Formulary Updates. Additional formulary updates may be found in the P & T Committee Review table beginning on page 4.
|
|
|
- Oswin Hill
- 10 years ago
- Views:
Transcription
1 SUPPORTING OUR PROVIDER PARTNERS THROUGH COMMUNICATION AND COLLABORATION. DATE JULY 2015 ISSUE 2 HELPFUL NUMBERS FOR PROVIDERS Passport Health Plan Magellan: Bin: Processor control: 747 HELPFUL NUMBERS FOR MEMBERS Passport Health Plan WEBSITE NEW IN THIS ISSUE Formulary Updates Pharmacy Tips & Reminders Recent FDA Advisories P & T Committee Review Formulary Updates Growth Hormone: Passport has revised its criteria concerning the use of Growth Hormone products. Norditropin (somatropin) is Preferred; Omnitrope and Tev-tropin are now Non-preferred. Oral Anticoagulants: Passport has revised its criteria concerning the use of Oral Anticoagulants. Xarelto (rivaroxaban) and Pradaxa (dabigatran) are Preferred; Eliquis (apixiban) is now Non-preferred. Any member currently maintained on Eliquis will be grandfathered and allowed to continue therapy without disruption New Generics: Abilify tablets are now available as a generic formulation called aripiprazole. Aripiprazole has been added as a Preferred generic on the Passport formulary and Brand name Abilify is now. Bromfed DM is now available as a generic formulation. The generic formulation has been added as a Preferred generic on the Passport formulary, while Brand name Bromfed DM will remain. Focalin XR 5mg and 10mg are now available in a generic formulation called dexmethlyphenidate ER. Dexmethylphenidate ER 5 & 10mg join strengths 15, 30, and 40mg as Preferred generics. Additional formulary updates may be found in the P & T Committee Review table beginning on page 4. Suboxone Prior Authorization Criteria Changes: Passport Health Plan has updated its prior authorization (PA) process for buprenorphine-containing medications (e.g., Suboxone, Zubsolv). The clinical criteria have been revised to incorporate the recent Kentucky Board of Medical Licensure (KBML) regulations, 201 KAR 9:270. Guidance from the National Institute of Health, the Substance Abuse and Mental Health Services Administration (SAMHSA), and the U.S. Department of Health and Human Services Clinical Guidelines for the use of Buprenorphine in the Treatment of Opioid Addiction were also sourced in developing changes to our current criteria. Some criteria changes include: 2015 PASSPORT HEALTH PLAN All medications may be subject to edits to limit quantities dispensed, day s supply, and drug-drug interactions at the point of service. Pharmacy and Therapeutic Committee decisions are based upon relevant medical literature that is evidence based and peer reviewed. Price(s) listed are calculated based on Wholesale Acquisition Cost (WAC) published by First Data Bank. The cost of therapy is calculated based on a 30 days supply unless otherwise indicated. This information is to be used as a reference and/or a learning tool for providers.
2 A Statement of Understanding Form to be discussed and completed by both the provider and member. This form will be found on the last page of the updated PA document. KASPER reports must be obtained and reviewed for a 12-month period immediately preceding the initial prescription and then on a monthly basis for reauthorization. Monthly drug screens must be performed, documented, and attached to requests. Every six months, at least one random drug screen should be coupled with a pill count. Please note Passport covers medication-assisted treatment (MAT) of those with substance abuse disorders (medications, behavioral therapies, medical office visits, and follow-ups). Therefore, a Medicaid provider who is providing one of these services must bill Passport or our business partner Beacon Health Strategies for these individual services and cannot charge the member for them. If you have any questions about the updated criteria, or to request a copy of the criteria, please contact Passport Pharmacy Department at (502) Lock-In Reminder: Some Passport members are enrolled in the Passport Lock-In program. If any member requires a change to their assigned Pharmacy provider or Controlled Substance Provider, it is that members responsibility to contact the Lock-In Coordinator at to make the request. Overrides for Drug to Drug and Drug to Gender Interactions, and Therapeutic Duplications will require NCPDP standard codes If you receive claim rejections for Drug-to-Drug, Drug-to-Gender Interactions, and Therapeutic Duplication, you may override select categories with the following NCPDP standard codes: Professional Service Code/Description 00 / No Intervention CC / Coordination of Care M0/Prescriber Consulted PE / Patient Education/instruction PH / Patient Medicaid History P0/Patient Consulted R0/Physician Consulted Other Result of Service Code/Description 1A/filled as is, false positive 1B/filled prescription as is 1C/filled, with different dose 1D/filled, different direction 1F/filled, different quantity 1G/filled, prescriber approved 2A/prescription not filled 3B/recommendation not accepted 3C/discontinued drug Providers: Please remember that expired credentials with Kentucky Medicaid will result in prescription denials. Passport will deny all prescriptions written by prescribers who are not enrolled as a provider with Kentucky Medicaid. To enroll or update credentials, contact DMS Provider Enrollment at (877) (Monday to Friday, 8 a.m. to 4:30 p.m.). You may also download an application for participation (MAP-811) on the DMS web site at Please note, the process may take up to 90 days. 2
3 Recent Federal Drug Administration (FDA) Advisories Affecting Network Pharmacies and Providers The FDA recently issued the following advisories: 3/9/15 FDA updates label for Chantix. These updates include information on a potential alcohol interaction and rare risk of seizures. Reviews of case series submitted by Pfizer and the FAERS (FDA Adverse Event Reporting System) database by the FDA revealed that some patients experienced decreased tolerance to alcohol, including unusual or aggressive behavior and no recollection of events that happened. After similar reviews, it was discovered that some patients with no previous history of seizures or a seizure disorder that had not been well-controlled had seizures, usually within the first month of starting Chantix. The FDA has updated the Warnings and Precautions section of the drug label and medication guide with information about these risks. 03/24/15 FDA warns of serious slowing of the heart rate when antiarrhythmic drug amiodarone is used with hepatitis C treatments containing sofosbuvir (Sovaldi ) or Harvoni in combination with another Direct Acting Antiviral drug. FDA reviews of postmarketing adverse event reports found that patients can develop symptomatic bradycardia when amiodarone is combined with either Harvoni or Sovaldi and another direct-acting antiviral. The FDA is recommending avoiding this combination of drugs. If alternate treatment options are unavailable, inpatient hospital heart monitoring is recommended for the first 48 hours. Daily heart monitoring at a doctor s office and/or self-monitoring should be done for two weeks thereafter. Patients should seek medical attention right away if experiencing signs or symptoms of symptomatic bradycardia such as: fainting, dizziness, malaise, weakness, shortness of breath, chest pains, and confusion. Information about symptomatic bradycardia has been added to the Harvoni and Sovaldi labels. 04/22/15 Mucinex Fast-MAX Products: Recall - Incorrect Labeling. RB (formerly Reckitt Benckiser) has announced the recall of certain lots of liquid bottles of Mucinex Fast-MAX Night Time Cold & Flu; Mucinex Fast-MAX Cold & Sinus; Mucinex Fast-MAX Severe Congestion & Cough; and Mucinex Fast-MAX Cold, Flu & Sore Throat because the over-thecounter medications, which correctly label the product on the front of the bottle and lists all active ingredients, may not have the correct corresponding drug facts label on the back. Lot numbers of affected products and additional information can be found at 5/15/15 FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. The U.S. Food and Drug Administration (FDA) is warning that the type 2 diabetes medicines canagliflozin, dapagliflozin, and empagliflozin may lead to ketoacidosis. 5/20/15 FDA cautions about dose confusion and medication errors for antibacterial drug Zerbaxa (ceftolozane and tazobactam). The US Food and Drug Administration (FDA) is warning the risk for dosing errors with the antibacterial drug Zerbaxa (ceftolozand and tazobactam) due to confusion about the drug strength displayed on the vial and carton labeling. Zerbaxa s vial label was initially approved with a strength that reflects each individual active ingredient; however, the product is dosed based on the sum of these ingredients. Please visit for more information. 3
4 The Passport Health Plan Pharmacy and Therapeutics Committee Reviewed the Following Medications in April 2015: BRAND NAME GENERIC NAME/ DOSAGE FORMS INDICATIONS FORMULARY ALTERNATIVES PASSPORT HEALTH PLAN STATUS Evzio Bunavail naloxone hydrochloride buprenorphine/ naloxone An opioid antagonist approved for: Emergency treatment of opioid overdose, either known or suspected, as demonstrated by respiratory and/or central nervous system depression. Not intended as a substitute for emergency medical care but for immediate administration as emergency therapy when opioids may have been used. CIII substance indicated for the maintenance treatment of opioid dependence naloxone hcl 1 mg/ml buprenorphinenaloxone Suboxone film, tablet QL: 4 per claim (2 boxes 1.6 ml) QL 60 films/30 days Izba travoprost Ophthalmic prostaglandin analog solution approved for: Reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. latanoprost travoprost QL One bottle/30 days Jublia efinaconazole An azole antifungal indicated for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. terbinafine hcl 250 mg tablet ciclopirox 8% solution QL 1 bottle/month Sivextro tedizolid phosphate Tedizolid phosphate (Sivextro) is an oxazolidinone antibacterial agent indicated in adults for the treatment of acute bacterial skin and skin structure infection (ABSSSI). Tedizolid should only be used to treat ABSSSI that have proven susceptibility to the following Gram-positive microorganisms: Staphylococcus aureus (incuding methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Streptococcus anginosus Group (Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus) and Enterococcus faecalis.. Zyvox 100 mg/5 ml suspension Zyvox 600 mg tablet QL 6 tablets for 6 days supply 4
5 BRAND NAME GENERIC NAME/ DOSAGE FORMS INDICATIONS FORMULARY ALTERNATIVES PASSPORT HEALTH PLAN STATUS Targiniq ER Oxycodone/ naloxone Targiniq ER is a combination of oxycodone hydrochloride, an opioid agonist, and naloxone hydrochloride, an opioid antagonist, in an extended-release abuse-deterrent formulation. This schedule II controlled substance, is indicated for pain management when the pain is severe enough to require daily, around-the-clock, long-term treatment with an opioid for which alternative treatments are inadequate. The product is not indicated to be used as needed. oxycontin QL 2 tablets per day (60 tabs/30 days) Vogelxo Testosterone Topical testosterone gel approved for: Androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone: primary hypogonadism (congenital or acquired) and hypogonadotropic hypogonadism (congenital or acquired). Androgel QL Maxiumum dose of 100 mg/day. Two gel tubes or packets/ day (10 g) or one metered dose pump/ week Plegridy peginterferon beta- 1A Pegylated interferon injection approved for: Treatment of patients with relapsing forms of multiple sclerosis (MS). Avonex Rebif Extavia QL Maxiumum dose: 2 pens or syringes per 28 days Viekira Pak ombitasvir/ paritaprevir/ ritonavir with dasabuvir Ombitasvir, paritaprevir, ritonavir; dasabuvir (Viekira Pak) with or without ribavirin (RBV) is indicated for the treatment of patients with genotype 1 chronic hepatitis C virus (HCV) infection including those with compensated cirrhosis. Viekira Pak is not recommended for use in patients with decompensated liver disease. Viekira Pak is preferred PREFERRED with PA. QL: One dasabuvir + ombitasvir/ paritaprevir/ ritonavir pack per 28 days. 5
6 BRAND NAME GENERIC NAME/ DOSAGE FORMS INDICATIONS FORMULARY ALTERNATIVES PASSPORT HEALTH PLAN STATUS Oral Anticoagulants Eliquis Xarelto Apixaban Rivaroxaban Dabigatran To reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. For the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients who have undergone hip or knee replacement surgery. Xarelto Pradaxa Eliquis will move to status. Current patients on Eliquis will be able to continue therapy. Pradaxa For the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy Growth Hormones Somatropin Pediatric: Treatment of children with growth failure due to growth hormone deficiency (GHD), short stature associated with Noonan syndrome, short stature associated with Turner syndrome and short stature born SGA with no catch-up growth by age 2 to 4 years. Adult: Treatment of adults with either adult onset or childhood onset GHD. Norditropin Omnitrope and Tev-Tropin will move to status Hepatitis C Viekira Pak Harvoni Olysio Sovaldi Dasabuvir/ Ombitasvir/ Paritaprevir/ Ritonavir Ledipasvir and Sofosbuvir Simeprevir Sodium Sofosbuvir Indicated for the treatment of patients with genotype 1 chronic hepatitis C virus (HCV) infection including those with compensated cirrhosis. VIEKIRA PAK includes ombitasvir, a hepatitis C virus NS5A inhibitor, paritaprevir, a hepatitis C virus NS3/4A protease inhibitor, ritonavir, a CYP3A inhibitor and dasabuvir, a hepatitis C virus non-nucleoside NS5B palm polymerase inhibitor. Viekira pak preferred Harvoni nonpreferred Olysio preferred Sovaldi - preferred Viekira Pak added as a preferred agent for genotype 1. Must have T/F of Viekira Pak for genotype 1 before Harvoni, Olysio, or Sovaldi, however, patients currently on therapy should be grandfathered as we do not want to interrupt therapy. Respiratory Intranasal Steroids: Nasonex Mometasone Furoate Treatment of Nasal Symptoms of Allergic Rhinitis in patients 2 years of age Treatment of Nasal Congestion Associated with Seasonal Allergic Rhinitis in patients 2 years of age Prophylaxis of Seasonal Allergic Rhinitis in patients 12 years of age Treatment of Nasal Polyps in patients 18 years of age Triamcinolone acetonide Nasonex is ; Nasonex: Remove the PA bypass for children under 4 years of age, since generics are available 6
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 5/21/2014 3/24/2016 3/24/2015 Policy Name Policy Number Hepatitis C Oral SRx-0003 Medical Policy Statements
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 5/21/2014 3/24/2016 3/24/2015 Policy Name Policy Number Hepatitis C Oral SRx-0003 Medical Policy Statements
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT
 PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants which meet any of the following conditions
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants which meet any of the following conditions
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
Update on Hepatitis C. Sally Williams MD
 Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
Clinical Criteria for Hepatitis C (HCV) Therapy
 Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
Diagnosis Clinical Criteria for Hepatitis C (HCV) Therapy Must have chronic hepatitis C (HCV infection > 6 months), genotype and sub-genotype specified to determine the length of therapy; Liver biopsy
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE June 22, 2015 SUBJECT EFFECTIVE DATE MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical
ISSUE DATE June 22, 2015 SUBJECT EFFECTIVE DATE MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical
HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information. Diagnosis Acute Hep C Chronic Hep C Hepatocellular Carcinoma
 HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information Recipient: MA#: Date of Birth: Phone #: Body Weight: Treatment Plan Sovaldi (sofosbuvir) 400mg: Take once daily for weeks Olysio
HEPATITIS C THERAPY PRIOR AUTHORIZATION FORM: Page 1 of 3 Patient Information Recipient: MA#: Date of Birth: Phone #: Body Weight: Treatment Plan Sovaldi (sofosbuvir) 400mg: Take once daily for weeks Olysio
July 2015 Preferred Drug List Review and Other Pharmacy Policy Changes
 Update June 2015 No. 2015-27 Affected Programs: BadgerCare Plus, Medicaid, SeniorCare To: Blood Banks, Dentists, Federally Qualified Health Centers, Hospital Providers, Nurse Practitioners, Nursing Homes,
Update June 2015 No. 2015-27 Affected Programs: BadgerCare Plus, Medicaid, SeniorCare To: Blood Banks, Dentists, Federally Qualified Health Centers, Hospital Providers, Nurse Practitioners, Nursing Homes,
How To Treat Anorexic Addiction With Medication Assisted Treatment
 Medication Assisted Treatment for Opioid Addiction Tanya Hiser, MS, LPC Premier Care of Wisconsin, LLC October 21, 2015 How Did We Get Here? Civil War veterans and women 19th Century physicians cautious
Medication Assisted Treatment for Opioid Addiction Tanya Hiser, MS, LPC Premier Care of Wisconsin, LLC October 21, 2015 How Did We Get Here? Civil War veterans and women 19th Century physicians cautious
A Proposal for Managing the Harvoni Wave June 22, 2015
 A Proposal for Managing the Harvoni Wave June 22, 2015 Clinical Background Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV) that damages the liver over time. The disease affects
A Proposal for Managing the Harvoni Wave June 22, 2015 Clinical Background Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV) that damages the liver over time. The disease affects
MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets
 MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets What is the most important information I should know about ELIQUIS? For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation
MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets What is the most important information I should know about ELIQUIS? For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation
Newer Anticoagulants and Newer Diabetic Drug Classes. Nicole N. Nguyen, PharmD Senior Clinical Pharmacist Health Care Services August 21, 2013
 Newer Anticoagulants and Newer Diabetic Drug Classes Nicole N. Nguyen, PharmD Senior Clinical Pharmacist Health Care Services August 21, 2013 Apixaban Newer Anticoagulants Dabigatran etexilate Rivaroxaban
Newer Anticoagulants and Newer Diabetic Drug Classes Nicole N. Nguyen, PharmD Senior Clinical Pharmacist Health Care Services August 21, 2013 Apixaban Newer Anticoagulants Dabigatran etexilate Rivaroxaban
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: CSD - Suboxone Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Analgesics and Antipyretics (Opiate Partial Agonists) Client: County of San
Prior Authorization Guideline Guideline: CSD - Suboxone Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Analgesics and Antipyretics (Opiate Partial Agonists) Client: County of San
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Opiate Dependence Treatments, Oral Buprenorphine Agents - Pharmacy
ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Opiate Dependence Treatments, Oral Buprenorphine Agents - Pharmacy
Committee Approval Date: September 12, 2014 Next Review Date: September 2015
 Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Hepatitis C Second Generation Antivirals Page 1 of 22 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Hepatitis C Second Generation Antivirals Through Preferred
Hepatitis C Second Generation Antivirals Page 1 of 22 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Hepatitis C Second Generation Antivirals Through Preferred
What You Need to KnowWhen Taking Anticoagulation Medicine
 What You Need to KnowWhen Taking Anticoagulation Medicine What are anticoagulant medicines? Anticoagulant medicines are a group of medicines that inhibit blood clotting, helping to prevent blood clots.
What You Need to KnowWhen Taking Anticoagulation Medicine What are anticoagulant medicines? Anticoagulant medicines are a group of medicines that inhibit blood clotting, helping to prevent blood clots.
Three new/novel oral anticoagulants (NOAC) have been licensed in Ireland since 2008:
 Key Points to consider when prescribing NOACs Introduction Three new/novel oral anticoagulants (NOAC) have been licensed in Ireland since 2008: Dabigatran Etexilate (Pradaxa ) 75mg, 110mg, 150mg. Rivaroxaban
Key Points to consider when prescribing NOACs Introduction Three new/novel oral anticoagulants (NOAC) have been licensed in Ireland since 2008: Dabigatran Etexilate (Pradaxa ) 75mg, 110mg, 150mg. Rivaroxaban
Rivaroxaban to prevent blood clots for patients who have a lower limb plaster cast. Information for patients Pharmacy
 Rivaroxaban to prevent blood clots for patients who have a lower limb plaster cast Information for patients Pharmacy Your doctor has prescribed a tablet called rivaroxaban. This leaflet tells you about
Rivaroxaban to prevent blood clots for patients who have a lower limb plaster cast Information for patients Pharmacy Your doctor has prescribed a tablet called rivaroxaban. This leaflet tells you about
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE SUBJECT EFFECTIVE DATE MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Opiate Dependence Treatments Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical
ISSUE DATE SUBJECT EFFECTIVE DATE MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Opiate Dependence Treatments Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical
Information for Pharmacists
 Page 43 by 42 CFR part 2. A general authorization for the release of medical or other information is NOT sufficient for this purpose. Information for Pharmacists SUBOXONE (buprenorphine HCl/naloxone HCl
Page 43 by 42 CFR part 2. A general authorization for the release of medical or other information is NOT sufficient for this purpose. Information for Pharmacists SUBOXONE (buprenorphine HCl/naloxone HCl
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Hepatitis C Second Generation Antivirals (2015) Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Hepatitis C Second Generation Antivirals Through
Hepatitis C Second Generation Antivirals (2015) Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Hepatitis C Second Generation Antivirals Through
How To Take Xarelto
 A patient's guide Your clinic's contact details are: Name: Contact number: Contents 2 Why have I been prescribed Xarelto? 2 What is Xarelto? 3 How do I take Xarelto? 3 What should I do if I miss a dose
A patient's guide Your clinic's contact details are: Name: Contact number: Contents 2 Why have I been prescribed Xarelto? 2 What is Xarelto? 3 How do I take Xarelto? 3 What should I do if I miss a dose
Ultram (tramadol), Ultram ER (tramadol extended-release tablets); Conzip (tramadol extended-release capsules), Ultracet (tramadol / acetaminophen)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.02.35 Subject: Tramadol Acetaminophen Page: 1 of 8 Last Review Date: September 18, 2015 Tramadol Acetaminophen
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.02.35 Subject: Tramadol Acetaminophen Page: 1 of 8 Last Review Date: September 18, 2015 Tramadol Acetaminophen
ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR DEEP VEIN THROMBOSIS (DVT)
 ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR DEEP VEIN THROMBOSIS (DVT) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet
ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR DEEP VEIN THROMBOSIS (DVT) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet
Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary
 Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary This program applies to Health Insurance Marketplace, FlexRx
Hepatitis C Second Generation Antivirals (Harvoni, Technivie TM, Viekira Pak ) Prior Authorization - Through Preferred Agent(s) Program Summary This program applies to Health Insurance Marketplace, FlexRx
Opioid Treatment Services, Office-Based Opioid Treatment
 Optum 1 By United Behavioral Health U.S. Behavioral Health Plan, California Doing Business as OptumHealth Behavioral Solutions of California ( OHBS-CA ) 2015 Level of Care Guidelines Opioid Treatment Services,
Optum 1 By United Behavioral Health U.S. Behavioral Health Plan, California Doing Business as OptumHealth Behavioral Solutions of California ( OHBS-CA ) 2015 Level of Care Guidelines Opioid Treatment Services,
A PATIENT S GUIDE TO DEEP VEIN THROMBOSIS TREATMENT
 A PATIENT S GUIDE TO DEEP VEIN THROMBOSIS TREATMENT This medicine is subject to additional monitoring. This will allow quick identification of new safety information. If you get any side effects, talk
A PATIENT S GUIDE TO DEEP VEIN THROMBOSIS TREATMENT This medicine is subject to additional monitoring. This will allow quick identification of new safety information. If you get any side effects, talk
Buprenorphine/Naloxone Maintenance Treatment for Opioid Dependence
 Buprenorphine/Naloxone Maintenance Treatment for Opioid Dependence Information for Family Members Family members of patients who have been prescribed buprenorphine/naloxone for treatment of opioid addiction
Buprenorphine/Naloxone Maintenance Treatment for Opioid Dependence Information for Family Members Family members of patients who have been prescribed buprenorphine/naloxone for treatment of opioid addiction
A COLLABORATIVE PRACTICE AGREEMENT FOR OPIOID OVERDOSE PREVENTION AND RESPONSE NALOXONE OVERDOSE KIT DISTRIBUTION PROTOCOL
 A COLLABORATIVE PRACTICE AGREEMENT FOR OPIOID OVERDOSE PREVENTION AND RESPONSE NALOXONE OVERDOSE KIT DISTRIBUTION PROTOCOL Purpose: To reduce morbidity and mortality from opioid overdose. Policy: Under
A COLLABORATIVE PRACTICE AGREEMENT FOR OPIOID OVERDOSE PREVENTION AND RESPONSE NALOXONE OVERDOSE KIT DISTRIBUTION PROTOCOL Purpose: To reduce morbidity and mortality from opioid overdose. Policy: Under
Failure or significant adverse effects to all of the alternatives: Eliquis and Xarelto
 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutics
This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutics
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below
 Name: generic (trade) Rivaroxaban (Xarelto ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below What it is Indications
Name: generic (trade) Rivaroxaban (Xarelto ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below What it is Indications
What you should know about treating your pain with opioids. Important information on the safe use of opioid pain medicine.
 What you should know about treating your pain with opioids Important information on the safe use of opioid pain medicine. If your healthcare provider has determined that opioid therapy is right for you,
What you should know about treating your pain with opioids Important information on the safe use of opioid pain medicine. If your healthcare provider has determined that opioid therapy is right for you,
Living with a Non-Vitamin K Antagonist Oral Anticoagulant (NOAC)
 Living with a Non-Vitamin K Antagonist Oral Anticoagulant (NOAC) dabigatran (Pradaxa ) rivaroxaban (Xarelto ) apixaban (Eliquis ) Information for patients Produced and made available by the Western Australian
Living with a Non-Vitamin K Antagonist Oral Anticoagulant (NOAC) dabigatran (Pradaxa ) rivaroxaban (Xarelto ) apixaban (Eliquis ) Information for patients Produced and made available by the Western Australian
Prior Authorization Guideline
 Guideline Guideline Name Formulary Xarelto (rivaroxaban) UnitedHealthcare Community & State Approval Date 0/0/203 Revision Date 8//204 Technician Note: CPS Approval Date: /5/20; CPS Revision Date: 8/20/204
Guideline Guideline Name Formulary Xarelto (rivaroxaban) UnitedHealthcare Community & State Approval Date 0/0/203 Revision Date 8//204 Technician Note: CPS Approval Date: /5/20; CPS Revision Date: 8/20/204
Guide to Dabigatran, Rivaroxaban and Apixaban
 Page 1 of 8 Guide to Dabigatran, Rivaroxaban and Apixaban Medicines to Prevent Blood Clots If you are deaf or hard of hearing, please let us know. We provide many free services including sign language
Page 1 of 8 Guide to Dabigatran, Rivaroxaban and Apixaban Medicines to Prevent Blood Clots If you are deaf or hard of hearing, please let us know. We provide many free services including sign language
TSOAC Initiation Checklist
 Task Establish appropriate dose based on anticoagulant selected, indication and patient factors such as renal function. Evaluate for medication interactions that may necessitate TSOAC dose adjustment.
Task Establish appropriate dose based on anticoagulant selected, indication and patient factors such as renal function. Evaluate for medication interactions that may necessitate TSOAC dose adjustment.
PRIOR AUTHORIZATION POLICY
 PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
PRIOR AUTHORIZATION POLICY Harvoni (sofosbuvir/ledipasvir tablets Gilead) To initiate a Coverage Review, Call 1-800-417-1764 OVERVIEW Harvoni is a fixed-dose combination of ledipasvir, a hepatitis C virus
2015 Travelers Prescription Drug Plan Blue Cross Blue Shield Plan and United Healthcare Choice Plus Plan
 2015 Travelers Prescription Drug Plan Blue Cross Blue Shield Plan and United Healthcare Choice Plus Plan Plan Details, Programs, and Policies Table of Contents Click on the links below to be taken to that
2015 Travelers Prescription Drug Plan Blue Cross Blue Shield Plan and United Healthcare Choice Plus Plan Plan Details, Programs, and Policies Table of Contents Click on the links below to be taken to that
Considerations in Medication Assisted Treatment of Opiate Dependence. Stephen A. Wyatt, D.O. Dept. of Psychiatry Middlesex Hospital Middletown, CT
 Considerations in Medication Assisted Treatment of Opiate Dependence Stephen A. Wyatt, D.O. Dept. of Psychiatry Middlesex Hospital Middletown, CT Disclosures Speaker Panels- None Grant recipient - SAMHSA
Considerations in Medication Assisted Treatment of Opiate Dependence Stephen A. Wyatt, D.O. Dept. of Psychiatry Middlesex Hospital Middletown, CT Disclosures Speaker Panels- None Grant recipient - SAMHSA
Medication-Assisted Addiction Treatment
 Medication-Assisted Addiction Treatment Molly Carney, Ph.D., M.B.A. Executive Director Evergreen Treatment Services Seattle, WA What is MAT? MAT is the use of medications, in combination with counseling
Medication-Assisted Addiction Treatment Molly Carney, Ph.D., M.B.A. Executive Director Evergreen Treatment Services Seattle, WA What is MAT? MAT is the use of medications, in combination with counseling
treat nasal congestion that happens with seasonal allergic rhinitis in adults and children 2 years of age and older.
 Patient Information NASONEX [nā-zə-neks] (mometasone furoate monohydrate) Nasal Spray, 50 mcg FOR INTRANASAL USE ONLY Read the Patient Information that comes with NASONEX before you start using it and
Patient Information NASONEX [nā-zə-neks] (mometasone furoate monohydrate) Nasal Spray, 50 mcg FOR INTRANASAL USE ONLY Read the Patient Information that comes with NASONEX before you start using it and
Growth Hormone Deficiency
 Growth Hormone Deficiency What is growth hormone deficiency? 1,2 Growth hormone deficiency is when your body doesn t make enough growth hormone. Growth hormone is one of many hormones made by the pituitary
Growth Hormone Deficiency What is growth hormone deficiency? 1,2 Growth hormone deficiency is when your body doesn t make enough growth hormone. Growth hormone is one of many hormones made by the pituitary
Prescriber Guide. 20mg. 15mg. Simply Protecting More Patients. Simply Protecting More Patients
 Prescriber Guide 20mg Simply Protecting More Patients 15mg Simply Protecting More Patients 1 Dear Doctor, This prescriber guide was produced by Bayer Israel in cooperation with the Ministry of Health as
Prescriber Guide 20mg Simply Protecting More Patients 15mg Simply Protecting More Patients 1 Dear Doctor, This prescriber guide was produced by Bayer Israel in cooperation with the Ministry of Health as
Coventry Health Care of Georgia, Inc. Coventry Health and Life Insurance Company
 Coventry Health Care of Georgia, Inc. Coventry Health and Life Insurance Company PRESCRIPTION DRUG RIDER This Prescription Drug Rider is an attachment to the Coventry Health Care of Georgia, Inc. ( Health
Coventry Health Care of Georgia, Inc. Coventry Health and Life Insurance Company PRESCRIPTION DRUG RIDER This Prescription Drug Rider is an attachment to the Coventry Health Care of Georgia, Inc. ( Health
PHARMACY PRIOR AUTHORIZATION
 PHARMACY PRIOR AUTHORIZATION Hepatitis C Clinical Guideline Harvoni (sofosbuvir/ledipasvir), Sovaldi (sofosbuvir), Viekira PAK (ombitsavir, paritapravir/ritonavir, dasubavir), and Olysio (simeprevir) Authorization
PHARMACY PRIOR AUTHORIZATION Hepatitis C Clinical Guideline Harvoni (sofosbuvir/ledipasvir), Sovaldi (sofosbuvir), Viekira PAK (ombitsavir, paritapravir/ritonavir, dasubavir), and Olysio (simeprevir) Authorization
FDA Approved Oral Anticoagulants
 FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000
 UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000 Articaine hydrochloride and adrenaline hydrochloride Consumer Medicine Information WHAT IS IN THIS LEAFLET Please read this leaflet carefully before you
UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000 Articaine hydrochloride and adrenaline hydrochloride Consumer Medicine Information WHAT IS IN THIS LEAFLET Please read this leaflet carefully before you
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
Prior Authorization of buprenophine/naloxone (Suboxone ) or buprenorphine (Subutex )
 June 2010 April 2009 Prior Authorization of buprenophine/naloxone (Suboxone ) or buprenorphine (Subutex ) Effective August 1, West Virginia Medicaid will require prior authorization for all Suboxone and
June 2010 April 2009 Prior Authorization of buprenophine/naloxone (Suboxone ) or buprenorphine (Subutex ) Effective August 1, West Virginia Medicaid will require prior authorization for all Suboxone and
Introduction. Background to this event. Raising awareness 09/11/2015
 Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Treatment with Rivaroxaban
 UW MEDICINE PATIENT EDUCATION Treatment with Rivaroxaban Xarelto This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto)
UW MEDICINE PATIENT EDUCATION Treatment with Rivaroxaban Xarelto This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto)
Human Normal Immunoglobulin Solution for Intravenous Infusion.
 CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM is available in single use bottles of 20 ml, 50 ml, 100 ml and 200 ml. OCTAGAM contains
CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM is available in single use bottles of 20 ml, 50 ml, 100 ml and 200 ml. OCTAGAM contains
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Review: How to work up your patient with Hepatitis C
 Review: How to work up your patient with Hepatitis C You screened your patient, and now the HCV antibody test is positive. What do you do next? The antibody test only means they have been exposed to HCV.
Review: How to work up your patient with Hepatitis C You screened your patient, and now the HCV antibody test is positive. What do you do next? The antibody test only means they have been exposed to HCV.
Medication Policy Manual. Topic: Eliquis, apixaban Date of Origin: July 12, 2013. Committee Approval Date: July 11, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru313 Topic: Eliquis, apixaban Date of Origin: July 12, 2013 Committee Approval Date: July 11, 2014 Next Review Date: July 2015 Effective Date: August 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru313 Topic: Eliquis, apixaban Date of Origin: July 12, 2013 Committee Approval Date: July 11, 2014 Next Review Date: July 2015 Effective Date: August 1, 2014 IMPORTANT
Updates to the Alberta Drug Benefit List. Effective January 1, 2016
 Updates to the Alberta Drug Benefit List Effective January 1, 2016 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone Number: (780)
Updates to the Alberta Drug Benefit List Effective January 1, 2016 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone Number: (780)
MEDICATION GUIDE. PROCRIT (PRO KRIT) (epoetin alfa)
 MEDICATION GUIDE PROCRIT (PROKRIT) (epoetin alfa) Read this Medication Guide: before you start PROCRIT. if you are told by your healthcare provider that there is new information about PROCRIT. if you are
MEDICATION GUIDE PROCRIT (PROKRIT) (epoetin alfa) Read this Medication Guide: before you start PROCRIT. if you are told by your healthcare provider that there is new information about PROCRIT. if you are
How To Treat Aneuricaagulation
 Speaker Introduction Jessica Wilhoite, PharmD, BCACP Doctor of Pharmacy: Purdue University Postgraduate Residency Training: PGY1 Pharmacy Practice St. Vincent Hospital PGY2 Ambulatory Care St. Vincent
Speaker Introduction Jessica Wilhoite, PharmD, BCACP Doctor of Pharmacy: Purdue University Postgraduate Residency Training: PGY1 Pharmacy Practice St. Vincent Hospital PGY2 Ambulatory Care St. Vincent
DVT/PE Management with Rivaroxaban (Xarelto)
 DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
Sudden dizziness, trouble walking, loss of balance or coordination
 rivaroxaban (Xarelto) Patient Education Information Sheet Veterans Health System Pharmacy Service 119 What is rivaroxaban (Xarelto)? A new anticoagulant ( blood thinner ) that reduces the risk of stroke
rivaroxaban (Xarelto) Patient Education Information Sheet Veterans Health System Pharmacy Service 119 What is rivaroxaban (Xarelto)? A new anticoagulant ( blood thinner ) that reduces the risk of stroke
A PATIENT S GUIDE TO PULMONARY EMBOLISM TREATMENT
 A PATIENT S GUIDE TO PULMONARY EMBOLISM TREATMENT This medicine is subject to additional monitoring. This will allow quick identification of new safety information. If you get any side effects, talk to
A PATIENT S GUIDE TO PULMONARY EMBOLISM TREATMENT This medicine is subject to additional monitoring. This will allow quick identification of new safety information. If you get any side effects, talk to
Package leaflet: Information for the patient. Naloxone Hydrochloride 20 micrograms / ml Solution for Injection Naloxone hydrochloride
 A leaflet will be included in each pack. The leaflet will consist of a Technical Information Leaflet and a Patient Information Leaflet. The two leaflets will be easily separatable. The text of the Technical
A leaflet will be included in each pack. The leaflet will consist of a Technical Information Leaflet and a Patient Information Leaflet. The two leaflets will be easily separatable. The text of the Technical
NEW DRUGS FOR THE TREATMENT OF HEPATITIS C. Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15)
 NEW DRUGS FOR THE TREATMENT OF HEPATITIS C Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15) Objectives Determine initial treatment options for patients with
NEW DRUGS FOR THE TREATMENT OF HEPATITIS C Marcella Honkonen, PharmD, BCPS AzPA Annual Convention. Sunday, June 29 th, 2014 (1:15-2:15) Objectives Determine initial treatment options for patients with
Treating Chronic Hepatitis C. A Review of the Research for Adults
 Treating Chronic Hepatitis C A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have chronic hepatitis C.
Treating Chronic Hepatitis C A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have chronic hepatitis C.
Devang M. Desai, MD, FACC, FSCAI Chief of Interventional Cardiology Director of Cardiac Catheterization Lab St. Mary s Hospital and Regional Medical
 Devang M. Desai, MD, FACC, FSCAI Chief of Interventional Cardiology Director of Cardiac Catheterization Lab St. Mary s Hospital and Regional Medical Center A.Fib affects 2.2 million Americans. The lifetime
Devang M. Desai, MD, FACC, FSCAI Chief of Interventional Cardiology Director of Cardiac Catheterization Lab St. Mary s Hospital and Regional Medical Center A.Fib affects 2.2 million Americans. The lifetime
Updates to the Alberta Human Services Drug Benefit Supplement
 Updates to the Alberta Human Services Drug Benefit Supplement Effective January 1, 2016 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone
Updates to the Alberta Human Services Drug Benefit Supplement Effective January 1, 2016 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone
It is important that you tell your family and the people closest to you of this increased sensitivity to opioids and the risk of overdose.
 MEDICATION GUIDE VIVITROL (viv-i-trol) (naltrexone for extended-release injectable suspension) Read this Medication Guide before you start receiving VIVITROL injections and each time you receive an injection.
MEDICATION GUIDE VIVITROL (viv-i-trol) (naltrexone for extended-release injectable suspension) Read this Medication Guide before you start receiving VIVITROL injections and each time you receive an injection.
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP. Primary Care Provider:
 Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
How To Get A Tirf
 Transmucosal Immediate Release Fentanyl (TIRF) Products Risk Evaluation and Mitigation Strategy (REMS) Education Program for Prescribers and Pharmacists Products Covered Under This Program Abstral (fentanyl)
Transmucosal Immediate Release Fentanyl (TIRF) Products Risk Evaluation and Mitigation Strategy (REMS) Education Program for Prescribers and Pharmacists Products Covered Under This Program Abstral (fentanyl)
An Approach to the Diagnosis and Treatment of Hepatitis C Virus Infection in 2015. Matthew McMahon, MD
 An Approach to the Diagnosis and Treatment of Hepatitis C Virus Infection in 2015 Matthew McMahon, MD In the United States, 2.2-3.2 million people are infected with the hepatitis C virus (HCV) Half unaware
An Approach to the Diagnosis and Treatment of Hepatitis C Virus Infection in 2015 Matthew McMahon, MD In the United States, 2.2-3.2 million people are infected with the hepatitis C virus (HCV) Half unaware
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Integrating Medication- Assisted Treatment (MAT) for Opioid Use Disorders into Behavioral and Physical Healthcare Settings
 Integrating Medication- Assisted Treatment (MAT) for Opioid Use Disorders into Behavioral and Physical Healthcare Settings All-Ohio Conference 3/27/2015 Christina M. Delos Reyes, MD Medical Consultant,
Integrating Medication- Assisted Treatment (MAT) for Opioid Use Disorders into Behavioral and Physical Healthcare Settings All-Ohio Conference 3/27/2015 Christina M. Delos Reyes, MD Medical Consultant,
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
MEDICATION GUIDE SYLATRON (SY-LA-TRON) (Peginterferon alfa-2b)
 MEDICATION GUIDE SYLATRON (SY-LA-TRON) (Peginterferon alfa-2b) Read this Medication Guide before you start taking SYLATRON, and each time you get a refill. There may be new information. This Medication
MEDICATION GUIDE SYLATRON (SY-LA-TRON) (Peginterferon alfa-2b) Read this Medication Guide before you start taking SYLATRON, and each time you get a refill. There may be new information. This Medication
NnEeWw DdEeVvEeLlOoPpMmEeNnTtSs IiıNn OoRrAaLl AaNnTtIiıCcOoAaGgUuLlAaTtIiıOoNn AaNnDd RrEeVvEeRrSsAaLl
 NnEeWw DdEeVvEeLlOoPpMmEeNnTtSs IiıNn OoRrAaLl AaNnTtIiıCcOoAaGgUuLlAaTtIiıOoNn AaNnDd RrEeVvEeRrSsAaLl Mikele Wissing, RN June 2014 Introduction until recently, was the unrivaled medication for treatment
NnEeWw DdEeVvEeLlOoPpMmEeNnTtSs IiıNn OoRrAaLl AaNnTtIiıCcOoAaGgUuLlAaTtIiıOoNn AaNnDd RrEeVvEeRrSsAaLl Mikele Wissing, RN June 2014 Introduction until recently, was the unrivaled medication for treatment
New Oral Anticoagulants. How safe are they outside the trials?
 New Oral Anticoagulants How safe are they outside the trials? Objectives The need for anticoagulant therapy Indications for anticoagulation Traditional anticoagulant therapies Properties of new oral anticoagulants
New Oral Anticoagulants How safe are they outside the trials? Objectives The need for anticoagulant therapy Indications for anticoagulation Traditional anticoagulant therapies Properties of new oral anticoagulants
Daclatasvir (Daklinza)
 FACTSHEET Daclatasvir (Daklinza) Summary Daclatasvir (Daklinza) is a medication used to treat hepatitis C. It is taken with sofosbuvir (Sovaldi) and sometimes ribavirin. This combination is approved in
FACTSHEET Daclatasvir (Daklinza) Summary Daclatasvir (Daklinza) is a medication used to treat hepatitis C. It is taken with sofosbuvir (Sovaldi) and sometimes ribavirin. This combination is approved in
Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery
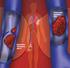 medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
Andrew Stoessel, PharmD PGY-1 Pharmacy Practice Resident Jackson Memorial Hospital
 Andrew Stoessel, PharmD PGY-1 Pharmacy Practice Resident Jackson Memorial Hospital Objectives Discuss rationale in analyzing prescribing practices for direct oral anticoagulants Outline current prescribing
Andrew Stoessel, PharmD PGY-1 Pharmacy Practice Resident Jackson Memorial Hospital Objectives Discuss rationale in analyzing prescribing practices for direct oral anticoagulants Outline current prescribing
MEDICATION GUIDE. What is Morphine Sulfate Oral Solution?
 MEDICATION GUIDE Morphine Sulfate (mor-pheen) (CII) Oral Solution IMPORTANT: Keep Morphine Sulfate Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and
MEDICATION GUIDE Morphine Sulfate (mor-pheen) (CII) Oral Solution IMPORTANT: Keep Morphine Sulfate Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and
HEPATITIS C DISCUSSION GUIDE:
 HEPATITIS C DISCUSSION GUIDE: INFORMATION AND ANSWERS TO AID YOUR COUNSELING OF PATIENTS INDICATION HARVONI is indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection in adults. Warnings
HEPATITIS C DISCUSSION GUIDE: INFORMATION AND ANSWERS TO AID YOUR COUNSELING OF PATIENTS INDICATION HARVONI is indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection in adults. Warnings
The TIRF REMS Access program is a Food and Drug Administration (FDA) required risk management program
 Subject: Important Drug Warning Announcement of a single shared REMS (Risk Evaluation and Mitigation Strategy) program for all Transmucosal Immediate Release Fentanyl (TIRF) products due to the potential
Subject: Important Drug Warning Announcement of a single shared REMS (Risk Evaluation and Mitigation Strategy) program for all Transmucosal Immediate Release Fentanyl (TIRF) products due to the potential
Opioid overdose can occur when a patient misunderstands the directions
 Facts About Opioid Overdose How Does an Overdose Occur? Opioid overdose can occur when a patient misunderstands the directions for use, accidentally takes an extra dose, or deliberately misuses a prescription
Facts About Opioid Overdose How Does an Overdose Occur? Opioid overdose can occur when a patient misunderstands the directions for use, accidentally takes an extra dose, or deliberately misuses a prescription
Inpatient Anticoagulation Safety. To provide safe and effective anticoagulation therapy through a collaborative approach.
 Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
The Role of the Newer Anticoagulants
 The Role of the Newer Anticoagulants WARFARIN = Coumadin DAGIBATRAN = Pradaxa RIVAROXABAN = Xarelto APIXABAN = Eliquis INDICATION DABIGATRAN (Pradaxa) RIVAROXABAN (Xarelto) APIXABAN (Eliquis) Stroke prevention
The Role of the Newer Anticoagulants WARFARIN = Coumadin DAGIBATRAN = Pradaxa RIVAROXABAN = Xarelto APIXABAN = Eliquis INDICATION DABIGATRAN (Pradaxa) RIVAROXABAN (Xarelto) APIXABAN (Eliquis) Stroke prevention
Treatments to Restore Normal Rhythm
 Treatments to Restore Normal Rhythm In many instances when AF causes significant symptoms or is negatively impacting a patient's health, the major goal of treatment is to restore normal rhythm and prevent
Treatments to Restore Normal Rhythm In many instances when AF causes significant symptoms or is negatively impacting a patient's health, the major goal of treatment is to restore normal rhythm and prevent
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Hepatitis C Agents
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Hepatitis C Agents that meet any of the following
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Hepatitis C Agents A. Prescriptions That Require Prior Authorization Prescriptions for Hepatitis C Agents that meet any of the following
DRUG INTERACTIONS: WHAT YOU SHOULD KNOW. Council on Family Health
 DRUG INTERACTIONS: WHAT YOU SHOULD KNOW Council on Family Health Drug Interactions There are more opportunities today than ever before to learn about your health and to take better care of yourself. It
DRUG INTERACTIONS: WHAT YOU SHOULD KNOW Council on Family Health Drug Interactions There are more opportunities today than ever before to learn about your health and to take better care of yourself. It
Share the important information in this Medication Guide with members of your household.
 MEDICATION GUIDE BUPRENORPHINE (BUE-pre-NOR-feen) Sublingual Tablets, CIII IMPORTANT: Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical
MEDICATION GUIDE BUPRENORPHINE (BUE-pre-NOR-feen) Sublingual Tablets, CIII IMPORTANT: Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical
Ever wish you could... Quit using heroin? Protect yourself from HIV infection? Get healthier?
 Ever wish you could... Quit using heroin? Protect yourself from HIV infection? Get healthier? Good News: Medical treatments called opioid (oh-pee-oyd) maintenance can help you! Injecting heroin puts you
Ever wish you could... Quit using heroin? Protect yourself from HIV infection? Get healthier? Good News: Medical treatments called opioid (oh-pee-oyd) maintenance can help you! Injecting heroin puts you
Anticoagulant therapy
 Anticoagulation: The risks Anticoagulant therapy 1990 2002: 600 incidents reported 120 resulted in death of patient 92 deaths related to warfarin usage 28 reports related to heparin usage Incidents in
Anticoagulation: The risks Anticoagulant therapy 1990 2002: 600 incidents reported 120 resulted in death of patient 92 deaths related to warfarin usage 28 reports related to heparin usage Incidents in
Out with the Old and in with the New? Target Specific Anticoagulants for Atrial Fibrillation
 Out with the Old and in with the New? Target Specific Anticoagulants for Atrial Fibrillation Goal Statement Pharmacists and technicians will gain knowledge in the use of target specific oral anticoagulants
Out with the Old and in with the New? Target Specific Anticoagulants for Atrial Fibrillation Goal Statement Pharmacists and technicians will gain knowledge in the use of target specific oral anticoagulants
ORAL ANTICOAGULANTS RIVAROXABAN (XARELTO) FOR PULMONARY EMBOLISM (PE)
 ORAL ANTICOAGULANTS RIVAROXABAN (XARELTO) FOR PULMONARY EMBOLISM (PE) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet has
ORAL ANTICOAGULANTS RIVAROXABAN (XARELTO) FOR PULMONARY EMBOLISM (PE) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet has
Blueprint for Prescriber Continuing Education Program
 CDER Final 10/25/11 Blueprint for Prescriber Continuing Education Program I. Introduction: Why Prescriber Education is Important Health care professionals who prescribe extended-release (ER) and long-acting
CDER Final 10/25/11 Blueprint for Prescriber Continuing Education Program I. Introduction: Why Prescriber Education is Important Health care professionals who prescribe extended-release (ER) and long-acting
