NATIONAL DRUG FILE Version 4.0 PHARMACY DATA MANAGEMENT Version 1.0
|
|
|
- Griffin Taylor
- 8 years ago
- Views:
Transcription
1 NATIONAL DRUG FILE Version 4.0 PHARMACY DATA MANAGEMENT Version 1.0 Analysis to Prevent Creation of Supra-therapeutic Dosages RELEASE NOTES April 2011 Department of Veterans Affairs Product Development
2
3 Table of Contents Introduction... 1 National Drug File V Creation of New Fields PSN*4* Display of New Fields PSN*4* Pharmacy Data Management V Create Default Possible Dosage = Yes Create Default Possible Dosage = No April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes i
4 (This page included for two-sided copying.) ii Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
5 Introduction The Analysis to Prevent Creation of Supra-therapeutic Dosages release includes patches PSN*4*261, PSN*4*262, and PSS*1*155. The scope of the Analysis to Prevent Creation of Supra-therapeutic Dosages project is to correct specific Patient Safety Issues and to address specific functional enhancements to the software as approved by the Health Provider Systems Enterprise Systems Manager. These patches provide functionality to prevent the inadvertent creation of supra-therapeutic possible dosages for high risk medications during the dosage creation segment of Pharmacy Data Management and National Data File updates. A supra-therapeutic dosage is one that far exceeds the normal therapeutic range. A sub-therapeutic dosage is one that is far below the normal therapeutic range. Certain drugs with an automatically calculated dosage of one time or two times the base strength that is supra- or sub-therapeutic will be adjusted to not auto-create those default possible dosages during the matching and rematching process. The monthly National Data File update patch will flag those drugs that are identified as supra-therapeutic. Installation instructions are included in the patch descriptions. Below is a list of the applications involved in this project along with their associated patch number: APPLICATION/VERSION PATCH 1. NATIONAL DRUG FILE V. 4.0 PSN*4*261 (released October 2010) 2. NATIONAL DRUG FILE V.4.0 PSN*4* PHARMACY DATA MANAGEMENT V. 1.0 PSS*1*155 The patches should be installed in the order outlined above. April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 1
6 National Drug File V. 4.0 Patches PSN*4*261 and PSN*4*262 include the following enhancements. The items listed below describe the features that will be active upon installation of both National Drug File patches. PSN*4*261 created three new fields in the Data Dictionary for the VA PRODUCT file (#50.68). PSN*4*262 contains changes to the Inquire to National Files [PSNACT] and Inquire to VA Product Info For Local Drug [PSNLOOK] options to include the display of the new fields. NOTE Patch PSN*4*262 is being released in conjunction with Pharmacy Data Management patch PSS*1*155, which has the functionality in place to determine if possible dosages should be auto-created or not during the match/re-match process. 2 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
7 Creation of New Fields PSN*4*261 Patch PSN*4*261 was released in October 2010 in preparation for the Supra-therapeutic Dosages project. It contains the following three new fields that were added to the VA PRODUCT file (#50.68). CREATE DEFAULT POSSIBLE DOSAGE Field (#40) Field Description Indicates whether Possible Dosages should be auto-created based on Dosage Form/Dispense Unit (Default) or if you want to control the Possible Dosage auto-creation process for the Dispense Drugs matched/re-matched to this VA Product. YES Possible Dosages will be auto-created based upon Dosage Form/Dispense Unit combination (Default). NO Possible Dosages will be auto-created according to the rule set by the POSSIBLE DOSAGES TO CREATE (#41) and PACKAGE (#42) fields. POSSIBLE DOSAGES TO CREATE Field (#41) Field Description Indicates which Possible Dosages or if no Possible Dosages should be auto-created for Dispense Drugs matched/re-matched to this VA Product. PACKAGE Field (#42) N No Possible Dosages are auto-created for Dispense Drugs matched to this VA PRODUCT entry during the match/re-match process. O Only 1x Possible Dosage is auto-created for Dispense Drugs Dispense Drugs matched to this VA PRODUCT entry during the match/re-match process. B Both 1x and 2x Possible Dosages are auto-created for Dispense Drugs Dispense Drugs matched to this VA PRODUCT entry during the match/re-match process. Field Description Indicates the Package(s) use for the Possible Dosage(s) auto-created. I Possible Dosages auto-created for Dispense Drugs matched/re-matched to this VA PRODUCT entry will be available for the Inpatient application only. O Possible Dosages auto-created for Dispense Drugs matched/re-matched to this VA PRODUCT entry will be available for the Outpatient application only. IO Possible Dosages auto-created for Dispense Drugs matched/re-matched to this VA PRODUCT entry will be available for both, Outpatient and Inpatient applications. April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 3
8 Display of New Fields PSN*4*262 Patch PSN*4*262 contains changes to the Inquire to National Files [PSNACT] and Inquire to VA Product Info For Local Drug [PSNLOOK] options to include the display of the new fields. Below are screen captures of how the new fields will display to the screen for both options. [PSNLOOK] Auto-Create Default Possible Dosage=Yes Select OPTION NAME: PSNLOOK This option will allow you to look up entries in your local DRUG file. It will display National Drug File software match information. Select DRUG GENERIC NAME: NIFEDIPINE 20MG CAPS CV200 DRUG Generic Name: NIFEDIPINE 20MG CAPS VA Product Name: NIFEDIPINE 20MG CAP VA Generic Name: NIFEDIPINE Dosage Form: CAP,ORAL Strength: 20 Units: MG National Formulary Name: NIFEDIPINE CAP,ORAL VA Print Name: NIFEDIPINE 20MG CAP VA Product Identifier: N0042 Transmit To CMOP: YES VA Dispense Unit: CAP PMIS: NIFEDIPINE - ORAL Active Ingredients: NIFEDIPINE Str: 20 Unt: MG Press Return to Continue: Primary Drug Class: CV200 CS Federal Schedule: None Single/Multi Source Product: Max Single Dose: Max Daily Dose: Max Cumulative Dose: National Formulary Indicator: No Min Single Dose: Min Daily Dose: Override DF Exclude from Dosage Checks: No Auto-Create Default Possible Dosage? Yes Restriction: Refer to PBM/MAP criteria for use of long-acting dihydropyridine calcium antagonists Press Return to Continue: [PSNLOOK] Auto-Create Default Possible Dosage=No, and No Possible Dosages Created National Formulary Indicator: No Override DF Exclude from Dosage Checks: No Auto-Create Default Possible Dosage? No Possible Dosages To Auto-Create: No Possible Dosages 4 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
9 [PSNLOOK] Auto-Create Default Possible Dosage=No, and Create One Possible Dosage National Formulary Indicator: No Override DF Exclude from Dosage Checks: No Auto-Create Default Possible Dosage? No Possible Dosages To Auto-Create: 1x Possible Dosages Package: Inpatient [PSNLOOK] Auto-Create Default Possible Dosage=No, and Create Two Possible Dosages National Formulary Indicator: No Override DF Exclude from Dosage Checks: No Auto-Create Default Possible Dosage? No Possible Dosages To Auto-Create: 2x Possible Dosages Package: Inpatient and Outpatient April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 5
10 [PSNACT] NDF Inquiry by VA Product Name, Auto-Create Possible Dosage=Yes LOOKUP BY (VA) PRODUCT, (N)DC, OR (C)MOP ID? VA VA PRODUCT Select VA PRODUCT NAME: hydrocortisone 1 HYDROCORTISONE 0.1% CREAM,TOP 2 HYDROCORTISONE 0.25% CREAM,TOP 3 HYDROCORTISONE 0.25% LOTION 4 HYDROCORTISONE 0.25%/NEOMYCIN SO4 0.5% CREAM,TOP 5 HYDROCORTISONE 0.5% AEROSOL,TOP Press <RETURN> to see more, '^' to exit this list, OR CHOOSE 1-5: 1 HYDROCORTISONE 0.1% CREAM,TOP VA Product Name: HYDROCORTISONE 0.1% CREAM,TOP VA Generic Name: HYDROCORTISONE Dose Form: CREAM,TOP (Exclude from Dosing Cks) Strength: 0.1 Units: % National Formulary Name: HYDROCORTISONE CREAM,TOP VA Print Name: HYDROCORTISONE 0.1% CREAM VA Product Identifier: H0161 Transmit to CMOP: Yes VA Dispense Unit: GM PMIS: None Active Ingredients: HYDROCORTISONE Strength: 0.1 Units: % Primary VA Drug Class: DE200 Secondary VA Drug Class: CS Federal Schedule: National Formulary Indicator: Yes National Formulary Restriction: Exclude Drg-Drg Interaction Ck: Yes (No check for Drug-Drug Interactions) Override DF Exclude from Dosage Checks: No Auto-Create Default Possible Dosage? Yes Press return to continue or '^' to exit: Auto-Create Default Possible Dosage is set to Yes. NDC: UPN: VA Product Name: HYDROCORTISONE 0.1% CREAM,TOP Manufacturer: MILL MARK PHARM Trade Name: HYDROCORTISONE Route: TOPICAL Package Size: 20 GM Package Type: TUBE NDC: UPN: VA Product Name: HYDROCORTISONE 0.1% CREAM,TOP Manufacturer: MALLARD Trade Name: HYDROCORTISONE Route: TOPICAL Package Size: 454 GM Package Type: JAR 6 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
11 [PSNACT] NDF Inquiry by NDC, Auto-Create Possible Dosages=No, and No Possible Dosages Auto-Created LOOKUP BY (VA) PRODUCT, (N)DC, OR (C)MOP ID? n NDC NDC (N) or UPN (U)? n NDC Enter NDC with or without Dashes (-): OK? Yes// <ENTER> (Yes) NDC: UPN: VA Product Name: PLACEBO TAB Manufacturer: CONSOLIDATED MC Trade Name: PLACEBO Route: ORAL Package Size: 1000 Package Type: BOTTLE VA Product Name: PLACEBO TAB VA Generic Name: PLACEBO Dose Form: TAB Strength: Units: National Formulary Name: PLACEBO TAB VA Print Name: PLACEBO CAP/TAB VA Product Identifier: P0256 Transmit to CMOP: Yes VA Dispense Unit: CAP/TAB PMIS: None Active Ingredients: LACTOSE Strength: 10 Units: %WW CELLULOSE Strength: 3 Units: %WW STARCH Strength: 87 Units: %WW Primary VA Drug Class: XX000 Secondary VA Drug Class: CS Federal Schedule: Press return to continue or '^' to exit: National Formulary Indicator: No National Formulary Restriction: Exclude Drg-Drg Interaction Ck will only display if it is set to Yes. Override DF Exclude from Dosage Checks: Yes (No dosage checks performed) Auto-Create Default Possible Dosage? No Possible Dosages To Auto-Create: No Possible Dosages Press return to continue or '^' to exit: Auto-Create Default Possible Dosage is set to No and no Possible Dosages are auto-created. April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 7
12 [PSNACT] NDF Inquiry by CMOP ID Number, Auto-Create Possible Dosages=No, and Possible Dosages Auto-Created for Inpatient and Outpatient Packages LOOKUP BY (VA) PRODUCT, (N)DC, OR (C)MOP ID? c CMOP ID CMOP ID: c0504 COD LIVER OIL VA Product Name: COD LIVER OIL VA Generic Name: COD LIVER OIL Dose Form: OIL (Exclude from Dosing Cks) Strength: Units: National Formulary Name: COD LIVER OIL OIL VA Print Name: COD LIVER OIL VA Product Identifier: C0504 Transmit to CMOP: Yes VA Dispense Unit: ML PMIS: None Active Ingredients: COD LIVER OIL Strength: Units: Primary VA Drug Class: VT801 Secondary VA Drug Class: CS Federal Schedule: National Formulary Indicator: No National Formulary Restriction: Override DF Exclude from Dosage Checks: Yes (Dosage Checks shall be performed) Auto-Create Default Possible Dosage? No Possible Dosages To Auto-Create: 1x and 2x Possible Dosages Package: Both Inpatient and Outpatient Press return to continue or '^' to exit: NDC: UPN: VA Product Name: COD LIVER OIL Manufacturer: WALMEAD Trade Name: COD LIVER OIL Route: ORAL Package Size: 120 ML Package Type: BOTTLE Auto-Create Default Possible Dosage is set to No and Possible Dosages are auto-created for both Inpatient and Outpatient packages. 8 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
13 Pharmacy Data Management V. 1.0 Patch PSS*1*155 retires the Auto Create Dosages [PSS DOSAGE CONVERSION] option. This option is retired because, if it is run inadvertently, it will delete all existing Possible Dosages and Local Possible Dosages and will reset the Possible Dosages back to NDF settings. The changes that were entered locally by the site for Possible Dosages and Local Possible Dosages will be lost. Upon patch installation, this option will be removed from the Dosages [PSS DOSAGES MANAGEMENT] menu. This patch uses the three new fields that were added to the VA PRODUCT file (#50.68) as part of patch PSN*4*261. These fields will be used during the match/rematch process of the Drug Enter/Edit [PSS DRUG ENTER/EDIT] and the Enter/Edit Dosages [PSS EDIT DOSAGES] options to determine if possible dosages should be auto-created or not. The three fields are: CREATE DEFAULT POSSIBLE DOSAGE field (#40) POSSIBLE DOSAGES TO CREATE field (#41) PACKAGE field (#42) When the CREATE DEFAULT POSSIBLE DOSAGE field is YES, then the existing functionality of auto-creating possible dosages will be retained. When this field is NO, it will be used in combination with the value in the POSSIBLE DOSAGES TO CREATE field to determine the auto-creation of possible dosages. The following conditions will apply when the CREATE DEFAULT POSSIBLE DOSAGE field is NO : If POSSIBLE DOSAGES TO CREATE field is NO, no possible dosages will be auto-created and the following message will be displayed: Due to National Drug File settings no possible dosages were auto-created. If POSSIBLE DOSAGES TO CREATE field is O, 1x possible dosage will be auto-created for the package specified by the new PACKAGE field and the following message will be displayed: Due to National Drug File settings only ONE possible dosage will be auto-created. If other dosages are needed, create POSSIBLE DOSAGES or LOCAL POSSIBLE DOSAGES as appropriate. If POSSIBLE DOSAGES TO CREATE field is B, 1x and 2x possible dosages will be autocreated for the package specified by the new PACKAGE field. April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 9
14 Create Default Possible Dosage = Yes The following screen captures illustrate the conditions when the CREATE DEFAULT POSSIBLE DOSAGE field in the VA PRODUCT file is set to Yes. Drug Enter/Edit option -- Message displayed when no possible dosages are auto-created Do you wish to match/rematch to NATIONAL DRUG file? No// YES (Yes) Deleting Possible Dosages... Match local drug INSULIN NPH U-100 INJ ORDER UNIT: VI DISPENSE UNITS/ORDER UNITS: 1 DISPENSE UNIT: I will try to match NDC: to NDF. I will attempt to match the NDCs from your SYNONYMS. ORDER UNIT: VI Match made with INSULIN NPH U-100 INJ Now select VA Product Name 18 INSULIN NPH HUMAN 100 U/ML INJ HUMULIN N INJ HS501 I INSULIN NPH HUMAN 100 U/ML INJ INNOLET 3ML INJ HS501 I INSULIN NPH HUMAN 100 U/ML INJ NOVOLIN N INJ HS501 I0161 Enter your choice or press return to continue: 18 Is this a match < Reply Y, N or press return to continue > : y CHOOSE FROM: 1 10 ML VIAL 2 OTHER OTHER Enter Package Size & Type Combination: 1 Local drug INSULIN NPH U-100 INJ matches INSULIN NPH HUMAN 100 U/ML INJ HUMULIN N PACKAGE SIZE: 10 ML PACKAGE TYPE: VIAL < Enter "Y" for yes > < Enter "N" for no > OK? : Y LOCAL DRUG NAME: INSULIN NPH U-100 INJ ORDER UNIT: VI DISPENSE UNITS/ORDER UNITS: 1 DISPENSE UNIT: VA PRODUCT NAME: INSULIN NPH HUMAN 100 U/ML INJ HUMULIN N VA PRINT NAME: INSULIN NPH HUMAN 100 UNIT/ML HUMULIN N CMOP ID: I0160 VA DISPENSE UNIT: VI MARKABLE FOR CMOP: YES PACKAGE SIZE: 10 ML PACKAGE TYPE: VIAL VA CLASS: HS501 INSULIN CS FEDERAL SCHEDULE: INGREDIENTS: INSULIN,NPH,HUMAN/rDNA 100 UNT/ML NATIONAL FORMULARY INDICATOR: NO NATIONAL FORMULARY RESTRICTION: < Enter "Y" for yes, "N" for no > Is this a match? Y... continued on next page Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
15 You have just VERIFIED this match and MERGED the entry. Resetting Possible Dosages.. Press Return to continue: Just a reminder...you are editing INSULIN NPH U-100 INJ. Strength from National Drug File match => 100 Strength currently in the Drug File => 100 UNT/ML Strength => 100 Unit => Press Return to continue,'^' to exit: POSSIBLE DOSAGES: LOCAL POSSIBLE DOSAGES: Due to National Drug File settings no possible dosages were auto-created. Do you want to manually enter possible dosages? N// YES Changing the strength will update all possible dosages for this Drug. STRENGTH: 100// Select DISPENSE UNITS PER DOSE:? You may enter a new POSSIBLE DOSAGES, if you wish Type a Number between 0 and , 4 Decimal Digits Select DISPENSE UNITS PER DOSE: 1 Are you adding '1' as a new POSSIBLE DOSAGES (the 1ST for this DRUG)? No// Y (Yes) Dosage = 100 POSSIBLE DOSAGES DOSE: 100// (No Editing) DISPENSE UNITS PER DOSE: 1// 0.1 PACKAGE: IO BCMA UNITS PER DOSE: Select DISPENSE UNITS PER DOSE: NOTE The prompt Do you want to manually enter possible dosages? N// is displayed only when no possible dosages were auto-created. April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 11
16 Drug Enter/Edit option -- Message displayed when 1 possible dosage is auto-created Do you wish to match/rematch to NATIONAL DRUG file? No// YES (Yes) Deleting Possible Dosages... Match local drug LOMUSTINE 100MG CAP ORDER UNIT: BT DISPENSE UNITS/ORDER UNITS: 20 DISPENSE UNIT: I will try to match NDC: to NDF. Local drug LOMUSTINE 100MG CAP matches LOMUSTINE 100MG CAP PACKAGE SIZE: 20 PACKAGE TYPE: BOTTLE Is this a match? Enter Yes or No: YES// YES LOCAL DRUG NAME: LOMUSTINE 100MG CAP ORDER UNIT: BT DISPENSE UNITS/ORDER UNITS: 20 DISPENSE UNIT: VA PRODUCT NAME: LOMUSTINE 100MG CAP VA PRINT NAME: LOMUSTINE 100MG CAP VA DISPENSE UNIT: CAP PACKAGE SIZE: 20 PACKAGE TYPE: BOTTLE VA CLASS: AN100 ANTINEOPLASTICS,ALKYLATING AGENTS CS FEDERAL SCHEDULE: INGREDIENTS: LOMUSTINE 100 MG NATIONAL FORMULARY INDICATOR: YES NATIONAL FORMULARY RESTRICTION: CMOP ID: L0055 MARKABLE FOR CMOP: YES < Enter "Y" for yes, "N" for no > Is this a match? Y You have just VERIFIED this match and MERGED the entry. Resetting Possible Dosages.. Press Return to continue: Just a reminder...you are editing LOMUSTINE 100MG CAP. Strength from National Drug File match => 100 MG Strength currently in the Drug File => 100 MG Strength => 100 Unit => MG Press Return to continue,'^' to exit: POSSIBLE DOSAGES: DISPENSE UNITS PER DOSE: 1 DOSE: 100MG PACKAGE: IO... continued on next page Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
17 LOCAL POSSIBLE DOSAGES: Due to National Drug File settings only ONE possible dosage was auto-created. If other dosages are needed, create POSSIBLE DOSAGES or LOCAL POSSIBLE DOSAGES as appropriate. Do you want to edit the dosages? N// YES Changing the strength will update all possible dosages for this Drug. STRENGTH: 100// Select DISPENSE UNITS PER DOSE:? Answer with POSSIBLE DOSAGES DISPENSE UNITS PER DOSE Choose from: IO You may enter a new POSSIBLE DOSAGES, if you wish Type a Number between 0 and , 4 Decimal Digits Select DISPENSE UNITS PER DOSE: Drug Enter/Edit option -- Message displayed when 2 possible dosages are auto-created Do you wish to match/rematch to NATIONAL DRUG file? No// Y (Yes) Deleting Possible Dosages... Match local drug DACARBAZINE 200MG INJ ORDER UNIT: BX DISPENSE UNITS/ORDER UNITS: 12 DISPENSE UNIT: I will try to match NDC: to NDF. Local drug DACARBAZINE 200MG INJ matches DACARBAZINE 200MG/VIL INJ PACKAGE SIZE: 12 X 200 MG PACKAGE TYPE: VIAL Is this a match? Enter Yes or No: YES// YES LOCAL DRUG NAME: DACARBAZINE 200MG INJ ORDER UNIT: BX DISPENSE UNITS/ORDER UNITS: 12 DISPENSE UNIT: VA PRODUCT NAME: DACARBAZINE 200MG/VIL INJ MARKABLE FOR CMOP: NOT MARKED PACKAGE SIZE: 12 X 200 MG PACKAGE TYPE: VIAL VA CLASS: AN900 ANTINEOPLASTIC,OTHER CS FEDERAL SCHEDULE: INGREDIENTS: DACARBAZINE 200 NATIONAL FORMULARY INDICATOR: YES NATIONAL FORMULARY RESTRICTION:... continued on next page... April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 13
18 < Enter "Y" for yes, "N" for no > Is this a match? Y You have just VERIFIED this match and MERGED the entry. Resetting Possible Dosages.. Press Return to continue: Just a reminder...you are editing DACARBAZINE 200MG INJ. Strength from National Drug File match => 200 Strength currently in the Drug File => 200 MG/VIAL Strength => 200 Unit => Press Return to continue,'^' to exit: POSSIBLE DOSAGES: DISPENSE UNITS PER DOSE: 1 DOSE: 200MG/1VIAL PACKAGE: IO DISPENSE UNITS PER DOSE: 2 DOSE: 400MG/2VIAL PACKAGE: IO LOCAL POSSIBLE DOSAGES: Due to National Drug File settings TWO possible dosages were auto-created. Do you want to edit the dosages? N// 14 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
19 Create Default Possible Dosage = No The following screen captures illustrate the conditions when the CREATE DEFAULT POSSIBLE DOSAGE field in the VA PRODUCT file is set to No. Drug Enter/Edit option -- Message displayed when NDF is set to not auto-create Possible Dosages Do you wish to match/rematch to NATIONAL DRUG file? No// Just a reminder...you are editing LOMUSTINE 10MG CAP (No) Strength from National Drug File match => 10 MG Strength currently in the Drug File => 10 MG Strength => 10 Unit => MG POSSIBLE DOSAGES: LOCAL POSSIBLE DOSAGES: This drug has been set within the National Drug File to not auto create possible dosages. Do you want to manually enter possible dosages? N// Drug Enter/Edit option -- Message displayed when NDF is set to auto-create 1 Possible Dosage Do you wish to match/rematch to NATIONAL DRUG file? No// Just a reminder...you are editing LOMUSTINE 10MG CAP (No) This drug can have Possible Dosages, but currently does not have any. This drug has been set within the National Drug File to auto create only one possible dosage. Create Possible Dosages for this drug? N// YES Resetting Possible Dosages.. Due to National Drug File settings only ONE possible dosage was auto-created. If other dosages are needed, create POSSIBLE DOSAGES or LOCAL POSSIBLE DOSAGES as appropriate. Press Return to continue: Strength from National Drug File match => 10 MG Strength currently in the Drug File => 10 MG Strength => 10 Unit => MG POSSIBLE DOSAGES: DISPENSE UNITS PER DOSE: 1 DOSE: 10 MG PACKAGE: IO LOCAL POSSIBLE DOSAGES: This drug has been set within the National Drug File to auto create only one possible dosage. Do you want to edit the dosages? N// April 2011 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes 15
20 Drug Enter/Edit option -- Message displayed when NDF is set to auto-create 2 Possible Dosages Do you wish to match/rematch to NATIONAL DRUG file? No// Just a reminder...you are editing LOMUSTINE 10MG CAP (No) This drug can have Possible Dosages, but currently does not have any. This drug has been set within the National Drug File to auto create two possible dosages. Create Possible Dosages for this drug? N// YES Resetting Possible Dosages.. Due to National Drug File settings TWO possible dosages were auto-created. Press Return to continue: Strength from National Drug File match => 10 MG Strength currently in the Drug File => 10 MG Strength => 10 Unit => MG POSSIBLE DOSAGES: DISPENSE UNITS PER DOSE: 1 DOSE: 10 MG PACKAGE: IO DISPENSE UNITS PER DOSE: 2 DOSE: 20 MG PACKAGE: IO LOCAL POSSIBLE DOSAGES: This drug has been set within the National Drug File to auto create two possible dosages. Do you want to edit the dosages? N// NOTE When using the Enter/Edit Dosages option for drugs that have been matched/rematched, the functionality and messages are similar to the Drug Enter/Edit option information illustrated above. 16 Analysis to Prevent Creation of Supra-therapeutic Dosages Release Notes April 2011
Medication Safety- Insulin U-500 Safety Enhancements. Item: AL09-15 March 3, 2009. Specific Incident:
 AL09-15 March 3, 2009 Item: Specific Incident: General Information: Medication Safety- Insulin U-500 Safety Enhancements South West VA CMOP received an order for 40 units of U-500 insulin and questioned
AL09-15 March 3, 2009 Item: Specific Incident: General Information: Medication Safety- Insulin U-500 Safety Enhancements South West VA CMOP received an order for 40 units of U-500 insulin and questioned
DRUG ACCOUNTABILITY/ INVENTORY INTERFACE
 DRUG ACCOUNTABILITY/ INVENTORY INTERFACE USER MANUAL Version 3.0 October 1997 (Revised April 2008) Department of Veterans Affairs VistA Health Systems Design & Development Revision History Each time this
DRUG ACCOUNTABILITY/ INVENTORY INTERFACE USER MANUAL Version 3.0 October 1997 (Revised April 2008) Department of Veterans Affairs VistA Health Systems Design & Development Revision History Each time this
Pharmacy Data Management
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Data Management (PDM) User Manual Version 1.0 Information Technology Support Center Division of Information Resource Albuquerque, New Mexico Table of Contents
RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Data Management (PDM) User Manual Version 1.0 Information Technology Support Center Division of Information Resource Albuquerque, New Mexico Table of Contents
PHARMACY DATA MANAGEMENT
 PHARMACY DATA MANAGEMENT USER MANUAL Version 1.0 September 1997 (Revised December 2013) Department of Veterans Affairs Product Development Revision History Each time this manual is updated, the Title
PHARMACY DATA MANAGEMENT USER MANUAL Version 1.0 September 1997 (Revised December 2013) Department of Veterans Affairs Product Development Revision History Each time this manual is updated, the Title
PHARMACY DATA MANAGEMENT
 PHARMACY DATA MANAGEMENT USER MANUAL Version 1.0 September 1997 (Revised December 2008) Department of Veterans Affairs Office of Information & Technology Office of Enterprise Development Revision History
PHARMACY DATA MANAGEMENT USER MANUAL Version 1.0 September 1997 (Revised December 2008) Department of Veterans Affairs Office of Information & Technology Office of Enterprise Development Revision History
MEDICATION ORDER CHECK HEALTHCARE APPLICATION (MOCHA) V1.0
 MEDICATION ORDER CHECK HEALTHCARE APPLICATION (MOCHA) V1.0 RELEASE NOTES April 2011 Department of Veterans Affairs Product Development (This page included for two-sided copying.) Table of Contents 1. Introduction...
MEDICATION ORDER CHECK HEALTHCARE APPLICATION (MOCHA) V1.0 RELEASE NOTES April 2011 Department of Veterans Affairs Product Development (This page included for two-sided copying.) Table of Contents 1. Introduction...
RN Finish Guide. RN Finish an Order. 1. Click the Resources tab. 2. Select RPMS Session from the RPMS menu (Figure 1).
 RN Finish an Order 1. Click the Resources tab. 2. Select RPMS Session from the RPMS menu (Figure 1). Figure 1: Resources tab, RPMS Session logon 3. Log on to RPMS: a. At the ACCESS CODE prompt, type your
RN Finish an Order 1. Click the Resources tab. 2. Select RPMS Session from the RPMS menu (Figure 1). Figure 1: Resources tab, RPMS Session logon 3. Log on to RPMS: a. At the ACCESS CODE prompt, type your
Inpatient Pharmacy Order Processing. January 2012
 Inpatient Pharmacy Order Processing January 2012 1 Learning Objectives Explain the difference between inpatient and outpatient medication orders Explain why inpatient and outpatient medication orders must
Inpatient Pharmacy Order Processing January 2012 1 Learning Objectives Explain the difference between inpatient and outpatient medication orders Explain why inpatient and outpatient medication orders must
VISTA TO MOCHA V1.0 INTERFACE
 VISTA TO MOCHA V1.0 INTERFACE INSTALLATION GUIDE PSS*1.0*136 April 2011 Department of Veterans Affairs Product Development Revision History Each time this manual is updated, the Title Page lists the new
VISTA TO MOCHA V1.0 INTERFACE INSTALLATION GUIDE PSS*1.0*136 April 2011 Department of Veterans Affairs Product Development Revision History Each time this manual is updated, the Title Page lists the new
Parenteral Dosage of Drugs
 Chapter 11 Parenteral Dosage of Drugs Parenteral Route of administration other than gastrointestinal Intramuscular (IM) Subcutaneous (SC) Intradermal (ID) IV Parenteral Most medications prepared in liquid
Chapter 11 Parenteral Dosage of Drugs Parenteral Route of administration other than gastrointestinal Intramuscular (IM) Subcutaneous (SC) Intradermal (ID) IV Parenteral Most medications prepared in liquid
Dear Valued Customer,
 Dear Valued Customer, Welcome to the comprehensive one-stop pharmacy management solution. The Digital Rx Pharmacy Management System features include: Retail Pharmacy module; Long-Term Care; Retail Point-of-Sale;
Dear Valued Customer, Welcome to the comprehensive one-stop pharmacy management solution. The Digital Rx Pharmacy Management System features include: Retail Pharmacy module; Long-Term Care; Retail Point-of-Sale;
State Prescription Monitoring Program (SPMP) Patch PSO*7*408. Release Notes
 State Prescription Monitoring Program (SPMP) Patch PSO*7*408 August 2014 Department of Veterans Affairs Product Development Table of Contents 1. Introduction... 1 1.1 Purpose... 1 1.2 Menu with Options...
State Prescription Monitoring Program (SPMP) Patch PSO*7*408 August 2014 Department of Veterans Affairs Product Development Table of Contents 1. Introduction... 1 1.1 Purpose... 1 1.2 Menu with Options...
Pharmacy Operating Guidelines & Information
 Pharmacy Operating Guidelines & Information RxAMERICA PHARMACY BENEFIT MANAGEMENT Pharmacy Operating Guidelines & Information Table of Contents I. Quick Reference List...3 C. D. E. Important Phone Numbers...
Pharmacy Operating Guidelines & Information RxAMERICA PHARMACY BENEFIT MANAGEMENT Pharmacy Operating Guidelines & Information Table of Contents I. Quick Reference List...3 C. D. E. Important Phone Numbers...
How to Consolidate Members
 How to Consolidate Members August 2008 (last update) SOS consolidation feature, allows the user, with the right security, to move data from one member to another. Then the original member is deleted. To
How to Consolidate Members August 2008 (last update) SOS consolidation feature, allows the user, with the right security, to move data from one member to another. Then the original member is deleted. To
Members covered under the Extended Family Planning (EFP) plan may not be eligible for all services. EFP is not a comprehensive benefit package.
 PHARMACEUTICALS NDC BILLING REQUIREMENTS POLICY This policy applies to Participating and Non-participating providers who render services to Neighborhood Health Plan of Rhode Island (Neighborhood) subscribers
PHARMACEUTICALS NDC BILLING REQUIREMENTS POLICY This policy applies to Participating and Non-participating providers who render services to Neighborhood Health Plan of Rhode Island (Neighborhood) subscribers
TECHNICIAN S USER MANUAL
 OUTPATIENT PHARMACY TECHNICIAN S USER MANUAL Version 7.0 December 1997 (Revised May 2013) Department of Veterans Affairs Product Development Revision History Each time this manual is updated, the Title
OUTPATIENT PHARMACY TECHNICIAN S USER MANUAL Version 7.0 December 1997 (Revised May 2013) Department of Veterans Affairs Product Development Revision History Each time this manual is updated, the Title
STATEMENT OF BELINDA J
 STATEMENT OF BELINDA J. FINN ASSISTANT INSPECTOR GENERAL FOR AUDITS AND EVALUATIONS OFFICE OF INSPECTOR GENERAL DEPARTMENT OF VETERANS AFFAIRS BEFORE THE SUBCOMMITTEE ON HEALTH COMMITTEE ON VETERANS AFFAIRS
STATEMENT OF BELINDA J. FINN ASSISTANT INSPECTOR GENERAL FOR AUDITS AND EVALUATIONS OFFICE OF INSPECTOR GENERAL DEPARTMENT OF VETERANS AFFAIRS BEFORE THE SUBCOMMITTEE ON HEALTH COMMITTEE ON VETERANS AFFAIRS
PHYSICIAN-ADMINISTERED MEDICATION: BILLING REQUIREMENTS
 PHYSICIAN-ADMINISTERED MEDICATION: BILLING REQUIREMENTS Policy For physician-administered medication, effective April 1, 2012, Neighborhood Health Plan requires National Drug Codes (NDC) on claims in addition
PHYSICIAN-ADMINISTERED MEDICATION: BILLING REQUIREMENTS Policy For physician-administered medication, effective April 1, 2012, Neighborhood Health Plan requires National Drug Codes (NDC) on claims in addition
Billing with National Drug Codes (NDCs) Frequently Asked Questions
 Billing with National Drug Codes (NDCs) Frequently Asked Questions NDC Overview Converting HCPCS/CPT Units to NDC Units Submitting NDCs on Professional Claims Reimbursement Details For More Information
Billing with National Drug Codes (NDCs) Frequently Asked Questions NDC Overview Converting HCPCS/CPT Units to NDC Units Submitting NDCs on Professional Claims Reimbursement Details For More Information
Ensemble Interface Engine Auto-Finish Setup
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Ensemble Interface Engine Auto-Finish Setup EHR Patch 9 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico
RESOURCE AND PATIENT MANAGEMENT SYSTEM Ensemble Interface Engine Auto-Finish Setup EHR Patch 9 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico
Pharmacy Auto Refill System
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Auto Refill System (BEX) Version 1.0 Information Technology Support Center Division of Information Resources Albuquerque, New Mexico Preface This document
RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Auto Refill System (BEX) Version 1.0 Information Technology Support Center Division of Information Resources Albuquerque, New Mexico Preface This document
Electronic Health Record
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Electronic Health Record (EHR) Version 1.1 Patch 11 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico Table
RESOURCE AND PATIENT MANAGEMENT SYSTEM Electronic Health Record (EHR) Version 1.1 Patch 11 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico Table
PRESCRIPTION MONITORING PROGRAM (PMP)
 PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
Average Sale Price (ASP) Data Collection Template/Data Validation Macro User Manual
 Centers for Medicare & Medicaid Services Center for Medicare (CM) Hospital and Ambulatory Policy Group 7500 Security Blvd Baltimore, MD 21244-1850 Average Sale Price (ASP) Data Collection Template/Data
Centers for Medicare & Medicaid Services Center for Medicare (CM) Hospital and Ambulatory Policy Group 7500 Security Blvd Baltimore, MD 21244-1850 Average Sale Price (ASP) Data Collection Template/Data
Summary of New Plans and Plan Sponsor changes Effective January 1, 2011
 Medco Health Solutions, Inc. 100 Parsons Pond Drive Franklin Lakes, NJ 07417 www.medco.com/rph Summary of New Plans and Plan Sponsor changes Effective January 1, 2011 New Plan Sponsors Plan sponsor: See
Medco Health Solutions, Inc. 100 Parsons Pond Drive Franklin Lakes, NJ 07417 www.medco.com/rph Summary of New Plans and Plan Sponsor changes Effective January 1, 2011 New Plan Sponsors Plan sponsor: See
RHODE ISLAND PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL
 RHODE ISLAND PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL Effective Date: October 13, 2014 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 Fax: 866-282-7076 RIRxReport@otech.com
RHODE ISLAND PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL Effective Date: October 13, 2014 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 Fax: 866-282-7076 RIRxReport@otech.com
MEDITECH TRAINING MANUAL PHARMACY APPLICATION. General Information
 MEDITECH PHARMACY TRAINING MANUAL ELLSWORTH COUNTY MEDICAL CENTER September 2004 MEDITECH TRAINING MANUAL PHARMACY APPLICATION General Information Meditech is a fully integrated system consisting of applications
MEDITECH PHARMACY TRAINING MANUAL ELLSWORTH COUNTY MEDICAL CENTER September 2004 MEDITECH TRAINING MANUAL PHARMACY APPLICATION General Information Meditech is a fully integrated system consisting of applications
OFFICE OF COMMUNICATIONS. Date: September 19, 2014. To: All Medicare Part D Plans. Director, Web and New Media Group
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Office of Communications 7500 Security Boulevard, Mail Stop S1-01-26 Baltimore, Maryland 21244-1850 OFFICE OF COMMUNICATIONS
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Office of Communications 7500 Security Boulevard, Mail Stop S1-01-26 Baltimore, Maryland 21244-1850 OFFICE OF COMMUNICATIONS
Using Electronic Medical Records Data for Health Services Research Case Study: Development and Use of Ambulatory Adverse Event Trigger Tools
 Using Electronic Medical Records Data for Health Services Research Case Study: Development and Use of Ambulatory Adverse Event Trigger Tools Hillary Mull VA Boston Healthcare System Boston University School
Using Electronic Medical Records Data for Health Services Research Case Study: Development and Use of Ambulatory Adverse Event Trigger Tools Hillary Mull VA Boston Healthcare System Boston University School
Meaningful use. Meaningful data. Meaningful care. The 3M Healthcare Data Dictionary (HDD): Implemented with a data warehouse
 Meaningful use. Meaningful data. Meaningful care. The 3M Healthcare Data Dictionary (HDD): Implemented with a data warehouse Executive summary A large academic research institution uses the 3M Healthcare
Meaningful use. Meaningful data. Meaningful care. The 3M Healthcare Data Dictionary (HDD): Implemented with a data warehouse Executive summary A large academic research institution uses the 3M Healthcare
Using WHI Current Medication Data. Jill Shupe May 6, 2009
 Using WHI Current Medication Data Jill Shupe May 6, 2009 Current Medications Presentation Outline Data Collection Medi-Span Data Files Examples Current Medication (Form 44) Data Collection When and on
Using WHI Current Medication Data Jill Shupe May 6, 2009 Current Medications Presentation Outline Data Collection Medi-Span Data Files Examples Current Medication (Form 44) Data Collection When and on
RESOURCE AND PATIENT MANAGEMENT SYSTEM. e-prescribing (BEPR) Pre-Deployment Guide. Version 1.0 October 2011
 RESOURCE AND PATIENT MANAGEMENT SYSTEM e-prescribing (BEPR) Version 1.0 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico Table of Contents 1.0
RESOURCE AND PATIENT MANAGEMENT SYSTEM e-prescribing (BEPR) Version 1.0 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico Table of Contents 1.0
IDEXX Cornerstone* Single Location Inventory 8.3
 IDEXX Cornerstone* Single Location Inventory 8.3 Proprietary Rights Notice Information in this document is subject to change without notice. Companies, names and data used in examples are fictitious unless
IDEXX Cornerstone* Single Location Inventory 8.3 Proprietary Rights Notice Information in this document is subject to change without notice. Companies, names and data used in examples are fictitious unless
Eclipse Palm Sales Force Automation. Release 8.6.2 (Eterm)
 Eclipse Palm Sales Force Automation Release 8.6.2 (Eterm) Legal Notices 2007 Activant Solutions Inc. All rights reserved. Unauthorized reproduction is a violation of applicable laws. Activant and the Activant
Eclipse Palm Sales Force Automation Release 8.6.2 (Eterm) Legal Notices 2007 Activant Solutions Inc. All rights reserved. Unauthorized reproduction is a violation of applicable laws. Activant and the Activant
Electronic Health Record (RPMS-EHR)
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Electronic Health Record (RPMS-EHR) Version 1.1 Patch 7 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico
RESOURCE AND PATIENT MANAGEMENT SYSTEM Electronic Health Record (RPMS-EHR) Version 1.1 Patch 7 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico
FDA Medication Guides Project
 FDA Medication Guides Project Java Component (Automatic Printing) INSTALLATION GUIDE XU*8*566 PSN*4*264 PSO*7*367 PSX*2*70 PSS*1*177 PSO*7*428 March 2012 (Revised December 2014) Department of Veterans
FDA Medication Guides Project Java Component (Automatic Printing) INSTALLATION GUIDE XU*8*566 PSN*4*264 PSO*7*367 PSX*2*70 PSS*1*177 PSO*7*428 March 2012 (Revised December 2014) Department of Veterans
Table of Contents. User Request for Access... 3. User Login... 3. Upload a Data File... 4
 Table of Contents User Request for Access... 3 User Login... 3 Upload a Data File... 4 Add Patient Records Direct Dispenser Users... 5 Pharmacy... 8 Search for Patient Records... 10 Search for Error Records...
Table of Contents User Request for Access... 3 User Login... 3 Upload a Data File... 4 Add Patient Records Direct Dispenser Users... 5 Pharmacy... 8 Search for Patient Records... 10 Search for Error Records...
COMPUTERIZED PROVIDER ORDER ENTRY: AD-HOC ORDERS
 COMPUTERIZED PROVIDER ORDER ENTRY: AD-HOC ORDERS Find Field Enter the desired order in the Find field. You may press Enter or select the Binoculars Icon to view all existing orderables. Orderable This
COMPUTERIZED PROVIDER ORDER ENTRY: AD-HOC ORDERS Find Field Enter the desired order in the Find field. You may press Enter or select the Binoculars Icon to view all existing orderables. Orderable This
Supply Management Getting Started Guide. Product Name: Pharmaserv Release Version: General Release 6.3.0
 Product Name: Pharmaserv Release Version: General Release 6.3.0 1008612 Copyright 2013 McKesson Corporation and/or one of its subsidiaries. All Rights Reserved. This documentation is an unpublished work
Product Name: Pharmaserv Release Version: General Release 6.3.0 1008612 Copyright 2013 McKesson Corporation and/or one of its subsidiaries. All Rights Reserved. This documentation is an unpublished work
In support of a number of our Plan Sponsors, Medco offers the attached year-end communications in preparation for 2012.
 LT42423M Medco 100 Parsons Pond Drive Franklin Lakes, NJ 07417 www.medco.com/rph December 2011 Dear Provider: In support of a number of our Plan Sponsors, Medco offers the attached year-end communications
LT42423M Medco 100 Parsons Pond Drive Franklin Lakes, NJ 07417 www.medco.com/rph December 2011 Dear Provider: In support of a number of our Plan Sponsors, Medco offers the attached year-end communications
DECISION SUPPORT SYSTEM (DSS) FY 2016 EXTRACTS USER MANUAL
 DECISION SUPPORT SYSTEM (DSS) FY 2016 EXTRACTS USER MANUAL Software Version 3.0 Revised: November 2015 Department of Veterans Affairs Office of Information and Technology (OIT) Product Development Revision
DECISION SUPPORT SYSTEM (DSS) FY 2016 EXTRACTS USER MANUAL Software Version 3.0 Revised: November 2015 Department of Veterans Affairs Office of Information and Technology (OIT) Product Development Revision
Prescribing a new medication
 Pulse Rx Solutions Prescribing a new medication The core of Pulse s Rx solution, the Rx Pad is designed to make the prescribing of patient medications straightforward, precise, and convenient. pulseinc.com
Pulse Rx Solutions Prescribing a new medication The core of Pulse s Rx solution, the Rx Pad is designed to make the prescribing of patient medications straightforward, precise, and convenient. pulseinc.com
Billing with National Drug Codes (NDCs) Frequently Asked Questions
 Billing with National Drug Codes (NDCs) Frequently Asked Questions NDC Overview Converting HCPCS/CPT Units to NDC Units Submitting NDCs on Professional/Ancillary Claims Reimbursement Details For More Information
Billing with National Drug Codes (NDCs) Frequently Asked Questions NDC Overview Converting HCPCS/CPT Units to NDC Units Submitting NDCs on Professional/Ancillary Claims Reimbursement Details For More Information
IT requirement specification Safety alert. Oral methotrexate 2.5mg and 10mg tablets
 IT requirement specification Safety alert Oral methotrexate 2.5mg and 10mg tablets Synopsis This document sets out the IT requirements for addressing methotrexate-related patient safety incidents that
IT requirement specification Safety alert Oral methotrexate 2.5mg and 10mg tablets Synopsis This document sets out the IT requirements for addressing methotrexate-related patient safety incidents that
Provider Notification
 Provider Notification Date of Notification June 1, 2012 Revision Date N/A Plans Affected All Lines of Business Subject Notice of Change to Billing Requirements for Drugs Administered in Outpatient Clinical
Provider Notification Date of Notification June 1, 2012 Revision Date N/A Plans Affected All Lines of Business Subject Notice of Change to Billing Requirements for Drugs Administered in Outpatient Clinical
Electronic Medical Record (EMR) Safety Results of CAH Testing. Tom Johns, PharmD Shands at the University of Florida
 Electronic Medical Record (EMR) Safety Results of CAH Testing Tom Johns, PharmD Shands at the University of Florida Vendors Evaluated CPSI Healthland Cerner Test Patients Test Patient A Age: 76 y/o Sex:
Electronic Medical Record (EMR) Safety Results of CAH Testing Tom Johns, PharmD Shands at the University of Florida Vendors Evaluated CPSI Healthland Cerner Test Patients Test Patient A Age: 76 y/o Sex:
Release Notes Version 200.0.0.58816(R7.5) Release Date: 31/01/2014
 Release Notes Version 200.0.0.58816(R7.5) Release Date: 31/01/2014 1 Contents.Net and Pro Flow... 3 Qicscript.Net to Qicscript Plus Importer... 3 Follow Up To/Do Lists:... 3 How to Create a Follow Up/To
Release Notes Version 200.0.0.58816(R7.5) Release Date: 31/01/2014 1 Contents.Net and Pro Flow... 3 Qicscript.Net to Qicscript Plus Importer... 3 Follow Up To/Do Lists:... 3 How to Create a Follow Up/To
Participate. to inpatients. Yes No. Team. Leader. Team. Name Degree(s) Title Department Address 1 Address 2. City State Zip Office Phone Fax E mail
 This printable application is provided for your reference only. Please enter yourr application information online at www.onepenonepatient.org. Completed applications must be submitted on or before August
This printable application is provided for your reference only. Please enter yourr application information online at www.onepenonepatient.org. Completed applications must be submitted on or before August
Laser Printed Prescription Labels with PMI Sheets
 Laser Printed Prescription Labels with PMI Sheets Phase I RELEASE NOTES April 2003 Department of Veterans Affairs VISTA System Design & Development Table of Contents 1. Introduction...1 1.1. Enhancements...
Laser Printed Prescription Labels with PMI Sheets Phase I RELEASE NOTES April 2003 Department of Veterans Affairs VISTA System Design & Development Table of Contents 1. Introduction...1 1.1. Enhancements...
6.0 Block Scheduling Guide
 6.0 Block Scheduling Guide 1 2 Table of Contents Accessing Dictionary to Create Blocks Adding Blocks in the Operating Room Dictionary Creating Blocks for Individual Physician or Physician Group Creating
6.0 Block Scheduling Guide 1 2 Table of Contents Accessing Dictionary to Create Blocks Adding Blocks in the Operating Room Dictionary Creating Blocks for Individual Physician or Physician Group Creating
ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes
 ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes # scored items 1. Regulations and Pharmacy Duties 35 A. Overview of technician
ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes # scored items 1. Regulations and Pharmacy Duties 35 A. Overview of technician
VistA Financial Annual Enhancements #1 eii RELEASE NOTES
 VistA Financial Annual Enhancements #1 eii RELEASE NOTES IB*2*457 June 2012 Department of Veterans Affairs VISTA Health Systems Design & Development Table of Contents 1. Introduction... 3 2. Patch Description
VistA Financial Annual Enhancements #1 eii RELEASE NOTES IB*2*457 June 2012 Department of Veterans Affairs VISTA Health Systems Design & Development Table of Contents 1. Introduction... 3 2. Patch Description
Pharmacy Point of Sale
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Point of Sale (ABSP) Version 1.0 Patch 42 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico Table
RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Point of Sale (ABSP) Version 1.0 Patch 42 Office of Information Technology (OIT) Division of Information Resource Management Albuquerque, New Mexico Table
PROVIDER BULLETIN NO. 08-03
 PROVIDER BULLETIN NO. 08-03 January 31, 2008 TO: Physicians, Mid-level Practitioners, and Clinics Administering Medications FROM: RE: Vivianne M. Chaumont, Director Division of Medicaid and Long-Term Care
PROVIDER BULLETIN NO. 08-03 January 31, 2008 TO: Physicians, Mid-level Practitioners, and Clinics Administering Medications FROM: RE: Vivianne M. Chaumont, Director Division of Medicaid and Long-Term Care
Frequently Asked Questions
 This document provides answers to some common questions regarding the reporting of controlled substances to the Hawaii (HI PDMP) database. A list of the questions answered in this document is provided
This document provides answers to some common questions regarding the reporting of controlled substances to the Hawaii (HI PDMP) database. A list of the questions answered in this document is provided
PHARMACY. billing module
 PHARMACY billing module Pharmacy Billing Module Coding...2 Basic Rules...2 Before You Begin...2 Reimbursement and Copayment...3 Point of Sale Billing...4 Billing for Split Prescriptions...5 Billing of
PHARMACY billing module Pharmacy Billing Module Coding...2 Basic Rules...2 Before You Begin...2 Reimbursement and Copayment...3 Point of Sale Billing...4 Billing for Split Prescriptions...5 Billing of
PC-ACE Pro32 Claim Management
 This document is a guide to assist PC-ACE Pro32 users in entering and managing Durable Medical Equipment (DME) claim information. This document includes: Managing Code Lists... 2 Claim Entry... 5 Managing
This document is a guide to assist PC-ACE Pro32 users in entering and managing Durable Medical Equipment (DME) claim information. This document includes: Managing Code Lists... 2 Claim Entry... 5 Managing
IDEXX Cornerstone* Initiating Inventory Management 8.3. Participant Workbook
 IDEXX Cornerstone* Initiating Inventory Management 8.3 Participant Workbook Proprietary Rights Notice Information in this document is subject to change without notice. Companies, names and data used in
IDEXX Cornerstone* Initiating Inventory Management 8.3 Participant Workbook Proprietary Rights Notice Information in this document is subject to change without notice. Companies, names and data used in
D( desired ) Q( quantity) X ( amount ) H( have)
 Name: 3 (Pickar) Drug Dosage Calculations Chapter 10: Oral Dosage of Drugs Example 1 The physician orders Lasix 40 mg p.o. daily. You have Lasix in 20 mg, 40 mg, and 80 mg tablets. If you use the 20 mg
Name: 3 (Pickar) Drug Dosage Calculations Chapter 10: Oral Dosage of Drugs Example 1 The physician orders Lasix 40 mg p.o. daily. You have Lasix in 20 mg, 40 mg, and 80 mg tablets. If you use the 20 mg
ARCIS/MRS MTF Customer WEB Portal MTF User Guide. Version: 20141011
 ARCIS/MRS MTF Customer WEB Portal MTF User Guide Version: 20141011 Table of Contents Overview:... - 3 - Chapter 1: Creating the SDF and RI ASCII Files in CHCS (Performed by Medical Records Technicians)..
ARCIS/MRS MTF Customer WEB Portal MTF User Guide Version: 20141011 Table of Contents Overview:... - 3 - Chapter 1: Creating the SDF and RI ASCII Files in CHCS (Performed by Medical Records Technicians)..
Email Setup Guide. network support pc repairs web design graphic design Internet services spam filtering hosting sales programming
 Email Setup Guide 1. Entourage 2008 Page 2 2. ios / iphone Page 5 3. Outlook 2013 Page 10 4. Outlook 2007 Page 17 5. Windows Live Mail a. New Account Setup Page 21 b. Change Existing Account Page 25 Entourage
Email Setup Guide 1. Entourage 2008 Page 2 2. ios / iphone Page 5 3. Outlook 2013 Page 10 4. Outlook 2007 Page 17 5. Windows Live Mail a. New Account Setup Page 21 b. Change Existing Account Page 25 Entourage
ODB Expanded Services Billing
 ODB Expanded Services Billing Contents Description of Services... 1 Reasons for a Clinical Intervention... 1 Outcomes... 2 Documentation Requirements... 2 Running the Update to Add Expanded Services PINs...
ODB Expanded Services Billing Contents Description of Services... 1 Reasons for a Clinical Intervention... 1 Outcomes... 2 Documentation Requirements... 2 Running the Update to Add Expanded Services PINs...
Pharmacist Quick Tips
 Pharmacist Quick Tips Kroll 9.1 Kroll 9.1 Contents Technician and Pharmacist Association... 1 Associating Technicians with Pharmacists... 1 Disassociating Technicians from Pharmacists... 1 Patients...
Pharmacist Quick Tips Kroll 9.1 Kroll 9.1 Contents Technician and Pharmacist Association... 1 Associating Technicians with Pharmacists... 1 Disassociating Technicians from Pharmacists... 1 Patients...
MEDICINES MANAGEMENT STANDARD OPERATING PROCEDURE (MMSOP018) Preparation of Medication Administration Record (MAR) Charts
 MEDICINES MANAGEMENT STANDARD OPERATING PROCEDURE (MMSOP018) Preparation of Medication Administration Record (MAR) Charts Any deviation in practice from this procedure must be discussed with the Community
MEDICINES MANAGEMENT STANDARD OPERATING PROCEDURE (MMSOP018) Preparation of Medication Administration Record (MAR) Charts Any deviation in practice from this procedure must be discussed with the Community
Login. 1. Enter your User ID into the User ID text box using the keyboard. 2. Use the Tab or Enter key to move to the PIN text box.
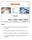 Login 1. Enter your User ID into the User ID text box using the keyboard. 2. Use the Tab or Enter key to move to the PIN text box. 3. Enter your PIN into the PIN text box using the keyboard. 4. Touch the
Login 1. Enter your User ID into the User ID text box using the keyboard. 2. Use the Tab or Enter key to move to the PIN text box. 3. Enter your PIN into the PIN text box using the keyboard. 4. Touch the
Drug Pricing System in Japan
 Reference Chuikyo - 1 June 6, 2012 Drug Pricing System in Japan April 2012 (Underlined phrases in red in this text: New features and modifications to the current pricing system adopted by the 2012 system
Reference Chuikyo - 1 June 6, 2012 Drug Pricing System in Japan April 2012 (Underlined phrases in red in this text: New features and modifications to the current pricing system adopted by the 2012 system
DAISY 4.2. New features. Electronic prescribing with erx Integrated credit card processing with DAISY InCharge
 DAISY 4.2 New features Electronic prescribing with erx Integrated credit card processing with DAISY InCharge DAISY 4.2 Update Release Notes 1 What s New in DAISY 4.2... 1 New Features... 1 Other Enhancements
DAISY 4.2 New features Electronic prescribing with erx Integrated credit card processing with DAISY InCharge DAISY 4.2 Update Release Notes 1 What s New in DAISY 4.2... 1 New Features... 1 Other Enhancements
Chapter 3.16: Archiving/Removing Terminated Customers
 Chapter 3.16: Archiving/Removing Terminated Customers Overview The chapter will explain how to archive and remove customers from your system that have become inactive and/or terminated. The customers can
Chapter 3.16: Archiving/Removing Terminated Customers Overview The chapter will explain how to archive and remove customers from your system that have become inactive and/or terminated. The customers can
BUSINESS AUTO ONLINE
 AMERICAN SERVICE INSURANCE COMPANY COMMERCIAL BUSINESS UNIT BUSINESS AUTO ONLINE TRAINING GUIDE WEBSITE: http://atlas-fin.com For your convenience, here is our Contact Information: PHONE #: (847) 472-6700
AMERICAN SERVICE INSURANCE COMPANY COMMERCIAL BUSINESS UNIT BUSINESS AUTO ONLINE TRAINING GUIDE WEBSITE: http://atlas-fin.com For your convenience, here is our Contact Information: PHONE #: (847) 472-6700
Accounts Receivable Service Charges
 Overview Accounts Receivable Service Charges Data Plus has the functionality to calculate and post service charges to client accounts based on certain settings in the system. The Terms Types must be set
Overview Accounts Receivable Service Charges Data Plus has the functionality to calculate and post service charges to client accounts based on certain settings in the system. The Terms Types must be set
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
Prior Authorization Guideline Guideline: PDP IBT Inj - Vivitrol Therapeutic Class: Central Nervous System Agents Therapeutic Sub-Class: Opiate Antagonist Client: 2007 PDP IBT Inj Approval Date: 2/20/2007
PHARMACEUTICAL MANAGEMENT PROCEDURES
 PHARMACEUTICAL MANAGEMENT PROCEDURES THE FORMULARY The purpose of Coventry Health Care s formulary is to encourage use of the most cost-effective drugs. The formulary is necessary because the cost of prescription
PHARMACEUTICAL MANAGEMENT PROCEDURES THE FORMULARY The purpose of Coventry Health Care s formulary is to encourage use of the most cost-effective drugs. The formulary is necessary because the cost of prescription
Completing your CTM Online - RESX Profile
 Completing your CTM Online - RESX Profile Your CTM Online profile information is used to complete online bookings and agent-assisted bookings. Note! Whether booking travel through Enterprise, or directly
Completing your CTM Online - RESX Profile Your CTM Online profile information is used to complete online bookings and agent-assisted bookings. Note! Whether booking travel through Enterprise, or directly
DRUG INFORMATION SYSTEM REFERENCE GUIDE. Department of Health and Wellness Province of Nova Scotia
 DRUG INFORMATION SYSTEM REFERENCE GUIDE Department of Health and Wellness Province of Nova Scotia Table of Contents EDUCATION AND TRAINING...1 EDUCATION... 1 THE DRUG INFORMATION SYSTEM...2 IMPLEMENTATION
DRUG INFORMATION SYSTEM REFERENCE GUIDE Department of Health and Wellness Province of Nova Scotia Table of Contents EDUCATION AND TRAINING...1 EDUCATION... 1 THE DRUG INFORMATION SYSTEM...2 IMPLEMENTATION
OUTLOOK 2010 TIPS TABLE OF CONTENTS 1. SEND A BLIND CARBON COPY MARQUETTE UNIVERSITY IT SERVICES
 OUTLOOK 2010 TIPS TABLE OF CONTENTS 1.Send a Blind Carbon Copy... 1 2. Change the view of the Outlook window... 2 3. Use Out of Office Assistant... 2 4. Create Rules... 4 5. Use Autocomplete... 5 6. Request
OUTLOOK 2010 TIPS TABLE OF CONTENTS 1.Send a Blind Carbon Copy... 1 2. Change the view of the Outlook window... 2 3. Use Out of Office Assistant... 2 4. Create Rules... 4 5. Use Autocomplete... 5 6. Request
INSTRUCTIONS FOR USE: OA-RX
 P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
Colorado Medical Assistance Program Web Portal Dental Claims User Guide
 Colorado Medical Assistance Program Web Portal Dental Claims User Guide The Dental Claim Lookup screen (Figure 1) is the main screen from which to manage Dental claims. It consists of different sections
Colorado Medical Assistance Program Web Portal Dental Claims User Guide The Dental Claim Lookup screen (Figure 1) is the main screen from which to manage Dental claims. It consists of different sections
Automating the Pharmacy Medication Cycle in Acute Care Settings
 Automating the Pharmacy Medication Cycle in Acute Care Settings Costs, Benefits and Potential Unintended Consequences Enterprise Information Systems Steering Committee Nursing Informatics Committee and
Automating the Pharmacy Medication Cycle in Acute Care Settings Costs, Benefits and Potential Unintended Consequences Enterprise Information Systems Steering Committee Nursing Informatics Committee and
Items. Gain detailed knowledge of the item record and learn how to work with items in SuccessFactors Learning.
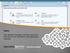 Items Gain detailed knowledge of the item record and learn how to work with items in SuccessFactors Learning. Items: Main Concepts An item is an assignable unit that can be tracked in the SuccessFactors
Items Gain detailed knowledge of the item record and learn how to work with items in SuccessFactors Learning. Items: Main Concepts An item is an assignable unit that can be tracked in the SuccessFactors
NOTE: You will need to receive the trade item into your inventory through the receiving system to complete any trade-in activity.
 RETAIL POINT OF SALE PROGRAM NAME: PSENTRY MENU OPTION TITLE: Retail Point Of Sale MAIN MODULE: SALES/SHIPPING HELP KEY ACTIVE: YES PROGRAM OVERVIEW The Point of Sale System is used to process "over the
RETAIL POINT OF SALE PROGRAM NAME: PSENTRY MENU OPTION TITLE: Retail Point Of Sale MAIN MODULE: SALES/SHIPPING HELP KEY ACTIVE: YES PROGRAM OVERVIEW The Point of Sale System is used to process "over the
Frequently Asked Questions
 This document provides answers to some common questions regarding the reporting of controlled substances to the New Hampshire (PDMP) database. A list of the questions answered in this document is provided
This document provides answers to some common questions regarding the reporting of controlled substances to the New Hampshire (PDMP) database. A list of the questions answered in this document is provided
Cerner PowerChart/FirstNet Home Medication History
 Cerner PowerChart/FirstNet Home Medication History Training Script Training and Education Services, IST Division Table of Contents INTRODUCTION...5 ICONS...6 TERMINOLOGY...6 SIGNING IN...8 OPENING THE
Cerner PowerChart/FirstNet Home Medication History Training Script Training and Education Services, IST Division Table of Contents INTRODUCTION...5 ICONS...6 TERMINOLOGY...6 SIGNING IN...8 OPENING THE
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PC (CO) - Insulin Delivery Systems Therapeutic Class: Hormones and Synthetic Substitutes Therapeutic Sub-Class: Insulin Delivery Systems Client: CO Approval Date:
Prior Authorization Guideline Guideline: PC (CO) - Insulin Delivery Systems Therapeutic Class: Hormones and Synthetic Substitutes Therapeutic Sub-Class: Insulin Delivery Systems Client: CO Approval Date:
Pharmacy Point of Sale
 RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Point of Sale (ABSP) Version 1.0 July 2001 Office of Information Technology (OIT) Division of Information Resources Albuquerque, New Mexico Pharmacy Point
RESOURCE AND PATIENT MANAGEMENT SYSTEM Pharmacy Point of Sale (ABSP) Version 1.0 July 2001 Office of Information Technology (OIT) Division of Information Resources Albuquerque, New Mexico Pharmacy Point
DRUG ACCOUNTABILITY/ INVENTORY INTERFACE (DA)
 DRUG ACCOUNTABILITY/ INVENTORY INTERFACE (DA) TECHNICAL MANUAL/ SECURITY GUIDE Version 3.0 October 1997 (Revised May 2009) Department of Veterans Affairs Office of Enterprise Development Revision History
DRUG ACCOUNTABILITY/ INVENTORY INTERFACE (DA) TECHNICAL MANUAL/ SECURITY GUIDE Version 3.0 October 1997 (Revised May 2009) Department of Veterans Affairs Office of Enterprise Development Revision History
CPOE Medication Order Entry
 Ordering Specific Medications Any medication can be ordered individually. These can be searched by either the brand or generic name. NOTE: The search field setting of either starts with or contains can
Ordering Specific Medications Any medication can be ordered individually. These can be searched by either the brand or generic name. NOTE: The search field setting of either starts with or contains can
Meditech 6.0 BMV Training Manual: ED Introduction to BMV Process
 Meditech 6.0 BMV Training Manual: What is the emar? The emar (Electronic Medication Administration Record) is utilized to capture and edit documentation for medication administrations in an electronic
Meditech 6.0 BMV Training Manual: What is the emar? The emar (Electronic Medication Administration Record) is utilized to capture and edit documentation for medication administrations in an electronic
IHS Pharmacy-Automated Dispensing Interface System (BOP)
 RESOURCE AND PATIENT MANAGEMENT SYSTEM IHS Pharmacy-Automated Dispensing Interface System (BOP) User Manual Version 1.0 July 2005 Office of Information Technology Albuquerque, New Mexico User Manual i
RESOURCE AND PATIENT MANAGEMENT SYSTEM IHS Pharmacy-Automated Dispensing Interface System (BOP) User Manual Version 1.0 July 2005 Office of Information Technology Albuquerque, New Mexico User Manual i
This exhibit describes how to upload project information from Estimator (PC) to Trns.port PES (server). Figure 1 summarizes this process.
 Facilities Development Manual Chapter 19 Plans, Specifications and Estimates Section 5 Estimates Wisconsin Department of Transportation Exhibit 10.5 Uploading project from Estimator to Trns port PES September
Facilities Development Manual Chapter 19 Plans, Specifications and Estimates Section 5 Estimates Wisconsin Department of Transportation Exhibit 10.5 Uploading project from Estimator to Trns port PES September
Fully Integrated, Automated Employee Time Clock
 TimeClock Fully Integrated, Automated Employee Time Clock RentWorks Version 4 TimeClock User Guide Table of Contents Overview...1 Revision History...2 Installation...3 Clock Setup...4 Preferences...7 Employee
TimeClock Fully Integrated, Automated Employee Time Clock RentWorks Version 4 TimeClock User Guide Table of Contents Overview...1 Revision History...2 Installation...3 Clock Setup...4 Preferences...7 Employee
PATIENT ADVOCATE TRACKING SYSTEM (PATS) USER GUIDE
 PATIENT ADVOCATE TRACKING SYSTEM (PATS) USER GUIDE Version 1.5 May 2007 Revised June 2009; Revised October 2009; Revised July 2010; Revised July 2012 Revised March 2013 Department of Veterans Affairs Office
PATIENT ADVOCATE TRACKING SYSTEM (PATS) USER GUIDE Version 1.5 May 2007 Revised June 2009; Revised October 2009; Revised July 2010; Revised July 2012 Revised March 2013 Department of Veterans Affairs Office
Service Programs. Requirements
 Requirements The Service Programs feature allows you to keep track of multiple services that need to be done at varying intervals during the course of a year using a range type of either eligibility dates
Requirements The Service Programs feature allows you to keep track of multiple services that need to be done at varying intervals during the course of a year using a range type of either eligibility dates
April 10, 2008 Montana Healthcare Programs Update
 April 10, 2008 Montana Healthcare Programs Update THE FOLLOWING INFORMATION UPDATES AND REPLACES THE PROVIDER NOTICE DATED MARCH 10, 2008. NEW OR REVISED INFORMATION IS MARKED WITH A CHANGE BAR IN THE
April 10, 2008 Montana Healthcare Programs Update THE FOLLOWING INFORMATION UPDATES AND REPLACES THE PROVIDER NOTICE DATED MARCH 10, 2008. NEW OR REVISED INFORMATION IS MARKED WITH A CHANGE BAR IN THE
Survey of Medical Care Activities in Public Health Insurance
 Survey of Medical Care Activities in Public Health Insurance Outline of Survey 1 Objective This survey is to perceive the situation of medical treatment, diseases and injuries, dispensing, and use of drugs
Survey of Medical Care Activities in Public Health Insurance Outline of Survey 1 Objective This survey is to perceive the situation of medical treatment, diseases and injuries, dispensing, and use of drugs
Resident s Guide to Inpatient Diabetes
 Resident s Guide to Inpatient Diabetes 1. All patients with diabetes of ANY TYPE, regardless of reason for admission, must have a Hemoglobin A1C documented in the medical record within 24 hours of admission
Resident s Guide to Inpatient Diabetes 1. All patients with diabetes of ANY TYPE, regardless of reason for admission, must have a Hemoglobin A1C documented in the medical record within 24 hours of admission
Eastern Bank TreasuryConnect Money Transfer User Manual
 Eastern Bank TreasuryConnect Money Transfer User Manual This user manual provides instructions for setting up new users and accessing services found within the Money Trans Admin group and the Money Transfer
Eastern Bank TreasuryConnect Money Transfer User Manual This user manual provides instructions for setting up new users and accessing services found within the Money Trans Admin group and the Money Transfer
Calculating Drug Dosages
 Calculating Drug Dosages Calculating Doses from Prepared Strength Liquids, Tablets, and Capsules Calculating With Proportions 1. Convert to a consistent unit of measure. 2. Set up a proportion: Original
Calculating Drug Dosages Calculating Doses from Prepared Strength Liquids, Tablets, and Capsules Calculating With Proportions 1. Convert to a consistent unit of measure. 2. Set up a proportion: Original
Humulin R (U500) insulin: Prescribing Guidance
 Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Types of insulin and How to Use Them
 Diabetes and Insulin Pumps Amy S. Pullen Pharm.D ISHP Spring Meeting April 2012 Objectives Describe the different types of insulin used in diabetes Identify the types of insulin that are compatible with
Diabetes and Insulin Pumps Amy S. Pullen Pharm.D ISHP Spring Meeting April 2012 Objectives Describe the different types of insulin used in diabetes Identify the types of insulin that are compatible with
SHINE Study Ordering Instructions for Investigators SUNY Downstate
 General Directions SHINE Study Ordering Instructions for Investigators SUNY Downstate 1) Call Spiro Demetis to get patient admitted to ICU ASAP. Cell: 646-261-5730; Pager: 917-760-1653. Let him know that
General Directions SHINE Study Ordering Instructions for Investigators SUNY Downstate 1) Call Spiro Demetis to get patient admitted to ICU ASAP. Cell: 646-261-5730; Pager: 917-760-1653. Let him know that
