Dabigatran (Pradaxa) for stroke prevention in patients with non-valvular atrial fibrillation (da-big-a-tran)
|
|
|
- Silas Baldwin
- 7 years ago
- Views:
Transcription
1 Dabigatran (Pradaxa) 1 Dabigatran (Pradaxa) for stroke prevention in patients with non-valvular atrial fibrillation (da-big-a-tran) Similar rate of major bleeding to warfarin KEY POINTS Dabigatran is an oral anticoagulant It is a direct thrombin inhibitor. Dose titration and monitoring of prothrombin time are not required Dabigatran is formulated as a capsule (150 mg or 110 mg) taken twice daily. Dabigatran 150 mg twice daily reduced the absolute risk of stroke or systemic embolism by 0.6% per year compared with warfarin The incidence of stroke or systemic embolism did not differ between dabigatran 110 mg twice daily and warfarin. Important differences in effectiveness were related to the quality of INR control in clinical trial patients Consistently therapeutic warfarin may be as effective as dabigatran. The overall rate of major bleeding did not differ between dabigatran 150 mg twice daily and warfarin Dabigatran 150 mg twice daily increased the risk of gastrointestinal bleeding, but reduced the risk of intracranial bleeding. There is no antidote to dabigatran-induced bleeding As with all anticoagulants, tell patients to seek prompt medical attention for unexplained bruising, blood in the urine or black stools. Dabigatran is contraindicated in severe hepatic and renal impairment (creatinine clearance 30 ml/min) Consider dabigatran 110 mg twice daily in patients with moderate renal impairment (creatinine clearance ml/min). PBS listing Dabigatran is PBS-listed for the prevention of stroke or systemic embolism in moderate-to-high risk patients with non-valvular atrial fibrillation who meet certain criteria outlined in the listing restriction. What is it? Dabigatran is a direct thrombin (factor IIa) inhibitor oral anticoagulant. It is formulated as dabigatran etexilate, a prodrug converted to dabigatran after administration. For information about dabigatran s pharmacokinetic profile, read the Australian Prescriber article New oral anticoagulant drugs mechanisms of action.
2 2 Dabigatran (Pradaxa) EVIDENCE SNAPSHOT WHAT IS KNOWN ABOUT THIS DRUG? Dabigatran 150 mg twice daily reduced the absolute risk of stroke or systemic embolism by 0.6% per year compared with warfarin (relative risk [RR] 0.65, 95% confidence interval [CI] 0.52 to 0.81). Major bleeding rates were similar for dabigatran 150 mg twice daily and warfarin (3.32% vs 3.57%, respectively; RR 0.93, 95% CI 0.81 to 1.07). AREAS OF UNCERTAINTY The safety and efficacy of dabigatran has not been established in patients at high risk of bleeding (Box 1). Safety data for dabigatran are limited to 2 years of follow-up. WHAT DOES NPS SAY? Consider individual risks and potential benefits when choosing an oral anticoagulant. Patients with an INR consistently in the therapeutic range may not benefit from switching to dabigatran therapy. Dabigatran may be an option for: warfarin-treated patients who find it difficult to maintain a therapeutic INR those at increased risk of drug drug or drug food interactions with warfarin those for whom regular INR monitoring is difficult or impractical. Who is it for? From September 1st 2013 Dabigatran is PBS-listed for preventing stroke and systemic embolism in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. 2 Both the indication and patient population are consistent with those evaluated in the main clinical trial (see How does it compare?). In April 2010, dabigatran was PBS listed for the prevention of venous thromboembolism after orthopaedic surgery. Dabigatran (150 mg once daily or 220 mg once daily) is taken for 10 days after knee replacement surgery and for days after hip replacement surgery. For further information refer to the NPS RADAR review Dabigatran (Pradaxa) for preventing venous thromboembolism after hip or knee replacement surgery.
3 Dabigatran (Pradaxa) 3 Review current anticoagulant control when considering dabigatran for patients with atrial fibrillation Warfarin-treated patients with unstable or poor INR control (for reasons other than nonadherence) may benefit if switched to dabigatran. Factors that contribute to the suboptimal use of warfarin, including drug interactions and difficulty monitoring INR, may also inform the decision to switch therapy. Patients with consistently therapeutic INRs while taking warfarin, who are at low risk of stroke, may be less likely to benefit from a switch to dabigatran. Some of these patients may also find frequent anticoagulant monitoring reassuring. Consider dabigatran for patients who are unwilling to take warfarin because of drug interactions, dietary restrictions or the need for regular monitoring. Dabigatran may be unsuitable for patients with serious comorbidities There are likely to be important differences between the dabigatran clinical trial population and patients treated in the community. For example, severe renal impairment (CrCl 30 ml/min) was among the exclusion criteria 3, and warfarin should therefore continue to be the preferred drug for this patient population. Patients with conditions associated with an increased risk of bleeding (Box 1) or active liver disease were also ineligible and should not receive dabigatran. Box 1: Bleeding risk factors that excluded people from the RE-LY trial 3 Major surgery in the previous month Planned surgery or intervention in the next 3 months History of intracranial, intraocular, spinal, retroperitoneal or atraumatic intraarticular bleeding Gastrointestinal haemorrhage within the past year Symptomatic or endoscopically documented gastroduodenal ulcer disease in the previous 30 days Haemorrhagic disorder or bleeding diathesis Need for anticoagulant treatment of disorders other than atrial fibrillation Fibrinolytic agents within 48 hours of study entry Uncontrolled hypertension (systolic blood pressure > 180 mmhg and/or diastolic blood pressure > 100 mmhg) Recent malignancy or radiation therapy ( 6 months) and not expected to survive for 3 years Avoid dabigatran in patients with a history of poor medication adherence Clinical trial investigators excluded patients thought unlikely to adhere to dosing instructions. 3 If patient adherence is an issue in clinical practice, the longer terminal half-life of warfarin is likely to provide a more consistent anticoagulant effect. Furthermore, prothrombin time cannot be used to guide management or monitor adherence in dabigatrantreated patients.
4 4 Dabigatran (Pradaxa) * A measure of the risk of stroke in which congestive heart failure, hypertension, age 75 years, and diabetes mellitus are each assigned 1 point and previous stroke or transient ischaemic attack is assigned 2 points; the score is calculated by summing all the points for a given patient. Where does it fit? The absolute risk of stroke and risk of major bleeding guide the choice of antithrombotic therapy in patients with non-valvular atrial fibrillation (see NPS News 62: Using antithrombotics wisely in stroke prevention). Patients at moderate to high risk of stroke are currently prescribed warfarin. It reduces the relative risk of stroke in non-valvular atrial fibrillation by 64% compared with placebo or no treatment. 4 Fixed-dose dabigatran is an alternative to warfarin for the primary and secondary prevention of stroke. Before prescribing dabigatran consider that: patients with an INR consistently in the therapeutic range may not benefit from switching therapy (see Who is it for?) safety has not been established in patients at high risk of bleeding (Box 1) renal impairment increases bleeding risk. How does it compare? Dabigatran has been compared with warfarin in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. 5 The RE-LY study randomised more than 18,000 patients at risk of stroke (mean CHADS2* score 2.1) to dabigatran 150 mg twice daily, dabigatran 110 mg twice daily or dose-adjusted warfarin. 3,5 The dabigatran dose was blinded whereas warfarin was administered in an openlabel fashion. To minimise bias, outcomes were adjudicated by investigators blinded to treatment assignment. The mean age of study participants was 71 years and the median follow-up period was 2 years. Half the patients enrolled in the RE-LY study were naïve to oral anticoagulants. Dabigatran reduces stroke and systemic embolism Dabigatran 150 mg twice daily reduced risk more than dabigatran 110 mg twice daily. 5,6 The higher dabigatran dose reduced the annual absolute risk of stroke or systemic embolism by 0.6% compared with warfarin (RR 0.65, 95% CI 0.52 to 0.81) and by 0.43% compared with dabigatran 110 mg twice daily (Table 1). Both haemorrhagic and ischaemic stroke occurred less often in the dabigatran 150 mg twice-daily group than in the warfarin group. Dabigatran 110 mg twice daily was non-inferior to warfarin. That is, a similar number of patients in each treatment group had a stroke or systemic embolism. Compared with warfarin, dabigatran significantly reduced the incidence of haemorrhagic, but not ischaemic, stroke. Warfarin-treated patients with an INR consistently in the therapeutic range may not benefit from switching to dabigatran The INR in the warfarin group was within the therapeutic range ( ) for 64% of the RE-LY study period, but the quality of INR control varied between countries and study sites. 5,7 Study centres in Australia reported a mean time in therapeutic range of 74 %; a figure within the upper quartile (top 25%) of participating study sites. 7 Post-hoc analyses found that dabigatran 150 mg twice daily reduced the rate of composite cardiovascular events at centres where INR control was poor (bottom 25%), but not at sites where INR control was better (Figure 1). Patients with an INR consistently in the therapeutic range may therefore be less likely to experience a net clinical benefit by switching to dabigatran therapy. The RE-LY study specified a composite net clinical benefit outcome of stroke, systemic embolism, pulmonary embolism, myocardial infarction, death and major bleeding.
5 Dabigatran (Pradaxa) 5 Table 1. Annual incidence of study outcomes in the RE-LY trial 5,6 Event Warfarin Dabigatran 150 mg Dabigatran 110 mg %/year %/year Absolute risk reduction versus warfarin (%/ year) %/year Absolute risk reduction versus warfarin (%/ year) Stroke or systemic embolism* Stroke Haemorrhagic stroke Ischaemic or unspecified stroke * Primary study outcome Includes haemorrhagic, ischaemic or unspecified, non-disabling and disabling or fatal stroke p < All values are for superiority over warfarin Figure 1: Rate of composite cardiovascular events relative to mean time in therapeutic range 7 Composite cardiovascular events (rate per 100 person years) * Dabigatran 150 mg twice daily Warfarin < > 72.6 Mean time in therapeutic range (%) by quartile * p < 0.05 versus warfarin Stroke, systemic embolism, pulmonary embolism, myocardial infarction, death and major bleeding Mean time in therapeutic range was used as a proxy for INR control Safety issues Serious bleeding is the greatest safety concern for dabigatran-treated patients and clinicians. However, unlike for warfarin, there is currently no specific antidote to dabigatran s effect. Rates of discontinuation were significantly higher in dabigatran- than warfarin-treated patients in the RE-LY study (21% vs 17%, respectively); gastrointestinal (GI) disorders were the most common adverse effect leading to discontinuation of dabigatran (see Gastrointestinal disorders may affect adherence). 5,8 Report suspected adverse reactions to the Therapeutic Goods Administration (TGA) online ( or by using the Blue Card distributed three times a year with Australian Prescriber. For information about reporting adverse reactions, see the TGA website (
6 6 Dabigatran (Pradaxa) Table 2. Annual incidence of major and lifethreatening bleeding in the RE-LY trial 5,6 Warfarin Dabigatran 150 mg Dabigatran 110 mg Any major bleeding* 3.57% 3.32% 2.87% Life-threatening bleeding 1.85% 1.49% 1.24% * Defined as a reduction in the haemoglobin level of at least 20 g/l, transfusion of at least two units of blood, or symptomatic bleeding in a critical area or organ A subcategory of major bleeding consisting of fatal bleeding, symptomatic intracranial bleeding, bleeding with a decrease in the haemoglobin level of at least 50 g/l, or bleeding requiring transfusion of at least four units of blood or inotropic agents or necessitating surgery p < All values are for superiority over warfarin Figure 2: Rate of major bleeding relative to mean time in therapeutic range 7 Major bleeding rate did not differ between dabigatran 150 mg twice daily and warfarin Patients at risk of bleeding were excluded from the RE-LY study (Box 1). 3 Major bleeding rates were similar for dabigatran 150 mg twice daily and warfarin (RR 0.93, 95% CI 0.81 to 1.07) in the total study population Major bleeding events (rate per 100 person years) * Dabigatran 150 mg twice daily Warfarin (Table 2). 5,6 However, the incidence of major bleeding was lower with dabigatran 110 mg twice daily than with warfarin (0.7% per year; RR 0.8, 95% CI 0.7 to 0.93). Warfarin was associated with a higher rate of lifethreatening bleeds than either dabigatran dose. Poor INR control was associated with increased bleeding risk Post-hoc analyses of RE-LY data suggest that there are differences in bleeding risk between dabigatran and warfarin when the quality of INR control is poor. 7 At study centres where INR control was poor, dabigatran 150 mg twice daily reduced the incidence of major bleeding compared with warfarin (Figure 2). However, there was no significant difference in risk with better INR control (mean time in therapeutic range 57.1%). 0 < > 72.6 Mean time in therapeutic range (%) by quartile * p < 0.05 versus warfarin Mean time in therapeutic range was used as a proxy for INR control
7 Dabigatran (Pradaxa) 7 Table 3. Annual incidence of GI bleeding in the RE-LY trial 2 Warfarin Dabigatran 150 mg Dabigatran 110 mg Major GI bleeding* 1.07% 1.57% 1.14% Any GI bleeding 4.02% 6.13% 5.41% * A subset of major bleeding Significantly greater incidence of gastrointestinal bleeding compared with warfarin (p < 0.05) More dabigatran-treated patients had a gastrointestinal bleed Compared with warfarin, the rate of major GI bleeding (Table 3) was significantly higher in patients treated with dabigatran 150 mg twice daily (RR 1.47; 95% CI 1.17 to 1.85). 2 The risk increased with age and was greatest in patients aged 75 years (hazard ratio [HR] 1.79, 95% CI 1.32 to 2.42). 8 Dabigatran 150 mg twice daily caused significantly more major GI bleeds than warfarin at study centres where INR control was above the median (mean time in therapeutic range > 65.5%). 7 Intracranial bleeding was uncommon, but higher with warfarin Dabigatran 150 mg twice daily reduced the absolute risk of intracranial bleeding compared with warfarin (0.32 % vs 0.76 %, respectively; RR 0.41, 95% CI 0.28 to 0.6). 6 Gastrointestinal disorders may affect adherence Gastrointestinal adverse events, including dyspepsia and gastritis-like symptoms, were common in dabigatran-treated patients (35% vs 24% in the warfarin group). 2 In the RE-LY study, gastrointestinal disorders frequently led to drug discontinuation (7%, 6.5% and 3.9% in the dabigatran 150 mg twice-daily, dabigatran 110 mg twice-daily and warfarin groups, respectively). 8 There is no antidote to dabigatran-induced bleeding Stopping dabigatran may be sufficient to manage minor bleeding in patients with normal renal function because dabigatran binds reversibly to thrombin and has a relatively short mean terminal half-life (12 14 hours). 2,9 The Pradaxa (dabigatran) product information suggests supportive strategies, including transfusion of fresh whole blood or fresh frozen plasma, for severe bleeding. 2 Take care combining dabigatran with antiplatelet drugs or NSAIDs Combining dabigatran with other anticoagulants or antiplatelet agents, including clopidogrel, is not recommended. 2 Seek expert advice for patients with an indication for ongoing antiplatelet therapy. Patients in the RE-LY study were permitted to take concomitant aspirin (< 100 mg/day) and/or clopidogrel. 5 Each drug, or the combination thereof, contributed to an increase in the annual rate of major bleeding events. 2,8 NSAIDs increased the relative risk of major bleeding by up to 50% when given in conjunction with dabigatran or warfarin. 2
8 8 Dabigatran (Pradaxa) For the first 3 days of verapamil therapy. 2 A direct thrombin inhibitor voluntarily withdrawn from the market after reports of severe liver damage. 11,12 Dabigatran has a different drug interaction profile to that of warfarin Use dabigatran cautiously in patients taking amiodarone, HIV-protease inhibitors or verapamil. These and other inhibitors of P-glycoprotein can increase dabigatran plasma levels and, therefore, the risk of bleeding. Starting dabigatran and verapamil simultaneously is contraindicated. 2 Verapamil can be added to dabigatran therapy, but dabigatran must be given 2 hours before verapamil to avoid interaction. Consider other treatment options for patients unlikely to be able to comply with these dosing instructions. The strong P-glycoprotein inhibitors cyclosporin, tacrolimus and itraconazole are not recommended in patients taking dabigatran; systemic ketoconazole is contraindicated. 2 An increased risk of myocardial infarction cannot be excluded Myocardial infarction occurred at a numerically, but not statistically significantly, greater rate in dabigatran- than warfarin-treated patients (annual event rate in the RE-LY study: dabigatran 150 mg twice daily: 0.81%; dabigatran 110 mg twice daily 0.82%; warfarin 0.64%). 5,6 The imbalance did not appear to be related to dabigatran dose. 5,10 Avoid dabigatran in patients with hepatic impairment Dabigatran should not be used by patients with liver disease or severe hepatic impairment expected to impact on survival. 2 Active liver disease, elevated liver enzyme levels at baseline (more than twice the upper limit of normal) or after previous exposure to ximelagatran were exclusion criteria in the RE-LY study. 3,5 The Pradaxa product information recommends that each patient has a liver function test before beginning treatment with dabigatran. 2 Dabigatran caused similar rates of elevated liver enzyme levels to those seen with warfarin in the RE-LY study. 5 Dosing issues The recommended dose of dabigatran is 150 mg twice daily, taken with or without food. The capsule should be swallowed whole with water. Breaking, chewing or emptying the contents increases the dose absorbed and the risk of bleeding. 2 Reduce the dose to 110 mg twice daily for patients aged 75 years. Consider prescribing the lower dose for patients with moderate renal impairment (CrCl 30 50mL/min) or those at higher risk of major bleeding. Dabigatran is contraindicated in patients: with severe renal impairment (CrCl < 30 ml/min) predisposed to bleeding with conditions associated with an increased risk of bleeding (Box 1) with GI haemorrhage within the last year with liver disease or hepatic impairment expected to impact on survival. This list is not exhaustive. Review the product information for additional contraindications and further information regarding those listed above. Switching patients from warfarin to dabigatran Stop warfarin and wait until the patient s INR is < 2.0 before starting dabigatran. 2
9 Dabigatran (Pradaxa) 9 Switching patients from dabigatran to warfarin Use CrCl to determine when warfarin should be started. For patients with CrCl: > 50 ml/min, start warfarin 3 days before stopping dabigatran ml/min, start warfarin 2 days before stopping dabigatran. Discontinue dabigatran before surgery Dabigatran should be stopped at least 1 day (CrCl 50mL/min) or 3 days (CrCl < 50mL/min) before elective surgery or an invasive procedure. 2,13 Discontinue dabigatran 2 4 days before surgery if complete haemostasis is required. Store dabigatran in its original packaging Dabigatran capsules should be dispensed and stored in the manufacturer s original packaging. 14,15 Repackaging dabigatran capsules increases the risk of exposure to moisture or humidity, causing product breakdown and loss of potency. MEDICINE UPDATE An NPS Medicine Update article on dabigatran for preventing stroke is available for consumers. Medicine Update helps consumers to ask the right questions about new medicines, and helps them compare the potential benefits and harms of a new medicine with those of other medicines. Information for patients Patients and their carers should clearly understand the risks and potential benefits of taking an anticoagulant to prevent stroke. Refer to NPS News 62: Using antithrombotics wisely in stroke prevention for a discussion of effective risk communication. Advise patients and carers: to take one dabigatran capsule at about the same time each morning and one dabigatran capsule at about the same time each evening. Swallow the capsule whole with water that a missed dabigatran dose can be taken up to 6 hours before the next scheduled dose. After this time, the missed dose should be omitted do not take a double dose to make up for it to consult a doctor if they have any prolonged or excessive bleeding or signs of internal bleeding, such as unexplained bruising, blood in the urine or black stools to consult a doctor before using nonprescription medicines containing aspirin or NSAIDs. Paracetamol can be used for minor ailments that dyspepsia is common; taking dabigatran capsules with food may help to tell their health professional at each consultation that they are taking dabigatran to keep dabigatran capsules in a cool, dry place. Dabigatran should be stored in the manufacturer s original packaging to protect the capsules from moisture. Discuss the Pradaxa consumer medicine information (CMI) leaflet with the patient.
10 10 Dabigatran (Pradaxa) REFERENCES 1. Pharmaceutical Benefits Branch. Dabigatran etexilate, capsules, 110 mg and 150 mg (as mesilate), Pradaxa March 2011 Canberra: Australian Government Department of Health and Ageing, publishing.nsf/content/pbac-psd-dabigatranmarch11 (accessed 6 July 2011). 2. Boehringer-Ingelheim Pty Ltd. Pradaxa product information. 29 April Ezekowitz MD, et al. Am Heart J 2009;157: , 10 e Hart RG, et al. Ann Intern Med 2007;146: Connolly SJ, et al. N Engl J Med 2009;361: Connolly SJ, et al. N Engl J Med 2010;363: Wallentin L, et al. Lancet 2010;376: US Food and Drug Agency (FDA). Center for Drug Evaluation and Research (CDER): Medical review of dabigatran; application no drugsatfda/index.cfm?fuseaction=search.label_ ApprovalHistory#apphist (accessed 28 April 2011). 9. van Ryn J, et al. Thromb Haemost 2010;103: US Food and Drug Administration (FDA). Division of Cardiovascular and Renal products: Addendum to Clinical Review for NDA ; Risk of myocardial infarction fda.gov/downloads/advisorycommittees/ CommitteesMeetingMaterials/Drugs/ CardiovascularandRenalDrugsAdvisoryCommittee/ UCM pdf (accessed 26 April 2011). 11. Agnelli G, et al. Thromb Res 2009;123: European Medicines Agency. Press release: AstraZeneca withdraws its application for Ximelagatran 36-mg film-coated tablets document_library/press_release/2010/02/ WC pdf (accessed 3 May 2011). 13. Rossi S, ed. Australian Medicines Handbook Adelaide: Australian Medicines Handbook Pty Ltd. 14. US Food and Drug Administration (FDA). FDA Drug Safety Communication: Special storage and handling requirements must be followed for Pradaxa (dabigatran etexilate mesylate) capsules ucm htm (accessed 27 April 2011). 15. Boehringer-Ingelheim Pty Ltd. Pradaxa consumer medicine information. 17 February US Food and Drug Administration (FDA). Center for Drug Evaluation and Research (CDER): Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules Drugs/DrugSafety/UCM pdf (accessed 27 April 2011). Updated mid-august 2013 to reflect PBS listing for this indication. First published: August 2011 (as PBAC recommended for PBS listing for this indication). The information contained in NPS RADAR is derived from a critical analysis of a wide range of authoritative evidence and is current at the time of publication. Any treatment decisions based on the information provided in NPS RADAR should be made in the context of the clinical circumstances of each patient. NPS RADAR articles may be updated when there is new evidence about safety or efficacy, or in case of regulatory or PBS listing changes. Please refer to for the most recent version as well as any supplementary information.
The Role of the Newer Anticoagulants
 The Role of the Newer Anticoagulants WARFARIN = Coumadin DAGIBATRAN = Pradaxa RIVAROXABAN = Xarelto APIXABAN = Eliquis INDICATION DABIGATRAN (Pradaxa) RIVAROXABAN (Xarelto) APIXABAN (Eliquis) Stroke prevention
The Role of the Newer Anticoagulants WARFARIN = Coumadin DAGIBATRAN = Pradaxa RIVAROXABAN = Xarelto APIXABAN = Eliquis INDICATION DABIGATRAN (Pradaxa) RIVAROXABAN (Xarelto) APIXABAN (Eliquis) Stroke prevention
Rivaroxaban (Xarelto) for preventing venous thromboembolism after hip or knee replacement surgery
 for preventing venous thromboembolism after hip or knee replacement surgery (riv-ah-rocks-ah-ban) Summary Rivaroxaban is an oral anticoagulant and the first direct factor Xa inhibitor. Rivaroxaban has
for preventing venous thromboembolism after hip or knee replacement surgery (riv-ah-rocks-ah-ban) Summary Rivaroxaban is an oral anticoagulant and the first direct factor Xa inhibitor. Rivaroxaban has
Dabigatran (Pradaxa) Guidelines
 Dabigatran (Pradaxa) Guidelines Dabigatran is a new anticoagulant for reducing the risk of stroke in patients with atrial fibrillation. Dabigatran is a direct thrombin inhibitor, similar to warfarin, without
Dabigatran (Pradaxa) Guidelines Dabigatran is a new anticoagulant for reducing the risk of stroke in patients with atrial fibrillation. Dabigatran is a direct thrombin inhibitor, similar to warfarin, without
TA 256: Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals TA 256: Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation 1 Name of Commissioning
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals TA 256: Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation 1 Name of Commissioning
New Treatments for Stroke Prevention in Atrial Fibrillation. John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013
 New Treatments for Stroke Prevention in Atrial Fibrillation John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013 Classification Paroxysmal atrial fibrillation (AF) Last < 7
New Treatments for Stroke Prevention in Atrial Fibrillation John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013 Classification Paroxysmal atrial fibrillation (AF) Last < 7
Long term anticoagulant therapy in patients with atrial fibrillation at high risk of stroke: a new scenario after RE-LY trial
 Long term anticoagulant therapy in patients with atrial fibrillation at high risk of stroke: a new scenario after RE-LY trial Camillo Autore Università di Roma Sapienza II Facoltà di Medicina e Chirurgia
Long term anticoagulant therapy in patients with atrial fibrillation at high risk of stroke: a new scenario after RE-LY trial Camillo Autore Università di Roma Sapienza II Facoltà di Medicina e Chirurgia
Appendix C Factors to consider when choosing between anticoagulant options and FAQs
 Appendix C Factors to consider when choosing between anticoagulant options and FAQs Choice of anticoagulant for non-valvular* atrial fibrillation: Clinical decision aid Patients should already be screened
Appendix C Factors to consider when choosing between anticoagulant options and FAQs Choice of anticoagulant for non-valvular* atrial fibrillation: Clinical decision aid Patients should already be screened
Devang M. Desai, MD, FACC, FSCAI Chief of Interventional Cardiology Director of Cardiac Catheterization Lab St. Mary s Hospital and Regional Medical
 Devang M. Desai, MD, FACC, FSCAI Chief of Interventional Cardiology Director of Cardiac Catheterization Lab St. Mary s Hospital and Regional Medical Center A.Fib affects 2.2 million Americans. The lifetime
Devang M. Desai, MD, FACC, FSCAI Chief of Interventional Cardiology Director of Cardiac Catheterization Lab St. Mary s Hospital and Regional Medical Center A.Fib affects 2.2 million Americans. The lifetime
AHA/ASA Scientific Statement Oral Antithrombotic Agents for the Prevention of Stroke in Atrial Fibrillation
 AHA/ASA Scientific Statement Oral Antithrombotic Agents for the Prevention of Stroke in Atrial Fibrillation A Statement for Healthcare Professionals from the American Heart Association/American Stroke
AHA/ASA Scientific Statement Oral Antithrombotic Agents for the Prevention of Stroke in Atrial Fibrillation A Statement for Healthcare Professionals from the American Heart Association/American Stroke
Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Rivaroxaban (Xarelto) for stroke prevention in non-valvular atrial fibrillation (riv-ah-rocks-ah-ban)
 1 for stroke prevention in non-valvular atrial fibrillation (riv-ah-rocks-ah-ban) An oral anticoagulant for the prevention of stroke in people with non-valvular atrial fibrillation KEY POINTS Rivaroxaban
1 for stroke prevention in non-valvular atrial fibrillation (riv-ah-rocks-ah-ban) An oral anticoagulant for the prevention of stroke in people with non-valvular atrial fibrillation KEY POINTS Rivaroxaban
FULL REVIEW. PBS listing. Rivaroxaban (Xarelto) NPS RADAR AUGUST 2013 KEY POINTS
 1 FULL REVIEW for treatment of deep vein thrombosis and pulmonary embolism, and for prevention of venous thromboembolism recurrence (riv-ah-rocks-ah-ban) An alternative to parenteral anticoagulation with
1 FULL REVIEW for treatment of deep vein thrombosis and pulmonary embolism, and for prevention of venous thromboembolism recurrence (riv-ah-rocks-ah-ban) An alternative to parenteral anticoagulation with
Committee Approval Date: September 12, 2014 Next Review Date: September 2015
 Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
rivaroxaban 15 and 20mg film-coated tablets (Xarelto ) SMC No. (756/12) Bayer PLC
 rivaroxaban 15 and 20mg film-coated tablets (Xarelto ) SMC No. (756/12) Bayer PLC 13 January 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS
rivaroxaban 15 and 20mg film-coated tablets (Xarelto ) SMC No. (756/12) Bayer PLC 13 January 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS
Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013
 Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013 Family physician with Rivergrove Medical Clinic Practice in the north end since 1985 Medical Director of the Wellness
Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013 Family physician with Rivergrove Medical Clinic Practice in the north end since 1985 Medical Director of the Wellness
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below
 Name: generic (trade) Rivaroxaban (Xarelto ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below What it is Indications
Name: generic (trade) Rivaroxaban (Xarelto ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below What it is Indications
Three new/novel oral anticoagulants (NOAC) have been licensed in Ireland since 2008:
 Key Points to consider when prescribing NOACs Introduction Three new/novel oral anticoagulants (NOAC) have been licensed in Ireland since 2008: Dabigatran Etexilate (Pradaxa ) 75mg, 110mg, 150mg. Rivaroxaban
Key Points to consider when prescribing NOACs Introduction Three new/novel oral anticoagulants (NOAC) have been licensed in Ireland since 2008: Dabigatran Etexilate (Pradaxa ) 75mg, 110mg, 150mg. Rivaroxaban
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation
 NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016 Version 1.4 EQIA Yes 01/06/2012
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016 Version 1.4 EQIA Yes 01/06/2012
New Anticoagulation Options for Stroke Prevention in Atrial Fibrillation. Joy Wahawisan, Pharm.D., BCPS April 25, 2012
 New Anticoagulation Options for Stroke Prevention in Atrial Fibrillation Joy Wahawisan, Pharm.D., BCPS April 25, 2012 Stroke in Atrial Fibrillation % Stroke 1991;22:983. Age Range (years) CHADS 2 Risk
New Anticoagulation Options for Stroke Prevention in Atrial Fibrillation Joy Wahawisan, Pharm.D., BCPS April 25, 2012 Stroke in Atrial Fibrillation % Stroke 1991;22:983. Age Range (years) CHADS 2 Risk
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation
 NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 06/06/2012 Review Date 06/06/2014 Version 1.1 EQIA Yes /
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 06/06/2012 Review Date 06/06/2014 Version 1.1 EQIA Yes /
Birmingham, Sandwell and Solihull Cardiac and Stroke Network. Rivaroxaban or warfarin for treatment of Atrial Fibrillation: Position statement
 Birmingham, Sandwell and Solihull Cardiac and Stroke Network Rivaroxaban or warfarin for treatment of Atrial Fibrillation: Position statement Introduction This guidance informs prescribers and commissioners
Birmingham, Sandwell and Solihull Cardiac and Stroke Network Rivaroxaban or warfarin for treatment of Atrial Fibrillation: Position statement Introduction This guidance informs prescribers and commissioners
NORTH WEST LONDON GUIDANCE ANTITHROMBOTIC MANAGEMENT OF ATRIAL FIBRILLATION
 North West London CardioVascular & Stroke Network NORTH WEST LONDON GUIDANCE ON ANTITHROMBOTIC MANAGEMENT OF ATRIAL FIBRILLATION Key Messages 1. Efforts should be made to identify patients with Atrial
North West London CardioVascular & Stroke Network NORTH WEST LONDON GUIDANCE ON ANTITHROMBOTIC MANAGEMENT OF ATRIAL FIBRILLATION Key Messages 1. Efforts should be made to identify patients with Atrial
Novel Anticoagulation Agents DISCLOSURES. Objectives ATRIAL FIBRILLATION TRIALS. NOAC Comparison 6/12/2015
 Novel Anticoagulation Agents DISCLOSURES James W. Haynes, MD Department of Family Medicine Univ of TN Health Science Center (Chattanooga) Objectives Understand mechanism of action behind the NOAC agents
Novel Anticoagulation Agents DISCLOSURES James W. Haynes, MD Department of Family Medicine Univ of TN Health Science Center (Chattanooga) Objectives Understand mechanism of action behind the NOAC agents
The author has no disclosures
 Mary Bradbury, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Surgery September 18, 2012 Mary.bradbury@inova.org This presentation will discuss unlabeled and investigational use of products The author
Mary Bradbury, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Surgery September 18, 2012 Mary.bradbury@inova.org This presentation will discuss unlabeled and investigational use of products The author
Xarelto (Rivaroxaban)
 Xarelto (Rivaroxaban) Hightly selective, reversible, direct oral FXa inhibitor Maxium concentratiion after 2 to 4 hrs High bioavailability(66%),increase with food ( suggest with food) 1/3 from renal excretion,
Xarelto (Rivaroxaban) Hightly selective, reversible, direct oral FXa inhibitor Maxium concentratiion after 2 to 4 hrs High bioavailability(66%),increase with food ( suggest with food) 1/3 from renal excretion,
STROKE PREVENTION IN ATRIAL FIBRILLATION
 STROKE PREVENTION IN ATRIAL FIBRILLATION OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention of ischemic stroke and arterial thromboembolism in patients
STROKE PREVENTION IN ATRIAL FIBRILLATION OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention of ischemic stroke and arterial thromboembolism in patients
FOR THE PREVENTION OF ATRIAL FIBRILLATION RELATED STROKE
 www.bpac.org.nz keyword: warfarinaspirin FOR THE PREVENTION OF ATRIAL FIBRILLATION RELATED STROKE Key Concepts In atrial fibrillation (AF) warfarin is more effective than aspirin for stroke prevention.
www.bpac.org.nz keyword: warfarinaspirin FOR THE PREVENTION OF ATRIAL FIBRILLATION RELATED STROKE Key Concepts In atrial fibrillation (AF) warfarin is more effective than aspirin for stroke prevention.
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
4/9/2015. Risk Stratify Our Patients. Stroke Risk in AF: CHADS2 Scoring system JAMA 2001; 285: 2864-71
 Anticoagulation in the 21 st Century Adam Karpman, D.O. Saint Francis Medical Center/Oklahoma State University Medical Center Disclosures: None Atrial Fibrillation Most common arrhythmia in clinical practice.
Anticoagulation in the 21 st Century Adam Karpman, D.O. Saint Francis Medical Center/Oklahoma State University Medical Center Disclosures: None Atrial Fibrillation Most common arrhythmia in clinical practice.
Breadth of indications matters One drug for multiple indications
 Breadth of indications matters One drug for multiple indications Sylvia Haas, MD, PhD Formerly of the Technical University of Munich Munich, Germany Disclosures: Sylvia Haas 1 Novel oral anticoagulants:
Breadth of indications matters One drug for multiple indications Sylvia Haas, MD, PhD Formerly of the Technical University of Munich Munich, Germany Disclosures: Sylvia Haas 1 Novel oral anticoagulants:
FDA Approved Oral Anticoagulants
 FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
RR 0.88 (95% CI: 0.77 1.00) P=0.051 (superiority) 3.75
 ALL-CAUSE MORTALITY RR 0.88 (95% CI: 0.77 1.00) P=0.051 (superiority) Rate per year (%) 5.0 4.0 3.0 2.0 1.0 0 3.64 D150 mg BID 3.75 D110 mg BID RR 0.91 (95% CI: 0.80 1.03) P=0.13 (superiority) 4.13 Warfarin
ALL-CAUSE MORTALITY RR 0.88 (95% CI: 0.77 1.00) P=0.051 (superiority) Rate per year (%) 5.0 4.0 3.0 2.0 1.0 0 3.64 D150 mg BID 3.75 D110 mg BID RR 0.91 (95% CI: 0.80 1.03) P=0.13 (superiority) 4.13 Warfarin
Clinical guide to managing patients treated with Pradaxa
 Clinical guide to managing patients treated with Pradaxa Updated August 2013 Treatment with Pradaxa (dabigatran etexilate) requires no routine laboratory monitoring. 1 Routine coagulation tests (aptt,
Clinical guide to managing patients treated with Pradaxa Updated August 2013 Treatment with Pradaxa (dabigatran etexilate) requires no routine laboratory monitoring. 1 Routine coagulation tests (aptt,
NEWER ANTICOAGULANTS: FOCUS ON STROKE PREVENTION IN ATRIAL FIBRILLATION AND DEEP VEIN THROMBOSIS/PULMONARY EMBOLISM
 NEWER ANTICOAGULANTS: FOCUS ON STROKE PREVENTION IN ATRIAL FIBRILLATION AND DEEP VEIN THROMBOSIS/PULMONARY EMBOLISM Carol Lee, Pharm.D., Jessica C. Song, M.A., Pharm.D. INTRODUCTION For many years, warfarin
NEWER ANTICOAGULANTS: FOCUS ON STROKE PREVENTION IN ATRIAL FIBRILLATION AND DEEP VEIN THROMBOSIS/PULMONARY EMBOLISM Carol Lee, Pharm.D., Jessica C. Song, M.A., Pharm.D. INTRODUCTION For many years, warfarin
Cardiovascular Subcommittee of PTAC Meeting held 27 February 2014. (minutes for web publishing)
 Cardiovascular Subcommittee of PTAC Meeting held 27 February 2014 (minutes for web publishing) Cardiovascular Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cardiovascular Subcommittee of PTAC Meeting held 27 February 2014 (minutes for web publishing) Cardiovascular Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
1/7/2012. Objectives. Epidemiology of Atrial Fibrillation(AF) Stroke in AF. Stroke Risk Stratification in AF
 Objectives Atrial Fibrillation and Prevention of Thrombotic Complications: Therapeutic Update Andrea C. Flores Pharm.D Pharmacy Resident at the Miami VA Healthcare System Review the epidemiology, pathophysiology
Objectives Atrial Fibrillation and Prevention of Thrombotic Complications: Therapeutic Update Andrea C. Flores Pharm.D Pharmacy Resident at the Miami VA Healthcare System Review the epidemiology, pathophysiology
Analyzing Clinical Trial Findings of the Efficacy and Safety Profiles of Novel Anticoagulants for Stroke Prevention in Atrial Fibrillation
 Analyzing Clinical Trial Findings of the Efficacy and Safety Profiles of Novel Anticoagulants for Stroke Prevention in Atrial Fibrillation Drew Baldwin, MD Virginia Mason Seattle, Washington NCVH May 29,
Analyzing Clinical Trial Findings of the Efficacy and Safety Profiles of Novel Anticoagulants for Stroke Prevention in Atrial Fibrillation Drew Baldwin, MD Virginia Mason Seattle, Washington NCVH May 29,
Prevention of stroke in patients with atrial fibrillation
 www.sign.ac.uk Prevention of stroke in patients with atrial fibrillation A guide for primary care January 2014 Evidence Contents 1 Introduction... 1 2 Detection...2 3 Risk stratification... 3 4 Treatment
www.sign.ac.uk Prevention of stroke in patients with atrial fibrillation A guide for primary care January 2014 Evidence Contents 1 Introduction... 1 2 Detection...2 3 Risk stratification... 3 4 Treatment
Dabigatran in Atrial Fibrillation Question and Answers document to inform commissioners
 Dabigatran in Atrial Fibrillation Question and Answers document to inform commissioners West Midlands Commissioning Support Unit, Birmingham University /New Medicines Evaluation Unit, Keele University
Dabigatran in Atrial Fibrillation Question and Answers document to inform commissioners West Midlands Commissioning Support Unit, Birmingham University /New Medicines Evaluation Unit, Keele University
Prescriber Guide. 20mg. 15mg. Simply Protecting More Patients. Simply Protecting More Patients
 Prescriber Guide 20mg Simply Protecting More Patients 15mg Simply Protecting More Patients 1 Dear Doctor, This prescriber guide was produced by Bayer Israel in cooperation with the Ministry of Health as
Prescriber Guide 20mg Simply Protecting More Patients 15mg Simply Protecting More Patients 1 Dear Doctor, This prescriber guide was produced by Bayer Israel in cooperation with the Ministry of Health as
AF, Stroke Risk and New Anticoagulants
 Carmarthen Cardiac Update Course AF, Stroke Risk and New Anticoagulants Dr Hamsaraj Shetty, B.Sc, FRCP (London & Edinburgh) Consultant Physician & Honorary Senior Lecturer University Hospital of Wales,Cardiff
Carmarthen Cardiac Update Course AF, Stroke Risk and New Anticoagulants Dr Hamsaraj Shetty, B.Sc, FRCP (London & Edinburgh) Consultant Physician & Honorary Senior Lecturer University Hospital of Wales,Cardiff
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation
 Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Issued: May 2012 guidance.nice.org.uk/ta256 NICE has accredited the process used by the Centre for Health
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Issued: May 2012 guidance.nice.org.uk/ta256 NICE has accredited the process used by the Centre for Health
VOLUME No: 21 04 written by Sara Wilds & Kathryn Buchanan. Date of issue: June 2012 (updated November 2012 following NICE TA 256)
 Prescribing Points A NEWSLETTER FOR ALL HEALTH CARE PROFESSIONALS IN OXFORDSHIRE, WRITTEN BY THE MEDICINES MANAGEMENT TEAM, OXFORDSHIRE PCT, JUBILEE HOUSE, OXFORD BUSINESS PARK SOUTH, OXFORD, OX4 2LH.
Prescribing Points A NEWSLETTER FOR ALL HEALTH CARE PROFESSIONALS IN OXFORDSHIRE, WRITTEN BY THE MEDICINES MANAGEMENT TEAM, OXFORDSHIRE PCT, JUBILEE HOUSE, OXFORD BUSINESS PARK SOUTH, OXFORD, OX4 2LH.
Bios 6648: Design & conduct of clinical research
 Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Anticoagulation at the end of life. Rhona Maclean Rhona.maclean@sth.nhs.uk
 Anticoagulation at the end of life Rhona Maclean Rhona.maclean@sth.nhs.uk Content Anticoagulant Therapies Indications for anticoagulation Venous thromboembolism (VTE) Atrial Fibrillation Mechnical Heart
Anticoagulation at the end of life Rhona Maclean Rhona.maclean@sth.nhs.uk Content Anticoagulant Therapies Indications for anticoagulation Venous thromboembolism (VTE) Atrial Fibrillation Mechnical Heart
Rivaroxaban shared care guidelines for the prevention of stroke and embolism in adult patients with nonvalvular atrial fibrillation.
 South West Essex Rivaroxaban Shared Care Guideline (SCG) Rivaroxaban shared care guidelines for the prevention of stroke and embolism in adult patients with nonvalvular atrial fibrillation. Introduction
South West Essex Rivaroxaban Shared Care Guideline (SCG) Rivaroxaban shared care guidelines for the prevention of stroke and embolism in adult patients with nonvalvular atrial fibrillation. Introduction
2. Background This indication of rivaroxaban had not previously been considered by the PBAC.
 PUBLIC SUMMARY DOCUMENT Product: Rivaroxaban, tablets, 15mg and 20mg, Xarelto Sponsor: Bayer Australia Ltd Date of PBAC Consideration: March 2013 1. Purpose of Application The application requested the
PUBLIC SUMMARY DOCUMENT Product: Rivaroxaban, tablets, 15mg and 20mg, Xarelto Sponsor: Bayer Australia Ltd Date of PBAC Consideration: March 2013 1. Purpose of Application The application requested the
NnEeWw DdEeVvEeLlOoPpMmEeNnTtSs IiıNn OoRrAaLl AaNnTtIiıCcOoAaGgUuLlAaTtIiıOoNn AaNnDd RrEeVvEeRrSsAaLl
 NnEeWw DdEeVvEeLlOoPpMmEeNnTtSs IiıNn OoRrAaLl AaNnTtIiıCcOoAaGgUuLlAaTtIiıOoNn AaNnDd RrEeVvEeRrSsAaLl Mikele Wissing, RN June 2014 Introduction until recently, was the unrivaled medication for treatment
NnEeWw DdEeVvEeLlOoPpMmEeNnTtSs IiıNn OoRrAaLl AaNnTtIiıCcOoAaGgUuLlAaTtIiıOoNn AaNnDd RrEeVvEeRrSsAaLl Mikele Wissing, RN June 2014 Introduction until recently, was the unrivaled medication for treatment
Oral Anticoagulants: What s New?
 Oral Anticoagulants: What s New? Sallie Young, Pharm.D., BCPS (AQ-Cardiology) Clinical Pharmacy Specialist, Cardiology Penn State Hershey Medical Center syoung1@hmc.psu.edu August 2012 Oral Anticoagulant
Oral Anticoagulants: What s New? Sallie Young, Pharm.D., BCPS (AQ-Cardiology) Clinical Pharmacy Specialist, Cardiology Penn State Hershey Medical Center syoung1@hmc.psu.edu August 2012 Oral Anticoagulant
TSOAC Initiation Checklist
 Task Establish appropriate dose based on anticoagulant selected, indication and patient factors such as renal function. Evaluate for medication interactions that may necessitate TSOAC dose adjustment.
Task Establish appropriate dose based on anticoagulant selected, indication and patient factors such as renal function. Evaluate for medication interactions that may necessitate TSOAC dose adjustment.
New Anticoagulants: Are we Ready to Replace Warfarin? Carole Goodine, RPh Horizon Health Network Stroke Conference 2011
 New Anticoagulants: Are we Ready to Replace Warfarin? Carole Goodine, RPh Horizon Health Network Stroke Conference 2011 Warfarin Decreases stroke risk by 60-70% Superior to ASA and ASA plus clopidogrel
New Anticoagulants: Are we Ready to Replace Warfarin? Carole Goodine, RPh Horizon Health Network Stroke Conference 2011 Warfarin Decreases stroke risk by 60-70% Superior to ASA and ASA plus clopidogrel
New Oral Anticoagulants. How safe are they outside the trials?
 New Oral Anticoagulants How safe are they outside the trials? Objectives The need for anticoagulant therapy Indications for anticoagulation Traditional anticoagulant therapies Properties of new oral anticoagulants
New Oral Anticoagulants How safe are they outside the trials? Objectives The need for anticoagulant therapy Indications for anticoagulation Traditional anticoagulant therapies Properties of new oral anticoagulants
22-Oct-14. Oral Anticoagulation Which Drug for Which Patient in the era of New Oral Anti-coagulants. Atrial Fibrillation. AF as an embolic risk factor
 Oral Anticoagulation Which Drug for Which Patient in the era of New Oral Anti-coagulants Dr Scott McKenzie BSc MBBS FRACP FCSANZ Cardiologist, Vascular Physician, Telehealth Specialist, Advanced Heart
Oral Anticoagulation Which Drug for Which Patient in the era of New Oral Anti-coagulants Dr Scott McKenzie BSc MBBS FRACP FCSANZ Cardiologist, Vascular Physician, Telehealth Specialist, Advanced Heart
STROKE PREVENTION IN ATRIAL FIBRILLATION. TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: ABBREVIATIONS: BACKGROUND:
 STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
Antiplatelet and Antithrombotics From clinical trials to guidelines
 Antiplatelet and Antithrombotics From clinical trials to guidelines Ashraf Reda, MD, FESC Prof and head of Cardiology Dep. Menofiya University Preisedent of EGYBAC Chairman of WGLVR One of the big stories
Antiplatelet and Antithrombotics From clinical trials to guidelines Ashraf Reda, MD, FESC Prof and head of Cardiology Dep. Menofiya University Preisedent of EGYBAC Chairman of WGLVR One of the big stories
Medicines Management Programme. Oral anticoagulants for stroke prevention in non-valvular atrial fibrillation
 Medicines Management Programme Oral anticoagulants for stroke prevention in non-valvular atrial fibrillation Drugs in this review include: Warfarin Apixaban Dabigatran Rivaroxaban Approved by Prof. Michael
Medicines Management Programme Oral anticoagulants for stroke prevention in non-valvular atrial fibrillation Drugs in this review include: Warfarin Apixaban Dabigatran Rivaroxaban Approved by Prof. Michael
How To Use Novel Anticoagulants In Cornwall
 PENINSULA NETWORK GUIDANCE ON NOVEL ANTICOAGULANTS FOR STROKE AND TIA MANAGEMENT 1. Aim/Purpose of this Guideline The aim of this document to guide clinicians on use of novel anticoagulants for stroke
PENINSULA NETWORK GUIDANCE ON NOVEL ANTICOAGULANTS FOR STROKE AND TIA MANAGEMENT 1. Aim/Purpose of this Guideline The aim of this document to guide clinicians on use of novel anticoagulants for stroke
Antiplatelet and Antithrombotic Therapy. Dr Curry Grant Stroke Prevention Clinic Quinte Health Care
 Antiplatelet and Antithrombotic Therapy Dr Curry Grant Stroke Prevention Clinic Quinte Health Care Disclosure of Potential for Conflict of Interest Dr. F.C. Grant Atrial Fibrillation FINANCIAL DISCLOSURE:
Antiplatelet and Antithrombotic Therapy Dr Curry Grant Stroke Prevention Clinic Quinte Health Care Disclosure of Potential for Conflict of Interest Dr. F.C. Grant Atrial Fibrillation FINANCIAL DISCLOSURE:
Anticoagulants in Atrial Fibrillation
 Anticoagulants in Atrial Fibrillation Starting and Stopping Them Safely Carmine D Amico, D.O. Overview Learning objectives Introduction Basic concepts Treatment strategy & options Summary 1 Learning objectives
Anticoagulants in Atrial Fibrillation Starting and Stopping Them Safely Carmine D Amico, D.O. Overview Learning objectives Introduction Basic concepts Treatment strategy & options Summary 1 Learning objectives
Failure or significant adverse effects to all of the alternatives: Eliquis and Xarelto
 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutics
This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutics
How To Treat Aneuricaagulation
 Speaker Introduction Jessica Wilhoite, PharmD, BCACP Doctor of Pharmacy: Purdue University Postgraduate Residency Training: PGY1 Pharmacy Practice St. Vincent Hospital PGY2 Ambulatory Care St. Vincent
Speaker Introduction Jessica Wilhoite, PharmD, BCACP Doctor of Pharmacy: Purdue University Postgraduate Residency Training: PGY1 Pharmacy Practice St. Vincent Hospital PGY2 Ambulatory Care St. Vincent
Rivaroxaban (Xarelto) in the management of stroke and DVT
 Rivaroxaban (Xarelto) in the management of stroke and DVT Steve Chaplin MSc, MRPharmS, Victoria Haunton BM, DGM, MRCP, Thompson Robinson BMedSci, MD, FRCP and Catherine Bagot MD, FRCPath KEY POINTS is
Rivaroxaban (Xarelto) in the management of stroke and DVT Steve Chaplin MSc, MRPharmS, Victoria Haunton BM, DGM, MRCP, Thompson Robinson BMedSci, MD, FRCP and Catherine Bagot MD, FRCPath KEY POINTS is
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants which meet any of the following conditions
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants which meet any of the following conditions
The New Anticoagulants: Which one is for You?
 The New Anticoagulants: Which one is for You? by Hans R. Larsen Although there is no evidence that otherwise healthy lone afibbers have an increased risk of ischemic stroke, it is clear that atrial fibrillation
The New Anticoagulants: Which one is for You? by Hans R. Larsen Although there is no evidence that otherwise healthy lone afibbers have an increased risk of ischemic stroke, it is clear that atrial fibrillation
All Wales Risk/Benefit Assessment Tool for Oral Anticoagulant Treatment in People with Atrial Fibrillation
 All Wales Risk/Benefit Assessment Tool for Oral Anticoagulant Treatment in People with Atrial Fibrillation October 2013 This report has been prepared by a multiprofessional collaborative group, with support
All Wales Risk/Benefit Assessment Tool for Oral Anticoagulant Treatment in People with Atrial Fibrillation October 2013 This report has been prepared by a multiprofessional collaborative group, with support
NHS DORSET CLINICAL COMMISSIONING GROUP POSITION STATEMENT ON ORAL ANTICOAGULANTS IN ATRIAL FIBRILLATION
 Version 3 August 2014 NHS DORSET CLINICAL COMMISSIONING GROUP POSITION STATEMENT ON ORAL ANTICOAGULANTS IN ATRIAL FIBRILLATION Dorset CCG commissions the use of newer oral anti-coagulants in accordance
Version 3 August 2014 NHS DORSET CLINICAL COMMISSIONING GROUP POSITION STATEMENT ON ORAL ANTICOAGULANTS IN ATRIAL FIBRILLATION Dorset CCG commissions the use of newer oral anti-coagulants in accordance
New Oral Anticoagulants in the Management of Atrial Fibrillation June, 2012 By Deborah K Brokaw, Pharm.D.
 New Oral Anticoagulants in the Management of Atrial Fibrillation June, 2012 By Deborah K Brokaw, Pharm.D. Introduction Since the 1950 s, the only orally available anticoagulant has been the vitamin K antagonist
New Oral Anticoagulants in the Management of Atrial Fibrillation June, 2012 By Deborah K Brokaw, Pharm.D. Introduction Since the 1950 s, the only orally available anticoagulant has been the vitamin K antagonist
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION RIVAROXABAN (Xarelto Bayer Inc.) Indication: Stroke Prevention in Atrial Fibrillation This recommendation supersedes the Canadian Drug Expert Committee (CDEC) recommendation for
CDEC FINAL RECOMMENDATION RIVAROXABAN (Xarelto Bayer Inc.) Indication: Stroke Prevention in Atrial Fibrillation This recommendation supersedes the Canadian Drug Expert Committee (CDEC) recommendation for
NICE TA 275: Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 275: Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation 1
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 275: Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation 1
Management of Antithrombotics with Procedures. Jordan Weinstein, MD
 Management of Antithrombotics with Procedures Jordan Weinstein, MD Presenter Disclosure Information Cardiology Update 2013 I have no relevant financial interest and/or arrangement with industry. Novel
Management of Antithrombotics with Procedures Jordan Weinstein, MD Presenter Disclosure Information Cardiology Update 2013 I have no relevant financial interest and/or arrangement with industry. Novel
ABOUT XARELTO CLINICAL STUDIES
 ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
Dorset Cardiac Centre
 P a g e 1 Dorset Cardiac Centre Patients with Atrial Fibrillation/Flutter undergoing DC Cardioversion or Ablation procedures- Guidelines for Novel Oral Anti-coagulants (NOACS) licensed for this use February
P a g e 1 Dorset Cardiac Centre Patients with Atrial Fibrillation/Flutter undergoing DC Cardioversion or Ablation procedures- Guidelines for Novel Oral Anti-coagulants (NOACS) licensed for this use February
DVT/PE Management with Rivaroxaban (Xarelto)
 DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
Recommendations on Use of Dabigatran in Atrial Fibrillation
 Recommendations on Use of Dabigatran in Atrial Fibrillation Developed by participants from the Section of Hematology/Oncology and Section of Cardiology, and Faculty of Pharmacy, University of Manitoba
Recommendations on Use of Dabigatran in Atrial Fibrillation Developed by participants from the Section of Hematology/Oncology and Section of Cardiology, and Faculty of Pharmacy, University of Manitoba
Therapeutic Class Overview Oral Anticoagulants
 Therapeutic Class Overview Oral Anticoagulants Therapeutic Class Overview/Summary: The oral anticoagulants, dabigatran etexilate mesylate (Pradaxa ), rivaroxaban (Xarelto ), and warfarin (Coumadin, Jantoven
Therapeutic Class Overview Oral Anticoagulants Therapeutic Class Overview/Summary: The oral anticoagulants, dabigatran etexilate mesylate (Pradaxa ), rivaroxaban (Xarelto ), and warfarin (Coumadin, Jantoven
New Oral Anticoagulants
 New Oral Anticoagulants Tracy Minichiello, MD Associate Professor of Medicine Chief, San FranciscoVA Anticoagulation and Thrombosis Service Ansell, J. Hematology Copyright 2010 American Society of Hematology.
New Oral Anticoagulants Tracy Minichiello, MD Associate Professor of Medicine Chief, San FranciscoVA Anticoagulation and Thrombosis Service Ansell, J. Hematology Copyright 2010 American Society of Hematology.
Comparison between New Oral Anticoagulants and Warfarin
 Comparison between New Oral Anticoagulants and Warfarin Warfarin was the mainstay of oral anticoagulant therapy until the recent discovery of more precise targets for therapy. In recent years, several
Comparison between New Oral Anticoagulants and Warfarin Warfarin was the mainstay of oral anticoagulant therapy until the recent discovery of more precise targets for therapy. In recent years, several
Anticoagulants for stroke prevention in atrial fibrillation Patient frequently asked questions
 Anticoagulants for stroke prevention in atrial fibrillation Patient frequently asked questions What is atrial fibrillation?...2 What are dabigatran, rivaroxaban and apixaban and what are they used for?...2
Anticoagulants for stroke prevention in atrial fibrillation Patient frequently asked questions What is atrial fibrillation?...2 What are dabigatran, rivaroxaban and apixaban and what are they used for?...2
New therapeutic approaches for the protection of AF patients from stroke: Do aspirin or warfarin have a role anymore?
 New therapeutic approaches for the protection of AF patients from stroke: Do aspirin or warfarin have a role anymore? Dr Tina Biss Consultant Haematologist The Newcastle Hospitals NHS Foundation Trust
New therapeutic approaches for the protection of AF patients from stroke: Do aspirin or warfarin have a role anymore? Dr Tina Biss Consultant Haematologist The Newcastle Hospitals NHS Foundation Trust
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION RIVAROXABAN (Xarelto Bayer Inc.) New Indication: Atrial Fibrillation, Stroke Prevention Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that rivaroxaban be
CDEC FINAL RECOMMENDATION RIVAROXABAN (Xarelto Bayer Inc.) New Indication: Atrial Fibrillation, Stroke Prevention Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that rivaroxaban be
Enoxaparin for long term anticoagulation in patients unsuitable for oral anticoagulants
 Enoxaparin for long term anticoagulation in patients unsuitable for oral anticoagulants Traffic light classification- Amber 2 Information sheet for Primary Care Prescribers Relevant Licensed Indications
Enoxaparin for long term anticoagulation in patients unsuitable for oral anticoagulants Traffic light classification- Amber 2 Information sheet for Primary Care Prescribers Relevant Licensed Indications
Addendum to Clinical Review for NDA 22-512
 Addendum to Clinical Review for DA 22-512 Drug: Sponsor: Indication: Division: Reviewers: dabigatran (Pradaxa) Boehringer Ingelheim Prevention of stroke and systemic embolism in atrial fibrillation Division
Addendum to Clinical Review for DA 22-512 Drug: Sponsor: Indication: Division: Reviewers: dabigatran (Pradaxa) Boehringer Ingelheim Prevention of stroke and systemic embolism in atrial fibrillation Division
Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Rivaroxaban (Xarelto ) by
 Essentia Health Med Moment Short Video Tune-Up A brief overview of a new medication, or important new medication information Rivaroxaban (Xarelto ) by Richard Mullvain RPH BCPS (AQC) Current - August 2011
Essentia Health Med Moment Short Video Tune-Up A brief overview of a new medication, or important new medication information Rivaroxaban (Xarelto ) by Richard Mullvain RPH BCPS (AQC) Current - August 2011
Anticoagulation in Atrial Fibrillation
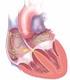 Anticoagulation in Atrial Fibrillation Parag P. Patel, MD FACC Disclosures Eliquis Speakers Bureau 1 Clinical Scenario Ms. L is a 76F admitted to the stroke service with a dense right sided hemiparesis
Anticoagulation in Atrial Fibrillation Parag P. Patel, MD FACC Disclosures Eliquis Speakers Bureau 1 Clinical Scenario Ms. L is a 76F admitted to the stroke service with a dense right sided hemiparesis
PHARMACOLOGICAL Stroke Prevention in Atrial Fibrillation STROKE RISK ASSESSMENT SCORES Vs. BLEEDING RISK ASSESSMENT SCORES.
 PHARMACOLOGICAL Stroke Prevention in Atrial Fibrillation STROKE RISK ASSESSMENT SCORES Vs. BLEEDING RISK ASSESSMENT SCORES. Hossam Bahy, MD (1992 2012), 19 tools have been identified 11 stroke scores 1
PHARMACOLOGICAL Stroke Prevention in Atrial Fibrillation STROKE RISK ASSESSMENT SCORES Vs. BLEEDING RISK ASSESSMENT SCORES. Hossam Bahy, MD (1992 2012), 19 tools have been identified 11 stroke scores 1
STARTING, SWITCHING OR STOPPING NEW ORAL ANTICOAGULANTS: A Practical Approach
 STARTING, SWITCHING OR STOPPING NEW ORAL ANTICOAGULANTS: A Practical Approach Jeffrey I Weitz, MD, FRCP(C), FACP Professor of Medicine and Biochemistry McMaster University Canada Research Chair in Thrombosis
STARTING, SWITCHING OR STOPPING NEW ORAL ANTICOAGULANTS: A Practical Approach Jeffrey I Weitz, MD, FRCP(C), FACP Professor of Medicine and Biochemistry McMaster University Canada Research Chair in Thrombosis
Oral Anticoagulants for Stroke Prevention in Atrial Fibrillation: A Brief Comparison of Four Agents
 Oral Anticoagulants for Stroke Prevention in Atrial Fibrillation: A Brief Comparison of Four Agents Abbreviations AF: Atrial fibrillation ARISTOTLE: Apixaban for Reduction in Stroke and Other Thromboembolic
Oral Anticoagulants for Stroke Prevention in Atrial Fibrillation: A Brief Comparison of Four Agents Abbreviations AF: Atrial fibrillation ARISTOTLE: Apixaban for Reduction in Stroke and Other Thromboembolic
1/12/2016. What s in a name? What s in a name? NO.Anti-Coagulation. DOACs in clinical practice. Practical aspects of using
 What s in a name? Practical aspects of using DOACs (Direct Oral Anticoagulants) James L. Sebastian, MD, MACP Professor of Medicine (GIM) Medical College of Wisconsin February 5, 2016 DOAC NOAC NOAC ODI
What s in a name? Practical aspects of using DOACs (Direct Oral Anticoagulants) James L. Sebastian, MD, MACP Professor of Medicine (GIM) Medical College of Wisconsin February 5, 2016 DOAC NOAC NOAC ODI
Cardiovascular Disease
 Cardiovascular Disease 1 Cardiovascular Disease 1. More target specific oral anticoagulants (TSOAC) 2. Vorapaxar (Zonivity) 3. Continued noise about a polypill 4. WATCHMAN 3 1 2 3 4 Left Atrial Appendage
Cardiovascular Disease 1 Cardiovascular Disease 1. More target specific oral anticoagulants (TSOAC) 2. Vorapaxar (Zonivity) 3. Continued noise about a polypill 4. WATCHMAN 3 1 2 3 4 Left Atrial Appendage
Rivaroxaban for acute coronary syndromes
 Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
THE BENEFITS OF RIVAROXABAN (XARELTO ) ACROSS MULTIPLE INDICATIONS AND THE RELEVANCE TO CARDIOLOGISTS
 THE BENEFITS OF RIVAROXABAN (XARELTO ) ACROSS MULTIPLE INDICATIONS AND THE RELEVANCE TO CARDIOLOGISTS Ingo Ahrens, Christoph Bode Cardiology and Angiology I, Heart Center Freiburg University, Freiburg,
THE BENEFITS OF RIVAROXABAN (XARELTO ) ACROSS MULTIPLE INDICATIONS AND THE RELEVANCE TO CARDIOLOGISTS Ingo Ahrens, Christoph Bode Cardiology and Angiology I, Heart Center Freiburg University, Freiburg,
The importance of adherence and persistence: The advantages of once-daily dosing
 The importance of adherence and persistence: The advantages of once-daily dosing Craig I. Coleman, PharmD Professor, University of Connecticut School of Pharmacy Storrs, CT, USA Conflicts of interest Dr
The importance of adherence and persistence: The advantages of once-daily dosing Craig I. Coleman, PharmD Professor, University of Connecticut School of Pharmacy Storrs, CT, USA Conflicts of interest Dr
3/25/14. To Clot or Not What s New In Anticoagulation? Clotting Cascade. Anticoagulant drug targets. Anita Ralstin, MS CNS CNP. Heparin.
 To Clot or Not What s New In Anticoagulation? Anita Ralstin, MS CNS CNP 1 Clotting Cascade 2 Anticoagulant drug targets Heparin XI VIII IX V X VII LMWH II Warfarin Fibrin clot 1 Who Needs Anticoagulation
To Clot or Not What s New In Anticoagulation? Anita Ralstin, MS CNS CNP 1 Clotting Cascade 2 Anticoagulant drug targets Heparin XI VIII IX V X VII LMWH II Warfarin Fibrin clot 1 Who Needs Anticoagulation
Thrombosis and Hemostasis
 Thrombosis and Hemostasis Wendy Lim, MD, MSc, FRCPC Associate Professor, Department of Medicine McMaster University, Hamilton, ON Overview To review the important developments in venous thromboembolism
Thrombosis and Hemostasis Wendy Lim, MD, MSc, FRCPC Associate Professor, Department of Medicine McMaster University, Hamilton, ON Overview To review the important developments in venous thromboembolism
Goals 6/6/2014. Stroke Prevention in Atrial Fibrillation: New Oral Anti-Coagulants No More INRs. Ashkan Babaie, MD
 Stroke Prevention in Atrial Fibrillation: New Oral Anti-Coagulants No More INRs Ashkan Babaie, MD Arrhythmia Service Providence Heart Clinic June 8 th, 2014 Goals Discuss the data behind approval of NOACs
Stroke Prevention in Atrial Fibrillation: New Oral Anti-Coagulants No More INRs Ashkan Babaie, MD Arrhythmia Service Providence Heart Clinic June 8 th, 2014 Goals Discuss the data behind approval of NOACs
CCPN SPAF Tool. www.ccpn.ca STROKE PREVENTION IN ATRIAL FIBRILLATION (SPAF): POCKET REFERENCE
 SEPTEMBER 2012 CCPN SPAF Tool STROKE PREVENTION IN ATRIAL FIBRILLATION (SPAF): POCKET REFERENCE Approximately 20% of all strokes are attributable to Atrial Fibrillation (AF). 1 Of these, 20% will result
SEPTEMBER 2012 CCPN SPAF Tool STROKE PREVENTION IN ATRIAL FIBRILLATION (SPAF): POCKET REFERENCE Approximately 20% of all strokes are attributable to Atrial Fibrillation (AF). 1 Of these, 20% will result
3/3/2015. Patrick Cobb, MD, FACP March 2015
 Patrick Cobb, MD, FACP March 2015 I, Patrick Cobb, MD, DO NOT have a financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict
Patrick Cobb, MD, FACP March 2015 I, Patrick Cobb, MD, DO NOT have a financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
Atrial Fibrillation: Stroke and Thromboprophylaxis. Derek Waller
 Atrial Fibrillation: Stroke and Thromboprophylaxis Derek Waller Atrial Fibrillation in the Elderly: Risk of Stroke Framingham study AGE 50-59 60-69 70-79 80-89 Prevalence of AF % Attributable Risk of AF
Atrial Fibrillation: Stroke and Thromboprophylaxis Derek Waller Atrial Fibrillation in the Elderly: Risk of Stroke Framingham study AGE 50-59 60-69 70-79 80-89 Prevalence of AF % Attributable Risk of AF
