tooth
IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS? Dental implants are a very successful and accepted treatment option to replace lost or missing teeth. A dental implant is essentially an artificial tooth
.org. Arthritis of the Hand. Description
 Arthritis of the Hand Page ( 1 ) The hand and wrist have multiple small joints that work together to produce motion, including the fine motion needed to thread a needle or tie a shoelace. When the joints
Arthritis of the Hand Page ( 1 ) The hand and wrist have multiple small joints that work together to produce motion, including the fine motion needed to thread a needle or tie a shoelace. When the joints
METAL HEMI. Great Toe Implant SURGICAL TECHNIQUE
 METAL HEMI Great Toe Implant SURGICAL TECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description Chapter 2 5 Intended Use 5 Indications 5 Contraindications Chapter 3 6 Surgical Technique
METAL HEMI Great Toe Implant SURGICAL TECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description Chapter 2 5 Intended Use 5 Indications 5 Contraindications Chapter 3 6 Surgical Technique
BRYAN. Cervical Disc System. Patient Information
 BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
Acetabular Wedge Augment System
 Orthopaedics Acetabular Wedge Augment System Stryker Hips. Implant with confidence. Restoration Acetabular Wedge Augment System Restoration Acetabular Wedge Augments, united with Tritanium, Trident, MDM
Orthopaedics Acetabular Wedge Augment System Stryker Hips. Implant with confidence. Restoration Acetabular Wedge Augment System Restoration Acetabular Wedge Augments, united with Tritanium, Trident, MDM
Total Shoulder Arthroplasty
 Specialists in Joint Replacement, Spinal Surgery, Orthopaedics and Sport Injuries Total Shoulder Arthroplasty Ms. Ruth Delaney Consultant Orthopaedic Surgeon www.sportssurgeryclinic.com Introduction Arthritis
Specialists in Joint Replacement, Spinal Surgery, Orthopaedics and Sport Injuries Total Shoulder Arthroplasty Ms. Ruth Delaney Consultant Orthopaedic Surgeon www.sportssurgeryclinic.com Introduction Arthritis
John M. Sigle, DPM, FACFAS Foot & Ankle Center of Illinois (217) 787-2700 www.myfootandanklecenter.com
 The MiToe implant is manufactured by Wright Medical, the recognized leader of surgical solutions for foot and ankle. Wright has been in business for more than 60 years and markets products in over 60 countries,
The MiToe implant is manufactured by Wright Medical, the recognized leader of surgical solutions for foot and ankle. Wright has been in business for more than 60 years and markets products in over 60 countries,
.org. Distal Radius Fracture (Broken Wrist) Description. Cause
 Distal Radius Fracture (Broken Wrist) Page ( 1 ) The radius is the larger of the two bones of the forearm. The end toward the wrist is called the distal end. A fracture of the distal radius occurs when
Distal Radius Fracture (Broken Wrist) Page ( 1 ) The radius is the larger of the two bones of the forearm. The end toward the wrist is called the distal end. A fracture of the distal radius occurs when
SALVATION. Fusion Bolts and Beams SURGICAL TECHNIQUE
 SALVATION Fusion Bolts and Beams SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 4 Intended Use Chapter 3 4 Device Description 4 Fusion Beams 5 Fusion Bolts Chapter 4 5 Preoperative Planning
SALVATION Fusion Bolts and Beams SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 4 Intended Use Chapter 3 4 Device Description 4 Fusion Beams 5 Fusion Bolts Chapter 4 5 Preoperative Planning
Bard * PerFix * Plug. Technique Guide. A Modified Technique with the. Open Inguinal Hernia Repair
 A Modified Technique with the Bard * PerFix * Plug A quick and simple preperitoneal underlay Modified Technique for the repair of groin hernias Technique Guide Open Inguinal Hernia Repair This technique,
A Modified Technique with the Bard * PerFix * Plug A quick and simple preperitoneal underlay Modified Technique for the repair of groin hernias Technique Guide Open Inguinal Hernia Repair This technique,
IMPLANT MENTOR EFFECTIVE JANUARY 2012 BREAST IMPLANTS NOT FOR DISTRIBUTION IN USA APPROVED FOR EMEA REGION. Mentor Worldwide LLC 2012 0907007 Rev C
 IMPLANT CATALOGUE EFFECTIVE JANUARY 2012 MENTOR BREAST IMPLANTS NOT FOR DISTRIBUTION IN USA APPROVED FOR EMEA REGION Mentor Worldwide LLC 2012 0907007 Rev C In 2002, we completed a new plant in Leiden,
IMPLANT CATALOGUE EFFECTIVE JANUARY 2012 MENTOR BREAST IMPLANTS NOT FOR DISTRIBUTION IN USA APPROVED FOR EMEA REGION Mentor Worldwide LLC 2012 0907007 Rev C In 2002, we completed a new plant in Leiden,
4052 Slimplicity Tech final_layout 1 6/29/15 3:29 PM Page 2 Surgical Technique
 Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
frequently asked questions Knee and Hip Joint Replacement Technology
 frequently asked questions Knee and Hip Joint Replacement Technology frequently asked questions Knee and Hip Joint Replacement Technology Recently, you may have seen advertisements from legal companies
frequently asked questions Knee and Hip Joint Replacement Technology frequently asked questions Knee and Hip Joint Replacement Technology Recently, you may have seen advertisements from legal companies
Titanium Wire with Barb and Needle. For canthal tendon procedures.
 Titanium Wire with Barb and Needle. For canthal tendon procedures. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Titanium Wire with Barb and Needle
Titanium Wire with Barb and Needle. For canthal tendon procedures. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Titanium Wire with Barb and Needle
.org. Ankle Fractures (Broken Ankle) Anatomy
 Ankle Fractures (Broken Ankle) Page ( 1 ) A broken ankle is also known as an ankle fracture. This means that one or more of the bones that make up the ankle joint are broken. A fractured ankle can range
Ankle Fractures (Broken Ankle) Page ( 1 ) A broken ankle is also known as an ankle fracture. This means that one or more of the bones that make up the ankle joint are broken. A fractured ankle can range
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
Guidance for implant removal. Straumann Dental Implant System
 Guidance for implant removal Straumann Dental Implant System The ITI (International Team for Implantology) is academic partner of Institut Straumann AG in the areas of research and education. ContentS
Guidance for implant removal Straumann Dental Implant System The ITI (International Team for Implantology) is academic partner of Institut Straumann AG in the areas of research and education. ContentS
Knotilus TM. Anchor Instability Repair. Technique Guide
 Knotilus TM Anchor Instability Repair Technique Guide Instability Repair Using the Knotilus TM Anchor Introduction While the shoulder has more mobility than any other joint in the body, it is also the
Knotilus TM Anchor Instability Repair Technique Guide Instability Repair Using the Knotilus TM Anchor Introduction While the shoulder has more mobility than any other joint in the body, it is also the
THE REVERSE SHOULDER REPLACEMENT
 THE REVERSE SHOULDER REPLACEMENT The Reverse Shoulder Replacement is a newly approved implant that has been used successfully for over ten years in Europe. It was approved by the FDA for use in the U.S.A.
THE REVERSE SHOULDER REPLACEMENT The Reverse Shoulder Replacement is a newly approved implant that has been used successfully for over ten years in Europe. It was approved by the FDA for use in the U.S.A.
How To Fix A Radial Head Plate
 Mayo Clinic CoNGRUENT RADIAL HEAD PLATE Since 1988 Acumed has been designing solutions to the demanding situations facing orthopedic surgeons, hospitals and their patients. Our strategy has been to know
Mayo Clinic CoNGRUENT RADIAL HEAD PLATE Since 1988 Acumed has been designing solutions to the demanding situations facing orthopedic surgeons, hospitals and their patients. Our strategy has been to know
Instructions for Use. Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1).
 Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
Posterior Referencing. Surgical Technique
 Posterior Referencing Surgical Technique Posterior Referencing Surgical Technique INTRO Introduction Instrumentation Successful total knee arthroplasty depends in part on re-establishment of normal lower
Posterior Referencing Surgical Technique Posterior Referencing Surgical Technique INTRO Introduction Instrumentation Successful total knee arthroplasty depends in part on re-establishment of normal lower
LifeStent XL Biliary Stent System
 B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
Your Practice Online
 P R E S E N T S Your Practice Online Disclaimer This information is an educational resource only and should not be used to make a decision on Knee replacement or arthritis management. All decisions about
P R E S E N T S Your Practice Online Disclaimer This information is an educational resource only and should not be used to make a decision on Knee replacement or arthritis management. All decisions about
Labral Repair. Surgical Protocol by Ronald Glousman, M.D. and Nicholas Sgaglione, M.D.
 Labral Repair Surgical Protocol by Ronald Glousman, M.D. and Nicholas Sgaglione, M.D. It s small. It s strong. And it's all suture. The JuggerKnot Soft Anchor represents the next generation of suture anchor
Labral Repair Surgical Protocol by Ronald Glousman, M.D. and Nicholas Sgaglione, M.D. It s small. It s strong. And it's all suture. The JuggerKnot Soft Anchor represents the next generation of suture anchor
Scaphoid Fracture of the Wrist
 Page 1 of 6 Scaphoid Fracture of the Wrist Doctors commonly diagnose a sprained wrist after a patient falls on an outstretched hand. However, if pain and swelling don't go away, doctors become suspicious
Page 1 of 6 Scaphoid Fracture of the Wrist Doctors commonly diagnose a sprained wrist after a patient falls on an outstretched hand. However, if pain and swelling don't go away, doctors become suspicious
 Total Hip Replacement Hip replacement surgery, or arthroplasty, uses implants to resurface and replace the bones in the joint, re-creating the smooth gliding surfaces that were once intact. Hip replacement
Total Hip Replacement Hip replacement surgery, or arthroplasty, uses implants to resurface and replace the bones in the joint, re-creating the smooth gliding surfaces that were once intact. Hip replacement
The information contained in this document is intended for healthcare professionals only.
 The information contained in this document is intended for healthcare professionals only. Dall-Miles Cabling System Dall-Miles Recon and Trauma Cable System Trochanteric Reattachment Using the Trochanteric
The information contained in this document is intended for healthcare professionals only. Dall-Miles Cabling System Dall-Miles Recon and Trauma Cable System Trochanteric Reattachment Using the Trochanteric
Technique Guide. Norian SRS Fast Set Putty. Calcium phosphate bone void filler.
 Technique Guide Norian SRS Fast Set Putty. Calcium phosphate bone void filler. TableofContents Introduction Norian SRS Fast Set Putty 2 Indications and Contraindications 3 Basic Science 4 Surgical Technique
Technique Guide Norian SRS Fast Set Putty. Calcium phosphate bone void filler. TableofContents Introduction Norian SRS Fast Set Putty 2 Indications and Contraindications 3 Basic Science 4 Surgical Technique
Ankle Syndesmosis Surgical Protocol by Timothy Charlton, M.D.
 Featuring Ankle Syndesmosis Surgical Protocol by Timothy Charlton, M.D. ZipLoop Technology is a unique weave in which a single strand of braided polyethylene is woven through itself twice in opposite directions.
Featuring Ankle Syndesmosis Surgical Protocol by Timothy Charlton, M.D. ZipLoop Technology is a unique weave in which a single strand of braided polyethylene is woven through itself twice in opposite directions.
ON-Q * Catheters and Introducers
 Instructions For Use ON-Q * Catheters and Introducers MANUFACTURED BY: Kimberly-Clark 1400 Holcomb Bridge Road Roswell, GA 30076 USA Kimberly-Clark N.V. Da Vincilaan 1 1935 Zaventem, Belgium Figure 1 4
Instructions For Use ON-Q * Catheters and Introducers MANUFACTURED BY: Kimberly-Clark 1400 Holcomb Bridge Road Roswell, GA 30076 USA Kimberly-Clark N.V. Da Vincilaan 1 1935 Zaventem, Belgium Figure 1 4
SAMBA Screw System INSTRUCTIONS FOR USE TABLE OF CONTENTS. Description. Indications. Contraindications. Warnings. Potential Adverse Events
 SAMBA Screw System INSTRUCTIONS FOR USE SAMBA Screw System TABLE OF CONTENTS Description Indications Contraindications Warnings Potential Adverse Events How Supplied and Handled Inspection Cleaning Sterilization
SAMBA Screw System INSTRUCTIONS FOR USE SAMBA Screw System TABLE OF CONTENTS Description Indications Contraindications Warnings Potential Adverse Events How Supplied and Handled Inspection Cleaning Sterilization
Orthopaedic Spine Center. Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs
 Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
YOUR GUIDE TO TOTAL HIP REPLACEMENT
 A Partnership for Better Healthcare A Partnership for Better Healthcare YOUR GUIDE TO TOTAL HIP REPLACEMENT PEI Limited M50 Business Park Ballymount Road Upper Ballymount Dublin 12 Tel: 01-419 6900 Fax:
A Partnership for Better Healthcare A Partnership for Better Healthcare YOUR GUIDE TO TOTAL HIP REPLACEMENT PEI Limited M50 Business Park Ballymount Road Upper Ballymount Dublin 12 Tel: 01-419 6900 Fax:
Surgical technique. End Cap for TEN. For axial stabilization and simultaneous protection of soft tissue.
 Surgical technique End Cap for TEN. For axial stabilization and simultaneous protection of soft tissue. Table of contents Indications and contraindications 3 Implants 4 Instruments 4 Preoperative planning
Surgical technique End Cap for TEN. For axial stabilization and simultaneous protection of soft tissue. Table of contents Indications and contraindications 3 Implants 4 Instruments 4 Preoperative planning
Integra. Subtalar MBA Implant
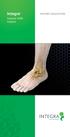 Integra Subtalar MBA Implant Patient EDUCATION Overview: What is Flatfoot? Flatfoot is a physical deformity where there is an absence of the arch that runs from the heel of the foot to the toes. A common
Integra Subtalar MBA Implant Patient EDUCATION Overview: What is Flatfoot? Flatfoot is a physical deformity where there is an absence of the arch that runs from the heel of the foot to the toes. A common
Exeter. Surgical Technique. V40 Stem Cement-in-Cement. Orthopaedics
 Exeter Orthopaedics V40 Stem Cement-in-Cement Surgical Technique Exeter V40 Stem Cement-in-Cement Surgical Technique Table of Contents Indications and Contraindications...2 Warnings and Precautions...2
Exeter Orthopaedics V40 Stem Cement-in-Cement Surgical Technique Exeter V40 Stem Cement-in-Cement Surgical Technique Table of Contents Indications and Contraindications...2 Warnings and Precautions...2
Your Practice Online
 P R E S E N T S Your Practice Online Disclaimer This information is an educational resource only and should not be used to make a decision on Revision Hip Replacement or arthritis management. All decisions
P R E S E N T S Your Practice Online Disclaimer This information is an educational resource only and should not be used to make a decision on Revision Hip Replacement or arthritis management. All decisions
BONE PRESERVATION STEM
 TRI-LOCK BONE PRESERVATION STEM Featuring GRIPTION Technology SURGICAL TECHNIQUE IMPLANT GEOMETRY Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
TRI-LOCK BONE PRESERVATION STEM Featuring GRIPTION Technology SURGICAL TECHNIQUE IMPLANT GEOMETRY Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
Anterior Hip Replacement
 Disclaimer This movie is an educational resource only and should not be used to manage Orthopaedic health. All decisions about the management of hip replacement and arthritis management must be made in
Disclaimer This movie is an educational resource only and should not be used to manage Orthopaedic health. All decisions about the management of hip replacement and arthritis management must be made in
Plasmax Plasma Concentration System BIOLOGICS
 Plasmax Plasma Concentration System BIOLOGICS Plasmax Plasma Concentration System Plasmax Plasma Concentrate System Contains Plasmax concentrator GPS III separator Provides 16ml of autologous output, 6ml
Plasmax Plasma Concentration System BIOLOGICS Plasmax Plasma Concentration System Plasmax Plasma Concentrate System Contains Plasmax concentrator GPS III separator Provides 16ml of autologous output, 6ml
Degenerative Spine Solutions
 Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Answers to commonly asked questions from patients with metal-on-metal hip replacements / resurfacings. Contents
 Answers to commonly asked questions from patients with metal-on-metal hip replacements / resurfacings John Skinner 1 and Alister Hart 2, Consultant Orthopaedic Surgeons and Directors of the London Implant
Answers to commonly asked questions from patients with metal-on-metal hip replacements / resurfacings John Skinner 1 and Alister Hart 2, Consultant Orthopaedic Surgeons and Directors of the London Implant
Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery.
 Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by
Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by
.org. Shoulder Pain and Common Shoulder Problems. Anatomy. Cause
 Shoulder Pain and Common Shoulder Problems Page ( 1 ) What most people call the shoulder is really several joints that combine with tendons and muscles to allow a wide range of motion in the arm from scratching
Shoulder Pain and Common Shoulder Problems Page ( 1 ) What most people call the shoulder is really several joints that combine with tendons and muscles to allow a wide range of motion in the arm from scratching
Foot and Ankle Technique Guide Proximal Inter-Phalangeal (PIP) Fusion
 Surgical Technique Foot and Ankle Technique Guide Proximal Inter-Phalangeal (PIP) Fusion Prepared in consultation with: Phinit Phisitkul, MD Department of Orthopedics and Rehabilitation University of Iowa
Surgical Technique Foot and Ankle Technique Guide Proximal Inter-Phalangeal (PIP) Fusion Prepared in consultation with: Phinit Phisitkul, MD Department of Orthopedics and Rehabilitation University of Iowa
PERIPROSTHETIC IMPLANTS
 PERIPROSTHETIC IMPLANTS PRODUCT OVERVIEW CLINICAL SOLUTIONS Periprosthetic fractures present unique challenges, such as how to gain fixation when the medullary canal is occupied. Special techniques and
PERIPROSTHETIC IMPLANTS PRODUCT OVERVIEW CLINICAL SOLUTIONS Periprosthetic fractures present unique challenges, such as how to gain fixation when the medullary canal is occupied. Special techniques and
Chapter 30. Rotational deformity Buddy taping Reduction of metacarpal fracture
 Chapter 30 FINGER FRACTURES AND DISLOCATIONS KEY FIGURES: Rotational deformity Buddy taping Reduction of metacarpal fracture Because we use our hands for so many things, finger fractures and dislocations
Chapter 30 FINGER FRACTURES AND DISLOCATIONS KEY FIGURES: Rotational deformity Buddy taping Reduction of metacarpal fracture Because we use our hands for so many things, finger fractures and dislocations
Standard Internal Hex
 Standard Internal Hex Touareg TM -OS Touareg TM -S Swell TM Touareg -S Touareg -OS Swell About ADIN Adin Dental Implant Systems Ltd., designs, manufactures and markets state of the art, technologically
Standard Internal Hex Touareg TM -OS Touareg TM -S Swell TM Touareg -S Touareg -OS Swell About ADIN Adin Dental Implant Systems Ltd., designs, manufactures and markets state of the art, technologically
Achilles Tendon Repair, Operative Technique
 *smith&nephew ANKLE TECHNIQUE GUIDE Achilles Tendon Repair, Operative Technique Prepared in Consultation with: C. Niek van Dijk, MD, PhD KNEE HIP SHOULDER EXTREMITIES Achilles Tendon Repair, Operative
*smith&nephew ANKLE TECHNIQUE GUIDE Achilles Tendon Repair, Operative Technique Prepared in Consultation with: C. Niek van Dijk, MD, PhD KNEE HIP SHOULDER EXTREMITIES Achilles Tendon Repair, Operative
Shoulder Arthroscopy
 Copyright 2011 American Academy of Orthopaedic Surgeons Shoulder Arthroscopy Arthroscopy is a procedure that orthopaedic surgeons use to inspect, diagnose, and repair problems inside a joint. The word
Copyright 2011 American Academy of Orthopaedic Surgeons Shoulder Arthroscopy Arthroscopy is a procedure that orthopaedic surgeons use to inspect, diagnose, and repair problems inside a joint. The word
Tibial Intramedullary Nailing
 Tibial Intramedullary Nailing Turnberg Building Orthopaedics 0161 206 4898 All Rights Reserved 2015. Document for issue as handout. Procedure The tibia is the long shin bone in the lower leg. It is a weight
Tibial Intramedullary Nailing Turnberg Building Orthopaedics 0161 206 4898 All Rights Reserved 2015. Document for issue as handout. Procedure The tibia is the long shin bone in the lower leg. It is a weight
What Dental Implants Can Do For You!
 What Dental Implants Can Do For You! Putting Smiles into Motion About Implants 01. What if a Tooth is Lost and the Area is Left Untreated? 02. Do You Want to Restore Confidence in Your Appearance? 03.
What Dental Implants Can Do For You! Putting Smiles into Motion About Implants 01. What if a Tooth is Lost and the Area is Left Untreated? 02. Do You Want to Restore Confidence in Your Appearance? 03.
Anatomic Percutaneous Ankle Reconstruction of Lateral Ligaments (A Percutaneous Anti ROLL)
 Anatomic Percutaneous Ankle Reconstruction of Lateral Ligaments (A Percutaneous Anti ROLL) Mark Glazebrook James Stone Masato Takao Stephane Guillo Introduction Ankle stabilization is required when a patient
Anatomic Percutaneous Ankle Reconstruction of Lateral Ligaments (A Percutaneous Anti ROLL) Mark Glazebrook James Stone Masato Takao Stephane Guillo Introduction Ankle stabilization is required when a patient
Zimmer DeNovo NT Natural Tissue Graft
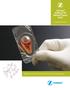 Zimmer DeNovo NT Natural Tissue Graft Surgical Technique Biologic treatment for early intervention and cartilage repair. Overview DeNovo NT Natural Tissue Graft (Fig. 1) is an off-the-shelf human tissue,
Zimmer DeNovo NT Natural Tissue Graft Surgical Technique Biologic treatment for early intervention and cartilage repair. Overview DeNovo NT Natural Tissue Graft (Fig. 1) is an off-the-shelf human tissue,
1 of 6 1/22/2015 10:06 AM
 1 of 6 1/22/2015 10:06 AM 2 of 6 1/22/2015 10:06 AM This cross-section view of the shoulder socket shows a typical SLAP tear. Injuries to the superior labrum can be caused by acute trauma or by repetitive
1 of 6 1/22/2015 10:06 AM 2 of 6 1/22/2015 10:06 AM This cross-section view of the shoulder socket shows a typical SLAP tear. Injuries to the superior labrum can be caused by acute trauma or by repetitive
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
OPERATION:... Proximal tibial osteotomy Distal femoral osteotomy
 AFFIX PATIENT DETAIL STICKER HERE Forename.. Surname NHS Organisation. Responsible surgeon. Job Title Hospital Number... D.O.B.././ No special requirements OPERATION:..... Proximal tibial osteotomy Distal
AFFIX PATIENT DETAIL STICKER HERE Forename.. Surname NHS Organisation. Responsible surgeon. Job Title Hospital Number... D.O.B.././ No special requirements OPERATION:..... Proximal tibial osteotomy Distal
Total elbow joint replacement for rheumatoid arthritis: A Patient s Guide
 www.orthop.washington.edu TABLE OF CONTENTS 1 Overview 2 Review of the condition 3 Considering surgery 5 Preparing for surgery 6 About the procedure 8 Recovering from surgery 9 Convalescence and Rehabilitation
www.orthop.washington.edu TABLE OF CONTENTS 1 Overview 2 Review of the condition 3 Considering surgery 5 Preparing for surgery 6 About the procedure 8 Recovering from surgery 9 Convalescence and Rehabilitation
Calcaneus (Heel Bone) Fractures
 Copyright 2010 American Academy of Orthopaedic Surgeons Calcaneus (Heel Bone) Fractures Fractures of the heel bone, or calcaneus, can be disabling injuries. They most often occur during high-energy collisions
Copyright 2010 American Academy of Orthopaedic Surgeons Calcaneus (Heel Bone) Fractures Fractures of the heel bone, or calcaneus, can be disabling injuries. They most often occur during high-energy collisions
Wrist Fracture. Please stick addressograph here
 ORTHOPAEDIC UNIT: 01-293 8687 /01-293 6602 UPMC BEACON CENTRE FOR ORTHOPAEDICS: 01-2937575 PHYSIOTHERAPY DEPARTMENT: 01-2936692 GUIDELINES FOR PATIENTS FOLLOWING WRIST FRACTURE Please stick addressograph
ORTHOPAEDIC UNIT: 01-293 8687 /01-293 6602 UPMC BEACON CENTRE FOR ORTHOPAEDICS: 01-2937575 PHYSIOTHERAPY DEPARTMENT: 01-2936692 GUIDELINES FOR PATIENTS FOLLOWING WRIST FRACTURE Please stick addressograph
FINAL DOCUMENT. Global Harmonization Task Force. Title: Label and Instructions for Use for Medical Devices
 GHTF/SG1/N70:2011 FINAL DOCUMENT Global Harmonization Task Force Title: Label and Instructions for Use for Medical Devices Authoring Group: Study Group 1 of the Global Harmonization Task Force Endorsed
GHTF/SG1/N70:2011 FINAL DOCUMENT Global Harmonization Task Force Title: Label and Instructions for Use for Medical Devices Authoring Group: Study Group 1 of the Global Harmonization Task Force Endorsed
The Right Choice. Exeter. total hip system
 The Right Choice Exeter total hip system Exeter The Right Choice Anatomic Reconstruction Offset The objectives of total hip replacement are to: relieve pain increase mobility and function Achieving a correct
The Right Choice Exeter total hip system Exeter The Right Choice Anatomic Reconstruction Offset The objectives of total hip replacement are to: relieve pain increase mobility and function Achieving a correct
Patient Information for Lumbar Spinal Fusion. What is a lumbar spinal fusion? Page 1 of 5
 Patient Information for Lumbar Spinal Fusion What is a lumbar spinal fusion? You have been offered surgery to the lumbar region of your spine, your lower back. The operation is called a lumbar spinal fusion.
Patient Information for Lumbar Spinal Fusion What is a lumbar spinal fusion? You have been offered surgery to the lumbar region of your spine, your lower back. The operation is called a lumbar spinal fusion.
Plasmax Plasma Concentration System for Laparoscopic Roux-en-Y Gastric Bypass
 Plasmax Plasma Concentration System for Laparoscopic Roux-en-Y Gastric Bypass BIOLOGICS The Digestive System Duodenum Stomach Large Intestine (colon) Small Intestine Ileum Jejunum Gastric Bypass Procedure
Plasmax Plasma Concentration System for Laparoscopic Roux-en-Y Gastric Bypass BIOLOGICS The Digestive System Duodenum Stomach Large Intestine (colon) Small Intestine Ileum Jejunum Gastric Bypass Procedure
.org. Shoulder Joint Replacement. Anatomy
 Shoulder Joint Replacement Page ( 1 ) Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was
Shoulder Joint Replacement Page ( 1 ) Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was
INJURIES OF THE HAND AND WRIST By Derya Dincer, M.D.
 05/05/2007 INJURIES OF THE HAND AND WRIST By Derya Dincer, M.D. Hand injuries, especially the fractures of metacarpals and phalanges, are the most common fractures in the skeletal system. Hand injuries
05/05/2007 INJURIES OF THE HAND AND WRIST By Derya Dincer, M.D. Hand injuries, especially the fractures of metacarpals and phalanges, are the most common fractures in the skeletal system. Hand injuries
it s time for rubber to meet the road
 your total knee replacement surgery Steps to returning to a Lifestyle You Deserve it s time for rubber to meet the road AGAIN The knee is the largest joint in the body. The knee is made up of the lower
your total knee replacement surgery Steps to returning to a Lifestyle You Deserve it s time for rubber to meet the road AGAIN The knee is the largest joint in the body. The knee is made up of the lower
Case 3:14-cv-02361-P Document 1 Filed 06/30/14 Page 1 of 9 PageID 1 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF TEXAS DALLAS DIVISION
 Case 3:14-cv-02361-P Document 1 Filed 06/30/14 Page 1 of 9 PageID 1 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF TEXAS DALLAS DIVISION JAMES MICHAEL CLINE, Plaintiff VS. Civil Action No. 3:14-cv-2361
Case 3:14-cv-02361-P Document 1 Filed 06/30/14 Page 1 of 9 PageID 1 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF TEXAS DALLAS DIVISION JAMES MICHAEL CLINE, Plaintiff VS. Civil Action No. 3:14-cv-2361
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery
 Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE:
 James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
Cormet Hip Resurfacing System
 Cormet Hip Resurfacing System Patient Product Information 325 Corporate Drive Mahwah, NJ 07430 t: 1-888-STRYKER www.aboutstryker.com The information presented in this brochure is for educational purposes
Cormet Hip Resurfacing System Patient Product Information 325 Corporate Drive Mahwah, NJ 07430 t: 1-888-STRYKER www.aboutstryker.com The information presented in this brochure is for educational purposes
External Fixation Systems IMPORTANT MEDICAL INFORMATION
 External Fixation Systems IMPORTANT MEDICAL INFORMATION EN SPECIAL NOTE External fixation should be used only under the directions of physicians who have a thorough knowledge of the anatomy, physiology
External Fixation Systems IMPORTANT MEDICAL INFORMATION EN SPECIAL NOTE External fixation should be used only under the directions of physicians who have a thorough knowledge of the anatomy, physiology
Options for Cervical Disc Degeneration A Guide to the M6-C. clinical study
 Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
Understanding Dental Implants
 Understanding Dental Implants Comfort and Confidence Again A new smile It s no fun when you re missing teeth. You may not feel comfortable eating or speaking. You might even avoid smiling in public. Fortunately,
Understanding Dental Implants Comfort and Confidence Again A new smile It s no fun when you re missing teeth. You may not feel comfortable eating or speaking. You might even avoid smiling in public. Fortunately,
Product Reference Guide
 Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
