ELIDEL (pimecrolimus)
|
|
|
- Sandra Harper
- 7 years ago
- Views:
Transcription
1 ELIDEL (pimecrolimus) NAME F THE DRUG The active ingredient of Elidel is pimecrolimus. Chemical name: (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-12-[(1E)-2- {(1R,3R,4S)-4-chloro-3-methoxycyclohexyl}-1-methylvinyl]-17-ethyl-1,14-dihydroxy- 23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[ ,9]octacos- 18-ene-2,3,10,16-tetraone Chemical structure: Cl H H H N H DESCRIPTIN Elidel (pimecrolimus) cream 1% contains the compound pimecrolimus, the 33-epi-chloroderivative of the macrolactam ascomycin. Pimecrolimus is a white to off-white fine crystalline powder. It is very soluble in methanol and ethanol and practically insoluble in water. Each gram of Elidel cream 1% contains 10 mg of pimecrolimus in a whitish cream base of benzyl alcohol, cetyl alcohol, citric acid, mono- and di-glycerides, oleyl alcohol, propylene glycol, sodium cetostearyl sulfate, sodium hydroxide, stearyl alcohol, medium chain triglycerides and water. Molecular formula: C 43 H 68 ClN 11 Relative molecular mass: CAS number: Page 1 of 14
2 PHARMACLGY Pharmacodynamics The actual mechanism of action of pimecrolimus in atopic dermatitis is not known. Pimecrolimus is an ascomycin macrolactam derivative. In vitro studies showed that pimecrolimus binds with high affinity to macrophilin-12 and inhibits the calcium-dependent phosphatase, calcineurin. As a consequence, it inhibits T-cell proliferation and prevents the transcription and release of both T helper type 1 cell (TH1) and T helper type 2 cell (TH2) inflammatory cytokines such as interleukin-2, interferon-γ, interleukin-4, interleukin-5, interleukin-10, tumour necrosis factor alpha and granulocyte macrophage colony-stimulating factor. Pimecrolimus inhibits recall antigen responses in human T-helper cell clones, isolated from the skin of an atopic dermatitis patient. Pimecrolimus also prevents the release of inflammatory cytokines and mediators from mast cells in vitro after stimulation by antigen/ige. In contrast to corticosteroids, Pimecrolimus does not impair the differentiation, maturation, functions and viability of murine Langerhans cells and human monocyte-derived dendritic cells, thus, underlining its cell-selective mode of action in vitro, but it affects the maturation of human Langerhans cells in vitro. The clinical significance of the in vitro findings in atopic dermatitis is unclear. In studies using various topical formulations, including the pimecrolimus cream, pimecrolimus penetrates similarly into, but permeates less through skin in vitro than corticosteroids, suggesting a lower systemic exposure to pimecrolimus after topical application as compared to corticosteroids. Pimecrolimus exhibits high anti-inflammatory activity in animal models of skin inflammation after topical and systemic application. Pimecrolimus is effective in the pig model of allergic contact dermatitis (ACD). Topical pimecrolimus also inhibits the inflammatory response to irritants, as shown in murine models of irritant contact dermatitis. Furthermore, topical and oral pimecrolimus effectively reduces skin inflammation and pruritis and normalises histopathological changes in hypomagnesemic hairless rats, a model that mimics acute aspects of atopic dermatitis. Topical pimecrolimus does not affect epidermal Langerhans cells in mice. In contrast, treatment with standard topical corticosteroids, including hydrocortisone, resulted in a reduction in Langerhans cells by %. A recent analysis of skin biopsies of atopic dermatitis patients has confirmed that treatment with the corticosteroid betamethasone 0.1%, but not Elidel 1% cream, for 3 weeks results in depletion of Langerhans cells, while both drugs significantly reduce T cells. From preclinical studies, the potential of pimecrolimus for affecting systemic immune responses appears to be lower than that of cyclosporin A, as shown in models of systemic immunosuppression and based on the dose comparison. Subcutaneous injections of cyclosporin A suppress the localised graft-versus-host reaction in rats 8-fold more potently than pimecrolimus. In contrast to cyclosporin A, oral treatment of mice with pimecrolimus neither impairs the primary immune response nor decreases lymph node weight and cellularity in ACD. Page 2 of 14
3 Pharmacokinetics Absorption in adults: Systemic exposure to pimecrolimus was investigated in 12 adult patients treated with Elidel 1% cream twice daily for 3 weeks. These patients had atopic dermatitis (eczema) lesions affecting 15-59% of their body surface area (BSA). 77.5% of pimecrolimus blood concentrations were below 0.5 ng/ml, the assay limit of quantitation (LoQ), and 99.8% of the total samples were below 1 ng/ml. The highest blood concentration of pimecrolimus measured in one patient was 1.4 ng/ml. In 40 adult patients treated for up to 1 year with Elidel, having 14-62% of their BSA affected at baseline, 98% of pimecrolimus blood concentrations were consistently low, mostly below the LoQ. A maximum blood concentration of 0.8 ng/ml was measured in only 2 patients in week 6 of treatment. There was no increase in blood concentration over time in any patient during the 12 months of treatment. In 13 adult patients with hand dermatitis treated with Elidel twice daily for 3 weeks (palmar and dorsal surfaces of hands treated, overnight occlusion), the maximum blood concentration of pimecrolimus measured was 0.91 ng/ml. Given the high proportion of pimecrolimus blood levels below the LoQ after topical application, the AUC could only be calculated from a few individuals. In 8 adult AD patients presenting with at least three quantifiable blood levels per visit day, the AUC(0-12h) values ranged from 2.5 to 11.4 ng x h/ml. Absorption in children: Systemic exposure to pimecrolimus was investigated in 58 paediatric patients aged 3 months to 14 years, who had atopic dermatitis (eczema) lesions involving 10-92% of the total body surface area. These children were treated with Elidel 1% cream twice daily for 3 weeks and five out of them were treated for up to 1 year on an as needed basis. The blood concentrations measured in these paediatric patients were consistently low, regardless of the extent of lesions treated or duration of therapy. They were in a range similar to that measured in adult patients treated under the same dosing regimen. 60% of pimecrolimus blood concentrations were below 0.5 ng/ml (LoQ) and 97% of all samples were below 2 ng/ml. The highest blood concentrations measured in 2 paediatric patients aged 8 months to 14 years of age were 2.0 ng/ml. In the youngest patients (aged 3 to 23 months), the highest blood concentration measured in one patient was 2.6 ng/ml. In the 5 children treated for 1 year, blood concentrations were consistently low, and the maximum blood concentration measured was 1.94 ng/ml (one patient). In these five patients, there was no increase in blood concentration over time in any patient during the 12 months of treatment. In 8 paediatric patients aged 2-14 years presenting at least three measurable blood concentrations per visit day, AUC(0-12h) ranged from 5.4 to 18.8 ng x h/ml. AUC ranges observed in patients with < 40% BSA affected at baseline were comparable to those in patients with 40% BSA. Distribution: In vitro plasma protein binding studies have shown that 99.5% of pimecrolimus in plasma is bound to proteins. The major fraction of pimecrolimus in plasma is bound to different lipoproteins. Page 3 of 14
4 Metabolism: Consistent with its skin selectivity, after topical application pimecrolimus blood levels are very low. Therefore, pimecrolimus metabolism could not be determined after topical administration. After single oral administration of radiolabelled pimecrolimus in healthy subjects, unchanged pimecrolimus was the major drug-related component in blood and there were numerous minor metabolites of moderate polarity that appeared to be products of -demethylations and oxygenation. No drug metabolism was observed in human skin in vitro. Elimination: Drug-related radioactivity was excreted principally via the faeces (78.4%) and only a small fraction (2.5%) was recovered in urine. Total mean recovery of radioactivity was 80.9%. Parent compound was not detected in urine and less than 1% of radioactivity in faeces was accounted for by unchanged pimecrolimus. Comparison to oral pharmacokinetic data: In psoriatic patients treated with oral pimecrolimus doses ranging from 5 mg once daily to 30 mg twice daily for 4 weeks, the highest dose was associated with an AUC(0-12h) of ng x h/ml. This exposure is approximately 26 and 16 times higher, respectively, than the highest systemic exposure observed in adult and paediatric atopic dermatitis (eczema) patients treated topically with Elidel twice daily for 3 weeks (AUC(0-12h) of 11.4 ng x h/ml and 18.8 ng x h/ml, respectively). CLINICAL TRIALS Short-term (acute) treatment in paediatric patients: Children and Adolescents: Two 6-week, vehicle-controlled trials were conducted in which a total of 403 paediatric patients 2-18 years old were treated twice daily with Elidel (pimecrolimus) cream 1%. The data of both studies were pooled. The physician s global evaluation of atopic dermatitis (i.e. The Investigator s Global Assessment, or IGA score) was used to evaluate the overall disease severity at baseline and throughout the duration of the trial. The IGA scores and descriptions are presented in the following table. Investigator s Global Assessment (IGA) score and description Score Description 0 = Clear No inflammatory signs of atopic dermatitis 1 = Almost clear Just perceptible erythema, and just perceptible papulation/infiltration 2 = Mild disease Mild erythema and mild papulation/infiltration 3 = Moderate disease Moderate erythema and moderate papulation/infiltration 4 = Severe disease Severe erythema and severe papulation/infiltration 5 = Very severe disease Severe erythema and severe papulation/infiltration with oozing/crusting Page 4 of 14
5 The primary endpoint in the short-term trials was the achievement of clear or almost clear disease (IGA 0 or 1) at 6 weeks. At study entry, about 31% of patients had mild disease (IGA=2), 59% of patients had moderate disease (IGA=3) and 8% had severe disease (IGA=4). The percentage mean body surface area (BSA) affected was 26% (range 5-96%). About 75% of patients had atopic dermatitis affecting the face and/or neck region. In these studies, patients applied either Elidel 1% cream or vehicle cream twice daily for up to 6 weeks. After 6 weeks of treatment, 34.8% of patients treated with Elidel were clear or almost clear of signs of atopic dermatitis compared to only 18.4% of vehicle-treated patients. More Elidel patients (56.6%) had mild or no pruritus at 6 weeks compared to vehicle patients (33.8%). The improvement in pruritus occurred in conjunction with the improvement of the signs and symptoms of atopic dermatitis. Infants: A similar 6-week study was conducted in 186 patients aged 3 to 23 months. At baseline, two-thirds of these patients had moderate disease (IGA = 3) and one-third had mild disease (IGA=2). At 6 weeks, the proportion of patients whose atopic dermatitis was assessed as clear or almost clear was significantly greater in the Elidel-treated group (54.5%) compared with the vehicle-treated group (23.8%). In addition, more Elidel patients (72.4%) had no or mild pruritus at 6 weeks compared with vehicle-treated patients (33.3%). In these three 6-week studies, the efficacy results at the end of the study were as follows: Endpoint Criteria Elidel 1% (N=267) Children and adolescents Vehicle (N=136) Infants p-value Elidel 1% (N=123) Vehicle (N=63) p-value IGA*: Clear or almost clear # 34.8% 18.4% < % 23.8% < Pruritus Absent or mild 56.6% 33.8% < % 33.3% < EASI : verall (mean % change) EASI : Head/Neck (mean % change) < < < < * Investigators Global Assessment (IGA) Eczema Area Severity Index (EASI) : mean % change in clinical signs (erythema, infiltration, excoriation, lichenification) and body surface area involved # Treatment success was defined as an IGA score of 0 (clear) or 1 (almost clear) at the end of 6 weeks 1 : p-value based on Cochran Mantel Haenszel (CMH) test stratified by centre 2 : p-value based on Analysis of Co-variance (ANCVA) model of EASI at Day 43 endpoint, with centre and treatment as factors and baseline (Day 1) EASI a covariate; In the three paediatric studies, a significant improvement in pruritus was observed within the first week of treatment with Elidel 1% cream in 44% of children and adolescents, and in 70% of infants. Long-term treatment: Paediatric patients: Elidel 1% cream was also evaluated in two double-blind studies of longterm management of atopic dermatitis in 713 children and adolescents (2-17 years) and 251 infants (3-23 months). The Elidel group used Elidel 1% cream twice daily at the first signs of itching and redness to prevent progression to flares of atopic dermatitis. Patients in the control group used the vehicle in place of Elidel cream to maintain the studies blind. In both groups, treatment with medium potency topical corticosteroids was initiated only in case of flare not controlled by trial medication (i.e. IGA score of at least 4). Patients in both groups were advised to use emollients throughout the course of the study. Page 5 of 14
6 The primary efficacy endpoint in these studies was the number of flares of atopic dermatitis in the first 6 months, although 12 month data are also presented. Both studies showed a reduction in the incidence of flares (p < 0.001) in favour of Elidel 1% cream first-line treatment; Elidel 1% cream first-line treatment showed better efficacy in all secondary assessments (Eczema Area Severity Index, Investigator Global Assessment, subject assessment); pruritus was controlled within a week with Elidel 1% cream. Significantly more patients on Elidel 1% cream completed 6 months and 12 months of treatment with no flares and did not use topical corticosteroids as rescue therapy (see Table below). The efficacy of Elidel 1% cream was maintained over time, with the ability to prevent disease progression to severe flares. % patients with no flare* % patients not using topical corticosteroids After 6 months of treatment After 12 months of treatment children infants children infants Elidel control Elidel control Elidel control Elidel control 61% 34% 70% 33% 51% 28% 57% 28% 65% 37% 70% 39% 57% 32% 63% 35% *Between group (Elidel vs. control) comparison p <0.001 In Studies 313 and 315, approximately 20% and 10% of patients, respectively, had used a potent topical corticosteroid at some stage before entry into the studies. However, no studies were conducted to specifically examine the effects of pimecrolimus cream in patients with a history of severe, recalcitrant atopic dermatitis who require management with potent topical corticosteroids and/or systemic therapies such as cyclosporin. Adult patients: A 6-month, randomised, double-blind, parallel group, vehicle-controlled study of similar design to the paediatric long-term studies was performed in 192 adults with moderate (IGA =3) to severe (IGA=4) atopic dermatitis at baseline. Topical corticosteroid medication was used on an average of 14.2 ± 24.2 % of the days of the 24-week treatment period in the Elidel group and on 37.2 ± 34.6 % of the days in the control group (p<0.001). A total of 50.0 % of the patients treated with Elidel did not experience any flare compared with 24.0 % of the patients randomised to the control group. Exposure to study medication in long-term trials: As shown in the following table, the overall exposure to study medication was higher in the Elidel group than in the vehicle group. This was due in part to higher rates of discontinuation and corticosteroid use in the vehicle group. Similarities in number of treatment days (i.e. the number of days the subject received study medication irrespective of the number of times the medication was applied during the day) and drug days (i.e. days assigned if study medication was applied at least twice daily), within groups indicate good overall compliance with the twice daily regimen. The frequency of use of Elidel 1% cream decreased over the duration of the study. Page 6 of 14
7 verall exposure to study medication by median number of treatment days in long-term clinical trials 1 Study period Infants (3-23 months) Elidel (N=204) Vehicle (N=46) lder children (2-17 years) Elidel (N=473) Vehicle (N=235) Adults Elidel (N=96) Vehicle (N=96) Total 6 months End of study : Treatment days refer to the number of days the subject received study medication, as recorded on diary cards 2 : "Total 6 months" represents median treatment data up to day 183 for infants and older children and up to day 168 for adults ther studies: Tolerability studies demonstrated that Elidel 1% cream is devoid of any irritation, contact sensitising, phototoxic or photosensitising potential. The atrophogenic potential of Elidel 1% cream in humans was tested in comparison to medium and highly potent topical steroids (betamethasone-17-valerate 0.1% cream, triamcinolone acetonide 0.1% cream) and vehicle in sixteen healthy volunteers treated for 4 weeks. Both topical corticosteroids induced a significant reduction in skin thickness measured by echography, as compared to Elidel 1% cream and vehicle, which did not induce a reduction of skin thickness. INDICATINS Elidel 1% cream is indicated for patients 3 months of age and older with atopic dermatitis (eczema) for: short term treatment of signs and symptoms intermittent long-term treatment of emerging and resolving lesions in atopic dermatitis where the use of a topical corticosteroid is not yet warranted, no longer needed, or is inadvisable (according to the usage restrictions in the respective topical corticosteroid Product Information). CNTRAINDICATINS Elidel cream 1% is contraindicated in individuals with a history of hypersensitivity to pimecrolimus or any of the components of the cream. PRECAUTINS Long-term safety of Elidel 1% cream has not been established. Although a causal relationship has not been established, rare cases of malignancy (e.g, skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including Elidel 1% cream. Page 7 of 14
8 Elidel cream should not be applied to areas affected by cutaneous pre-malignant changes (e.g. actinic keratoses) as caused, for example, by excessive sun exposure or phototherapy, or to areas where skin cancers have been removed. The safety of Elidel 1% cream has not been established in patients with Netherton s syndrome and generalised erythroderma. Elidel 1% cream is not recommended in patients with Netherton s syndrome or severely inflamed or damaged skin (e.g. erythroderma) where there is a potential for increased absorption. The safety and efficacy of Elidel 1% cream in immunocompromised patients have not been studied. The use in immunocompromised patients is therefore not recommended. In clinical studies, 14 cases of lymphadenopathy (0.9%) were reported while using Elidel cream. These cases were usually related to infections and were noted to resolve upon appropriate antibiotic therapy. f these 14 cases, the majority had either a clear aetiology or were known to resolve. Patients who receive Elidel cream and who develop lymphadenopathy should have the aetiology of their lymphadenopathy investigated. In the absence of a clear aetiology or in the presence of infectious mononucleosis, discontinuation of Elidel cream should be considered. Patients who develop lymphadenopathy should be monitored to ensure it resolves. Elidel 1% cream should not be applied to areas affected by acute cutaneous viral infections. In the presence of a dermatological bacterial or fungal infection, the use of an appropriate antimicrobial agent should be instituted. If resolution of the infection does not occur, Elidel 1% cream should be discontinued until the infection has been adequately controlled. Use of Elidel 1% cream may cause mild and transient reactions at the site of application, such as a feeling of warmth and/or burning sensation. Patients should see a physician if an application site reaction is severe. Patients with atopic dermatitis are predisposed to superficial skin infections including eczema herpeticum (Kaposi's varicelliform eruption). Treatment with Elidel cream may be associated with an increased risk of eczema herpeticum. In the presence of this skin infection, the balance of risks and benefits associated with Elidel cream use should be evaluated. Pimecrolimus per se was neither phototoxic nor photocarcinogenic in animal studies, but the cream base was found to slightly enhance the development of skin tumours induced by UV radiation in hairless mice. Care should be taken to avoid exposure to the sun of skin areas treated with Elidel cream (see ADVERSE REACTINS Post-marketing data). Patients should be advised to wear protective clothing, hats, low irritant sunscreens when Elidel is used. Elidel is to be applied first. Elidel should not be used in patients who are receiving phototherapy. Care should be taken to avoid contact with eyes and mucous membranes. If accidentally applied to these areas, the cream should be thoroughly wiped off and rinsed off with water. Effects on ability to drive and use machines: No data exist on the effects of pimecrolimus on the ability to drive and use machines. Page 8 of 14
9 Patients with altered renal or hepatic function: Although no formal studies have been carried out in patients with altered renal or hepatic function, there is no evidence to suggest reduced tolerability or the need to alter dosage requirements in this specific patient population. Use in children: Studies on the safety and efficacy of Elidel in paediatric patients below the age of 3 months have not been conducted. Use in the elderly: Atopic dermatitis (eczema) is rarely observed in patients aged 65 and over. Nine (9) patients 65 years old received Elidel cream in phase III studies. Clinical studies of Elidel did not include sufficient numbers of patients aged 65 and over to assess efficacy and safety. Use in patients with weakened immune systems Elidel should not be used in children and adults with weakened immune systems. Carcinogenicity, mutagenicity, impairment of fertility Carcinogenicity: In a 2-year dermal carcinogenicity study in rats using Elidel 1% cream, no cutaneous or systemic carcinogenic effects were observed at doses up to 10 mg/kg/day associated with a mean blood AUC0-24h for pimecrolimus of 3.3 times the maximum value observed in paediatric patients during clinical trials. In a mouse dermal carcinogenicity study using pimecrolimus in ethanolic solutions, no increase in incidence of neoplasms was observed in the skin or other organs at doses up to 4 mg/kg/day corresponding to a mean blood AUC(0-24h) of approximately 30 times the maximum value observed in paediatric patients during clinical trials. In a dermal photocarcinogenicity study in hairless mice using Elidel 1% cream, no photocarcinogenic effect versus vehicle-treated animals was noted at pimecrolimus doses up to 10 mg/kg/day. However, the topical cream base was found to enhance the development of skin tumours induced by UV-radiation. Care should be taken to avoid exposure of Elidel 1% cream treated skin area to the sun (also see PRECAUTINS ). In an oral carcinogenicity study in mice, an increased incidence of malignant lymphoma along with signs of systemic immunosuppression was evident in both males and females treated with 45 mg/kg/day pimecrolimus. No carcinogenic effect was apparent at 15 mg/kg/day associated with a blood AUC(0-24h) of times the maximum value observed in paediatric patients during clinical trials. In an oral carcinogenicity study in rats, an increased incidence of benign thymoma was observed in males treated with 5 mg/kg/day pimecrolimus and in both males and females treated with 10 mg/kg/day pimecrolimus. No oncogenic effect was apparent in males dosed at 1 mg/kg/day or females dosed at 5 mg/kg/day, associated with a blood AUC(0-24h) of up to 1.1 and 21 times the maximum value observed in paediatric patients during clinical trials, respectively. In a repeat-dose toxicity study in Cynomolgous monkeys, pimecrolimus given orally for weeks was associated at all doses tested with lymphoproliferative disorders, including lymphomas and leukaemias. As a no effect dose was not established, a margin of safety cannot be determined for these disorders. A chronic monkey study using topical dermal administration of pimecrolimus has not been performed. Page 9 of 14
10 Lymphoproliferative disorders and other neoplasm have been observed in humans and animals administered oral immunosuppressive therapies. Examples include calcineurin inhibitors such as tacrolimus and cyclosporin; inhibitors of m-tr (a critical kinase for cell progression) such as sirolimus and everolimus; and glucocorticoids and azathioprine. Mutagenicity: Pimecrolimus was not genotoxic, as assessed in vitro for gene mutations and chromosomal aberrations. An in vivo assay of chromosomal damage (micronucleus test in mice) was also negative. Effects on fertility: There are no clinical data on the effects of pimecrolimus on male or female fertility. Two oral fertility and embryofoetal developmental studies in rats revealed disturbance of oestrous cycles and post implementation loss in females at 45 mg/kg/day (associated with blood AUC of > 23 times the maximum observed human value during clinical trials). ne of the studies also revealed reduced testicular and epididymal weights, reduced testicular sperm counts and motile sperm at 45 mg/kg/day and testicular degeneration and oligospermia of the epididymis at 10 and at 45 mg/kg/day in males (associated with blood AUC of > 24 times the maximum observed human value), and decreased fertility rate, corpora lutea and implementations in females at 45 mg/kg/day with NELs of 2 mg/kg/day for effects in males and 10mg/kg/day in females (associated with blood AUC of about 1.2 and 8 times the maximum observed human value, respectively). Reduced serum sex hormone levels and morphological changes in reproductive organs (reduced ovarian activity, atrophy of the uterine and vaginal epithelium, decreased prostate weight with prostate atrophy and/or epithelial atrophy in seminal vesicles) have been observed in mice and rats following repeated administration of pimecrolimus, but these effects usually occurred at systemic exposures significantly higher (8-28 times) than that anticipated in humans. Use in Pregnancy (Category B3) There are no adequate data from the use of Elidel 1% cream in pregnant women. The effects of pimecrolimus on embryofoetal development have not been adequately assessed in animals following dermal administration. Following oral administration, pimecrolimus and at least some of its metabolites crossed the placenta of rats and rabbits. ral administration of pimecrolimus to rats and rabbits produced no evidence of teratogenicity at respective doses up to 45 and 20 mg/kg/day (associated with blood AUC of 27 and 3 times the maximum value observed in adult patients during clinical trials, respectively). However, evidence of embryofoetal toxicity (increased post-implantation loss, reduced live litter size, decreased placental weight, decreased fetal body weight and increased fetal retardation) was observed in rats at oral doses of >10 mg/kg/day. No maternal or embryofoetal toxicity was observed in rats dosed at 2 mg/kg/day (corresponding to blood AUC below the maximum value observed in adult patients during clinical trials) or in rabbit doses at 20 mg/kg/day (corresponding to an AUC of 3 times the maximum value observed in adult patients during clinical trials). Elidel cream should not be used in pregnant women. Use in Lactation Animal studies have not been conducted to investigate the milk excretion of pimecrolimus. However, reduced postnatal growth of offspring was observed in rats treated orally with pimecrolimus at a maternal dose of 40 mg/kg/day, with a NEL of 10 mg/kg/day. It is not known whether pimecrolimus is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse effects on nursing infants, Page 10 of 14
11 caution should be exercised when Elidel 1% cream is to be used in a breastfeeding woman. In particular, breastfeeding women should not apply Elidel 1% cream onto the breast. Interactions with ther Drugs Potential interactions between Elidel 1% cream and other drugs have not been systematically evaluated. Based on its minimal extent of absorption, interactions of Elidel 1% cream with systemically administered drugs are unlikely to occur. A vaccination response survey was conducted in 76 children aged 3-23 months who were treated with pimecrolimus 1% cream for up to 2 years. These children had moderate to severe atopic dermatitis with an average of 27.6% total body surface affected. The results showed that the proportions of children who had protective antibodies titres were in accordance with the seropositivity rates of age-matched children reported in literature. Application of Elidel 1% cream to vaccination sites, as long as local reactions persist, was not studied and is therefore not recommended. ADVERSE REACTINS Adverse Events Reported in Clinical Trials The safety profile of Elidel 1% cream has been established in more than 2000 patients, including infants ( 3 months), children, adolescents and adults enrolled in phase II and III studies. ver 1500 of these patients were treated with Elidel 1% cream and over 500 were treated with control treatment i.e. either Elidel vehicle and/or topical corticosteroids. The most common adverse events were application site reactions which were reported by approximately 19% of the patients treated with Elidel 1% cream and 16% of patients in the control group. These reactions generally occurred early in treatment, were mild/moderate in severity and were of short duration. Adverse reactions (Table 1) are ranked under heading of frequency, the most frequent first, using the following convention: very common ( 1/10); common ( 1/100, < 1/10); uncommon ( 1/1,000, < 1/100); rare ( 1/10,000, < 1/1,000); very rare (< 1/10,000), including isolated reports. Table 1 Skin and subcutaneous tissue disorders Very common Common Uncommon application site burning application site reactions (irritation, pruritus and erythema), skin infections (folliculitis) impetigo, condition aggravated, herpes simplex, herpes simplex dermatitis (eczema herpeticum), molluscum contagiosum, application site disorders such as rash, pain, paraesthesia, desquamation, dryness, oedema, skin papilloma, furuncle Page 11 of 14
12 Post-marketing data The following adverse reactions have been reported during post-marketing experience. The frequency has been estimated from the reporting rates. Because these reactions are reported voluntarily from a population of uncertain size, the frequency reflects only an estimate. Immune system disorders Very rare Metabolism and nutrition disorders anaphylactic reactions Rare alcohol intolerance 1) Skin and subcutaneous tissue disorders Rare allergic reactions (e.g. rash, urticaria, angioedema), skin discoloration (e.g. hypopigmentation, hyperpigmentation) 1) In most cases, flushing, rash, burning, itching or swelling occurred shortly after the intake of alcohol. Worldwide, there have been rare reports of cutaneous malignancies (squamous cell carcinoma, basal cell carcinoma) in adults and lymphomas in paediatric and adult patients treated with Elidel cream. Causality has not been established (see PRECAUTINS ). DSAGE AND ADMINISTRATIN Apply a thin layer of Elidel 1% cream to the affected skin twice daily and rub in gently and completely. Elidel 1% cream may be used on all skin areas, including the head and face, neck and intertriginous areas. Elidel cream should only be applied to areas of eczema (see PRECAUTINS ). The amount of cream to apply can be limited by using the fingertip dosing unit : a fingertip unit is the amount of cream expressed from a tube and applied from the distal skin crease to the tip of the palmar aspect of an adult index finger. A fingertip unit of cream is sufficient to cover the surface corresponding to two hand areas of eczema. Carers should wash their hands after application of Elidel to their children. Emollients can be applied after using Elidel 1% cream. Elidel 1% cream should be initiated at the first sign of itching (pruritus), such as persistent scratching or rubbing, and/or persistent redness (erythema), and/or thickening of the skin (infiltration), to prevent progression to flares of atopic dermatitis. Treatment should be discontinued when there is no longer evidence of the disease apart from dry skin. Treatment should be resumed at the first signs of recurrence. In short-term clinical trials, Elidel cream was used twice daily for up to 6 weeks, or until resolution of signs and symptoms, if this occurred before 6 weeks. In long-term clinical trials Elidel was used intermittently for up to 12 months in infants and children and 6 months in adults (see Clinical Trials section for more information on the extent of drug exposure during the long-term trials). Elidel treatment was initiated at the first signs of itching, redness and/or skin thickening. Treatment with a moderately potent topical corticosteroid was initiated if the disease progressed to flares (severe erythema, papulation/infiltration, with or without oozing/crusting). When topical corticosteroid therapy was initiated for the treatment of flares, Elidel therapy was discontinued. Elidel therapy was recommenced to treat residual disease or Page 12 of 14
13 if topical corticosteroid therapy was no longer appropriate or inadvisable because of potential risks. In general, the duration of treatment with Elidel cream for each eczema episode is up to 6 weeks (see also Use in Infants below). If no improvement in the signs and symptoms occurs after 6 weeks, or in case the condition worsens, Elidel should be stopped and the patient should be re-evaluated. Long-term continuous usage of Elidel cream is not recommended. Therapy should be intermittent, in conjunction with other therapies (see INDICATINS ). Use in infants (3-23 months): In the absence of safety data beyond 12 months, application of Elidel cream in infants (3-23 months) should be limited to the smallest practicable body surface area and treatment of each episode should generally be limited to no more than 3 weeks. Use in babies under 3 months of age has not been evaluated. Use in the elderly: Clinical studies with Elidel 1% cream did not include a sufficient number of patients in this age range to determine whether they respond differently from younger patients. Use in patients with hepatic and renal impairment: There is no evidence to suggest the need to alter dosage requirements in this specific patient population (see PRECAUTINS ). VERDSAGE There has been no experience of overdose with Elidel 1% cream. From the company s experience with developing orally administered pimecrolimus, the maximal systemic exposure in humans was achieved when 30 mg was administered twice daily for 4 weeks. At this dosage level, the drug was generally well tolerated. By comparison, each gram of Elidel 1% cream contains 10 mg pimecrolimus. The excipients used in Elidel 1% cream are not known to be toxic via the oral route. Hence, the accidental ingestion of Elidel 1% cream is unlikely to be a clinical concern. Contact the Poisons Information Centre on for advice on management. PRESENTATIN Elidel is available in aluminium tubes with an epoxy protective inner lacquer and polypropylene screw cap. Tubes of 15, 30 and 100 grams. Storage: Store below 25 C. Do not freeze. Keep out of reach and sight of children. nce opened, the contents of the tube should be used within 12 weeks. Page 13 of 14
14 SPNSR NVARTIS Pharmaceuticals Australia Pty Limited ABN Waterloo Road NRTH RYDE NSW 2113 = Registered Trademark Approved by Therapeutic Goods Administration: 30 November 2007 Date of most recent amendment: 5 June 2008 Page 14 of 14
Elidel (pimecrolimus 1% w/w) cream contains the compound pimecrolimus, the 33-epichloro-derivative
 ELIDEL pimecrolimus 1% w/w Cream PRDUCT INFRMATIN NAME F THE MEDICINE The active ingredient of Elidel is pimecrolimus. Chemical name: (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-12-[(1E)-2- {(1R,3R,4S)-4-chloro-3-methoxycyclohexyl}-1-methylvinyl]-17-ethyl-1,14-dihydroxy-23,25-
ELIDEL pimecrolimus 1% w/w Cream PRDUCT INFRMATIN NAME F THE MEDICINE The active ingredient of Elidel is pimecrolimus. Chemical name: (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-12-[(1E)-2- {(1R,3R,4S)-4-chloro-3-methoxycyclohexyl}-1-methylvinyl]-17-ethyl-1,14-dihydroxy-23,25-
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
PACKAGE LEAFLET: INFORMATION FOR THE USER. Elidel 10 mg/g Cream. pimecrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
D.RIGOPOULOS, D.IOANNIDES,* D.KALOGEROMITROS, S.GREGORIOU AND A.KATSAMBAS
 British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Pimecrolimus cream (Elidel) for facial atopic dermatitis
 for facial atopic dermatitis (pi-me-kro-ly-mus) Summary Pimecrolimus 1% cream is PBS listed for treating facial atopic dermatitis in adults and children when topical corticosteroids are contraindicated
for facial atopic dermatitis (pi-me-kro-ly-mus) Summary Pimecrolimus 1% cream is PBS listed for treating facial atopic dermatitis in adults and children when topical corticosteroids are contraindicated
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
PRODUCT INFORMATION. Silver sulfadiazine is a sulfonamide and has broad antimicrobial activity against both Grampositive and Gram-negative organisms.
 PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS
 39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%
![MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%](/thumbs/27/10084771.jpg) MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
FLAMAZINE CREAM 1.0% w/w
 PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/35/17235501.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w
 NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION
 NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION NIZORAL (ketoconazole) 2% Shampoo is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in
NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION NIZORAL (ketoconazole) 2% Shampoo is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
The clinical studies have been performed in children, adolescents and adults, from 4 years up to 55 years of age.
 NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
Professor Andrew Wright,
 Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
Material Safety data sheet
 EMERGENCY OVERVIEW METFORMIN HYDROCHLORIDE TABLET contain Metformin and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions of normal occupational
EMERGENCY OVERVIEW METFORMIN HYDROCHLORIDE TABLET contain Metformin and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions of normal occupational
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray
 PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B
 PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Guidance for Industry
 Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
PROTOPIC (tacrolimus) Ointment 0.03% Ointment 0.1%
 PROTOPIC (tacrolimus) Ointment 0.03% Ointment 0.1% FOR DERMATOLOGIC USE ONLY NOT FOR OPHTHALMIC USE Rx Only Prescribing Information See boxed WARNING concerning long-term safety of topical calcineurin
PROTOPIC (tacrolimus) Ointment 0.03% Ointment 0.1% FOR DERMATOLOGIC USE ONLY NOT FOR OPHTHALMIC USE Rx Only Prescribing Information See boxed WARNING concerning long-term safety of topical calcineurin
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
Member State Marketing Authorisation Holder Invented name Strength Pharmaceutical Route of administration
 ANNEX I LIST OF THE INVENTED NAMES, PHARMACEUTICAL FORMS, STRENGTHS OF THE MEDICINAL PRODUCTS, ROUTE OF ADMINISTRATION AND MARKETING AUTHORISATION HOLDERS IN THE MEMBER STATES 1 Member State Marketing
ANNEX I LIST OF THE INVENTED NAMES, PHARMACEUTICAL FORMS, STRENGTHS OF THE MEDICINAL PRODUCTS, ROUTE OF ADMINISTRATION AND MARKETING AUTHORISATION HOLDERS IN THE MEMBER STATES 1 Member State Marketing
NERIDERM Cream, Ointment, Fatty Ointment
 PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT
 NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd.
 RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
PSORIASIS AND ITS. Learn how vitamin D medications play an important role in managing plaque psoriasis
 PLAQUE PSORIASIS AND ITS TREATMENTS Learn how vitamin D medications play an important role in managing plaque psoriasis 2 Understanding Plaque Psoriasis WHAT CAUSES PLAQUE PSORIASIS? No one knows exactly
PLAQUE PSORIASIS AND ITS TREATMENTS Learn how vitamin D medications play an important role in managing plaque psoriasis 2 Understanding Plaque Psoriasis WHAT CAUSES PLAQUE PSORIASIS? No one knows exactly
1. NAME OF THE MEDICINAL PRODUCT. Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION. 1 capsule contains:
 1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (TRADITIONAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
Guidance for Industry Safety Testing of Drug Metabolites
 Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor)
 EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
See 17 for PATIENT COUNSELING INFORMATION and FDAapproved. Revised: 4/2015
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DUAC Gel safely and effectively. See full prescribing information for DUAC Gel. DUAC (clindamycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DUAC Gel safely and effectively. See full prescribing information for DUAC Gel. DUAC (clindamycin
September 19, 1984 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM
 Agriculture Canada September 19, 1984 T-1-245 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM RE: Guidelines for Developing
Agriculture Canada September 19, 1984 T-1-245 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM RE: Guidelines for Developing
Session 6 Clinical Trial Assessment Phase I Clinical Trial
 L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
PRODUCT INFORMATION. NAME OF THE MEDICINE SOOLANTRA (ivermectin) 10mg/g cream
 PRODUCT INFORMATION NAME OF THE MEDICINE SOOLANTRA (ivermectin) 10mg/g cream Australian Approved Name (AAN): ivermectin Chemical Names: 5-O-demethyl-22,23-dihydroavermectin A 1a ; 5-O-demethyl-25-de(1-
PRODUCT INFORMATION NAME OF THE MEDICINE SOOLANTRA (ivermectin) 10mg/g cream Australian Approved Name (AAN): ivermectin Chemical Names: 5-O-demethyl-22,23-dihydroavermectin A 1a ; 5-O-demethyl-25-de(1-
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
X-Plain Psoriasis Reference Summary
 X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
Core Topic 2. The immune system and how vaccines work
 Core Topic 2 The immune system and how vaccines work Learning outcome To be able to describe in outline the immune system and how vaccines work in individuals and populations Learning objectives Explain
Core Topic 2 The immune system and how vaccines work Learning outcome To be able to describe in outline the immune system and how vaccines work in individuals and populations Learning objectives Explain
Report series: General cancer information
 Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
LEFLUNOMIDE (Adults)
 Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis
 For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
Hydrozole Cream Hydrocortisone (microfine) 1% w/w and Clotrimazole 1% w/w
 CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
Technological platforms
 Advitech Advitech is a life sciences & technology company Its mission is to discover and commercialize proprietary and evidence-based natural health products Focus on milk, whey and bovine colostrum R&D,
Advitech Advitech is a life sciences & technology company Its mission is to discover and commercialize proprietary and evidence-based natural health products Focus on milk, whey and bovine colostrum R&D,
Facts About Chickenpox and Shingles for Adults
 Facts About Chickenpox and Shingles for Adults What is chickenpox? Chickenpox, also known as varicella, is a very contagious disease caused by the varicella-zoster virus. It is spread easily through the
Facts About Chickenpox and Shingles for Adults What is chickenpox? Chickenpox, also known as varicella, is a very contagious disease caused by the varicella-zoster virus. It is spread easily through the
Is Monotherapy Treatment of Etanercept Effective Against Plaque Psoriasis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
Leukocytoclastic Vasculitis and Stasis Dermatitis With Id Reaction
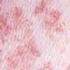 Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
Hull & East Riding Prescribing Committee
 Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Efficacy and Safety of Calcipotriol Ointment in Psoriasis Vulgaris - Experiences in Hong Kong
 ORIGINAL ARTICLES Efficacy and Safety of Calcipotriol Ointment in Psoriasis Vulgaris - Experiences in Hong Kong Drs. C. W. Fung, L.Y. Chong, C.Y. Leung, C. N. Look, K.K. Lo, K. M. Ho Social Hygiene Service
ORIGINAL ARTICLES Efficacy and Safety of Calcipotriol Ointment in Psoriasis Vulgaris - Experiences in Hong Kong Drs. C. W. Fung, L.Y. Chong, C.Y. Leung, C. N. Look, K.K. Lo, K. M. Ho Social Hygiene Service
A topic dermatitis is an itching inflammatory skin
 969 ORIGINAL ARTICLE Systemic exposure, tolerability, and efficacy of pimecrolimus cream 1% in atopic dermatitis patients B R Allen, M Lakhanpaul, A Morris, S Lateo, T Davies, G Scott, M Cardno, M-E Ebelin,
969 ORIGINAL ARTICLE Systemic exposure, tolerability, and efficacy of pimecrolimus cream 1% in atopic dermatitis patients B R Allen, M Lakhanpaul, A Morris, S Lateo, T Davies, G Scott, M Cardno, M-E Ebelin,
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
VOLTAREN OPHTHA EYE DROPS
 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) REFLECTION PAPER
 European Medicines Agency London 23 April 2009 EMEA/CHMP/BMWP/102046/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) REFLECTION PAPER NON-CLINICAL AND CLINICAL DEVELOPMENT OF SIMILAR MEDICINAL
European Medicines Agency London 23 April 2009 EMEA/CHMP/BMWP/102046/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) REFLECTION PAPER NON-CLINICAL AND CLINICAL DEVELOPMENT OF SIMILAR MEDICINAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE EXTENT OF POPULATION EXPOSURE TO ASSESS CLINICAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE EXTENT OF POPULATION EXPOSURE TO ASSESS CLINICAL
Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide)
 EMA/467911/2014 Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide) This is a summary of the risk management plan (RMP) for Xultophy, which details the measures to be
EMA/467911/2014 Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide) This is a summary of the risk management plan (RMP) for Xultophy, which details the measures to be
Treatments for allergy are usually straightforward, safe and effective. Common treatments include:
 Allergy Medications The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient
Allergy Medications The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
PACKAGE LEAFLET: INFORMATION FOR THE USER PROPECIA 1 mg film-coated Tablets (finasteride)
 PACKAGE LEAFLET: INFORMATION FOR THE USER PROPECIA 1 mg film-coated Tablets (finasteride) This medicine is for use in men only Read all of this leaflet carefully before you start to take this medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER PROPECIA 1 mg film-coated Tablets (finasteride) This medicine is for use in men only Read all of this leaflet carefully before you start to take this medicine.
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
Package Leaflet: Information for the user Propecia 1 mg film-coated Tablets Finasteride
 Package Leaflet: Information for the user Propecia 1 mg film-coated Tablets Finasteride This medicine is for use in men only Read all of this leaflet carefully before you start taking this medicine because
Package Leaflet: Information for the user Propecia 1 mg film-coated Tablets Finasteride This medicine is for use in men only Read all of this leaflet carefully before you start taking this medicine because
FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN
 FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN General Pantarhei Bioscience B.V. is an emerging specialty pharmaceutical company with a creative approach towards drug development. The Company
FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN General Pantarhei Bioscience B.V. is an emerging specialty pharmaceutical company with a creative approach towards drug development. The Company
Basics of Immunology
 Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Transient Hypogammaglobulinemia of Infancy. Chapter 7
 Transient Hypogammaglobulinemia of Infancy Chapter 7 An unborn baby makes no IgG (antibody) and only slowly starts producing it after birth. However, starting at about the sixth month of pregnancy, the
Transient Hypogammaglobulinemia of Infancy Chapter 7 An unborn baby makes no IgG (antibody) and only slowly starts producing it after birth. However, starting at about the sixth month of pregnancy, the
