A New Spinal Cord Stimulation System. Effectively Relieves Chronic, Intractable Pain: A Multicenter Prospective Clinical Study
|
|
|
- Augustine Montgomery
- 7 years ago
- Views:
Transcription
1 NEUROMODULATION: TECHNOLOGY AT THE NEURAL INTERFACE Volume 10 Number ORIGINAL ARTICLE A New Spinal Cord Stimulation System Blackwell Publishing Inc Effectively Relieves Chronic, Intractable Pain: A Multicenter Prospective Clinical Study John C. Oakley, MD* Elliot S. Krames, MD Joshua P. Prager, MD John Stamatos, MD Allison M. Foster, PhD Richard Weiner, MD** Ralph R. Rashbaum, MD Jaimie Henderson, MD *Yellowstone Neurosurgical Associates, Billings, MT, USA. Pacific Pain Treatment Center, San Francisco, CA, USA; California Pain Medicine Centers, Los Angeles, CA, USA; North Shore University Hospital, Syosset, NY, USA; Advanced Bionics/Boston Scientific, Valencia, CA, USA; **Dallas Neurosurgical Associates, Dallas, TX, USA; Texas Back Institute, Plano, TX, USA; and Stanford Medical Center, Stanford, CA, USA ABSTRACT Objectives. A prospective, open label, multicenter clinical trial confirmed the functionality of a new spinal cord stimulation (SCS) system for the treatment of chronic, intractable pain of the trunk and/or limbs. Materials and Methods. Sixty-five subjects tested a rechargeable 16-channel SCS system with individual current control of each contact on one or two percutaneous eight-contact epidural leads. After baseline measurements, subjects were tracked on pain ratings and complication rates for up to 18 months. Results. After a trial period, 75% of subjects underwent permanent implantation of the entire SCS system. More than one-half the implanted subjects experienced 50% or greater relief of pain after permanent implantation; some subjects reported relief of 90% or more of their pain. The most common complications after permanent implantation were lead migration, uncomfortable stimulation, and component failure; most resolved after reprogramming or device replacement. Conclusions. The new SCS system provided good pain relief to a majority of subjects, and the results confirm a favorable safety and efficacy profile for the SCS system. KEY WORDS: Back pain, electrical stimulation therapy, programming, SCS, treatment efficacy. Introduction Debilitating chronic pain is estimated to affect between 2% and 40% of the American population between the ages of 18 and 75 years, based on a review of the epidemiological literature (1). Injuries to the peripheral or central nervous system can result in the loss of normal inhibition or the loss of spontaneous neural activity. The Submitted: September 11, 2006; accepted: January 26, Address correspondence and reprint requests to: Allison M. Foster, PhD, Advanced Bionics/Boston Scientific, Rye Canyon Loop, Valencia, CA 91355, USA. afoster@advancedbionics.com. Dr. Oakley died on April 18, 2006 and is greatly missed International Neuromodulation Society, /07/$15.00/0
2 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 263 resulting neuropathic pain is oftentimes resistant to medical or surgical intervention. When treating chronic pain, treatments should be prescribed according to a carefully considered continuum of care, based on the principle of less invasive and less costly first (2). Pain refractory to more conservative management using cognitive and behavioral therapies, functional restorative therapies, complementary medical practice, or the use of analgesic medications may require more aggressive therapies such as nerve blocks, neuroablation, surgery to stabilize the spine, and implantable drug pumps (3 7). For patients who fail conservative pain management strategies, spinal cord stimulation (SCS) has proved to be an effective therapy for the treatment of chronic, intractable pain in the trunk and/or limbs (8,9). In the United States, SCS is most commonly used for treating back and leg pain arising from failed back surgery syndrome (FBSS), and pain associated with complex regional pain syndrome (CRPS) (10 12). Worldwide, indications commonly treated with SCS include angina pectoris, peripheral vascular disease, low back pain, spinal and nerve root lesions, spinal stenosis, perineal pain, and urological diseases (10). The gate control theory in which noxious afferent activity is masked by the non-noxious sensations produced by stimulation, is often used to explain the mechanism behind the efficacy of spinal cord stimulation (13). However, this theory inadequately describes the mechanism by which non-nociceptive pain is relieved by SCS, which may involve changes in neurotransmitter release or receptor expression (14,15). Modern SCS systems utilize a multicontact lead or leads placed over the dorsal columns of the spinal cord within the epidural space. Lead placement is guided by fluoroscopy and patient feedback regarding the topography of paresthesias. Patient-perceived concordant paresthesia overlapping the area of pain is essential for success of this therapy. Prior to 2004, SCS systems utilized current- or voltage-control that was split across all contacts. This study is the first efficacy report of a multicenter, prospective, open-label clinical trial using a new SCS system, the Precision (Advanced Bionics/Boston Scientific, Valencia, CA, USA). Portions of these data have been published previously in abstract form (16). Methods Device Description The implantable pulse generator (IPG) is mm and can accept one or two eightcontact percutaneous lead(s) or a paddle-type lead (however, for this study, all subjects used percutaneous leads). The system features 8 or 16 individually controlled current-driven contacts, and it has the capacity to deliver up to 12.7 ma current-controlled, asymmetrical, biphasic, chargebalanced stimulation pulses to each contact with a total of 20 ma across all contacts. The available stimulation rates range from 2 to 1200 Hz, and available pulse widths range from 10 to 1000 µsec. Patients implantated with Precision periodically recharge the implant battery with a radio frequency charging unit, and control their stimulation with a remote control unit. This SCS system s independent current control of each contact theoretically allows current to be fractionalized across contacts; that is, a percentage of the total current is delivered across each of multiple contacts to vary the volumes of activation (17). In this way, electrical fields can be steered to specific regions of the spinal cord using a joystick principle based on patient feedback (18). In all SCS systems, lead orientation and contact array geometries can determine the extent of nerve fibers activated by spinal cord stimulation (19). By electrically producing a variety of electrode configurations, current fractionation in this system is hypothesized to allow more precise overlap of paresthesia with painful areas. However, this study addresses only the pain relief afforded by the new system and does not support any claims specific to the technology. Subjects Sixty-five subjects were recruited from investigators practices under ethics committee approval at seven geographically diverse clinical
3 264 OAKLEY ET AL. pain-management sites in the United States. All subjects were assigned unique identification codes for confidentiality. The safety and health of the subjects was of primary concern in conducting this study, which was conducted pursuant to the Declaration of Helsinki. At all times, investigators followed the approved study plan and abided by all Good Clinical Practice requirements and any Institutional Review Board regulations. To be considered for study entry, subjects were required to be 18 years old or older, be diagnosed with chronic, intractable pain of the trunk and/or limbs (e.g., FBSS, intractable low back pain, and leg pain) as determined by a physician, have pain of a known and identifiable cause as determined by a physician, have a minimum pain score of 5 on the visual analog scale (VAS), be refractory to more conservative therapies as determined by a physician (e.g., biofeedback, nonimplanted stimulator devices, behavioral therapy, pharmacologic therapy, functional restoration, etc.), be an appropriate candidate for surgery, be capable of giving informed consent, and be capable and willing to follow all study related procedures. Additionally, the following criteria were exclusionary: being unable to operate the system either by self or caregiver, pregnancy or plans to become pregnant during the study, a history of coagulopathy or bleeding disorder, participation in any clinical investigation that could conflict with the requirements of this study, another active implantable device regardless of whether stimulation is on or off, or life expectancy of less than one year. All subjects underwent a temporary trial period. Each subject was required to demonstrate adequate (i.e., 50% or greater) pain relief during the temporary trial period in order to continue on in the study and to receive a permanent implant. Study Design Baseline All subjects completed baseline assessments after giving informed consent and meeting entry criteria, and within 30 days prior to any surgical procedures. A medical history review and brief physical examination were performed. Subjects rated the average level of pain they had experienced within the previous seven days, as measured on a 10-cm VAS for pain (0 cm = no pain, 10 cm = worst imaginable pain). The VAS was used for pain assessment in this study due to its straightforward nature, reproducibility, common use, and sensitivity to treatment effects (20). Temporary Trial Period All subjects underwent surgery to implant a temporary trial Precision system. Briefly, one or two percutaneous leads were inserted into the epidural space as appropriate to produce paresthesia in painful areas, as determined clinically by each implanting physician. Lead impedances were tested to confirm electrical continuity of the system, and the distal ends of the leads (or lead extensions, if used) were externalized and attached to an external trial stimulator (ETS). The ETS was activated and programmed to produce optimal pain-paresthesia overlap. Up to four programs could be selected for use by the subject at any time during the trial period. Additionally, the intensity of the stimulation could be adjusted or the stimulation could be turned off. The ETS was worn outside of the body for a minimum of 48 hours. At a visit at the end of the temporary trial period, subjects discussed their outcomes with their physician; success was defined as a 50% or greater reduction in pain from baseline during the trial period. Average VAS scores for the time since the last study visit, lead impedances, and stimulation parameter settings were recorded at the activation and end of the temporary trial period. Permanent Implantation and Activation Permanent lead implantation involved either replacing the temporary trial lead(s) and/or extension(s) with permanent leads, or internalizing the leads used during the temporary trial, thus making the trial leads permanent. Both implantation techniques are considered standard medical practice; which technique was used with
4 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 265 each subject was at the implanting physician s discretion or as clinically indicated (21). Additionally, at permanent implantation, physicians implanted single or dual percutaneous leads based on the hardware needed to adequately address individual subjects pain patterns. Lead(s) were then tunneled subcutaneously to a surgically produced pocket for the IPG either at the upper buttock or the abdominal wall. The leads were connected to the IPG and all incisions were surgically closed. Within 48 hours of implantation, the IPG was activated. The IPG was programmed to provide the optimal pain-paresthesia overlap for each subject. IPG stimulation parameters, lead impedances, and average VAS scores for the time since the last study visit were recorded at activation of the permanent SCS system. Follow-up Visits Activation of the IPG was counted as day 0 for study purposes. Post-activation visits were scheduled for two weeks, three months, six months, and every six months thereafter until study closure. Study procedures were the same at each visit: average VAS scores over the previous week (with and without stimulation) were obtained, lead impedances were gathered, and device settings were recorded. Additional Visits Unscheduled visits were possible at any time to address possible adverse events (AEs), to reprogram the device for optimal therapy, or for any other issues that could not be resolved in a telephone consultation. Addition, Reposition, or Replacement of a Lead During the Clinical Study During the course of the study, if any lead(s) was/were implanted, repositioned or replaced, timelines were then restarted. Pain rating data gathered after surgical revisions were analyzed as a separate subset. Additional Therapy Subjects were required to not participate in other interventional therapies for pain management for the duration of the study. While changes in medication dosing, either up or down, were allowed, new prescriptions of pain medications were required to be reported. Magnetic resonance imaging procedures were contraindicated. Data Management All data were gathered on preprinted case report forms. The forms were collected and their information verified at regularly scheduled monitoring visits to each site performed by sponsor staff. In addition, continued compliance with study procedures and regulatory requirements was confirmed at each monitoring visit. For all data, descriptive statistics were calculated including the number of observations, mean, standard error of the mean, range, and percent change. Analysis of pain rating data was performed using two-tailed paired t-tests with significance levels set at p = A safety analysis reflecting relatedness of AEs was performed by examining the cumulative frequency of events for all subjects from enrollment through withdrawal or study closure. Results Subjects Demographics A total of 65 subjects were enrolled. The sample included 39 men (60%) and 26 women (40%). The average age was 52.0 years (range: years old), and the average duration of chronic pain symptoms was 6.4 years (range: < 1 29 years old). The most common etiology of pain was FBSS. Table 1 shows the demographics of all enrolled subjects. Subject Implants and Disposition All 65 enrolled subjects underwent surgery for implantation with temporary trial leads; of these, 49 subjects (75.3%) proceeded to subsequent surgery for permanent implantation with the Precision system. Two lead implantation techniques were used at permanent implantations; 20 subjects underwent replacement of leads
5 266 OAKLEY ET AL. TABLE 1. Demographics of All Enrolled Subjects Including Sex, Age at Implant, Symptom Duration at Implant, and Etiology of Pain Enrolled Male 39 60% subjects Female 26 40% Mean age at implant (years) 52 Mean symptom duration at implant (years) 6.4 Etiology of pain Failed back surgery syndrome (FBSS) 40* 62% Complex regional pain syndrome (CRPS) 9 14% Radiculopathy/neuropathy 4 6% Scoliosis, spinal stenosis 1 1% Amputation pain 1 1% Vascular disease 1 1% Unknown 9 14% Total % *Three FBSS subjects each had one additional etiology of pain, although FBSS was listed as the primary causative factor. The other complaints were: postoperative pain, cauda equina syndrome, and coccydynia. after the temporary trial, while 29 subjects had their trial leads internalized. Most implanting physicians used one implant technique or another. We stratified the implanting technique by diagnosis, and found that approximately two-thirds of the subjects with FBSS and other etiologies of pain (68% and 62%, respectively) underwent internalizations of their leads after the trial period, while only a quarter of subjects with CRPS (25%) did. No significant differences in pain relief outcome based on permanent lead implant techniques, however, were found at any of the time points (p > 0.05). Additionally, we assessed the number of leads that were used for each subject. Of the 49 permanently implanted subjects, 26 used two leads and 23 used one lead. The majority of subjects with CRPS (75%) and pain of other etiologies (62%) underwent implantation with a single lead, while most subjects with FBSS (68%) received two leads. Four of the subjects originally underwent implantation with one lead (two subjects with FBSS, one with CRPS, and one with pain of unknown origins) had a subsequent surgical procedure to add a second lead; this will be discussed in greater detail in a later section. At later time points, in particular the 12-month and 18-month visits, fewer subjects completed data collection activities. This diminution in numbers was not due to subject withdrawal (although 11 subjects withdrew from the study following permanent implantation), but was instead related to fulfillment of regulatory goals. The study was opened on February 26, 2003, and the study protocol called for permanently implanted subjects to be followed at regular intervals. On April 27, 2004, the Food and Drug Administration granted Premarket Approval for the device under study. A final study report was submitted to the Food and Drug Administration in December 2004, and the study was formally closed shortly thereafter; thus, the study participation of enrolled subjects ended at that time. Lower subject representation at extended time points is therefore a reflection of study truncation, rather than loss to or intolerance of the study therapy. Indeed, we compared the withdrawal rates among the subjects implantated early enough to theoretically reach the 12-month point with the withdrawal rates among the subjects who enrolled later and thus were not able to participate at later time points. Ratios of withdrawals to subjects were equal in both groups (0.21 vs. 0.23). The present report describes all data received via monitoring visits through January 25, No additional data were received after that date. Pain Relief Outcomes During Trial Period This section summarizes efficacy data obtained during the trial period. All 65 enrolled subjects underwent surgical procedures to place trial leads in the epidural space. Following the trial period, 16 subjects withdrew from the study and 49 subjects proceeded to surgery for a permanent Precision system. We will first present the outcomes of the cohort that withdrew from the study after the trial period. Next, we will summarize the effectiveness of stimulation during the trial period for those subjects who proceeded to surgery for permanent implants. In all analyses, baseline scores were calculated independently for each cohort. Subjects Not Proceeding to Permanent Implant For the 16 subjects who withdrew from the study after the trial period, the average baseline VAS
6 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 267 TABLE 2. Effectiveness of Stimulation During the Trial Period for Subjects Who Did Not Proceed to Permanent Implant VAS scores during trial period (N = 16) Activation for temporary trial period End of temporary trial period Visit Baseline Without paresthesia With paresthesia Without paresthesia With paresthesia Average VAS score (±SEM) 7.47 (0.40) 8.28 (0.34) 3.95 (0.74) 8.02 (0.46) 5.86 (0.79) Numbers of subjects experiencing improvement of VAS scores during stimulation VAS score improvement (with vs. without paresthesia) Activation for temporary trial period Number of subjects End of temporary trial period Number of subjects % % % % % % % 1 2 < 30% 4 5 Missing data 2 7 Paresthesia was associated with pain relief at all time points; however, seven subjects (50% of assessed subjects) achieved 50% or better relief of pain at the activation of the temporary trial system, while only one subject (10% of assessed subjects) achieved 50% or better relief of pain at the end of the temporary trial period. score was 7.47 (± 0.40, standard error of the mean [SEM]). Trial implant surgery was not completed for two of the 16 subjects; at activation of the trial system for the remaining subjects, the average VAS score was 8.28 (± 0.34) with stimulation off and 3.95 (± 0.74) with stimulation on, a 52% average decrease in pain scores between off and on. At the end of the temporary trial period, a further five subjects did not complete VAS scoring. Nonetheless, the improvement in pain ratings was markedly less: the average VAS score was 8.02 (± 0.46) with stimulation off and 5.86 (± 0.79) with stimulation on, a 32% average decrease in pain scores. We performed paired t-tests to determine whether the stimulation-off VAS scores during the temporary trial period varied from the baseline VAS score. All pain ratings in the absence of paresthesia were statistically comparable (ps > 0.10). At activation for the temporary trial period, VAS scores with stimulation on were significantly lower than the VAS scores with stimulation off (p < 0.001). The VAS scores with stimulation on also were lower than the VAS scores at baseline (p = 0.001). At the end of the temporary trial period, VAS scores with stimulation on were significantly lower than the VAS scores with stimulation off (p = 0.033). Importantly, stimulation-on VAS scores at the end of the temporary trial period did not vary significantly from baseline VAS scores (p > 0.05) for the subjects who withdrew from the study after the temporary trial period (Table 2 and Fig. 1). The most common reason for withdrawal in the temporary trial period was ineffective therapy (9 of 16 subjects). Other cited reasons for withdrawal included surgery ending early (two subjects), medical decision to seek other forms of treatment (one subject), subject/family request (one subject), and dislike of paresthesia (one subject). Two subjects withdrew from the study in the trial period without citing a reason. Subjects Proceeding to Permanent Implant after Trial Period Forty-nine of the 65 enrolled subjects (75.3%) continued to permanent implant after the trial
7 268 OAKLEY ET AL. FIGURE 1. Trial period visual analog scale (VAS) scores with paresthesia (Stim ON) and without paresthesia (Stim OFF) at baseline, initial activation of the temporary trial leads (Temporary Activation), and the visit at the end of the trial (End of Temporary Trial) for the 16 subjects who did not continue to permanent implant. Better pain relief with paresthesia was observed at activation than at the end of the temporary trial period. Column heights represent means ± standard error of the mean (SEM). Asterisks indicate statistically significant differences between measurements; * indicates a score significantly lower than Stim OFF at the same visit, while ** denotes a score significantly lower than Baseline. FIGURE 2. Trial period visual analog scale (VAS) scores with paresthesia (Stim ON) and without paresthesia (Stim OFF) at baseline, initial activation of the trial leads (Temporary Activation), and the visit at the end of the trial (End of Temporary Trial) for the 49 subjects who continued to permanent implant. Pain relief of 50% or more was observed at activation and at the end of the temporary trial period. Column heights represent means ± standard error of the mean (SEM). Asterisks indicate statistically significant differences between measurements; * indicates a score significantly lower than Stim OFF at the same visit, while ** denotes a score significantly lower than Baseline. stimulation. Below, we present pain ratings (VAS) from these 49 subjects during the trial period. The average baseline VAS score was 7.97 (± 0.18). At the initial activation of the trial system, the average VAS score was 8.18 (± 0.17) with stimulation off and 3.20 (± 0.33) with stimulation on, a 61% average decrease in pain scores. The VAS scores of one subject were missing at this time point. At the end of the temporary trial, pain relief was again substantial: the average VAS score was 7.92 (± 0.28) with stimulation off and 2.64 (± 0.34) with stimulation on, a 67% average decrease in pain. We performed paired t-tests to determine whether the stimulation-off VAS scores during the trial period varied from the baseline VAS score. All pain ratings in the absence of paresthesia were statistically comparable (ps > 0.28). At the activation visit for the temporary SCS system, VAS scores with stimulation on were significantly lower than the VAS scores with stimulation off (p < 0.001). The VAS scores with stimulation on also were lower than the VAS scores at baseline (p < 0.001). At the end of the temporary trial period, VAS scores with stimulation on were significantly lower than the VAS scores with stimulation off (p < 0.001). The VAS scores with stimulation on also were lower than the VAS scores at baseline (p < 0.001). See Table 3 and Fig. 2 for data summaries representing the trial period for subjects who later received permanent implants. Pain Relief Outcomes After Permanent Implantation After the temporary trial period, 49 subjects went on to surgery for a permanent implant. Subjects participated in the study for an average of 10.9 months after activation: as described in a previous section, the majority of subjects ended their participation due to study closure, not voluntary withdrawal. Below, we present pain ratings (VAS) from these 49 subjects. Six of
8 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 269 TABLE 3. Effectiveness of Stimulation During the Trial Period for Subjects Who Later Received a Permanent Implant VAS scores during trial period (N = 49) Activation for temporary trial period End of temporary trial period Visit Baseline Without paresthesia With paresthesia Without paresthesia With paresthesia Average VAS score (±SEM) 7.97 (0.18) 8.18 (0.17) 3.20 (0.33) 7.92 (0.28) 2.64 (0.34) Numbers of subjects experiencing improvement of VAS scores during stimulation VAS score improvement (with vs. without paresthesia) Activation for temporary trial period Number of subjects End of temporary trial period Number of subjects % % % % % % % 3 2 < 30% 8 7 Missing Data 1 Thirty-two subjects (67% of assessed subjects) achieved 50% or better relief of pain at activation of the temporary system, and 37 subjects (75% of assessed subjects) achieved 50% or better relief of pain at the end of the temporary trial period. the 49 subjects underwent surgical revisions of their permanent implants, after which the study clock was reset. The data presented in this section reflect VAS scores reported before any revision surgeries. Outcomes after revisions will be presented in a separate section. The average baseline VAS score for the 49 subjects before surgery was 7.97 (± 0.18). At the initial activation of the permanent system (N = 47), the average VAS score was 8.10 (± 0.26) with stimulation off and 2.24 (± 0.28) with stimulation on, a 72% average decrease in pain scores. At the two-week visit (N = 47), pain relief was again substantial: the average VAS score was 8.39 (± 0.18) with stimulation off and 2.52 (± 0.27) with stimulation on, a 70% average decrease in pain. At the three-month visit (N = 38), the average VAS score was 8.10 (± 0.28) with stimulation off and 3.22 (± 0.33) with stimulation on, a 60% average decrease in pain. At the six-month visit (N = 34), the average VAS score was 8.28 (± 0.25) with stimulation off and 3.91 (± 0.43) with stimulation on, a 53% average decrease in pain. At the 12-month visit (N = 12), the average VAS score was 8.30 (± 0.78) with stimulation off and 2.23 (± 0.54) with stimulation on, a 73% average decrease in pain. At the 18-month visit (N = 4), the average VAS score was 8.15 (± 0.89) with stimulation off and 1.10 (± 0.56) with stimulation on, an 86% average decrease in pain. We performed paired t-tests to determine whether the stimulation-off VAS scores after permanent implant varied from the baseline VAS score. All pain ratings in the absence of paresthesia were statistically comparable (ps > 0.12). At all visits, including activation of the permanent system, VAS scores during stimulation were significantly lower than the VAS scores with stimulation off, and also were lower than the VAS scores at baseline (ps < 0.005). See Table 4 and Fig. 3 for data summaries representing the VAS data for subjects with permanent implants. Reasons for withdrawal after permanent implant included ineffective therapy (2 of 11 subjects), an AE (two subjects), surgery ended early (two subjects), and subject death (two subjects). Other cited reasons for withdrawal
9 270 OAKLEY ET AL. TABLE 4. Effectiveness of Stimulation for Subjects With Permanent Implants VAS scores with permanent implant (N = 49) Permanent activation 2-Week 3-Month 6-Month 12-Month 18-Month Visit Baseline OFF ON OFF ON OFF ON OFF ON OFF ON OFF ON Average VAS score (±SEM) (0.18) (0.26) (0.28) (0.18) (0.27) (0.28) (0.33) (0.25) (0.43) (0.78) (0.54) (0.89) (0.56) Numbers of subjects experiencing improvement of VAS scores during stimulation VAS score improvement (ON vs. OFF) Baseline Permanent activation 2-Week 3-Month 6-Month 12-Month 18-Month % % % % % % % < 30% Missing data VAS scores without paresthesia (OFF) and with paresthesia (ON) at each time point are shown. In summary, 50% or better relief of pain was achieved by 38 (81% of the 47 assessed subjects) at activation of the permanent system, 40 (85% of the 47 assessed subjects) at the 2-week, 24 (63% of the 38 assessed subjects) at the 3-month, 18 (55% of the 33 assessed subjects) at the 6-month, 9 (75% of the 12 assessed subjects) at the 12-month, and 4 (100% of the 4 assessed subjects) at the 18-month. included loss of contact with subject (one subject), subject noncompliance with study visits (one subject), and subject/family request (one subject). Outcomes Following Revision Surgeries Because the study clock was reset to day 0 if a revision surgery was performed, only data points prior to revision surgeries are included in the above section. Here, we present summary data for the subset of subjects undergoing revision surgery. All subjects continued in the study for a minimum of three months following surgical revision. Six subjects required a total of nine surgical revisions of their permanent implant. Reasons for revision included the addition of a second lead (N = 4), system replacement (N = 2), lead revision following migration (N = 1), IPG replacement (N = 1), and IPG reposition (N = 1). Before revision surgery, the average VAS score with stimulation off was 8.38 (± 0.20), and 3.17 (± 0.40) with stimulation on. After revision surgery, the average VAS score with stimulation off was 8.32 (± 0.35), and 4.91 (± 0.58) with stimulation on. VAS scores were averaged across all time points due to the variable duration between initial and revision surgeries across subjects. One subject withdrew after revision surgery due to ineffective therapy, while the other five subjects included in this subset continued to participate until study closure. Reprogramming Sessions There were 31 extraprotocol visits among the 49 permanently implanted subjects during the course of the study that were explicitly described for purposes of reprogramming (or improve program, or adjustment, or the like). Some
10 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 271 FIGURE 3. Visual analog scale (VAS) scores with paresthesia (Stim ON) and without paresthesia (Stim OFF) at baseline, initial activation of the permanent system (Permanent Activation), and visits at 2 weeks, 3 months, 6 months, 12 months, and 18 months postactivation for those subjects with permanent implants. With paresthesia, pain was relieved by 50% or more at all time points. Column heights represent means ± standard error of the mean (SEM). Asterisks indicate statistically significant differences between measurements; * indicates a score significantly lower than Stim OFF at the same visit, while ** denotes a score significantly lower than Baseline. subjects were reprogrammed more than once. For the subjects requesting reprogramming, the average VAS score with stimulation off was 8.46 (± 0.20). The average VAS score immediately after reprogramming, with stimulation on, was 4.01 (± 0.51), a 53% average improvement in pain. The difference in VAS scores between stimulation off and stimulation on after reprogramming was statistically significant (p < 0.001). Adverse Events In total, 40 AEs were reported across 31 subjects. The overall incidence rate was 29.8%, based on 34 device-related AEs among 114 implants (including 65 temporary and 49 permanent implants). The duration of study participation (i.e., from enrollment through last study visit) was determined for each subject; in total, 41.5 patient-years were represented in this study. The complication rate of device-related AEs per patient-year was The most common AEs were lead migration (7%), brief uncomfortable stimulation (5%), and component failure (4%; damaged leads noted at surgery before implantation [N = 2], IPG failure to stimulate [N = 1], and charger device malfunction [N = 1]). Lead migration and component failure occurred more often with permanent implants than temporary systems. Brief uncomfortable stimulation was reported more often in temporary systems than with permanent implants. In Table 5, we describe all AEs that were related to the Precision SCS system and those that occurred without relation to the device. Two subjects died while enrolled in the study. One subject died due to complications of a broken hip. A second subject died in his sleep and the toxicology evaluation was negative. Medical investigations determined that the deaths were not related to the device, surgical procedure, or study participation. Discussion Spinal cord stimulation has been used successfully since 1967 (9). This investigational device exemption clinical trial used the Precision (Advanced Bionics/Boston Scientific) system for spinal cord stimulation to treat chronic, intractable pain of the back and legs. Precision is a new SCS system that utilizes up to 16 individually currentcontrolled contacts. Outcomes were tested with a temporary system; following a successful trial in which pain was relieved by 50% or more, subjects underwent implantation with a permanent pulse
11 272 OAKLEY ET AL. TABLE 5. Summary of All Adverse Events (AEs) Occurring During the Study Based on Relation to the Device AEs related to device or procedure Number of events Incidence rate (of 114 implants; 65 trial, 49 permanent) Temporary or permanent system Comments Lead migration % 3 Temporary; All but one instance of migration was surgically revised. 5 permanent Brief uncomfortable stimulation % 5 Temporary; 1 permanent One instance required IPG replacement. All others were resolved with reprogramming or subject education. Component failure (implanted device) % 1 Temporary; 3 permanent Failed components were replaced during initial surgery (2 instances), or requiring additional surgery (2 instances). Infection % 1 Temporary; 3 permanent All instances were resolved with antibiotics; three instances required hospitalization and explantation of system. Pain % 3 Permanent Two instances of pain at implant site were surgically resolved. One instance of pain in a new location was resolved by addition of a second lead. Cerebrospinal fluid leak % 2 Temporary; 1 permanent Two instances resolved without medical intervention. In one instance the surgery was aborted and the subject chose to withdraw from the study. Telemetry failure (implanted device) % 2 Permanent Communication with IPG was restored via reprogramming or relocation of IPG. Telemetry failure (external component) % 2 Permanent Remote control was replaced (1 instance), and system reprogrammed (1 instance) Component failure % 1 Permanent Charger system was replaced. (external component) Seroma % 1 Permanent Seroma at incision site resolved over 3 months without medical intervention. AEs not related to device or procedure Movement-related pain % Temporary A subject experienced pain in the low back following activity, and the surgeon explanted the lead. Seizure % Permanent Subject experienced a grand mal seizure due to changing medications and dosage without medical supervision. Nausea % Permanent Subject experienced nausea, vomiting, headache, and chills and was given antibiotic prophylaxis. Symptoms resolved without further intervention. Headache % Temporary Subject developed a daily headache and sought management with primary care physician. Abstinence syndrome % Permanent Subject discontinued all opioid medications without medical supervision and developed a typical abstinence syndrome. Symptoms resolved with the addition of opioids to her medication regimen. Skin irritation % Temporary An allergy to the tape used to secure components to skin resolved without medical intervention. Thirty-four device-related AEs were reported across 114 implants, an incidence rate of 29.8%. generator. Subjects with permanent implants were tracked for up to 18 months. Subjects The average age of the study s subjects was 52 years old, and 60% were male. FBSS and CRPS comprised 76% of subjects pain etiologies. Taken together, the subject cohort was representative of the chronic pain population and typical patients receiving SCS (22). The study was initiated for the purpose of fulfilling regulatory requirements for public release of the Precision system. When the requirements were met, the study was closed. Because enrollment was graduated across sites, subjects were at varying stages of study completion at the time of closure. At closure, 12 and 4 subjects had attained the temporally advanced time points of 12 and 18 months, respectively. Instead of attrition, the apparent
12 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 273 diminution in participation at later time points was an artifact of a hard cut-off for study activities. Subjects participating at these later time points were among the first to enroll in the study, because the entire duration of the study was approximately 22 months. Because inclusion in the later time points was a function of enrollment date, it is unlikely that these data were biased through a self-selection process by which subjects with poor outcomes withdraw from the study. It also should be noted that subjects withdrawing from participation in the study did not necessarily undergo device explantation. Furthermore, it is commonly accepted that the minimum clinically meaningful change for chronic low back pain on a 0 10 VAS scale is 2 points (23). To detect a 2-point change with 90% power, assuming a standard deviation of 1.25 (representative of this study), only five subjects are needed. Thus, we are confident that the data presented in the 12- and 18-month time points are likely representative of the entire cohort (24). Statistically detectable differences with small sample sizes also indicate a large effect (25). Thus, we assert that these time points contain statistically defensible data. Nevertheless, we acknowledge that small sample sizes are associated with lower statistical power, and as such, the potential for stringent hypothesis testing at the later time points that included fewer subjects may be limited. These data, in particular from the 18-month, may thus be most conservatively viewed as trend data suggestive of long-term therapy. Further testing of the system at extended time points is needed before definitive statements of long-term efficacy can be made. Outcomes of Trial An SCS trial allows the patient and physician to assess the benefits and safety of the therapy to the individual before implantation of a permanent device (26). In most patients, SCS trials are easy to perform, result in low morbidity, and emulate the permanent application of the therapy. Additionally, an unsuccessful trial may be terminated without significant risk through simple lead removal (27). Forty-nine of 65 subjects proceeded to receive permanent implants, a 75.3% success rate during the trial period. This success rate is similar to published reports (e.g., 85% of a sample of 254; 80% of a sample of 410; 80% of a sample of 235) (22,28,29). Sixteen of the 65 enrolled subjects (24.6%) elected to withdraw from the study after the trial period. The individual outcome of each subject, not group outcomes, predicated his or her choice to withdraw. Eleven of the 16 withdrawing subjects (68.7%) cited inadequate, or dislike for, therapy with SCS during the trial period as the primary reason for withdrawal. Indeed, only one of the withdrawing subjects achieved 50% or greater pain relief during the trial period and still chose to discontinue his or her SCS treatment. Indeed, as summarized in Figs 1 and 2, despite initially good pain relief in both groups, those that withdrew after the trial had worse outcomes by the end of the trial than those who proceeded to permanent implant. The underlying cause of inadequate therapy for some subjects, whether related to the stimulation, lead placement, or some other mechanism, is unclear. For a review of the bases of SCS efficacy, see Oakley and Prager (2002) or Linderoth and Foreman (2006) (28,30). Implantation Techniques About 60% of the 49 permanently implanted subjects underwent surgical internalization of the leads after the temporary trial, while the remainder of subjects had their leads replaced. Removing and replacing temporary leads may create extensive fibrotic changes and concomitant resistivity variability within the epidural space (31). Alternately, internalizing leads after the trial necessitates a more extensive surgical implantation procedure at temporary trial lead placement than if percutaneous leads are removed and replaced. More than 50% of subjects underwent implantation with two leads at permanent implant; of those receiving only one lead, four subjects underwent a subsequent procedure to implant a second lead. It is not clear from the data gathered during the study the rationale of the implanters for having placed one vs. two leads. However, the majority of subjects with CRPS and pain of other etiologies received
13 274 OAKLEY ET AL. one lead, while most subjects with FBSS received two leads. It is possible that subjects with CRPS and other pain (including nonspecific neuropathy and amputation pain) may have experienced primarily unilateral pain, which could be adequately managed with a single lead. Subjects with FBSS, on the other hand, may have experienced pain on both sides of their body and required two leads. The subsequent placement of a second lead in four subjects may indicate that these subjects painful areas were inadequately covered by the paresthesia generated by a single lead, or that a progression of their pain symptoms enlarged their painful areas. However, we acknowledge that while the etiology of pain may account for some physicians choices of implantation technique, this remains an assumption without confirmation from the implanters. Regardless, contradictory evidence in published reports shows better, or worse, clinical outcomes with a single lead compared to dual leads (32,33). Ultimately, the choice of one vs. two leads may be determined simply by the degree of pain-paresthesia overlap attainable with the available hardware, anatomical limitations, and/or a programmer s skill at achieving coverage. Outcomes After Permanent Implantation Forty-nine of the 65 enrolled subjects received a permanent Precision SCS implant. After permanent implant, subjects were followed up at 2 weeks, 3 months, 6 months, 12 months, and 18 months postactivation. At all time points, average stimulation-on VAS scores were significantly better than both average stimulation-off VAS scores (within time points) and average baseline VAS scores. The majority of subjects experienced good pain relief. At each interval, the percentage of assessed subjects experiencing 50% or better reduction in pain was between 55% and 100%. Other published articles report similar levels of pain relief in 60 74% of subjects (29,34). On average, subjects reported that their pain was relieved by between 53% at six months postactivation and 86% at 18 months postactivation. In fact, 10 20% of assessed subjects experienced relief of more than 90% of their pain. During the course of the study, only two permanently implanted subjects withdrew from the study due to inadequate pain relief. Reprogramming and Revisions Six subjects required a total of nine revision surgeries after permanent implantation. Surgical revision is a known complication with any SCS system. In this study, most revisions were for the purpose of adding a second lead. As noted above, the etiology of pain may have contributed to the implanters medical decision to implant one vs. two leads. Ultimately, however, investigators in this study were given considerable leeway in their surgical technique in order to provide the best therapy for a particular subject s pain pattern. North et al. (2005) have identified clinical benefits and lower amplitude requirements when using a single midline lead, and a recent modeling study showed that single leads are likely to recruit more fibers in the dorsal column than dual leads (19,35). However, dual leads may better compensate for imprecise lead placement or migration within the epidural space (32). In this study, lead migration was cited only once as a reason to restart the study clock. We observed three cases in which the IPG was replaced due to telemetry, stimulation, or location problems. Importantly, we observed no instances of lead breakage or battery failure. Overall, the revision rate of this study is within published values (29). Reprogramming also is expected during treatment with SCS. Coverage patterns may change over time due to minor shifts in lead placement or fibrous tissue encapsulation of the lead that may be uneven and cause different impedance values at each contact (36). Lead impedance also may be affected by lead location and cerebrospinal fluid layer thickness (37). Reprogramming involves recapture of the painful areas by programming contacts as anodes, cathodes, or off, as needed. Subjects who requested reprogramming rated their pain as 53% relieved with stimulation immediately after
14 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 275 reprogramming. Surgery was undertaken only if reprogramming was found to be inadequate to regain the concordant paresthesia necessary for stimulation efficacy. The subset of subjects undergoing revision surgery experienced 36% pain relief after the procedure. These findings indicate that patients can reasonably expect clinically meaningful relief of pain, even if complications necessitate reprogramming and revisions. Adverse Events A total of 34 device-related AEs were reported across 114 implants (65 trial and 49 permanent; 29.8%). This study included 41.5 patient-years and the rate of device-related AEs per patientyear was 0.82, suggesting that a typical patient may expect to experience less than one devicerelated complication per year of device use. Lead migration, uncomfortable stimulation, and component failure were the most common adverse events observed during this clinical trial. All are known complications of SCS systems and are well-documented in the medical literature (11). Complication rates observed in this study were similar to, or lower than, complication rates observed in recent retrospective analyses of SCS failure modes (38,39). Deviceor procedure-related adverse events were not life-threatening and were resolved with either standard medical care, reprogramming of the device, or, in some cases, surgical revision. In only one case was subject withdrawal a direct result of an adverse event. Other documented device- or procedure-related complications during this clinical study were telemetry failure, infection, pain (at implant site, and development of new painful areas), cerebrospinal fluid leak, and seroma. Lead breakage, hematoma, paralysis, and skin erosion were not observed during this clinical study, nor were any serious complications such as spinal cord compression or injury, or life-threatening infection. Eight lead migrations were reported, an incidence rate of 7%. This incidence rate is similar to the published rate of migrations across the literature (3 12% with percutaneous leads) (29,35,40). While the design of percutaneous leads allows for easy placement of the leads into the epidural space based on feedback from the conscious subject, these leads also are more prone to migration than surgical leads, either over time or as a result of sudden flexing of the spine. This can change the location and nature of paresthesia and the required stimulation intensity, both of which can require reprogramming of the device and/or surgical correction. However, the advent of multicontact leads has significantly reduced the rates of migration (26). Device migration or failure also can be caused by changes in the tensile load on the lead between the anchor and the IPG during movement of the spine, whether through flexion/ extension or twisting motion. It is important to note that in this study, lead damage or in vivo lead fracture were not observed. Four instances of infections were reported; this 3.5% incidence rate is consistent with the literature, which ranges between 2.5% and 9% (41). As with most infections that occur as a result of an SCS implantation, infections during this clinical study were resolved either with antibiotic therapy or with the removal of the SCS unit followed by antibiotic therapy. A previous analysis of 114 cases of infections with SCS systems indicated that 40% were treated with antibiotics, and SCS components were partially or completely explanted in 94% of cases. Treatments undertaken for infections in this study were similar to those used in standard clinical practice (41). We observed cerebrospinal fluid leaks three times during the course of the study (2.6%); dural punctures were diagnosed by the presence of a wet tap and/or severe positional headaches. Dural puncture is a risk with any epidural procedure: epidural anesthesia administration carries a % risk of postdural puncture headache, depending on the technique and needle used (42). Pain at the implant site also is a possible risk with this type of procedure, and was observed in 2.6% of cases in this study. Whenever there is a disruption of body tissue, temporary pain due to the healing process results. Implant site pain is reported in
15 276 OAKLEY ET AL. the literature as more commonly occurring in patients suffering from CRPS; however, none of the subjects reporting such pain in this study suffered from CRPS (43). Limitations and Future Directions A recent review of SCS literature strongly encourages the adoption of thoughtful study design and endpoints for future studies (44). We suggest that future study designs include bias-reduction strategies, measures of painparesthesia overlap, and nonpain outcomes measures. Clinical data may be biased if the person collecting the data also is responsible for providing subjects with therapy; subjects may feel compelled to modify their answers to please the clinician. It has been demonstrated, for instance, that patients report different outcomes to their physician as compared to a disinterested third-party (45). Thus, a possible source of bias may exist in this study because VAS scores were recorded by research/nursing staff at each of the investigational sites. Although this person was trained according to Good Clinical Practice guidelines and did not influence medical care to the same degree as the subjects physician, it is possible that their involvement in the study may have influenced subject reports. Indeed, biased clinician statements readily induce group differences in pain reports (46). Data collection by a disinterested third party, never involved in patient care, has been implemented in some well-designed pain studies, and we support this design inclusion as a compelling means of reducing bias (32). Overlap of pain and paresthesia with SCS is significantly correlated with pain relief (35,47). This study did not record the location of paresthesias produced by stimulation, although investigators employed standard medical practices for SCS therapy that optimize the overlap. In future studies, we believe it is important to assess whether paresthetic sensations cover the painful areas; it is likely that patients with good overlap will experience better outcomes. Similarly, VAS ratings were global and made no distinction between back pain, leg pain, etc. Although the effect of stimulation on global VAS was marked, it may have been more informative to distinguish the location of pain (48). Furthermore, it is possible that limiting the inclusion criteria to a single diagnosis, rather than the variety of diagnoses in this study, may provide more homogeneous data. We are preparing a subsequent analysis of these data that will individually assess outcomes in homogeneous subject populations. Outcomes of these analyses will be discussed in a future published report. Relief from chronic neuropathic pain can improve many aspects of a patient s life including psychologic function, disability, self-care, social interactions, and work status (49). For instance, subjects treated with neuromodulation often report being able to reduce or altogether stop their pain medication consumption (27,50). In fact, one reported adverse event in this study was due to abstinence syndrome following complete cessation of opioid medications; clearly, medication intake was significantly reduced for that subject. Due to the average 50 80% reduction in pain across subjects, we hypothesize that many subjects in this study may have reduced their pain medication intake after implantation. Reducing narcotic consumption has many implications for improving a patient s psychologic state (50). We recommend that future studies include assessment of concomitant medications, and measures of quality of life, including activities of daily living. Conclusions Spinal cord stimulation is a minimally invasive therapy for pain when compared to conventional surgical techniques. Relative to ablative neurosurgical pain procedures, SCS is remarkably benign. Furthermore, it is completely reversible because stimulation can be turned off at any time, and the device can be explanted through a minor surgical procedure. The results of this clinical trial illustrate that spinal cord stimulation with the Advanced Bionics/Boston Scientific Precision system is safe and highly effective for Boston scientific a majority
16 NEW SCS SYSTEM IS EFFECTIVE FOR PAIN 277 of subjects across etiologies of pain, including FBSS and CRPS. The Precision system provides substantial benefits for patients with chronic pain and is likely to provide pain relief for those in whom conventional therapies have failed. Acknowledgments This study was sponsored by Advanced Bionics/ Boston Scientific. This clinical trial was performed in compliance with the US Food and Drug Administration Code of Federal Regulations (21 CFR 812) and Investigational Device Exemption (FDA IDE G030004, approved 01/ 30/2003). Dr. Foster is an employee of Advanced Bionics/Boston Scientific. References 1. Verhaak PFM, Kerssens JJ, Dekker J et al. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain 1998;77: Krames ES. Interventional pain management. Appropriate when less invasive therapies fail to provide adequate analgesia. Med Clin North Am 1999;83: Stanton-Hicks M. Nerve blocks in chronic pain therapy are there any indications left? Acta Anaesthesiol Scand 2001;45: Pierron D, Robine D, Cornejo M, Dubeaux P. Chronic low back pain and lumbar rhizotomy. Agressologie 1991;32: Fritzell P, Hagg O, Wessberg P, Nordwall A. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 2001;26: Rainov NG, Heidecke V, Burkert W. Longterm intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage 2001;22: Pertoldi S, Di Benedetto P. Shoulder hand syndrome after stroke. A complex regional pain syndrome. Eur Med 2005;41: Burchiel KJ, Anderson VC, Wilson BJ et al. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery 1995;36: Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 1967;46: Costantini A. Spinal cord stimulation. Minerva Anestesiol 2005;71: Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine 2004;30: Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20- year literature review. J Neurosurg 2004;100: Nathan PW. The gate-control theory of pain. A critical review. Brain 1976;99: Meyerson BA, Linderoth B. Mechanisms of spinal cord stimulation in neuropathic pain. Neurol Res 2000;22: Stanton-Hicks M, Salamon J. Stimulation of the central and peripheral nervous system for the control of pain. J Clin Neurophysiol 1997;14: Oakley JC, Krames E, Prager J et al. Usefulness of stimulation and demographic parameters for prediction of response to SCS [abstract]. American Associate of Neurological Surgeons 73rd annual meeting. CNS Section on Pain #807, New Orleans, LA, April 16 21, Moffitt J, Bradley K, Peterson D. Incremental movement of the Volume of activation in spinal cord stimulation via fractionalization of current between contacts [abstract]. Proceedings from the 9th annual meeting of the North American Neuromodulation Society. Neuromodulation 2006;9: Oakley J, Varga C, Krames E, Bradley K. Real-time paresthesia steering using continuous electric field adjustment. Part I: intraoperative performance. Neuromodulation 2004;7: Manola L, Holsheimer J, Veltink P. Technical performance of percutaneous leads for spinal cord stimulation: a modeling study. Neuromodulation 2005;8: Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine 2000;25: Precision. Physician lead implantation manual. Sylmar, CA: Advanced Bionics Corporation, Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain some predictors of success. A 15-year experience. Surg Neurol 1998;50: Ostelo RWJG, de Vet HCW. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 2005;19: Machin D, Campbell M, Fayers P, Pinol A. Sample size tables for clinical studies, 2nd ed. Malden, MA: Blackwell Science, 1997.
17 278 OAKLEY ET AL. 25. Hays WL. Statistics, 9th ed. New York: Holt, Rinehart and Winston Inc., North RB, Wetzel FT. Spinal cord stimulation for chronic pain of spinal origin: a valuable longterm solution. Spine 2002;27: Van Buyten J-P, Van Zundert J, Vueghs P, Vanduffel L. Efficacy of spinal cord stimulation: 10 years of experience in a pain centre in Belgium. Eur J Pain 2001;5: Oakley JC, Prager JP. Spinal cord stimulation: mechanisms of action. Spine 2002;27: Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006;58: Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006;7 (Suppl. 1):S14 S Grill WM, Mortimer JT. Electrical properties of implant encapsulation tissue. Ann Biomed Eng 1994;22: North RB, Kidd DH, Olin J, Sieracki JM et al. Spinal cord stimulation for axial low back pain. A prospective, controlled trial comparing dual with single percutaneous electrodes. Spine 2005;30: Alo KM, Redko V, Charnov J. Four year followup of dual electrodes spinal cord stimulation for chronic pain. Neuromodulation 2002;5: Devulder J, De Laat M, Van Bastelaere M, Rolly G. Spinal cord stimulation: a valuable treatment for chronic failed back surgery patients. J Pain Symptom Manage 1997;13: North RB, Kidd DH, Farroki F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005;56: Richardson RR, Nunez C, Siqueira EB. Histological reaction to percutaneous epidural neurostimulation: initial and long-term results. Med Prog Technol 1979;6: Alo K, Varga C, Krames E et al. Factors affecting impedance of percutaneous leads in spinal cord stimulation. Neuromodulation 2006;9: Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine 2006;5: Rosenow JM, Stanton-Hicks M, Rezai AR, Henderson JM. Failure modes of spinal cord stimulation hardware. J Neurosurg Spine 2006;5: Ohnmeiss DD, Rashbaum RF. Patient satisfaction with spinal cord stimulation for predominant complaints of chronic, intractable low back pain. Spine 2001;1: Follett KA, Boortz-Marx RL, Drake JM et al. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology 2004;100: Vallejo MC, Mandell GL, Sabo DP, Ramanathan S. Postdural puncture headache: a randomized comparison of five spinal needles in obstetric patients. Anesth Analg 2000;91: Barolat G. Spinal cord stimulation for chronic pain management. Arch Med Res 2000;31: Mailis-Gagnon A, Furlan AD, Sandoval JA, Taylor R. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev 2004; Issue 3, Art. No.: CD doi: / North RB, Kidd DH, Zahurak M, James CS, Long DM. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery 1993;32: Branch MA, Carlson CR, Okeson JP. Influence of biased clinician statements on patient report of referred pain. J Orofac Pain 2000;14: Holsheimer J. Effectiveness of spinal cord stimulation in the management of chronic pain: analysis of technical drawbacks and solutions. Neurosurgery 1997;40: Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery 1995;37: Arnstein P. Chronic neuropathic pain: issues in patient education. Pain Manag Nurs 2004;5 (4 Suppl. 1): Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain 2003;7:
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet
 Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal cord stimulation
 Spinal cord stimulation This leaflet aims to answer your questions about having spinal cord stimulation. It explains the benefits, risks and alternatives, as well as what you can expect when you come to
Spinal cord stimulation This leaflet aims to answer your questions about having spinal cord stimulation. It explains the benefits, risks and alternatives, as well as what you can expect when you come to
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Neurostimulation: Orthopaedic Institute of Ohio 801 Medical Drive Lima, Ohio 45804 419-222-6622
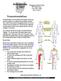 801 Medical Drive Lima, Ohio 45804 419-222-6622 Neurostimulation is the stimulation of the spinal cord by tiny electrical impulses. An implanted lead (a flexible insulated wire), which is powered by an
801 Medical Drive Lima, Ohio 45804 419-222-6622 Neurostimulation is the stimulation of the spinal cord by tiny electrical impulses. An implanted lead (a flexible insulated wire), which is powered by an
Department of Neurosciences Dorsal Root Ganglion (DRG) Stimulation Information for patients
 Oxford University Hospitals NHS Trust Department of Neurosciences Dorsal Root Ganglion (DRG) Stimulation Information for patients We have recently seen you in clinic as you have had pain for a long period
Oxford University Hospitals NHS Trust Department of Neurosciences Dorsal Root Ganglion (DRG) Stimulation Information for patients We have recently seen you in clinic as you have had pain for a long period
 Epimed Would Like To Congratulate The 20th Annual Budapest Conference The New Shape of Pain Relief SmalleST. ThiNNeST. CoNTouRed design. Precision Novi is the world s smallest, thinnest 16 contact primary
Epimed Would Like To Congratulate The 20th Annual Budapest Conference The New Shape of Pain Relief SmalleST. ThiNNeST. CoNTouRed design. Precision Novi is the world s smallest, thinnest 16 contact primary
MEDICAL CONTESTED CASE HEARING NO 12090 M6-12-38043-01 DECISION AND ORDER
 MEDICAL CONTESTED CASE HEARING NO 12090 M6-12-38043-01 DECISION AND ORDER This case is decided pursuant to Chapter 410 of the Texas Workers Compensation Act and Rules of the Division of Workers Compensation
MEDICAL CONTESTED CASE HEARING NO 12090 M6-12-38043-01 DECISION AND ORDER This case is decided pursuant to Chapter 410 of the Texas Workers Compensation Act and Rules of the Division of Workers Compensation
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery
 Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
.org. Herniated Disk in the Lower Back. Anatomy. Description
 Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
EXHIBIT C BOSTON SCIENTIFIC PRODUCTS AVAILABLE FOR NIH BRAIN INITIATIVE Page 1 of 9
 Page 1 of 9 NOTE: All systems and products should be considered as investigational-use only in the context of the NIH BRAIN Initiative. VERCISE DEEP BRAIN STIMULATION SYSTEM Description: 16-channel rechargeable
Page 1 of 9 NOTE: All systems and products should be considered as investigational-use only in the context of the NIH BRAIN Initiative. VERCISE DEEP BRAIN STIMULATION SYSTEM Description: 16-channel rechargeable
Open Discectomy. North American Spine Society Public Education Series
 Open Discectomy North American Spine Society Public Education Series What Is Open Discectomy? Open discectomy is the most common surgical treatment for ruptured or herniated discs of the lumbar spine.
Open Discectomy North American Spine Society Public Education Series What Is Open Discectomy? Open discectomy is the most common surgical treatment for ruptured or herniated discs of the lumbar spine.
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
X Stop Spinal Stenosis Decompression
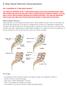 X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg
 Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Spinal Cord Stimulation
 Spinal Cord Stimulation Relieving Chronic Pain If chronic pain restricts your life, you may have tried many ways to find relief. This may include medications, nerve blocks, physical therapy, and surgery.
Spinal Cord Stimulation Relieving Chronic Pain If chronic pain restricts your life, you may have tried many ways to find relief. This may include medications, nerve blocks, physical therapy, and surgery.
Contractor Number 11302. Oversight Region Region IV
 Local Coverage Determination (LCD): Spinal Cord Stimulators for Chronic Pain (L32549) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11302 Contractor Type MAC
Local Coverage Determination (LCD): Spinal Cord Stimulators for Chronic Pain (L32549) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11302 Contractor Type MAC
Intrathecal Baclofen for CNS Spasticity
 Intrathecal Baclofen for CNS Spasticity Last Review Date: November 13, 2015 Number: MG.MM.ME.31bC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Intrathecal Baclofen for CNS Spasticity Last Review Date: November 13, 2015 Number: MG.MM.ME.31bC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Sample Treatment Protocol
 Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Y O U R S U R G E O N S. choice of. implants F O R Y O U R S U R G E R Y
 Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Your Surgeon Has Chosen the C 2 a-taper Acetabular System The
Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Your Surgeon Has Chosen the C 2 a-taper Acetabular System The
Back & Neck Pain Survival Guide
 Back & Neck Pain Survival Guide www.kleinpeterpt.com Zachary - 225-658-7751 Baton Rouge - 225-768-7676 Kleinpeter Physical Therapy - Spine Care Program Finally! A Proven Assessment & Treatment Program
Back & Neck Pain Survival Guide www.kleinpeterpt.com Zachary - 225-658-7751 Baton Rouge - 225-768-7676 Kleinpeter Physical Therapy - Spine Care Program Finally! A Proven Assessment & Treatment Program
6/3/2011. High Prevalence and Incidence. Low back pain is 5 th most common reason for all physician office visits in the U.S.
 High Prevalence and Incidence Prevalence 85% of Americans will experience low back pain at some time in their life. Incidence 5% annual Timothy C. Shen, M.D. Physical Medicine and Rehabilitation Sub-specialty
High Prevalence and Incidence Prevalence 85% of Americans will experience low back pain at some time in their life. Incidence 5% annual Timothy C. Shen, M.D. Physical Medicine and Rehabilitation Sub-specialty
INFORMED CONSENT DERMABRASION
 INFORMED CONSENT DERMABRASION INSTRUCTION This is an informed-consent document which has been prepared to help your plastic surgeon inform you about dermabrasion, its risks, and alternative treatments.
INFORMED CONSENT DERMABRASION INSTRUCTION This is an informed-consent document which has been prepared to help your plastic surgeon inform you about dermabrasion, its risks, and alternative treatments.
X-Plain Trigeminal Neuralgia Reference Summary
 X-Plain Trigeminal Neuralgia Reference Summary Introduction Trigeminal neuralgia is a condition that affects about 40,000 patients in the US every year. Its treatment mostly involves the usage of oral
X-Plain Trigeminal Neuralgia Reference Summary Introduction Trigeminal neuralgia is a condition that affects about 40,000 patients in the US every year. Its treatment mostly involves the usage of oral
Interscalene Block. Nancy A. Brown, MD
 Interscalene Block Nancy A. Brown, MD What is an Interscalene Block? An Interscalene block is a form of regional anesthesia used in conjunction with general anesthesia for surgeries of the shoulder and
Interscalene Block Nancy A. Brown, MD What is an Interscalene Block? An Interscalene block is a form of regional anesthesia used in conjunction with general anesthesia for surgeries of the shoulder and
Study Protocol Template
 Study Protocol Template (Chart Reviews) Instructions: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. It
Study Protocol Template (Chart Reviews) Instructions: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. It
INFORMED CONSENT FOR SLEEVE GASTRECTOMY
 INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
Spinal Surgery Functional Status and Quality of Life Outcome Specifications 2015 (01/01/2013 to 12/31/2013 Dates of Procedure) September 2014
 Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
A Phase 2 Study of Interferon Beta-1a (Avonex ) in Ulcerative Colitis
 A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy
 Pain Physician 2007; 10:313-318 ISSN 1533-3159 Case Series Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy R. Allen Hooper
Pain Physician 2007; 10:313-318 ISSN 1533-3159 Case Series Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy R. Allen Hooper
SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS?
 SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS? The spinal canal is best imagined as a bony tube through which nerve fibres pass. The tube is interrupted between each pair of adjacent
SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS? The spinal canal is best imagined as a bony tube through which nerve fibres pass. The tube is interrupted between each pair of adjacent
Local Coverage Determination (LCD): Spinal Cord Stimulation (Dorsal Column Stimulation) (L34705)
 Local Coverage Determination (LCD): Spinal Cord Stimulation (Dorsal Column Stimulation) (L34705) Contractor Information Contractor Name Novitas Solutions, Inc. LCD Information Document Information LCD
Local Coverage Determination (LCD): Spinal Cord Stimulation (Dorsal Column Stimulation) (L34705) Contractor Information Contractor Name Novitas Solutions, Inc. LCD Information Document Information LCD
Electroconvulsive Therapy - ECT
 Electroconvulsive Therapy - ECT Introduction Electroconvulsive therapy, or ECT, is a safe and effective treatment that may reduce symptoms related to depression or mental illness. During ECT, certain parts
Electroconvulsive Therapy - ECT Introduction Electroconvulsive therapy, or ECT, is a safe and effective treatment that may reduce symptoms related to depression or mental illness. During ECT, certain parts
Economic aspects of Spinal Cord Stimulation (SCS)
 Economic aspects of Spinal Cord Stimulation (SCS) Burden of Chronic Pain Chronic pain affects one in five adults in Europe 1. Up to 10 per cent of chronic pain cases are neuropathic in origin. 2 Chronic
Economic aspects of Spinal Cord Stimulation (SCS) Burden of Chronic Pain Chronic pain affects one in five adults in Europe 1. Up to 10 per cent of chronic pain cases are neuropathic in origin. 2 Chronic
The Pharmacological Management of Cancer Pain in Adults. Clinical Audit Tool
 The Pharmacological Management of Cancer Pain in Adults Clinical Audit Tool 2015 This clinical audit tool accompanies the Pharmacological Management of Cancer Pain in Adults NCEC National Clinical Guideline
The Pharmacological Management of Cancer Pain in Adults Clinical Audit Tool 2015 This clinical audit tool accompanies the Pharmacological Management of Cancer Pain in Adults NCEC National Clinical Guideline
Effects of Acupuncture on Chronic Lower Back Pain. Audience: Upper Division IPHY Majors
 1 Effects of Acupuncture on Chronic Lower Back Pain Audience: Upper Division IPHY Majors Introduction: Lower back pain is the leading cause of limited physical activity and the second most frequent reason
1 Effects of Acupuncture on Chronic Lower Back Pain Audience: Upper Division IPHY Majors Introduction: Lower back pain is the leading cause of limited physical activity and the second most frequent reason
SYNOPSIS. Risperidone: Clinical Study Report CR003274
 SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
CONSENT FORM 12/19/08
 12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
NEUROMODULATION THERAPY ACCESS COALITION POSITION STATEMENT ON SPINAL CORD NEUROSTIMULATION
 NEUROMODULATION THERAPY ACCESS COALITION POSITION STATEMENT ON SPINAL CORD NEUROSTIMULATION Introduction: Spinal cord stimulation (SCS) is an established treatment for chronic neuropathic pain, which can
NEUROMODULATION THERAPY ACCESS COALITION POSITION STATEMENT ON SPINAL CORD NEUROSTIMULATION Introduction: Spinal cord stimulation (SCS) is an established treatment for chronic neuropathic pain, which can
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS
 Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS Operation Manual Read Before Using LUMI-TENS-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS Operation Manual Read Before Using LUMI-TENS-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Herniated Disk in the Lower Back
 Nader M. Hebela, MD Fellow of the American Academy of Orthopaedic Surgeons http://orthodoc.aaos.org/hebela Cleveland Clinic Abu Dhabi Cleveland Clinic Abu Dhabi Neurological Institute Al Maryah Island
Nader M. Hebela, MD Fellow of the American Academy of Orthopaedic Surgeons http://orthodoc.aaos.org/hebela Cleveland Clinic Abu Dhabi Cleveland Clinic Abu Dhabi Neurological Institute Al Maryah Island
Herniated Disk. This reference summary explains herniated disks. It discusses symptoms and causes of the condition, as well as treatment options.
 Herniated Disk Introduction Your backbone, or spine, has 24 moveable vertebrae made of bone. Between the bones are soft disks filled with a jelly-like substance. These disks cushion the vertebrae and keep
Herniated Disk Introduction Your backbone, or spine, has 24 moveable vertebrae made of bone. Between the bones are soft disks filled with a jelly-like substance. These disks cushion the vertebrae and keep
sound or ringing in the ears.
 (Idiopathic Intracranial Hypertension) Sashank Prasad, MD www.brighamandwomens.org/neuro-ophthalmology A Patient s Guide Symptoms Diagnosis Treatment Prognosis Symptoms The symptoms of include: Headaches
(Idiopathic Intracranial Hypertension) Sashank Prasad, MD www.brighamandwomens.org/neuro-ophthalmology A Patient s Guide Symptoms Diagnosis Treatment Prognosis Symptoms The symptoms of include: Headaches
ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System
 ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91035972-01
ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91035972-01
Orthopaedic Spine Center. Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs
 Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY
 YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY What is functional endoscopic sinus surgery (FESS)? Functional endoscopic sinus surgery
YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY What is functional endoscopic sinus surgery (FESS)? Functional endoscopic sinus surgery
Special Control Guidance for Premarket Notifications for Totally Implanted Spinal Cord Stimulators for Pain Relief
 . na,:: t;. _ f _ 3.:. Druft 7 Not fvr Implementation Guidance for Industry Special Control Guidance for Premarket Notifications for Totally Implanted Spinal Cord Stimulators for Pain Relief Draft Guidance
. na,:: t;. _ f _ 3.:. Druft 7 Not fvr Implementation Guidance for Industry Special Control Guidance for Premarket Notifications for Totally Implanted Spinal Cord Stimulators for Pain Relief Draft Guidance
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants
 Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Quick Facts about Breast Augmentation with IDEAL IMPLANT Saline-filled Breast Implants Important Factors Breast Augmentation Patients Should Consider October 2015 Caution: Federal law restricts this device
Outcome of Drug Counseling of Outpatients in Chronic Obstructive Pulmonary Disease Clinic at Thawangpha Hospital
 Mahidol University Journal of Pharmaceutical Sciences 008; 35(14): 81. Original Article Outcome of Drug Counseling of Outpatients in Chronic Obstructive Pulmonary Disease Clinic at Thawangpha Hospital
Mahidol University Journal of Pharmaceutical Sciences 008; 35(14): 81. Original Article Outcome of Drug Counseling of Outpatients in Chronic Obstructive Pulmonary Disease Clinic at Thawangpha Hospital
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
33 % of whiplash patients develop. headaches originating from the upper. cervical spine
 33 % of whiplash patients develop headaches originating from the upper cervical spine - Dr Nikolai Bogduk Spine, 1995 1 Physical Treatments for Headache: A Structured Review Headache: The Journal of Head
33 % of whiplash patients develop headaches originating from the upper cervical spine - Dr Nikolai Bogduk Spine, 1995 1 Physical Treatments for Headache: A Structured Review Headache: The Journal of Head
Image-guided Spine Procedures for Relief of Severe Lower Back Pain:
 Image-guided Spine Procedures for Relief of Severe Lower Back Pain: A Guide to Epidural Steroid Injection, Facet Joint Injection, and Selective Nerve Root Block. PETER H TAKEYAMA MD HENRY WANG MD PhD SVEN
Image-guided Spine Procedures for Relief of Severe Lower Back Pain: A Guide to Epidural Steroid Injection, Facet Joint Injection, and Selective Nerve Root Block. PETER H TAKEYAMA MD HENRY WANG MD PhD SVEN
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
Lumbar Laminectomy and Interspinous Process Fusion
 Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Laser Treatment Policy
 Laser Treatment Policy Pursuant to federal law 21 CFR 812.2(c)7 and 812.3(b), physician(s) at this pain center may advise and use unapproved laser s on patients under one or more of the following conditions:
Laser Treatment Policy Pursuant to federal law 21 CFR 812.2(c)7 and 812.3(b), physician(s) at this pain center may advise and use unapproved laser s on patients under one or more of the following conditions:
Normal bladder function requires a coordinated effort between the brain, spinal cord, and the bladder.
 .. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
.. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
Epidurals for pain relief after surgery
 Epidurals for pain relief after surgery This information leaflet is for anyone who may benefit from an epidural for pain relief after surgery. We hope it will help you to ask questions and direct you to
Epidurals for pain relief after surgery This information leaflet is for anyone who may benefit from an epidural for pain relief after surgery. We hope it will help you to ask questions and direct you to
CLINICAL PRACTICE GUIDELINES FOR MANAGEMENT OF LOW BACK PAIN
 CLINICAL PRACTICE GUIDELINES FOR MANAGEMENT OF LOW BACK PAIN Low back pain is very common, up to 90+% of people are affected by back pain at some time in their lives. Most often back pain is benign and
CLINICAL PRACTICE GUIDELINES FOR MANAGEMENT OF LOW BACK PAIN Low back pain is very common, up to 90+% of people are affected by back pain at some time in their lives. Most often back pain is benign and
X-Plain Inguinal Hernia Repair Reference Summary
 X-Plain Inguinal Hernia Repair Reference Summary Introduction Hernias are common conditions that affect men and women of all ages. Your doctor may recommend a hernia operation. The decision whether or
X-Plain Inguinal Hernia Repair Reference Summary Introduction Hernias are common conditions that affect men and women of all ages. Your doctor may recommend a hernia operation. The decision whether or
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp. 1800 Bridge Parkway
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp. 1800 Bridge Parkway
Welcome to the July 2012 edition of Case Studies from the files of the Institute for Nerve Medicine in Santa Monica, California.
 Welcome to the July 2012 edition of Case Studies from the files of the Institute for Nerve Medicine in Santa Monica, California. In this issue, we focus on a 23-year-old female patient referred by her
Welcome to the July 2012 edition of Case Studies from the files of the Institute for Nerve Medicine in Santa Monica, California. In this issue, we focus on a 23-year-old female patient referred by her
X-Plain Sinus Surgery Reference Summary
 X-Plain Sinus Surgery Reference Summary Introduction Sinus surgery is a very common and safe operation. Your doctor may recommend that you have sinus surgery. The decision whether or not to have sinus
X-Plain Sinus Surgery Reference Summary Introduction Sinus surgery is a very common and safe operation. Your doctor may recommend that you have sinus surgery. The decision whether or not to have sinus
URINARY INCONTINENCE
 URINARY INCONTINENCE What is urinary incontinence? Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only a few drops when you cough or sneeze to entirely
URINARY INCONTINENCE What is urinary incontinence? Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only a few drops when you cough or sneeze to entirely
PAIN MANAGEMENT AT UM/SYLVESTER
 PAIN MANAGEMENT AT UM/SYLVESTER W HAT IS THE PURPOSE OF THIS BROCHURE? We created this brochure for patients receiving care from the University of Miami Sylvester Comprehensive Cancer Center and their
PAIN MANAGEMENT AT UM/SYLVESTER W HAT IS THE PURPOSE OF THIS BROCHURE? We created this brochure for patients receiving care from the University of Miami Sylvester Comprehensive Cancer Center and their
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Temple Physical Therapy
 Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Denver Spine Surgeons David Wong, MD, Sanjay Jatana, MD, Gary Ghiselli, MD
 Cervical and Lumbar Spine Health History Name: Today s Date: Referring Provider: How did you find us: (Please circle) Primary care physician, Google search, Facebook, Friend or Family member, Website (JatanaSpine
Cervical and Lumbar Spine Health History Name: Today s Date: Referring Provider: How did you find us: (Please circle) Primary care physician, Google search, Facebook, Friend or Family member, Website (JatanaSpine
Bionic Navigator 1.2 Software Guide
 Bionic Navigator 1.2 Software Guide SC-7150, SC-7150-4, SC-7150-4R, NM-7153-12, NM-7153-12R, NM-7151-30, NM-7151-30R, NM-7153-30, NM-7153-30R Clinician Programmer CAUTION: Federal law restricts this device
Bionic Navigator 1.2 Software Guide SC-7150, SC-7150-4, SC-7150-4R, NM-7153-12, NM-7153-12R, NM-7151-30, NM-7151-30R, NM-7153-30, NM-7153-30R Clinician Programmer CAUTION: Federal law restricts this device
Breast Augmentation. If you are dissatisfied with your breast size, augmentation surgery is a choice to consider. Breast augmentation can:
 Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery
 Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
Post-operative Pain Management
 Post-operative Pain Management Total Hip Replacement www.ormc.org A member of the Greater Hudson Valley Health System Post-operative Pain MANAGEMENT Post-operative pain management after total joint replacement
Post-operative Pain Management Total Hip Replacement www.ormc.org A member of the Greater Hudson Valley Health System Post-operative Pain MANAGEMENT Post-operative pain management after total joint replacement
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998
 Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Osteoporosis and Vertebral Compression (Spinal) Fractures Fact Sheet
 Osteoporosis and Vertebral Compression (Spinal) Fractures Fact Sheet About Osteoporosis Osteoporosis is estimated to affect 200 million women worldwide. 1 Worldwide, osteoporosis causes more than nine
Osteoporosis and Vertebral Compression (Spinal) Fractures Fact Sheet About Osteoporosis Osteoporosis is estimated to affect 200 million women worldwide. 1 Worldwide, osteoporosis causes more than nine
Spinal Cord Stimulation Business Case for
 Spinal Cord Stimulation Business Case for Failed Back Surgery Syndrome Understanding How Various Stakeholders Gauge Success in Treating Chronic Pain FBSS is a subset of chronic low back pain, which is
Spinal Cord Stimulation Business Case for Failed Back Surgery Syndrome Understanding How Various Stakeholders Gauge Success in Treating Chronic Pain FBSS is a subset of chronic low back pain, which is
A Patient s Guide to Artificial Cervical Disc Replacement
 A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
Lumbar Spinal Stenosis
 Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Failed back spine surgery
 Failed back spine surgery Presentation Definition Etiology Prevalence Evaluation Treatment Case study FBSS: Definition A constellation of conditions that describes persistent or recurring low back pain,
Failed back spine surgery Presentation Definition Etiology Prevalence Evaluation Treatment Case study FBSS: Definition A constellation of conditions that describes persistent or recurring low back pain,
Sacral Nerve Neuromodulation/Stimulation
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Sacral Nerve Neuromodulation/Stimulation Number 7.01.69 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Sacral Nerve Neuromodulation/Stimulation Number 7.01.69 Effective
Uterine Fibroid Symptoms, Diagnosis and Treatment
 Fibroids and IR Uterine Fibroid Symptoms, Diagnosis and Treatment Interventional radiologists use MRIs to determine if fibroids can be embolised, detect alternate causes for the symptoms and rule out misdiagnosis,
Fibroids and IR Uterine Fibroid Symptoms, Diagnosis and Treatment Interventional radiologists use MRIs to determine if fibroids can be embolised, detect alternate causes for the symptoms and rule out misdiagnosis,
VNS Therapy for Epilepsy
 VNS Therapy for Epilepsy This pamphlet provides general information for the public. It is not intended to cover all possible uses, directions, precautions, interactions or adverse effects involving any
VNS Therapy for Epilepsy This pamphlet provides general information for the public. It is not intended to cover all possible uses, directions, precautions, interactions or adverse effects involving any
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
FREEDOM INGUINAL Hernia Repair System TECHNIQUE GUIDE
 FREEDOM INGUINAL Hernia Repair System TECHNIQUE GUIDE The following describes the open surgical preparation and implantation technique for the Freedom Inguinal Hernia Repair System. 1) Anesthesia can be
FREEDOM INGUINAL Hernia Repair System TECHNIQUE GUIDE The following describes the open surgical preparation and implantation technique for the Freedom Inguinal Hernia Repair System. 1) Anesthesia can be
Neck Pain Overview Causes, Diagnosis and Treatment Options
 Neck Pain Overview Causes, Diagnosis and Treatment Options Neck pain is one of the most common forms of pain for which people seek treatment. Most individuals experience neck pain at some point during
Neck Pain Overview Causes, Diagnosis and Treatment Options Neck pain is one of the most common forms of pain for which people seek treatment. Most individuals experience neck pain at some point during
June 2010 Case Study: 33-year-old Male with Pudendal Pain Tuesday, 01 June 2010 13:44
 Welcome to the June 2010 Neurography Institute Case Study. In this issue, we learn how MR Neurography (MRN) and Interventional MR (IMR) helped a thirtysomething male regain his active lifestyle without
Welcome to the June 2010 Neurography Institute Case Study. In this issue, we learn how MR Neurography (MRN) and Interventional MR (IMR) helped a thirtysomething male regain his active lifestyle without
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Neural Tube Defects - NTDs
 Neural Tube Defects - NTDs Introduction Neural tube defects are also known as NTDs. They happen when the spine and brain do not fully develop while the fetus is forming in the uterus. Worldwide, there
Neural Tube Defects - NTDs Introduction Neural tube defects are also known as NTDs. They happen when the spine and brain do not fully develop while the fetus is forming in the uterus. Worldwide, there
Department of Pharmacy, Kaiser Permanente San Francisco Medical Center, San Francisco 94115, California, USA
 Journal of Pharmacy and Pharmacology 3 (2015) 33-38 doi: 10.17265/2328-2150/2015.01.005 D DAVID PUBLISHING Evaluation of Glycemic Control with a Pharmacist-Managed Post-Cardiothoracic Surgery Insulin Protocol
Journal of Pharmacy and Pharmacology 3 (2015) 33-38 doi: 10.17265/2328-2150/2015.01.005 D DAVID PUBLISHING Evaluation of Glycemic Control with a Pharmacist-Managed Post-Cardiothoracic Surgery Insulin Protocol
Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: An Observational Study
 Bartolomeo Operti, MD and Dr Tiziano Tealdo Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: Bartolomeo Operti, MD Chief of Anesthesia Department
Bartolomeo Operti, MD and Dr Tiziano Tealdo Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: Bartolomeo Operti, MD Chief of Anesthesia Department
MEDICAL/DENTAL MALPRACTICE
 Date of Trial: 4/7/06 MEDICAL/DENTAL MALPRACTICE Sex and Age: Plaintiff: Male and Female ages unknown/ Defendant: N/A Last demand before trial: unknown Last offer before trial: unknown Specifics: Repair
Date of Trial: 4/7/06 MEDICAL/DENTAL MALPRACTICE Sex and Age: Plaintiff: Male and Female ages unknown/ Defendant: N/A Last demand before trial: unknown Last offer before trial: unknown Specifics: Repair
PATIENT INFORMATION SHEET KEY FACTS
 PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Clinical and Therapeutic Cannabis Information. Written by Cannabis Training University (CTU) All rights reserved
 Clinical and Therapeutic Cannabis Information Written by Cannabis Training University (CTU) All rights reserved Contents Introduction... 3 Chronic Pain... 6 Neuropathic Pain... 8 Movement Disorders...
Clinical and Therapeutic Cannabis Information Written by Cannabis Training University (CTU) All rights reserved Contents Introduction... 3 Chronic Pain... 6 Neuropathic Pain... 8 Movement Disorders...
User Manual Table of Contents. Instructions for Using the LaserTouchOne Charging the Unit... 5 Holding the Device... 5 Treatment Steps...
 User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
The LifeWave ALAVIDA Non-transdermal Antiaging Patch Improves Cellular Functional Status in Different Organs
 The LifeWave ALAVIDA Non-transdermal Antiaging Patch Improves Cellular Functional Status in Different Organs Research Performed by: Sherry Blake-Greenberg MA, HMD Health Integration Therapy, Palos Verdes
The LifeWave ALAVIDA Non-transdermal Antiaging Patch Improves Cellular Functional Status in Different Organs Research Performed by: Sherry Blake-Greenberg MA, HMD Health Integration Therapy, Palos Verdes
